1
Strategic Plan for Preventing and Mitigating
Drug Shortages
Food and Drug Administration
October 2013

2
TABLE OF CONTENTS
EXECUTIVE SUMMARY .................................................................................................3
INTRODUCTION...............................................................................................................7
I. UNDERSTANDING AND RESPONDING TO DRUG SHORTAGES ...............8
A. R
ECENT CHANGES TO FDA OVERSIGHT OF DRUG SHORTAGES ............................... 9
B. R
OOT CAUSES OF DRUG SHORTAGES .....................................................................
11
C. C
URRENT FDA EFFORTS TO PREVENT AND MITIGATE DRUG SHORTAGES............
12
D. I
NTERNAL AND EXTERNAL COMMUNICATION AND ENGAGEMENT ........................
15
II. CONTINUING PROGRESS: FDA’S STRATEGIC PLAN...............................17
A. GOAL
#1: STRENGTHEN MITIGATION RESPONSE ................................................. 18
B. GOAL
#2: DEVELOP LONG-TERM PREVENTION STRATEGIES............................... 20
III. ACTIONS OTHER STAKEHOLDERS SHOULD CONSIDER ........................21
A. M
ANUFACTURING INCENTIVES ...............................................................................
22
B. U
SE OF QUALITY DATA ..........................................................................................
22
C. R
EDUNDANCY, CAPABILITY, AND CAPACITY .........................................................
23
D. T
HE GRAY MARKET................................................................................................
23
CONCLUSION .................................................................................................................23
APPENDIX A: FEDERAL REGISTER NOTICE .........................................................25
APPENDIX B: STATUTORY ELEMENTS...................................................................28
APPENDIX C:
COLLECTING INFORMATION ON DRUG SHORTAGES..............29
APPENDIX D: FDA OFFICES ENGAGED IN DRUG SHORTAGE EFFORTS......
31
APPENDIX E: COORDINATING DRUG SHORTAGE EFFORTS IN CDER ..........
34
APPENDIX F: COMMUNICATING WITH EXTERNAL GROUPS...........................
37

3
Strategic Plan for Preventing and Mitigating
Drug Shortages
Executive Summary
On July 9, 2012, the President signed into law the Food and Drug Administration Safety and
Innovation Act (FDASIA).
1
Among other things, Title X of FDASIA directs the Food and Drug
Administration (FDA or the Agency) to establish a task force on drug shortages to develop and
submit to Congress a Strategic Plan to enhance FDA’s response to preventing and mitigating
drug shortages.
Understanding and Responding to Drug Shortages
Shortages of drugs and biologics pose a significant public health threat, delaying, and in some
cases even denying, critically needed care for patients. Preventing drug shortages remains a top
priority for FDA.
Although FDA cannot directly affect many of the business and economic decisions that
contribute to drug shortages, FDA is well positioned to play a significant role as manufacturers
work to restore lost production of life-saving medications. FDA can be most effective when
there is time to plan; thus, it is critical that manufacturers notify FDA as soon as possible when
manufacturing disruptions are expected. Early notification about possible shortages, as
requested in the President’s Executive Order 13588 and then codified into law by Congress, has
enabled FDA to work with manufacturers to restore production of many lifesaving therapies.
There has been a 6-fold increase in notifications to FDA since the Executive Order. These
increased notifications combined with allocation of additional FDA resources have resulted in
real progress in addressing shortages―FDA helped prevent close to 200 drug shortages in 2011
and more than 280 in 2012. The total number of new shortages decreased from 251 in 2011 to
117 in 2012.
If notified of a potential disruption in production, FDA can take a number of steps to help
prevent or mitigate a shortage, including:
Determine if other manufacturers are willing and able to increase production
Expedite inspections and reviews of submissions
Exercise temporary enforcement discretion for new sources of medically necessary drugs
Work with the manufacturer to ensure adequate investigation into the root cause of the
shortage
1
Public Law 112-144.
4
Review possible risk mitigation measures for remaining inventory
When selecting specific regulatory tools, FDA works closely with manufacturers and chooses
tools that are appropriate to the specific situation. FDA also makes certain that drug shortages
are considered before taking an enforcement action or issuing a Warning Letter. Additionally,
FDA communicates up-to-date information about a shortage to affected stakeholders and
continues to monitor a shortage until it is resolved.
FDA’s efforts to date demonstrate that FDA is an important part of the solution to the drug
shortages problem. By working closely with manufacturers experiencing problems, as well as
potential alternative manufacturers, and by exercising regulatory flexibility in appropriate cases,
FDA has had a substantial positive impact on the shortage situation. However, FDA cannot
address the drug shortage threat alone. An examination of FDA’s response to drug shortages
underscores the importance of strong collaboration and constant communication between FDA,
industry, health professionals, and patients. In addition, ensuring that critical drugs are available
to the patients who need them will require further action by manufacturers and other
stakeholders.
FDA’s Drug Shortage Strategic Plan
In its Drug Shortage Strategic Plan (Strategic Plan), FDA identifies two central goals to address
drug shortages: improving our mitigation response to imminent or existing shortages, and
implementing strategies for the long-term prevention of shortages by focusing on the root causes
of shortages. The Strategic Plan outlines specific tasks under each goal.
Goal #1: STRENGTHEN MITIGATION RESPONSE
Improve and streamline FDA’s current mitigation activities once the
Agency is notified of a supply disruption or shortage
TASK 1.1
Revise and standardize procedures to more accurately reflect and
Develop and/or Streamline enhance the interactions between units within FDA and maximize the
Internal FDA Processes efficiency of FDA’s response to a notification of a disruption in supply.
TASK 1.2
Improve Data and Response
Tracking
Improve Agency databases related to shortages and the tracking
procedures FDA uses to manage shortages. Improved tracking will
enable FDA to better assess progress on preventing and mitigating
shortages.
TASK 1.3
Clarify Roles/Responsibility
of Manufacturers
Clarify roles/responsibilities of manufacturers by finalizing the
proposed rule explaining when and how to notify FDA
of a
discontinuance or interruption in manufacturing, working with
manufacturers on remediation efforts, and encouraging manufacturers
to engage in best practices to avoid or mitigate shortages.
5
TASK 1.4
Enhance Public
Communications about Drug
Shortages
Continue to improve FDA’s public communications about drug
shortages by developing a smartphone application so that individuals
can instantaneously access drug shortage information, updating the
website to include the therapeutic category(ies) for shortage products,
and improving the functionality of the website by adding sort and
search capabilities.
Goal #2: DEVELOP LONG-TERM PREVENTION STRATEGIES
Develop long-term prevention strategies to address the underlying causes of supply disruptions
and prevent drug shortages
TASK 2.1
Develop Methods to
Incentivize and Prioritize
Manufacturing Quality
Identify ways FDA can implement positive incentives to promote and
sustain manufacturing and product quality improvements.
TASK 2.2
Use Regulatory Science to
Identify Early-Warning
Signals of Shortages
Continue to develop risk-based approaches to identify early warning
signals for manufacturing and quality problems to prevent supply
disruptions.
TASK 2.3
Increase Knowledge to
Develop New Strategies to
Address Shortages
Continue to work with stakeholders to further develop our
understanding of issues related to shortages, including whether a
Qualified Manufacturing Partner Program would be feasible and
beneficial. This additional information could help inform new
strategies to address shortages.
Actions for Other Stakeholders to Consider
Stakeholders outside FDA have a significant role to play in mitigating and preventing drug
shortages. Because there are limits to what FDA can do on its own to address shortages, any
comprehensive drug shortages plan must also discuss other stakeholders’ roles and potential
contributions. FDA has identified four specific areas that merit external stakeholder attention:
Manufacturing incentives: Many shortages are caused by manufacturing quality issues.
FDA is exploring ways to use its existing authorities to promote and sustain quality
manufacturing. However, our ability to offer financial or other economic incentives for
innovation and new investments in high-quality manufacturing is limited. Given the
importance of quality and its link to shortages, payers might explore financial or
economic incentives to encourage high-quality manufacturing that could help reduce the
occurrence and severity of shortages.
Use of data on manufacturing quality in purchasing decisions: FDA makes certain
information publicly available about manufacturers’ historical ability to produce quality
products. However, FDA cannot influence whether or how these quality data are used by
buyers, such as hospitals, pharmacies, and other group purchasing organizations, when
they make purchasing decisions. Better use of this information could help incentivize
manufacturers to focus on quality and, ultimately, prevent shortages.
6
Redundancy, capability, and capacity: A disruption in supply is exacerbated if there is
limited manufacturing capacity and capability, market concentration, or just-in-time
inventory practices that result in minimal product inventory being on hand at any given
time. However, FDA cannot prevent manufacturing concentration or require redundancy
of manufacturing capability and capacity. Nor can FDA require a company to
manufacture a drug, maintain a certain level of inventory of drug product, or reverse a
business decision to cease manufacturing. Manufacturers could consider opportunities
for building redundant manufacturing capacity, holding spare capacity, or increasing
inventory levels to lower the risks of shortages; and other stakeholders might explore
how to incentivize such practices.
Gray market: In the context of drug shortages, the term gray market is used to reference
the downstream distribution of approved drug products at significantly marked-up prices.
FDA has limited data on the gray market and limited influence on its workings (for
example, we have no authority regarding product pricing). However, there is evidence
that gray market activities can worsen the impact of drug shortages. Actions on the part
of other stakeholders to minimize gray market activities when such activities exacerbate
the impact of drug shortages could play a role in mitigating the impact of shortages and
reducing risks to patients.
Conclusion
Drug shortages remain a significant public health issue in the United States, and addressing
shortages remains a top priority for FDA. Early and open dialogue between FDA and
manufacturers is critical to successfully mitigating and preventing shortages. Recent important
actions by the President and Congress have enabled FDA to learn more about possible shortages
before they occur. These actions, combined with an increase in the resources FDA is devoting to
drug shortages, have helped prevent numerous recent shortages—more than 280 in 2012.
Nevertheless, substantial challenges remain, and more work by all relevant stakeholders is
needed. This Strategic Plan identifies a number of activities to improve the Agency’s ability to
address drug shortages, focusing on two goals:
Strengthening FDA’s ability to respond to notices of a disruption in supply, including
improving our mitigation tools
Developing long-term prevention strategies to address the underlying causes of supply
disruptions and prevent drug shortages
Recognizing that manufacturers and other stakeholders have important roles to play in ensuring
that drugs are available to the patients who need them, the Strategic Plan also identifies actions
others can consider that show promise in helping to prevent shortages.
FDA looks forward to implementing this Strategic Plan as part of a collaborative and sustained
effort to address drug shortages in the United States.

7
FDA’s Strategic Plan for Preventing and
Mitigating Drug Shortages
Introduction
On July 9, 2012, the President signed into law the Food and Drug Administration Safety and
Innovation Act (FDASIA). Title X of FDASIA pertains to drug shortages and defines a shortage
or drug shortage as a period of time when the demand or projected demand for a drug within the
United States exceeds the supply of the drug.
2
Among other things, FDASIA directed FDA to
establish a task force on drug shortages to develop and submit to Congress a Strategic Plan to
enhance FDA’s response to preventing and mitigating drug shortages. FDASIA specifically
required the Drug Shortages Strategic Plan (Strategic Plan) to include the following:
Plans for enhanced inter- and intra-agency coordination, communication,
and decision-making
Plans to ensure drug shortages are considered when FDA initiates a
regulatory action that could precipitate or exacerbate a drug shortage
Plans for effective communication with external stakeholders
Plans for considering the impact of drug shortages on clinical trials
An examination of whether to establish a “qualified manufacturing partner
program” as further described in FDASIA
Appendix B includes a table indicating where each statutory element is discussed in this
document.
Since the passage of FDASIA, FDA has convened a Drug Shortages Task Force (Task Force),
representing multiple disciplines, centers, and offices within FDA.
3
This Strategic Plan
4
is the
culmination of the efforts of the Task Force over the last several months. The Plan also reflects
input received from a wide variety of sources, including discussions with outside stakeholders
2
Section 506C(h)(2) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 USC 356c(h)(2), as amended by
Title X of FDASIA.
3
Information on drug shortages, including the members of the Task Force can be found at
http://www.fda.gov/drugs/drugsafety/drug
shortages/default.htm (drug shortages). Information about biologics
shortages can be found at http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Shorta
ges/default.htm
(biologics shortages).
4
The Strategic Plan encompasses both drugs and biologics—although shortages of drugs outnumber shortages of
biologics, both can have a similar and equally troubling impact on patient care. Unless otherwise indicated, we use
the term drug to refer to both drugs and biologics.

8
and comments responding to a Federal Register notice FDA published on February 12, 2013
(Appendix A).
5
FDA also recognizes that numerous other groups are examining drug shortages,
including the Government Accountability Office (GAO) and several private organizations.
Following this Introduction, the Strategic Plan consists of three sections. The first section
explains FDA’s oversight of drug shortages, examines what is known about the causes of
shortages, and describes FDA’s current efforts to resolve existing shortages and respond to
disruptions in supply. The second section is the Strategic Plan: it explains the actions FDA is, or
will be, taking to strengthen and expand its efforts to address shortages. Recognizing that FDA
cannot address this problem alone, the third section outlines potential actions for other
stakeholders to consider.
I. Understanding and Respondi
ng to Drug Shortages
Drug shortages pose a significant public health threat, affecting critically important drugs
including those intended for intravenous administration (e.g., chemotherapy, nutritional support,
and antibiotics). A shortage can result in delaying or denying needed care to patients and may
cause practitioners to prescribe an alternative therapy that may be less effective for the patient or
that poses greater risk.
6
Drug shortages have even disrupted clinical trials, potentially delaying
research on important new therapies.
The number of new drug shortages quadrupled from approximately 60 in 2005 to more than 250
in 2011. These statistics reflect the number of new shortages reported in a given year, but
because shortages typically continue for extended amounts of time, the actual number of
shortages at a given point in time is likely to be higher. As a result, although the number of new
shortages significantly decreased in 2012 to 117 (from 251 in 2011), more than 300 shortages
remained active at the end of 2012.
7
Behind these statistics are individual patients from across the United States who are in need of
drugs to treat life-threatening diseases, including cancer and serious infections. Preventing drug
shortages has been, and continues to be, a top priority for FDA. Working within the confines of
the current statutory and regulatory framework and in partnership with manufacturers and other
stakeholders, FDA helped prevent close to 200 drug shortages in 2011 and more than 280
shortages in 2012.
5
The Federal Register notice and the submitted comments can be found online at http://www.regulations.gov,
Docket No. FDA-2013-N-0124.
6
Berg N, Kos K, et al. Report on the ISPE 2013 Drug Shortages Survey. June 2013. Tampa, FL: International
Society for Pharmaceutical Engineering. Available at www.ispe.org/drugshortages/2013JuneReport
.
7
FDA drug shortage statistics can be found at
http://www.fda.gov/Drugs/DrugSafety/DrugSho
rtages/ucm050796.htm.
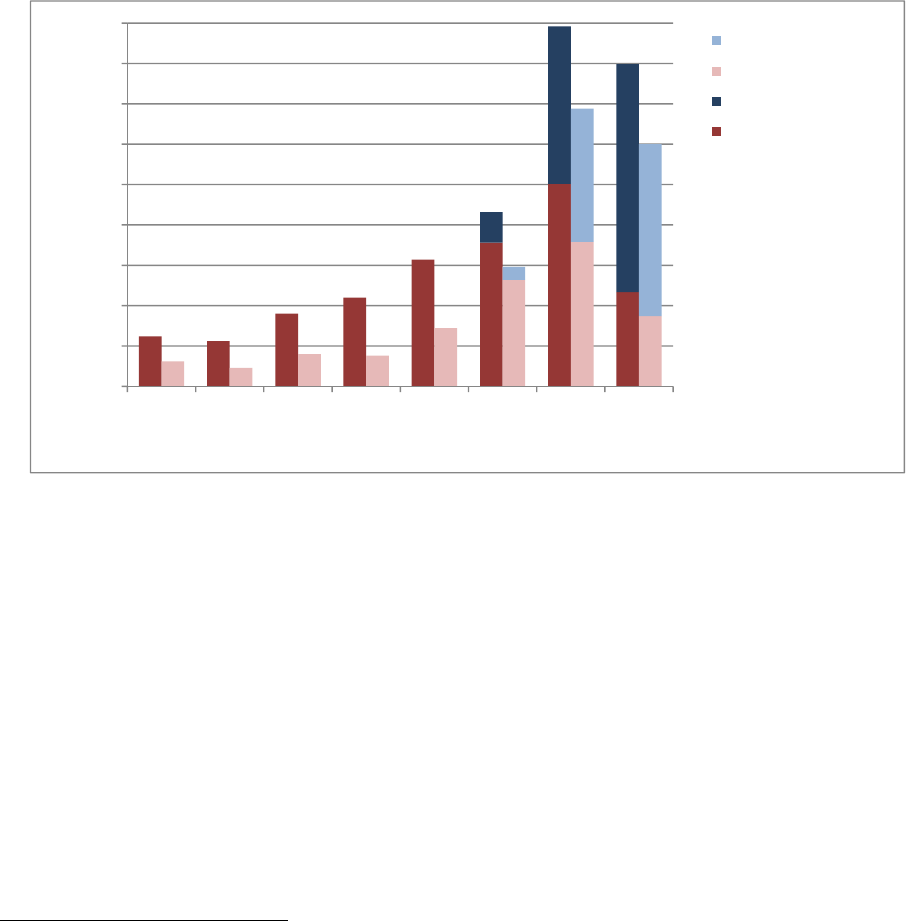
Figure 1 illustrates the number of new drug shortages by year from 2005-2012 and the number of
prevented shortages by year from 2010-2012 (FDA began tracking prevented shortages in 2010).
Figure 1 also shows that shortages predominantly affect sterile injectable products and reflects
FDA’s focus on preventing nationwide shortages of these critical drugs
8
.
Figure 1. Number of New and Prevented Shortages by Dosage Form, 2005-2012
62
56
90
110
157
178
251
117
38
195
282
31
23
40
38
72
132
179
87
16
165
213
0
50
100
150
200
250
300
350
400
450
2005 2006 2007 2008 2009 2010 2011 2012
NumberofShortages
CalendarYear
Injectables‐Prevented
Injectables‐New
All‐Prevented
All‐New
Source:DatafromFDA’s
internaldrugandbiologics
shortagesdatabases.
A. Recent Changes to FDA Oversight of Drug Shortages
Authorities granted by Congress as part of the Federal Food, Drug, and Cosmetic Act (FD&C
Act) have enabled FDA to coordinate with manufacturers to help prevent or mitigate drug
shortages. However, FDA’s ability to take effective action depends on the relevant manufacturer
notifying FDA in a timely fashion of a disruption or possible disruption in supply. FDA has at
its disposal a variety of mitigation tools (more fully described in section C.3) that the Agency
can use when working with a manufacturer (or manufacturers) to prevent a possible shortage or
to take appropriate remedial actions once a shortage has occurred; but these tools are only useful
to the extent that FDA receives early notification about the supply disruption. Lack of, or late,
notification severely limits FDA’s ability to coordinate a timely response with manufacturers.
8
There are regional or local shortages of certain products. However, these are often the result of distribution issues
that can be resolved locally and are not indicative of a national supply and demand imbalance. In general, FDA
focuses its resources on nationwide drug shortages of medically necessary products that have the most significant
impact on public health across the country.
9
10
Recently, the White House and Congress have taken important and welcome steps to expand
early notification of interruptions and discontinuations, enhancing FDA’s ability to address drug
shortages. Before FDASIA, notification under section 506C of the FD&C Act was limited in
scope, applying only to sole manufacturers; only to a discontinuance (which could be read to
imply a permanent shutdown); and only to certain approved drugs (e.g., unapproved drugs and
biologics were excluded).
9
The following actions have expanded the scope of early notification
requirements and the rate of early reporting:
October 31, 2011, Executive Order 13588 – Reducing Prescription Drug
Shortages
10
: acknowledged the need for a “multifaceted approach” to address the many
different factors that contribute to drug shortages, and, among other things, directed FDA
to “[u]se all appropriate administrative tools” to require drug manufacturers to provide
advance notice of manufacturing discontinuances that could lead to shortages.
11
December 19, 2011, Interim Final Rule (IFR)
12
: published in response to the Executive
Order, the IFR amended FDA’s regulations related to early notification to improve the
likelihood of FDA receiving advance notification of a potential drug shortage.
13
July 9, 2012, FDASIA: broadens the scope of the early notification provisions by
requiring all manufacturers of certain medically important prescription drugs
14
(approved
9
See 21 CFR 314.80(b)(3)(iii) (as amended by the Interim Final Rule of December 19, 2011).
10
Executive Order 13588, available at http://www.whitehouse.gov/the-press-office/2011/10/31/executive-order-
reducing-prescription-drug-shortages.
11
On October 31, 2011, FDA also released a review of the Agency’s approach to drug shortages, available at
http://www.fda.gov/AboutFDA/Repo
rtsManualsForms/Reports/ucm277749.htm and sent a letter to drug and
biologic manufacturers, encouraging them to voluntarily report potential shortages to FDA, beyond what was
required under the FD&C Act.
12
76 FR 78530 (December 19, 2011). The IFR is a final rule implementing the pre-FDASIA section 506C.
FDASIA significantly amended section 506C and requires FDA to initiate a new rulemaking process to implement
the amended section 506C. FDA is in the process of developing a proposed rule for public comment implementing
the new drug shortages provisions of FDASIA. Once final, the rule will supersede the IFR.
13
As a complement to the IFR, the Agency also published a draft guidance for industry on drug shortages on
February 21, 2012, available at
http://www.fda.gov/downloads/Drugs/GuidanceComplian
ceRegulatoryInformation/Guidances/UCM292426.pdf.
The draft guidance was issued for public comment, and: (1) further discussed the Agency’s interpretation of the
mandatory early notification requirements in the IFR; (2) explained a policy of encouraging additional voluntary
reporting; and (3) discussed the role that manufacturers play in preventing or responding to drug shortages,
including that many shortages arise from quality or other issues experienced during the manufacturing process.
14
Specifically, the requirement currently applies to prescription drugs that are not biologics, and that are: (1) life-
supporting; life-sustaining; or intended for use in the prevention or treatment of a debilitating disease or condition,
including any such drug used in emergency medical care or during surgery; and “(2) … not a radio pharmaceutical
drug product….” FD&C Act § 506C(a).

11
or unapproved) to notify FDA of a permanent discontinuance or (temporary) interruption
in manufacturing. FDASIA also allows FDA to require, by regulation, early notification
of discontinuances or interruptions in the manufacturing of biologics, and requires FDA
to send a noncompliance letter to firms that fail to notify the Agency in accordance with
FDASIA.
15
B. Root Causes of Drug Shortages
In addition to understanding the legal and regulatory authorities related to drug shortages, it is
important to explain why drug shortages occur. In most cases, a shortage is preceded by a
production disruption (i.e., a discontinuance or interruption in manufacturing). Once a
manufacturer experiences a discontinuance or interruption in manufacturing, a shortage will
occur if there is no other manufacturer to step in to fill the gap in supply, or if other
manufacturers cannot increase production quickly enough to make up the loss.
16
A production disruption can be triggered by several factors, including a natural disaster or other
unexpected event not within a manufacturer’s control, or a business decision to permanently
discontinue production of a drug (e.g., because the product is no longer profitable or is less
profitable than other products that could be produced with the limited production capacity
available to the firm) (Figure 2).
More often, however, failures in product or facility quality are the primary factor leading to
disruptions in manufacturing (Figure 2). In 2012, for example, based on information collected
from manufacturers, FDA determined that the majority of production disruptions (66%) resulted
from either (1) efforts to address product-specific quality failures (31%, labeled Quality:
Manufacturing Issues in Figure 2) or (2) broader efforts to remediate or improve a problematic
manufacturing facility (35%, labeled Quality: Remediation Efforts in Figure 2). Quality or
manufacturing concerns can involve compromised sterility, such as roof leakage; mold in
manufacturing areas; or unsterilized vials or containers to hold the product—issues that could
pose extreme safety risks to patients.
Since 2011, the number of shortages associated with shutdowns or slowdowns in manufacturing
to address overall facility quality (remediation) has not changed significantly, although there has
been a decline in the number of shortages due to quality concerns related to a specific product
(e.g., particulates, contamination). Remediation efforts can lead to a short-term risk of a
shortage for specific products while facility upgrades are made, but this risk is balanced by the
15
Under FDASIA, FDA must issue a final rule implementing certain of the FDASIA shortages provisions by
January 9, 2014.
16
See Woodcock J, Wosinska, M. Economic and technological drivers of generic sterile injectable drug shortages.
Clin Pharmacol Ther. 93:170-176 (2013), pp. 174-75 (discussing why one manufacturer’s disruption in supply may
result in a market-wide shortage).
expectation that improvements will lead to long-term, stable, high-quality manufacturing
capacity, thus reducing the long-term risk of shortages.
17
Figure 2. Drug Shortages by Primary Reason for Disruption in Supply in 2012
35%
31%
14%
8%
2%
6%
4%
Quality:Facility
RemediationEfforts
Quality:Product
ManufacturingIssues
Discontinuationof
Product
RawMaterials(API)
Shortage
OtherComponent
Shortage
IncreasedDemand
LossofManufacturing
Site
Source:Datafrom
FDA’sinternaldrug
andbiologics
shortagesdatabases.
C. Current FDA Efforts to Prevent and Mitigate Drug Shortages
Preventing or mitigating drug shortages requires a careful and coordinated response by FDA,
which begins with a notification to FDA of a potential disruption in supply. This notification
allows FDA to assess the risk of a shortage developing and subsequently deploy one or more
regulatory tools, as appropriate, to prevent or mitigate the shortage. FDA largely focuses its
efforts on ensuring that a disruption in production does not result in a shortage, or on mitigating
the impact of the shortage should it become unavoidable.
1. Notifying FDA of a Disruption in Supply
The most important way to prevent or mitigate a drug shortage is for FDA to learn about a
disruption in production as soon as possible. The sooner FDA is notified of a discontinuance or
interruption in manufacturing, the more time FDA has between the disruption and the potential
shortage to help coordinate the manufacturers’ response. Notification of a disruption in
production can come from a variety of sources outside the agency, including a drug
manufacturer, a professional organization, interest groups, patients, and health care
professionals, or through internal channels at FDA. Since the Executive Order was issued in
17
Berg N, Kos K, et al. Report on the ISPE 2013 Drug Shortages Survey. June 2013. Tampa, FL: International
Society for Pharmaceutical Engineering. Available at http://www.ispe.org/drugshortages/2013JuneReport
.
12

13
October 2011, FDA has seen a six-fold increase in the num
ber of notifications from
manufacturers (from 10 notifications per month prior to the Executive Order to an average of 60
per month since the Executive Order). These early notifications have had a direct impact on the
number of shortages FDA has been able to prevent.
2. Assessing the Risk of Shortage
Once FDA’s shortage staff has been alerted to a discontinuance or disruption in production, they
first verify if an actual shortage exists or may occur. The shortage staff may take the following
actions to do this:
Use a market research database to collect initial information to determine whether or not
the current supply of product across manufacturers is stable
Contact product manufacturer(s) to collect up-to-date inventory information, rate of
demand (units/month), manufacturing schedules, and any changes in ordering patterns.
Although manufacturers are not required to provide this information to FDA, voluntarily
sharing this information greatly facilitates the management of shortages
Evaluate product inventory in the distribution chain to the extent possible. This
information can help predict how quickly a shortage may develop (if at all)
Additional information on how FDA collects and uses data to assess the risk of a shortage can be
found in Appendix C.
When the shortage staff determines that a shortage either exists or is likely to occur imminently,
shortage staff members lead and coordinate the mitigation efforts with multiple other offices
within FDA. Working with FDA’s drug review division and/or professional organizations, they
determine if the drug is medically necessary.
18
FDA uses the information about whether or not a
drug is medically necessary to prioritize its response to a shortage overall and to inform the risk-
benefit assessment for the specific product in question.
3. Mitigating an Actual or Imminent Shortage
Mitig
ation efforts begin once FDA has confirmed that a shortage exists or could occur and that
the drug is medically necessary. The actions FDA can take to mitigate a shortage include, as
appropriate:
Identify the extent of the shortfall and determine if other manufacturers are willing and
able to increase production to make up the gap
18
A medically necessary drug product is a product that is used to treat or prevent a serious disease or medical
condition for which there is no alternative drug in adequate supply that is judged by medical staff to be an
appropriate substitute. Off-label uses are taken into account when making medical necessity determinations. CDER
MAPP 6003.1, Drug Shortage Management at 2, available at
http://www.fda.gov/downloads/AboutFDA/Cen
tersOffices/CDER/ManualofPoliciesProcedures/ucm079936.pdf.
14
Expedite FDA inspections and reviews of submissions from manufacturers attempting to
restore production
Expedite FDA inspections and reviews of submissions from competing manufacturers
who are interested in starting new production or increasing existing production of
products in shortage
Exercise temporary enforcement discretion for new sources of medically necessary drugs
Work with the manufacturer to ensure adequate investigation into the root cause of the
shortage
Develop risk mitigation measures for a batch(es) of product initially not meeting
established standards
FDA may use one or more of these mitigation tools, or may seek to develop other options,
depending on the severity of the shortage and the circumstances surrounding the shortage. When
selecting specific tools, FDA continues to work with the manufacturer to tailor its response to the
specific situation. FDA also frequently communicates available information about a shortage to
affected stakeholders and monitors a shortage until it is resolved. Additional information on how
FDA collects and uses data to manage a shortage can be found in Appendix C.
It is important to note that FDA’s standards of safety, efficacy, and quality do not change in a
shortage situation. FDA’s preferred solution to a shortage is a supply of approved drugs
sufficient to meet patient demand. However, FDA recognizes that there can also be risks to
patients when treatment options are not available for critical conditions, and understands the
importance of using the appropriate tools to prevent or mitigate a shortage. FDA also makes
certain that drug shortages are considered before taking an enforcement action or issuing a
Warning Letter. In appropriate cases, temporary exercise of regulatory flexibility has proven to
be an important tool in ensuring access to treatment options for health care practitioners and
patients in critical need.
For example, when particulate matter (including glass and metal particles) was found in an
injectable drug product that was medically necessary and vulnerable to shortage, FDA exercised
discretion to allow distribution of the product along with a letter, included in the drug’s
packaging, warning health care professionals to use a filter when administering the drug. The
exercise of discretion was temporary, and was conditioned on the manufacturer’s ability to
demonstrate to FDA that the filter did not affect the way the drug works and could successfully
remove the particulate. FDA also worked with the manufacturer while the manufacturer
identified and addressed the root cause of the problem, so that it could resume producing a drug
product that did not need the work-around involving the filter. In the last two years, FDA has
exercised temporary regulatory flexibility involving the use of filters for eight important drugs,
including life-saving components of IV nutrition for newborns, children, and other patients who
are unable to eat or drink by mouth.
In contrast, a drug that is contaminated with bacteria or fungi presents a more extreme risk to
patients, one that cannot be mitigated through a work-around such as the one described above.
In such cases, the manufacturer must correct the conditions leading to the contamination before
the product is safe for use, even if correcting the conditions ultimately leads to a shortage. Each
situation must be carefully evaluated to determine the public health impact, keeping in mind that
a given action may have unintended, and potentially long-term, consequences.
19
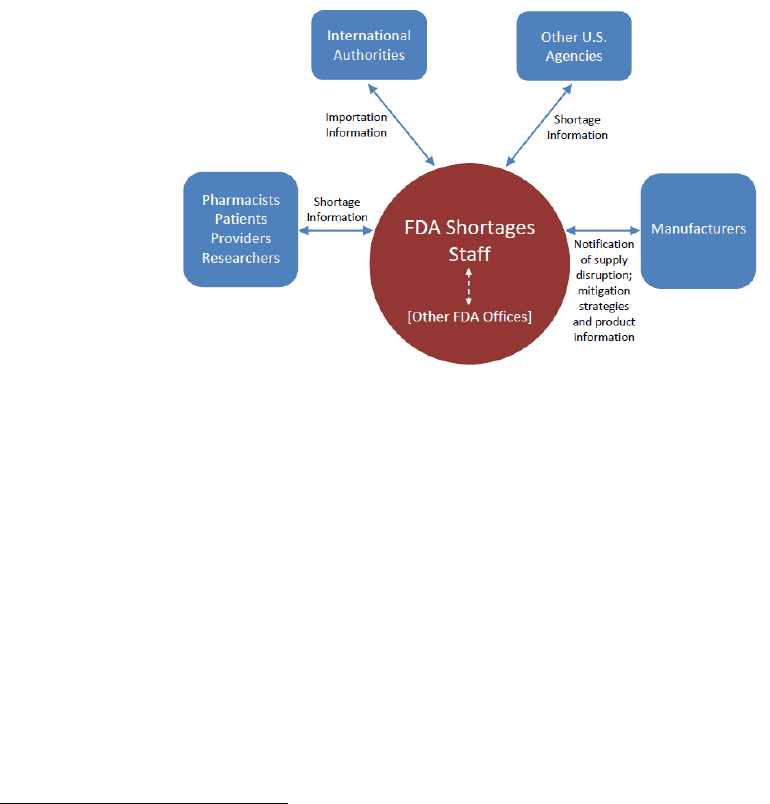
D. Internal and External Communication and Engagement
FDA communication and collaboration processes are essential to effectively prevent and manage
drug shortages. These types of interactions, both internally and externally, occur in a variety of
ways and with a variety of stakeholders (Figure 3).
Figure 3. Internal and External Communication During Shortage Management
1. Internal Coordination
The Agency’s response to a notification of a discontinuance or production disruption involves
multiple offices within FDA and often requires a cross-functional team of up to 25 individuals to
respond to a specific shortage or potential shortage. Appendix D lists examples of specific
offices and their roles in managing drug shortages. Appendix E lists specific examples of the
types of communication that occur within the Center for Drug Evaluation and Research (CDER)
to manage a drug shortage.
In response to the recent increase in drug shortages, CDER has nearly tripled the number of staff
directly responsible for shortages, from 4 employees in 2011 to 11 employees in 2013. The
office has also been elevated within the Center to indicate the priority FDA has placed on this
topic. Although this staff serves as the primary point of contact for drug shortage-related issues,
shortage staff members work with a large number of other offices and divisions within FDA.
19
For example, if the use of enforcement discretion signals to industry that FDA is willing to exercise flexibility to
ensure the availability of any critical product, this could create a long-term disincentive for manufacturers to invest
in manufacturing upgrades or other quality improvements to avoid disruptions in supply, exacerbating the risk of
shortages over the long term.
15

16
2. External Communication and Engagement
In addition to internal communication and coordination to facilitate FDA decision-making, it is
essential for FDA to adequately maintain communication with the many external groups affected
by drug shortages, including patients, health professionals, federal partners, and international
groups. Appendix F describes these communications in greater detail.
One straightforward and accessible method for enhancing communication with these groups has
been through FDA’s website. In response to feedback from numerous stakeholders, including
those who responded to the Federal Register notice, FDA has significantly improved its drug
and biologics shortage websites in the past year. For example, CDER’s website now includes
the following features:
More frequent updates to the CDER shortage website, including biweekly updates on
postings from manufacturers about progress on specific shortages
Icons to highlight new listings and update dates
Improved layout for easier navigation, including the creation of a Current Drug Shortage
Index, and separating the shortage list into sections of the alphabet for each page
Information about the causes of shortages
A page for additional news and information, such as extensions of expiry date for a
specific lot of product to prevent shortage
Another important shortage website is maintained by the American Society of Health-System
Pharmacists (ASHP) and the Drug Information Service at the University of Utah.
20
FDA, the
Drug Information Service, and ASHP exchange information on a routine basis, sharing
notifications and public information on the status of the drug supply. This collaborative effort
has greatly improved FDA’s ability to monitor product disruptions. ASHP’s website also
provides recommendations about therapeutic alternatives.
Communication with manufacturers is also a key component of FDA’s coordination efforts.
FDA’s drug shortages staff interact with manufacturers on an ongoing basis to understand
specific issues related to possible or ongoing shortages. For example, when the shortage staff
hears about a potential disruption in production, FDA may contact other manufacturers to see if
they have experienced an increase in demand; determine the status of their on-hand supply; and
if a shortage appears imminent, determine whether the manufacturer is willing to initiate or
increase production. FDA then works with those manufacturers as they increase production.
During a shortage, FDA also continues to work with the manufacturer causing the shortage to aid
in recovery efforts. For instance, when a firm shuts down to address manufacturing and/or
quality problems, FDA works with the firm as it remediates the problem to restore or maintain
the availability of critical medicines. To maintain some level of supply during remediation, FDA
may recommend creating a priority list that emphasizes continued manufacture of shortage
20
Available at http://www.ashp.org/shortages.
products; encourage allocation programs to hold back supply of shortage products; or
recommend third-party quality review for release of manufactured batches.
Finally, FDA understands the important role that the public plays in discussing and identifying
solutions to drug shortage issues. FDA is actively involved in meetings with outside
stakeholders to help guide FDA’s regulatory and policy decision making, as well as to increase
our understanding of the issues facing these stakeholders. In 2012, FDA staff participated in
more than 45 such meetings with academics, patient groups, pharmacy organizations,
distributors, and manufacturers.
II. Continuing Progress: FDA’s Strategic Plan
As described previously, Title X of FDASIA requires FDA to establish a task force to develop
and submit to Congress a Strategic Plan to enhance FDA’s ability to address drug shortages. In
response, FDA convened a Task Force representing multiple centers, offices, and disciplines
within FDA. The Task Force has identified two central goals and related tasks for FDA to focus
on to address drug shortages, stated below. Specific tasks are discussed in further detail in the
sections that follow.
STRENGTHEN MITIGATION RESPONSE.
Goal #1
Improve and streamline FDA’s current mitigation activities once the
Agency is notified of a supply disruption or shortage.
DEVELOP LONG-TERM PREVENTION STRATEGIES.
Goal #2
Develop prevention strategies to address the underlying causes of
production disruptions to prevent drug shortages.
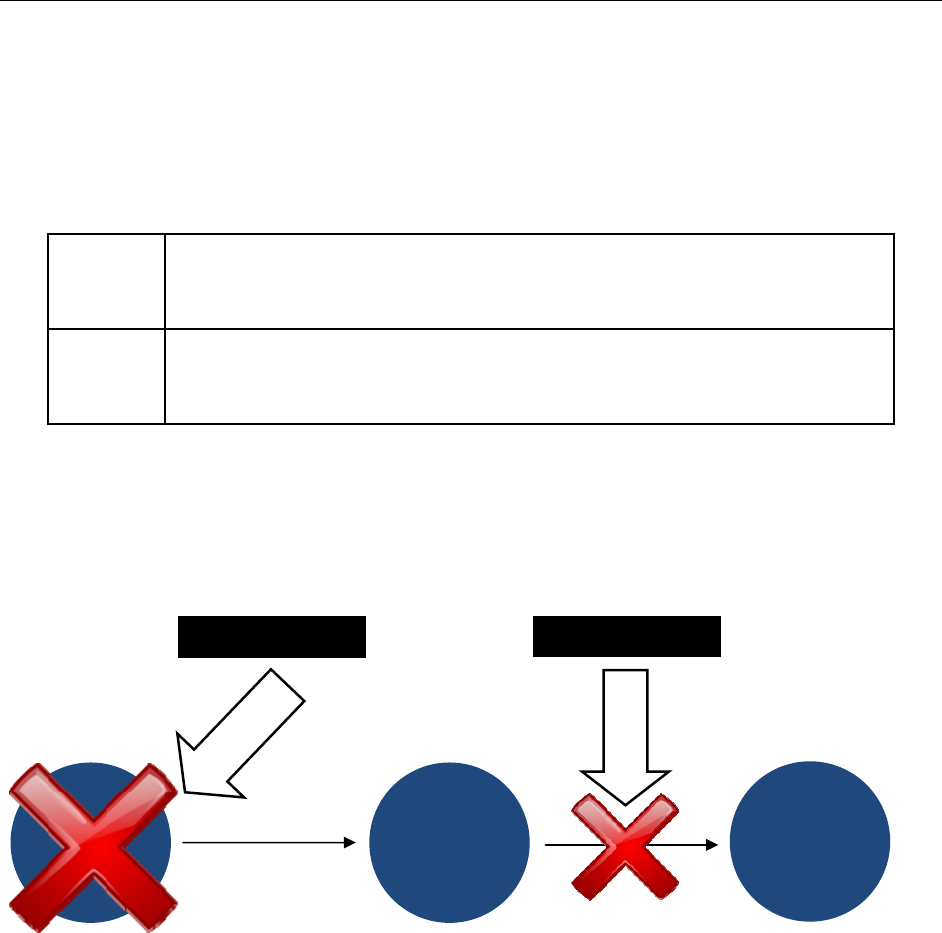
As depicted in the Figure 4, mitigation activities are directed at preventing supply disruptions
from turning into actual shortages. Long-term prevention strategies are intended to address the
underlying causes of shortages to prevent supply disruptions from occurring in the first place.
Figure 4. Addressing Drug Shortages: Mitigation Activities and Long-Term Prevention
17
Supply
Disruption
MITIGATION
Quality
Problems &
Other Factors
PREVENTION
Drug
Shortage

18
A. GOAL #1: Strengthen Mitigation Response
The Task Force identified the following tasks to strengthen FDA’s ability to respond to a
notification of a production disruption to prevent a shortage or to mitigate an unavoidable
shortage.
TASK 1.1: Develop and/or Streamline Internal FDA Processes
While coordinating the increased number of drug shortages over the last few years, FDA has
continued to improve its approaches to the management of shortages. Revising and
standardizing procedures will more accurately reflect and enhance the working interactions
between units within FDA and will maximize the efficiency of FDA’s response to a shortage.
As a part of this work, the Center for Biologics Evaluation and Research (CBER) is undertaking
a review of its internal procedures to address shortages, including revising its standard operating
policy and procedure on CBER-regulated product shortages.
21
In addition, CDER is revising its
Manual of Policies and Procedures (MAPP) on Drug Shortage Management.
22
Specifically, FDA
intends to:
Standardize the risk-benefit assessment process prior to compliance actions
23
Update the process for interacting with the Drug Enforcement Administration (DEA) in
the event of a shortage of a controlled substance, including updating a memorandum of
understanding between the two agencies that will improve the sharing of important
information
Develop and implement a process for issuing a noncompliance letter for failure to notify
FDA as required by FDASIA
Explore new approaches to extend expiration dating to temporarily mitigate a shortage
TASK 1.2: Improve Data and Response Tracking
FDA is working to improve its databases related to shortages and the tracking procedures it uses
to manage them. FDA has several databases that it uses to support efforts to address shortages,
but they were not created specifically for the purposes of assisting shortage-related activities.
24
21
CBER Standard Operating Policy and Procedure 8506, Management of Shortages of CBER-Regulated Products,
available at
http://www.fda.gov/BiologicsBloodVacci
nes/Guidan
ceComplianceRegulatoryInformation/ProceduresSOPPs/ucm29
9304.htm.
22
CDER MAPP 6003.1, Drug Shortage Management.
23
This work is also responsive to FDASIA, which directed FDA to evaluate certain risks and benefits prior to an
enforcement action or issuance of a warning letter that could reasonably be expected to lead to a meaningful
disruption in the supply of a drug covered by section 506C. FD&C Act § 506D(c).
24
Examples include databases supporting the Orange and Red Books, and the databases that collect information
about the inspections of manufacturing facilities.
19
In addition to these more general databases, CDER has created a dedicated database that focuses
solely on collecting data related to shortages. This improved tracking will enable FDA to better
assess progress on preventing and mitigating shortages and will enhance FDA’s ability to
compile the information necessary for the required annual report to Congress on drug shortages.
TASK 1.3: Clarify Roles/Responsibility of Manufacturers
Patients expect and deserve high-quality drugs, and it is the manufacturer’s responsibility to
ensure that its products are safe, effective, and of high quality. FDA is committed to working
with industry to resolve quality or manufacturing problems that arise, or avoid them if possible.
Where justified, FDA may exercise regulatory flexibility to prevent or mitigate a shortage. To
facilitate its work with industry, FDA intends to:
Finalize the proposed rule on Notification to FDA of a Discontinuance or Interruption in
Supply of Certain Drug or Biological Products
Work with manufacturers as needed to remediate manufacturing problems, facilitate their
return to full production, and discuss how to prioritize drugs in shortage as they address
manufacturing deficiencies
Encourage manufacturers to engage in practices to avoid or mitigate shortages. These
practices are listed in the table below:
Practices to Avoid or Mitigate Shortages
Create Allocation Plans Design an allocation plan in advance, in the event that a product
shortage occurs.
Communicate with
Contract Manufacturing
Organizations
Communicate frequently with contract manufacturers to ensure up-to-
date knowledge of their manufacturing processes and facilities and to
anticipate potential problems that might lead to a shortage
Manage Inventory Build robust inventories before major manufacturing changes, such as
upgrades to manufacturing facilities or transfer of facility ownership
Develop Short- and
Long-Term Proposals
Provide short- and long-term proposals to address issues that could
cause a shortage, either as part of the notification to FDA of a
potential shortage or shortly thereafter
Communicate with FDA Engage in dialogue with FDA to work on a long-term solution to a
shortage, including remediation efforts
Investigate Root Causes Provide a realistic timeline for investigation of product defects and
scheduled restart after shut downs
Consider Clinical Trials Consider the possibility of shortages during initial clinical trial design
and developing and implementing contingency plans for handling a
shortage during the clinical trial, should one arise (see Appendix F for
additional discussion of this point).
TASK 1.4: Enhance Public Communications about Drug Shortages
Comments received in response to the Federal Register notice made clear that ongoing efforts by
FDA to improve external communications have enhanced our response to shortages or potential
20
shortages. Additionally, groups requested up-to-the-minute and customized information about
shortages. FDA will continue to improve our public communications about drug shortages by:
Developing a smartphone application so that individuals can access the drug shortage
information currently posted online instantaneously from their mobile phones or tablets
Updating the website to include the therapeutic category (or categories) for the products
listed in shortage
Improving the functionality of the drug shortages website so that users will be able to sort
by therapeutic or other categories and view the relevant products confirmed to be in
shortage nationally
B. GOAL #2: Develop Long-Term Prevention Strategies
Developing long-term strategies focused on the underlying causes of production disruptions can,
ultimately, prevent drug shortages. Efforts to build on existing tools to mitigate or prevent
existing shortages are an important piece of the Strategic Plan. However, a comprehensive
strategy must also recognize the importance of addressing the underlying causes of shortages,
including sustaining manufacturing quality. While keeping in mind the role other stakeholders
play in ensuring manufacturing quality, FDA is also exploring actions it can take, both alone and
in collaboration with other groups.
TASK 2.1: Develop Methods to Incentivize and Prioritize Manufacturing Quality
The majority of drug shortages are the result of production disruptions caused by manufacturing
problems, particularly problems that affect product quality (see Figure 2). Ultimately,
prevention of future drug shortages means improving the quality of manufacturing facilities and
manufactured products. In addition to regulatory enforcement, FDA is exploring what it can do
to provide more positive incentives for quality improvements and to make manufacturing quality
a priority, taking into account the responses we received to the Federal Register notice. For
example, FDA is:
Examining the broader use of manufacturing quality metrics to assist in the evaluation of
product manufacturing quality
Implementing internal organizational improvements to focus on quality, including the
creation of an Office of Pharmaceutical Quality within CDER
Considering public recognition of manufacturers who have demonstrated a consistent
record of high quality manufacturing
Continuing expedited review to mitigate shortages, including the review of submissions
for facility upgrades to improve quality
TASK 2.2: Use Regulatory Science to Identify Early Warning Signals of Shortages
Understanding the factors contributing to supply disruptions and drug shortages and identifying
potential warning signals of future production disruptions could help FDA and manufacturers in
their efforts to prevent them. However, identifying these factors and signals is a challenging

21
undertaking. To further this effort, FDA will explore risk-based approaches to identifying the
early warning signals of manufacturing and quality problems that could lead to production
disruptions. In addition, to gain a better understanding of the factors and forces contributing to
manufacturing and quality problems, FDA will work with stakeholders to identify vulnerabilities
at manufacturing sites that could put the production of quality drugs at that facility at risk, and
thereby contribute to shortages.
TASK 2.3: Increase Knowledge to Develop New Strategies to Address Shortages
A wide variety of stakeholders have collected and analyzed data on drug shortages and potential
approaches to their prevention, and it is essential that FDA continue to work with these
stakeholders to fill gaps in our understanding of issues around shortages. This additional
information could help inform new strategies to address shortages. Specifically, FDA intends to:
Work with the International Society for Pharmaceutical Engineering (ISPE) to analyze
data from a recent global survey ISPE conducted on the technical, scientific,
manufacturing, quality, and compliance issues that have resulted in drug shortages
Join with other stakeholder groups, such as groups convened by the American Society of
Anesthesiologists and ASHP, and with individual companies, patient groups, and group
purchasing organizations to discuss shortages
Work with manufacturers to identify best practices for avoiding disruptions in production
Continue to explore (through the Department of Health and Human Services) the
potential benefit, and assess the identified challenges, of establishing a Qualified
Manufacturing Partner Program (QMPP)
25
III. Actions Other Stakeholders Should Consider
FDA can play a significant role in addressing drug shortages and has identified several additional
ways to enhance its efforts. It is clear, however, that FDA cannot address the threat of drug
shortages alone. Success in addressing drug shortages requires a collective effort by all
stakeholders—manufacturers, federal partners, researchers, professional organizations, and
patients. In preparing this Strategic Plan, FDA has identified four key areas that merit
consideration by the broader community for their potential to reduce drug shortages. FDA
limitations in these areas and possible roles for others are discussed in the following sections.
25
FDASIA requires FDA to examine the value of establishing a QMPP. FDA asked for public input on the
feasibility and advisability of a QMPP in the Federal Register notice. We asked commenters to address several
significant challenges FDA identified with such a program, including lack of funding to incentivize participation and
a potentially unlimited scope of applicable products. Many public comments supported the idea of a QMPP, but did
not address the challenges FDA raised. In follow up to these comments, the Department of Health and Human
Services will explore further the feasibility of creating a QMPP.

22
A. Manufacturing Incentives
Advances in drug discovery and development have been and continue to be encouraged and
supported through a variety of economic incentives, such as listed patent protection, various
forms of statutory market exclusivity, and tax credits. Yet, the need for innovation goes beyond
drug discovery and approval—manufacturing processes and technologies must keep pace with
advances in drug research and development. In many cases pharmaceutical manufacturing
processes, facilities, and equipment lag behind innovation in drug development. Some processes
and facilities have become outdated, resulting in quality problems that can lead to drug
shortages.
FDA Limitations: FDA is exploring ways to use its existing authorities to promote and sustain
quality manufacturing. However, our ability to offer financial or other economic means to
promote innovation in quality manufacturing is limited.
Opportunities for Others: Given the importance of quality and its link to shortages, other
stakeholders might explore economic, financial, or other means to incentivize innovation and
new investments in manufacturing quality drugs to reduce the occurrence and severity of
shortages.
B. Use of Data on Manufacturing Quality
Within the limits set by disclosure law, FDA makes certain information publicly available about
the historical ability of manufacturers to produce quality products. Several indicators of
historical quality, including a manufacturer’s history of inspection outcomes and classification,
recalls, and shortages, are publicly available. Nevertheless, numerous comments to the Federal
Register notice suggested that buyers (e.g., hospitals, health maintenance organizations, group
purchasing organizations, and others) do not consider or value this potentially important
information. This decoupling of quality considerations from purchasing decisions makes cost
the major factor in purchasing decisions, most likely intensifying price competition, leading
manufacturers to focus more on reducing costs than on maintaining quality, and potentially
contributing to shortages.
FDA Limitations: Although FDA makes certain quality information public, buyers ultimately
decide how or whether they will use this data when they make purchasing decisions.
Opportunities for Others: An effort by buyers to use publicly available information to take
quality into account when making drug purchasing decisions—for example, by buying only from
manufacturers with a history of good quality, or including “failure to supply” clauses in
purchasing contracts—could further incentivize manufacturers to invest in quality improvements,
and ultimately prevent drug shortages.

23
C. Redundancy, Capability, and Capacity
A disruption in supply is exacerbated by limited manufacturing capacity and capability, market
concentration, and just-in-time inventory practices that result in minimal product inventory being
on hand at any given time. If these factors are present, a disruption in supply is more likely to
result in a nationwide shortage. Many stakeholders, including commenters to the Federal
Register notice, have suggested that building redundancy, holding spare capacity, and increasing
inventory levels could lower the risks of shortages.
FDA Limitations: FDA does not regulate manufacturing concentration and cannot require
redundancy of manufacturing capability or capacity. Nor can FDA require a company to
manufacture a drug, maintain a certain level of inventory of drug product, or reverse a business
decision to cease manufacturing.
Opportunities for Others: Manufacturers could explore building redundant manufacturing
capacity, holding spare capacity, or increasing inventory levels to lower the risks of shortages.
Other stakeholders could consider how to incentivize such practices.
D. The Gray Market
In the context of drug shortages, the term gray market is often used to reference the downstream
distribution of approved drug products at significantly marked-up prices. A shortage offers a
unique opportunity for this to occur, because large-scale buyers are often willing to pay any price
to obtain a much-needed product in short supply. When a gray market distributor handles a
product that it normally would not distribute, there can also be safety issues that put patients at
risk – if, for example, the product is not stored or handled appropriately. These activities do not
cause shortages, but may exacerbate the impact of an existing shortage.
FDA Limitations: FDA has limited data on the gray market and limited influence on its
workings (including no authority regarding product pricing). However, there is evidence that
gray market activities exacerbate the impact of drug shortages.
Opportunities for Others: Actions on the part of other stakeholders to minimize gray market
activities could play a role in mitigating the impact of shortages and reducing risks to patients.
Conclusion
Drug shortages remain a significant public health issue in the United States and a top priority for
FDA. FDA works with manufacturers to help prevent shortages from occurring. In this respect,
early and open dialogue between FDA and manufacturers is critical to successfully preventing a
shortage. Because of recent important actions by the President and Congress, FDA has been able
to learn of many possible shortages before they occur. These actions, along with increases in the
24
resources FDA is devoting to drug shortages, have helped prevent numerous recent shortages—
more than 280 in 2012.
Despite these achievements, substantial challenges remain, and more work is needed on the part
of manufacturers and other relevant stakeholders. This Strategic Plan identifies a number of
activities to improve the Agency’s ability to address drug shortages. Activities center around
two key goals: (1) strengthening FDA’s ability to respond to notices of a disruption in supply,
including improving our mitigation tools, and (2) developing long-term strategies to prevent drug
shortages by addressing the underlying causes of shortages.
FDA recognizes that manufacturers and other stakeholders have important roles to play in
ensuring that drugs are available to the patients who need them. Thus, the Task Force has also
identified actions outside stakeholders can consider that show promise in helping to prevent
shortages.
FDA looks forward to implementing this Strategic Plan as part of a collaborative and sustained
effort to address drug shortages in the United States.
25
APPENDIX A: Federal Register Notice
http://www.gpo.gov/fdsys/pkg/FR-2013-02-12/html/2013-03198.htm
[Federal Register Volume 78, Number 29 (Tuesday, February 12, 2013)]
[Notices]
[Pages 9928-9929]
From the Federal Register Online via the Government Printing Office
[www.gpo.gov]
[FR Doc No: 2013-03198]
-----------------------------------------------------------------------
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Food and Drug Administration
[Docket No. FDA-2013-N-0124]
Food and Drug Administration Drug Shortages Task Force and
Strategic Plan; Request for Comments
AGENCY: Food and Drug Administration, HHS.
ACTION: Notice; request for comments.
-----------------------------------------------------------------------
SUMMARY: To assist the Food and Drug Administration (FDA or Agency) in
drafting a strategic plan on drug shortages as required by the Food and
Drug Administration Safety and Innovation Act, the Agency is seeking
public comment from interested persons on certain questions related to
drug and biological product shortages.
DATES: Submit either electronic or written comments by March 14, 2013.
ADDRESSES: You may submit comments, identified by Docket No. FDA-2013-
N-0124, by any of the following methods:
Electronic Submissions:
Submit electronic comments in the following way:
Federal eRulemaking Portal: http://www.regulations.gov.
Follow the instructions for submitting comments.
Written Submissions:
Submit written submissions in the following way:
Mail/Hand delivery/Courier (for paper or CD-ROM
submissions): Division of Dockets Management (HFA-305), Food and Drug
Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852.
Instructions: All submissions received must include the Agency name
and Docket No. FDA-2013-N-0124. All comments received may be posted
without change to http://www.regulations.gov, including any personal
information provided. For additional information on submitting
comments, see the ``Comments'' heading of the SUPPLEMENTARY INFORMATION
section of this document.
Docket: For access to the docket to read background documents or
comments received, go to http://www.regulations.gov and insert the
docket number, found in brackets in the heading of this document, into
the ``Search'' box and follow the prompts and/or go to the Division of
Dockets Management, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852.
FOR FURTHER INFORMATION CONTACT: Kalah Auchincloss, Center for Drug
Evaluation and Research, Food and Drug Administration, 10903 New
Hampshire Ave., Bldg. 51, rm. 6208; Silver Spring, MD 20993, 301-796-
0659.
SUPPLEMENTARY INFORMATION:
I. Background
On July 9, 2012, the President signed into law the Food and Drug
Administration Safety and Innovation Act (FDASIA) (Pub. L. 112-144).
Section 1003 of FDASIA adds section 506D to the Federal Food, Drug, and
Cosmetic Act (the FD&C Act) to require the formation of a task force to
26
develop and implement a strategic plan for enhancing the Agency's
response to preventing and mitigating drug shortages. Section 506D of
the FD&C Act (21 U.S.C. 356D) requires that the drug shortages
strategic plan include the following:
-Plans for enhanced interagency and intra-agency coordination, communication,
and decisionmaking;
-Plans for ensuring that drug shortages are considered when the Secretary
initiates a regulatory action that could precipitate a drug shortage or
exacerbate an existing drug shortage;
-Plans for effective communication with outside stakeholders, including who
the Secretary should alert about potential or actual drug shortages, how the
communication should occur, and what types of information should be shared;
-Plans for considering the impact of drug shortages on research and clinical
trials; and
-An examination of whether to establish a ``qualified
manufacturing partner program'' as described in section 506D(a)(1)(C)
of the FD&C Act.
II. Scope of Public Input Requested
Per the directive in section 506D, FDA has formed an internal Drug
Shortages Task Force (Task Force) to develop and implement the drug
shortages strategic plan. The Task Force is seeking comments from the
public on issues related to the development of this strategic plan.
Importantly, although FDASIA refers only to a drug shortages strategic
plan, we anticipate that the strategic plan will consider prevention
and mitigation of both drug and biological product shortages.
Accordingly, we are interested in receiving comments on these questions
from all parties, including those with an interest in biological
products. The Task Force is specifically interested in seeking public
input on the following questions:
1. In an effort to address the major underlying causes of drug and
biological product shortages, FDA is seeking new ideas to encourage
high-quality manufacturing and to facilitate expansion of manufacturing
capacity.
a. To assist in the evaluation of product manufacturing quality,
FDA is exploring the broader use of manufacturing quality metrics. With
that in mind, FDA would like input on the following issues: What
metrics do manufacturers currently use to monitor production quality?
To what extent do purchasers and prescribers use information about
manufacturing quality when deciding how to purchase or utilize
products? What kinds of manufacturing quality metrics might be valuable
for purchasers and prescribers when determining which manufacturers to
purchase from or which manufacturers' products to prescribe? What kinds
of manufacturing quality metrics might be valuable for manufacturers
when choosing a contract manufacturer? How frequently would such
metrics need to be updated to be meaningful?
b. The use of a qualified manufacturing partner program similar to
one used under the Biomedical Advanced Research and Development
Authority (BARDA) has been suggested as a potentially useful approach
to expanding manufacturing capacity and preventing shortages. FDA
recognizes that there are important potential differences between the
BARDA program and the use of a parallel program to address shortages.
For example, the BARDA program covers a relatively stable and limited
number of products, but drugs at risk of shortage are many, may change
rapidly over time, and are difficult to predict in advance. In
addition, FDA does not have funding to pay manufacturers to participate
in a drug shortages qualified manufacturing partner program or to
27
guarantee purchase of the end product. With these differences in mind,
is it possible to design a qualified manufacturing partner program that
would have a positive impact on shortages?
c. Are there incentives that FDA can provide to encourage
manufacturers to establish and maintain high-quality manufacturing
practices, to develop redundancy in manufacturing operations, to expand
capacity, and/or to create other conditions to prevent or mitigate
shortages?
2. In our work to prevent shortages of drugs and biological
products, FDA regularly engages with other U.S. Government Agencies.
Are there incentives these Agencies can provide, separately or in
partnership with FDA, to prevent shortages?
3. When notified of a potential or actual drug or biological
product shortage, FDA may take certain actions to mitigate the impact
of the shortage, including expediting review of regulatory submissions,
expediting inspections, exercising enforcement discretion, identifying
alternative manufacturing sources, extending expiration dates based on
stability data, and working with the manufacturer to resolve the
underlying cause of the shortage. Are there changes to these existing
tools that FDA can make to improve their utility in managing shortages?
Are there other actions that FDA can take under its existing authority
to address impending shortages?
4. To manage communications to help alleviate potential or actual
shortages, FDA uses a variety of tools, including posting information
on our public shortages Web sites and sending targeted notifications to
specialty groups. Are there other communication tools that FDA should
use or additional information the Agency should share to help health
care professionals, manufacturers, distributors, patients, and others
manage shortages more effectively? Are there changes to our public
shortage Web sites that would help enhance their utility for patients,
prescribers, and others in managing shortages?
5. What impact do drug and biological product shortages have on
research and clinical trials? What actions can FDA take to mitigate any
negative impact of shortages on research and clinical trials?
6. What other actions or activities should FDA consider including
in the strategic plan to help prevent or mitigate shortages?
III. Comments
Interested persons may submit either electronic comments regarding
this document to http://www.regulations.gov or written comments to the
Division of Dockets Management (see ADDRESSES). It is only necessary to
send one set of comments. Identify comments with the docket number
found in brackets in the heading of this document. Received comments
may be seen in the Division of Dockets Management between 9 a.m. and 4
p.m., Monday through Friday, and will be posted to the docket at
http://www.regulations.gov.
Dated: February 7, 2013.
Leslie Kux,
Assistant Commissioner for Policy.
[FR Doc. 2013-03198 Filed 2-11-13; 8:45 am]
28
APPENDIX B: Statutory Elements
26
The following table indicates where in this Strategic Plan the reader can find discussion of
specific topics required by FDASIA.
Element Section&Task
Enhancedinter‐agencyandintra‐agencycoordination,
communication,anddecision‐making
Task1.1
SectionI.C.2
SectionII.A
Ensuredrugshortagesareconsideredbeforeinitiating
aregulatoryactionthatcouldprecipitateor
exacerbateadrugshortage
Task1.1
Section1.C.1
Effectivecommunicationwithexternalstakeholders Task1.4
Task2.3
SectionI.C.2
SectionII.A
Impactofdrugshortagesonresearchandclinicaltrials Task1.3
SectionI.C.2
Examinationofestablishinga“qualifiedmanufacturing
partnerprogram”
Task2.3
SectionII.B
26
Section 506D(a)(1)(B) (added by FDASIA) requires FDA to consider certain elements when developing the
Strategic Plan.
29
APPENDIX C: Collecting Information on Drug Shortages
Recognizing the complexity of drug manufacturing and the importance of having up-to-date data
to manage shortages, FDA draws on a variety of sources for information on whether a shortage
exists and how best to manage a shortage. The table below lists various sources of information
and the type of information obtained from each source. Figure 5 illustrates some of these
sources of information in the context of the drug supply chain.
Source TypeofInformation
OrangeBookorDrugs@FDA Informationontherapeuticequivalents
NationalDrugCode(NDC)product
directory
IdentificationofNDCsforproducts
InternalFDAdatabases
Informationonmanagingandtracking
drugandbiologicslicenseapplications;
inspectionsandregisteredmanufacturing
establishments;andlotdistributionand
releasedata
IMSHealth(healthcareanalyticsfirm)
Informationonthepharmaceutical
market,includinghistoricalsupplyand
demand
RedBook
Informationoncurrentsuppliersof
products
Emailsfromprescribers,patients,
pharmacies,manufacturers,andother
stakeholders
Notificationsofadisruptioninsupplyand
informationontheimpactofashortage
ASHPshortagewebsite
Additionalinformationonexisting
shortagesandtherapeuticalternatives
Otheragencies,suchDEA,theCenters
forDiseaseControlandPrevention
(CDC),andNIH
Dataonotherfactorsthatmaybe
influencingtheshortage
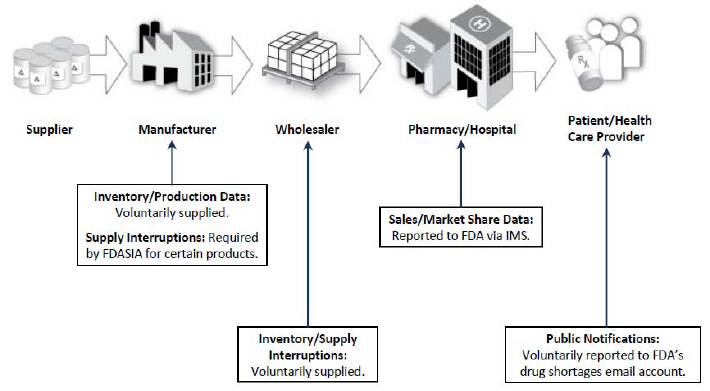
Figure 5. FDA’s Key Supply Chain Information Sources
However, although extremely useful, these data provide only part of the picture. For example,
key aspects of drug manufacture and distribution are not transparent, such as production
schedules, distribution of production volume across various contract manufacturing facilities, the
amount of inventory stored by a manufacturer, and wholesaler and pharmacy/hospital supply and
purchasing practices. Additionally, the role that other sources of products (e.g., gray market
distribution, compounding, unapproved drugs) play in contributing to shortages or in the
reactions to shortages is not clearly understood.
30

31
APPENDIX D: FDA Offices Engaged in Drug Shortage Efforts
1. FDA’s Drug Shortage Task Force
This overarching group, which includes individuals from across CBER, CDER, and the Office of
Regulatory Affairs (ORA), was formed in 2012, per the directive in FDASIA. The Task Force
coordinates the development of consistent policy with regard to FDA’s handling of drug
shortages, facilitates intra- and inter-agency communications around shortages, and provides a
forum for individuals working on shortage issues within FDA to discuss policy issues related to
shortages, including the development of this Strategic Plan.
2. CBER’s Product Shortage Coordinator
The Product Shortage Coordinator within CBER’s Office of Compliance and Biologics Quality,
Immediate Office of the Director, is the primary contact for potential or actual shortages of
biologics regulated by CBER. The relative infrequency of biologics shortages has enabled
CBER to manage the workload with a shortage coordinator and staff support. When a potential
or actual shortage is reported, CBER responds by gathering information through communications
with manufacturers, other CBER and FDA offices, and external entities, such as ASHP, as well
as through market research data when available. The CBER Product Shortage Coordinator is
generally in close communication with other Department of Health and Human Services
agencies, including the Biomedical Advanced Research and Development Authority (BARDA)
27
and the CDC. CBER can draw from an array of policy options when addressing a shortage
situation, including: encouraging other manufacturers of the product (if available) to increase
their production, expediting inspections of manufacturing facilities, and expediting review of
biologics license applications, new drug applications, or abbreviated new drug applications.
To respond to the recent increase in shortages, CBER has revised its standard operating policy
and procedure titled, Management of Shortages of CBER-Regulated Products
28
; created a new,
robust database for internal tracking purposes; and significantly revised the CBER shortage
website.
27
Contracts for the procurement of medical countermeasures against chemical, biological, nuclear and radiological
threat agents (e.g. smallpox and anthrax vaccines) are administered by BARDA, part of the Office of the Assistant
Secretary for Preparedness and Response in the U.S. Department of Health and Human Services (HHS). See
http://www.hhs.gov/aspr
.
28
See CBER Standard Operation Policy and Procedure 8506.
32
3. CDER’s Drug Shortage Staff
The CDER Drug Shortage Staff (DSS) is the primary contact for potential or actual shortages of
products regulated by CDER. This group was established in 1999 and is located in the Office of
the Center Director. DSS coordinates FDA’s response to drug shortages and engages in the day-
to-day management of specific shortages. DSS also works to integrate up-to-date information
across products, manufacturers, review activities, and pending compliance actions to monitor
shortages, and vulnerability for shortage, on an ongoing basis. Although the staff serves as the
primary point of contact for shortage-related issues, it works with a large number of other offices
and divisions within CDER and FDA.
To respond to the recent increase in shortages, CDER has made two major changes to the DSS.
First, during the last two years, CDER has nearly tripled the number of staff directly responsible
for shortages, from 4 employees in 2011 to 11 employees in 2013. Second, CDER elevated DSS
from its original location within the Office of New Drugs to its current location in the Immediate
Office of the Center Director. Now, DSS reports to the Deputy Director for Regulatory
Programs. This location reflects the priority nature of responding to drug shortages for CDER
and the reality that shortages affect both new drugs and generic products and require cross-
functional coordination across CDER.
4. Office of Regulatory Affairs District Drug Shortage Coordinators
Communication between ORA and FDA’s medical product centers is critical in effectively
managing product shortages. District Drug Shortage Coordinators are the points of contact in
each of the district offices. They are responsible for notifying the relevant center of any issue
that has the potential to lead to a product shortage (e.g., information obtained during an
inspection or other field activities). The creation of Field Management Directive (FMD) #15,
Product Shortage Communication, in July 2012, established a mutual understanding of
communication roles, responsibilities, and expectations involving both potential and current
product shortage situations between ORA and the centers. Effective sharing of information and
coordination of efforts has optimized the outcomes and use of resources by both ORA and center
staff (e.g., when there is an emergency need to conduct an inspection to resolve a shortage issue).
5. CDER and CBER Offices of Compliance
Interaction and coordination with th
e Offices of Compliance in CDER and CBER (and ORA, as
needed) are integral to the prevention and management of potential shortages. Located within
CDER’s Office of Compliance is the Office of Drug Security, Integrity and Recalls (ODSIR),
and within ODSIR is the Recalls and Shortages Branch Shortage Coordination Team. This team
ensures that the relevant CDER Compliance and ORA personnel are involved in a shortage issue
and works diligently to coordinate efforts for the evaluating and determining whether to use
enforcement discretion, as warranted by medical necessity, clinical and chemistry review, and
shortage need.
33
6. Other Center Offices
The drug shortages staff in FDA centers also communicate extensively with other offices within
CBER and CDER to determine the medical necessity of a product in shortage or at risk of
shortage to assess whether there are pending applications in FDA that may address a shortage, to
obtain information from clinicians and scientists to inform the risk–benefit calculation, and to
work with communication specialists to alert the public about important drug shortage
developments.
34
APPENDIX E: Coordinating Drug Shortage Efforts in CDER
The following table highlights the extensive coordination and interaction that takes place in
CDER to assess and address a drug shortage.
CoordinatingOffices ExamplesofInformationShared ExamplesofDecisionsMade
Officeof
DSS
NewDrugs(OND)
DSS
→OND
29
Shortageevaluation
Marketingstatus,including
whetheralternatemanufacturers
areavailable
OND
→DSS
Evaluationofmedicalnecessity,
includingoff‐labeluseand
potentialalternativetherapies
Clinicalperspectiveofrisk–benefit
calculationtoinformwhether
enforcementdiscretionis
appropriate
Informationonreviewof
supplementsrelatedtothedrugin
shortage
Medicalnecessity
Revisionstoproposedlabeling
andDearHealthcareProvider
lettertomitigateshortage
Officeof
DSS
Compliance(OC)
OC
→DSS
Compliancestatusofaforeignor
domesticsite
Complianceperspectiveofrisk–
benefitcalculus,whichmay
includeaHealthHazardEvaluation
DSS
→OC
Medicalnecessitydetermination
fromOND
Shortageevaluation
Marketingstatus,including
WarningLetterorotheraction
Needforproductrecall
Revisionstoproposedlabeling
andDearHealthcareProvider
lettertomitigateshortage
Expeditinginspectionsto
addressshortage
Temporaryenforcement
whetheralternatemanufacturers
areavailable
Shortagememo,whichprovides
themedicalnecessity
determinationandwhatmaybe
discretiontomaintain
availabilityofmedically
necessarydrugs
29
Arrows specify the direction of information flow. For example, in the first row, DSS → OND indicates that DSS
shared with OND the shortage evaluation and marketing status.
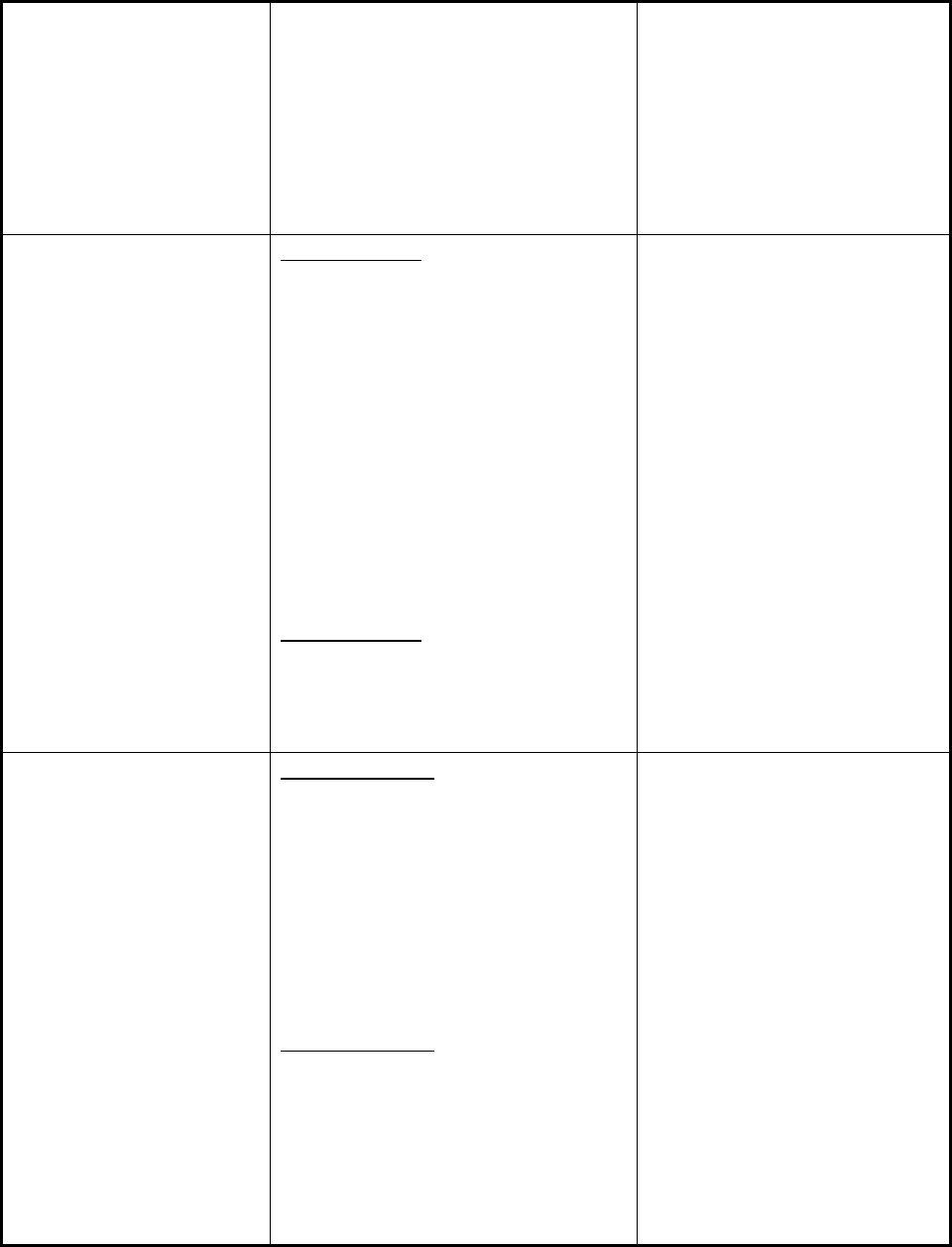
35
theimpactofanenforcement
action
DSS
OC
OfficeofRegulatoryAffairs
(ORA)
ORA/OC→DSS
Upcomingfirmdecisionstorecall
thatmayimpactanexistingor
potentialdrugshortage
Numberoflotsinvolvedinrecall,
estimatedimpacttomarketof
recall
Alternativeproductavailability
Manufacturerassessmentofrisk
involvedwithproductand
proposedriskmitigationstrategy
Alternativestoproductrecall,if
any
DSS→ORA/OC
Shortageevaluation
Marketingstatus,including
whetheralternatemanufacturers
areavailable
Needforproductrecall
DSS
OfficeofGenericDrugs
(OGD)
OfficeofPharmaceutical
Science(OPS)
DSS→OGD/OPS
Shortageevaluation
Marketingstatus,including
whetheralternatemanufacturers
areavailable
Medicalnecessitydetermination
fromOND
Pendingshortageduetoproduct
expiration(ifapplicable)
OGD/OPS→DSS
Assessmentofpending
applicationsin‐houseforproducts
inshortage
Chemistryandmicrobiology
perspectiveofrisk–benefit
calculus
Assessmentofpotentialextension
Expeditingreview
Enforcementdiscretionon
supplementfilingcategory
Enforcementdiscretiononpost‐
marketcommitments
Extensionofshelflife/expiration
dating
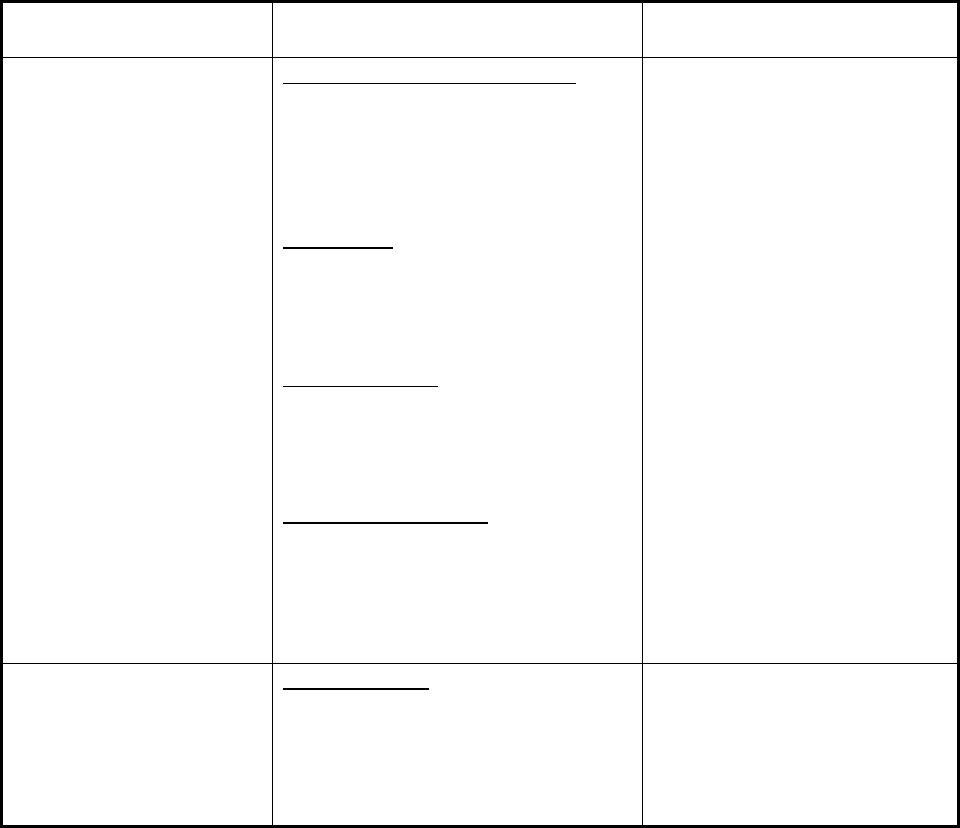
36
ofshelflifebasedondatafrom
manufacturer
DSS
OND
OGD
OPS
OC
ORA
DSS→OND/OGD/OPS/OC/ORA
Shortageevaluation
Marketingstatus,including
whetheralternatemanufacturers
areavailable
OND→DSS
Medicalnecessitydetermination
Clinicalperspectiveofrisk–benefit
calculus
OGD/OPS→DSS
Chemistryandmicrobiology
perspectiveofriskinvolvedand
howtoaddress
OCand/orORA→DSS
Compliancestatusofaforeignor
domesticsite
ComplianceandORAperspective
ofriskinvolvedandhowto
address
Actionsonimport‐relatedissues
DSS
OfficeofCommunications
(OCOMM)
DSS→OCOMM
Informationrelevanttoshortagethat
mayneedtobebroadly
communicatedtopublic,suchas
recallsorresolutionoftheshortage
Pressrelease,FrequentlyAsked
Questions(FAQ)document,or
otherformofcommunicationto
thepublictoconvey necessary
information(inadditionto
postingonDSSwebsite)

37
APPENDIX F: Communicating With External Groups
1. Patients, Prescribers, and Pharmacists
Keeping the public informed about shortages is essential to help support the fullest access
possible to drugs in shortage and to ensure that patients, prescribers, and others are aware of
progress in resolving each drug shortage. CBER and CDER maintain shortage websites as key
resources for external stakeholders.
30
Since they were first launched, these FDA websites have
served as a vehicle for making publicly available the latest information provided by
manufacturers on each shortage, including information about the reasons for the shortage, places
where additional information can be found (e.g., company contacts for allocations of products in
shortage), and any estimates on when a given shortage might be resolved. FDA updates this
information daily. The websites also include links to resolved shortages (once FDA determines
that the shortage is over).
FDA also employs a wide range of other tools to communicate information about drug shortages
to specific populations. These include collaborating with manufacturers on information sent
directly to health care professionals in the form of Dear Healthcare Provider letters, press
releases, and communications with groups representing specific patient populations affected by a
particular drug shortage. For example, FDA worked closely with the American Society of
Parenteral and Enteral Nutrition on recent shortages of components of total parenteral nutrition,
including trace elements, which provide essential nutrients for patients (including neonates) who
are unable to eat or drink by mouth.
2. Clinical Researchers and Sponsors of Clinical Trials
One particularly challenging issue that has confronted industry and the academ
ic community is
consistent access to products for use in clinical trials. FDA recognizes that shortages may affect
clinical research and the conduct of clinical trials. Generally, shortages affect trials designed to
compare a new product with an existing product. For example, according to comments in
response to the Federal Register notice, many oncology trials and studies of new antibiotics use
such a design—i.e., comparing a candidate drug to one already approved, rather than using a
placebo controlled clinical trial. If the approved drug goes into shortage, the trial may need to be
delayed or stopped, halting research on important new products. FDA is continuing to explore
how shortages affect clinical trials and how best to address this issue. While FDA works to
identify long-term solutions, we encourage trial sponsors to consider the possibility of shortages
during initial trial design and to develop and implement contingency plans for handling a
shortage, should one arise.
30
For drugs and therapeutic biologics regulated in CDER, see
http://www.fda.gov/Drugs/DrugSafety/DrugSho
rtages/default.htm. For products regulated by CBER, see
http://www.fda.gov/BiologicsBloodVacci
nes/SafetyAvailability/Shortages/default.htm.

38
3. Manufacturers
Ultimately, shortages cannot be resolved until one or more manufacturers commit to fill in for
lost production. FDA is in a unique position to coordinate such a response.
FDA’s drug shortages staff interacts with manufacturers on an ongoing basis to understand
specific issues related to possible or ongoing shortages. For example, when the shortage staff
hears about a potential disruption in production, FDA contacts other manufacturers to see if they
have experienced an increase in demand; determines the status of their on-hand supply; and
when a shortage looms, determines if they are willing to initiate or increase production to cover
expected gaps in supply. FDA then works with those manufacturers in their efforts to increase
production.
During a shortage, FDA continues to work with manufacturers to aide in recovery efforts. For
instance, when a firm shuts down to address manufacturing and/or quality problems, FDA works
with the firm as it remediates to maintain the availability of critical medicines.
To maintain some level of supply during remediation, FDA may recommend creating a priority
list that emphasizes continued manufacture of shortage products; encourage allocation programs
to hold back supply of shortage products; or recommend third-party quality review for release of
manufactured batches. FDA may also work with the manufacturer to assist it in providing drop
shipments
31
of available supply to hospitals to speed the delivery of the drug to patients. If new
raw materials are needed, FDA can facilitate the qualification of new or additional sources of
raw materials.
4. Other Governmental Agencies
FDA recognizes the important roles that other U.S. governmental agencies play in shortage
mitigation efforts. For example, FDA worked closely with NIH to address a shortage of
doxorubicin, a drug needed for clinical cancer trials. In another recent case, FDA alerted CDC
as soon as we were informed of the potential shortage of rifampin injection, a drug used in the
treatment of seriously ill patients with tuberculosis who require intravenous treatment. In
addition, under the President’s Executive Order 13588 (October 2011), FDA has been sending
information to the Department of Justice that could be indicative of stockpiling or exorbitant
pricing.
One particularly important interaction related to shortages is between FDA and DEA. Among
other things with input from FDA, DEA is responsible for setting aggregate limits on the amount
of each basic class of controlled substance that may be manufactured. DEA is also responsible
for approving requests by manufacturers for a specific amount of the aggregate limit (a quota).
This tight control over the manufacturing of controlled substances requires FDA and DEA to
31
A drop shipment is a shipment directly from a manufacturer to the end purchaser or user (e.g., hospital or
pharmacist) that bypasses the intermediate distributor from whom the end purchaser originally bought the product.

39
coordinate when there is a shortage of a controlled substance. Recognizing this need, FDASIA
includes new provisions requiring improved coordination and communication between FDA and
DEA when there is a shortage of a controlled substance.
5. International Authorities
FDA interac
tions with international authorities have been critical to successfully mitigating drug
shortages. One important area of interaction has been around the importation of drugs from
other countries to address shortages in the United States.
32
Numerous comments to the Federal
Register notice asked FDA to expand the use of temporary importation of unapproved drugs to
address shortages. FDA has used this tool to address shortages a number of times in recent
years. For example, in 2012, FDA worked to facilitate the importation of propofol injection,
which was in critical shortage when two firms discontinued the drug because of quality
problems, including contamination with particulates and endotoxin.
Working through FDA’s Office of International Programs, FDA has established ties with many
regulatory agencies in other countries and maintains ongoing dialogues with those agencies
through existing confidentiality agreements. FDA has worked with these agencies on a variety
of issues related to importation, for example, requesting recent documents from foreign
regulatory authorities who have audited manufacturing sites and tested products. An example of
the value of this international cooperation is the recent importation of sodium bicarbonate
injection, a critical drug for use in the intensive care unit that was in shortage. With cooperation
of the Australian authorities, this critical drug was temporarily imported by a firm willing and
able to supply their Australian product to the United States to address the U.S. shortage.
Not all drugs that are manufactured by foreign firms are appropriate for importation, and setting
up a process to explore the possibility of importation required the cooperation of many
stakeholders. When a foreign-registered drug manufacturer has been willing to supply a drug in
shortage, FDA has worked closely with the company and assembled an internal cross-functional
team to evaluate the drug and the facility in which it is made. Where the requested information
has been found acceptable, considering risks associated with the drug and the risk to patients if it
is not available, FDA has exercised enforcement discretion for temporary importation to provide
treatment options for patients in critical need during the shortage.
33
In addition to contacting foreign authorities for assistance with importation to address a specific
shortage, FDA has begun discussing shortages more broadly with regulatory agencies in other
regions. For example, to harmonize the international approach to shortage communications,
32
FDA is aware of the recent decision in Cook v. FDA (D.C. Circuit, Case No. 12-5176). We are currently
reviewing the decision in the context of our drug shortages program.
33
See also FDA’s Regulatory Procedures Manual Chapter 9, Section 2 available at
http://www.fda.gov/downloads/ICECI/ComplianceMan
uals/RegulatoryProceduresManual/UCM074300.pdf; and
CPG Sec. 440.100 Marketed New Drugs Without Approved NDAs and ANDAs available at
http://www.fda.gov/ICECI/ComplianceManuals/Comp
liancePolicyGuidanceManual/ucm074382.htm
40
FDA meets regularly with the European Medicines Agency (EMA) to discuss various drug
shortage issues. FDA has also spoken with the Canadian health authority about its approach to
shortages, particularly those shortages that may cross U.S./Canadian borders and affect both
countries.
