Evidence of Physical verses
Chemical Change
VOCABULARY – physical properties,
chemical properties, physical change,
chemical change, chemical reaction,
Law of Conservation of Mass
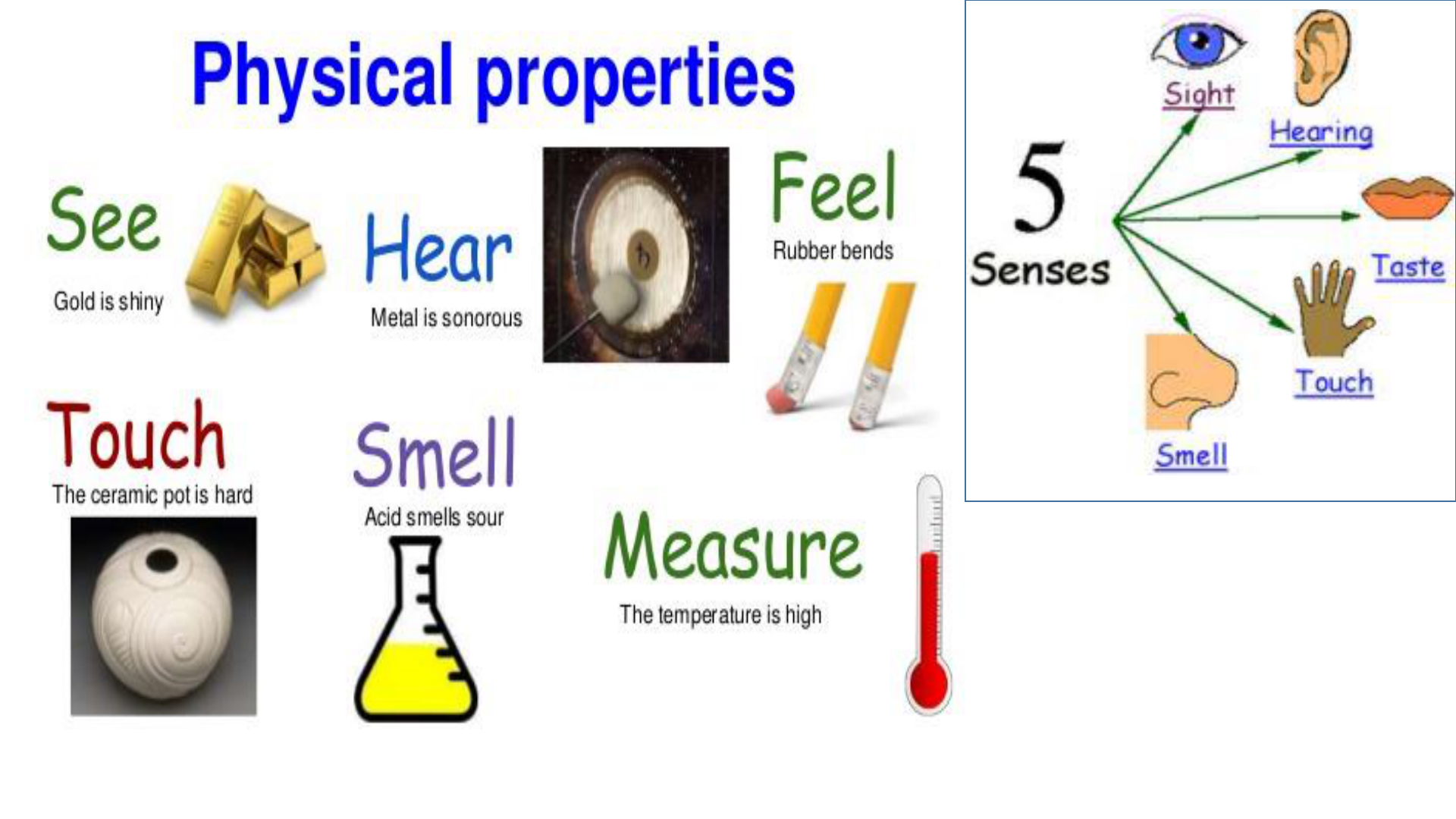
Physical Properties
All matter has both physical and chemical
properties useful to scientists in the
classification of it.
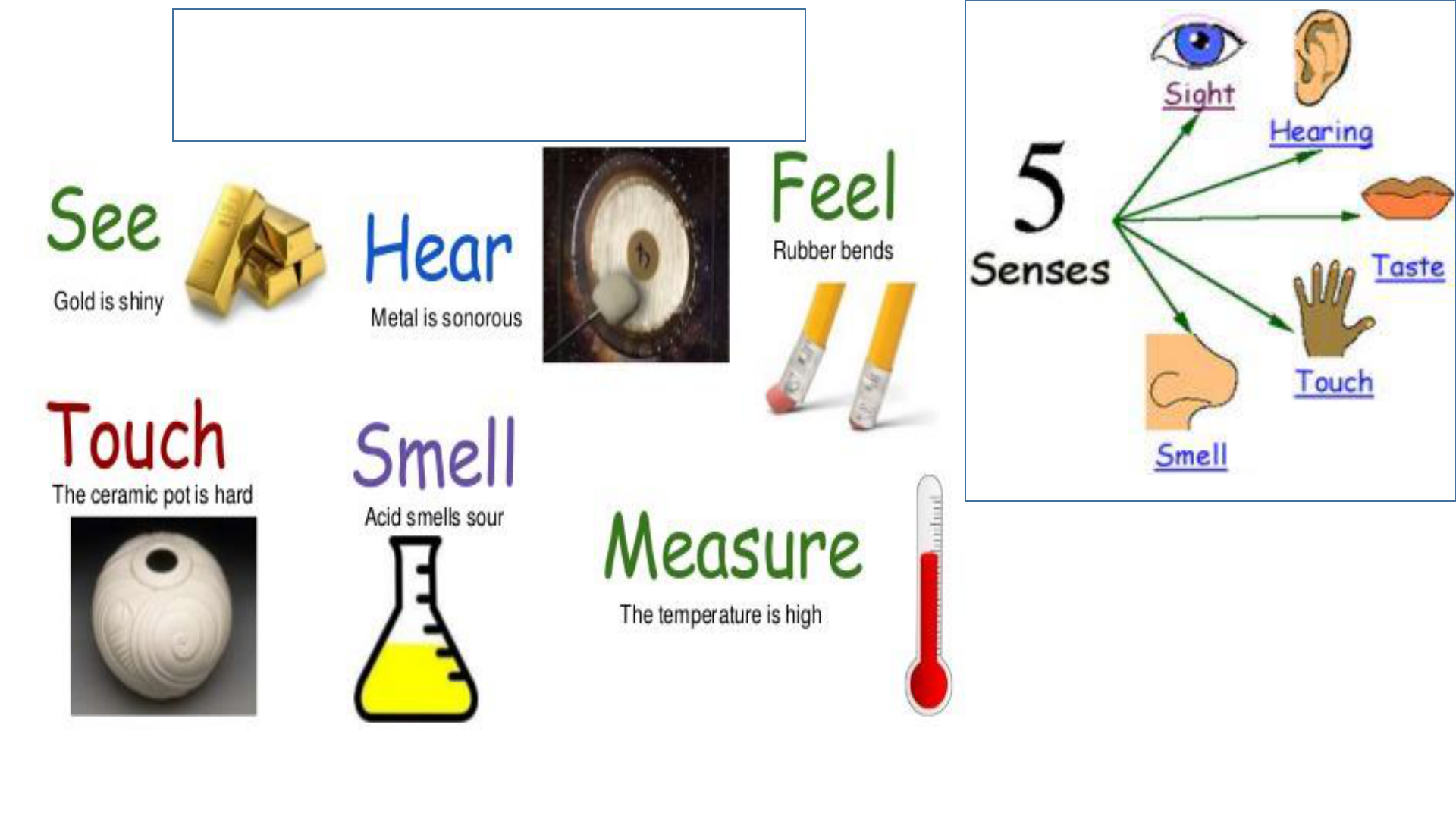
Typical physical properties we will consider
are: color, odor, density, hardness,
solubility, phase of matter, melting points
or boiling points.
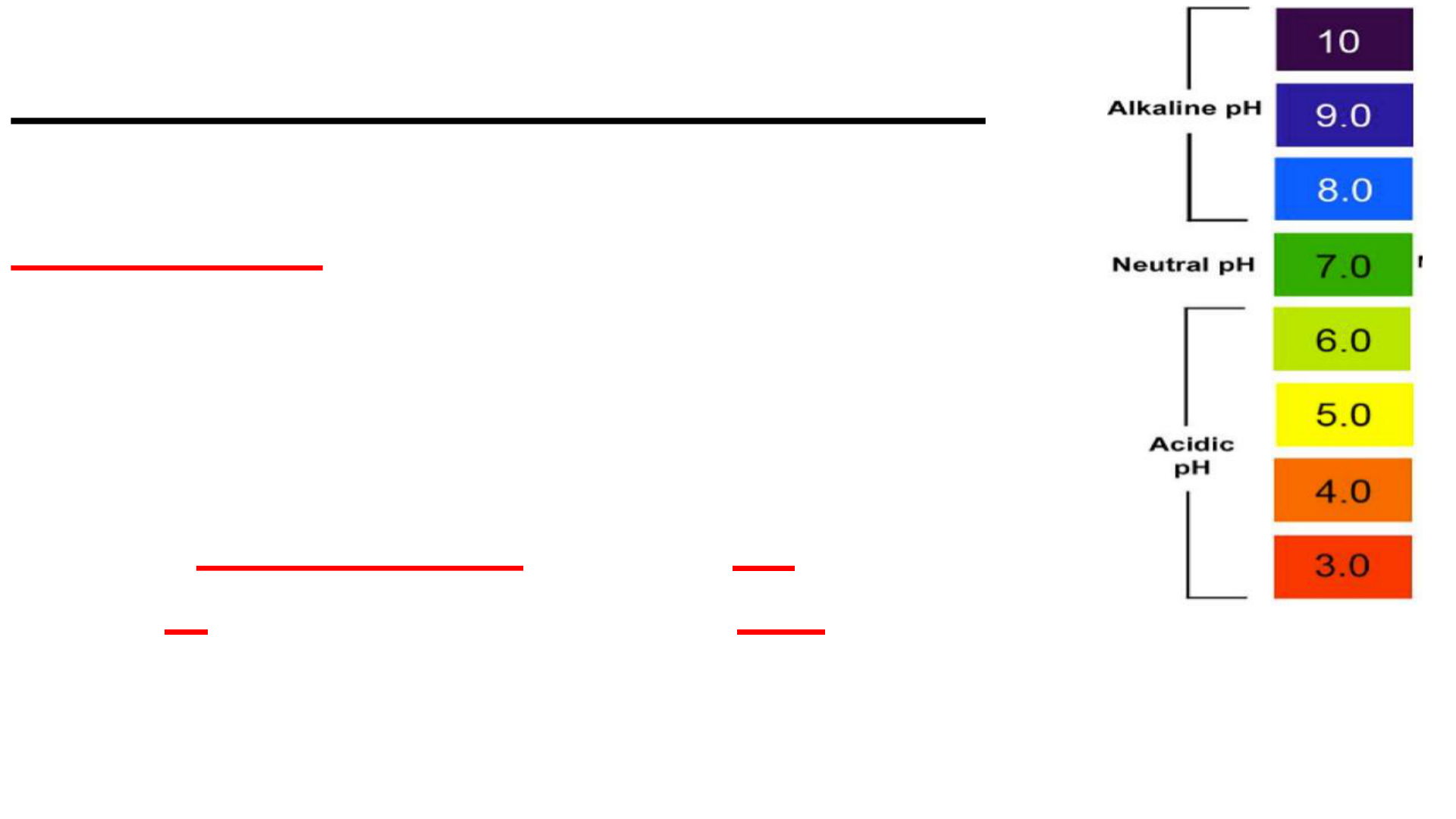
Chemical Properties
These are determined by the
reactivity of a substance with
another substance.
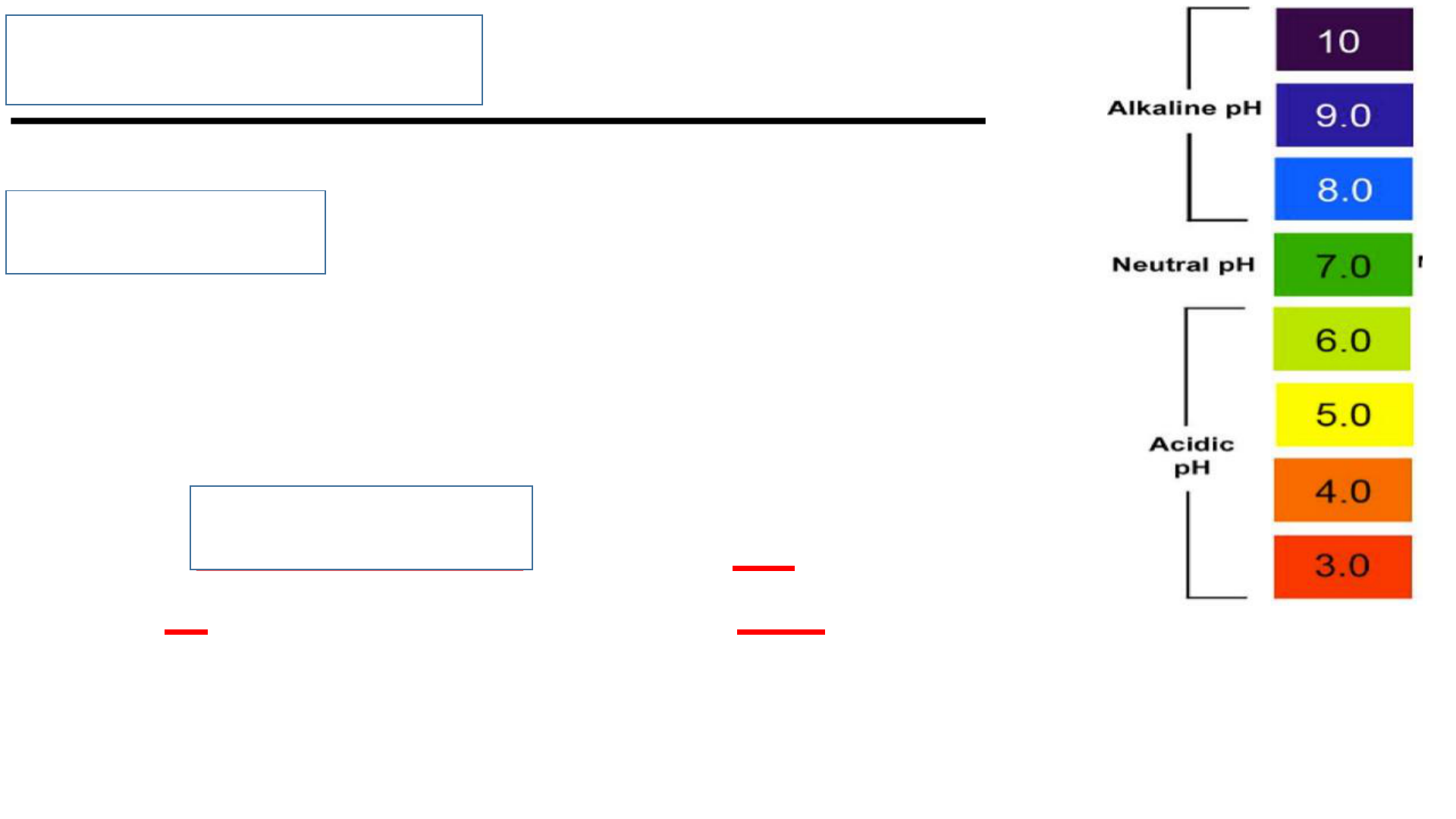
Examples: acidity or basicity
reactions with oxygen or other gases
What properties cause A to react
with B to possibly form AB?
Caution: do not get hung up on the nuisances between these two.
Simply refer to the definition as defined here in class.
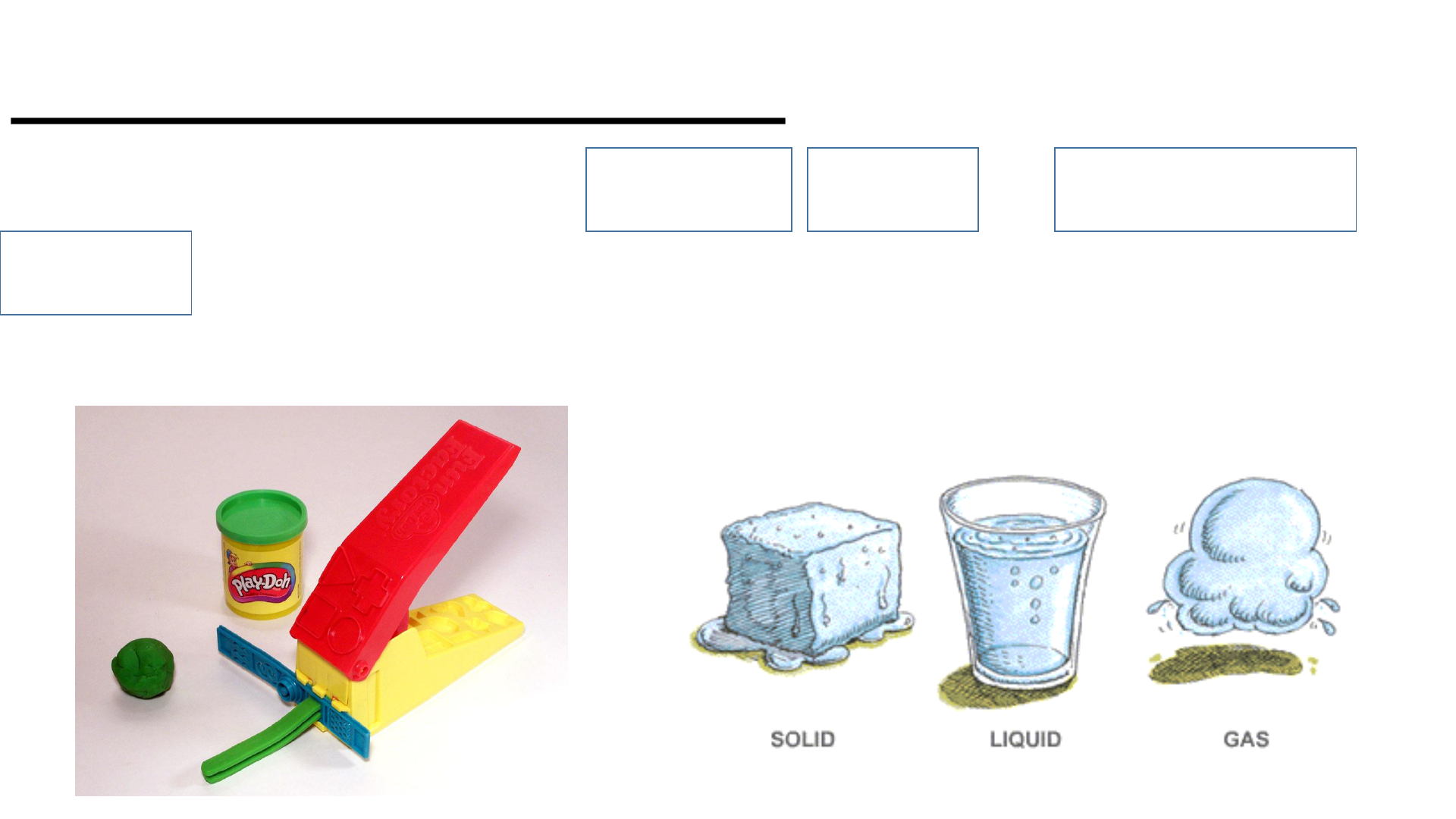
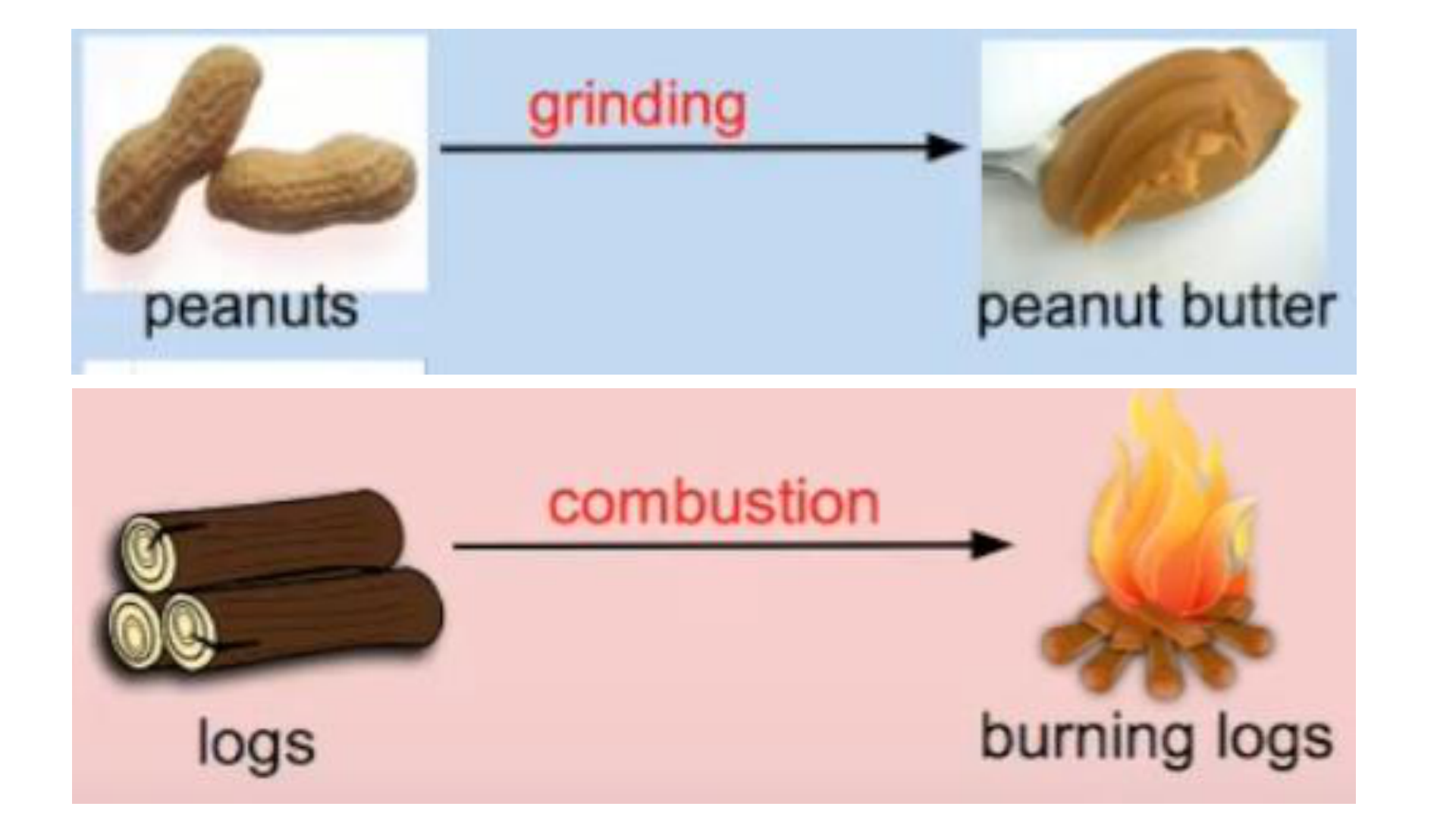
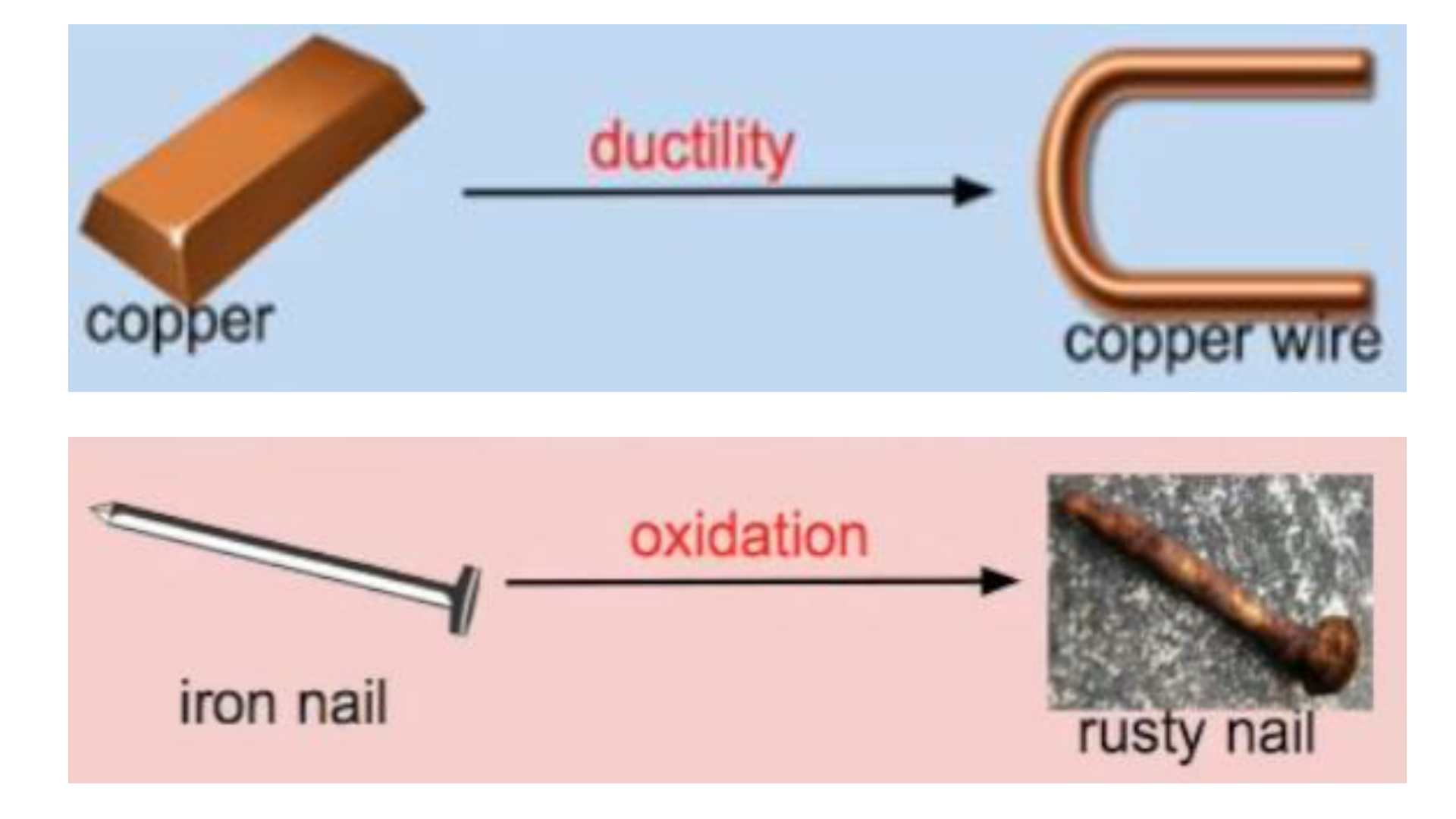
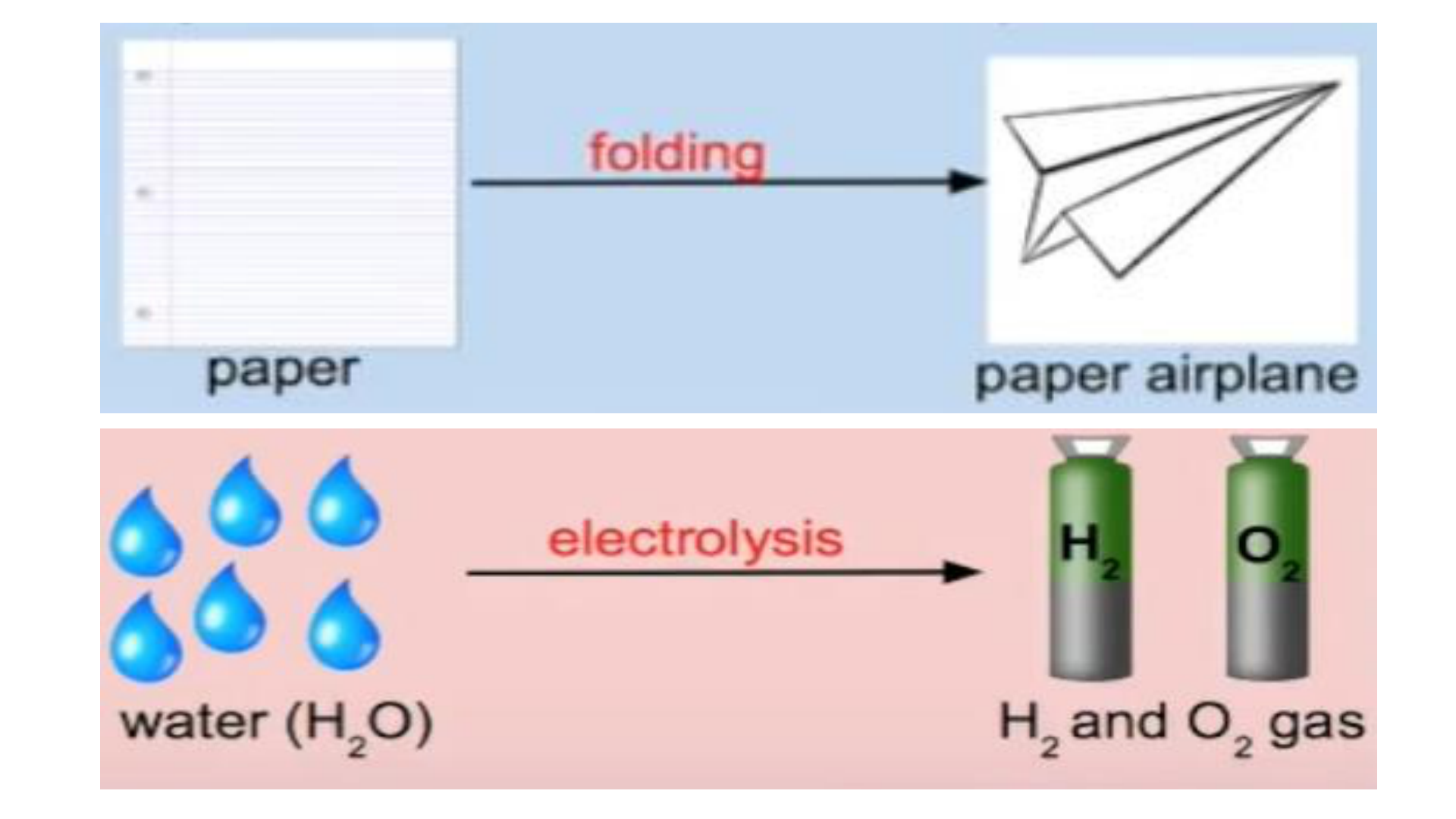
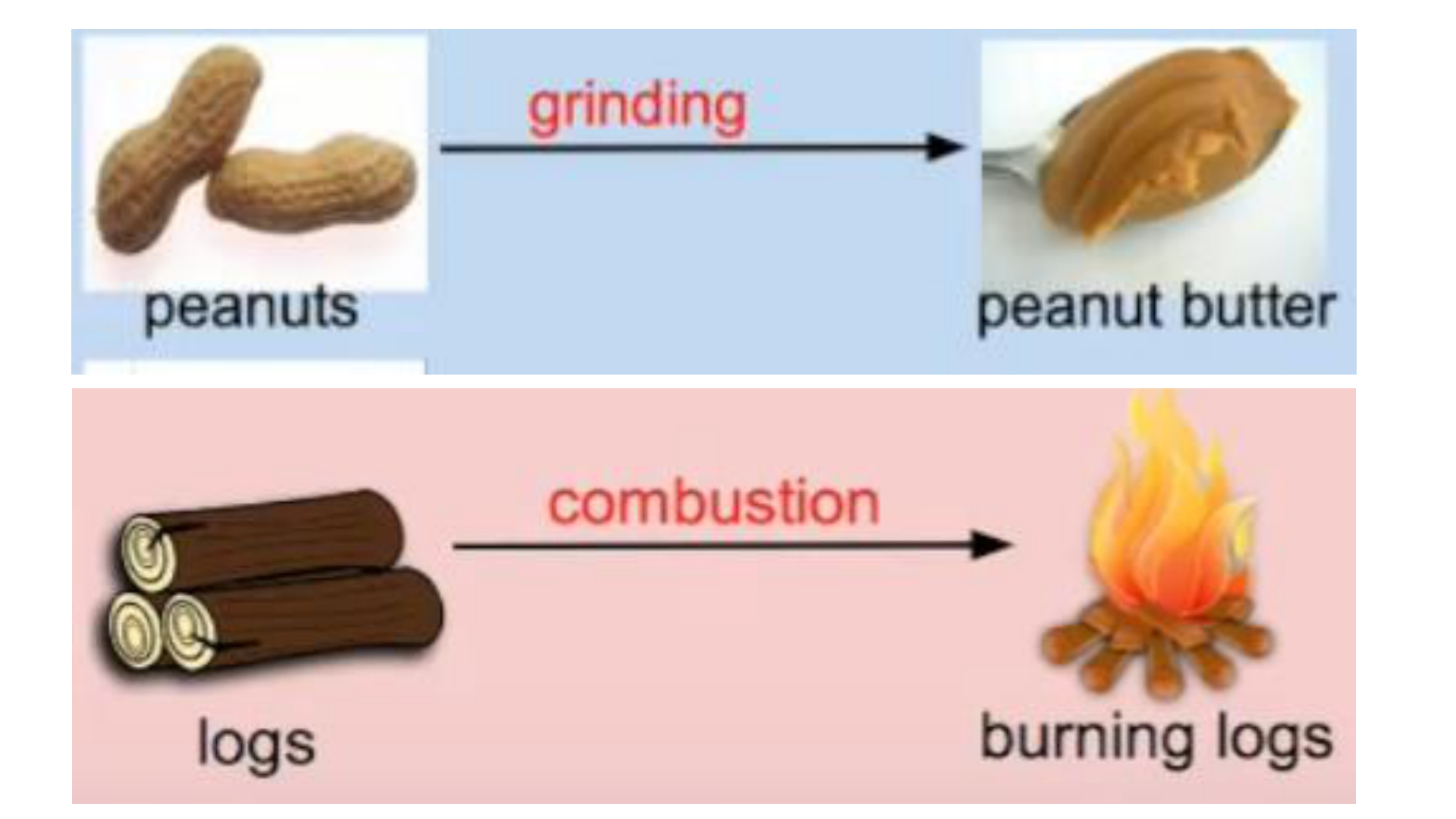
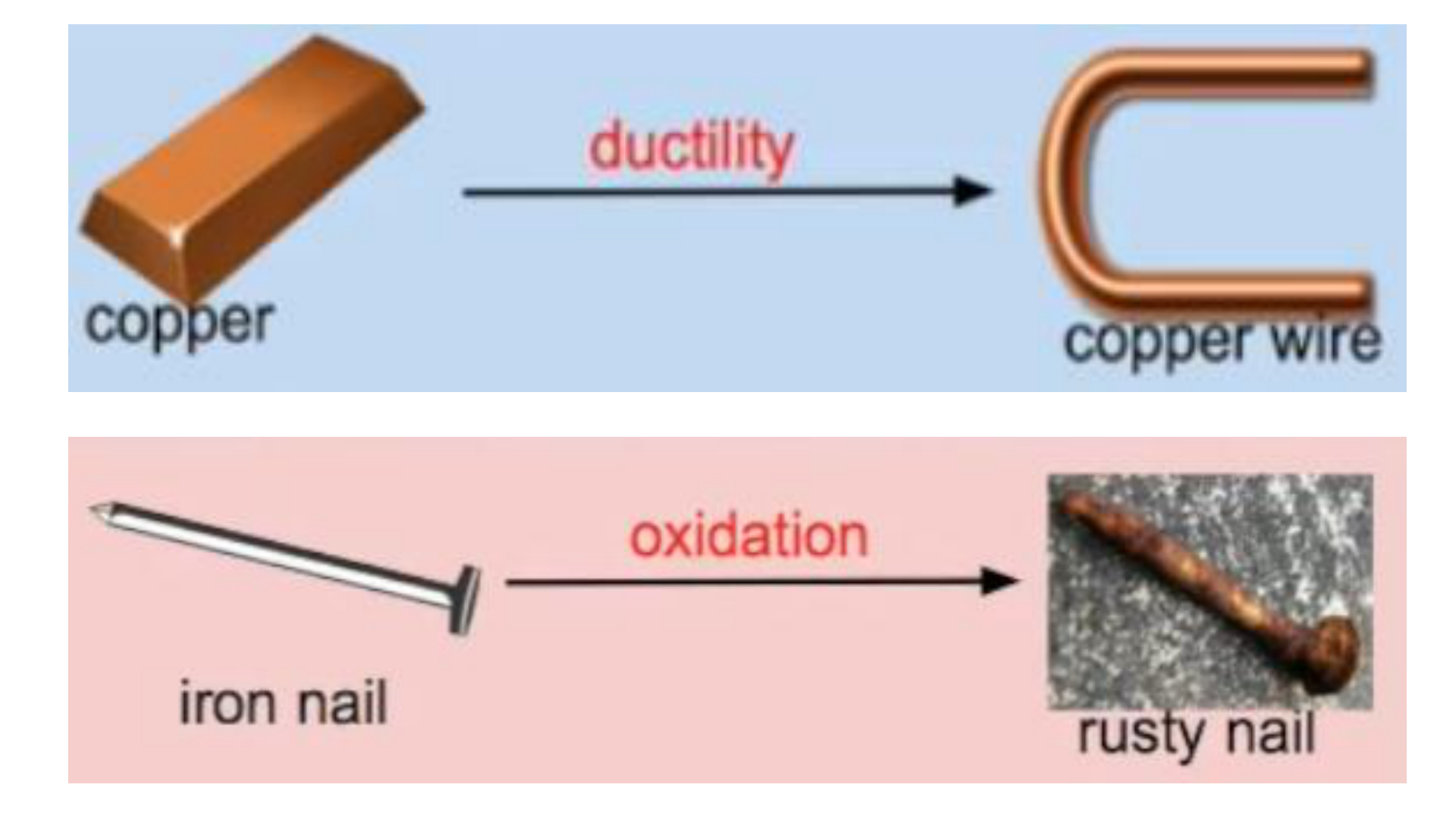
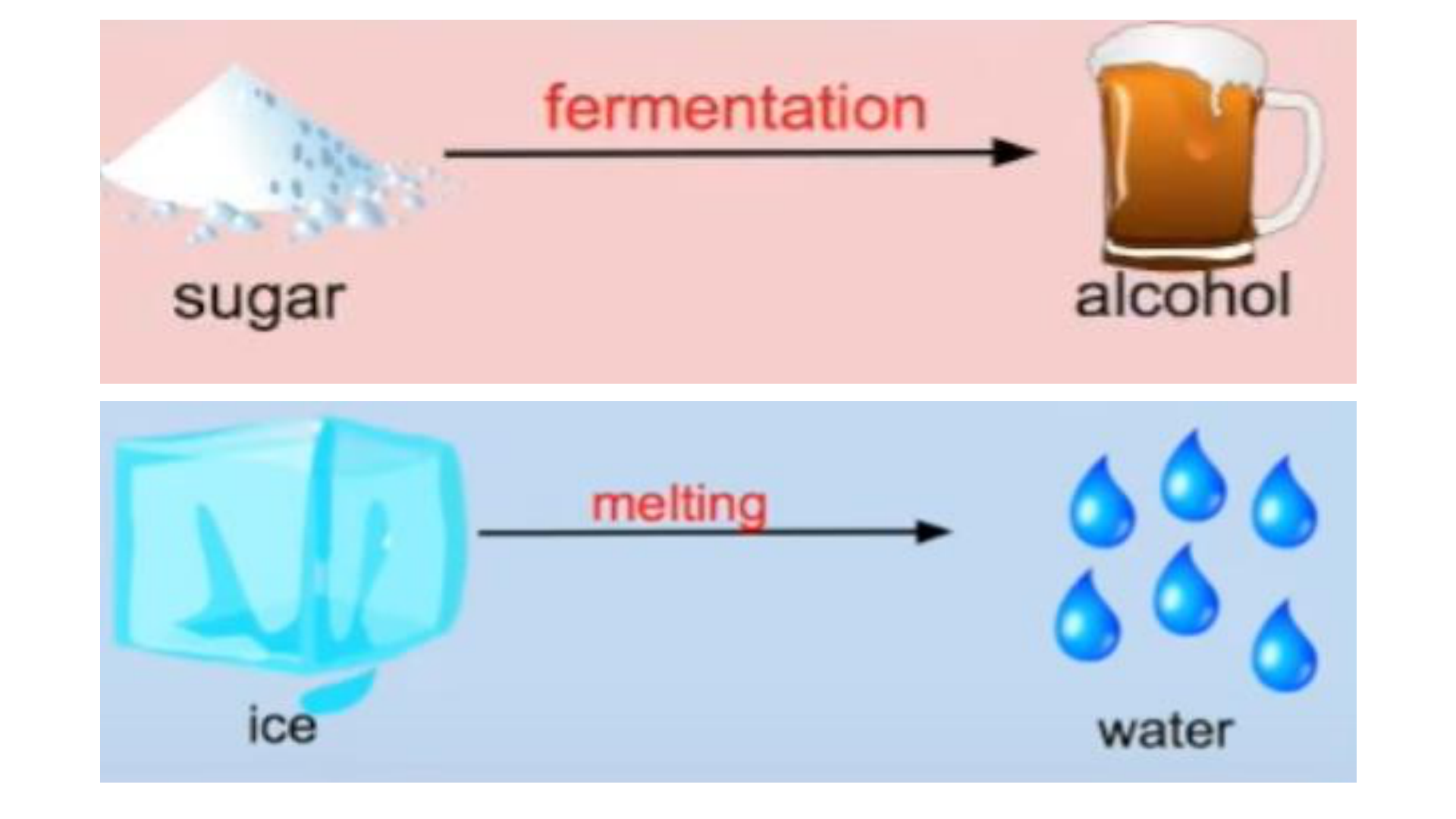
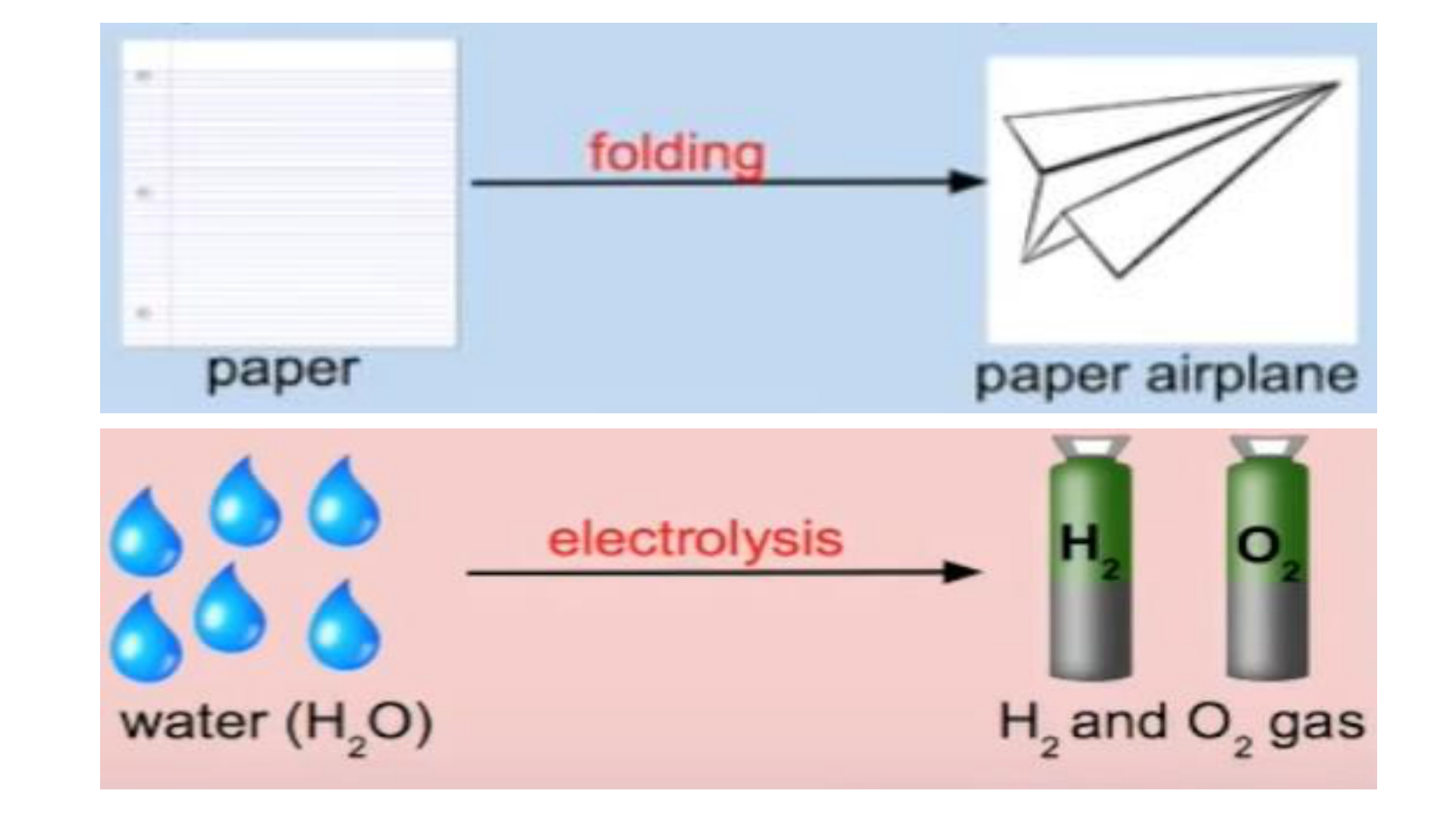
Physical Change
This occurs if the shape, size or physical
state is changed, but the chemical
composition remains the same.
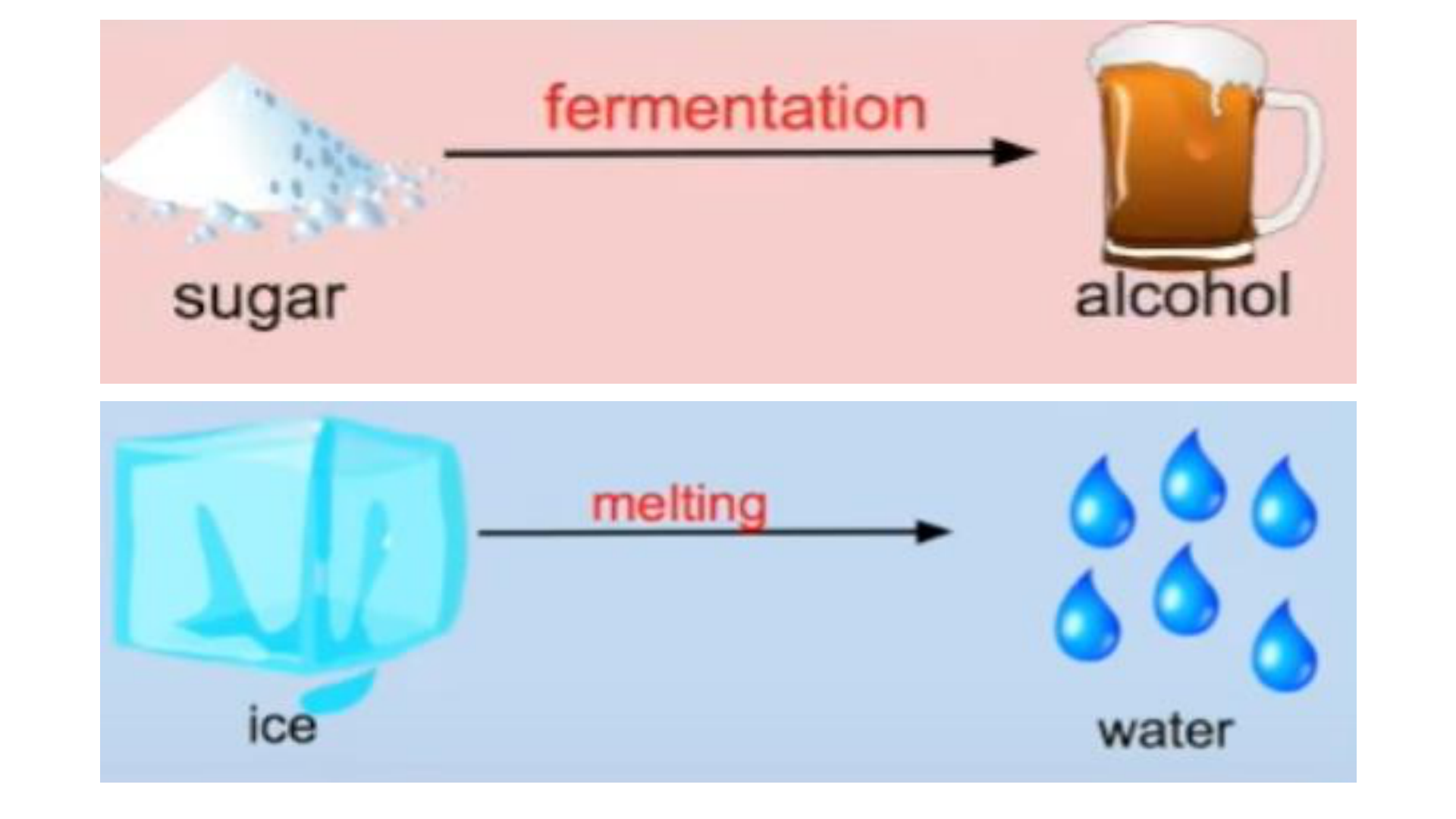
Chemical Change (reaction)
any change that results in the formation of
new chemical substances. At the
molecular level, chemical change involves
making or breaking of bonds between
atoms. Ex: iron rusting (iron oxide forms)
Note: All chemical changes are also physical changes
Evidence of a Chemical Reaction
Signs of chemical change are: 5 examples
change in color (pigmentation loss or gain)
rust formation (oxidation of some metals)
bubbling or fizzing (gas being produced)
light or heat produced (release of energy)
formation of a solid (called a precipitate)
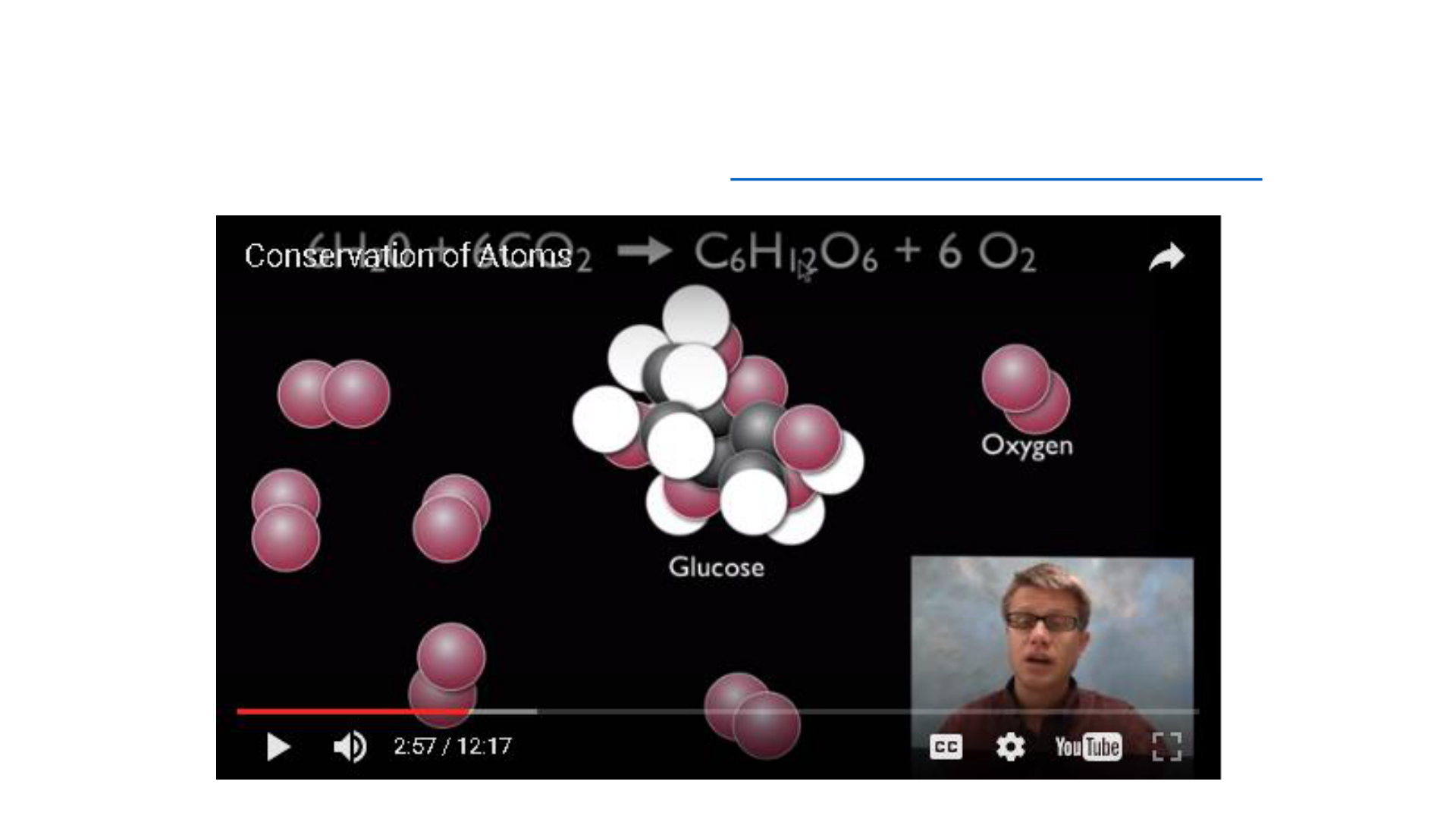
Law of Conservation of Mass
Mass or matter can never be destroyed or
created during a chemical reactions or
physical change. Mass of all substances
present before a chemical change is equal
to the mass of all new substances
produced after the chemical change.
History of the Law of the Conservation of Mass
The ancient Greeks first proposed the idea
that the total amount of matter in the universe
is constant. Later, Antoine Lavoisier described
this with The Law of Conservation of Mass as a
fundamental principle of physics in 1789. He
demonstrated it with the following experiment:
Source: Boundless. “The Law of Conservation of Mass.” Boundless Chemistry. Boundless, 26 May. 2016.
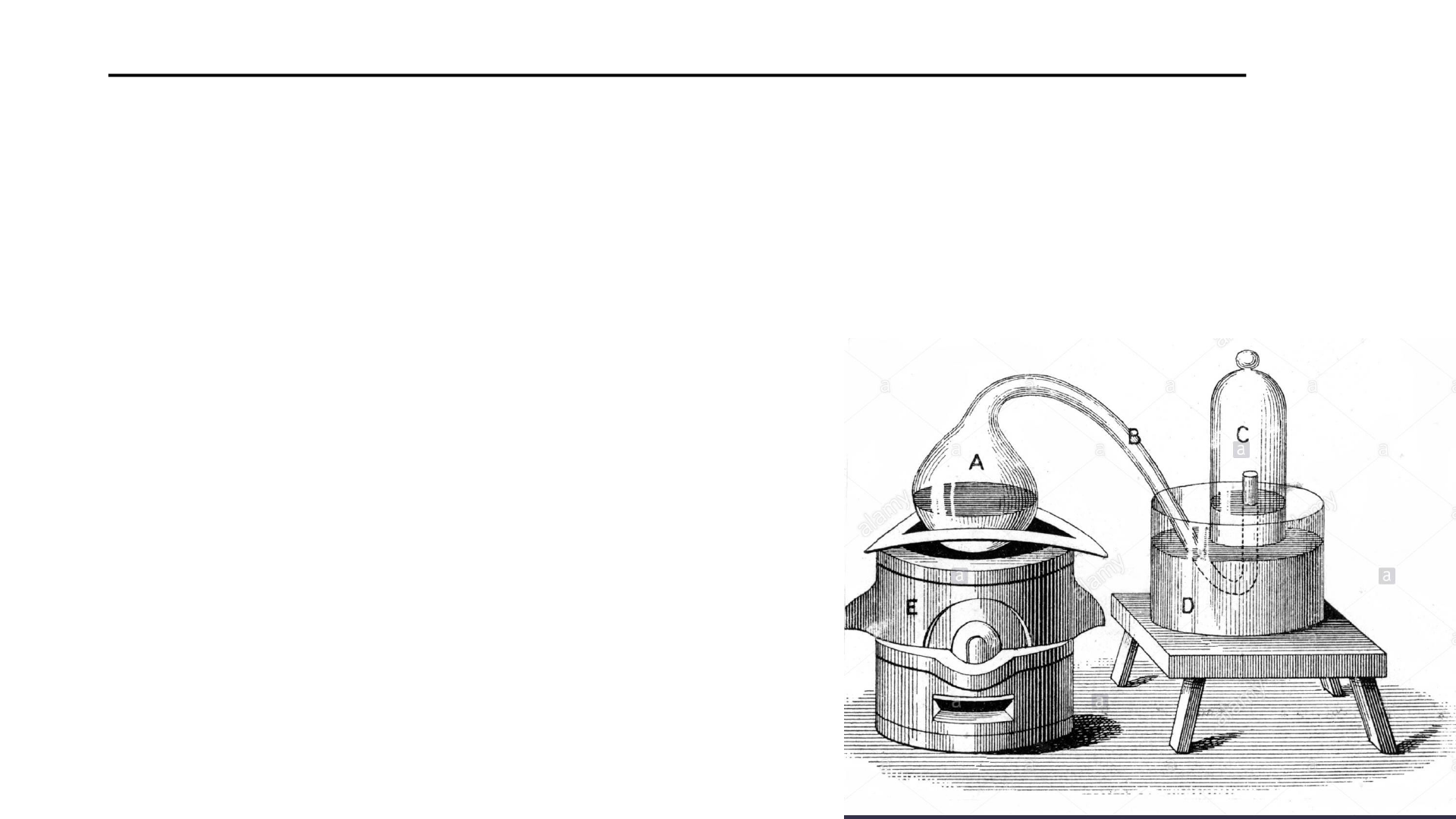
History of the Law of the Conservation of Mass
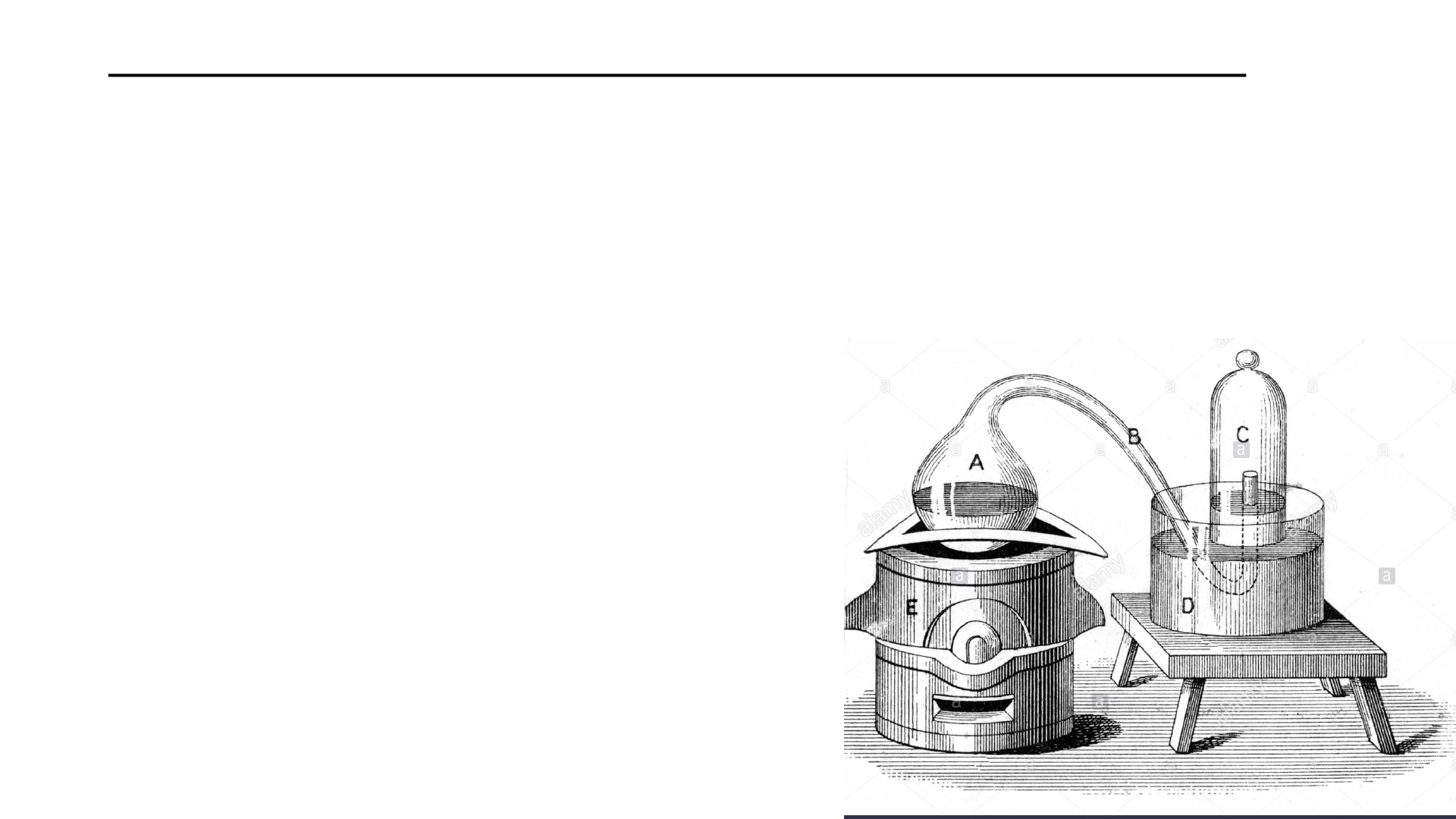
The reaction is reversible too.
Heating HgO produces O and Hg.
Lavoisier was able to account for
all mass on either side of the
reactions
The heating of mercury (Hg) liquid causes it to
react with oxygen forming mercury calx (HgO).
Evidence of Physical verses
Chemical Change
VOCABULARY – physical properties,
chemical properties, physical change,
chemical change, chemical reaction,
Law of Conservation of Mass
Physical Properties
All matter has both physical and chemical
properties useful to scientists in the
classification of it.
Typical physical properties we will consider
are: color, odor, density, hardness,
solubility, phase of matter, melting points
or boiling points.
Chemical Properties
These are determined by the
reactivity of a substance with
another substance.
Examples: acidity or basicity
reactions with oxygen or other gases
What properties cause A to react
with B to possibly form AB?
Caution: do not get hung up on the nuisances between these two.
Simply refer to the definition as defined here in class.
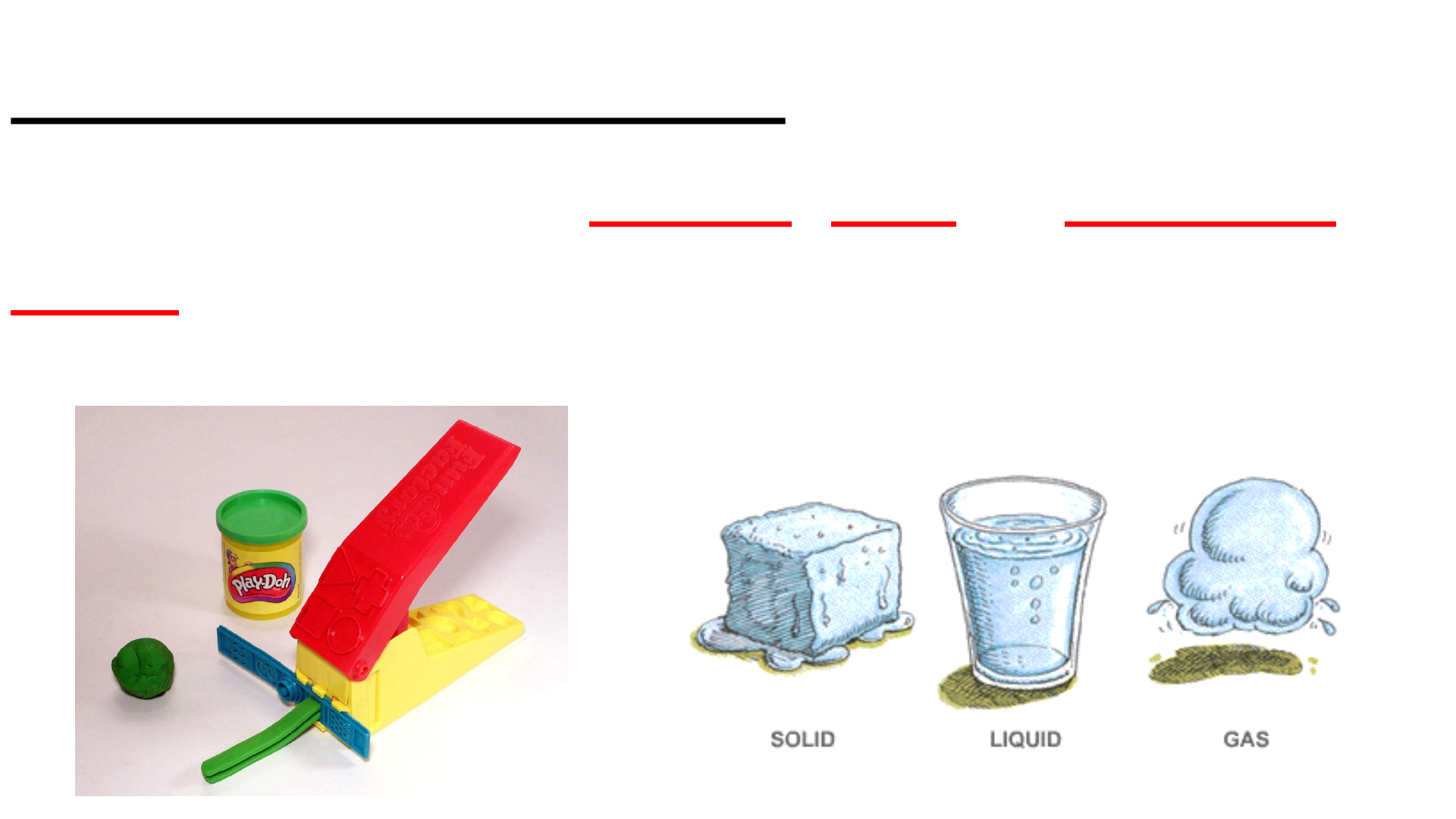
Physical Change
This occurs if the shape, size or physical
state is changed, but the chemical
composition remains the same.
Chemical Change (reaction)
any change that results in the formation of
new chemical substances. At the
molecular level, chemical change involves
making or breaking of bonds between
atoms. Ex: iron rusting (iron oxide forms)
Note: All chemical changes are also physical changes
Evidence of a Chemical Reaction
Signs of chemical change are: 5 examples
change in color (pigmentation loss or gain)
rust formation (oxidation of some metals)
bubbling or fizzing (gas being produced)
light or heat produced (release of energy)
formation of a solid (called a precipitate)
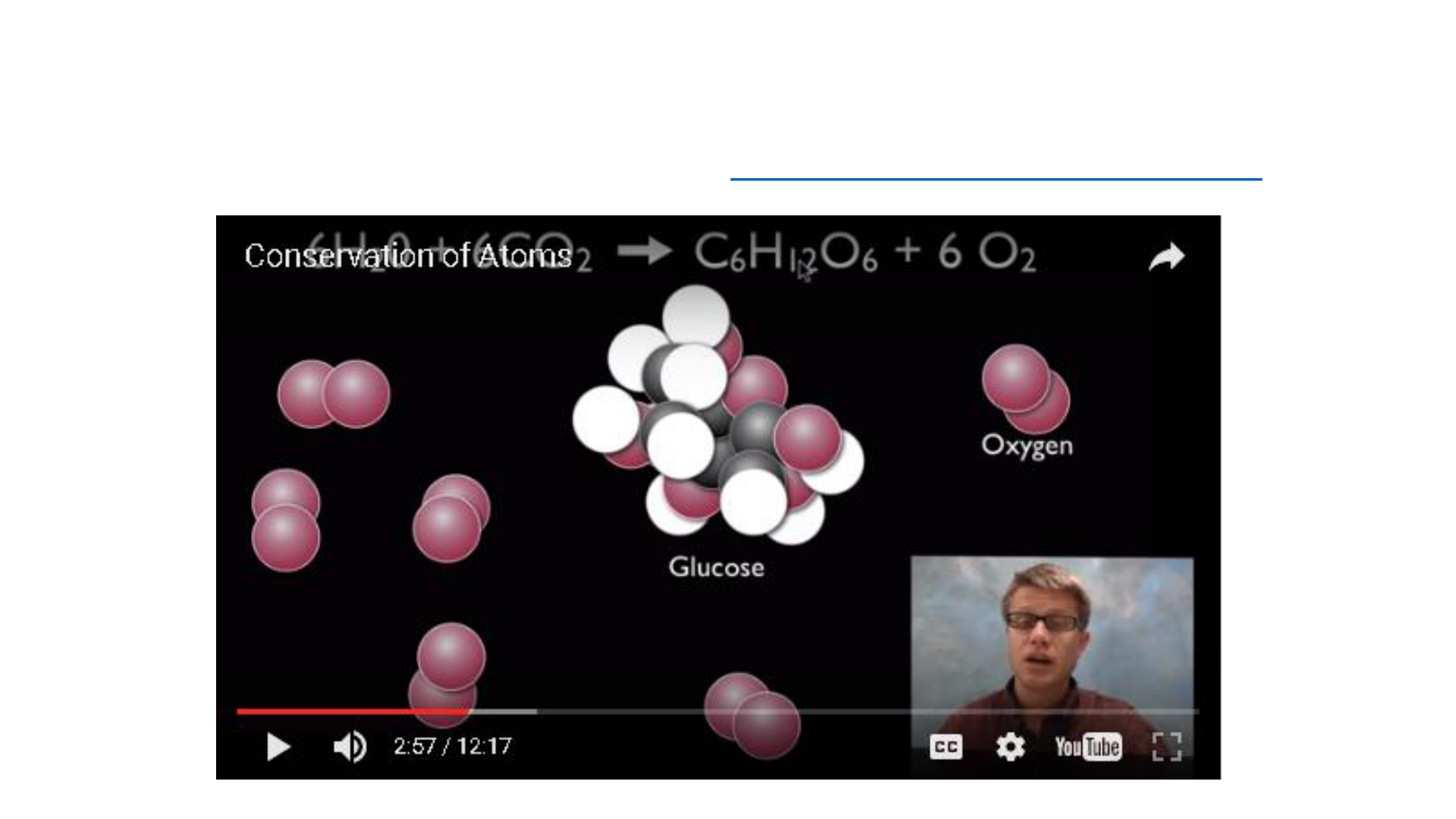
Law of Conservation of Mass
Mass or matter can never be destroyed or
created during a chemical reactions or
physical change. Mass of all substances
present before a chemical change is equal
to the mass of all new substances
produced after the chemical change.
History of the Law of the Conservation of Mass
The ancient Greeks first proposed the idea
that the total amount of matter in the universe
is constant. Later, Antoine Lavoisier described
this with The Law of Conservation of Mass as a
fundamental principle of physics in 1789. He
demonstrated it with the following experiment:
Source: Boundless. “The Law of Conservation of Mass.” Boundless Chemistry. Boundless, 26 May. 2016.
History of the Law of the Conservation of Mass
The reaction is reversible too.
Heating HgO produces O and Hg.
Lavoisier was able to account for
all mass on either side of the
reactions
The heating of mercury (Hg) liquid causes it to
react with oxygen forming mercury calx (HgO).

Evidence of Physical or Chemical Changes Lab
1. Read and highlight pg 1
2. Complete Physical Properties notes pg 2
3. Complete hands on portion of lab
4. Complete pg 3 prep. Reference reading pg 1
to find the answers.

Physical Properties
Baking soda: NaHCO
3
solid – powder, fine, white
Vinegar: CH
3
COOH (acetic acid)
liquid – transparent, clear, sour acidic odor
acid

Baking Soda & Vinegar
1. Observe the physical properties of baking soda and
vinegar. Record observations.
2. Put 1 spoonful of baking soda into the petri dish.
3. Place a pipette full of vinegar onto baking soda in
the petri dish.
4. Observe and record any changes

Physical Properties
Corn Starch: NaHCO
3
solid – powder, fine, white
Iodine (povidone-iodine): I-NCHOCH
2
liquid - colloid, opaque, brownish/red tint
Water: H
2
O - liquid, transparent, polar, wet 😊

Cornstarch, Water and Iodine
1. Observe the physical properties of cornstarch and
iodine. Record observations.
2. Put 1 spoonful of cornstarch into water. Stir
3. Using the dropper, drip one drop at a time of
iodine into the mixture and stir after each.
4. Observe and record any changes

Epsom Salt, Washing soda and water in solution
1. Observe the physical properties of Epsom Salt and
Washing soda . Record observations.
2. Put 1 spoonful of each into water. Stir
3. Then pipette several samples of each onto a petri
dish.
4. Observe and record any changes
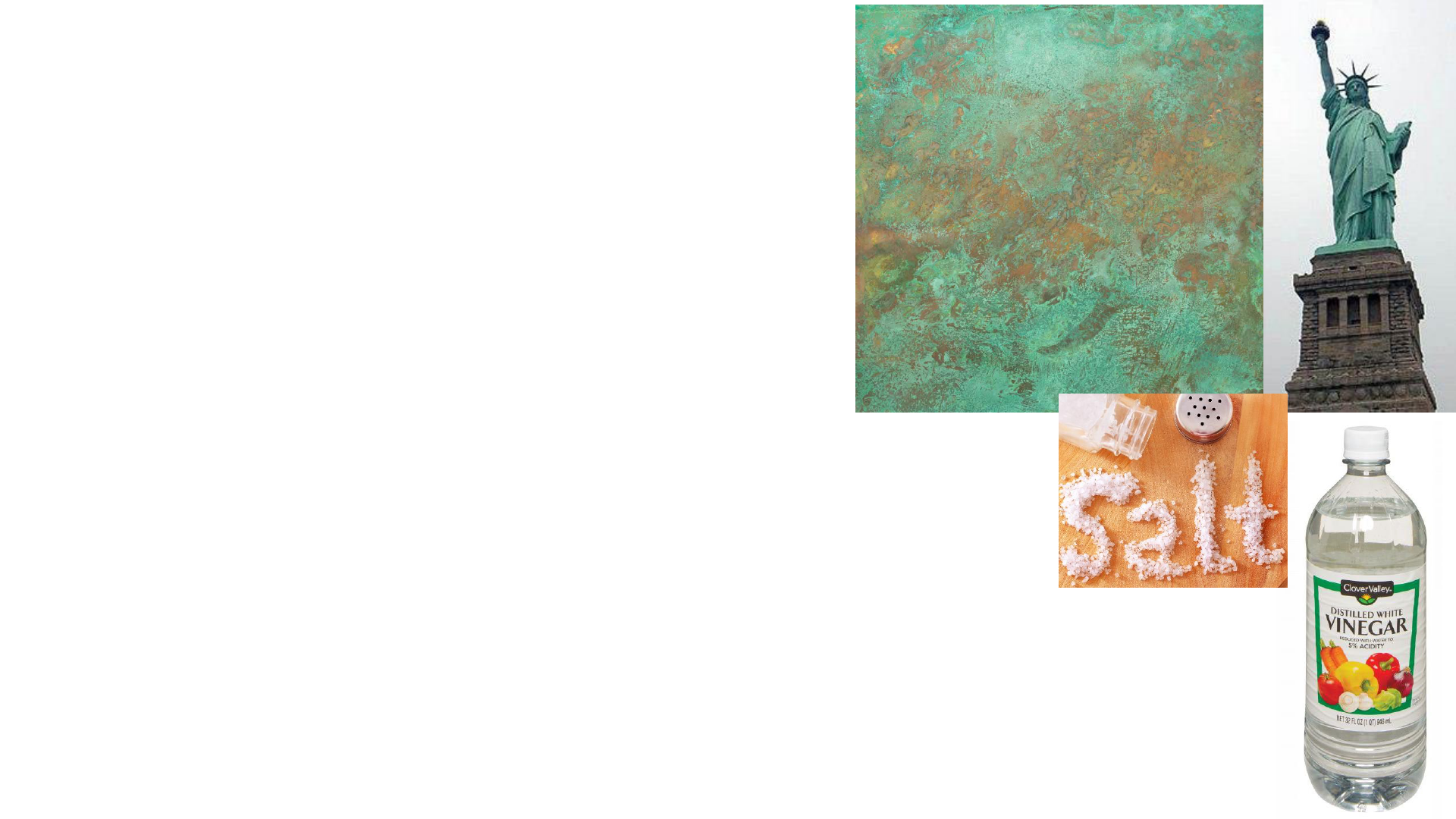
Physical Properties
Copper penny: Cu …. CuO
2
H
solid – metallic, Abe Lincoln,
patina or tarnish greenish tinge
Table Salt: NaCl
solid - crystalline granular, cuboidal, white
Vinegar – liquid, transparent, clear, sour acidic odor

Sugar and Water
1. Observe the physical properties of sugar and
water. Record observations.
2. Put 1 spoonful of sugar into water. Stir
3. Observe and record any changes
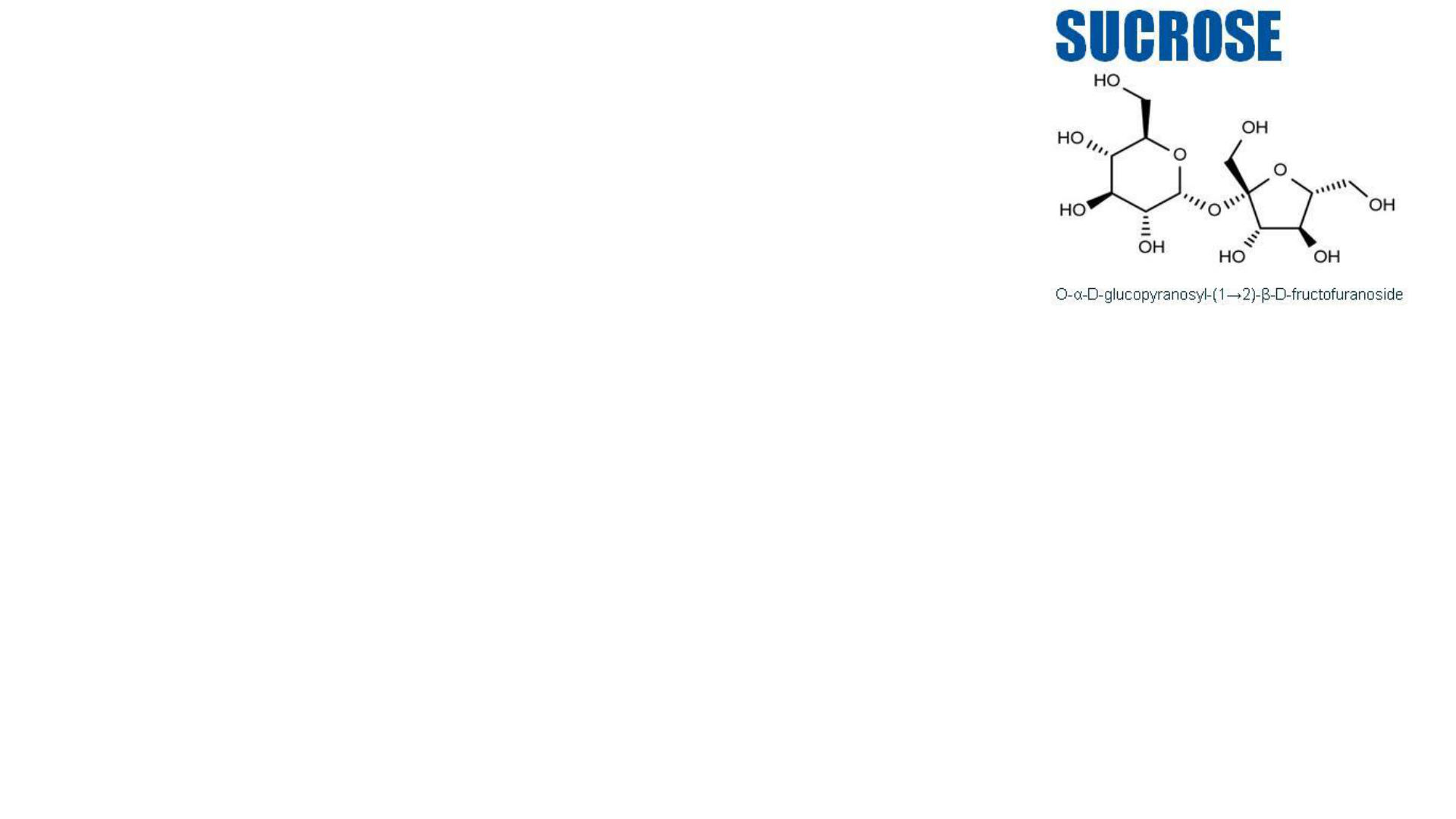
Physical Properties
Sugar : C
6
H
12
O
6
solid – solid, granular crystalline, pale white
Water: H
2
O - liquid, transparent, polar, wet 😊
Form a solution – observe