
Annual operational plan for
immunization services
Guidelines for development or optimization
Finalized version August 2021
Eric Laurent (laurent.emj@gmail.com)
Ulla Griffiths (ugriffiths@unicef.org)

AOP guidelines - Finalized version August 2021
2
Table of content
1
I. Rationale for new annual operational plan guidance .................................................. 3
II. Definitions and concepts ............................................................................................... 4
Objectives, strategies, activities definitions ................................................................... 4
Operational planning definition .................................................................................... 4
Operational planning environment ................................................................................ 4
III. Principles for operational planning ........................................................................... 5
Which kind of planning “instrument” do countries need? ............................................. 5
Best practices ............................................................................................................... 5
IV. Structure of a standard AOP ..................................................................................... 6
Headings and contents .................................................................................................. 6
Software or template .................................................................................................... 6
Rolling AOP ................................................................................................................... 7
Best practices ................................................................................................................ 8
V. Planning cycle and process – actors, alignment and timetable .................................... 8
Planning cycle................................................................................................................ 8
Planning process ........................................................................................................... 8
Actors involved .............................................................................................................. 9
Alignment of plans ........................................................................................................ 9
Best practices .............................................................................................................. 10
VI. Costing and budgeting process ............................................................................... 10
Resource requirement ................................................................................................. 10
Budgeting .................................................................................................................... 11
Financing ..................................................................................................................... 11
Best practices .............................................................................................................. 11
VII. Monitoring and evaluation ..................................................................................... 12
Activities monitoring ................................................................................................... 12
Best practices .............................................................................................................. 12
Annexes .............................................................................................................................. 13
Acronyms .................................................................................................................... 13
Reference documents ................................................................................................. 14
1
The “Guidelines for development or optimization of annual operational plan for immunization services”
comprise the following materials:
- A planning narrative (this document)
- A planning template (an Excel template, but could be Access-based, Google Sheet, DHIS2)
AOP guidelines - Finalized version August 2021
3
I. Rationale for new annual operational plan guidance
- Global partners highlighted the needs for upgrading guidelines and tools for
immunization strategic and operational planning in several reports: Rapid
stocktaking and support to revising the cMYP (Mott MacDonald 2017); Strategic and
operational planning, a review of best practices (UNICEF 2018); Landscape analysis in
30 Gavi eligible countries (UNICEF 2019); Immunization Agenda 2030 (WHO 2020)
- Assessments and reports demonstrated the current fragmentation in operational
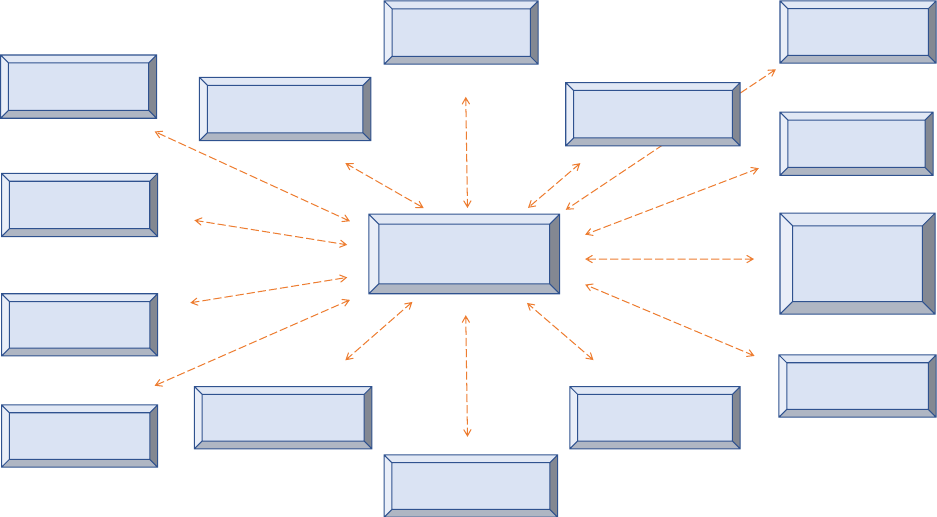
planning, with a multiplicity of plans developed in recent years, as shown in figure 1.
Immunization services are sometimes “lost in planning”
- There is no effective alignment between immunization AOP and National Health Plan
(NHP) and a lack of integration of immunization planning within MCH and PHC
services planning
- Immunization AOP budget and national health budget cycle are often not linked nor
interconnected
- The current multiplicity and diversity of plan formats make it impossible to recover
simple information (what, how, who, when, at what cost)
- There are multiple reasons for all above listed problems, but the main one is the lack
of standard AOP process and format for “driving” all immunization operational
planning, budgeting, implementation, monitoring, making it the “reference
operational plan” for all stakeholders, including partners
Figure 1: Example of fragmented planning for immunization
HSS plan
Data quality
implementation plan
TCA plan
Transition plan
CCEOP plan
EVM implementation
plan
VPD surveillance
plan
Communication plan
NIS / cMYP
New vaccine
introduction plan
Sub-national
plans
MR elimination
plan
Polio eradication
plan
COVID vaccine
roll-out plan
Annual operational
plan
AOP guidelines - Finalized version August 2021
4
II. Definitions and concepts
Objectives, strategies, activities definitions
To make sure strategic and operational planning are properly positioned, we need to
recall some key definitions:
- Objective is defined as “where to go”, as “what to achieve”
- Strategy is defined as “how to go, to achieve, to reach objective”, as a “series of
broad lines of action intended to achieve program goal and target”
2
- Activity is defined as “what to concretely do to achieve objective”, as a “series of
detailed actions to enable the strategy”
- The range of activities could be broad. Often, we find that operational plans use
“group/set of activities”, “core/key activities”, “activities”, “tasks”, “sub-tasks”. All
should be referring to concrete and detailed activity
Operational planning definition
3
- Operational planning is the link between the objectives and strategies of the national
strategic plan and the implementation of the program. It is about transforming the
strategic plan into actionable tasks. Operational planning will identify the activities to
be carried out to achieve the objectives of the strategic plan
- An operational plan should specify “what” needs to be done, by “whom”, at “which
cost” and “when”
- Operational planning is managerial and short term, as opposed to strategic planning,
and deals with month-by-month or quarter-by-quarter activities, and often has a
one-year time horizon (annual operational plan)
Operational planning environment
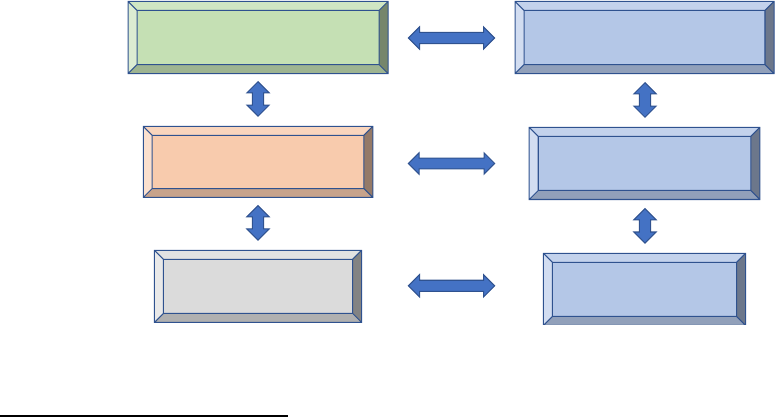
Figure 2: Example of a national planning environment
2
https://www.who.int/healthsystems/hss_glossary/en/
3
Strategizing national health in the 21st century: A handbook, WHO 2016
Immunization annual
operational plan (AOP)
MCH action plan
Family health action plan
National health action planHealth sector strategy
MCH strategy
Family health strategy
National immunization
strategy (NIS)

AOP guidelines - Finalized version August 2021
5
- Annual operational plan (AOP) environment:
ð Not a stand-alone document
ð Part of a planning “environment”: NHP, MCH, program plans, etc.
ð AOP connected and aligned with strategic plan
III. Principles for operational planning
Which kind of planning “instrument” do countries need?
- An instrument which is:
ð “Essential” for planning, budgeting, implementing, coordinating and monitoring
activities
ð “Central” to the immunization program and wider MCH program
ð “Active and lively” working instrument, not to “put on the shelves”
- An instrument which is in line with the national authority planning cycle, as well as
subnational operational plans developed at intermediate health levels
- An instrument which is consistent with operational plans of other communicable
disease, family, maternal and child health programs
- An instrument whose format is:
ð Simple, easy to use, saving program officers precious time
ð Structured, providing essential information, not a “shopping list”
ð Compatible, aligned and integrated with other plans
- An instrument which is developed, owned and managed by a core planning team,
e.g. MCH/NIP manager and technical staff at national and subnational levels
- An instrument which will require commitment and accountability from:
ð MCH/NIP manager and staff at national and subnational levels
ð All stakeholders involved in immunization, including development partners
Best practices
4
Ensure the AOP is a “central” planning instrument that all stakeholders refer too
Ensure the AOP is an “action-oriented” instrument, transforming the strategic plan into
annual activities to be carried out to achieve the objectives
Ensure the AOP is developed, owned and managed by the core planning team, at national
and subnational levels
Ensure the AOP is simple, easy to use, structured, compatible, aligned and integrated with
other plans
Planning success = Ownership + Team work + Accountability + Prioritization + Simplicity
4
National strategic and operational planning for immunization - A review of best practices for optimized
planning, UNICEF 2019

AOP guidelines - Finalized version August 2021
6
IV. Structure of a standard AOP
Headings and contents
- An annual operational plan typically includes the following headings:
ð What: Description of activities for each objective of the strategic plan
ð Who: Person(s) and/or institution(s) responsible for the activities
ð What cost: Resources required and origins of resources
ð When: Timing and sequencing of all activities
ð How: A method of measuring progress (monitoring)
- Additional important information could be included in the headings:
ð Priority levels (colour coded, e.g. red for high, yellow for medium, green for low)
ð Information and link to other plans (plans reference code)
- Standard immunization program components could be used as AOP categories
5
1. Program management and financing
2. Human resources management
3. Vaccine supply, quality and logistics
4. Service delivery, including new vaccine introduction
5. Immunization coverage, including AEFI monitoring
6. Disease surveillance, control and outbreak response
7. Advocacy, communication and demand generation
- Level of details of the AOP needs to be defined (set of activities, activities, tasks)
considering usage. Individual workplans could be derived from the AOP
Software or template
- In case of an existing AOP software or template already in use in the country, it
should be assessed whether it is a comprehensive AOP for immunization (it may also
be an MCH AOP or an overall MoH/Health AOP, where immunization is integrated),
and if there is a need for issuing any immunization AOP or not
- AOP could be an Excel-based or an Access-based instrument. It could also be set-up
online, like Google Sheets or DHIS2
- An AOP must be a “manageable” instrument:
ð Not a huge Excel workbook with dozens of complex worksheets
ð Should be easily printable in an A3 format for sharing/posting on NIP office walls
ð Activities should be simply described or summarized to avoid Excel cells to
become “large and unreadable”
5
A guide for conducting an Expanded Program on Immunization (EPI) Review, WHO 2017

AOP guidelines - Finalized version August 2021
7
Figure 3: Example of AOP Excel template (provided as an annex to these guidelines)
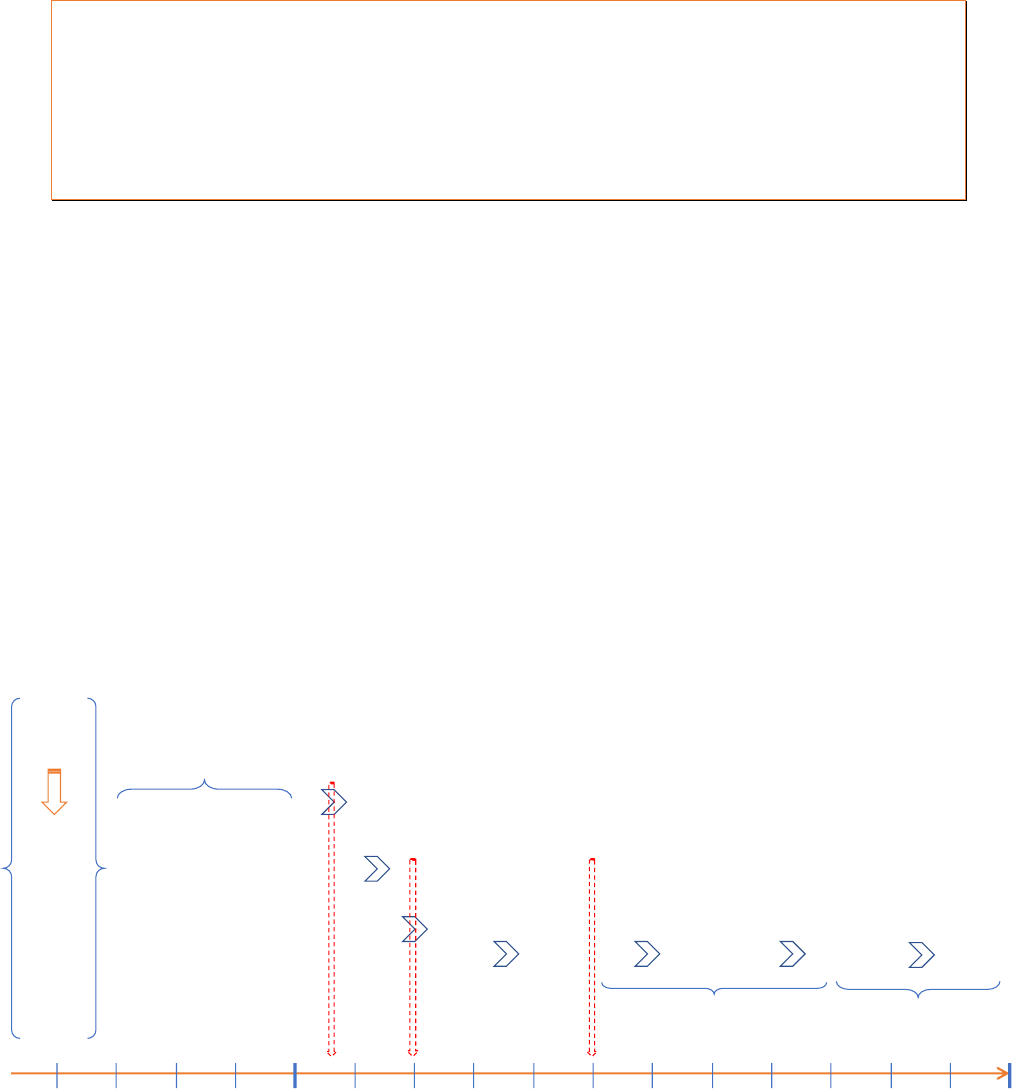
Rolling AOP
- In addition to the standard AOP, there could be a shorter description of activities that
are expected to be implemented in the period of 1-2 years beyond the AOP period
- The reason for capturing these additional activities in a “rolling AOP” is to increase the
visibility of upcoming activities and improve the planning of activities that require
sequencing of implementation over a multi-year period (e.g. vaccine introduction)
- Rolling AOP should only capture prioritised activities, both routine and non-routine,
including HSS, CCEOP, SIAs, etc. Activities that are considered low priority should not
be included in the rolling AOP
Figure 4: Example of a rolling AOP
AOP guidelines - Finalized version August 2021
8
Best practices
Ensure that the AOP answers the questions: Which activities? Linked to which objectives
and strategies? Who will be responsible? How much will it cost? When to be implemented?
How to monitor the activities?
Ensure AOP will be an “easy manageable” instrument, Excel, Access, Google Sheets, DHIS2
Consider the possibility to extend the AOP to a 2-3 years rolling AOP
V. Planning cycle and process – actors, alignment and timetable
Planning cycle
- The existing planning system, process and procedures in place in the country should
be considered in order to optimize the immunization operational planning process
- Submissions and deadlines are important to be respected by all stakeholders when
developing an AOP. That annual exercise remains a “priority” for any program
- A typical planning cycle, from early start of planning to budget approval and
execution of programs AOP, covers a period of 12-15 months. Therefore, it will be
important that all submissions and deadlines be respected by the programs, as well
as donors to align with a country’s planning and budgeting cycle
Figure 5: Example of a planning and budgeting cycle in Lao PDR
Acronyms: HSDP: Health Sector Development Plan; RMNCAH: Reproductive Maternal Neonatal Child
Adolescent Health; DHHP: Department of Hygiene and Health Promotion; DPC: Department of Planning and
Cooperation; DOF: Department of Finance; MPI: Ministry of Planning and Investment
Planning process
- The AOP process could be completed, consolidated and agreed through a workshop,
however much preparation will have to take place before
January
February
March
April
May
June
July
August
September
October December
National
Assembly
Sub-national & national
NIP/MCH AOP drafting
HSDP
+
RMNCAH
+
cMYP/NIS
+
DHIS-2 data
+
Reviews &
assessments
NIP/MCH AOP
submission
DHHP
review
DPC
consolidation
DOF
Budget
MOF
Budget
Programs
AOP
revision
Submission
to DPC
Submission
to MPI
National Assembly to
approve requested budget
Programs to review AOP
based on approved budget
Budget
approval
NovemberSeptember
October December
November
Available
information
for planning
AOP guidelines - Finalized version August 2021
9
- The operational planning process needs to be streamlined, limited in time and
presented in a concise format. The process, including filling in the template, should
not go beyond a few weeks. The final AOP document should be limited to the Excel
instrument (or any other format), and there is no need for any Word narrative
- All existing plans, action plans, workplans specific to immunization and development
partners-driven (e.g. HSS, CCEOP, EVMIP, DQIP, NVI, TCA) should ultimately come
“under” the immunization AOP, and be considered as “annexes”
- Operational planning needs to consider the following issues:
ð Operational planning is still needed even if there is no strategic plan
ð Commitment and rigorousness are important when developing an AOP
ð Operational planning could be a bottom-up or/and top-down process
ð Consider developing sub-national AOPs for decentralized country
Actors involved
- An operational plan is best done by those who are responsible for the plan. Ideally,
all of those who are responsible for related activities in the health sector will be
involved in operational planning, either directly or through having their interests
represented by someone involved in the formal planning process
- Operational planning involves many stakeholders, and thus negotiating between
various government departments, programs, donors, and non-state actors is
important. Key stakeholders are national and sub-national health authorities and
health service providers
- The actors developing the operational plan should be organized under a “core
planning team” and should function together when drafting the AOP. Any division of
stakeholders, such as component by component, will bring the risk of a fragmented
AOP. Ultimately, people responsible for a specific component can prepare the work
upstream, but consolidation should be done together
- Leadership, management and coordination (LMC) for AOP development is essential.
Whatever the core planning team configuration, there should a “captain in the ship”
Alignment of plans
• Alignment with the national immunization strategic plan (NIS)
- The annual operational plan should follow and come from the national immunization
strategy (NIS), as it will operationalize the strategies. It is essential that all activities
of the operational plan are aligned with strategies of the NIS
• Alignment with the MCH and/or NHP operational plan
- The immunization annual operational plan should be aligned as much as possible
with the MCH operational plan and/or the overall MoH/health operational plan
• Alignment with subnational operational plans
- AOPs are often developed at the national level, but when required (e.g. decentralized
health system), subnational AOPs will be developed too. The harmonization of the
national AOP with subnational AOPs will be important as some functions still remain
at the national level (e.g. vaccine supply)
AOP guidelines - Finalized version August 2021
10
- At the lower levels of the health system, i.e. health facilities, microplans are a kind of
operational plan. At this level too, alignment should be respected, i.e. between
subnational AOPs and microplans
• Alignment with the immunization subsets of plans
- The annual operational plan and the immunization subsets of plans (Covid vaccine
roll-out plan, HSS, Polio & MR plans, CCEOP, EVM plan, NVI plan, etc.) should be
harmonized. Ideally all immunization subsets of plans should ultimately come
“under” the immunization AOP
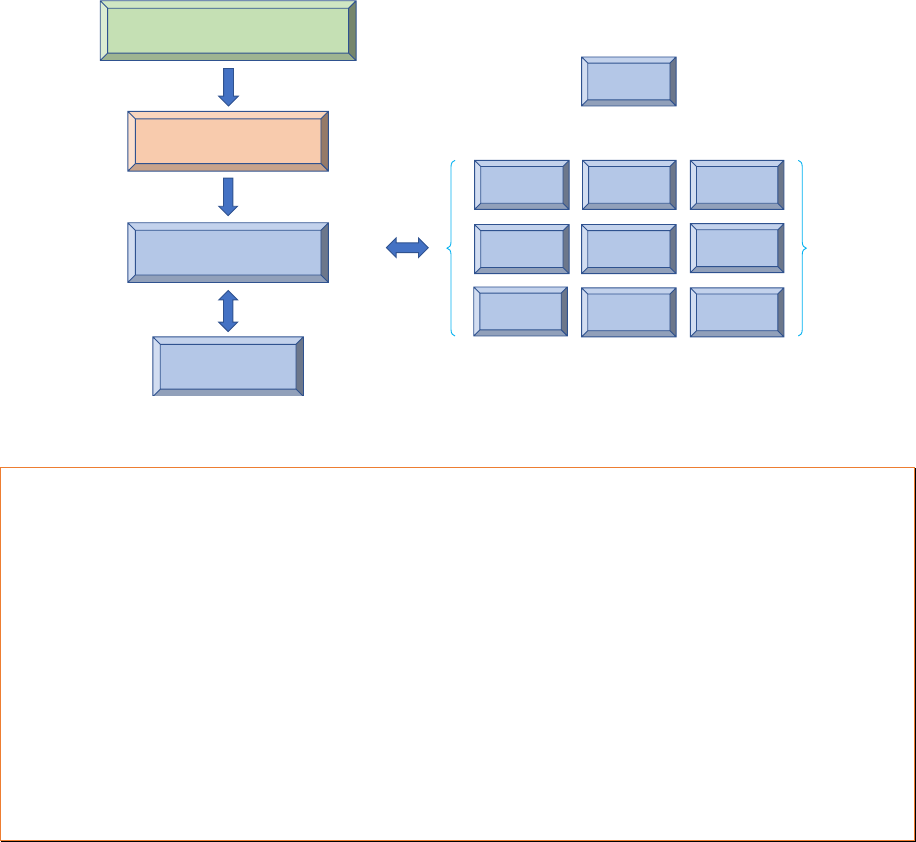
Figure 6: Example of planning alignment
Best practices
Ensure all stakeholders are fully committed when developing the AOP and dedicate the
required time necessary to the exercise
Ensure AOP involves all those who will be responsible for implementation, organized in a
“core planning team” and coordinated by a leader
Ensure the AOP process be streamlined, limited in time and the AOP presented in a
concise format (Excel, Access, Google Sheet, DHIS2)
Ensure the activities of the AOP are aligned with the objectives and strategies of the
immunization strategic plan (NIS)
Ensure strong alignment of all activities between the national operational plan and the
subnational operational plans
VI. Costing and budgeting process
Resource requirement
- An AOP is an instrument to support implementation of activities and hence it is
critical for it to include the resources requirement for the activities
National Health Plan 2021-2030
National Immunization
Strategy 2021-2025
Immunization Annual
Operational Plan
Sub-National Plans
HSS plan
Data quality
plan
TCA plan
CCEOP plan EVM plan
Surveillance
VPD plan
Communica-
tion plan
New vaccine
intro. plan
Covid vaccine
roll-out plan
Polio & MR
plans

AOP guidelines - Finalized version August 2021
11
- However, detailed calculation of the resource requirements (e.g. unit costs, number
of staffs, of items, of days, etc.) should not be in the AOP final worksheet, but in a
separate calculation spreadsheet
- AOP budgeting process requires reasonable estimation of the cost of activities,
avoiding on one side too rough estimations, and on the other side spending days to
get accurate amounts
- "Shared costs" associated with activities at the PHC level should be estimated by
those responsible to deliver PHC and supposedly not NIP. Nonetheless, NIP may
provide support to those responsible to deliver PHC to undertake this costing
- The NIS new guidelines will have a costing, budgeting and financing instrument,
called “NIS.COST”. Wherever possible, the use of that instrument could help the AOP
resources requirement estimations
Budgeting
- As previously shown in the planning graph, the national planning and budgeting
cycle should be considered. The AOP development cycle, including budgeting, needs
to align with the broader MoH planning and budgeting
- In this regard, the AOP budget information should be fed upwards and should be
aligned with the broader budget envelope provided to the immunization program
- Ideally, the sector budget ceiling as well as the exact allocations to the budget
centers should be clear before developing an AOP. If the public budget negotiation
process is still not completely concluded at the time of operational planning, the
approximate sector budget allocation as well as the national strategic plan
disaggregated costing can be used as an approximate ceiling within which to plan
Financing
- The AOP should describe a realistic picture on how the program is financed.
Therefore, in the AOP headings, it will be important to inform the source of funding
- Beyond MoH financing, funds provided by development partners should also be
listed in the AOP
- The AOP is mainly used as a planning and managing instrument and not so much as
an accountability or grant management tool
Best practices
Ensure the AOP instrument contains a reasonable estimation of the cost of the activities
Ensure a strong alignment between the AOP and the broader MoH planning and
budgeting process, including the budget envelope provided to the NIP
Ensure the AOP describes how activities are financed, including funds from MoH, but also
development partners

AOP guidelines - Finalized version August 2021
12
VII. Monitoring and evaluation
Activities monitoring
- An AOP is an instrument to manage implementation of activities and hence it is
critical to include monitoring the status of activity implementation (done, not done,
in progress, percentage of achievements)
- There will be a monitoring and evaluation (M&E) framework attached to the NIS,
including, impact, outcome, output indicators, providing key information for the
performance of the program. Therefore, there is no need for another M&E
framework for the AOP. Monitoring the status of AOP activity implementation will
be sufficient
Best practices
Ensure the AOP contain a column with the status of implementation of each activity
(done, not done, in progress) and an overall estimate of percentage of activities completed
AOP guidelines - Finalized version August 2021
13
Annexes
Acronyms
AEFI Adverse Events Following Immunization
AFP Acute Flaccid Paralysis
AFR Acute Fever & Rash
AOP Annual Operational Plan
BCG Bacillus Calmette Guerin (TB vaccine)
bOPV Bivalent Oral Polio Vaccine
US CDC US Centres for Disease Control & Prevention
cMYP Comprehensive Multi-Year Plan
CRS Congenital Rubella Syndrome
cVDPV Circulating Vaccine Derived Poliovirus
DHIS District Health Information System
DHO District Health Office
DQA Data Quality Assessment
DQIP Data Quality Improvement Plan
DT/Td Diphtheria-Tetanus Vaccine
eIR Electronic Immunization Registry
EPI Expanded Program on Immunization
EVM Effective Vaccine Management
EVMIP Effective Vaccine Management Improvement
Plan
FETP Field Epidemiologist Training Program
Gavi Gavi, The Vaccine Alliance
GDP Gross Domestic Product
GPEI Global Polio Eradication Initiative
HepB Hepatitis B Vaccine
HIV Human Immunodeficiency Virus
HPV Human Papillomavirus Vaccine
HSS Health System Strengthening
HCW Health Care Worker
ICC Inter-agency Coordination Committee
IEC Information Education Communication
IIP Immunization in Practice
IPV Inactivated Polio Vaccine
JE Japanese Encephalitis Vaccine
JRF Joint Reporting Form
KABP Knowledge Attitude Behaviour Practice
MCH Mother and Child Health
MCV Measles Containing Vaccine
MICS Multiple Indicator Coverage Survey
MLM Mid-Level Management
MR Measles and Rubella Vaccine
MOE Ministry of Education
MOH Ministry of Health
MOF Ministry of Finance
MPI Ministry of Planning and Investment
NCC National Certification Committee
NGO Non-Governmental Organization
NHI National Health Insurance
NVC National Verification Committee
NIP National Immunization Program
NIS National Immunization Strategy
NITAG National Technical Advisory Committee
NRA National Regulatory Authority
NVI New Vaccine Introduction
OOP Out of Pocket Payment
OPV Oral Polio Vaccine
PCV Pneumococcal Conjugate Vaccine
Penta Pentavalent Vaccine (DPT-HepB-Hib)
PIE Post Introduction Evaluation
PHC Primary Health Care
QCM Quarterly Committee Meeting
RRL Regional Reference Laboratory
RV Rotavirus Vaccine
SDG Sustainable Development Goals
SIA Supplementary Immunization Activities
SOP Standard Operating Procedures
TB Tuberculosis
TOR Terms of Reference
TWG Technical Working Group
UNFPA United Nations Population Fund
UNICEF United Nations International Children's
Emergency Fund
VPD Vaccine Preventable Disease
VVM Vaccine Vial Monitor
WB World Bank Group
WHO World Health Organization
AOP guidelines - Finalized version August 2021
14
Reference documents
1.
National Immunization Strategy (NIS) guidance document – WHO – May 2020
2.
National strategic and operational planning for immunization – A review of best practices for optimized
planning – UNICEF – August 2019
3.
Strategic and operational planning for immunization – Landscape analysis in 30 Gavi eligible countries –
UNICEF – October 2019
Afghanistan; Armenia; Benin; Burkina Faso; Burundi; Cambodia; Cameroon; Central African Republic; Chad;
Comoros; Ethiopia; Ghana; Guinea-Bissau; Honduras; Indonesia; Korea DPR; Lao PDR; Liberia; Madagascar; Mali;
Mozambique; Myanmar; Papua New Guinea; Sierra Leone; Sudan; Tanzania; Togo; Uganda; Vietnam; Zimbabwe
4.
Optimal strategic and operational planning and budgeting for immunization – Country case study – UNICEF
- Lao PDR, November 2019
- Haiti, September 2019
5.
Comprehensive multi-year plans assessments and roadmaps
- Roadmap for the next phase of comprehensive multi-year plans for immunization – BMGF; GAVI Secretariat;
UNICEF; WHO; World Bank – 1
st
December 2017
- Rapid Stocktaking and Support to Revising the cMYP – Mott MacDonald – 31 July 2017
- Report on cMYP translated into 2011 Annual Immunization Plan in GAVI eligible countries – WHO – April 2012
6.
Guidelines on strategic and operational planning (health and immunization)
- Instructions for Developing Annual Plan of Action of Expanded Program on Immunization – PAHO – 2014
- Instructions for Costing Annual Plan of Action of Expanded Program on Immunization – PAHO – 2014
- Guidelines for Comprehensive Multi-Year Planning for Immunization – WHO-UNICEF – September 2013
- Planning for Immunization (online course) – UNICEF – 2014
- A Tool and User Guide for cMYP Costing and Financing (Excel tool) – WHO – 2014
- A guide for conducting an Expanded Program on Immunization (EPI) Review – WHO – 2017
- A framework for National Health Policies, Strategies and Plans – WHO – June 2010
- Operational Planning for HIV/AIDS: A Guidance Note – ASAP UNAIDS – May 2009
- Planning guide for the health sector response to HIV/AIDS – WHO – 2011
- Strategizing national health in the 21st century: a handbook – WHO – 2016
- Toolkit to develop a national strategic plan for TB prevention, care and control – WHO – 2015
