
PHD PROGRAMS
Student and Faculty
Handbook
LEADERSHIP & STAFF
Michael H. Overholtzer, Ph.D., Dean
Thomas G. Magaldi, Ph.D., Associate Dean
Julie Nadel, Ph.D., Assistant Dean
David L. McDonagh, Senior Registrar/Curriculum Specialist
Stacy De La Cruz, Program Coordinator
Yuliya Masen, Senior Project Coordinator
Oluwakemi Y. Adeyemo, Administrative Data Coordinator
Stacey Lara, Administrative Assistant
REVISED June 2024
The Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer Center,
is accredited by the New England Commission of Higher Education (formerly the Commission on Institutions of
Higher Education of the New England Association of Schools and Colleges, Inc.).
Inquiries regarding the accreditation status by the Commission should be directed to the administrative staff of
the institution. Individuals may also contact: New England Commission of Higher Education 3 Burlington Woods
Drive, Suite 100, Burlington, MA 01803-4514 Phone: (781) 425 7785 E-Mail: [email protected]

GSK Student & Faculty Handbook
v. 6/2024 2
Table of Contents
Introduction..................................................................................................................... 4
The Cancer Biology and Engineering P h. D. Programs .......................................................... 5
Admissions ...................................................................................................................... 6
Notice of Non-Discrimination............................................................................................. 6
Transfer of Credit ............................................................................................................ 7
GSK Transfer Policy ........................................................................................................ 7
Financial Support ............................................................................................................. 8
Stipend and Tuition ......................................................................................................... 8
Supplemental Stipend Support .......................................................................................... 8
Applying for External Fellowships ................................................................................... 8
Useful Resources at MSK ............................................................................................. 8
Common External Fellowships ....................................................................................... 9
Travel Allowance to Attend Scientific Conferences ..............................................................10
Reimbursement for Special Courses .................................................................................10
The Academic Program....................................................................................................12
General Program Requirements .......................................................................................12
Laboratory Rotation........................................................................................................12
Choice of Thesis Mentor .................................................................................................13
Change of Thesis Mentor .............................................................................................13
Seminars and Journal Clubs ............................................................................................13
Special Courses ............................................................................................................14
Observing in the Clinic ....................................................................................................14
Clinical Apprenticeship....................................................................................................14
Advancement to Candidacy .............................................................................................14
Course Descriptions .......................................................................................................14
GSK Cancer Biology and Cancer Engineering Core Courses .............................................14
Logic and Critical Analysis (Cancer Biology and Engineering Students)...............................17
Responsible Conduct of Research (RCR) (Cancer Biology and Engineering Students) ..........17
President's Research Seminar Series Journal Club (Cancer Biology and Engineering Students) .... 18
Chalk Talks (Cancer Biology and Engineering Students) ...................................................18
Graduate Student Seminar (Cancer Biology and Engineering Students) ..............................18
Current Topics Journal Club (Cancer Biology and Engineering Students) ............................18
Opportunity to Apply for a Teaching Fellowship ..................................................................19
Progress Points ................................................................................................................20
General Program Requirements .......................................................................................20
Typical Cancer Biology First Year ..................................................................................20
Typical Cancer Engineering First Year ...........................................................................21
Typical Program Schedule for All PhD Students ..............................................................21
Thesis Proposal Examination...........................................................................................22
Registering for the TPE and key deadlines:.....................................................................22
Evaluation of the Written Proposal: ................................................................................23
Evaluation of the Oral Examination: ...............................................................................24
Deadline: ...................................................................................................................25
Guidelines for preparing the TPE Specific Aims page. ......................................................26
Guidelines for preparing the written proposal. ..................................................................26
Dissertation...................................................................................................................28
The Written Document .................................................................................................29
The Committee ...........................................................................................................29
The Defense and Seminar............................................................................................30
Dissertation Deposit ....................................................................................................30
Degree Conferral ........................................................................................................30
Academic Policies and Procedures .....................................................................................31
Grading, Course Examinations.........................................................................................31
GPA.............................................................................................................................31
Advisory Mechanisms.....................................................................................................32

GSK Student & Faculty Handbook
v. 6/2024 3
Satisfactory Progress .....................................................................................................33
Student Publications and Citation of Student Affiliation ........................................................34
Academic Dis hones ty , Plagiarism and Artificial Intelligence ...................................................34
Grievance Policy............................................................................................................34
Appeals Policy ...............................................................................................................34
Core Course Attendance Policy........................................................................................35
Vacation Policy ..............................................................................................................35
Parental Leave ..............................................................................................................35
Leave of A bs enc e and Withdrawal ....................................................................................35
Involuntary Student Leave ...............................................................................................36
Request for Re-enrollment ..............................................................................................36
Policy for Return from Medical Leave ................................................................................37
Maximum Time for Completion of All Degree Requirements .................................................37
Student Services ..............................................................................................................38
Career and Professional Development ..............................................................................38
Internship and Externship Policies ....................................................................................39
Other Facilities ..............................................................................................................39
Student Benefits...............................................................................................................41
Medical Insurance ..........................................................................................................41
Mental Health ................................................................................................................41
Short-Term Counseling (up to six sessions for each resource) ...........................................41
Long-Term Counseling and/or Psychiatric Prescription Management, if needed....................41
Dental Insurance ...........................................................................................................42
Vision Coverage ............................................................................................................42
Housing and Cost of Living ..............................................................................................42
The GSK and MSK Hardship Fund ...................................................................................42
Student Progress and Outcomes ........................................................................................44
Compliance Policies .........................................................................................................45
Memorial Sloan Kettering Cancer Center Code of Conduct ...................................................45
Endorsement Policy .......................................................................................................45
Special Considerations for GSK Students Working on Conflicted Faculty/Mentor Research................. 45
Prohibition on the Marketing of Credit Cards ......................................................................46
Policy Against Harassment and Discrimination ...................................................................46
S c ope .......................................................................................................................46
Introduction ................................................................................................................47
Policy ........................................................................................................................47
Definitions:.................................................................................................................47
Retaliation .................................................................................................................48
Confidentiality ............................................................................................................48
Complaint and Investigation Procedures.........................................................................48
Bystander Intervention .................................................................................................49
Anti-Discrimination, Harassment and Retaliation Training..................................................50
Policy f or the P rev ention of and Res pons e to Sexual Misconduct ..........................................51
Title IX Coordinators. ..............................................................................................54
Amendments to the Educational Law .................................................................................67
Campus Security Rules and Regulations ...........................................................................68
Drug-Free Schools And Communities Act (DFSCA) Substance Abuse Policy ..........................70
Family Education Ri ght s and Priv acy Act
(FERPA)...............................................................77
Student Resources ...........................................................................................................80
Mental Health Resources Contact Information ....................................................................81
Short-Term Counseling ................................................................................................81
Long-Term Counseling ................................................................................................81
MSK Professional and Mentoring Resources...................................................................82
Other Resources:........................................................................................................82
Our F acult y ....................................................................................................................84
Our Special Contributing Faculty ......................................................................................89
Appendix 1: GSK Student Tax Treatment.........................................................................90

GSK Student & Faculty Handbook
v. 6/2024 4
Introduction
The Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan
Kettering Cancer Center (GSK) is the degree-granting arm of Memorial Sloan Kettering
Cancer Center (MSK). Our website can be found here.
In addition to GSK, MSK is comprised of the Memorial Hospital for Cancer and Allied
Diseases (MH), the clinical treatment arm of MSK that houses patient-oriented research,
and the Sloan Kettering Institute for Cancer Research (SKI), which houses most of the
laboratory research programs of MSK.
The mission of GSK is to advance the frontiers of knowledge by providing to gifted and
creative students in an interactive, innovative, and collegial environment the education and
training they need to make new discoveries in the biological sciences.
GSK strives to recruit a diverse student population and to support the development of
scientists from underrepresented backgrounds in our community. Equity, diversity and
inclusion, and the recruitment and support of underrepresented students, are a central
focus of our mission to educate and train the next generation of leaders in the biomedical
sciences.
The graduate school is housed in the Zuckerman Research Center (ZRC) located at 417
68th Street, Manhattan, on the Upper East Side in New York City. The administrative offices
are located on the sixth floor, ZRC-669 adjacent to the GSK Student Center that includes a
state-of-the-art classroom, study space for journal clubs, discussion groups, and student
meetings.
The School
GSK was chartered by the New York State Board of Regents on September 10, 2004.
Absolute Charter was granted on March 18, 2008 and initial accreditation was granted in
May 2008 and renewed in March 2015. In January 2023, GSK became accredited by the
New England Commission of Higher Education and relinquished its accreditation with New
York State. GSK has matriculated eighteen classes of graduate students since July 2006.
GSK alumni now include over 110 graduates holding the Ph.D. degree. GSK grants only
graduate degrees, there are no undergraduates enrolled. The Board of Trustees is chaired
by Alan D. Schnitzer, JD, the President is Selwyn M. Vickers, MD, the Provost is Joan
Massaguè, Ph.D., the Dean is Michael H. Overholtzer, Ph.D., the Associate Dean is
Thomas G. Magaldi, Ph.D. and the Assistant Dean is Julie Nadel, Ph.D.
In addition to offering PhD programs in Cancer Biology and Cancer Engineering, GSK also
offers a Master of Science (M.S.) degree program in Clinical and Translational Cancer
Research (CTCR) for MSK appointed physicians. The handbook and information regarding
this program are presented in a separate document.
There are currently 173 f ull-time faculty, all of whom hold either a Ph.D. or M.D. degree, or
both. These faculty serve in the Cancer Biology PhD, Cancer Engineering, PhD, and/or
CTCR MS programs.

GSK Student & Faculty Handbook
v. 6/2024 5
The Cancer Biology and Engineering Ph.D. Programs
Our students are an integral part of our research effort and receive the most significant part
of their doctoral training in the student-mentor relationship leading to the Ph.D. dissertation.
Prior to the full-time research focus of their training, students participate in a year-long
course, special seminars, journal clubs, and an exploration of the faculty through up to three
laboratory rotations and organized meetings during the first year.
All students enter the program without formal commitment to a particular laboratory.
Students may change their initial areas of interests as they participate in the first-y ear
curriculum and learn about new areas of research through meetings with the faculty,
laboratory rotations, seminars, and journal clubs. During the first year, each student
consults with the Deans and a First Year Mentor (FYM) who provide advice and guidance
during the intense coursework, as well as assist with the selection of laboratory rotation
mentors. Following the end of the spring semester in June, students are expected to
formally choose a research mentor and begin full-time dissertation research in July of their
second year. Students who decide to join one of their first two rotation laboratories may be
exempted from the third rotation upon approval by the Dean.
All students must be enrolled full-time from matriculation to graduation. All students are
required to develop a research project, under the supervision of one or more mentors who
are members of the GSK faculty that will result in a dissertation that reports new findings
and is presented, defended and approved before the faculty. All students must successfully
complete all other degree requirements that are part of the training program before the
degree is awarded. The maximum time limit for the completion of all requirements for the
Ph.D. degree is six years.
The Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer
Center, a non-profit and private graduate school, is accredited by the New England Commission of Higher
Education (formerly the Commission on Institutions of Higher Education of the New England Association
of Schools and Colleges, Inc.).
Inquiries regarding the accreditation status by the Commission should be directed to the administrative
staff of the institution. Individuals may also contact New England Commission of Higher Education:
• Address: 3 Burlington Woods Drive, Suite 100, Burlington, MA 01803-4514
• Phone: (781) 425-7785
• Email: info@neche.org
Cancer Biology and Cancer Engineering are approved by New York State Education Department as
official programs offered by GSK. These programs are both registered as biological science degrees
under HEGIS code: 0499.00 (biological sciences, other) and are listed on the Department’s Inventory of
Registered Programs.
The school can award five graduate degrees:
• Master of Science (MS) in Cancer Biology: Program code 29204
• Master of Science (MS) in Cancer Engineering: Program code 42788*
• Master of Science (MS) in Clinical and Translational Cancer Research: Program code 40200
• Doctor of Philosophy (PhD) in Cancer Biology: Program code 29205
• Doctor of Philosophy (PhD) in Cancer Engineering: Program code 42789*
*The Cancer Engineering degrees are not registered under engineering and will not lead to a
professional license.

GSK Student & Faculty Handbook
v. 6/2024 6
Admissions
Students who wish to pursue a Ph.D. in Cancer Biology or Cancer Engineering at GSK
and who have, or will have, completed a baccalaureate program at an accredited institution,
should submit all components of an online application for consideration for admission to
GSK by the deadline. Letters from research mentors and written comments from the
applicant about prior research are major components of the admissions file.
In most cases, successful applicants to the Cancer Biology program will have completed
the following courses: general biology, general chemistry, general physics, organic
chemistry (2 semesters), mathematics (through integral calculus), introductory
biochemistry, and additional advanced science and math courses. Significant basic science
research experience is essential.
Successful applicants to the Cancer Engineering program will have completed the following
courses: general biology, general chemistry, general physics (two semesters), mathematics
(four semesters, including calculus), and extensive upper division coursework in either
engineering, physics, or computer science. Coursework in linear algebra and differential
equations are strongly encouraged. Significant science and engineering research
experience is essential.
The deadline for receipt of completed applications is December 1, but early submission is
highly recommended. As part of the application form, the applicant must include a personal
statement, official transcripts from all institutions attended, and three letters of
recommendation. Final transcripts may be requested upon completion of programs to which
a student is currently enrolled. The submission of GRE (Graduate Record Examinations)
scores (verbal, quantitative and analytical writing) is optional, and is not required to apply
to the school. Applicants for whom English is not the first language must submit official
scores of the TOEFL (Test of English as a Foreign Language). This requirement is
automatically waived for applicants who earned their undergraduate or graduate degrees
in either the United States or a United States Territory, and can be waived upon request if
degrees were earned in a country where English is the official language or at an institution
where the primary language of instruction is English.
Applicants who, on the basis of their submitted application materials, are being seriously
considered for acceptance will be invited for interviews. The GSK Admissions Committees
will consider all data on each applicant before making its decision.
N.B. Upon matriculation, students must remain enrolled in the program (Cancer Biology or
Cancer Engineering) to which they were accepted.
Notice of Non-Discrimination
In accordance with institutional policy and in compliance with the requirements of the Civil
Rights Act, the Education Amendments, the Rehabilitation Act, the Age Discrimination Act,
and the Americans with Disabilities Act, Gerstner Sloan Kettering Graduate School of
Biomedical Sciences (GSK) does not discriminate on the basis of age, gender identity,
gender expression, race, color, creed, religion, national origin, disability, veteran status,
marital status, sex, sexual orientation, citizenship status or any other status protected by
law in the administration of its educational policies, admission policies, employment or any
other GSK program or activity.

GSK Student & Faculty Handbook
v. 6/2024 7
Any inquiries regarding the non-discrimination policies can be addressed to GSK’s Title IX
Coordinators:
• Leslie Ballantyne, Esq., MSK HR Legal & Regulatory Affairs (tel: 646-227-
2742; ballantl@mskcc.org
)
• Lindsay Cornacchia, Esq., Associate Director of Compliance & Assistant General
Counsel (Tel: (212) 639-2496; cornaccl@mskcc.org
)
For further information on notice of non-discrimination, you can contact the appropriate
federal office by visiting the website
for the address and phone number of the office that
serves your area, or call (800) 421-3481.
Transfer of Credit
Matriculated students can apply for transfer of credit. Supporting materials including
complete syllabus, reading materials, and exams should be submitted to the registrar with
a cover letter detailing the request. Such requests will be reviewed by the curriculum
committee for content, topic coverage, and comparison to the Gerstner Sloan Kettering
Graduate School curriculum.
GSK Transfer Policy
GSK does not accept transfer students. Any student who would like to enroll in GSK must
first apply and be accepted into the program under the normal PhD recruitment and
application cycle.

GSK Student & Faculty Handbook
v. 6/2024 8
Financial Support
Stipend and Tuition
All matriculated students are offered a fellowship package consisting of a stipend, the full
cost of tuition, and a comprehensive health insurance plan including medical, dental, and
vision benefits. Insurance benefits also cover students’ spouses, domestic partners and
dependents. Student progress is evaluated each semester and continuation of the
fellowship is contingent upon satisfactory progress (see section on Satisfactory Progress)
in the program. The school reserves the right to terminate the fellowship in the absence of
satisfactory progress. The annual stipend rate for the 2023-24 academic year is $48,600.
Federal and state taxes are not withheld from student stipends in accordance with relevant
tax laws, but students are responsible for reporting and paying taxes on their stipends as
these are taxable income. If a spouse, domestic partner, and/or dependent child is added
to your insurance plan, the value of this additional coverage is also considered taxable as
imputed income (see student tax regulations in Appendix 1
).
Supplemental Stipend Support
Students are encouraged to apply for external fellowships. All students who are awarded
external individual fellowships above the amount of $25,000 per year are provided with a
supplement of $5,000 per year that is added to the standard annual stipend amount for
the duration of the award. GSK or MSK fellowships awarded to students are not
considered external fellowships.
N.B. There is only one stipend bonus per year for external fellowship(s).
Applying for External Fellowships
Students who intend to apply for fellowships should:
1) Contact MSK’s Grants and Contracts team at fdt@mskc.org
. This team can
help identify appropriate fellowship opportunities, ensure that you are eligible for
fellowships you have identified on your own, provide proposal tips and
strategies, and offer proposal review services.
2) Once students have identified a fellowship that they intend to target, contact
Alana Zbaren , GSK’s Grants Management Specialist, at zbarena@mskcc.org
.
The Grants Management Specialist can review budgets and obtain institutional
approval. In some cases, the GMS must submit the application on your behalf.
3) Contact GSK Associate Dean who can help with any necessary letters of
support from the Graduate School.
4) Students who receive a grant or award should notify the Graduate School and
the Grants Management Specialist immediately.
Useful Resources at MSK
For tips on developing fellowship proposals as well as successful examples, please visit
the Grants and Contracts Proposal Development Page.
The Office of Scientific Training and Education also provides additional tips on searching
for fellowships. https://education.mskcc.org/OSET/Home/Fellowship
GSK Student & Faculty Handbook
v. 6/2024 9
Common External Fellowships
US Citizens and Permanent Residents Only
NIH (NRSA) for Individual Pre-Doctoral and MD/PhD Fellowships (F30 and F31)
https://www.cancer.gov/grants-training/training/funding/f31
https://www.cancer.gov/grants-training/training/funding/f30
NSF Graduate Research Fellowship
www.nsfgrfp.org
Department of Defense (DoD) National Defense Science and Engineering Graduate
Fellowship
ndsegfellowships.org
Department of Energy (DoE) Computational Science Graduate Fellowship
krellinst.org/csgf
The PhRMA Foundation Predoctoral Fellowship in Pharmacology/Toxicology and
Informatics
phrmafoundation.org/awards
The Hertz Foundation Graduate Fellowship
hertzfoundation.org
For All Students
The NCI Predoctoral to Postdoctoral Fellow Transition Award (F99/K00) (Must be
nominated by GSK)
cancer.gov/grants-training/training/funding/f99
Thermo Fisher Scientific Antibody Scholarship Program
goo.gl/9N2Wf7
For Underrepresented Minority Students
NRSA (F31) to Promote Diversity in Health-Related Research
researchtraining.nih.gov/programs/fellowships/F31
Ford Foundation Pre-Doctoral Fellowship
nationalacademies.org/ford
For International Students
Boehringer Ingelheim Fonds PhD Fellowship (European Citizens)
bifonds.de
American Association of University Women (AAUW) International Pre-Doctoral Fellowship
aauw.org/

GSK Student & Faculty Handbook
v. 6/2024 10
Travel Allowance to Attend Scientific Conferences
Each GSK student is eligible for one award per academic year (July 1 to June 30) of up to
$1,200 to cover fees and travel to attend a scientific conference at which the student is
either giving a talk or presenting a poster. The award is only applicable for travel that occurs
while the student is matriculated and in good academic standing in the GSK. In advance of
travel, students must complete the Travel Award Application located on the SIS student
portal under Forms and have it signed by their Thesis Mentor and upload their abstract in
in SIS. Copies of the signed form and abstract must be submitted to the GSK office for
approval by the Associate Dean prior to the meeting. Students must subsequently submit
proof of the poster/talk presentation at the time that reimbursement is requested. Travel
funds will not be given in advance of the meeting. Students are required to access Egencia
to book, air/rail, travel, hotel and ground transportation. Students must register to use
Egencia
. Questions regarding travel can be addressed to GSK’s Administrative Data
Coordinator.
Travel Checklist:
1. Complete the Graduate Student Travel Award Application in SIS before
your trip. (MSK requires 1 month for domestic travel and 2 months
minimum for international travel).
2. Should you expect total expenses to be over $1,200, you must provide
your mentor’s Cost Center/Fund number that will cover the additional
expense charges.
3. Submit original proof of purchase for all items (registration and/or fees,
an abstract or picture of the poster; flight, hotel, etc.). Remember to book
travel and hotel using Egencia as per MSK policy. NOTE: MSK will not
reimburse for AIRBNB reservations.
4. Submit receipts for reimbursement within two weeks of return. All
receipts must be original and itemized for food. MSK will not reimburse
for any alcoholic beverages purchased.
Reimbursement for Special Courses
Academic courses: The Graduate School will cover the cost of tuition for one special course
per student offered outside of GSK that the mentor approves in advance. A letter should be
submitted to the Associate Dean from the student’s mentor stating why the course is
required to provide foundation knowledge for the student's dissertation project. A detailed
invoice and receipt of payment showing the cost of tuition, as well as proof of course
completion (in the form of a certificate) are required for reimbursement. GSK will only pay
for the cost of tuition and it will be the responsibility of the student to ensure any additional
fees or payments to the institution are made. Students may request advance payment for
course tuition to be paid directly to the institution where the course is offered. Proof of
course completion is required. Additional courses can be taken outside of GSK upon special
request and approval by the Dean. Note that tuition costs covered by GSK for outside
courses are considered taxable income for GSK students. Please see Appendix 1
f or a
summary of relevant tax regulations.

GSK Student & Faculty Handbook
v. 6/2024 11
Professional and career development courses: Students in years four and above are eligible
to request reimbursement of up to $750 per year for registration fees or tuition to attend
professional/career development course(s) approved in advance. Students must submit a
full course description in advance of taking the course for approval to the Associate Dean.
If the course is approved, students must submit a detailed invoice and receipt of payment
along with proof of course completion (in the form of either a certificate or signed letter from
the instructor) for reimbursement. Reimbursement requests must be made in writing no later
than thirty days after completion of the course. Reimbursement is limited to a one-t ime
award of $750 and can only be made for the cost of tuition or registration fees. Travel and
other associated expenses to attend the course will not be reimbursed. Reimbursed
registration fees or tuition for professional and career development courses are also
considered taxable income for GSK students (see Appendix 1
).

GSK Student & Faculty Handbook
v. 6/2024 12
The Academic Program
General Program Requirements
All students must register for both fall and spring terms each year and are expected to fulfill
the following requirements for the Ph.D. degree:
• Core Cancer Biology Course
Students enrolled in the Cancer Biology program complete this year-long
course during the first year of study. The core course consists of four sections:
Experimental Biology, Mechanistic Biology I and II, and Cancer Biology.
• Core Cancer Engineering Course
Students enrolled in the Cancer Engineering program complete this year-long
course during the first year of study. The core course consists of five sections:
Experimental Biology including an additional week of advanced statistics,
Cancer Engineering, Entrepreneurship, Immunology, and Cancer Biology.
• Laboratory Rotation
All students enroll in three laboratory rotations in the first year prior to selecting their
thesis laboratory. Students may join their thesis laboratory after the second rotation
and be exempted from the third rotation upon approval by the Dean.
• Logic and Critical Analysis
All students complete this five-week course at the beginning of the first year.
• Responsible Conduct of Research (RCR)
All students complete this course in the first and fifth years.
• President's Research Seminar Series Journal Club
All students complete two semesters of this course during the first year.
• Graduate Student Seminar
All students in years three and four complete this course by presenting research
seminars, and all students from years one through four are required to attend.
• Chalk Talks
All first-year students register for this course and students in year two complete this
course by presenting research chalk talks.
• Current Topics Journal Club
All students complete this course each semester beginning in their second year and
continuing through their fifth year of study.
• Thesis Proposal
All students are expected to present and defend their thesis proposal by April 15 of
their second year in the Program.
• Dissertation Defense
All students are expected to defend their dissertation by the end of the sixth year in
the Program.
N.B. - Late registration or failure to register may result in suspension of stipend.
Laboratory Rotation
Laboratory rotations are an important part of the first-year curriculum of the graduate
program at GSK. The rotations provide students with the opportunity to experience different
research projects, as well as different laboratory and mentoring styles. In addition, the
rotations provide the faculty with the opportunity to assess the interests and aptitude of the
students. There are three, five-week laboratory rotations that are typically scheduled during

GSK Student & Faculty Handbook
v. 6/2024 13
the months of August, January, and June. Students choose their rotations by directly
contacting faculty of interest and may consult with the Dean and the First Year Mentors.
Once a rotation is agreed upon by a student and faculty member, students must register for
the rotation in SIS. After each rotation, students prepare and submit a two-page written
summary, and student performance in the rotation is assessed by written evaluation by the
rotation mentor. Rotation mentors must also sign the cover page that is attached to the
student summary. Following each rotation, all first-year students also present a short (10
minute) power point-based presentation based on their rotation project in a seminar for all
GSK students and their rotation mentors. Students and rotation mentors should also meet
separately to discuss the rotation experience.
Students are required to complete at least the first two rotations before they can declare a
research mentor. Students wishing to join their thesis laboratory after the second rotation
can do so upon approval by the Dean and be exempted from the third rotation.
Choice of Thesis Mentor
The choice of a thesis mentor is a major decision for each student at the end of the f irst
year of the program. Students are urged to take full advantage of their rotation
experiences and the guidance of the Deans and First Year Mentors. First year students
also have the opportunity to meet the f aculty in special sessions held throughout the f all.
Rotation mentors are urged to present a realistic state of funding for their laboratory, the
nature of the ongoing projects, how work is assigned or monitored, laboratory policies
(such as publication/authorship, laboratory journal clubs and attendance at conferences),
and the extent of direct contact to be expected with the mentor.
Each student should complete a Thesis Mentor Declaration Form (found in Student
Information System) at the end of the first year in the program after they have been
approved for promotion to the second year by the Curriculum Committee. In addition, each
student, in consultation with their thesis mentor, selects a two- or three-member Advisory
Committee from the GSK faculty who will be most helpful with their dissertation project.
Students are expected to declare their mentor at the end of the first year in the
program, and their Advisory Committee by October 31 of their second year.
Change of Thesis Mentor
In some instances, a student may contemplate a change in mentor. Students who are
thinking about such a change must contact the Deans to discuss the issue and get
approval to proceed. The student must also discuss this issue fully with the current
mentor before approaching other faculty members. Students are urged to discuss such a
change with the members of their Advisory Committee as well to seek their advice for a
new thesis mentor. Upon approval by the Dean, students may have the opportunity to rotate
in a new lab, for up to five weeks, before formally declaring a new mentor. It is the
student's responsibility to explicitly discuss this change of advisor with the new thesis
mentor.
S
eminars and Journal Clubs
Seminars and journal clubs are central components of the educational mission of the
program. It is important that students take advantage of the many opportunities to regularly
meet scientists and build critical and presentation skills. All students are expected to
participate in seminar and journal club activities each semester that they are matriculated
in the program. (See General_Program_Requirements
for more information.)

GSK Student & Faculty Handbook
v. 6/2024 14
Special Courses
GSK will cover the cost of tuition for a special course that the mentor certifies, in a letter to
the graduate school, is required to provide foundation knowledge for a student's
dissertation project. (See Reimbursement for Special Courses
for more information.)
Observing in the Clinic
An important aspect of integrating basic and clinical sciences is to develop an appreciation
for the human side of disease (cancer in this case), to observe medical challenges faced
by clinical practitioners, and to understand the gap between a good idea and its execution.
During the first year in the program, under the supervision of a clinician, students visit
various MSK clinics as observers. These visits, coordinated by the GSK Registrar, are held
in December and May of the first year in the Program. An orientation to the clinics is held
prior to the start of the clinic visits.
Clinical Apprenticeship
Students are encouraged to develop a clinical perspective as to how bench work can be
applied in the clinic. At the end of the second year in the program and in consultation with
the Thesis Committee and physician-scientist members of the GSK faculty, students can
select a clinical mentor after consulting with the GSK clinical faculty representing the
curriculum committee. The individual choice is guided by the student's thesis project.
Clinical Apprenticeships typically span two years of a student’s study at GSK. The clinical
mentor will guide the student in hospital-based academic activities such as Grand Rounds,
Pathology Conference and Disease Management Team (DMT) Conferences.
Upon being advanced to candidacy, students are encouraged to meet individually with a
designated member of the GSK faculty to discuss their research interests and the decision
of whether to commit to a Clinical Apprenticeship. Although students are encouraged to
pursue this apprenticeship, it is an optional part of the program. Students who choose a
clinical mentor will input the name into SIS and arrange to meet with the Associate Dean to
review the guidelines.
Advancement to Candidacy
Students will be advanced to candidacy after successful completion of the thesis proposal
examination. Students must successfully complete both the written and oral parts before
they are advanced to candidacy.
Course Descriptions
•
GSK Cancer Biology and Engineering Core Courses
•
Logic and Critical Analysis
•
Responsible Conduct of Research (RCR)
•
President's Research Seminar Series Journal Club
•
Chalk Talks
•
Graduate Student Seminar
•
Current Topics Journal Club
GSK Cancer Biology and Cancer Engineering Core Courses
GSK students take one “core” course in the first year. Some courses are shared by the
Biology and Engineering students: Experimental Biology and Cancer Biology. Others are
taken by the just Biology Students (Mechanistic Biology I and II) or the Engineering Students

GSK Student & Faculty Handbook
v. 6/2024 15
(Cancer Engineering, Immunology, and Entrepreneurship)
Experimental Biology (Cancer Biology and Engineering Students) teaches conceptual
and practical aspects of five different research disciplines: imaging, genetics, biochemistry,
genomics, and quantitative biology. Each topic is considered for one week through a
combination of workshops, research paper discussions, and lectures. Questions that are
considered include:
- How is imaging performed at different length scales, and what can be learned
through different techniques?
- How have imaging technologies pushed the boundaries of knowledge?
- How are genetic principles and applied technologies used to make new discoveries?
- What techniques allow for the experimental manipulation of DNA, RNA, and protein,
and how do they work?
- How do the “kits” on my research bench actually work?
- How can I think quantitatively about different approaches and data sets?
After completing Experimental Biology, Cancer Engineering students will take an additional
week of advanced statistics.
Mechanistic Biology I and II (Cancer Biology Students) teach what is understood about
how cells are constructed and maintained, how groups of cells collaborate to achieve
normal development, and how the immune system works. In this class a research paper is
dissected every day with one of our GSK faculty members who is at the cutting edge of their
research field. Over 15 weeks the class will consider:
- Genome biology, gene expression, and proteins
- Cellular architecture: from the cytoskeleton to organelles
- Cell cycle control, cell division, and cell death
- Cell signaling
- Stem cells and pluripotency
- Tissue and organismal development
- Innate and adaptive immunity
Cancer Engineering (Cancer Engineering Students)
Cancer Engineering provides students with a foundation in engineering principles that can
be used to solve challenges in cancer biology and oncology. The curriculum includes 9
weeks of classes organized into three sections: Molecular and Nanoengineering; Cancer
Imaging; and Genetic Engineering.
• Foundations and Pharmacology. This three-week section starts with a focus on the
basic principles of pharmacology. It then teaches basic molecular, biomolecular, and
nanoengineering methods needed for success in a research lab, including: drug
delivery, nanomaterials, instrumentation, and tissue engineering. The course will
focus on molecular and nanoengineering from the perspective of solving problems
in cancer biology and oncology.
• Cancer Imaging. This three-week section introduces basic and advanced concepts
in molecular imaging in the context of cancer biology. It includes methods for optical
(including microscopy and intravital) and acoustic imaging (including ultrasound),
nuclear imaging (PET, SPECT, and CT) as well as magnetic resonance imaging
(MRI/MRS).
• Genetic Engineering. This three-week section provides a foundation in genetic

GSK Student & Faculty Handbook
v. 6/2024 16
engineering tools and concepts that can be applied to laboratory research. It also
provides a greater awareness of the benefits and risks of genetic engineering and
an overview of the latest research and technologies advancing the science of genetic
principles explored during the Experimental Biology course that are essential to
understanding genetic engineering.
Entrepreneurship (Cancer Engineering Students)
This one-week course teaches the processes involved in developing a technology for the
market, including:
• Understanding intellectual property
• Evaluating the market for a technology
• Building a basic financial model
• Establishing funding mechanisms
• Assessing regulatory issues
• Developing a business plan.
Immunology (Cancer Engineering Students)
This four-week course familiarizes students with cellular, molecular, and biochemical
aspects of the immune system and how immune responses function in physiology. It
focuses on the development of the immune system and the biological functions of its major
components. It ends with an overview of recent developments in cancer immunotherapies
and how engineering principles were applied. This section of the Core Course will be shared
with PhD students enrolled in the Cancer Biology Graduate Program.
Cancer Biology (Cancer Biology and Engineering Students) teaches how to think about
cancer as a disease and also as a biological problem. This course leverages the world-
class research and clinical expertise at Memorial Sloan Kettering. The course lasts for ten
weeks and considers both the biology of cancer and also clinical approaches to combatting
this disease. Ten different, week-long topics are considered, including:
- Cancer as a disease
- Genetic and epigenetic mechanisms
- Computational biology and oncology
- Cancer signaling
- Cancer metabolism
- Metastasis
- Tumor modeling and heterogeneity
- Cancer types and microenvironments
- Therapeutic strategies
- Immunotherapeutic approaches
All students are expected to attend the GSK Core Class regularly. A student must notify
the Registrar via email prior to class if they are going to be absent. A student is allowed a
total of 3 absences from the Core Class over the course of the semester. Any absences in
excess of 3 will result in 2 percentage points being subtracted from a student’s
participation grade PER ABSENCE.
Additionally, students are expected to be ON TIME for class - being late to class can be
considered an absence at the discretion of the teaching fellow and could affect your

GSK Student & Faculty Handbook
v. 6/2024 17
participation and final grade.
N.B. – Students may not switch core course requirements outside of their enrolled
program. For example, a Cancer Biology student may not take Cancer Engineering in
place of Mechanistic Biology and vice versa. However, students may explore taking
courses that are part of the other program in later years.
Logic and Critical Analysis (Cancer Biology and Engineering Students)
All first year students complete this course during the first rotation period. Papers from the
scientific literature are used to help set the foundation for students to develop their ability to
think along a logical path, to critically analyze information and data, and to present scientific
results to a group. Students are encouraged to develop an approach to understanding the
scientific literature that includes asking the following questions about each experiment
considered: What is the question that the authors asked? How was the experiment
performed? What techniques were used and why? What is the nature of the data produced?
What represents a significant result? What were the conclusions made by the authors?
Does the author's data justify the conclusions made? And, what conclusions would the
student make? This course is organized by the Dean and GSK faculty members.
Responsible Conduct of Research (RCR) (Cancer Biology and Engineering Students)
First and fifth year GSK students are required to take the formal RCR course that is
managed by Memorial Sloan Kettering Cancer Center. This course is closely aligned with
and meets the requirements of the National Institutes of Health and National Science
Foundation guidelines for education and training in this area.
The goals of this course are to heighten awareness of ethical considerations that are
important to the conduct of research, to inform of federal, state and institutional policies
and procedures, and to provide critical analysis and problem-solving skills for ethical
decision making.
The course will be offered in its entirety twice a year — in the fall from September
to December and in the spring from January to April. Participants may register for one or
the other, but it must be completed within a single semester. Those who start and don’t
finish successfully will be required to repeat the course in its entirety the following
semester.
Participants are required to complete the online modules and participate in a series of in-
person and virtual workshop and discussion sessions.
Topics Include:
• Research Integrity and Research Misconduct (including whistleblowing and
dispute resolution)
• Data Acquisition, Management, Sharing and Ownership
• Rigor and Reproducibility
• Saf e Laboratory Practices
• Animal Welfare
• Protection of Human Subjects
• Conflicts of Interest and Commitment

GSK Student & Faculty Handbook
v. 6/2024 18
• Disclosure and Reporting Requirements associated with Export Control and
Research Security matters
• Authorship and Responsible Publication Practices
• Peer Review
• Collaboration and Mentoring
• The Scientist and Social Responsibility
• Saf e Research Environments (e.g., those that promote inclusion and are free of
sexual, racial, ethnic, disability and other forms of discriminatory harassment)
Visit the RCR course website for more information: https://www.mskcc.org/rcr
President's Research Seminar Series Journal Club (Cancer Biology and Engineering
Students)
The President's Research Seminar Series brings the leading and most distinguished
scientists in the world to MSK. The topics represented are wide-ranging and cover some of
the most exciting fields in modern biology, thus regular attendance by the students will
encourage them to broaden their viewpoint. Students participate in a journal club the day
before the President's Research Seminar Series to review some of the published works of
the speaker. The class is conducted by the faculty member that is hosting the speaker.
Some students meet with the speaker on the day of the seminar. Students may nominate
and host one President's Research Seminar Series speaker each year.
Chalk Talks (Cancer Biology and Engineering Students)
Chalk talks are presented by second year students on Thursday afternoons during the fall
term. These provide second year students an opportunity to discuss the specific aims of
their thesis projects, and first year students a chance to discuss research from one of their
first two rotations. All first and second year students are required to present chalk talks
and attend these sessions. Students must attend 75 percent of the sessions in a given
semester to pass.
Graduate Student Seminar (Cancer Biology and Engineering Students)
One important feature of becoming a successful scientist is being able to present the
results of your research in a coherent and logical form. Graduate Student Seminars ( GSS)
provide the opportunity for graduate students to develop their presentation skills in a formal
seminar environment. Each student from the third and fourth year classes presents a 30
minute seminar in this course, and all students from the first through the fourth years are
required to attend. The seminars are held on Thursday afternoons during f all and spring
terms. GSS does not meet during the summer. A student must attend 75 percent of the
sessions in a given semester to pass.
Current Topics Journal Club (Cancer Biology and Engineering Students)
Students participate in this student-run course beginning in the second year and continuing
throughout their fifth year in the graduate program. A journal club of this type is important
in that it helps prevent the tunnel vision that can sometimes develop as students focus on
their thesis research. There are eight sections of roughly five participants each. Each
section has a leader chosen by the Dean who chooses a topic that will serve as a guideline

GSK Student & Faculty Handbook
v. 6/2024 19
for the papers to be discussed. Topics are reviewed by the Dean before being published to
the student cohort. Students must sign up for one section per semester. Each student in a
section is expected to lead the discussion of one paper. The participants of each section,
as organized by the Section Leader, are responsible for deciding how and when to conduct
“in class” discussion sessions. Section leaders are responsible for the logistics of the
meeting including informing members of the group of the assigned papers.
Students are required to sign in for each paper that is discussed. Signatures will be collected
by the Section Leader and forwarded to the GSK Registrar.
In order to receive a passing grade, students must attend and participate in at least f our of
the five discussions. Any student who does not meet this requirement will receive a grade
of F for the course that semester. In order to be approved by the Graduate School to proceed
to thesis defense, students must have satisfactorily completed all corresponding sections of
Journal Club in the semesters preceding the one in which they register for their defense up
to a total of eight sections. This requirement will be pro-rated for students currently in the
program as of September 1, 2016.
Opportunity to Apply for a Teaching Fellowship
GSK offers a teaching opportunity for graduate students and postdoctoral researchers in
the GSK Core Course that runs from September through May each year. The role of a GSK
Teaching Fellow is to attend the classes, read the papers and facilitate discussion, lead one
Special Topics class, lead one to two review sessions, and provide feedback to the students
and Section Leaders about the students’ participation and presentations. GSK Teaching
Fellows will be expected to commit to blocks of the course; each block is approximately two
weeks. Application for fall is announced in the summer and the early winter for the spring
course. A letter of support from the mentor is required as part of the application. Teaching
Fellows attend an orientation and receive an honorarium for their participation.

GSK Student & Faculty Handbook
v. 6/2024 20
Progress Points
General Program Requirements
The maximum time for completion of all requirements for the Ph.D. degree is six years.
Students must defend and deposit their dissertation by June 30 of the sixth year in the
Program. Students who do not deposit by April 15 will participate in the graduation
ceremony of the following year. All students must be enrolled full time. All students are
required to develop a research project, under the supervision of their mentor, which results
in a dissertation that reports new findings, and is presented and defended before the faculty.
All students must successfully complete 68 degree credits (which are fulfilled with a
combination of coursework and research credits) and meet all other degree requirements
that are part of the program to receive the Ph.D. degree.
Typical Cancer Biology First Year

GSK Student & Faculty Handbook
v. 6/2024 21
Typical Cancer Engineering First Year
Typical Program Schedule for All PhD Students

GSK Student & Faculty Handbook
v. 6/2024 22
Thesis Proposal Examination
The Thesis Proposal Examination (TPE) is required to be completed by April 15 of the
second year. It consists of three parts: (1) a committee meeting that is held in January of
the second year, (2) a written proposal, and (3) an oral examination.
The Examination Committee for the TPE consists of the two members of the Advisory
Committee that are chosen by the student, and two additional members that are
appointed by the Dean. One of these additional members serves as the Examination
Committee chair.
Registering for the TPE and key deadlines:
- By December 15 of the second year, students must pre-register for the TPE by
submitting a Specific Aims page to the graduate school via upload to the student
portal, and the Dean will then appoint the additional two members of the Examination
Committee. Formatting guidelines for the Specific Aims page are included below
under “Guidelines for preparing the TPE Specific Aims page”.
- By the end of January, the committee meeting must be held with the student’s
Advisory Committee and mentor.
- F
our weeks prior to the oral examination, the oral exam must be scheduled with all
members of the Examination Committee, and registered with the GSK office and
Dean
- T
hree weeks prior to the oral examination, the written proposal must be uploaded to
the student portal; it will then be submitted to all members of the Examination
Committee by the Registrar.
- O
ne week prior to the oral examination, the members of the Examination Committee
must vote to approve the written proposal and submit the Evaluation of Written
Proposal Form. Approval of the written proposal advances the student to the oral
examination.
- By
April 15, the TPE must be completed, unless revisions or a retake of the oral exam
are required.
1. The Committee Meeting is held with the student’s Advisory Committee, which includes
the major sponsor and two members who are recruited by the student. This meeting is held
in January of the second year. It provides an opportunity for students to organize their ideas
for the thesis proposal and to receive feedback from their committee members about their
Specific Aims, preliminary data, and the approaches that will be used. This meeting is not
a pre-test of the TPE and is meant to help the student identify and address deficiencies in
their Specific Aims prior to writing the full TPE proposal.
2. The Written Proposal is prepared after the committee meeting and must be submitted
to GSK for distribution to the Examination Committee at least three weeks prior to the
scheduled oral examination date. Examination Committee members may reschedule the
oral examination if not given the appropriate amount of time to read and evaluate the
proposal. The written document is prepared with the same format as an NIH F31 student
fellowship application. Detailed guidelines for the formatting of this document are included
in the “Guidelines for preparing the written proposal” section below.

GSK Student & Faculty Handbook
v. 6/2024 23
The written proposal should be:
- based on the student’s ideas and not exclusively the mentor’s, although the ideas
should be informed by discussions with the mentor as part of the normal advising
process.
- written by the student and not the mentor, although mentors are encouraged to
read the proposal and to provide guidance throughout the TPE process.
Evaluation of the Written Proposal:
After submission of the written proposal, the Examination Committee will read and evaluate
the document, and each member must submit the Evaluation of Written Proposal Form
within two weeks to the committee chair. On this form each member of the Examination
Committee will indicate whether they find the written proposal to be “Satisfactory” or
“Unsatisfactory”. Three out of the four members of the Examination Committee (or four out
of five, if applicable) must find the written proposal to be “Satisfactory” prior to the student
proceeding to the oral examination.
A satisfactory written proposal will be considered “passed” and will not be subject
to re-writing or further editing after the oral examination, unless revisions of the written
document are considered to be helpful for students needing to retake the oral exam, as
decided by the Examination Committee chair.
If two or more of the four members of the Examination Committee find the written document
to be “Unsatisfactory”, the oral examination will be postponed, and a revised version of the
written proposal must be prepared. The committee members will include specific critiques
on the Evaluation of Written Proposal Form to allow the student to respond with a revised
proposal. The extent of revisions that will be required will be communicated to the student
and to GSK by the Examination Committee chair, and an appropriate timeline for preparing
the revised written document will be established, in consultation with the Dean. The student
is allowed only one cycle of revision for the written proposal. Upon approval of the revised
written proposal by at least three of the four members of the Examination Committee, the
student may proceed to the oral examination.
3. The Oral Examination is scheduled after the January committee meeting and must be
held by April 15. Students are responsible for scheduling the exam with the members of
their Examination Committee at least four weeks ahead of the exam date, for reserving a
conference room for two hours on the date of the exam, and for registering the date and
location of the exam with the GSK office via the student portal.
The oral exam provides members of the Examination Committee the opportunity to assess
the student’s ability to evaluate and synthesize the relevant literature, articulate and
elaborate on the research project, show and evaluate preliminary data, and discuss
experimental design. Students should prepare a presentation that includes the background
and preliminary data, specific aims, experimental approaches, potential outcomes, and
alternative approaches where necessary.
Students should be prepared to discuss in detail any aspect of what is shown on their slides,
and they are advised that the discussion flow of the oral examination can be unpredictable.
Generally, the student-examination committee dialogue during the exam should evolve into
a broadly based discussion that establishes the breadth of the student’s grasp of key issues
in the field. As a practical point, the student should expect as “fair game” any question

GSK Student & Faculty Handbook
v. 6/2024 24
pertinent to points they raise:
For example, if a student is studying protein phosphorylation, it is reasonable to be
expected to know the structure of phosphorylated amino acids. If a student is
transfecting a gene whose product functions in the nucleus, it is fair game for the
committee to ask how proteins are imported into the nucleus. If a drug or inhibitor is
used as a key reagent, the committee may ask the student to discuss in detail the
mechanism of action. And so forth... One common mistake that students make is to
anticipate questioning only on the hypothesis and the technical aspects of the
proposed experiments; the committee members are also likely to probe a student’s
more general knowledge and critical thinking skills.
During the oral examination, the student’s mentor is not a member of the Examination
Committee and is not present during the exam. The mentor is encouraged to attend the
start of the exam to meet with the Examination Committee with the student excused from
the room, to introduce the student and the research project, and to share any other
information that might be relevant to the examination. The mentor must then leave the room
before the examination begins. If the mentor cannot be present on the day of the
examination, they are encouraged to send the Examination Committee chair any
introductory information that they would like presented to the committee, and the chair will
read this to the committee prior to the start of the exam with the student out of the room.
Evaluation of the Oral Examination:
The duration of the oral examination can be variable, and it is at the discretion of the
Examination Committee chair, in consultation with the other committee members, to
determine when the examination is finished. At the conclusion of the exam, the chair will
excuse the student and the committee will discuss the outcome of the exam. Three out of
the four (or four out of five) Examination Committee members must agree to vote
“Satisfactory” for the student to pass the exam. If two or more members out of the four vote
“Unsatisfactory”, the student does not pass and a retake of the oral examination must be
scheduled. A final outcome of either “Satisfactory” or “Unsatisfactory” will be entered on the
Oral Examination Voting Form. The Examination Committee will then invite the student back
into the room to discuss the outcome of the exam. If a retake is required, the Examination
Committee should discuss the deficiencies that led to the “Unsatisfactory” decision, and
also discuss a plan for the student to retake the exam. This could involve retaking the entire
exam, or only particular sections. The extent of the retake that will be required will be
decided by the Examination Committee chair in consultation with the other committee
members.
A student is permitted only one retake of the oral examination. The student and the GSK
office should receive, in writing from the Examination Committee chair, a description of the
extent of the retake that will be required, and the expected timetable for completion of the
oral examination. The timing will depend in part on the extent of the deficiencies that were
noted, and the appropriate timeframe will be decided in consultation with the student’s
mentor and with the Dean. The maximum allowable time for a retake of the exam is four
months. The committee may also ask that revisions to sections of the written proposal be
submitted and approved prior to retaking of the oral exam, when this is determined to be
helpful for the student to reconsider the background information or experimental aims in the
proposal.
A committee may refuse a student the opportunity to be re-examined if the student has

GSK Student & Faculty Handbook
v. 6/2024 25
failed to show sufficient research progress and ability. In such as instance, the Examination
Committee will meet with the Dean before the decision is officially recorded with GSK. A
student whose outcome of re-examination is deemed unsatisfactory will be dismissed from
the school.
Students who satisfactorily complete both the written and oral presentations will be
Advanced to Candidacy.
Deadline:
All students must complete the Thesis Proposal Examination by April 15th of the second
year in the program. If there are revisions and/or re-examination, these must be successfully
completed within four months of the date of the initial examination. Individual students may
request an extension of these deadlines under special circumstances by writing to the Dean
at least one month prior to the deadline. Failure to meet this timetable can result in loss of
good standing in the program and may result in Administrative Withdrawal from the
program.

GSK Student & Faculty Handbook
v. 6/2024 26
Guidelines for preparing the TPE Specific Aims page.
By December 15 of the second year, students must pre-register for the TPE by submitting
a Specific Aims page describing their proposed research. The scope of the proposal
should typically be limited to two specific aims that are listed sequentially.
Typically, a strong Specific Aims page includes the following parts:
Introductory Paragraph – Introduces the scientific problem and the critical need to solve
this problem. The central hypothesis should be articulated, as well as the overall
objectives of the proposed research.
Specific Aims – The title and objective of each aim, as well as some information for
experimental design and the methods for accomplishing the specific aims, should be
described concisely under each heading.
Guidelines for preparing the written proposal.
Document formatting:
The document should be 8 pages in length (including figures but excluding the
references). The first page is the title page, the second page is the Specific Aims page,
and the remaining 6 pages form the body of the Research Strategy, which should follow
the format of an NIH F31 fellowship proposal. This TPE format is designed to encourage
eligible students to submit their thesis proposals as NIH fellowships following successful
completion of the TPE exam. Formatting, fonts, and margins should adhere to NIH
guidelines found here.
Title Page
This is self-explanatory.
Specific Aims
Length: 1 page
See “Guidelines for preparing the TPE Specific Aims page” above.
Research Strategy
Length: 6 pages
Must be organized into two main sections:
A. Significance
B. Approach
A. Significance
(Recommended length: 2 to 3 pages (including figures))
In this section, the student should explain the importance of the problem or critical barrier
to progress in the field that the proposed project addresses. Explain how the proposed
project will improve scientific knowledge, technical capability, and/or clinical practice in one
or more broad fields. The student should provide background information involving a review
and critical evaluation of the literature pertinent to the proposed research. The discussion
of the literature should identify unresolved issues or gaps in knowledge that the proposed

GSK Student & Faculty Handbook
v. 6/2024 27
experiments are intended to address.
The model or hypothesis to be tested should also be explicitly stated and described in detail;
the student should make clear how the model/hypothesis is consistent with available
information. The use of diagrams and/or figures can be very effective, and a diagram of the
proposed hypothesis/model should be included if possible. The source of any reproduced
or adapted figures/diagrams must be cited. Division of the text into discrete subsections, if
possible, can also make the background material easier to read; it also focuses attention
on the key themes conveyed by the subsection titles. The significance of each Specific Aim
should be clearly stated.
Students who need help identifying publications that will support the background section of
the written document are welcome to consult the resources and services offered by the
MSK library.
B. Approach
(Recommended length: 3 to 4 pages (including figures))
This section should describe the experimental strategies and procedures that will be used
to evaluate the central hypothesis or model under study in the proposal. Each specific aim
should be presented as a separate section, with a series of sub-sections entitled:
A. Rationale
B. Proposed Experiments
C. Possible Results & Interpretation
D. Potential Problems & Alternative Approaches
Consider that the following aspects should be included in the Approach:
• Discussion of why a particular experimental approach was chosen, including its
advantages and limitations.
• A concise description of available reagents; if desired reagents are unavailable, a
brief description of how the reagents will be developed and validated.
• Consideration of proper controls, which is an integral element of experimental design.
• Prediction of possible outcomes.
• Discussion of how the results will be interpreted in the context of the working
hypothesis/model.
• An indication of what will be done to follow up on the observations.
• Discussion of what alternative approaches will be considered if the proposed
experimental strategy is unsuccessful
• Most importantly, a clear explanation of how the experimental results will distinguish
between the proposed hypothesis/model and alternative competing
hypothesis/model(s).
It is important that students entertain more than one outcome for each experiment.
Students should not confine discussion of the outcomes to those that will merely confirm
the initial hypothesis.
High rigor often entails designing experiments that explicitly aim to disprove the
hypothesis/model; negative results of such experiments may be more convincing to
skeptics than positive results that are merely “consistent with” a model but that do not
exclude other models.
Diagrams may be included and can be helpful for illustrating the rationale behind the

GSK Student & Faculty Handbook
v. 6/2024 28
design and interpretation of an experiment. The source of any reproduced or adapted
figure/diagram must be cited.
References
In the list of references, please include the names of all authors and the full title. There is
no page limit for the References.
Some additional points to consider:
To merit approval, the written proposal should present a well-defined hypothesis that is
soundly based on scientific knowledge and experimental observations reported in the
literature. The logic underlying the experimental design should be clearly articulated and
critical features of the experimental approaches explained so that faculty from different
fields of biomedical research can readily follow and review the written proposal.
The primary objective of evaluating the written proposal at this stage is to prevent a
student from advancing to the oral examination with an indefensible proposal.
Deficiencies that can lead to an unsatisfactory proposal generally fall into six areas:
1. Faulty logic
2. Poor articulation of the central hypothesis
3. Poor articulation of the rationale underlying the experimental strategy
4. Failure to design experimental approaches that test the proposed hypothesis/model
5. Experimental approaches that cannot yield a predictable outcome
6. A lack of understanding of the quantitative aspects of the data being obtained
7. Specific aims that are co-dependent on each other. For example, you cannot achieve
aim 2 if aim 1 is not achieved.
8. A project that is beyond the scope of a PhD thesis project.
Dissertation
Students should follow the GSK Dissertation Guidelines available from the GSK
Registrar for preparing the Doctoral Dissertation. Students are encouraged to meet
with the Associate Dean and/or the GSK Registrar to receive advice regarding the timing
and logistics of planning the formal dissertation. The first step in the dissertation process is
to submit the signed “Dissertation Registration Approval Form”, which is signed by all
members of the advisory committee at the final committee meeting. Students are expected
to defend the dissertation within 6 months of submitting the approval form.
• The Written Document
• The Committee
• The Defense and Seminar
• Dissertation Deposit
• Degree Conferral

GSK Student & Faculty Handbook
v. 6/2024 29
The Written Document
Any data presented that was not obtained by the student should be identified clearly and
attributed properly. Presentation of such data should be limited to that which is essential to
develop the story. Similarly, work performed for the student by any of the Core Facilities of
the Center should be identified clearly and attributed properly. Descriptions of the methods
for such work should be limited to that which is necessary to understand the data.
While a publication is not required for graduation, students who wish to compile published
manuscripts as the backbone of the dissertation text may do so with the following
guidelines:
•
a general introduction, literature review, and summary will be written for the
dissertation;
•
the relevant publisher must grant permission for the use of the published paper
as a dissertation chapter;
•
the student must be first author on the paper;
•
the publication represents the writing and the scientific work of the student;
•
multi-author publications must include an explanation of the work not actually
performed by the student. It would be appropriate to omit those experiments that
were not done by the student and cite them.
If a published paper is used in the dissertation, copyright approval must be secured from
the journal. A note should be made on the paper indicating that copyright approval was
granted.
Electronic copies of theses from GSK graduates can be found on the MSK Library Page.
The Committee
The Dissertation Committee is composed of five members, including the mentor, who must
be a silent observer during the defense. If the student has co-mentors, both may be on the
committee and both must be silent observers during the defense. The four voting members
will consist of the two members of the Advisory Committee, an External Examiner, and an
additional member appointed by the Dean, who will also serve as the Chair of the
Dissertation Committee. External Examiners cannot be members of either the GSK or MSK
faculty and must be full-time, tenure track faculty of an accredited graduate school. The
External Examiner may not be a present or recent (last three years) collaborator of either
the student or the student’s Thesis Mentor, a recent (last five years) GSK graduate, nor a
person in whose laboratory the student intends to pursue postdoctoral training. In addition,
the External Examiner may not have been present at any of the student’s Thesis Committee
Meetings prior to the dissertation defense. Students must submit the CV of prospective
External Examiners for approval by the Dean.
For External Examiners, GSK will pay an honorarium of $400 and fully reimburse travel
expenses. This includes transportation, (economy air f are), overnight accommodation for
1-2 nights, and meals. Students are encouraged to find local examiners if at all
possible.
The Dissertation Committee must read and approve the dissertation prior to the oral
defense. The student should upload the dissertation as early as possible, but no later than
two weeks before the defense. If the committee finds that there is sufficient reason to
postpone the defense, the chair must communicate the committee’s decision to the student
and the GSK Registrar at least one week prior to the scheduled defense date. The
committee members may also reschedule the defense if not given the appropriate amount

GSK Student & Faculty Handbook
v. 6/2024 30
of time to prepare for it.
The Defense and Seminar
All students must present a 45-55 minute seminar on their work, which is open to the Tri-
Institutional community, in addition to the closed door oral defense. The Dissertation
Committee members attend the seminar, but should refrain from asking questions, as their
questions should be reserved for the closed door defense.
Dissertation Deposit
The dissertation must be deposited within eight weeks from the date of the defense for the
student to be eligible for the degree.
The following deposit deadlines and enrollment requirements will determine the date of the
conferral of the degree.
Degree Conferral
Degrees are conferred on September 30, January 31, and the date of the GSK
Commencement in May. Diplomas are prepared for distribution only at the May
commencement. A student can request an interim letter testifying to the completion of the
degree requirements after they have deposited their dissertation. Commencement
information will be sent during the Spring semester to the last email address recorded with
the GSK office.
Contingent upon the defense outcome, students maintain their student status, including
stipends and housing for eight weeks.
The dissertation must be deposited in final form by the end of the indicated time period,
otherwise the degree will not be awarded.
Exceptions to this schedule will be considered only under extenuating circumstances.

GSK Student & Faculty Handbook
v. 6/2024 31
Academic Policies and Procedures
Grading, Course Examinations
All credited courses are assigned grades A(-), B(+/-), C(+/-), F, P (Pass), I (Incomplete), S
(Satisfactory), or U (Unsatisfactory). In general, seminars and journal clubs are assigned
P/F grades and ongoing research courses are assigned S/U grades.
The Core Course is the only letter-graded course at GSK. Students who have not
completed the required work for a course may be given a grade of Incomplete. Students
must fulfill the obligation to resolve an incomplete grade within the next two semesters
that they are registered. Af ter one year, unresolved incomplete grades will appear
permanently as incomplete on the student's record and will accrue no credit toward the
degree. Letter grades (A, B, C, or F) may not be changed af ter they have been recorded
in the GSK records, unless there was a clerical error by the course director in the
submission of the grade. If a course director agrees to consider additional work from a
student, then an initial grade of incomplete should be recorded. Students are expected
to monitor their progress toward completion of the program and the degree requirements.
If a student cannot complete course requirements by the established deadline because
of health or personal issues, the instructor must be notified on or before the due
date, or as soon thereafter as possible. A doctor's note must be submitted to the course
director and the GSK office, and arrangements must be made for a new deadline.
GPA
All students must complete at least 68 graduate credits (a combination of course work and
research credits) and maintain a GPA of 3.0 or higher.
To compute the grade point average:
1. For each course with a letter grade that counts toward the GPA, the number
of credits is multiplied by the appropriate quality point value, as indicated
in the table below.
2. The quality point values are added for all the courses to determine the
total quality points.
3. The total quality points are divided by the total number of credits.
The resulting figure is the GPA/cumulative index. GPA is computed to two decimal
points.
Although credits with grades of P/F or S/U count toward the degree requirement, they do
not figure into the computation of the GPA.
Students who do not meet the requirements of the New York State Immunization Law will

GSK Student & Faculty Handbook
v. 6/2024 32
not be permitted to register. Student Health Services is required by New York State Law
to maintain up-to-date records for each student. Students who fail to comply with this
requirement will not be permitted to register and may be Administratively Withdrawn from
the program.
Advisory Mechanisms
The GSK has developed an extensive advisory system to help students optimize their
tenure at GSK. This advisory system is multi-pronged, and students are strongly
encouraged to take full advantage of the opportunities available to them, and to discuss
their decisions and progress in the program with their advisors. Together with the GSK
faculty members, the Deans serve as ad hoc advisors to students throughout their tenure
at GSK.
After matriculation, each student is assigned a First Year Mentor. Students can meet with
their First Year Mentor to discuss rotations, courses, or any other aspects relating to their
experience at GSK. Students are also encouraged to meet with the Deans, as a cohort and
individually as they are fulfilling the didactic requirements of the course and moving towards
identifying and selecting a thesis mentor. Students may seek the advice of their First Year
Mentor and the Deans as they are deciding on their laboratory rotations and can meet to
discuss the rotation experience following each rotation.
Once students have formally declared their thesis mentor in July, they will subsequently
select a two-member Advisory Committee from the GSK faculty, which, with the mentor, will
constitute the Thesis Committee. The Advisory Committee members should be those
faculty members who are most likely to be helpful in the student's area of research.
Students may add an additional f aculty member from either the GSK f aculty or another
graduate school. External Members of the Advisory Committee must be full-time, tenure
track f aculty members of an accredited graduate school. Students should submit the CV of
prospective External Members to the Dean for approval.
The Thesis Committee, chaired by the mentor, is expected to meet with the
student according to the schedule below. Students are required to provide the Thesis
Committee with a completed Progress Report (updated and downloaded from the GSK
Student Information System) and a two-page report showing progress and changes f rom
the last meeting (sent to the committee at least one week prior to the scheduled
meeting). Students are required to prepare a PowerPoint presentation for these meetings.
Thesis Committee Meeting Deadlines
• Second year students: meeting I: January 31
• T
hird year students: meeting I: December 31
• Fourth year students: meeting I: September 30 and meeting II: May 31
• Fifth year students: meeting I: January 31 and meeting II: July 31
• Sixth year students: meeting I: January 31 and meeting II: July 31.
Second year students will have a planning meeting no later than January 31. The purpose
of this meeting is to bring the advisors up-to-date on plans for the thesis project: what has
been done, preliminary data, and future plans. The advisors are expected to provide
feedback and discuss the merits and feasibility of the project. The Thesis Committee should
help the student troubleshoot the project. In preparation for this meeting, students must
send to the thesis committee a copy of their Specific Aims page (which was submitted to
the graduate school by December 15, see TPE guidelines). In this first committee meeting

GSK Student & Faculty Handbook
v. 6/2024 33
students can discuss the specific aims of their thesis project with the Advisory Committee.
Formal progress reports must be filed by GSK students in year two and beyond
according to the schedule indicated above. Prior to meeting with the Committee, the
student should review their information in the Progress Report Form in the GSK Student
Information System and correct/update as necessary. Student’s should download the
individualized Progress Report Form and provide it to the members of the Thesis Committee
prior to the scheduled meeting. The Committee should use the Report to evaluate the
student's progress, indicate strengths and weaknesses, and discuss the student’s Individual
Development Plan (IDP). Meetings should conclude with a discussion between the advisors
and the student, in the absence of the mentor.
The Thesis Committee for second year students will only be asked to certify that the
committee met, not to evaluate student progress. A Progress Report Form, signed by the
Thesis Committee members and the student, must be submitted to the GSK office
immediately following each meeting. Students should convene the Thesis Committee as
needed, but Progress Reports must be submitted according to the schedule above.
Beginning in the fourth year, the committee meetings should be focused on timely
completion of the thesis research and the preparation of manuscripts for publication. In
addition to the overall progress, the committee members will closely monitor student’s
progress to timely completion of the degree.
Timely meetings with the deadlines above are required and occur to maintain satisf actory
academic progress. Students are encouraged to take the initiative in scheduling meetings
with the Thesis Committee or the Deans, as necessary. Both students and faculty are urged
to view the advisory system as crucial in identifying and resolving problems, in maintaining
realistic expectations for progress, and as a source of new ideas and approaches.
Satisfactory Progress
It is essential that students and the Thesis Committee monitor student progress for the
duration of the Program. Continued financial support is contingent upon
maintaining satisfactory progress at all times. Additionally, failure to achieve and
maintain satisfactory progress, after advice is sought from the Thesis Committee and/or
the Deans, can result in academic probation and ultimately, dismissal from the program.
A student who fails to maintain satisfactory progress may be placed on an
Administrative Leave of Absence by the Dean, following consultation with the
student's mentor and Advisory Committee.
Satisfactory progress is achieved and maintained by meeting the following requirements:
•
matriculation on a full-time basis
•
for first year students: timely completion of all course requirements,
demonstration of research potential and of timely progress toward the choice of
thesis mentor through rotation activities and meetings with the f aculty
•
timely submission of a completed Laboratory Rotation Agreement Form, Rotation
Evaluation Form, Rotation Abstract and Rotation Report for each rotation
•
maintaining a 3.0 GPA or higher
•
no more than two Incomplete grades are allowed in any given semester, unless
they resulted from an approved leave of absence that began before a f inal grade
was assigned

GSK Student & Faculty Handbook
v. 6/2024 34
•
successful and timely completion of the Thesis Proposal Examination
•
meetings with the Thesis Committee according to the schedule indicated in the
section on Advisory Mechanisms and submitting a Progress Report immediately
following each meeting
•
demonstration of progress in the research project and the ability to demonstrate
growth in research skills
•
completion of at least 68 graduate credits (a combination of coursework and
research credits)
•
completion of all dissertation requirements, including defense and timely deposit
Student Publications and Citation of Student Affiliation
Students should indicate their affiliation on all publications and abstracts as: Louis V.
Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer
Center, New York, NY.
Students are required to update their official publications with the GSK office and also on
their student portal in SIS. All publications authored by GSK students will be placed on the
GSK website.
Academic Dishonesty, Plagiarism and Artificial Intelligence
Students are expected to understand all standard rules associated with plagiarism.
Resources available to further inform the student of what constitutes plagiarism can be
found in the MSK Code of Conduct, the content of the Responsible Conduct of Research
course as well as in many guides offered to explain the seriousness of any breach of not
submitting one’s own work for credit. Note: Students may not use Artificial Intelligence tools
such as ChatGPT to complete or enhance their work including problem sets, thesis proposal
exams, and theses.
Any instance of suspected plagiarism or academic dishonesty by a student will be brought
to the attention of the Dean for further inquiry and action. Proven instances of plagiarism
can result in dismissal from GSK.
Grievance Policy
Students who have a concern about a matter related to the PhD program, whether it
concerns a course, instructor, or mentorship, are encouraged to discuss their concern with
their thesis committee and/or First Year Mentor. If the issue cannot be resolved in session
with these mentors or if these mentors have a conflict of interest, the student may choose
to present the grievance to the Associate Dean and Dean. Matters that require additional
focus will be elevated to the Curriculum Committee or a special Ad Hoc Committee called
by the Dean.
Appeals Policy
Issues concerning the grading and evaluation of student performance in courses will be
discussed with the course director. If the student f eels there is a need for further resolution,
the issue should be brought to the attention of the Dean. The Dean may discuss the
issue with the Executive Committee, if needed.
Students who have been placed on probationary status or asked to leave the program
based on lack of Satisf actory Progress may appeal these decisions by petitioning the

GSK Student & Faculty Handbook
v. 6/2024 35
Dean to have an ad hoc Academic Appeals Committee convened. The student and the
student's mentor (and/or First Year Mentor) will have an opportunity to speak with the
Academic Appeals Committee. The committee will make a recommendation to the Dean;
the student must abide by this decision.
A student will be permitted to maintain student status, with some student privileges,
during the appeals process or until June 30 of that academic year, whichever comes
first.
Core Course Attendance Policy
All students are expected to attend the Core Class regularly. If a student is going to be
absent from the Core Class, they must notify the Registrar/Curriculum Specialist prior to
the absence. A student is allowed a total of three absences from the Core Class over the
course of the semester. Any absences in excess of three will result in two percentage
points being subtracted from a student’s participation grade per absence.
Additionally, students are expected to be ON TIME for class - being late to class can be
considered an absence at the discretion of the teaching fellow, and could affect your
participation and final grade.
Vacation Policy
In general, GSK anticipates that students in the second year and on will take up to three
weeks of vacation each year, exclusive of travel to scientific meetings. Individual
circumstances may dictate that a student along with the mentor design a vacation plan that
is appropriate given the nature of the student's efforts over a period of time, in particular
family circumstances, parental leave, etc. Students must be sensitive to their obligation to
inform the mentor (or the rotation mentor, Deans and the First Year Mentor) of planned
absences to minimize any disruption of work in the laboratory.
In the event of an unanticipated absence, students should make every effort to
communicate with the laboratory and the Deans as soon as possible. Any unexplained
absence will constitute lack of Satisf actory Progress in the program and can result in
academic probation, administrative Leave of Absence, and ultimately, dismissal from the
program.
Parental Leave
Thesis mentors are expected to allow students to take up to eight weeks of paid time off for
parental leave for the birth or adoption of a child.
Leave of Absence and Withdrawal
A student wishing to interrupt doctoral study for one academic year or less, for serious
illness or compelling personal reasons, may request a leave of absence. If the leave is
approved by the Dean, the student will be reassured of readmission at the end of the
approved leave. Such approval can only be obtained if the student is in good academic
standing and has the approval of the mentor. Students who fail to follow any conditions of
the approval of the leave of absence will be administratively withdrawn from the program. If
the student wishes to return at a later date, that student must apply for re- admission to the
program.
Students on a personal leave of absence are not eligible for the benefits associated with
maintenance of student status, including stipend, health insurance, travel award, and
student housing for the duration of the leave. Students on a medical leave of absence

GSK Student & Faculty Handbook
v. 6/2024 36
who have completed at least one semester in the Graduate School may petition for
continuation of benefits associated with student status; this request will be considered on
an individual basis.
Students must submit a Request for a Leave of Absence Form with the appropriate
documentation to the Dean. A student requesting a personal leave of absence must
include a written statement that explains a "compelling personal reason." A student
requesting a medical leave of absence must submit documentation from a physician.
Once the letter requesting leave is submitted, the Associate Dean will provide the student
with a protocol for submitting necessary documentation from their treating physician as
well as the process for connecting with MSK Benefits to apply for continuation of benefits.
N.B. – The period of an authorized leave is included in the program time limit. A student
who was on an approved leave of absence may petition the Dean for an extension of the
program time limit, to include the time period of the leave of absence. This petition must
have the support of the student's mentor and Advisory Committee. Such requests will be
considered under special circumstances.
International students can only request a leave of absence for medical reasons.
Request for voluntary withdrawal from the program must be made by submission of the
Request for Withdrawal Form. To resume doctoral study a former student must apply for
re-admission.
NOTE: Students who are out of school for any extended period of time are responsible for
ensuring that they abide by f inancial aid (such as loans) regulations as stipulated by the
relevant agencies/institutions.
Involuntary Student Leave
The Graduate School is committed to the health, safety and well-being of all students and
members of the community. In situations where a student is unable to care for themselves
on a regular basis, is unable to perform their duties as a student, or presents a substantial
risk of self-harm or harm to others, and the student declines to take a voluntary leave, the
dean of the Graduate School has the authority to place the student on an involuntary leave.
A
n involuntary leave is not a disciplinary sanction and may not be intended or interpreted
as punitive in nature. The leave does not count towards the statute of limitations for
obtaining a degree. Operating under the umbrella of MSK, the Graduate School will consult
certain MSK departments including Employee Health, HR Legal & Regulatory Affairs,
Environmental Health & Safety, and Security in assessing an individual student’s situation.
The Dean will form an ad hoc committee to determine the appropriate action and issue an
official letter to the student of its decision. The student will have 5 days to submit a rebuttal.
Subject to approval by the Dean, a student placed on involuntary leave may remain in
Graduate School housing, and for a period of three (3) months may continue to receive
medical coverage, and may access available services including counseling and the
Employee Assistance Program. At the end of the three-month period if the student does
not submit a formal request for re-enrollment as set forth below, the ad hoc committee will
review their case to determine next steps.
Request for Re-enrollment
A formal request for re-enrollment must be submitted to the Dean. The student’s re-
enrollment request will be reviewed by the Dean, who, in consultation with the relevant

GSK Student & Faculty Handbook
v. 6/2024 37
MSK departments, must approve the re-enrollment and may impose such conditions as
they may recommend to ensure the student’s successful return to the Graduate School.
Upon receiving the student’s formal request for re-enrollment, the student will be notified
of any applicable eligibility requirements, including medical clearance or other
confirmation of their fitness to return/re-enroll. A student denied re-enrollment and return
from an involuntary leave of absence may submit an appeal to be re-considered by the
Dean in consultation with the relevant MSK departments. The final decision will be made
by the Dean and communicated to the student.
Policy for Return from Medical Leave
Students who wish to return from medical leave of absence should submit a formal letter
to the Associate Dean along with the appropriate documentation from a physician clearing
them to return as a student. Students must also be cleared to return as a student by
MSK’s Employee Health and Wellness unit.
Maximum Time for Completion of All Degree Requirements
GSK allows students to be matriculated for a maximum of six years. Failure to complete all
degree requirements within this time period may result in loss of good academic standing.
Extension of this time period may be granted by the Dean upon consideration of a Petition
for Extension by the student. Such petition, which may be downloaded from the student
portal, must be co-signed by the student and Thesis Mentor and include a description of
progress to degree, explanation of any extenuating circumstances, and an estimate of the
additional time required for completion. Petitions must be filed by April 15th of the student’s
sixth year in the program for extension to a seventh year of residence. Additional petitions
for extension of residence must be filed by February 1 of the student’s seventh year and
will be reviewed by the Curriculum Committee.

GSK Student & Faculty Handbook
v. 6/2024 38
Student Services
Career and Professional Development
GSK actively supports career guidance for its students. It organizes regular seminars
and workshops to increase students' awareness of the wide range of career options
available to them. Invited speakers include individuals f rom a wide range of careers.
GSK also participates in the activities and events of the New York Academy of Sciences.
Paid memberships to the NYAS for all students are renewed each year. Students are
encouraged to join the membership of the Academy to take advantage of its many scientific
and career development programs. In particular, students can participate in the Science
Alliance for Graduate Students and Postdocs. The Academy has partnered with the major
educational institutions in New York City to provide a number of services, including:
mentoring, networking, and career development. This is accomplished through local events
and via a dynamic website
.
The Office of Career and Professional Development
SKI’s Office of Career and Professional Development (OCPD) works to enhance the
professional development of our graduate students and postdoctoral researchers while
assisting the f aculty in training the next generation of leading biomedical scientists. The
goal of the OCPD is to prepare the students for success during their graduate training
and to help in planning for fulfilling careers in academia, industry, business, or
government.
OCPD’s services are offered in a number of ways, including professional development
workshops, seminars, and courses to complement the training at the bench. Individual
guidance on interview processes and different career paths is readily available for GSK
students, and individual career counseling, are provided through panel discussions and
networking events.
Complete information about the services offered by the OCPD and on how to schedule an
Individual appointment is available on the Office of Career and Professional Development
website.
International Student Services
The GSK office coordinates services for international students with the MSK Immigration
Office. Upon acceptance into GSK, students will be guided through the process of
obtaining an F-1 student visa.
In addition to the issuance of an F-1 visa, students will be directed to resources on
professional advisement on immigration, financial, employment, and other matters
through personal appointments, specialized orientations, and workshops.
Students will be provided with information on Government Regulations and Procedures
on issues such as maintaining legal status, employment options, travel and re-entry, and
other matters.
All international students are required to adhere to the laws of the Department of
Homeland Security and the US State Department. Students must provide a validated I- 20
form before their fellowship package can be activated. Students are responsible to make

GSK Student & Faculty Handbook
v. 6/2024 39
sure that they are always in compliance as mandated by the Department of Homeland
Security and the US State Department.
All questions regarding the visa status should be addressed to the GSK Designated
School Officials (DSO) in the MSK Immigration Office, or to the GSK office.
Internship and Externship Policies
GSK students in their third year or beyond in good standing and firmly established in their
laboratory research are allowed to pursue full-time internships or part-time externships to
enhance their development as scientists. Students who wish to enhance their scientific
training outside of MSK may pursue Internships, which are full-time, temporary (up to
three months) positions that will contribute to the student’s scientific development and
advance their thesis research.
Moreover, some students may wish to learn about non-research career paths for
scientists or develop their transferable skills by pursuing Externships, which are paid or
unpaid, part-time experiences that are pursued on a student’s own personal time and do
not need to benefit a student’s thesis research.
Students should note that internships and externships are a privilege and not a right.
Students interested in pursuing either an Internship or Externship should initiate contact
with the Dean to discuss their plans and to request the written guidelines and application
f orm.
N.B. - All employment agreements will be reviewed by MSK’s Vice President (VP), Human
Resources (HR) Legal & Regulatory Affairs. All technology transfer agreements must be
approved by MSK’s Chief Intellectual Property (IP) Counsel. Students with questions
about internships or externships should consult with the Deans in advance.
The Library
The MSK Library's mission is to proactively partner with Library users by delivering
innovative services and targeted published content in support of quality patient care,
research excellence, and ongoing learning for the progressive control and cure of cancer.
The MSK Library subscribes to a full range of abstracting and indexing databases
covering key science, medical and healthcare information. Library clients have access to
large number of journal titles and textbooks that reflect the research and medical activities
of the Center with over 85% of these titles available electronically.
Most of the Library’s content is available 24x7 via the MSK Library Website.
These online
resources can be accessed either on campus or remotely. Students are encouraged to
become familiar with the wide range of services available to them and to consult with
library staff should they need assistance.
The MSK Library has expanded support in response to the NIH Public Access Policy
and
the mandate for compliance. Students who publish and associate their manuscripts with
MSK can learn more about compliance by contacting us.
Other Facilities
Memorial Sloan Kettering's main campus, home to the Gerstner Sloan Kettering Graduate
School, is located on Manhattan's Upper East Side. Its research space totals

GSK Student & Faculty Handbook
v. 6/2024 40
approximately 618,500 net square feet of research space divided between the Mortimer
B. Zuckerman Research Center, the Schwartz Research Building, and the Rockefeller
Research Laboratories. The Rockefeller Laboratories house the 4 Basic Research
Programs in state-of-the-art facilities. Although the building was originally opened in 1992,
it is currently undergoing a floor-by-floor renovation. Among the
upgrades are a CryoElectron Microscopy Facility, a dedicated epigenetics research
center, and addition of computational biology workspace on 3 floors. To facilitate the
expansion in the Bridge Research Programs, the Mortimer B. Zuckerman Research
Center was built on the site of the original Kettering Research building. It was completed
in 2014 and is home to the 4 Bridge Research Programs as well as the laboratories of the
Clinical Research Program. It also has dry lab space for computational biology, a GMP
cell engineering facility, and additional vivarium space. The Schwartz Building
accommodates clinical and translational research laboratories, including members of
Cancer Biology and Experimental Pathology, Imaging and Radiation Sciences, and
Experimental Therapeutics Programs. A new 3,700 net-square foot cyclotron facility was
completed in 2014 in its basement that supports the development of new imaging,
therapeutic, and theranostic probes.
Dozens of Research Core Facilities
— ranging from bioinformatics to high-throughput
drug screening and x-ray crystallography — serve both basic and clinical research
needs. These shared facilities offer state-of-the-art instruments and technical staff
support to graduate students as they train and conduct research projects.
The Student Center, equipped with communications and audio-visual equipment, is the
hub of activities for graduate students. It is adjacent to the offices of the graduate school.
A student lounge is designed to accommodate student meetings, journal clubs and small
seminars.
Memorial Sloan Kettering's Student and Faculty Club offers students an informal setting
to interact with colleagues, including fellow students, faculty, postdoctoral fellows, and
students from joint programs with Weill Cornell Medical College and The Rockefeller
University.
GSK Student & Faculty Handbook
v. 6/2024 41
Student Benefits
Medical Insurance
All students are provided with a comprehensive health insurance package as part of the
fellowship package. Insurance benefits also cover students’ spouses, domestic partners
and dependents. Medical insurance coverage begins on the first day of the subsequent
month in which the student matriculates and will end on the last day of the month in which
the student status is no longer effective. If a spouse, domestic partner, and/or dependent
child is added to your insurance plan, the value of this additional coverage is also
considered taxable as imputed income (see student tax regulations in Appendix 1
).
Mental Health
All students are offered full mental health coverage as part of their health insurance plan.
In addition to benefits offered through health insurance, GSK and MSK have invested in
additional resources to make counseling services more accessible.
Short-Term Counseling (up to six sessions for each resource)
The Magellan Employee Assistance Program (EAP)
o Offers free, confidential short-term counseling and supportive services 24/7/365
to all students and members of their household. Licensed counselors are
available in person, by text message, live chat, phone or video conference to
support students and household members with challenges such as stress,
anxiety, grief, substance misuse, relationships, parenting and more.
o Magellan’s services also include access to Dr. Penni Morganstein, Clinical
Psychologist, who is dedicated to serving all MSK postdocs and students. Dr.
Morganstein offers free, in-person and virtual counseling services exclusively to
support MSK/GSK trainees with challenges of stress, anxiety, adjustment
challenges, grief, relationship concerns, parenting, substance misuse and more.
Daytime sessions are available. To speak confidentially with Dr. Morganstein
and to schedule an appointment, please use the following link
or contact her via
morgansteinp@magellanhealth.com.
o Note, all discussions with Dr. Morganstein remain confidential and are not
shared with MSK/GSK.
GSK students can also seek short term counseling from Dr. Chanchal Sharma,
Assistant Attending, Clinical Psychologist. Dr. Sharma offers free, confidential, on-site
and virtual short-term psychotherapy and interventions as well as assistance with
linkage to external clinical SW, psychologists and psychiatrists. For more information,
please see the mental health contact information page below
.
Long-Term Counseling and/or Psychiatric Prescription Management, if needed
Students have access to long-term counselors through their health insurance
provider.
Students who need assistance identifying healthcare providers and long-term mental
health counselors who are within their insurance network can either contact their health

GSK Student & Faculty Handbook
v. 6/2024 42
insurance provider directly or use MSK’s Health Advocate program.
Health Advocate helps you navigate the healthcare system and make the most of
your MSK medical plan. Turn to them throughout the year at (866) 695-8622 for help:
• Finding doctors, hospitals, and other providers in your plan network
• Scheduling appointments and tests, and transferring medical records
• Resolving billing and insurance claims issues
https://mskbenefits.mskcc.org/keyword/health-advocate/
Telemedicine for Mental Health Through Your MSK Medical Plan: If you are
enrolled in any of the MSK medical plans, you and your covered dependents have
access to free mental health services through your medical carrier’s telemedicine
provider. You can schedule virtual visits with licensed psychiatrists, psychologists,
counselors, and social workers. Depending on which MSK medical plan you’re enrolled
in, you can use Teladoc, LiveHealth Online, or Virtual Visits to schedule confidential
telemedicine visits with licensed mental health clinicians. You have the freedom to
choose your healthcare provider, and you may continue your future visits with the same
provider, if you choose. And Teladoc, LiveHealth Online, and Virtual Visits adhere to all
HIPPA requirements, so you can feel confident knowing your health information is
secure.
Dental Insurance
All students are provided with dental coverage through MetLife. The plan pays up to
$1,000 in benefits for each covered person in a calendar year for preventive, diagnostic and
restorative service.
Vision Coverage
All students are provided with the VSP Vision Care Plan, which provides an annual benefit
for eye exams, glasses or contacts.
Housing and Cost of Living
Students who matriculate in GSK are offered affordable housing owned by MSK. The
most available housing options will be studio or shared apartments with a 2024 monthly
rent of $1,617 or $1,320, respectively. The cost of housing is very competitive for this
area.
The occupancy agreement should be read carefully because it is a contract between MSK
and the student. The housing contract is in effect for the duration of study and is
automatically renewed each year. If a student wishes to vacate MSK housing before
completion of the program, the student must abide by the vacate policy of the Housing
Division.
Students who are finishing their degrees must comply with the GSK sign-out process,
which includes a 30-day notice for termination of the occupancy agreement with MSK.
The GSK and MSK Hardship Fund
GSK and MSK are committed to providing support to the many scientists engaged in
cancer research, endeavoring to maximize their potential and facilitate groundbreaking
discoveries in the field. Through dedicated resources and collaborative opportunities,
MSK and GSK aim to empower its scientists to push the boundaries of cancer research.

GSK Student & Faculty Handbook
v. 6/2024 43
To this end, the MSK and GSK Emergency Hardship Fund has been established to offer
financial support to MSK trainees and GSK students who are facing sudden and
temporary financial challenges resulting from personal or family emergencies. This GSK
portion of the fund was made possible by Mr. Louis V. Gerstner Jr.
, Chair Emeritus of the
GSK Board and the namesake of the graduate school.
This fund is intended to provide aid to GSK students who are unable to resolve immediate
financial difficulty through fellowships, loans, or personal resources, and to eventually help
them return to financial stability.
To learn more about eligible hardship expenses and the application process, visit the
Hardship fund OneMSK page.
If you have further questions, please email mskhardshipfund@mskcc.org
.
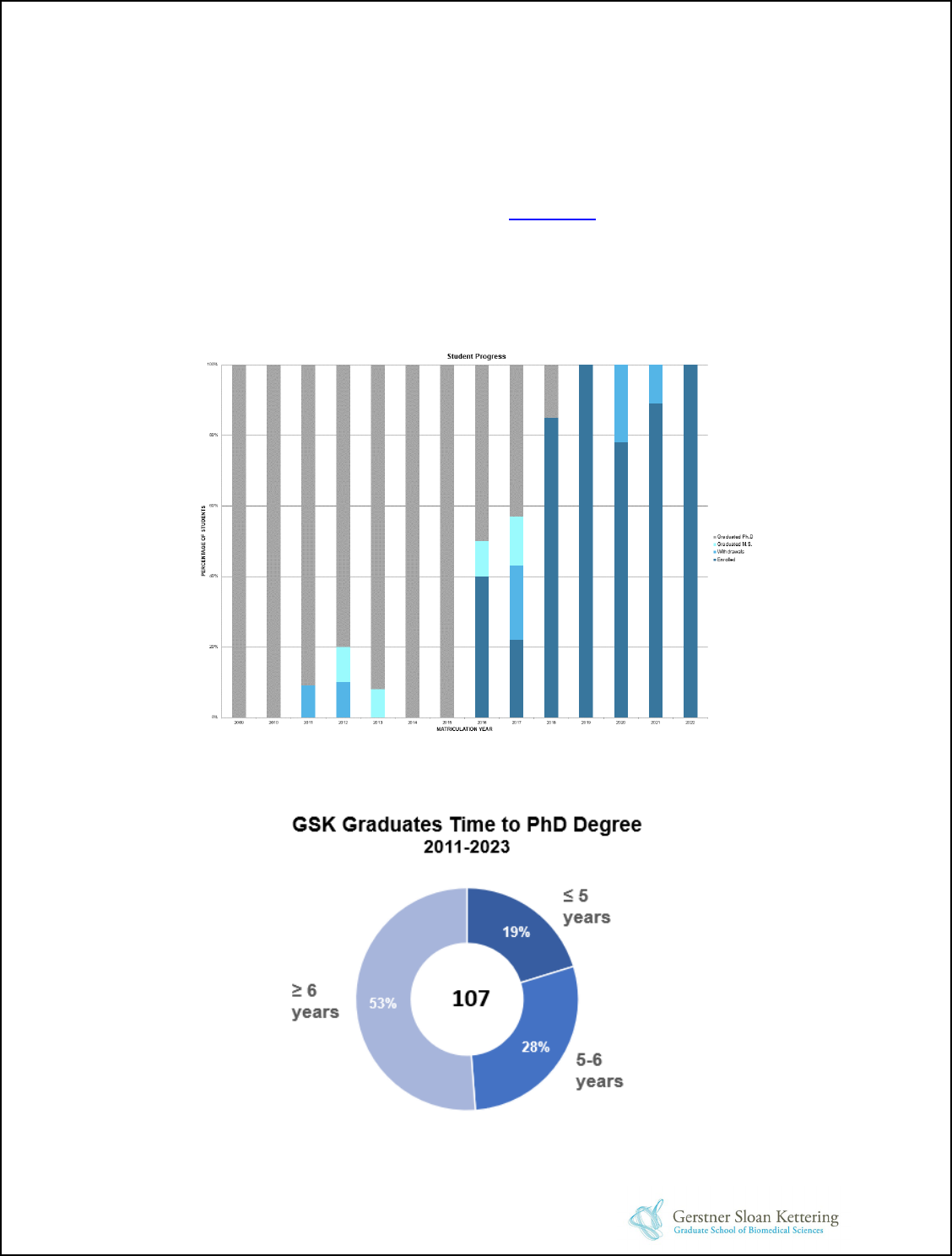
GSK Student & Faculty Handbook
v. 6/2024 44
Student Progress and Outcomes
Gerstner Sloan Kettering Graduate School has matriculated 220 students since our first
class enrolled in 2006. We have a retention rate of 91% and the average time to degree is
6 years. Our students have been very successful, publishing over 480 papers and
competing successfully for 69 independent graduate research fellowships such as those
from the NIH and NSF. Directly after graduation, our alumni
have pursued postdoctoral and
full-time research positions in academia and industry as well as science-related careers in
business and finance including positions as a biotech analyst, science writer, biotech firm
manager, and consultant.
*As of Fall 2023

GSK Student & Faculty Handbook
v. 6/2024 45
Compliance Policies
•
Code of Conduct
•
Endorsement Policy
•
Special Considerations for GSK Students Working on Conflicted Faculty/Mentor
Research
•
Prohibition on the Marketing of Credit Cards
•
Policy Against Harassment and Discrimination
•
Policy for the Prevention of and Response to Sexual Misconduct
•
Amendments to the Educational Law
•
Campus Security Rules & Regulations
•
Drug-Free Schools And Communities Act (DFSCA) Substance Abuse Policy
•
Family Education Rights and Privacy Act (FERPA)
•
Student Resources
•
Compliance Hotline
N.B., MSK’s Required Regulatory Training, which all MSK employees are subject to,
includes the following online courses that cover a subset of regulatory and compliance-
related topics:
1. Workplace Conduct
2. Safety & Emergency Preparedness
3. Information Technology (IT) Security
4. Privacy Basics
Memorial Sloan Kettering Cancer Center Code of Conduct
The Code of Conduct describes the governing values and standards of conduct for
everyone associated with MSK. All MSK workforce members are required to follow the
standards described in this Code when performing work in support of the Center’s mission.
The full Code of Conduct may be accessed via MSK website: Code of Conduct
. A hard
copy is distributed to incoming students.
Endorsement Policy
Gerstner Sloan Kettering will not promote events or provide resources including finances,
staff, facilities, and the use of GSK/MSK branding in support of any product, cause, political
party, movement, or candidate unrelated to our scientific and educational mission.
Special Considerations for GSK Students Working on Conflicted Faculty/Mentor
Research
MSK has adopted the Policy on Conflict of Interest and Commitment
(“MSK Individual COI
Policy”), which encompasses GSK, to identify, review, and manage outside interests and
relationships that may give rise to conflicts of interest (“COI”) in the context of an
individual’s institutional responsibilities. GSK students engaged in research at MSK are
considered “Covered Persons” under the MSK Individual COI Policy and are therefore
subject to the training, disclosure, and other requirements outlined in this policy.
GSK student involvement in research related to a company in which a GSK faculty
m
ember maintains a financial interest, including a start-up company founded by that faculty

GSK Student & Faculty Handbook
v. 6/2024 46
member, requires special considerations from a COI perspective to ensure that both the
student and educational mission are protected. Particular challenges arise when a GSK
faculty member also serves as a mentor to a GSK student (including First Year Mentor,
Rotation Mentor, or Thesis Mentor), and directs that student on a project that appears to
benefit a company that the mentor founded or maintains a financial interest in.
To manage such potential conflicts, the following strategies should be employed:
1. Disclosure of Faculty Member Interests under COI Management. GSK faculty
members, including mentors, under a COI Management Plan issued by the MSK COI
Office, must provide full disclosure of their financial interests and relationship with any
company that they have founded and/or maintain a financial interest in, to the
following:
Any students working on research related to the company (i.e. research
involving the company as a funder, collaborator, or sub-contractee, and/or
involving intellectual property owned by, licensed to, or intended to be
commercialized by the company);
Any students for whom the faculty member serves as mentor; and
The Thesis Committees of students working in that faculty member’s lab.
2. Notification of Interests to GSK Dean. The GSK Dean’s Office will be notified by
the MSK COI Office of any faculty member covered under a COI Management Plan
and whose interests may impact GSK students working on related research or under
that faculty member’s supervision.
3. Q
uestions/Concerns. Students may direct questions or concerns related to the
effect of a faculty member’s conflict on their work to the GSK deans and/or the MSK
COI Office (
ecoi@mskcc.org), and/or the Office of the Ombudsperson
(ombudsiac@mskcc.org).
Prohibition on the Marketing of Credit Cards
In accordance with New York State Law (Article 129-A, Section 6437), the advertising,
marketing or merchandising of credit cards on the GSK campus to students is prohibited.
Policy Against Harassment and Discrimination
Scope
This policy applies to all applicants for employment, MSK employees, remote workers,
contingent workers, contractors, vendors, and students. This policy prohibits harassment,
discrimination and retaliation, whether engaged in by fellow employees/students, by a
manager, or by someone not directly connected to MSK (e.g., an outside vendor,
consultant, customer conducting business with the institution, patient, or visitor who uses
MSK’s resources or who visits patients). Conduct prohibited by this policy is unacceptable
in the workplace as well as in any work- related setting outside the workplace, such as
during business trips or at business meetings and business-related social events, or in
any other situations that might adversely impact the workplace.

GSK Student & Faculty Handbook
v. 6/2024 47
Introduction
MSK is committed to a work environment in which all individuals are treated with respect
and dignity. Everyone has the right to work in a professional atmosphere that prohibits
harassment, discrimination and retaliation. MSK expects that all work relationships among
employees/students or between employees/students and persons outside the institution
will be business-like and free of discrimination, harassment and retaliation.
Policy
MSK abides by all applicable federal, state and local laws which prohibit discrimination or
harassment on the basis of race, color, religion, creed, gender, age, sex (including sexual
harassment), national or ethnic origin, marital, caregiver, familial or partnership status,
sexual orientation, gender identity or expression or transgender status, pregnancy, sexual
and reproductive health choices, citizenship status or alienage, disability, status in the
uniformed services of the United States (including veteran status), credit and salary
history, unemployment status, genetic pre-disposition or carrier status, status as a victim
of domestic violence, sexual violence or stalking, arrest and conviction record or any other
status protected by law in any employment program, policy, or practice of MSK. The
prohibition of discrimination on the basis of race, color, religion or creed also prohibits
grooming or appearance policies that ban, limit, or otherwise restrict natural hair or
hairstyles associated with racial, ethnic, or cultural identities, or restrictions on attire,
clothing or facial hair that would require an employee/student to violate or forego a
sincerely held practice of their religion.
In accordance with these laws, MSK also prohibits retaliation against anyone who has
complained about harassment or discrimination or has otherwise exercised rights
guaranteed by these laws. All employees/students of MSK have an obligation to
cooperate in the application of this policy and the investigation into alleged violations of
this policy. Employees/students found to have engaged in acts of discrimination,
harassment and/or retaliation will be subject to corrective action, up to and including
termination.
Definitions:
Sexual harassment is a form of gender-based discrimination and is defined as unwelcome
sexual advances, requests for sexual favors and other verbal or physical conduct of a
sexual nature when, for example: (i) submission to such conduct is made either explicitly
or implicitly a term or condition of an individual’s employment; (ii) submission to or
rejection of such conduct by an individual is used as the basis for employment decisions
affecting such individual; or (iii) such conduct has the purpose or effect of unreasonably
interfering with an individual’s work performance or creating an intimidating, hostile or
offensive working environment.
Discrimination based on sex stereotypes, gender expression and perceived identity are all
forms of sexual harassment. The gender spectrum is nuanced, but the three most
common ways people identify are cisgender, transgender, and non-binary. A cisgender
person is someone whose gender aligns with the sex they were assigned at birth. A
transgender person is someone whose gender is different than the sex they were
assigned at birth. A non-binary person does not identify exclusively as male or female.
Sexual harassment may include a range of subtle and not so subtle behaviors and may
involve individuals of the same or different gender. Harassment does not need to be
severe or pervasive to be illegal. Depending on the circumstances, these behaviors may
include, but are not limited to: unwanted sexual advances or requests for sexual favors;
sexual jokes and innuendo; intentional misuse of an individual's preferred pronouns;

GSK Student & Faculty Handbook
v. 6/2024 48
verbal abuse of a sexual nature; commentary about an individual’s body, sexual prowess
or sexual deficiencies; leering, catcalls or touching; insulting or obscene comments or
gestures; display or circulation of pictures, videos or electronic communications; and other
physical, verbal or visual conduct of a sexual nature.
Harassment or discrimination on the basis of any other protected characteristic is defined
as verbal or physical conduct that denigrates or shows hostility or aversion toward an
individual because of his or her race, color, religion, creed, sex, national or ethnic origin,
age, disability, citizenship status or alienage, sexual orientation, veteran status, marital,
caregiver, familial or partnership status, gender identity or expression or transgender
status, pregnancy, sexual and reproductive health choices, credit and salary history,
unemployment status, genetic pre-disposition or carrier status, status in the uniformed
services of the United States (including veteran status), status as a victim of domestic
violence, sexual violence or stalking, arrest and conviction record or any other status
protected by law or that of his or her relatives, friends or associates, and that: (i) has the
purpose or effect of creating an intimidating, hostile or offensive work environment; (ii) has
the purpose or effect of unreasonably interfering with an individual’s work performance; or
(iii) otherwise adversely affects an individual’s employment opportunities (such as a
promotion, pay increase or performance evaluation).
Harassing conduct includes, but is not limited to: epithets, slurs or stereotyping;
threatening, intimidating or hostile acts; denigrating jokes; and display, circulation, or
electronic communication in the workplace of written or graphic material that denigrates or
shows hostility or aversion toward an individual or group. Intent does not affect whether
conduct is considered harassment.
Retaliation
MSK prohibits retaliation against any individual who reports discrimination or harassment
or participates in an investigation of such reports. Retaliation against an individual for
reporting discrimination or harassment or for participating in an investigation of a claim of
discrimination or harassment is a serious violation of this policy and, like harassment or
discrimination, will be subject to corrective action, up to and including termination.
Confidentiality
Cases involving discrimination, harassment and/or retaliation are particularly sensitive,
and confidentiality is taken very seriously. Dissemination of information relating to the
case will be limited to individuals who have a legitimate “need to know” or who have
information regarding the matter.
Complaint and Investigation Procedures
MSK strongly urges the prompt reporting of all incidents of harassment, discrimination
and/or retaliation, regardless of the offender’s identity or position, so that rapid and
appropriate action can be taken. Generally, if practical, an employee/student who believes
he or she is being harassed, discriminated and/or retaliated against should inform the
individual who is the source of the perceived harassment, discrimination and/or retaliation
that the employee/student is offended by the behavior and request that it be stopped.
If, for any reason, the employee/student does not feel comfortable discussing the
perceived harassment, discrimination and/or retaliation directly with the individual who is
the source of the perceived conduct, or if the employee/student has requested that the
behavior stop and it has not stopped, the employee/student should immediately report the
conduct to his or her manager, any other management-level employee of MSK, an
Employee Relations Advisor, any representative of the HR Legal & Regulatory Affairs

GSK Student & Faculty Handbook
v. 6/2024 49
Department or the MSK Compliance Hotline (866-568-5421 or
https://mskcc.ethicspoint.com
). Employees may complain verbally or in writing by
submitting a complaint using the complaint form available here. There is no requirement
to use the complaint form.
If the harassment, discrimination and/or retaliation complaint is reported to someone other
than a representative of the Human Resources Department, the individual hearing the
complaint must report it to an Employee Relations Advisor or the HR Legal & Regulatory
Affairs Department. The HR Representative will promptly and thoroughly investigate the
complaint, ensuring confidentiality to the extent possible throughout the investigation
process. The investigation may include individual interviews with parties involved and,
where necessary, with individuals who may have observed the alleged conduct or may
have other relevant knowledge.
Based on the findings of the investigation, the HR Representative will recommend
appropriate action, if any, to be taken. Such action may include training, referral to
counseling and/or corrective action, such as a warning, reprimand, withholding of a
promotion or pay increase, reassignment, temporary suspension with or without pay, or
termination of employment, as MSK determines is appropriate under the circumstances.
An HR Representative, in connection with appropriate management, will inform both the
employee/student complaining of harassment, discrimination and/or retaliation and the
individual alleged to have engaged in said behavior of the results of the investigation, and
will ensure that any agreed-upon action is carried out. Managers and supervisors have a
responsibility to address the needs of those who have experienced harassment or
discrimination to ensure the workplace is safe, supportive, and free from retaliation.
Bystander Intervention
Any employee/student witnessing harassment, discrimination and/or retaliation as a
bystander is encouraged to report it. A manager or supervisor that is a bystander is
required to report it. There are five standard methods of bystander intervention that can
be used when anyone witnesses harassment or discrimination: (i) interrupt the
harassment by engaging with the individual being harassed; (ii) ask a third party to help
intervene; (iii) record or take notes on the harassment to benefit a future investigation; (iv)
check in with the person who experienced harassment or discrimination; (v) if safe,
confront the harasser(s) and name the behavior as inappropriate.
Legal Protections and External Remedies
Discrimination, harassment and retaliation are prohibited not only by MSK, but also by
state, federal and local law. Employees/students who bring claims in these external
venues and prevail on those claims will be entitled to remedies, which could include a
monetary award.
Aside from MSK’s internal processes, employees/students also may choose to pursue
legal remedies with the following governmental entities.
New York State Division of Human Rights
The Human Rights Law (HRL), codified as N.Y. Executive Law, art. 15, § 290 et seq.,
applies to employers in New York State regarding discrimination, harassment and
retaliation, and protects employees/students, paid or unpaid interns and non-employees
regardless of immigration status. A complaint alleging violation of the Human Rights Law
may be filed either with New York State Division of Human Rights (DHR) or in New York
State Supreme Court.

GSK Student & Faculty Handbook
v. 6/2024 50
Complaints with DHR may be filed any time within one year of the discrimination,
harassment or retaliation. If an individual did not file at DHR, they can sue directly in state
court under the HRL, within three years of the alleged discrimination, harassment or
retaliation. An individual may not file with DHR if they have already filed an HRL complaint
in state court. In addition, New York State has established a free and confidential hotline
(1-800-HARASS-3) to connect with pro bono attorneys on sexual harassment issues, or
submit a complaint.
United States Equal Employment Opportunity Commission (EEOC)
The EEOC enforces federal anti-discrimination laws, including Title VII of the 1964 federal
Civil Rights Act (codified as 42 U.S.C. § 2000e et seq.). An individual can file a complaint
with the EEOC anytime within 300 days from the discrimination, harassment or retaliation.
There is no cost to file a complaint with the EEOC. The EEOC will investigate the
complaint and determine whether there is reasonable cause to believe that discrimination,
harassment or retaliation has occurred, at which point the EEOC will issue a Right to Sue
letter permitting the individual to file a complaint in federal court.
Local Protections
Many localities enforce laws protecting individuals from discrimination, harassment and
retaliation. An individual should contact the county, city or town in which they live to find
out if such a law exists. For example, employees /students who work in New York City
may file complaints of discrimination, harassment or retaliation with the New York City
Commission on Human Rights.
Anti-Discrimination, Harassment and Retaliation Training
All MSK employees/students and contingent workers must complete anti-discrimination,
harassment and retaliation training on an annual basis. Training will be provided through
MSK’s Required Regulatory Training.

GSK Student & Faculty Handbook
v. 6/2024 51
Policy for the Prevention of and Response
to Sexual Misconduct
To the extent that this Policy overlaps with Gerstner Sloan Kettering Graduate School’s
Non- Discrimination, Anti-Harassment, and Anti-Retaliation Policies, this Policy will
control in cases involving sexual misconduct, including but not limited to sex
discrimination (to the extent described below), sexual harassment, and/or sexual
violence, sexual assault, domestic violence, dating violence, and stalking against a
student.
1. Policy Statement
The Policy for the Prevention of and Response to Sexual Misconduct (“Policy”) is for the
benefit of students at the Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences
(“GSK” or “the School”), Memorial Sloan Kettering Cancer Center (“MSK”). GSK is
committed to maintaining an educational environment for students that is free from sexual
misconduct. GSK does not discriminate on the basis of sex, gender, parental, family or
marital status in its education programs and activities, and it is required by Title IX of the
U.S. Education Amendments of 1972 not to discriminate in such a manner.
GSK strongly encourages every member of our community who is a victim of, or has
knowledge of sexual misconduct, including but not limited to sex discrimination, sexual
harassment, and/or sexual violence, sexual assault, domestic violence, dating violence,
and/or stalking, against a student to report that conduct as set forth below. GSK is
committed to responding to such reports promptly, with sensitivity for all concerned, and
with a fair and equitable process.
2. Controlling Law
A. Title IX. GSK complies with Title IX of the Educational Amendments of 1972 (“Title
IX”), which is a federal law that prohibits any person in the United States from being
discriminated against on the basis of sex in seeking access to any educational
program or activity receiving federal financial assistance. The U.S. Department of
Education, which enforces Title IX, has determined that Title IX’s prohibition on sex
discrimination includes various forms of sexual harassment and sexual violence
that may interfere with a student’s ability to equally access education programs or
activities.
B. Article 129-B. Article 129-B of the New York Education Law (“Article 129-B) is a
state law that also provides protections for students who are the victims of sexual
misconduct, including the right to report the incident to GSK or law enforcement, to
be protected by GSK from retaliation for reporting an incident, and to receive
assistance and resources from GSK.
3. Individuals and Conduct Covered
This Policy covers any occurrence of sexual misconduct, including but not limited to
sex discrimination, sexual harassment, and/or sexual violence, sexual assault,

GSK Student & Faculty Handbook
v. 6/2024 52
domestic violence, dating violence, and stalking, regardless of whether the accused is
a student, employee or third party, or whether the prohibited conduct occurred on or off
campus.
GSK will address reported sexual misconduct against a student whether the report is
made by the alleged victim or a reporting individual. GSK will also ensure that a
student who is the victim of sexual misconduct is afforded the protections outlined in
the Students’ Bill of Rights, which appears at the end of this Policy, including the right
to make a report to local law enforcement and to be protected from retaliation.
4. Definitions
“Affirmative Consent” is a knowing, voluntary, and mutual decision among all participants
to engage in sexual activity. Consent can be given by words or actions, as long as those
words or actions create clear permission regarding willingness to engage in the sexual
activity. Silence or lack of resistance, in and of itself, does not demonstrate consent. The
definition of consent does not vary based upon a participant’s sex, gender, sexual
orientation, gender identity, or gender expression. The following principles are provided as
guidance for the GSK community regarding the concept of Affirmative Consent:
a) Consent to any sexual act or prior consensual sexual activity between or with any
party does not necessarily constitute consent to any other sexual act.
b) Consent is required regardless of whether the person initiating the act is under the
influence of drugs and/or alcohol.
c) Consent may be initially given but withdrawn at any time.
d) Consent cannot be given when a person is incapacitated, which occurs when an
individual lacks the ability to knowingly choose to participate in sexual activity.
Incapacitation may be caused by the lack of consciousness or being asleep, being
involuntarily restrained, or if an individual otherwise cannot consent. Depending on
the degree of intoxication, someone who is under the influence of alcohol, drugs, or
other intoxicants may be incapacitated and therefore unable to consent.
e) Consent cannot be given when it is the result of any coercion, intimidation, force, or
threat of harm.
f) When consent is withdrawn or can no longer be given, sexual activity must stop.
“Complainant” is any individual who has reported being or is alleged to be the victim of
conduct that could constitute sexual misconduct. A complainant may also be referred to as
a Reporting Individual.
“Dating Violence” is violence committed by a person who is or has been in a social
relationship of a romantic or intimate nature with the victim. Dating violence includes, but is
not limited to, sexual or physical abuse or the threat of such abuse. The existence of such
a relationship shall be determined based on the complainant’s statement and with
consideration of the length of the relationship, the type of relationship, and the frequency of
interaction between the persons involved in the relationship.
“Domestic Violence” is also considered relationship violence and includes any felony or
misdemeanor crimes of violence committed by a current or former spouse or intimate
partner of the victim, by a person with whom the victim shares a child in common, by a

GSK Student & Faculty Handbook
v. 6/2024 53
person who is cohabitating with or has cohabitated with the victim as a spouse or intimate
partner, by a person similarly situated to a spouse of the victim under New York domestic
or family violence laws or by any other person against an adult or youth victim who is
protected from that person's acts under the domestic or family violence laws of New York.
A “Hostile Environment” is created when prohibited conduct is sufficiently severe or
pervasive as to limit or deny a student’s ability to participate in or benefit from GSK’s
educational programs or activities.
“Respondent” is an individual who has been reported to be the perpetrator of conduct that
could constitute sexual misconduct under this Policy.
“Sex Discrimination” is inequitable treatment of an individual on the basis of the
individual’s actual or perceived gender or sex.
“Sexual Assault” is any type of sexual contact or behavior that occurs without the explicit
consent of the recipient, including instances where the victim is incapable of giving
consent. Examples of such contact or behavior include rape, fondling, incest, and statutory
rape.
“Sexual Harassment as defined by Title IX” conduct that includes one or more of the
following: (1) an employee of the School conditioning the provision of an aid, benefit, or
service of the School on an individual’s participation in unwelcome sexual conduct (i.e.,
quid pro quo); (2) unwelcome conduct determined by a reasonable person to be so severe,
pervasive, and objectively offensive that it effectively denies a person equal access to the
School’s education program or activity; or (3) sexual assault, domestic violence, dating
violence and stalking. (Sexual harassment is defined more broadly under other laws and
under other School policies, including the MSK Policy Against Harassment and
Discrimination).
“Sexual Misconduct” includes sexual discrimination, sexual harassment, sexual violence,
sexual assault, domestic violence, dating violence and stalking. For sexual misconduct to
fall under Title IX, it must: (1) meet the definition of sexual harassment as defined by Title
IX; (2) occur in GSK’s education program or activity; and (3) within the United States.
Incidents of sexual violence, sexual assault, domestic violence, dating violence and
stalking that occur off campus or outside of the United States, in which a student is a
complainant or respondent, violate New York State law, and will follow the procedures set
forth below, except there is no live hearing process for such claims – the investigator’s
Investigative Report will be reviewed by the Designated Arbiter, who will determine whether
there has been a violation of this Policy. Sexual harassment that occurs outside of the
School’s education program or activities, or incidents that do not meet the definition of
sexual harassment in this Policy, may fall under other GSK and MSK policies and will be
addressed consistent with those policies.
“Sexual Violence” is an actual or attempted physical sexual act performed against a
person’s will or without a person’s affirmative consent, including where the person is
incapable of giving consent due to a disability or the use of drugs and/or alcohol.
“Stalking” is a course of conduct directed at a specific person that would cause a
reasonable person to fear for their safety or the safety of others or suffer substantial
emotional distress.

GSK Student & Faculty Handbook
v. 6/2024 54
5. Reporting a Violation of this Policy
A. Title IX Coordinators. GSK has designated and authorized the following
individuals as Title IX Coordinators to address concerns or inquiries regarding
discrimination on the basis of sex, including sexual harassment, sexual assault,
dating violence, domestic violence, stalking, and gender-based harassment:
Leslie Ballantyne, Esq.
Vice-President, HR Legal &
Regulatory Affairs
633 Third Avenue
Telephone: (646) 227-2742
Email: ballantl@mskcc.org
Lindsay Cornacchia, Esq.
Associate Director of
Compliance & Assistant
General Counsel
1275 York Avenue
Telephone: (212) 639-2496
Email: cornaccl@mskcc.org
Any individual may report a violation of this Policy (whether or not the person reporting is
the person alleged to be the victim of conduct that could constitute sex misconduct) to the
Title IX Coordinators at any time, including during non-business hours, by mail, phone, or
email.
The Title IX Coordinators oversee implementation of GSK’s Policies and must be informed
of all reports and complaints of sexual misconduct, including but not limited to sex
discrimination, sexual harassment, and/or sexual violence, sexual assault, domestic
violence, dating violence, and stalking against a student, even if the report or complaint
was initially made to another individual or if the investigation will be conducted by another
individual or office.
The Title IX Coordinators are responsible for:
• Activating GSK’s Title IX grievance fact-finding, hearing, and determination
procedures;
• Evaluating confidentiality requests;
• Determining the resources required to conduct an investigation, if warranted;
• Conducting and/or managing a grievance investigation and appeal, including
designating other GSK employees or third parties to assist, as needed and working
with law enforcement when necessary;
• Determining appropriate interim measures for a complainant, including providing
support and counseling resources, and taking steps to protect public safety during
the course of an investigation;
• Determining appropriate sanctions against an offender and remedies for the
complainant;
• Enforcing sanctions with the assistance of GSK and MSK’s administrative
leadership; and
• Recommending necessary changes to GSK’s policies or procedures, as needed.
B. Other Options for Reporting. In addition to the Title IX Coordinators, reports can
also be made to:

GSK Student & Faculty Handbook
v. 6/2024 55
• Reporting to Law Enforcement: Members of the GSK community have the option
of reporting sexual misconduct to law enforcement. Formal complaints of sexual
misconduct can be made to local law enforcement authorities by contacting the
NYPD (by calling 911 or reporting the crime to a local police precinct), the District
Attorney of New York Sex Crimes Unit at (212) 335-9373, or the New York State
Police Sexual Assault Victims Unit.
• Reporting to the MSK Compliance Hotline (by calling 866.568.5421 or by going
to https://mskcc.ethicspoint.com
). The MSK Compliance Hotline is available 24
hours a day, every day of the year, and is managed by an outside company.
Reports can be made anonymously.
• Reporting to the School Security Department or Vice-President of Security:
The School Security Department can be reached by dialing (212) 639- 7866. Mark
Moodie, the Vice-President of Security, can be reached at (212) 639-7863 or
moodiem@mskcc.org
.
• Reporting to the MSK Human Resources Department: Melissa Maxwell-Avila,
MSK Human Resources Associate Director, Business Talent Consulting, can be
reached at (646)227-3888 or maxwellm@mskcc.org
and Nina Kosch, MSK Senior
Employee Relations Advisor, can be reached at (646) 952-1498 or
koschn@mskcc.org.
6. Confidentiality & Privacy
GSK values the privacy of its community members, and individuals should be able to
seek the assistance they need without fear that the information they provide will be
shared more broadly.
A. Confidentiality. Some resources on campus are confidential and will not share
any identifying information with others, except as required by law in emergency
circumstances. If an individual complainant wishes to keep their identity
anonymous, they can use a confidential resource. Confidential resources can and
will maintain the confidentiality of information provided to them by a complainant,
accused, or reporting individual. A list of available confidential resources can be
found on the GSK website.
B. Privacy. Other resources are not confidential but will protect students’ privacy to
the greatest extent possible and share information with others only on a need-to-
know basis to comply with the School’s obligations under the law and School
policies. This includes the Title IX Coordinators, as well as certain GSK and MSK
personnel (including officials with authority) who have a duty to report conduct
prohibited under this Policy to the Title IX Coordinator or take action to redress such
conduct. These personnel will share a complaint or report only as necessary for the
Title IX Coordinators to investigate and/or seek a resolution and, if required, with
law enforcement.
C. Requests for Confidentiality. Where a victim of sexual misconduct wishes to
maintain confidentiality, GSK must weigh the request(s) against its obligation to
provide a safe, non-discriminatory environment for all members of the community,
including the victim, and its legal obligations. The Title IX Coordinators will evaluate
requests for confidentiality once the Title IX Coordinators are on notice of alleged

GSK Student & Faculty Handbook
v. 6/2024 56
sexual misconduct.
7. Complainant’s Rights
A. A complainant has the right to make a complaint, withdraw a complaint at any time,
or not report a complaint. A complainant also has the right to file a criminal
complaint or to pursue his or her rights under Title VII of the Civil Rights of 1964,
before, during, or after (i) reporting a Title IX complaint, or (ii) activating GSK’s
internal Title IX investigation or appeal process.
B. Individuals that choose to make a complaint of sexual misconduct, including but not
limited to sex discrimination, sexual harassment, and/or sexual violence, sexual
assault, domestic violence, dating violence, and stalking are afforded the right to:
Notify GSK’s Vice President of Security, local law enforcement, and/or
state police;
Have emergency access to the Title IX Coordinators who will provide
information regarding options to proceed, the importance of preserving
evidence, and detailing the criminal justice process and the standards of
proof that apply;
Disclose confidentially the incident to institution representatives who may
assist in obtaining services;
Disclose confidentially the incident and obtain services from the state or
local government;
Disclose the incident to GSK or MSK representatives who can offer
privacy or confidentiality, as appropriate;
File a report of sexual assault, domestic violence, dating violence, and/or
stalking and the right to consult the Title IX Coordinators and other
appropriate GSK or MSK representatives;
Disclose the incident to MSK’s Human Resources division or the right to
request that a confidential or private employee assist in reporting to the
appropriate human resources representative; and
Withdraw a complaint or involvement from the GSK or MSK process at
any time.
C. Supportive Measures
Complainants who report allegations that fall under this Policy, have the right to
receive supportive measures from GSK regardless of whether they desire to file a
complaint. Supportive measures are non-disciplinary and non-punitive. They may
be made available free of charge and kept confidential except as necessary to
facilitate the supportive measure. Supportive measures may include, but are not
limited to, the following:
Counseling

GSK Student & Faculty Handbook
v. 6/2024 57
Extensions of deadlines or other course/resource-related adjustments
Modifications of work or class schedules
Campus security escort services
Restrictions on contact between the parties (no contact orders)
Changes in work or housing locations
Leaves of absence
Increased security and monitoring of certain areas of the campus
8. Grievance Process for Addressing Complaints of Sex Discrimination
Any complaint of sex discrimination alleged by a student – i.e., alleged unfavorable
treatment of a student on the basis of the student’s gender – shall be subject to the
complaint procedures set forth in GSK’s Non-Discrimination, Anti-Harassment, and
Anti-Retaliation Policy, including prompt investigation and responsive action, if
appropriate.
9. Grievance Process for Addressing Complaints of Sexual Misconduct
1
A. Initial Assessment
1. Following receipt of a report of sexual misconduct, the Title IX Coordinator
will conduct an initial assessment. As part of the initial assessment, the Title
IX Coordinator will take the following steps:
a) Assess the nature and circumstances of the complaint/report.
b) Address the immediate needs and concerns of the complainant,
including physical safety and emotional well-being needs.
c) Provide copies of or direct the complainant to GSK’s Policy and
accompanying procedures.
d) Provide the complainant with information about resources, including
information about intervention, mental health counseling and medical
services, sexually transmitted infections, and sexual assault forensic
examinations.
e) Provide information regarding options to proceed, the importance of
1
On May 19, 2020, the U.S. Department of Education issued a Final Rule under Title IX that: (1) defines the meaning of
“sexual harassment”; (2) addresses how this institution must respond to reports of misconduct falling within that definition of
sexual harassment; and (3) mandates a grievance process that this institution must follow to comply with the law in these
specific covered cases before issuing a disciplinary sanction against a person accused of sexual harassment. Under the
Final Rule, GSK must narrow both the geographic scope of its authority to act under Title IX and the types of “sexual
harassment” that it must subject to its Title IX adjudication process. Incidents that fall outside the definition of sexual
harassment under Title IX will follow the procedures set forth below, except there is no live hearing process for such claims –
the final Investigative Report will be reviewed by the Designated Arbiter, who will determine whether there has been a
violation of this Policy.

GSK Student & Faculty Handbook
v. 6/2024 58
preserving evidence, and detailing the criminal justice process and the
standards of proof that apply.
f) Direct the complainant to information regarding on-campus and off-
campus resources and the range of appropriate and available supportive
and protective measures.
g) Explain GSK’s policy prohibiting retaliation.
2. If the Title IX Coordinator determines that the circumstances warrant
proceeding to an investigation, the School will ask for consent from the
complainant before commencing an investigation, and whether the
complainant wants to file a formal complaint. A formal complaint is a
document filed by the complainant (in person, by mail, or by electronic mail)
with the Title IX Coordinator alleging sexual misconduct against a
respondent. At the time of filing a formal complaint, a complainant must be
participating in or attempting to participate in the education program or
activity of the School.
3. A complainant may decline to file a formal complaint and request that GSK
not investigate or take action. GSK will honor the complainant’s request
unless the School, through the Title IX Coordinator, determines in good faith
that it is necessary to proceed in order to limit the risk of harm to the
complainant or other members of the School Community, in which case the
Title IX Coordinator may sign the formal complaint. The School will inform
the complainant of this decision in writing, and the complainant need not
participate in the process further but will receive all notices issued under this
Policy and Process. Nothing in this Policy prevents a complainant from
seeking the assistance of state or local law enforcement alongside the
appropriate on-campus process.
4. The following factors will be used when determining whether to honor a
request to not investigate or take action:
a) Whether the accused has a history of violent behavior or is a repeat
offender;
b) Whether the incident represents escalation in unlawful conduct on behalf
of the accused from previously noted behavior;
c) The increased risk the accused will commit additional acts of violence;
d) Whether the accused used a weapon or force;
e) Whether the complainant is a minor; and
f) Whether the institution possesses other means to obtain evidence such
as security footage, and whether available information reveals a pattern
of perpetration at a given location or by a particular group.

GSK Student & Faculty Handbook
v. 6/2024 59
B. Determining Jurisdiction under Title IX or other GSK Policies
1. In the first instance, the Title IX Coordinator or designee shall determine
whether the alleged conduct falls under the definition of sexual misconduct
under Title IX – including the requirement that the conduct occur in the School’s
educational program or activities and within the United States.
2. If the Title IX Coordinator determines that a complaint does not fall under the
definition of sexual misconduct under Title IX – for example, if the alleged
incident did not occur in the School’s education program or activity – the School
will promptly send a Notice of Dismissal of the action under Title IX to both
parties. Dismissal at this stage may be reviewed on appeal.
3. Where the complainant or respondent is a student, the Title IX Coordinator or
designee will then assess whether the complaint alleges sexual misconduct
under this Policy, outside of the Title IX definition, including sexual assault,
stalking, domestic violence and/or dating violence that occurred off campus.
Conduct in this category will be investigated and assessed pursuant to the
procedures set forth below, except that there will not be hearings in such
matters – rather, the Investigative Report will be reviewed by the Designated
Arbiter, and the Designated Arbiter will make a determination as to whether a
violation of this Policy occurred.
4. If the conduct alleged does not constitute sexual misconduct as defined by this
policy, it may still violate other School policies, and it may be investigated
consistent with applicable School policies and procedures. If the actions alleged
in a complaint do not fall under this policy, but they may fall under another
policy, the Title IX Coordinator will inform the appropriate individual(s) of the
matter for further investigation and consideration.
C. Notice of Allegations
1. The Title IX Coordinator will draft and provide the Notice of Allegations to any
party (complainants and respondents) to the allegations of sexual misconduct.
Notice will go out to the parties promptly after complainant files a formal
complaint or the School decides to proceed with a formal complaint.
2. Parties will be provided sufficient time to review the Notice of Allegations and
prepare a response before any initial interview. The Title IX Coordinator may
determine that the formal complaint must be dismissed under Title IX on the
mandatory grounds identified above and will issue a Notice of Dismissal. If such
a determination is made, any party to the allegations of sexual misconduct
identified in the formal complaint will receive the Notice of Dismissal in
conjunction with, or in separate correspondence after, the Notice of Allegations.
3. Contents of Notice. The Notice of Allegations will include the following:
a) Notice of GSK’s Grievance Process and a hyperlink to a copy of the
process.
b) Description of the date, time, location and factual allegations concerning
the violation – including who was involved.

GSK Student & Faculty Handbook
v. 6/2024 60
c) A reference to the specific rules and/or code of conduct/policy provisions
alleged to have been violated.
d) A statement that the respondent is presumed not responsible for the
alleged conduct and that a determination regarding responsibility is
made at the conclusion of the grievance process.
e) A statement that the parties may have an advisor of their choice, who
may be, but is not required to be, an attorney.
f) A statement that the parties can request to inspect and review evidence,
and the parties will be provided an opportunity to do so.
g) A statement that information protected by legal privilege – e.g., attorney-
client privilege or doctor-patient privilege – cannot be used during the
investigation unless the person holding that privilege waives it.
h) A statement that prohibits knowingly making false statements or
knowingly submitting false information during the grievance process.
4. Updated Notice. An updated notice will be provided if GSK decides to
investigate allegations about the respondent or complainant that are not included
in the initial notice.
5. Interim Measures. Prior to and during an investigation, interim measures may
be issued based on a party’s request or at GSK’s own initiative. Potential interim
measures include but are not limited to:
a) Following an individualized safety and risk analysis, GSK may seek to
remove a respondent from campus on an emergency basis if GSK
determines that there is an immediate threat to the health or safety of
any student or other individual arising from the allegations. The
respondent will be afforded the opportunity to challenge the decision
immediately following the removal.
b) GSK may impose reasonable accommodations that effect a change in
academic class, work schedules, housing arrangements, employment,
transportation, and other applicable arrangements in order to help
ensure safety, prevent retaliation, and avoid an ongoing hostile
environment.
c) When the respondent is a GSK student, the complainant may request a
“no contact order” consistent with GSK and MSK policies and
procedures.
d) Both parties may (i) request any of the above protections or
accommodations, (ii) request to be afforded a reasonably prompt review
by the Title IX Coordinator or designee of the need for and terms of any
of the above protections or accommodations (even if the victim does not
file or continue to pursue a complaint), including potential modification,
and (iii) will be allowed to submit evidence in support or defense of the
request. The Title IX Coordinator will be responsible for coordinating
with appropriate offices on campus to implement appropriate measures.

GSK Student & Faculty Handbook
v. 6/2024 61
D. Investigation Process
1. The Title IX Coordinator and/or an investigator designated by the Title IX
Coordinator (“Title IX investigator”) will perform an investigation of the conduct
alleged to constitute sexual misconduct after issuing the Notice of Allegations.
2. The sexual misconduct investigation may involve, but is not limited to: (1)
conducting interviews of the complainant, the respondent, and any witnesses or
other third-parties who may have information or evidence regarding the
allegations; (2) reviewing documents and records, including law enforcement
investigation documents, student and personnel files, and written statements
regarding the allegations; and (3) gathering and examining other relevant
documents and evidence, including video, audio, photographs, e-mails, text-
messages or social media posts that may be relevant to the allegations.
3. The following principles will guide all investigations of alleged sexual
misconduct:
a) The investigation will be fair, impartial, timely, and thorough, taking
into consideration any request by the complainant or reporting
individual for confidentiality and/or privacy.
b) GSK, and not the parties, has the burden of proof and the burden of
gathering evidence, i.e., the responsibility of showing a violation of
this Policy has occurred.
c) GSK cannot access, consider, or disclose medical records without a
waiver from the party (or parent, if applicable) to whom the records
belong or of whom the records include information.
d) Equal opportunity will be provided for the parties to present
witnesses, including fact and expert witnesses, and other inculpatory
and exculpatory evidence, (i.e., evidence that tends to prove and
disprove the allegations) as described below. All parties must
submit any evidence they would like included in the investigation
prior to beginning of the inspection and review period.
4. Timeframe. To the extent possible and consistent with a full and fair process,
the School will seek to resolve complaints within approximately 100 calendar
days of an initial report, not including the time for any appeal. The School will
seek to resolve appeals within 50 calendar days. Time frames will vary
depending on the complexity of the investigation and the severity and extent of
the alleged misconduct. The School will give the parties periodic status
updates.
5. A
dvisor of Choice. Parties will be allowed equal access to advisors and
support persons; any restrictions on advisor participation will be applied equally.
Students participating as complainant or respondent in this process may be
accompanied by an Advisor of Choice to any meeting or hearing to which they
are required or are eligible to attend.
6. N
otice of Meetings and Interviews. The School will provide, to a party whose
participation is invited or expected, written notice of the date, time, location,

GSK Student & Faculty Handbook
v. 6/2024 62
participants, and purpose of all hearings, investigative interviews, or other
meetings with a party, with sufficient time for the party to prepare to participate.
7. Investigative Report.
a) The Title IX Coordinator or Title IX investigator will create an
Investigative Report (“Report”) that fairly summarizes relevant evidence
discovered during the course of the investigation, and the investigator’s
findings.
b) The parties will receive a draft of the full Report and any evidence
directly related to the complaint. The parties will have ten (10) calendar
days to respond to the Report and the evidence in writing. Witnesses
will also be given a copy of a summary of their witness interview in the
draft Report and be given least ten (10) calendar days to confirm its
accuracy or make any necessary corrections.
c) The Title IX Coordinator or Title IX investigator will consider any written
response to the Report or evidence by the parties before finalizing the
Report. The respondent and complainant will receive a final copy of the
Report at least ten (10) calendar days before the hearing or review
before a Designated Arbiter.
d) The final Report will be submitted to the appropriate Designated Arbiter.
The appropriate Designated Arbiter will be determined on a case-by-
case basis and will be appointed by the Dean of GSK or designee.
E. Hearing Procedures for Allegations of Sexual Misconduct under Title IX
1. General Hearing Rules. For allegations of sexual misconduct under Title IX,
upon receipt and consideration of the Investigative Report, the Designated
Arbiter will hold a live hearing. The live hearing may be conducted with all
parties physically present in the same geographic location, or—at GSK’s
discretion—any or all parties, witnesses, and other participants may appear at
the live hearing virtually through secure remote video conferencing as
prescribed by GSK. All reasonable measures will be taken to ensure that
proceedings are conducted in a manner that does not inflict additional trauma
on the complainant. A recording or transcript will be made of the hearing and
will be made available for the parties to review.
2. P
articipants in Hearing. Live hearings are not public, and the only individuals
permitted to participate in the hearing are as follows:
a) Complainant and Respondent (The Parties). The parties cannot
waive the right to a live hearing. If a party does not appear at the
hearing or is not subject to questioning/cross-examination, the
Designated Arbiter will exclude and not rely on that party’s
statements in reaching a determination regarding responsibility. The
decision-maker cannot draw an inference about the determination
regarding responsibility based solely on a party’s absence from the
live hearing or refusal to answer cross examination or other
questions.
b) Designated Arbiter. A hearing body will consist of a single

GSK Student & Faculty Handbook
v. 6/2024 63
Designated Arbiter. No member of the hearing body will also have
served as the Title IX Coordinator, Title IX investigator, or advisor to
any party in the case, nor may any member of the hearing body
serve on the appeals body in the case. No member of the hearing
body will have a conflict of interest or bias in favor of or against
complainants or respondents generally, or in favor or against the
parties to the particular case. During the hearing, the Designated
Arbiter will determine the order of witnesses and has the discretion
to ask the witnesses questions.
c) Advisor of choice. The parties have the right to select an advisor
of their choice, who may be, but does not have to be, an attorney.
The advisor of choice may accompany the parties to any meeting or
hearing they are permitted to attend, but may not speak for the party,
except for the purpose of cross-examination. The parties are not
permitted to conduct cross-examination; it must be conducted by the
advisor. As a result, if a party does not select an advisor, GSK will
select an advisor to serve in this role for the limited purpose of
conducting the cross-examination at no fee or charge to the party. If
a party does not attend the live hearing, the party’s advisor may
appear and conduct cross-examination on their behalf. If neither a
party nor their advisor appear at the hearing, GSK will provide an
advisor to appear on behalf of the non-appearing party.
d) Witnesses. Witnesses cannot be compelled to participate in the live
hearing and have the right not to participate in the hearing free from
retaliation. If a witness does not appear at the hearing or is not
subject to questioning/cross-examination, the Designated Arbiter will
exclude and not rely on that witness’ statements in reaching a
determination regarding responsibility.
3. Cross-Examination Procedure. Each party’s advisor will conduct live cross-
examination of the other party or parties and witnesses. During this live-cross
examination the advisor will ask the other party or parties and witnesses
relevant questions and follow-up questions, including those challenging
credibility. Questions must be relevant and must not pertain to complainant’s
past sexual behavior or sexual predisposition – with two exceptions – where
evidence of prior sexual behavior is offered to prove someone other than the
respondent committed the alleged offense, or where prior sexual behavior
evidence is specifically about the complainant and the respondent and is
offered to prove consent. Before any cross-examination question is answered,
the Designated Arbiter will determine if the question is relevant. A lawyer from
the Office of General Counsel will serve as counsel to the Designated Arbiter
and may consult with the Designated Arbiter concerning such determinations.
F. Determination Regarding Responsibility
1. Standard of Proof. The preponderance of the evidence standard will apply for
investigations and determinations regarding responsibility of formal complaints
covered under this Policy. This means that the investigation and hearing will
determine whether it is more likely than not that a violation of this Policy
occurred.
2. W
ritten Determination. The Designated Arbiter will issue a written

GSK Student & Faculty Handbook
v. 6/2024 64
determination regarding responsibility with findings of fact, conclusions about
whether the alleged conduct occurred, rationale for the result as to each
allegation, any disciplinary sanctions imposed on the respondent, and whether
remedies will be provided to the complainant. Designated Arbiters are
empowered to impose what they believe to be the appropriate sanctions and/or
remedial actions following a determination that this Policy was violated. Such
sanctions and remedies include, but are not limited to:
a) Disciplining the respondent, up to and including expulsion and
discharge/termination;
b) Providing counseling for complainants, respondents, and other
parties as appropriate;
c) Issuing “No Contact” orders;
d) Providing effective escorts to ensure that the complainant can move
safely between classes and activities;
e) Ensuring that the complainant and the respondent do not share
classes, workspaces, or extracurricular activities;
f) Moving the complainant (if the complainant requests to be moved) or
respondent to a different residence hall or housing assignment; and
g) Placing notations on the respondent’s transcript regarding the
subject violations.
3. Notification of Outcome. The written determination will be sent
simultaneously along with information on how to file an appeal.
4. R
ecord Retention. Records of each formal complaint of alleged sexual
misconduct under Title IX, including any actions taken, including supportive
measures, in response to a report or formal complaint of sexual misconduct
under Title IX, will be maintained for seven years.
G. Appeals.
1. Each party may appeal the dismissal of a formal complaint or any included
allegations and/or a determination regarding responsibility. An appeal may be
based on the following grounds:
a) Procedural irregularity that affected the outcome of the matter;
b) New evidence discovered that was not reasonably available at the
time the Designated Arbiter made the determination; or
c) Conflict of interest on the part of the Title IX Coordinator,
investigator(s) or Designated Arbiter that affected the outcome of the
matter.
2. To appeal, a party must submit their written appeal to the Title IX Coordinator
within ten (10) calendar days of being notified of the decision, indicating the
grounds for the appeal. The submission of appeal stays any sanctions for the

GSK Student & Faculty Handbook
v. 6/2024 65
pendency of an appeal. Supportive measures and remote learning opportunities
remain available during the pendency of the appeal.
3. If a party appeals, the institution will as soon as practicable notify the other party
in writing of the appeal.
4. Appeals will be decided by an Appeals Panel who will be free of conflict of
interest and bias, and will not serve as investigator, Title IX Coordinator, or
hearing decisionmaker in the same matter. The outcome of appeal will be
provided in writing simultaneously to both parties and include rationale for the
decision.
10. Prohibition Against Retaliation
GSK prohibits retaliation against any individual who reports sexual misconduct, including
but not limited to sex discrimination, sexual harassment, and/or sexual violence, sexual
assault, domestic violence, dating violence, and stalking, or participates in an investigation
of such reports.
11. Amnesty Policy for Alcohol And/or Drug Use by Reporting Individuals
The health and safety of every student at GSK is of utmost importance. GSK recognizes
that students who have been drinking and/or using drugs (whether such use is voluntary or
involuntary) at the time that sexual misconduct, including but not limited to sex
discrimination, sexual harassment, and/or sexual violence, sexual assault, domestic
violence, dating violence, and stalking occurs may be hesitant to report such incidents due
to fear of potential consequences for their own conduct. GSK strongly encourages
students to report sexual misconduct to the Title IX Coordinator, GSK administration, MSK
Security and/or any other reporting/compliance channels. A bystander, complainant, or
reporting individual who in good faith discloses any incident to MSK officials or law
enforcement will not be subject to disciplinary action for violation of GSK’s Substance
Abuse Policy occurring at or near the time of the commission of the act.
12. Students’ Bill of Rights Concerning Response to Sexual Misconduct
All students have the right to:
a) Make a report to local law enforcement and/or state police;
b) Have disclosures of sexual violence, including domestic violence,
dating violence, stalking, and sexual assault treated seriously;
c) Make a decision about whether or not to disclose a crime and/or
violation and to participate in GSK/MSK’s investigation, hearing, and
decision-making process and/or criminal justice process free from
pressure by GSK/MSK;
d) Participate in a process that is fair, impartial, and provides adequate
notice and a meaningful opportunity to be heard;
e) Be treated with dignity and receive from GSK/MSK information
concerning access to courteous, fair and respectful health care and
counseling services;

GSK Student & Faculty Handbook
v. 6/2024 66
f) Be free from any suggestion that the complainant is at fault when
these crimes and/or violations are committed, or should have acted
in a different manner to avoid such crimes and/or violations;
g) Describe the incident to as few GSK representatives as practicable
and not be required to unnecessarily repeat a description of the
incident;
h) Be protected from retaliation by GSK, any student and/or the
accused, and/or their family, friends and acquaintances within the
jurisdiction of the institution;
i) Access at least one level of appeal of a determination;
j) Be accompanied by an advisor of choice who may assist and advise
a complainant, reporting individual, accused or respondent
throughout the investigation process, including during all meetings
and hearings related to such process; and
k) Exercise civil rights and practice of religion without interference by
the grievance investigation, hearing, and decision-making process of
GSK/ MSK.
Policy Adopted as Revised March 2021

GSK Student & Faculty Handbook
v. 6/2024 67
Amendments to the Educational Law
Current New York State law requires colleges and universities to report violent felonies
within 24 hours, with the exception of sexual offenses, and to report missing students.
In the event of a sexual assault, students are advised that a Special Victims Division
exists for them to call directly at
Hotline:
Tel: 646-610-7272
24 hours a day 7 days a week
The hotline will provide the victim direct access to policy professionals who are experts
in this field.
In the event of emergency, students are reminded not to hesitate to
Call 911
The President of MSK and GSK has signed a Memo of Understanding with the Police
Commissioner of the City of New York in April 2015 that a f ormal agreement exists
between the two entities stipulating that GSK will follow the procedures set f orth by the
law.

GSK Student & Faculty Handbook
v. 6/2024 68
Campus Security Rules and Regulations
In case of emergency, individuals may contact the MSK Security Department, or the New
York Police Department at 911.
MSK Security Department
Bobst Basement RM C-G43 (open 24/7)
Primary: (212) 639- 7866
Secondary: (212) 639-7867
Email: securitd@mskcc.org
Security Webpage
(Link accessible via OneMSK while on the MSK Network)
Overview
The MSK Security Department is dedicated to prioritizing the well-being and safety of
all MSK patients, employees, and visitors, and seeks to prevent any action or situation
which recklessly or intentionally endangers the mental or physical health of a member
of the community. Additional information, including guidance for reporting complaints,
relevant MSK security policies, and crime prevention tips, may be found on the
Security Webpage
. In addition, MSK has established the Office of Threat
Management to further address and resolve any incidences of incivility occurring on
MSK premises. For information on reporting an incident of incivility, please contact the
Threat Management Department (securethreatfac@mskcc.org; 646-888-8555) or visit
the
Threat Management Webpage.
General Provisions Pertaining to Crimes of Hazing
The MSK Security Department will investigate any violations pertaining to and
surrounding reports of conduct that support any reckless or intentional situation that
endangers the mental health, physical health or forced consumption of liquor or
controlled substance for the purpose of initiation into or affiliation with any
organization. The Security Department will assist in the prosecution of any crimes
consistent with the crime of Hazing or related crimes as described in the Penal Law
under section 120-16.
Campus Security Advisory Committee
The Campus Security Advisory Committee is comprised of a broad representation of
constituents that have a stake in promoting the health and safety of the GSK community,
including representatives from GSK leadership, MSK Compliance, MSK Security, GSK
faculty, and current GSK students. The Committee will meet as required under
applicable law to review current campus security policies and procedures and solicit
recommendations for potential improvement from all stakeholders. The Commit te e
shall report, in writing, to the college president on its findings and recommendations at least
once each academic year, and such report shall be available upon request.
Security Orientation
Upon arrival on campus, GSK students will receive an orientation by the Security
Department that will include:

GSK Student & Faculty Handbook
v. 6/2024 69
• An overview of applicable security-related policies and procedures;
• Information on bias related and hate crime prevention measures;
• How to report an incident or concern;
• Fire safety and other personal safety tips;
• The importance of securing personal property and wearing identification
badges while on campus; and
• The availability of on-campus and off-campus student resources dedicated
to student mental health, safety, and wellness.
In addition, members of the GSK community are required to take Required Regulatory
Training courses, including Workplace Conduct and Safety & Emergency Preparedness, on
an annual basis. Additional training and education on security-related topics is available via
the MyLearning portal in Workday, including Active Shooter Training and Threat
Management Course.
Crime Statistics
The Campus Security Advisory Committee or the Security Department (212-639-7866)
will provide all campus crime statistics upon request. Information may also be
obtained via the U.S. Department of Education’s website
for campus crime statistics.
Violent Felony Offenses and Related Crimes Investigation
Violent offenses, and related crimes, including a report of any missing student, must
be immediately reported to the MSK Security Department. The Security Department
will make an initial assessment and conduct an investigation to determine the need for
further notification to local law enforcement agencies, and where appropriate, make such
notification as soon as practicable.
Bias-Related and Hate Crimes Information
GSK is committed to a diverse, safe, and inclusive learning environment. Bias-related
incidents, including hate crimes, as defined by New York Penal Law 485, should be
immediately reported to the MSK Security Department for further investigation and
appropriate handling in accordance with institutional procedures. Counseling and other
resources are available to assist members of the community who have experienced
or are experiencing bias-related or hate crime incidents.
MSK Security Officers License and Authority
All MSK Security Officers are licensed by the State of New York and have received 16
hours of classroom instruction and 120 hours of field training with a superior officer. All
Security Officers also receive an additional eight hours of in-service training annually, in
addition to roll-call training daily on matters of importance that directly impact the saf ety
of the institution. The campus is patrolled during non-business hours. Security officers
will respond to and investigate all crimes and serious incidents.
Violation of Campus Security Rules and Regulations
Violations of such rules may result in ejection of a violator from campus, or other
appropriate disciplinary action, in addition to any penalty pursuant to the penal law or any
other law to which a violator may be subject.

GSK Student & Faculty Handbook
v. 6/2024 70
Drug-Free Schools And Communities Act
(DFSCA) Substance Abuse Policy
The Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences (“GSK”) is committed
to a drug-free environment and protecting the safety, health and well-being of all employees
and students. In accordance with the Drug-Free Schools and Campuses Regulations (34
CFR Part 86) of the Drug-Free Schools and Communities Act (“DFSCA”), GSK has adopted
and implemented a program to prevent the use of illicit drugs and abuse of alcohol by
students and employees. As part of its program, GSK has adopted this policy which sets
forth the standards of conduct for employees and students related to unlawful possession,
use or distribution of illegal drugs, as well as the range of sanctions that can be imposed for
violation of GSK’s policies regarding substance abuse. GSK is the degree-granting arm of
Memorial Sloan Kettering Cancer Center (“MSK”), and both institutions equally apply and
enforce the standards, policies and sanctions set forth below.
1. Standards of Conduct
Student use, misuse, or abuse of alcohol, any illegal drug, or any controlled substance on
MSK premises or while acting in any capacity as a representative of MSK, shall be
considered misconduct, and will subject the student to a drug test and/or disciplinary action,
up to and including dismissal from the GSK. Such misconduct includes, but is not limited
to, being under the influence of alcohol, any illegal drug, or any controlled substance.
“Under the influence” includes, but is not limited to, the presence of a physically detectable
quantity of alcohol, any illegal drug, or any controlled substance in the body considered
significant by MSK.
MSK prohibits the unlawful manufacture, possession, use, sale, and distribution of drugs in
the workplace. MSK also prohibits workforce members f rom being under the influence of
alcohol, any illegal drug, or any non-prescribed controlled substance while at work or
conducting business as a representative of MSK. MSK reserves the right to search
employees’/students’ belongings while they are on MSK premises. This helps to ensure the
saf ety and protection of our employees/students, as well as of patients and visitors.
A violation of either of these policies shall be grounds for immediate discipline, up to and
including dismissal from the GSK and/or termination of employment from MSK.
Conviction for a drug-related offense on or off MSK premises shall be grounds for
disciplinary action, up to and including dismissal from the GSK and/or termination of
employment from MSK.
Students and employees are expected to cooperate fully in any required testing or
prescribed treatment program, and with all monitoring requirements. Failure to cooperate
may result in disciplinary action, up to and including dismissal from the GSK and/or
termination of employment from MSK.
Requirements and procedures related to academic and/or job performance and conduct
continue to be applicable when a student or employee:
• h
as been referred for drug testing and/or treatment;
• is treated for substance abuse; or
• has re-enrolled or return to work after treatment for substance abuse
GSK Student & Faculty Handbook
v. 6/2024 71
MSK and GSK substance abuse policies do not apply to the student's or employee’s use of
prescription drugs in accordance with a physician's orders, the consumption of reasonable
amounts of alcohol at MSK or GSK-sponsored activities or the consumption of reasonable
and appropriate amounts of alcohol by a student or employee when they are acting as a
representative of MSK.
2. Legal Sanctions
MSK complies with and abides by all federal, state and local laws and regulations pertaining
to drug and alcohol abuse and trafficking. A student or employee who violates substance
abuse policies may be subject to criminal sanctions provided by federal, state, and local law
in addition to any sanctions MSK may impose.
As required by the Drug-Free Workplace Act of 1988, an employee working on projects
funded through federal contracts or grants must notify Human Resources or the Dean’s Office
of a conviction of a criminal drug violation that occurred on MSK property within five (5) days
of such conviction. The Institution is required to notify the relevant federal contracting or
granting agency within ten (10) days and to take the appropriate personnel action within (30)
days of receipt of the notice. A conviction includes: a plea or finding of guilty, any plea of
“nolo contendere,” or an imposition of a fine or penalty.
Federal Sanctions:
Federal law has numerous penalties for the illegal possession of controlled substances,
possession of crack cocaine, and trafficking in methamphetamine, heroin, cocaine, cocaine
base, PCP, LSD, fentanyl and fentanyl analogue. An up to date list of federal drug trafficking
penalties (by schedule) can be found online. See http//www.dea.gov/druginfo/ftp3.shtml for
a complete listing of drugs by schedule.
Possession sentences range from up to one-year imprisonment and $1,000 fine to 20 years
imprisonment and fines up to $250,000. Forfeiture of personal and real property used to
possess or to facilitate possession of a controlled substance can be a sanction for
convictions. Sanctions can also include denial of federal benefits, such as student loans,
grants, contracts, public housing tenancy, eligibility to receive or purchase firearms, and
professional and commercial licenses.
New York State Sanctions:
New York State law also forbids the possession, use, or distribution of illicit drugs and
imposes criminal penalties, which may include imprisonment. The penalty imposed for a
conviction will generally depend upon the specific drug and the amount of the drug held or
sold, as well as the individual’s history of prior convictions. Judges have some discretion
to consider the circumstances in sentencing. The following are a few examples of potential
criminal penalties for drug infractions under New York law:
• The possession of less than 500 mg of cocaine is classified as criminal
possession in the 7th degree, a Class A misdemeanor which carries a penalty
of up to one year in jail.
• The possession of one-half an ounce of cocaine or more is a Class B felony
punishable by up to 9 years in prison.
This list is not intended to be exhaustive and is subject to change. The full list of New York
State drug crimes and their penalties can be found in the New York Penal Code.

GSK Student & Faculty Handbook
v. 6/2024 72
Penalties for Unlawful Distribution of Alcohol:
Under both Federal and New York State laws, selling or otherwise furnishing alcohol to an
individual under the age of 21 is a misdemeanor punishable by fine and/or imprisonment.
Selling alcohol without a license or permit is unlawful and punishable by a fine and/or
imprisonment. See New York State Laws and Regulations:
http://www.health.ny.gov/professionals/narcotic/laws_and_regulations/
3. Health Risks of Substance Abuse
The health consequences of alcohol abuse and substance use may be immediate and
unpredictable, such as fatalities associated with alcohol poisoning and drug overdose, or
more subtle and long-term, such as liver and brain damage associated with prolonged use
of alcohol.
In addition to health-related problems, alcohol abuse and substance use are associated
with financial difficulties, interpersonal conflicts, domestic violence, deterioration of the
family structure, accidental injuries or fatality, and may significantly impact academic and
work performance.
Selected drugs and their effects
Alcohol and Other Depressants (barbiturates, sedatives, and tranquilizers)
• Alcohol, tranquilizers, and sedatives are all considered depressants. These drugs
depress the central nervous system by mimicking either the brain’s natural
sedating chemicals or by diminishing the brain’s natural ability to produce
stimulating chemicals.
• Short-term effects: Alcohol consumption causes a number of marked changes in
behavior; even low doses significantly impair judgment and coordination.
Moderate to high doses cause significant impairments in higher mental functions,
severely altering a person’s ability to learn and remember information. Very high
doses can cause respiratory depression and death. The effects of other
depressants are similar to those of alcohol: large doses can cause slurred
speech, poor motor coordination, altered perception, psychosis, hallucinations
and paranoid delusions, coma, or death.
• Long-term effects: Long-term effects of using alcohol include addiction,
depression, accidents as a result of impaired ability, ulcers, gastritis, pancreatitis,
fatty liver, alcoholic hepatitis, chronic active hepatitis, and cirrhosis. Long-term
use of other depressants can also lead to addiction, including both physical and
psychological dependence. Regular use over time may result in a tolerance to
the drug. Withdrawal symptoms may range from restlessness, insomnia, and
anxiety, to convulsions and death.
Nicotine
• Nicotine, one of more than 4,000 chemicals found in the smoke from tobacco
products, is the primary component in tobacco that acts on the brain. Nicotine is
absorbed through the skin and mucosal lining of the mouth and nose or by
inhalation in the lungs. Nicotine increases the levels of dopamine in the brain.
The acute effects of nicotine dissipate in a few minutes, causing the smoker to
continue dosing frequently throughout the day to maintain the drug’s pleasurable
effects and prevent withdrawal.
• E
ffects: Select effects include addiction, high blood pressure, emphysema, heart
and lung disease, and cancer.

GSK Student & Faculty Handbook
v. 6/2024 73
Marijuana
• THC (delta-9-tetrahydrocannabinol) stores itself in the fatty tissue of the brain,
reproductive organs, liver, lungs, and spleen, where it causes tissue damage and
hinders normal body f unction. In the brain, THC widens the gaps between nerve
cells causing decreased transmission of impulses.
• Effects: Use can result in speech, memory and learning problems, physical
impairment, and can interfere with judgment, and cause difficulty thinking and
solving problems. Use can also elevate anxiety and cause a panic reaction.
Long-term use can cause permanent memory problems. There is also an
increased risk of developing respiratory problems including, but not limited to,
cancer.
Stimulants (cocaine, amphetamines, “speed, “uppers”)
• Cocaine use interferes with reabsorption of dopamine causing euphoria, which
constricts blood vessels, dilates pupils, and increases heart rate and blood
pressure.
• Effects: Acute cardiovascular or cerebrovascular emergencies such as heart
attack or stroke can result from use, regardless of frequency. Cocaethylene,
created by the liver when cocaine and alcohol are used, increases the chance of
sudden death. Addiction, lung damage, depression, paranoia, and toxic
psychosis are also possible. Similar risks are presented by the use of speed and
uppers.
Ecstasy (MDMA, Molly)
• Ecstasy is a synthetic drug, and is similar to both methamphetamine and
mescaline, which is a hallucinogenic.
• Effects: The drug mainly affects the body by affecting neurons that use the
chemical serotonin, which can greatly affect mood, aggression, sexual activity,
sleep, and sensitivity to pain. In high doses, MDMA can interfere with the body’s
ability to regulate temperature, which can lead to a sharp increase in body
temperature (hyperthermia), resulting in liver, kidney, and cardiovascular system
failure.
Hallucinogens (LSD, PCP)
• PCP is a white powder that is readily soluble in water or alcohol. LSD [lysergic
acid diethylamide] is manufactured from lysergic acid, which is found in ergot, a
fungus that grows on rye and other grains. The effects of these substances are
unpredictable, and depend on the amount taken, the user’s personality and
mood, and the surroundings in which the drug is used.
• Short-term effects: These drugs alter users’ perception of time and space by
changing the way the brain interprets stimuli. They also increase heart rate and
blood pressure, which can lead to coma, or heart and lung failure. High doses
can cause symptoms that mimic schizophrenia, such as delusions, hallucinations,
paranoia, disordered thinking, a sensation of distance from one’s environment,
and catatonia. Speech is often sparse and garbled. PCP can be addictive.
• Long-term effects: Flashbacks can occur days, months, or even years af ter use.
Users can also experience decreased motivation, prolonged depression,
increased anxiety, increased delusions and panic, and psychosis such as
schizophrenia or severe depression.
Narcotics (opium, morphine, codeine, heroin)
• Narcotics include opium, opium derivatives, and semi-synthetic substitutes of
opium derivatives. Narcotic use is associated with a variety of unwanted effects

GSK Student & Faculty Handbook
v. 6/2024 74
including drowsiness, inability to concentrate, apathy, lessened physical activity,
constriction of the pupils, dilation of the subcutaneous blood vessels causing
flushing of the f ace and neck, constipation, nausea and vomiting, and most
significantly, respiratory depression. As the dose is increased, the subjective,
analgesic (pain relief), and toxic effects become more pronounced.
• Short-term effects: Short-term effects include restlessness, irritability, loss of
appetite, nausea, tremors, and drug craving.
• Long-term effects: Long term effects include addiction, accidental overdose, risk
of hepatitis and AIDS infection from contaminated needles.
Prescription Drug Abuse
• The most commonly misused prescription drugs are: painkillers (codeine,
Oxycontin, Vicodin, Demerol), CNS depressants (Nembutal, Valium, Xanax), and
stimulants (Ritalin, Dexedrine, Adderall).
• Short-term effects: Stimulants and CNS depressants present risks for irregular
heartbeat, greatly reduced heart rate, seizures, dangerously increased body
temperature, and can cause aggressive or paranoid behavior.
• Long-term effects: The greatest risk from these drugs is the significant chance for
dependence. This can lead to greater doses and increased frequency of use.
Attempting to cease use without proper medical help af ter dependence has been
established can be dangerous and even f atal.
Inhalants (gas, aerosols, glue, nitrites, nitrous oxide)
• Inhalants are breathable chemical vapors that produce psychoactive effects. A
variety of products common in the home and the workplace contain substances
that can be inhaled:
o Solvents: paint thinners or removers, degreasers, dry-cleaning fluids,
gasoline, and glue Art or office supply solvents: correction fluids, felt-tip-
marker fluid, and electronic contact cleaners
o Gases (used in household or commercial products): butane lighters and
propane tanks, whipped cream aerosols (whip-its), and refrigerant gases
o Household aerosol propellants: contained in items such as spray paints,
hair or deodorant sprays, f abric protector sprays, and aerosol computer
cleaning products Medical anesthetic gases: ether, chloroform, halothane,
and nitrous oxide
o Nitrites: volatiles including cyclohexyl, butyl, and aryl nitrites, commonly
known as “poppers”. Volatile nitrites are often sold in small brown bottles
and labeled as “video head cleaner,” “room odorizer,” “leather cleaner,” or
“liquid aroma.”
• Short-term effects: These chemicals slow down the body’s functions, and can
cause momentary intoxication which, if continued, can lead to stimulation,
reduced inhibition, and ultimately loss of consciousness. Using solvents or
aerosol sprays can induce heart failure and death, known as “sudden sniffing
death.” This effect is mostly associated with butane, propane, and chemicals in
aerosols.
• Long-term effects: These chemicals can cause severe damage to the brain, liver,
and kidneys. Specifically, they can cause hearing loss, peripheral neuropathies
(limb spasms), central nervous system damage, and even bone marrow damage.
GHB
• GHB (gamma hydroxybutyrate) is a central nervous system depressant. It is
made from gamma butyrolactone and sodium or potassium hydroxide, which
means that it is essentially degreasing solvent or floor stripper combined with

GSK Student & Faculty Handbook
v. 6/2024 75
drain cleaner. In liquid form it is usually clear and looks like water. GHB and two
of its precursors, gamma butyrolactone (GBL) and 1,4 butanediol (BD) have been
characterized as predatory drugs used to commit acts of sexual violence.
• Effects: Abuse of GHB can cause amnesia, coma and/or seizures, inability to
move, or impaired speech. There is also a risk of death, especially when
combined with alcohol or other drugs.
Substance abuse, whether alcohol or drug, is harmful to your health. Please take the time
to read more information about the harmf ul effects of alcohol and drug abuse cited below:
National Institute on Alcohol Abuse and Alcoholism
Website: https://www.niaaa.nih.gov/alcohol-health
National Institute on Drug Abuse
Website: https://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs-charts
More information about controlled substances can be found in Title 21 United
States Code (USC) Controlled Substances Act, Section 811.
4. Drug and Alcohol Programs- Available Resources
If you have questions or issues about alcohol and other drugs, there are resources at MSK
where you can get help. You may contact the Magellan Employee Assistance Program
(EAP) and/or Employee Health & Wellness Services (“EH&WS”). These services are
readily available to students and employees who voluntarily seek counseling and
rehabilitation. The EAP offers free, professional, confidential counseling and referrals for
a broad range of issues. On demand resources are also available. Go to
MagellanAscend.com to sign up. For in-person counseling and Telehealth support, you
may call (800) 327-7893 or visit MagellanAscend.com to find care online. Students,
employees and family members are eligible to use the service at no cost. Counselors are
available to assist 24/7.
For convenience and privacy, Memorial Sloan Kettering Cancer Center’s Magellan EAP
can be reached by calling 1-800-327-8793.
Employee Health & Wellness Services is located at 222 E. 70th Street, has a satellite
office in room MG-03 at 1275 York Avenue, and can be reached at (646) 888-4000.
If a student or staff member shows signs or symptoms of illness or impairment, you should
notify your supervisor, Human Resources, EH&WS, or the confidential MSK Compliance
Hotline (by calling 866.568.5421 or by going to https://mskcc.ethicspoint.com
).
MSK Main Security
212-639-7866
Administrator on Call
Beeper:1521: iPhone: 646-581-3582
Page Operator:
212-639-2000 (main line) 212-639-6680
Emergency Dial
212-639-6000
Alcoholics Anonymous
212-647-1680
Women for Sobriety
215-536-8026
Smart Recovery Self Help
212-631-1198
Narcotics Anonymous
212-929-6262
Cocaine Anonymous
212-262-2463

GSK Student & Faculty Handbook
v. 6/2024 76
5. Disciplinary Sanctions
Procedures
Anyone observing something in an individual's performance or behavior that indicates that
this individual may be violating GSK’s Substance Abuse policy should refer the matter to
the Dean of the GSK. The Dean will contact an HR Legal & Regulatory representative for
evaluation and advice and may contact EH&WS when appropriate.
If the observation occurs outside of normal business hours, the available Administrator on
Call and/or Nursing Supervisor should be contacted. In consultation with the Dean, the
AOC and/or Nursing Supervisor will determine whether or not to refer the employee to the
Urgent Care Center. The Nursing Supervisor can be contacted by calling the Page Operator
at telephone 639-7900 and asking to speak to the Nursing Supervisor. The AOC can be
contacted at the above numbers.
The student exhibiting concerning behavior or performance may be placed on academic
probation and informed not to return to class/laboratory pending a discussion with the
mentor, an HR Legal & Regulatory representative, and the EH&WS Medical Director of
Employee Health Service (or designee). The employee demonstrating this behavior or
performance may be suspended with or without pay and subject to additional corrective
action up to and including termination of employment.
The student or employee may be asked to undergo drug and alcohol testing to determine
whether or not GSK’s policy has been violated. At this time the student or employee will be
required to sign a release giving EH&WS permission to release drug and alcohol test results
to HR, if such testing is done. The signing of this release is mandatory and a condition of
continued enrollment and/or employment. If the student or employee refuses to sign the
release, it should be so indicated on the Consent Form, and EH&WS will contact HR to
discuss next steps.
If it is suspected or known that a student or employee has violated GSK’s Substance Abuse
policy, including refusal to be tested for drugs and alcohol, to release the results of those
tests or to cooperate in a required or recommended treatment program, the matter should
be referred to the Dean of the GSK or HR Legal & Regulatory Affairs. A determination will
then be made as to the action to be taken. This determination will result from discussion
between the student’s mentor, the Dean of the GSK, an HR Legal & Regulatory
representative and, when appropriate, a representative from EH&WS, and actions that may
be required include, but are not limited to:
• referral to the EAP;
• referral to an outside program for either in patient or out-patient treatment;
periodic medical follow-up by EH&WS or EAP;
• disciplinary action, up to and including dismissal from the GSK and/or termination
of employment; and
• referral for prosecution
Revised October 2021

GSK Student & Faculty Handbook
v. 6/2024 77
Family Education Rights and Privacy Act
(FERPA)
Gerstner Sloan Kettering Graduate School of Biomedical Sciences Notification of Rights
under FERPA
The Family Educational Rights and Privacy Act (FERPA) affords eligible students certain
rights with respect to their education records. An “eligible student” under FERPA is a
student who is 18 years of age or older or who attends a postsecondary institution at any
age.
These rights include:
The right to inspect and review the student's education records within 45 days af ter the
day the School receives a request for access. A student should submit to the
registrar/curriculum specialist, dean, associate dean or other appropriate official, a
written request that identifies the record(s) the student wishes to inspect. The school
official will make arrangements for access and notify the student of the time and place
where the records may be inspected. If the records are not maintained by the school
official to whom the request was submitted, that official shall advise the student of the
correct official to whom the request should be addressed.
The right to request the amendment of the student’s education records that the student
believes is inaccurate, misleading, or otherwise in violation of the student’s privacy rights
under FERPA.
A student who wishes to ask the School to amend a record should write to the School
official responsible for the record, clearly identify the part of the record the student wants
changed and specify why it should be changed.
If the School decides not to amend the record as requested, the School will notify the
student in writing of the decision and the student’s right to a hearing regarding the
request for amendment. Additional information regarding the hearing procedures will be
provided to the student when notified of the right to a hearing.
The right to provide written consent before the School discloses personally identifiable
information (PII) from the student's education records, except to the extent that FERPA
authorizes disclosure without consent. The School discloses education records without a
student’s prior written consent under the FERPA exception for disclosure to school
officials with legitimate educational interests. A school official is typically includes a
person employed by the School in an administrative, supervisory, academic, research, or
support staff position (including law enforcement unit personnel and health staff); a
person serving on the board of trustees; or a student serving on an official committee,
such as a disciplinary or grievance committee. A school official also may include a
volunteer or contractor outside of the School who performs an institutional service of

GSK Student & Faculty Handbook
v. 6/2024 78
function for which the school would otherwise use its own employees and who is under the
direct control of the school with respect to the use and maintenance of PII from education
records, such as an attorney, auditor, or collection agent or a student volunteering to assist
another school official in performing his or her tasks. A school official typically has a
legitimate educational interest if the official needs to review an education record in order
to fulfill his or her professional responsibilities for the School.
The right to file a complaint with the U.S. Department of Education concerning alleged
failures by the [School] to comply with the requirements of FERPA. The name and address
of the office that administers FERPA is:
Family Policy Compliance Office
U.S. Department of Education 400 Maryland Avenue, SW Washington, DC 20202
Schools may disclose appropriately designated “directory information” without written
consent, unless you have advised the School to the contrary in accordance with School
procedures. The primary purpose of directory information is to allow the School to include
information f rom your education records in certain school publications.
Examples include:
A lecture poster, showing your role in the presentation; The annual yearbook;
Honor roll or other recognition lists; Graduation programs; and Directory information, which
is information that is generally not considered harmful or an invasion of privacy if released,
can also be disclosed to outside organizations without prior written consent. Outside
organizations include, but are not limited to, companies that manufacture class rings or
publish yearbooks. In addition, two federal laws require local educational agencies (LEAs)
receiving assistance under the Elementary and Secondary Education Act of 1965, as
amended (ESEA) to provide military recruiters, upon request, with the following inf ormation
– names, addresses and telephone listings – unless parents have advised the LEA that
they do not want their student’s information disclosed without their prior written consent.
If you do not want the School to disclose any or all of the types of information designated
below as directory information from your education records without your prior written
consent, you must notify the School in writing within 10 days of the first day of the semester
of enrollment. The School has designated the following information as directory
information:
Student's name Address Telephone listing
Electronic mail address Photograph
Date and place of birth Major field of study Dates of attendance Grade level
Participation in officially recognized activities and sports
Degrees, honors, and awards received
The most recent educational agency or institution attended
Student ID number, user ID, or other unique personal identifier used to communicate in
electronic systems but only if the identifier cannot be used to gain access to education
records except when used in conjunction with one or more f actors that authenticate the
user’s identity, such as a PIN, password, or other factor known or possessed only by the
authorized user

GSK Student & Faculty Handbook
v. 6/2024 79
A student ID number or other unique personal identifier that is displayed on a student ID
badge, but only if the identifier cannot be used to gain access to education records except
when used in conjunction with one or more f actors that authenticate the user's identity,
such as a PIN, password, or other factor known or possessed only by the authorized user.

GSK Student & Faculty Handbook
v. 6/2024
80
Student Resources
Michael H. Overholtzer, PhD
Dean
Tel: 212-639-6536
overhom1@mskcc.org
Thomas G. Magaldi, PhD
Associate Dean
Tel: 646-888-8436
magaldit@mskcc.org
Julie Nadel, PhD
Assistant Dean
Tel: 646-888-6636
nadelj1@mskcc.org
Ushma S. Neill, PhD
Vice President, Scientific Education and Training
Tel: 646-888-2011
neillu@mskcc.org
Yaihara Fortis-Santiago, PhD
Associate Director of Postdoctoral Affairs & Trainee
Diversity Initiatives
Tel: 646-888-3705
fortissy@mskcc.org
Leslie Ballantyne, Esq.
MSK HR Legal & Regulatory Affairs
Tel: 646-227-2742
BallantL@mskcc.org
Lindsay Cornacchia, Esq.
Associate Director of Compliance & Assistant General Counsel
Tel: 212-639-2496
CornaccL@mskcc.org
Melissa Maxwell-Avila
MSK Human Resources Associate
Director, Business Talent Consulting
Tel: 646-227-3888
maxwellm@mskcc.org
Nina Kosch
MSK Senior Employee Relations
Advisor
Tel: 646-952-1498
koschn@mskcc.org

GSK Student & Faculty Handbook
v. 6/2024 81
Mental Health Resources Contact Information
GSK is committed to safety and respect within the campus community, and to the support
of those in need of help. The following are resources available both on and off campus to
members of the GSK community.
Short-Term Counseling
Magellan Employee Assistance Program (EAP)
Tel: (800) 327-8793
Website: MagellanAscend.com
Penni Morganstein, PsyD
Magellan counselor dedicated to MSK trainees
Free, no copays, no use of insurance, Magellan Employee Assistance Program
morgansteinp@magellanhealth.com
Appointments available in-person and virtually: http://mskbookme.magellanhealth.com/
Available during the day
Chanchal Sharma, Psy.D; MS.Ed
Employee Health Psychologist
646-888-4128
sharmac1@mskcc.org
Available by appointment: Monday – Friday, 9 AM – 5 PM
Faith-Based Counseling
Protestant, Catholic, Muslim, Jewish
212-639-5982
Social Workers at MSK
212-639-7020
National Suicide Prevention Line (non-MSK)
1-800-273-8255
Available: 24/7
Long-Term Counseling
Health Insurance Providers
• Aetna: www.aetna.com
• Empire: www.empireblue.com
• UHC: mskcc.welcometouhc.com
To help find a counselor who is takes your insurance and is seeing new patients,
you can use either contact your health insurance provider directly
Health Advocate helps you navigate the healthcare system and make the most of
your MSK medical plan. Turn to them throughout the year for 24/7 at (866) 695-8622
https://mskbenefits.mskcc.org/keyword/health-advocate/.

GSK Student & Faculty Handbook
v. 6/2024 82
Telemedicine for Mental Health: If you are enrolled in any MSK medical plan, you and
your covered dependents have access to telemedicine through your respective medical
plan carrier at no cost to you.
• Aetna EPO, Aetna POS: To get started, sign in or register at teladoc.com/aetna,
download the app, or call (855) 835-2362.
• Empire POS: To get started, sign in or register at livehealthonline.com or download the
app.
• UnitedHealthcare (UHC) EPO, UHC CDHP: To get started, sign in or register
at uhc.com/virtualvisits.
https://mskbenefits.mskcc.org/keyword/telemedicine/
MSK Professional and Mentoring Resources
Office of Scientific Education and Training
oset@mskcc.org
Office of Career and Professional Development
opa@mskcc.org
Gerstner Sloan Kettering Graduate School of Biomedical Sciences
gradstudies@sloankettering.edu
HR Business Talent Consultants and Employee Relations Advisors
646-227-3456
Other Resources:
Victim Intervention Program (New York-Presbyterian Hospital/Weill Cornell
Medical Center): Free, confidential services for survivors of crime, including
sexual assault and domestic violence, as well as for family and friends. The
Victim Prevention Program is located at 525 East 68th Street, New York, NY
10065,
NYPVIP@nyp.org, (212) 746-9414.
New York State Domestic and Sexual Violence Hotline: 800-942-6906
New York State Office of Victim Services: 800-247-8035 or www.ovs.ny.gov
Safe Horizon’s Rape/Sexual Assault and Domestic Violence Hotline: 800-621-
HOPE (4673)
Anyone observing something in an individual’s performance or behavior that indicates that
this individual may be violating GSK or MSK’s Code of Conduct policies should ref er the
matter to the Dean of GSK. The Dean should contact an HR representative for evaluation
and advice. HR will contact Employee Health Service when appropriate.
Other resources:
MSK Main Security
212-639-7866
Administrator on Call
Beeper:1521: iPhone: 646-581-3582
Pag e Operator:
212-639-2000 (main line) 212-639-6680 (private line)
Emergency Dial
212-639-6000
Alcoholics Anonymous (AA)
212-647-1680

GSK Student & Faculty Handbook
v. 6/2024 83
Women for Sobriety
215-536-8026
Smart Recovery Self Help
212-631-1198
Narcotics Anonymous
212-929-6262
Cocaine Anonymous
212-262-2463
Compliance Hotline
If you are not comfortable raising an issue in your department, or if you have raised a
concern and feel that it has not been addressed, you can call the MSK Compliance Hotline.
It provides a way for employees as well as vendors and contractors to report concerns
about how MSK does business. You can report anonymously – without identifying yourself
– when you make a report to the hotline.
The MSK Compliance Hotline is available 24 hours a day, every day of the year, and is
managed by an outside company. You can submit a concern by calling 866.568.5421 or
by going to https://mskcc.ethicspoint.com
.
If you make a report through the Compliance Hotline, you will be asked to call back in ten
days for a status report. It’s important to remember to call back – especially if you did not
give your name – because we may need more information in order to complete our review.

GSK Student & Faculty Handbook
v. 6/2024 84
Our Faculty
Omar I. Abdel-Wahab
Professor
MD, Duke University School of Medicine
Heeseon An
Assistant Professor
PhD, Northwestern University
Cristina Antonescu
Professor
MD, Carol Davila Faculty of Medicine and Pharmacy
Daniel Bachovchin
Associate Professor
PhD, The Scripps Research Institute
Samuel F. Bakhoum
Associate Professor
MD, Dartmouth Medical School
PhD, Dartmouth College
Vinod P. Balachandran
Associate Professor
MD, SUNY Stony Brook School of Medicine
Zhirong Bao
Professor
PhD, Washington University
Mary K. Baylies
Professor
PhD, The Rockefeller University
Robert Benezra
Professor
PhD, Columbia University
Michael F. Berger
Professor
PhD, Harvard University
Adrienne A. Boire
Associate Professor
MD, University of Chicago
PhD, Tufts University
Chrysothemis C. Brown
Assistant Professor
MBBS, Royal Free and University College London
Medical School (UK)
PhD, Kings College (UK)
Jian Carrot-Zhang
Assistant Professor
PhD, McGill University (Canada)
Joseph M. Chan
Assistant Professor
MD, PhD, Columbia University
Sarat Chandarlapaty
Professor
MD, Wake Forest School of Medicine
PhD, University of North Carolina
Jayanta Chaudhuri
Professor
PhD, Albert Einstein College of Medicine
Yu Chen
Associate Professor
MD, Weill Cornell Medical College
PhD, The Rockefeller University
Emily H. Cheng
Professor
MD, Taipei Medical University
PhD, Johns Hopkins University School of Medicine
Nai-Kong V. Cheung
Professor
MD, Harvard Medical School
PhD, Harvard University
Ping Chi
Professor
MD, Weill Cornell Medical College
PhD, The Rockefeller University
Gabriela Chiosis
Professor
PhD, Columbia University
John D. Chodera
Associate Professor
PhD, University of California San Francisco
Junhong Choi
Assistant Professor
PhD, Stanford University
Arvin Dar
Professor
PhD, University of Toronto (Canada)
Yael David
Associate Professor
PhD, The Weizmann Institute of Science
K
ushal Dey
Assistant Professor
PhD, University of Chicago
Gretchen Diehl
Associate Professor
PhD, University of California, Berkeley

GSK Student & Faculty Handbook
v. 6/2024 85
Melinda M. Diver
Assistant Professor
PhD, Weill Cornell Graduate School of Medical
Sciences
Kojo S. J. Elenitoba-Johnson
Professor
MD, University of Lagos
James A. Fagin
Professor
MD, University of Buenos Aires School of Medicine
(Argentina)
Robert V. Farese, Jr.
Professor
MD, Vanderbilt Medical School
Lydia W. Finley
Associate Professor
PhD, Harvard University
Karuna Ganesh
Assistant Professor
MD, PhD, University of Cambridge
Frederic Geissmann
Professor
MD, University of Paris, Paris VI Pierre et Marie Curie
PhD, University o f Paris, Paris V Ren e-Descartes
Alexander Gitlin
Assistant Professor
MD, Weill Cornell Medical College
PhD, The Rockefeller University
Michael S. Glickman
Professor
MD, Columbia University College of Physicians and
Surgeons
Jonathan D. Goldberg
Professor
PhD, Imperial College (London)
Benjamin D. Greenbaum
Associate Professor
PhD, Columbia University
Jan Grimm
Professor
MD, University of Hamburg (Germany)
PhD, University of Kiel (Germany)
Anna-Katerina Hadjantonakis
Professor
PhD, Imperial College (London)
Alan M. Hanash
Associate Professor
MD, PhD, University of Miami
Daniel A. Heller
Professor
PhD, University of Illinois at Urbana-Champaign
Richard Hite
Associate Professor
PhD, Harvard University
Tobias M. Hohl
Professor
MD, Weill Medical College of Cornell University
PhD, Weill Graduate School of Medical Sciences of
Cornell University
Hedvig Hricak
Professor
MD, University of Zagreb School of Medicine
(Yugoslavia)
Dr. Med. Sc., Karolinska Institute (Sweden)
Katharine C. Hsu
Professor
MD, PhD, Cornell University Medical College
Danwei Huangfu
Professor
PhD, Cornell University
Morgan Huse
Professor
PhD, The Rockefeller University
Christine A. Iacobuzio-Donahue
Professor
MD, PhD, Boston University
Andrew M. Intlekofer
Assistant Professor
MD, PhD, University of Pennsylvania School of
Medicine
Prasad V. Jallepalli
Professor
MD, PhD, Johns Hopkins University School of
Medicine
Maria Jasin
Professor
PhD, Massachusetts Institute of Technology
Xuejun Jiang
Professor
PhD, University of Texas Southwestern Medical
Center at Dallas
Alexandra L. Joyner
Professor
PhD, University of Toronto
Scott N. Keeney
Professor
PhD, University of California, Berkeley

GSK Student & Faculty Handbook
v. 6/2024 86
Alex Kentsis
Associate Professor
MD, Mount Sinai School of Medicine
PhD, New York University
Kayvan R. Keshari
Professor
PhD, University of North Carolina Chapel Hill
Michael G. Kharas
Professor
PhD, University of California, Irvine
Christopher A. Klebanoff
Associate Professor
MD, Emory University School of Medicine
Andrew Ko ff
Professor
PhD, State University of New York at Stony Brook
Richard N. Kolesnick
Professor
MD, University of Chicago School of Medicine
Marc Ladanyi
Professor
MD, McGill University
Eric C. Lai
Professor
PhD, University of California, San Diego
Caleb Lareau
Assistant Professor
PhD, Harvard University
Christina S. Leslie
Professor
PhD, University of California, Berkeley
Ross L. Levine
Professor
MD, Johns Hopkins School of Medicine
Jason S. Lewis
Professor
PhD, The University of Kent (UK)
Ming Li
Professor
PhD, Columbia University
Yueming Li
Professor
PhD, University of California, Berkeley
Christopher D. Lima
Professor
PhD, Northwestern University
Piro Lito
Associate Professor
MD, PhD, Michigan State University
Stephen B. Long
Professor
PhD, Duke University
Scott W. Lowe
Professor
PhD, Massachusetts Institute of Technology
Minkui Luo
Professor
PhD, Princeton University
John Maciejowski
Associate Professor
PhD, Gerstner Sloan Kettering
Kenneth J. Marians
Professor
PhD, Cornell University
Joan Massagué
Pro fessor & Director, SKI, GSK Provo st
PhD, University of Barcelona
Christine Mayr
Professor
MD, Free University Berlin
PhD, Humboldt University Berlin
Ingo K. Mellinghoff
Professor
MD, Technical University Munich (Germany)
Quaid Morris
Professor
PhD, Massachusetts Institute of Technology
Rachel E. Niec
Assistant Professor
MD, PhD, Weill Cornell Medical College
Philipp M. Niethammer
Professor
PhD, European Molecular Biology Laboratories
Dimitar B. Nikolov
Professor
PhD, The Rockefeller University
Thomas M. Norman
Assistant Professor
PhD, Harvard University
Richard J. O’Reilly
Professor
MD, University of Rochester School of Medicine

GSK Student & Faculty Handbook
v. 6/2024 87
Kenneth Offit
Professor
MD, Harvard Medical School
Alban S. Ordureau
Assistant Professor
PhD, University of Dundee (UK)
Ricardo Otazo
Professor
PhD, University of New Mexico
Michael H. Overholtzer
Professor & Dean, GSK
PhD, Princeton University
Elli Papaemmanuil
Associate Professor
PhD, University of London
Luis F. Parada
Professor
PhD, Massachusetts Institute of Technology
Dinshaw J. Patel
Professor
PhD, New York University
Nikola P. Pavletich
Professor
PhD, Johns Hopkins University
Dana Pe’er
Professor
PhD, Hebrew University
Jonathan Peled
Assistant Professor
MD, PhD, Albert Einstein College of Medicine
Justin S. Perry
Assistant Professor
PhD, Washington University in St. Louis
Alexandros Pertsinidis
Professor
PhD, Brown University
John H. J. Petrini
Professor
PhD, University of Michigan Ann Arbor
Simon N. Powell
Professor
PhD, MD, University of London
Dirk Remus
Professor
PhD, University of California, Berkeley
Eduard Reznik
Assistant Professor
PhD, Boston University
Gabrielle Rizzuto
Assistant Professor
MD, PhD, Weill Cornell Graduate School of Medical
Sciences
Neal X. Rosen
Pro fessor
MD, PhD, Albert Einstein College of Medicine
Alexander Y. Rudensky
Professor
PhD, Gabrichevsky Institute for Microbiology and
Epidemiology
Charles M. Rudin
Professor
MD, PhD, University of Chicago Pritzker School of
Medicine
Michel W. Sadelain
Professor
MD, University of Paris PhD, University of Alberta
Charles L. Sawyers
Professor
MD, Johns Hopkins University School of Medicine
David A. Scheinberg
Professor
MD, PhD, Johns Hopkins School of Medicine
Andrea Schietinger
Associate Professor
PhD, University of Chicago
N
ikolaus D. Schultz
Professor
PhD, Freie Universitat Berlin
Agnel Sfeir
Professor
PhD, UT Southwestern Medical Center, Dallas
Sohrab P. Shah
Professor
PhD, University of British Columbia (Canada)
Ronglai Shen
Professor
PhD, University of Michigan
Mara Sherman
Associate Professor
PhD, University of California, Los Angels
Stewart H. Shuman
Professor
MD, PhD, Albert Einstein College of Medicine

GSK Student & Faculty Handbook
v. 6/2024 88
Samuel Singer
Professor
MD, Harvard Medical School
Ruslan Soldatov
Assistant Professor
PhD, Institute for Information Transmission Problems
David B. Solit
Professor
MD, University of Pennsylvania
Lorenz P. Studer
Professor
MD, PhD, Universitat Bern
Joseph C. Sun
Professor
PhD, University of Washington
Viviane S. Tabar
Professor
MD, American University of Beirut
Tuomas Tammela
Assistant Professor
MD, PhD, University of Helsinki, Finland
Derek S. Tan
Professor
PhD, Harvard University
Wesley Tansey
Assistant Professor
PhD, University of Texas at Austin
Paul J. Tempst
Professor
PhD, Universiteit Gent
Craig B. Thompson
Professor
MD, University of Pennsylvania
Meng-Fu Bryan Tsou
Professor
PhD, University of California, Davis
Asmin Tulpule
Assistant Professor
MD, PhD, Harvard University
Santosha A. Vard hana
Assistant Professor
MD, PhD, New York University School of Medicine
Andrea Ventura
Professor
MD, Catholic University of Rome (Italy)
PhD, European Institute of Oncology (Milan Italy),
Open University (London, UK)
Thomas S. Vierbuchen
Assistant Professor
PhD, Stanford University School of Medicine
Tobias C. Walther
Professor
PhD, European Molecular Biology Laboratory
(Germany)
Hans-Guido Wendel
Professor
MD, Medical School of the Technical University of
Aachen
Iestyn Whitehouse
Professor
PhD, The University of Dundee
Joao D. Xavier
Associate Professor
PhD, Universidade Nova de Lisboa
Rona Yaeger
Associate Professor
MD, New York University
Xinbo Yang
Assistant Professor
PhD, University of Maryland
Jennifer A. Zallen
Professor
PhD, University of California, San Francisco
Xiaolan Zhao
Professor
PhD, Columbia University

GSK Student & Faculty Handbook
v. 6/2024 89
Our Special Contributing Faculty
Faculty members who do not serve as dissertation mentors but who make contributions
to the education of our students by teaching or serving as clinical mentors are appointed
as Gerstner Sloan Kettering Special Contributing Faculty.
The following is a list of current members of the contributing faculty:
Cameron W. Brennan
MD, Cornell University Medical College
Murray F. Brennan
MD, University of Otago
Jacqueline F. Bromberg
MD, PhD, The University of North Carolina at
Chapel Hill
Marinela Capanu
PhD, University of Florida
Paul Chapman
MD, Cornell University Medical College
Aimee M. Crago
MD, Harvard Medical School
PhD, Cambridge University (UK)
Hironori Funabiki
PhD, Kyoto University
Christian Grommes
MD, RWTH Aachen Medical School (Germany)
John L. Humm
PhD, South Bank University
Mary Louise Keohan
MD, St. George's University School of Medicine
Mark G. Kris
MD, Cornell University Medical College
Robert J. Mo tzer
MD, University of Michigan Medical School
Larry Norton
MD, Columbia University College of Physicians
and Surgeons
Jae H. Park
MD, Johns Hopkins School of Medicine
Philip B. Paty
MD, Stanford University School of Medicine
Mi
chael A. Postow
MD, New York University School of Medicine
Diane Reidy-Lagunes
MD, State University of New York Downstate
Medical Center
Victo r E. Reuter
MD, Pedro Henriquez Urena National
University (Dominican Republic)
Howard I. Scher
MD, New York University School of Medicine
Eytan M. Stein
MD, Northwestern University Medical School
William P. Tew
MD, University of Rochester School of
Medicine and Dentistry
Tiffany A. Traina
MD, Weill Cornell Medical College
Agnes Viale
PhD, Universite de Nice-Sophia Antipolis
Phillip A. Watson
PhD, University of Wisconsin - Madison
Harel Weinstein
DSc, Technion-Israel Institute of Technology
James W. Young
MD, Vanderbilt University
Pat Zanzonico
PhD, Cornell University
Appendix 1 GSK Students Tax Treatment
Page 1 of 5 f:\payroll\gsk grad student reclassification project\gsk students tax treatment 03232020.docx
Last Updated 1/28/2023
Introduction
The Louis V. Gerstner Jr. Graduate School of Biomedical Sciences, Memorial Sloan Kettering (GSK) provides PhD candidates with the following:
• An annual award of full tuition
• A stipend to cover living and incidental expenses
• Health, Vision and Dental insurance for individual students and spouse/children/domestic partners, if requested
• Reimbursement for relocation costs
• A laptop
• Annual membership to the New York Academy of Sciences ($35 per year)
GSK PhD students do not perform any services in exchange for the amounts they receive.
The purpose of this document is to explain how payments made to students are classified for tax purposes.
Payment Types:
The types of cash payments made to GSK students include:
• Scholarships and fellowships to pay for fees, books, supplies, computers, equipment
• Stipends (both regular stipend and bonus of $5000 per year for anyone who receives external fellowship)
• Prizes and awards
• Other reimbursements
• Travel reimbursements
• Moving Expense reimbursements
• One-time payments for students who are appointed as Teaching Fellows
Other noncash benefits that GSK students are recipients of are:
• Full tuition which is waived by Memorial Sloan Kettering
• Health, Vision and Dental insurance which is paid by MSK on behalf of the student (for individual and spouse/children/domestic partner, if requested)
90
Appendix 1 GSK Students Tax Treatment
Page 2 of 5 f:\payroll\gsk grad student reclassification project\gsk students tax treatment 03232020.docx
Last Updated 1/28/2023
Payment Categories:
The Scholarship payments made to GSK students or on behalf of GSK students are classified as either Qualified Expenses or Nonqualified Expenses for US tax and reporting
purposes.
Qualified Scholarship Expenses – A "qualified scholarship" is the amount of a scholarship or fellowship grant that can be excluded from the recipient's income and is
limited to the amount used for:
• tuition and fees
• books
• supplies
• equipment required for courses
These items must be required of all students in a course of instruction for the scholarship or fellowship grant to be tax-free (Prop. Reg. 1.117-6(c)(2)).
These amounts are not taxable.
Non-Qualified Scholarship Expenses – A portion of the scholarship/fellowship paid may be considered non-qualified. A non-qualified scholarship or fellowship is a
nonqualified payment when it is used for expenses that are not qualified tuition or related expenses. Some examples of non-qualified scholarship expenses are stipends
used to pay for:
• biweekly living allowance
• prizes and awards
• travel reimbursements (see section “e” below to determine taxation)
• moving expense reimbursements
• Medical, Vision and Dental Benefits provided to student and spouse/children/domestic partner
• personal expenses
• Non-MSK course reimbursement
• optional fees
All non-qualified scholarship/fellowship payments may be subject to income tax withholding. Determination of whether there will be withholding on the non-qualified
portion of the scholarship is based on the student’s immigration status. Once the immigration status is determined the student will receive either Form 1098-T, Form
1042-S or Form 1099-MISC. The student should maintain the documentation necessary to support information reported to the Internal Revenue Service (IRS).
91

Appendix 1 GSK Students Tax Treatment
Page 3 of 5 f:\payroll\gsk grad student reclassification project\gsk students tax treatment 03232020.docx
Last Updated 1/28/2023
a. Stipend: Stipend paid by Memorial Sloan Kettering biweekly is taxable. It is not payment for services. Stipends are considered taxable income to the recipient
and are reported by Memorial Sloan Kettering on Forms 1098-T or 1042-S (reporting for Non-Resident Aliens, “NRAs”).
b. Prizes and Awards: Prizes and awards are sometimes given to GSK students and are always taxable. If the prize or award is equal to or exceeds $600,
Memorial Sloan Kettering reports the prize or award to the IRS and the student on Form 1099-MISC, Miscellaneous Income:
(http://www.irs.gov/instructions/i1099msc/ar02.html), or on Form 1042-S, Foreign Person’s U.S. Source Income Subject to Withholding. The form sent to each
student is determined based on the immigration status of the student. (Income Subject to Withholding: http://www.irs.gov/pub/irs-pdf/f1042s.pdf
.)
c. Other Payments: Payments for travel that do not meet the criteria listed in section “g” below, cash, non-required fees, books, supplies, equipment and
reimbursement for courses taken outside of MSK. The expenses are considered taxable income to the recipient and reported as taxable income by Memorial Sloan
Kettering on Forms 1098T, 1042-S (reporting for Nonresident Aliens, “NRAs”) or 1099-MISC.
d. Teaching Fellows Payments: One-time payments for students who are appointed as Teaching Fellows. These expenses are considered taxable income to the
recipient and reported as taxable income by Memorial Sloan Kettering on Forms 1042-S (reporting for Nonresident Aliens, “NRAs”) or 1099-MISC.
e. Moving and Relocation Expenses: Moving expenses and Relocation reimbursements provided to GSK student are taxable and not considered part of
qualified scholarship. These expenses are considered taxable income to the recipient and reported as taxable income by Memorial Sloan Kettering on Forms 1042-S
(reporting for Nonresident Aliens, “NRAs”) or 1099-MISC.
f. Medical, Vision and Dental benefits provided by MSK to the student and spouse/children/domestic partner: The cost to cover the student is not
taxable. The costs to cover the student’s spouse/children/domestic partner are considered taxable income to the recipient and reported as taxable income by
Memorial Sloan Kettering on Forms 1042-S (reporting for Nonresident Aliens, “NRAs”) or 1099-MISC.
g. Travel Expenses: Travel expense reimbursements are non-taxable if the following criteria are met:
- Travel must be for legitimate training purposes.
- Students must adequately account for the expenses within a reasonable time period, generally within 60 days.
- If the student receives payment in advance of the expenses, the students must return any excess reimbursement or allowance within a reasonable
time period, generally within 120 days.
92

Appendix 1 GSK Students Tax Treatment
Page 4
of 5
f:\payroll\gsk grad student reclassification project\gsk students tax treatment 03232020.docx
Last Updated 1/28/2023
The chart below outlines the different categories of payments made to GSK students and how their taxation and reporting will be handled depending on which of the following
four categories they fall into:
• US citizens
• US Resident Aliens: not eligible for a tax treaty
• US Resident Aliens: tax treaty eligible
• Non-Resident Alien: tax treaty eligible
• Non-Resident Alien: not eligible for a tax treaty
GSK Students - Tax Treatment for Payment Categories
Residency Status
Qualified Scholarship Expenses :
Tuition, fees, books, supplies and equipment
required for courses of instruction.
Cost of Student’s MSK provided Medical, Vision
and Dental Coverage
Non-Qualified Scholarship Expenses:
Biweekly Stipend Paid by MSK, room and board, and
misc. expenses.
Other Non-Qualified Payments:
Prizes and Awards, Te ac hing Fe llow and Other
Payments, Moving Expenses, Non-MSK course
reimbursement, MSK provided Medical, Vision and
Dental Coverage to the student’s
Spouse/Children/Domestic Partner
Taxation
Tax
Withholding
by MSK
Tax Forms
received at
Year end
Taxation*
Tax
Withholding
by MSK
Tax Forms
received at
Year end
Taxation
Tax Withholding
by MSK
Tax Forms
received at
Year end
US Citizen
Not
Taxable
No Withholding No Tax Forms Taxable Income No Withholding 1098-T
Taxable
Income
No Withholding 1099-MISC
US Resident Alien: not
eligible for tax treaty
Not
Taxable
No Withholding No Tax Forms Taxable Income No Withholding 1098-T
Taxable
Income
No Withholding 1099-MISC
US Resident Alien: tax treaty
eligible
Not
Taxable
No Withholding No Tax Forms
Not Taxable up to
treaty limits
No Withholding
1042-S (income
code 16)
Taxable
Income
No Withholding 1099-MISC
Non-Resident Alien: tax
treaty eligible
Not
Taxable
No Withholding No Tax Forms
Not Taxable up to
treaty limits
No Withholding
1042-S (income
code 16)
Taxable
Income
Subject to
Withholding
1
1042-S ( in co me
code 23)
Non-Resident Alien: not
eligible for a tax treaty
Not
Taxable
No Withholding No Tax Forms Taxable Income
Subject to
Withholding
1
1042-S (income
code 16)
Taxable
Income
Subject to
Withholding
1
1042-S ( in co me
code 23)
To determine your Tax Treaty eligibility, please reach out to Payroll Department at mskpayroll@mskcc.org. Certain Tax Treaties are subject to income limits and amounts that exceed the limit are
considered taxable.
*For all categories that are considered taxable income, students are required to self-report taxable income to the IRS as a part of individual US tax return.
1
MSK will withhold at predefined rates stipulated by the IRS. The rates are dependent on individual’s status.
93

Appendix 1
Resources for Filing Individual Tax Returns and Making Estimated Payments
The following resources are for general information purposes only and should not be considered
advice given by or sanctioned by MSK/GSK. Since individual circumstances vary based on
citizenship, tax treaty, income, year of study, and more, you may want to consult with a tax
advisor regarding your individual circumstances.
Filling Taxes:
To determine if and how to file a return and pay taxes you may consult the IRS website for
federal taxes and New York State Department of Taxation and Finance for state taxes.
Nonresident and resident aliens may also consult IRS Publication 519, US Tax Guide for Aliens
for additional information.
Estimated Tax Payments:
You may be required to make federal and/or state quarterly estimated income tax payments.
You may refer to guidance from the IRS website for federal estimated taxes and the
New York
State Department of Taxation and Finance for state estimated taxes to determine if and when
estimated taxes must be made.
Domestic Student Resources:
US Citizens and US resident aliens (who are not eligible for a tax treaty) may choose to use the
IRS’s Free File
to file their individual taxes if they meet the eligibility requirements. Students are
also eligible for discounted tax preparation assistance and filing services offered to all MSK staff
and trainees through Lifemart and Magellan. Check the MSK
employee discounts page to learn
more.
International Student Resources:
Nonresident aliens and resident aliens (who are eligible for a tax treaty) may consider using
Sprintax, a tax preparation software designed by persons unrelated to MSK/GSK. Students will
be notified of how to use this software during tax filling season. Students who wish to use this
software will be asked to sign a waiver acknowledging that the guidance provided is not from or
validated by MSK.
Pa
ge 5 of 5
94

Office Location:
Zuckerman Research Center, Suite 669
417 E. 68
th
Street
New York, New York
Mailing Address:
Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences
Memorial Sloan Kettering Cancer Center
1275 York Ave, Box 441, New York, New York 10065
gradstudies@sloankettering.edu
646.888.6639
www.sloankettering.edu
