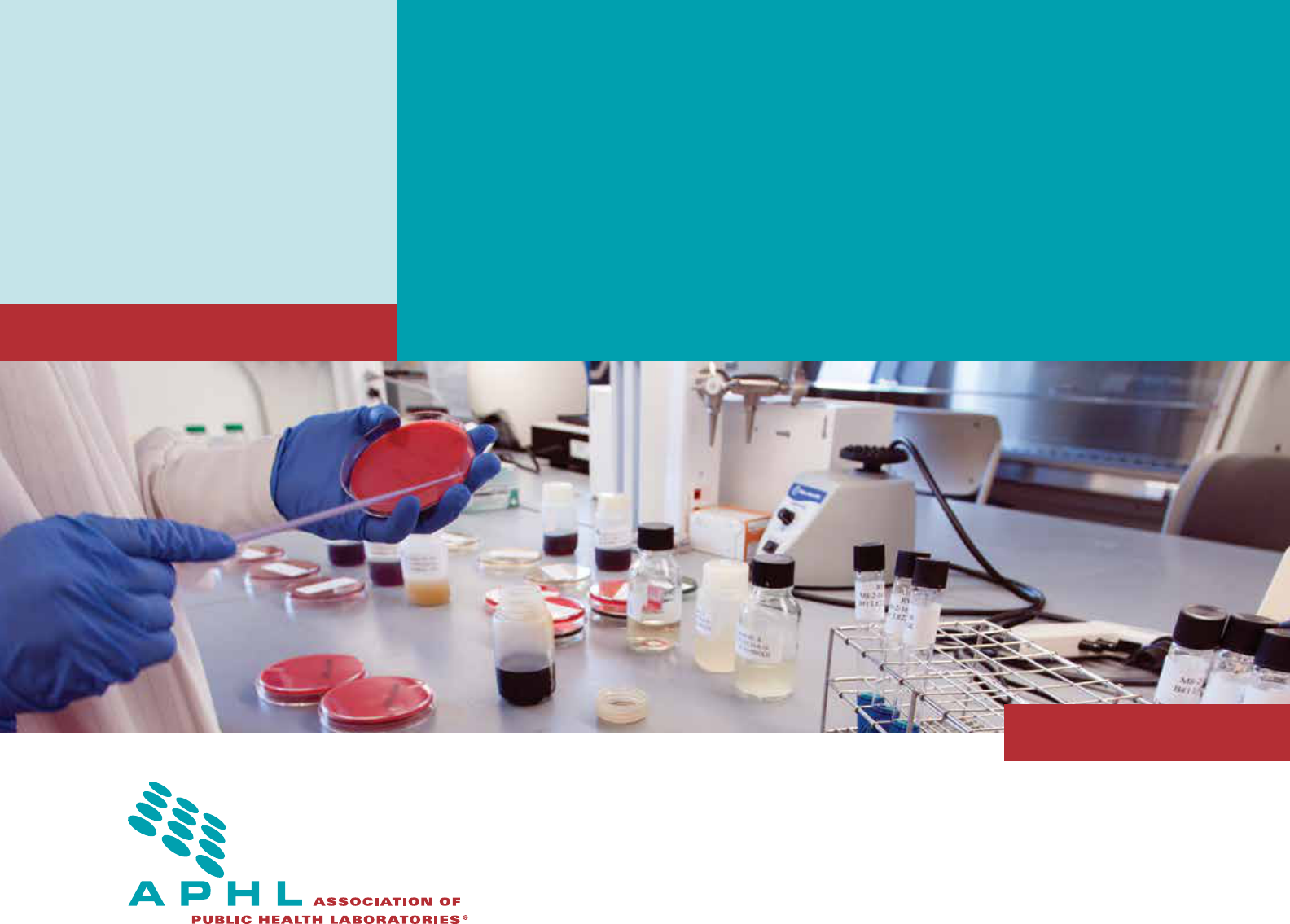
Laboratory
Internal Audit Plan
MAY 2017

APHL Laboratory Internal Audit Plan | 2
Acknowledgements
The following APHL workgroup members contributed to this document:
Charlene Thomas, BS, MT(ASCP), manager, microbiologist, Alabama Bureau of Clinical Laboratories (workgroup lead)
Lauren Farnsworth, MS, QA & Safety Program manager, Colorado Department of Public Health & Environment
Steve Gradus, PhD, D(ABMM), CLIA director, City of Milwaukee Health Department
Sheri Hearn, MPH, Quality Management ofcer, Oregon State Public Health Laboratory
Lorelei Kurimski, MS, director, Ofce of Organizational Development, State Hygienic Laboratory at the University of Iowa
Deborah Severson, BS MT(ASCP), director of Laboratory Services, Fairfax County Health Department Laboratory
Bertina Su, MPH, senior specialist, Quality Systems, Association of Public Health Laboratories

APHL Laboratory Internal Audit Plan | 3
APHL Internal Laboratory Audit Plan
A laboratory audit program is critical to ensuring the institution meets applicable requirements. Follow the guidelines below to develop appropriate
documents/forms/statements for each heading of your internal audit plan. Add more headings as necessary.
Purpose
This section should describe the purpose of the internal audit including any dened goals. For example:
• To determine overall compliance with internal policy or regulatory requirements in preparation for an external audit
• To conduct a follow-up internal audit in response to a complaint, poor external inspection or a nonconforming event
• To ensure a new method is compliant with regulatory requirements
• To provide assurance to management that the management system is implemented as intended
• To serve training purposes, learn the requirements of accreditation bodies (standards)
• To improve processes
• To verify compliance with the requirements of the quality management system, including analytical methods, SOPs,
the quality manual, ethics policies, data integrity, internal laboratory policies, and regulatory requirements such as
AIHA-LAP, LLC, CLIA and TNI
• To compile the appropriate documentation to include:
• Auditor reviews of corrective actions
• Auditee/section response if necessary
• Final report reviewed by management
• Follow-up if needed
Scope
This section should describe the range of the audit. For example:
• Ensuring that appropriate test procedures, equipment, controls, calibrations methods are established
• Ensuring that appropriate laboratory tests are ordered
• Procuring test samples in an efcient and timely manner
• Producing accurate laboratory test results
• Disseminating laboratory test information to clients/providers in a timely manner
• Utilizing qualied laboratory personnel
APHL Laboratory Internal Audit Plan | 4
Activities to
Be Audited
This section should dene the activities to be audited. For example:
• Preanalytical
Review of:
• Temperature logs
• Sample receipt logs
• Sample rejection logs
• Sample quality logs
Policies and procedures for:
• Sample or patient identication verication
• Sample collection, handling, and transportation
• Sample receipt, preparation, and storage
• Sample acceptance and rejection criteria
• Chain of custody
• Data entry and review
• Postanalytical
Review of:
• Non-conforming events/corrective actions
▫ Report completeness
▫ Turn-around-time
▫ Corrected reports
Policies and procedures for:
• Result release
• Report preparation and delivery
• Sample archiving and management
• Sample storage and disposal requirements
• Demographic and results corrections (corrected reports)
• Analytical
Review of:
• Temperature logs
• Review of instrument runs
• Reagent and standard logs
• Instrument/equipment maintenance logs
• Prociency tests
• Failed runs
Policies and procedures for:
• Testing and examinations
• Results review and follow-up
• Critical values
• Method verication and validation
• Quality control requirements; type, frequency, and
corrective action for out of range QC
• Calibration and calibration verication
APHL Laboratory Internal Audit Plan | 5
Reference
Requirements
This section should reference documents/regulations pertinent to the performance of the laboratory test. For example:
• Clinical Laboratory Improvement Amendments of 1988. 42 CFR Parts 493.
• EPA Manual for the Certication of Laboratories Analyzing Drinking Water Criteria and Procedures Quality Assurance
Fifth Edition
• ISO/IEC 17025 General Requirements for the Competence of Testing and Calibration Laboratories, Second edition
2005-05-15
APHL Laboratory Internal Audit Plan | 6
Assigned
Auditors(s)
This section should establish the general qualications, responsibilities, training and continuing education of the
auditors. For example:
• Personnel are trained and qualied
• Personnel are independent of the activity to be audited.
• Training
• Internal training provided by quality managers or a designated quality group
• External training available through accrediting bodies such as A2LA
• Resources available on web (be sure these are from qualied organizations)
• Training certicates maintained in auditors’ training les
• Auditor responsibilities
• Reviews prior documents and audit reports
• Performs the audit
• Asks objective questions (e.g., “What is your process?”)
• Reviews documents and records
• Seeks lead auditor help when necessary
• Completes audit form and summary
• Reviews non-conformances with management
• Submits review documentation to lead auditor for review
• Attends meetings
• Discusses ndings and answers questions
• Lead auditor responsibilities (usually the quality manager)
• Plans and schedules
• Forms the team
• Provides documents and reviews with team
• Assigns standards segments to team
• Assists with the audit
• Reviews the audit
• Discusses obstacles and challenges with leadership
• Helps with audit report
• Manages meetings

APHL Laboratory Internal Audit Plan | 7
Schedule
This section should give an overview of the timetable for the audits.
An internal audit is a formal laboratory activity that must be performed in accordance with a documented procedure and
on a regular schedule. Laboratories may choose to conduct a full laboratory audit annually or biannually, or to audit parts
of their system every month. Generally it is recommended that a laboratory audit every part of its management system,
including its testing and/or calibration activities, at least once every twelve months.
Steps may include:
• Determine and document with a planning calendar regulators’ inspection cycles, e.g., biennial CLIA inspections.
• Plan “off year” internal inspections in preparation for regulators’ inspections. At a minimum, allow 3-6 months lead
time for internal inspection prior to “ofcial” inspection.
• Develop a pre-determined plan for walk-through inspections by supervisors/management using checklists. Use
regulators’ checklists if available.
• Throughout the year, plan regular (e.g., monthly, quarterly, etc.) inspections of the laboratory. Depending on the size
and complexity of the facility, this could be by section, workbench or the entire laboratory.
Sampling Plan
This section should cover activities required to complete the laboratory test. Horizontal audits usually capture processes
from beginning to end (e.g., a training program). Vertical audits usually provide a deep look into a specic process
(e.g., sample receipt acceptability and rejection).
For example:
• Sample tracking (Horizontal)
• Nonconformance program (Horizontal)
• Sample receipt and login (Vertical)
• Turnaround time (Vertical)
APHL Laboratory Internal Audit Plan | 8
Report of Findings
(Nonconforming
Activities/Actions)
This section should explain how nonconforming events are documented, including follow-up actions. For example:
• Some checklists are available through regulatory bodies for use in internal audits and reports
• Verbal
▫ Begin with what was positive in the audit
▫ Cite the standard used to identify any non-conformances (regulation/accreditation, method, policy,
procedure, checklist, etc.)
▫ Identify any actions required (immediate correction, timeline for corrective action or recommendation)
• Written Report (generally provided within 2 weeks of audit)
▫ Capture individuals interviewed
▫ Provide checklist with ndings (include dates of audit)
▫ Begin with what was positive in the audit
▫ Provide summary of ndings (mention documents, records, equipment, etc.)
▫ Cite the standard used to identify any non-conformances (regulation/accreditation, method, policy,
procedure, checklist, etc.)
▫ Identify any action required (immediate correction, timeline for corrective action or recommendation)
▫ Submit overall conclusions and recommendations
▫ Examples of terminology to use
■ There is no evidence…
■ There is insufcient evidence…
■ Current practice does not reect…
■ Current documentation…
■ Not all…
■ The procedure for ____ is not fully implemented

APHL Laboratory Internal Audit Plan | 9
Report of Findings
(Nonconforming
Activities/Actions)
• Reports go to section audited (supervisor and/or section manager) with a copy to management
• Auditee responds to audit with any required corrective actions
▫ Response and/or results of root cause analysis with a statement of action: Detail any corrections made while
identifying the underlying cause. Include a statement of the corrective action for each nonconformity/deciency.
Perform a root cause analysis of the problem or use another form of analysis such as Dene, Analyze, Resolution,
Action(s) taken, also known as “The Five Whys;” cause mapping; shbone diagrams; or Plan, Do, Check, Act (PDCA)
▫ Proof of commitment: Indicate changes in documentation. If there was a written procedure that was not being
followed, then provide other evidence (e.g., document name and section if updated)
▫ Objective evidence of compliance: Provide copies of laboratory records demonstrating compliance or provide a
timeline/deadline of when the compliance will be achieved.
■ Auditor reviews corrective actions and responds
■ Auditee/section responds if necessary
■ Final report reviewed by management
■ Follow-up if needed
Quality Function
Approval and Date
This section should include the signatures/dates of the management staff accountable for the audit ndings. It is
recommended that nonconformances and corrective actions be shared with all staff in the relevant laboratory section.
Effectiveness
of Actions
(taken over time)
This section should describe the effectiveness of actions taken over time in response to identied internal audit
nonconformance(s). The specic nonconforming activities determine whether immediate actions, corrective actions or
both are executed. For example:
• Review immediate correction for effectiveness. This may include reviewing specic logs (after a period of time
determined by the remediation plan) to ensure nonconformances are appropriately documented.
• Review corrective actions for effectiveness. This may include updating an SOP, retraining and monitoring periodically
over a longer period of time (typically 3 months to 1 year).
Documentation of ndings from the review(s) should indicate whether the actions taken were effective. If so, the results
should be shared with staff and acknowledgements signed by appropriate management.
If the results were not effective, a root cause analysis should be performed if not completed already, and further
corrective actions identied, with a new plan and another review for follow-up effectiveness.
APHL Laboratory Internal Audit Plan | 10
Tracking/
Monitoring
This section should describe the process for correction of a documented nonconformance activity. For example:
• Non-con
formance/deficiencies should be documented and tracked by management or delegated to staff as
appropriate.
• Assessments are written whenever an audit finds a non-conformance/deficiency
• Assessments include timelines for expected action
• Assessments should be documented using an assessment tool such as a root cause analysis form
• Non-conformance/deficiencies should be monitored for trends over time

Association of Public Health Laboratories
The Association of Public Health Laboratories (APHL) works to strengthen laboratory systems serving the public’s health in the US
and globally. APHL’s member laboratories protect the public’s health by monitoring and detecting infectious and foodborne diseases,
environmental contaminants, terrorist agents, genetic disorders in newborns and other diverse health threats.
This project was 100% funded with federal funds from a federal program of $459,900. This publication was supported by Cooperative
Agreement # NU60OE000103 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the
authors and do not necessarily represent the ofcial views of CDC or the Department of Health and Human Services.
8515 Georgia Avenue, Suite 700
Silver Spring, MD 20910
Phone: 240.485.2745
Fax: 240.485.2700
Web: www.aphl.org
© Copyright 2017, Association of Public Health Laboratories. All Rights Reserved.
