
P.S./CHEMISTRY
P.S./CHEMISTRY
The University of the State of New York
REGENTS HIGH SCHOOL EXAMINATION
PHYSICAL SETTING
CHEMISTRY
Friday, January 27, 2023 — 9:15 a.m. to 12:15 p.m., only
The possession or use of any communications device is strictly prohibited when
taking this examination. If you have or use any communications device, no matter how
brie y, your examination will be invalidated and no score will be calculated for you.
This is a test of your knowledge of chemistry. Use that knowledge to answer all
questions in this examination. Some questions may require the use of the 2011 Edition
Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all
parts of this examination according to the directions provided in this examination
booklet.
A separate answer sheet for Part A and Part B–1 has been provided to you. Follow
the instructions from the proctor for completing the student information on your
answer sheet. Record your answers to the Part A and Part B–1 multiple-choice
questions on this separate answer sheet. Record your answers for the questions in
Part B–2 and Part C in your separate answer booklet. Be sure to ll in the heading on
the front of your answer booklet.
All answers in your answer booklet should be written in pen, except for graphs and
drawings, which should be done in pencil. You may use scrap paper to work out the
answers to the questions, but be sure to record all your answers on your separate
answer sheet or in your answer booklet as directed.
When you have completed the examination, you must sign the statement printed
on your separate answer sheet, indicating that you had no unlawful knowledge of the
questions or answers prior to the examination and that you have neither given nor
received assistance in answering any of the questions during the examination. Your
answer sheet and answer booklet cannot be accepted if you fail to sign this declaration.
Notice. . .
A four-function or scienti c calculator and a copy of the 2011 Edition Reference Tables for
Physical Setting/Chemistry must be available for you to use while taking this examination.
DO NOT OPEN THIS EXAMINATION BOOKLET UNTIL THE SIGNAL IS GIVEN.

P.S. Chem.–Jan. ’23 [2]
1 Which conclusion was developed as a result of
the gold foil experiment?
(1) Atoms are mostly empty space.
(2) All atoms are hard, indivisible spheres.
(3) Atoms have different volumes.
(4) All atoms have the same volume.
2 Which two particles each have a mass
approximately equal to one atomic mass unit?
(1) positron and proton
(2) positron and electron
(3) neutron and electron
(4) neutron and proton
3 An excited potassium atom emits a speci c
amount of energy when one of its electrons
moves from
(1) the rst shell to the fourth shell
(2) the second shell to the fourth shell
(3) the fourth shell to the fth shell
(4) the fourth shell to the second shell
4 Which list of elements includes a metal, a
metalloid, and a noble gas?
(1) Rb, Cl, Ne (3) Rn, Cl, Ne
(2) Sr, Si, Rn (4) Si, Rb, Sr
5 Which element has the lowest density at 298 K
and 101.3 kPa?
(1) argon (3) nitrogen
(2) uorine (4) oxygen
6 Which phrase describes the crystal structure and
properties of two different forms of solid carbon
called diamond and graphite?
(1) same crystal structure and same properties
(2) same crystal structure and different proper-
ties
(3) different crystal structures and different
properties
(4) different crystal structures and same proper-
ties
7 Which element has chemical properties most
similar to sodium?
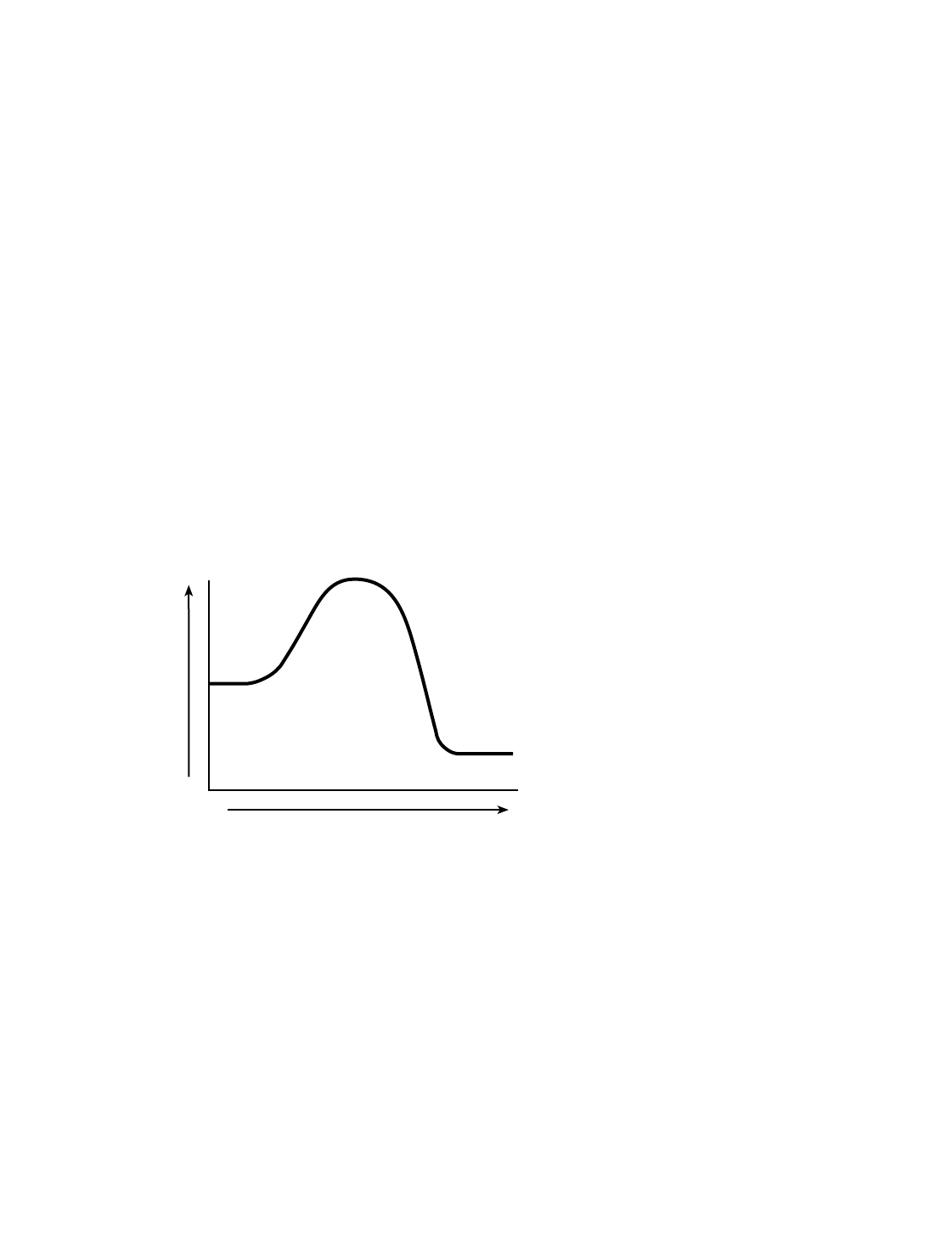
(1) magnesium (3) phosphorus
(2) oxygen (4) rubidium
8 Which substance contains elements chemically
combined in a xed proportion?
(1) manganese (3) silicon
(2) methane (4) strontium
9 Which property can be used to differentiate
between a 50.-gram sample of solid potassium
nitrate at STP and a 50.-gram sample of solid
silver chloride at STP?
(1) mass (3) phase
(2) temperature (4) solubility
10 Which type of bond forms when electrons are
equally shared between two atoms?
(1) a polar covalent bond
(2) a nonpolar covalent bond
(3) a hydrogen bond
(4) an ionic bond
Part A
Answer all questions in this part.
Directions (1–30): For each statement or question, record on your separate answer sheet the number of the
word or expression that, of those given, best completes the statement or answers the question. Some questions
may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.

P.S. Chem.–Jan. ’23 [3] [OVER]
11 Which statement describes the changes in
bonding and energy that occur when a molecule
of iodine, I
2
, forms two separate atoms of iodine?
(1) A bond is formed as energy is absorbed.
(2) A bond is formed as energy is released.
(3) A bond is broken as energy is absorbed.
(4) A bond is broken as energy is released.
12 The degree of polarity in the bond between a
hydrogen atom and an oxygen atom in a molecule
of water can be assessed using the difference in
(1) densities
(2) electronegativities
(3) melting points
(4) intermolecular forces
13 Which substance can not be broken down by a
chemical change?
(1) ammonia (3) krypton
(2) ethanol (4) water
14 Which sample of matter is a mixture?
(1) CO
2
(g) (3) MgCl
2
(aq)
(2) CCl
4
( ) (4) Sn(s)
15 Which term is used to express the concentration
of an aqueous solution?
(1) parts per million (3) pressure at 0°C
(2) heat of fusion (4) volume at 0°C
16 The particles in which sample have the lowest
average kinetic energy?
(1) 50. g of sulfur at 273 K
(2) 40. g of aluminum at 298 K
(3) 30. g of sulfur at 303 K
(4) 20. g of aluminum at 323 K
17 Which process represents a chemical change?
(1) Iodine sublimes.
(2) Water evaporates.
(3) An ice cube melts.
(4) A candle burns in air.
18 Which equation represents a physical equilibrium?
(1) NaCl(s)
H
2
O
Na
1
(aq) 1 Cl
2
(aq)
(2) 2SO
2
(g) 1 O
2
(g) 2SO
3
(g)
(3) 3O
2
(g) → 2O
3
(g)
(4) N
2
( ) N
2
(g)
19 Systems in nature tend to undergo changes toward
(1) higher energy and higher entropy
(2) higher energy and lower entropy
(3) lower energy and higher entropy
(4) lower energy and lower entropy
20 Which formula represents a hydrocarbon?
(1) C
2
H
6
(3) C
2
H
5
Cl
(2) C
2
H
5
OH (4) C
2
H
6
O
21 Which statement describes the bonding in an
alkyne molecule?
(1) There is at least one carbon-to-carbon
double bond.
(2) There is at least one carbon-to-carbon
triple bond.
(3) There is at least one carbon-to-oxygen
single bond.
(4) There is at least one carbon-to-oxygen
double bond.
22 Which compound has a functional group that
contains two oxygen atoms?
(1) 1-propanamine (3) methyl propanoate
(2) 2-chloropropane (4) methyl ethyl ether
23 Which term identi es a type of organic reaction?
(1) deposition (3) polymerization
(2) distillation (4) vaporization
24
In an electrochemical cell, oxidation occurs at the
(1) anode (3) salt bridge
(2) cathode (4) switch

P.S. Chem.–Jan. ’23 [4]
25 Which energy conversion occurs in an operating
electrolytic cell?
(1) chemical energy to electrical energy
(2) electrical energy to chemical energy
(3) nuclear energy to electrical energy
(4) electrical energy to nuclear energy
26 One acid-base theory states that a base is an
(1) H
2
donor (3) H
1
donor
(2) H
2
acceptor (4) H
1
acceptor
27 The acidity or alkalinity of a solution can be
measured by its
(1) pH value
(2) electronegativity value
(3) boiling point
(4) freezing point
28 When the nucleus of an atom of neon-19 decays,
which particle is emitted?
(1)
4
2
He (3)
1
0
n
(2)
0
21
e (4)
0
11
e
29 Which nuclear emission has the greatest mass?
(1) positron (3) beta particle
(2) gamma ray (4) alpha particle
30 Which statement describes the net change that
occurs during nuclear ssion?
(1) Electrons are converted to protons.
(2) Protons are converted to electrons.
(3) Mass is converted to energy.
(4) Energy is converted to mass.

P.S. Chem.–Jan. ’23 [5] [OVER]
31 What is the net charge of a monatomic ion that
has 15 protons, 16 neutrons, and 18 electrons?
(1) 21 (3) 31
(2) 22 (4) 32
32 The table below shows the atomic masses and
natural abundances of the two naturally occurring
isotopes of rhenium.
Naturally Occurring Isotopes of Rhenium
Isotope
Atomic Mass
(u)
Natural
Abundance
(%)
Re-185 184.95 37.40
Re-187 186.96 62.60
Which numerical setup can be used to calculate
the atomic mass of rhenium?
(1) (184.95 u)(37.40) 1 (186.96 u)(62.60)
(2) (184.95 u)(0.3740) 1 (186.96 u)(0.6260)
(3) (184.95 u)(37.40) 1 (186.96 u)(62.60)
2
(4) (184.95 u)(0.3740) 1 (186.96 u)(0.6260)
2
33 Which general trend is observed as the elements
in Period 2 are considered from left to right?
(1) Atomic mass decreases.
(2) Melting point increases.
(3) Electronegativity increases.
(4) First ionization energy decreases.
34 Which formula represents chromium(III) oxide?
(1) CrO
3
(3) Cr
2
O
3
(2) Cr
3
O (4) Cr
3
O
2
35 Given the balanced equation representing a
reaction:
2KClO
3
1 energy → 2KCl 1 3O
2
What is the mass of KCl produced when 24.51
grams of KClO
3
reacts completely to produce
9.60 grams of O
2
?
(1) 5.31 g (3) 34.11 g
(2) 14.91 g (4) 43.71 g
36 Which equation represents conservation of atoms?
(1) TiO
2
1 2Al → 2Al
2
O
3
1 Ti
(2) TiO
2
1 4Al → 2Al
2
O
3
1 Ti
(3) 3TiO
2
1 2Al → 2Al
2
O
3
1 3Ti
(4) 3TiO
2
1 4Al → 2Al
2
O
3
1 3Ti
37 One mole of bromine gas, Br
2
, has a mass of
(1) 35.0 g (3) 79.9 g
(2) 70.0 g (4) 159.8 g
38 Given the equation representing a reaction:
2NaCl → 2Na 1 Cl
2
Which type of reaction does this equation
represent?
(1) double replacement
(2) decomposition
(3) synthesis
(4) single replacement
Part B–1
Answer all questions in this part.
Directions (31–50): For each statement or question, record on your separate answer sheet the number of the
word or expression that, of those given, best completes the statement or answers the question. Some questions
may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.

P.S. Chem.–Jan. ’23 [6]
39 Which statement describes the charge and the
radius of the magnesium ion formed when a
magnesium atom loses two electrons?
(1) The Mg ion is positive and has a radius
larger than the Mg atom.
(2) The Mg ion is negative and has a radius
larger than the Mg atom.
(3) The Mg ion is positive and has a radius
smaller than the Mg atom.
(4) The Mg ion is negative and has a radius
smaller than the Mg atom.
40 An oxide ion, O
22
, has the same electron
con guration as an atom of which noble gas?
(1) helium (3) argon
(2) neon (4) krypton
41 What is the vapor pressure of propanone at
45°C?
(1) 21 kPa (3) 70. kPa
(2) 60. kPa (4) 79 kPa
42 Based on Table G, what is the mass of KCl that
must be dissolved in 200. grams of H
2
O at 10.°C
to make a saturated solution?
(1) 15 g (3) 60. g
(2) 30. g (4) 120. g
43 Based on Table I, which chemical equation
represents a reaction with a heat of reaction that
indicates a net release of energy?
(1) N
2
(g) 1 O
2
(g) → 2NO(g)
(2) N
2
(g) 1 2O
2
(g) → 2NO
2
(g)
(3) 2C(s) 1 3H
2
(g) → C
2
H
6
(g)
(4) 2C(s) 1 2H
2
(g) → C
2
H
4
(g)
44 The greatest increase in entropy occurs when a
1.00-gram sample of water changes from
(1) solid to liquid (3) gas to liquid
(2) solid to gas (4) liquid to solid
45 Which particle diagram represents one substance,
only?
Key
= atom of one element
= atom of a different element
( 1 )
( 2 )
( 4 )
( 3 )
46 Based on Table J, atoms of which metal will lose
electrons to Ca
21
ions?
(1) aluminum (3) nickel
(2) lead (4) potassium
47 Which aqueous solution is the best conductor of
an electrical current?
(1) 0.1 M NaNO
3
(3) 0.01 M NaNO
3
(2) 0.2 M NaNO
3
(4) 0.02 M NaNO
3
48 Given the equation representing a reaction:
2
1
H 1
3
1
H →
4
2
He 1
1
0
n
This equation represents
(1) sublimation (3) ssion
(2) condensation (4) fusion

P.S. Chem.–Jan. ’23 [7] [OVER]
49 Which formula represents 2-butene?
H
H
C
C C
H
H
H
C
H
H
H
( 1 )
HC
C C
H
H
C
H
H
H
( 3 )
H
H
C
C C
HH
C
H
H
H
( 2 )
HC
C C
C
H
H
H
( 4 )
H H
H
50 Given a formula representing a compound:
H
H
H
CCC
HH
HH
OH
Which formula represents an isomer of the compound?
( 3 )
H
H
C
C C
O
H
H
H
H
C
C O
H
C
H
H
H
( 1 )
H
H
C
C C
H
( 2 ) ( 4 )
H
H H
H
H
H
H
H
C
C C
H
H H
O
OH
O
H

P.S. Chem.–Jan. ’23 [8]
51 Explain, in terms of neutrons and protons, why P-32 and P-31 are different isotopes of
phosphorus. [
1]
52 Determine the oxidation state of chromium in K
2
CrO
4
. [1]
Base your answers to questions 53 and 54 on the information below and on your knowledge of chemistry.
The rst four elements in Group 14 are carbon, silicon, germanium, and tin. These
elements form compounds with chlorine that have similar formulas. Two examples of these
formulas are silicon tetrachloride, SiCl
4
, and germanium tetrachloride, GeCl
4
.
53 State the general trend in atomic radius as these four elements are considered in order
of increasing atomic number. [1]
54 State, in terms of electron con guration, why silicon and germanium both form
tetrachloride compounds. [
1]
Base your answers to questions 55 through 57 on the information below and on your knowledge of chemistry.
The equation below represents the reaction between ammonia and hydrogen chloride.
NH
3
(g) 1 HCl(g) → NH
4
Cl(s)
compound 1 compound 2
55 Explain, in terms of distribution of charge, why a molecule of compound 1 is polar. [
1]
56 Draw a Lewis electron-dot diagram for a molecule of compound 2. [
1]
57 Identify the two types of chemical bonds in the product of this reaction. [
1]
Part B–2
Answer all questions in this part.
Directions (51-65): Record your answers in the spaces provided in your answer booklet. Some questions may
require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.

P.S. Chem.–Jan. ’23 [9] [OVER]
Base your answers to questions 58 through 60 on the information below and on your knowledge of chemistry.
A sample of helium gas, He(g), is placed in a rigid cylinder sealed with a movable piston.
The temperature of the helium is 25.0°C. The volume of the helium is 300. milliliters and
the pressure is 0.500 atmosphere.
58 State, in terms of the average distance between the helium atoms, why the density of the
gas increases when the piston is pushed farther into the rigid cylinder. [
1]
59 Determine the volume of the helium gas when the pressure is increased to 1.50 atm and
the temperature remains at 25.0°C. [
1]
60 Compare the number of helium atoms in the cylinder at a pressure of 0.500 atm to the
number of helium atoms in the cylinder when the pressure is increased to 1.50 atm by
pushing the piston in. [
1]
Base your answers to questions 61 and 62 on the information below and on your knowledge of chemistry.
During a laboratory activity, a student places 21.0 mL of hydrochloric acid solution,
HCl(aq), of unknown concentration into a ask. The solution is titrated with 0.125 M
NaOH(aq) until the acid is exactly neutralized. The volume of NaOH(aq) added is
18.5 milliliters. During this laboratory activity, appropriate safety equipment is used and
safety procedures are followed.
61 Explain, in terms of ions, why the hydrochloric acid solution can conduct an electric
current. [
1]
62 Determine the concentration of the HCl(aq) solution, using the titration data. [
1]

P.S. Chem.–Jan. ’23 [10]
Base your answers to questions 63 through 65 on the information below and on your knowledge of chemistry.
The table below lists the hydronium ion concentration and pH values of four different
solutions and distilled water. The pH value is missing for sample 2.
Hydronium Concentration and pH Value for Five Samples
Sample
Number
Sample Description
Hydronium Ion
Concentration
(M)
pH
Value
1 0.1 M HCl(aq) 1
3 10
–1
1.0
2 0.01 M HCl(aq) 1
3 10
–2
?
3 distilled H
2
O( )1 3 10
–7
7.0
4 0.01 M NaOH(aq) 1
3 10
–12
12.0
5 0.1 M NaOH(aq) 1
3 10
–13
13.0
63 Determine the pH value of sample 2. [1]
64 Identify the ion released by the compound dissolved in sample 4 that allows the
compound to be classi ed as an Arrhenius base. [
1]
65 State how many times greater the hydronium ion concentration is in sample 4 than
it is in sample 5. [
1]

P.S. Chem.–Jan. ’23 [11] [OVER]
Base your answers to questions 66 through 68 on the information below and on your knowledge of chemistry.
Boric acid, H
3
BO
3
, is heated to produce tetraboric acid, H
2
B
4
O
7
, and water. The equation
below represents the reaction to form tetraboric acid.
4H
3
BO
3
(s) H
2
B
4
O
7
(s) 1 5H
2
O(g)
boric acid tetraboric acid
The tetraboric acid is then used to make borax, which is used as a cleaning agent. Borax,
Na
2
B
4
O
7
•
10H
2
O, is a hydrate with a gram-formula mass of 381 grams per mole. A hydrate
is a compound with water within its crystal structure. Borax has ten moles of water for every
mole of Na
2
B
4
O
7
.
66 Explain why the formula for tetraboric acid is an empirical formula. [
1]
67 Determine the number of moles of boric acid that react in the equation to produce
10 moles of water. [
1]
68 Show a numerical setup for calculating the mass, in grams, of a 0.200-mole sample of
borax. [
1]
Part C
Answer all questions in this part.
Directions (66-85): Record your answers in the spaces provided in your answer booklet. Some questions may
require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.

P.S. Chem.–Jan. ’23 [12]
Base your answers to questions 69 through 71 on the information below and on your knowledge of chemistry.
During a laboratory activity, appropriate safety equipment is used and safety procedures
are followed. A student uses the lab equipment shown in the diagram below to determine
the heat of combustion of candle wax.
Can with water
Iron ring with wire gauze
Ring stand
Candle
Heat of combustion is de ned as the amount of heat released when a known mass of
a substance is burned and can be measured in joules per gram. At the start of the activity,
the mass of the candle and the mass of the water are measured. The starting temperature
of the water is 5.0°C, and the air temperature in the room is 22.0°C. The candle is lit, and
the water is stirred with a stirring rod. Several minutes later, the candle is extinguished, and
the student measures the temperature of the water in the can. When the candle is cool, the
student measures the nal mass of the candle. Lab activity results are shown in the table
below.
Lab Activity Results
Mass of Candle
Wax Burned
(g)
Mass of Water
in the Can
(g)
Calculated
Temperature
Change of Water
(°C)
Heat Absorbed by
the Water
(J)
Calculated Heat
of Combustion of
Candle Wax
(J/g)
0.83 190. 39 ? 37 000
69 State the number of signi cant gures used to express the value for the mass of the water
in the can. [
1]
70 State the direction of the heat ow between the air and the water in the can before the
candle is lit. [
1]
71 Determine the amount of heat absorbed by the water. [
1]

P.S. Chem.–Jan. ’23 [13] [OVER]
Base your answers to questions 72 through 76 on the information below and on your knowledge of chemistry.
A process was developed in 1912 to produce ammonia gas from atmospheric nitrogen
gas and hydrogen gas. Iron can be used as a catalyst. The equation representing this system
at equilibrium is shown below.
N
2
(g) 1 3H
2
(g) 2NH
3
(g) 1 91.8 kJ
72 State evidence from the equation that the forward reaction is exothermic. [
1]
73 Compare the rate of the forward reaction to the rate of the reverse reaction at
equilibrium. [
1]
74 On the labeled axes in your answer booklet, draw a potential energy diagram for the
forward reaction represented in this equation. [
1]
75 State, in terms of moles of gases, why the equilibrium shifts to the right due to an
increase in pressure on the system at constant temperature. [
1]
76 State what happens to the rate of forward reaction when the iron is added to this
system. [
1]
Base your answers to questions 77 through 79 on the information below and on your knowledge of chemistry.
Before the year 1828, it was thought that organic compounds were produced only by
living organisms and that inorganic compounds were made from nonliving substances. Urea
is an organic compound. In 1828, a chemist heated ammonium cyanate and produced urea,
which is very soluble in water. The equation below represents this reaction.
NH
4
OCN H
2
NCONH
2
ammonium urea
cyanate
77 Identify the element present in urea that is present in all organic compounds. [
1]
78 Compare the formula mass of the two compounds in the equation. [
1]
79 State, in terms of molecular polarity, why urea is very soluble in water. [
1]

Base your answers to questions 80 and 81 on the information below and on your knowledge of chemistry.
When a voltmeter is connected in the circuit of a voltaic cell, an electrical measurement
called voltage can be read on the meter. The voltage of the cell is affected if the concentration
of the solute in the half-cells is changed. The diagram, the ionic equation, and the graph
below represent a copper-zinc cell. When the switch is closed, electricity ows through the
circuit as the cell operates at constant temperature.
Cu(s) + Zn
2+
(aq)
Cu(s) electrode Zn(s) electrode
Salt
bridge
Cu(NO
3
)
2
(aq) Zn(NO
3
)
2
(aq)
Switch
Voltmeter
V
Wire
Cu
2+
(aq) + Zn(s)
0.5 1.0 1.5 2.0 2.5
Cell Voltages at Different Cu
2+
Concentrations
Voltage (V)
0
1.13
Concentration of Cu
2+
(M)
1.12
1.11
1.10
1.09
1.08
80 Based on the graph, determine the voltage of the cell if the Cu(NO
3
)
2
(aq) concentration
is 1.5 M. [
1]
81 Write a balanced half-reaction equation for the oxidation of zinc that occurs in this
operating cell. [
1]
P.S. Chem.–Jan. ’23 [14]

P.S. Chem.–Jan. ’23 [15] [OVER]
Base your answers to questions 82 through 85 on the information below and on your knowledge of chemistry.
Synthetic radioisotopes may be made by bombarding other nuclides with neutrons.
The equations below represent a sequence of reactions converting stable iron–58 to
cobalt–60, which is used in medical treatments.
Equation 1:
58
26
Fe 1
1
0
n →
59
26
Fe
Equation 2:
59
26
Fe →
59
27
Co 1
0
21
e
Equation 3:
59
27
Co 1 X →
60
27
Co
82 State the neutron to proton ratio for an atom of the
58
Fe in equation 1. [1]
83 State, in terms of elements, why equation 2 represents a transmutation reaction. [
1]
84 Identify the particle represented by X in equation 3. [
1]
85 Determine the fraction of an original sample of Co-60 that remains unchanged after
15.813 years. [
1]
P.S./CHEMISTRY
P.S./CHEMISTRY
Printed on Recycled Paper

The University of the State of New York
REGENTS HIGH SCHOOL EXAMINATION
PHYSICAL SETTING
CHEMISTRY
Friday, January 27, 2023 — 9:15 a.m. to 12:15 p.m., only
ANSWER BOOKLET
Student . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Teacher. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
School . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Grade . . . . . . . . .
Record your answers for Part B–2 and Part C in this booklet.
51
52
53
54
Part B–2

55
56
57 __________________________________________ and __________________________________________
58
59 mL
60
P.S./Chem. Answer Booklet–Jan. ’23 [2]

61
62 M
63
64
65
P.S./Chem. Answer Booklet–Jan. ’23 [3] [OVER]

P.S./Chem. Answer Booklet–Jan. ’23 [4]
66
67 mol
68
69
70 From ______________________________________ to ___________________________________________
71 J
72
73
Part C

P.S./Chem. Answer Booklet–Jan. ’23 [5] [OVER]
74
75
76
77
78
Reaction Coordinate
Potential Energy

79
80 V
81
82
83
84
85
P.S./Chem. Answer Booklet–Jan. ’23 [6]

P.S./CHEMISTRY
P.S./CHEMISTRY
Printed on Recycled Paper

Examination Date
Question
Number
Scoring
Key
Question
Type
Credit Weight
Physical Setting/Chemistry
January '23
1 1
MC 1 1
Physical Setting/Chemistry
January '23
2 4
MC 1 1
Physical Setting/Chemistry
January '23
3 4
MC 1 1
Physical Setting/Chemistry
January '23
4 2
MC 1 1
Physical Setting/Chemistry
January '23
5 3
MC 1 1
Physical Setting/Chemistry
January '23
6 3
MC 1 1
Physical Setting/Chemistry
January '23
7 4
MC 1 1
Physical Setting/Chemistry
January '23
8 2
MC 1 1
Physical Setting/Chemistry
January '23
9 4
MC 1 1
Physical Setting/Chemistry
January '23
10 2
MC 1 1
Physical Setting/Chemistry
January '23
11 3
MC 1 1
Physical Setting/Chemistry
January '23
12 2
MC 1 1
Physical Setting/Chemistry
January '23
13
3
MC 1 1
Physical Setting/Chemistry
January '23
14 3
MC 1 1
Physical Setting/Chemistry
January '23
15
1
MC 1 1
Physical Setting/Chemistry
January '23
16 1
MC 1 1
Physical Setting/Chemistry
January '23
17 4
MC 1 1
Physical Setting/Chemistry
January '23
18 4
MC 1 1
Physical Setting/Chemistry
January '23
19 3
MC 1 1
Physical Setting/Chemistry
January '23
20 1
MC 1 1
Physical Setting/Chemistry
January '23
21 2
MC 1 1
Physical Setting/Chemistry
January '23
22 3
MC 1 1
Physical Setting/Chemistry
January '23
23 3
MC 1 1
Physical Setting/Chemistry
January '23
24 1
MC 1 1
Physical Setting/Chemistry
January '23
25 2
MC 1 1
Physical Setting/Chemistry
January '23
26 4
MC 1 1
Physical Setting/Chemistry
January '23
27 1
MC 1 1
Physical Setting/Chemistry
January '23
28 4
MC 1 1
Physical Setting/Chemistry
January '23
29 4
MC 1 1
Physical Setting/Chemistry
January '23
30 3
MC 1 1
Physical Setting/Chemistry
January '23
31 4
MC 1 1
Physical Setting/Chemistry
January '23
32 2
MC 1 1
Physical Setting/Chemistry
January '23
33 3
MC 1 1
Physical Setting/Chemistry
January '23
34 3
MC 1 1
Physical Setting/Chemistry
January '23
35 2
MC 1 1
Physical Setting/Chemistry
January '23
36 4
MC 1 1
Physical Setting/Chemistry
January '23
37 4
MC 1 1
Physical Setting/Chemistry
January '23
38 2
MC 1 1
Physical Setting/Chemistry
January '23
39 3
MC 1 1
Physical Setting/Chemistry
January '23
40 2
MC 1 1
Physical Setting/Chemistry
January '23
41 3
MC 1 1
Physical Setting/Chemistry
January '23
42 3
MC 1 1
Physical Setting/Chemistry
January '23
43 3
MC 1 1
Physical Setting/Chemistry
January '23
44 2
MC 1 1
Physical Setting/Chemistry
January '23
45 1
MC 1 1
Physical Setting/Chemistry
January '23
46 4
MC 1 1
Physical Setting/Chemistry
January '23
47 2
MC 1 1
Physical Setting/Chemistry
January '23
48 4
MC 1 1
Physical Setting/Chemistry
January '23
49 2
MC 1 1
Physical Setting/Chemistry
January '23
50 1
MC 1 1
The State Education Department / The University of the State of New York
Regents Examination in Physical Setting/Chemistry – January 2023
Scoring Key: Parts A and B-1 (Multiple-Choice Questions)
P.S./Chemistry Scoring Key 1 of 2

Examination Date
Question
Number
Scoring
Key
Question
Type
Credit Weight
Physical Setting/Chemistry
January '23
51 -
CR 1 1
Physical Setting/Chemistry
January '23
52 -
CR 1 1
Physical Setting/Chemistry
January '23
53 -
CR 1 1
Physical Setting/Chemistry
January '23
54 -
CR 1 1
Physical Setting/Chemistry
January '23
55 -
CR 1 1
Physical Setting/Chemistry
January '23
56 -
CR 1 1
Physical Setting/Chemistry
January '23
57 -
CR 1 1
Physical Setting/Chemistry
January '23
58 -
CR 1 1
Physical Setting/Chemistry
January '23
59 -
CR 1 1
Physical Setting/Chemistry
January '23
60
-
CR 1 1
Physical Setting/Chemistry
January '23
61 -
CR 1 1
Physical Setting/Chemistry
January '23
62 -
CR 1 1
Physical Setting/Chemistry
January '23
63 -
CR 1 1
Physical Setting/Chemistry
January '23
64
-
CR 1 1
Physical Setting/Chemistry
January '23
65 -
CR 1 1
Physical Setting/Chemistry
January '23
66 -
CR 1 1
Physical Setting/Chemistry
January '23
67 -
CR 1 1
Physical Setting/Chemistry
January '23
68 -
CR 1 1
Physical Setting/Chemistry
January '23
69 -
CR 1 1
Physical Setting/Chemistry
January '23
70 -
CR 1 1
Physical Setting/Chemistry
January '23
71 -
CR 1 1
Physical Setting/Chemistry
January '23
72 -
CR 1 1
Physical Setting/Chemistry
January '23
73 -
CR 1 1
Physical Setting/Chemistry
January '23
74 -
CR 1 1
Physical Setting/Chemistry
January '23
75 -
CR 1 1
Physical Setting/Chemistry
January '23
76 -
CR 1 1
Physical Setting/Chemistry
January '23
77 -
CR 1 1
Physical Setting/Chemistry
January '23
78 -
CR 1 1
Physical Setting/Chemistry
January '23
79 -
CR 1 1
Physical Setting/Chemistry
January '23
80 -
CR 1 1
Physical Setting/Chemistry
January '23
81
-
CR 1 1
Physical Setting/Chemistry
January '23
82
-
CR 1 1
Physical Setting/Chemistry
January '23
83 -
CR 1 1
Physical Setting/Chemistry
January '23
84 -
CR 1 1
Physical Setting/Chemistry
January '23
85 -
CR 1 1
Key
MC = Multiple-choice question
The chart for determining students' final examination scores for the January 2023 Regents
Examination in Physical Setting/Chemistry will be posted on the Department’s web site at
https://www.nysedregents.org/Chemistry/
on the day of the examination. Conversion charts provided
for the previous administrations of the Physical Setting/Chemistry examination must NOT be used to
determine students’ final scores for this administration.
Regents Examination in Physical Setting/Chemistry – January 2023
Scoring Key: Parts B-2 and C (Constructed-Response Questions)
CR = Constructed-response question
P.S./Chemistry Scoring Key 2 of 2

FOR TEACHERS ONLY
The University of the State of New York
REGENTS HIGH SCHOOL EXAMINATION
PHYSICAL SETTING/CHEMISTRY
Friday, January 27, 2023 — 9:15 a.m. to 12:15 p.m., only
RATING GUIDE
Directions to the Teacher:
Refer to the directions on page 2 before rating student papers.
Updated information regarding the rating of this examination may be posted on the New York
State Education Department’s web site during the rating period. Check this web site
at: http://www.nysed.gov/state-assessment/high-school-regents-examinations
and select the link
“Scoring Information” for any recently posted information regarding this examination. This site
should be checked before the rating process for this examination begins and several times
throughout the Regents Examination period.

P.S./Chem. Rating Guide–Jan. ’23 [2]
Directions to the Teacher
Follow the procedures below for scoring student answer papers for the Regents Examination in
Physical Setting/Chemistry. Additional information about scoring is provided in the publication
Information Booklet for Scoring Regents Examinations in the Sciences.
At least two science teachers must participate in the scoring of the Part B–2 and Part C open-ended
questions on a student’s paper. Each of these teachers should be responsible for scoring a selected number
of the open-ended questions on each answer paper. No one teacher is to score more than approximately
one-half of the open-ended questions on a student’s answer paper. Teachers may not score their own
students’ answer papers.
Students’ responses must be scored strictly according to the Rating Guide. For open-ended questions,
credit may be allowed for responses other than those given in the rating guide if the response is a scientifically
accurate answer to the question and demonstrates adequate knowledge, as indicated by the examples in the
rating guide. Do not attempt to correct the student’s work by making insertions or changes of any kind. On
the student’s separate answer sheet, for each question, record the number of credits earned and the
teacher’s assigned rater/scorer letter.
Fractional credit is not allowed. Only whole-number credit may be given for a response. If the student
gives more than one answer to a question, only the first answer should be rated. Units need not be given
when the wording of the questions allows such omissions.
For hand scoring, raters should enter the scores earned in the appropriate boxes printed on the
separate answer sheet. Next, the rater should add these scores and enter the total in the box labeled
“Total Raw Score.” Then the student’s raw score should be converted to a scale score by using the
conversion chart that will be posted on the Department’s web site at: http://www.nysed.gov/state-assessment/
high-school-regents-examinations
on Friday, January 27, 2023. The student’s scale score should be entered
in the box labeled “Scale Score” on the student’s answer sheet. The scale score is the student’s final examination
score.
Schools are not permitted to rescore any of the open-ended questions on this exam after each
question has been rated once, regardless of the final exam score. Schools are required to ensure
that the raw scores have been added correctly and that the resulting scale score has been
determined accurately.
Because scale scores corresponding to raw scores in the conversion chart may change from one
administration to another, it is crucial that, for each administration, the conversion chart provided for that
administration be used to determine the student’s final score.

P.S./Chem. Rating Guide–Jan. ’23 [3]
51 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
An atom of P-32 has 15 protons and 17 neutrons. An atom of P-31 also has 15 protons but has
16 neutrons.
These two atoms have the same number of protons but a different number of neutrons.
same number of p, different number of n
52 [1] Allow 1 credit for 16 or 61.
53 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
As atomic number increases, atomic radius increases.
Atomic radius increases.
Radius increases going down the group.
From top to bottom in Group 14, radius increases.
54 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Both germanium and silicon have 4 valence electrons in the ground state.
The Si and Ge atoms have the same number of outermost shell electrons.
55 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
A molecule of NH
3
is polar because the distribution of charge is asymmetrical.
The molecule has an uneven charge distribution.
The center of positive charge and the center of negative charge do not coincide.
Part B–2
Allow a total of 15 credits for this part. The student must answer all questions in this part.

P.S./Chem. Rating Guide–Jan. ’23 [4]
56 [1] Allow 1 credit.
Examples of 1-credit responses:
Note: Do not allow credit for or or for a bond because each represents
one electron and each represents two electrons.
57 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
polar covalent bonds and ionic bonds
ionic and covalent
ionic and coordinate covalent
58 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The gas atoms are closer together, so the volume of the gas is smaller. A smaller volume means
a greater density because the mass remained the same.
The atoms have a smaller average distance between them.
Average distance decreases.
59 [1] Allow 1 credit for 100. mL or 100 mL.
Cl H
ClH
ClH
Cl H

60 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The number of helium atoms at 0.50 atm is equal to the number of helium atoms at 1.50 atm.
The number of atoms is the same.
equal
same
61 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The HCl(aq) has ions in water, which are mobile.
The ions in the solution move freely.
Hydrochloric acid solution contains H
1
(aq) and Cl
2
(aq) ions.
62 [1] Allow 1 credit for 0.110 M or any value from 0.11 M to 0.11012 M, inclusive.
63 [1] Allow 1 credit for 2.0 or 2 or two.
64 [1] Allow 1 credit for OH
2
ion or hydroxide.
Note: Do not allow credit for OH or hydroxyl or hydroxyl ion.
65 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
10
ten
tenfold
10 times
P.S./Chem. Rating Guide–Jan. ’23 [5]

P.S./Chem. Rating Guide–Jan. ’23 [6]
66 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The formula is the simplest whole number ratio of atoms of the elements in the compound.
The formula Na
2
B
4
O
7
cannot be reduced.
67 [1] Allow 1 credit for 8 mol.
68 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
0.200 mol 5
x
_________
381 g/mol
(0.200 mol)(381 g/mol)
(0.2)(381)
0.200 mol 3
381 g
_____
1 mol
Note: Allow credit for a setup using a gram-formula mass for borax (Na
2
B
4
O
7
•10H
2
O) with any
value from 381 g/mol to 382 g/mol, inclusive.
69 [1] Allow 1 credit for 3 or three.
70 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
from the air to the water
from air to H
2
O
71 [1] Allow 1 credit for 31 000 J or any value from 30 700 J to 31 122 J, inclusive.
Part C
Allow a total of 20 credits for this part. The student must answer all questions in this part.
72 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The heat term is on the right side of the equation.
The 91.8 kJ of energy is a product.
The energy term is on the product side.
Note: Do not accept “Heat is released.” without stating supporting evidence from the equation.
73 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The rate of the forward reaction is equal to the rate of the reverse reaction.
The rates are the same.
equal
same
74 [1] Allow 1 credit for showing that the PE of the products is lower than the PE of the reactants.
Example of a 1-credit response:
75 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
There are 4 moles of gas on the left side and 2 moles of gas on the right side of the equation,
so the shift to the right relieves the increased pressure.
There are more moles of gas on the left side of the equation than on the other.
fewer moles, less pressure
Reaction Coordinate
Potential Energy
P.S./Chem. Rating Guide–Jan. ’23 [7]

76 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Adding the iron will increase the rate of production of the NH
3
(g).
The rate of the forward reaction would increase.
Forward rate increases.
The NH
3
(g) will be produced faster.
77 [1] Allow 1 credit for C or carbon.
78 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The formula mass of ammonium cyanate is equal to the formula mass of urea.
The formula masses of the two compounds are the same.
Ammonium cyanate and urea both have a formula mass of 60. u.
equal
same
79 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Urea and water have similar molecular polarities.
Water molecules and urea molecules are both polar.
Urea is polar.
80 [1] Allow 1 credit for any value from 1.102 V to 1.108 V, inclusive.
81 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Zn(s) → Zn
21
(aq) 1 2e
2
Zn → 2e
2
1 Zn
12
Note: Do not allow credit for the e without the minus sign (2).
P.S./Chem. Rating Guide–Jan. ’23 [8]

82 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
32:26
32
___
26
16 to 13
83 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
An atom of iron is changed to an atom of cobalt.
The Fe-59 has an atomic number of 26 and becomes Co-59 with an atomic number of 27.
One element changed to a different element.
84 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
1
0
n
n
neutron
85 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
1
__
8
0.125
12.5%
P.S./Chem. Rating Guide–Jan. ’23 [9]

P.S./Chem. Rating Guide–Jan. ’23 [10]
The Chart for Determining the Final Examination Score for the January 2023
Regents Examination in Physical Setting/Chemistry will be posted on the
Department’s web site at: http://www.nysed.gov/state-assessment/high-school-
regents-examinations on Friday, January 27, 2023. Conversion charts provided
for previous administrations of the Regents Examination in Physical
Setting/Chemistry must NOT be used to determine students’ final scores for
this administration.
Regents Examination in Physical Setting/Chemistry
January 2023
Chart for Converting Total Test Raw Scores to
Final Examination Scores (Scale Scores)
Online Submission of Teacher Evaluations of the Test to the Department
Suggestions and feedback from teachers provide an important contribution to the test
development process. The Department provides an online evaluation form for State
assessments. It contains spaces for teachers to respond to several specific questions and to
make suggestions. Instructions for completing the evaluation form are as follows:
1. Go to https://www.surveymonkey.com/r/8LNLLDW
.
2. Select the test title.
3. Complete the required demographic fields.
4. Complete each evaluation question and provide comments in the space provided.
5. Click the SUBMIT button at the bottom of the page to submit the completed form.

P.S./Chem. Rating Guide–Jan. ’23 [11]
Map to Core Curriculum
# $$!""!" $
#!"# !
(%$"$ !!&"$% $& $& $&
"
&(
&(
&(
!!#'$((
!!#'$((
!!#'$((
!!$!%!(
"
(
(
(
"
(
(
(
(
(
"
(
(
" !!!
(
(
(
"
(
(
(
!
&"!

Raw Scale Raw Scale Raw Scale Raw Scale
Score Score Score Score Score Score Score Score
85
100
63
74
41
59
19
38
84
98
62
73
40
58
18
37
83
96
61
72
39
57
17
36
82
95
60
72
38
57
16
34
81
93
59
71
37
56
15
33
80
92
58
70
36
55
14
31
79
90
57
69
35
54
13
30
78
89
56
69
34
54
12
28
77
87
55
68
33
53
11
26
76
86
54
67
32
52
10
24
75
85
53
67
31
51
9
22
74
84
52
66
30
50
8
20
73
83
51
66
29
49
7
18
72
82
50
65
28
48
6
16
71
81
49
64
27
47
5
14
70
80
48
63
26
46
4
11
69
79
47
63
25
45
3
9
68
78
46
62
24
44
2
6
67
77
45
61
23
43
1
3
66
76
44
61
22
42
0
0
65
75
43
60
21
41
64
75
42
59
20
40
Schools are not permitted to rescore any of the open-ended questions on this exam after each question has
been rated once, regardless of the final exam score. Schools are required to ensure that the raw scores have
been added correctly and that the resulting scale score has been determined accurately.
Because scale scores corresponding to raw scores in the conversion chart change from one administration to
another, it is crucial that for each administration the conversion chart provided for that administration be used to
determine the student’s final score. The chart above is usable only for this administration of the Regents Examination
in Physical Setting/Chemistry.
The State Education Department / The University of the State of New York
Regents Examination in Physical Setting/Chemistry – January 2023
Chart for Converting Total Test Raw Scores to Final Examination Scores (Scale Scores)
To determine the student’s final examination score, find the student’s total test raw score in the column labeled “Raw
Score” and then locate the scale score that corresponds to that raw score. The scale score is the student’s final
examination score. Enter this score in the space labeled “Scale Score” on the student’s answer sheet.
P.S./Chemistry 1 of 1
