
Current Biology 19, 436–441, March 10, 2009 ª2009 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2009.01.056
Report
Specialized Sugar Sensing
in Diverse Fungi
Victoria Brown,
1,
*
Jeffrey Sabina,
1
and Mark Johnston
1
1
Department of Genetics
Center for Genome Sciences
Washington University School of Medicine
St. Louis, MO 63108
USA
Summary
S. cerevisiae senses glucose and galactose differently.
Glucose is detected through sensors that reside in the
cellular plasma membrane. When activated, the sensors
initiate a signal-transduction cascade that ultimately inacti-
vates the Rgt1 transcriptional repressor by causing degrada-
tion of its corepressors Mth1 and Std1 [1, 2]. This results in
the expression of many HXT genes encoding glucose
transporters [3]. The ensuing flood of glucose into the cell
activates Mig1, a transcriptional repressor that mediates
‘‘glucose repression’’ of many genes, including the GAL
genes; hence, glucose sensing hinders galactose utilization
[4–6]. Galactose is sensed in the cytoplasm via Gal3. Upon
binding galactose (and ATP), Gal3 sequesters the Gal80
protein, thereby emancipating the Gal4 transcriptional acti-
vator of the GAL genes [7]. Gal4 also activates expression
of MTH1, encoding a corepressor critical for Rgt1 function
[8]. Thus, galactose inhibits glucose assimilation by encour-
aging repression of HXT genes. C. albicans senses glucose
similarly to S. cerevisiae but does not sense galactose
through Gal3-Gal80-Gal4 [9]. Its genome harbors no GAL80
ortholog, and the severely truncated CaGal4 does not
regulate CaGAL genes [9, 10]. We present evidence that
C. albicans senses galactose with its Hgt4 glucose sensor,
a capability that is enabled by transcriptional ‘‘rewiring’’ of
its sugar-sensing signal-transduction pathways. We suggest
that galactose sensing through Hgt4 is ancestral in fungi.
Results and Discussion
Hgt4 Affects Cell Growth and Filamentation on Galactose
C. albicans Dhgt4 mutants cannot grow on glucose in the pres-
ence of the respiration inhibitor antimycin A [11], which forces
cells to ferment glucose and demands a high rate of glucose
influx. Because galactose and glucose are structurally similar,
it seemed plausible that the Hgt4 glucose sensor might sense
galactose (Figure 1). Indeed, Dhgt4 cells have a marked growth
defect on galactose with antimycin A (Figure S1A, available
online), suggesting that Hgt4 is required for galactose utiliza-
tion (see Table S3 for strains used in this study). Galactose
induces robust filamentation (yeast-to-hyphal morphogen-
esis) of C. albicans cells, and the Dhgt4 cells are also defective
in this response (Figure S1B). Thus, in the absence of Hgt4,
C. albicans cells display growth and morphological defects
in galactose.
Galactose and Glucose Induce Expression
of the Same Genes
Expression
of 49 genes increased by R 2-fold (Table 1, Groups
I–III) in response to 2% galactose (compared to glycerol). Most
of these galactose-induced genes (40, or 82%) are also signif-
icantly induced by 2% glucose (Table 1, Group I). Six of the
nine genes that were not induced by 2% glucose are in fact
induced by low glucose levels (<0.2%) but have been shown
to be repressed in cells exposed to the high level of glucose
used here (Table 1, Group II) [11–15]. Only three genes are
modestly induced by galactose but not induced by glucose
(Table 1, Group III). Therefore, 94% (46/49) of the characterized
genes that are induced in response to galactose are also
induced in response to low or high levels of glucose.
Hgt4 Affects the Transcriptional Response to Galactose
Expression of five of the top genes listed in Table 1 (Group I)
was reexamined by RT-PCR analysis. In cells grown on glyc-
erol, these genes are either not expressed (HGT7, QDR1,
AOX2) or expressed at low levels (CMK1, HXK2), and all five
are induced in response to galactose in an Hgt4-dependent
manner (Figure 2). HGT12, encoding a glucose transporter
related to Hgt4 [11, 16], does not affect the expression of
these genes. Induction of GAL1 expression by galactose is
significantly diminished in the Dhgt4 mutant (Figure S2),
consistent with the previous observation that the Hgt4 signal
increases GAL1 and GAL7 expression 2-fold [11, 12]. Galac-
tose still induces GAL1 expression in Dhgt4 cells, indicating
that another signaling pathway contributes to GAL1 expres-
sion, possibly by acting upon Cph1 (a C. albicans homolog
of S. cerevisiae Ste12) [9].
The CaHGT7 gene, encoding a hexose transporter, is highly
induced—over 30-fold—by both galactose and glucose (Table
1). HGT7 expression is activated by low levels (0.04%) of
glucose, fructose, or mannose (Figure 3A, top) and by a high
level (1.6%) of galactose (Figure 3A, bottom).
HGT7 expression
in
response
to sugars is entirely dependent on HGT4
(Figure 3B), and Hgt4 mediates the dose-dependent galactose
induction of HGT7 expression at concentrations as low as
0.6% (Figure S3).
Galactose-Induced Genes Have Rgt1-Binding Sites
Of the 50 genes most highly induced by galactose, 34 (68%)
contain at least one consensus Rgt1 DNA-binding motif (5
0
-
CGGANNA-3
0
) within 1 kb upstream of the translational start
codon (Table S1). This is a significant enrichment (p <
10
23
)—only 46% of promoters genome-wide harbor an Rgt1
motif—that is similar to the enrichment of consensus Rgt1-
binding sites upstream of genes regulated by glucose via
Hgt4 and CaRgt1 (66%, p < 10
25
) (see Supplemental Experi-
mental Procedures for statistical methods). The CaCph1 tran-
scription factor has also been implicated in the expression of
the CaGAL genes in response to galactose [9], but its
binding-site is not enriched in other galactose-induced genes
(Table S1). The promoters of the GAL1-10 and GAL7 genes
(encoding the enzymes for galactose metabolism) each
contain both a perfect Cph1 response element (5
0
-TGTAAC
GTTACA-3
0
) [9] and two Rgt1 recognition-sequence motifs,*Correspondence: vbrownk@wustl.edu
brought to you by COREView metadata, citation and similar papers at core.ac.uk
provided by Elsevier - Publisher Connector
consistent with the idea that Rgt1 and Cph1 coordinately regu-
late these genes in response to galactose.
Hgt4 Senses Galactose in S. cerevisiae
In S. cerevisiae, HXT genes are induced by glucose, fructose,
and mannose but not by galactose, ostensibly because Snf3
and Rgt2 do not bind galactose [17]. If Hgt4 binds galactose,
then expressing it in S. cerevisiae should cause galactose
induction of HXT genes. The HGT4 sugar-binding domain
(codon optimized) was expressed in S. cerevisiae from the
RGT2 promoter (see Supplemental Experimental Procedures).
Because the C-terminal cytoplasmic tails of the glucose
sensors have diverged almost completely in the w200 million
years since the C. albicans and S. cerevisiae lineages
diverged, the Hgt4 sugar-binding domain was fused to the
Rgt2 tail to enable coupling of the sensor to the S. cerevisiae
signal-transduction pathway (Figure S4) [11]. Exchanging the
intracellular signaling tails of glucose sensors does not affect
their response to glucose (V.B., unpublished data; V. Brachet,
unpublished data), so we are confident that the Hgt4-Rgt2
chimera retains the sugar-sensing specificity of Hgt4. In
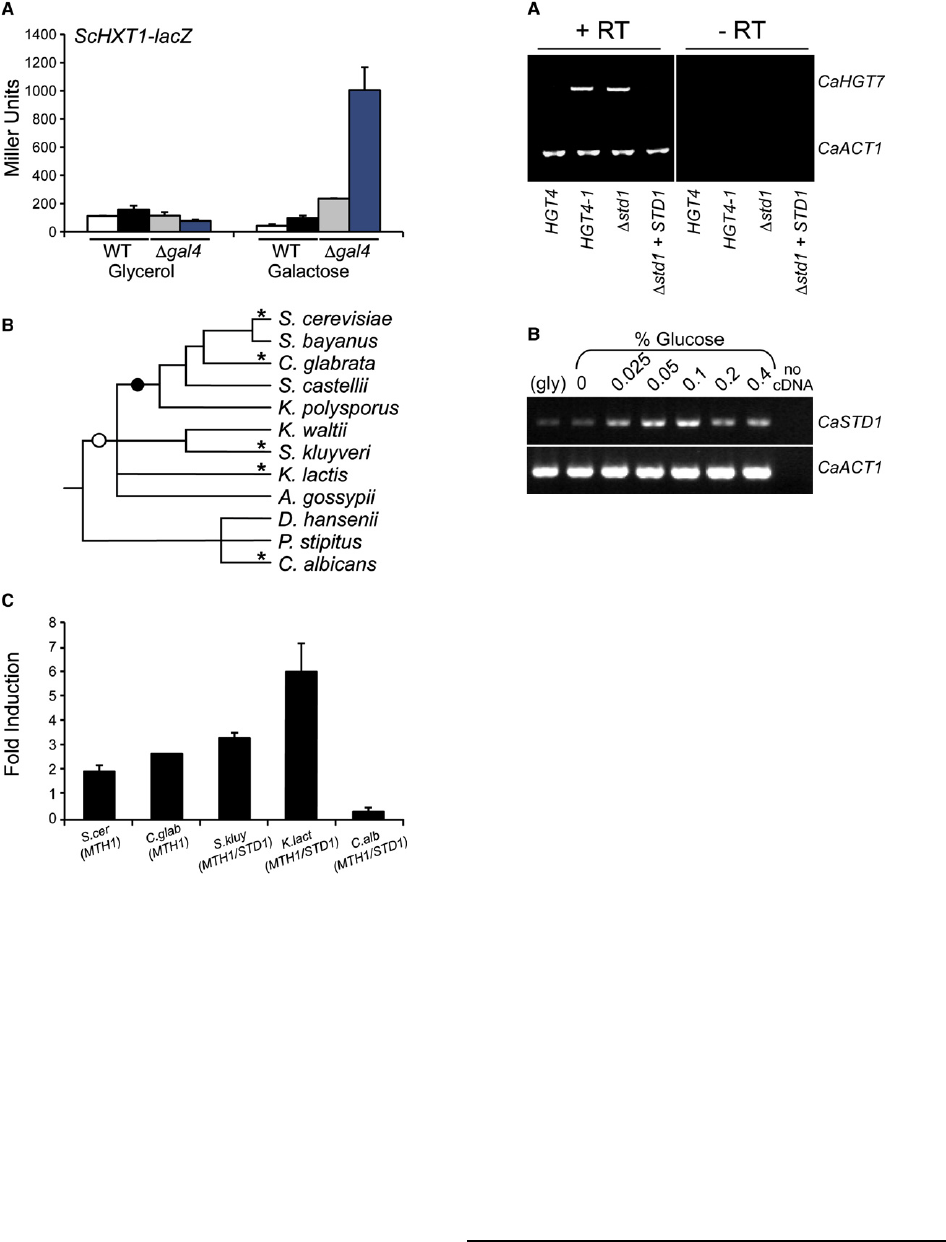
S. cerevisiae cells expressing the Hgt4 chimera, HXT1 is not
induced by galactose (Figure 4A, black bars; Figure S5, third
row). However, galactose induces MTH1 expression in
S. cerevisiae via Gal4 [8], and the resulting increase in Mth1
levels would be expected to reinforce Rgt1-mediated repres-
sion of HXT1, effectively masking any galactose signal gener-
ated by Hgt4 in S. cerevisiae. Deleting ScGAL4 eliminates this
control element and reveals robust activation of the HXT1-lacZ
reporter in response to galactose in cells expressing the Hgt4
chimera (Figure 4A, blue bars; Figure S5, bottom row) [18].In
contrast, neither Rgt2 nor Snf3 (which are present in these
strains) respond to galactose (indicated by cells with the
vector control; Figure 4A, gray bars; Figure S5, first column).
Thus, expression of the Hgt4 sugar-binding domain in
S. cerevisiae confers a novel galactose response upon baker’s
yeast.
Galactose Induces MTH1 Expression in Diverse Fungi
C. albicans did not undergo a whole-genome duplication, so it
has one homolog of the
S. cerevisiae MTH1 and STD1 paralogs
(CaSTD1).
CaStd1
(orf19.6173) is 27% identical both to the
S. cerevisiae Std1 (43% similar) and to Mth1 (41% similar)
and harbors a conserved motif (SxSxxSSIFS, residues 62–71)
that is critical for glucose-induced phosphorylation of ScStd1
and ScMth1 (which leads to their degradation) [19].We
surmised that since Hgt4 functions as a galactose sensor in
C. albicans, CaSTD1 expression must not be induced by
galactose. Indeed, CaSTD1 expression is unaffected by galac-
tose (Table 1 and data not shown), a result confirmed by
RT-PCR and RT-qPCR analyses (Figure S6 and Figure 4C,
respectively). To assess the evolutionary conservation of this
galactose response, we measured expression of MTH1 ortho-
logs in a diverse sampling of fungi spanning w200 million
years of evolution (Figure 4B). In all species tested except
C. albicans, expression of MTH1 is induced in response to
galactose (Figure 4C and Figure S6). Induction occurs even
in C. glabrata, which has lost the GAL4 gene, as well as in
K. lactis, which lacks canonical Gal4-binding sites in the
promoter of its MTH1 ortholog (Table S2). Galactose-induced
activation of ScMTH1 expression by ScGal4 in S. cerevisiae
[8] appears to antagonize the galactose signal generated by
Hgt4, and such antagonism is likely in the four fungi in the
S. cerevisiae to K. lactis clade that we analyzed.
These data illuminate the evolution of galactose sensing in
fungi. Sensing galactose through both the Gal4 and the
Hgt4-Snf3-Rgt2 type pathways seems imprudent, because it
would lead to crossrepression of genes in both pathways
(see Summary and Figure 1). Within the Ascomycetes,
Candida glabrata, Kluyveromyces waltii, and Ashbya gossypii
have no canonical galactose sensor because GAL4 and/or
GAL80 is absent, but they have also lost all galactose-utiliza-
tion-pathway enzymes (GAL1, GAL7, and GAL10) and thus
cannot utilize galactose in any case [10, 20, 21]. The Gal4-
mediated galactose-sensing pathway is intact in a few yeasts
that diverged before the duplication, such as K. lactis
and
S.
kluyveri [22–24]. Debaromyces
hansenii and Pichia stipitus
have GAL4 homologs but no obvious GAL80 homologs. In
contrast, all of the Candida species that we surveyed (except
C. glabrata), as well as Yarrowia lipolytica and Lodderomyces
elongisporus, harbor genes encoding the enzymes for galac-
tose metabolism, but their GAL4 genes are more similar to
CaGAL4 (than to ScGAL4), and all lack a GAL80 functional
homolog. The implication is that the Ascomycetes that can
metabolize galactose but have no Gal4 or Gal80 regulators
utilize an Hgt4-like sensing pathway to control galactose-
response genes. This supports the notion that the Gal4-
Gal80 control circuit arose prior to the origin of the
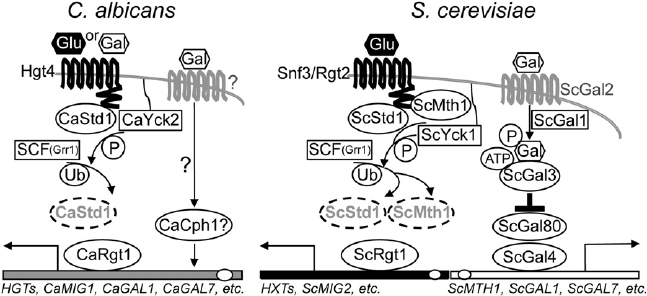
Figure 1. Sugar-Sensing Pathways in C. albicans
and S. cerevisiae
Glucose signaling begins at the cell surface with
the sensors (CaHgt4, or ScSnf3 and ScRgt2)
and ends in the nucleus, with deactivation of
the Rgt1 transcriptional repressor [1, 11]. The
keystone proteins are the transcriptional core-
pressors (CaStd1, or ScStd1 and ScMth1), which
associate with both the sensor and the transcrip-
tional repressor, and it is the levels of these
proteins that translate the environmental signal
into gene-expression changes. Sugar binding to
a sensor activates yeast casein kinase (Yck),
which then phosphorylates Std1 and Mth1,
thereby marking them for ubiquitylation by the
SCF
Grr1
complex and dooming them to destruc-
tion by the proteasome. Depletion of the core-
pressors renders Rgt1 impotent, which results
in transcriptional derepression of downstream genes. In S. cerevisiae, galactose enters the cell, is phosphorylated, and binds (with ATP) to the Gal3 protein.
This complex binds and sequesters Gal80 and relieves the inhibition of the Gal4 transcriptional activator. In C. albicans, CaGal4 does not regulate the GAL
genes. Instead, galactose is sensed by the Hgt4 glucose sensor and probably also through Cph1 (a homolog of the S. cerevisiae Ste12).
Fungal Sugar Sensing
437

S. cerevisiae–K. lactis clade but after this clade and Candida,
species diverged from their common ancestor (Figure 4B,
white dot), and it suggests that Hgt4 represents an ancestral
sensor of galactose. In C. albicans, the altered specificity of
the Hgt4 glucose sensor, in combination with the absence of
a canonical Gal4 pathway, has enabled this fungus to sense
galactose through Hgt4.
C. albicans Std1 Functions in the Sugar-Sensing Pathway
Because the Gal4 signaling pathway is structured differently in
C. albicans, it was possible that the Hgt4 pathway had also
changed. If sugar sensing by C. albicans is analogous to
S. cerevisiae glucose sensing, the CaStd1 corepressor
should be a key protein in the pathway (Figure 1). Indeed it
is, because homozygous Dstd1 null mutant cells have the
Table 1. Genes Induced in Response to Sugars
Name ORF ID Galactose Fold Up Glucose Fold Up Annotation
GROUP I
a
HGT7 orf19.2023 32.2 30.8 Putative glucose transporter
QDR1 orf19.508 18.6 56.1 Antibiotic resistance transporter
AOX2 orf19.4773 13.7 8 Alternative oxidase
CRZ2 orf19.2356 10.1 12.8 Putative transcription factor
FET99 orf19.4212 10 19.1 Multicopper oxidase family
RHR2 orf19.5437 9.2 25.1 Putative glycerol 3-phosphatase
RNR22 orf19.1868 8.1 25.4 Ribonucleoside di-Phosphate reductase
TPO3 orf19.4737 7.4 16.4 Possible polyamine transporter
PDC11 orf19.2877 6.2 9.1 Similar to pyruvate decarboxylase
HGT6 orf19.2020 5.3 3.2 Putative glucose transporter
MNN22 orf19.3803 5.3 9.1 Golgi alpha-1, 2-mannosyltxferase
FMA1 orf19.6837 5.2 9.9 Membrane-assoc. protein
HAK1 orf19.6249 5.1 3.4 Putative potassium transporter
HXK2 orf19.542 5 8 Hexokinase II
TYE7 orf19.4941 4.4 5.7 Putative bHLH transcription factor
GDH3 orf19.4716 4.3 20.2 NADP-glutamate dehydrogenase
CMK1 orf19.5911 4.1 5 Ca2+/Calmodulin-dependent kinase
FET34 orf19.4215 3.8 6.8 Similar to multicopper ferroxidase
STP4 orf19.909 3.8 5.7 Putative transcription factor
AOX1 orf19.4774 3.7 3.3 Alternative oxidase
EHT1 orf19.3040 3.4 8.4 Similar to Eht1p
PFK1 orf19.3967 3.2 7 a-subunit of phosphofructokinase
CRP1 orf19.4784 3.2 6.9 Copper transporter
PFK2 orf19.6540 3.1 5.7 b-subunit of phosphofructokinase
PHO15 orf19.4444 3 5.8 4-nitrophenyl phosphatase
UBC15 orf19.5337 2.9 3 Ub-conjugation, DNA repair
MIG1 orf19.4318 2.9 3.2 Transcriptional repressor
AHP1 orf19.2762 2.9 6.3 Putative alkyl hydroperoxide reductase
ARG1 orf19.7469 2.7 4 Similar to argininosuccinate synthase
PHO113 orf19.2619 2.6 3.6 Constitutive acid phosphatase
NDE1 orf19.339 2.5 2.7 Putative NADH dehydrogenase
GPX2 orf19.85 2.5 2.25 Similar to glutathione peroxidase
ROD1 orf19.1509 2.4 3.8 Drug tolerance; Rgt1-repressed
FCR1 orf19.6817 2.3 2.8 Put. Zn-cluster transcription factor
OPT9 orf19.2584 2.1 3.1 Probable pseudogene
EBP7 orf19.5816 2 1.9 Stress-induced via Cap1p
ARG5 orf19.4788 2 5 Arginine biosynthetic enzyme
ARG4 orf19.6689 2 3.9 Argininosuccinate lyase
DOG1 orf19.3392 1.9 2.6 Put. 2-deoxygluc-6-phosphatase
YIM1 orf19.847 1.9 1.8 Similar to mitochondrial protease
GROUP II
b
HGT12 orf19.7094 6.74 0.07 Glucose, fructose, mannose transporter
HXT10 orf19.4384 2.6 0.21 Sugar transporter
HGT2 orf19.3668 2 0.02 Putative glucose transporter
GAL1 orf19.3670 6.1 0.8 Galactokinase
GAL10 orf19.3672 6 0.7 UDP-glucose 4-epimerase
GAL7 orf19.3675 5.9 0.9 UDP-hexose-1-P uridylyltransferase
GROUP III
c
PGA37 orf19.3923 3.2 1.1 Putative GPI-anchored protein
HSP30 orf19.4526 2.9 0.48 Similar To heat shock protein
STB3 orf19.203 2.3 1.4 Predicted Sin3 Binding protein
Fold induction indicates gene expression in each sugar relative to its expression in glycerol cultures. For all genes listed, p < 0.05 (Student’s t test). Unchar-
acterized open reading frames induced in galactose and glucose are shown in Table S4.
a
Genes induced in 2% galactose and in 2% glucose.
b
Genes induced in 2% galactose but repressed in 2% glucose.
c
Genes induced in 2% galactose, not induced in 2% glucose.
Current Biology Vol 19 No 5
438

same hyperfilamented morphology as Drgt1 mutant cells
(Figure S7A) and cells carrying the constitutively signaling
HGT4-1 mutation (Figure S7B), and this phenotype is reversed
by reintroduction of one wild-type allele into these cells
(Figure S7B). This result supports previous observations that
implicated the Hgt4 pathway in C. albicans filamentation [11,
12]. Furthermore, HGT7 expression is constitutive in the
Dstd1 mutant (just like in the HGT4-1 mutant), and reintroduc-
tion of one copy of CaSTD1 into this mutant reverses this
phenotype (Figure 5A). Thus, although the galactose-sensing
pathways are completely different between C. albicans and
S. cerevisiae, the glucose-sensing pathway remains the
same (Hgt4-CaStd1-CaRgt1).
Examining CaStd1 function in C. albicans sheds light on the
separate functions of the S. cerevisiae paralogs. CaSTD1
expression resembles that of ScSTD1, not ScMTH1:itis
induced by glucose but not by galactose (Figure 5B and
Figure 4C, respectively) [25]. This implies that ScStd1 has
a more ancestral role, and ScMth1 a more derived role, in
this signal-transduction pathway. The Hgt4-Snf3-Rgt2 sugar-
sensing pathway may be universally involved in fungal
morphology; disrupting ScMTH1 represses filamentous
growth in baker’s yeast in the S1278b pseudohyphal strain
[26]. Additional studies on pre- and postduplication yeast
species will be necessary for determining whether Mth1 and
Std1 function redundantly, cooperatively, or in opposition to
each other and whether they affect fungal morphogenesis
throughout this kingdom.
It seems clear that the glucose- and galactose-sensing
systems in fungi work together as a network to regulate tran-
scription of genes such as GAL1 in C. albicans and HXT1 in
S. cerevisiae. In fact, transcriptional regulation of the HXT
genes in S. cerevisiae is the result of at least seven intercon-
nected signal-transduction cascades: (1) glucose sensing
through Snf3 or Rgt2 [27], (2) sugar sensing through the Gpr1
G protein-coupled receptor [28], (3) osmosensing through
the Hog1 MAP kinase pathway [29], (4) glucose repression
mediated by Mig1 and Mig2 [6],
(5)
the TOR1 protein kinase
pathway [30], (6) oxygen availability [31], and, finally, (7)
galactose sensing through Gal4. These signal-transduction
Figure 2. Hgt4 Regulates Galactose-Induced Genes
Log-phase cultures of wild-type (BWP17), Dhgt4 (CM9 and CM10), or
Dhgt12 (CM64) cells were split and incubated in fresh media containing
5% galactose or 5% glycerol at 30
C for 2 hr. Total RNA was reverse tran-
scribed and PCR amplified with primers for HGT7 (orf19.2023), QDR1
(orf19.508), AOX2 (orf19.4773), CMK1 (orf19.5911), HXK2 (orf19.542), and
ACT1 (orf19.5007). Control reactions lacking reverse transcriptase yielded
no products (not shown).
Figure 3. HGT7 Is Induced in Response to Galactose
(A) The HGT7 promoter was fused to Streptococcus thermophilus lacZ
gene, and this construct was integrated into the C. albicans genome at
the native HGT7 locus. Cells with HGT7::HGT7-lacZ (CM79 and CM80)
were grown in glycerol media, split, and incubated at 30
C for 2 hr in fresh
media containing glycerol or 0.04% (top panel) or 1.6% (bottom panel) of
the sugars indicated. Cells were lysed and assayed for b-galactosidase
activity (in quadruplicate [top] or in triplicate [bottom]), and the results
were normalized to the lacZ activity in the glycerol media. Data are pre-
sented as mean 6 1 SD.
(B) Cells with HIS1::HGT7-lacZ (HGT4 strains CM230 and CM231 and Dhgt4
strains CM232 and CM233) were grown in media with glycerol as carbon
source, split, and incubated at 30
C for 2 hr in fresh media lacking histidine
but containing glycerol or the sugar indicated (n = 10 for HGT4; n = 10 for
Dhgt4). Black bar: 0.04% glucose; gray bar: 1.6% glucose; striped bar:
0.04% galactose; white bar: 1.6% galactose. All values were normalized
to activity in glycerol and expressed as the percentage of the maximum
response in 0.04% glucose. Data are presented as mean 6 1 SD.
Fungal Sugar Sensing
439
pathways provide a malleable framework for response to
extracellular nutrients.
Supplemental Data
Supplemental Data include Supplemental Experimental Procedures, seven
figures, and four tables and can be found with this article online at http://
www.current-biology.com/supplemental/S0960-9822(09)00628-9.
Acknowledgments
We thank Chris Todd Hittinger and Chandra Tucker for critical reading of this
manuscript and Jim Dover and Christine Carle for technical assistance. This
work was supported by a grant from the National Institute of Diabetes and
Digestive and Kidney Diseases (K01DK077878 to V.B.), by grants from the
National Institute of General Medical Sciences (F32GM076967 to J.S. and
R01GM32540 to M.J.), and by funds provided to M.J. by the James
S. McDonnell Foundation.
Received: October 1, 2008
Revised: January 12, 2009
Accepted: January 15, 2009
Published online: February 26, 2009
Figure 4. C. albicans HGT4 Confers a Novel Galactose Response
upon S. cerevisiae
(A) S. cerevisiae strains were grown in media containing glycerol, cell densi-
ties were normalized, and the culture was split and incubated overnight at
30
C in fresh media containing 5% glycerol or 5% galactose, then lysed,
and b-galactosidase activity was assayed (see Supplemental Experimental
Procedures). Data are the average of biological duplicates. White bars:
wild-type cells + pRS316 vector (YM7642); black bars: wild-type cells
+Hgt4-chimera (YM7643); gray bars: Dgal4 cells + pRS316 vector (YM7644);
blue bars: Dgal4 cells + Hgt4-chimera (YM7645). Data are presented as
mean 6 1 SD.
(B) MTH1 orthologs are galactose-induced in diverse fungi. A phylogenetic
tree showing the relationship of yeasts spanning w200 million years of
evolution is shown [32–35]. Characteristics of the galactose-sensing path-
ways in these species are described in Table S2. The black circle represents
a whole-genome duplication event, the white circle represents the
proposed appearance of the Gal4-Gal80 gene-regulatory mechanism, and
asterisks indicate the species analyzed in (C).
(C) Each species was grown overnight in glycerol media and incubated in
fresh media containing 5% glycerol or 5% galactose at 30
C for 3 hr.
RT-PCR was performed on total RNA with the use of species-specific
primers for either ACT1 or the MTH1/STD1 ortholog (fungal strains are
described in Supplemental Experimental Procedures). First-strand cDNAs
served as templates for quantitative PCR. Each MTH1/STD1 signal was
normalized to the ACT1 signal in that sample, and the DDCt values are
expressed as ‘‘Fold Induction’’ of expression in galactose relative to expres-
sion in glycerol (2
DDCt
). Data are presented as mean 6 1 SD. Separate exper-
iments were performed with the use of semiquantitative PCR for confirma-
tion of the results (see Figure S6).
Figure 5. CaSTD1 and ScSTD1 Function Similarly
(A) CaSTD1 plays a role in the HGT4 pathway. Isogenic strains [HGT4 (CM87)
compared to HGT4-1 (CM36) and Dstd1 (CM222) compared to STD1
(CM224)] were grown at 30
C to log phase in media containing glycerol.
Cells were harvested and snap frozen, and total RNA was purified for
RT-PCR analysis of HGT7 (orf19.2023) or ACT1 (orf19.5007).
(B) CaSTD1 is glucose-induced. C. albicans cells (SC5314) were grown to
log phase in media containing glycerol, then incubated at 30
Cfor2hrin
fresh media with glycerol (gly) or with the indicated concentrations of
glucose (0 indicates no carbon source). Cells were harvested and snap
frozen, and total RNA was purified for RT-PCR analysis with the use of
primers for CaSTD1 (orf19.6173) or ACT1 (orf19.5007).
Current Biology Vol 19 No 5
440
References
1. Johnston, M., and Kim, J.H. (2005). Glucose as a hormone: receptor-
mediated glucose sensing in the yeast Saccharomyces cerevisiae.
Biochem. Soc. Trans. 33, 247–252.
2. Santangelo, G.M. (2006). Glucose Signaling in Saccharomyces cerevi-
siae. Microbiol. Mol. Biol. Rev. 70, 253–282.
3. Ozcan, S., and Johnston, M. (1999). Function and regulation of yeast
hexose transporters. Microbiol. Mol. Biol. Rev. 63, 554–569.
4. Johnston, M., Flick, J.S., and Pexton, T. (1994). Multiple mechanisms
provide rapid and stringent glucose repression of GAL gene expression
in Saccharomyces cerevisiae. Mol. Cell. Biol. 14, 3834–3841.
5. De Vit, M.J., Waddle, J.A., and Johnston, M. (1997). Regulated nuclear
translocation of the Mig1 glucose repressor. Mol. Biol. Cell 8, 1603–
1618.
6. Lutfiyya, L.L., Iyer, V.R., DeRisi, J., DeVit, M.J., Brown, P.O., and John-
ston, M. (1998). Characterization of three related glucose repressors
and genes they regulate in Saccharomyces cerevisiae. Genetics 150,
1377–1391.
7. Johnston, M. (1987). A model fungal gene regulatory mechanism: the
GAL genes of Saccharomyces cerevisiae. Microbiol. Rev. 51, 458–476.
8. Ren, B., Robert, F., Wyrick, J.J., Aparicio, O., Jennings, E.G., Simon, I.,
Zeitlinger, J., Schreiber, J., Hannett, N., Kanin, E., et al. (2000). Genome-
wide location and function of DNA binding proteins. Science 290, 2306–
2309.
9. Martchenko, M., Levitin, A., Hogues, H., Nantel, A., and Whiteway, M.
(2007). Transcriptional rewiring of fungal galactose-metabolism
circuitry. Curr. Biol. 17, 1007–1013.
10. Braun, B.R., van Het Hoog, M., d’Enfert, C., Martchenko, M., Dungan, J.,
Kuo, A., Inglis, D.O., Uhl, M.A., Hogues, H., Berriman, M., et al. (2005). A
human-curated annotation of the Candida albicans genome. PLoS
Genet 1, 36–57.
11. Brown, V., Sexton, J.A., and Johnston, M. (2006). A glucose sensor in
Candida albicans. Eukaryot. Cell 5, 1726–1737.
12. Sexton, J.A., Brown, V., and Johnston, M. (2007). Regulation of sugar
transport and metabolism by the Candida albicans Rgt1 transcriptional
repressor. Yeast 24, 847–860.
13. Murad, A.M., d’Enfert, C., Gaillardin, C., Tournu, H., Tekaia, F., Talibi, D.,
Marechal, D., Marchais, V., Cottin, J., and Brown, A.J. (2001). Transcript
profiling in Candida albicans reveals new cellular functions for the tran-
scriptional repressors CaTup1, CaMig1 and CaNrg1. Mol. Microbiol. 42,
981–993.
14. Singh, V., Satheesh, S.V., Raghavendra, M.L., and Sadhale, P.P. (2007).
The key enzyme in galactose metabolism, UDP-galactose-4-epimerase,
affects cell-wall integrity and morphology in Candida albicans even in
the absence of galactose. Fungal Genet. Biol. 44 , 563–574.
15. Fan, J., Chaturvedi, V., and Shen, S.H. (2002). Identification and phylo-
genetic analysis of a glucose transporter gene family from the human
pathogenic yeast Candida albicans. J. Mol. Evol. 55, 336–346.
16. Luo, L., Tong, X., and Farley, P.C. (2007). The Candida albicans gene
HGT12 (orf19.7094) encodes a hexose transporter. FEMS Immunol.
Med. Microbiol. 51, 14–17.
17. Ozcan, S., and Johnston, M. (1995). Three different regulatory mecha-
nisms enable yeast hexose transporter (HXT) genes to be induced by
different levels of glucose. Mol. Cell. Biol. 15, 1564–1572.
18. Kelly, D.E., Lamb, D.C., and Kelly, S.L. (2001). Genome-wide generation
of yeast gene deletion strains. Comp. Funct. Genomics 2, 236–242.
19. Moriya, H., and Johnston, M. (2004). Glucose sensing and signaling in
Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein
kinase I. Proc. Natl. Acad. Sci. USA 101, 1572–1577.
20. Byrne, K.P., and Wolfe, K.H. (2005). The Yeast Gene Order Browser:
combining curated homology and syntenic context reveals gene fate
in polyploid species. Genome Res. 15, 1456–1461.
21. Hittinger, C.T., Rokas, A., and Carroll, S.B. (2004). Parallel inactivation of
multiple GAL pathway genes and ecological diversification in yeasts.
Proc. Natl. Acad. Sci. USA 101, 14144–14149.
22. Cliften, P.F., Fulton, R.S., Wilson, R.K., and Johnston, M. (2006). After
the duplication: gene loss and adaptation in Saccharomyces genomes.
Genetics 172, 863–872.
23. Kellis, M., Birren, B.W., and Lander, E.S. (2004). Proof and evolutionary
analysis of ancient genome duplication in the yeast Saccharomyces
cerevisiae. Nature 428, 617–624.
24. Langkjaer, R.B., Cliften, P.F., Johnston, M., and Piskur, J. (2003). Yeast
genome duplication was followed by asynchronous differentiation of
duplicated
genes.
Nature 421, 848–852.
25. Kim, J.H., Brachet, V., Moriya, H., and Johnston, M. (2006). Integration of
transcriptional and posttranslational regulation in a glucose signal
transduction pathway in Saccharomyces cerevisiae. Eukaryot. Cell 5,
167–173.
26. Suzuki, C., Hori, Y., and Kashiwagi, Y. (2003). Screening and character-
ization of transposon-insertion mutants in a pseudohyphal strain of
Saccharomyces cerevisiae. Yeast 20, 407–415.
27. Kaniak, A., Xue, Z., Macool, D., Kim, J.H., and Johnston, M. (2004).
Regulatory network connecting two glucose signal transduction path-
ways in Saccharomyces cerevisiae. Eukaryot. Cell 3, 221–231.
28. Kim, J.H., and Johnston, M. (2006). Two glucose-sensing pathways
converge on Rgt1 to regulate expression of glucose transporter genes
in Saccharomyces cerevisiae. J. Biol. Chem. 281, 26144–26149.
29. Tomas-Cobos, L., Casadome, L., Mas, G., Sanz, P., and Posas, F.
(2004). Expression of the HXT1 low affinity glucose transporter requires
the coordinated activities of the HOG and glucose signalling pathways.
J. Biol. Chem. 279, 22010–22019.
30. Tomas-Cobos, L., Viana, R., and Sanz, P. (2005). TOR kinase pathway
and 14–3-3 proteins regulate glucose-induced expression of HXT1,
a yeast low-affinity glucose transporter. Yeast 22, 471–479.
31. Rintala, E., Wiebe, M.G., Tamminen, A., Ruohonen, L., and Penttila, M.
(2008). Transcription of hexose transporters of Saccharomyces cerevi-
siae is affected by change in oxygen provision. BMC Microbiol. 8, 53.
32. Dujon, B. (2006). Yeasts illustrate the molecular mechanisms of eukary-
otic genome evolution. Trends Genet. 22, 375–387.
33. Dujon, B., Sherman, D., Fischer, G., Durrens, P., Casaregola, S., Lafon-
taine, I., De Montigny, J., Marck, C., Neuveglise, C., Talla, E., et al. (2004).
Genome evolution in yeasts. Nature 430, 35–44.
34. Scannell, D.R., Frank, A.C., Conant, G.C., Byrne, K.P., Woolfit, M., and
Wolfe, K.H. (2007). Independent sorting-out of thousands of duplicated
gene pairs in two yeast species descended from a whole-genome dupli-
cation. Proc. Natl. Acad. Sci. USA 104, 8397–8402.
35. Scannell, D.R., Byrne, K.P., Gordon, J.L., Wong, S., and Wolfe, K.H.
(2006). Multiple rounds of speciation associated with reciprocal gene
loss in polyploid yeasts. Nature 440, 341–345.
Fungal Sugar Sensing
441
