
Vanderbilt University Law School Vanderbilt University Law School
Scholarship@Vanderbilt Law Scholarship@Vanderbilt Law
Vanderbilt Law School Faculty Publications Faculty Scholarship
2023
Education and Electronic Medical Records and Genomics Education and Electronic Medical Records and Genomics
Network, Challenges and Lessons Learned from a Large-Scale Network, Challenges and Lessons Learned from a Large-Scale
Clinical Trial Using Polygenic Risk Scores Clinical Trial Using Polygenic Risk Scores
Ellen Wright Clayton
John J. Connolly
et al.
Follow this and additional works at: https://scholarship.law.vanderbilt.edu/faculty-publications
Part of the Medical Jurisprudence Commons

REVIEW
Education and electronic medical records and
genomics network, challenges, and lessons learned
from a large-scale clinical trial using polygenic risk
scores
ARTICLE INFO
Article history:
Received 2 February 2023
Received in revised form
17 May 2023
Accepted 18 May 2023
Available online 26 May 2023
Keywords:
Education
eMERGE
Genome-Informed Risk Report
Polygenic risk score
PRS
ABSTRACT
Polygenic risk scores (PRS) have potential to improve health care by identifying individuals that
have elevated risk for common complex conditions. Use of PRS in clinical practice, however,
requires careful assessment of the needs and capabilities of patients, providers, and health care
systems. The electronic Medical Records and Genomics (eMERGE) network is conducting a
collaborative study which will return PRS to 25,000 pediatric and adult participants. All par-
ticipants will receive a risk report, potentially classifying them as high risk (~2-10% per con-
dition) for 1 or more of 10 conditions based on PRS. The study population is enriched by
participants from racial and ethnic minority populations, underserved populations, and pop-
ulations who experience poorer medical outcomes.
All 10 eMERGE clinical sites conducted focus groups, interviews, and/or surveys to understand
educational needs among key stakeholders—participants, providers, and/or study staff.
Together, these studies highlighted the need for tools that address the perceived benefit/value of
PRS, types of education/support needed, accessibility, and PRS-related knowledge and under-
standing. Based on findings from these preliminary studies, the network harmonized training
initiatives and formal/informal educational resources.
This paper summarizes eMERGE’s collective approach to assessing educational needs and
developing educational approaches for primary stakeholders. It discusses challenges encoun-
tered and solutions provided.
© 2023 American College of Medical Genetics and Genomics.
Published by Elsevier Inc. All rights reserved.
Introduction
The electronic Medical Records and Genomics (eMERGE)
network was established in 2006 and aims to develop,
disseminate, and apply approaches to research that combine
biorepositories with electronic medical record (EMR) sys-
tems for genomic discovery and genomic medicine
implementation research. In July 2020, Phase IV of the
eMERGE program was launched with the objective of
recruiting 25,000 participants across 10 clinical sites—all of
whom will receive a risk report that includes polygenic risk
scores (PRS), monogenic risk (adults only), family history,
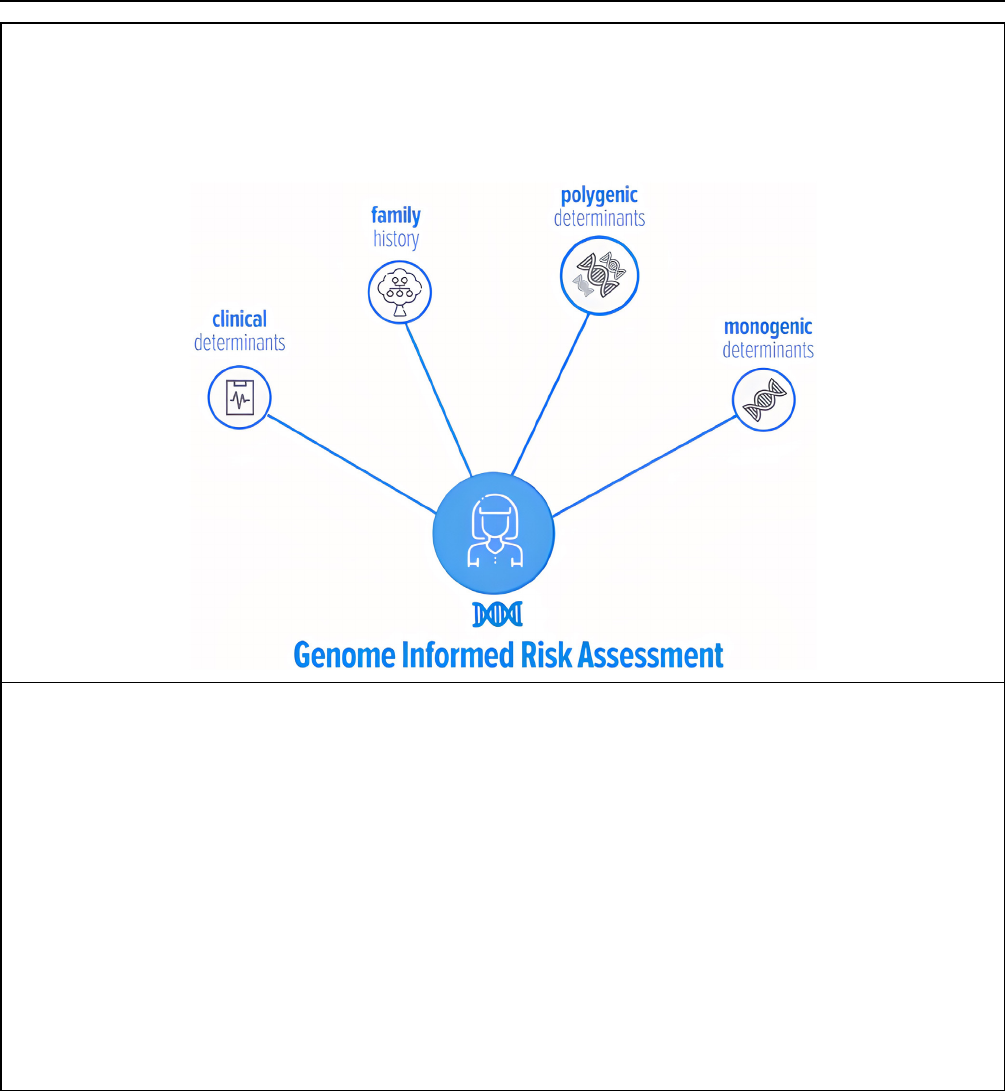
and clinical risk factors. The report is called the Genome
Informed Risk Assessment (GIRA).
*
Correspondence and requests for materials should be addressed to John J. Connolly, Center for Applied Genomics, 3615 Civic Center Blvd, Abramson
Building Suite 1216F, Philadelphia, PA 19104. Email address: [email protected] OR Maya Sabatello, Center for Precision Medicine & Genomics,
Department of Medicine, Columbia University Irving Medical Center, New York, NY 10032. Email address: [email protected]
A full list of authors and affiliations appears at the end of the paper.
Genetics in Medicine (2023) 25, 100906
www.journals.elsevier.com/genetics-in-medicine
doi: https://doi.org/10.1016/j.gim.2023.100906
1098-3600/© 2023 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.

Unlike a single research study focused on a specific
condition or population, the results of the eMERGE study
will inform not only how PRS can be used to identify people
at high risk for common conditions but, importantly, if
receiving this risk information helps patients and their pro-
viders make health care decisions to improve the patient’s
overall health. With minimal stated exclusion criteria (eg,
unable to consent, recent bone marrow transplant, or no
medical record at recruitment site), participants are not
selected for presence/absence of disease or susceptibility to
disease. They span a wide spectrum of ages (3-75 years) and
are geographically diverse, recruited from health care cen-
ters across the United States (though biased tow ard prox-
imity to recruitment sites in large urban areas in which
eMERGE sites are based).
In selecting phenot ypes for PRS-based return of results
(RoR), netw ork leaders prioritized the need to generat e
feasible, actionable, and translatable scores for individuals
across 4 groups: African, Asian, European, and Hispanic/
Latino. PRS for a range of phenotypes are promising for
their potential to improve health care/outcomes and have
been shown to outperform clinical predictors for several
diseases.
1-3
Supplemental Table 1 summarizes the final
conditions and thresholds/logic for return. Although condi-
tions such as breast cancer and coronary heart disease are
relatively mature in terms of the validity and potential for
clinical application of relevant PRS, other conditions are
less well established. For this reason, the network collec-
tively conducted an extensive process of PRS validation for
all phenotypes. Phenotype selection and validation pro-
cesses are summarized elsewhere.
4
Returning PRS for multiple conditions, in a clinical
environment, is relatively novel. Best practices are only
beginning to take shape, though forerunners such as the
Polygenic Risk Score Reporting Standards, a joint collab-
oration between the Clinical Genome Resource (ClinGen)
Complex Disease Working Group,
5
have been influential.
As outlined in Box 1, eMERGE’s custom GIRA report
classifies participants at “high risk” (or not) for 11 condi-
tions, based on a range of risk factors. For 10 of the 11
conditions, PRS are the primary driver of high-risk reporting
and for most are prerequisite to returning other risk factors
such as family history and/or clinical history for most con-
ditions (ie, for most conditions, high clinical/family history
risk is not retur ned in the absence of high risk PRS—see
Supplemental Table 1 for more details). The relative novelty
of returning and integrating PRS for multiple conditions
represents an exciting research opportunity but also poses
educational challenges.
Importantly, PRS performance diff ers by group. Scores
for many condition s were originally developed using ge-
notypes from individuals of European ancestries and
therefore tended to perform better in European cohorts.
6-8
A
major goal of the eMERGE network is to tackle a long-
documented bias in who participates in genomics resear ch.
In the US where health disparities are significantly higher
than in other high- and middle-income countries,
9,10
it is
especially important that PRS-linked phenotypes be vali-
dated in historically underrepresented cohorts. Per the rele-
vant National Institutes of Health Requests for Application
(HG-19-014), “Minority” (also non-European ancestry) re-
fers to individuals from the following populations: African
American or Black, Asian, American Indian or Alaska
Native, Native Hawaiian or other Pacific Islander, or Latino/
Hispanic. Six of the 10 eMERGE sites aim to recruit at least
75% of their participants from racial or ethnic minority
populations, underserved populations, or populations that
experience poorer medical outcomes. The remaining 4 sites
have a 35% recruitment target for minority/underserved
communities. PRS for all conditions implemented in
eMERGE-IV were validated across at least 2 of 4 groups
(African, Asian, European, and Hispanic/Latino). From an
educational perspective, network members agreed upon the
importance of both acknowledging the historical short-
comings of the genomics community in delivering equitable
resources and the urgent effort to address this imbalance.
Multiple clinical sites led independent projects engaging
local communities and underrepresented populations to
assist in designing educational materials and approaches,
recruitment strategies, and offer guidance on study imple-
mentation. Additionally, the eMERGE network has been
transparent about the fact that not all PRS are validated for
all 4 groups .
In addition to marginalized racial and ethnic populations,
eMERGE has made efforts toward inclusion of people with
disability, in which health disparities are among the largest
in the United States.
11
Despite research indicating that many
people with disability express high interest in participation
in precision medicine resear ch, they often also experience
numerous barriers for participation in mainstream
research.
12
Key barriers include accessibility of facilities
and study materials and limited knowledge among re-
searchers and research staff about how to design inclusive
studies. To address some of these challenges, eMERGE
collects self-reported disability status information among
other demographic questions in its baseline survey. The
development of educational material for the network
similarly considered issues of accessibility.
Overall, PRS will be returned to a large cohort (N =
25,000), therefore requiring scalable educational resources.
Across all sites, approximately 24% of research participants
are expected to be classified as high risk for at least 1
condition (see Supplemental Table 1), necessitating coor-
dination between clinical sites for consistency in study
design and risk reporting.
The latest phase of the eMERGE program constitutes a
model of genomic medicine that is potentially generalizable
and scalable to a large proportion of the US population and
other countries with genomics capacity and access to EMRs.
Education will be a key component in helping to develop a
comprehensive suite of resources and know-how that sup-
port the use of genomic information in clinical care of
diverse populations. It will also be critical for promoting
team-oriented genomic care, encompassing patients,
2 J.J. Connolly et al.
research participant, health care provider, or study team
member. The experiences, processes, and lessons learn ed
from eMERGE IV can shed light on these issues and inform
other consortia that are considering simil ar issues or related
work.
A crucial component of this coordinated approach is the
need to unders tand and subsequently address the educa-
tional requirements of eMERGE participants, providers, and
site representatives (eg, study coordinators, recruiters, and
clinical staff returning results). For this purpose, an Edu-
cation Subgroup was created to develop and coordinate the
work surrounding educational material. The Education
Subgroup comprises members from all eMERGE sites and
diverse expertise. This paper will review the network’s
collective approach to education through needs assessment
and subsequent resource development. It specifically
Box 1. Main elements of the genome-informed risk assessment (GIRA). Genetic results, along with family and medical
history are used to estimate participants’ risk of developing 11 common conditions (Supplemental Table 1). The GIRA can
classify participants at “high risk” of developing a condition based on several factors. For 2 conditions (break cancer and
CHD), an integrated absolute risk score is presented; for the others, the information is displayed separately.
4
Note, in
addition to the GIRA, a PRS report and monogenic report (if relevant) are produced separately and appended to the GIRA.
Polygenic results: In eMERGE, PRS are calculated for 10 of the 11 conditions implemented. Each phenotype has a specific PRS
percentile cut-off (2%-10%) that is associated with high risk. The participant’s exact percentile is not provided. For breast cancer
and coronary heart disease, PRS are integrated with other risk factors to achieve a global score, incorporating the other risk
elements discussed below. Breast cancer used the BOADICEA model.
20
Monogenic results: Adult participants (18+ YO) provide an additional biosample, which is assessed for monogenic susceptibility across
a 16-gene panel. Relevant conditions tested are the following: Constitutional mismatch repair deficiency syndrome (CMMR-D), Lynch
syndrome, BRCA1/BRCA2-Associated Hereditary breast and ovarian cancer (HBOC) syndrome, PALB2-related conditions, PTEN-related
conditions, Li Fraumeni syndrome (LFS), Peutz-Jeghers syndrome (PJS), Familial hypercholesterolemia (FH), and LMNA-related
conditions.
21
Family history: Participants are asked to provide their family health history for all conditions using an online tool called MeTree,
developed at Duke University.
22
For some conditions (eg, coronary heart disease), positive family history alone can trigger high risk.
For other conditions (eg, hypercholesterolemia) positive family history is returned only if a participant is at high PRS/monogenic
risk for the same condition.
Clinical risk factors: Data from participant self-report surveys and the EMR will be used to calculate risk, or contextualize high genetic
risk. Examples of these clinical risk factors include most recent hemoglobin A1C, reported physical activity, and BMI, when
available. Similar to family history, high risk for any given condition is only returned if a participant is at high PRS/monogenic risk
for the same condition.
J.J. Connolly et al. 3

addresses (1) assessment of educational needs around PRS,
(2) the process of developing educational resources, and (3)
challenges and lessons learned.
Assessment of educational needs around PRS
To facilitate RoR and study coordination, eMERGE leaders
developed a range of initiatives to understand and address
educational requirements for its many constituents as sum-
marized in Table 1. Key to these were Ethical, Legal, and
Social Implication (ELSI) studies conducted at member sites
in the first 2 years of the eMERGE study. These ELSI
studies differed in scope and methods used (eg, inte rviews
or focus groups) but all explored perspe ctives and needs
related to study implementation. Ultimately, these studies
informed the development of educational resources, which
are summarized in Table 2. The resources address
eMERGE’s primary stakeholders: patients/participants,
providers, and study teams.
In addition to the wi de-range of ELSI studies that
informed study design and relevant educational initiatives, a
huge effort was undert aken across the network to solicit
input from a broad spectrum of individuals. Sites directly
engaged with different community groups and advisory
boards, including those representing Black/African Amer-
ican community members, pediatric- and adult-specific
subcohorts, health care providers with a range of back-
grounds, and hospital leadership. Collectively, these
generated feedback on study design, knowledge of genetics,
and interest in participating in the study. Feedback from
these groups, though not (necessarily) captured in “formal”
research design, provided important discussion points for
consideration by eMERGE education leadership.
Development of educational resources
Design of the GIRA
All 25,000 participants across the eMERGE network will
receive a novel report - the GIRA. This includes approxi-
mately 6000 participants expected to be identified as high
risk. The GIRA was custom-developed for returning results
to participants, with relevant features including PRS,
monogenic risk, family history, and clinical risk.
In developing the network-wide risk report, several fea-
tures specific to the eMERGE study were important to
consider, including the novelty of the study objectives
(particularly PRS), the diversity of participants, (particularly
in terms of race, ethnicity, and age), and the different
backgrounds/needs of providers and patients in interacting
with relevant content. The goals of the GIRA were to inform
participants of their study results, to provide educational
material about the associated condition, and to provide care
recommendations for the participant and their provider. In-
dividual eMERGE sites drafted risk report language and
recommendations for the condition(s) led by that site.
Relevant material included educational content to help pa-
tients/providers understand the conditions, the implications
of their risk result, and available options for next steps to
reduce high risk. This approach was the most pragmatic
because it allowed the network to leverage local expertise
and resources specific of the site(s) leading respective con-
ditions. However, it yielded substantial variability in the
requisite detail and language level . Once drafts wer e created,
a GIRA development subgroup worked to standardize the
language and graphics for all 11 eMERGE conditions.
Educational resources for research participants
Concepts such as “monogenic” and “polygenic” risk are
unlikely to be familiar to this broadly healthy research
cohort, presenting a challenge for returning
results—particularly for individuals not identified as high
risk, who may not have the opportunity to discuss results
with a health professional. Informal education resources can
play a major role in addressing this issue. Surveys show that
the majority of internet users in the United States use online
sources as their primary source of health information.
23,24
However, the lack of reliable infor mation has been well
documented, sowing distrust among users.
25
Authoritative
print, video, and internet multimedia developed by univer-
sities, hospitals, and science centers can help to address the
communication gap between research/medical communities
and the general public. A large consensus in the scientific
community favors the free dissemination of information as
widely as possible.
26
To this end, the eMERGE network
clinical sites developed educational materials for online
dissemination, aiming to engage participants to be fully
informed decision makers throughout their participation.
Study website. Much of the educational content pro-
duced for the GIRA, along with many other efforts across
the network, was repurposed for a participant-facing website
www.emerge.study. This website organizes the broad
spectrum of participant-facing educational materials in one
location for current and prospective participants to refer-
ence. Participants can use this website throughout their
participation in eMERGE, from finding contact information
for their site to register for eMERGE, all the way up to
viewing the GIRA frequently asked questions (FAQs) and
condition-specific education pages while waiting for their
results.
In creating the web resource, an eMERGE web team dis-
cussed scenarios most likely to be relevant to users, agreeing
upon 4: (1) prospective participants interested in learning
more about the study, (2) enrolled participants interested in
learning about next steps or discu ssing questions, (3) in-
dividuals looking to contact a local eMERGE site for any
reason, and (4) providers with questions about the eMERGE
study and/or GIRA recommendations. A dedicated section
was built around each of these scenarios with separate FAQs
for providers/participants, and similarly distinct discussion of
study protocols tailored to respective users.
All material was created in both English and Spanish and
made openly-available to the general public, with no re-
quirements for registration. Before launch, usability-testing
4 J.J. Connolly et al.

Table 1 Key eMERGE stakeholders and respective educational objectives. A one-size-fits-all approach to education is unsuitable for a
program involving heterogeneous stakeholders with disparate educational objectives. Acknowledging the numerous constituents across the
research clinical-spectrum, the eMERGE Education Subgroup identified 3 primary target groups: research participants, research staff, and
health care providers. These groups have necessarily different competencies and priorities, which require targeted approaches to capturing
learning needs and developing educational resources. This table summarizes the main research considerations considered for each group and
the respective educational objectives addressed:
A. General public: Broadly healthy population. Non-medically oriented individuals.
B. Health care providers: Medically-trained individuals working in the clinical domain. The individual will have direct contact with the
eMERGE participant as a patient at their clinical site. However, 2 important criteria are consistent across the network: (1) High risk reports
are returned in-person; (2) Reports that do not include a high-risk result are returned to the participant without in-person consultation.
C. Study staff: Medical- and/or research-orientated individual that is a member of an eMERGE
a
clinical or support center
b
.
Unless explicitly stated, the points delineated below arose from eMERGE ’ s preliminary ELSI studies.
A. Research Participants:
Research Considerations
Objective(s) of Educational
Resources Relevant Findings and Lessons Learned
Develop a novel risk report (the
GIRA) that integrates PRS,
monogenic risk, family history,
and clinical risk factors.
Ensure patient-participants
understand the risk report
(GIRA).
• Patients may struggle to understand PRS-based reports.
13
• Misinterpreting percentiles as absolute risk is a common
mistake.
13
• Patients prefer absolute risk information and a continuous
result (vs, high risk/not high risk) report design.
13
• Patients have a preference for visualization.
13
• Patients/community members express a need for simple
language,
13
including Spanish versions among target sub-
cohorts.
All 25,000 participants will
receive a GIRA risk report. Of
these, ~6000 will be identified
as high-risk for 1 or more
conditions
Avoid harm by worry.
Avoid false reassurance for
individuals not at high risk.
• Clarify that “high risk” and “low risk” are not indicative of a
diagnosis.
• For high-risk participants, results should be returned directly
by a genetic counselor, physician, or trained clinical research
professional.
• Provide system navigation for those who are at high risk For
participants not at high-risk for any conditions, results can be
returned to the EMR and/or by letter. In this instance, edu-
cation is reliant upon the clarity and comprehensiveness of
the risk report, and supplemented by informal educational
resources including brochures, video, and study website.
Facilitate easy access to reliable
information about PRS and the
eMERGE study
Develop accessible study website
to engage participants to be
fully informed decision makers
throughout their entire
participation.
• Delineate subsections likely to be relevant to users. For
eMERGE this includes:
○
Prospective participants interested in learning more about
the study.
○
Enrolled participants with questions about next steps.
○
Individuals looking to contact a local eMERGE site.
○
Providers with questions about the eMERGE study and/or
GIRA.
• Translate content if targeting a sub-cohort with different
language capacity.
• Apply usability testing to address functionality, clarity, and
relevance.
• Capture and review analytics.
Minority Enriched: Six of the 10
eMERGE sites aim to recruit at
least 75% of their participants
from racial or ethnic minority
populations, underserved
populations, or populations
who experience poorer medical
outcomes.
Provide cohort-appropriate
educational material and
approaches.
• Leverage off-line forms of communication, including
community-based newspapers, magazines, and radio.
• Opportunities for interpersonal conversations are important
for participants of racial or ethnic minority populations for
responding to questions and concerns.
• Per above, Spanish versions of educational material can help
target sub-cohorts.
(continued)
J.J. Connolly et al. 5

Table 1 Continued
A. Research Participants:
Research Considerations
Objective(s) of Educational
Resources Relevant Findings and Lessons Learned
Age Spectrum: Participants span
a pediatric-adult age spectrum
(3-75 years).
Pediatric and adult participants
receive different GIRA reports,
with different conditions
relevant to each. In addition,
adults will receive a
monogenic risk report for a
discrete number of conditions.
Understand needs of different
sub-cohorts in terms of
expectations.
Communicate clearly implications
of respective phenotypes.
• Parents face barriers in acting on recommendations,
14
which
reflect previously-reported limitations for minority and un-
derrepresented communities.
15,16
Perhaps the most notable
barrier that participants reported was existing behavioral
health issues in their children. Given the high prevalence of
conditions such as ADHD (9.4%),
17
behavioral conduct
(7.4%), and anxiety problems (7.1%),
18
behavioral health
clearly needs to be taken into consideration when strategizing
risk reduction in children.
• Younger participants (20s and 30s) may be lower resourced
and/or less motivated to address a risk in the future (vs older
participants).
B. Healthcare Providers
Research Considerations Educational Objective(s) Relevant Findings and Lessons Learned
Large-scale return of results
leveraging PRS is novel.
Improve genomic knowledge by
providing templates and
resources to facilitate return
of results and
recommendations for follow-
up care.
Provide educational resources
that can be shared with
patients.
• Providers probed for their reactions to PRS reports of different
designs, reported that
13
:
○
The report is more than just communicating a result, but
integral to the whole interaction with the patient.
○
The Limitations section of the GIRA report should make it
very clear what these mean for the patient.
○
Education can help in becoming comfortable with their
“spiel.”
• Providers request different types of educational
materials—some that would enhance their own knowledge
and some that could be shared with patients.
Primary care providers and health
care providers may not be
aware of the eMERGE study
Provide as-needed resources to
support encounters in which
the program may be
unfamiliar.
Provide an online resource that
clearly outlines program goals
and report implications.
• Many PCPs are inexperienced with PRS and may feel
uncomfortable using it in clinical practice. In particular,
clinicians may not be comfortable returning results that are
not within their immediate expertise and they have limited
time to do so.
• PCPs express a preference for easy to use, non-time-
consuming material—preferably, info that can be found by
clicking a link and consumed quickly (eg, versus watching a
video).
• Many providers would prefer forewarning of pending genetic
report information
• Endorsement or actual guidelines from relevant specialty
societies would promote trust in test reports.
• Some providers were interested in technical information
regarding GIRA development, available outcome data, and
other published research. Links to peer reviewed journal
articles would promote trust in the test reports.
• Online resource should include the following:
○
Sources for guidelines and recommendations.
○
Contact details for local teams.
• Supporting educational initiatives preferred by providers
include:
○
An online webinar with CME.
○
Available online recording.
○
Separate online webinar to practices where recruitment will
be high.
○
The option to provide in-person “lunch and learn” sessions.
○
Modeling of results return by a PCP for both high- and low-
risk scores.
(continued)
6 J.J. Connolly et al.
was performed on a small convenience sample of in-
dividuals (N = 5), which addressed functionality, clarity,
and appropriateness of resources, yielding minor revisions
in content structure and language. To monitor usage of the
site, analytics are captured and reviewed on a monthly basis,
with the opportunity to formatively adapt to relevance/
popularity of content on an ongoing basis. (However, it is a
requirement of the central institutional review board [IRB]
that all study material on the website be approved by the
IRB, necessarily limiting the speed with which any content
can be updated.) The site follows Section 504 of the 1973
Rehabilitation Act to prevent discrimination on the basis of
disability. Care was taken to ensure broad representation in
images, including diversity in race, ethnicity, disability,
gender, and age. This approach was similarly applied in
developing other content for dissemination across eMERGE
stakeholders (brochures, flyers, etc.).
Education resources for health care providers
In addition to receiving the GIRA directly (either through a
patient portal or by mail), high-risk participants are contacted
by a genetic counselor, clinician, or trained health profes-
sional, who recommend that the high-risk participant follows
up with their local primary care provider (PCP) where addi-
tional testing/referral may be required. Importantly, the
majority of PCPs across eMERGE will likely be naive to the
eMERGE study and may encounter the GIRA for the first
time during a clinical encounter with a participant-patient. A
range of educational approaches are therefore required to
support providers in discussing and acting upon GIRA health
care recommendations.
We learned from our ELSI studies that, though
comfortable explaining risk to patients, many PCPs are
inexperienced with PRS technology and may feel uncom-
fortable using it as a tool in clinical practice. They also
expressed concerns about being caught “off guard,” not
having heard about the study in advance, and they had
questions and concerns about the validity of the PRS and
implications for prevention and treatment. This gap between
knowledge and practice with PRS is a potential obstacle for
uptake among providers, with downstream ramifications for
(possible difficulty in) implementing care recommendations.
Moreover, clinicians may not be comfortable discussing
results that are beyond thei r immediate expertise, in partic-
ular when time constrained. Rather, they expressed a pref-
erence for easy to use, non-time-consuming material,
Physicians were also interested in accessible fact sheets for
sharing with patients. eMERGE education initiatives that
address these findings include the provision of clinical de-
cision support (CDS), development of custom web content,
Table 1 Continued
B. Healthcare Providers
Research Considerations Educational Objective(s) Relevant Findings and Lessons Learned
Provide clinical decision support:
(CDS) to facilitate reporting
and downstream health care
implementation
A well-designed CDS
development and validation
plan can facilitate
implementation of PRS-based
recommendations.
• Reflecting a theme from previous work,
19
PCPs expressed a
desire for CDS targeted to primary care that may include
actionable steps in the EMR such as the following:
○
Modification of the problem list.
○
Reviewing provider education.
○
Provision of patient-family education.
○
Referrals to specialists, and/or
○
Ordering a preventive medication.
C. Study Staff
Research Considerations Educational Objective(s) Relevant Findings and Lessons Learned
Recruiters, Coordinators,
Resource Developers, and
Other Research Staff are highly
diverse in terms of
background, experience, and
training. Staff require answers
regarding study components
and key research objectives.
Need for accessible educational
resources that address
misperceptions.
Disseminate standard resources/
messaging across the network.
Collective need to coordinate and share know-how and resources.
Core elements required for centralized access:
• (Electronic) data capture and tracking.
• (Electronic) consent driven by a single IRB.
• Standard operating procedures (SOPs) for sample management
and ordering, and engagement.
• Provision of regular training.
A centralized database accessible to clinical sites can streamline
and standardize Network supports (eMERGE utilized REDCap for
this purpose):
• Support data integration from third-party providers for shared
SOP development and to facilitate collective testing and
training.
a
Clinical sites: Children’s Hospital of Philadelphia (CHOP), Cincinnati Children’s Hospital Medical Center (CCHMC), Columbia University, Mass General
Brigham, Mayo Clinic, Mount Sinai, Northwestern University, University of Alabama At Birmingham, University of Washington, and Vanderbilt University
Medical Center.
b
Support Sites: Coordinating Center: Vanderbilt University Medical Center; Genotyping Center: Broad; Monogenic Sequencing: Invitae; Family History:
MeTree (Duke University).
J.J. Connolly et al. 7
Table 2 Resources developed by eMERGE network sites. Unless otherwise stated in Column 1, relevant resources are used network-wide by
all eMERGE sites (resources delineated as site-specific were nevertheless shared with network partners).
Resource Stakeholder Description
GIRA Report Participant and Provider The GIRA (Genome Informed Risk Assessment), the novel result report issued in this
study, caters to both patients and providers through its accessible phrasing and
diagrams, as well as separate FAQ sections for participants and providers.
Infographics Participant Infographics were developed through an iterative process, including 10 “think
aloud” sessions in English and Spanish with community members. The
infographics explain the components of a GIRA, the meaning of genetic
predisposition for a disease, and general recommendations for both high-risk and
not at high-risk individuals.
Participant and Provider
facing website
(www.emerge.study)
Participant and Provider The website is a resource for participants to learn about the goals of the study, the
timeline of participation, and information about results. A subgroup led by CHOP
was formed to spearhead the development of the website. They used the services
of third-party vendor Culture Shift to design the website and translate it into
Spanish. In addition, the website follows requirements for disability accessibility
Participant and provider
FAQ’s/patient
education pages
Participant and Provider Centrally-developed educational material included a plain-language brochure,
common FAQs, and patient/provider education pages for use across the network.
Clinician education
resources (site-specific)
Provider Several sites developed local CDS to facilitate on-demand education, focused on
implementing GIRA guidelines. Although network members shared expertise and
methodologies, individual sites developed individualized CDS to align with local
institutional infrastructure and policy.
Bilingual content Participant Although not a “resource” in the traditional sense, the provision of educational
material in Spanish as well as English was important to addressing representation
and included here as a core component of Education strategy.
Animated recruitment
video to explain
eMERGE
Prospective participant Animated 2-minute video developed by collaborators across the Network and made
available to all prospective and existing participants.
Participant-facing online
presentations/webinars
[site-specific]
Participant Two sites made short (~7-minute) presentations available to (prospective)
participants. These focused on explaining PRS, their clinical use, and current
limitations, including increased accuracy in populations with origins from Europe
compared with other populations. They also defined genetic terms, such as 'DNA',
'gene', and 'genetic variants'. Additionally, a vignette where a patient was offered
a PRS for hypercholesterolemia was incorporated to help contextualize the
application of PRS for common health conditions
Provider-facing in-person
presentations
[site-specific]
Provider Provider-facing presentations introducing eMERGE study, implementation, and
basic concepts around PRS were given at primary care recruitment sites (a) at the
start of recruitment and (b) leading up to return of results. Presentations also
directed providers to the eMERGE.study website and staff contact information.
Presentations were given at grand rounds, lab-meetings, departmental meetings,
and scheduled informational sessions.
Provider-facing online
presentations/webinars
[site-specific]
Provider Following the same format as in-person presentations, 15 to 20-minute webinars
were recorded and made available online for provider access.
Provider-facing PRS online
media
Provider Easily navigable online resource containing practical information about the study,
specifically designed for use during routine patient care activity.
Clinical hotline
[site-specific]
Provider One site developed an expert clinician hotline to connect clinicians with study
experts to address any questions related to study results.
Staff-facing PowerPoint Study Team PowerPoint presentation given to all eMERGE research staff members. Disseminated
Network-wide over a series of “classes” to introduce the basics of eMERGE and
create a shared baseline of knowledge amongst staff members. Creating these
slides was a network wide effort undertaken by the education workgroup. The
presentation was given by multiple members of this workgroup.
(continued)
8 J.J. Connolly et al.
and creation of a range of learning sessions, and are dis-
cussed immediately below.
CDS. Interviews with providers at several eMERGE
sites indicated a preference for CDS to help implement GIRA
recommendations. CDS is an important component of edu-
cation strategy, addressing the need for continuous and cur-
rent knowledge in daily practice
27
and can also address the
complex sociotechnical factors that arise from genomic test
results.
19
Reflecting a theme from previous work,
19,28
in-
terviews with providers underline a desire for CDS targeted
at primary care that may include actionable steps in the EMR,
such as modification of the problem list, reviewing provider
education, provision of patient-family education, referrals to
specialists, and/or ordering a preventive medication. PCPs
welcomed CDS for positive results only, with a strong
preference for clear recommendations and next steps.
Custom web content for providers. To facilitate easy
access to reliable information about PRS and the eMERGE
study, the emerge.study website was updated with provider-
specific content. Interviews with physicians and clinical
leaders indicated that links to peer-reviewed papers and
endorsement or actual guidelines from relevant specialty
societies would make them more like ly to trust and use these
tests. The site was updated with links to source material and
guidelines, on which GIRA recommendations are based. In
addition, provider-specific FAQs were published, in which a
range of themes incl uded a program overview, the role of
the health care provider in eMERGE, the patient/participant
experience, and data privacy.
Webinars and other learning sessions. Although
recognizing a preference for informal, just-in-time, resources,
several initiatives were developed for providers preferring
more increased engagement. These are particularly pertinent
to health care practices/clinics enriched for eMERGE patient-
participants and include the following:
• An online webinar with Continuing Medical Educa-
tion—several sites offered Continuing Medical Edu-
cation credits centered on PRS and the GIRA
• Targeted online webinars in practices in which
recruitment will be highest
• Provision of webinar recordings available to PCPs
online
• In-person “lunch and learn” sessions at practices with a
high level of eMERGE participation.
These initiatives were considered necessary to supplement
resources that are intentionally not time consuming and
providing a more in-depth perspective on the goals and impli-
cations of the eMERGE program. Webinars and presentations
focused on explaining PRS, their clinical use, and current
limitations, including increased accuracy in populations with
origins from Europe compared with other populations. Sites
focused on recruiting Hispanic individuals offered relevant
educational resources in both Spanish and English.
RoR training sessions were developed to help coordinate
RoR and to ensure that individuals felt confident to discuss
this new form of results. A content guide was developed that
collected network resources, including, lecture series, articles,
and tools to aid return. Talking points for high-risk pediatric
PRS results, high-risk adult PRS results, monogenic results,
and edge cases were also generated. The network also pro-
vided staff the option to participate in mock roleplay sessions.
Potential scenarios and mock GIRAs were generated, and
those who wanted the additional practice had the opportunity
to gain feedback on ways to better manage the RoR session.
Understanding the need for ongoing communication and
Table 2 Continued
Resource Stakeholder Description
Research assistant office
hours
Study Team Biweekly meetings open to any Network member focused on answering the
questions and addressing the concerns of coordinators/recruiters/research
assistants. Open space to solicit advice from others and collaboratively solve
problems and share tips.
Centralized staff support
and training
Study Team The Network Coordinating Center hosted frequent education/training program for
staff at all sites and developed several standard operating procedure documents
consisting relevant to study implementation and general utilization of the
interface.
Return of return of result
staff training materials
Study Team Several tools were created by the Network for return of result staff members to
utilize and prepare for the sessions. A content guide was developed to provide
staff with educational resources needed to efficiently explain results to
participants. Talking points for the various return of result sessions were created
to help outline the sessions and to guide sessions. Finally, mock roleplay sessions
were held among various members across the Network to allow individuals to
practice returning results scenarios in a training environment.
Return of results round
tables
Study Team Biweekly meetings open to any Network member who is involved in the return of
results process to discuss shared experiences. Opportunity to gain feedback on
how the sessions went and ways to improve the discussion of results.
CDS, clinical decision support; CHOP, Children’s Hospital of Philadelphia; eMERGE, electronic Medical Records and Genomics; FAQs, frequently asked
questions; GIRA, Genome Informed Risk Assessment; PRS, plygenic risk scores.
J.J. Connolly et al. 9

feedback about RoR sessions, the network also hosted
biweekly round tables for staff members to share experiences
and learn from the experiences of other s.
Education resources for recruiters and study coordinators
Any large program will require delegation of workload
across several subgroups. Although essential from an
organizational perspective, an unwelcome consequence is
the potential for respective workgroups to become siloed
and detached from the broader mission. In the context of
eMERGE, this is of particular concern to team members
directly interacting with participants and who are required to
cogently address participants’ questions and concerns. To
provide the requisite resources for participant engagement,
the network developed and conducted a series of training
sessions for coordinator and recruitment teams, which
reviewed program goals and approaches and anticipated
questions from (prospective) participants. The goal was to
ensure study members meet a standard minimum in terms of
baseline knowledge of the eMERGE program and imple-
mentation requi rements.
The primary staff education initiative was developed and
coordinated to begin 1 to 2 months before the first partici-
pants were enrol led. A 2.5-hour training curriculum was
developed for recruiters and study coordinators across all 10
clinical sites and focused on program goals and relevant
study themes such as genetics; PRS; disease risk; and lim-
itations of disease risk prediction for certain groups study
privacy, study partners, and consent language.
Importantly, a series of form ative assessments were in-
tegrated into the session, facilitating real-time feedback from
attendees, with the opportunity to pivot accordingly. A set
of multiple-choice questions addressing the major topics
covered in the training sessions were prepared in advance
and integrated into a polling feature, which allo ws the
audience members to answ er questions and have their an-
swers recorded and displayed for the group. These questions
were asked after each topic was presented and used to assess
whether the audience had unders tood the content, reinforce
major points, and add additional explanations if indicated.
Coordination. Additional suppor t focused on know-
how related to the use of genomic information in clinical
care. This leveraged a centralized data capture portal built in
REDCap,
29,30
which allowed participants to electronically
consent and enter survey data, while also providing sites the
ability to track participants and send/receive data from
Network data partners. The Network Coordinating Center
(Vanderbilt University Medical Center [VUMC]) hosted
frequent education/training programs for staff at all sites and
developed several standard operating procedure docum ents
consisting relevant to study implementation and general
utilization of the interface.
Challenges and Lessons Learned
Although each clinical site necess arily has distinct de-
mographic and institutional priorities, common themes from
eMERGE’s coll ective research and approaches have been
critical in driving network-wide collaboration. We hope that
our shared initiatives can be instructive to other consortia
and/or individual sites similarly involved in operationaliz-
ing/expanding precision medicine programs and propose
several discussion points.
Need for harmonization
Among the biggest challenges, educationally, for eMERGE
has been to harmonize the efforts of 10 different sites,
operating from 10 different funded proposals, albeit with
shared goals. Clinical sites in eMERGE are individually
focused on disparate target populations (eg, African, Asian,
European, and Hispanic), age groups (adult/pediatric), and
institutional priorities. These align with different language
or communication preferences. An important part of edu-
cation strategy is making sure these priorities are all repre-
sented and messaging is consistent across groups. Virtually
all eMERGE sites used community engagement and input
from relevant community groups was important in high-
lighting requirements, as well as potential gaps in under-
standing. The importance of consultation with these groups
early in the planning process should be stressed because
relevant feedback was adopted into educational materials
through FAQs, videos, and presentations.
Centralized coordination was c ritical to the harmoniza-
tion process. Although somewhat unwieldy from a logistical
perspective, the network’s use of a single IRB greatly
facilitated harmonization of educational material—ensuring
all sites were working from a common resource set. Simi-
larly, using a single site (VUMC) to generate the GIRA
ensured sites were working from a level playing field in
terms of the validity of the final product being returned. This
further ensured that content created for patients and pro-
viders was applicable to all sites. Similarly, training and
educational material for study teams was the same for all
sites and based on a report (GIRA) that is consistent for all
research participants.
Targeting different stakeholders
Aone-size-fits-all approach to education is unsuitable for a
program with heterogeneous stakeholders who have disparate
educational objectives. Acknowledging the numerous con-
stituents across the research-clinical spectrum, the eMERGE
Education Subgroup identified 3 primary target groups:
research participants, research staff, and health care pro-
viders. These groups have necessarily different competencies
and priorities, which require targeted approaches to capturing
learning needs and developing educational resources.
Awareness of barriers for participants
A common theme in genetic literature is the difficulty pa-
tients/participants have in inte rpreting risk, and this theme is
10 J.J. Connolly et al.

borne out again in relation to PRS.
31,32
From an educational
perspective, it is clear that resources should be spent
determining the extent to which participants understand risk
as reported to them, to plainly stress that they are at higher
than normal risk where this is relevant, and to simulta-
neously avoid genetic determinism. Including next steps and
ways to reduce risk are practical educational steps that may
facilitate implementation.
Relatedly, several ELSI studies in eMERGE identified
obstacles participants face in following through on health
care recommendations. Collectively, these obstacles stress
that the risk result does not exist in a vacuum and that
numerous factors can affect a participant’s likelihood of
following recommendations. For example, for parents of
pediatric participants, a high-risk report may need to
“compete” with existing behavioral health issues in chil-
dren.
14
Similarly, adults face competing priorities and as is-
sues of access that influence the feasibility of some
recommendations. It is critical that health care providers be
aware of such barriers (eg, other medical/behavioral problems
or resource/time scarcity) and address them where relevant.
Providers interacting with risk reports should be reminded of
these factors, whereas recommendations for patients/partici-
pants need to be cognizant of the wider psychosocial context.
Finally, these findings underscore the importance of trust
between patients and providers. Participants in this study
emphasized the importance of access to a trusted resource
with whom they could review the results and ask questions.
33
Cognizance of implementation barriers for
providers
Studies from several sites found that clinicians may not be
comfortable returning results that are not within their im-
mediate expertise and have limited time to do so. Given the
novelty of PRS in the clinical environment, this presents an
educational challenge to provider support for RoR, and also
prompts ELSI-related discussions on the readiness of pro-
viders to interact with these types of results. As mentioned
above, the language and formatting of the GIRA is critical to
the patient-provider interaction and may be the centerpiece
of the clinical encounter. This stresses the need for plain and
uncomplicated language, as well as an awareness of diffi-
culties in risk-perception among many participants. Simi-
larly, from a practical educat ional perspective, the
limitations section of the risk report should be very clear on
implications for the patient.
The provision of effective CDS can facilitate provider
interactions with the GIRA and was highlighted as a
requirement among providers in interviews at 2 sit es.
Visualization and risk preferences matter
Health and genomic literacy can affect genomic utilization
and participation in genomic research, but studies indicate
that genomic literacy in the United States is limited.
34,35
Challenges are pronounced for individuals with limited
oral, print, or graph-reading literacy and further augmented
for individuals whose first language is other than English.
Infographics can be an important tool in increasing health
literacy
36-38
and in particular addressing deficits in under-
standing complex health concepts,
37
including genomics
and genetic risk factors.
39
For eMERGE, infographics were
developed through an iterative process, including modified
“think aloud” sessions with English and Spanish-speakers
(overall n = 10) to explain the components of a GIRA
and the meaning of genetic predisposition for a disease, as
well as recommendations for both high-risk and not at high-
risk individuals.
Centralized training for study teams
Finally, given the novelty, large scale , and wide scope of the
project, centralized training of study teams is strongly rec-
ommended as an educational output. Clinical and research
teams at diff erent sites differ greatly in terms of knowledge,
experience, and understanding of study goals and health care
best practices. A major effort was undertaken by the coordi-
nating center to standardize enrollment tools and reporting
resources, requiring the generation and implementation of
SOPs for all major aspects of the program (including, but not
limited to, education). Although this effort involves a massive
investment of time and human resources, it ultimately yields a
standardized protocol, which should greatly facilitate out-
comes analyses later in the project.
Assessment
A key component of the eMERGE study is to assess the
impact of return of PRS and GIRA results on health care be-
haviors and outcomes for participants and their providers. It
will be essential to re-evaluate relevant educational methods
and the clinical utility of the information once outcomes data
are available. Toward the end of the eMERGE study timeline,
a wide range of studies are planned that address the efficacy
and acceptability of the tools and approaches used. Analyses
of EHR and self-report data will assess differences in clinical
care for participants who received high-risk reports versus
those who did not. In tandem, a series of studies across the
network will assess outcomes that speak more directly to the
efficacy of educational approaches delineated herein. These
include interviews with participants (or their parents in the
case of pediatric participants), interviews with providers and a
network-wide provider survey. Leveraging these approaches,
we will assess the extent to which participants and providers
struggled to understand reports and act upon relevant infor-
mation. Although several of the ELSI studies outlined above
utilized hypothetical risk report aimed to replicate the RoR
scenario, the real-world “ road test” will be most critical to
relevant outcomes. This will yield formative data and the
opportunity to further update educational material/methods
for the utility of future PRS-based programs.
J.J. Connolly et al. 11

A limitation of the eMERGE program pertains to the
representativeness of the pilot study participants to the full
cohort of 25,000 participants. It may be that findings from
the preliminary ELSI studies are biased given that they re-
flected the views of participants in these small-scale studies
rather than the general population. However, similar to the
eMERGE network more generally, these preliminary studies
comprised primarily of individuals from minority/un der-
represented racial and ethnic communities, reflecting a
recruitment goal across the 10 clinical eMERGE sites.
Conclusions
In developing a comprehensive education strategy, consid-
eration should be given to (1) the necessity of thinking
through the educational needs of different stakeholders, (2)
the necessity for different approaches to ascertaining those
needs, including targeted studies and stakeholder engage-
ment, (3) that this all takes a huge amount of resources, and
(4) that much of what is learnt and developed can be shared
across projects, lowering a potentially massive barrier for
other projects. For its part, eMERGE is committed to
sharing the resources it has put together, which continues to
be consolidated as the study progresses, which can be
accessed via the network’s coordinating center.
40
Data Availability
Data used in this manuscript are available upon request by
contacting the corresponding author. Requests should be sent
to the corresponding author with a description of the reason for
the request and the qualifications of those requesting the data.
Acknowledgments
The network would like to thank our eMERGE participants and
their families for graciously taking part in this study and the
patient and physician advisory groups that helped shape study
design, report content, and education materials. Because of the
structure of the U01 cooperative agreement, the recruitment
sites, coordinating center, and funding agency collaborated on
the study design, as well as collection, management, analysis,
and interpretation of the data. This trial was registered with
clinicaltrials.gov under identifier NCT05277116.
Funding
This phase of the eMERGE network was initiated and funded
by the NHGRI through the following grants: U01HG011172
(Cincinnati Children’s Hospital Medical Center),
U01HG011175 (Children’s Hospital of Philadelphia),
U01HG008680 (Columbia University), U01HG011166
(Duke University), U01HG011176 (Icahn School of Medi-
cine at Mount Sinai), U01HG008685 (Mass General Brig-
ham), U01HG006379 (Mayo Clinic), U01HG011169
(Northwestern University), U01HG011167 (University of
Alabama at Birmingham), U01HG008657 (University of
Washington), U01HG011181 (Vanderbilt University Medi-
cal Center), and U01HG011166 (Vanderbilt University
Medical Center serving as the Coordinating Center).
Author Information
Conceptualization: J.J.C., E.S.B., M.Sm., M.Sa.; Data Cura-
tion: J.J.C., S.L.; Formal Analysis: J.J.C., E.S.B., M.Sm., S.L.,
S.T., M.Har., M.Sa.; Funding Acquisition: J.J.C., E.S.B.,
M.Sm., M.Har., S.S., I.A.H., K.D., C.N., A.K., R.L.C.,
W.-Q.W., H.T.B., E.W.C., J.E.L., N.A.L., L.A.O., J.W., H.H.,
M.Sa.; Investigation: J.J.C., E.S.B., M.Sm., S.L., S.T., M.Har.,
D.K., S.S., I.A.H., K.D., C.N., A.K., R.L.C., A.A., W.-Q.W.,
H.T.B., E.W.C., E.R.S., J.E.L., N.A.L., A.M., S.N., H.B.,
M.Ham., A.S., A.C.F.L., E.P., L.A.O., M.A.-D., S.C., J.W.,
H.H., M.Sa. Methodology: J.J.C., E.S.B., M.Sm., S.L., S.T.,
M.Har., D.K., S.S., I.A.H., K.D., C.N., A.K., R.L.C., A.A.,
W.-Q.W., H.T.B., E.W.C., E.R.S., J.E.L., N.A.L., A.M., S.N.,
H.B., M.Ham., A.S., A.C.F.L., E.P., L.A.O., M.A.-D., S.C.,
J.W., H.H., M.Sa.; Project Administration: J.J.C., C.N., A.K.,
H.T.B., E.W.C., E.R.S., A.M., E.P., T.K.R.-B.; Writing-
Original Draft: J.J.C., E.S.B., M.Sm., S.L., S.T., M.Har., D.K.,
C.N., A.M., E.P., L.A.O., T.K.R.-B., J.W., M.Sa.; Writing-
Review & Editing: J.J.C., E.S.B., M.Sm., S.L., S.T., M.Har.,
D.K., S.S., I.A.H., K.D., C.N., A.K., R.L.C., A.A., W.-Q.W.,
H.T.B., E.W.C., E.R.S., J.E.L., N.A.L., A.M., S.N., H.B.,
M.Ham., A.S., A.C.F.L., E.P., L.A.O., T.K.R.-B., M.A.-D.,
S.C., C.L.S., J.W., H.H., M.Sa.
Ethics Declaration
The studies discussed in this reviewed were all conducted
according to the guidelines of the Declarat ion of Helsinki
and approved by the Institutional Rev iew Board of VUMC
and/or local Institutional Review Boards. Informed consent
is required from all enrolled individuals.
Conflict of Interest
Emma Perez is a paid consultant for Allelica Inc. Lori
Orlando and Tejinder Rakhra-Burris are founders of a
company developing MeTree. Maureen Smith is a Section
Editor for the Journal of Genetic Counseling. Maya
Sabatello serves as IRB member of the All of Us
Research Program. All other authors declare no conflicts of
interest.
12 J.J. Connolly et al.

Additional Information
The online version of this article (http s://doi.org/10.1016/j.
gim.2023.100906) contains supplemental material, which
is available to authorized users.
Authors
John J. Connolly
1,*
, Eta S. Berner
2
, Maureen Smith
3
,
Samuel Levy
1
, Shannon Terek
1
, Margaret Harr
1
,
Dean Karavite
4
, Sabrina Suckiel
5
, Ingrid A. Holm
6
,
Kevin Dufendach
7
, Catrina Nelson
8
, Atlas Khan
9
,
Rex L. Chisholm
10
, Aimee Allworth
11
, Wei-Qi Wei
12
,
Harris T. Bland
12
, Ellen Wright Clayton
13,14,15
,
Emily R. Soper
5,16
, Jodell E. Linder
17
, Nita A. Limdi
18
,
Alexandra Miller
19,20
, Scott Nigbur
19
, Hana Bangash
19
,
Marwan Hamed
19
, Alborz Sherafati
19
,
Anna C.F. Lewi s
21,22
, Emma Perez
23
, Lori A. Orlando
24
,
Tejinder K. Rakhra-Burris
24
, Mustafa Al-Dulaimi
25
,
Selma Cifric
25
, Courtney Lyn am Scherr
26
, Julia Wynn
27
,
Hakon Hakonarson
1,28,29,30
, Maya Sabatello
31,32,*
Affiliations
1
Center for Applied Genomics, Children’s Hospital of
Philadelphia, PA;
2
Department of Health Services Admin-
istration, University of Alabama at Birmingham, Birming-
ham, AL;
3
Center for Genetic Medicine, Department of
Medicine, Northwestern University, Chicago, IL;
4
Depart-
ment of Biomedical and Health Informatics, Children’s
Hospital of Philadelphia, PA;
5
The Institute for Genomi c
Health, Department of Medicine, Icahn School of Medicine
at Mount Sinai, New York, NY;
6
Division of Genetics and
Genomics, Boston Children’s Hospital; Department of Pe-
diatrics, Harvard Medical School, Boston, MA;
7
Depart-
ment of Pediatrics, University of Cincinnati, Cincinnati,
OH;
8
Center for Autoimmune Genomics and Etiology,
Cincinnati Children’s Hospital Medical Center, Cincinnati,
OH;
9
Division of Nephrology, Dept of Medicine, Vagelos
College of Physic ians & Surgeons, Columbia University,
New York, NY;
10
Center for Genetic Medicine, Feinberg
School of Medicine, Northwestern University, Chicago, IL;
11
Department of Medical Genetics, University of Wash-
ington, Seattle, WA;
12
Department of Biomedical Infor-
matics, Vanderbilt University Medical Center, Nas hville,
TN;
13
Division of Genetic Medicine, Department of Medi-
cine, Vanderbilt University Medical Center, Nashville, TN;
14
Center for Biomedical Ethics and Society, Vanderbilt
University, Nashville, TN;
15
Vanderbilt University Law
School, Nashville, TN;
16
Division of Genomic Medicine,
Department of Medicine, Icahn School of Medicine at
Mount Sinai, New York, NY;
17
Vanderbilt Institute for
Clinical and Translational Research, Vanderbilt University
Medical Center, Nashville, TN;
18
Department of Neurology,
Heersink School of Medicine, University of Alabama at
Birmingham, Birmingham, AL;
19
Department of Cardio-
vascular Medicine, Mayo Clinic, Rochester, MN;
20
Department of Clinical Genomics, Mayo Clinic, Roches-
ter, MN;
21
Edmond and Lily Safra Center for Ethics, Har-
vard, MA;
22
Brigham and Women's Hospital, Boston, MA;
23
Mass General Brigham Personalized Medicine, Brigham
and Wome n’s Hospital, Boston, MA;
24
Department of
Medicine, Duke University, Durham, NC;
25
Department of
Biology, The College of Idaho, Caldwell, ID;
26
School of
Communication | Department of Communication Studies,
Northwestern University, Chicago, IL;
27
Department of
Pediatrics, Columbia University Irving Medical Center,
New York, NY;
28
Department of Pediatrics, The Perelman
School of Medicine, University of Pennsylvania, Philadel-
phia, PA;
29
Division of Human Genetics, Children's Hos-
pital of Philadelphia, Philadelphia, PA;
30
Division of
Pulmonary Medicine, Children's Hospital of Philadelphia,
Philadelphia, PA;
31
Center for Precision Medicine & Ge-
nomics, Department of Medicine, Columbia University
Irving Medical Center, New York, NY;
32
Division of
Ethics, Department of Medical Humanities & Ethics,
Columbia University Irving Medical Center, New York, NY
References
1. Maas P, Barrdahl M, Joshi AD, et al. Breast cancer risk from modifi-
able and nonmodifiable risk factors among white women in the United
States. JAMA Oncol. 2016;2(10):1295-1302. http://doi.org/10.1001/
jamaoncol.2016.1025
2. Schumacher FR, Al Olama AA, Berndt SI, et al. Association analyses
of more than 140,000 men identify 63 new prostate cancer suscepti-
bility loci. Nat Genet. 2018;50(7):928-936. http://doi.org/10.1038/
s41588-018-0142-8
3. Sharp SA, Rich SS, Wood AR, et al. Development and standardization
of an improved Type 1 diabetes genetic risk score for use in newborn
screening and incident diagnosis. Diabetes Care. 2019;42(2):200-207.
http://doi.org/10.2337/dc18-1785
4. Linder JE, Allworth A, Bland HT, et al. Returning integrated genomic
risk and clinical recommendations: the eMERGE study. Genet Med.
2023;25(4):100006. http://doi.org/10.1016/j.gim.2023.100006
5. Wand H, Lambert SA, Tamburro C, et al. Improving reporting stan-
dards for polygenic scores in risk prediction studies. Nature.
2021;591(7849):211-219. http://doi.org/10.1038/s41586-021-03243-6
6. Kessler MD, Yerges-Armstrong L, Taub MA, et al. Challenges and
disparities in the application of personalized genomic medicine to
populations with African ancestry. Nat Commun. 2016;7:12521. http://
doi.org/10.1038/ncomms12521
7. Martin AR, Gignoux CR, Walters RK, et al. Human demographic
history impacts genetic risk prediction across diverse populations. Am J
Hum Genet. 2017;100(4):635-649. http://doi.org/10.1016/j.ajhg.2017.
03.004
8. Kim MS, Patel KP, Teng AK, Berens AJ, Lachance J. Genetic disease
risks can be misestimated across global populations. Genome Biol.
2018;19(1):179. http://doi.org/10.1186/s13059-018-1561-7
9. Hero JO, Zaslavsky AM, Blendon RJ. The United States leads other
nations in differences by income in perceptions of health and health
care. Health Aff (Millwood). 2017;36(6):1032-1040. http://doi.org/10.
1377/hlthaff.2017.0006
10. Williams DR, Priest N, Anderson NB. Understanding associations
among race, socioeconomic status, and health: patterns and prospects.
Health Psychol. 2016;35(4):407-411. http://doi.org/10.1037/hea0000242
J.J. Connolly et al. 13

11. Krahn GL, Walker DK, Correa-De-Araujo R. Persons with disabilities
as an unrecognized health disparity population. Am J Public Health.
2015;105(suppl 2):S198-S206. http://doi.org/10.2105/AJPH.2014.
302182
12. Sabatello M, Chen Y, Zhang Y, Appelbaum PS. Disability inclusion in
precision medicine research: a first national survey. Genet Med.
2019;21(10):2319-2327. http://doi.org/10.1038/s41436-019-0486-1
13. Lewis ACF, Perez EF, Prince AER, et al. Patient and provider per-
spectives on polygenic risk scores: implications for clinical reporting
and utilization. Genome Med. 2022;14(1):114. http://doi.org/10.1186/
s13073-022-01117-8
14. Terek S, Del Rosario MC, Hain HS, et al. Attitudes among parents
towards return of disease-related polygenic risk scores (PRS) for their
children. J Pers Med. 2022;12(12). http://doi.org/10.3390/
jpm12121945
15. Fisher EB, Fitzgibbon ML, Glasgow RE, et al. Behavior matters. Am J
Prev Med. 2011;40(5):e15-e30. http://doi.org/10.1016/j.amepre.2010.
12.031
16. Patel N, Ferrer HB, Tyrer F, et al. Barriers and facilitators to healthy
lifestyle changes in minority ethnic populations in the UK: a narrative
review. J Racial Ethn Health Disparities. 2017;4(6):1107-1119. http://
doi.org/10.1007/s40615-016-0316-y
17. Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD,
Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and
associated treatment among U.S. Children and adolescents, 2016.
J Clin Child Adolesc Psychol. 2018;47(2):199-212. http://doi.org/10.
1080/15374416.2017.1417860
18. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treat-
ment of depression, anxiety, and conduct problems in US children.
J Pediatr. 2019;206:256-267.e3. http://doi.org/10.1016/j.jpeds.2018.
09.021
19. Pennington JW, Karavite DJ, Krause EM, Miller J, Bernhardt BA,
Grundmeier RW. Genomic decision support needs in pediatric primary
care. J Am Med Inform Assoc. 2017;24(4):851-856. http://doi.org/10.
1093/jamia/ocw184
20. Lee A, Mavaddat N, Wilcox AN, et al. Boadicea: a comprehensive
breast cancer risk prediction model incorporating genetic and nonge-
netic risk factors. Genet Med. 2019;21(8):1708-1718. http://doi.org/10.
1038/s41436-018-0406-9
21. invitae.com. Invitae eMERGE Panel. Invitae. Accessed January 18,
2023.
22. Orlando LA, Buchanan AH, Hahn SE, et al. Development and vali-
dation of a primary care-based family health history and decision
support program (MeTree). N C Med J. 2013;74(4):287-296. http://doi.
org/10.18043/ncm.74.4.287
23. Brown SN, Jouni H, Marroush TS, Kullo IJ. Effect of disclosing ge-
netic risk for coronary heart disease on information seeking and
sharing: the MI-GENES study (myocardial infarction genes). Circ
Cardiovasc Genet. 2017;10(4). http://doi.org/10.1161/CIRCGE-
NETICS.116.001613
24. Saks E, Tyson A. Americans Report More Engagement with Science
News than in 2017. Pew Research Center. https://www.pewresearch.
org/fact-tank/2022/11/10/americans-report-more-engagement-with-
science-news-than-in-2017/
25. Funk C, Hefferon M, Kennedy B, Johnson C. Trust and mistrust in
Americans’ views of scientific experts; 2019. Pew Research Center.
https://www.pewresearch.org/science/2019/08/02/trust-and-mistrust-
in-americans-views-of-scientific-experts/
26. De Castro P, Marsili D, Poltronieri E, Calder
´
on CA. Dissemination of
public health information: key tools utilised by the NECOBELAC
network in Europe and Latin America. Health Info Libr J.
2012;29(2):119-130. http://doi.org/10.1111/j.1471-1842.2012.00977.x
27. Konstantinidis ST, Bamidis PD. Why decision support systems are
important for medical education. Healthc Technol Lett. 2016;3(1):56-
60. http://doi.org/10.1049/htl.2015.0057
28. Pet DB, Holm IA, Williams JL, et al. Physicians’ perspectives on
receiving unsolicited genomic results. Genet Med. 2019;21(2):311-318.
http://doi.org/10.1038/s41436-018-0047-z
29. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.
Research Electronic Data Capture (REDCap)—a metadata-driven
methodology and workflow process for providing translational
research informatics support. J Biomed Inform. 2009;42(2):377-381.
http://doi.org/10.1016/j.jbi.2008.08.010
30. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building
an international community of software platform partners. JBiomed
Inform. 2019;95:103208. http://doi.org/10.1016/j.jbi.2019.103208
31. Nightingale SD. Risk preference and laboratory test selection. J Gen
Intern Med. 1987;2(1):25-28. http://doi.org/10.1007/BF02596246
32. Huhn JM III, Potts CA, Rosenbaum DA. Cognitive framing in action.
Cognition. 2016;151:42-51. http://doi.org/10.1016/j.cognition.2016.02.
015
33. Suckiel SA, Braganza GT, Agui
˜
niga KL, et al. Perspectives of diverse
Spanish- and English-speaking patients on the clinical use of polygenic
risk scores. Genet Med. 2022;24(6):1217-1226. http://doi.org/10.1016/
j.gim.2022.03.006
34. Lea DH, Kaphingst KA, Bowen D, Lipkus I, Hadley DW. Commu-
nicating genetic and genomic information: health literacy and
numeracy considerations. Public Health Genomics. 2011;14(4-5):279-
289. http://doi.org/10.1159/000294191
35. Hurle B, Citrin T, Jenkins JF, et al. What does it mean to be genom-
ically literate?: National Human Genome Research Institute Meeting
Report. Genet Med. 2013;15(8):658-663. http://doi.org/10.1038/gim.
2013.14
36. Damman OC, Vonk SI, van den Haak MJ, van Hooijdonk CMJ,
Timmermans DRM. The effects of infographics and several quantita-
tive versus qualitative formats for cardiovascular disease risk, including
heart age, on people’s risk understanding. Patient Educ Couns.
2018;101(8):1410-1418. http://doi.org/10.1016/j.pec.2018.03.015
37. McCrorie AD, Donnelly C, McGlade KJ. Infographics: healthcare
communication for the digital age. Ulster Med J. 2016;85(2):71-75.
38. Jain S, Dhaon SR, Majmudar S, et al. Empowering health care workers
on social media to bolster trust in science and vaccination during the
pandemic: making impact using a place-based approach. J Med
Internet Res. 2022;24(10):e38949. http://doi.org/10.2196/38949
39. Lindberg NM, Gutierrez AM, Mittendorf KF, et al. Creating accessible
Spanish language materials for Clinical Sequencing Evidence-
Generating Research consortium genomic projects: challenges and
lessons learned. Pers Med. 2021;18(5):441-454. http://doi.org/10.2217/
pme-2020-0075
40. The electronic medical records and genomics (eMERGE) network.
Resource Center. Accessed January 20, 2023. https://emerge-network.
org/resourcelibrary/
14 J.J. Connolly et al.
