
Ecient Labeling of Vesicles with Lipophilic Fluorescent Dyes via
the Salt-Change Method
Minkwon Cha,
#
Sang Hyeok Jeong,
#
Seoyoon Bae,
#
Jun Hyuk Park, Yoonjin Baeg, Dong Woo Han,
Sang Soo Kim, Jaehyeon Shin, Jeong Eun Park, Seung Wook Oh, Yong Song Gho,* and Min Ju Shon*
Cite This: https://doi.org/10.1021/acs.analchem.2c05166
Read Online
ACCESS
Metrics & More Article Recommendations
*
sı
Supporting Information
ABSTRACT: Fluorescent labeling allows for imaging and tracking of vesicles down to single-
particle level. Among several options to introduce fluorescence, staining of lipid membranes
with lipophilic dyes provides a straightforward approach without interfering with vesicle
content. However, incorporating lipophilic molecules into vesicle membranes in an aqueous
solution is generally not ecient because of their low water solubility. Here, we describe a
simple, fast (<30 min), and highly eective procedure for fluorescent labeling of vesicles
including natural extracellular vesicles. By adjusting the ionic strength of the staining buer with
NaCl, the aggregation status of DiI, a representative lipophilic tracer, can be controlled
reversibly. Using cell-derived vesicles as a model system, we show that dispersion of DiI under
low-salt condition improved its incorporation into vesicles by a factor of 290. In addition,
increasing NaCl concentration after labeling induced free dye molecules to form aggregates,
which can be filtered and thus eectively removed without ultracentrifugation. We consistently
observed 6- to 85-fold increases in the labeled vesicle count across dierent types of dyes and
vesicles. The method is expected to reduce the concern about o-target labeling resulting from the use of high concentrations of
dyes.
■
INTRODUCTION
Fluorescent labeling of extracellular vesicles (EVs) oers a
unique approach to study physical and functional properties of
vesicles. For example, tracking diusion of EVs under
fluorescence microscopes can measure the size distribution
of EV population, similarly to nanoparticle tracking analysis by
light scattering.
1,2
More sophisticated techniques also exist for
super-resolution imaging, multiplexed measurements, or flow
cytometry of EVs.
3,4
Lastly, docking of EVs on live cell
membranes and their subsequent uptake can be followed in a
quantitative manner.
5−7
Regardless of the specific properties
under investigation, the results can be often confounding due
to the underlying heterogeneity in vesicles, in which case the
analysis must be conducted at the single-vesicle level to
faithfully reconstruct the ensemble properties.
8
Therefore,
eective and unbiased, homogeneous labeling with bright
fluorescent dyes that visualizes individual vesicles is a
prerequisite for EV analysis by fluorescence.
Several methods to fluorescently label EVs are available:
immunostaining of surface proteins, internal protein tagging
with membrane-permeable dyes, use of water-soluble dyes
inside vesicles, or membrane staining with lipophilic dyes.
9
Unfortunately, vesicle labeling via proteins would be biased by
the abundance of proteins and potentially interfere with the
following functional characterization dependent on the
targeted proteins. Water-soluble dyes behave largely independ-
ent of the vesicle content, but cannot be internalized into
preformed vesicles such as purified EVs due to the membrane
barrier. Although some nonfluorescent, membrane-permeant
molecules, such as carboxyfluorescein diacetate succinimidyl
ester (CFDA-SE), can passively diuse into vesicles and then
become fluorescent,
3
they only work with vesicles containing
active esterases and therefore will be biased by the vesicle
content. Membrane staining with lipophilic tracers oers
unbiased and bright labeling: a variety of cyanine-derivative
dyes with single-molecule sensitivity are developed across the
entire spectrum of visible light.
10
Since a typical, 100 nm
vesicle carries ∼80000 lipid molecules, introducing only 0.01
mol % of lipophilic dyes will yield ∼8 dye molecules per vesicle
on average, sucient for single-particle tracking. Additionally,
these molecules naturally exhibit a large increase in
fluorescence upon partitioning into the membrane, further
contributing to the high signal-to-noise ratio against free dye
molecules.
Notably, however, vesicle staining with lipophilic dyes often
suers from confounding factors, such as the complications
from o-target labeling of lipoproteins and free-dye aggregates
Received: November 19, 2022
Accepted: March 15, 2023
Technical Notepubs.acs.org/ac
© XXXX The Authors. Published by
American Chemical Society
A
https://doi.org/10.1021/acs.analchem.2c05166
Anal. Chem. XXXX, XXX, XXX−XXX
Downloaded via 211.200.107.101 on April 3, 2023 at 00:47:08 (UTC).
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
that are similar in size to EVs.
11−13
These problems are
associated with the use of high concentrations of dyes that are
used to force the incorporation of lipophilic molecules into
closed, preformed vesicles. Although slightly soluble in water,
most of the lipophilic molecules are kinetically trapped in large
aggregates, so the passage from aggregates to membranes is
unfavorable. One can drive the equilibrium toward vesicles by
increasing dye concentrations, but at the expense of additional
sample processing to remove the large amount of free dyes,
which is frequently accompanied by severe loss of vesicles.
Moreover, a recent work suggests that the incorporation of
large dye aggregates may also lead to a significant increase in
vesicle size.
14
Consequently, an eective method to fluo-
rescently label vesicles with high sensitivity, high specificity,
and simplicity to preserve the quality and quantity of vesicle
samples is desirable.
Here, we describe an improved method for the fluorescent
labeling of vesicle samples, including EVs. This method is
simple, fast, and eective both in improving the labeling
eciency and in removing unwanted free dyes. By character-
izing the aggregation status of a lipophilic tracer DiI (Figure 1)
as a function of NaCl concentration using total internal
reflection fluorescence (TIRF) microscopy, we observed that
∼150 mM NaCl, a typical constituent of standard phosphate-
buered saline (PBS), induces a pronounced aggregation of
DiI molecules that potentially inhibit their incorporation into
vesicle membranes. The aggregation was reversible, as shown
by the dispersion of aggregates at a lower concentration of
NaCl. Therefore, to improve labeling eciency, we performed
labeling and free-dye removal in separate steps with distinct
NaCl concentrations. The free-dye aggregates, due to their
large size, were completely removed by simple filtering without
requiring ultracentrifugation, along with minimal vesicle loss.
The resulting eciency of labeling was consistently improved
across multiple types of lipophilic dyes and vesicle samples by
at least an order of magnitude compared with a standard
labeling procedure. The increased eciency in turn allows
labeling with lower concentrations of dyes than usual, and
therefore reduces the concern about free-dye aggregates that
are preferentially formed at high concentrations. The wide
applicability of the proposed method will likely facilitate the
use of fluorescently labeled vesicles in many dierent types of
EV analysis.
■
MATERIALS AND METHODS
Fluorescent Labeling of Vesicles. Preparation of vesicles
are described in the Supporting Information. For the salt-
change labeling, vesicle samples in PBS were diluted to 10
10
particles/mL with ultrapure water to lower NaCl concentration
to below 20 mM and reacted with 2 μM DiI or DiD for 20 min
at 37 °C. A small volume of PBS concentrate (10×) was then
added to increase NaCl concentration to ∼150 mM. For direct
staining, the same concentration of dyes was directly applied to
vesicles in regular PBS with 150 mM NaCl. The mixture was
filtered through a 0.2 μm syringe filter (Advantec,
03CP020AS) to remove aggregates of dye molecules and
subjected to further experiments. Labeling results were verified
by imaging of either free-floating or anti-CD63-bound vesicles
on a TIRF microscope (see Video S1 for the representative
results) as described in the Supporting Information.
Figure 1. Chemical structures of lipophilic fluorescent probes for
vesicles. Lipophilicity (log P) values for octanol/water partition
coecient were calculated using a web tool (SwissADME
15
) with a
XLOGP3 model.
23
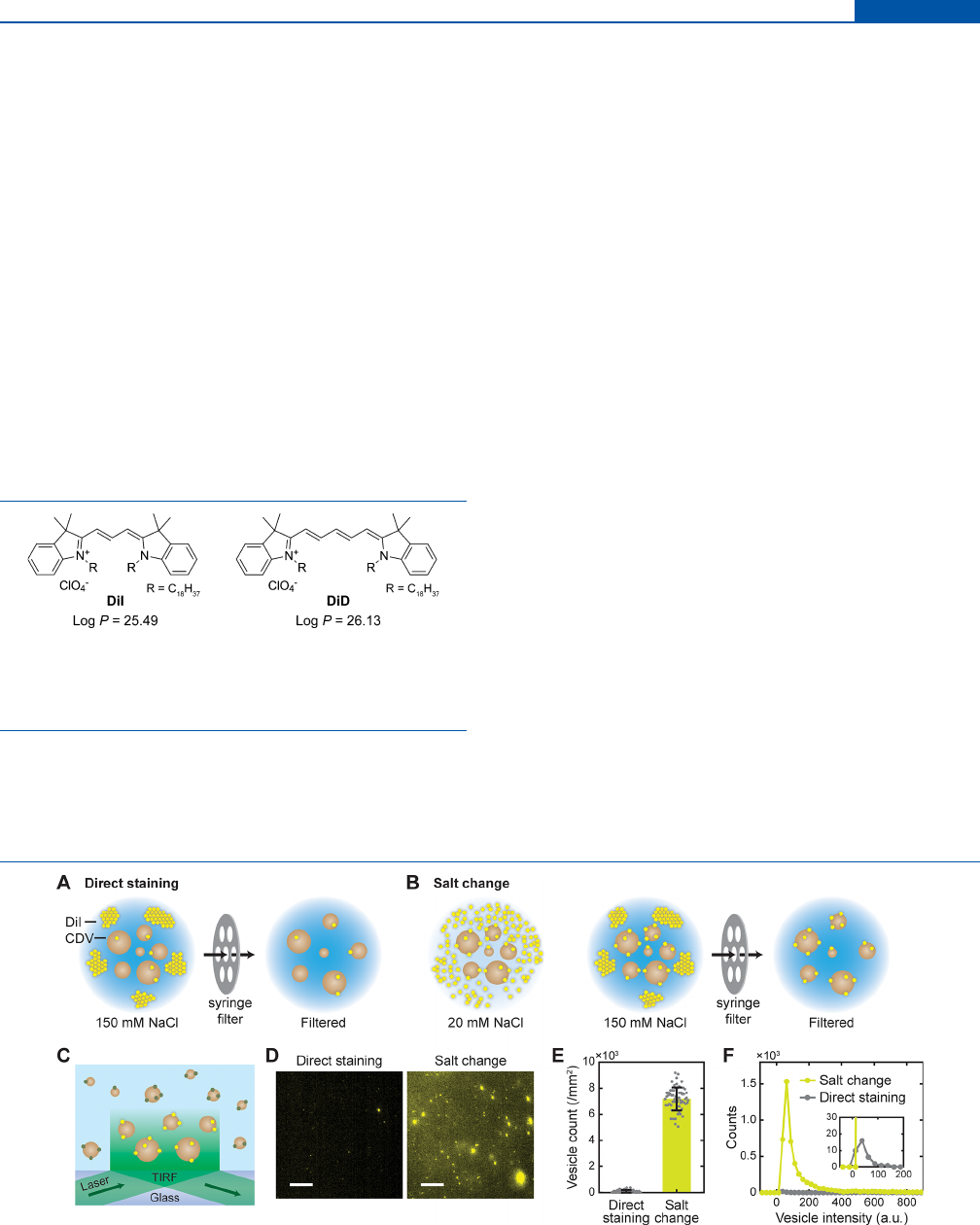
Figure 2. Fluorescent labeling of vesicles by salt-change method. (A, B) Schematics of direct staining (A) and salt-change (B) method for the
labeling of cell-derived vesicles (CDVs) with DiI in an aqueous buer. In the salt-change method, staining was performed in a low-salt buer, then
[NaCl] was increased to 150 mM to promote aggregation of DiI, and the aggregates were removed by filtration. (C) Schematic of single-vesicle
imaging of DiI-labeled CDVs by TIRF microscopy. (D) Representative fluorescent images of DiI-labeled vesicles. Scale, 10 μm. (E) Numbers of
DiI-labeled vesicles obtained by direct staining and salt-change method observed in TIRF images. Error bars, mean ± s.d. of n = 63 images. (F)
Distributions of fluorescence intensity for the labeled vesicles. Inset, close-up view of the same curves; n = 56 (direct staining), n = 4671 (salt
change) spots.
Analytical Chemistry pubs.acs.org/ac Technical Note
https://doi.org/10.1021/acs.analchem.2c05166
Anal. Chem. XXXX, XXX, XXX−XXX
B

■
RESULTS
NaCl Dependence of DiI Aggregation. Although
lipophilic dyes are commercially available as powders or as
solutions in organic solvents such as ethanol, dimethylforma-
mide (DMF), or dimethyl sulfoxide (DMSO), they need to be
transferred to aqueous solutions for vesicle labeling not to
disrupt vesicles and the embedded proteins. This requirement
poses a challenge because the water solubility of lipophilic
molecules is generally low, as shown by their high lipophilicity
(calculated log P values for the octanol/water partition
coecient
15
are given in Figure 1). Therefore, we first directly
examined DiI molecules dissolved in aqueous solutions by
single-molecule TIRF microscopy
16
(Figure S1A). After
introducing DiI solutions into a glass flow cell, the fluorescent
particles floating by near the glass surface were illuminated.
We first imaged 2 μM DiI solution in a buer with ∼150
mM NaCl, a physiological and typical condition for common
buers including PBS. A small number of bright, slowly
moving particles were detected (Figure S1A), which are likely
large aggregates of DiI rather than single molecules. Since most
fluorescent dyes including DiI exhibits aggregation-caused
quenching of fluorescence intensity, the brightness of the
particles would actually underestimate the number of dye
molecules per particle. Indeed, these aggregates were
completely removed by filtering the solution through 0.2 μm
pores (Figure S1A,B), implying that they are mostly micron-
sized. These large aggregates are likely inecient at labeling
vesicular membranes and may cause an increase in vesicle size
after labeling.
14
We therefore attempted to improve the solubilization of DiI
by decreasing NaCl concentration. The aggregates gradually
dispersed as NaCl concentration was lowered to ∼20 mM, as
shown by the increase of relatively dim particles (Figure S1A−
C). Importantly, we checked that these changes to particles
occurred while the total amount of dye molecules and their
fluorescence remained constant: After solubilizing the dye
aggregates completely with detergent (0.1% Triton X-100), the
overall fluorescence intensity from the solution was measured
to be the same across the concentrations of NaCl we tested
(Figure S1D). In contrast, the fluorescence from the buer
with 155 mM NaCl almost completely disappeared after
micropore filtering (Figure S1D; ∼5 nM DiI left from the
original 2 μM solution), suggesting that most of the dye
molecules in this condition were trapped in the aggregates and
subsequently removed.
Improvement of Fluorescent Labeling by the Salt-
Change Method. The above results suggest that dispersion of
DiI in a buer with a low concentration of NaCl can potentiate
membrane partitioning of DiI and that the excess dye can be
removed by inducing its aggregation at a higher concentration
of NaCl. We therefore exploited this reversible aggregation of
DiI to improve the labeling of vesicles (Figure 2). To verify the
general applicability of labeling procedures, we employed cell-
derived vesicles (CDVs) as model EVs that are similar in size
to large exosomes and small microvesicles.
17
CDVs from
human natural killer cells (hereafter called NK-CDVs) were
prepared by using a published procedure,
18
and labeled them
with DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocya-
nine; DiIC
18
(3)), a lipophilic fluorescent tracer for labeling
lipid membranes.
For the side-by-side comparison of labeling eciency, two
labeling methods were applied to NK-CDVs: (a) “Direct
staining” performed with 150 mM NaCl, that is, in regular PBS
(Figure 2A), and (b) “Salt-change” approach in which the
labeling with and removal of DiI were performed separately in
buers with distinct ionic strength (Figure 2B). In the latter
method, after staining NK-CDVs in a low-salt buer ([NaCl] <
20 mM), we raised NaCl concentration to ∼150 mM to induce
aggregation of free dye molecules, then filtered the solution
using a regular syringe filter. This filtering step was applied also
to the direct staining procedure for a fair comparison of
labeling results and vesicle yield. The labeled CDVs were then
visualized using a TIRF microscope
16
(Figure 2C).
CDVs after direct staining displayed only a small number of
dim particles (Figure 2D; see also Video S1). Since the CDVs
were prepared at a fairly high concentration (∼10
10
particles/
mL), we expected much more particles to be present in the
field of view, and therefore, it was very unlikely that all CDVs
were successfully labeled by the direct staining method.
Although the observed level of labeling eciency might be
suitable for bulk assays that probe many vesicles at the same
time (e.g., cellular uptake of vesicles), the labeled CDVs were
neither suciently abundant nor suciently bright for
quantitative measurements at the single-vesicle level. Accord-
ing to our observations of NaCl concentration-dependent DiI
aggregation, we argued that the low labeling eciency would
stem from poor solubilization of lipophilic dyes in the staining
buer.
19
In stark contrast, the salt-change method increased
the number of bright fluorescent vesicles 85 ± 10 times
(Figure 2D,E; see also Video S1), and their average brightness
also increased 2.3 times compared to vesicles stained in PBS
with 150 mM NaCl (Figure 2F). The simultaneous increase in
number and brightness of fluorescent vesicles implies that the
overall DiI incorporation (estimated from the areas under the
curves in Figure 2F) was improved by a factor of 290.
To accurately measure the labeling density (i.e., number of
DiI molecules per vesicle), the labeled NK-CDVs were stably
captured on a surface and their fluorescence intensity was
measured (Figure S2A; see Supporting Information for the
method). We estimated the number of DiI molecules in each
vesicle from the ratio of the initial fluorescence to photo-
bleaching step size (Figure S2B−D). Each CDV typically
carried 1−3 molecules of DiI, and these numbers followed a
Poisson distribution as expected (Figure S2E). The results
imply that only a small fraction of the CDVs remained
unlabeled and, at the same time, that the mole fraction of DiI
in vesicle membranes was <10
−4
(less than 10 dye molecules vs
∼10
5
lipid molecules; see Supporting Information for the full
calculation). Therefore, the labeling density we achieved was
sucient for single-vesicle imaging, but unlikely to disrupt the
native properties of the membrane.
Applications to Other Vesicles and Dyes. To test
whether the salt-change labeling method can be applied to
other vesicles, we first prepared another sample of CDVs from
umbilical cord mesenchymal stem cells (UCMSC-CDVs) and
labeled them with DiI. Again, the salt-change method showed a
dramatic improvement in labeling eciency (Figure S3A,B),
consistent with the results for NK-CDVs. It is remarkable that
the proposed method was much more eective than adding
dimethyl sulfoxide (DMSO) to the staining buer (Figure
S3A,B), a common approach to improve the solubility of
lipophilic dyes. Also, if the syringe filters for dye removal were
not rinsed with buer (2 mL of PBS) before use, we noticed
that the vesicle yield decreased slightly (by 17%; Figure
Analytical Chemistry pubs.acs.org/ac Technical Note
https://doi.org/10.1021/acs.analchem.2c05166
Anal. Chem. XXXX, XXX, XXX−XXX
C
S3C,D), possibly due to the trace wetting agents in the o-the-
shelf cellulose acetate filter membranes.
Importantly, the labeling method was also successfully
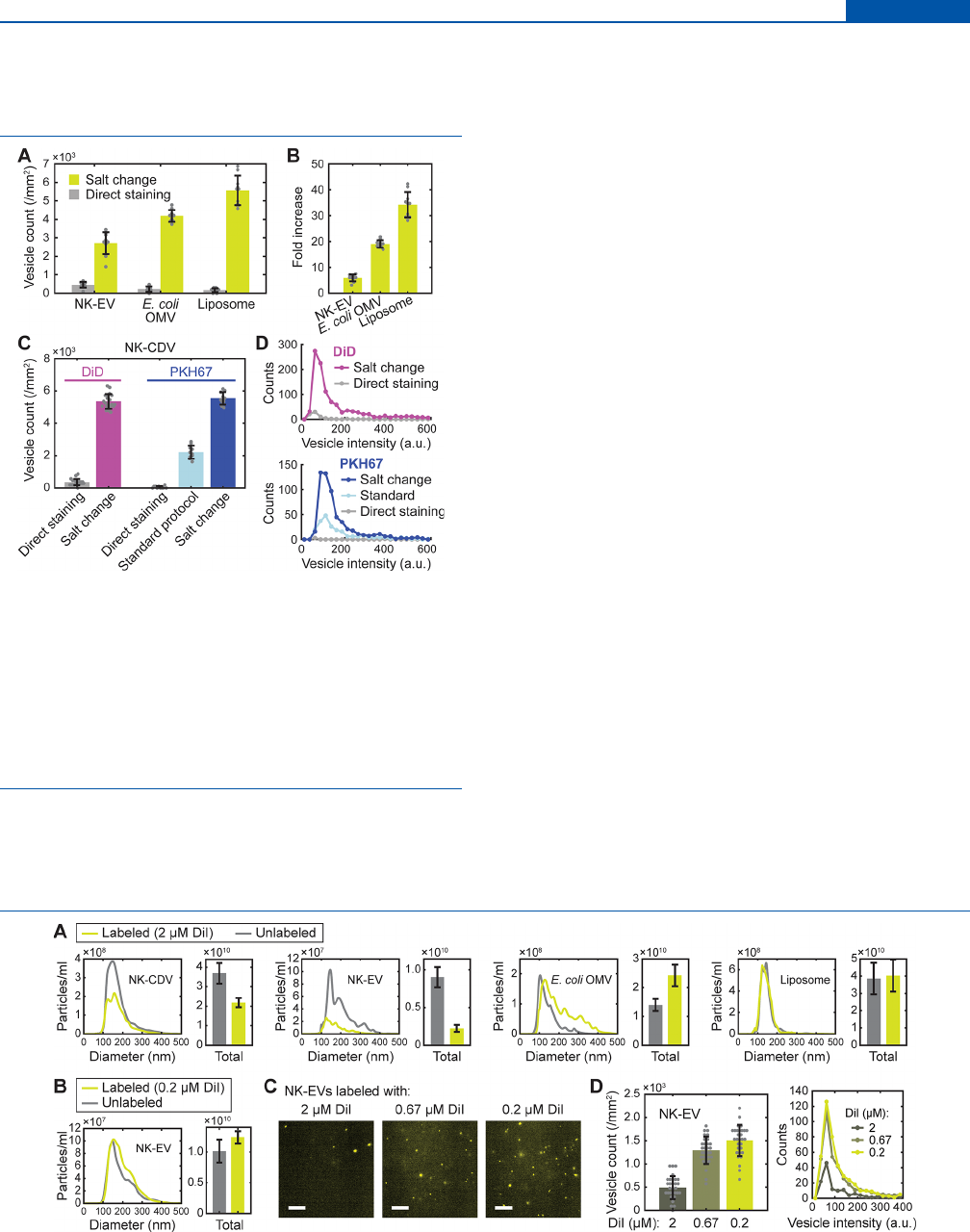
applied to naturally occurring EVs (Figure 3A,B). We prepared
two types of EVs: mammalian EVs from human natural killer
cells (NK-92; “NK-EV”) and bacterial outer-membrane
vesicles (OMVs) from Escherichia coli W3110 (a widely used
wild-type strain) (“E. coli OMV”), following a published
procedure.
20,21
Applying the salt-change labeling to these
vesicles, we again obtained a great improvement in labeling
eciency over direct staining in a high-salt buer (6-fold and
19-fold for NK-EVs and E. coli OMVs, respectively; Figure
3A,B). Furthermore, the method also proved to be useful in
labeling preformed liposomes consisting purely of synthetic
POPC (palmitoyloleoylphosphatidylcholine) lipids (Figure
3A), with the greatest (34-fold) increase in vesicle count
(Figure 3B). Since CDVs, mammalian and bacterial EVs, and
liposomes fairly dier in lipid and protein composition and size
distribution, our results clearly suggest that the proposed salt-
change method will be generally applicable to most types of
native and synthetic vesicles.
Experiments with two other lipophilic dyes DiD (1,1′-
dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine,
DiIC
18
(5); Figure 1) and PKH67 showed similar results in
CDV staining (Figure 3C,D). Strikingly, the salt-change
method applied to PKH67 dyes performed better than a
recommended standard protocol (from Sigma-Aldrich) that
used 3 times more vesicles and 6 times more dyes for
comparable results, improving both the number and brightness
of the stained vesicles. The same vesicles were barely detected
after labeling by direct staining with PKH67. The moderate
improvement with the standard protocol over direct staining
can be explained by the use of Diluent C, a commercial salt-
free isotonic solution supplied for general membrane labeling
(from Sigma-Aldrich). Although the exact structure of PKH67
is unpublished, it is reported (in the product description by
Sigma-Aldrich) to contain an aliphatic tail longer than PKH2
that has a C
22
tail. It is therefore expected to be highly
lipophilic, and presumably, the labeling strategy proved
successful similarly to DiI and DiD. Together, these results
demonstrate the broad applicability of the salt-change method
for the fluorescent labeling of preformed vesicles with
lipophilic dyes.
Integrity and Recovery of Vesicles after Labeling.
Consistent with the small amount of dye molecules per vesicle,
the size distribution of NK-CDVs, as measured by nanoparticle
tracking analysis (median diameter of ∼150 nm), was not
distorted by the salt-change labeling (Figure 4A). Although the
Figure 3. Applications of salt-change labeling. (A) Fluorescent
labeling of mammalian EVs from NK-92 cells (NK-EV), bacterial
outer-membrane vesicles from E. coli W3110 (E. coli OMV), and
synthetic liposomes with comparison of the labeling methods. (B)
Fold increase in labeling eciency (vesicle count) calculated from
(A). (C) Comparison of labeling methods for DiD and PKH67. For
PKH67, results from a standard protocol (Supporting Information) is
also shown. Error bars, mean ± s.d. of n = 20 (DiD) and 10 (PKH67)
images. (D) Distributions of fluorescence intensity for the DiD- and
PKH67-labeled vesicles shown in (C).
Figure 4. Size distribution and recovery of vesicles after salt-change labeling. (A) Nanoparticle tracking analysis (NTA) of vesicle size distribution
for the unlabeled (gray) and 2 μM DiI-labeled (green) vesicles via the salt-change method. The size distributions (left panels) are shown with the
corresponding total particle concentrations on right (bars). (B) NTA results for the salt-change labeling of NK-EVs with 0.2 μM DiI. In (A) and
(B), error bars represent mean ± s.d. of n = 26−29 measurements. (C) Representative images of DiI-labeled EVs prepared by salt-change labeling;
scale, 20 μm. (D) Number of DiI-labeled vesicles prepared with the indicated concentrations of DiI. Vesicle counts (left) from images such as
shown in (C) are shown with the corresponding intensity distribution (right). Error bars, mean ± s.d. of n = 30 images.
Analytical Chemistry pubs.acs.org/ac Technical Note
https://doi.org/10.1021/acs.analchem.2c05166
Anal. Chem. XXXX, XXX, XXX−XXX
D
nominal pore size (0.2 μm) of the syringe filter for dye removal
was close to the size of CDVs, the actual pore sizes in the
cellulose acetate filter membrane are heterogeneous and allow
the passage of vesicles slightly larger than 0.2 μm, so the
vesicles between 200 and 400 nm were not appreciably cut o.
Overall, 60% of the vesicles were recovered after salt-change
labeling of NK-CDVs (Figure 4A). It is remarkable that free
DiI molecules were almost completely removed by the same
filtering process (Figure S1D), thus, the size dierence
between vesicles and free-dye aggregates could be successfully
exploited to purify labeled vesicles.
Vesicle sizes were largely maintained for the other types of
vesicles (NK-EVs, E. coli OMVs, and synthetic liposomes), too,
but the yield somewhat depended on vesicle type (Figure 4A).
While synthetic liposomes were most reliably recovered with a
minimal change in size distribution, NK-EVs and E. coli OMVs
showed opposite results in the obtained numbers of vesicles
(25% and 170%, respectively). The increase in OMV number
can be rationalized by the suboptimal detection of small
particles in NTA measurements, which became detected upon
labeling. Notably, although the recovery of NK-EV was
relatively low when 2 μM DiI was used, the yield was
completely restored by using 0.2−0.67 μM DiI (Figure 4B). In
fact, this condition improved the labeling eciency as well
(Figure 4C,D and Video S2), suggesting that an optimal dye
concentration needs to be determined empirically for a given
sample of vesicles.
For downstream uses, retaining the native structures and
functions of vesicle proteins after salt-change labeling would be
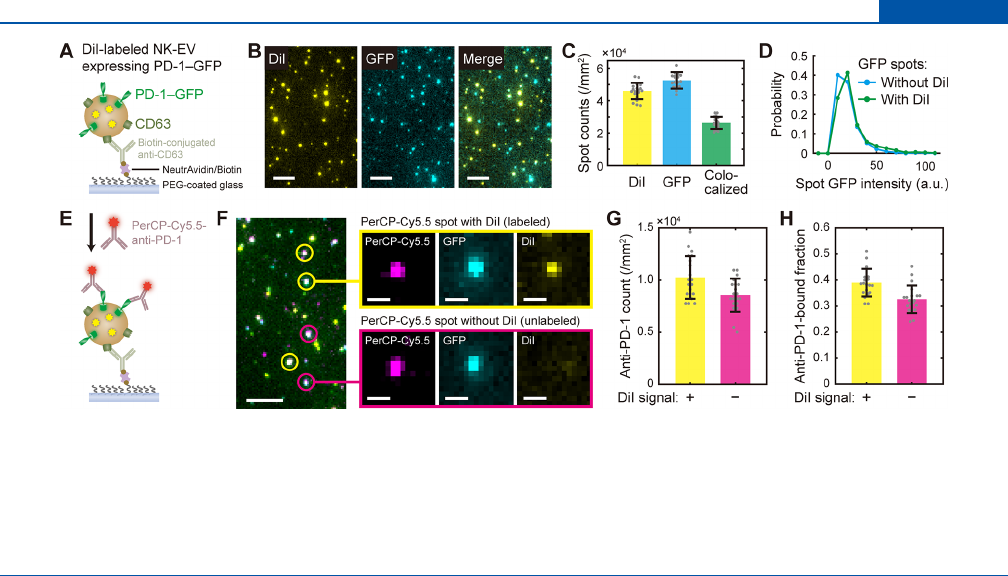
important. To this end, we tested whether the labeled vesicles
can be captured by antibodies to a common component of
EVs, CD63, and then detected by another antibody toward a
cargo protein.
22
We prepared EVs from NK-92 cells that
overexpressed PD-1-GFP, labeled them with DiI by using the
salt-change method, and then pulled them down onto a
polyethylene glycol (PEG)-coated glass surface with anti-
CD63 (Figure 5A). The resulting surface showed bright DiI
spots that are colocalized (57%) with GFP spots (Figure
5B,C), indicating successful capturing of NK-EVs via CD63
and therefore the presence of intact CD63 molecules on EV
membranes after labeling. By comparing the GFP spots with
and without DiI, we noticed that the distribution of GFP
intensity was not altered by the presence of DiI (Figure 5D),
suggesting that the amount of PD-1-GFP per vesicle was not
perturbed (e.g., from leakage or vesicle fusion) during the
labeling procedure. Additionally, when a PD-1 antibody was
introduced over the captured EVs (Figure 5E), the
fluorescence from anti-PD-1 colocalized with GFP spots with
a high eciency (Figure 5F,G), verifying the presence of PD-1.
Since the detection eciency (as measured by anti-PD-1-
bound fraction) did not depend on the presence of DiI label
(Figure 5H), we conclude that the incorporation of DiI
molecules did not change the anity between PD-1 and its
antibody, and suggest that such native interactions can be
preserved after salt-change labeling.
■
DISCUSSION
Successful labeling of vesicles with bright fluorescent dyes is a
prerequisite for quantitative analysis of vesicles via fluo-
rescence. Vesicles are commonly labeled by targeting surface
proteins, but this method not only depends on protein
composition but also interfere with downstream measure-
ments. The membrane staining procedure introduced here
addresses many challenges associated with vesicle labeling:
labeling was unbiased and eective for all types of vesicles and
dyes we tested because the dyes target generic lipid bilayers
(Figures 2 and 3); virtually all free-dye particles were removed
by NaCl-induced aggregation and subsequent filtering (Figure
S1); and the recovery of input vesicles was satisfactory (Figure
4).
The two-orders-of-magnitude improvement in labeling
eciency with the salt-change method is impressive given its
simple steps, and therefore can potentially substitute complex
labeling and purification protocols (such as the standard
protocol for PKH67 we used for comparison), even for
researchers with access to an ultracentrifugation system.
3
The
Figure 5. Integrity of vesicle proteins after salt-change labeling (A) Schematic of single-vesicle pull-down and imaging of DiI-labeled NK-EVs
containing PD-1-GFP. (B) Representative fluorescence images from the experiments described in (A). Scale, 5 μm. (C) Colocalization of DiI and
GFP spots from the NK-EV images such as shown in (B). (D) GFP intensity distribution for the spots with DiI (labeled) and without DiI
(unlabeled). (E) Schematic of NK-EV detection with PerCP-Cy5.5-conjugated PD-1 antibody. (F) Representative fluorescence images from the
experiments described in (E). Insets show magnified views of the selected spots with and without DiI. Scale, 5 μm (on left) and 1 μm (insets). (G)
Numbers of anti-PD-1 (PerCP-Cy5.5) spots with (yellow) and without (magenta) DiI signal. (H) Fraction of GFP spots detected by anti-PD-1 as
a function of the presence of DiI.
Analytical Chemistry pubs.acs.org/ac Technical Note
https://doi.org/10.1021/acs.analchem.2c05166
Anal. Chem. XXXX, XXX, XXX−XXX
E

method also does not involve any proprietary formulation (e.g.,
diluent C used with PKH dyes
12
) and can be finished within
30 min in a regular wet lab. Improving the labeling eciency
also allows the use of lower concentrations of dyes and vesicles
and thereby reduce the possibility of nonspecific labeling. We
expect this method to apply to clinically obtained EVs from
liquid biopsy, which will particularly benefit from eective
labeling because they are usually limited in amount.
One concern with the salt-change labeling is that ionic
strength change may induce osmotic stress on vesicles and
membrane proteins. We verified that the selected proteins
(endogenous CD63 and overexpressed PD-1) were still
recognized by antibodies after labeling (Figure 5). Although
these were minimal tests, we think the salt-change labeling
method does not seriously sacrifice the functionality of vesicles
given that the incorporated numbers of dye molecules were
small (∼2 on average) and the size and shape of the vesicles
were largely maintained. Also, it would be important to be
aware of potential contamination from the syringe filters,
especially because some filter membranes contain wetting
surfactants that can destroy vesicles. We showed that
prerinsing of the filter to remove aqueous extractables can
gently improve the recovery of vesicles (Figure S3C,D), but
the results will likely depend on the specific filter models in
use.
■
CONCLUSION
In this study, we investigated fluorescent labeling of vesicles
with lipophilic dyes, revealing a critical dependence on NaCl
concentration. We exploited the reversible aggregation of DiI
molecules both to improve labeling eciency and to remove
free dye molecules from the vesicle solution. The salt-change
labeling method was shown to be widely applicable to many
types of vesicles and dyes, without noticeably degrading the
functional properties of vesicle samples and content proteins.
We expect that this protocol will be useful in a broad spectrum
of fluorescence-based assays interrogating natural EVs and
engineered nanovesicles.
■
ASSOCIATED CONTENT
*
sı
Supporting Information
The Supporting Information is available free of charge at
https://pubs.acs.org/doi/10.1021/acs.analchem.2c05166.
Supplementary Methods for the preparation of vesicles,
NTA measurements, PKH67 labeling, TIRF microscopy
and image analysis including labeling eciency;
Supplementary Figures on NaCl dependence of DiI
aggregation (Figure S1), estimation of labeling eciency
(Figure S2), and comparison of CDV labeling eciency
(Figure S3) (PDF)
NK-CDVs labeled by direct staining (Video S1) (AVI)
Eect of DiI concentration on salt-change labeling of
NK-EVs (Video S2) (AVI)
■
AUTHOR INFORMATION
Corresponding Authors
Min Ju Shon − Department of Physics, Pohang University of
Science and Technology (POSTECH), Pohang 37673,
Republic of Korea; School of Interdisciplinary Bioscience and
Bioengineering, Pohang University of Science and Technology
(POSTECH), Pohang 37673, Republic of Korea;
orcid.org/0000-0002-0333-1150; Email: mjshon@
postech.ac.kr
Yong Song Gho − Department of Life Sciences, Pohang
University of Science and Technology (POSTECH), Pohang
Authors
Minkwon Cha − Department of Physics, Pohang University of
Science and Technology (POSTECH), Pohang 37673,
Republic of Korea; POSTECH Biotech Center, Pohang
University of Science and Technology (POSTECH), Pohang
37673, Republic Korea
Sang Hyeok Jeong − Department of Physics, Pohang
University of Science and Technology (POSTECH), Pohang
37673, Republic of Korea
Seoyoon Bae − Department of Life Sciences, Pohang
University of Science and Technology (POSTECH), Pohang
37673, Republic of Korea
Jun Hyuk Park − Department of Physics, Pohang University of
Science and Technology (POSTECH), Pohang 37673,
Republic of Korea
Yoonjin Baeg − Biodrone Research Institute, MDimune Inc.,
Seoul 04790, Republic of Korea
Dong Woo Han − Biodrone Research Institute, MDimune Inc.,
Seoul 04790, Republic of Korea
Sang Soo Kim − Department of Life Sciences, Pohang
University of Science and Technology (POSTECH), Pohang
37673, Republic of Korea
Jaehyeon Shin − Department of Physics, Pohang University of
Science and Technology (POSTECH), Pohang 37673,
Republic of Korea
Jeong Eun Park − Biodrone Research Institute, MDimune Inc.,
Seoul 04790, Republic of Korea
Seung Wook Oh − Biodrone Research Institute, MDimune
Inc., Seoul 04790, Republic of Korea
Complete contact information is available at:
https://pubs.acs.org/10.1021/acs.analchem.2c05166
Author Contributions
#
These authors contributed equally to this work.
Author Contributions
M.C., S.H.J., J.H.P., and M.J.S. designed experiments and
analyzed data. M.C., S.H.J., J.H.P., and J.S. verified vesicle
labeling results with TIRF microscopy. S.H.J., J.H.P., and
S.S.K. performed NTA measurements. S.B. prepared extrac-
ellular vesicles and Y.S.G. supervised the process. Y.B. and
D.W.H. prepared CDVs and J.E.P. and S.W.O. supervised the
process. M.C. and M.J.S. wrote the manuscript with inputs
from S.H.J., S.B., S.W.O., and Y.S.G.
Notes
The authors declare the following competing financial
interest(s): M.C., Y.B., D.W.H., J.E.P., S.W.O., and M.J.S.
filed a patent on the vesicle labeling method described in this
study.
■
ACKNOWLEDGMENTS
We thank Dr. Cherlhyun Jeong for helpful discussions. This
work was supported by the BioDrone Award funded by
MDimune Inc.. This work was also supported by the National
Research Foundation of Korea (NRF) grant funded by the
Korea government (MSIT; NRF-2022R1C1C1012176, NRF-
2021R1A4A1031754, and NRF-2021R1A6A1A10042944 to
Analytical Chemistry pubs.acs.org/ac Technical Note
https://doi.org/10.1021/acs.analchem.2c05166
Anal. Chem. XXXX, XXX, XXX−XXX
F

M.J.S.; 2021R1I1A1A01059751 to M.C.). M.C. was also
supported by Korea Initiative for fostering University of
Research and Innovation Program (2020M3H1A1075314).
■
REFERENCES
(1) Wyss, R.; Grasso, L.; Wolf, C.; Grosse, W.; Demurtas, D.; Vogel,
H. Anal. Chem. 2014, 86 (15), 7229−7233.
(2) Cho, S.; Yi, J.; Kwon, Y.; Kang, H.; Han, C.; Park, J. ACS Nano
2021, 15 (7), 11753−11761.
(3) van der Vlist, E. J.; Nolte-’t Hoen, E. N. M.; Stoorvogel, W.;
Arkesteijn, G. J. A.; Wauben, M. H. M. Nat. Protoc. 2012, 7 (7),
1311−1326.
(4) Görgens, A.; Bremer, M.; Ferrer-Tur, R.; Murke, F.; Tertel, T.;
Horn, P. A.; Thalmann, S.; Welsh, J. A.; Probst, C.; Guerin, C.;
Boulanger, C. M.; Jones, J. C.; Hanenberg, H.; Erdbru
̈
gger, U.;
Lannigan, J.; Ricklefs, F. L.; El-Andaloussi, S.; Giebel, B. J. Extracell.
Vesicles 2019, 8 (1), 1587567.
(5) Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. J. Cell. Biochem.
2010, 111 (2), 488−496.
(6) Franzen, C. A.; Simms, P. E.; Van Huis, A. F.; Foreman, K. E.;
Kuo, P. C.; Gupta, G. N. BioMed. Res. Int. 2014, 2014, e619829.
(7) Carpintero-Fernández, P.; Fafián-Labora, J.; O’Loghlen, A. Front.
Mol. Biosci. 2017, 4, 79.
(8) Chiang, C.; Chen, C. J. Biomed. Sci. 2019, 26 (1), 9.
(9) Colombo, F.; Norton, E. G.; Cocucci, E. Biochim. Biophys. Acta
BBA - Gen. Subj. 2021, 1865 (4), 129752.
(10) Levitus, M.; Ranjit, S. Q. Rev. Biophys. 2011, 44 (1), 123−151.
(11) Takov, K.; Yellon, D. M.; Davidson, S. M. J. Extracell. Vesicles
2017, 6 (1), 1388731.
(12) Puz
̌
ar Dominkus
̌
, P.; Stenovec, M.; Sitar, S.; Lasic
̌
, E.; Zorec,
R.; Plemenitas
̌
, A.; Z
̌
agar, E.; Kreft, M.; Lenassi, M. Biochim. Biophys.
Acta BBA - Biomembr. 2018, 1860 (6), 1350−1361.
(13) Simonsen, J. B. J. Extracell. Vesicles 2019, 8 (1), 1582237.
(14) Dehghani, M.; Gulvin, S. M.; Flax, J.; Gaborski, T. R. Sci. Rep.
2020, 10 (1), 9533.
(15) Daina, A.; Michielin, O.; Zoete, V. Sci. Rep. 2017, 7 (1), 42717.
(16) Lee, H.-W.; Choi, B.; Kang, H. N.; Kim, H.; Min, A.; Cha, M.;
Ryu, J. Y.; Park, S.; Sohn, J.; Shin, K.; Yun, M. R.; Han, J. Y.; Shon, M.
J.; Jeong, C.; Chung, J.; Lee, S.-H.; Im, S.-A.; Cho, B. C.; Yoon, T.-Y.
Nat. Biomed. Eng. 2018, 2 (4), 239−253.
(17) Jang, S. C.; Kim, O. Y.; Yoon, C. M.; Choi, D.-S.; Roh, T.-Y.;
Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y. S. ACS Nano 2013,
7 (9), 7698−7710.
(18) Zhang, C.; Mok, J.; Seong, Y.; Lau, H.; Kim, D.; Yoon, J.; Oh,
S. W.; Park, T. S.; Park, J. Nanomedicine Nanotechnol. Biol. Med. 2021,
37, 102448.
(19) Mousseau, F.; Berret, J.-F.; Oikonomou, E. K. ACS Omega
2019, 4 (6), 10485−10493.
(20) Choi, D.; Go, G.; Kim, D.-K.; Lee, J.; Park, S.-M.; Di Vizio, D.;
Gho, Y. S. J. Extracell. Vesicles 2020, 9 (1), 1757209.
(21) Kim, O. Y.; Park, H. T.; Dinh, N. T. H.; Choi, S. J.; Lee, J.;
Kim, J. H.; Lee, S.-W.; Gho, Y. S. Nat. Commun. 2017, 8 (1), 626.
(22) Han, C.; Kang, H.; Yi, J.; Kang, M.; Lee, H.; Kwon, Y.; Jung, J.;
Lee, J.; Park, J. J. Extracell. Vesicles 2021, 10 (3), e12047.
(23) Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.;
Wang, R.; Lai, L. J. Chem. Inf. Model. 2007, 47 (6), 2140−2148.
Analytical Chemistry pubs.acs.org/ac Technical Note
https://doi.org/10.1021/acs.analchem.2c05166
Anal. Chem. XXXX, XXX, XXX−XXX
G
