DE GRUYTER Current Directions in Biomedical Engineering 2020;6(3): 20203114
Open Access. © 2020 Paula Rosam et al., published by De Gruyter. This work is licensed under the Creative Commons Attribution 4.0 License.
Paula Rosam*, Michael Stiehm, Finja Borowski, Jonas Keiler, Andreas Wree, Alper Öner,
Klaus-Peter Schmitz and Wolfram Schmidt
Development of an in vitro measurement
method for improved assessment of the side
branch expansion capacity
Abstract: The expansion capacity and accessibility of the
side branch is essential for the stenting of complex
bifurcations. Since previous measurement methods only
provide limited information based on geometrical data of
stent cells, a new measurement approach was developed
which considers the mechanical deformation capacity of the
stent design. This approach provides essential information on
the stent with regard to the application of bifurcation
stenting. Four different commercially available coronary
stents (nominal diameter 3.0 mm) were dilated and a central
strut cell was over-expanded by means balloon catheters of
increasing nominal diameter (2.0 to 5.0 mm). After balloon
inflation, the remaining cell size was investigated for
maximum cell diameter and strut fractures. Large expansion
capacity without cell damage is taken as a measure of the
accessibility of the side branch. In none of the expansion
experiments the desired target size could be achieved, which
is due to the elastic recoil of the stent cells. Deviations from
the target diameter between 14-38% were determined.
However, larger diameters also showed a constriction of the
balloon, so that in some cases the target diameter could not
be achieved at all. No strut fractures occurred even at
maximum balloon diameter and pressure (5.0 mm non-
compliant balloons). As a result the side branch accessibility
differs depending on the individual stent designs. No
particular risk for the stent was found by extensive over-
dilatation.
Keywords: stents, side branch accessibility, expansion
capacity, strut deformation, bifurcation
https://doi.org/10.1515/cdbme-2020-3114
1 Introduction
The term over-expansion capacity of stent designs is used to
evaluate how the stent geometry changes when over-
dilatation is performed [1,2]. This parameter is also
suggested in the context of bifurcation stenting for the
selection of suitable stents [3], including a description of how
side branch accessibility changes when over-dilatation occurs
(cell opening).
This is a common approach in various kinds of
bifurcation stenting, in which the side branch has to be
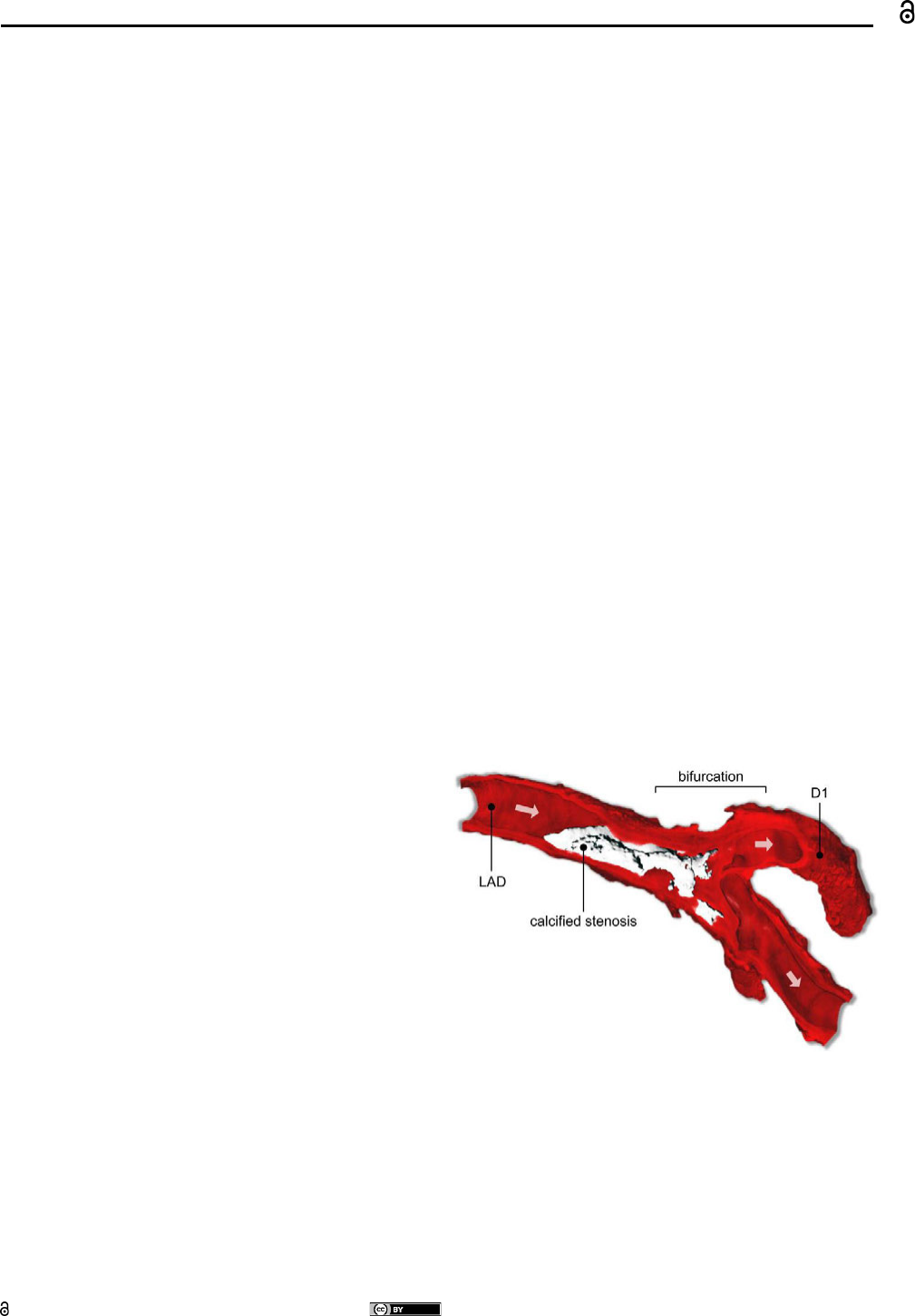
stented additionally. High grade stenoses as seen in figure 1
obstruct the blood flow extensively along the bifurcation and
must be treated.
Figure 1: MicroCT scan of an anatomical bifurcation preparation
from the Department of Anatomy (Rostock). Arrows are indicating
flow direction. LAD: left anterior descending artery, D1: (diagonal)
side branch.
This side-branch accessibility was tested by an approach
similar to above-mentioned, namely without extra
mechanical stress by a penetrating balloon / stent system
(catheter) for the “side-branch-struts”.
_____
*Corresponding author: Paula Rosam: Institute for
ImplantatTechnology and Biomaterials e.V., Friedrich-Barnewitz-
Str. 4, 18119 Rostock-Warnemünde, Germany,
mail: paula.rosam@uni-rostock.de
Finja Borowski, Michael Stiehm, Klaus Peter Schmitz: Institute
for ImplantatTechnology and Biomaterials e.V., 18119 Rostock-
Warnemünde, Germany
Jonas Keiler, Andreas Wree: Rostock University Medical Center,
Department of Anatomy, Rostock, Germany
Alper Öner: Rostock University Medical Center, Department for
Cardiology, Rostock, Germany
Wolfram Schmidt: Institute for Biomedical Engineering, Rostock
University Medical Center, 18119 Rostock-Warnemünde,
Germany

Paula Rosam et al., Development of an in vitro measurement method for improved assessment of the side branch expansion capacity — 2
However, the result is only one criterion for success. If the
passage with wire and catheter is successful, the dilatation of
the side branch also causes a considerable deformation of the
cell. The question arises how far a stent cell can be dilated
without breaking and thus not becoming a risk to balloon and
vessel. Balloon ruptures at sharp-edged strut fractures
prevent the expansion, but also the safe evacuation and
withdrawal of the balloon. This aspect has already been
investigated for bioresorbable scaffolds (BVS Absorb,
Abbott Vascular) and has been evaluated as critical [4].
There is a lack of systematic studies for permanent stents.
2 Material and method
For the following coronary permanent drug eluting stents
DES (each n = 1)
▪ Abbott Xience Sierra 3.0/15
▪ Boston Scientific Promus PREMIER Select 3.0/16
▪ BIOTRONIK Orsiro 3.0/15
▪ Medtronic Resolute Integrity 3.0/15
the resulting cell sizes were determined after probing one
mesh each with a guide wire (ChoiCE
TM
PT 0.014" x 182 cm,
REF H74912160011, Boston Scientific). This mesh was then
dilated using ascending diameter balloon catheters (see Table
1). The balloon pressure corresponds to the nominal pressure
of the balloon (NP) and the expected maximum balloon
diameter corresponds to the nominal diameter of the balloon.
The used non-compliant balloons for larger test diameters
allow at the same time maximum pressure and represent a
highly challenging case. The procedure is shown in Figure 2.
Table 1: Balloon catheters used for over-expansion of the stent
cells; balloon parameters are: dimension, balloon pressure and
compliance
Ballon catheter
Dimension
[mm]
Ballon pressure
[atm]
comment
Biotronik Pantera
2.0/20
7
semi-
compliant
Biotronik Pantera
3.0/20
7
semi-
compliant
Biotronik Pantera
3.5/25
7
semi-
compliant
Biotronik Pantera
LEO
4.0/30
14
non-
compliant
Biotronik Pantera
LEO
5.0/30
14
non-
compliant
Figure 2: Xience Sierra 3.0/15 with guide wire ChoiCE PT and
balloon Pantera 2.0/20 in a Y-silicone model
After balloon expansion and retraction, the cell size was
determined optically (light microscope SZX16, magnification
1.25x, Olympus). The maximum inscribed circle was
measured with image analysis software (Stream Start, V1.7,
Olympus). The diameter of this circle was used as a measure
of the achieved cell size (see Figure 3).
Figure 3: Xience Sierra 3.0/15 - Mesh expanded with balloon
Pantera Leo 5.0/30. Diameter measurement of the inscribed circle
was used as a measure for the mesh size
Due to the three-dimensional deformation of the stent, a strict
vertical top view was not possible. Horizontal distortions
may occur due to the position of the stents under the
microscope. As long as the inscribed circle is vertically
limited by the stent structure, the diameter of the circle is still
correct, as no distortions occur in this direction.
3 Results
During diameter increase of the dilating balloon, an
increasing constriction of the balloon through the mesh was
detected. This effect was strongest at the maximum diameter
(d = 5.0 mm)
Paula Rosam et al., Development of an in vitro measurement method for improved assessment of the side branch expansion capacity — 3
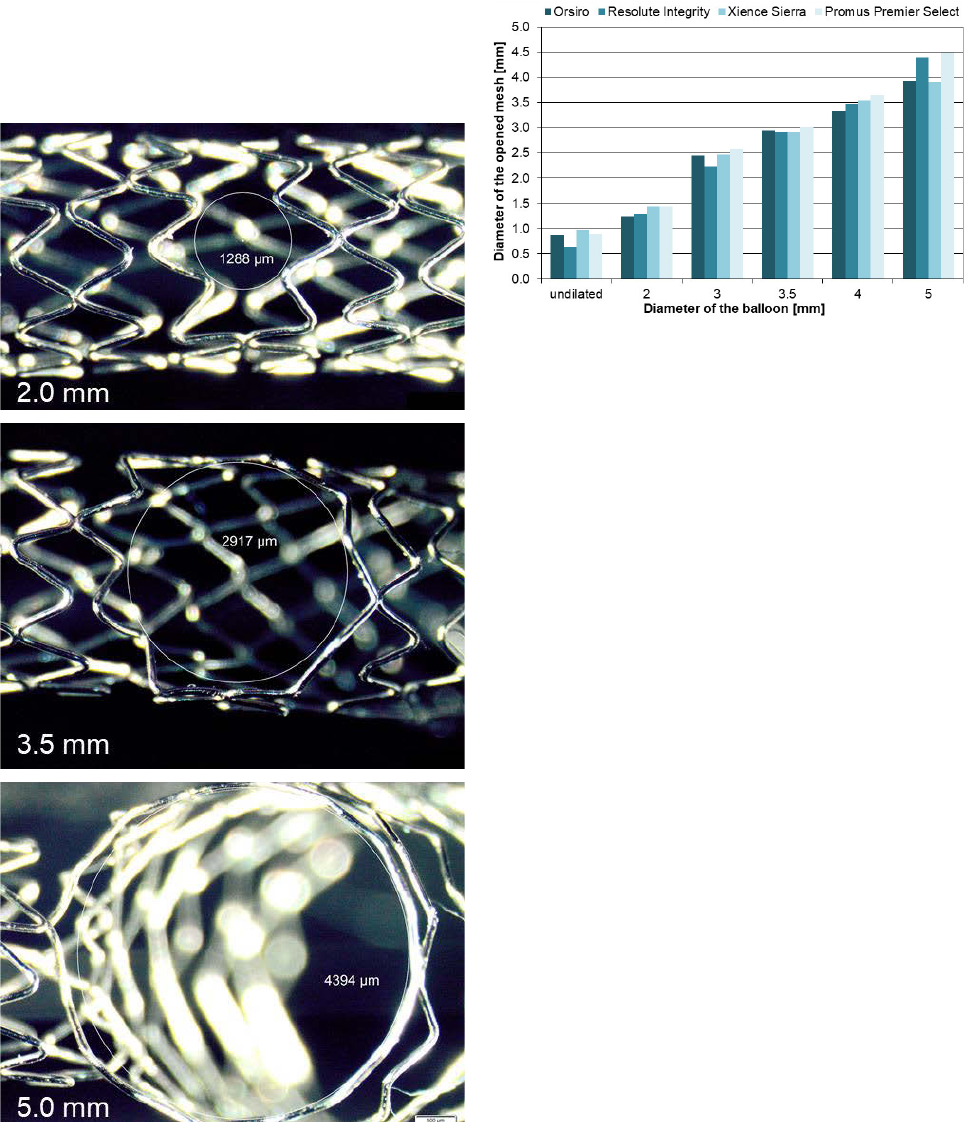
The following Figure 4 shows an example of the increasing
deformation of a stent mesh when stretched from 2.0 to
5.0 mm. As a result of the severe deformation of the cell, in
none of the investigated stents were torn. However, at
maximum expansion an almost circular cell without visible
reserve for further diameter increase was seen. A strong
deformation of adjacent cells also occurred.
Figure 4: Side branch expansion of the Resolute Integrity 3.0/15.
Increasing deformation of a stent mesh when stretched with
balloons from 2.0 to 5.0 mm.
The cell diameters reached during the over-expansion process
of all stents are shown in Figure 5.
Figure 5: Stent cell diameter in relation to the diameter of the
expanding balloon.
4 Discussion
The achieved cell diameters were mostly about 0.5 mm
smaller than the targeted diameters. On the one hand this is
due to the constriction of the balloons which was especially
seen with large balloon diameters. This is remarkable
because especially for the diameters 4.0 and 5.0 mm non-
compliant balloons were used, which were dilated with high
balloon pressure (14 atm). The result indicates considerable
mechanical stress on the cell. On the other hand, every plastic
deformation is accompanied by an elastic component, which
leads to a restoring movement after mechanical loading.
Thus, the cell size also shows elastic recoil.
At the target diameter of 2.0 mm, the difference to the
achieved mesh size was maximal (28 - 38%). The mesh
structure was only slightly deformed and the proportion of
elastic deformation dominates. As the target diameter
increases, the difference initially becomes smaller (at
3.5 mm: 14 - 17%) since the proportion of plastic
deformation increases. At the largest target diameter of
5.0 mm, there is a clear obstruction of the balloon expansion
(constriction), so that the stent mesh was not expanded to this
target diameter.
Figure 6 provides an overview of the measured
deviations from the target diameter.
Over-expansion of stent cells larger than the diameter of
the main branch is of little relevance to clinical practice.
However, since the stents investigated were examined at
nominal diameter, they have a capacity for over-dilatation up
to 3.5 - 4.75 mm according to the manufacturer's
specifications [5–8]

Paula Rosam et al., Development of an in vitro measurement method for improved assessment of the side branch expansion capacity — 4
Figure 6: Difference between target diameter and achieved mesh
size as a function of the diameter of the expanding balloon
On the other hand since it is assumed that secondary
branches are smaller than the main vessel, dilatation up to
5.0 mm is a very extreme case. The absence of strut failure is
a sign of high stent quality and material strength. It facilitates
low-risk use in bifurcation stenting from mechanical point of
view. The deformation of adjacent cells including the kinking
of the stent would probably have been less severe in a closed
vascular model, thus representing a worst-case scenario. In
such a model, however, the measurement could not have
been performed with the same accuracy.
The novel investigation shows a new relevance
compared to previous measurements of side branch
accessibility. In a common benchmark of different stents, as
it is also performed in our test laboratory, only the size of the
expanded mesh is measured after conventional stent
dilatation to nominal pressure. The corresponding
measurement results are shown in Table 2.
Table 2: Tested stents and their side branch accessibility
Tested Stents
side branch accessibility [mm]
Abbott Xience Sierra
3.0/15
0.967
Boston Scientific Promus
PREMIER Select 3.0/16
0.891
BIOTRONIK Orsiro
3.0/15
0.871
Medtronic Resolute Integrity
3.0/15
0.640
These values show no correlation with the new in vitro
measurements. They are much lower than needed for probing
the side branch.
However, since an additional widening and deformation
of the meshes occurs, especially with regard to the stenting of
bifurcations, the side branch expansion capacity should also
be measured in the future.
Author Statement
Research funding: Financial support by the European
Regional Development Fund (ERDF) and the European
Social Fund (ESF) within the collaborative research between
economy and science of the state Mecklenburg-Vorpommern
within the project “TheraMagna” is gratefully acknowledged.
Conflict of interest: Authors state no conflict of interest.
References
[1] Foin N, Sen S, Allegria E et al. Maximal expansion capacity
with current DES platforms: a critical factor for stent selection
in the treatment of left main bifurcations? EuroIntervention
2013; 8: 1315–1325; DOI: 10.4244/EIJV8I11A200
[2] Ng J, Foin N, Ang HY et al. Over-expansion capacity and
stent design model: An update with contemporary DES
platforms. Int J Cardiol 2016; 221: 171–179; DOI:
10.1016/j.ijcard.2016.06.097
[3] Foin N, Alegria E, Sen S et al. Importance of knowing stent
design threshold diameters and post-dilatation capacities to
optimise stent selection and prevent stent
overexpansion/incomplete apposition during PCI. Int J
Cardiol 2013; 166: 755–758; DOI:
10.1016/j.ijcard.2012.09.170
[4] Foin N, Lee R, Bourantas C et al. Bioresorbable vascular
scaffold radial expansion and conformation compared to a
metallic platform: insights from in vitro expansion in a
coronary artery lesion model. EuroIntervention 2016; 12:
834–844; DOI: 10.4244/EIJV12I7A138
[5] Medtronic Inc. Resolute Integrity™ Zotarolimus-Eluting
Coronary Stent System. Instructions for Use. 2015
[6] BIOTRONIK. Orsiro: Ultrathin struts. Superior patient
outcomes.; 2019
[7] Boston Scientific. Promus PREMIER™ - Directions for Use (if
your product does not contain CLIPIT™ Hypotube Clips):
91054414. Aufl.; 2015
[8] Abbott Vascular. Abbott Vascular XIENCE Xpedition,
XIENCE Xpedition SV, and XIENCE Xpedition LL Everolimus
Eluting Coronary Stent System - U.S. 2015
0
5
10
15
20
25
30
35
40
2 3 3.5 4 5
Deviation of the opened meshes from
the target diameter in %
Target diameter of the balloon [mm]
Orsiro Resolute Integrity Xience Sierra Promus Premier Select
