
Applied Soil Ecology 19 (2002) 147–160
Organic and synthetic fertility amendments influence soil
microbial, physical and chemical properties on organic
and conventional farms
L.R. Bulluck III
a,1
, M. Brosius
b
, G.K. Evanylo
b
, J.B. Ristaino
a,∗
a
Department of Plant Pathology, North Carolina State University, P.O. Box 7616, Raleigh, NC 27695-7616, USA
b
Department of Crop and Soil Environmental Sciences, 421 Smyth Hall, Virginia Tech, Blacksburg, VA 24061, USA
Received 22 May 2001; accepted 1 November 2001
Abstract
Field experimentswereconductedtoexamine the effectsoforganicandsyntheticsoilfertilityamendmentsonsoilmicrobial
communities and soil physical and chemical properties at three organic and three conventional vegetable farms in Virginia and
Maryland in 1996 and 1997. Two treatments, including either an alternative organic soil amendment (composted cotton-gin
trash, composted yard waste, or cattle manure) or synthetic soil amendment (fertilizer) were applied to three replicated
plots at each grower field location. Production history and time affected propagule densities of Trichoderma species which
remained higher in soils from organic farms. Propagule densities of Trichoderma species, thermophilic microorganisms, and
enteric bacteria were also detected in greater numbers in soils amended with alternative than synthetic amendments, whereas
propagule densities of Phytophthora and Pythium species were lower in soils amended with alternative than synthetic fertility
amendments. Concentrations of Ca, K, Mg, and Mn were higher in soils amended with alternative than synthetic fertility
amendments. Canonical correlations and principle component analyses indicated significant correlation between these soil
chemical factors and the biological communities. First-order canonical correlations were more negative in fields with a
conventional history, and use of synthetic fertilizers, whereas canonical correlations were more positive in fields with a
history of organic production and alternative soil amendments. In the first year, yields of corn or melon were not different in
soil amended with either synthetic or organic amendments at four of six farms. In the second year, when all growers planted
tomatoes, yields were higher on farms with a history of organic production, regardless of soil amendment type. Alternative
fertility amendments, enhanced beneficial soil microorganisms reduced pathogen populations, increased soil organic matter,
total carbon, and cation exchange capacity (CEC), and lowered bulk density thus improving soil quality. © 2002 Elsevier
Science B.V. All rights reserved.
Keywords: Soil chemical and physical factors; Organic agriculture; Sustainable agriculture; Soil microbial communities
∗
Corresponding author. Tel.: +1-919-515-3257;
fax: +1-919-515-7716.
E-mail address: Jean
1
Present address. Postdoctoral Research Associate Department
of Plant Pathology, University of California, One Shields Avenue,
Davis, CA 95616, USA.
1. Introduction
Demand for organically produced food has increa-
sed 24% yearly in the US in the 1990s, as many con-
sumers have expressed concern over pesticide residues
on foods (Govindasamy and Italia, 1998; Thompson,
1998). Food and environmental safety are often-cited
0929-1393/02/$ – see front matter © 2002 Elsevier Science B.V. All rights reserved.
PII: S0929-1393(01)00187-1
148 L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160
reasons for the use of alternative soil amendments, but
increasingly, economic considerations are becoming
important with a rise in popularity of organically pro-
duced foods (Govindasamy and Italia, 1998; Klonsky
and Tourte, 1998; Thompson, 1998). A premium of
12–60% is often obtained from organic produce (Lohr,
1998). Since this premium exists, organic agriculture
has become more attractive to farmers (Langley et al.,
1983; Klonsky and Tourte, 1998; Thompson, 1998).
The use of organic soil amendments has been
associated with desirable soil properties including
higher plant available water holding capacity and
CEC and lower bulk density, and can foster benefi-
cial microorganisms (Doran, 1995; Drinkwater et al.,
1995). Benefits of compost amendments to soil also
include pH stabilization and faster water infiltration
rate due to enhanced soil aggregation (Stamatiadis
et al., 1999). Soil chemical characteristics are affected
by soil amendment and production system. For exam-
ple, at the Rodale Institute, long-term legume-based
and organic production systems have resulted in an
increase in soil organic matter and reduced nitrate
runoff (Drinkwater et al., 1998). Soils in organic pro-
duction systems lost less nitrogen into nearby water
systems than did conventional production systems
(Liebhardt et al., 1989). The amount of soil nitro-
gen in fields under conventional production systems
has been negatively correlated with soil microbial
components, whereas soil nitrogen in fields under
organic production was positively correlated with soil
microbial components (Gunapala and Scow, 1998).
Yields of crops grown in organic and conventional
production systems can be equivalent. Vegetable fields
under organic production in California produced
yields equal to those under conventional production
(Drinkwater et al., 1995; Stamatiadis et al., 1999).
Long-term research in Pennsylvania has also demon-
strated little difference in yields between conventional
and organic production systems (Drinkwater et al.,
1998).
Limited field studies have been conducted to
determine the impact of soil amendments on micro-
bial communities in actual organic and conventional
production systems in the fields (Drinkwater et al.,
1995; Gunapala and Scow, 1998). However, it has
been shown that microbial activity and biomass
is higher in fields with organic amendments than
fields with conventional fertilizers (Drinkwater et al.,
1995). Many studies on soil microbial communities,
as affected by organic amendments, have examined
functional groups, or classes of organisms, while few
studies have examined the impact on community com-
position and genera within these groups. One such
study in organic tomato fields in California found
that suppression of corky root disease was associated
with increased actinomycete activity (Workneh et al.,
1993; Workneh and van Bruggen, 1994).
Organic production systems have increased in
recent years in the southeastern United States, but we
know little about the soil microbial communities in
these fields or the impact of these production practices
on yield. We examined microorganisms in soil that
were either beneficial (compost organisms that decom-
pose organic matter, organisms that parasitize plant
pathogens, or beneficial rhizosphere microorganisms),
or potential pathogens that have a significant impact
on soil ecology, plant and human health. The objective
of our research was to examine the effects of either
synthetic fertilizers or alternative soil amendments,
including composted animal manures and plant mate-
rials on specific soil microbial communities, soil phys-
ical and chemical properties and yield on farms with
a history of either conventional or organic production.
2. Materials and methods
2.1. Experimental design
Field experiments were conducted in 1996 and 1997
at three farms with a history of either conventional
or organic production. Five of the six experimental
sites were located in Virginia, and one was located
in Maryland. The three conventionally-managed sites
had a history of at least 5 years of vegetable or field
crop monoculture, synthetic fertilizers, and pesticide
use. The three organically-managed sites had a history
of at least 3 consecutive years of organic soil fertil-
ity amendment, winter cover crops, mulch for weed
control, and biologically-based pest control. Pesticides
were not used during this study at any of the exper-
imental sites. Grower field soil types, amendments
used, and crops grown each year are shown in Table 1.
The experimental design consisted of a randomized
complete block with three replicates per field. Two
treatments, consisting of either a blended synthetic
L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160 149
Table 1
Summary of soil series, amendments, crops, and production history for field experimental sites for 1996 and 1997
Grower Soil series
a
Location Productivity
a
(mean corn
yield, t/ha)
Alternative
amendment
b
Crop Production
history
c
1996 1997
1 Eunola loamy fine sand (fine-
loamy, siliceous, semiactive,
thermic Aquic Hapludults)
Suffolk VA 6.92 Cotton-gin trash Melons Tomato Conventional
2 Eunola loamy fine sand (fine-
loamy, siliceous, semiactive,
thermic Aquic Hapludults)
Suffolk VA 6.92 Cotton-gin trash Melons Tomato Conventional
3 Eunola loamy fine sand (fine-
loamy, siliceous, semiactive,
thermic Aquic Hapludults)
Suffolk VA 6.92 Cotton-gin trash Melons Tomato Conventional
4 Westphalia fine sandy loam
(coarse-loamy, siliceous,
semiactive, mesic Inceptic
Hapludults)
Upper
Marlboro MD
4.09 Cattle manure Corn Tomato Organic
5 Chester loam (fine-loamy,
mixed, mesic Typic
Hapludults)
Leesburg VA 9.43 Hay-manure compost Corn Tomato Organic
6 Glenelg loam (fine-loamy,
mixed, semiactive, mesic
Typic Hapludults)
Blacksburg VA 6.92 Yard waste compost Corn Tomato Organic
a
Classified according to the Virginia Agronomic Land Use Evaluation System, VALUES (Simpson et al., 1993) and based primarily
on plant available water-holding capacity.
b
Soil amendments were added to plots at least 1 week prior to planting.
c
Production histories were either conventional production systems with synthetic fertilizer and pesticide use, or organic production
systems that had at least 3 years of organic amendments and no pesticide use.
fertilizer or an organic waste, were applied at each of
the six locations (Table 2). Alternative amendments
consisting of organic wastes that were used as fertil-
ity sources were either composted cotton-gin trash,
mixed yard waste-poultry litter compost, uncom-
posted cattle manure, or mixed hay-cattle manure
compost. Synthetic fertilizers consisted of mixtures of
nitrogen as ammonium nitrate (35.5-0-0), phosphorus
as triple superphosphate (0-46-0), and potassium as
muriate of potash (0-0-60).
Nutrient requirements for each crop and field were
based on Virginia Cooperative Extension recommen-
dations (Donohue and Heckendorn, 1994) following
routine soil testing laboratory analysis performed
in the Department of Crop and Soil Environmen-
tal Sciences (CSES) at Virginia Tech, Blacksburg,
Virginia (Donohue, 1992). Synthetic fertilizers were
applied to all farms according to Soil Test Labora-
tory analyses and recommendations. Organic wastes
were applied at rates designed to provide required
plant available nitrogen (PAN) on the historically
conventional farms. On the historically organic farms,
organic amendments were applied according to the
recommendations of the farmers based on their expe-
riences with anticipated residual nitrogen from con-
tinuous annual applications of compost and the use
of green manure cover crops. The rates of compost
used by organic farmers were generally lower than
the rates calculated to provide the required PAN for
each crop and soil (Table 2).
PAN contents of the organic wastes were estimated
from analyses of the inorganic and organic forms of
nitrogen in the composts according to the following
equation:
PAN = [Org-N] × A + [NH
4
-N] × B + [NO
3
-N]
where Org-N is the concentration of organic-bound N
in the waste as calculated by [TKN]-[NH
4
-N]; TKN
the total Kjeldahl nitrogen concentration; NH
4
-N the
ammonium nitrogen concentration; NO
3
-N the nit-
rate nitrogen concentration; A the fraction of Org-N
expected to mineralize or become plant available in
150 L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160
Table 2
Estimated rates of plant available nutrients from plots amended with synthetic or alternative soil amendments at six locations in 1996 and
1997
Grower Amendment
type
a
Amendment
rate (Mg/ha)
b
Cover
crop N
c
Available nutrients from
amendment (kg/ha)
Total PAN
d
NPK
1996
1 Alternative 33.6 0 92 165 346 92
Synthetic 0 101 28 73 101
2 Alternative 33.6 0 92 165 346 92
Synthetic 0 101 56 171 101
3 Alternative 33.6 0 92 165 346 92
Synthetic 0 101 112 78 101
4 Alternative 28.0 40 29 71 188 74
Synthetic 40 157 91 99 165
5 Alternative 8.7 60 63 22 86 130
Synthetic 0 157 45 99 157
6 Alternative 20.0 0 72 176 250 72
Synthetic 0 157 45 39 157
1997
1 Alternative 69.5 0 133 224 926 133
Synthetic 0 101 24 187 101
2 Alternative 51.3 0 113 166 684 113
Synthetic 0 101 49 187 101
3 Alternative 51.8 0 115 169 697 115
Synthetic 0 101 37 187 101
4 Alternative 42.1 40 50 109 462 95
Synthetic 40 101 37 94 146
5 Alternative 17.3 60 24 22 48 91
Synthetic 0 101 24 94 101
6 Alternative 30.0 0 66 138 288 66
Synthetic 0 101 0 0 101
a
Alternative refers to alternative amendments given in Table 1 for each grower. Synthetic amendments are ammonium nitrate (35.5-0-0),
triple superphosphate (0-46-0), and muriate of potash (0-0-60).
b
Rate of dry compost applied in metric tons per hectare (Mg/ha). Synthetic amendment rates are given in each row under available
nutrients.
c
Estimated nitrogen supplied by winter cover crop green manure (kg/ha).
d
Total estimated PAN (kg/ha).
the year of application, generally estimated to be ap-
proximately 0.10–0.15 for compost and 0.35 for beef
cattle manure in the mid-Atlantic region of the US
(Evanylo, 1994); B the fraction of NH
4
-N expected to
be plant available in the year of application, generally
estimated to be 1.0 for compost and 0.85 for beef
manure incorporated within 24h of application.
Nitrogen contents of the organic wastes were
determined in the CSES Department at Virginia Tech
through analysis for TKN (Bremner and Mulvaney,
1982) and NH
4
-N and NO
3
-N (Keeney and Nelson,
1982). Most fields were allowed to remain fallow
over winter, but organic growers 4 and 5 planted
a rye cover crop in the winters of 1995 and 1996
(Table 2). All soil fertility amendments were applied
between April and June of each year and immediately
incorporated into soil.
Plots were 7.6m × 7.6m and consisted of four
rows that were 1.6 m wide. Planting occurred within 1
week of soil amendment. In the first season, conven-
tional growers planted melons (Cucumus melo L. var.
reticulatum), and organic growers planted sweet corn
(Zea mays L. var. Silver Queen) (Table 1). In the sec-
ond season, all growers planted tomatoes (Lycopersi-
con escelentum L. var. Celebrity or Mountain Spring)
(Table 1).
L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160 151
2.1.1. Soil sampling
Soil samples were removed from each field approx-
imately 2 weeks after planting and at harvest in both
years and subjected to physical and chemical analyses
and assays for selected microbiological populations.
Twelve soil cores (30cm in length and 1.9 cm in di-
ameter) were removed in a serpentine pattern from
each of two center rows of each plot resulting in 24
soil cores per plot. Subsequently, all cores taken from
a single plot were pooled. Samples were removed
from the root zone around plants in the rows. Soil
cores were placed in a large (4 l) plastic bag and
stored on ice in coolers. In the laboratory, samples
were stored at 5
◦
C and analyses were accomplished
within 3 weeks of sampling. All soils were handled
similarly so relative comparisons between soils from
different farming systems were possible.
2.2. Propagule densities of selected soil
microorganisms
Numbers of culturable bacteria, fluorescent pseudo-
monad bacteria, enteric bacteria, total fungi, ther-
mophilic microorganisms, Trichoderma, Fusarium,
Phytophthora and Pythium species were quantified.
Soil samples were analyzed for selected soil micro-
Table 3
Media, dilution factors, organisms, and incubation conditions for microorganisms isolated from soils in organic and conventional field soils
Medium Dilution
factor
a
Organism(s)
cultured
Culture conditions Reference
Temperature
(
◦
C)
Incubation
(days)
Light
conditions
Masago’s
b
10
−1
,10
−2
Pythium and
Phytophthora spp.
22 5–7 Dark Masago et al. (1977)
Trichoderma
medium E
10
−2
,10
−3
Trichoderma spp. 22 7 Light Papavizas and Lumsden (1982)
YGA 10
−2
,10
−3
Thermophilic
microorganisms
45 2–4 Dark Stevens (1974)
GYRBA 10
−3
Fusarium spp. 22 5–7 Dark Newhouse (1980)
King’s
medium B
10
−4
,10
−5
Fluorescent
Pseudomonas spp.
20–25 5–7 Dark Sands and Rovira (1970)
Endo 10
−5
,10
−6
Enteric bacteria 37 1–2 Dark Difco manual
PDA
c
10
−4
,10
−5
Total fungi 20–25 3–5 Dark Stevens (1974)
TSA
d
10
−6
,10
−7
Culturable bacteria 20–25 1–2 Dark Difco manual
a
Dilution factor number is the 1:10 serial dilution from each sample which was plated in triplicate.
b
Media not amended with hymexazol to allow growth of Pythium and Phytophthora species.
c
Potato dextrose agar with 100mg/ml streptomycin sulfate (Fisher Scientific, Pittsburgh) to inhibit bacterial growth.
d
Tryptic soy agar with 100mg/ml nystatin (Sigma, St. Louis) to inhibit fungal growth.
organisms using 10-fold serial dilutions of soil and
eight different selective media. The 10 g of soil was
diluted in 90 ml sterile water agar (w/v, 0.25%, Difco,
Detroit). Serial 10-fold dilutions were made to 10
−7
(Table 3). Triplicate plates for each medium were used
for each sample, and several media required different
soil dilutions for statistically accurate propagule esti-
mation (Table 3). Colonies were counted from plates
containing 30–300 colonies. Variance in count data
was normalized using log
10
(x + 1) transformation
prior to analysis where x equals the average number of
propagules of each type of microorganism per gram
dry soil. Percent soil moisture content for each sample
was determined gravimetrically. Data are expressed as
number of colony forming units (CFUs)/g of dry soil.
2.3. Soil chemical and physical parameters
Soil samples for physical and chemical parameters
were collected concurrently with microbial samples,
and were quantified for Mehlich I-extractable P, K,
Ca, Mg, Mn, Zn, Cu, and B; pH (Donohue, 1992);
total Kjeldahl N (Bremner and Mulvaney, 1982);
NH
4
-N, and NO
3
-N (Keeney and Nelson, 1982).
Additional soil chemical and physical properties:
bulk density by core method (Blake, 1965); organic
152 L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160
matter by Walkley-Black; total organic carbon by
dry combustion (Nelson and Sommers, 1982); CEC
(Rhoades, 1982); exchangeable cations (Thomas,
1982) and plant available water holding capacity by
pressure plate method (Klute, 1986) were conducted
on samples collected at harvest in 1996 and 1997.
2.3.1. Yield
Yield of marketable vegetables were taken from
the center, 4m of two center rows, in each plot and
weighed by growers on a weekly basis once fruit
began to ripen. Weights from each plot from each week
were tallied, analyzed, and presented as average total
per plot in metric tons per hectare (Mg/ha).
2.4. Statistical analyses of data
Statistical analyses were performed on all the data
using the GLM procedure from PC SAS 6.2 and 7.0
(SAS Institute, Cary, NC). Analyses of microbial
data were conducted using the transformed and arith-
metic means and are presented in figures. Analyses of
variance for both microbial and chemical parameters
were performed. Principle components (PRINCOMP)
partial correlations analyses and canonical correla-
tions (CANCORR) were calculated between selected
chemical and biological parameters. Variation occu-
rred between experimental sites, climatic features,
soil types, and weather conditions between years,
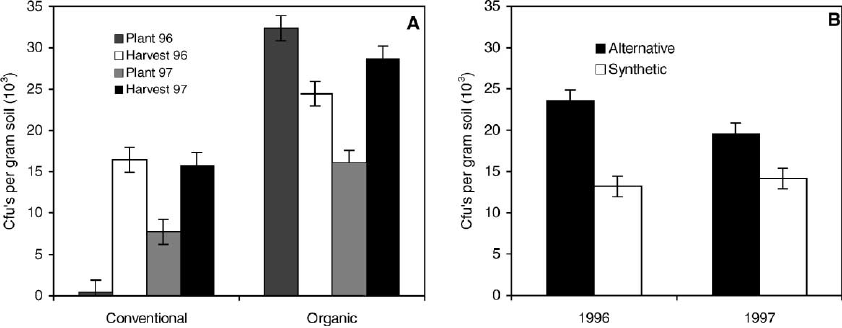
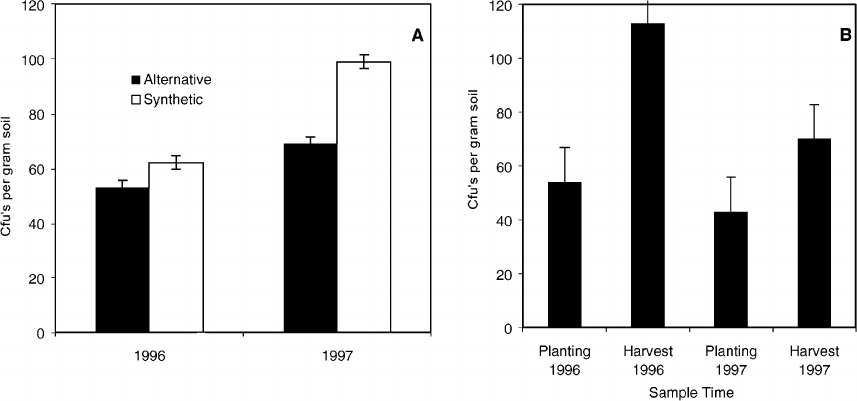
Fig. 1. (A) Impact of grower production history and time on propagule densities of Trichoderma species in grower field soils from three
organic and three conventional field locations in 1996 and 1997 (Lsd = 1499 CFUs/g soil). (B) Effects of alternative and synthetic soil
amendments on propagule densities of Trichoderma species in field soils from 1996 and 1997 (Lsd = 1223 CFUs/g soil).
so only interactions that were statistically significant
(P ≤ 0.05) for each year, and overall (combined data
from 1996 and 1997) are presented in this paper.
3. Results
3.1. Soil microbial populations
Production history and time affected propagule den-
sities of beneficial soil fungi in the genus Trichoderma
in soil in both years (P = 0.02, Fig. 1A). Numbers of
Trichoderma species were higher initially in 1996, in
soils from fields with a history of organic than con-
ventional production. Propagule densities increased
over time in fields with a conventional history, but
remained higher over time in soils from organic com-
pared to conventional fields. Soil amendment also
affected propagule densities of Trichoderma species
in both years (P = 0.01, Fig. 1B). Soils with alter-
native amendments had higher propagule densities of
Trichoderma species than soils amended with syn-
thetic fertilizers in both years regardless of production
system history (Fig. 1B).
Propagule densities of thermophilic microorgan-
isms were significantly higher in soils amended with
alternative amendments than in soils amended with
synthetic fertilizers in both years. In 1996, propagules
densities of thermophilic organisms were 2.1 × 10
4
L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160 153
CFUs/g dry soil in soils with alternative amendments
and 1.47× 10
4
CFUs/g dry soil in soils with synthetic
fertilizers (P<0.01). In 1997, propagules densities
of thermophilic microorganisms were 5.07 × 10
4
and
1.94× 10
4
CFUs/g dry soil in soils with alternative or
synthetic amendments, respectively (P = 0.01).
Enteric bacteria were also affected by soil fertil-
ity amendments in both years. Soils with alterna-
tive fertility amendments had nearly twice as many
propagules of enteric bacteria than soils with synthetic
fertilizers in each year. In 1996, propagules densities
of enteric bacteria were 1.85 × 10
7
CFUs/g dry soil
in soils amended with alternative amendments and
1.08 × 10
7
CFUs/g dry soil in soils with synthetic
fertilizers in 1996 (P = 0.05). In 1997, propagules
densities of enteric bacteria were 3.88 × 10
7
CFUs/g
dry soil in soils amended with alternative fertility
amendments and 1.94 × 10
7
CFUs/g dry soil in soils
amended with synthetic fertilizers (P = 0.03).
Propagule densities of Phytophthora plant patho-
genic and Pythium species were affected by soil
amendment and time in both years. Propagule den-
sities of Phytophthora and Pythium species were
lower in soils with alternative fertility amendments
than in soils amended with synthetic fertilizers in
both years (P = 0.03, Fig. 2A). In addition, propa-
Fig. 2. (A) Impact of the alternative and synthetic soil amendments on propagule densities of Phytophthora and Pythium species in grower
field soils from 1996 and 1997 (Lsd = 2.48 CFUs/g soil). (B) Effects of sampling time on propagule densities of Phytophthora and
Pythium species in grower field samples from 1996 and 1997 (Lsd = 12.8 CFUs/g soil).
gule densities of these pathogens increased over time
and were higher at harvest than at planting in both
years (P = 0.02, Fig. 2B). Orthogonal contrast com-
parisons reveal that treatment effects on propagule
densities of Phytophthora and Pythium species were
not different between years (P = 0.07).
Production systems and soil fertility amendments
did not affect propagule densities of Fusarium species
at any location in either year. Initially, fluorescent
pseudomonads were more abundant in 1996 in soils
from fields under organic production than those in
conventional production (P = 0.05), but these dif-
ferences were not maintained over time. Total fungi
and culturable bacteria were more abundant in soils
with alternative than synthetic fertility amendments
fertilizers in 1997 but not in 1996.
3.2. Soil chemical components
Several soil chemical factors were affected by soil
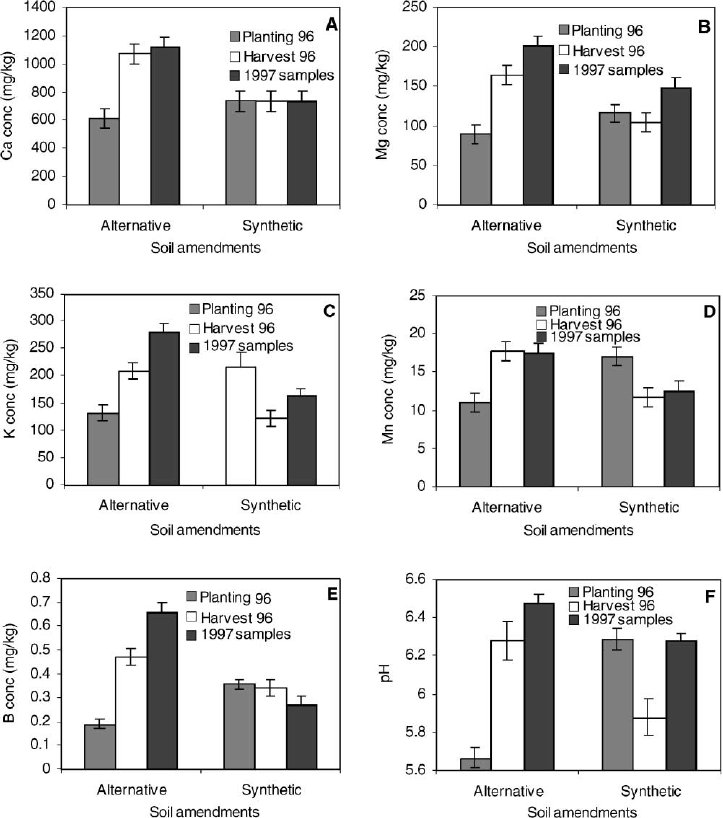
fertility amendments and time (Fig. 3). Calcium con-
centrations in soils with alternative fertility amend-
ments were increased two-fold over the 2-year period.
In contrast, no increase in calcium concentrations
occurred in soils with the synthetic fertilizers (P<
0.01, Fig. 3A). Similarly, magnesium concentrations
154 L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160
Fig. 3. Effects of alternative and synthetic soil fertility amendments and time on concentrations of (A) calcium (Lsd = 70mg/kg
soil); (B) magnesium (Lsd = 12 mg/kg soil); (C) potassium (Lsd = 14 mg/kg soil); (D) manganese (Lsd = 1.2 mg/kg soil); (E) Boron
(Lsd = 0.06mg/kg soil); and (F) pH (Lsd = 0.09 pH), respectively, from field soils in 1996 and 1997.
more than doubled in soils amended with alternative
fertility amendments, whereas only slight increases
in magnesium concentrations were observed in soils
with synthetic fertilizers over the same time period
(P<0.01, Fig. 3B). Potassium concentrations in
soils amended with alternative fertility amendments
increased by a factor of 3, and were higher at the end
of the second year in soils with alternative amend-
ments than in soils with synthetic fertility amend-
ments, whereas potassium concentrations decreased
over time in soils with synthetic fertilizers (P = 0.01,
Fig. 3C). Soil manganese concentrations increased
over time in soils amended with alternative fertility
amendments, but decreased in soils with synthetic
fertilizers (P = 0.02, Fig. 3D). Boron increased in
soils with alternative fertility amendments over time

L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160 155
Table 4
Chemical and physical parameters as affected by alternative or
synthetic soil fertility amendments at six grower locations after 2
years
Chemical or physical
factor
Soil amendment
Alternative
a
Synthetic
b
Lsd
c
Organic matter (%) 2.83 2.00 0.25
Total C (%) 1.90 1.17 0.29
CEC (cmol/kg) 7.97 6.05 0.84
Bulk density (g/cm
3
) 1.01 1.17 0.07
a
Alternative soil amendments were either cotton-gin trash, hay
manure compost or yardwaste.
b
Synthetic soil amendments were commercial fertilizers.
c
Lsd from ls-means procedure in SAS 7.0.
whereas no differences in boron concentration were
observed in soils with synthetic fertilizers (P = 0.003,
Fig. 3E). Soil fertility amendments also affected soil
pH (P = 0.05, Fig. 3F). Soils with alternative fertility
amendments initially had a lower soil pH than soils
with synthetic fertilizers, but over time soil pH in-
creased in soils with alternative amendments to higher
levels than pH in soils with synthetic fertilizers. Levels
of other soil nutrients (zinc, iron and aluminum) were
not affected by soils amendment, sample time, produc-
tion history or interactions of these components in
1996 and 1997. However, in 1997, copper and phos-
phorus levels were higher in soils with alternative
than synthetic soil fertility amendments.
Mean soil organic matter, total C, and CEC were
higher and bulk density was lower in plots with the
alternative soil amendments compared to synthetic
fertilizers after 2 years (Table 4). Continuous annual
applications of compost are typically required to
cause significant enhancements in these soil prop-
erties (Mays et al., 1973; Shiralipour et al., 1992),
Table 5
Partial correlation matrix (r
2
) from microbial data, and soil chemical data from principle components analysis in 1996 and 1997
Partial correlation matrix (r
2
)
Trichoderma
species
Thermophilic
microorganisms
Phytophthora
and Pythium spp.
Enteric
bacteria
Calcium 0.483
a
0.328
a
0.316 0.109
Magnesium 0.451
a
0.418
a
0.286 0.144
Potassium 0.317 0.256 0.110 0.123
Manganese 0.359
a
0.245 0.279 0.028
a
Significant correlations (P<0.05) from principle components procedure in SAS 7.0.
thus, it was not surprising that these changes were not
observed at all locations after the first year.
3.3. Canonical correlations and principle
components of soil chemical and microbial
parameters
A positive correlation of principle components was
detected between the levels of calcium, magnesium
and manganese, and propagule densities of Tricho-
derma species in soils (Table 5). Significant positive
correlation exists when the r
2
was above 0.32. Propag-
ule densities of thermophilic microorganisms were
also positively correlated with levels of calcium and
magnesium in soils (Table 5). Other correlations were
not significant.
Analyses of canonical correlations of soil chemical
components and soil microbial propagule densities
revealed that specific chemical components of the soil
and some propagule densities of soil microorganisms
were highly correlated (Fig. 4). For each sample time,
a correlation coefficient of 0.89–0.98 was observed
for the interaction of the first-order canonical cor-
relations. Cumulative correlations of 88–97% were
observed in the first four canonical correlations over
time. Clustering of correlations existed with more
negative correlations associated with conventional
production systems and synthetic fertilizers, with 27
of 36 canonical correlations in the fourth quadrant
(−X, −Y) (Fig. 4). More positive correlations were as-
sociated with organic production systems and organic
amendments, with 20 of 36 canonical correlations in
the first quadrant (+X, +Y) (Fig. 4). Organic pro-
duction systems with synthetic fertilizers had 19 of
36 canonical correlations in the fourth quadrant, and
12 of 36 canonical correlations in the first quadrant
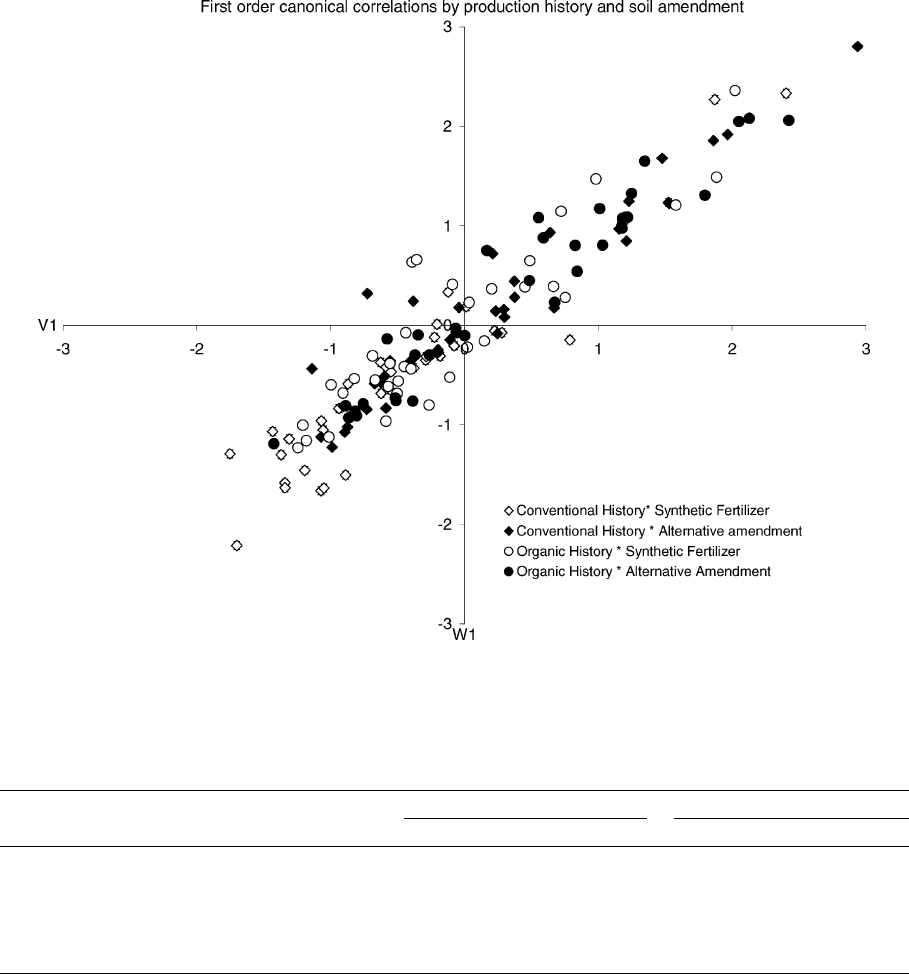
156 L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160
Fig. 4. First-order canonical of all chemical parameters (W1) with all microbial propagule densities (V1) over time (first-order canonical
correlations coefficient of correlation = 0.98). Canonical correlations are identified by production history and soil amendment.
Table 6
Impact of alternative and synthetic fertility amendments on yield of vegetables from grower fields with a history of organic or conventional
production in 1996 and 1997
Grower Production history
a
Crops 1996/1997
b
Yield 1996 (Mg/ha) Yield 1997 (Mg/ha)
Alternative
c
Synthetic
d
Lsd
e
Alternative Synthetic Lsd
d
1 Conventional Melon/tomatoes 14.88 23.09 5.63 28.07 26.30 13.22
2 Conventional Melon/tomatoes 16.25 15.42 3.29 6.32 3.72 4.65
3 Conventional Melon/tomatoes 3.97 4.82 1.90 14.35 17.28 5.41
4 Organic Sweet corn/tomatoes 6.66 10.96 2.16 40.93 28.54 6.49
5 Organic Sweet corn/tomatoes 6.90 8.81 4.01 39.50 47.86 11.40
6 Organic Sweet corn/tomatoes 2.53 2.78 1.17 32.82 37.84 9.54
a
Fields under conventional production included monoculture of vegetable or field crops for several years and a history of synthetic
fertilizers and pesticide use, while fields under organic production included 3 years of organic amendments and no chemical pesticide use.
b
Melon (Cucumis melo var. reticulatus or Citrullus lanatus) or sweet corn (Zea mays var. “Silver Queen”) were planted in 1996 and
tomatoes (L. esculentum var. Celebrity or Mountain Spring was planted in 1997.
c
Alternative amendments were either composted cotton-gin trash, composted yard waste, composted hay-manure, or composted cattle
manure.
d
Conventional amendments were synthetic fertilizers.
e
Least significant difference from 95% confidence intervals from SAS 7.0.
L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160 157
(Fig. 4). Conventional production systems with or-
ganic amendments had 17 of 36 canonical correlations
in both the first and fourth quadrants (Fig. 4).
3.4. Yield
Differences in yield of melon, corn or tomatoes
were not detected on four of six farms in plots
amended with alternative or synthetic fertilizer in
1996 and five of six farms in 1997 (Table 6). Yields
from different growers could not be compared sta-
tistically because growers grew different crops and
used different production practices. Nevertheless, in
1997 when all growers grew tomatoes, growers with
a history of organic production had higher yields than
growers with a history of conventional production,
regardless of soil amendments.
4. Discussion
Specific components of the soil microbial commu-
nity were changed by the addition of synthetic or alter-
native fertility amendments to soil in this experiment.
The addition of alternative soil amendments led to
increased propagule densities of Trichoderma species,
thermophilic microorganisms, enteric bacteria, and
decreased numbers of plant-pathogenic microorgan-
isms, such as Phytophthora and Pythium species in
soil. These changes were observed regardless of previ-
ous production history on a particular farm. Therefore,
soil quality on conventional farms was significantly
improved over a 2-year period by the addition of
organic fertility amendments. Furthermore, little yield
difference was observed in on-farm comparisons.
Production history influenced initial propagule
densities of Trichoderma species. Soils with a history
of organic production had higher initial populations
of these fungi than soils with a conventional history.
Conventional field soils amended with alternative fer-
tility amendments had significantly higher propagule
densities of Trichoderma at the end of the second
year, but these levels remained lower than in soils
with a history of organic production.
Trichoderma species are known biological control
agents of many different plant-pathogenic fungi
(Punja et al., 1982; Papavizas and Lewis, 1989;
Abada, 1994; Benhamou and Chet, 1996). Because
propagule densities of Trichoderma species were
higher in soils amended with organic than synthetic
soil amendments, lower propagule densities of soil-
borne plant pathogens might be expected in the
organically-amended soils. Soils may have indige-
nous populations of Trichoderma species, but these
fungi also can be added to soils through the applica-
tion of composted organic materials, as they are able
to quickly colonize compost during curing (Hoitink,
1986). We found similar results in soils amended with
composted plant materials in experiment station plots
(Bulluck and Ristaino, 2002).
Numbers of thermophilic microorganisms were also
higher in soils amended with organic amendments
than soils amended with synthetic fertilizers. Actino-
mycetes were a major constituent of the thermophilic
microorganisms detected in our study. Greater propag-
ule densities of actinomycetes in tomato field soils
under organic production compared with conventional
production systems in California have also been
reported (Drinkwater et al., 1995). Actinomycetes
present in alternative fertility amendments used in
avocado plantations were suppressive to Phytoph-
thora species (You and Sivasithamparam, 1995; You
et al., 1996). Since thermophilic microorganisms were
more abundant in soils with organic amendments, this
may explain lower propagule densities of Phytoph-
thora and Pythium species in soils in our study with
alternative fertility amendments.
In this study, we observed higher numbers of enteric
bacteria in soils with organic amendments than in soils
with synthetic fertilizers. However, enteric bacteria
were also present in soils with synthetic fertilizers at
densities greater than 1.0 × 10
7
CFU/g soil. Research
has shown that E. coli that was released in water was
killed in 10 days, and those released to soil were
reduced by 8 orders of magnitude in 60 days
(Bogosian et al., 1996). Because E. coli, Salmonella
spp., and other enteric bacteria are adapted to an envi-
ronment with a constant nutrient supply and temper-
ature, their survival rates in soils are minimal. Most
cases of food-related illness in the US are caused
by enteric bacterial pathogens in undercooked meat,
eggs, poultry, or contaminated deli meats and are not
linked to contaminated produce (Mead et al., 1999;
Food Safety and Inspection Service, 1998).
Much of the research that compares different
types of production systems is conducted in fields at
158 L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160
experimental stations, because of the inherent diffi-
culty associated with using grower fields for com-
parisons. Tomato agroecosystems were studied under
conventional or organic production systems in grower
fields in a California study (Workneh et al., 1993;
Workneh and van Bruggen, 1994; Drinkwater et al.,
1995). Microbial activity and nitrogen mineraliza-
tion rates were higher under organic production than
under conventional production practices in experi-
mental plots (Workneh et al., 1993; Workneh and van
Bruggen, 1994).
In our study, differences in chemical properties of
the soil were more related to amendment type than
to production history. Calcium, potassium, magne-
sium and manganese increased in the soils in our
study that received organic amendments, but not in
those soils receiving synthetic fertilizers. Clark et al.
(1998) found that concentrations of carbon, phospho-
rus, potassium, calcium, and magnesium were greater
in soils with incorporated manures and cover crops,
and soil carbon, phosphorus, and potassium declined
after manure applications ceased. Soils with alterna-
tive fertility amendments initially had a lower soil
pH than soils with synthetic fertilizers, but over time
pH increased in soils with alternative amendments
to higher levels than pH in soils with synthetic fer-
tilizers. Despite the soil pH-lowering mineralization
that occurs upon addition of composted N-containing
organic wastes to soil (Bevacqua and Mellano, 1994;
Sikora and Yakovchenko, 1996), compost additions
typically raise the pH of acid soils by complexing
Al and increasing base saturation (Shiralipour et al.,
1992; Van den Berghe and Hue, 1999).
Organic amendments provide advantages beyond
the benefits of increased organic matter content on
soil physical and chemical properties since nutrients
that are seldom applied by farmers (e.g. manganese,
zinc, and sulfur) are added as insurance against
potential yield limitations. Furthermore, nutrients that
are normally applied in commercial fertilizers (e.g.
potassium) and liming sources (i.e. calcium, magne-
sium) are supplemented in organic amendments and
permitted to accrue in the soil.
Yield increases in fields transitioning from conven-
tional to organic production systems usually require
3–5 years to detect (Parr et al., 1992; Altieri, 1995).
The sustainability of organic production systems has
been questioned recently (Trewavas, 2001). However,
in a recent study, yield of apples under organic, in-
tegrated, and conventional production systems were
equal (Reganold et al., 2001). In addition, lower neg-
ative environmental impact, higher profitability, and
higher apple fruit quality were demonstrated in the
organic farming systems (Reganold et al., 2001). No
differences in the yields of tomato were observed
between organic and conventional production in
California (Drinkwater et al., 1995). Similarly, soy-
bean yields were as high in fields undergoing transition
from conventional to low-input production as in fields
under conventional production practices (Liebhardt
et al., 1989). In our study, yields were higher in fields
under organic production than conventional produc-
tion in the second year, and these differences were
not related to soil amendment type used in a given
year at a given location. Field soils on organic farms
were more productive than conventional fields prob-
ably due to the beneficial effects on soil properties of
long-term organic amendments. Few statistically sig-
nificant differences in yields were observed between
soils amended with alternative amendments and soils
amended with synthetic fertilizers regardless of pro-
duction system. Therefore, the argument that organic
farming is equivalent to low yield farming is not
supported by our data (Avery, 1995).
The use of recycled organic wastes as alterna-
tive soil fertility amendments can result in increased
organic matter and biological activity in soils. Our
results demonstrate that alternative soil amendments
can enhance soil biological, chemical, and physical
attributes of soil compared with synthetic fertilizers
and improve plant yield. The use of alternative soil
amendments can result in a higher quality soil and
greater plant disease suppressiveness (Bulluck and
Ristaino, 2002).
Acknowledgements
The authors would like to thank committee
members, Dr. Frank Louws and Dr. Ken Barker of
the Department of Plant Pathology, and Dr. Mary
Barbercheck, of the Department of Entomology for
comments and suggestions and Dr. Marcia Gumpertz,
Department of Statistics for statistical consulting on
this research. This research was funded by a sub-
contract from the USDA Southern SARE grant no.
L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160 159
LS95-70. The contributions of the six grower collab-
orators including Mr. Joel Copeland, Ms. Nell Faulk,
Mr. Michael Heller (Claggett Farms, Chesapeake Bay
Foundation), Ms. Ellen Polishuk, Mr. John Smith and
Mr. Arthur Whitener are greatly appreciated.
References
Abada, K.A., 1994. Fungi causing damping-off and root-rot
on sugar-beet and their biological control with Trichoderma
harzianum. Agric. Ecosyst. Environ. 51, 333–337.
Altieri, M.A., 1995. Agroecology. The Science of Sustainable
Agriculture. Westview Press, Boulder, CO, p. 433.
Avery, D.T., 1995. Saving the Planet with Pesticides and Plastics:
the Environmental Triumph of High-Yield Farming. Hudson
Institute, Indianapolis, IN, p. 432.
Benhamou, N., Chet, I., 1996. Parasitism of sclerotia of
Sclerotium rolfsii by Trichoderma harzianum: ultrastructural
and cytochemical aspects of the interaction. Phytopathology 86,
405–416.
Bevacqua, R.F., Mellano, V.J., 1994. Cumulative effects of sludge
compost on crop yields and soil properties. Commun. Soil Sci.
Plant Anal. 25, 395–406.
Blake, G.R., 1965. Bulk density. In: Black, C.A. et al. (Eds.),
Methods of Soil Analysis. Part 1, 1st Edition. ASA, Madison,
WI, Agron. Monogr. 9, 374–390.
Bogosian, G., Sammons, L.E., Morris, P.J.L., O’Neil, J.P.,
Heitkamp, M.A., Weber, D.B., 1996. Death of E. coli K-12
strain w3110 in soil and water. Appl. Environ. Microbiol. 62,
4114–4120.
Bulluck, L.R., Ristaino, J.B., 2002. Synthetic and organic
amendments affect southern blight, soil microbial communities
and yield of processing tomatoes, Phytopathology 92, in press.
Bremner, J.M., Mulvaney, C.S., 1982. Nitrogen-total. In: Page,
A.L., et al. (Eds.), Methods of Soil Analysis. Part 2, 2nd Edition.
ASA and SSSA, Madison, WI, Agron. Monogr. 9, 595–624.
Clark, M.S., Horwath, W.R., Shennan, C., Scow, K.M., 1998.
Changes in soil chemical properties resulting from organic and
low-input farming practices. Agron. J. 90, 662–671.
Donohue, S.J., 1992. Reference soil and media diagnostic proce-
dures for the southern region of the United States. Southern
Coop. Series Bull. No. 374. Virginia Agricultural Experiment
Station, Virginia Tech, Blacksburg, VA, p. 47.
Donohue, S.J., Heckendorn, S.E., 1994. Soil Test Recommenda-
tions for Virginia. Virginia Cooperative Extension, Blacksburg,
VA, p. 155.
Doran, J., 1995. Building soil quality. In: Proceedings of the
1995 Conservation Workshop on Opportunities and Challen-
ges in Sustainable Agriculture. Red Deer, Alta., Canada,
Alberta Conservation Tillage Society and Alberta Agriculture
Conservation, Development Branch, pp. 151–158.
Drinkwater, L.E., Letourneau, D.K., Workneh, F., van Bruggen,
A.H.C., Shennan, C., 1995. Fundamental differences between
conventional and organic tomato agroecosystems in California.
Ecol. Appl. 5, 1098–1112.
Drinkwater, L.E., Wagoner, P., Sarrantonio, M., 1998. Legume-
based cropping systems have reduced carbon and nitrogen
losses. Nature 396, 262–265.
Evanylo, G.K., 1994. Mineralization and availability of nitrogen
in organic waste-amended mid-Atlantic soils. In: Nelson, S.,
Elliott, P. (Eds.), Perspectives on Chesapeake Bay, 1994:
Advances in Estuarine Sciences. Chesapeake Bay Program.
Scientific and Technical Advisory Committee, CRC Publication
No. 147, pp. 77–103.
Food, Safety, and Inspection and Service, 1998. Report to
Congress: Food Net: An active surveillance system for bacterial
foodborne diseases in the United States. Washington, DC,
USDA, Food Safety and Inspection Service, p. 17.
Govindasamy, R., Italia, J., 1998. A willingness-to-purchase
comparison of integrated pest management and conventional
produce. Agribusiness 14, 403–414.
Gunapala, N., Scow, K., 1998. Dynamics of soil microbial biomass
and activity in conventional and organic farming systems. Soil
Biol. Biochem. 30, 805–816.
Hoitink, H.A.J., 1986. Basis for the control of soilborne plant
pathogens with composts. Ann. Rev. Phytopathol. 24, 93–114.
Keeney, D.R., Nelson, D.W., 1982. Nitrogen-inorganic forms. In:
Page, A.L., et al. (Eds.), Methods of Soil Analysis. Part 2,
2nd Edition. ASA and SSSA, Madison, WI, Agron. Monogr.
9, 643–698.
Klonsky, K., Tourte, L., 1998. Organic agricultural production in
the United States: debates and directions. Am. J. Agric. Econ.
80, 1119–1124.
Klute, A., 1986. Water retention: laboratory methods. In: Klute,
A., et al. (Eds.), Methods of Soil Analysis. Part 1, 2nd Edition.
ASA and SSSA, Madison, WI, Agron. Monogr. 9, 635–662.
Langley, J.A., Heady, E.O., Olson, K.D., 1983. The macro-
implications of a complete transformation of US agricultural
production to organic farming practices. Agric. Ecosyst.
Environ. 10, 323–333.
Liebhardt, W.C., Andrews, R.W., Culik, M.N., Harwood, R.R.,
Janke, R.R., Radke, J.K., Rieger-Schwartz, S.L., 1989. Crop
production during conversion from conventional to low-input
methods. Agron. J. 81, 150–159.
Lohr, L., 1998. Implications of organic certification for market
structure and trade. Am. J. Agric. Econ. 80, 1125–1133.
Masago, H., Yoshikawa, M., Fukada, M., Nakanishi, N., 1977.
Selective inhibition of Pythium spp. on a medium for
direct isolation of Phytophthora spp. from soils and plants.
Phytopathology 67, 425–428.
Mays, D.A., Terman, G.L., Duggan, J.C., 1973. Municipal
compost: effects on crop yields and soil properties. J. Environ.
Qual. 2, 89–92.
Mead, P.S., Slutsker, L., Dietz, V., McCaig, L.F., Bresee, J.S.,
Griffin, P.M., Tauxe, R.V., 1999. Food-related illness and death
in the United States. Emerg. Infect. Dis. 5, 607–625.
Nelson, D.W., Sommers, L.E., 1982. Total carbon, organic carbon,
and organic matter. In: Page, A.L., et al. (Eds.), Methods of
Soil Analysis. Part 2, 2nd Edition. ASA and SSSA, Madison,
WI, Agron. Monogr. 9, 539–581.
Newhouse, J.R., 1980. The biology of Cylindrocladium, taxonomy,
antagonism, and methods of isolation. MS Thesis, Department
of Biology, California State College, CA, p. 156.
160 L.R. Bulluck III et al. /Applied Soil Ecology 19 (2002) 147–160
Papavizas, G.C., Lewis, J.A., 1989. Effect of Gliocladium and
Trichoderma on damping-off and blight of snapbean caused by
Sclerotium rolfsii in the greenhouse. Plant Pathol. 38, 277–286.
Papavizas, G.C., Lumsden, R.D., 1982. Improved medium for iso-
lation of Trichoderma spp. from soil. Plant Dis. 66, 1019–1020.
Parr, J.F., Pappendick, R.I., Hornick, S.B., Meyer, R.E., 1992. Soil
quality: attributes and relationship to alternative and sustainable
agriculture. Am. J. Alt. Agric. 7, 5–11.
Punja, Z.K., Grogan, R.G., Unruh, T., 1982. Comparative control
of Sclerotium rolfsii on golf greens in northern California with
fungicides, inorganic salts, and Trichoderma spp.. Plant Dis.
66, 1125–1128.
Reganold, J.P., Glover, J.D., Andrews, P.K., Hinman, H.R., 2001.
Sustainability of three apple production systems. Nature 410,
926–930.
Rhoades, J.D., 1982. Cation exchange capacity. In: Page, A.L.,
et al. (Eds.), Methods of Soil Analysis. Part 2, 2nd Edition.
ASA and SSSA, Madison, WI, Agron. Monogr. 9, 149–158.
Sands, D.C., Rovira, A.D., 1970. Isolation of fluorescent
pseudomonads with a selective medium. Appl. Microbiol. 20,
513–514.
Shiralipour, A., McConnell, D.B., Smith, W.H., 1992. Physical
and chemical properties of soils as affected by municipal solid
waste compost application. Biomass and Bioenergy 3, 261–266.
Sikora, L.J., Yakovchenko, V., 1996. Soil organic matter minera-
lization after compost amendment. Soil Sci. Soc., Am. J. 60,
1401–1404.
Simpson, T.W., Donohue, S.J., Hawkins, G.W., Monnett, M.M.,
Baker, J.C., 1993. The development and implementation of
the Virginia agronomic land use evaluation system (VALUES).
Department of Crop and Soil Environmental Sciences, Virginia
Tech, Blacksburg, VA, p. 83.
Stamatiadis, S., Werner, M., Buchanan, M., 1999. Field assessment
of soil quality as affected by compost and fertilizer application
in a broccoli field (San Benito County, California). Appl. Soil
Ecol. 12, 217–225.
Stevens, R., 1974. Mycology Guidebook: University of Washington
Press, Seattle, p. 677.
Thomas, G.W., 1982. Exchangeable cations. In: Page, A.L., et al.
(Eds.), Methods of Soil Analysis. Part 1, 2nd Edition. ASA
and SSSA, Madison, WI, Agron. Monogr. 9, 159–166.
Thompson, G.T., 1998. Consumer demand for organic foods: what
we know and what we need to know. Am. J. Agric. Econ. 80,
1113–1118.
Trewavas, A., 2001. Urban myths of organic farming. Nature 410,
409–410.
Van den Berghe, C.H., Hue, N.V., 1999. Limiting potential of
composts applied to an acid oxisol in burundi. Compost Sci.
Utiliz. 7, 40–46.
Workneh, F., van Bruggen, A.H.C., 1994. Suppression of corky
root of tomatoes in soils from organic farms associated with
soil microbial activity and nitrogen status of soil and tomato
tissue. Phytopathology 84, 688–694.
Workneh, F., van Bruggen, A.H.C., Drinkwater, L.E., Shennan, C.,
1993. Variables associated with corky root and Phytophthora
root rot of tomatoes in organic and conventional farms.
Phytopathology 83, 581–589.
You, M.P., Sivasithamparam, K., 1995. Changes in microbial
populations of an avocado plantation mulch suppressive to
Phytophthora cinnamomi. Appl. Soil Ecol. 2, 33–43.
You, M.P., Sivasithamparam, K., Kurtböke, D.I., 1996.
Actinomycetes in organic mulch used in avocado plantations
and their ability to suppress Phytophthora cinnamomi. Biol.
Fertil. Soils 22, 237–242.
