
3dmoleculardesigns.com
Osmosis Teacher Key - Page 1
© Copyright 2013. All rights reserved.
. . .where molecules become real
TM
Water Kit
©
Osmosis Lesson
Objectives
Students will:
• Dene osmosis as the diffusion of water through a membrane.
• Construct and explain a physical representation of osmosis in hypertonic, hypotonic and isotonic
environments.
• Compare the movement of water molecules through a membrane in hypertonic, hypotonic and
isotonic environments.
• Recognize and account for the necessity of aquaporins in water transport across a membrane.
• Conceptualize the scaling factor for the water molecule models.
• Quantify the relative size of a water molecule in relation to a typical human cheek cell.
Materials
• 1 Water Kit
©
cup per small group
• 1 copy of this packet per person
Osmosis
Living things must perform vital activities in order to maintain their existence including
exchanging gases like CO
2
and O
2
; taking in water, minerals and food, and eliminating wastes. These tasks
occur at the cellular level and require that molecules move through a membrane that surrounds the cell.
The cell membrane is a complex structure that is responsible for separating the contents of the cell from its
surrounding environment and for controlling the movement of materials into and out of the cell.
It is important to understand how water ows in and out of a cell through the membrane as it will
directly impact a cell’s ability to survive. The passive transport of water across a selectively permeable
membrane is called osmosis. The net ow of water is in the direction toward the highest
concentration of solute.

. . .where molecules become real
TM
3dmoleculardesigns.com
Osmosis Teacher Key - Page 2
© Copyright 2013. All rights reserved.
Directions
You will explore osmosis by making models of the hypertonic, hypotonic and isotonic
states of osmosis and predicting the ow of water in each state.
You will use the water molecule and ion models in the Water Kit
©
and the graphic image of a cell on
page 10 to make your models. After exploring each state, you will document your ndings by drawing
your model on the smaller cheek cell image of a cell and answering the questions in the blue boxes.
1. Note that the water molecules and ions are at a different scale than the image of a cell on page
10. Answer the questions below to explain the differences in scale.
Water Kit
©
Osmosis
Questions
1. Based on the size of the water molecule models, how large would the image of the cell
be, if they were at the same scale?
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
2. Explain your process in determining what the size the cell image would be, if it was at
the same scale as the water molecular models.
_____________________________________________________________________
_____________________________________________________________________
3. What source(s) did you use to determine the relative proportion of a water molecule
and a cheek cell?
_____________________________________________________________________
_____________________________________________________________________
4. Are all cells the same size? _______________________________________________
5. What does this imply about your calculations?
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
Answers will vary based on sources used. Average cheek cell = 50 microns. Water
Molecule = .1nm (1x10
-10
m). Model water = 2.7cm (.027m).
0.027/(1x10
-10
) = 2,700,000,000 x’s bigger.
50 microns x 2,700,000,000 = 135,000,000,000 microns (divide by 1,000,000) =
135,000 meters in lengh.
Answers will vary.
No.
The calculations are an approximation for an average sized cell.

. . .where molecules become real
TM
3dmoleculardesigns.com
Osmosis Teacher Key - Page 3
© Copyright 2013. All rights reserved.
Questions
1. Identify the solute. Where is the solute located?_______________________________
2. Water may pass through the membrane but the solute may not. Predict the direction
of the net ow of the water by drawing arrows to indicate this on your diagram.
Explain why the water would ow in this direction.
_____________________________________________________________________
_____________________________________________________________________
3. When water ows in the direction you predicted, what happens to the volume of the cell?
_____________________________________________________________________
_____________________________________________________________________
When the concentration of solutes outside the cell is higher than the concentration of
solutes inside the cell, the net ow of water will be out of the cell. This type of a solution is
referred to as hypertonic.
Hypertonic
Water Kit
©
Osmosis
Cheek Cell Phospholipid Bilayer
2. Place your sodium (Na
+
) and chloride (Cl
-
) ion models on the outside of the cell image
(page 10). Place four water molecules (H
2
O) on the inside of the cell and four water
molecules on the outside of the cell.
In the image below, draw how you placed the molecules and ions on the large image. Use H
2
O to indicate
water, Na to indicate sodium and Cl to indicate chloride. Draw a circle around the solute.
The solute is sodium chloride (Na
+
, Cl
-
).
See diagram for arrow. The highest concentration of solute is outside the cell. Net flow
of water is towards the hightest concentration of solute.
The volume of the cell will decrease and the cell will shrink.
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Na
+
Cl
-
H
2
O

. . .where molecules become real
TM
3dmoleculardesigns.com
Osmosis Teacher Key - Page 4
© Copyright 2013. All rights reserved.
Water Kit
©
Osmosis
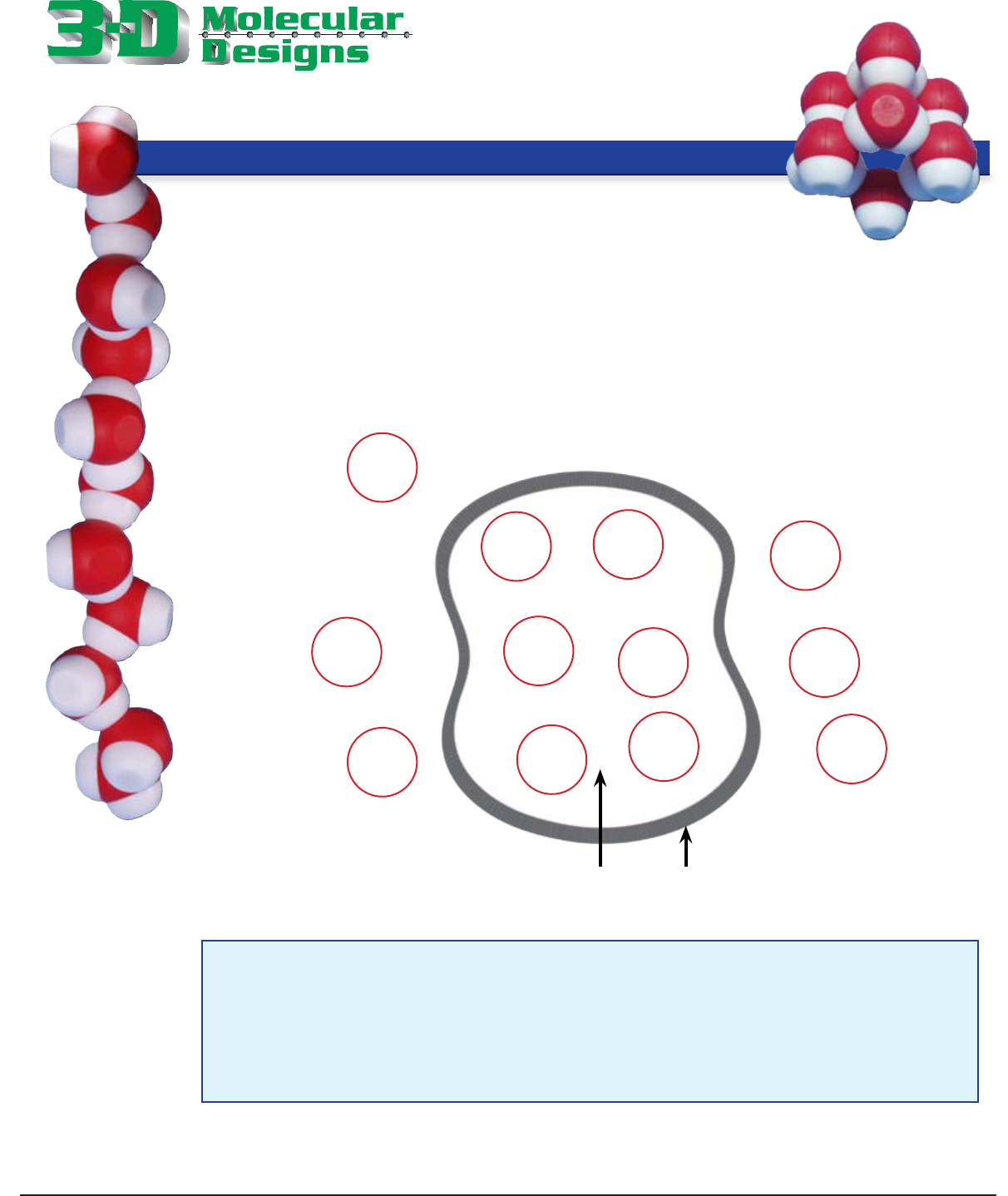
3. Again, using the molecules and image on page 10, set up a physical representation
where the concentration of solutes is higher inside the cell than outside. This type
of solution is referred to as hypotonic.
Sketch your placement of the water and solute molecules in the diagram below. Indicate the net
ow of water in this system.
Hypotonic
Questions
1. Where is the initial concentration of solute molecules higher?
_____________________________________________________________________
2. Predict the direction of the net ow of the water by drawing arrows to indicate
this on your diagram. Explain why the water would ow in this direction.
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
3. What happens to the volume of the cell in this system?
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
Cheek Cell Phospholipid Bilayer
The solute molecules have a higher concentration inside the cell.
See diagram for arrow. Because the highest concentration of solute is inside the cell,
water flows into the cell
The volume of the cell will increase and the cell will expand.
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Na
+
Cl
-
. . .where molecules become real
TM
3dmoleculardesigns.com
Osmosis Teacher Key - Page 5
© Copyright 2013. All rights reserved.
Water Kit
©
Osmosis
Equilibrium
4. Next, with the models, create a model of a system where equilibrium has been reached.
You will have to work with another group in order to use two sodium and chloride models.
Place one Na
+
both inside and outside the cell. Place one Cl
-
both inside and outside of the cell.
Place an equal amount of water molecules inside and outside of the cell. Sketch the placement of
the water and solute molecules in the diagram below. Indicate the direction of the net ow of water.
When the concentration of solutes is equal on either side of the cell membrane, a state of equilibrium
has been reached. Water still continues to ow through the membrane but at an equal rate in and
out of the cell. This type of solution is said to be isotonic.
Questions
1. Explain what happens to the ow of water in an isotonic solution.
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
Questions Continued on Next page
Cheek Cell Phospholipid Bilayer
Water will flow inside and out at an equal rate when equilibrium has been reached.
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Na
+
Na
+
Cl
-
Cl
-

. . .where molecules become real
TM
3dmoleculardesigns.com
Osmosis Teacher Key - Page 6
© Copyright 2013. All rights reserved.
Water Kit
©
Osmosis
Questions
2. Using the vocabulary of osmosis, explain what may happen to the vegetation along the
side of a road when excessive amounts of salt are used during the winter.
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
3. Thinking osmotically, explain why grocery stores spray water on their fresh vegetables.
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
4. Explain what will happen to a blood cell if it is placed in a 1.5% salt solution when
normal blood has a salt concentration of 0.9%. Sketch a model of this system in the
space below.
_____________________________________________________________________
_____________________________________________________________________
The high concentration of salt would create a hypertonic environment for the plant cells
causing the water to flow out of the cells.
The water would flow into the vegetables due to a higher concentration of solutes. The
cells would expand, giving the vegetables a plump look to consumers.
Water wull flow out of the cell in this hypertonic solution to dilute the higher
concentration of the salt outside the cell. The blood cell will shrink in volume.
Normal Isotonic 1.5% Salt Solution
Blood Cell Blood Cell
0.9% Salt
0.9% Salt
1.5% Salt
0.9% Salt

. . .where molecules become real
TM
3dmoleculardesigns.com
Phospholipid Bilayer
Water molecules are small enough to diffuse
across the phospholipid bilayer (left photo), but the middle zone
of the cell membrane (bottom photo) is highly hydrophobic, since
it consists of compact carbon atoms. Given the nature of water,
the hydrophobia of the middle zone impedes the passage of water
across the phosphilipid bilayer.
Discovery of Aquaporin
The movement of the water molecules through cell membranes is too rapid to be explained by unaided
diffusion alone. Transport proteins called aquaporins facilitate the diffusion of water across the cell
membrane. While studying Rh factors in red blood cells, Peter Agre made the serendipitous discovery
of a protein that later became known as aquaporin 1. The 1992 discovery was considered so important
that Agre was awarded the 2003 Noble Prize in Chemistry. To date, 13 variants of aquaporins have
been discovered in humans.
Osmosis Teacher Key - Page 7
© Copyright 2013. All rights reserved.
Aquaporin
This space lled model of a
phospholipid bilayer is printed
on a 3-D ZCorp Printer by 3D
Molecular Designs.
Passage of the water molecules.

. . .where molecules become real
TM
3dmoleculardesigns.com
Osmosis Teacher Key - Page 8
© Copyright 2013. All rights reserved.
Aquaporin
This alpha carbon backbone model of aquaporin is printed on a 3-D ZCorp
Printer by 3D Molecular Designs. It is based on 1J4N.pdb and features the
six alpha helices and two half-alpha helices of the structure and the two
asparagine involved in selectively moving water through the channel. From
this perspective you can see portions of the six alpha helices (red, orange,
dark green, light green, blue and yellow), two half-alpha helices (magenta and
purple) and one of the two asparagines.
Asparagine
Side Chain
Color Key
oxygen
nitrogen
carbon
Aquaporin Structure
Aquaporin consists of six alpha helices and two
half-alpha helices.
Two asparagine (ASN) amino acids – ASN 78 and ASN
194 – are found at the turns of the two half alpha helices
(colored magenta and purple in the photo). These are
located at the narrowest part of the hour-glass shaped
channel and form the lter that allows water to pass
through aquaporin.
Asparagine

. . .where molecules become real
TM
3dmoleculardesigns.com
Function
Water molecules rapidly ow in single le through the aquaporin channel. The ability
of aquaporin to selectively bind water molecules and prevent other molecules from
entering the channel is referred to as the aromatic /arginine selectivity lter.
While the process is not fully understood, many researchers
1
believe that water molecules
roll over as they reach the narrowest part of the channel, where the arginine are located.
In computer simulations the oxygen (red) atom of each
water molecule points down as it moves through the
channel toward the two asparagine. To pass through the
narrow opening each water molecule binds rst to one
asparagine and then to the second. In this process each
water molecule rolls over so that the oxygen points up
toward the asparagine — now from the opposite side of the
passageway — and passes through the remaining portion
of the channel. (See illustration right.)
Note: Water molecules form hydrogen bonds with
asparagine. The partially negative oxygen atom forms a
hydrogen bond with the partially positive nitrogen (blue)
atom of the asparagine amino acid.
Most of the amino acids in the aquaporin channel are
hydrophobic, which enables water molecules to move
freely within the channel until binding with asparagine.
For an animation and explanation from the National
Institutes of Health (NIH) Center for Macromolecular
Modeling & Bioinformatics and the University of Illinois at
Urbana-Champaign, go to http://www.ks.uiuc.edu/Gallery/
Movies/aquaporin-movie-explanation.html
Osmosis Teacher Key - Page 9
© Copyright 2013. All rights reserved.
Aquaporin
1
Tajkhorshid E, Nollert P, Jensen MØ, Miercke LJ, O’Connell J, Stroud RM, Schulten K (2002). “Control of the selectivity of the aquaporin
water channel family by global orientational tuning”. Science 296 (5567): 525–30. doi:10.1126/science.1067778. PMID 11964478.
Water Channel
Questions
1. What factors may inuence the passage of water through a membrane?
___________________________________________________________________
___________________________________________________________________
2. Water is reabsorbed in the cells of the kidneys. What would happen to the rate of diffusion
of water if the number of aquaporin protiens decreased? Explain your answer
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
Answers may include the type of solution (hypotonic, hypertonic, isotonic) into which
the cell is placed and the number of aquaporin protiens present in the cell membrane.
The rate would greatly decrease as the water would have difficulty passing though
the lipid bilayer of the cell.

Osmosis Teacher Key - Page 10
© Copyright 2013. All rights reserved.
. . .where molecules become real
TM
Cheek Cell Phospholipid Bilayer

3dmoleculardesigns.com
Osmosis Teacher Key - Page 11
© Copyright 2013. All rights reserved.
. . .where molecules become real
TM
National Framework
Connections to: A Framework for K-12 Science Education
Practices, Crosscutting Concepts, and Core Ideas*
*The NSTA Reader’s Guide to A Framework for K-12 Science Education, National Research Council (NRC), 2011. A Framework for K-12
Science Education: Practices, Crosscutting Concepts, and Core Ideas. Washington, D.C.: National Academies Press.
Dimension 1: Scientic and Engineering Practices
2. Developing and Using Models
6. Constructing Explanations and Designing Solutions
Dimension 2: Cross Cutting Concepts
1. Patterns
3. Scale, Proportion and Quantity
4. Systems and System Models
5. Energy and Matter: Flows, Cycles, and Conservation
6. Structure and Function
7. Stability and Change
Dimension 3: Disciplinary Core Ideas
Physical Sciences
HS-PS1 Matter and Its Interactions
HS-PS1-2. Construct and revise an explanation for the outcome of a simple chemical
reaction based on the outermost electron states of atoms, trends in the periodic table,
and knowledge of the patterns of chemical properties.
Life Sciences
HS-LS1 From Molecules to Organisms: Structures and Processes
HS-LS1-2. Develop and use a model to illustrate the hierarchical organization of
interacting systems that provide specic functions within multicellular organisms.
