
The
EMBO
Journal
vol.9
no.8
pp.2431
-2438,
1990
Molecular
cloning
and
expression
of
glycogen
synthase
kinase-3/Factor
A
James
Robert
Woodgett
Ludwig
Institute
for
Cancer
Research,
91
Riding
House
Street,
London
WIP
8BT,
UK
Communicated
by
M.Waterfield
Glycogen
synthase
kinase-3
(GSK-3)
is
a
protein-serine
kinase
implicated
in
the
hormonal
control
of
several
regulatory
proteins
including
glycogen
synthase
and
the
transcription
factor
c-jun.
Two
classes
of
rat
brain
cDNA
for
this
enzyme
have
been
isolated
termed
GSK-3a
and
GSK-3(.
The
c-type
encodes
a
51
kd
polypeptide,
the
sequence
of
which
includes
all
of
the
tryptic
peptides
determined
by
protein
sequence
analysis
of
purified
skeletal
muscle
GSK-3.
The
novel
3-type
cDNA
has
the
potential
to
encode
a
47
kd
protein
with
85%
amino
acid
identity
to
GSK-3a.
The
two
types
of
cDNA
are
the
products
of
distinct
genes
as
determined
by
genomic
organization
and
nucleic
acid
sequence
analysis.
Both
a
and
,B
clones
exhibit
kinase
activity
when
expressed
in
COS-1
cells
and
type-specific
antibodies
to
GSK-3
a
and
(3
detect
proteins
of
51
and
47
kd,
respectively,
in
a
variety
of
rat
tissue
extracts,
with
highest
levels
of
both
in
brain.
Partial
purification
of
GSK-3
activity
from
bovine
brain
results
in
the
isolation
of
active
a
and
(
proteins.
The
physiological
importance
of
these
two
proteins
in
cellular
signal
transduction
is
discussed.
Key
words:
cDNA/c-jun/kinase/PCR/serine
Introduction
Studies
of
the
normal
role
of
proto-oncogenes
in
cell
growth
and
differentiation
have
implicated
this
class
of
proteins
in
a
number
of
signalling
pathways
from
growth
factor
receptors
at
the
plasma
membrane
to
transcription
factors
in
the
nucleus.
In
an
effort
to
identify
the
mechanisms
via
which
signals
at
the
cell
surface
are
transmitted
to
nuclear
transcription
factors,
we
have been
studying
the
processes
by
which
two
nuclear
proto-oncogenes
c-myb
and
c-jun
are
normally
regulated
(Klempnauer
et
al.,
1982;
Boyle
et
al.,
1983;
Bohmann
et
al.,
1987;
Angel
et
al.,
1988).
Both
proteins
act
as
transcription
factors:
c-jun
is
a
component
of
AP-1,
mediating
gene
activation
by
agents
such
as
phorbol
esters
and
insulin
(Lee
et
al.,
1987;
reviewed
in
Mitchell
and
Tjian,
1989);
c-myb
regulates
gene
expression
in
cells
of
the
myeloid
lineage
(Ness
et
al.,
1989).
In
vivo,
these
two
proteins
are
phosphorylated
on
serine
and
threonine
residues
and
in
an
attempt
to
identify
the
protein-serine
kinase(s)
responsible
for
their
phosphorylation,
we
surveyed
a
selection
of
characterized
enzymes.
Only
one
protein
kinase,
glycogen
synthase
kinase-3
(GSK-3),
phosphorylated
jun
and
myb
exclusively
at
sites
identified
in
vivo
by
phospho-
peptide
mapping
(W.Boyle,
T.Smeal,
L.Defize,
P.Angel,
J.R.Woodgett,
M.Karin
and
T.Hunter,
in
preparation).
Furthermore,
the
site
phosphorylated
in
c-jun
by
GSK-3
is
regulated
in
cells;
phorbol
ester
treatment
causes
specific
dephosphorylation.
In
vitro,
phosphorylation
of
c-jun
by
GSK-3
inhibits
DNA
binding,
suggesting
that
agonist-
induced
dephosphorylation
in
cells
is
involved
in
c-jun
activation
(Boyle
et
al.,
in
preparation).
GSK-3
was
first
identified
as
one
of
the
protein
kinases
that
phosphorylates
glycogen
synthase,
the
rate-limiting
enzyme
of
glycogen
deposition
(Embi
et
al.,
1980;
Hemmings
et
al.,
1982).
Insulin
causes
site-specific
dephosphorylation
of
glycogen
synthase
specifically
at
residues
targeted
by
GSK-3,
causing
activation
of
the
enzyme
(Parker
et
al.,
1983;
Cohen,
1985;
Cohen
et
al.,
1985;
Poulter
et
al.,
1988).
GSK-3
is
identical
to
Factor
A
(FA),
the
activator
protein
of
an
inactive
MgATP-dependent
form
of
a
broad
specificity
protein-serine/threonine
phosphatase,
termed
phosphatase-l
(Vandenheede
et
al.,
1980;
Hemmings
et
al.,
1982;
reviewed
in
Cohen,
1989).
Since
this
phosphatase
dephosphorylates
substrates
of
GSK-3,
this
protein
kinase
potentially
catalyses
opposing
reactions.
However,
the
physiological
significance
of
FA
activity
is
presently
unclear
(Cohen,
1989).
Agonist-induced
dephosphorylation
of
c-jun
and
glycogen
synthase
could
occur
by
either
inhibition
of
GSK-3
or
activation
of
a
protein
phosphatase
(or
both).
Although
GSK-3/FA
has
been
previously
purified
by
several
groups
(Hemmings
et
al.,
1982;
Woodgett
and
Cohen,
1984;
Tung
and
Reed,
1989),
its
mode
of
regulation
in
vivo
is
unknown.
As
a
first
step
in
assessing
the
role
of
GSK-3
in
hormonal
regulation
we
have
isolated
cDNA
clones
of
this
protein
kinase,
revealing
it
to
comprise
a
multigene
family.
The
structure,
expression
and
enzymatic
function
of
two
GSK-3
proteins
are
described,
and
their
potential
involvement
in
the
mechanism
of
action
of
insulin
and
regulation
of
proto-
oncogenes
is
discussed.
Results
Peptide
sequence
determination
and
amplification
of
GSK-3
cDNA
Purification
of
GSK-3
from
2
kg
rabbit
skeletal
muscle
resulted
in
the
isolation
of
25-30
,ug
of
a
single
polypeptide
of
51
kd
as
described
previously
(Woodgett,
1989).
Reverse-
phase
HPLC
of
a
tryptic
digest
of
this
material
resolved
several
peptides
which,
following
rechromatography,
were
subjected
to
peptide
sequence
analysis.
In
all,
11
peptides
yielded
information.
A
further
peptide
sequence
was
generated
from
digestion
with
Staphylococcus
aureus
V8
protease.
Comparison
of
the
peptide
sequences
with
the
Swissprot
database
(September,
1989)
revealed
no
exact
matches,
although
visual
alignment
of
two
peptides
(DIKPQN...
and
VLGTPT)
revealed
similarity
to
sequences
within
other
protein-serine
kinases
(Hanks
et
al.,
1987).
Whilst
the
peptides
were
being
sequenced,
a
six
amino
©
Oxford
University
Press
2431

J.R.Woodgett
acid
region
of
one
peptide
(EMNPNY)
was
used
to
design
designed
to
hybridize
to
a
sequence
motif
conserved
among
a
fully
representative,
32-fold
degenerate,
antisense
protein-serine
kinases,
H/Y-R-D-L/I-K-P-E/Q-N,
that
we
oligonucleotide
for
PCR
amplification.
This
was
paired
with
have
used
for
the
amplification
of
novel
protein
kinase
a
sense
oligonucleotide
primer
that
had
previously
been
cDNAs
(P.Coffer
and
J.R.Woodgett,
unpublished).
Despite
A
1-
GGGCACGCCGGAGCCCGAACGGCGCAGCCTGGAAGAGGCCGGAGCCCAAGGGAGGCGGCGGTAGAGGCAGCGGCGGGGGCAGCCCAGGCAGCCCGAGCC
CCGCGGCCTGGGCCTGCGCTC
M
S
G
G
G
P S
G
G
G
P
G
G
S
G R
A
R
T
S
S
F
A
E
P
G
G G
G
G
G
G
G G
G
P
G
G
-
38
121-GGCGCCATGAGCGGCGGCGGGCCTTCGGGAGGTGGCCCTGGGGGCTCGGGCCGGGCGCGGACCAGCTCGTTCGCGGAGCCAGGCGGCGGAGGCGGAGGCGGTGGCGGCGGCCCCGGGGGC
S
A
S
G
P
G
G
T
G
G
G
K
A
S
V
G
A
M
G
G
G
V
G
A
S
S
S
G
G
G
P
S
G
S
G
G
G
G
S
G
-
78
241-TCGGCCTCCGGCCCAGGAGGCACTGGCGGCGGGAAGGCGTCAGTCGGGGCTATGGGTGGGGGCGTGGGAGCCTCGAGCTCTGGGGGTGGCCCCAGCGGCAGCGGCGGAGGAGGCAGCGGT
G
P
G
A
G
T
S
F
P
P P
G
V
K
L
G
R
D
S
G
K
V
T T
V
V
A
T
L
G
Q
G
P
E
R
S
0
E
V
A
-118
361-GGCCCCGGCGCGGGCACTAGCTTCCCGCCGCCCGGAGTGAAGCTGGGCCGTGACAGCGGGAAGGTGACCACAGTGGTAGCCACTCTAGGCCAAGGCCCAGAGCGTTCCCAAGAGGTGGCT
Y
T
D
I
K
V
I
G
N
G
S
F
G
V
V
Y
0
A
R
L
A
E T
R E
L
V
A
I
K
K
V
L
Q
D
K
R
F
K N
-158
4
81-
TACAC
CGACATCAAAGTGATTGGCAATGGCT
CATTCGGAGTAGTGTACCAGGCACGGCTGGCAGAAACGAGGGAACTGGTGGCCATCAAGAAGGTTCTTCAGGACAAAAGGTTCAAGAAC
R
E
L
0
I
M
R
K
L
D
H
C
N
I
V
R
L
R
Y
F
F
Y
S
S
G
E
K
K
D
E
L
Y
L
N
L
V
L
E
Y
V
-198
6
01-
CGAGAGC
TGCAGATTATGC
GTAAGCTGGACCAC
TGCAATATCGTGAGGCTGCGGTACTTTTTC
TACTCCAGTGGGGAGAAGAAAGATGAGCTGTATTTAAATCTGGTGCTGGAATATGTG
P
E
T
V
Y
R
V
A
R
H
F
T
K
A
K
L
I
I
P
I
I
Y
V
K
V
Y
M
Y
Q
L
F
R
S
L
A
Y
I
H
S
Q
-238
721-CCCGAGACCGTGTACCGAGTGGCCCGTCACTTTACCAAGGCCAAGTTGATCATCCCTATCATCTATGTCAAGGTGTACATGTACCAGCTCTTCCGGAGCTTGGCCTACATCCACTCCCAA
G
V
C
H
R
D
I
K
P
Q
N
L
L
V
D
P
D T
A
V
L
K
L
C
D F
G
S
A
K
Q
L
V
R
G
E
P
N
V
S
-278
841
-GGTGTGTGTC
Y
I
C
S
R Y
Y R
A
P
E
L
I
F G
A
T
D
Y
T
S
S
I
D
V
W
S
A
G
C
V
L
A
E
L
L
L
G
Q
P
-318
961-TACATCTGTTCTCGGTACT
GCTCCGG
CTCATCTTTGGAGCCACAGATTACACCTCGTCCATCGATGT
TGGTCAGC
GGCTGTGT
GCTGCTTCTTGGCCAGCC
I
F
P
G D
S
G
V
D
0
L
V
E
I
I
K
V
L
G
T
P
T
R
E
Q
I
R
E
M
N
P
N
Y
T
E
F
K
F
P
Q
-358
1081-ATCTTCCC
GGGACAGTGGGGTAGACCAGCTTGTGGAGATCATCAAGGTACTAGGr.ACr-CCAACCAGGGAGCAAA
GAACCCTAACTACACAGAATTCAAGTTCCCCCAG
I
K
A
H
P
W
T
K
V
F
K
S
R
T
P
P
E
A
I
A
L
C
S
S
L
L
E
Y
T
P
S
S
R
L
S P
L
E
A
C
-398
1201-ATCAAAGCTCACCCCTGGACAAAGGTGTTCAAATCTCGGACACCACCTGAGGCCATCGCACTCTGCTCTAGCCTGCTGGAGTACACTCCATCCTCAAGGCTCTCCCCACTAGAGGCCTGT
A
H
S
F
F
D
E
L
R
S
L
G
T
Q
L
P
N
N R
P
L
P
P
L
F
N
F
S
P
G
E
L
S
I
Q
P
S
L
N
A
-438
1321-GCCCACAGTTTCTTTGATGAACTGCGGAGTCTCGGAACCCAGCTCCCCAACAACCGCCCGCTTCCCCCCCTCTTCAACTTCAGTCCTGGTGAACTTTCCATCCAACCGTCTCTCAATGCC
I
L
I
P
P
H
L
R
S
P
S
G
P
A
T
L
T
S
S
S
Q
A
L
T
E
T
Q
T
G
Q
D
W
Q
A
P
D
A
T
P
T
-478
1441-ATTCTCATCCCTCCTCACTTGAGGTCCCCATCAGGCCCTGCTACCCTCACCTCGTCCTCACAAGCTTTAACTGAGACTCAGACTGGCCAAGACTGGCAGGCACCTGATGCCACACCTACC
L
T N
S
S
-483
15
61-
CT
CACTAACTCTTCCT
GAGGGC
CCTACCGACTACCC
CTCACGCTCACTCGGAAGGCTCAAGTGGGCTGGAAAGGGCCATAGCCCATCCAGCTCCTGCCTGCTGGCCCTAGACTGAGGGCA
16
81-
GAGGTAAATGAACTACCCAGCATCTAGGC
CTCC
CTCACCAGCCTCACCTTGTGGTGGCTTTTAAAGAGGATT
TAACTGTTTGTGGGGGAGGGAAGGGAAGAGAAGGAC
GGACGGGGTTTG
18 01
-GGGTATGAGGAC
CTTCTACCCCCGTGGTC
CCCTCCCCTCCCCCAGGCTAC
CACTCCTCCCCCCCCTCCCATGTCCCTTGTAAATAGAACCAGCCCAGCCGTATCCTCTTCCTGGCCCTTG
19
21-
GGTGTAAATAGATTGTTATAATTTTTTTC
TTAAAGAAAACGTCGATTCACACCATCCAACCT
GCCCTCCCCCTCAGCTGTACCCCCCTC
TTGTCCTCTGCTCCCAAGGCTTCCTCCCTCT
2
041-
CCCCATCCCAAGGAGGGGAGTAGGGAGAGCCCCTGGTGTCTTAGTTTCCACAGTAAGGTTTGCCTGTGTACAGACCTCCGTTCAATAAATTATTGGCATGAAA
AAAAAA
B
M
S
-2
1-
TTTT
TTCTTCGCGGGAGAACTTAATGCTGCATTTATTATTAACCTAGTACCCTAACATAAAACAAAAGGAAGAAAAGGACCAAGGAAGGAAAAGGTGAATCGAGAAGAGC
CAT
CAT
GTCG
G
R
P
R
T
T
S
F
A
E
S
C
K
P
V
Q
Q
P
S
A
F
G
S
M
K
V
S
R
D
K
D
G
S
K
V
T
T
V
V
A
-42
121
-GGGC
GACCGAGAACCACCTCCTTTGCGGAGAGCTGCAAGCCAGTGCAGCAGC
CTTCAGCTTTTGGTAGCATGAAAGTTAGCAGAGATAAAGATGGCAGCAAGGTAACCACAGT
GGTGGCA
T
P
G
Q
G
P
D
R
P
Q
E
V
S
Y
T
D
T
K
V
I
G
N
G
S
F
G
V
V
Y
Q
A
K
L
C
D
S
G
E
L
V
-82
2
41
-ACTC
CAGGACAGGGTC
CTGACAGGCCACAGGAAGTCAGTTACACAGACACTAAAGTCATTGGAAATGGGTCATTTGGTGTGGTATATCAAGCCAAACTTTGTGACTCAGGAGAACTGGTG
A
I
K
K
V
L
Q
D
K R
F
K N
R
E
L
Q
I
M
R
K
L
D
H
C
N
I
V
R
L
R
Y
F F
Y
S
S
G
E
K
-122
3
61
-GC
CATCAAGAAAGTTCTTCAGGACAAGCGATTTAAGAACCGAGAGCTCCAGATCATGAGAAAGCTAGATCACTGTAACATAGTCCGATTGCGGTATTTC
TTCTACTCGAGTGGCGAGAAG
K
D
E
V
Y
L
N
L
V
L
D
Y
V
P
E
T
V
Y
R
V
A
R
H
Y
S
R
A
K
Q
T
L
P
V
I
Y
V
K
L
Y
M
-162
4
81
-AAAGAT
GAGGTCTACC
TTAACCTGGTGCTGGACTATGT
TCCGGAAACAGTGTACAGAGTC
GCCAGACAC
TATAGTCGAGCCAAGCAGACACTCCCTGTGATCTAT
GTCAAGTT
GTATATG
Y
Q
L
F
R
S
L
A
Y
I
H
S
F
G
I
C
H
R D
I
K
P
Q
N
L
L
L
D
P
D
T
A
V
L
K
L
C
D
F
G
-202
6
0
1-TACCAGCTGTTCAGAAGTCTAGCCTATATCCATTCCTTTGGGATCTGCCATCGAGACATTAAACCACAGAACCTCTTGCTGGATCCTGATACAGCTGTATTAAAMCTCTGCGACTTTGGA
S
A
K
Q
L
V
R
G
E
P
N
V
S
Y
I
C
S
R
Y Y
R
A
P
E
L
I
F
G
A
T
D
Y
T
S
S
I
D
V
W
S
-242
7
21
-AGTGCAAAGCAGCTGGTCCGAGGAGAGCCCAATGTTTCATATATCTGTTCTCGGTACTACAGGGCACCAGAGCTGATCTTTGGAGCCACCGATTACACGTCTAGTATAGATATGTGGTCT
A
G
C
V
L
A
E
L
L
L
G
Q
P
I
F
P
G
D
S
G
V
D
Q
L
V
E
I
I
K
V
L
G
T
P
T
R
E
Q
I
R
-282
8
41
-GCAGGC
TGTGTGTTGGCTGAATTGTTGCTAGGACAACCAATATTTCCTGGGGACAGTGGTGTGGATCAGTTGGTGGAAATAATAAAGGTCCTAGGAACACCAACAAGGGAGCAAATTAGA
E
M
N
P
N
Y
T
E
F
K
F
P
Q
I
K
A
H
P
W
T
K
V
F
R
P
R
T
P
P
E
A
I
A
L
C
S
R
L
L
E
-322
9
61
-GAAATGAACCCAAATTATACAGAATTCAAATTCCCCCAAATCAAGGCACATCCTTGGACGAAGGTCTTTCGGCCCCGAACTCCACCAGAGGCAATCGCACTGTGTAGCCGTCTCC
TGGAG
Y
T
P
T
A
R
L
T
P L
E
A
C
A
H
S
F
F
D
E
L
R
D
P
N
V
K
L
P
N
G
R
D
T
P
A
L
F
N
F
-362
10
81-TACACGCCGACCGC
CCGGCTAACACCACTGGAAGCTTGTGCACATTCATTTTTTGATGAATTACGGGACCCAAATGTCAAACTACCAAATGGGCGAGACACACCTGCCCTCTTCAACTTT
T
T
O
E
L
S
S
N
P
P
L
A
T
I
L
I
P
P
H
A
R
I
0
A
A
A
S
P
P
A
N
A
T
A
A
S
D
T
N
A
-402
1201-ACCACTCAAGAACTGTCAAGTAACCCACCCCTGGCCACCATCCTTATCCCTCCTCACGCTCGGATTCAGGCAGCTGCTTCACCTCCTGCAAACGCCACAGCAGCCTCAGATACTAATGCT
G
D
R
G
Q
T
N
N
A A
S
A
S
A
S
N
S
T
-420
1321-GGAGACCGTGGACAGACCAATAACGCCGCTTCTGCATCAGCCTCCAACTCTACCTGAACAGCCCCAAGTAGCCAGCTGCGCAGGGAAGACCAGCACTTACTTGAGTGCCACTCAGCAACA
1441
-CTGGTCACGTTTGGAAAGAAAATTAAAAAAAAAA
2432

Glycogen
synthase
kinase-3/FA
c
PPKAS'P1A.'PP,'7'ASPSS
,
S
,S
,
II,SGJPIA-TSFPF
''
.15K
,R.
.
Th/'AT
(
.MSGRPRTTSFAES-sKPVQQPSAFGSMJ'SREYx'
,SYK%'TT7".ATP
p
ELOIM
GQGPERSQEVAYTDIKVIGNGSFG''VYQARLAETRELVAItKKVLQDKRFKRELQ:MY,L
U
GQGPDPPQEF'7SY%T'KTVIGNGSFG-NVYQAKLCDSGELVAIK%'LQDY
KNRF->NRF.Q-MPRsL
13
YFFYS
DHCNI'v'RLRY'FFYSSEKKYDEY'L''NLVLEYVPETVYRVARHFTYAIl.
-PI
1Y
PVfvYMIY-
(
DHCNI'/RLRYFFYSSG,EKKYFDEVYLI;L'wrLDYVIPE7.'YRVARHYS7RAKQ,TL
'2'IYfwYKLY!*IYQ
i
DI
KPONL_VDPDTAVLK
LFRSLAYIHSQGVCHRDIKPQNLLVDPDTAVL-KLCDFGSAKQLVRGEPN;SYP:
SRYYR,
u
LFRSLAYIHSFGICHRDIKPQNLLLDPDTAVLKLCDFGSAPQLVRGEP>*ISYICSRYYRA
0
PEL
:FCA
T:Y
:S
P:Y,WzSA
PC.PrAPLLL'GQP
1FPIDSG
'IIQ
L.a
E
1K.L
TPT
.
'EC%.FM
PELIF
GAT%Y7SS
D1W'SAGC.
7
E!,LGQP1FP%SGVQ'E:I
NPNYTEFK
POIAHPFTK
NPNYTEFKFPQIYKAHPWTKVFKSRTPPEAIALCSSLLEYTPSSRLSPLEACAHSFFDELR
NPNYTEFKFPQIKAHPWTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELR
5
SPAGSTPLSOSSOALTEA=-C-
SLGTQLPNNRPLPPLFNFSPGELSIQPSLNAILIPPHLRSPSGPATLTSSSQALTETQT.
DPNVKLPNGRDTPALFNFTTQELSSNPPLATILIPPHARIQAAASPPA1;ATAASDTNAGCD
:
SDWOS
.EATPOL
QDWQAPDATPTLTNSS
a
RGQTNNXAASASASNST
Fig.
1.
Nucleotide
and
predicted
protein
sequences
of
the
two
rat
GSK-3
cDNA
clones.
The
complete
nucleotide
sequences
of
the
longest
GSK-3
a
and
cDNAs
are
shown
in
(A)
and
(B)
respectively,
with
the
corresponding
predicted
amino
acid
residues
indicated
above
(in
single
letter
code).
Nucleotides
are
numbered
on
the
left;
amino
acids,
starting
with
the
first
methionine
as
+
1,
on
the
right.
The
sequence
of
the
PCR-amplified
fragment
is
underlined
in
(A).
In
(C)
the
predicted
protein
sequences
of
rat
GSK-3cx
and
GSK-3,B
are
aligned
with
the
purified
peptide
sequences
from
rabbit
skeletal
muscle
(underlined).
Dots
between
GSK-3
a
and
indicate
amino
acid
identities.
the
high
degeneracy
of
the
sense
oligonucleotide
(62
000-fold),
a
single
fragment
of
340
bp
was
amplified
from
both
HeLa
and
rat
brain
cDNAs.
The
nucleotide
sequences
of
three
independent
clones
of
these
fragments
contained
the
same
potential
open
reading
frame
that,
at
the
5'
end,
corresponded
to
the
C-terminal
portion
of
the
DIKPQN...
peptide.
The
amino
acid
juxtaposing
the
sequence
encoded
by
the
3'
oligonucleotide
was
an
arginine
consistent
with
the
cleavage
specificity
of
trypsin.
Further-
more,
the
VLGTPT
peptide
sequence
was
contained
within
the
fragment
and
was
preceded
by
a
lysine
residue;
this
confirms
that
the
PCR
product
was
derived
from
GSK-3
cDNA.
The
nucleotide
differences
between
the
brain
and
HeLa
fragments
were
silent
with
respect
to
the
amino
acid
sequence.
Sequence
analysis
reveals
two
genes
for
GSK-3
Since
brain
has
been
reported
to
be
a
rich
source
of
GSK-3
(Tung
and
Reed,
1989),
a
rat
brain
cDNA
library
was
screened
with
the
brain
PCR
fragment
and
10
positive
plaques
isolated.
Nucleotide
sequence
analysis
of
these
clones
delineated
two
types
of
cDNA.
Five
of
the
isolates
were
derived
from
one
gene
and
three
of
these
contained
an
open
reading
frame
of
483
amino
acids,
with
the
potential
to
encode
a
protein
of
50.89
kd
(Figure
IA).
Within
this
predicted
sequence
were
all
of
the
purified
peptides,
preceded
by
lysine/arginine
(tryptic
peptides)
or
glutamic
acid
(V8
peptide)
(Figure
IC).
The
regions
corresponding
to
three
of
the
peptides
(AHPFTK,
SPAGSTP...,
AQTGS...)
contained
several
differences
(1/6,
7/18
and
5/15
respectively)
possibly
due
to
species
variation:
the
peptides
were
derived
from
rabbit
protein
and
the
cDNA
from
rat.
The
first
ATG
codon
of
the
open
reading
frame,
occurring
at
nucleotides
127-129,
was
preceded
by
a
typical
eukaryotic
translational
start
sequence
(Kozak,
1987),
although
no
stop
codons
were
present
upstream.
This
region
consisted
of
80%
G
+
C,
as
indeed
did
the
first
400
nucleotides.
The
other
five
clones
were
derived
from
a
distinct
gene.
Two
of
these
contained
an
open
reading
frame
of
420
amino
acids,
with
the
potential
to
encode
a
protein
of
46.5
kd
(Figure
1B).
The
putative
initiating
methionine
residue,
occurring
at
nucleotides
114-116,
was
preceded
by
in-frame
termination
codons
and
by
a
typical
translational
start
sequence.
Comparison
of
the
amino
acid
sequences
of
the
two
open
reading
frames
revealed
considerable
identity
(Figure
IC).
This
was
highest
(98%)
in
the
region
containing
motifs
common
to
all
protein
kinases
which
form
the
'kinase
domain'
(Hanks
et
al.,
1987).
Regions
at
the
N-
and
C-
termini
were
considerably
more
divergent.
Only
36
%
of
the
C-terminal
76
residues
were
conserved
between
the
two
cDNAs:
this
region
also
contained
the
two
rabbit
tryptic
peptides
that
exhibited
several
amino
acid
differences
with
the
cDNA
clones.
The
4
kd
variation
between
the
predicted
molecular
masses
was
accounted
for
by
a
glycine-rich,
N-
terminal
extension
to
the
longer
clone.
Since
a
portion
of
this
sequence
was
present
in
one
of
the
tryptic
peptides
(ASVGAM...),
the
extension
was
not
due
to
a
cloning
artifact.
As
the
longer
form
exhibited
greater
identity
with
the
peptides
derived
from
purified
GSK-3,
agreeing
also
with
the
purified
proteins'
molecular
mass
(51
kd),
this
form
has
been
designated
GSK-3a
and
the
shorter
protein
GSK-33.
The
three
sequenced
rat
brain-derived
PCR
fragments
were
all
derived
from
GSK-3ci
cDNA.
Comparison
of
both
GSK-3
sequences
with
the
Swissprot
database
revealed
predictable
identity
with
other
protein
kinases.
The
closest
identity,
35%,
was
exhibited
with
the
cdc2/kin28
sub-family
(Simon
et
al.,
1986;
Lee
and
Nurse,
1987),
as
compared
with
a
general
kinase
identity
of
-
25%.
Expression
of
GSK-3
cDNAs
Translation
of
synthetic
RNA
derived
from
the
a-
and
1-
type
cDNAs
in
vitro
resulted
in
the
synthesis
of
major
proteins
of
51
and
47
kd,
respectively,
in
agreement
with
those
predicted
from
the
nucleotide
sequences
(Figure
2A).
The
efficiency
of
translation
of
the
GSK-3a
RNA
was
only
10%
of
1,B
possibly
caused
by
the
high
G+C
content
surrounding
the
translational
start
site
of
GSK-3a.
A
smaller
difference
in
the
synthesis
of
the
two
clones
was
observed
upon
transient
transfection
of
COS-1
cells.
Immunoblotting
with
antibodies
specific
for
GSK-3ca
or
13
(see
below)
revealed
the
presence
of
a
major
51
kd
protein
and
a
minor
59
kd
protein
in
cells
transfected
with
GSK-3a
DNA
and
a
47
kd
protein
in
GSK-3f3
transfectants
(Figure
2B).
Since
the
59
kd
band
was
immunologically
related
to
GSK-3,
it
is
likely
to
be
derived
from
an
upstream
CTG
codon
in
the
GSK-3a
cDNA
since
there
are
no
other
ATG
codons
in
the
5'
untranslated
region.
Fractionation
of
cell
lysates
of
monoS
FPLC
revealed
a
3-fold
increase
in
2433
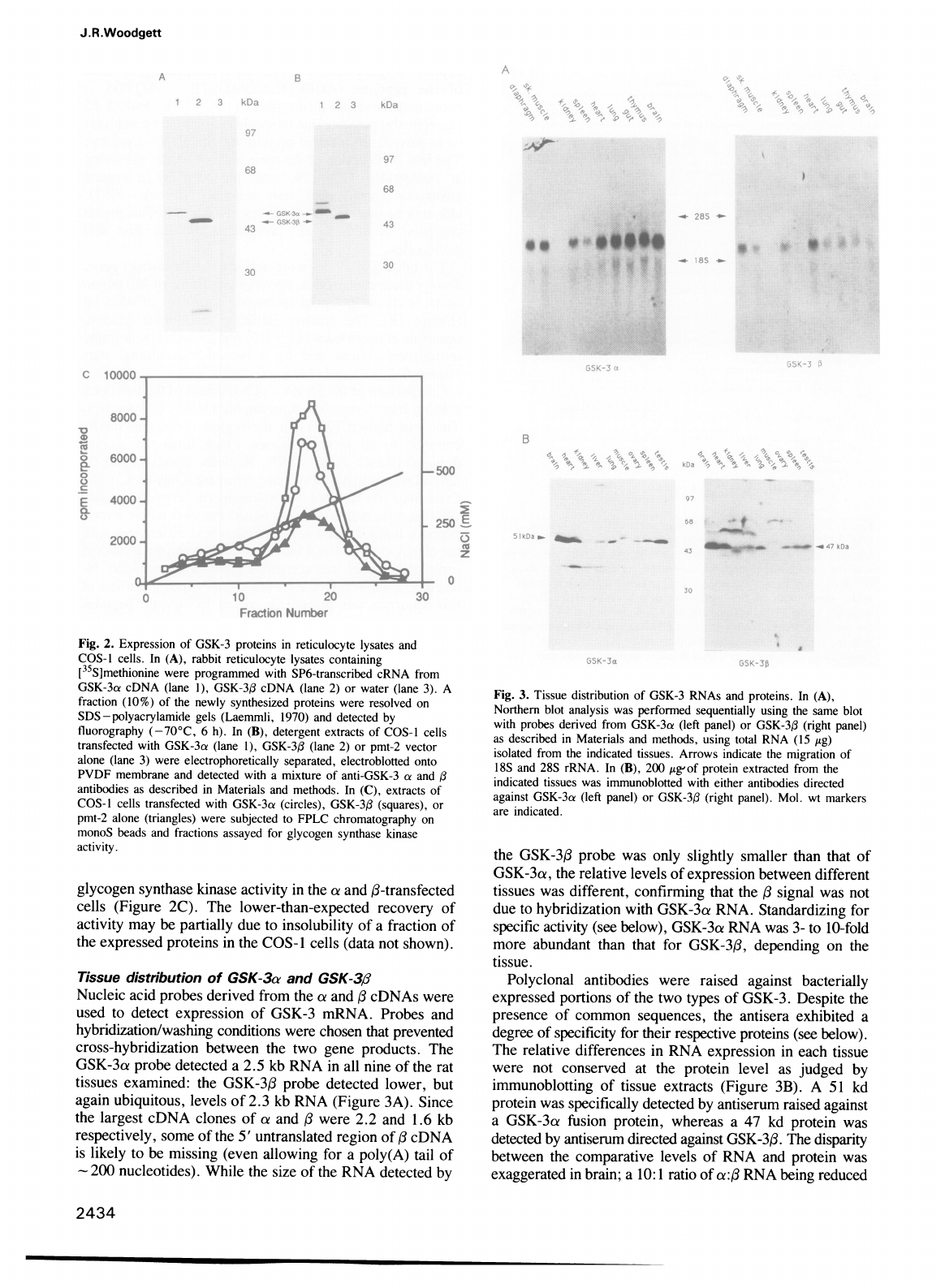
J.R.Woodgett
..
O.
0:
Fig.
2.
Expression
of
GSK-3
proteins
in
reticulocyte
lysates
and
COS-1
cells.
In
(A),
rabbit
reticulocyte
lysates
containing
[35S]methionine
were
programmed
with
SP6-transcribed
cRNA
from
GSK-3a
cDNA
(lane
1),
GSK-3,B
cDNA
(lane
2)
or
water
(lane
3).
A
fraction
(10%)
of
the
newly
synthesized
proteins
were
resolved
on
SDS-polyacrylamide
gels
(Laemmli,
1970)
and
detected
by
fluorography
(-70°C,
6
h).
In
(B),
detergent
extracts
of
COS-1
cells
transfected
with
GSK-3a
(lane
1),
GSK-3j3
(lane
2)
or
pmt-2
vector
alone
(lane
3)
were
electrophoretically
separated,
electroblotted
onto
PVDF
membrane
and
detected
with
a
mixture
of
anti-GSK-3
ca
and
(3
antibodies
as
described
in
Materials
and
methods.
In
(C),
extracts
of
COS-1
cells
transfected
with
GSK-3a
(circles),
GSK-3f3
(squares),
or
pmt-2
alone
(triangles)
were
subjected
to
FPLC
chromatography
on
monoS
beads
and
fractions
assayed
for
glycogen
synthase
kinase
activity.
glycogen
synthase
kinase
activity
in
the
a
and
,B-transfected
cells
(Figure
2C).
The
lower-than-expected
recovery
of
activity
may
be
partially
due
to
insolubility
of
a
fraction
of
the
expressed
proteins
in
the
COS-1
cells
(data
not
shown).
Tissue
distrbution
of
GSK-3a
and
GSK-3,B
Nucleic
acid
probes
derived
from
the
a
and
,B
cDNAs
were
used
to
detect
expression
of
GSK-3
mRNA.
Probes
and
hybridization/washing
conditions
were
chosen
that
prevented
cross-hybridization
between
the
two
gene
products.
The
GSK-3ao
probe
detected
a
2.5
kb
RNA
in
all
nine
of
the
rat
tissues
examined:
the
GSK-3,B
probe
detected
lower,
but
again
ubiquitous,
levels
of
2.3
kb
RNA
(Figure
3A).
Since
the
largest
cDNA
clones
of
a
and
,B
were
2.2
and
1.6
kb
respectively,
some
of
the
5'
untranslated
region
of
,B
cDNA
is
likely
to
be
missing
(even
allowing
for
a
poly(A)
tail
of
-
200
nucleotides).
While
the
size
of
the
RNA
detected
by
Fig.
3.
Tissue
distribution
of
GSK-3
RNAs
and
proteins.
In
(A),
Northern
blot
analysis
was
performed
sequentially
using
the
same
blot
with
probes
derived
from
GSK-3a
(left
panel)
or
GSK-3fl
(right
panel)
as
described
in
Materials
and
methods,
using
total
RNA
(15
ytg)
isolated
from
the
indicated
tissues.
Arrows
indicate
the
migration
of
18S
and
28S
rRNA.
In
(B),
200
tg'of
protein
extracted
from
the
indicated
tissues
was
immunoblotted
with
either
antibodies
directed
against
GSK-3ca
(left
panel)
or
GSK-3,B
(right
panel).
Mol.
wt
markers
are
indicated.
the
GSK-3(
probe
was
only
slightly
smaller
than
that
of
GSK-3a,
the
relative
levels
of
expression
between
different
tissues
was
different,
confirming
that
the
(
signal
was
not
due
to
hybridization
with
GSK-3a
RNA.
Standardizing
for
specific
activity
(see
below),
GSK-3a
RNA
was
3-
to
10-fold
more
abundant
than
that
for
GSK-3(,
depending
on
the
tissue.
Polyclonal
antibodies
were
raised
against
bacterially
expressed
portions
of
the
two
types
of
GSK-3.
Despite
the
presence
of
common
sequences,
the
antisera
exhibited
a
degree
of
specificity
for
their
respective
proteins
(see
below).
The
relative
differences
in
RNA
expression
in
each
tissue
were
not
conserved
at
the
protein
level
as
judged
by
immunoblotting
of
tissue
extracts
(Figure
3B).
A
51
kd
protein
was
specifically
detected
by
antiserum
raised
against
a
GSK-3ae
fusion
protein,
whereas
a
47
kd
protein
was
detected
by
antiserum
directed
against
GSK-3(.
The
disparity
between
the
comparative
levels
of
RNA
and
protein
was
exaggerated
in
brain;
a
10:1
ratio
of
a:
3
RNA
being
reduced
2434
4
4-%Ww-.
411baft,
..
41
..:,.,.,-.
-
Glycogen
synthase
kinase-3/FA
to
an
cz:
protein
ratio
of
1.5:
1.
It
should
be
noted
that
the
high
concentration
of
actin
(45
kd)
in
skeletal
muscle
extracts
distorts
the
migration
of
other
proteins
in
the
40-50
kd mass
range.
Immunoreactive
staining
for
GSK-3a
and
3
is
present
in
these
extracts
but
is
diffused
by
the
actin.
However,
the
total
absence
of
GSK-3,B
from
preparations
of
skeletal
muscle
GSK-3
is
somewhat
surprising.
It
is
possible
that
the
(3
RNA
is
not
translated
in
muscle
or
that
the
protein
is
lost
during
purification
(but
see
below).
Copunification
of
GSK-3a
and,5
from
bovine
brain
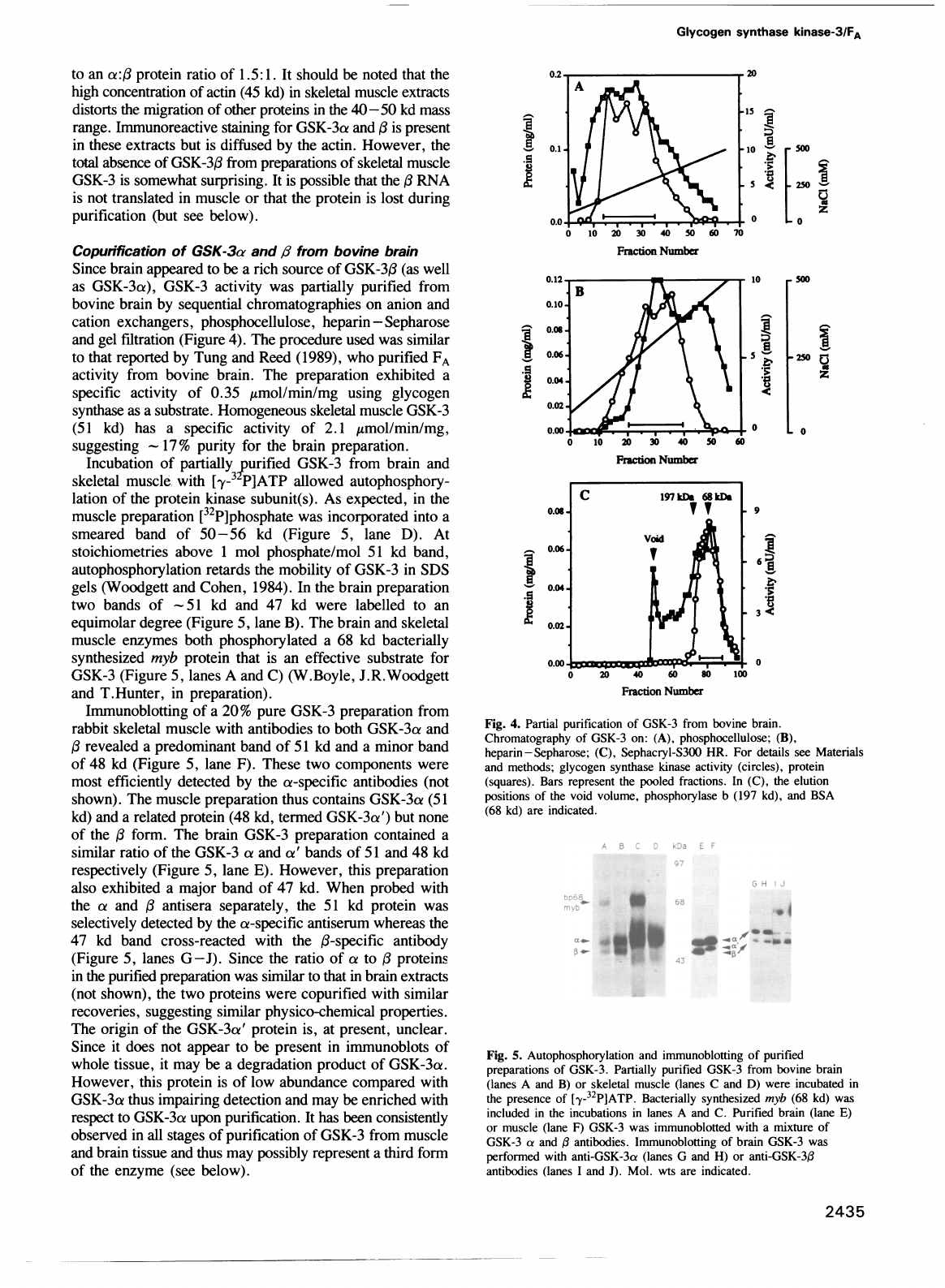
Since
brain
appeared
to
be
a
rich
source
of
GSK-3,B
(as
well
as
GSK-3a),
GSK-3
activity
was
partially
purified
from
bovine
brain
by
sequential
chromatographies
on
anion
and
cation
exchangers,
phosphocellulose,
heparin
-
Sepharose
and
gel
filtration
(Figure
4).
The
procedure
used
was
similar
to
that
reported
by
Tung
and
Reed
(1989),
who
purified
FA
activity
from
bovine
brain.
The
preparation
exhibited
a
specific
activity
of
0.35
Amol/min/mg
using
glycogen
synthase
as
a
substrate.
Homogeneous
skeletal
muscle
GSK-3
(51
kd)
has
a
specific
activity
of
2.1
Amol/min/mg,
suggesting
-
17%
purity
for
the
brain
preparation.
Incubation
of
partially
purified
GSK-3
from
brain
and
skeletal
muscle
with
[y-3
P]ATP
allowed
autophosphory-
lation
of
the
protein
kinase
subunit(s).
As
expected,
in
the
muscle
preparation
[32P]phosphate
was
incorporated
into
a
smeared
band
of
50-56
kd
(Figure
5,
lane
D).
At
stoichiometries
above
1
mol
phosphate/mol
51
kd
band,
autophosphorylation
retards the
mobility
of
GSK-3
in
SDS
gels
(Woodgett
and
Cohen,
1984).
In
the
brain
preparation
two
bands
of
-51
kd
and
47
kd
were
labelled
to
an
equimolar
degree
(Figure
5,
lane
B).
The
brain
and
skeletal
muscle
enzymes
both
phosphorylated
a
68
kd
bacterially
synthesized
myb
protein
that
is
an
effective
substrate
for
GSK-3
(Figure
5,
lanes
A
and
C)
(W.Boyle,
J.R.Woodgett
and
T.Hunter,
in
preparation).
Immunoblotting
of
a
20%
pure
GSK-3
preparation
from
rabbit
skeletal
muscle
with
antibodies
to
both
GSK-3a
and
,
revealed
a
predominant
band
of
51
kd
and
a
minor
band
of
48
kd
(Figure
5,
lane
F).
These
two
components
were
most
efficiently
detected
by
the
a-specific
antibodies
(not
shown).
The
muscle
preparation
thus
contains
GSK-3a
(51
kd)
and
a
related
protein
(48
kd,
termed
GSK-3a')
but
none
of
the
(3
form.
The
brain
GSK-3
preparation
contained
a
similar
ratio
of
the
GSK-3
a
and
a'
bands
of
51
and
48
kd
respectively
(Figure
5,
lane
E).
However,
this
preparation
also
exhibited
a
major
band
of
47
kd.
When
probed
with
the
a
and
(
antisera
separately,
the
51
kd
protein
was
selectively
detected
by
the
a-specific
antiserum
whereas
the
47
kd
band
cross-reacted
with
the
(3-specific
antibody
(Figure
5,
lanes
G
-J).
Since
the
ratio
of
a
to
(
proteins
in
the
purified
preparation
was
similar
to
that
in
brain
extracts
(not
shown),
the
two
proteins
were
copurified
with
similar
recoveries,
suggesting
similar
physico-chemical
properties.
The
origin
of
the
GSK-3a'
protein
is,
at
present,
unclear.
Since
it
does
not
appear
to
be
present
in
immunoblots
of
whole
tissue,
it
may
be
a
degradation
product
of
GSK-3a.
However,
this
protein
is
of
low
abundance
compared
with
GSK-3a
thus
impairing
detection
and
may
be
enriched
with
respect
to
GSK-3a
upon
purification.
It
has
been
consistently
observed
in
all
stages
of
purification
of
GSK-3
from
muscle
and
brain
tissue
and
thus
may
possibly
represent
a
third
form
of
the
enzyme
(see
below).
0.1.
.s
1500
I
-
*
250
al0
Fraction
Number
0.10-
0.08
0.06-
o.os.
0.04
0.02.
-
A
6
10
20
30
40
50
Fraction
Number
40
60
80
Fraction
Number
10
I
a
,0
-250
-0
9
6{
3
Fig.
4.
Partial
purification
of
GSK-3
from
bovine
brain.
Chromatography
of
GSK-3
on:
(A),
phosphocellulose;
(B),
heparin-Sepharose;
(C),
Sephacryl-S300
HR.
For
details
see
Materials
and
methods;
glycogen
synthase
kinase
activity
(circles),
protein
(squares).
Bars
represent
the
pooled
fractions.
In
(C),
the
elution
positions
of
the
void
volume,
phosphorylase
b
(197
kd),
and
BSA
(68
kd)
are
indicated.
S-
_.
b
dB
_~~.4
..r
Fig.
5.
Autophosphorylation
and
immunoblotting
of
purified
preparations
of
GSK-3.
Partially
purified
GSK-3
from
bovine
brain
(lanes
A
and
B)
or
skeletal
muscle
(lanes
C
and
D)
were
incubated
in
the
presence
of
[-y-32P]ATP.
Bacterially
synthesized
myb
(68
kd)
was
included
in
the
incubations
in
lanes
A
and
C.
Purified
brain
(lane
E)
or
muscle
(lane
F)
GSK-3
was
immunoblotted
with
a
mixture
of
GSK-3
ca
and
,B
antibodies.
Immunoblotting
of
brain
GSK-3
was
performed
with
anti-GSK-3a
(lanes
G
and
H)
or
anti-GSK-3(3
antibodies
(lanes
I
and
J).
Mol.
wts
are
indicated.
2435
B
J.R.Woodgett
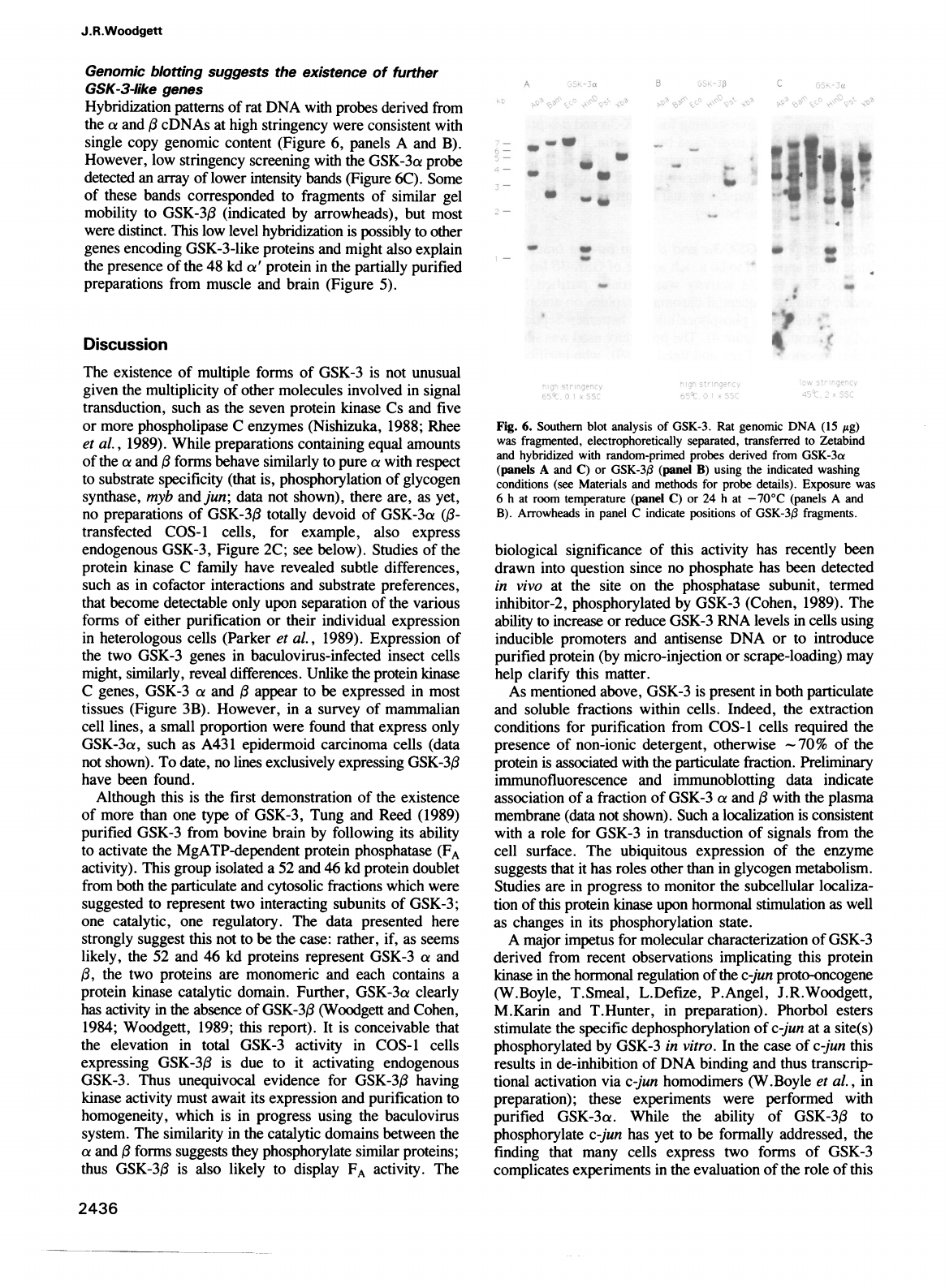
Genomic
blotting
suggests
the
existence
of
further
GSK-3-like
genes
Hybridization
patterns
of
rat
DNA
with
probes
derived
from
the
a
and
,B
cDNAs
at
high
stringency
were
consistent
with
single
copy
genomic
content
(Figure
6,
panels
A
and
B).
However,
low
stringency
screening
with
the
GSK-3a
probe
detected
an
array
of
lower
intensity
bands
(Figure
6C).
Some
of
these
bands
corresponded
to
fragments
of
similar
gel
mobility
to
GSK-3,B
(indicated
by
arrowheads),
but
most
were
distinct.
This
low
level
hybridization
is
possibly
to
other
genes
encoding
GSK-3-like
proteins
and
might
also
explain
the
presence
of
the
48 kd
a'
protein
in
the
partially
purified
preparations
from
muscle
and
brain
(Figure
5).
Discussion
The
existence
of
multiple
forms
of
GSK-3
is
not
unusual
given
the
multiplicity
of
other
molecules
involved
in
signal
transduction,
such
as
the
seven
protein
kinase
Cs
and
five
or
more
phospholipase
C
enzymes
(Nishizuka,
1988;
Rhee
et
al.,
1989).
While
preparations
containing
equal
amounts
of
the
a
and
forms
behave
similarly
to
pure
oa
with
respect
to
substrate
specificity
(that
is,
phosphorylation
of
glycogen
synthase,
myb
and
jun;
data
not
shown),
there
are,
as
yet,
no
preparations
of
GSK-3,B
totally
devoid
of
GSK-3ai
((3-
transfected
COS-1
cells,
for
example,
also
express
endogenous
GSK-3,
Figure
2C;
see
below).
Studies
of
the
protein
kinase
C
family
have
revealed
subtle
differences,
such
as
in
cofactor
interactions
and
substrate
preferences,
that
become
detectable
only
upon
separation
of
the
various
forms
of
either
purification
or
their
individual
expression
in
heterologous
cells
(Parker
et
al.,
1989).
Expression
of
the
two
GSK-3
genes
in
baculovirus-infected
insect
cells
might,
similarly,
reveal
differences.
Unlike
the
protein
kinase
C
genes,
GSK-3
a
and
appear
to
be
expressed
in
most
tissues
(Figure
3B).
However,
in
a
survey
of
mammalian
cell
lines,
a
small
proportion
were
found
that
express
only
GSK-3a,
such
as
A431
epidermoid
carcinoma
cells
(data
not
shown).
To
date,
no
lines
exclusively
expressing
GSK-3,
have
been
found.
Although
this
is
the
first
demonstration
of
the
existence
of
more
than
one
type
of
GSK-3,
Tung
and
Reed
(1989)
purified
GSK-3
from
bovine
brain
by
following
its
ability
to
activate
the
MgATP-dependent
protein
phosphatase
(FA
activity).
This
group
isolated
a
52
and
46
kd
protein
doublet
from
both
the
particulate
and
cytosolic
fractions
which
were
suggested
to
represent
two
interacting
subunits
of
GSK-3;
one
catalytic,
one
regulatory.
The
data
presented
here
strongly
suggest
this
not
to
be
the
case:
rather,
if,
as
seems
likely,
the
52
and
46
kd
proteins
represent
GSK-3
a
and
(,
the
two
proteins
are
monomeric
and
each
contains
a
protein
kinase
catalytic
domain.
Further,
GSK-3a
clearly
has
activity
in
the
absence
of
GSK-3(3
(Woodgett
and
Cohen,
1984;
Woodgett,
1989;
this
report).
It
is
conceivable
that
the
elevation
in
total
GSK-3
activity
in
COS-l
cells
expressing
GSK-3,B
is
due
to
it
activating
endogenous
GSK-3.
Thus
unequivocal
evidence
for
GSK-3,
having
kinase
activity
must
await
its
expression
and
purification
to
homogeneity,
which
is
in
progress
using
the
baculovirus
system.
The
similarity
in
the
catalytic
domains
between
the
a
and
forms
suggests
they
phosphorylate
similar
proteins;
thus
GSK-3,B
is
also
likely
to
display
FA
activity.
The
Fig.
6.
Southern
blot
analysis
of
GSK-3.
Rat
genomic
DNA
(15
jig)
was
fragmented,
electrophoretically
separated,
transferred
to
Zetabind
and
hybridized
with
random-primed
probes
derived
from
GSK-3a
(panels
A
and
C)
or
GSK-3(3
(panel
B)
using
the
indicated
washing
conditions
(see
Materials
and
methods
for
probe
details).
Exposure
was
6
h
at
room
temperature
(panel
C)
or
24
h
at
-70°C
(panels
A
and
B).
Arrowheads
in
panel
C
indicate
positions
of
GSK-3,B
fragments.
biological
significance
of
this
activity
has
recently
been
drawn
into
question
since
no
phosphate
has
been
detected
in
vivo
at
the
site
on
the
phosphatase
subunit,
termed
inhibitor-2,
phosphorylated
by
GSK-3
(Cohen,
1989).
The
ability
to
increase
or
reduce
GSK-3
RNA
levels
in
cells
using
inducible
promoters
and
antisense
DNA
or
to
introduce
purified
protein
(by
micro-injection
or
scrape-loading)
may
help
clarify
this
matter.
As
mentioned
above,
GSK-3
is
present
in
both
particulate
and
soluble
fractions
within
cells.
Indeed,
the
extraction
conditions
for
purification
from
COS-1
cells
required
the
presence
of
non-ionic
detergent,
otherwise
-
70%
of
the
protein
is
associated
with
the
particulate
fraction.
Preliminary
immunofluorescence
and
immunoblotting
data
indicate
association
of
a
fraction
of
GSK-3
ca
and
,8
with
the
plasma
membrane
(data
not
shown).
Such
a
localization
is
consistent
with
a
role
for
GSK-3
in
transduction
of
signals
from
the
cell
surface.
The
ubiquitous
expression
of
the
enzyme
suggests
that
it
has
roles
other
than
in
glycogen
metabolism.
Studies
are
in
progress
to
monitor
the
subcellular
localiza-
tion
of
this
protein
kinase
upon
hormonal
stimulation
as
well
as
changes
in
its
phosphorylation
state.
A
major
impetus
for
molecular
characterization
of
GSK-3
derived
from
recent
observations
implicating
this
protein
kinase
in
the
hormonal
regulation
of
the
c-jun
proto-oncogene
(W.Boyle,
T.Smeal,
L.Defize,
P.Angel,
J.R.Woodgett,
M.Karin
and
T.Hunter,
in
preparation).
Phorbol
esters
stimulate
the
specific
dephosphorylation
of
c-jun
at
a
site(s)
phosphorylated
by
GSK-3
in
vitro.
In
the
case
of
c-jun
this
results
in
de-inhibition
of
DNA
binding
and
thus
transcrip-
tional
activation
via
c-jun
homodimers
(W.Boyle
et
al.,
in
preparation);
these
experiments
were
performed
with
purified
GSK-3a.
While
the
ability
of
GSK-303
to
phosphorylate
c-jun
has
yet
to
be
formally
addressed,
the
finding
that
many
cells
express
two
forms
of
GSK-3
complicates
experiments
in
the
evaluation
of
the
role
of
this
2436
w
_ _
_
0
w
*
-.
.,,
,
-m
.w
w
_MO

Glycogen
synthase
kinase-3/FA
protein
kinase
in
c-jun
regulation.
However,
availability
of
nucleic
acid
probes
and
antibodies
will
help
in
the
elucidation
of
the
molecular
mechanisms
by
which
these
protein
kinases
are
regulated
in
cells,
as
well
as
the
processes
which
they,
in
turn,
regulate.
Materials
and
methods
Purification
of
skeletal
muscle
GSK-3
GSK-3
was
purified
from
rabbit
skeletal
muscle
essentially
as
described
(Woodgett,
1989),
except
that
following
chromatography
on
monoS,
the
peak
of
GSK-3
activity
was
subjected
to
reverse
phase
HPLC
using
a
C4
column
(2.1
mm,
wide-pore,
Vydac)
in
0.1
%
trifluoroacetic
acid
and
eluted
with
acetonitrile
(at
-
35%).
Proteolysis
of
muscle
GSK-3
and
separation
of
peptides
The
purified
protein
(20-25
jg)
was
fragmented
either
by
trypsin
(5%
by
weight,
Worthington,
TPCK-treated)
at
370C
for
18
h,
or
with
Saureus
V8
protease
(5%
by
weight,
Boehringer
Glu-C
protease)
using
standard
conditions.
The
resulting
peptides
were
resolved
on
a
C8-RP300
reverse-
phase
column
in
50
mM
ammonium
acetate
pH
6.8
with
acetonitrile
elution
(0-50%)
(Tempste
et
al.,
1986).
Single
peaks
were
rechromatographed
on
a
small
scale
column
of
the
same
media
but
using
0.08%
trifluoroacetic
acid,
pH
2.1.
Peptides
(25-70
pmol)
were
sequenced
using
an
Applied
Biosystems
477A
sequencer
(Hewick
et
al.,
1981).
In
total,
peptide
sequence
data
were
obtained
from
four
different
preparations
of
GSK-3.
Amplification
of
GSK-3
cDNA
Two
oligonucleotides
corresponding
to
two
of
the
tryptic
peptide
sequences
(H/Y-R-D-L/I-K-P-E/Q-N
and
E-M-N-P-N-Y)
were
designed
as
primers
for
the
polymerase
chain
reaction
(PCR).
Amplification
was
performed
with
7hermus
aquaticus
DNA
polymerase
(Perkin-Elmer
Cetus)
according
to
the
suppliers
instructions
(Mullis
et
al.,
1986).
30
cycles
consisting
of
denaturation
at
93°C
for
1
min,
annealing
at
50°C
for
2
min,
and
polymeriza-
tion
at
70°C
for
5
min
were
performed
using
1
jig
of
HeLa
cDNA
(in
Okayama
and
Berg
vector
linearized
with
BamHI,
complexity
of
1
x
106;
Hanks,
1987)
or
1
Ag
of
rat
brain
cDNA
(in
XZAPII
vector,
complexity
2
x
106,
Stratagene)
in
a
programmable
heating
block.
The
amplified
products
of
340
bp
were
gel-purified,
ligated
to
EcoRI
adaptors
(Promega),
and
inserted
into
Bluescript
(Stratagene)
for
sequence
analysis.
Isolation
of
full-length
clones
A
random-primed
probe
(5
x
108
c.p.m.I/g,
106
c.p.m./ml;
Feinberg
and
Vogelstein,
1983)
was
generated
from
the
340
bp
PCR
fragment
and
used
to
screen
106
recombinants
of
a
rat
brain
cDNA
library
in
a
XZAPH
vector
(Stratagene).
Hybridization
conditions
were:
5
x
SSPE,
100
Ag/ml
sonicated
and
denatured
salmon
sperm
DNA,
5
x
Denhardts,
1%
SDS,
50%
formamide
at
42°C
for
18
h.
The
filters
were
washed
with
2
x
SSPE,
0.1%
SDS
at
42°C
for
2
h,
followed
by
0.5
x
SSPE,
0.1%
SDS
at
550C
for
2
x
20
min
and
autoradiography
for
18
h
at
-700C
with
intensifying
screens.
Ten
positive
plaques
were
purified,
pBluescript
plasmids
rescued
by
super-infection
with
R408
helper
phage
(Short
et
al.,
1988),
and
subjected
to
DNA
sequence
analysis
on
both
strands
by
use
of
exonuclease
III-nested
deletions,
restriction
fragment
cloning
into
M13
and
specific
oligonucleotide
primers.
The
10
clones
fell
into
two
classes:
XC
(1.2
kb),
XD
(1.6
kb),
XG2
(2.2
kb),
XH
(1.4
kb),
XN5
(2.2
kb)
were
derived
from
one
gene
and
XI
(1.5
kb),
XJ
(1.6
kb),
XK1
(1.6
kb),
XK2
(1.1
kb)
and
XN1
(1.5
kb)
from
a
second
gene.
DNA
and
RNA
analysis
Total
RNA
was
isolated
from
various
rat
tissues
(Chirgwin
et
al.,
1979),
and
10
Ftg
of
each
electrophoretically
separated
on
formaldehyde-agarose
gels
and
transferred
to
GeneScreen
(Du
Pont).
Blot
hybridizations
and
washes
were
performed
as
described
above
using
a
0.75
kb
BamHI
fragment
from
clone
XJ
(GSK-3,B,
nucleotide
681
-3'polylinker).
Following
autoradiography,
the
membrane
was
stripped
and
hybridized
with
a
1
kb
EcoRI
fragment
from
clone
XD
(GSK-3ca,
nucleotide
I
1
80-3
'polylinker).
For
Southern
blots,
15
/Ag
rat
genomic
DNA
was
digested
with
the
indicated
restriction
enzymes,
electrophoretically
separated,
transferred
to
Zetabind
(CUNO)
and
hybridized
according
to
the
manufacturers'
protocol
with
the
probes
used
for the
RNA
analysis.
Low
stringency
washing
was
for
2
h
with
five
changes
of 2
x
SSPE,
0.1%
SDS
at
42°C.
The
high
stringency
washes
contained
0.1
x
SSPE,
0.1%
SDS
at
65°C
for
2
x
20
min.
In
vitro
transcription
and
translation
An
EcoRV-BamHI
fragment
of
GSK-3a
(5'
polylinker-3'
polylinker
containing
entire
cDNA)
and
a
RsaI-EcoRV
fragment
of
GSK-3,B
(nucleotide
47-3'
polylinker)
were
cloned
into
the
BgIH
site
of
pSP64T
(Melton
et
al.,
1984).
These
fragments
contained
the
entire
open
reading
frames
of
the
two
cDNAs.
Capped
RNA
was
transcribed
from
XbaI-
linearized
plasmid
with
SP6
RNA
polymerase
and
I
Mg
used
to
program
a
message-dependent
rabbit
reticulocyte
lysate
(Promega)
in
the
presence
of
100
MCi
[3'S]methionine.
After
incubation
for
1
h
at
30°C,
the
proteins
were
denatured
by
heating
at
95°C
in
1
%
SDS,
resolved
on
a
15%polyacrylamide
gel
and
radioactivity
detected
by
fluorography.
Transfection
of
COS-1
cells
The
same
DNA
fragments
of
GSK-3a
and
i3
used
for
in
vitro
transcription
were
ligated
into
the
EcoRI
site
of
pmt-2
(Wong
et
al.,
1985).
Plasmid
DNA
(20
Mg)
was
transfected
into
two
6
mm
dishes
of
COS-1
cells
by
calcium
phosphate
precipitation
in
the
presence
of
chloroquine
(Luthman
and
Magnusson,
1983)
or
by
lipofection
(Gibco)
according
to
the
supplier.
Cells
were
washed
with
ice-cold
Tris-buffered
saline
48
h
after
introduction
of
DNA
and
lysed
at
4°C
in
20
mM
HEPES-OH,
1
mM
EDTA,
1
mM
dithiothreitol
(buffer
A)
containing
1%
Triton
X-100
and
10
mM
NaCl.
After
brief
sonication,
the
lysate
was
centrifuged
at
10
000
g
for
30
min
and
the
supernatant
applied
to
a
monoS
FPLC
column
equilibrated
in
buffer
A
containing
0.05%
Triton
X-100.
Proteins
were
eluted
with
a
20
ml
0-500
mM
NaCl
gradient.
Fractions
were
assayed
for
glycogen
synthase
kinase
activity
as
described
(Woodgett
and
Cohen,
1984).
Partial
purification
of
GSK-3
from
bovine
brain
The
procedure
is
modified
from
Tung
and
Reed
(1989).
All
procedures
were
performed
at
4°C.
Briefly,
fresh
bovine
brain
(500
g)
was
homogenized
in 11
of
50
mM
Tris-HCI,
4
mM
EDTA,
2
mM
EGTA,
15
mM
2-mercaptoethanol,
0.1
mM
phenylmethylsulphonyl
fluoride,
1
mM
benzamidine,
pH
7.5
and
centrifuged
at
6000
g
for
40
min.
The
supernatant
was
passed
through
DEAE-Sepharose
FF
(5
x
30
cm)
and
the
flow-through
fractions
directly
applied
to
CM-Sepharose
FF
(both
columns
equilibrated
in
25
mM
Tris-HCI,
1
mM
EDTA,
1
mM
EGTA,
15
mM
2-mercaptoethanol,
5%
glycerol,
pH
7.5;
buffer
A).
GSK-3
was
eluted
with
buffer
A
+
200
mM
NaCI.
The
active
fractions
were
diluted
2-fold
and
applied
to
phosphocellulose
(7
x
7
cm)
in
buffer
A.
After
washing
with
buffer
A
+
100
mM
NaCl,
GSK-3
was
eluted
with
a
100-500
mM
NaCl
gradient
(Figure
4A).
The
active
fractions
were
dialysed
against
buffer
A
and
applied
to
heparin-Sepharose
(2.6
x
5
cm)
and
eluted
with
a
gradient
of
100-500
mM
NaCI
(Figure
4B).
Active
fractions
were
dialysed
against
buffer
A
and
concentrated
by
chromatography
on
S-Sepharose
(1
x
10
cm),
eluting
with
buffer
A
+
250
mM
NaCl.
The
protein
peak
was
size
fractionated
by
Sephacryl
S-300HR
(2.6
x
90
cm)
in
buffer
A
+
200
mM
NaCl
(Figure
4C).
The
activity
eluted
in
a
symmetrical
peak
corresponding
to
70
kd.
The
active
fractions
were
concentrated
and
dialysed
against
buffer
A
containing
200
mM
NaCI
and
50%
glycerol
and
stored
at
-20°C.
The
preparation
had
a
specific
activity
of
0.35
Amol
phosphate
transferred
into
glycogen
synthase
per
min
per
mg
at
30°C.
Immunological
methods
The
C-terminal
regions
of
the
two
forms
of
GSK-3
were
fused
to
TrpE
or
glutathione
S-transferase
for
bacterial
expression.
Briefly,
for
TrpE
fusions:
a
660 bp
ClaI
fragment of
GSK-3a
extending
from
amino
acid
302
to
beyond
the
C-terminus
was
cloned
into
the
unique
ClaI
site
of
pATH-3
(constructed
by
T.J.Koerner
and
A.Tzagaloff;
Angel
et
al.,
1988);
a
750
bp
BamHI
fragment
of
GSK-3,B
extending
from
amino
acid
189
to
beyond
the
C-terminus
was
ligated
into
the
unique
BwmHI
site
of
pATH-2.
The
resulting
plasmids,
pATHa
and
pATH,B
respectively,
were
transformed
into
C600
bacteria.
TrpE
fusion
proteins
of 53
kd
(pATHct)
and
58
kd
(pATH,B)
were
expressed
and
purified
from
inclusion
bodies
by
standard
procedures
(Sambrook
et
al.,
1989).
For
glutathione
S-transferase
fusions:
a
PvuII
fragment of
GSK-3a
from
amino
acid
306
to
the
C-terminus
was
filled
in
and
ligated
into
the
SnaI
site
of
pGEX1
(Smith
and
Johnson,
1988)
to
form
pGEXca;
a
750
bp
BamHI
fragment
of
GSK-313
amino
acid
189
to
C-
terminus)
was
ligated
into
the
BamHI
site
of
pGEX
1
to
form
pGEX(3.
Fusion
proteins
of
45
kd
(pGEXa)
and
50
kd
(pGEX,3)
were
purified
by
chromatography
on
glutathione-agarose
(Pharmacia)
(Smith
and
Johnson,
1988).
The
two
TrpE
fusion
proteins
(200
Mg)
were
separately
mixed
with
Freunds
adjuvant
and
each
subcutaneously
injected
into
two
rabbits.
The
animals
were
boosted
every
4-6
weeks
and
serum
collected
after
7
days.
The
glutathione
S-transferase
fusion
proteins
were
used
for
antisera
characterization
and
quantitation.
It
should
be
noted
that
although
the
regions
of
the
two
proteins
used
as
antigens
contained
stretches
of
highly
homologous
peptide
sequence
(Figure
IC),
the
antibodies
raised
were
specific
for
the
2437

J.R.Woodgett
respective
antigen,
especially
in
later
bleeds.
Presumably
the
common
peptides
are
less
antigenic
than
the
distinct
peptides.
The
antibodies
recognized
denatured
and,
with
lower
efficiency,
native
GSK-3
protein.
For
immunoblotting,
various
rat
tissues
were
homogenized
on
ice
in
4
volumes
of
20
mM
HEPES-OH,
1
mM
EDTA,
0.1
mM
phenylmethyl-
sulphonyl
fluoride,
1
mM
dithiothreitol,
pH
7.5
using
a
Polytron
cell
disrupter.
An
equal
volume
of
2
x
Laemmli
sample
buffer
(Laemmli,
1970)
was
added
and
the
samples
boiled
for
5
min.
After
brief
sonication,
tissue
extracts
(100
jig)
were
separated
on
12.5%
polyacrylamide
gels
and
electrophoretically
transferred
to
PVDF
membranes
(Millipore).
Blots
were
processed
using
standard
procedures
(Harlow
and
Lane,
1988);
antiserum
passed
through
DEAE-Affigel
Blue
(Bio-Rad)
was
used
at
50-
to
100-fold
dilutions
and
detection
was
with
[125I]protein
A
(Amersham).
Acknowledgements
I
am
indebted
to
the
assistance
and
encouragement
of
the
members
of
the
Ludwig
Institute,
London.
I
would
particularly
like
to
thank:
Nick
Totty
and
Rob
Philp
for
their
invaluable
help
in
the
isolation
and
sequencing
of
peptides;
Dick
Schaap,
for
sharing
his
RNA
blots;
Peter
Parker,
Jeremy
Brockes
and
Mike
Waterfield
for
'red-inking'
the
manuscript;
Bill
Boyle,
Tony
Hunter,
Kathy
Gould
and
Caroline
for
their
guidance.
Simon,M.,
Seraphin,B.
and
Faye,G.
(1986)
EMBO
J.,
5,
2697-2701.
Smith,D.B.
and
Johnson,K.S.
(1988)
Gene,
67,
31-40.
Tempste,P.,
Woo,D.D.-L.,
Teplow,D.B.,
Aebersold,R.,
Hood,L.E.
and
Kent,S.B.H.
(1986)
J.
Chromatogr.,
359,
403-412.
Tung,H.Y.L.
and
Reed,L.J.
(1989)
J.
Biol.
Chem.,
264,
2985-2990.
Vandenheede,J.R.,
Yang,S.-D.,
Goris,J.
and
Merlevede,W.
(1980)
J.
Biol.
Chem.,
255,
11768-11774.
Wong,G.G.
et
al.
(1985)
Science,
228,
810-815.
Woodgett,J.R.
(1989)
Anal.
Biochem.,
180,
237-241.
Woodgett,J.R.
and
Cohen,P.
(1984)
Biochim.
Biophys.
Acta.,
788,
339-347.
Received
on
April
18,
1990;
revised
on
May
11,
1990
Note
added
in
proof
The
nucleotide
sequence
data
reported
here
will
appear
in
the
EMBL,
GenBank
and
DDBJ
Nucleotide
Sequence
Databases
under
the
accession
numbers
X53427
(GSK-3c)
and
X53428
(GSK-3,B).
References
Angel,P.,
Allegretto,E.A.,
Okino,S.T.,
Hattori,K.,
Boyle,W.J.,
Hunter,T.
and
Karin,M.
(1988)
Nature,
332,
166-171.
Bohmann,D.,
Bos,T.J.,
Loliger,C.,
Nagata,L.P.,
Nishimura,T.,
Vogt,P.K.
and
Tjian,R.
(1987)
Science,
238,
1386-1392.
Boyle,W.J.,
Lipsick,J.S.,
Reddy,E.P.
and
Baluda,M.A.
(1983)
Proc.
Natl.
Acad.
Sci.
USA,
80,
2834-2838.
Chirgwin,J.M.,
Przybyla,A.E.,
MacDonald,R.J.
and
Rutter,W.J.
(1979)
Biochemistry,
18,
5294-5299.
Cohen,P.
(1985)
Eur.
J.
Biochem.,
151,
439-448.
Cohen,P.
(1989)
Annu.
Rev.
Biochem.,
58,
453-508.
Cohen,P.,
Parker,P.J.
and
Woodgett,J.R.
(1985)
In
Czech,M.P.
(ed.),
Molecular
Basis
of
Insulin
Action.
Plenum
Press,
New
York,
pp.
213-233.
Embi,N.,
Rylatt,D.B.
and
Cohen,P.
(1980)
Eur.
J.
Biochem.,
107,
519-527.
Feinberg,A.J.
and
Vogelstein,B.
(1983)
Anal.
Biochem.,
132,
6-13.
Hanks,S.K.
(1987)
Proc.
Natl.
Acad.
Sci.
USA,
84,
388-392.
Hanks,S.K.,
Quinn,A.M.
and
Hunter,T.
(1987)
Science,
241,
42-52.
Harlow,E.
and
Lane,D.P.
(1988)
Antibodies:
A
Laboratory
Manual.
Cold
Spring
Harbor
Laboratory
Press,
Cold
Spring
Harbor,
NY.
Hemnmings,B.A.,
Yellowlees,D.,
Kemohan,J.C.
and
Cohen,P.
(1982)
Eur.
J.
Biochem.,
119,
443-451.
Hewick,R.M.,
Hunkapillar,M.W.,
Hood,L.E.
and
Dreyer,W.J.
(1981)
J.
Biol.
Chem.,
256,
7990-7997.
Klempnauer,K.-H.,
Gonda,T.J.
and
Bishop,J.M.
(1982)
Cell,
31,
453-463.
Kozak,M.
(1987)
Nucleic
Acids
Res.,
15,
8125-8131.
Laemmli,U.K.
(1970)
Nature,
227,
680-685.
Lee,M.G.
and
Nurse,P.
(1987)
Nature,
327,
31-35.
Lee,W.,
Mitchell,P.
and
Tjian,R.
(1987)
Cell,
49,
741-752.
Luthman,H.
and
Magnusson,G.
(1983)
Nucleic
Acids
Res.,
11,
1295-
1308.
Melton,D.A.,
Krieg,P.A.,
Rebagliati,M.R.,
Maniatis,T.,
Zinn,K.
and
Green,M.R.
(1984)
Nucleic
Acids
Res.,
12,
7035-7056.
Mitchell,P.J.
and
Tjian,R.
(1989)
Science,
245,
371-378.
Mullis,K.,
Faloona,F.,
Scharf,S.,
Saiki,R.,
Horn,G.
and
Erlich,H.
(1986)
Cold
Spring
Harbor
Symp.
Quant.
Biol.,
51,
263-273.
Ness,S.A.,
Marknell,A.
and
Graf,T.
(1989)
Cell,
59,
1115-1125.
Nishizuka,Y.
(1988)
Nature,
334,
661-665.
Parker,P.J.,
Caudwell,F.B.
and
Cohen,P.
(1983)
Eur.
J.
Biochem.,
130,
227-234.
Parker,P.J.,
Kour,G.,
Marais,R.M.,
Mitchell,F.,
Pears,C.,
Schaap,D.,
Stabel,S.
and
Webster,C.
(1989)
Mol.
Cell.
Endocrinol.,
65,
1-11.
Poulter,L.,
Ang,S.G.,
Gibson,B.W.,
Williams,D.H.,
Holmes,C.F.B.,
Caudwell,F.B.,
Pitcher,J.
and
Cohen,P.
(1988)
Eur.
J.
Biochem.,
175,
497-510.
Rhee,S.G.,
Suh,P.-G.,
Ryu,S.-H.
and
Lee,S.Y.
(1989)
Science,
244,
546-550.
Sambrook,J.,
Fritsch,E.G.
and
Maniatis,T.
(1989)
Molecular
Cloning:
A
Laboratory
Manual.
Cold
Spring
Harbor
Laboratory
Press,
Cold
Spring
Harbor,
NY.
Short,J.M.,
Fernandez,J.M.,
Sorge,J.A.
and
Huse,W.D.
(1988)
Nucleic
Acids
Res.,
16,
7583-7600.
2438
