
Epidemiology and Public Health Preparedness Division
Epidemiology and Disease Surveillance Unit
5202 E. Ben White Blvd. Ste 600
Austin, TX 78741
Reporting Communicable
Diseases in Travis County
2023

Contents
Letter to Reporting Agencies .......................................................................................................... 1
Letter about Health Insurance Portability and Accountability Act (HIPAA) ................................... 2
Texas Administrative Code Section 97.2 (Communicable Disease Control) .................................. 3
Reporting Phone Numbers and Other Useful Numbers ................................................................. 4
List of Texas Notifiable Conditions .............................................................................................. 5-6
List of Texas Notifiable Conditions by Time Frame ........................................................................ 7
Important Notice about Bacterial Isolates or Specimens ............................................................... 8
Important Notice about Controlled Substance Overdoses………………………………………………………….9
Reporting Forms............................................................................................................................ 10
Infectious Disease Reporting Form ........................................................................................... 10
Varicella (Chickenpox) Reporting Form ..................................................................................... 11
STD Reporting Form ............................................................................................................. 12-13
STD-28 ....................................................................................................................................... 14
Perinatal Hepatitis B Reporting Form ....................................................................................... 15

Letter to Reporting Agencies
March 17, 2023
Dear Reporting Agency,
Thank you for reporting notifiable health conditions to Austin Public Health. Timely reporting allows
Austin Public Health to respond to potential disease outbreaks, mitigate transmission of disease, and
monitor health trends in Travis County.
The purpose of this Reporting Packet is to provide you with the 2022 list of notifiable conditions,
reporting forms, and other helpful information. The packet includes:
1. Letter about Health Insurance Portability and Accountability Act (HIPAA)
2. Texas Administrative Code Section 97.2 (Communicable Disease Control)
3. Reporting Phone Numbers
4. List of Notifiable Conditions in Texas
5. Important Notice about Bacterial Isolates or Specimens
6. Important Notice about Controlled Substance Overdoses
7. Reporting Forms
a. General Infectious Disease
b. Varicella (Chickenpox)
c. STD Reporting Form
d. Perinatal Hepatitis B OB/GYN
e. Perinatal Hepatitis B Labor/Delivery & Postpartum
Reports of disease and reporting forms may be faxed to 512-972-5772.
To report diseases over phone, especially those requiring immediate attention, please call 512-972-5555.
This number is answered during business hours, Monday through Friday, 8 a.m. to 5 p.m, and serves as
our 24/7 emergency on-call line after hours.
Thank you again for your assistance.
Sincerely,
Desmar Walkes, MD Janet Pichette, MS, CEM
Medical Director / Health Authority Chief Epidemiologist
1

March 17, 2023
To Whom It May Concern:
We understand that there may be some confusion regarding the Health Insurance Portability
and Accountability Act (HIPAA) and release of protected health information to public health
authorities. This letter will clarify the relationship between HIPAA and public health functions.
The Epidemiology and Disease Surveillance Unit is a program within Austin Public Health, the
local health department for the City of Austin and Travis County. Local health departments are
authorized by state law to conduct disease surveillance activities (Texas Health and Safety
Code, Title 2. Health. Chapter 81. Communicable Diseases). Disease surveillance activities or
monitoring the health status to identify and solve community health problems is an essential
function of public health. HIPAA permits use and disclosure of protected health information,
without an individual’s authorization or permission, for 12 national priority purposes. Public
Health Activities is one of the priority purposes.
As a HIPAA covered entity, you may disclose protected health information for public health
activities and purposes to a public health authority that is authorized by law to collect and
receive such information for preventing and controlling disease, injury, or disability. This
includes but is not limited to, the reporting of diseases, injury, vital events such as birth or
death, and the conduct of public health surveillance, public health investigations, and public
health interventions. See 45 Code of Federal Regulations (CFR) 164.512(b)(1).
If you have any questions, please contact me at the Epidemiology and Disease Surveillance Unit
at (512) 972-5555. Thank you for efforts in preventing diseases, promoting health, and
protecting the people of Austin and Travis County.
Kindest Regards,
Janet Pichette, MS, CEM
Chief Epidemiologist
Epidemiology and Disease Surveillance Unit
Austin Public Health
2

Texas Administrative Code
TITLE 25
HEALTH SERVICES
PART 1
DEPARTMENT OF STATE HEALTH SERVICES
CHAPTER 97
COMMUNICABLE DISEASES
SUBCHAPTER A
CONTROL OF COMMUNICABLE DISEASES
RULE §97.2
Who Shall Report
(a) A physician, dentist, veterinarian, chiropractor, advanced practice nurse, physician assistant, or person
permitted by law to attend a pregnant woman during gestation or at the delivery of an infant shall report, as
required by these sections, each patient (person or animal) he or she shall examine and who has or is
suspected of having any notifiable condition, and shall report any outbreak, exotic disease, or unusual group
expression of illness of any kind whether or not the disease is known to be comm
unicable or reportable. An
employee from the clinic or office staff may be designated to serve as the reporting officer. A physician,
dentist, veterinarian, advanced practice nurse, physician assistant, or chiropractor who can assure that a
designated or appointed person from the clinic or office is regularly reporting every occurrence of these
diseases or health conditions in their clinic or office does not have to submit a duplicate report.
(b) The chief administrative officer of a hospital shall appoint one reporting officer who shall be
responsible for reporting each patient who is medically attended at the facility and who has or is suspected
of having any notifiable condition. Hospital laboratories may report through the reporting officer or
independently in accordance with the hospital's policies and procedures.
(c) Except as provided in subsection (b) of this section, any person who is in charge of a clinical laboratory,
blood bank, mobile unit, or other facility in which a laboratory examination of a
ny specimen derived from a
human body yields microscopic, bacteriologic, virologic, parasitologic, serologic, or other evidence of a
notifiable condition, shall report as required by this section.
(d) School authorities, including a superintendent, principal, teacher, school health official, or counselor of
a public or private school and the administrator or health official of a public or private institution of higher
learning should report as required by these sections those students attending school who are suspected of
having a notifiable condition. School administrators who are not medical directors meeting the criteria
described in §97.132 of this title (relating to Who Shall Report Sexually Transmitted Diseases) are exempt
from reporting sexually transmitted diseases.
(e) Any person having knowledge that a person(s) or animal(s) is suspected of having a notifiable condition
should notify the local health authority or the department and provide all information known to them
concerning the illness and physical condition of such person(s) or animal(s).
(f) Sexually transmitted diseases including HIV and AIDS shall be reported in accordance with Subchapter
F of this chapter (relating to Sexually Transmitted Diseases Including Acquired Immunodeficiency
Syndrome (AIDS) and Human Immunodeficiency Virus (HIV)).
(g) Failure to report a notifiable condition is a Class B misdemeanor under the Texas Health and Safety
Code, §81.049.
(h) The Health Insurance Portability and Accountability Act (HIPAA) allows reporting without
authorization for public health purposes and where required by law. Title 45 Code of Federal Regulations
§164.512(a) and (b).
Source Note: The provisions of this §97.2 adopted to be effective March 16, 1994, 19 TexReg 1453;
amended to be effective March 5, 1998, 23 TexReg 1954; amended to be effective January 1, 1999, 23
TexReg 12663; amended to be effective March 26, 2000, 25 TexReg 2343; amended to be effective
December 20, 2000, 25 TexReg 12426; amended to be effective August 5, 2001, 26 TexReg 5658;
amended to be effective June 5, 2007, 32 TexReg 2997; amended to be effective December 20, 2012, 37
TexReg 9777; amended to be effective April 3, 2016, 41 TexReg 2317
3

REPORTING PHONE NUMBERS
Reportable diseases/conditions occurring in Travis County shall be reported to Austin Public Health. Refer to the Texas
Department of State Health Services (TDSHS) listing for names of reportable diseases/conditions and other information.
Disease/Condition
Phone
Fax
General Communicable Diseases
(512) 972-5555
(512) 972-5772
HIV/AIDS
(512) 972-5144 or
(512) 972-5142 or
(512) 972-5583
(512) 972-5772
Perinatal Hepatitis B Program
(512) 972-6218
(512) 972-6287
STD Reporting Syphilis
Chlamydia & Gonorrhea
(512) 972-5310
(512) 972-5313 or
(512) 972-5314 or
(512) 972-5829 or
(512) 972-5809
(512) 972-5772
Tuberculosis Reporting
(512) 972-5448
(512) 972-5451
OTHER USEFUL PHONE NUMBERS
Department
Phone
Animal Control
311
Environmental Health
311
Immunizations
(512) 972-5520
Refugee Screening Clinic
(512) 972-6210
STI Clinic
(512) 972-5430
TB Clinic
(512) 972-5460
Vaccines for Children Program
(512) 972-5414
4

E59-11364 (Rev. 1/08/23) Expires 12/31/23 -- Go to http://www.dshs.texas.gov/idcu/investigation/conditions/ or call your local or regional health department for
updates.
A – L
When to Report
L – Y
When to Report
*Acquired immune deficiency syndrome (AIDS)
1
Within 1 week
Legionellosis
2
Within 1 week
Amebic meningitis and encephalitis
2
Within 1 week
Leishmaniasis
2
Within 1 week
Anaplasmosis
2
Within 1 week
Listeriosis
2, 3
Within 1 week
Anthrax
2, 3, 25
Call Immediately
Lyme disease
2
Within 1 week
Arboviral infections
2, 4, 5
Within 1 week
Malaria
2
Within 1 week
*Asbestosis
6
Within 1 week
Measles (rubeola)
2
Call Immediately
Ascariasis
2
Within 1 week
Meningococcal infection, invasive (Neisseria meningitidis)
2, 3
Call Immediately
Babesiosis
2,5
Within 1 week
Mumps
2
Within 1 work day
Botulism (adult and infant)
2, 3, 7, 25
Call Immediately
7
Paragonimiasis
2
Within 1 week
Brucellosis
2,
3, 25
Within 1 work day
Pertussis
2
Within 1 work day
Campylobacteriosis
2
Within 1 week
*Pesticide poisoning, acute occupational
8
Within 1 week
*Cancer
9
See rules
9
Plague (Yersinia pestis)
2, 3, 25
Call Immediately
Candida auris
2, 3, 10
Within 1 work day
Poliomyelitis, acute paralytic
2
Call Immediately
Carbapenem-resistant Enterobacteriaceae (CRE)
2, 11
Within 1 work day
Poliovirus infection, non-paralytic
2
Within 1 work day
Chagas disease
2, 5
Within 1 week
Prion disease such as Creutzfeldt-Jakob disease (CJD)
2, 12
Within 1 week
*
Chancroid
1
Within 1 week
Q fever
2
Within 1 work day
*Chickenpox (varicella)
13
Within 1 week
Rabies, human
2
Call Immediately
*
Chlamydia trachomatis infection
1
Within 1 week
Rubella (including congenital)
2
Within 1 work day
*
Contaminated sharps injury
14
Within 1 month Salmonellosis, including typhoid fever
2, 3
Within 1 week
*Controlled substance overdose
15
Report Immediately
Shiga toxin-producing Escherichia coli
2, 3
Within 1 week
Coronavirus, novel
2, 16
Call Immediately
Shigellosis
2
Within 1 week
Coronavirus Disease 2019 (COVID-19)
2
Within 1 week
*Silicosis
17
Within 1 week
Cryptosporidiosis
2
Within 1 week
Smallpox
2, 25
Call Immediately
Cyclosporiasis
2
Within 1 week
*Spinal cord injury
18
Within 10 work days
Cysticercosis
2
Within 1 week
Spotted fever rickettsiosis
2
Within 1 week
Diphtheria
2, 3
Call Immediately
Streptococcal disease (S. pneumo.
2, 3
), invasive
Within 1 week
*Drowning/near drowning
18
Within 10 work days
*Syphilis – primary and secondary stages
1, 19
Within 1 work day
Echinococcosis
2
Within 1 week
*Syphilis – all other stages including congenital syphilis
1,19
Within 1 week
Ehrlichiosis
2
Within 1 week Taenia solium and undifferentiated Taenia infection
2
Within 1 week
Fascioliasis
2
Within 1 week
Tetanus
2
Within 1 week
*Gonorrhea
1
Within 1 week
Tick-borne relapsing fever (TBRF)
2
Within 1 week
Haemophilus influenzae, invasive
2, 3
Within 1 week
*Traumatic brain injury
18
Within 10 work days
Hansen’s disease (leprosy)
20
Within 1 week
Trichinosis
2
Within 1 week
Hantavirus infection
2
Within 1 week
Trichuriasis
2
Within 1 week
Hemolytic uremic syndrome (HUS)
2
Within 1 week
Tuberculosis (Mycobacterium tuberculosis complex)
3, 21
Within 1 work day
Hepatitis A
2
Within 1 work day
Tuberculosis infection
22
Within 1 week
Hepatitis B, C, and E (acute)
2
Within 1 week
Tularemia
2, 3, 25
Call Immediately
Hepatitis B infection identified prenatally or at delivery (mother)
2
Within 1 week
Typhus
2
Within 1 week
Hepatitis B, perinatal (HBsAg+ < 24 months old) (child)
2
Within 1 work day
Vancomycin-intermediate Staph aureus (VISA)
2, 3
Call Immediately
Hookworm (ancylostomiasis)
2
Within 1 week
Vancomycin-resistant Staph aureus (VRSA)
2, 3
Call Immediately
*Human immunodeficiency virus (HIV), acute infection
1, 23
Within 1 work day
Vibrio infection, including cholera
2,
3
Within 1 work day
*Human immunodeficiency virus (HIV), non-acute infection
1, 23
Within 1 week
Viral hemorrhagic fever (including Ebola)
2, 25
Call Immediately
Influenza-associated pediatric mortality
2
Within 1 work day
Yellow fever
2
Call Immediately
Influenza, novel
2
Call Immediately
Yersiniosis
2
Within 1 week
*Lead, child blood, any level & adult blood, any level
24
Call/Fax Immediately
In addition to specified reportable conditions, any outbreak, exotic disease, or unusual group expression of disease that may be of
public health concern should be reported by the most expeditious means available. This includes any case of a select agent
25
See select agent list at https://www.selectagents.gov/selectagentsandtoxinslist.html
*See condition-specific footnotes for reporting contact information
Texas Notifiable
Conditions - 2023
Report all Confirmed and Suspected cases
Unless noted by*, report to your local or regional health department using number above or
find contact information at http://www.dshs.texas.gov/idcu/investigation/conditions/contacts/
24/7 Number for Immediately Reportable – 1-800-705-
5

E59-11364 (Rev. 1/08/23) Expires 12/31/23 -- Go to http://www.dshs.texas.gov/idcu/investigation/conditions/ or call your local or regional health department for
updates.
Texas Notifiable Conditions Footnotes - 2023
1
Please refer to specific rules and regulations for HIV/STD reporting and who to report to at:
http://www.dshs.texas.gov/hivstd/healthcare/reporting.shtm.
2
Reporting forms are available at http://www.dshs.texas.gov/idcu/investigation/forms/ and investigation forms at
http://www.dshs.texas.gov/idcu/investigation/. Call as indicated for immediately reportable conditions.
3
Lab samples of the following must be sent to the Department of State Health Services, Laboratory Services Section, 1100 West 49th Street, Austin, Texas
78756-3199 or other public health laboratory as designated by the Department of State Health Services: Bacillus anthracis isolates (also requested-
Bacillus cereus isolates that may contain anthrax toxin genes from patients with severe disease or death), Clostridium botulinum isolates, Brucella
species isolates, Candida auris isolates, Corynebacterium diphtheriae isolates, Haemophilus influenzae isolates from normally sterile sites in children
under five years old, Listeria monocytogenes isolates, Neisseria meningitidis isolates from normally sterile sites or purpuric lesions, Yersinia pestis
isolates, Salmonella species isolates (also requested - specimens positive for Salmonella by culture-independent diagnostic testing (CIDT) methods),
Shiga toxin-producing Escherichia coli (all E.coli O157:H7 isolates and any E.coli isolates or specimens in which Shiga toxin activity has been
demonstrated), isolates of all members of the Mycobacterium tuberculosis complex, Staphylococcus aureus with a vancomycin MIC greater than 2
µg/mL (VISA and VRSA), Streptococcus pneumoniae isolates from normally sterile sites in children under five years old, Francisella tularensis isolates,
and Vibrio species isolates (also requested - specimens positive for Vibrio by culture-independent diagnostic testing (CIDT) methods). Pure cultures (or
specimens) should be submitted as they become available accompanied by a current department Specimen Submission Form. See the
Texas
Administrative Code (TAC) Chapter 97: §97.3(a)(4), §97.4(a)(6), and §97.5(a)(2)(C). Call 512-776-7598 for specimen submission information.
4
Arboviral infections including, but not limited to, those caused by California serogroup viruses, chikungunya virus, dengue virus, Eastern equine
encephalitis (EEE) virus, St. Louis encephalitis (SLE) virus, Western equine encephalitis (WEE) virus, West Nile (WN) virus, and Zika virus.
5
All blood collection centers should report all donors with reactive tests for West Nile virus, Zika virus, Babesia species, and Trypanosoma cruzi (Chagas
disease) to the DSHS Zoonosis Control Branch. If your center uses a screening assay under an IND protocol, please include results of follow-up testing as
well. To report, send a secure email to W[email protected]exas.gov
or fax the report to 512-776-7454. Providing the following: Collection Agency; Unique BUI
#; Test Name, Collection Date; Last Name, First Name, Donor Phone Number, Donor Address, Date of Birth, Age, Sex, Race, and Hispanic Ethnicity (Y/N).
If your location has a city or county health department, DSHS recommends that you also share this same information with them.
6
For asbestos reporting information see http://www.dshs.texas.gov/epitox/Asbestosis-and-Silicosis-Surveillance/.
7
Report suspected botulism immediately by phone to 888-963-7111.
8
For pesticide reporting information see https://www.dshs.texas.gov/sites/default/files/epitox/pestrptfrm.pdf
9
For more information on cancer reporting rules and requirements go to http://www.dshs.texas.gov/tcr/reporting.shtm.
10
See additional Candida auris reporting information at https://www.dshs.texas.gov/IDCU/health/antibiotic_resistance/Cauris-Home.aspx.
11
See additional CRE reporting information at http://www.dshs.texas.gov/IDCU/health/antibiotic_resistance/Reporting-CRE.doc.
12
For purposes of surveillance and notification, Prion disease such as Creutzfeldt-Jakob disease (CJD) also includes Kuru, Gerstmann-Sträussler-Scheinker (GSS)
disease, fatal familial insomnia (FFI), sporadic fatal insomnia (sFI), Variably Protease-Sensitive Prionopathy (VPSPr), familial CJD (fCJD) or genetic CJD (gCJD),
variant CJD (vCJD), iatrogenic CJD (iCJD) and any novel prion disease affecting humans.
13
Call your local health department for a copy of the Varicella Reporting Form with their fax number. The Varicella (Chickenpox) Reporting Form should be
used instead of an Epi-1 or Epi-2 morbidity report.
14
Applicable for governmental entities. Not applicable to private facilities. (TAC §96.201) Initial reporting forms for Contaminated Sharps at
http://www.dshs.texas.gov/idcu/health/infection_control/bloodborne_pathogens/reporting/.
15
To report a Controlled Substance Overdose, go to https://odreport.dshs.texas.gov/.
16
Novel coronavirus causing severe acute respiratory disease includes Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome
(SARS). Call immediately for SARS, MERS, or any other novel coronavirus cases.
17
For silicosis reporting information see http://www.dshs.texas.gov/epitox/Asbestosis-and-Silicosis-Surveillance/.
18
Please refer to specific rules and regulations for injury reporting and who to report to at http://www.dshs.texas.gov/injury/rules.shtm.
19
Laboratories should report syphilis test results within 3 work days of the testing outcome.
20
Reporting forms are available at https://www.dshs.texas.gov/idcu/disease/hansens/forms.shtm.
21
Reportable tuberculosis disease includes the following: suspected tuberculosis disease pending final laboratory results; positive nucleic acid
amplification tests; clinically or laboratory-confirmed tuberculosis disease; and all Mycobacterium tuberculosis (M. tb) complex including
M. tuberculosis, M. bovis, M. africanum, M. canettii, M. microti, M. caprae, and M. pinnipedii. See rules and reporting information at
http://www.dshs.texas.gov/idcu/disease/tb/reporting/
.
22
TB infection is determined by a positive result from an FDA-approved Interferon-Gamma Release Assay (IGRA) test such as T-Spot TB or
QuantiFERON - TB GOLD In-Tube Test or a tuberculin skin test, and a normal chest radiograph with no presenting symptoms of TB disease.
See rules and reporting information at http://www.dshs.texas.gov/idcu/disease/tb/reporting/
. Please report skin test results in millimeters.
23
Any person suspected of having HIV should be reported, including HIV exposed infants.
24
For lead reporting information see http://www.dshs.texas.gov/lead/Reporting-Laws-Administrative-Code.aspx.
25
Please secure select agent isolates and specimens in accordance with the guidance in the Select Agent Regulation, and immediately initiate a
consultation with public health regarding need for further testing or sequencing. Notify any transfer facilities of any test results of high
consequence/interest.
6

Texas Notifiable Conditions 2023 (by reporting timeframe)
Both suspected and confirmed cases are IMMEDIATELY Reportable!
CALL 512-972-5555 (365/24/7)
• Anthrax
• Botulism (adult & infant)
• Controlled substance overdose (see rules)
• Coronavirus, novel
• Diphtheria
• Influenza, Novel
• Lead, childhood/adult, any level (see rules)
• Measles (rubeola)
• Meningococcal infection, invasive (Neisseria
meningitidis)
• Plague (Yersinia pestis)
• Poliomyelitis, acute paralytic
• Rabies, human
• Smallpox
• Staph. Aureus, vancomycin-resistant (VISA &
VRSA)
• Tularemia
• Viral hemorrhagic fever (including Ebola)
• Yellow fever
Report within ONE WORKING DAY
• Brucellosis
• Candida auris
• Carbapenem resistant Enterobacteriaceae (CRE)
• Hepatitis A (acute)
• Hepatitis B, perinatal (HBsAg+ <24 months) (child)
• Human immunodeficiency virus (HIV), acute infection
• Influenza-associated pediatric mortality
• Mumps
•
• Pertussis
• Poliovirus infection, non-paralytic
• Q fever
• Rubella (including congenital)
• Syphilis, primary & secondary stages
• Tuberculosis disease (M. tuberculosis complex)
• Vibrio infection, including cholera
Report within ONE WEEK
• Acquired immune deficiency
syndrome (AIDS)
• Amebic Meningitis & Encephalitis
• Anaplasmosis
• Arboviral Infection
• Asbestosis
• Ascariasis
• Babesiosis
• Campylobacteriosis
• Cancer
• Chagas Disease
• Chancroid
• Chickenpox (Varicella)
• Chlamydia trachomatis infection
• Coronovirus Disease 2019 (COVID-
19)
• Cryptosporidiosis
• Cyclosporiasis
• Cysticercosis
• Echinococcosis
• Ehrlichiosis
• Fascioliasis
• Gonorrhea
• Haemophilus influenza, invasive
• Hansen’s disease (Leprosy)
• Hantavirus infection
• Hemolytic Uremic Syndrome
(HUS)
• Hepatitis B, C, and E (acute)
• Hepatitis B identified prenatally
or at delivery (mother)
• Hookworm (ancylostomiasis)
• Human immunodeficiency virus
(HIV) non-acute infection
• Legionellosis
• Leishmaniasis
• Listeriosis
• Lyme disease
• Malaria
• Paragonimiasis
• Pesticide poisoning, acute
occupational
• Prion disease such as Creutzfeldt-
Jakob disease (CJD)
• Salmonellosis, including typhoid
fever
• Shiga toxin-producing
Escherichia coli
• Shigellosis
• Silicosis
• Spotted fever group rickettsioses
• Streptococcal disease (S.
pneumo), invasive
• Syphilis, all other stages
including congenital syphilis
• Taenia solium & undifferentiated
Taenia infection
• Tetanus
• Tick-borne relapsing fever
(TBRF)
• Trichinosis
• Trichuriasis
• Tuberculosis infection
• Typhus
• Yersiniosis
Report within 10 WORKING DAYS (See Rules)
• Drowning/Near Drowning
• Spinal Cord Injury
• Traumatic brain injury
Report within ONE MONTH
• Contaminated sharps injury
Report by the most expeditious means available
• In addition to specified reportable conditions, any outbreak, exotic disease, or unusual group expression of disease that
may be of public health concern should be reported by the most expeditious means available
7

Important Notice about Bacterial Isolates or Specimens
Pure cultures (or specimens) of the following must be submitted as they become available
accompanied by a current department Specimen Submission Form to:
Department of State Health Services
Laboratory Services Section
1100 West 49th Street, Austin, Texas 78756-3199
• Arboviral infections including, but not limited, those caused by California serogroup viruses,
chikungunya virus, dengue virus, Eastern equine encephalitis (EEE), St. Louis encephalitis (SLE) virus,
Western equine encephalitis (WEE) virus, West Nile (WN) virus, and Zika virus
• Bacillus anthracis isolates (also requested—Bacillus cereus isolates that may contain anthrax toxin
genes from patients with severe disease or death)
• Brucella species isolates
• Candida auris isolates
• Clostridium botulinum isolates
• Corynebacterium diphtheria isolates
• Francisella tularensis isolates
• Haemophilus influenzae isolates from normally sterile sites in children under five years old
• Listeria monocytogenes isolates
• Mycobacterium tuberculosis complex isolates
• Neisseria meningitidis isolates from normally sterile sites or purpuric lesions
• Salmonella species isolates (also requested – specimens positive for Salmonella by culture-
independent diagnostic testing (CIDT) methods)
• Shiga toxin-producing Escherichia coli (all E.coli O157:H7 isolates and any E.coli isolates or specimens in
which Shiga toxin activity has been demonstrated)
• Staphylococcus aureus with a vancomycin MIC greater than 2 μg/mL (VISA and VRSA)
• Streptococcus pneumoniae isolates from normally sterile sites in children under five years old
• Vibrio species isolates (also requested - specimens positive for Vibrio by culture-independent
diagnostic testing (CIDT) methods)
• Yersinia pestis isolates
All blood collection centers should report all donors with reactive tests for West Nile virus, Zika virus,
Babesia species, and Trypanosoma cruzi (Chagas disease)
See the Texas Administrative Code (TAC) Chapter 97: §97.3(a)(4), §97.4(a)(6), and §97.5(a)(2)(C). Call 512-
776-7598 for specimen submission information.
Lab Test/Specimen Submission Instructions
Laboratory Services Section Forms, Including G-2A and G-2B
Last updated March 17, 2023
8

Important Notice about Controlled Substance Overdoses
By Texas state law, Penalty Group 1 controlled substance overdoses shall be reported to the
Department of State Health Services immediately.
Texas Health and Safety Code §161.042 requires health care providers, or the administrator,
superintendent, or other person in charge of a hospital, sanatorium, or other institution in which
an overdose of a controlled substance listed in Penalty Group 1 is attended, treated, or in which
attention or treatment is requested, report all overdoses from substances listed in Penalty Group 1.
An overdose is defined as an accidental or intentional Penalty Group 1 drug effect, direct or
indirect, resulting in an unfavorable health event.
Penalty Group 1 drugs are classified by Texas Health and Safety Code §481.102 as opiates, opioids,
cocaine, opiate and opium derivatives, and other drugs. The complete list of reportable Penalty
Group 1 drugs as defined by the
Texas Health and Safety Code §481.102.
Failing to report is a misdemeanor punishable by confinement in jail for not more than six months,
or by a fine of not more than $100.
To report a controlled substance overdose, please use the reporting link below.
Please do not include any identifiable patient information, such as, patient name, address, or any
other information concerning the patient's identity.
Controlled Substance Overdose Reporting Form
Last updated March 14, 2022
9

Revised 1/2020
Infectious Disease Report
This form may be used to report suspected cases and cases of notifiable conditions in Texas, as listed on the current Texas
Notifiable Conditions List (http://www.dshs.state.tx.us/idcu/investigation/conditions
). In addition, any outbreak, exotic
disease, or unusual group expression of disease that may be of public health concern should be reported by the most
expeditious means available. You may be contacted to further investigate this Infectious Disease Report.
Report cases to Austin Public Health by faxing this form to (512) 972-5772 or calling (512) 972-5555
PATIENT INFORMATION
Last Name
First Name
Phone (Primary)
Phone (Secondary)
Date of Birth
Age
Sex
☐
Male
☐ Female
Ethnicity
☐
Hispanic
☐ Not Hispanic
Race
☐
White
☐ Asian
☐
Black
☐ Other
☐
Unknown
Address
City
State
Zip Code
County
CLINICAL INFORMATION
Disease or Condition
Illness Onset Date
Test Name/Type
Date of Collection
Specimen Source
☐
Blood
☐ Nose
☐
Throat
☐ Stool
☐
Urine
☐ Other ______
Result (attach copy)
Treatment Name
Treatment Start Date
Treatment Duration
REPORTING INFORMATION
Reporter Name
Date Reported
Reporter Phone
Healthcare Provider Name
Provider Address
Provider Phone
PATIENT INFORMATION
Last Name
First Name
Phone (Primary)
Phone (Secondary)
Date of Birth
Age
Sex
☐
Male
☐ Female
Ethnicity
☐
Hispanic
☐ Not Hispanic
Race
☐
White
☐ Asian
☐
Black
☐ Other
☐
Unknown
Address
City
State
Zip Code
County
CLINICAL INFORMATION
Disease or Condition
Illness Onset Date
Test Name/Type
Date of Collection
Specimen Source
☐
Blood
☐ Nose
☐
Throat
☐ Stool
☐
Urine
☐ Other ______
Result (attach copy)
Treatment Name
Treatment Start Date
Treatment Duration
REPORTING INFORMATION
Reporter Name
Date Reported
Reporter Phone
Healthcare Provider Name
Provider Address
Provider Phone
10

NBS ID:_______________ Case Investigation ID: CAS_________________TX01
VARICELLA (chickenpox) Reporting Form
Please use this form to report cases of Varicella to your local health office. Please complete as many fields as possible and fax completed
forms to APH at (512) 972-5772 at the end of every week. A report can still be submitted if all questions cannot be answered.
PATIENT INFORMATION:
Last Name: ________________ First: _____________________
DOB: ___/___/____ Age: ____ Sex: ____
Address: ___________________ City: _______________
Zip Code: __________ Phone: ________________
DEMOGRAPHICS:
Race: White Black or African-American Asian
Pacific Islander Native American/Alaskan Unknown
Hispanic: Yes No Unknown
Place of Birth: U.S.A. Other___________________
Is the patient pregnant? Yes No Unknown
REPORTING INFORMATION:
Name of Person Reporting: __________________________
Agency/Organization Name: _________________________
Phone: __________________________________________
Address: _________________________________________
City: ___________ Zip: _________ County: _____________
Date Reported: ___/___/_____
Health Department: ________________________________
Was the patient hospitalized for this disease?
Yes* No *If yes, please send medical records
Did patient visit a healthcare provider during this illness?
Yes Date: ___/___/_____ No
Physician: __________________________
Did the patient develop any complications? Yes No
Specify: ___________________________________
Is the patient immunocompromised? Yes No
Treated with any antiviral for this illness? Yes No
If yes, specify: ______________________ Start date: ____/____/_____
Hospital: _________________________________________
Admit date: ____/___/____ Discharge date: ___/___/____
Is this patient a contact to another known varicella or
shingles case? Yes No Unknown
Name of contact: __________________ Phone: _____________
Outbreak? Yes** No (*complete the Varicella Outbreak
Report Form, one per outbreak)
**NEDSS Outbreak Name: ____________________________
CLINICAL DATA:
Illness Onset Date ____/____/_____ Illness duration: ____ days
Rash Onset Date ____/____/_____
Rash Location: Generalized Focal Unknown
If generalized, first noted: (check all that apply)
Face/head Legs Trunk Arms Inside Mouth
Other (specify) _______________________
If focal, specify dermatome: _______________
Number of lesions:
<50 (specify)___________ 50-249 250- 499 500+
If <50, how many of each:
Macules #_____ Papules #____ Vesicles #____
Did the rash crust? Yes, rash lasted _____ days before crusting
No, rash lasted ____days Unknown
Fever?Yes, temperature ______F
Date of Fever onset: ____/____/______ No. of days ______
No
Unknown
Character of Lesions:
Mostly Macular/Papular?
Mostly Vesicular?
Hemorrhagic?
Itchy?
Scabs?
Crops/Waves?
Yes / No / Unknown
Yes / No / Unknown
Yes / No / Unknown
Yes / No / Unknown
Yes / No / Unknown
Yes / No / Unknown
LABORATORY DATA: Testing done? Yes No Unknown
Ordering Facility: ___________________
DFA Result: ________ Date of test: ___/___/____
PCR Result: ________ Date of test: ___/___/____
Culture Result: ________ Date of test: ___/___/____
IgM Result: ________ Date of test: ___/___/____
IgG Acute Result: ________ Date of test: ___/___/____
Conv Result: ________ Date of test: ___/___/____
Previous History of Disease? Yes No
Date of Disease ____/____/_____ Age at diagnosis: _____ years
Diagnosed by whom:
Parent/friend Physician/Health Care Provider Other
Varicella Vaccination? Yes No
Number of Doses Received? 1 2 3
Date(s) of Varicella Vaccine:
1
st
Dose: ____/____/____ Type: MMRV Varicella
2
nd
Dose: ____/____/____ Type: MMRV Varicella
Did the patient attend: School Day Care Work College Other___________________
Name of institution: __________________________ City: ________________
Transmission Setting (Setting of Exposure): Athletics College Community Correctional Facility Day Care Doctor’s office Home
Hospital ER Hospital Outpatient Clinic Hospital Ward International Travel Military Place of Worship School Work Unknown
Other ________________________
TEXAS DEPARTMENT OF STATE HEALTH SERVICES STOCK NO. EF11-11046
EMERGING AND ACUTE INFECTIOUS DISEASE BRANCH REVISED 03/2021
11
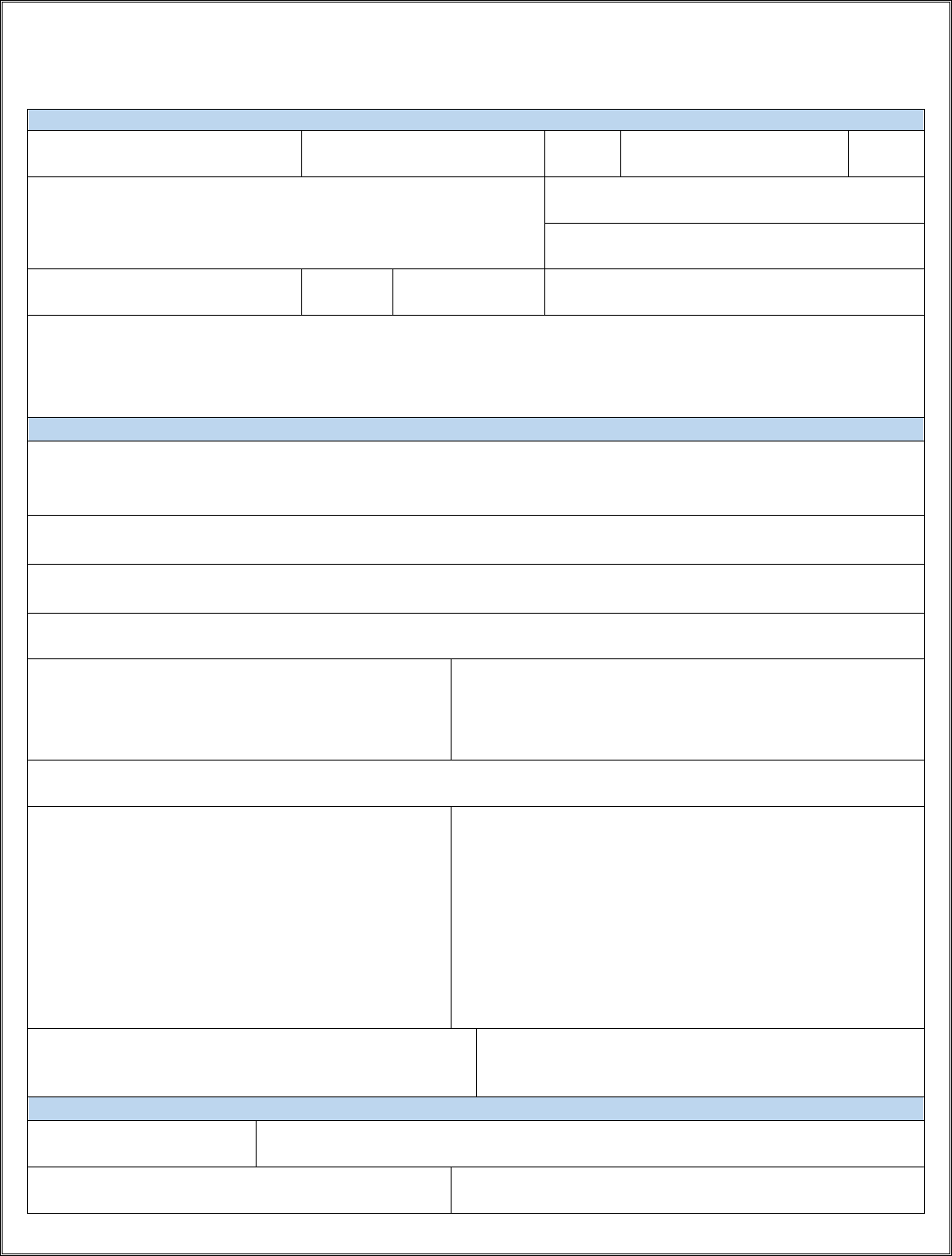
CONFIDENTIAL STD CASE REPORT FORM
AUSTIN PUBLIC HEALTH DEPARTMENT, 5202 E. Ben White, Ste 600, Austin, TX 78741
PHONE: (512) 972-5555 | FAX: (512) 972-5772
APH-EDSU-S-27
Revised March 2021
PATIENT INFORMATION
Last Name
First Name
MI
Date of Birth:
Age
Address:
Phone Number:
Work Number:
City:
State:
Zip code:
Emergency Contact Number:
Sex: ☐ Male ☐ Female Marital Status:
☐
S ☐ M ☐ W ☐ Is patient pregnant? ☐ Yes ☐ No Weeks: _______
Race (check all that apply): ☐ American Indian or Alaskan Native ☐ Black or African American ☐ Asian
☐ White ☐ Native Hawaiian or Pacific Islander ☐ Unknown
Ethnic Origin: ☐ Hispanic or Latino ☐ Not Hispanic or Latino
CLINICAL INFORMATION
Exam Reason:
☐ Partner Referral ☐ Referred by Partner ☐ Screening Jail/Prison ☐ STD Exposure ☐ Prenatal
☐ Delivery ☐ Volunteer ☐ Referred by Another Provider
☐
Other: ______________________________
Site / Specimen (check all that apply):
☐ Cervix ☐ Pharynx ☐ Rectum ☐ Urethra ☐ Urine
☐
Vagina
☐
Blood
Clinical Information (check all that apply):
☐
Asymptomatic
☐
Symptomatic
☐
Rash
☐
Chancre (sore/lesion ☐ Condyloma ☐ Alopecia
STD Lab Result(s): (Please fax lab results with report)
Performing laboratory: ______________________________ Date of Collection: ____________
☐ Chancroid
☐
positive
☐
negative
☐ Chlamydia ☐ positive ☐ negative
☐ Gonorrhea ☐ positive ☐ negative
☐
Pelvic Inflammatory Disease (Syndrome)
Treated:
☐
Yes
☐
No Date: _________________
☐ Azithromycin ☐ 1 g ☐ 2 g
☐ Ceftriaxone 500 mg in a single dose
☐ Other: _______________________
Syphilis Lab Result(s): (Please fax lab results with report)
Performing laboratory: ______________________________ Date of Collection: ____________
☐
700 – Syphilis
☐ 710 – Primary Syphilis (lesions)
☐ 720 – Secondary Syphilis (symptoms)
☐ 730 – Early latent Syphilis (<1 Year)
☐ 745 – Late Latent Syphilis (<1 year)
☐ 750 – Latent Syphilis w/ clinical manifestations
☐ 790 – Congenital Syphilis
Neurological Involvement: ☐ Yes ☐ No ☐ Unknown
Confirmatory Lab (i.e TPPA): ☐ positive
☐
negative
Titer (RPR/VDRL): ☐ Not reactive ☐ 1: _____________
History (Last RPR) DOC: __________ Titer: ________
Treated:
☐
Yes
☐
No
Date(s): ___________ ___________ ___________
☐ Bicillin 250 MU IM ☐ X1 ☐ X3
☐ Doxycycline 100 mg BID ☐ X7 ☐ X14 ☐ X28
☐ Ceftriaxone (Rocephin) ☐ 250 mg ☐ 500mg __
☐ Other ___________________________
Please call (512)-972-5144 or 5145
Report HIV/AIDS, including test performed during
prenatal visits.
Notes:
FACILITY INFORMATION
Physician or Facility Name
Facility Address
Contact Person:
Phone Number:
12

CONFIDENTIAL STD CASE REPORT FORM
AUSTIN PUBLIC HEALTH DEPARTMENT, 5202 E. Ben White, Ste 600, Austin, TX 78741
PHONE: (512) 972-5555 | FAX: (512) 972-5772
APH-EDSU-S-27
Revised March 2021
Please use form S-27 to report all notifiable Sexually Transmitted Diseases. Please complete all sections of this
form using available data. If a response is unknown, please leave that value blank. Reporting rules mandate that
positive lab results and disease diagnoses must be reported within the indicated time frames, regardless of
treatment status. A second report should be sent as needed to document successful treatment.
Codes for form STD-27
100 – Chancroid
200 – Chlamydia
300 – Gonorrhea
490 – Pelvic Inflammatory Disease (Syndrome)
600 – Lymphogranuloma Venereum (LGV)
700 – Syphilis
710 – Primary Syphilis (lesions)
720 – Secondary Syphilis (symptoms)
730 – Early latent Syphilis (<1 Year)
745 – Late Latent Syphilis (<1 year)
750 – Latent Syphilis with Symptomatic Manifestations
790 – Congenital Syphilis
900 – HIV (non-AIDS)
950 – AIDS (Syndrome)
Special Instructions
• Please use the provided “Notes/Symptoms” section to document all symptoms of 710/720, both observed
and as reported by patient, as this will assist in properly staging this infection.
• Please document the last known RPR titer, or any previous negative testing for 700.
• Please note all other STD laboratory results (including non-reactive results) when positive lab is collected in
conjunction with additional STD testing.
• Please document all lab results (including non-reactive results) when positive lab was ordered as part of a
comprehensive testing algorithm (e.g.: 700 RPR + 700 Confirmatory).
• While reporting on this document serves as proof of timely report, additional information is required on
900 patients. Please call 512-972-5145 or 512-972-5144, and staff will assist you with reporting all of the
required information.
• It is normal for various representatives of the Health Department to contact you during all stages of the
Public Health Follow-up process to obtain additional patient information.
Please call 512-972-5555 with any additional questions regarding HIV/STD reporting.
Please fax all completed forms to 512-972-5772. Alternately, this form may be mailed to:
Austin Public Health
5202 E. Ben White, Ste 600
Austin, Texas 78741
Attn: Surveillance Program
13
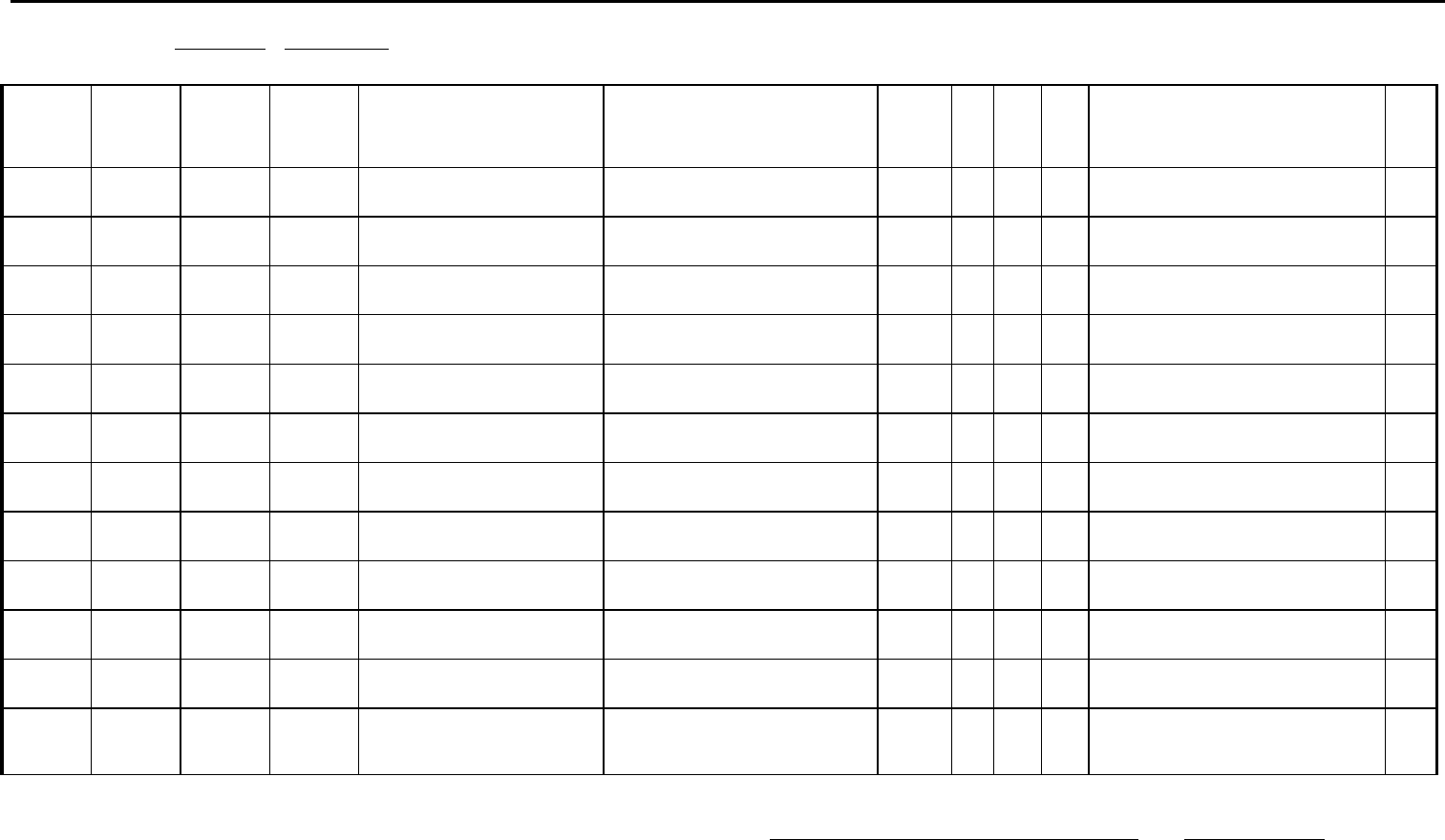
Austin Public Health
5202 E Ben White Bldg 600, Austin, TX 78741
Phone: (512) 972-5555 Fax: (512) 972-5772
General Reporting Form
(Name of Laboratory) (Address) (City) (State) (Zip) (Phone Number)
REPORT PERIOD: FROM TO .
Subm
it form weekly to local or regional health departments.
Test
Name
Results
(Titer if
applicable)
Date of
Specimen
Collection
Date of Lab
Analysis
Patient’s Name (Last, First, MI): Patient’s Address
(Including, City, County & Zip)
DOB Sex Race Hisp
Y/N
Physician/Facility’s
Name, Address, City, Zip & Phone No.
Preg/
Mat *
NOTIFICATION OF LABORATORY TEST FINDINGS INDICATING PRESENCE OF
CHLAMYDIA TRACHOMATIS, GONORRHEA, SYPHILIS, CHANCROID, HIV INFECTIONS
OR SUPRESSED CD4 COUNTS
Laboratory Supervisor Date
STD-28
Updated 04/1/2021
14

PERINATAL HBV HOSPITAL & PROVIDER
PORTAL
PAPER FORMS WILL NOT BE ACCEPTED AS OF
AUGUST 1, 2022.
Labs, hospitals, and providers should be provided with the
following link:
https://txhhs.force.com/DSHSPeriHepBPreventionPortal/s/
The public portal is intended to take the place of emails and faxes from labs, hospitals,
and providers. All submissions will be live upon entry into the database.
Submitters will also be able to upload supporting documents with their submissions. All
submitters will be required to include an email address as they will receive an automated
confirmation email notifying them that their submission was successful.
• Submitters will click on the tab that is needed.
• Submitters will complete all fields with an asterisk and may upload
supplemental documents as needed.
• Submitters then click Submit.
15
