6.6(A) compare metals, nonmetals, and metalloids using physical properties such as
luster, conductivity, or malleability;
Classify
Sort or group together based on shared
characteristics,
physical properties, or chemical properties.
classify
to put things into groups according to their
characters
conductivity
- ability of an object to transfer heat or
electricity to another object.
corrosion
- the gradual wearing away of a metal
element due to a chemical reaction.
ductile
- material that can be pulled out into a
long wire.
Element
A pure substance composed of the
same type of atom throughout
freezing point
The temperature at which a liquid changes
to a solid.
gas
a form of matter that does not have a
definite shape or a definite volume
Graduated
Cylinder
1. A tall thin container marked with lines
that is used to accurately measure the
volume of liquids. 2. The peice of lab
equipment best suited for finding liquid
volume.
Irregular
shaped object
1. Any object that has no definite shape
such as a rock or paper clip. Its volume is
measured by water displacement. 2. An
object whose sides or any parts are
impossible to measere with a metric ruler.
liquid
a state of matter that has no definite shape
but has a definite volume
magnetism
force of attraction (pulling) or repulsion
(pushing) by magnetic materials; property
of matter
malleable
- material that can be pounded into
shapes.
Mass
A measure of the amount of matter in an
object
measure
To determine the size, amount, or degree of
an object by comparison with a standard
unit
melting point
The temperature at which a solid becomes
a liquid
metal
- class of elements characterized by
physical properties including shininess,
malleability, ductility, & conductivity.
nonmetal
- element lacking most of the
properties of a metal.
- poor conductor of electricity and heat
- reactive with other elements
- solid nonmetals are dull and brittle
observe
to be aware of or notice something by
looking or watching/use your five senses

particle
a very small piece of matter
•
Physical
Properties of
Matter
how matter is described according to
physical characteristics; characteristics that
can be observed without changing the
substance into something else; ex. luster
Metals vs Non
Metals
a characteristic of a substance that can be
observed without changing it into another
substance
examples: density, color, solubility,
magnetism, size
physical state
form of matter at a given temperature
examples: solid, liquid, or gas
reactivity
- the ease and speed with which an
element combines, or reacts, with other
elements and compounds.
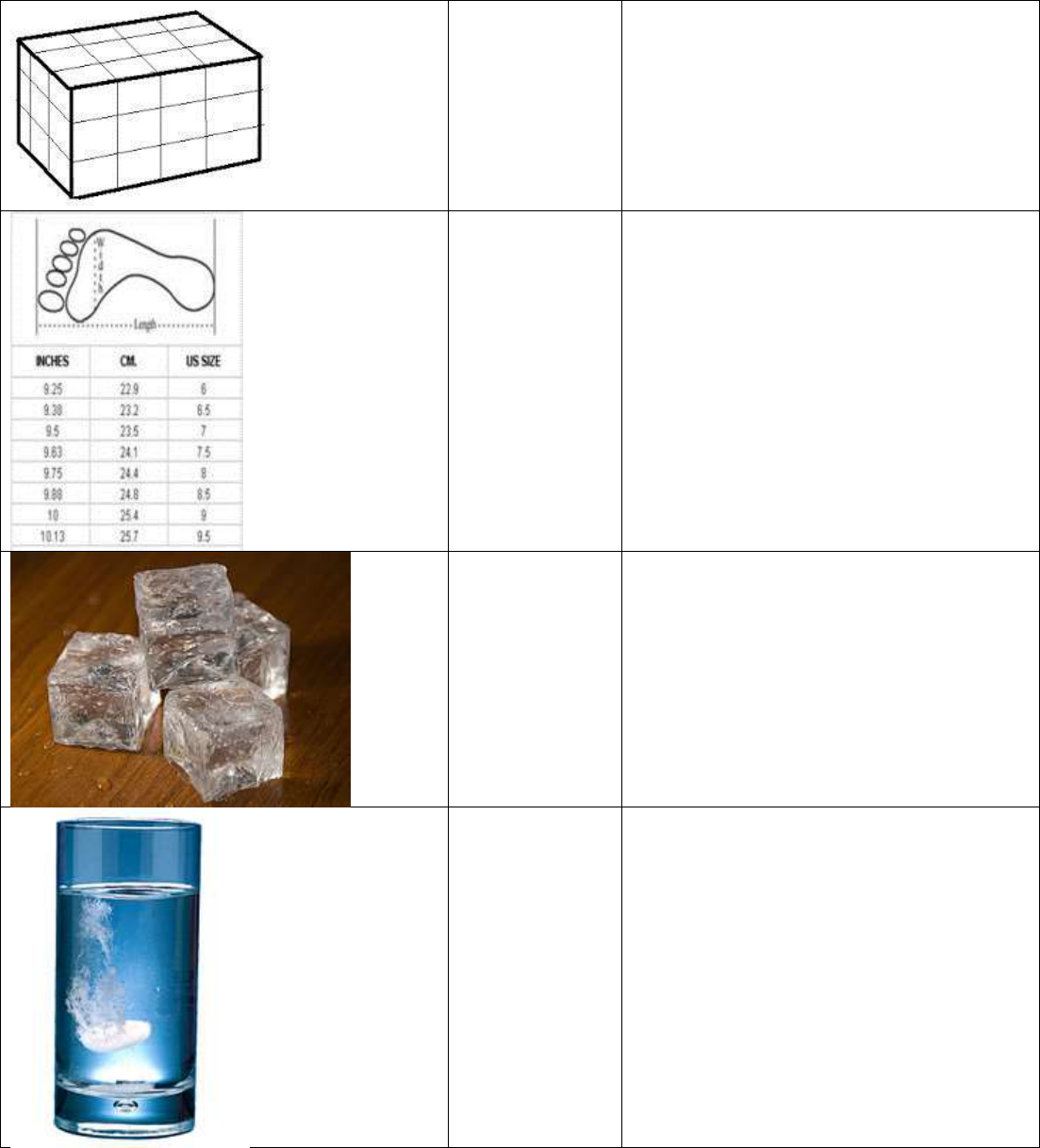
Regular shaped
object
1.Any three dimensional object such as a
cone, cube sphere or pyramid. Its volume
can be determined using a ruler and
mathematical formula. 2. An object whose
volume can be determined by a math
formula.
size
how big or small something is
solid
a state of matter that has a definite shape
and volume
solubility
the ability of one substance to dissolve in
another at a given temperature and pressure
surface tension
a force that occurs at the surface of a
liquid.
boiling point
The temperature at which a liquid changes
to a gas
temperature
a measure of the amount of heat an object
has
Volume
1. A measure of the size of a body or
region in three-dimensional space. 2. The
amount of space an object takes up. 3
Length x Width x H
volume
amount of space an object takes up
Water
Displacement
1. A method used to determine the volume
of an irregular solid. 2. The movement of
water when an object is added to a
graduated cylinder; it is a way to find the
volume of an irregular shaped solid.
