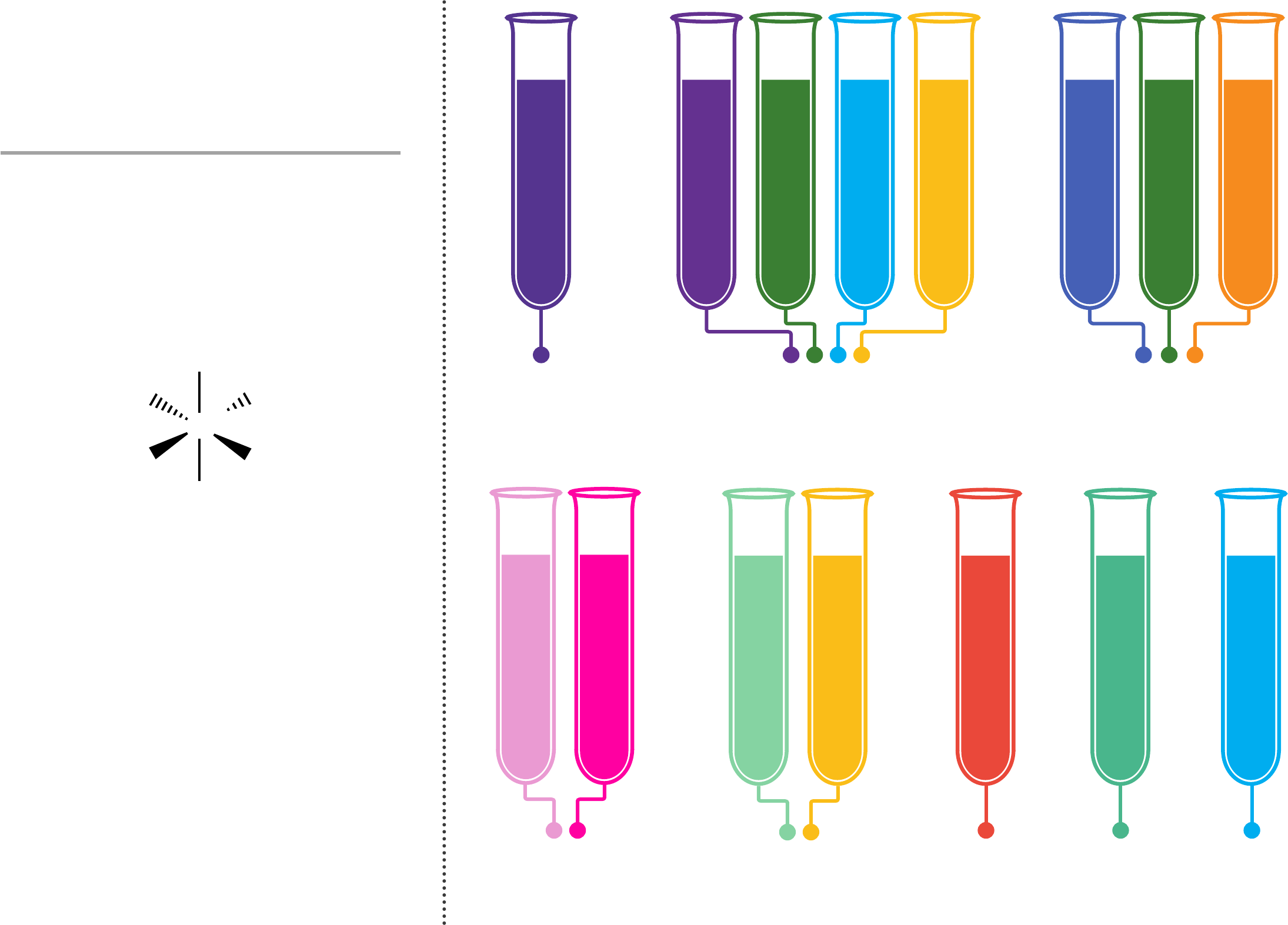
Transition metals form coloured compounds
and complexes. These colours can vary
depending on the charge on the metal ion,
and the number and type of groups of atoms
(called ligands) attached to the metal ion. In
aqueous solutions, the ions form complexes
with the colours shown to the right.
Electrons are arranged around the nucleus of
the metal atom in orbitals. Transition metals,
unlike other metals, have partially lled d
orbitals, which can hold up to 10 electrons.
When ligands are present, some d orbitals
become higher in energy than before, and
some become lower. Electrons can then move
between these higher and lower d orbitals by
absorbing a photon of light. This absorption
of light aects the percieved colour of the
compound or complex. The wavelength of the
light absorbed is aected by the size of the
energy gap between the d orbitals, which is
in turn aected by the type of ligand and the
charge on the metal ion.
TRANSITION METAL
ION COLOURS
2014 COMPOUND INTEREST WWW.COMPOUNDCHEM.COM
3+ 2+ 3+ 4+ 5+ 2+ 3+ 6+
2+ 7+ 2+ 3+ 2+ 2+ 2+
TITANIUM
Ti
VANADIUM
V
CHROMIUM
Cr
MANGANESE
Mn
IRON
Fe
COBALT
Co
NICKEL
Ni
COPPER
Cu
M
n+
OH
2
OH
2
H
2
O OH
2
H
2
O OH
2
HYDRATED TRANSITION METAL ION
