
DEPARTMENT OF HEALTH & HUMAN SERVICES
Centers for Medicare & Medicaid Services
7500 Security Boulevard, Mail Stop S2-26-12
Baltimore, Maryland 21244-1850
SHO# 23-002
RE: Medicaid Continuous
Enrollment Condition Changes,
Conditions for Receiving the
FFCRA Temporary FMAP
Increase, Reporting Requirements,
and Enforcement Provisions in the
Consolidated Appropriations Act,
2023
January 27, 2023
Dear State Health Official:
On December 29, 2022, the Consolidated Appropriations Act, 2023 (P.L. 117-328) (CAA, 2023)
was enacted.
1
This state health official (SHO) letter discusses section 5131 of subtitle D of title
V of division FF of the CAA, 2023 (hereinafter referred to as “section 5131”). This section
makes significant changes to the continuous enrollment condition and availability of the
temporary increase in the Federal Medical Assistance Percentage (FMAP) under section 6008 of
the Families First Coronavirus Response Act (FFCRA) (hereinafter referred to as “temporary
FMAP increase”) and establishes new state reporting requirements and enforcement authorities
for the Centers for Medicare & Medicaid Services (CMS). Specifically, as discussed in further
detail in this letter, section 5131:
(1) Separates the end of the FFCRA continuous enrollment condition from the end of the
COVID-19 public health emergency (COVID-19 PHE), and ends that condition on
March 31, 2023, thus enabling states to terminate Medicaid enrollment of individuals
who no longer meet Medicaid eligibility requirements on or after April 1, 2023;
(2) Amends the conditions states must meet to claim, and extends the availability of, the
temporary FMAP increase beginning April 1, 2023, gradually phasing down the
increase until December 31, 2023;
(3) Adds new reporting requirements for all states under section 1902(tt) of the Social
Security Act (the Act); and
(4) Creates new enforcement authorities for CMS related to the new reporting
requirements and to state renewal activities during the period that begins on April 1,
2023 and ends on June 30, 2024 (a time frame that will overlap with states’
unwinding periods).
2
The newly enacted CAA, 2023 does not address the end date of the COVID-19 PHE. On
1
https://www.congress.gov/117/bills/hr2617/BILLS-117hr2617enr.pdf.
2
As described in prior CMS guidance, states will have up to 12 months to initiate, and 14 months to complete, a
renewal for all individuals enrolled in Medicaid, CHIP, and the Basic Health Program (BHP) following the end of
the continuous enrollment condition—this process has commonly been referred to as “unwinding.”
The contents of this document do not have the force and effect of law and are not meant to bind the public in any
way, unless specifically incorporated into a contract. This document is intended only to provide clarity to the public
regarding existing requirements under the law.

Page 2 – State Health Official Letter
January 11, 2023, the Secretary renewed the COVID-19 PHE, which is still in effect as of the
date of this letter.
3
On January 5, 2023, CMS released a Center for Medicaid and CHIP Services Informational
Bulletin (CIB), Key Dates Related to the Medicaid Continuous Enrollment Condition Provisions
in the Consolidated Appropriations Act, 2023,
4
which is the first in a series of guidance on the
changes made by section 5131 to the continuous enrollment condition and the impact of these
changes on prior CMS guidance.
5
The January 5, 2023, CIB relays key dates and deliverables
for states as they resume renewals and other eligibility and enrollment actions following the end
of the continuous enrollment condition (referred to in prior guidance as the “unwinding period”).
This SHO letter is the next in this series of guidance and builds on the guidance provided in the
January 5, 2023, CIB. CMS also expects to provide additional guidance on the CAA, 2023 in
the future.
CMS will be reviewing data, state activity, and other information to ensure all states comply with
federal eligibility renewal requirements and the new section 1902(tt) reporting requirements in
preparation for and during the state’s unwinding period. States may need to make programmatic
and operational changes to eligibility and enrollment policies, procedures, systems, and
operations and consider adopting alternative strategies and mitigation plans to ensure
compliance. CMS is available to consult with states as they prepare for and resume renewals and
other eligibility determinations. States may contact their CMS state lead for assistance.
Changes to the FFCRA Continuous Enrollment Condition and Temporary FMAP Increase
Section 5131(a)(2)(C) separates the end of the continuous enrollment condition from the end of
the COVID-19 PHE by amending section 6008(b)(3) of the FFCRA to end continuous Medicaid
enrollment as a condition for claiming the temporary FMAP increase on March 31, 2023. This
means that, on or after April 1, 2023, states claiming the temporary FMAP increase will no
longer be required to maintain the enrollment of a Medicaid beneficiary for whom the state
completes a renewal and who no longer meets Medicaid eligibility requirements. With the
changes made in section 5131, states must end the enrollment of ineligible beneficiaries on or
after April 1, 2023, after a full renewal is conducted during the state’s unwinding period, no
matter when the COVID-19 PHE ends.
Consistent with the March 3, 2022, SHO #22-001, RE: Promoting Continuity of Coverage and
Distributing Eligibility and Enrollment Workload in Medicaid, the Children’s Health Insurance
Program (CHIP), and Basic Health Program (BHP) Upon Conclusion of the COVID-19 Public
Health Emergency (hereinafter SHO #22-001), and as explained in the January 5, 2023, CIB:
3
The Secretary renewed the COVID-19 PHE, effective January 11, 2023. See:
https://aspr.hhs.gov/legal/PHE/Pages/covid19-11Jan23.aspx.
4
CMS. (January 5, 2023). Key Dates Related to the Medicaid Continuous Enrollment Condition Provisions in the
Consolidated Appropriations Act, 2023 [CMCS Informational Bulletin]. Available at
https://www.medicaid.gov/sites/default/files/2023-01/cib010523_1.pdf.
5
CMS. (March 3, 2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload
in Medicaid, the Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon
Conclusion of the COVID-19 Public Health Emergency [State Health Official Letter #22-001]. Available at
https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.

Page 3 – State Health Official Letter
• States can begin their unwinding period as early as February 1, 2023, by initiating
renewals that may result in eligibility terminations on or after April 1, 2023. States must
begin their unwinding period by initiating renewals no later than April 2023.
• For states that initiate renewals prior to April 1, 2023, terminations of Medicaid
eligibility may not be effective earlier than April 1, 2023.
• States must initiate renewals for all individuals enrolled in Medicaid, CHIP, and BHP
within 12 months of the beginning of the state’s unwinding period and must complete
renewals for all individuals within 14 months of the beginning of the state’s unwinding
period.
6
A renewal is considered initiated when the state begins the renewal process by attempting to
renew eligibility on an ex parte basis – i.e., based on available reliable information without
contacting the individual. CMS encourages states to distribute renewals in a reasonable manner
and recommends that states initiate no more than 1/9 of their total caseload of Medicaid and
CHIP renewals in a given month during the unwinding period for several reasons discussed in
SHO #22-001, including to ensure states have a renewal schedule that is sustainable in future
years. States may refer to SHO #22-001 (pages 14-21) for information on strategies states may
use to prioritize and distribute workload during the unwinding period.
7
Section 5131 also separates the end of the temporary FMAP increase from the end of the
COVID-19 PHE. Specifically, section 5131(a) amends section 6008(a) of the FFCRA to
continue the temporary FMAP increase through December 31, 2023 (instead of through the end
of the quarter in which the COVID-19 PHE ends), and phases down the amount of the FMAP
increase beginning April 1, 2023. Table 1 displays the amount of the FMAP increase that will be
available in each quarter of calendar year (CY) 2023, provided that the state claiming the FMAP
increase meets the applicable conditions in subsections (b) and (f) of section 6008 of the FFCRA,
as amended by section 5131 (discussed below). If the state meets the applicable conditions, the
FMAP increase applies to the same match rates to which it applied prior to the enactment of the
CAA, 2023.
8
6
CMS. (March 3, 2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload
in Medicaid, the Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon
Conclusion of the COVID-19 Public Health Emergency [State Health Official Letter #22-001] (pages 7-9).
Available at https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.
7
CMS. (March 3, 2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload
in Medicaid, the Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon
Conclusion of the COVID-19 Public Health Emergency [State Health Official Letter #22-001] (pages 14-21).
Available at https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.
8
In general, the FFCRA FMAP increase is available for allowable Medicaid medical assistance expenditures for
which federal matching is paid ordinarily at the state-specific FMAP defined in the first sentence of section 1905(b)
of the Act. For additional guidance on the match rates that can be temporarily increased under section 6008 of the
FFCRA (as amended), see Section IV.F in: CMS. (2021). COVID-19 Frequently Asked Questions (FAQs) for State
Medicaid and Children’s Health Insurance Program (CHIP) Agencies. Available at:
https://www.medicaid.gov/state-resource-center/downloads/covid-19-faqs.pdf.

Page 4 – State Health Official Letter
Table 1. CAA, 2023 FMAP Increase Phase Out
2023 Calendar Overview
Temporary FMAP Increase Available
Q1: January 1-March 31, 2023
6.2 percentage points
Q2: April 1-June 30, 2023
5.0 percentage points
Q3: July 1-September 30, 2023
2.5 percentage points
Q4: October 1-December 31, 2023
1.5 percentage points
Conditions for Receipt of Temporary FMAP Increase
The conditions for claiming the temporary 6.2 percentage point FMAP increase remain
unchanged for the first quarter of CY 2023. States claiming the temporary FMAP increase for
quarter 2, 3, or 4 of CY 2023 (that is, for any quarter in the period beginning April 1, 2023, and
ending December 31, 2023) must continue to meet three of the conditions originally set forth in
section 6008(b) of the FFCRA during each quarter in which the state claims the FMAP increase:
(1) section 6008(b)(1) (related to maintenance of effort); (2) section 6008(b)(2) (related to
premiums), with one modification made by section 5131 (discussed below); and (3) section
6008(b)(4) (related to coverage of COVID vaccination, testing, and treatment services, without
cost-sharing). The applicability of these conditions during quarters 2, 3, and 4 of CY 2023 is
discussed in this section of the SHO.
Section 5131 also amended section 6008 of the FFCRA to establish new conditions for states to
claim the temporary FMAP increase during quarters 2, 3, and 4 of CY 2023 under a new section
6008(f) of the FFCRA. States that claim the temporary FMAP increase during quarter 2, 3, or 4
of CY 2023 must comply with the new FFCRA section 6008(f) conditions for Medicaid renewals
that are conducted during each quarter for which the state claims the FMAP increase. Guidance
on the new conditions established in section 6008(f) of the FFCRA is provided later in this SHO
letter. As noted above, states will continue to have 12 months to initiate their work on renewals,
redeterminations based on changes in circumstances, and post-enrollment verifications, and 14
months to complete this work once the state’s unwinding period begins, consistent with the
guidance in the January 5, 2023, CIB and SHO #22-001.
In preparation for and during the unwinding period, CMS will review data, state activities, and
other information to ensure states are complying with federal eligibility renewal requirements
and, for states claiming the FFCRA temporary FMAP increase, the applicable conditions for
claiming that increase. States may need to make programmatic and operational changes to
eligibility and enrollment policies, procedures, systems, and operations and consider adopting
alternative strategies to ensure compliance. States that are not compliant with federal
redetermination requirements, regardless of whether they continue to claim the FFCRA section
6008 FMAP increase, may be subject to corrective action and penalties under section 1904 or
section 1902(tt)(2)(B) of the Act (see discussion below). States not in compliance with all
9
9
For additional information on the condition under section 6008(b)(3) of the FFCRA to maintain enrollment of
individuals effective through March 31, 2023, see: 42 CFR § 433.400, and CMS. (2021). COVID-19 Frequently
Asked Questions (FAQs) for State Medicaid and Children’s Health Insurance Program (CHIP) Agencies. Available
at: https://www.medicaid.gov/state-resource-center/downloads/covid-19-faqs.pdf.
Page 5 – State Health Official Letter
redetermination requirements may work with CMS to develop and implement an approved
mitigation plan.
Maintenance of Effort Condition through December 31, 2023
Under section 6008(b)(1) of the FFCRA, states may not claim the temporary FMAP increase for
a quarter if, during that quarter, they impose eligibility standards, methodologies, or procedures
that are more restrictive than those in effect on January 1, 2020. Section 5131 did not change
this condition, and states must continue to meet it for any quarter in which they claim the
temporary FMAP increase, through December 31, 2023. See previous guidance in Section IV.F.
in the COVID-19 Frequently Asked Questions (FAQs) for State Medicaid and Children’s Health
Insurance Program (CHIP) Agencies
10
for additional guidance on this condition.
Maintenance of Medicaid Premium Levels through December 31, 2023 (Modified by Section
5131)
Prior to the enactment of the CAA, 2023, section 6008(b)(2) of the FFCRA provided that states
could not claim the temporary FMAP increase if they imposed any premium “with respect to an
individual” enrolled under the state plan (or a waiver of the plan) that exceeded the amount of
such premium as of January 1, 2020. CMS interpreted this condition to mean that a state
claiming the temporary FMAP increase could not increase the amount of any premium charged
to an individual enrollee (even if that person’s income increased).
11
Beginning April 1, 2023 (that is, beginning in quarter 2 of CY 2023), section 5131(a)(2)(B)
amends section 6008(b)(2) of the FFCRA to remove the language “with respect to an individual
enrolled under such plan (or waiver).” Under the amended section 6008(b)(2) condition, states
claiming the temporary FMAP increase for quarters beginning on or after April 1, 2023, must
continue to ensure that the amounts in their Medicaid premium schedule do not exceed the
amounts that were in place under the state plan or any waiver of the plan (including a section
1115 demonstration) as of January 1, 2020. However, beginning April 1, 2023, states may,
under the amended section 6008(b)(2) of the FFCRA, increase the premium amount that is
imposed on a given individual (e.g., put an individual in a higher premium band if their income
has increased, or newly charge an individual a premium if they are moved into an eligibility
group that is subject to premiums) without jeopardizing the state’s ability to claim the temporary
FMAP increase, subject to the following three conditions:
1) The increase must be consistent with the state’s Medicaid premium schedule.
2) The premium schedule amounts must not have increased over the amounts in effect as of
January 1, 2020.
10
See: CMS. (2021). COVID-19 Frequently Asked Questions (FAQs) for State Medicaid and Children’s Health
Insurance Program (CHIP) Agencies. Available at: https://www.medicaid.gov/state-resource-
center/downloads/covid-19-faqs.pdf.
11
For guidance on the condition described in section 6008(b)(2) in effect through March 31, 2023, see FAQ #II.B.9-
10, 12-13 of: CMS. (2021). COVID-19 Frequently Asked Questions (FAQs) for State Medicaid and Children’s
Health Insurance Program (CHIP). Available at: https://www.medicaid.gov/state-resource-
center/downloads/covid-19-faqs.pdf.
Page 6 – State Health Official Letter
3) The state must comply with redetermination requirements prior to resumption of
Medicaid premiums, as discussed in response to Q24-25 of the COVID-19 Public Health
Emergency Unwinding Frequently Asked Questions for State Medicaid and CHIP
Agencies.
12
The state also must comply with all premium requirements and limitations specified at sections
1916 and 1916A of the Act and 42 CFR §§ 447.50 through 447.57; all applicable advance notice
and fair hearing requirements at 42 CFR § 435.917 and 42 CFR Part 431, Subpart E; public
notice requirements at 42 CFR § 447.57; state plan amendment (SPA) effective date
requirements at 42 CFR § 430.20; and tribal consultation requirements at section 1902(a)(73) of
the Act. CMS plans to provide additional guidance on the CAA, 2023 amendment to section
6008(b)(2) of the FFCRA, including information about the implications of this change for state
decisions about rescinding disaster relief SPAs.
States may request authority under section 1902(e)(14)(A) of the Act to delay the resumption of
Medicaid premiums during the unwinding period until a full redetermination is completed for
beneficiaries who are subject to premiums under the State plan or a waiver, including through a
section 1115 demonstration project. States can refer to SHO #22-001
13
for details on how to
request approval of section 1902(e)(14)(A) waivers. States may also contact their CMS state
lead for more information.
Coverage without Cost Sharing for COVID-19 Testing, Vaccines, and Treatment through
December 31, 2023
States claiming the temporary FMAP increase for any quarter in the period beginning April 1,
2023, and ending December 31, 2023, must continue to meet the condition in section 6008(b)(4)
of the FFCRA, under which the state must provide coverage, without cost sharing, for any testing
services and treatments for COVID-19, including vaccines, specialized equipment, and
therapies.
14
This condition was not changed by section 5131. However, it is important to note
12
See previous guidance regarding redetermination requirements prior to resumption of Medicaid premiums,
including individualized notice requirements and when a state must obtain updated income information before
reinstatement, at Q24-25 of: CMS. (2022). COVID-19 Public Health Emergency Unwinding Frequently Asked
Questions for State Medicaid and CHIP Agencies. Available at: https://www.medicaid.gov/federal-policy-
guidance/downloads/covid-19-unwinding-faqs-oct-2022.pdf.
13
CMS. (March 3, 2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload
in Medicaid, the Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon
Conclusion of the COVID-19 Public Health Emergency [State Health Official Letter #22-001]. Available at
https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.
14
For information regarding implementation of this condition, see previous guidance at FAQ #II.B.2, 4,14-15 of:
CMS. (2021). COVID-19 Frequently Asked Questions (FAQs) for State Medicaid and Children’s Health Insurance
Program (CHIP) Agencies. Available at: https://www.medicaid.gov/state-resource-center/downloads/covid-19-
faqs.pdf. See also the discussions of section 6008(b)(4) of the FFCRA in the interim final rule with request for
comments entitled “Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health
Emergency,” 85 FR 71142, 71148-50 (Nov. 6, 2020) (available at:
https://www.federalregister.gov/documents/2020/11/06/2020-24332/additional-policy-and-regulatory-revisions-in-
response-to-the-covid-19-public-health-emergency), and in CMS. (2022). Coverage and Reimbursement of COVID-
19 Vaccines, Vaccine Administration, and Cost-Sharing under Medicaid, the Children’s Health Insurance Program,
and Basic Health Program. Available at https://www.medicaid.gov/state-resource-center/downloads/covid-19-
vaccine-toolkit.pdf.

Page 7 – State Health Official Letter
that section 9811 of the American Rescue Plan Act of 2021 (ARP) (P.L. 117-2) amended various
provisions of the Act to require states to provide coverage, without cost sharing, of: COVID-19
vaccinations; COVID-19 testing; treatments for COVID-19, including specialized equipment and
therapies (including preventive therapies); and, when certain conditions are met, treatment of
conditions that may seriously complicate the treatment of COVID-19. These requirements under
section 9811 of the ARP remain in place until the last day of the first calendar quarter that begins
one year after the last day of the COVID-19 PHE. The requirements under section 9811 of the
ARP generally overlap with, are in many circumstances broader than, and will extend longer
than the coverage and cost-sharing condition under section 6008(b)(4) of the FFCRA, and they
apply even if the state is not claiming the FFCRA temporary FMAP increase. CMS therefore
expects the CAA, 2023’s amended sunset date for section 6008(b)(4) of the FFCRA will not
have much practical impact on states’ coverage of these services without cost sharing. For
additional information, see the June 3, 2021, CIB,
15
SHO #21-003,
16
SHO #21-006,
17
SHO #22-
002,
18
and the CMCS COVID-19 Vaccine Toolkit.
19
Overview of New Conditions for Receipt of the Temporary FMAP Increase in Effect April 1,
2023, through December 31, 2023
Section 5131 added a new subsection (f) to section 6008 of the FFCRA. States claiming the
temporary FMAP increase for quarter 2, 3, and/or 4 of CY 2023 must satisfy the conditions
under sections 6008(b)(1), 6008(b)(2), and 6008(b)(4), as described above, in addition to the
following new conditions under section 6008(f) of the FFCRA:
• Conduct Medicaid eligibility redeterminations in accordance with all applicable federal
requirements, including renewal strategies authorized under section 1902(e)(14)(A) of the
Act or other alternative processes and procedures approved by CMS (section
6008(f)(2)(A));
• Attempt to ensure that they have up-to-date contact information for a beneficiary before
redetermining eligibility for such beneficiary (section 6008(f)(2)(B)); and
• Undertake a good-faith effort to contact an individual using more than one modality prior
to terminating their enrollment on the basis of returned mail (section 6008(f)(2)(C)).
These new conditions apply to Medicaid renewals that are conducted during any quarter during
the period beginning April 1, 2023, and ending December 31, 2023, in states claiming the
temporary FMAP increase for that quarter. In addition to being a condition of receiving the
15
CMS. (June 3, 2021). Medicaid, Children’s Health Insurance Program (CHIP), and Basic Health Program
(BHP) Related Provisions in the American Rescue Plan Act of 2021 [CMCS Informational Bulletin]. Available at:
https://www.medicaid.gov/federal-policy-guidance/downloads/cib060321.pdf
16
CMS. (August 30, 2021). Medicaid and CHIP Coverage and Reimbursement of COVID-19 Testing under the
American Rescue Plan Act of 2021 and Medicaid Coverage of Habilitation Services [State Health Official Letter
#21-003]. Available at: https://www.medicaid.gov/federal-policy-guidance/downloads/sho-21-003.pdf
17
CMS. (October 22, 2021). Mandatory Medicaid and CHIP Coverage of COVID-19-Related Treatment under the
American Rescue Plan Act of 2021 [State Health Official Letter #21-006]. Available at:
https://www.medicaid.gov/federal-policy-guidance/downloads/sho102221.pdf.
18
CMS. (May 12, 2022). Medicaid and CHIP Coverage of Stand-alone Vaccine Counseling [State Health Official
Letter #22-002]. Available at: https://www.medicaid.gov/federal-policy-guidance/downloads/sho22002.pdf.
19
CMS. (2022). Coverage and Reimbursement of COVID-19 Vaccines, Vaccine Administration, and Cost-Sharing
under Medicaid, the Children’s Health Insurance Program, and Basic Health Program. Available at
https://www.medicaid.gov/state-resource-center/downloads/covid-19-vaccine-toolkit.pdf.
Page 8 – State Health Official Letter
temporary FMAP increase beginning April 1, 2023, implementation of these conditions will help
states minimize procedural denials and reduce the administrative burden associated with churn.
These new conditions and how states may comply with them are described in detail below.
We understand that states may be concerned about whether their plan to comply with one or
more conditions for receiving the temporary FMAP increase beginning April 1, 2023, is
sufficient. CMS is available to work with states to evaluate the sufficiency of their plans and
identify additional measures that states can consider to ensure satisfaction of all conditions.
Compliance with Federal Renewal Requirements
As indicated above, in order to claim the temporary FMAP increase after March 31, 2023, under
section 6008(f)(2)(A) of the FFCRA, states must conduct Medicaid redeterminations consistent
with federal requirements, including any renewal strategy approved under section
1902(e)(14)(A) of the Act or other CMS-authorized processes and procedures.
Federal requirements related to redeterminations of eligibility are described at 42 CFR §
435.916. Under federal regulations at § 435.916, states must comply with the following
requirements:
• Ex Parte Renewals: Begin the renewal process for all beneficiaries, including both those
whose financial eligibility is based on modified adjusted gross income (MAGI) (“MAGI-
based beneficiaries”) and those whose financial eligibility is not based on MAGI (“non-
MAGI beneficiaries”), by redetermining eligibility without requiring information from
the individual, if the state is able to do so based on reliable information contained in the
individual’s account or more current reliable information available to the state. This
information may include, but is not limited to, information accessed through data sources,
consistent with the state’s verification plan;
• Renewal Form: Provide a renewal form that requests only information needed to
determine eligibility
20
when eligibility cannot be renewed on an ex parte basis. This
form must be pre-populated for MAGI-based beneficiaries;
• Reasonable Timeframe and Modalities to Return Form: Provide MAGI-based
beneficiaries with a minimum of 30 days to return their pre-populated renewal form and
any requested information. Provide non-MAGI beneficiaries with a reasonable period of
time to do so. Beneficiaries must be able to return their renewal form through any of the
modes of submission described at § 435.907(a) (online, by phone, by mail, or in-person);
• Determine Eligibility on All Bases: Consider all bases of Medicaid eligibility prior to
determining an individual is ineligible for Medicaid and terminating coverage;
21
• Advance Notice and Fair Hearing Rights: Provide a minimum of 10 days’ advance
notice
22
and fair-hearing rights prior to terminating or reducing Medicaid eligibility, in
20
For example, a form that requests that a beneficiary provides their Social Security Number, citizenship or
immigration status would not satisfy the requirement. Such information is only needed once and, thus, would not be
needed to renew eligibility.
21
42 CFR §§ 435.916(f)(1) and 435.930(b).
22
42 CFR § 431.211 requires the state to send a notice at least 10 days before the date of action, which is defined at
§ 431.201 as “the intended date on which a termination, suspension, reduction, transfer or discharge becomes
effective.”

Page 9 – State Health Official Letter
accordance with § 435.917 and 42 CFR Part 431, Subpart E;
• Assess Eligibility for Other Insurance Affordability Programs (IAPs) and Transfer
Accounts as Appropriate: For individuals determined ineligible for Medicaid, assess
eligibility for other IAPs (including CHIP, BHP, and qualified health plans (QHPs)
offered through a Health Insurance Marketplace
®23
with advance payments of premium
tax credits or cost-sharing reductions), and transfer the individual’s account to the
appropriate program. States with Marketplaces that use the federal eligibility and
enrollment platform are reminded that they should only transfer accounts to the
Marketplace for individuals about whom the state has sufficient information to determine
Medicaid and CHIP ineligibility. States with Marketplaces that use the federal eligibility
and enrollment platform should not transfer accounts to the Marketplace for individuals
whose Medicaid or CHIP coverage is terminated for procedural reasons, such as failure to
return a renewal form or other requested information needed to determine eligibility.
24
States that operate State-based Marketplaces using their own platform may, at state
option, transfer accounts to the Marketplace for a determination of advance payments of
premium tax credits or cost-sharing reductions for individuals whose coverage has been
terminated from Medicaid or CHIP for procedural reasons; and
• Reconsideration Period: Reconsider eligibility without requiring a new application for
MAGI-based beneficiaries whose coverage is terminated for failure to return their
renewal forms or necessary information if the individual’s renewal form or information is
returned within 90 days (or longer if elected by the state) after coverage is terminated.
States may, at their option, apply this policy to non-MAGI beneficiaries.
In evaluating compliance with § 435.916, states are directed to the CIB, Medicaid and
Children’s Health Insurance Program Renewal Requirements,
25
released on December 4, 2020,
for more information on federal redetermination requirements.
States that are unable to comply with one or more of the requirements in § 435.916 may satisfy
the condition in section 6008(f)(2)(A) of the FFCRA, as added by section 5131(a), by
implementing renewal strategies authorized under section 1902(e)(14)(A) of the Act or other
alternative policies and procedures that CMS authorizes and approves as sufficient for purposes
of satisfying section 6008(f)(2)(A) and claiming the temporary FMAP increase. Depending on
the state’s systems and processes, states that are unable to comply with all requirements set forth
in § 435.916 may need to adopt multiple alternative strategies in order to claim the temporary
FMAP increase after March 31, 2023. States can refer to SHO #22-001
26
or the section
23
Health Insurance Marketplace® is a registered service mark of the U.S. Department of Health & Human Services.
24
CMS. (July 25, 2016). Coordination of Eligibility and Enrollment Between Medicaid, CHIP and the Federally
Facilitated Marketplace (FFM or “Marketplace”) [CMCS Informational Bulletin]. Available at
https://www.medicaid.gov/federal-policy-guidance/downloads/cib072516.pdf.
25
CMS. (December 4, 2020). Medicaid and Children’s Health Insurance Program (CHIP) Renewal Requirements
[CMCS Informational Bulletin]. Available at: https://www.medicaid.gov/federal-policy-
guidance/downloads/cib120420.pdf.
26
CMS. (March 3, 2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload
in Medicaid, the Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon
Conclusion of the COVID-19 Public Health Emergency [State Health Official Letter #22-001]. Available at
https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.

Page 10 – State Health Official Letter
1902(e)(14)(A) waivers portion of the CMS unwinding website
27
for details on section
1902(e)(14)(A) alternative renewal strategies that CMS can authorize and how to submit a
request for such authorization to CMS. States may also reach out to their CMS state lead for
more information and to obtain technical assistance. CMS is available to work with states that
are unsure of whether they meet a given regulatory renewal requirement and to provide technical
assistance on section 1902(e)(14)(A) waivers or other CMS authorized alternative processes that
may be implemented to satisfy section 6008(f)(2)(A) of the FFCRA for purposes of claiming the
temporary FMAP increase after March 31, 2023.
We remind states that, regardless of whether a state is claiming the temporary FMAP increase,
all states must comply with federal renewal requirements. States that do not meet federal
renewal requirements may be subject to corrective action under section 1904 and, if the
noncompliance occurs during the period that begins on April 1, 2023, and ends on June 30, 2024,
may be subject to corrective action under section 1902(tt)(2)(B) of the Act (added by section
5131). See discussion in the Monitoring State Progress and Corrective Action section of this
letter for additional information on corrective action under section 1902(tt)(2)(B) of the Act.
Up-to-Date Contact Information
As a condition of claiming the temporary FMAP increase after March 31, 2023, under section
6008(f)(2)(B) of the FFCRA, as added by section 5131(a), a state must attempt to ensure that it
has up-to-date contact information for each individual for whom it conducts a renewal.
28
This
condition requires that states use the United States Postal Service (USPS) National Change of
Address (NCOA) database, information maintained by state health and human services agencies,
or other reliable sources of contact information. For purposes of compliance with this condition,
the types of contact information a state must attempt to update include a beneficiary’s mailing
address, phone number, and email address. States may need to use multiple data sources and/or
adopt multiple strategies in order to update all types of beneficiary contact information.
The NCOA database information is limited to mailing address information and does not provide
email addresses or phone numbers. Additionally, for some beneficiaries, the NCOA database
will not provide up-to-date mailing address information, and other state agency databases may
not have such contact information either. Therefore, to satisfy this condition, states will need to
take other reasonable actions in an effort to obtain up-to-date information. Managed care
organizations (MCOs) are a particularly effective source of reliable contact information for
beneficiaries, and CMS strongly encourages states that contract with MCOs to work with them to
obtain up-to-date contact information. States could also look to beneficiaries themselves as a
reliable source of contact information and use beneficiary outreach initiatives encouraging
beneficiaries to update their contact information.
Implementing a robust plan to obtain up-to-date contact information for multiple modes of
communication will also assist states in meeting the returned-mail condition under section
27
CMS. (2022). COVID-19 PHE Unwinding Section 1902(e)(14)(A) Waiver Approvals. Available at
https://www.medicaid.gov/covid-19-phe-unwinding-section-1902e14a-waiver-approvals/index.html.
28
As noted above, the conditions in section 6008(f) of the FFCRA apply to Medicaid renewals that are conducted
during any quarter during the period beginning April 1, 2023, and ending December 31, 2023, in states claiming the
temporary FMAP increase for that quarter.

Page 11 – State Health Official Letter
6008(f)(2)(C) of the FFCRA, described below, under which states must make a good-faith effort
to contact an individual through more than one modality prior to terminating coverage on the
basis of returned mail.
In order to comply with the up-to-date contact information condition, states should consider the
following:
• Sources of Up-to-Date Contact Information: States must use the NCOA database,
information maintained by a state health and human services agency, or other reliable
sources of contact information. States have broad discretion to determine which other
sources of contact information are reliable for the purposes of this provision but must
document the sources and other processes used in their unwinding operational plan. For
example, many states have implemented beneficiary outreach strategies using direct
phone calls, mass mailings, social media, or other mass communication strategies to
prompt beneficiaries to provide up-to-date information themselves. Contact information
received directly from beneficiaries in response to an outreach campaign could
reasonably be considered reliable. States have also worked with other entities to obtain
up-to-date beneficiary contact information, in addition to accessing the NCOA. Updated
contact information received from an MCO under contract with the state, for example, is
generally considered to be a reliable source of information. Further, states can request
section 1902(e)(14)(A) waiver authority to update an individual’s case record with up-to-
date contact information from one of its MCOs without first having to confirm the change
with the individual.
• Attempt to Ensure Up-To-Date Contact Information: States must attempt to ensure they
have up-to-date contact information for each individual for whom they will conduct an
eligibility redetermination. We interpret this condition to mean that a state must
implement a comprehensive plan for confirming that they have up-to-date information on
beneficiaries before their eligibility is redetermined, including a plan that uses reliable
data sources and adopts other reasonable strategies that apply broadly to the state’s
Medicaid population (e.g., routinely obtaining updated contact information from
managed care organizations or broad outreach campaigns).
• Timing of Attempt to Ensure Up-to-Date Contact Information: States must have recent
and reliable information, or have recently attempted to obtain up-to-date contact
information as described immediately above, prior to initiating a renewal for an
individual to minimize the possibility that the information in the case record has become
outdated. For example, CMS would consider quarterly data matches with NCOA or
adoption of the section 1902(e)(14)(A) strategy involving MCOs providing updated
contact information about a beneficiary whenever they obtain it, to be a recent attempt.
• Document Strategies to Update Contact Information in Unwinding Operational Plan:
States must document in their unwinding operational plan the strategies and processes for
obtaining up-to-date contact information for beneficiaries in order to demonstrate
compliance with the condition for claiming the temporary FMAP increase described in
section 6008(f)(2)(B) of the FFCRA.
Page 12 – State Health Official Letter
In implementing policies and procedures to satisfy this condition, states may want to review
pages 36-40 of SHO #22-001,
29
in which CMS discusses several strategies for states to
reestablish communication with beneficiaries, such as working with MCOs, social services
organizations, and other entities. States are strongly encouraged to implement several strategies
to ensure an attempt is made to obtain up-to-date contact information for all beneficiaries,
including strategies that target hard-to-reach, homeless, rural, or Tribal populations for whom
many strategies may be less effective. For instance, due to limited postal delivery and broadband
services in Tribal communities, we encourage states to engage with the Indian Health Service
(IHS), Tribes and Tribal organizations, and urban Indian organizations (collectively, ITU) to
help with updated contact information for Tribal Medicaid beneficiaries, including sharing
enrollment and renewal data with ITUs. We remind states that such data sharing must be
consistent with Medicaid confidentiality standards under section 1902(a)(7)(A) of the Act and 42
CFR part 431, subpart F, and applicable privacy laws.
30
Further, we remind states that if they have received approval under a section 1902(e)(14)(A)
waiver related to updating beneficiary contact information,
31
there may be several sources of up-
to-date contact information they can rely on without first confirming a change with beneficiaries.
The waiver(s) could make it easier for the state to ensure that it has up-to-date beneficiary
contact information and complies with this condition. We encourage states that do not currently
have such an approved waiver to consider submitting a request to CMS for approval.
32
Contact Beneficiaries Using More Than One Modality Prior to Terminating Enrollment on the
Basis of Returned Mail
In order to claim the FFCRA temporary FMAP increase after March 31, 2023, states must
comply with section 6008(f)(2)(C) of the FFCRA, as added by section 5131(a), which provides
that when states receive returned mail in response to a redetermination of eligibility, they must
undertake a good-faith effort to contact an individual using more than one modality prior to
disenrollment on the basis of returned mail (“returned-mail condition”).
Nothing in the CAA, 2023 changes federal Medicaid rules regarding the steps that states are
required to take upon receipt of returned mail. These rules apply regardless of whether a state is
conducting redeterminations during its unwinding period. We remind states that there are
29
CMS. (March 3, 2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload
in Medicaid, the Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon
Conclusion of the COVID-19 Public Health Emergency [State Health Official Letter #22-001]. Available at
https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.
30
See “Presentation: Strengthening Tribal and State Partnerships to Prepare for Unwinding” (Posted 8/30/2022)
Available at https://www.medicaid.gov/sites/default/files/2022-08/strengthening-tribal-partner-to-prepare-
unwinding-081822.pdf.
31
See the COVID-19 PHE Unwinding Section 1902(e)(14)(A) Waiver Approvals page for more information on
these strategies at https://www.medicaid.gov/covid-19-phe-unwinding-section-1902e14a-waiver-
approvals/index.html.
32
To request authority to implement one or more of these strategies, states can request such waiver authority under
section 1902(e)(14)(A) of the Act on a time-limited basis during the unwinding period. CMS is available to provide
technical assistance and can provide sample language the state can use to craft a letter requesting the waiver
authority. States interested in one or more of the temporary waiver authorities described above should contact their
CMS State Lead.
Page 13 – State Health Official Letter
generally three types of beneficiary mail that could be returned to the Medicaid agency: (1) mail
with an in-state forwarding address; (2) mail with an out-of-state forwarding address; and (3)
mail that does not include a forwarding address. States must continue to follow existing
requirements for processing each of these types of returned mail including confirming whether
the address information on the piece of returned mail is complete and consistent with the address
information the state has on file.
33
The new returned-mail condition under section 6008(f)(2)(C) of the FFCRA applies specifically
to situations in which the state sends a notice to a beneficiary, instructing them to return a
renewal form. For the purposes of meeting this returned mail condition, we define a good faith
effort to contact an individual using more than one modality to mean: (1) the state has a process
in place to obtain up-to-date mailing addresses and additional contact information (i.e., telephone
numbers, email addresses) for all beneficiaries; and (2) the state attempts to reach an individual
whose mail is returned through at least two modalities using the most up-to-date contact
information the state has for the individual, which could include a forwarding address if one is
provided on the returned mail.
States also should consider the following:
• Up-to-Date Contact Information: The requirement to obtain up-to-date contact
information for purposes of complying with the returned mail condition will be satisfied
if the state has met the condition described in section 6008(f)(2)(B) of the FFCRA to
attempt to obtain an up-to-date mailing address, phone number, and email address for all
beneficiaries due for a renewal. In addition to the data sources described in section
6008(f)(2)(B) of the FFCRA, MCOs are a particularly effective source of reliable contact
information for beneficiaries, and CMS strongly encourages states that contract with
MCOs to work with them to obtain up-to-date contact information.
• Permissible Modalities: States have discretion in the types of modalities they rely upon
to satisfy the returned mail condition. Such modalities may include mail, telephone,
email, text messaging, communication through an online portal, or other commonly
available electronic means.
34
The returned-mail condition under section 6008(f)(2)(C) of the FFCRA applies whenever
beneficiary mail is returned to the state agency in response to a redetermination of eligibility,
thus prompting states to process the returned mail and requiring states to make a good faith effort
to attempt to contact the beneficiary using more than one modality. States should follow these
steps to meet the returned mail condition of attempting to make a good faith effort to contact the
beneficiary through two modalities.
• Returned Mail has Complete Information: Generally, when beneficiary mail is returned,
states must ensure that the mail was sent to the intended address by comparing the
33
For a detailed discussion on the requirements for processing returned mail, see Appendix C of: CMS. (March
2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload in Medicaid, the
Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon Conclusion of the COVID-
19 Public Health Emergency [State Health Official Letter #22-001]. Available at
https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.
34
Phone calls and text messages, initiated either directly by the state agency or through a state contractor or partner,
must be compliant with Federal communications laws such as the Telephone Consumer Protection Act. For more
information, see: https://www.fcc.gov/document/fcc-provides-guidance-enable-critical-health-care-coverage-calls.

Page 14 – State Health Official Letter
completeness and accuracy of the address on the returned mail against the information in
the beneficiary’s record. If the address listed on the original mailing contains an error or
is missing information, such as an apartment number, the state must resend the returned
notice to a completed address. If the subsequent mailing to the beneficiary’s correct
address is not returned, the state is no longer required to take additional steps to meet the
returned mail condition by attempting to contact the beneficiary using an additional
modality. If the subsequent mailing to a corrected address is returned, the state would
proceed as indicated below based on whether the returned mail has a forwarding address
or not.
• Returned Mail with No Forwarding Address: When a state receives returned mail with
no forwarding address, it must attempt to contact the beneficiary through two modalities,
including by phone, email, text, or other available modalities. If the state only has one
other mode of contact available, such as only an email address, the state must attempt to
contact the beneficiary using that one modality; in this instance, the state will be
compliant with the returned mail condition provided that it has complied with the up-to-
date contact information condition. Similarly, if the state has no other modalities
available to contact the beneficiary, the state similarly will be compliant with the returned
mail condition if it has satisfied the up-to-date contact information condition.
• Returned Mail with a Forwarding Address:
States are not required to send the returned mail to the forwarding address, but
doing so would represent one attempt to contact the beneficiary for purposes of
the returned mail condition.
If the beneficiary’s returned mail contains a forwarding address, and the state
sends the original mailing to the forwarding address,
35
except as noted below, the
state would need to attempt to contact the beneficiary through at least one other
modality, if available, to comply with the returned mail condition. If the state has
no other modalities available to contact the beneficiary, the state will be
compliant with the returned mail condition if it has satisfied the condition to
obtain up-to-date contact information from all beneficiaries. Importantly, if the
notice is mailed to the forwarding address and is not returned to the state, the
returned mail condition no longer applies because the original mailing has been
completed and is no longer considered to be returned. Under these circumstances,
no additional outreach is required.
If a state is unable to or does not send the correspondence to the forwarding
address, the state must attempt to contact the beneficiary through two modalities,
if available, prior to termination. If the state only has one other mode of contact
available, such as only an email address, the state must make a good faith effort to
contact the beneficiary using that one modality. If the state does not send the
notice to the forwarding address and does not have two other modes of contact,
the state will need to document in their unwinding operational plan why it is
unable to send notices to a forwarding address.
• Lack of Alternative Contact Information: A state that has satisfied the condition
described in section 6008(f)(2)(B) of the FFCRA to attempt to obtain up-to-date contact
information for other modalities but was not able to obtain such alternative contact
35
States may request section 1902(e)(14)(A) waiver authority to update a beneficiary’s address information from the
USPS without first reaching out to confirm the change with the individual.
Page 15 – State Health Official Letter
information for a given beneficiary will not violate the returned mail condition due to not
reaching out to the beneficiary through another modality as long as the state has taken the
steps outlined above to reach the individual by mail (i.e., resending the notice to a
corrected address, if applicable, and sending the notice to a forwarding address, if
provided and the state is able to do so). For example, if after complying with the
condition described in section 6008(f)(2)(B), the only contact information available to the
state is the address in the individual’s case record, and the state does not have a phone
number, email address or other means to contact the individual, the state would be in
compliance with section 6008(f)(2)(C). If a state has documented in its unwinding
operational plan why it is unable to mail a notice to a forwarding address and has no
other modalities available, the state has complied with the returned mail condition.
• Document Returned Mail Policies in Unwinding Operational Plan: States must document
in their unwinding operational plans their process for undertaking a good-faith effort to
contact individuals using more than one modality prior to disenrollment on the basis of
returned mail. States must also document their inability to send mail to a forwarding
address, if applicable.
Attempting to reach beneficiaries through other modalities after beneficiary mail is returned in
response to a redetermination of eligibility is necessary to satisfy the returned mail condition,
and it may also help reduce procedural denials and churn. In addition, MCOs are an important
tool for conducting outreach to individuals when a state is not able to reach a beneficiary. CMS
strongly encourages states that contract with MCOs to work with them on other avenues to reach
and support eligible beneficiaries in maintaining coverage through Medicaid or the
Marketplace.
36
To ensure beneficiaries are able to complete the renewal process, states should
ensure that, if they successfully contact an individual after receiving beneficiary returned mail,
the beneficiary receives any necessary renewal forms at their correct address and has sufficient
time to return the renewal form and complete the renewal process.
Additional Considerations and Process for Claiming the Temporary FMAP Increase
State Attestation of Compliance
As discussed above, states may claim federal financial participation (FFP) associated with the
temporary FMAP increase under section 6008 of the FFCRA, as amended by section 5131(a),
after March 31, 2023, only if they meet the conditions described in subsections 6008(b)(1), (2),
(4), and 6008(f). While states are ineligible for the temporary FMAP increase unless they meet
these conditions, CMS will not require that states submit a demonstration of compliance prior to
drawing FFP associated with the temporary FMAP increase. Each state should be aware that
when it draws FFP associated with the temporary FMAP increase in the Payment Management
System (PMS), it is attesting that:
(1) It is eligible for the temporary FMAP increase;
(2) The expenditures for which it is drawing FFP are those for which the temporary FMAP
increase is applicable; and
36
For a detailed discussion on engaging managed care plans, see: CMS. (January 2023). Strategic Approaches to
Engaging Managed Care Plans to Maximize Continuity of Coverage as States Resume Normal Eligibility and
Enrollment Operations.
Available at: https://www.medicaid.gov/resources-for-states/downloads/health-plan-
strategy.pdf.

Page 16 – State Health Official Letter
(3) It meets the applicable conditions in section 6008(b) and (f) of the FFCRA for claiming
the temporary FMAP increase.
To minimize the need for separate review, avoid state burden, and expedite providing federal
matching funds to states, CMS will indicate in each grant award letter it sends to states that, by
drawing down the funds, the state is attesting that it is in compliance with all federal
requirements, including that the state meets all applicable conditions for receiving the temporary
FMAP increase, subject to expenditure review. This is the same process states have been using
to draw FFP associated with the temporary FMAP increase under FFCRA section 6008 since the
temporary FMAP increase became available.
Further, CMS intends to engage states to provide technical assistance regarding compliance and,
as necessary, will request additional information to assess compliance. States may propose
additional strategies and mitigation plans to achieve compliance with section 6008(f)(2) of the
FFCRA for purposes of claiming the temporary FMAP increase. For example, we understand
that many states face challenges in conducting ex parte renewals for some or all of their
populations. These states can adopt alternative strategies, such as section 1902(e)(14)(A) waiver
strategies, including using the Supplemental Nutrition Assistance Program (SNAP)-based
income enrollment strategy, in order to satisfy the conditions described in section 6008(f)(2) of
the FFCRA. As noted above, states can refer to SHO #22-001 or the section 1902(e)(14)(A)
waivers portion of the CMS unwinding website for details on section 1902(e)(14)(A) alternative
renewal strategies that CMS can authorize and how to submit a request for such authorization to
CMS.
CMS is also considering additional strategies to support states seeking to adopt alternative CMS-
approved strategies to satisfy the conditions in section 6008(f)(2) of the FFCRA needed to claim
the temporary FMAP increase between April 1, 2023, and December 31, 2023. States interested
in using any alternative strategies should contact their CMS state lead.
If CMS determines that a state that drew down FFP associated with the temporary FMAP
increase did not satisfy the conditions under section 6008(b) and (f) of the FFCRA, as modified
by section 5131, during a quarter in which those conditions applied, the state will be required to
return the FFP associated with the temporary FMAP increase for any quarter when it did not
qualify.
Process for Claiming FFP Associated with the Temporary FMAP Increase
Qualifying states can claim the FFP associated with the temporary FMAP increase for qualifying
expenditures on a quarterly basis using the Form CMS-64 submission in the automated Medicaid
Budget and Expenditure System (MBES).
37
States should ensure that their quarterly and, if
applicable, supplemental budget request on the Form CMS-37 for the relevant period clearly
indicates whether the state is requesting FFP associated with the temporary FMAP increase and
the amount of FFP. CMS will ensure that the MBES and Medicaid and CHIP Financial System
37
In addition to the attestation outlined above within the grant award, states certify on the Form CMS-64 that they
are claiming expenditures under the Medicaid program (and as applicable, under CHIP) only if the expenditures are
allowable in accordance with applicable implementing federal, state, and local statutes, regulations, policies, and the
state plan.

Page 17 – State Health Official Letter
(MACFin) are updated to enable state reporting, as applicable.
Beginning April 1, 2023, the temporary FMAP increase is available for each quarter’s qualifying
medical assistance expenditures until (and including) December 31, 2023, for states that have
met conditions in section 6008(b) and the new section 6008(f) of the FFCRA (discussed above).
States should follow existing federal policy regarding the particular match rates that are available
for a given quarter. Per 45 CFR § 95.13(b), the applicable FMAP is based on date of payment,
not date of service, for current quarter original expenditures. The FMAP applicable to
expenditures for all prior period adjustments should be the FMAP at which the original
expenditure was claimed, consistent with 45 CFR § 95.13(b). States must report overpayments
and collections at the same match rate at which the expenditures were originally claimed,
including when the original rate incorporated the FFCRA FMAP increase. Therefore, if a
Medicaid expenditure was claimed using the FFCRA FMAP increase, the returned federal share
of any recoveries associated with that expenditure would reflect that same FMAP increase.
Consistent with existing requirements at 42 CFR § 433.32(a), states must document expenditures
to ensure a clear audit trail, including by isolating expenditures that are matched at increased
FMAPs. CMS will conduct oversight to ensure that state expenditures are allowable and
accurate, including with respect to the matching rate claimed.
Please note that under the timely claims filing requirement at section 1132 of the Act and 45
CFR part 95 subpart A, states must claim federal Medicaid matching funds for qualifying
expenditures within the two-year time limit unless they meet one of the four exceptions (see 45
CFR § 95.19).
Reporting Requirements
States regularly provide a variety of data about Medicaid and CHIP applications, enrollment, and
call-center activity through the submission of the Medicaid and CHIP Eligibility and Enrollment
Performance Indicator data and the Transformed Medicaid Statistical Information System (T-
MSIS) data, as well as other reporting mechanisms. SHO #22-001 announced that states would
be required to submit additional data through a new reporting tool as they restore routine
eligibility and enrollment operations, including resuming renewals, after the Medicaid
continuous enrollment condition ends. In March 2022, CMS released a new Unwinding
Eligibility and Enrollment Data Reporting Template (“Unwinding Data Report”)
38
that states
will use to submit a baseline data report and ongoing monthly reports during a state’s unwinding
period.
39
States that operate a State-based Marketplace (SBM) with its own eligibility and
enrollment platform also submit QHP and BHP enrollment metrics (“State-based Marketplace
(SBM) Priority Metrics”) to CMS, as required under 45 CFR 155.1200(a)(3). CMS anticipates
38
The Unwinding Data Report and Unwinding Data Report Specifications are available at
www.Medicaid.gov/unwinding. States will submit their baseline and monthly Unwinding Data Reports through the
same portal used to submit Performance Indicator data, which is available at https://sdis.medicaid.gov/user/login.
39
For an explanation that states must submit baseline unwinding data by February 8, 2023, March 8, 2023, or April
8, 2023, depending on when a state initiates renewals, see: CMS. (2023). Key Dates Related to Medicaid Continuous
Enrollment Condition Provisions in the Consolidated Appropriations Act, 2023 [CMCS Informational Bulletin].
Available at: https://www.medicaid.gov/federal-policy-guidance/downloads/cib010523.pdf.

Page 18 – State Health Official Letter
that SBMs with their own eligibility and enrollment platforms will use these existing reports to
submit monthly data during a state’s unwinding period.
New section 1902(tt)(1) of the Act, as added by section 5131(b), requires that, during the period
that begins on April 1, 2023, and ends on June 30, 2024, states submit to CMS, and CMS makes
public, certain monthly data about activities related to eligibility determinations and
redeterminations conducted during that same period. CMS currently believes that all the data
states must report under these new reporting requirements are included in existing data sources,
including the unwinding data report and SBM priority metrics. As such, CMS does not
anticipate that states will need to submit a separate report (or additional reporting) to CMS to
comply with section 1902(tt)(1) of the Act. Rather, CMS anticipates that states will be able to
submit the data metrics required under section 1902(tt)(1) through the appropriate existing CMS
data reporting tool. CMS reserves the right to add reporting metrics, if needed, in the future. As
needed, CMS will provide states additional guidance and information on the data specifications
for metrics reported to satisfy the reporting requirements under section 1902(tt)(1) of the Act.
While CMS currently believes that states can meet the reporting requirements in section
1902(tt)(1) of the Act through existing reports and reporting tools, the timing of certain state
reporting may need to change to satisfy the timeline under section 1902(tt)(1) of the Act.
Specifically, states will need to submit monthly unwinding data reports through June 30, 2024.
This timeline reflects a change from previous guidance regarding unwinding data, as some states
may submit monthly unwinding data for a period that exceeds 14 months, depending on when
the state initiates its first batch of renewals.
40
As noted, with regard to the reporting of data under section 1902(tt)(1)(D), CMS anticipates that
states with Marketplaces that operate their own eligibility and enrollment platform will use
existing reports to satisfy this requirement. Additionally, for states with Marketplaces that use
the Federal eligibility and enrollment platform, CMS expects to report the required data on
behalf of states, to the extent possible. CMS therefore does not anticipate that states with
Marketplaces that use the Federal eligibility and enrollment platform will need to report any
additional data to satisfy this requirement.
CMS will publish all data that is reported to comply with section 1902(tt)(1) of the Act.
However, for a number of reasons, Medicaid and CHIP data sent to Marketplaces that use the
Federal eligibility and enrollment platform
41
do not, on their own, specify when an individual’s
account has been transferred to the Marketplace because that person is not eligible for, or is
losing, Medicaid or CHIP coverage. CMS intends to explore using additional data sources to
determine and report which account transfers to Marketplaces that use the Federal eligibility and
enrollment platform result from Medicaid or CHIP redeterminations. To publish data that
identifies which of these account transfers result from Medicaid or CHIP redeterminations, CMS
might have to make estimates based on several administrative data sets, which limits both the
40
CMS. (March 3, 2022). Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload
in Medicaid, the Children’s Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon
Conclusion of the COVID-19 Public Health Emergency [State Health Official Letter #22-001]. Available at
https://www.medicaid.gov/federal-policy-guidance/downloads/sho22001.pdf.
41
All Marketplaces that use the Federal eligibility and enrollment platform have eligibility systems that are not
integrated with Medicaid or CHIP.
Page 19 – State Health Official Letter
accuracy and timeliness of public reporting. Working within these constraints, CMS expects to
make these account transfer data public as expeditiously as possible.
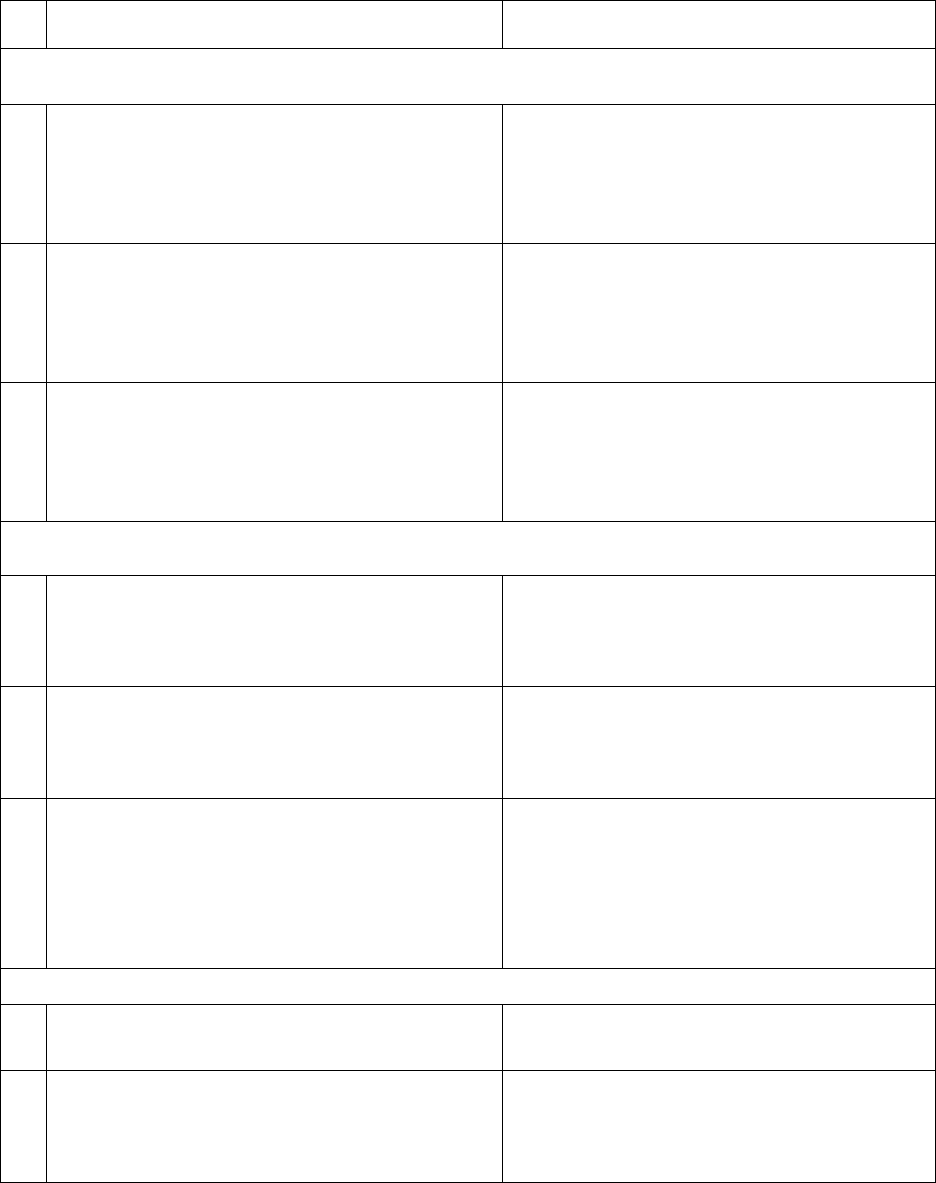
In Table 2, CMS identifies each reporting element under section 1902(tt)(1) of the Act and the
data source that CMS anticipates that states (or the Marketplaces that use the Federal platform)
will use to satisfy each element.
Table 2. Reporting Elements Under Section 1902(tt)(1) for the Period from April 1, 2023,
through June 30, 2024, and Corresponding Data Sources
Reporting Element
Data Source
Apply to All States
1
Total number of Medicaid and CHIP
beneficiaries for whom a renewal was
initiated
Unwinding Data Report, Monthly Metric
4
42
2
Total number of Medicaid and CHIP
beneficiaries whose Medicaid or CHIP
coverage is renewed
Unwinding Data Report, Monthly Metric
5a
3
Of the Medicaid and CHIP beneficiaries
whose Medicaid or CHIP coverage is
renewed, those whose coverage is renewed
on an ex-parte basis
Unwinding Data Report, Monthly Metric
5a(1)
4
Total number of individuals whose coverage
for Medicaid or CHIP was terminated
Unwinding Data Report, Monthly Metric
5b and Unwinding Data Report, Monthly
Metric 5c
5
Total number of individuals whose coverage
for Medicaid or CHIP was terminated for
procedural reasons
Unwinding Data Report, Monthly Metric
5c
6
Total number of beneficiaries who were
enrolled in a separate CHIP
T-MSIS, CHIP-CODE
43
7
For each state call center, total call center
volume
Medicaid and CHIP Eligibility and
Enrollment Performance, Indicator 1
44
8
For each state call center, average wait times
Medicaid and CHIP Eligibility and
Enrollment Performance, Indicator 2
9
For each state call center, average
abandonment rate
Medicaid and CHIP Eligibility and
Enrollment Performance, Indicator 3
42
Unwinding Data Report Specifications are available at: https://www.medicaid.gov/sites/default/files/2022-
12/unwinding-data-specifications-dec-2022.pdf.
43
The T-MSIS Data Dictionary is available at: https://www.medicaid.gov/medicaid/data-
systems/macbis/transformed-medicaid-statistical-information-system-t-msis/t-msis-data-dictionary/index.html.
44
The Performance Indicator Data Dictionary is available on the Performance Indicator Technical Assistance page
at: https://www.medicaid.gov/medicaid/national-medicaid-chip-program-information/medicaid-chip-enrollment-
data/performance-indicator-technical-assistance/index.html.
Page 20 – State Health Official Letter
Reporting Element
Data Source
Apply to Marketplaces that use the Federal eligibility and enrollment platform (Non-
Integrated Eligibility System)
10
Total number of individuals whose accounts
are received at the Marketplace from the
state Medicaid/CHIP agency due to a
Medicaid/CHIP redetermination
Marketplaces that use the Federal
eligibility and enrollment platform
(Federally-facilitated Marketplaces and
State-based Marketplaces on the Federal
platform); states do not need to report
11
In the accounts received at the Marketplace
due to a Medicaid/CHIP redetermination, the
number of individuals who apply for and are
determined eligible for a QHP
Marketplaces that use the Federal
eligibility and enrollment platform
(Federally-facilitated Marketplaces and
State-based Marketplaces on the Federal
platform); states do not need to report
12
In the accounts received at the Marketplace
due to a Medicaid/CHIP redetermination and
who apply for and are determined eligible
for a QHP, the number of individuals who
select a plan
Marketplaces that use the Federal
eligibility and enrollment platform
(Federally-facilitated Marketplaces and
State-based Marketplaces on the Federal
platform); states do not need to report
Apply to SBMs with their own platforms that use a Non-Integrated Eligibility System
13
Number of individuals whose accounts are
received by the SBM or BHP
SBM Priority Metrics,
Monthly Metrics 7a and 7b
14
Number of individuals whose accounts are
received by the SBM or BHP and are
determined eligible for a QHP or BHP
SBM Priority Metrics,
Monthly Metric 9a and 172a
15
Number of individuals whose accounts are
received by the SBM or BHP and are
determined eligible for a QHP or a BHP who
make a QHP plan selection or are enrolled in
a BHP
SBM Priority Metrics,
Monthly Metric 1a and 169a
Apply to SBMs with Integrated Eligibility System
16
Number of individuals who are determined
eligible for a QHP or a BHP
SBM Priority Metrics,
Monthly Metric 9a and 172a
17
SBMs with Integrated Eligibility System:
Number of individuals who are determined
eligible for a QHP or BHP and make a QHP
plan selection or are enrolled in a BHP
SBM Priority Metrics,
Monthly Metric 1a and 169a
As noted in SHO #22-001, states may be expected to report additional data and/or report
information more frequently in cases where additional CMS oversight or enforcement is needed,
such as when reported data indicate states are not meeting the timelines to initiate and complete
Page 21 – State Health Official Letter
renewals for total caseload during the unwinding period. CMS is available to provide technical
assistance to ensure states understand metric data specifications and are able to fulfill statutory
reporting requirements.
Monitoring State Progress and Corrective Action
In SHO #22-001, CMS announced that it will monitor states’ compliance with reporting required
data and meeting timelines relating to initiating and completing required eligibility and
enrollment actions during the state’s unwinding period. Where reported data or other
information indicates that states are not meeting unwinding timelines in SHO #22-001 and
subsequent guidance, or in circumstances where data or other information demonstrate other
potential compliance issues – including potential erroneous disenrollment of eligible
beneficiaries – states may be expected to report additional data, including the state’s unwinding
operational plan, and/or report information more frequently.
45
As explained in SHO #22-001,
states that do not resolve their pending eligibility and enrollment actions within the timelines
specified may be required to submit a corrective action plan (CAP) to CMS outlining strategies
and a timeline to come into compliance with federal requirements.
In addition to enforcement and oversight authorities CMS already has under existing laws and
regulations, section 1902(tt)(2) of the Act (added by section 5131(b)) includes new enforcement
mechanisms for CMS to apply if CMS determines that a state is not in compliance with the
reporting requirements in section 1902(tt)(1) of the Act, or if CMS determines that a state is non-
compliant with federal eligibility redetermination requirements during the period that begins on
April 1, 2023 and ends on June 30, 2024. Specifically:
• FMAP Reduction for Failure to Comply with Section 1902(tt)(1) Reporting
Requirements: If a state does not satisfy the reporting requirements in section 1902(tt)(1)
of the Act during any fiscal quarter that occurs during the period that begins on July 1,
2023, and ends on June 30, 2024, the FMAP determined for the state for the quarter
under section 1905(b) shall be reduced by the number of percentage points (not to
exceed 1 percentage point) equal to the product of 0.25 percentage points and the
number of fiscal quarters during such period for which the state has failed to satisfy such
requirements. CMS will apply FMAP reductions using MBES and intends to provide
more information in future guidance regarding the FMAP reduction process.
• Corrective Action for Failure to Comply with Section 1902(tt)(1) Reporting
Requirements or Federal Eligibility Redetermination Requirements: If a state has been
determined by CMS to be out of compliance with the reporting requirements in section
1902(tt)(1) and/or federal eligibility redetermination requirements during the period that
begins on April 1, 2023, and ends on June 30, 2024, CMS may require the state to submit
and implement a CAP. Under section 1902(tt)(2)(B) of the Act, not later than 14 days
45
For explanation that unwinding operational plans must be made available to CMS upon request, see both: CMS.
(December 22, 2020). Planning for the Resumption of Normal State Medicaid, Children’s Health Insurance
Program (CHIP), and Basic Health Program (BHP) Operations Upon Conclusion of the COVID-19 Public Health
Emergency [State Health Official Letter #20-004], and CMS. (March 3, 2022). Promoting Continuity of Coverage
and Distributing Eligibility and Enrollment Workload in Medicaid, the Children’s Health Insurance Program
(CHIP), and Basic Health Program (BHP) Upon Conclusion of the COVID-19 Public Health Emergency [State
Health Official Letter #22-001]. These guidance documents are available at https://www.medicaid.gov/federal-
policy-guidance/downloads/sho20004.pdf and https://www.medicaid.gov/federal-policy-
guidance/downloads/sho22001.pdf.

Page 22 – State Health Official Letter
after receiving a written notice that the state is out of compliance, the state must submit a
CAP to CMS. The state must receive CMS approval for its proposed CAP not later than
21 days after submitting it to CMS. The state must initiate implementation of the plan
not later than 14 days after CMS approves it.
• Suspension of Terminations for Procedural Reasons and Civil Monetary Penalties: If a
state fails to submit or implement an approved CAP in accordance with the timelines and
requirements described above, CMS may require a state under section 1902(tt)(2) to
suspend some or all terminations of Medicaid eligibility that are for procedural reasons
until the state takes appropriate corrective action (as determined by CMS), and/or may
impose on that state a civil money penalty of not more than $100,000 for each day that
the state is not in compliance.
Implications for Optional COVID-19 Group, Waivers Approved Under Section
1902(e)(14)(A) of the Act, and Other Authorities
Even though section 5131 does not address when the COVID-19 PHE will end, the changes in
the CAA, 2023 could have implications for certain COVID-19 PHE-related flexibilities and
authorities. We outline below certain implications that the changes made by section 5131 may
have for state redeterminations for the optional COVID-19 group and for waivers already
approved under section 1902(e)(14)(A) of the Act that states intended to have in place when the
continuous enrollment condition ends. Certain other COVID-19 PHE related flexibilities can
remain in effect for the duration of the COVID-19 PHE, including, but not limited to, Medicaid
and CHIP section 1135 waivers and disaster SPAs. As a reminder, the requirements under
sections 9811 and 9821 of the ARP apply until the last day of the first calendar quarter that
begins one year after the COVID-19 PHE ends. These ARP requirements include providing
coverage, without cost sharing, of: COVID-19 vaccinations; COVID-19 testing; treatments for
COVID-19, including specialized equipment and therapies (including preventive therapies); and,
when certain conditions are met, treatment of conditions that may seriously complicate the
treatment of COVID-19. The CAA, 2023 generally does not impact authorities tied to the end of
the COVID-19 PHE (e.g., 1135 waivers, disaster relief SPAs).
Optional COVID-19 Group
Section 6004(a)(3) of the FFCRA created a new optional COVID-19 Medicaid eligibility group
at section 1902(a)(10)(A)(ii)(XXIII) of the Act. Section 9811(a)(2) of the ARP amended the
language following section 1902(a)(10)(G) of the Act describing the coverage that must be
provided to the optional COVID-19 eligibility group. Federal authority for this eligibility group
expires on the last day of the COVID-19 PHE. States that adopted the optional COVID-19
group may continue to provide coverage to this eligibility group while the COVID-19 PHE is in
effect. While federal authority for the optional COVID-19 group was not amended in the CAA,
2023, the changes made by the CAA, 2023 make it possible for the continuous enrollment
condition to expire before the end of the COVID-19 PHE, which will have implications for
redeterminations of eligibility for beneficiaries enrolled in the optional COVID-19 group.
If the COVID-19 PHE ends after the continuous enrollment condition ends, states that opt to
continue covering the optional COVID-19 eligibility group after the continuous enrollment
condition ends on March 31, 2023, will need to establish a process to initiate renewals for
Page 23 – State Health Official Letter
individuals enrolled in the group following the end of the continuous enrollment condition.
CMS recognizes that states that adopted a streamlined enrollment process outside of their
eligibility system to implement the optional COVID-19 group (e.g., using a separate application
to facilitate enrollment) may face operational challenges initiating renewals during unwinding
and may not have sufficient information to complete a full renewal of eligibility, including
assessing all other bases of eligibility. These states may choose to deprioritize redeterminations
for this group during the unwinding period while they work to establish such a process. CMS is
available to provide technical assistance if needed.
When the COVID-19 PHE ends (or earlier if the state initiates a regular renewal for beneficiaries
in the optional COVID-19 group after the state begins its unwinding period and before the end of
the COVID-19 PHE), states must redetermine eligibility on all bases for beneficiaries who are
enrolled in the optional COVID-19 group and sunset the eligibility group. Guidance to assist
states with meeting the requirements to redetermine eligibility on all bases when authority for the
group expires can be found in the “Ending Coverage in the Optional COVID-19 Group Preparing
States for the End of the Public Health Emergency” slide deck.
46
States are reminded that they
must stop claiming FFP for services provided to individuals enrolled in this group once the
COVID-19 PHE ends.
CMS will provide states that adopted the optional COVID-19 group with additional guidance on
redetermining eligibility for these beneficiaries during the unwinding period if the COVID-19
PHE remains in effect. States that opt to end coverage for this optional eligibility group before
the COVID-19 PHE ends may submit a SPA to CMS. See the May 11, 2021, all-state call slides
for additional information.
47
Section 1902(e)(14)(A) Waiver Effective Dates
To support states facing significant operational issues with income and eligibility determination
systems and to protect eligible beneficiaries from inappropriate coverage losses during the
unwinding period, CMS has granted more than 40 states section 1902(e)(14)(A) waivers to
support unwinding efforts. The effective and/or expiration date of many of these approved
waivers is linked to the end of the COVID-19 PHE. Given that the CAA, 2023 has de-linked the
end of the continuous enrollment condition and the start of the unwinding period from the end of
the COVID-19 PHE, the effective dates of section 1902(e)(14)(A) waivers granted for the
purpose of assisting states in their unwinding efforts may no longer align with states’ unwinding
timelines. To minimize administrative burden on states as they begin their unwinding process,
CMS is providing the following guidance to allow states to implement modified effective dates,
without needing to submit a revised request to CMS. States should, however, document any
change in the effective date of a section 1902(e)(14)(A) waiver in their records and maintain a
copy of this guidance.
• Approved section 1902(e)(14)(A) waivers with a start date tied to the COVID-19 PHE:
Some states elected to make a section 1902(e)(14)(A) waiver effective in the month the
46
CMS. (October 2022). Ending Coverage in the Optional COVID-19 Group Preparing States for the End of the
Public Health Emergency. Available at https://www.medicaid.gov/resources-for-states/downloads/ending-covrg-
optnl-covid-grp-guidance.pdf.
47
CMS. (May 11, 2021). All State Medicaid and CHIP Call. Available at
https://www.medicaid.gov/sites/default/files/2021-05/allstatecall-20210511.pdf.

Page 24 – State Health Official Letter
COVID-19 PHE ends or the month following the end of the COVID-19 PHE. These
states can now use the date of the end of the continuous enrollment condition (March 31,
2023) in lieu of the end date of the COVID-19 PHE. For example, if a state received
CMS approval to begin a section 1902(e)(14)(A) waiver in the month after the end of the
COVID-19 PHE, the state’s new start date would be the month after the end of the
continuous enrollment provision (i.e., April 1, 2023).
• Approved section 1902(e)(14)(A) waivers with an expiration date tied to the COVID-19
PHE: Some states have section 1902(e)(14)(A) waivers that expire a certain number of
months after the COVID-19 PHE ends. These states can now replace the date that the
COVID-19 PHE ends with the date that the continuous enrollment conditions ends
(March 31, 2023) for purposes of these waivers. For example, if a state’s approved
section 1902(e)(14)(A) waiver ends 14 months after the month that the COVID-19 PHE
ends, the state can elect to change the end date to 14 months after the end of the
continuous enrollment condition (i.e., May 31, 2024).
• Approved section 1902(e)(14)(A) waivers with an effective and/or expiration date tied to
the state’s unwinding period: Some states have waivers that are effective when their
state-specific unwinding period begins. Additionally, some states have waivers that end
12 months or longer after the state-specific unwinding period, as defined in SHO #22-
001. These dates do not need to be adjusted as a result of the CAA, 2023.
ARP Section 9817 Maintenance of Effort Requirements
Section 9817 of the ARP provides qualifying states with a temporary 10 percentage point FMAP
increase for certain Medicaid home and community-based services (HCBS) expenditures
provided between April 1, 2021 and March 31, 2022. Under ARP section 9817, states must use
the federal funds attributable to the increased FMAP to supplement, not supplant, state funds
expended for Medicaid HCBS in effect as of April 1, 2021. As discussed in SMD #21-003
48
and
SMD #22-002,
49
states are expected to demonstrate compliance with this requirement by
maintaining HCBS eligibility, covered services, and payment rates until the state funds
equivalent to the amount of federal funds attributable to the increased FMAP are fully expended.
The CAA, 2023 did not change ARP section 9817, including the conditions related to receiving
that FMAP increase. However, as discussed in SMD #21-003, the conditions for the FMAP
increase under ARP section 9817 do not supersede other statutory or regulatory requirements
that apply to section 1915(c) waivers, or other requirements under other provisions authorizing
HCBS. As a result, if states have implemented temporary changes to HCBS eligibility, covered
services, and/or payment rates, states are expected to retain those changes for as long as
allowable under those authorities (e.g., according to the end date approved under an Appendix K
but no later than 6 months after the COVID-19 PHE ends), but CMS will not apply penalties or
non-compliance restrictions on the receipt of the increased FMAP once the authority for those
48
CMS. (May 13, 2021). Implementation of American Rescue Plan Act of 2021 Section 9817: Additional Support
for Medicaid Home and Community-Based Services during the COVID-19 Emergency [State Medicaid Director
Letter #21-003]. Available at: https://www.medicaid.gov/federal-policy-guidance/downloads/smd21003.pdf.
49
CMS. (June 3, 2022). Updated Reporting Requirements and Extension of Deadline to Fully Expend State Funds
Under American Rescue Plan Act of 2021 Section 9817 [State Medicaid Director Letter #22-002]. Available at:
https://www.medicaid.gov/federal-policy-guidance/downloads/smd22002.pdf.

Closing
Page 25 – State Health Official Letter
temporary changes has expired or if the state needs to implement changes to comply with other
federal statutory or regulatory requirements.
As states restore routine eligibility and enrollment operations, CMS shares states’ goals of
ensuring that eligible individuals remain enrolled in Medicaid, CHIP, and/or BHP coverage, and
that individuals who are no longer eligible are able to transition seamlessly to other coverage
options (if they are eligible for those other coverage options), including Marketplace coverage.
We are committed to providing states with updated guidance and resources, as appropriate, as
well as ongoing technical assistance, to better enable states to proceed with eligibility and
enrollment work in a manner that is consistent with the changes made under the CAA, 2023, as
described in this letter. For additional information and resources, states are encouraged to review
guidance and other information posted to the Medicaid.gov/Unwinding page. States may also
submit technical assistance questions directly to [email protected].
Sincerely,
Daniel Tsai
Deputy Administrator and Director
Cc:
National Association of Medicaid Directors
National Academy for State Health Policy
National Governors Association
American Public Human Services Association
Association of State and Territorial Health Officials
Council of State Governments
National Conference of State Legislatures
Academy Health
National Association of State Alcohol and Drug Abuse Directors
