
FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 1 of 26
For the most current and official copy, check QMiS.
Sections in This Document
1. Purpose .................................................................................................................................... 2
2. Scope ....................................................................................................................................... 2
3. Responsibility............................................................................................................................ 3
4. Background............................................................................................................................... 5
5. References ............................................................................................................................... 6
6. Procedure ................................................................................................................................. 6
6.1. Overview ........................................................................................................................ 6
6.2. Auditor Qualifications ..................................................................................................... 7
6.2.1. Contract Auditor Training Requirements .......................................................... 7
6.2.2.
Auditor Training and Verification
...................................................................... 7
6.3. Contract Audit Elective ................................................................................................... 8
6.3.1. Phase II ............................................................................................................ 9
6.3.2. FDA Verification Audits .................................................................................... 9
6.3.3. Verification Audit Failure ................................................................................ 10
6.4. Audit Requirements ...................................................................................................... 10
6.4.1. Minimum Audit Requirements ........................................................................ 10
6.4.2. Contract Audit Tracker ................................................................................... 11
6.4.3. Audit Selection ............................................................................................... 11
6.4.4. Audit Reduction Request ............................................................................... 12
6.4.5. Posting of Audit Completion Data .................................................................. 13
6.5. Audit Procedures.......................................................................................................... 13
6.5.1. Human and Animal Food Contract Audits ...................................................... 13
6.5.2. Human and Animal Food Verification Audits ................................................. 14
6.5.3. Egg, Medical Device, and Other State Inspection Programs ........................ 15
6.6. Reporting Audit Findings .............................................................................................. 16
6.6.1. Human and Animal Food Contracts ............................................................... 16
6.6.2. Egg, Medical Device, and Other State Inspection Programs ......................... 16
6.7. Audit Requirement Deficiencies ................................................................................... 16
6.8. Performance Deficiencies ............................................................................................ 17
6.8.1. Individual Inspector Performance Deficiencies .............................................. 17
6.8.2. Program Performance Deficiencies ............................................................... 18
6.8.3. Documenting Performance Deficiencies ........................................................ 18

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 2 of 26
For the most current and official copy, check QMiS.
6.9. Process for Contract Modifications for Program and Performance Deficiencies ......... 19
6.10. Dispute Resolution ....................................................................................................... 20
6.11. Quality Assurance ........................................................................................................ 20
6.11.1. State Program Quality Assurance .................................................................. 20
6.11.2. Contract Program System Audit .................................................................... 20
7. Glossary/Definitions ................................................................................................................ 20
8. Records .................................................................................................................................. 22
9. Supporting Documents ........................................................................................................... 22
10. Document History ................................................................................................................... 23
11. Change History ....................................................................................................................... 24
12. Attachments ............................................................................................................................ 26
1. Purpose
The purpose of this Field Management Directive (FMD) is to delineate the
required: (1) procedures for conducting audits of state contract inspections, (2)
frequency of audits, (3) auditor training, and (4) records documenting audits.
Specific audit procedures and forms, data reporting instructions, and audit
summary report forms are included as appendices.
This FMD-76 document governs the Food and Drug Administration (FDA)
Office of Regulatory Affairs’ (ORA’s) oversight of the state contract audit
program.
2. Scope
FMD-76 applies to contract inspections in these programs:
• Human Food
• Animal Food
• Egg, Medical Device, and Other State Inspection Programs
When a contract or contract program is suspended or terminated, the audit
requirements within this FMD are similarly suspended or terminated.
This FMD does not address training requirements and procedures for
oversight of states performing inspections of mammography facilities certified
by the FDA under the Mammography Quality Standards Act of 1992 (MQSA).
This FMD does not delineate the procedures for reviewing the quality of state
contract inspection documents. ORA program divisions are encouraged to

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 3 of 26
For the most current and official copy, check QMiS.
conduct a quality assurance review of state documents as part of their quality
assurance program. Refer to SOP-000115 Management of ORA State
Contract Inspection Process. Deficiencies in state inspection reports
completed under contract are also beyond this FMD’s scope.
For this directive, the term “state” refers to both state and territorial agencies
participating in the state contract inspection program.
In situations where this directive conflicts with the agency’s contract, the
contract takes precedence.
3. Responsibility
A. Associate Commissioner of Regulatory Affairs (ACRA), Assistant
Commissioner for Human and Animal Food Operations, Assistant
Commissioner for Medical Products and Tobacco Operations
1. Ensures Program Directors (PDs) comply with FMD-76 requirements
2. Initiates actions to correct national deficiencies
B. Program Director (PD)
1. Ensures that the PD’s respective program division complies with FMD-
76 requirements
2. Reviews contract modification requests
C. Program Division Director (PDD)
1. Ensures the required numbers of audits are completed
2. Ensures documented program and performance deficiencies are
corrected
3. Ensures adequate staff are assigned to accomplish Audit Program
responsibilities
D. FDA Auditor
1. Conducts audits of state inspectors performing contract inspections
2. Trains and verifies state auditors’ performance
3. Submits audit reports to the state liaison
E. State Liaison
1. Manages the Contract Inspection Audit Program for assigned state(s)
2. Informs program division management of contract audit performance

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 4 of 26
For the most current and official copy, check QMiS.
3. Works with management and the state agency to:
a. Assign audits to the FDA employees
b. Ensure the required numbers of audits are completed and that
identified inspectors are audited
c. Document and ensure correction of individual and program
performance deficiencies
d. Ensure required documentation, including audit reports, is
completed, maintained, and distributed, as needed
F. Director, Office of Partnerships (OP)
1. Has primary oversight of the administration of the contract inspection
and associated audit program
2. Resolves disputes in audit classification findings
3. Approves audit-rate reduction requests
G. Director, OP Division of Partnership Investments and Agreements
(DPIA)
1. Reviews changes to the contract proposed by the program division
H. Project Manager (PM), OP DPIA
1. Leads oversight of the contractor’s technical performance, in
conjunction with the Contracting Officer Representative (COR) and the
state liaison
2. Reviews proposals for corrective actions
I. Contracting Officer’s Representative (COR), Office of Regulatory
Management Operations (ORMO)
1. Works with the Project Manager and others to support the contract and
provide financial oversight of a specific contract
2. Recommends contract modifications
J. Audit Program Manager, OP DPIA
1. Conducts the national system audit
2. Coordinates the audit program
K. State Auditor (Phases II and III only)
1. Conducts audits of state inspectors performing contract inspections.
2. Trains and verifies the performance of state auditors.

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 5 of 26
For the most current and official copy, check QMiS.
3. Submits audit report or memorandum to the state liaison for review
through the state agency.
4. Background
The original FMD established procedures for joint ORA‒state inspections and
independent audit inspections for the human food, medicated feed (currently
animal food), and interstate travel programs. In 1982, ORA revised the FMD,
combining the general procedures for all current programs into one document.
In 1999, ORA added instructions for auditing food sanitation and medicated
feed contract inspections.
In June 2000, the Department of Health and Human Services, Office of
Inspector General (OIG), published the results of its evaluation of the FDA’s
oversight of food firm inspections conducted by states contracted to do so. The
report recommended that the FDA take steps to address shortcomings in its
system of oversight. In 2006, ORA revised this FMD to incorporate the OIG’s
recommendations and to improve the oversight of human food, animal food,
and other inspections done under contract by the states.
ORA updated this FMD in 2012, strengthening the processes for ensuring the
audit rates are met and identifying and correcting systemic problems identified
during the audits. The revision expanded the oversight of egg contract
inspections and added procedures and computer-automated forms to improve
reporting and tracking of completed audits.
ORA had based evaluations on audit rate (as a percentage of the total number
of contract inspections), but its May 2015 revision of this FMD changed to an
inspector-focused evaluation. This change makes this audit program
consistent with the requirements for Manufactured Food Regulatory Program
Standards (MFRPS) and the Animal Feed Regulatory Program Standards
(AFRPS) and ensures that each inspector performing contract work is
periodically evaluated.
ORA updated this FMD in March 2019 to reflect the organizational structural
changes stemming from the May 2017 implementation of Program Alignment,
which transformed ORA programs and offices from being geographic-district
focused to being regulated-commodity focused (i.e., specialized for specific
FDA-regulated commodities). Terminology was updated to reflect changes in
regulations and included an elective for the Animal Food programs to
participate in the audit phases, previously reserved for Human Food programs.
Additional changes were intended, where possible, to align the state contract
audit program with the other audit programs. In addition, to improve oversight,

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 6 of 26
For the most current and official copy, check QMiS.
ORA added audit questions — regarding Limited Scope and Modified
Preventive Controls — to the human and animal food program audit forms.
ORA also added an audit option for the egg program.
Current changes (Rev 05) are necessary to incorporate the revised human
food audit form (FDA Form 3610H) to incorporate new questions, develop an
electronic form, and to automate the audit form routing process. The form was
revised to have questions that are specific to each individual human food
inspection type and to include new questions that cover Full Scope Preventive
Control inspections.
5. References
SOP-000115 Management of ORA State Contract Inspection Process
Contract Statement of Work (SOW)
ORA Records Management Program
SOP-000321 OHAFO State Contract Inspection Report Review Process
Procedure
FORM-000585 OHAFO State Contract Report Quality Factor Checklist
6. Procedure
6.1. Overview
The FDA audits contract inspectors to ensure that the quality of state-
conducted inspections purchased through contracts is adequate and complies
with the contract requirements. The Contract Inspection Audit Program
(hereafter known as the Audit Program) is a standardized system of formal
field audits conducted by qualified FDA and state auditors at a minimum
frequency or audit rate.
The Audit Program is implemented in three phases:
1. Phase I: The program division is responsible for conducting the
minimum number of contract audits.
2. Phase II: The program division and state agency share responsibility for
conducting the minimum number of contract audits to meet the audit
rate.
3. Phase III: The state agency assumes full responsibility for conducting
the minimum number of contract audits to meet the audit rate.

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 7 of 26
For the most current and official copy, check QMiS.
NOTE: Phases II and III apply only to the human food and animal food
contracts. Section 6.3 provides instructions for implementing Phases II and III
of the Audit Program.
6.2. Auditor Qualifications
To conduct contract audits, the FDA or state auditor must have completed the
auditor training below, the training courses specified in the contract, and all
training course prerequisites, as required by Office of Training Education and
Development (OTED). The auditors must have experience in conducting
inspections in the program area and understand the relevant FDA compliance
program and regulations. Additional program qualifications for state auditors
are listed in Section 6.2.1.
6.2.1. Contract Auditor Training Requirements
A. All Human Food Contract Auditors
1. FD320W100 - State Food Contract Audit Course
2. Program specific training
B. All Animal Food Contract Auditors
1. VM212W100 - State Bovine Spongiform Encephalopathy (BSE)/Feed
Establishment Contract Audits course
2. Program specific training
6.2.2.
Auditor Training and Verification
A. The program division and state agency develop a plan to accomplish the
training and verification audits for those state inspectors who have
completed the training requirements in Section 6.2.1 and the SOW. If
requested by the program division, the state agency provides records to
verify that state auditors have completed the training requirements.
B. The state auditor must complete one training audit and one verification
audit for each type of inspection the auditor will be responsible for auditing.
For example, to conduct audits for current Good Manufacturing Practices
(cGMP) and Seafood Hazard Analysis Critical Control Points (HACCP) the
state auditor must complete at least one training and one verification audit
for cGMP and one training and one verification audit for Seafood HACCP.
A state auditor must pass a cGMP audit to qualify as a specialty area
auditor. An audit may cover multiple areas in one inspection depending on
the scope of the inspection.
C. The FDA auditors train and verify the performance of state auditor trainees.
States with one qualified auditor may conduct the training and verification

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 8 of 26
For the most current and official copy, check QMiS.
audits for new state auditor trainees. States with two qualified state auditors
may conduct verification audits of state auditors following the Phase III
audit procedures (See Section 6.3.2). The contract audits completed during
the training and verification audits are counted toward the audit obligation.
D. One auditor should train only one state auditor trainee during a contract
inspection. The state supervisor or additional state inspectors are not
permitted to accompany the auditor during a training or verification audit.
E. During the training audit, the state auditor trainee observes the FDA or
state auditor conducting a contract inspection audit. The auditor, not the
trainee, completes Form FDA 3610H (Appendix B) or the Animal Food
Audit Form (Appendix C).
F. During the verification audit, the FDA or state auditor observes the state
auditor trainee conducting a contract audit. The state auditor trainee
completes Form FDA 3610H or Animal Food Audit Form (not the
verification auditor).
G. The original audit forms are submitted to the state liaison no later than 30
business days after the audit. The auditor follows the guidelines in
Appendix D to document the state auditor trainee’s performance during the
verification audit. The FDA sends a copy of the memorandum to the state
agency when FDA conducts the audit, and vice versa.
H. Only the state inspector, not the state auditor, reports his/her time in the
electronic State Access to Field Accomplishment and Compliance Tracking
System (eSAF). The number of hours is reported as an audit, not an
inspection. At the time data is entered in eSAF, the state data entry user
changes the Inspection Type field on the Add/Update Inspection Operation
screen from "State" to "Audit.”
6.3. Contract Audit Elective
Full implementation of the Audit Program occurs when the state agency
assumes responsibility for auditing its food (human and animal) contract
inspections. This process begins in Phase II and is completed in Phase III.
Phases II and III of the Audit Program are offered to the state agency as an
elective under the Human Food & Animal Food Contract SOWs. If the state
agency bids on this elective, an agreement (Appendix H State Implementation
Agreement and Yearend Evaluation) must be completed and signed by the
PDD and the director of the state inspection program. The state must submit
this signed agreement with its contract quote/proposal prior to award of the
contract.
At the end of the contract performance period, the program division updates
the agreement to include a year-end evaluation and a summation of the

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 9 of 26
For the most current and official copy, check QMiS.
number of audits completed. The updated agreement is emailed to the director
of the state program and to the Contract Audits mailbox
the contract performance period.
6.3.1. Phase II
Phase II occurs when the contracting agency assumes partial responsibility for
auditing its human food and animal food contract inspections. Phase III
Phase III occurs when the state agency assumes full responsibility for auditing
its human food and animal food contract inspections. The state agency must
have a quality assurance program (QAP) that requires correcting inspection or
audit performance deficiencies. The QAP must describe the remedial training
process and an internal audit of an auditor who fails to recognize: (1) deficient
performance by an auditor or inspector or (2) an inspector’s performance that
should be rated as “needs improvement,” as discussed in Section 6.8 of this
FMD.
The state lists all state auditors who can conduct contract audits for the
contract performance period when completing Appendix H, (Section V). The
state agency must audit its own auditors every 36 months, considering the
inspection priorities listed in the human food and animal food contract SOW
and the inspections performed under contract. To meet this requirement, the
state agency must have a minimum of two qualified state auditors. If during the
contract year the state agency is unable to retain a qualified auditor for
contracted specialized inspections or a minimum of two auditors, the state
remains in Phase III for the remainder of the contract year. The state agency is
moved to Phase II the following contract year and remains in Phase II until it
has a minimum of two qualified auditors trained in all areas in which contract
audits will be conducted.
6.3.2. FDA Verification Audits
For Phase II states, the FDA conducts two verification audits per auditor every
36 months. For Phase III states, the FDA conducts one of the two verification
audits, and the state conducts the second. The FDA or state auditor evaluates
a state auditor performing an audit of a state contract inspector. The FDA
auditor prioritizes evaluation of new state auditors who have not previously
been audited by the FDA. Verification audits should be conducted in a
specialized area (e.g., Seafood HACCP, Juice HACCP), whenever possible.
States in Phases II and III may count new auditor verification audits toward the
verification audit rate. Verification audits of specialized inspection types count
toward the state auditor’s verification audit rate.

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 10 of 26
For the most current and official copy, check QMiS.
The performance and documentation of a verification audit follows the
procedures in Section 6.5.2 of this FMD.
6.3.3. Verification Audit Failure
If the verification auditor assigns an overall rating of “needs improvement” in a
specialized area (e.g., Seafood HACCP, Juice HACCP), the auditor is
considered to have failed and is removed from performing audits in that
specialty area. The auditor may continue to perform audits in the cGMP area, if
approved by the program division and state program. To determine an
appropriate course of action, the state liaison must notify the Audit Program
Manager at the Contract Audits mailbox (ContractAudits@fda.hhs.gov) within
10 business days and copy the Program Division Director if the failure may
impact the contract.
The “needs improvement” audit rating counts only toward the audit rate, not
the performance rating.
In the event an auditor fails a verification audit, the inspector must undergo
another audit.
6.4. Audit Requirements
6.4.1. Minimum Audit Requirements
The minimum audit requirements to be accomplished each contract year by
the inspection program are shown in Table 1. All human food and animal food
inspectors must be audited a minimum of twice in a 36-month period. The 36-
month period is distinct to each inspector. All inspectors within a state program
are not required to be on the same 36-month cycle.
A state must complete a separate Appendix H State Implementation
Agreement and Yearend Evaluation form for each contract program in Phases
II and III and for each contract type, human food or animal food. For states in
the audit program, a state implementation agreement (Appendix H) must be
submitted with the contract proposal or option year letter. At the end of the
contract year, the state liaison completes Section IV (Planned and Completed
Audits) and Section VII (Yearend Evaluation) for the contract performance
period.
FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 11 of 26
For the most current and official copy, check QMiS.
Table 1. Audit Rate for Contract Inspection Programs
Inspection Program
Minimum Audit Rate
Human Food
1
2 audits per role (inspector and auditor) every 36 months
Animal Food
2
2 audits per role (inspector and auditor) every 36 months
Egg
One joint audit inspection or audit option audit per
performance year
Medical Device, and
Other State Inspection
Programs
One joint audit inspection of each inspection program per
performance year
By the end of the second quarter of the contract performance period, if less
than 25 percent of the required audits of a state’s human food or animal food
contract inspections have been completed, the Audit Program Manager emails
a status reminder to the state liaison.
6.4.2. Contract Audit Tracker
The state liaison must enter the following information in the Contract Audit
Tracker for all human food and animal food contracts:
• Number of inspections to be performed
• Names of inspectors performing contract inspections
• Names of auditors performing contract audits (Phase II & III)
• Number of audits to be performed during the contract year
6.4.3. Audit Selection
The program division and the state agency managers develop an audit
schedule when assigning the firms to be inspected under contract by the state
agency. Firm selection should be based on the inspection priorities listed in the
SOW and the contractual obligation of the contractor including the state’s
implementation of the contract audit program.
The types of contract inspections conducted by an inspector must be
considered when scheduling an audit. The most complex inspections should
1
Includes low-acid canned foods and acidified foods, Preventive Controls, Seafood HACCP, and Juice HACCP inspections,
where appropriate.
2
Includes BSE only for inspections at licensed and non-licensed feed-mill inspections.

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 12 of 26
For the most current and official copy, check QMiS.
be audited. The state or program division must rotate inspection types to
ensure the state inspector is audited in all applicable program areas (i.e.,
Seafood HACCP, Juice HACCP, Low-Acid Canned Foods (LACF), Preventive
Controls (PC), medicated feed, BSE). If an inspector is trained in multiple
specialized inspection areas, at least one of the audits in the 36-month period
should be in a specialized area. Refer to Appendix D.
A training or verification audit is counted as one audit, because a single
contract audit is being performed during a training or verification audit of a
state auditor.
Program divisions may schedule joint inspections as needed for training
purposes. These approved joint inspections count toward the FMD-76 audit
requirement when an audit form is completed.
If a state auditor also performs inspections under the contract, the state auditor
must be audited as an inspector as well (see Table 1).
6.4.4. Audit Reduction Request
In limited circumstances, a state agency may request a reduction in the
number of audits to be conducted in a contract year. Reductions are not given
when a program division or state agency fails to conduct the required audits.
When evaluating such a request, OP considers the number and type of
contract inspections, the number of state inspectors conducting the contract
inspections, and previous individual and program performance. The OP
director has final discretion in granting a reduction. If the request is not
approved, OP provides an explanation and the program division and state
agency have an opportunity to provide additional information.
Audit reduction requests for human and animal food are requested using the
Request for Audit Reduction Form (see Appendix I). Audit reduction requests
for other contract types are made by memoranda.
The program division must submit the request for audit reduction via email to
of the contract performance period. Requests may be submitted later if
conditions change during the contract performance period. Submission of a
separate form for each program is required to request an audit reduction in
both human and animal food. A response will be provided by OP within 20
business days of receiving the request. The audit rate reduction is valid for the
specified contract performance period and can be canceled if conditions
change.

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 13 of 26
For the most current and official copy, check QMiS.
The state and program division understand that the audit reduction is valid for
the contract performance period specified in this agreement. If any of the
following conditions occur, the audit reduction is reevaluated:
1. The state changes the number of inspectors conducting contract
inspections.
2. An inspector/auditor receives an overall rating of “needs improvement”;
and/or
3. The contract is significantly modified (e.g., increases in the specialized
inspections or number of inspections).
The program division and state are responsible for reporting any changes to
the information provided on the request form (Appendix I). The state notifies
the program division of any changes within 10 working days. The program
division is responsible for reporting the changes to OP within 10 working days.
6.4.5. Posting of Audit Completion Data
The annual summary audit completion data for each state program is posted
by OP on the FDA internet site. Information includes:
• Number of contract inspections completed
• Number of audits completed
• State program overall audit performance rating (see Section 6.8.2)
6.5. Audit Procedures
This section describes the references, audit requirements, performance
documentation and factors, and timeframes for submitting performance
documents for all contract inspection programs.
6.5.1. Human and Animal Food Contract Audits
A. Audit Requirements - Every inspector must be audited a minimum of
twice in 36 months for each role they serve.
B. Timeframe for Submitting Performance Documentation
1. When the FDA conducts the audit, the FDA auditor sends a copy of the
audit form to the state liaison. The state liaison sends the audit
information to the state agency no later than 30 business days after the
audit is completed.
2. When the state agency conducts the audit, the state agency sends the
original audit form to the state liaison no later than 30 business days
after the audit is completed.

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 14 of 26
For the most current and official copy, check QMiS.
3. If a contract audit is rated as “needs improvement,” the state liaison or
state agency must notify the other party no later than 10 business days
after the audit is completed (see also Section 6.8).
6.5.1.1. Human Food Contract Audits
A. References
1. Appendix A - Instructions for Evaluating Contract Inspections
2. Appendix B.2 - Instructions for Reporting Human Food Contract Audits
B. Performance Documentation - Appendix B - Human Food Field
Inspection Audit Form (Form FDA 3610H) is used to evaluate the state
inspector’s performance.
C. Performance Factors - See Appendix B.
6.5.1.2. Animal Food Contract Audits
A. References
1. Appendix A - Instructions for Evaluating Contract Inspections
2. Appendix C.1 - Instructions for Completing the Animal Food Safety
Inspection Audit Form
3. Appendix C.2 - Instructions for Reporting Animal Food Contract Audits
B. Performance Documentation - Appendix C - Animal Food Safety
Inspection Audit Form
C. Performance Factors - See Appendix C.
6.5.2. Human and Animal Food Verification Audits
A. Audit Requirement - Each state auditor must be audited a minimum of
twice in 36 months. Verification Audits for programs in Phase II are
conducted by the FDA. Verification Audits for programs in Phase III have
one (1) of the two (2) required audits conducted by FDA.
B. Timeframe for Submitting Performance Documentation
1. The program division sends a copy of the audit memorandum to the
state liaison and state agency no later than 30 business days after the
audit is completed.
2. When the state agency conducts the audit, the state agency sends the
memorandum for the verification audit to the state liaison no later than
30 business days after the audit is completed.

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 15 of 26
For the most current and official copy, check QMiS.
3. If a verification audit is unacceptable, the program division or the state
agency should notify the other party no later than 10 business days
after the audit is completed (see also Section 6.8).
C. References - Appendix D - Instructions for Conducting Joint Audit
Inspections, Verification Audits for State Auditors, and Joint Inspections
D. Performance Documentation - The FDA or state auditor follows Appendix
D to document the state auditor’s performance.
E. Performance Factors - Follow instructions in Appendix D.
6.5.3. Egg, Medical Device, and Other State Inspection Programs
A. Audit Requirement - One joint audit inspection or audit (audit option
applies to egg program only) is required of each inspection program every
contract year.
B. Audit Option for Egg Program - The program division makes auditing
decisions, in consultation with the FDA auditor and state program, based
on the number of trained inspectors in the program. If the state program
has two or more trained inspectors, the annual requirement is an audit,
unless the division determines training is needed and elects to do a Joint
Audit Inspection. The division identifies the state inspectors to be audited,
based on past audit history and inspector performance. Each inspector is
assigned specific roles during the audit, determined at a pre-audit meeting.
The auditor evaluates each inspector on performance in those roles. A
separate audit memo is created for each inspector to document the
assigned areas and the inspector’s performance in those assigned areas.
The audit memo is also used to plan future audits to ensure inspectors are
audited in all aspects of an egg inspection.
The program division determines how many inspectors can be evaluated
during an audit. This decision should consider the firm’s requirements for
the number of personnel allowed in their facility. The designated lead
inspector creates one inspection report. Evaluation of the inspection report
is not part of this audit.
(NOTE: This process remains in place until the draft Egg Safety Regulatory
Program Standards are approved by Office of Management and Budget.)
C. Timeframe for Submitting Performance Documentation – The FDA
sends a copy of the audit memorandum to the state agency no later than
30 business days after the audit is completed. If the audit/joint audit
inspection is unacceptable, the program division should notify the state
agency no later than 10 business days after the audit is completed.
D. References

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 16 of 26
For the most current and official copy, check QMiS.
1. Refer to Relevant Contract: Statement of Work (SOW)
2. Appendix A - Instructions for Evaluating Contract Inspections
3. Appendix D - Instructions for Conducting Joint Audit Inspections,
Verification Audits for State Auditors, and Joint Inspections
E. Performance Documentation - The FDA or state auditor follows Appendix
D to document the state auditor’s performance.
F. Performance Factors for Joint Audit Inspections - See Appendix D.
6.6. Reporting Audit Findings
The state liaison reports the audit findings for each quarter. All audit results
must be reported, even when more than the required numbers are performed.
The state liaison ensures the audit results in the Contract Audit Tracker (CAT)
Tool are representative and accurate at least on a quarterly basis. The state
liaison confirms prior contract year audit data before the program division
conducts work planning with the state. This enables them to plan audits for
the current contract year. The CAT is used to:
1. Ensure state inspectors and auditors meet the minimum audit
requirements
2. Ensure verification audits are completed timely
3. Calculate an overall rating for the contract performance period
4. Evaluate the audit ratings for a single performance factor
5. Ensure the minimum audit requirement is being met
6.6.1. Human and Animal Food Contracts
The state liaison records the type of audit as joint inspection, contract audit, or
verification audit and the inspection type. Contract audits also contain a record
of the inspection individual performance factor results.
6.6.2. Egg, Medical Device, and Other State Inspection Programs
The state liaison records egg, medical device, and other state inspection
programs as joint audit inspections and records the overall rating of the audit.
6.7. Audit Requirement Deficiencies
When the minimum audit requirement is not met, the PDD must provide a
written explanation as to why the audit requirement was not met no later than
30 business days after the end of the contract performance period. The
memorandum is emailed to the Contract Audits mailbox
1. The number of inspections awarded in the contract and the number of
inspections for each type of inspection

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 17 of 26
For the most current and official copy, check QMiS.
2. The number of audits completed for each type of inspection
3. The number of audits not completed
4. Detailed reasons for not completing the required number of audits
5. Detailed recommendations for solving issues that caused the required
number of audits not to be met
6. Detailed proposal for meeting the required number of audits for the next
contract performance period
The OP Director reviews the memorandum and discusses the need to adjust
the state agency’s implementation phase with the PDD and the director of the
state commodity program.
In Phase I, the PDD prepares the memorandum.
In Phase II, the PDD and state agency work together to prepare the
memorandum. The PDD also documents how to increase oversight of the
program and, if necessary, implement action to assume increased
responsibility for completing the audits.
In Phase III, the director of the state agency prepares the memorandum and
sends it to the PDD for concurrence; the PDD forwards it to the PD. The
memorandum includes the following content:
1. Support of the memorandum submitted by the state agency
2. Summary of discussions held between PDD, state liaison, and state
agency to prevent program deficiencies from reoccurring
3. The proposal for increasing oversight of the audit program to ensure the
required number of audits are met in the next contract performance
period
6.8. Performance Deficiencies
6.8.1. Individual Inspector Performance Deficiencies
A. When there is an individual performance deficiency, the program division
or state agency notifies the other party no later than 10 business days after
the audit is completed. The program would be credited with completing a
contract inspection and receive payment.
B. An individual performance deficiency occurs when a:
1. Contract audit is rated as “needs improvement” by receiving an overall
score of less than 80%.
2. Verification audit is rated as “needs improvement” (this applies to
human food and animal food contracts only); or

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 18 of 26
For the most current and official copy, check QMiS.
3. Joint audit inspection of an inspector conducting an egg, medical
device, or other inspection done under contract is rated as
“unacceptable.” (Refer to Appendix D).
C. The program division or state agency follows these steps to address
individual performance deficiencies identified during audits. The state
inspector or state auditor cannot return to performing inspections or audits
until all these steps are completed and passed.
1. The program division and state agency discuss the deficiencies
identified during the audit.
2. The state inspector or state auditor discontinues conducting or auditing
that type of inspection, respectively, until remedial training is completed.
The state may be required to absorb the cost of the training.
3. State inspectors receiving an overall rating of “needs improvement”
must complete remedial training in deficient areas. The program
division and state agency managers agree on the remedial training
needed to allow the state inspector or state auditor to resume
conducting or auditing contract inspections, respectively. The remedial
training should directly address the deficiencies noted during the audit.
4. After remedial training is completed, the state agency conducts an
internal audit of the state inspector or state auditor while conducting or
auditing a non-contract inspection, respectively. The internal audit
should evaluate the effectiveness of the remedial training.
5. The program division audits the state inspector or state auditor while
conducting or auditing a contract inspection, respectively, once
remedial training and the internal audit has been completed.
6.8.2. Program Performance Deficiencies
When there is a program performance deficiency, the PDD or state or territorial
agency notifies the other party no later than 10 business days after the end of
the contract performance period.
A program performance deficiency occurs when the overall audit performance
rating is below 80 percent “acceptable” when averaged across the contract
performance period.
6.8.3. Documenting Performance Deficiencies
The program division and state agency follow these steps to address
individual or program performance deficiencies:
1. Develop a plan to correct the deficiencies. The plan must address:

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 19 of 26
For the most current and official copy, check QMiS.
a. The possible causes for the individual or program performance
deficiency
b. The corrective actions that will improve performance.
2. Complete the Corrective Action Plan for Program and Individual
Performance Deficiencies (Appendix J) form and submit to the Audit
Program Manager upon completion of the corrective action.
3. The program division records corrective actions taken by the state in the
Quality Management System (QMS) for national trending.
6.9. Process for Contract Modifications for Program and Performance
Deficiencies
A. The OP Audit Program Manager or state liaison immediately notifies the
Project Manager of any individual or program performance deficiency that
may affect a contractual requirement. The Project Manager works with the
Contracting Officer’s Representative (COR) to make any necessary
contract changes. The program division provides the Project Manager with
additional notification of all follow-up actions and copies of any written
correspondence to the state agency.
B. If the program division proposes a change to the contract, the PDD emails
a recommendation to change the contract to the PD and OP DPIA Director,
no later than 10 business days after the decision to propose a change to
the contract is identified.
C. The recommendation must contain the following information:
1. Documentation of the problem including attached copies of pertinent
state inspection reports and the FDA audit reports
2. A description of the steps taken by the state agency and the program
division to correct the problem
3. Copies of correspondence such as emails between the program
division and state agency documenting efforts to address and correct
the problem
4. An assessment by the program division of the cause of the problem
and suggested changes to the contract
D. The OP DPIA Director, Project Manager, and COR review the program
division’s proposal to determine if the recommended action is appropriate
and complies with contracting regulations and procedures. The OP DPIA
Director discusses with the program division any potential action to be
taken. OP requests the Office of Acquisitions and Grant Services (OAGS)
send an official notification of any action to the contractor. Any actions

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 20 of 26
For the most current and official copy, check QMiS.
pursued under this section are in accordance with the instructions provided
in the SOW regarding alteration of the contract and payment for work
conducted under the contract.
6.10. Dispute Resolution
The program division and the state agency must make every effort to resolve
disputes about audit findings and overall audit ratings. If, however, the
program division and state agency are unable to resolve a dispute, both
parties send a written summary of the situation and a proposed resolution to
the Director, OP. All related documents, including the FDA audit reports and
state inspection reports, shall be included. The OP Director reviews the reports
and works with the program division and the state agency to arrive at a
resolution. If the state agency fails to respond, the disposition of the contract
may be affected.
6.11. Quality Assurance
Quality assurance for the contract programs is a combined effort between the
state program, ORA program division, and OP. The inspection audits
referenced in this FMD ensure the quality assurance of individual inspector
performance in relation to meeting contract requirements.
6.11.1. State Program Quality Assurance
The program division conducts a performance audit of each state program
within the first quarter of the fiscal year for the completed contract year. The
internal audit evaluates program performance as described in Section 6.8.2
and the division’s management of the state contract inspection program. The
internal audit findings are be provided to the PDD, state liaison, and OP and
addressed per QMS procedures.
6.11.2. Contract Program System Audit
A comprehensive review and analysis are conducted by OP of the national
performance data and evaluation of state program performance and identifies
continuous improvement opportunities. The OP director provides a written
report of the audit findings, accomplishments, national trends, describes
systemic deficiencies, and recommends corrective actions or opportunities for
improvement to the ACRA, and to the manager of OHAFO and other
designated managers.
7. Glossary/Definitions
Terms relevant to audits and oversight of contract inspections are:

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 21 of 26
For the most current and official copy, check QMiS.
Audit Performance Rating - This is the comprehensive assessment of all audits
conducted in a contract program during a single contract performance period.
The Audit Performance Rating is presented as a percentage based on the
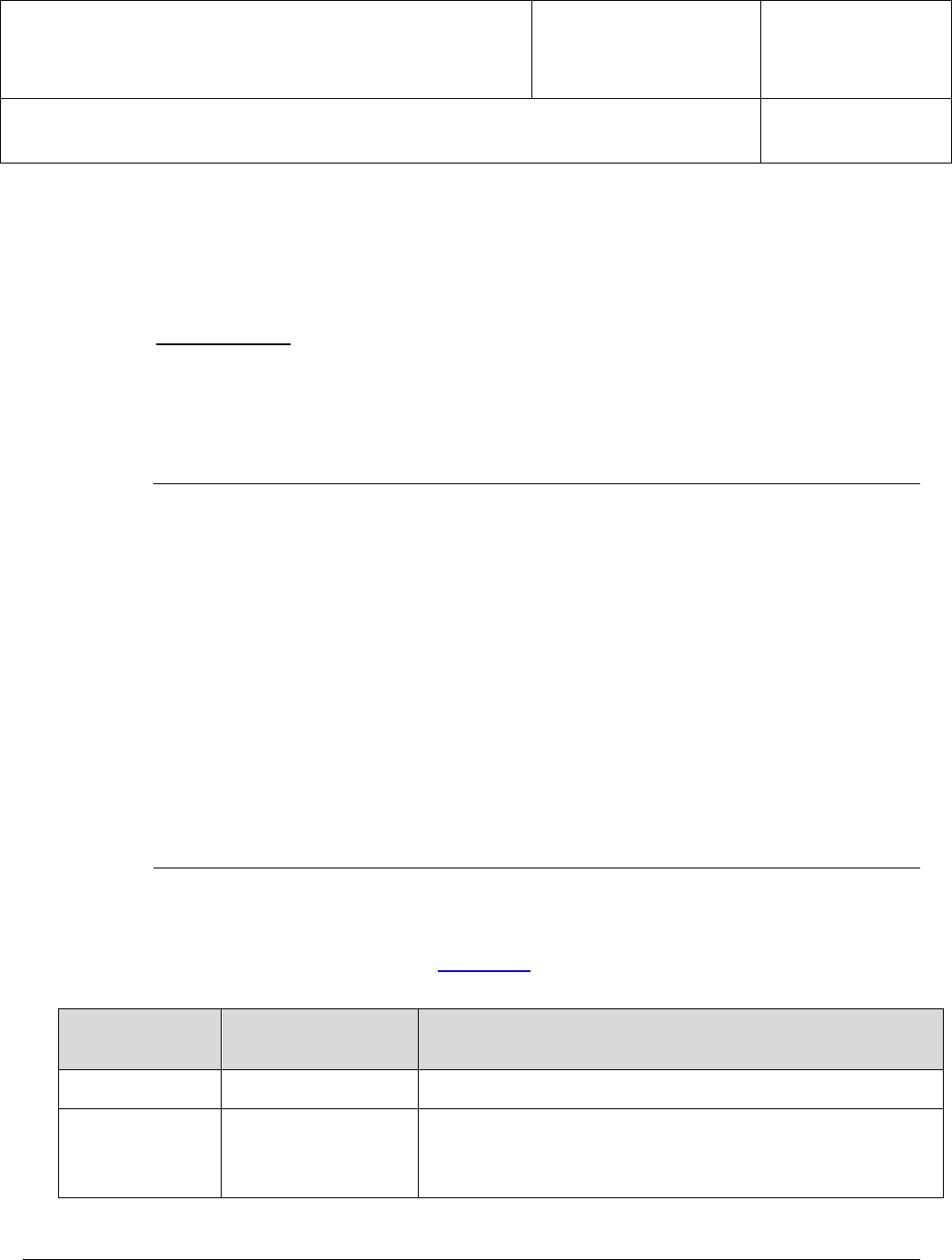
rating of all individual performance factors: Total rated as “Acceptable” divided
by (the Total “Acceptable” plus the Total “ Needs Improvement”) multiplied by
100.
Total rated as "Acceptable"
(
Total rated "" + Total rated " "
)
×100%
The Audit Performance Rating must be greater than or equal to 80 percent.
Contract Audit - This is an evaluation of a contract inspection in which a
qualified auditor accompanies a state inspector to document the inspector’s
performance. The FDA investigators or state personnel are qualified to
conduct a contract audit after all the requirements for the specific inspection
program listed in Sections 6.2 have been successfully completed.
Contract Year - This is the contract performance period for a state contract,
otherwise known as the period of performance. It is specific to the state
contract. This may or may not coincide with the calendar year or federal fiscal
year.
FDA Auditor – Program employee who has completed the required auditor
and program training and with appropriate program experience.
Joint Audit Inspection - This is an audit conducted by an FDA investigator
accompanying a state inspector and observing the latter’s performance. A joint
audit inspection may be used to assess the quality of contract inspections for
egg, medical device, and other industries that are not covered by an FDA
audit course. Appendix D provides guidelines for conducting and reporting
joint audit inspections.
Joint Inspection - This an inspection conducted jointly by the program division
and state inspectors for training. Joint inspections may be counted toward the
required number of audits when used to train state inspectors. Training may
be necessary when a new contract is negotiated, new industries are added to
an existing contract, or remedial training is needed. If authorized in the
contract, the state agency may count the joint inspection as a contract
inspection. Appendix D provides additional guidelines for conducting and
reporting joint inspections.
Overall Audit Rating - This is the comprehensive assessment for an individual
audit (see Appendices B and C).
Specialized Inspection - This refers to contract inspections that cover a
specialized area. Specialized inspection areas include Seafood HACCP, Juice
FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 22 of 26
For the most current and official copy, check QMiS.
HACCP, LACF/AF, BSE, Licensed and Non-licensed Medicated Feed, and
Preventive Controls. They are identified as electives in the contract. A state
program can elect to perform these inspections under the contract if it has
inspection staff with the required training and experience to perform the
inspections.
Training Audit - This is an audit in which a state auditor trainee accompanies
an FDA or state auditor and the state inspector during a contract inspection.
Its purpose is to teach the trainee how to conduct an audit by observing an
audit of a state inspector. The state auditor trainee must also meet the auditor
qualifications in Sections 6.2, 6.3, and relevant for the specific commodity.
8. Records
Contract Audit Forms
Contract Audit Tracker database
State Implementation Agreement and Yearend Evaluation Request for Audit
Reduction Forms
Corrective Action Plan for Program and Performance Factors Program Audit
Record
Contract Program System Audit
Annual State Contract Inspection Audit Summary
Supporting Documents
9. Supporting Documents
Users are responsible for ensuring they use the most up-to-date version of the
referenced documents. Please see the Contracts webpage on FDA.gov or QMIS
(internal use only) to access the appendices.
Appendix
Internal
Document #
Document Title
Appendix A JA-000024 Instructions for Evaluating Contract Inspections
Appendix B
FORM-000161
FORM FDA
3610H
Human Food Field Inspection Audit Form
FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 23 of 26
For the most current and official copy, check QMiS.
Appendix
Internal
Document #
Document Title
Appendix B.2 JA-000026 Instructions for Reporting Human Food Audits
Appendix C FORM-000162 Animal Food Safety Inspection Audit Form
Appendix C.1 JA-000027 Instructions for Completing the Animal Food Safety
Inspection Audit Form
Appendix C.2 JA-000028 Instructions for Reporting Animal Food Contract Audits
Appendix D JA-000029
Instructions for Conducting Joint Audit Inspections,
Verification Audits for State Auditors, and Joint
Inspections
Appendix H FORM-000163 State Implementation Agreement and Yearend Evaluation
Appendix I FORM-000164 Request for Audit Reduction Form and Instructions
Appendix J FORM-000165 Corrective Action Plan for Program and Performance
Factors
10. Document History
Revision
#
Status*
(D, I, R)
Date Author Name and Title
Approving Official Name
and Title
1.0 R 1/10/14
Beverly Kent, OP OIG
Working Group
Barbara Cassens,
Acting Director OP
2.0 R 4/30/15
Cathy Hosman, OP FMD 76
Working Group
Barbara Cassens,
Acting Director OP
03
R
03/05/2019
Cathy Hosman, OP ACSL Barbara Cassens,
Director OP
04 R 06/19/2020
SCIPI Working Group Barbara Cassens,
Director, OP
05 R See QMIS
Graham Giesen, OP Audit
Program Manager
Erik Mettler,
ACPP
* - D: Draft, I: Initial, R: Revision

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 24 of 26
For the most current and official copy, check QMiS.
11. Change History
Revision #
Change
1.0
Previous versions of this document exist and are archived, however version numbering was
not included. This is the first version in the new format.
2.0
Removed responsibility for Program Divisions to develop individual procedures for
implementing
this FMD. Added recommendation in 5.3.2 for feed auditors to have the VM213
BSE Inspection
Training. 5.4.1 - Revised audit rate to include minimum of two audits per
inspector every 36
months. 5.4.2 - Added clarification that audits should be conducted on the
most complex
program. 5.8.3 – Added requirement for submission of corrective action plans
to OP. 5.11 –
Added requirement to initiate a corrective action in QMS for national
performance deficiency
trends.
03
Document migrated to updated SOP/FMD template. Section 5 procedures moved to Section 6
in
new template.
Changed “Feed” to “Animal Food”.
Changed communication requirements from within 20 business days to 30 business days to be
consistent with the SOWs.
Updated FMD to correlate with changes realized by Program Alignment. District becomes
Program Division. DD becomes PDD. RFDD becomes PD. Separated state liaison role from
generic District reference.
Scope – added statement to suspend/terminate audit requirements for programs that have
been suspended/terminated.
Responsibilities – Change State Contract Liaison/Monitor role to state liaison role as the
Contract Technical Advisor, added description of COR role, added Program Manager role.
6.3 - Section moved from end of procedure.
6.3.3 – Content separated from 6.3.2 under new header
6.3.4 – Section added.
6.4.1 – Minimum audit rates- clarified frequency requirements. Required frequency unchanged.
6.4.2 Added description of requirements for complex inspection types.
6.4.4 - Clarified what data will be posted to the internet.
6.5 - Added Animal Food program ability to elect audit phases. Separated inspection audit and
verification audit requirements.
6.6 – Added audit data to be tracked by Contract Audit Tracker (CAT). Removal of Appendices
and Workbook E, F, & G. Replaced by CAT.
6.8.2 - Clarified the performance factors to be examined by the program division audit
6.8.3 – Added tracking of state corrective actions by ORA QMS.
Internal document number changed from FMD.076 to DIR-000033. Internal document numbers
applied to FMD appendices.
Continued next page

FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 25 of 26
For the most current and official copy, check QMiS.
Revision #
Change
04
Scope - Removed tissue residue from list of program areas.
Responsibilities - Removed District Director role since all state liaisons are now aligned with HAF
program divisions. Added Project Manager role to reflect change in OP DPIA role in the contract
separate from the COR role now held by OM. Changed title of Program Manager, DPIA to Audit
Program Manager to differentiate from Project Manager role.
Background – Added summary of changes from May 2015 revision of FMD to present.
References – Added reference to SOP-000115 Management of ORA State Contract Inspection
Process.
6.2 – Removed list of specialized training courses.
6.3 – Added clarification of who signs the Appendix H and align sequence to contract SOW.
6.3.2 & 6.3.3 – Clarified requirements.
6.4 – Added Preventative Controls program areas
6.5.2 – Clarified requirements.
6.5.3 – Added egg audit option.
6.8.1C – Added statement to clarify requirements “State inspector or state auditor cannot return
to performing inspections or audits until all of these steps are completed and passed.”
6.8.2 – Changed definition of “Needs improvement” for a single performance factor. Added “OR
a score of less than 80% conformance in a single performance factor” and provided the program
division to determine which is appropriate. Changed overall audit performance rating from 90%
to 80% to align with the audit program in the regulatory program standards.
6.9 – Revised to include COR role now with OM.
Appendix B FDA-3610 form-added questions covering audits of Limited Scope and Modified
Preventive Controls inspections.
Appendix B1 Guidance for Completing the Contract Audit Form-added examples of “Needs
Improvement” for new section.
Appendix C Animal Food Safety Inspection Audit Form
1. Renamed to “Animal Food Safety Inspection Audit Form”.
2. Added new section covering AFRPS inspections.
3. Revised wording of some questions to reflect changes in regulation.
Appendix C.1 Guidance for Completing the Animal Food Safety Inspection Audit Form-updated
questions to agree with audit form.
Appendix D Guidance for Conducting Joint Audit Inspections, Verification Audits for State
Auditors, and Joint Inspections.
1. Removed tissue residue audits since they are no longer contracted work.
2. Added description of findings that would warrant a “Needs Improvement” rating.
Appendix H State Implementation Agreement and Yearend Evaluation
1. Planned Resources-clarified the state personnel who could become qualified auditors.
2. Section I. Contact Information-updated FDA and state contact information to agree with
contract SOW.
3. Section IV. & V. Planned and Completed Audits-minor changes to form information
requested.
Appendix I Request for Audit Reduction-updated to reflect changes to audit requirement from
percentage of total contract inspections to inspector audit of 2 audits in 36 months
All - Edited for syntax, spelling, punctuation, grammar, adherence to the ORA STYLE GUIDE
and to the principles of Plain Language (K. Lee Herring, OCPM, DC, SCB) 2/24/2020
FOOD AND DRUG ADMINISTRATION
OFFICE OF REGULATORY AFFAIRS
Office of Partnerships
Document Number:
DIR-000033
Revision #: 05
Revised:
22 Aug 2023
Title:
FMD-76 State Contracts – Evaluation of Inspectional Performance
Page 26 of 26
For the most current and official copy, check QMiS.
05
Section 2: Added statement that when there is conflict between the FMD and the contract—the
contract takes precedence. “State” refers to state and territorial agencies participating in the
FDA contract inspection program.
Section 6.3.1: Created new subheading for Phase II utilizing existing language in FMD.
Section 6.4: Update steps/references for routing and documenting in CAT Tool to include
clarifying existing requirements to submit Appendix H within 30 days end of POP.
Section 6.4.2: Created subsection heading for existing CAT Tool data requirement updates.
Section 6.5.2: Clarified existing requirements for Phase III verification audit responsibilities.
Section 6.8: Update Performance Measures - Removed requirements on single performance
factors.
Section 6.9B: Change timeline for requesting contract modification to 10 business days after the
decision is made that it is necessary to modify the contract.
Glossary: Removed 4 or more needs improvement reference to individual performance.
Appendix B: Fundamental changes to 3610 to update audit questions to align with program
specific questions and to incorporate Full Scope PC questions, now 3610H.
Appendix B1: Delete appendix as the job aid on the new form replaces this guidance.
Appendix B2: Update PAC codes and reporting requirements
12. Attachments
See Section 9 for links to document appendices.
