
MEDICARE MODERNIZATION ACT FINAL GUIDELINES -- FORMULARIES
CMS Strategy for Affordable Access to Comprehensive Drug Coverage
Guidelines for Reviewing Prescription Drug Plan Formularies and Procedures
1.
Purpose of the Guidance
This paper is final guidance on how CMS will review Medicare prescription drug benefit plans
to assure that beneficiaries receive clinically appropriate medications at the lowest possible cost.
Two key requirements in the Medicare Modernization Act (MMA) are to assure that drug plans
provide access to medically necessary treatments for all and do not discriminate against any
particular types of beneficiaries, and to encourage and support the use of approaches to drug
benefit management that are proven and in widespread use in prescription drug plans today. The
goal is for plans to provide high-quality cost-effective drug benefits by negotiating the best
possible prices and using effective drug utilization management techniques. This goal can be
achieved through a CMS drug benefit review strategy that facilitates appropriate beneficiary
access to all medically necessary Part D covered drugs along with plan flexibility to develop
efficient benefit designs, thus bringing drug benefit strategies that are already providing effective
coverage to millions of seniors and people with a disability to the Medicare population. Our
formulary review process focuses on three areas:
1. Pharmacy and Therapeutics (P&T) committees. CMS will require P&T committees to rely
on widely-used best practices, reinforced by MMA standards. CMS oversight of these
processes will assure that plan formularies are designed to provide appropriate, up-to-date
access for beneficiaries and give plans the flexibility to offer benefit designs that provide
affordable access to medically necessary drugs.
2. Formulary lists. CMS’ review of plan formularies will look to best practices in existing
drug benefits serving millions of seniors and people with disabilities to ensure
appropriate access for Medicare beneficiaries. CMS will evaluate formulary classification
systems as well as the actual list of drugs included in the formulary, using existing widely
used classification systems and drug plans as checks.
3. Benefit management tools. CMS will compare plans’ use of benefit management tools,
like prior authorization, to the way these tools are used in existing drug plans, to ensure
that they are being applied in a clinically appropriate fashion. We have protected
beneficiary rights by putting appropriate appeals and exceptions standards in the final
regulations and by reviewing processes that plans use to provide timely access. In
developing this approach, CMS has looked to existing national drug benefit management
standards and guidelines that underlie drug plans that are currently providing effective
coverage, as well as a variety of examples of such drug plans.

CMS has developed the final rule for the new Medicare drug benefit based on extensive public
comments on how best to provide access to up-to-date medical treatments for all beneficiaries at
the lowest possible cost. This paper sets forth the approach that will be used in conjunction with
the final regulation to promote transparency, predictability, and effective implementation of the
law in conjunction with our final rule.
2.
Background
The addition of a prescription drug benefit to Medicare as a result of the MMA represents a
landmark change to the Medicare program, a change that will significantly improve the
healthcare coverage available to millions of Medicare beneficiaries. In the final regulation, we
have included policies, such as formulary requirements and exceptions and appeals processes, to
assure that beneficiaries have access to covered drugs that are medically necessary for their
condition while enabling plans to design and manage their formularies to provide the most
affordable benefit possible. We are also adjusting the payments to drug plans based on the
expected costs of their enrollees, as well as implementing many other steps to limit the financial
risk facing drug plans. Together, our goal is to provide a foundation for fair competition to offer
high-quality coverage at the lowest cost to all types of Medicare beneficiaries, and to reward
plans that focus on this critical policy goal.
The MMA is designed to encourage prescription drug plans and Medicare Advantage
prescription drug plans that meet the law’s requirements to offer comprehensive prescription
drug plan options for Medicare beneficiaries by providing flexibility for plan design and
management. This flexibility is modeled after the way most Americans, including millions of
seniors and people with disabilities, receive drug benefits today through federal and private-
sector retiree coverage, as well as State Medicaid programs. How much beneficiaries save
depends on how a plan’s formulary is structured and the benefit is operated. The goal of this
program, however, is not to save money on prescription drug costs at the expense of appropriate
medical care. Appropriate medical care would not be possible if plans actively sought to
discourage enrollment by beneficiaries with high, expected drug costs.
Consequently, CMS seeks to implement a strategy to ensure that formularies and utilization
management tools are consistent with effective practices in drug benefit management today, in
conjunction with many other steps we are implementing to appropriately compensate plans for
covering beneficiaries with relatively high expected drug costs. CMS oversight will ensure that
Part D plans operate in accordance with this strategy. We will compare proposed Part D
formularies using current best practices for developing and maintaining a formulary’s drug
categories and classes, and will support the use of USP model categories and classes for plans
that choose to use them (plans are not required to do so). However, because drug classes alone,
whether detailed or general, are not sufficient to determine whether beneficiaries have adequate
access, we also will review the drug plans’ formularies and benefits to identify discriminating
practices. Under Section 1860D-11(e)(2)(D) of the Social Security Act, a plan design will be
approved only if "the Secretary does not find that the design of the plan and its benefits
(including any formulary and tiered formulary structure) are likely to substantially discourage
enrollment by certain part D eligible individuals under the plan." Thus, even if CMS concludes
that a plan’s therapeutic categories and classes are robust, our review may find the plan design
violates this statutory provision if some other aspect of the plan’s benefit design is problematic.
CMS intends to encourage and approve formularies and benefit management approaches that are
already in widespread use to provide drug coverage to millions of seniors and people with
disabilities today. We will consider the structure and use of an organization’s P&T committee, as
well as the structure of the formulary and the policies and procedures for providing access to
both formulary and non-formulary drugs. Since drug utilization management activities are as
important as the list of drugs in the formulary in providing access to high quality pharmaceutical
care for all categories of beneficiaries, we will use checks based on commonly-used best
practices to review those policies and procedures to ensure beneficiary access to Part D covered
prescription drugs that are medically necessary for their course of treatment. Approved
formularies for Part D contractors will be available for beneficiaries in time for them to consider
their options prior to enrollment. We anticipate that drug plans that follow commonly used best
practices will have little difficulty with these checks.
3.
Guiding Principles for CMS Formulary and Benefit Review Strategy
A formulary is more than a list of approved medications. A formulary must consist of drugs that
will provide patients with a clinically appropriate medication for the course of treatment
established by the physician. Consistent with industry standards/practices, the formulary is
supported by a system of care management tools to consistently provide patients with access to
medications that have been demonstrated to be safe, effective, and affordable, while maintaining
and improving quality patient care. To ensure that Medicare prescription drug plans are
following best practices, the CMS formulary review will follow four important principles.
Principle #1 – Rely on Existing Best Practices: CMS’ review will rely on widely recognized
best practices for existing drug benefits serving millions of seniors and people with
disabilities, to ensure appropriate access for Medicare beneficiaries.
Principle #2 -- Provide Access to Medically Necessary Drugs: We will require that drug
plans provide access to Part D drugs determined to be medically necessary.
Principle #3 -- Flexibility: CMS will allow plans to be flexible in their benefit designs to
promote real beneficiary choice while protecting beneficiaries from discrimination.
Principle #4 – Administrative Efficiency: CMS will develop a streamlined process to conduct
effective reviews of plan offerings within a compressed period of time.

4. Strategic Approach
A. P & T Committee Review
We believe that current best practices for P&T committees should be applied when developing
and administering P&T committees for the Medicare drug benefit. Incorporating best practice
philosophies, along with inclusion of the MMA requirements, allows for a drug benefit that is
clinically robust.
Rationale
CMS oversight of the P&T committee process is an important requirement of the MMA to
ensure plans offer a comprehensive drug benefits. Operated under appropriate guiding principles,
a P&T committee is a forum for an evidence-based formulary review process that establishes
policies on the use of drug products and therapies, and identifies drug products and therapies that
are medically appropriate and cost-effective. P&T committees must meet best practices
consistent with those contained in several widely accepted guidelines for P&T management.
CMS standards and guidelines for the P&T activities will help ensure that formulary decisions
are based on scientific and economic considerations that achieve appropriate, safe and cost
effective drug therapy, and that the P&T committee has a key role in defining policies for
utilization management activities such as access to non-formulary drugs, prior authorization, step
therapy, quantity limitations, generic substitution, and therapeutic interchange protocols to assure
that products and therapies, such that these tools are used to drive medically appropriate and
cost-effective access to Part D covered drugs. The P&T committee will also be expected to
analyze and recommend, where appropriate, regional variations of national best practices.
These standards will be clearly articulated in the plan applications and our contracts with
Medicare prescription drug plans. They will also be integrated into the CMS management and
oversight of Part D plans after January 2006, to assure that the P&T rules are maintained and
followed.
Approach
CMS will require that plans assure the implementation and use of a P&T committee consistent
with the pharmacy benefit management principles outlined and expressed by the American
Society of Health System Pharmacists (ASHP Statement on the Pharmacy and Therapeutics
Committee, Am J Hosp Pharm. 1992, http://www.ashp.org/bestpractices/formulary-
mgmt/Form_St_PTComm.pdf), or the Principles of a Sound Drug Formulary System October
2000, www.amcp.org , a consensus document endorsed by the Academy of Managed Care
Pharmacy, American Association of Retired Persons, the Alliance of Community Health Plans,
the American Medical Association, the American Society of Health-System Pharmacists, the
Department of Veteran Affairs, the National Business Coalition on Health, and the U.S.
Pharmacopeia.

The requirements listed below are represented as ‘BP’ for best practice (or Industry Standard
Practice) where they have been drawn from commercial best practices consistent with these
nationally recognized P&T guidelines, and are represented as ‘MMA’ where the requirements
support the unique provisions of the MMA.
Membership
• P&T committee members must represent various clinical specialties that adequately
represent the needs of plans beneficiaries (i.e., include representation of “high
volume specialists” in the standard terminology of the industry). (BP)
• A majority of the P&T committee members must be practicing physicians,
practicing pharmacists or both. (BP)
• At least one P&T committee practicing pharmacist and one practicing physician
must be experts in the care of elderly or disabled persons. (MMA)
• At least one P&T committee practicing pharmacist and one practicing physician
must be independent and free of conflict with respect to the plan and
pharmaceutical manufacturers. (MMA)
Conflict of Interest
• P&T committee members should sign a conflict of interest statement revealing
economic or other relationships with entities affected by drug coverage decisions
that could influence committee decisions. (BP)
Meeting Administration
• P&T committee should meet on a regular basis, and not less frequently than on a
quarterly basis. (BP)
• P&T committee decisions regarding formulary development or revision must be
documented in writing. (BP)
Formulary Management
• P&T committee must review for clinical appropriateness, the practices and policies
for formulary management activities, such as prior authorizations, step therapies,
quantity limitations, generic substitutions and other drug utilization activities that
affect access. (BP)
• Formulary management decisions must be based on scientific evidence, and may
also be based on pharmacoeconomic considerations that achieve appropriate, safe
and cost effective drug therapy. (BP)
• The P&T committees will be required to establish and document procedures to
assure appropriate drug review and inclusion. (BP)
• Clinical decisions by the P&T committee should be based on scientific evidence and
standards of practice, including peer reviewed medical literature, well-established
clinical practice guidelines and pharmacoeconomic studies as well as other
sources of appropriate information. (BP)
• Drugs’ therapeutic advantages in terms of safety and efficacy must be considered
when selecting formulary drugs and placing them into formulary tiers. (MMA)
• The P&T committee will make a reasonable effort to review a new chemical entity
within 90 days, and will make a decision on each new chemical entity within 180

days of its release onto the market, or a clinical justification will be provided if
this timeframe is not met. These timeframes also include the review of products
for which new FDA indications have been approved. We set this timeframe in
response to public comment on our proposed guidance, but note that plans must
make access to new drugs available to enrollees when medically appropriate via
exceptions processes even before this deadline. (BP)
• P&T committee will approve inclusion or exclusion of the therapeutic classes in the
formulary on an annual basis. (MMA)
• Formulary therapeutic categories and classes may be changed only at the beginning
of each plan year or when new drugs or new drug therapeutic uses appear.
(MMA)
Formulary Exceptions
• P&T committees must review for clinical appropriateness protocols and procedures
for the timely use of and access to both formulary and non-formulary drug products.
A non-formulary drug may be needed, for example, when the formulary drug would
cause adverse effects or would not be as effective or both, based on scientific
evidence or medical necessity. (BP)
B. Formulary List Review
Rationale
The formulary list review will incorporate best practices from the private sector, Medicaid and
FEHB formularies. The MMA requires CMS to review Part D formularies to ensure that
beneficiaries have access to a broad range of medically appropriate drugs to treat all disease
states and to ensure that the formulary design does not discriminate or substantially discourage
enrollment by certain groups. We expect that the kinds of formularies in widespread use today,
which provide high-quality drug coverage to millions of Medicare beneficiaries, would receive a
straightforward approval under this approach with modifications to account for specific features
of Medicare’s benefit structure, (i.e., including home infusion products that may not be covered
under a pharmacy benefit in commercial benefit designs). Plans may also have to make
modifications to existing commonly used formularies to allow for coverage of commonly-used
vaccines and diabetic supplies as outlined in the MMA. Below we provide a series of checks that
CMS will use to confirm that plan formularies will provide the kind of effective, non-
discriminatory access available in drug benefit plans today.
Approach
We encourage plans to submit formularies similar to those in widespread use today. We will
check the formulary to ensure inclusion of a range of drugs in a broad distribution of therapeutic
categories and classes, to satisfy the MMA requirement that a plan’s categorization system does
not discourage enrollment to any group of beneficiaries. We also will consider the specific drugs,
tiering and utilization management strategies employed in each formulary. CMS will identify
outliers from common benefit management practices for further evaluation. Plans may be asked
to provide written clinical justification for unusual benefit features that are deemed as outliers.

Review of Categories and Classes
We will review all classification systems to assure that plans provide an appropriate
breadth of categories and classes that cover all disease states. CMS will not consider a
classification system in isolation from the subsequent steps in our formulary review; a
classification system with a smaller number of classes may be acceptable if it nonetheless
provides preferred access to a relatively broad range of widely used medicines.
As described in the MMA, plans that utilize a classification system that is consistent with
the USP classification system, available at
http://www.usp.org/pdf/drugInformation/mmg/finalModelGuidelines2004-12-31.pdf, will
satisfy a safe harbor and thus CMS will approve their formulary classification system.
For plans that choose to adopt an alternative to USP’s classification structure, CMS will
check the plan’s proposed classification system to determine if it is similar to USP or
other commonly used classification systems, such as the American Hospital Formulary
Service (AHFS) Pharmacologic-Therapeutic Classification, information available at
www.ashp.org/ahfs.
The minimum statutory requirement is that a formulary must include at least two drugs in
each approved category and class (unless only one drug is available for a particular
category or class), regardless of the classification system that is utilized. We view this
requirement as a floor rather than an absolute standard. CMS may require more than two
drugs per category or class in cases where additional drugs present unique and important
therapeutic advantages in terms of safety and efficacy, and their absence from the plan
formulary may substantially discourage enrollment in the plan by beneficiaries with
certain disease states.
Even though a formulary may pass the classification review and have a safe harbor for its
categories and classes, it still must undergo the drug list review and benefit management
tools review in order to analyze the depth and breadth of drugs and their restrictions.
Drug List Review
Regardless of the classification system chosen, CMS will review and approve drug lists
that are consistent with best practice formularies currently in widespread use today. The
following paragraphs describe the multiple checks that will be utilized as part of the drug
list review.
1. CMS will review formularies for at least one drug in each of the Formulary Key Drug
Types identified by USP as Attachment B in comments provided to CMS on the draft
formulary guidance, available at:
http://www.usp.org/pdf/drugInformation/mmg/attachmentstoUSPComments2004-12-
30.pdf. Best practice formularies commonly include at least one drug in each of the
Formulary Key Drug Types as a minimum on their formulary. Plans may present a
reasonable clinical justification for formularies that do not contain at least one drug
for each of the USP Formulary Key Drug Types.
2. CMS will review tier placement to provide an assurance that the formulary does not
discourage enrollment of certain beneficiaries. When developing their formulary tier
structure, plans should utilize standard industry practices. Tier 1 should be considered
the lowest cost-sharing tier available to beneficiaries. Any and all subsequent tiers
within the formulary structure will be higher cost-sharing tiers in ascending order. For
example, drugs in Tier 3 will have a higher cost-share for beneficiaries than drugs in
Tier 2. Best practices in existing formularies and Medicaid preferred drug lists
generally place drugs in a less preferable position only when drugs that are
therapeutically similar (i.e., drugs that provide similar treatment outcomes) are in
more preferable positions on the formulary. The CMS review will focus on
identifying drug categories that may discourage enrollment of certain beneficiaries by
placing drugs in non-preferred tiers in the absence of commonly used therapeutically
similar drugs in more preferred positions.
3. CMS will analyze formularies to determine whether appropriate access is afforded to
drugs addressed in the following widely accepted national treatment guidelines which
are indicative of general best practice: asthma, diabetes, chronic stable angina, atrial
fibrillation, heart failure, thrombosis, lipid disorders, hypertension, chronic
obstructive pulmonary disease, dementia, depression, bipolar disorder, schizophrenia,
benign prostatic hyperplasia, osteoporosis, migraine, gastroesophageal reflux disease,
epilepsy, Parkinson’s disease, end stage renal disease, hepatitis, tuberculosis,
community acquired pneumonia, rheumatoid arthritis, multiple sclerosis and HIV.
This list of conditions does not represent an exhaustive list, but merely serves as
another check in the review process. Drugs or drug classes included within these
widely accepted guidelines will not place undue burden on plans since these drugs are
usually placed in favorable positions on commonly used, best practice formularies.
4. CMS will use Medicare risk adjustment data to check proposed formularies to
determine whether the formularies include drugs that are most commonly used by the
Medicare population and are reflected across the Drug Hierarchical Condition
Categories (DHCC) used to determine Medicare risk adjustment. These DHCCs are
representative of more than 5,000 ICD-9 diagnostic codes. For each DHCC, both the
inclusion of the drug and its tier position will be checked against other Part D
formularies and commonly used drugs in the overall Medicare population, to avoid
drug selection and cost-sharing that discriminate against specific disease groups.
5. CMS’ expectations are that best practice formularies contain a majority of drugs
within the following classes: antidepressants, antipsychotics, anticonvulsants,
antiretrovirals, immunosuppressants, and antineoplastics. Following common best
practices, CMS will check to see that beneficiaries who are being treated with these
classes of medications have uninterrupted access to all drugs in that class via
formulary inclusion, utilization management tools, or exceptions processes. When
medically necessary, beneficiaries should be permitted to continue utilizing a drug
that is providing clinically beneficial outcomes. In cases where practices may deviate
from the above, plans must provide clinical documentation to justify their decisions.

6. CMS will analyze the availability and tier position of the most commonly prescribed
drug classes for the Medicare population in terms of cost and utilization (Appendix
A). This list is derived from the Medicare Current Beneficiary Survey (MCBS) data
from 2002. CMS understands that plans will not provide identical coverage of these
drug classes, and our review will focus on assuring that plans present a balanced
formulary. These drug classes will cover common diseases and conditions, and will
allow us to ensure that plans are covering the most widely used medications, or
therapeutically similar medications, for the most common conditions.
All formularies will be evaluated using the criteria outlined above. Outliers for each area
of review will be further evaluated by CMS to determine if the outlier is deemed
potentially discriminatory. Examples of this may include a lack of appropriate drug
classes to treat certain diseases, a lack of sufficient drugs in a therapeutic class,
inappropriate tier placement that would discriminate against a group of beneficiaries, or
missing drugs that would cause discrimination. If any of the outliers appear to create
problems of access, plans will have the opportunity to present reasonable clinical
justifications.
Long Term Care Accessibility
Part D plans will be required to provide medically necessary prescription drug treatments
to LTC facility residents. Well in advance of the application deadline, CMS will provide
additional LTC guidance that will reflect standard practices in LTC pharmacies.
C. Review of Benefit Management Tools that Affect Access
Rationale
CMS will review plans’ use of utilization management tools, including prior authorization, step
therapy, quantity limitations, and generic substitution to ensure that beneficiaries are given
appropriate access to drugs in a timely manner. We will also review plans’ drug utilization
review procedures and appeals, exceptions and grievances processes. Our review will focus on
ensuring that these plan systems reflect best practices that are commonly utilized in the private
sector, Medicaid and FEHB plans.
Approach
Prior Authorization, Step Therapy, Quantity Limitations, Generic Substitution
CMS will look to existing best practices to check that plans’ use of these utilization
management tools is consistent with such practices. We will look to current industry
standards as well as appropriate guidelines that might be found from expert organizations
such as NCQA, AMCP, and NAIC, and to the use of such standards in existing drug
plans that are widely used by seniors and people with disabilities. CMS will assure that

plans’ use of such tools is consistent with best practices. CMS will also compare
formularies amongst the applicants to analyze the comparative use of practices such as
prior authorization, step therapy, and quantity limits. Our expectation is that these
techniques will be used in Part D formularies consistently with the way they are applied
in existing formulary systems, both in terms of the situations in which they are used and
the timeliness of the processes. In cases where a plan may fall outside of best practices,
the plan will be asked to provide a reasonable justification for their practices.
Drug Utilization Review (DUR)
CMS will review plans’ DUR practices to confirm that they meet industry best practices
in terms of access to drugs and quality oversight. We will expect plans’ use of tools and
techniques currently in place in their commercial coverage business. These processes
may include concurrent review as well as prospective and/or retrospective utilization
review. These reviews will be expected to assure appropriate access to medically
necessary therapies as well as guard against inappropriate or dangerous utilization of
prescription medications.
Appeals, Exceptions and Grievances
The standards for handling appeals, exceptions, and grievances are specific and are
contained in the final rule. We believe the final rule reflects current best practices around
appeal and grievance timeframes. We are developing notice requirements to ensure that
beneficiaries understand their rights in this area. We also expect to require standardized
reporting from Part D plans on denial, reconsideration and appeals, and exceptions
processing, and we will use these data in our management and oversight activities. We
expect plans to make appropriate use of the data for internal quality initiatives, such as
those directed at managing excessive rates of overturned utilization management
decisions. Part D plan sponsors that provide prescription drug benefits for Part D drugs
and manage this benefit through the use of a tiered formulary must establish and maintain
reasonable and complete exceptions procedures subject to CMS’ approval for this type of
coverage determination.
5.
Formulary Submission Requirements
In support of the Medicare Modernization Act (MMA), CMS is establishing a systems interface
within the Health Plan Management System (HPMS) to enable MA-PD plans and PDPs to
submit their formularies electronically. This functionality will provide for the upload and receipt
of the formulary file, exceptions and notes file, prior authorization supplemental data and step
therapy supplemental data, as defined by CMS. It will also allow CMS to provide more timely,
systematic, and consistent feedback to plans regarding their formulary practices.
Using the HPMS formulary upload module, the user will submit one or more formulary files for
the MA-PDs or PDPs offered under a contract. This submission must occur prior to April 18,
2005 at 5:00pm EDT. Detailed technical user instructions will be forthcoming. The general
process that the user will complete in order to submit their plan’s drug formulary information
includes the following steps:
• General formulary-level data entry in a designated HPMS web page
o Plan will be required to complete data entry in an HPMS web interface for
information such as Plan name , formulary name, classification structure used,
etc.
• Attachment of an NDC-level Formulary file in a flat file text format
o Plan will attach their Formulary file submission. The Formulary file will be
created as a flat file in ASCII format by the MA-PD or PDP outside of the HPMS
prior to submission. The file must be created using National Drug Codes (NDCs)
as a proxy for drug name. Appendix B illustrates the required data fields for each
NDC record in the Formulary file.
• If relevant, attachment of a Step Therapy Algorithm file in MS-Word format
o During the general formulary-level data entry process, the user will be asked if the
NDCs in the formulary submission are associated with one or more Step Therapy
management programs. If the drugs in the formulary submission are associated
with Step Therapy management programs, then the user is required to submit an
attachment that provides the detailed algorithms for all Step Therapy management
programs in the formulary. The Step Therapy Management Algorithm file should
be submitted in HPMS as a Word file. The user should submit only one Step
Therapy Management Algorithm file attachment per formulary file submission.
• If relevant, attachment of a Prior Authorization Criteria file in MS-Word format
o During the general formulary-level data entry process, the user will be asked if the
NDCs in the formulary submission are subject to prior authorization. If the drugs
in the formulary submission are associated with prior authorization, then the user
is required to submit an attachment that provides the detailed criteria for all prior
authorization programs. The Prior Authorization Criteria file should be submitted
in HPMS as a Word file. The user should submit only one Prior Authorization
Criteria file attachment per formulary file submission.
• If relevant, attachment of an Exceptions and Formulary Notes file in MS-Word format
o The user will have the ability to attach a Word file that provides additional details
about any exceptions associated with the Formulary file. For example, when a
particular dosage form or strength has restrictions, such as prior authorization or
unique quantity limits, then the user should note these exceptions in a Word
attachment (e.g. Imitrex injection vs. tablets). Plan must also include in the notes
section, an explanation of its entire exceptions process.
Information concerning formularies will be made publicly available at some point prior to the
beneficiary enrollment period. Applicants can always seek to protect their information under the
Freedom of Information Act and label truly proprietary information "confidential" or
"proprietary." When information is so labeled, the Applicant is required to explain the

applicability of the FOIA exemption they are claiming. When there is a request for information
that is designated by the Applicant as confidential or that could reasonably be considered exempt
under Exemption 4, CMS is required by its FOIA regulation at 45 C.F.R. §5.65(d) and by
Executive Order 12,600 to give the submitter notice before the information is disclosed. To
determine whether the Applicant’s information is protected by Exemption 4, the Applicant must
show that— (1) disclosure of the information is likely to impair the government's ability to
obtain necessary information in the future; (2) disclosure of the information is likely to cause
substantial harm to the competitive position of the submitter; or (3) the records are considered
valuable commodities in the marketplace which, once released through the FOIA, would result in
a substantial loss of their market value. Consistent with our approach under the Medicare
Advantage program, we would not release information under the Medicare Part D program that
would be considered proprietary in nature.
6.
Formulary Maintenance Requirements
Under the MMA, plans may only change therapeutic categories and classes at the beginning of
each plan year unless new drugs or new therapeutic uses appear. However, plans must submit
changes to the formulary (additions, deletions or tier changes) as they occur. CMS will accept
changes to formulary drugs on a regular basis, within 30 days after a P&T committee makes
decisions. Plans shall submit any formulary drug list changes between the 1
st
day and the 7
th
day
of each month, beginning November 1, 2005. These submitted changes will be reviewed by
CMS to ensure that formularies remain nondiscriminatory and meet other minimum standards.
The effective dates of submitted formulary changes are subject to the time periods outlined in the
final rule.
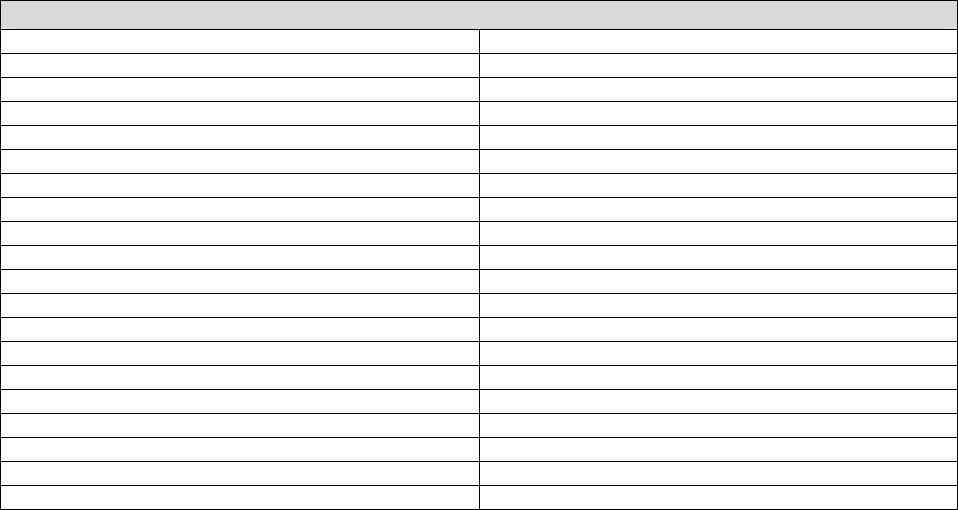
Appendix A
Top Drug Classes by Cost and Utilization
Top Drug Classes by Cost and Utilization
ACE inhibitors Nitrates
Alpha blockers Non-sedating antihistamines
Angiotensin receptor blockers Opioids
Anticoagulants Opioid / analgesic
Antigout Platelet aggregation inhibitors
Atypical antipsychotics Potassium
Beta-blockers Potassium sparing diuretic / thiazide diuretic
Biguanides Ophthalmic prostaglandins
Bisphosphonates Proton pump inhibitors
Calcium channel blockers Quinolones
Calcium channel blocker / ACE inhibitor Sedatives
Cardiac inotropes Selective estrogen receptor modifier
Cholinesterase inhibitors Short-acting beta agonists
Corticosteroids SSRIs
Cox-2 inhibitors Statins
Estrogen replacement Sulfonylureas
GABA Agents Thiazide diuretics
Leukotriene modifiers Thiazolidinediones
Long-acting beta agonist / inhaled corticosteroid Thyroid replacement
Loop diuretics Tricyclic antidepressants
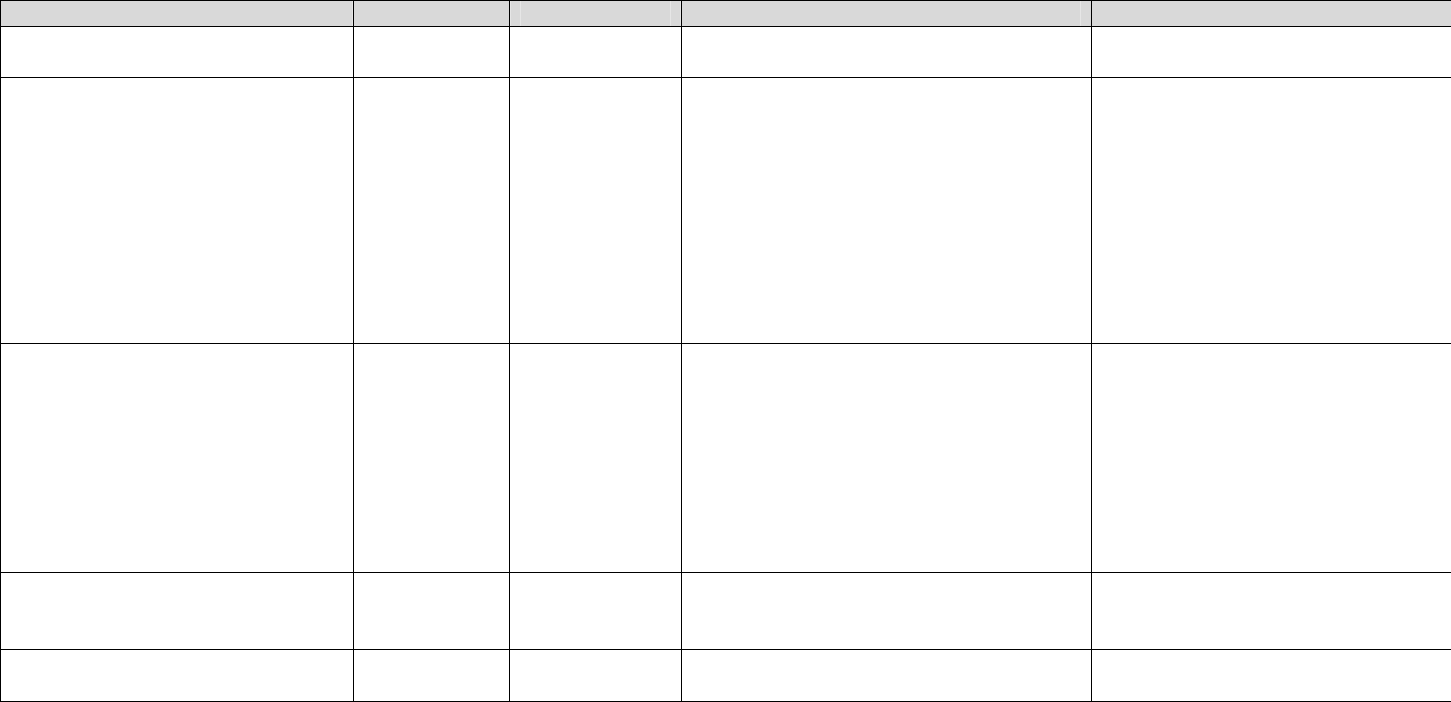
Appendix B
Draft Formulary File Record Layout
Required File Format = ASCII File
Field Name Field Type Field Length Field Description Sample Field Value(s)
NDC CHAR
NOT NULL
11 11-Digit National Drug Code 00000333800
Tier_Level_Value CHAR
2
NOT NULL
Defines the Cost Share Tier Level Value
Associated with the NDC. Assumption is
that the NDC is assigned to one tier value.
These values are consistent with the
selection of value options available to data
entry users in the Plan Benefit Package
software.
If no Tier Level Value applies, enter ‘1’ as
the value for this field.
1 = Tier Level 1
2 = Tier Level 2
3 = Tier Level 3
4 = Tier Level 4
5 = Tier Level 5
6 = Tier Level 6
7 = Tier Level 7
8 = Tier Level 8
9 = Tier Level 9
10 = Tier Level 10
Drug_Type_Label_Value CHAR
NOT NULL
1 Define the Drug Type Label Value for the
NDC. Enter the label value for the Drug
Type from the defined list of labels in the
instructions.
If Drug Type Label Value 6 = “Other” is
used, then the user must describe the
“Other” label description in the
Drug_Type_Label_Value_Other field.
1 = Generic
2 = Preferred Brand
3 = Non-Preferred Brand
4 = Non-Formulary
5 = Specialty
6 = Other
Drug_Type_Label_Value_Other CHAR
NULL
50 Describe the “Other” label description.
If “Other” does not apply, leave this field
blank.
Orphan
Quantity_Limit_Amount_YN CHAR
NOT NULL
1 Does the NDC have a quantity limit other
than a 30-day or 34-day limit?
1 = Yes
0 = No

Quantity_Limit_Amount NUM
NULL
3 If Yes to Quantity_Limit_Amount_YN,
enter the quantity limit unit amount. The
units for this amount may be defined as
number of pills, number of injections, etc.
If a limit other than 30 or 34 days does not
apply, leave this field blank.
9
Quantity_Limit_Days NUM
NULL
3 Enter the days associated with the quantity
limit.
If a limit other than 30 or 34 days does not
apply, leave this field blank.
60 (e.g., 9 pills every 60 days)
(e.g., 9 injections every 60 days)
Prior_Authorization_YN CHAR
NOT NULL
1 Is prior authorization required for the
NDC?
1 = Yes
0 = No
Therapeutic_Category_Name CHAR
NULL
100 If the Category/Class Database Source is
indicated as “OTHER” in the HPMS Data
Entry Web Interface (i.e., neither USP nor
AHFS is used by the plan), then the user
should enter the Therapeutic Category
Name for each NDC in the file.
If the drug is based on either USP or AHFS
Therapeutic Category Classes, then leave
this field blank.
Analgesics
Therapeutic_Class_Name CHAR
NULL
100 If the Category/Class Database Source is
indicated as “OTHER” in the HPMS Data
Entry Web Interface (i.e., neither USP nor
AHFS is used by the plan), then the user
should enter the Pharmacological Class
Name for each NDC in the file.
If the drug is based on either USP or AHFS
Pharmacological Classes, then leave this
field blank.
Opioid Analgesics
Formulary_Key_Drug_Type_Name CHAR
NULL
100 OPTIONAL: If the Category/Class
Database Source is indicated as “OTHER”
Opioid Analgesics, long-acting
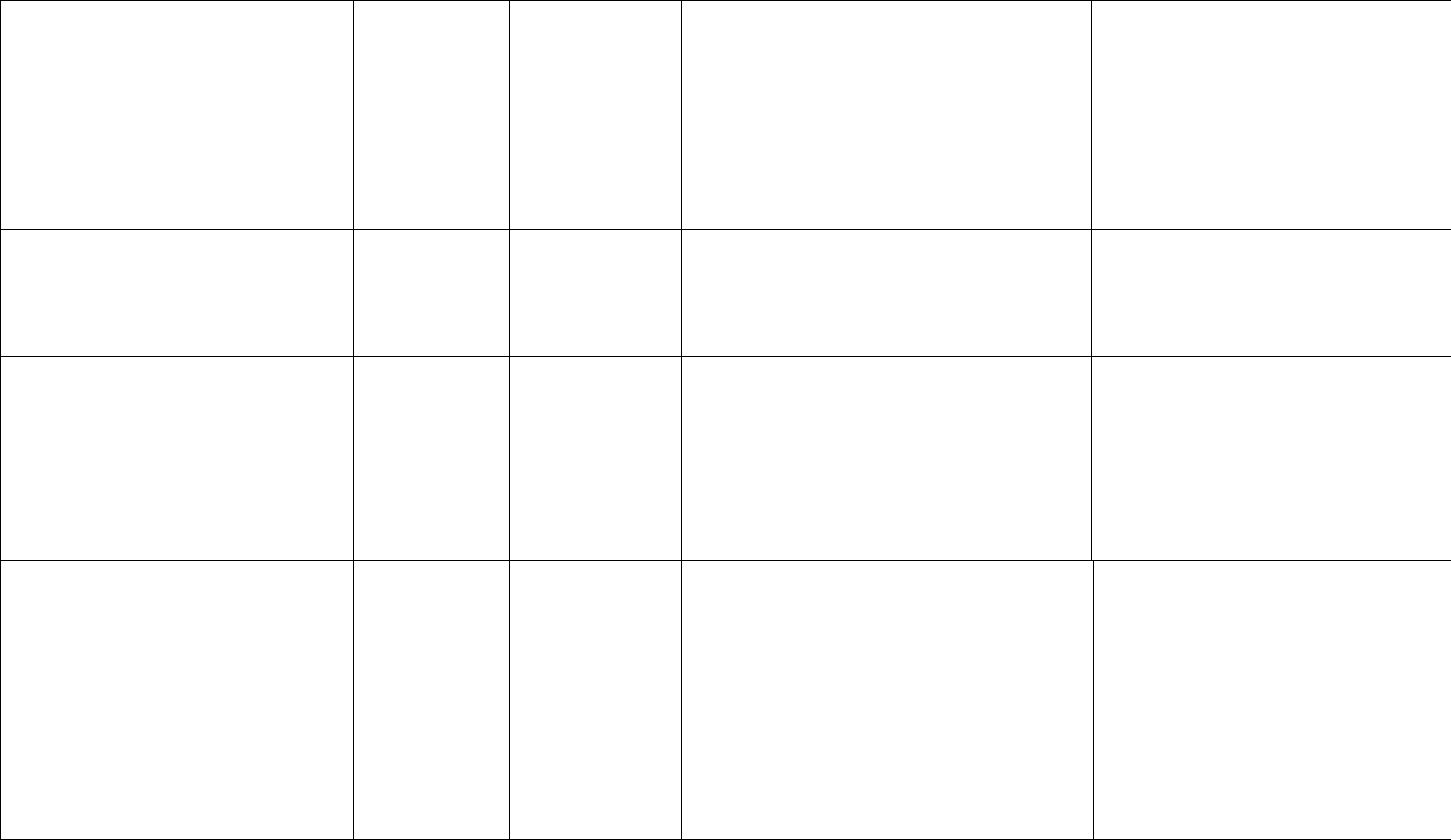
in the HPMS Data Entry Web Interface
(i.e., neither USP nor AHFS is used by the
plan), then the user has the option to enter
the Formulary Key Drug Type
(subdivision) Name for each NDC in the
file.
If the drug is based on either USP or
AHFS, then leave this field blank.
Step_Therapy_Type_Group_Num NUM
NULL
1 Number of step therapy drug treatment
groups, in which the NDC is included.
If Step Therapy does not apply to this drug,
then leave this field blank.
3
Step_Therapy_Type_Group_Desc_X CHAR
NULL
100 Description of step therapy drug treatment
group. Field should be repeated in the
record based upon number of groups
declared in
Step_Therapy_Type_Group_Num
If Step Therapy does not apply to this drug,
then leave this field blank.
Step_Therapy_Type_Group_Desc_1 = “C
H
Therapy”
Step_Therapy_Type_Group_Desc_2 = “An
Therapy”
Step_Therapy_Type_Group_Desc_3 = “C
V
Therapy”
Step_Therapy_Type_Group_Step_X CHAR
NULL
3 Step number within the sequence for the
Step Therapy Group. Field should be
repeated in the record based upon number
of groups declared in
Step_Therapy_Type_Group_Num
AND
in the same order as
Step_Therapy_Type_Group_Desc_X
If Step Therapy does not apply to this drug,
then leave this field blank.
Step_Therapy_Type_Group_Step_1 = 4 (e
Step 4 of 6)
Step_Therapy_Type_Group_Step_2 = 1 (e.
Step 1 of 3)
Step_Therapy_Type_Group_Step_3 = 5 (e.
Step 5 of 5)
