109/29/2023 CS321570-AO
6 Months Through 4 Years of Age
Updated (2023–2024 Formula)
Pfizer-BioNTech COVID-19 Vaccine
Standing Orders for Administering Vaccine
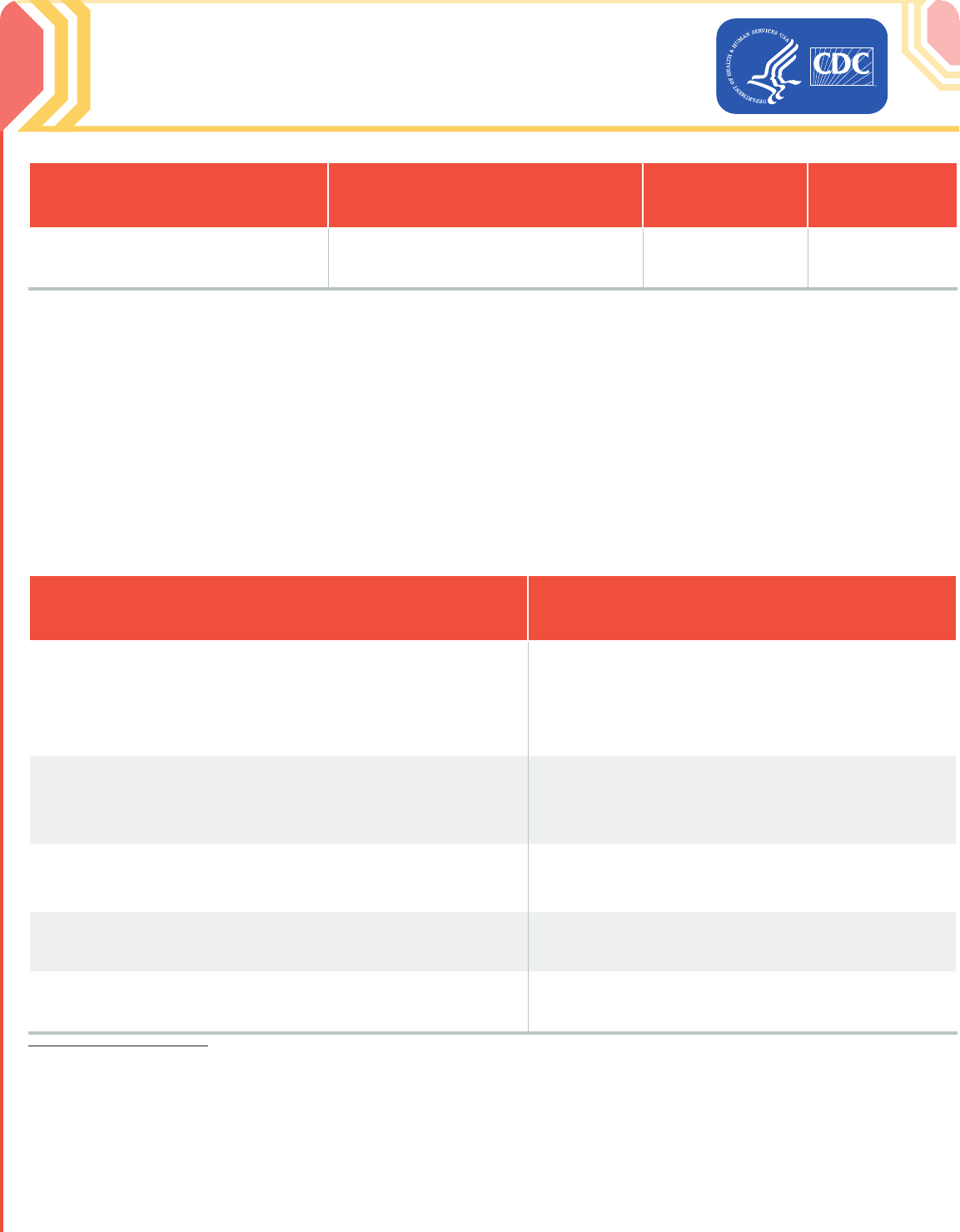
2023–24 Formula Vaccine
Presentation
Diluent
Dose/Injection
Amount
Route
Multidose vial with yellow cap and
yellow label
1.1 mL of 0.9% sodium chloride (normal
saline, preservative-free) diluent
0.3 mL/3 µg
Intramuscular
(IM) injection
Purpose
To reduce morbidity and mortality from coronavirus disease
2019 (COVID-19) by vaccinating persons who meet the criteria
established by the Centers for Disease Control and Prevention’s
Advisory Committee on Immunization Practices (ACIP).
Policy
Where authorized under state law, standing orders enable eligible
nurses and other health care professionals (e.g., pharmacists)
to assess and vaccinate persons who meet the criteria in the
"Procedure" section below without the need for clinician
examination or direct order from the attending provider at the
time of the interaction.
Procedure
Assess children 6 months through 4 years of age for vaccination with the 2023–24 Pfizer-BioNTech COVID-19 Vaccine based on the
following criteria:
People who are NOT moderately or severely immunocompromised
*
* Persons with a recent SARS-CoV-2 infection may consider delaying vaccination by 3 months from symptom onset or positive test (if infection was asymptomatic).
† COVID-19 vaccination history refers to previous receipt of dose(s) of Original monovalent mRNA, bivalent mRNA vaccine, Updated (2023–2024 Formula), or a combination of the
three, unless otherwise specified.
‡ An 8-week interval between the first and second COVID-19 vaccine (Moderna, Novavax, and Pfizer-BioNTech) doses might be optimal for some people as it might reduce the small
risk of myocarditis and pericarditis associated with these vaccines.
§ People who are recommended to receive a multidose mRNA series for initial vaccination (i.e., children ages 6 months–4 years and people who are moderately or severely
immunocompromised) should receive all doses from the same manufacturer. However, in the following exceptional situations a different age-appropriate COVID-19 vaccine product
may be administered: the same vaccine is not available, the person would otherwise not complete the vaccination series, or the person starts but is unable to complete a vaccination
series with the same vaccine due to a contraindication.
¶ For children who have received 1 Moderna and 1 Pfizer-BioNTech vaccines of any formulation, follow a 3-dose schedule. A third dose of either Moderna vaccine or Pfizer-BioNTech
vaccine should be administered at least 8 weeks after the second dose.
COVID-19 vaccination history
†
(regardless of COVID-19
vaccine formula)
Schedule for administration of 2023-24 Pfizer-
BioNTech COVID-19 Vaccine
Unvaccinated Give a 3-dose initial series. Administer:
Dose 1 now
Dose 2 at least 3–8 weeks after Dose 1
‡
Dose 3 at least 8 weeks after Dose 2
1 previous dose of any Pfizer-BioNTech COVID-19 Vaccine (Dose 1)
§
Complete series. Administer:
Dose 2 at least 3–8 weeks after Dose 1
‡
Dose 3 at least 8 weeks after Dose 2
2 doses of any Pfizer-BioNTech COVID-19 Vaccine (Doses 1 and 2)
§¶
Complete series. Administer:
Dose 3 at least 8 weeks after Dose 2
3 or more doses Pfizer-BioNTech COVID-19 Vaccine, NOT including
at least 1 dose of 2023–24 COVID-19 vaccine
§
Give 1 dose at least 8 weeks (2 months) after the previous
dose.
3 or more doses Pfizer-BioNTech COVID-19 Vaccine, INCLUDING at
least 1 dose of 2023–24 COVID-19 vaccine
§
No further doses are indicated.

209/29/2023 CS321570-AO
6 Months Through 4 Years of Age
Updated (2023–2024 Formula)
Pfizer-BioNTech COVID-19 Vaccine
Standing Orders for Administering Vaccine
People who ARE moderately or severely immunocompromised
COVID-19 vaccination history
*
(regardless of COVID-19
vaccine formula)
Schedule for administration of 2023-24 Pfizer-
BioNTech COVID-19 Vaccine
Unvaccinated Give a 3-dose initial series. Administer:
Dose 1 now
Dose 2 at least 3 weeks after Dose 1
Dose 3 at least 8 weeks after Dose 2
1 previous dose of any Pfizer-BioNTech COVID-19 Vaccine (Dose 1)
†
Complete series. Administer:
Dose 2 at least 3 weeks after Dose 1
Dose 3 at least 8 weeks after Dose 2
2 doses of any Pfizer-BioNTech COVID-19 Vaccine
(Doses 1 and 2)
†‡
Complete series. Administer:
Dose 3 at least 8 weeks after Dose 2
3 or more doses of Pfizer-BioNTech COVID-19 Vaccine, NOT
including at least 1 dose of 2023–24 COVID-19 vaccine
†
Give 1 dose at least 8 weeks (2 months) after the previous
dose.
3 or more doses of Pfizer-BioNTech COVID-19 Vaccine, INCLUDING
at least 1 dose of 2023–24 COVID-19 vaccine
†
People who are moderately or severely
immunocompromised have the option to receive 1
additional dose at least 8 weeks (2 months) following
the last recommended dose.
Further additional dose(s) may be administered,
informed by the clinical judgement of a health care
provider and personal preference and circumstances.
Any further additional doses should be administered
at least 8 weeks (2 months) after the last COVID-19
vaccine dose.
* COVID-19 vaccination history refers to previous receipt of dose(s) of Original monovalent mRNA, bivalent mRNA vaccine, Updated (2023–2024 Formula), or a combination of the
three, unless otherwise specified.
† People who are recommended to receive a multidose mRNA series for initial vaccination (i.e., children ages 6 months–4 years and people who are moderately or severely
immunocompromised) should receive all doses from the same manufacturer. However, in the following exceptional situations a different age-appropriate COVID-19 vaccine product
may be administered: the same vaccine is not available, the person would otherwise not complete the vaccination series, or the person starts but is unable to complete a vaccination
series with the same vaccine due to a contraindication.
‡ For children who have received 1 Moderna and 1 Pfizer-BioNTech vaccines of any formulation, follow a 3-dose schedule. A third dose of either Moderna vaccine or Pfizer-BioNTech
vaccine should be administered at least 8 weeks after the second dose.

309/29/2023 CS321570-AO
6 Months Through 4 Years of Age
Updated (2023–2024 Formula)
Pfizer-BioNTech COVID-19 Vaccine
Standing Orders for Administering Vaccine
Additional Clinical Considerations
2023–24 Pfizer-BioNTech COVID-19 Vaccine may be
simultaneously administered with other routinely
recommended vaccines. There are additional
considerations for simultaneous administration of an
orthopoxvirus vaccine and COVID-19 vaccine.
Persons who have received HCT or CAR-T-cell therapy
○ Revaccinate persons who received doses of COVID-19
vaccine prior to or during HCT or CAR-T-cell therapy,
following the current COVID-19 vaccination schedule.
Revaccination should start at least 3 months (12 weeks) after
transplant or CAR-T-cell therapy.
For additional details and all clinical considerations, see
Interim Clinical Considerations for Use of COVID-19 Vaccines.
Contraindications:
History of a severe allergic reaction (e.g., anaphylaxis) after a
previous dose or to a component of the COVID-19 vaccine
Precautions:
History of:
A diagnosed non-severe allergy to a component of the
COVID-19 vaccine
Non-severe, immediate (onset less than 4 hours) allergic
reaction after administration of a previous dose of one
COVID-19 vaccine type, if receiving the same vaccine type
Moderate to severe acute illness, with or without fever
Multisystem inflammatory syndrome in children (MIS-C) or
adults (MIS-A)
Myocarditis or pericarditis within 3 weeks after a dose of any
COVID-19 vaccine
Administration
Provide all recipients and/or parents/legal guardians with a
copy of the current Fact Sheet for Recipients and Caregivers.
Prepare to administer the vaccine following the manufacturer's
guidance.
Prepare to administer vaccine by IM injection.
○ Needle gauge and length: Use a 22–25 gauge, 1-inch
*
○ For children:
» 6 months through 2 years: Vastus lateralis muscle in the
anterolateral thigh
†
» 2 through 4 years: Deltoid muscle in the upper arm
‡
* A 5/8 inch needle may be used if administering the vaccine in the deltoid muscle AND the skin is stretched tightly and the subcutaneous tissue is not bunched for children ages 1–4
years.
† The deltoid muscle in the upper arm may be used if the muscle mass is adequate for ages 1 through 2 years
‡ The vastus lateralis muscle in the anterolateral thigh may be used as an alternate site.
○ Administer Pfizer-BioNTech COVID-19 Vaccine by
intramuscular (IM) injection using the vial with yellow cap
and yellow label. Administer 0.3 mL/3 μg
.
Document Vaccination
Document each recipient's vaccine administration information:
Medical record: The vaccine and the date it was administered,
manufacturer, lot number, vaccination site and route, name
and title of the person administering the vaccine.
Vaccination record for recipient: Date of vaccination,
product name/manufacturer, lot number, and name/location
of the administering clinic or health care professional.
Immunization information system (IIS): Report the
vaccination to the appropriate state/local IIS.
Be Prepared to Manage Medical Emergencies
Consider observing persons after vaccination to monitor for
allergic reactions and syncope:
30 minutes for persons with:
○ A history of a non-severe, immediate (onset within 4 hours)
allergic reaction after a previous dose of one COVID-19
vaccine type, if receiving the same vaccine type
○ A history of a diagnosed non-severe allergy to a component
of the COVID-19 vaccine, if receiving the same vaccine type
15 minutes: All other persons
Syncope may occur in association with injectable vaccines.
Procedures should be in place to avoid falling injuries and
manage syncopal reactions.
Have a written protocol to manage medical emergencies
following vaccination.
Health care personnel who are trained and qualified to recognize
the signs and symptoms of anaphylaxis as well as administer
intramuscular epinephrine should be available at the vaccination
location at all times.
Report Adverse Events to the Vaccine Adverse Event
Reporting System (VAERS)
Adverse events that occur in a recipient following administration
of any licensed or authorized COVID-19 vaccine should be
reported to VAERS, including:
Vaccine administration errors, whether or not associated with
an adverse event
Serious adverse events, irrespective of attribution to
vaccination

409/29/2023 CS321570-AO
6 Months Through 4 Years of Age
Updated (2023–2024 Formula)
Pfizer-BioNTech COVID-19 Vaccine
Standing Orders for Administering Vaccine
Cases of Multisystem Inflammatory Syndrome (MIS) in adults
and children
Cases of myocarditis
Cases of pericarditis
Cases of COVID-19 that result in hospitalization or death
Reporting is also encouraged for any other clinically significant
adverse event, even if it is uncertain whether the vaccine caused
the event. Information on how to submit a report to VAERS is
available at https://vaers.hhs.gov or by calling 1-800-822-7967.
For More Information, Please See:
Interim Considerations: Preparing for the Potential
Management of Anaphylaxis after COVID-19 Vaccination
CDC’s General Best Practice Guidelines for Immunization,
“Preventing and Managing Adverse Reactions,”
Medical Management of Vaccine Reactions in Children and
Teens in a Community Setting
Note: For more information/guidance, please contact the immunization program at your state or local health department or the
appropriate state body (e.g., state board of medical/nursing/pharmacy practice).
Standing Orders Authorization
This policy and procedure shall remain in effect for all patients of the
effective until rescinded or until .
Medical director (or other authorized practitioner)
/ / .
Adapted with appreciation from the immunize.org standing orders.
