
Clinical Audit
A Practical Guide 2023
HSE National Centre for Clinical Audit
Scan for National
Centre for Clinical
Audit website
HSE National Centre for Clinical Audit

Clinical Audit A Practical Guide
2
Reader Information
Title:
HSE National Centre for Clinical Audit
Clinical Audit – A Practical Guide
Purpose:
This document replaces the previously published “A Practical Guide to
Clinical Audit” (HSE 2013). It forms part of a series of resources being
developed to support services and all healthcare professionals to ensure
the continuation and development of effective clinical audit across the Irish
healthcare system as recommended by the National Review of Clinical
Audit (HSE 2019).
Clinical Audit – A Practical Guide should be read in conjunction with HSE
NCCA Nomenclature (see web link below).
Developed by: HSE National Centre for Clinical Audit
Approved by:
Chief Clinical Ofcer Dr. Colm Henry, Chair, National Steering Group for
Clinical Audit
National Clinical Director Dr. Orla Healy, NQPSD, Co-Chair National
Steering Group for Clinical Audit
Publication Date: March 2023
Version Number: 2
ISBN Number: 978-1-78602-220-2
Target Audience:
All HSE staff, clinical audit service providers and all agencies involved in
clinical audit
Cite this document as:
Health Service Executive (HSE), National Centre for Clinical Audit, Clinical
Audit – A Practical Guide, National Quality and Patient Safety Directorate,
March 2023.
Contact Details for further
information:
HSE National Centre for Clinical Audit
E-mail: [email protected]
https://www2.healthservice.hse.ie/organisation/ncca/
Related/ Associated
documents:
HSE National Centre for Clinical Audit Nomenclature
https://www.hse.ie/eng/about/who/nqpsd/ncca/nomenclature-a-glossa-
ry-of-terms-for-clinical-audit.pdf
Health Service Executive (HSE) National Review of Clinical Audit Report,
November 2019
https://www.hse.ie/eng/services/publications/national-review-of-clinical-au-
dit-report-2019.pdf
Revision date: March 2025
Access: https://www2.healthservice.hse.ie/organisation/ncca/
Creative Commons License and Legal Disclaimer
is work is licensed under an Attribution-Non Commercial-ShareAlike: CC BY-NC-SA 4.0 International License.
is publication was developed by the Health Service Executive (the “HSE”) specically for use in clinical audit and quality, patient
safety and improvement initiatives. e HSE shall have no liability, whether in contract, tort or otherwise, for any injury, loss damage
or claims whatsoever arising out of or in connection with, any third party’s use of the materials or any part thereof. Please contact the
National Centre for Clinical Audit team at [email protected] for more detailed information on the terms and conditions of use.

Clinical Audit A Practical Guide
3
1. Select Topic
2. Set Criteria &
Standards
5. Clinical Audit
Report
6. QI Plan & Action
7. Re-Audit
3. Design Audit Tool
& Collect Data
4. Analyse Data & Compare
Results with standards
The Seven Stages of Clinical Audit
Stage 1 Select Topic
Stage 2 Set Criteria and Standards
Stage 3 Design Clinical Audit Tool and Collect Data
Stage 4 Analyse Data and Compare Results with Standards
Stage 5 Clinical Audit Report
Stage 6 QI Plan and Action
Stage 7 Re-Audit

Clinical Audit A Practical Guide
4
Contents
Page
1.0 Background and Context 5
2.0 Introduction 5
3.0 The Seven Stages of Clinical Audit 6
3.1 Select Topic 7
3.2 Criteria and Standards 13
3.3 Design Audit Tool and Collect Data 18
3.4 Analyze Data and Compare Results with Standards 24
3.5 Clinical Audit Report 28
3.6 QI Plan and Action 31
3.7 Re-audit 34
4.0 Data Protection and Ethical issues for Clinical Audit 37
4.1 Data Protection Responsibilities 37
4.2 Data Protection Principles 37
4.3 Data Protection Guidelines 37
4.4 Ethical Issues 38
4.5 Is Ethical Review Required for Clinical Audit 39
4.6 Guidance Regarding Consent Requirements for Clinical Audit 40
4.7 Condentiality and Access to Service User Health Information 40
4.8 Anonymisation of Data 41
5.0 Organisational Requirements for Clinical Audit 43
5.1 Clinical Audit Strategy 43
5.2 Clinical Audit Policy 43
5.3 Clinical Audit Programme 43
5.4 Clinical Audit Leads 44
5.5 Fostering a Culture which is Supportive of Clinical Audit 44
5.6 Practical Supports for Clinical Audit 45
5.7 Clinical Audit Facilitation 45
References 46
Glossary of Terms 48
Resources 53
Resource 1: Checklist for Best Practice in Clinical Audit 53
Resource 2: Clinical Audit Sample Proposal Form 54
Resource 3: Clinical Audit Report Sample Template 59
Resource 4: Quality Improvement Plan / Action Plan Sample Template 61
Resource 5: Model for Improvement 62

Clinical Audit A Practical Guide
5
1. Background and Context
The Health Service Executive (HSE) National Centre for Clinical Audit (NCCA) was established within the
National Quality and Patient Safety Directorate (NQPSD) in 2022, following publication of the HSE National
Review of Clinical Audit Report 2019. Establishing the HSE NCCA marks an important step in the HSE’s
continued efforts to improve the quality and safety of healthcare for patients. This will strengthen the
development of an end-to-end process for clinical audit in accordance with the recommendations in the report
and meet the needs of clinical audit service providers and multidisciplinary stakeholders.
The NCCA is primarily responsible for implementing the HSE National Review of Clinical Audit Report
recommendations under ve key pillars:
• National Governance of Clinical Audit
• Local Governance of Clinical Audit
• Clinical Audit Training
• Clinical Audit Education Resources
• Legislative Changes affecting Clinical Audit (i.e. GDPR and Data Protection)
The National Review of Clinical Audit Report 2019 identied the importance of developing guidance for Clinical
Audit, one of which includes “A Practical Guide for Clinical Audit should be updated to reect best practice in
clinical audit and healthcare professionals should use this new guidance to design and develop their clinical
audits”. This will support and improve the consistency and quality of clinical audit across the health service to
support the planning and management of high-quality healthcare.
2. Introduction
Following the establishment of the NCCA and progression of the agreed recommendations and programme
of work, we are very pleased to present Clinical Audit – A Practical Guide, which contains the agreed seven
stages produced following the publication of the Report. The Practical Guide should be adopted by the HSE
and become the national standard for clinical audit for all agencies involved in clinical audit. The National
Review of Clinical Audit found that there were a number of different denitions for clinical audit across the
healthcare system, resulting in confusion around clinical audit design.
The denition contained in this document provides clarity with the stages of the clinical audit cycle;
(HSE NCCA Nomenclature document, A Glossary of Terms for Clinical Audit, 2022)
The aim of this guidance document is to assist and support healthcare staff in understanding the stages of the clinical
audit cycle, to help support best practice in clinical audit and to improve awareness of clinical audit as an essential
and integral component of clinical practice. Clinical audit provides the ability to improve the quality of patient care
in a collaborative and systematic way. When clinical audit is conducted well, it enables the quality of care to be
reviewed objectively within an approach which is supportive, developmental and focused on quality improvement.
“Clinical audit is a clinically-led quality improvement process that seeks to improve patient care
and outcomes through systematic review of care against explicit criteria and acting to improve
care when standards are not met. The process involves the selection of aspects of the structure,
processes and outcomes of care which are then systematically evaluated against explicit criteria.
If required, improvements should be implemented at an individual, team or organisation level and
then the care re-evaluated to conrm improvements.”
DOHC (2008, p.152)
Clinically-led includes the breadth of clinical professionals working in health and social care services.
Clinical Audit A Practical Guide
6
3. The Seven Stages of Clinical Audit
The agreed seven stages of clinical audit in this guidance document, produced as a result of the National
Review of Clinical Audit, should be adopted by the HSE, and become the national standard for clinical audit for
all agencies involved in clinical audit. Each of the seven stages of the Clinical Audit cycle has individual steps
involved which are detailed in sections 3.1 to 3.7 of this document.
Each stage of the clinical audit cycle must be undertaken to ensure that a clinical audit is systematic and
successful.
1. Select Topic
2. Set Criteria &
Standards
5. Clinical Audit
Report
6. QI Plan & Action
7. Re-Audit
3. Design Audit Tool
& Collect Data
4. Analyse Data & Compare
Results with standards
Figure 1: The Seven Stages of the Clinical Audit Cycle
Stage 1 Select Topic
Stage 2 Set Criteria and Standards
Stage 3 Design Clinical Audit Tool and Collect Data
Stage 4 Analyse Data and Compare Results with Standards
Stage 5 Clinical Audit Report
Stage 6 QI Plan and Action
Stage 7 Re-Audit
Clinical Audit A Practical Guide
7
Stage 1: Select Topic

Clinical Audit A Practical Guide
8
Stage 1: Select Topic
1
The clinical audit should focus on improving care, patient safety and service delivery for patients
and healthcare professionals
2
A clear singular aim stating why the clinical audit needs to be undertaken should be agreed,
documented, and shared with all key stakeholders
3
A clinical audit lead with the necessary clinical audit skills must be identied to plan, oversee
and co-ordinate the clinical audit
4
A sub-group of multi-disciplinary key stakeholders needs to be identied to support delivery of
the clinical audit
5
Any topic selected for clinical audit should ideally incorporate patient/service-user views
6
Stakeholders must consider local and national reporting arrangements for proposed clinical
audits
7
A clinical audit proposal form must be completed and submitted to the local Clinical Audit/QPS
Committee for review and approval
8
Following approval, the proposal form must be completed
1. Select Topic
Figure 2: Clinical Audit Cycle Stage 1– Select Topic

Clinical Audit A Practical Guide
9
3.1 Step 1: Involving Stakeholders
Anyone involved in providing or receiving care can be considered a stakeholder in clinical audit. Therefore, to
determine who should be involved in deciding on the topic and objectives of an audit, it is necessary to identify:
• Who is involved in the delivery of care?
• Who is in receipt of, uses or benet from the care or service?
• Who has the authority to support implementation of any identied changes?
When planning a clinical audit, the audit team should include service users in the audit process. For example,
would it be benecial to include their experience of receiving clinical care?
Common methods of involving service users in the clinical audit process are:
• Gathering service user feedback, for example letters of complaint or compliment
• Analysis of comments made at service user forums
• Interview with service users
• Service user surveys
• Focus groups
• Expert user groups
• Examining critical incidents
Where service users are involved in clinical audit, their roles need to be clearly dened and appropriate
support and guidance provided to them to enable delivery. (This should include the provision of information
and guidance in relation to data protection requirements).
Who has the authority to support implementation of any identied changes?
Commitment to the clinical audit process should be sought from those with the authority to approve changes
arising from audit recommendations, particularly if they have potential resource consequences or implications
for other service areas.
3.1 Step 2: Determining the clinical audit topic
Topics for clinical audit should be selected with a view to improving the quality or safety of care or of service
provision. The classication system of structure, process and outcome can be used to focus on areas of
practice from which a topic may be selected:
Structure
• Resources required to deliver care
• Environment in which care is delivered
• Equipment made available
• Documentation of policies, procedures, protocols and guidelines
Process
The procedures and practices implemented by staff in the prescription, delivery and evaluation of care

Clinical Audit A Practical Guide
10
Outcome
The effect of care received by service users as a result of healthcare provision and the costs to the service of
providing care, e.g., the result of clinical interventions
Questions to assist with determining an audit topic:
• Is the topic of high cost, high volume or high risk to staff and/or service users?
• Is there evidence of wide variation in clinical practice?
• Is good evidence available to inform clinical audit standards (systematic reviews or clinical guidelines)?
• Is the problem measurable against relevant standards?
• Is clinical auditing the problem likely to improve healthcare outcomes as well as process improvements?
• Is there evidence of a (serious) quality problem (e.g., service user complaints or high complication rates,
adverse outcomes or poor symptom control)?
• Is the topic of key professional or clinical interest?
• Are reliable sources of data readily available for data collection purposes?
• Can data be collected within a reasonable time period?
• Is the problem concerned amenable to change?
• Is the topic pertinent to national or local initiatives or priorities?
• Does the topic lend itself to the clinical audit process or is a different process more appropriate (e.g., look
back review, service evaluation)?
• How much scope is there for improvement and what are the potential benets of undertaking this clinical
audit?
3.1 Step 3: Planning the delivery of the clinical audit
Aims and objectives of the clinical audit
The audit team must understand the overall purpose of the audit they are about to perform. The purpose of the
audit must be outlined in the form of aims and objectives. Audit aims and objectives must be dened through
the use of verbs, such as:
• Improve
• Increase
• Enhance
• Ensure
• Change
Clinical audit is a quality improvement process so your audit objectives need to show the intent to improve. For
example, an audit of healthcare records might have as its overall aim:
‘To ensure the content of the healthcare record provides an accurate chronology of events’.
Verbs + aspects of quality can be used for the purpose of designing the clinical audit:
(Safe, Effective, Patient-Centred, Timely, Efcient and Equitable)

Clinical Audit A Practical Guide
11
‘Increase the timeliness of …’
‘Improve the effectiveness of …’
‘Ensure the safety of …’
Identifying the skills and people needed to carry out the audit
For a clinical audit to be successful and achieve its aim and purpose, it needs to involve the right people with
the right skills from the outset. Therefore, the identication of skills required and of individuals possessing
these skills should be a priority. The level of skill required for a clinical audit will also be dependent on the size
of the clinical audit.
Skills required for the clinical audit process:
• Leadership, organisational and management skills
• Clinical, managerial and other service input and leadership
• Project management skills
• Change management skills
• Clinical audit methodology expertise
• Understanding of data protection requirements
• Data collection and data analysis skills
• Facilitation skills
• Communication skills
• Interpersonal skills
The skills outlined should be drawn from all relevant groups involved in the delivery of care and the clinical
audit team should be multidisciplinary. To achieve the best possible results, all relevant staff groups should
have a degree of involvement in the performance of the clinical audit and in the implementation of quality
improvements.
For a clinical audit to be carried out effectively, all staff should be appropriately trained and briefed about their
role in the clinical audit. All members of the team should have:
• A basic understanding of clinical audit
• An understanding of and commitment to the plans and objectives of the clinical audit
• An understanding of what is expected of the clinical audit team and this should be claried at the outset
(Ashmore, Ruthven and Hazelwood, 2011a).
Providing the Necessary Structures
Appropriate structures and processes should be in place prior to the commencement of clinical audit work.
The clinical audit team should complete a Clinical Audit Proposal Form (see Resource 2). This ensures that
all aspects of the proposed clinical audit have been considered and that the clinical audit will be robust and of
high quality.
Completed forms along with supporting standards, clinical audit tool(s) and other documentation should be
submitted to the appropriate responsible clinical lead, directorate or governance committee for consideration
to ensure that the proposed clinical audit meets the requirements of the service provider through local clinical
audit channels.

Clinical Audit A Practical Guide
12
The team carrying out the clinical audit should ensure that appropriate resources are available with which
to perform the clinical audit and to implement quality improvements. Where there is an insufcient level of
resources available to carry out a clinical audit and the improvements, this issue should be raised through the
appropriate governance structures as and when they arise.
The structures should include a mechanism for the review of ndings and progress reporting to the appropriate
clinical lead, directorate or governance committee. Clear lines of accountability should be agreed at the outset
of the clinical audit.
It may be appropriate to consider and discuss the question of possible publication of clinical audit results via
conference proceedings, poster, oral presentation or journal article at the planning stage, particularly if the
planned clinical audit is large.
A timetable should be agreed for designing and carrying out the clinical audit. A simple Clinical Audit Checklist
may also be a useful tool (see Resource 1).

Clinical Audit A Practical Guide
13
Stage 2: Set Criteria and
Standards
Clinical Audit A Practical Guide
14
Stage 2: Set Criteria and Standards
1
The clinical audit must measure performance against standards
2
Standards for clinical audit must reect current best practice, be evidence-based and agreed
by all stakeholders
3
The clinical audit standards may measure structure, process and/or outcome
4
The clinical audit standards need to be clearly referenced to their source document
5
The clinical audit standards must be SMART (specic, measurable, achievable, realistic and
time-bound)
6
The clinical audit standards must be unambiguous and not be open to interpretation
7
The clinical audit standards must clearly state the target level of compliance that enables a
clear comparison to be made with current practice.
2. Set Criteria &
Standards
Figure 3: Clinical Audit Cycle Stage 2– Set Criteria and Standards

Clinical Audit A Practical Guide
15
3.2 Step 1: Selecting Best Practice Standards
When the clinical audit topic has been selected, the next essential step is to review the available evidence to
identify the standards and clinical audit criteria against which the clinical audit will be conducted.
Standards should be ‘robust’ and evidence based (Potter, Fuller & Ferris, 2010) and current.
When specifying standards, they are dened as structures and processes needed to identify, assess and
manage risks in relation to the subject area (for example, healthcare records management) (HSE, 2013).
What are clinical audit standards?
Clinical audit is a quality improvement cycle that involves measurement of the effectiveness of healthcare
against agreed and proven standards for high quality and taking action to bring practice in line with these
standards so as to improve the quality of care and health outcomes.
A standard is the desired and achievable level of performance against which performance can be measured.
Useful sources for standards include:
• Local standards, in the form of evidence-based guidelines
• Nationally endorsed clinical guidelines
• Standards and clinical guidelines from relevant quality and safety programmes, clinical care programmes
and professional bodies
• Clinical guideline development organisations such as NICE, SIGN, NCEC, Royal Colleges
If national or local guidelines are not available, a literature review can be carried out to identify the best and
most up to date evidence from which clinical audit standards and criteria may be generated.
For standards to be valid and lead to improvements in care, they should be consistent with SMART guidance.
• Specic (explicit statements, not open to interpretation)
• Measurable
• Achievable (of a level of acceptable performance agreed with stakeholder)
• Relevant (related to important aspects of care)
• Theoretically sound or timely (evidence based)
Structure Criteria
(What is needed), refers to those resources that are required to deliver care, including the numbers of staff and
skill mix, current knowledge, skills and attitudes, materials and drugs, equipment and space.
Standard Title: Summarises the area on which that particular standard focuses
Standard Statement: Explains the level of performance to be achieved
Standard Criteria: Provides the detail of what needs to be achieved for the standard to be reached

Clinical Audit A Practical Guide
16
Process Criteria
(What is done), refers to the actions and decisions taken by healthcare professionals together with service users
and includes communications, assessments and prescription of surgical and other therapeutic interventions.
Outcome Criteria
(What is expected to happen as a result), refers to the expected outcomes of care. Increasingly the measurement
of outcomes of care is being seen as the most appropriate measure of effectiveness.
Measuring Care
The measurement against criteria of care is at the heart of clinical audit. In order to compare actual care with
care that should be provided, each clinical audit criterion should have an expected level of performance’ or
‘target’ assigned to it (Ashmore, Ruthven and Hazelwood, 2011a).
3.2 Step 2: Set the Target/Level of Performance
Three factors should be considered and assessed when setting targets. These factors are clinical importance,
practicality, and acceptability. The expected level of performance or target can range from 0% (the criterion
is something that must never be done) to 100% (the criterion is something that must always be adhered to).
Where a criterion is critical to the safety of service users, targets may be set at 100% or 0%, for example, a
clinical audit relating to safe administration of medication could have a target of 100% for the following criterion
‘medication is not administered to a service user with a known allergy to the medication’.
However, where clinical importance is not as signicant, resources required to full the target performance
level should be considered and an acceptable performance level (one which is seen as both reasonable and
attainable by those delivering and receiving care) should be identied, for example, in a clinical audit relating to
the timeframe within which service users should be seen in a particular outpatient clinic, a target of 90% may
be deemed appropriate.
An optimum level of performance is set when the best care possible is identied, given the resources available
and normal conditions of caregiving. This will lie somewhere between the minimal acceptable level of care and
the highest possible level of care (possible under ideal conditions, with no restrictions on resources).
Target / Level of Performance:
A dened level or degree of expected compliance with clinical audit criteria may be expressed as a percentage
or proportion of cases.
3.2 Step 3: Consider Inclusion / Exclusion Criteria
In order to ensure that the clinical audit sample is representative of the target population and to collect data
which is t for purpose, it is necessary to dene what information should be collected and what information
should not be collected.

Clinical Audit A Practical Guide
17
Inclusion Criteria
Dene areas included in the remit of the clinical guideline/standard.
Exclusion Criteria
Dene areas outside the remit of the clinical guideline/standard.
Many evidence based clinical guidelines identify inclusion and exclusion criteria.
Step 4: Consider Exceptions
There may be a justiable reason why some cases from the identied sample may not comply with specic
clinical audit criterion. In these circumstances the case is not included in the data analysis, they are exceptions
in the clinical audit.
Exceptions
‘An exception is a clinically acceptable reason or circumstance for not complying with specic criteria’
(Dixon, 2009a)
*Consensus with the clinical audit team on exceptions should be agreed before the start of the audit.
Example
In a clinical audit of NICE clinical guideline CG61 (NICE, 2008; updated April 2017)
‘Irritable bowel syndrome in adults: diagnosis and management in primary care’, the following apply:
Inclusion criteria:
• Adults (18 years and over) who present to primary care with symptoms suggestive of irritable bowel
syndrome (IBS).
Exclusion criteria:
• Adults with other gastrointestinal disorders such as non-ulcer dyspepsia, coeliac disease or
inammatory bowel disease.
Example
A clinical audit on the previously referenced NICE CG61 ‘Irritable bowel syndrome in adults’ could have
the following criterion and exceptions:
Criterion:
• Percentage of service users with irritable bowel syndrome (IBS) advised how to adjust their doses
of laxative or anti-motility agent according to the clinical response.
Exceptions:
• Service users with IBS who are not using laxative or anti-motility agent.
Clinical Audit A Practical Guide
18
Stage 3: Design Clinical
Audit Tool and
Collect Data
Clinical Audit A Practical Guide
19
Stage 3: Design Audit Tool & Collect Data
1
Design a questionnaire or data collection tool
2
Agree the sample size. As a minimum, data collected must be adequate to determine if the
clinical audit standards are being achieved
3
Agree data collection methodology. There are a range of options for collecting clinical audit
data including: concurrent, retrospective and prospective data collection
4
Inclusion and exclusion criteria must be considered prior to the collection of data
5
Data collection must be in accordance with the agreed standards and sample size. Data not
required to measure if clinical audit standards are being met, should not be collected
6
The data collection tool must be piloted
7
The data collection tool must not capture patient identiable data. All data collected must be in
compliance with HSE Data Protection Policy and General Data Protection Regulation (GDPR).
3. Design Audit Tool
& Collect Data
Figure 4: Clinical Audit Cycle Stage 3– Design Audit Tool and Collect Data

Clinical Audit A Practical Guide
20
3.3 Step 1: Devise Audit Tool
The following principles apply to designing a data collection sheet:
• The data to be collected should be relevant to the objectives and criteria for the clinical audit and the
expected performance levels.
• Acronyms, jargon, and technical terms should be avoided.
• Denition of terms used should be included where necessary.
• There should be space to record exceptions.
• Questions should be episode-specic, they relate to a specic episode of care.
• Closed questions should be used, these should be clearly worded and contain no ambiguity to clarify the
format for the answer (for example, date: day/month/year).
• Limit the use of free text or open questions to clinical audits with qualitative elements as free text is difcult
to code and analysis is very time consuming.
• Data items should be presented in a logical order. The tool should not require the person collecting or
analysing the data to skip backwards and forwards.
There are 3 methods for data collection in a clinical audit: Retrospective, Concurrent and Prospective.
Retrospective Data Collection
Retrospective data is collected after completion of treatment/care to service users.
Advantage:
• The data already exists and may be gathered quickly.
Disadvantage:
• The data can quickly become out of date and the data available may not be complete and accurate.
Concurrent Data Collection
Concurrent data is collected while treatment / care is being provided.
Prospective Data Collection
If the data required is not routinely recorded, a prospective clinical audit must be undertaken.
Advantage:
• If the data required is not routinely recorded, a prospective clinical audit must be undertaken
Disadvantages:
• Time is required to collect the data
• There is a potential for bias.
• The care provided may be affected by the knowledge that a clinical audit is ongoing
3.3 Step 2: Data Collection Process
The overall objective of clinical audit is to improve the quality of care and outcomes by measuring current
practice against best practice. When the standards against which the clinical audit will be conducted have
been identied, the next step in the clinical audit process is the collection of relevant data about current
practice in order to facilitate comparison.

Clinical Audit A Practical Guide
21
It is important that data collected in the course of any clinical audit is precise and pertinent to the clinical audit
being performed. To ensure that data is collected appropriately, there are a number of details which need to be
established at the outset. These are:
• The population or sample to be included, with inclusion/exclusion criteria dened
• The consent required to access the population or sample information
• The healthcare professionals involved in the service user’s / patient’s care
• The time period over which the criteria apply
• The analysis to be performed
Resources should be used effectively to collect the minimum amount of data necessary to achieve the clinical
audit objectives. Resource utilisation decisions should be made at the outset of the clinical audit and revised, if
appropriate, during the clinical audit process. Due cognisance should be given to data protection requirements.
Planning data collection
Before data collection commences, a structured approach should be taken to the identication of relevant data
and to ensuring that the data collection process is efcient, effective, accurate and clear.
When standards of best practice, clinical audit criteria, expected compliance rates and known exceptions
have been identied, denitions and instructions for data collection should be compiled. This involves
dening terms in the clinical audit criteria and known exceptions for data collection purposes and also
dening where evidence should be obtained.
Sources of Data
The source of data for a clinical audit should be specied and agreed by the clinical audit team. The source
specied should provide the most accurate and complete data as readily as possible.
Questions to assist with preparing for data collection
• What type of data do I need to collect (quantitative and/or qualitative)?
• What data items will need to be used to show whether performance levels have been met for each
standard?
• What data sources will be used to nd the data?
• Will a data collection tool be piloted?
• Will I need to collect data concurrently, prospectively and/or retrospectively?
• What size is the target population, and will I need to take a sample?
• How will data be collected (manually and/or electronically)?
• How long will it take to collect the required amount of data?
• Who will be collecting the data?
• How will I ensure data quality?
(Adapted from Ashmore, Ruthven and Hazelwood (2011))
Collection of data which is not required for the purposes of measurement is more time consuming and
may infringe compliance with information governance requirements and practices.

Clinical Audit A Practical Guide
22
Clinical audit is not research. It is about evaluating care against best practice standards rather than creating
new knowledge; therefore sample sizes for data collection are often a compromise between the statistical
validity of the results and pragmatic issues around data collection such as time, access to data, and costs.
When determining the number of sample subjects, consideration should be given to the level of condence
required from clinical audit results and any constraints which may impact upon the clinical audit. For many
clinical audit topics, a small amount of data may be sufcient for the purposes of the clinical audit; however, if
a contentious issue is being audited, a larger sample size may be required.
Sample selection methods
It is often not possible or necessary to gather data on all patients/service users, events or items for clinical
audit purposes; therefore sampling is often required. It is important that any sample selected is representative
of the population under examination. There are numerous sampling methods which may be used; however
random sampling and convenience sampling tend to be the most commonly used methods.
The simplest form of random sampling involves selecting service users at random from an overall population
listing, for example every 3rd, 6th case etc. The Hospital Inpatient Enquiry System (HIPE) offers this facility
(HSE, 2008). Random number generation can also be used.
Convenience sampling is sometimes used as a simple and effective way of carrying out a sample survey. It
involves choosing the nearest and most convenient persons to act as respondents; it therefore does not produce
ndings that can be taken to be representative, for example, the rst 10 cases presenting after a specic time.
Interval sampling – is often determined by a time period. For example, all cases in a specic timeframe. The
sample size should be sufcient to generate meaningful results.
• Where necessary the sample should allow for adjustment for case mix
• The clinical audit should use pre-existing data sets where possible
Piloting the Data Collection Tool
Piloting a data collection tool and its methodology can provide evidence as to whether the proposed methodology
is feasible. A pilot may reveal problems such as a data collection tool which is difcult to understand or to
complete; or be used to identify themes in answers provided to open questions on data collection forms and
these in turn can be reformed as tick-box options for ease of analysis.
If the data collection takes too long, interest will be lost and data completeness will suffer.
• In numerical clinical audits, the number of cases selected should reect the most common of the condition
or therapy, but should be of a reasonable number to draw subsequent conclusions
• In time based clinical audits, one to three months should be adequate for the majority of clinical audits
(NHS Clinical Governance Support Team, 2005)
How long should it take to collect the required amount of data?
The time period chosen depends on the number of cases that are treated on a daily basis and the number
needed to make a condent judgment of the care provided. (NICE, 2002).
Sample Size
The sample should be small enough to allow for speedy data collection but large enough to be
representative.
Clinical Audit A Practical Guide
23
Timeframe for data collection is inuenced by:
• The sample (size and population)
• Inclusion and exclusion criteria
• Target date for clinical audit completion
A credible sample of subjects should be agreed with stakeholders. If the clinical audit intends to include the
perspective of service users, the aim should be to ensure that the sample of service users recruited to the
clinical audit is as representative of the relevant population as possible. In addition, different clinical audit
techniques might be needed to engage the views of different groups, such as a questionnaire/survey.
Who collects the data?
Depending on the clinical audit, data may be collected by more than one person or different people may be
responsible for completing different data sets.
There should be no confusion over terminology. A denition should be provided for each data item so that
it is collected consistently (inter-rater reliability). In addition, everyone involved in data collection should be
recorded.
Ensuring data quality
Data can be said to be of good quality when it does what it is needed to do. There should be clear denitions
for each data item to be collected to ensure that data collectors have a good understanding of what, how and
when data needs to be collected. There should also be routine data quality checks to minimise the occurrence
of reporting and input errors. (Health Information and Quality Authority, 2010)

Clinical Audit A Practical Guide
24
Stage 4: Analyse Data
and Compare
Results with
Standards

Clinical Audit A Practical Guide
25
Stage 4: Analyse Data and Compare Results with Standards
1
Clinical audit data should be analysed by the team within the agreed timeline and
comprehensively checked for accuracy
2
Clinical audit data must be analysed to determine if best practice standards have been
achieved
3
The clinical audit data analysis process should identify relevant trends/data that meet the
standard/patterns/variations
4
Consideration must be given to how clinical audit results will be generated via the data
analysis process. Clinical audit results must be shared in the most appropriate format to allow
key stakeholders to gain the most accurate and understandable picture of performance
5
Clinical audit data must not display named individual healthcare professionals
6
Clinical audit results must explain how missing or not applicable data has been managed
7
Clinical audit results must be effectively communicated and presented to all relevant
stakeholders, ideally including patients.
4. Analyse Data & Compare
Results with standards
Figure 5: Clinical Audit Cycle Stage 4– Analyse Data and Compare Results with Standards

Clinical Audit A Practical Guide
26
3.4 Step 1: Data Analysis
To compare actual practice and performance against the agreed standards, the clinical audit data must be
collated and analysed. The basic aim of data analysis is to convert a collection of facts (data) into useful
information. The main aim of data analysis is to answer the questions posed by the clinical audit objectives;
highlighting areas of good practice and areas that require particular attention or improvement.
It is often necessary to perform basic calculations on the raw data collected to get results from which conclusions
can be arrived. The type of data analysis depends on the type of information collected. This can range from
simple averages and percentages to other statistical techniques.
3.4 Step 2: Calculating compliance with clinical audit criteria
The basic requirement of a clinical audit is to identify whether or not required performance levels have been
reached. This requires working out the percentage of cases that have met each clinical audit criterion. In
order to calculate the percentage, it is necessary to identify both the total number of applicable cases for a
criterion (the denominator) and the total number within the sample that met the criterion (the numerator). The
percentage is then calculated by dividing the numerator by the denominator and multiplying the answer by 100.
This gure is obtained by subtracting cases that are agreed exceptions for a particular criterion, from the total
number of cases which meet the inclusion criteria minus exclusions.
Clinical Audit Criterion:
• Number of patients/service users with irritable bowel syndrome (IBS) advised on how to adjust their dose
of laxative or anti-motility agent according to clinical response.
• Inclusion criteria: Adults (18 years and over) who present to primary care with symptoms suggestive of
irritable bowel syndrome (IBS).
• Exclusion criteria: Adults with other gastrointestinal disorders such as non-ulcer dyspepsia, coeliac disease
or inammatory bowel disease
• Agreed exception: Service users with IBS who are not using a laxative or anti-motility agent
For most clinical audits, complex statistical analysis is not necessary or appropriate. A simple, clear and
concise analysis which can be easily understood by everyone involved in the provision of care is all that
is required to stimulate change.
Example
Using the previously referenced Clinical Guideline NICE CG61 (NICE, 2008; updated April 2017)
‘Irritable bowel syndrome in adults; diagnosis and management in primary care’
Displaying Data
To facilitate the drawing of conclusions from analysed data, the data should be displayed in the simplest,
clearest, and most effective way possible. There are many ways of displaying data, through comparing
data from one area against data from another area, to comparing results against expected level of
performance or current clinical audit results against previous clinical audit results.
Clinical Audit A Practical Guide
27
The nature of the clinical audit topic and the data measured will determine which type of descriptive statistic
will be most useful for presentation of information. Useful descriptive statistics include information on the
distribution of data, the mean or average, median, mode and measures of dispersion i.e. the range and possibly
the standard deviation.
3.4 Step 3: Drawing Conclusions
After results have been compiled and the data has been analysed, the nal step in the process is to identify if
the standard was met or not met.
To understand the reason(s) a standard was not met, the clinical audit team should carefully review all ndings to:
• Clearly identify and agree on areas for improvement identied by the clinical audit
• Analyse the areas for improvement, to identify what underlying, contributory or deep-rooted factors are
involved
• Have a clear understanding of the reasons why performance levels are not being reached, to enable
development of appropriate and effective solutions.
3.4 Step 4: Sharing Results
The aim of any presentation of results should be to maximise the impact of the clinical audit on the audience
to generate discussion and to stimulate and support action planning.
To facilitate the drawing of conclusions from analysed data, the data should be displayed in the simplest,
clearest and most effective way possible. Reading or listening to lots of facts and gures is not always an
effective way to convey information and may prove difcult for an audience to interpret and understand the
information being conveyed. Visual methods can make the point more effectively than data alone.
Data graphics are a good way of communicating this information to others, such as infographics. The most
commonly used form of data graphics in clinical audit are tables, graphs and charts, using Excel.
When deciding on which form of data graphics to use, consideration of the following may be helpful:
• What information is to be communicated?
• Who is the audience?
• What might prevent them from understanding this information?
*In compliance with Data Protection legislation, unless presentation of clinical audit results are conned to the
clinical care team, only irrevocably anonymized data should be disclosed
Clinical Audit A Practical Guide
28
Stage 5: Clinical Audit
Report

Clinical Audit A Practical Guide
29
Stage 5: Clinical Audit Report
1
All clinical audits should be written into a clinical audit report. The clinical audit report should
utilise relevant local or national clinical audit report templates
2
The clinical audit report should follow the required sub-headings: background, aim,
objectives,methodology, results, conclusion, recommendations, quality improvement plan
3
The clinical audit report should clearly state the rationale for undertaking the clinical audit
4
The clinical audit report must have a clearly described methodology
5
The clinical audit report must not include data for named individual healthcare professionals
6
A list of all specialist vocabulary, acronyms and abbreviations are included in the clinical audit report
7
The clinical audit report should be disseminated to all key stakeholders.
5. Clinical Audit
Report
Figure 6: Clinical Audit Cycle Stage 5– Clinical Audit Report

Clinical Audit A Practical Guide
30
3.5 Step 1: Layout of Report
Your clinical audit report should follow a standard Clinical Audit Report template (See Resource 3).
Additional items to include in a Re-Audit Report
Background – information should be provided about the previous clinical audit and the key actions that were
implemented as a result
Action plans – previous action plans must be evidenced when later re-auditing and an assessment made of
the success of any actions taken
Conclusion/Summary – a progress report and comparison to the previous clinical audit must be included.
This can be contained as a summary or a table.
Consider what has changed, either for the better or worse since the previous clinical audit. (Healthcare Quality
Improvement Partnership (HQIP), 2012.
3.5 Step 2: Reection
What has the team learned from completing this clinical audit? What went well? Looking back, what would
you have done differently? Has anything changed in practice? Has the clinical audit been of any benet to the
patients, the practice and/or the team?

Clinical Audit A Practical Guide
31
Stage 6:
QI Plan and
Action
Clinical Audit A Practical Guide
32
Stage 6: QI Plan and Action
1
Based on the ndings and conclusion, the clinical audit stakeholders should agree a QI plan
with actions that will be implemented to improve care
2
The QI plan will include ownership of actions and agreed timelines
3
The clinical audit QI plan should utilise agreed templates. This will ensure consistency.
4
The QI plan should be disseminated and communicated to all relevant stakeholders and
governance reporting lines
5
Implementation of the action(s) must be closely monitored, with progress reported to key
stakeholders
6
The re-audit should not commence until all actions have been completed
6. QI Plan & Action
Figure 7: Clinical Audit Cycle Stage 6– QI Plan and Action
3.6 Step 1: Development of a Quality Improvement Plan
Clinical audit results may show areas of excellent or notable practice and this should be acknowledged. Clinical
audit results may also identify areas for improvement where the required standards are not being met.The
Quality Improvement Plan is a fundamental part of the clinical audit cycle; without it, the clinical audit is not
effective. It is an important change management tool; however, to be effective a QI plan must explicitly contain
the following information:
1. Highlight what needs to change (recommendation)
2. Indicate the action(s) that must be taken in order to achieve change
3. Give a deadline by which time the actions must be carried out
4. Show who is responsible for making sure that the actions are carried out
5. Indicate the evidence required to prove that the actions have been implemented

Clinical Audit A Practical Guide
33
Implementing change is often the most difcult part of the clinical audit. QI plans are live documents and will
need to be updated and reviewed regularly to ensure progress is being made and maintained. (Healthcare
Quality Improvement Partnership, (HQIP) 2012)
The clinical audit loop is completed by developing and implementing the QI plan (the QI plan is often referred
to as an action plan).
3.6 Step 2: Action
Priorities for action should be identied and these should be clearly documented. All clinical audits should be
accompanied by an improvement plan which should be consistent with SMART guidance. QI Plans should be:
• Specic (explicit statements, not open to interpretation)
• Measurable
• Achievable (a level of acceptable performance agreed with stakeholders)
• Relevant (related to important aspects of care)
• Theoretically sound and timely (evidence based)
QI Toolkit
There are a number of QI tools that can be utilised to facilitate improvements: Process mapping, the ‘Five
Whys,’ Cause and Effect Diagram (Fishbone Diagramming) and the Model for Improvement (MFI), Plan, Do,
Study, Act (PDSA) cycles.
Process Mapping
This involves mapping out each step of a process in sequence so that areas for improvement can be identied.
Process maps are an effective way to identify constraints and ineffective or unnecessary process steps.
The Five Whys
Involves repeatedly asking the question ‘why?’ in order to drill down further into an issue, which can lead to the
cause of the problem. The reason for any problem can often lead to another question. Asking ‘why’ ve times is
only a guide as depending on the issue, the question may be asked a lesser or greater number of times before
reaching the origin of the problem.
Cause and Effect Diagram (Fishbone Diagram)
This process can be used independently or as part of a Cause and Effect Diagram (Fishbone Diagram). A
problem or an effect is written at the head of the ‘sh’, then a common set of major categories of causative
factors are written on diagonal lines branching from the main arrow, ‘the bones’. Examples include people,
procedures, materials, equipment and environment. In order to develop the various categories, it is necessary
to think in terms of each major step in the process.
A list of possible causes for each category should be generated through brainstorming by asking the question
‘why does this happen?’ in relation to each cause. The causes and sub-causes are then listed on branch bones
(branching off from the main branch/cause). This will highlight relationships among the causes. It is necessary
to keep asking ‘why?’ until a useful level of detail is reached and an appropriate solution may be developed.
By establishing the reasons why performance levels for specic criteria were not met, the team are then
enabled to discuss/lead discussions around recommendations for improvements.

Clinical Audit A Practical Guide
34
Stage 7: Re-Audit
Clinical Audit A Practical Guide
35
Stage 7: Re-Audit
1
If the clinical audit results demonstrate that all standards are being achieved, a re-audit is not
required
2
The methodology applied in the re-audit must be the same as in the rst cycle. For example,
best practice standards and data collection tools need to be replicated. The re-audit must
measure a comparable dataset to the rst clinical audit cycle in terms of number of patients/
cases, timeframes, etc.
3
The re-audit should be carried out in an agreed timeframe. The re-audit should not be carried
out prior to changes being implemented. Re-audit should take place within a maximum of 12
months of changes being recommended
4
The results of the re-audit are collated and disseminated to all stakeholders
5
If re-audit results demonstrate that care does not meet the agreed standard, an action plan/QI
plan should be developed to support the changes required and a re-audit done.
7. Re-Audit
Stage 7: Re-Audit

Clinical Audit A Practical Guide
36
3.7 Step 1: Completing the Clinical Audit Cycle
The clinical audit cycle is a continuous process. If the rst data collection cycle demonstrates that the required
standard was met, the clinical audit does not need to be re-audited.
If the clinical audit shows that the standard was not met, completing the clinical audit cycle involves two data
collections and a comparison of one with the other, following implementation of change after the rst clinical
audit completion, to determine whether the desired improvements have made a difference. Further cycles may
be necessary if performance still fails to attain the levels set at the outset of the clinical audit. At this stage,
there may be justication for adjusting the desired performance levels in light of the results obtained.
3.7 Step 2: Sharing Results
Completion of a clinical audit cycle will usually result in improvements in practice. This should be communicated
to all stakeholders.
A successful clinical audit in one service may be transferable to other parts of the service. Completed clinical
audits should be shared locally via the most appropriate mechanisms.
Consideration should also be given to sharing clinical audit work regionally and nationally through relevant
journals, conferences and other media.
See Resource 1 for Summary Checklist of Best Practice for steps involved in the clinical audit process.

Clinical Audit A Practical Guide
37
4.0 Data Protection, General Data Protection Regulation and Ethical
Issues for Clinical Audit
4.1 Data Protection and GDPR Responsibilities
Legislation around data protection and service user record condentiality must be complied with when
performing clinical audits. The Data Protection Acts 1988 and 2018 provide the legislative basis for the
approach of the Ofce of the Data Protection Commissioner with regard to personal data across all sectors of
society - public, private and voluntary.
4.2 Data Protection and GDPR Principles
Anyone processing personal data must comply with the eight rules of data protection in line with the Data
Protection Acts, 1988 - 2018:
• Obtain and process information fairly
• Keep data only for one or more specied, explicit and lawful purposes
• Use and disclose it only in ways compatible with these purposes
• Keep data safe and secure
• Keep it accurate, completed and up to date
• Ensure data is adequate, relevant and not excessive
• Retain it for no longer than is necessary for the purpose or purposes
• Give a copy of his/her personal data to that individual on request
4.3 Data Protection and GDPR Guidelines
Data Protection Guidelines on research in the Health Sector (Data Protection Commissioner, 2007, P.12)
states:
However, the Data Protection Act provides an exemption from obtaining consent from the service user for
processing for statistical, research or scientic purposes carried out by the data controller itself (i.e. the treating
healthcare professional/service provider) where there are no disclosures of personal data to any outside third
parties.
Where access to service user identiable information is not accompanied by explicit consent, the Data
Protection Act requires that access is necessary for medical purposes; and access is only given to:
• a health professional; or
• a person who, in the circumstances, owes a duty of condentiality to the service user that is equivalent to
that which would exist if that person were a health professional.
“Given the fundamental role played by clinical audit in patient care, implied consent is normally
all that is required when the clinical audit could likely be of benet to that patient. Implied consent
will also be considered as sufcient in those cases where no direct benet is likely to accrue to
the patient concerned and where the clinical audit is to be carried out by the health facility itself”

Clinical Audit A Practical Guide
38
The National Ofce of Clinical Audit (NOCA) in Ireland also provides guidance on understanding the GDPR
Denitions:
• Personal data includes identiable and pseudonymised data
• Pseudonymised data is data that has a link back to enable identication. This link is usually retained for
use by the Data Controller and not by the Data Processor
• Processing of personal data can include gathering, processing and use of personal data
• If the data in question can no longer be linked back to any identiable individual, then it will constitute
anonymised data, is not personal data and is not subject to the GDPR. For example, if NOCA has received
data for clinical audit purposes but does not have access to any other information which would enable it to
identify the underlying individuals, then this data will not constitute personal data (NOCA, 2019).
4.4 Ethical Issues
What is Ethics?
Ethics is the inquiry into the morality of an action. There should be consideration of ethical principles in relation
to all aspects of clinical care including clinical audit.
Clinical audit should be conducted within an ethical framework, i.e. the clinical audit process should:
• Respect each service user’s right to make choices concerning their own lives
• Benet service users and not cause harm
• Treat all service users fairly
At a practical level, this means ensuring service users and staff condentiality and ensuring data is collected
and stored appropriately (UH Bristol Clinical Audit Team, 2009b).
Clinical audits should not examine the work of another professional or specialty without their knowledge. All
those whom the clinical audit will directly affect should be informed of and, if possible, involved in, the clinical
audit.
Service users should be approached in a sensitive and respectful manner and it should be explained that they
are not obliged to be part of the clinical audit and declining to take part will not affect care in any way.
Service users should be assured about the condentiality of any responses given (for example, anonymization
of data) and the length of time for which their personal information will be held. Anyone conducting a clinical
audit that involves direct contact with service users for interview or to request completion of a questionnaire
should give a full written explanation to the service user, in relevant language, as to the purpose of the clinical
audit.
Clinical audits involving questionnaires should be accompanied by a written explanation of the purpose of the
questionnaire/clinical audit along with an identied contact name and number (usually the clinical audit lead
or a clinical audit facilitator). While encouraging participation for improvement purposes, the letter should also
state that recipients are under no obligation to take part in the clinical audit and that declining to take part will
not affect their care in any way.
The name and the telephone number of a contact point should be given in case any questions/issues arise in
connection with the questionnaire. No consent form is required for questionnaires as consent will be deemed
to have been given if the service user returns the questionnaire.

Clinical Audit A Practical Guide
39
4.5 Is Ethical Review Required for Clinical Audit?
Previously, decisions regarding whether an activity required ethical review related directly to whether the activity
was classed as clinical audit or research. If an activity was classed as clinical audit it was automatically deemed
not to require ethical review, whereas research proposals require ethical review and approval. However, due to
the many similarities between clinical audit and clinical research, the boundaries between them can be blurred.
As a result, Wade (2005) recommends that ‘Decisions about the need for ethical review should be based on
the morality of all actions rather than arbitrary distinctions between clinical audit and research’.
The National Ofce of Clinical audit (NOCA) provides guidance which states that clinical audit does not require
ethical approval but, as always and in line with best practice, ethical issues should still be considered while
also adhering to data protection principles (NOCA, 2019).
Possible screening questions to determine if ethical review may be required are outlined in the following table:
Will the Proposed Clinical Audit:
Infringe on the rights of any service user or risk breaching their condentiality or privacy?
Pose any risk for or burden on a service user beyond those of his or her routine care?
Involve any clinically signicant departure from usual clinical care?
Gather any information about any service user other than information that is ordinarily collected as part of
providing routine care for the patient?
Collect data directly from any service user and if so could collect the activity subject a service user to
more than a minimal burden or risk if it requests sensitive information or is it time consuming?
Collect or disclose any data that could be used to identify any service user or healthcare professional?
Have someone carrying out the activity who does not normally have access to service users’ records?
People who normally have access to service users records include clinical staff providing direct care and
staff employed to support clinical audit when a duty of condentiality is included in their job descriptions?
Involve a potential conict of obligation to individual or all service users such as if the activity involves a
trade-off between cost and quality?
Involve the use of any untested clinical or systems intervention or testing a hypothesis?
Allocate any interventions differently among groups of service users or staff, for example, in implementing
a change in practice?
Adapted from Dixon, N. (2009b)
If the clinical audit team is concerned about the ethicality of their clinical audit, ethical advice
should be sought.

Clinical Audit A Practical Guide
40
Exceptions to the healthcare profession’s duty of condentiality to the service user:
Where a service user gives explicit consent to the disclosure of information to third parties.
When disclosure is required by or under any enactment or by a rule of law or order of a court
When disclosure is necessary to protect the vital interests of the service user or of another individual (consent
should be obtained if possible in such situations)
(Data Protection Acts, 1988 and 2018).
Other exceptions provided for in legislation include:
Health (Provision of Information) Act 1997 allows for provision of information to the National Cancer Registry
without the consent of the service users concerned.
Infection Diseases Regulations (1981 and 2011) set out legal obligations to disclose details of notiable
diseases with or without consent.
4.6 Guidance Regarding Consent Requirements for Clinical Audit
In general, clinical audit does not require informed consent (HSE, 2013). Members of a healthcare team (or
their support staff, for example, clinical audit staff) delivering direct care to a service user can perform a review
of service user data without consent. However, it is good practice to inform service users that as part of normal
care processes, personal data may be used for clinical audit and quality improvement purposes and also about
the importance of the clinical audit function within the service. This may be achieved through informing service
users through a statement of information practices or leaets or posters which are clearly displayed/made
available by the service provider (HIQA, October 2012).
Consent is not required where the personal health information is irrevocably anonymised by the data controller
prior to disclosing to a third party. Care must be taken to ensure that the service user is completely unidentiable
even when the data is anonymized.
Where a clinical audit is carried out by persons who are not involved in service user care (i.e. persons who are
external to the data controller (the service provider)), informed consent is required to enable such persons to
access personal data.
If a service user gives consent to the disclosure of records to third parties, the health professional ensures
they understand the consequences of such disclosure, what will be disclosed, the reasons for the disclosure
and the consequences of giving consent. Service users’ healthcare records are only disclosed in accordance
with the conditions of their consent. Service users have the right to withdraw consent to disclosure of their
healthcare records information at any time.
4.7 Condentiality and Access to Service User Health Information
The clinical audit methodology should be designed so that the condentiality of personal health information
is not compromised. When reporting on clinical audits, data is completely anonymised in every case. No link
between clinical audit conclusions, service users or healthcare staff should be possible.
All healthcare staff must make every effort to preserve the condentiality of personal health information and
ensure that they work within the requirements of the Data Protection Acts 1988 and 2018:
Clinical Audit A Practical Guide
41
• Data is only accessible by appropriately authorised staff on a need-to-know basis
• Data collection sheets containing any personal identiable information should only be kept for the length of
time they are absolutely required (for the purposes of the clinical audit). Once they are no longer required,
they should be destroyed immediately
• Raw data is anonymised before it is entered into a computer database
• Data is checked to ensure condentiality and accuracy
• No service user identiable information is stored on a computer with raw data
• Anonymised data sheets/questionnaires should be kept only for as long as is necessary and destroyed as
soon as all information has been retrieved from the questionnaires
• Any waste material that contains personal, private or condential information should be eliminated in a
manner which ensures that privacy rights and condentiality obligations are not compromised
• There should be a designated point of storage for data in current use. This should be a locked ling
cabinet, to comply with data protection requirements
• All data should be stored together i.e. the physical raw data, the rst data input into the computer, any
subsequent analysis, and the nal draft
• The data must be archived, so that it remains available throughout the subsequent phases of the clinical
audit and for seven years afterwards
• Archived clinical audits should be stored on a secure computer
• All computers are password protected
• All devices used to store data are encrypted (for example, laptops and USB devices)
• If laptops are removed from the work location, the person responsible for that laptop must ensure that it is
secure at all times
• The service provider should have a central location for the storage of nal clinical audit reports (both
in hard and soft copy). It is also recommended that a log be maintained for traceability purposes of the
reports and where they are at any given time
• All data recorded for clinical audit purposes should be made anonymous by appropriately authorised
individuals before being made available for review and consideration by others
4.8 Anonymisation of Data
The anonymization of data involves removal of all data elements that could be used to identify an individual, for
example, name or healthcare record number. It is recommended that service user data be anonymised before
it is accessed for clinical audit purposes:
• Irrevocable anonymisation of personal data puts it outside data protection requirements as the data can no
longer be linked to an individual and therefore cannot be considered to be personal data
• Where service user data is anonymised, there is no need from a data protection perspective to seek
consent for the use of the data for clinical audit purposes
However, care needs to be taken when rendering data anonymous, as depending on the nature of the
illness and the prole of the service user, there may be instances in which the data may actually still be
identiable. Where this might possibly be the case, an extra effort should be made to further remove any
potential identifying information. Where this is not possible it would be advisable to either refrain from using
the identiable information or seek the consent of the person for such use.

Clinical Audit A Practical Guide
42
Equally, it is recognized that in some instances where there is a need to link episodes of care and prevent
duplication of data; information may need to be capable of being matched or linked. This can be achieved
through appropriate pseudonymisation (e.g., use of initials, coding) methods without the need to retain all
identifying characteristics with the data.
Pseudonymisation (or “reversible anonymisation”) involves the use of a coding system, for example, allocating
individuals with unique, reference numbers. The look-up list from which the true identities may be obtained is
then held securely and only accessed by authorised persons for specic, pre-dened purposes. Similar to the
advice in relation to anonymisation, where pseudonymisation methods are used, it is recommended that extra
efforts, beyond use of initials etc, be incorporated where a condition is particularly rare. Unique identifying
numbers should also be given to healthcare professional that may be involved in the clinical audits. Individuals
should not be named in any of the reports.
In certain cases where anonymising data may be impractical and detrimental to the clinical audit, such as
during the ongoing data collection to prevent duplication of data collection, the clinical audit team must ensure
that the data is kept purely for the purposes of analysis by those directly involved in the management of the
clinical audit. Identiable data must not be transferred to third parties without the permission of the service
user.
Further data protection information and advice is available from the Ofce of the Data Protection Commissioner
website www.dataprotection.ie.
To ensure that those involved in clinical audit are aware of and supported in their efforts to be compliant with
any legislative changes affecting clinical audit, clear and consistent information is provided across the HSE in
relation to legislation that supports both patient safety and advancing and improving care.
All HSE services, staff and clinical audit service providers carrying out clinical audit locally and nationally
should understand and comply with GDPR and the Patient Safety Bill 2019 requirements.
General Data Protection Regulation (2018) and clinical audit outcomes:
• Dedicated guidance on GDPR and Data Protection and its application to clinical audit
• The HSE will advise and share this guidance to ensure consistency across all clinical audit service providers
• The HSE will form a national healthcare Data Protection Ofcer (DPO) network to support the process of
consistent guidance to the system
• The HSE will provide timely guidance on any changes or updates to legislation and guidance which
affects clinical audit and this will be published on the dedicated clinical audit web portal that has been
recommended by the national clinical audit review report
• General Data Protection Regulation (GDPR) training on HSeLanD to be completed by all HSE staff
• HSE staff to follow the HSE_GDPR Policy (2019) when conducting any clinical audit activity
Clinical Audit A Practical Guide
43
5.0 Organisational Requirements to Support Clinical Audit
For clinical audit to be effective, it requires commitment and support throughout the service and the organisation,
which includes senior management. Clinical audit should be seen as a valued activity and should be included
as a priority in Quality and Patient Safety and service planning.
5.1 Clinical Audit Strategy
A clinical audit strategy is an operational plan primarily aimed at those with responsibility for overseeing the
direction and development of clinical audit within the service or organisation. For example, divisional/service/
department leads or quality/ safety and clinical audit committees/ governance groups.
Clinical audit strategies should begin with a statement of a service provider’s commitment to the process of
clinical audit and to delivering the objectives set out in the strategy in accordance with best practice. A strategy
on the development of clinical audit describes how a healthcare provider will implement the policy and increase
the impact of clinical audit on clinical services (Healthcare Quality Improvement Partnership, 2020).
Progress in delivering the quality improvement plan and meeting objectives, should be monitored on a regular
basis and reported to the relevant Quality and Safety and/or Clinical Audit Committee.
The strategy should be reviewed and updated annually.
Clinical audit strategies should be supported and underpinned by a clinical audit policy.
5.2 Clinical Audit Policy
A clinical audit policy should set out the procedure for the conduct of clinical audit within the service or
organisation, outlining best practice standards which should be met, processes and procedures to be followed
and how different issues are to be addressed.
A policy on the use and conduct of clinical audit sets out the principles, roles, responsibilities and practices
a healthcare provider will follow in auditing clinical practice, and improving the quality of services to meet
the needs of patients, healthcare commissioners, healthcare regulators and others (Healthcare Quality
Improvement Partnership, 2020).
5.3 Clinical Audit Programme / Forward Plan
Each service provider should have an agreed programme for clinical audit. This is a plan which species what
clinical audits will be carried out over the course of the programme duration (usually annually).
A clinical audit strategy should:
• Be a time limited document, i.e., covering a period of one or more years
• Connect clinical audit with the service provider’s governance and assurance systems and corporate
objectives
• Provide a medium to long term vision for the development of clinical audit, for example, 3 to 5 years
• Set out a number of service objectives for the period being covered by the strategy
(Healthcare Quality Improvement Partnership ( 2012)

Clinical Audit A Practical Guide
44
It should give direction and focus with regard to how and which clinical audit activity will be supported in the
service and should be based on the service provider’s priorities for clinical audit. Acknowledging that the
clinical audit cycle includes re-audit, a proportion of topics for re-audit should also be included in the annual
clinical audit plan.
As with all plans, the clinical audit programme is subject to change as priorities in service provision change.
Any changes to the clinical audit plan should be communicated to all stakeholders.
Supports for the Delivery of a Clinical Audit Programme/Forward Plan
Clinical audit committee with members who can provide expertise and experience with clinical audit
Clinical audit support staff who can provide advice and training and refer to other available resources
Clinical and educational leads
Healthcare records manager and staff who can facilitate access to service user records
Information systems access and advice
Training available related to the clinical audit process and how to design and carry out clinical audits
Advice on how to handle ethical issues related to clinical audits
Templates for planning and reporting on clinical audits
Advice on the technical aspects of carrying out a clinical audit
Access to reference materials on clinical audit
Technical support for clinical audit including a database of clinical audits
Proposed clinical audit programmes should be discussed at a meeting of relevant stakeholders (dependent on
whether the programme pertains to a particular clinical service or the service in its entirety).
5.4 Clinical Audit Leads
There are different levels of clinical audit leads, for example, at Service, Divisional or Specialty level. At service
level, the clinical lead’s responsibility is to organise, develop, improve and support the performance of clinical
audit within the service whereas the role of the lead for a specic clinical audit is to provide leadership in the
completion of the clinical audit cycle.
5.5 Fostering a Culture which is Supportive of Clinical Audit
Requirements of a culture which is supportive of clinical audit are:
• A common vision of the benets and resource requirements of clinical audit among managers and staff
• A service wide strategy with clear lines of responsibility and accountability
• An overall plan for clinical audit comprising of a comprehensive structured programme aimed at nurturing
effective clinical audits
Clinical Audit A Practical Guide
45
• Leadership and direction of clinical audit programmes including a designated lead whose responsibility is
to organize, develop, improve and support the performance of clinical audit within the service
• Strategy and planning in clinical audit programmes
• Resources and support for clinical audit programmes
• Monitoring and reporting of clinical audit activity
• Commitment to, participation in and high levels of clinical audit activity which by its nature and impact, is
seen by its participants to be involved and relevant and thus fosters positive attitudes for further participation.
5.6 Practical Support for Clinical Audit
Provision of practical support for clinical audit includes the provision of the following:
• Policies, procedures, protocols and guidelines (PPPGs) in relation to clinical audit which provide a vision
of the goals and purposes of clinical audit within the service and dening how a clinical audit should be
undertaken
• Effective training in clinical audit methods
• Dedicated staff to provide expertise and/or advice on clinical audit design and analysis, for example,
clinical audit facilitators
• Practical mechanisms to make data collection easier such as good quality information systems and support
from service information departments and information specialists
• Allocated (protected) time for clinical audit
• Support for required changes identied by the clinical audit process.
5.7 Clinical Audit Facilitation
Service providers should assess whether additional clinical audit support staff are required to provide hands
on help and design of clinical audits.
Clinical audit facilitators can provide support in all aspects of clinical audit, including:
• Project planning
• Form design
• Spreadsheet/database design
• Data checking and entry
• Data analysis
• Presentation design
• Report writing
• Action planning
Clinical audit facilitators should have skills in study design, data collection, and computing data analysis. The
training needs of clinical audit facilitators should be recognized and resources should be made available in
order to facilitate their attendance at appropriate courses.

Clinical Audit A Practical Guide
46
References
Ashmore, S., Ruthven, T., and Hazelwood, L. (2011). Stage 2: Measuring performance. In Burgess, R. (ed.)
NEW Principles of Best Practice in Clinical audit. Healthcare Quality Improvement Partnership (HQIP).
Abingdon, Radcliffe Medical Press, pp 59-79.
Dixon N. (2009a). Getting Clinical Audit Right to Benet Patients. Romsey: Healthcare Quality Quest. GDPR
Guidance for Clinical Audit, National Ofce for Clinical Audit (NOCA), 2019 www.http://noca.ie
Health Information and Quality Authority (HIQA) (2010) Data Quality Guide. https://www.hiqa.ie/sites/default/
les/2017-01/Data-Quality-Guide.pdf
Healthcare Quality Improvement Partnership (2012). Template for Clinical Audit Strategy. Available from: http://
www.hqip.org.uk/template-policy-strategy
Healthcare Quality Improvement Partnership (2020) Best Practice in Clinical Audit. Available from: https://
www.hqip.org.uk/resource/best-practice-in-clinical-audit/
Health Service Executive (HSE) Data Protection Policy V1.1, June 2019. Available from: https://www.hse.ie/
eng/gdpr/hse-data-protection-policy/hse-data-protection-policy.pdf
HSE National Centre for Clinical Audit Nomenclature – Glossary of Terms for Clinical Audit 2022 https://www.
hse.ie/eng/about/who/nqpsd/ncca/nomenclature-a-glossary-of-terms-for-clinical-audit.pdf
HSE National Review of Clinical Audit Report 2019 https://www.hse.ie/eng/services/publications/national-
review-of-clinical-audit-report-2019.pdf
HSE A Practical Guide to Clinical Audit. Dublin: Health Service Executive; 2013; Available at: https://www.hse.
ie/eng/about/who/qid/measurementquality/clinicalclinical audit/practicalguideclinical audit2013.pdf.
HSE National Centre for Clinical Audit Interim Web Page. https://preview.hse.ie/eng/about/who/nqpsd/ncca/
ncca.html
Healthcare Quality Improvement Partnership (HQIP), 2018: https://www.hqip.org.uk/about-us/our-gdpr-
statement/#.ZBBHgXbP02w
Irish Council of Bioethics (2004). Guidance on Operational Procedures for Research Ethics Committees. Irish
Council of Bioethics, Dublin.
Medical Council. Clinical Audit Guides. 2019; Available at: https://www.medicalcouncil.ie/news-and-
publications/videos/clinical-clinical audit-guides
National Health Service (NHS), UK. (NHS Clinical Governance Support Team, 2005).
National Institute for Health and Clinical Excellence (2008, updated 2017). Irritable bowel syndrome (CG61).
Available from: http://www.nice.org.uk/CG61
National Institute for Health and Clinical Excellence; Principles for Best Practice in Clinical audit, 2002.
https://www.pslhub.org/learn/improving-patient-safety/clinical-governance-and-audits/nice-principles-for-best-
practice-in-clinical-audit-2002-r2956/
Clinical Audit A Practical Guide
47
Potter, J., Fuller, C., and Ferris, M (2010). Local clinical audit: handbook for physicians. Healthcare Quality
Improvement Partnership. Available at: http://www.hqip.org.uk/assets/Guidance/Local-clinical-clinical audit-
handbook-for- physicians-August-2010-FINAL.pdf
RCPI Professional Competence guidance for Doctors conducting clinical audit and quality improvement: https://
www.rcpi.ie/Learn-and-Develop/Lifelong-Learning/Professional-Competence/Professional-Competence-
Guidelines-and-Resources/Your-Audit-or-Quality-Improvement-Project
UH Bristol Clinical audit Team (2009b). How to: Apply Ethics to Clinical Audit. Available at: https://www.uhbristol.
nhs.uk/les/nhs-ubht/10%20How%20To%20Ethics%20v3.pdf
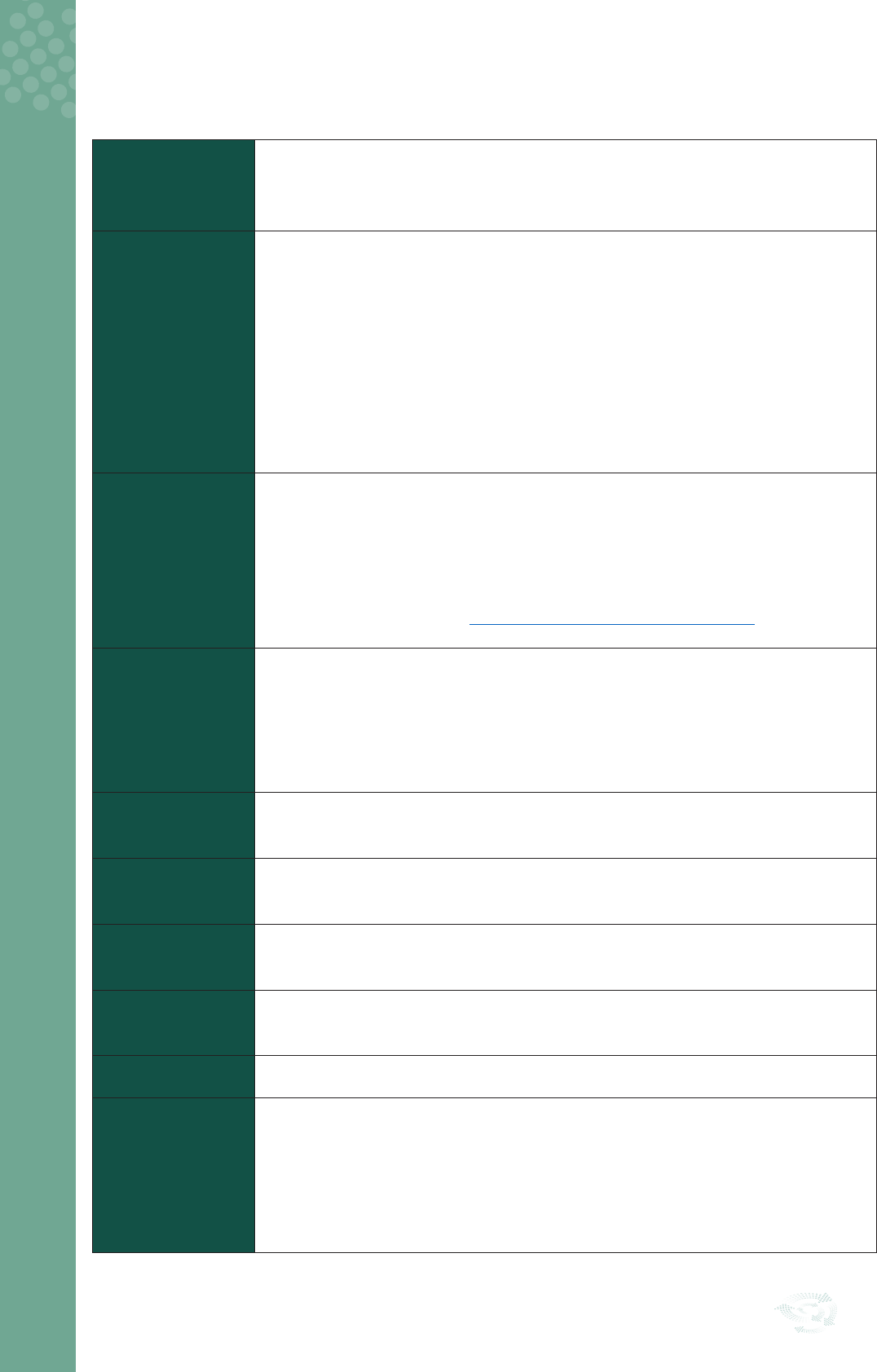
Clinical Audit A Practical Guide
48
Glossary of Terms
Clinical Audit
Criterion
The measurement of compliance against criteria of care is at the heart of clinical audit.
A clinical audit criterion is a criterion of care with an ‘expected level of performance’
or ‘target’ assigned to it.
Clinical Audit
“Clinical audit is a clinically-led quality improvement process that seeks to improve
patient care and outcomes through systematic review of care against explicit
criteria and acting to improve care when standards are not met. The process
involves the selection of aspects of the structure, processes and outcomes of
care which are then systematically evaluated against explicit criteria. If required,
improvements should be implemented at an individual, team or organisation level
and then the care re- evaluated to conrm improvements.”
DOHC (2008, p. 152)
“Clinically-led” includes all health and social care professionals.
Clinical
Governance
Clinical governance is the system through which healthcare teams are accountable
for the quality, safety and satisfaction of patients in the care they have delivered.
For health care staff this means; specifying the clinical standards you are going to
deliver and showing everyone the measurements you have made to demonstrate
that you have done what you set out to do. Further information on clinical
governance is available at: http://www.hse.ie/go/clinicalgovernance
Clinical
Guidelines
“Clinical guidelines are systematically developed statements, based on a thorough
evaluation of the evidence, to assist practitioner and patient decisions about
appropriate healthcare for specic clinical circumstances, across the entire clinical
spectrum.” NCEC/HIQA (2015, p. 7)
Data
Controller
Refers to a person who, either alone or with others, controls the contents and use
of personal data.
Data Item
A single unit of data for which the denition and permissible values are specied by
means of a set of attributes.
Data Quality
Refers to data that is accurate, valid, reliable, relevant, legible, timely and complete.
Data
Processor
A computer or person that carries out operations on data to retrieve, transform, or
classify information.
Data Set
A group of data items.
Health
Information
and Quality
Authority
(HIQA)
Reporting directly to the Minister for Health, this independent organisation has
legal power and responsibility for improving the quality, safety and value of health
and social care services in Ireland. HIQA has responsibility across health and
social care services (excluding mental health) for setting standards, monitoring
and inspecting the quality and safety of service provision, providing guidance on
health information and carrying out health technology assessments

Clinical Audit A Practical Guide
49
Healthcare
Audit
“Healthcare audit, in line with the design and practice of Internal audit, is an
independent, objective assurance activity designed to add value and improve an
organisation’s operations. It helps an organisation accomplish its objectives by
bringing a systematic, disciplined approach to evaluate and improve the effectiveness
of risk management, control, and governance processes.”
HSE (2019a, p. 2)
“Under the HSE’s Code of Governance, Healthcare Clinical audit sits alongside and
mirrors the organisation’s Internal Clinical audit function by providing ‘third line of
defence’
1
assurance in relation to risks and controls in care related activities
in both clinical and non-clinical settings. The HSE’s Healthcare clinical auditors
are members of the Chartered Institute of Internal Clinical auditors (CIIA) and are
required to comply with the professional and general standards set by the CIIA.”
HSE (2019a, p. 2)
Healthcare
Quality
Improvement
Partnership
(HQIP)
Body funded by the English Department of Health to promote best practice in
clinical audit and to re-invigorate clinical audit activity.
Healthcare
Record
Review
“A Healthcare Record Review is where pre-recorded, person-centred data are
used to answer one or more questions. The review is not part of direct patient care.
It may be carried out for a number of purposes, including clinical audit, research, or
incident review. The purpose will dictate the governance structures to be followed.
It can also be referred to as a chart review or case review.”
“A Healthcare Record Review for the purposes of clinical audit collects pre-agreed
datasets from a cohort of charts without reviewing the overall care or looking at
the context of that care. These datasets are used as inputs to a clinical audit which
aims to provide learning and subsequent quality improvement.”
Incident
Review
“An Incident Review takes place after an individual patient safety incident has
occurred. It involves a structured analysis and is conducted using best practice
methods, to determine what happened, how it happened, why it happened, and
whether there are learning points for the service, wider organisation, or nationally”
(HSE, 2018, p. 5).
Key
Performance
Indicators
(KPI)
Performance Indicators are specic and measurable elements of practice that
can be used to assess quality of care. Indicators are quantitative measures of
structures, processes or outcomes that may be correlated with the quality of care
delivered by the healthcare system.
Look Back
Review
“A Look Back Review is a process that is initiated where it has been determined that
a number of people have been exposed to a specic hazard. The process seeks
to identify if any of those exposed to the hazard have been harmed and what needs
to be done to ameliorate the harm. This process consists of three key stages:
Preliminary Risk Assessment, Clinical audit and Recall.”
HSE (2018, p. 29)
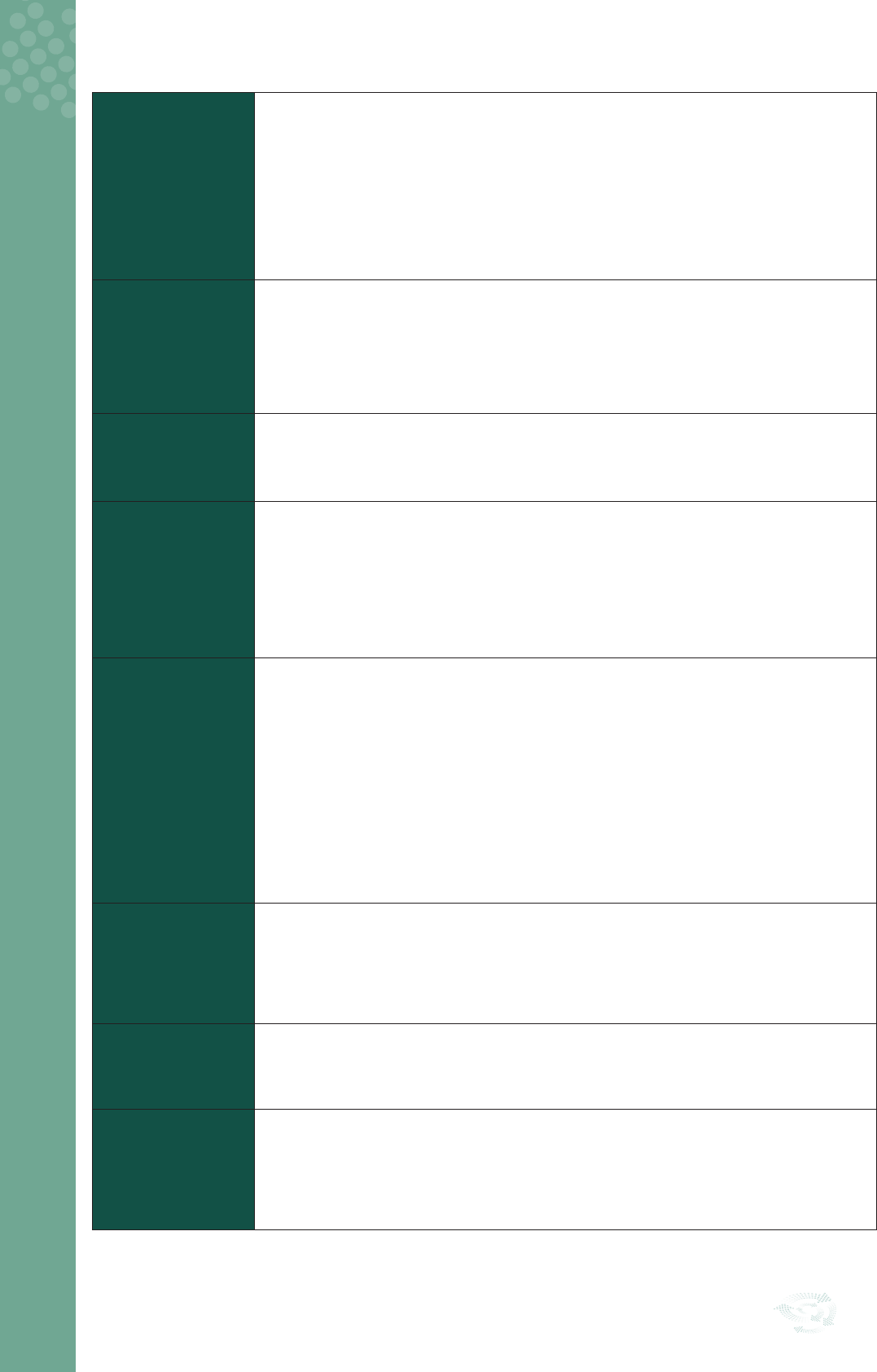
Clinical Audit A Practical Guide
50
National
Centre for
Clinical Audit
(NCCA)
The HSE National Centre for Clinical Audit (NCCA) established (April 2022)
within the QPSD, follows publication of the HSE National Review of Clinical
Audit Report in 2019, and is primarily responsible for implementing the report’s
recommendations. This step conrms the HSE’s commitment to developing clinical
audit as an essential quality and patient safety tool in Ireland, promoting improved
patient outcomes.
National
Clinical
Effectiveness
Committee
(NCEC)
A partnership between key stakeholders in service user safety in the Irish
health system. The aim of the committee is to provide a framework for national
endorsement of clinical guidelines and clinical audit to optimise patient care, within
the health system, both public and private.
National
Clinical
Guideline
A guideline that meet specic quality assurance criteria and has been mandated by
the designated national body – National Clinical Effectiveness Committee (NCEC).
National
Institute
for Health
and Clinical
Excellence
(NICE)
The National Institute for Health and Clinical Excellence was established as a
Special Health Authority by the UK Department of Health and is one of the key
elements of the NHS in England and Wales. It was set up to reduce variation in
the availability and quality of treatments and care in the National Health Service.
Its principal role is to provide authoritative, robust and reliable guidance on best
practice procedure.
National
Ofce of
Clinical Audit
(NOCA)
The National Ofce of Clinical Audit (NOCA) was established in 2012 through
the collaboration of the HSE’s Quality and Patient Safety Directorate and Clinical
Strategy and Programmes Directorate together with the Royal College of Surgeons
in Ireland (RCSI) and the College of Anaesthetists. NOCA manages a suite of
national clinical audits.
Each clinical audit focuses on a unique area of healthcare such as hip fracture,
major trauma, hospital mortality, ICU care and joint replacements. Governance
structures are established both in NOCA and locally in each hospital to oversee
the management and sustainability of the clinical audit. All NOCA clinical audits
are led at a hospital level by clinicians and supported by their management teams.
Peer Review
“Peer review is the professional assessment, against standards, of the organisation
of healthcare processes and quality of work, with the objective of facilitating its
improvement.”
McCormick (2012, p. 8)
Personal
Data
Data relating to a living individual who is or can be identied either from the data
or form the data in conjunction with other information that is in, or is likely to come
into, the possession of the data controller.
Quality
Assurance
“Quality assurance is dened as all those planned and systematic actions necessary
to provide adequate condence that a structure, system, component or procedure
will perform satisfactorily and comply with agreed standards.”
HSE (2019b)

Clinical Audit A Practical Guide
51
Quality
Improvement
“Quality improvement (QI) is the combined and unceasing efforts of everyone —
healthcare professionals, patients and their families, researchers, commissioners,
providers and educators — to make the changes that will lead to:
• better patient outcomes
• better experience of care
• continued development and supporting of staff in delivering quality care.”
HSE (2016b, p. 4)
“All methods highlight the importance of accessing the unique knowledge that
frontline staff possess and involving them in any change and improvement process.
Improving the quality of care, and sustaining it, requires all programmes to have a
theory of change that is based on the application of improvement science.”
HSE (2016b, p. 15)
Registry
“A clinical registry is described as a system which collects a dened minimum data
set from patients undergoing a particular procedure or therapy, diagnosed with a
disease or using a healthcare resource.” Hoque et al. (2019)
Research
“Research is designed and conducted to generate new generalisable or
transferrable knowledge. It includes both quantitative and qualitative studies that
aim to generate new hypotheses as well as studies that aim to test existing or new
hypotheses.”
Health Research Board (2018)
Sample
Some of the service users, events, cases, situations or items that are drawn from
the population on which the clinical audit is focused (a sub-set of the population).
Service
Anywhere health or social care is provided. Examples include but are not limited
to: acute hospitals, community hospitals, district hospitals, health centres, dental
clinics, GP surgeries, home care, etc.
Service
Evaluation
“Service evaluation seeks to assess how well a service is achieving its intended
aims. It is undertaken to benet the people using a particular healthcare service
and is designed and conducted with the sole purpose of dening or judging the
current service.”
Twycross and Shorten (2014, p. 65)
Unlike clinical audit, it does not compare the service to a predened standard.
Service
Provider
Any person, organisation, or part of an organisation delivering healthcare or social
care services – as described in the Health Act 2007 Section 8(1)(b)(i)–(ii).
Clinical Audit A Practical Guide
52
Service User
The term ‘service user’ is used in general throughout this document, but occasionally
the term ‘patient’ is used where it is more appropriate. The term ‘Service user’
includes:
• People who use health and social care services as patients
• Carers, parents and guardians
• Organisations and communities that represent the interests of people who use
health and social care services
• Members of the public and communities who are potential users of health
services and social care interventions
Stakeholder
A person, group, organisation, or system who affects or can be affected by an
organisation’s actions. Health service provider’s stakeholders, for example, include
its service users, employees, healthcare staff, government, insurers, industry and
the community.
Standard
Standards are dened as structures and processes needed to identify, assess
and manage specied risks in relation to the subject area (for example, healthcare
records management, decontamination etc).
“A standard is a denable measure against which existing structures, processes or
outcomes can be compared.” NCEC/HIQA (2015, p. 9)
Standard
Criteria
The standard statement is expanded in the section headed criteria, with different
criteria providing the detail of what needs to be achieved for the standard to be
reached.
Statement of
Information
Practices
A document, clearly displayed and accessible to all staff and service users that sets
out what information the service collects, how it is used, with whom it is shared and
for what purpose, the safeguards that are in place to protect it and how service
users can access information held about them.
Target/
Level of
Performance
A dened level or degree of expected compliance with clinical audit criteria; may
be expressed in percentage or proportion of cases.
Target
Population
All of the service users, events, cases, situations or items on which the standard or
clinical audit is focused. A population can range from a very small limited number
to a large or innite number.
Clinical Audit A Practical Guide
53
Resource 1
Checklist of Best Practice in Clinical Audit
Stage 1 Select Topic
Step 1 Involve stakeholders
Step 2 Determine the Clinical Audit Topic
Step 3 Plan the Delivery of the Clinical Audit
Stage 2 Set Criteria and Standards
Step 1 Select the Standard(s) to be used
Step 2 Set the Target / Level of Performance
Step 3 Consider Inclusion / Exclusion Criteria
Step 4 Consider Exceptions
Stage 3 Design Clinical Audit Tool and Collect Data
Step 1 Design Clinical Audit Tool
Step 2 Data Collection Process Discussed and Decided
Stage 4 Analyse Data and Compare Results with Standards
Step 1 Data Analysis
Step 2 Calculating Compliance with Clinical Audit Criteria
Step 3 Drawing Conclusions
Step 4 Sharing Results
Stage 5 Clinical Audit Report
Step 1 Layout of the Clinical Audit Report
Step 2 Write Report
Step 3 Reection
Stage 6 QI Plan and Action
Step 1 Development of a Quality Improvement Plan
Step 2 Actions discussed, decided and documented
Stage 7 Re-audit
Step 1 If re-audit is required, start the process again
Clinical Audit A Practical Guide
54
Adapted from King’s College Hospital https://www.kch.nhs.uk
1
CLINICAL AUDIT PROPOSAL FORM SAMPLE TEMPLATE
(*This is a sample Clinical Audit template which can be adapted for National/Local use)
For Further Information please see HSE NCCA Nomenclature/Glossary of Terms for Clinical Audit:
https://www.hse.ie/eng/about/who/nqpsd/ncca/nomenclature-a-glossary-of-terms-for-clinical-audit.pdf
Clinical Audit Topic –
Title of Clinical Audit –
CLINICAL AUDIT & PROJECT LEAD:
Clinical Audit Lead (Name/Job Title): ________________________________
Clinical Audit Project Lead (Name/Job Title): __________________________
WHY WAS THE TOPIC CHOSEN?
Under whose initiative was the audit instigated? (Tick all boxes that apply)
Local
National
Other Quality/Patient Safety Initiative, please specify: Triage Accuracy in EDs
Project source must be one or more of the following (Tick all boxes that apply)
High Cost Activity High Risk Activity High Volume Activity
Based on evidence based healthcare and clinical effectiveness issues (best practice)
Local initiative which centres on processes that may have a significant effect on provision of
patient care and/or outcome
Risk Management Issues
Re-audit of previously accepted project
Each clinical audit project must satisfy all of the following:
Resource 2
Clinical Audit Sample Proposal Form
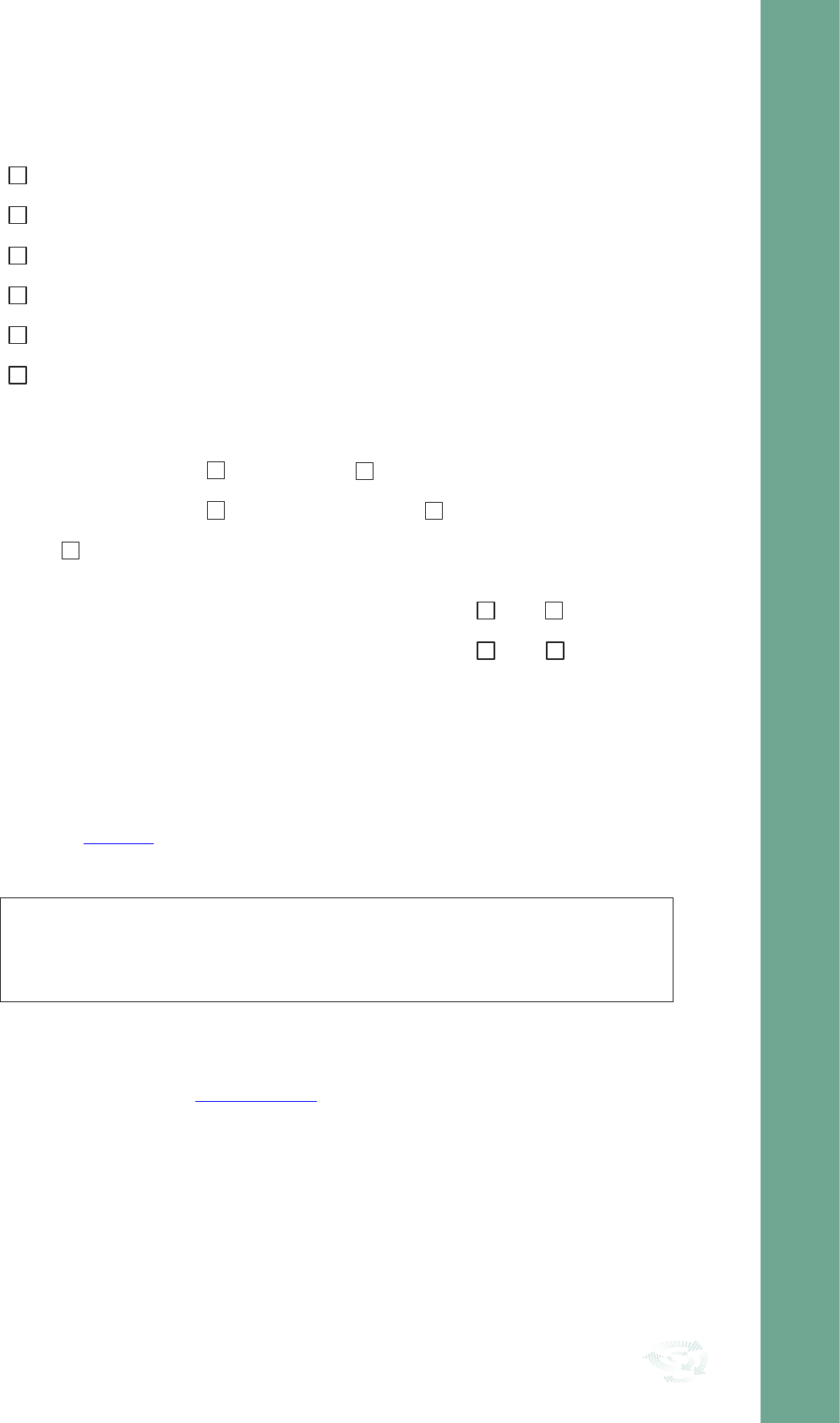
Clinical Audit A Practical Guide
55
Adapted from King’s College Hospital https://www.kch.nhs.uk
2
Aims must be realistic and achievable within available resources
Clear objectives
Multidisciplinary
Clinical and managerial support with a willingness to implement any changes
Agreed and approved standards for audit agreed by team
Willingness to agree recommendations from the audit and agreed action plan(s).
PARTICIPANTS:
Medical Nursing
Allied Health Professionals Other Speciality / Dept BIU
Other:
Add as required: _____________________________
Have all potential members of the project group been identified? Yes No
Has the clinical audit been discussed with them? Yes
No
Please note the relevant groups involved:
AIM OF CLINICAL AUDIT:
AIM Statement*:
(*An aim statement is the answer to the first question in the Model for Improvement: What Are We Trying to
Accomplish?) www.IHI.org
OBJECTIVE(s) OF CLINICAL AUDIT:
CRITERIA AND STANDARDS:
(*A Standard is a definable measure against which existing structures, processes or outcomes can be compared.
(NCEC/HIQA 2015, p. 9)

Clinical Audit A Practical Guide
56
Adapted from King’s College Hospital https://www.kch.nhs.uk
3
1.
2.
3.
4.
5.
6.
Web link (Please insert web link to Standards source):
METHOD:
Has a literature search been undertaken Yes No
Key words used in search and databases used:
INCLUSION/ EXCLUSION CRITERIA:
Inclusion Criteria:
The sample identified for the review must
Exclusion Criteria: These outside of the date ranges above
DATA COLLECTION: Concurrent Retrospective Prospective
How will cases be identified?
METHOD OF DATA COLLECTION: Chart Review
Patient Questionnaire
Staff Questionnaire Telephone Interview
Observation
Other:
SAMPLE SIZE:
Clinical Audit A Practical Guide
57
Adapted from King’s College Hospital https://www.kch.nhs.uk
4
Anticipated Time-scale Proposed: Target completion date:
RESOURCES:
Involvement or resources that you wish to request from your Local Clinical Audit Support Team
(where available locally)
Literature Search
Topic Selection
Proforma Design
Questionnaire Design
Data Analysis
Data Collection
Dissemination
Report Production
Other
ACKNOWLEDGMENT:
PATIENTS INCLUDED IN THE AUDIT:
a. Are all patients included in this audit under the care of the Audit Lead? Yes No
b. If no, please complete the form overleaf (Request for inclusion of patients in a Clinical
Audit)
c. Is there a patient representative on the clinical audit sub group? Yes No
If No, please state the reason for this.
PROJECT ORGINATOR/LEAD:
This proposal and its possible (clinical and managerial) implications have been discussed with the
relevant participants previously noted who undertake to support the audit and the implementation
of any necessary changes identified as a result of the audit.
Signed __________________ _______ Date ____________

Clinical Audit A Practical Guide
58
Adapted from King’s College Hospital https://www.kch.nhs.uk
5
Request for inclusion of patients in Clinical Audit
(Insert Title of Clinical Audit):_________________________________________
Dear Colleague,
I would be grateful if your patient’s anonymous details could be included in the clinical audit
proposed above.
This will be undertaken in conjunction with the Local Clinical Audit Support Team and with the
support/supervision of __________________________.
Yours sincerely,
Agreed (Signature & Date)
_____________________________ _________________
_____________________________ _________________
_____________________________ _________________
_____________________________ _________________
_____________________________ _________________
_____________________________ _________________
References and Further Information
https://www.hse.ie/eng/about/who/nqpsd/ncca/nomenclature-a-glossary-of-terms-for-clinical-audit.pdf

Clinical Audit A Practical Guide
59
Resource 3
Clinical Audit Report Sample Template
Clinical Audit
Lead/Author(s):
Service Provider:
Key Stakeholders:
Service/ Speciality:
Title of Clinical
Audit
This should be the same as the title on the Proposal Form
Date of Report
Distribution
Aim and
Objectives:
Standard(s) /
Criteria used:
Clinical audit must measure against standards / guidelines; these should be
identied and the source included
Background &
Introduction
This is essentially narration, clarifying why the clinical audit was done. For
example, was the clinical audit prompted by being a high volume, high risk,
problem prone topic? The background should explain the rationale for doing
the clinical audit. Summarise the evidence base for the clinical audit topic,
giving any references at the end. If a team was convened to undertake this
clinical audit, include how this was organised and who was involved.
Aim(s) of the
Clinical Audit
Explain what the clinical audit is trying to achieve; the aim and objectives
should have been identied in the planning stage of the clinical audit.
Methodology To include:
• Chosen population
• How sample selected
• Retrospective, concurrent or prospective
• Sample size
• Describe tool used
State the chosen population for this study (for example, ‘patients referred to
the one-stop breast clinic for suspected cancer’) and then how the sample
was selected for the clinical audit. Specify whether a retrospective, concurrent
or prospective approach was used (for example, for a prospective clinical
audit, ‘the rst 100 patients referred to the clinic starting from 01/10/20’, or for
a retrospective clinical audit, ‘all patients seen at the outpatient clinic during
July’). Describe how these patients were identied, the sample size, the time
period, and clarify how this was calculated or agreed upon.
The data collection method should also be included, for example, ‘Data was
collected from patients’ case notes using a data collection sheet, or ‘a query
was run in ICT’. List who was responsible for data collection, when this
was done, and mention briey the method of data input (if appropriate) and
analysis.
Clinical Audit A Practical Guide
60
Results State the results.
Start with total number (n=00). Data may be presented visually (graphs, tables)
The number of subjects (for example, patients) included in the clinical audit is
the initial ‘n’ number. If data is incomplete, explain why, for example, it might
not be possible to nd every set of patient notes.
How data is analysed depends upon the question/s to be answered. Ensure
to include the number and percentage of cases meeting each criteria of the
standard, making it clear what number is being taken a percentage of as the
‘n’ number may change at different points of the report, for example, 45/50
(90%) for criterion A and 81/90 (90%) for criterion B.
Conclusion(s) List key points that relate to the clinical audit ndings.
List the key points that ow from the clinical audit results - use bullet points
and avoid long paragraphs. Ensure conclusions are supported by the data,
or if the data points to no rm conclusions, say so - don’t make claims that
are not supported by the evidence. Make objective, factual statements, not
subjective ones.
Recommendations
& Quality
Improvement Plan
Quality improvement plan - with review date and person responsible for action.
Recommendations for change should be made. Make sure these are realistic
and achievable.
A quality improvement plan (action plan) should be agreed, stating what
changes will be implemented, who will be responsible for carrying them out
and when this will be done. If appropriate (i.e. changes are to be made), set a
date for a re-clinical audit to complete the clinical audit cycle.
References
Appendices
Clinical Audit A Practical Guide
61
Resource 4
Quality Improvement / Action Plan
No/Ref Recommendation
Person
Responsible
Target Date Status Comments
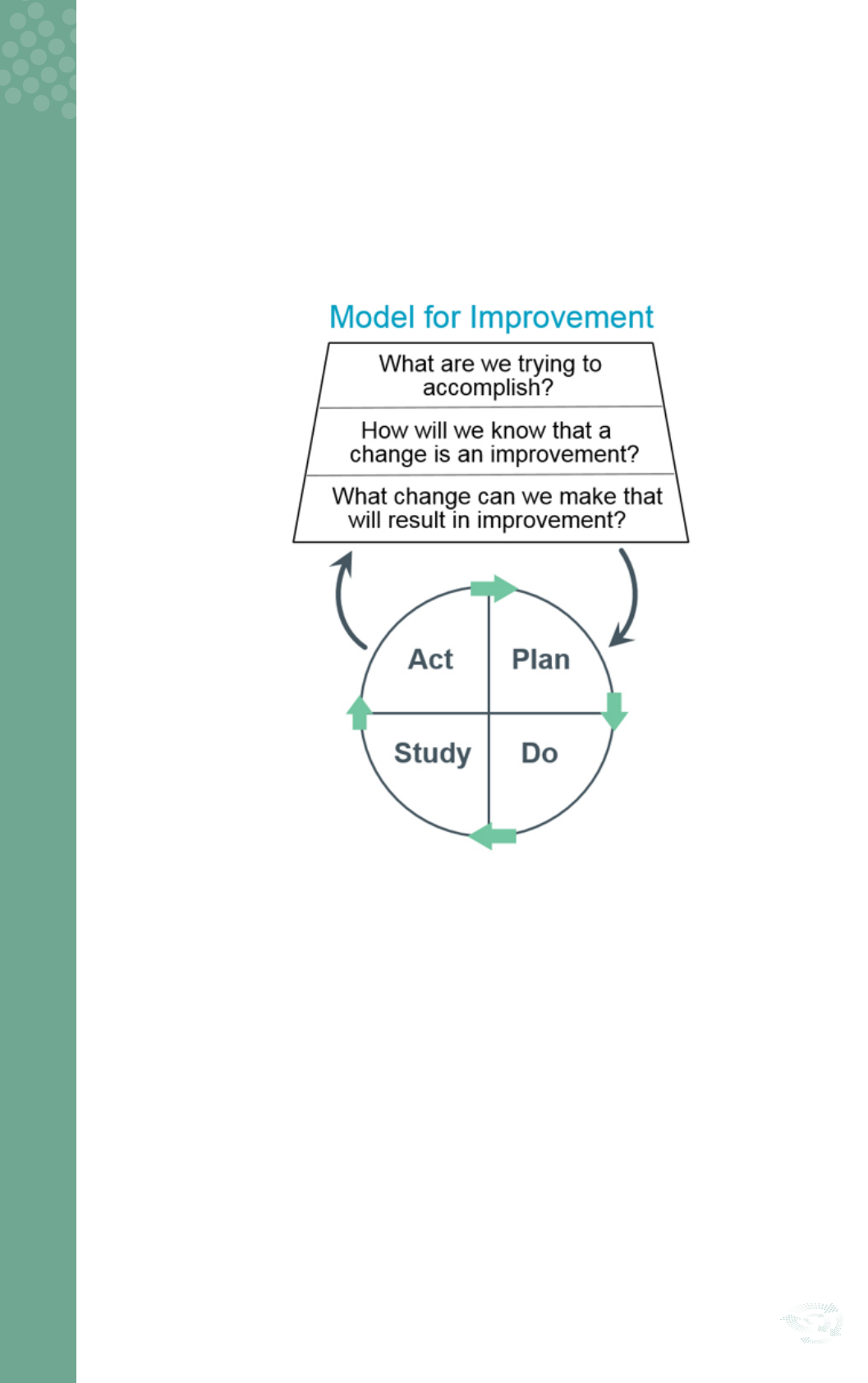
Clinical Audit A Practical Guide
62
Resource 5
Model for Improvement
Plan-Do-Study-Act (PDSA) Cycles
The Plan-Do-Study-Act (PDSA) cycle is shorthand for testing a change — by planning it, trying it, observing
the results, and acting on what is learned. This is the scientic method, used for action-oriented learning.
Steps in the PDSA Cycle
Step 1: Plan
Plan the test or observation, including a plan for collecting data.
• State the objective of the test.
• Make predictions about what will happen and why.
• Develop a plan to test the change. (Who? What? When? Where? What data need to be collected?)
Step 2: Do
Try out the test on a small scale.
• Carry out the test.
• Document problems and unexpected observations.
• Begin analysis of the data.
Clinical Audit A Practical Guide
63
Step 3: Study
Set aside time to analyze the data and study the results.
• Complete the analysis of the data.
• Compare the data to your predictions.
• Summarize and reect on what was learned.
Step 4: Act
Rene the change, based on what was learned from the test.
• Determine what modications should be made.
• Prepare a plan for the next test.
https://www.ihi.org/resources/Pages/HowtoImprove/ScienceofImprovementTestingChanges.aspx

About the HSE National Centre for Clinical Audit
National Quality and Patient Safety Directorate
The National Quality and Patient Safety Directorate (NQPSD) was established within the Ofce of the Chief
Clinical Ofcer in Summer 2021, following the HSE Corporate Centre review. It merged a number of functions
from the former national Quality Assurance and Verication Division (QAVD) and National Quality Improvement
Team (NQIT). National QPSD is anchored in the HSE Patient Safety Strategy 2019-2024. It works to embed
a culture of patient safety improvement at every level of the health and social care service. This is achieved
through developing a collaborative culture aimed at repeating and improving on positive outcomes and
minimizing adverse outcomes.
The HSE National Centre for Clinical Audit (NCCA) established within the QPSD, follows publication of the
HSE National Review of Clinical Audit Report in 2019, and will be primarily responsible for implementing
the report’s recommendations. This step conrms the HSE’s commitment to developing clinical audit as an
essential quality and patient safety tool in Ireland, promoting improved patient outcomes.
Clinical audit is an integral component of safety in all modern healthcare systems and the programme will
ensure delivery of a standardised approach. Establishing the HSE NCCA marks an important step in the
HSE’s continued efforts to improve the quality and safety of healthcare for patients. This will strengthen the
development of an end-to-end process for clinical audit in accordance with the recommendations in the report
and meet the needs of clinical audit service providers and multi-disciplinary stakeholders.
For further information, please contact:
HSE National Centre for Clinical Audit
Health Service Executive
Dr Steevens Hospital
Dublin D08 W2A8
w: https://www2.healthservice.hse.ie/organisation/ncca/
@hsencca
