PRODUCT MONOGRAPH
TAXOL
✶
(paclitaxel)
Injection, 6 mg/mL
ANTINEOPLASTIC AGENT
Bristol-Myers Squibb Canada
Montréal, Canada
*Registered TM of Bristol-Myers Squibb Company
used under licence by Bristol-Myers Squibb Canada
Date of Revision:
22 February 2010
Control number: 134380

PRODUCT MONOGRAPH
TAXOL
✶
(paclitaxel)
Injection, 6 mg/mL
THERAPEUTIC CLASSIFICATION
ANTINEOPLASTIC AGENT
TAXOL (PACLITAXEL) SHOULD BE ADMINISTERED UNDER THE SUPERVISION OF A
PHYSICIAN EXPERIENCED IN THE USE OF CANCER CHEMOTHERAPEUTIC AGENTS.
PATIENTS RECEIVING TAXOL SHOULD BE PRETREATED WITH CORTICOSTEROIDS,
ANTIHISTAMINES, AND H
2
ANTAGONISTS (SUCH AS DEXAMETHASONE,
DIPHENHYDRAMINE AND CIMETIDINE OR RANITIDINE) TO MINIMIZE
HYPERSENSITIVITY REACTIONS (SEE DOSAGE AND ADMINISTRATION). SEVERE
HYPERSENSITIVITY REACTIONS CHARACTERIZED BY DYSPNEA AND HYPOTENSION
REQUIRING TREATMENT, ANGIOEDEMA, AND GENERALIZED URTICARIA HAVE
OCCURRED IN PATIENTS RECEIVING TAXOL. THESE REACTIONS ARE PROBABLY
HISTAMINE MEDIATED. RARE FATAL REACTIONS HAVE OCCURRED IN PATIENTS
DESPITE PRE-TREATMENT. PATIENTS WHO EXPERIENCE SEVERE
HYPERSENSITIVITY REACTIONS TO TAXOL SHOULD NOT BE RECHALLENGED WITH
THE DRUG.
ACTIONS AND CLINICAL PHARMACOLOGY
TAXOL (paclitaxel) is a novel antimicrotubule agent that promotes the assembly of microtubules
from tubulin dimers and stabilizes microtubules by preventing depolymerization.
In vitro, TAXOL exhibits cytotoxic activity against a wide variety of both human and rodent tumor
cell lines including leukemia, non-small cell lung carcinoma, small cell lung carcinoma, colon
carcinoma, CNS carcinoma, melanoma, renal carcinoma, ovarian carcinoma and breast
carcinoma (see PHARMACOLOGY).
The pharmacokinetics of paclitaxel have been evaluated over a wide range of doses, up to
300 mg/m
2
, and infusion schedules ranging from 3 to 24 hours. Following intravenous
administration of TAXOL, the drug exhibited a biphasic decline in plasma concentrations. The
initial rapid decline represents distribution to the peripheral compartment and elimination of the
drug. The later phase is due, in part, to a relatively slow efflux of paclitaxel from the peripheral
compartment. In patients treated with doses of 135 and 175 mg/m
2
given as 3 and 24 hour
infusions, mean terminal half-life has ranged from 3.0 to 52.7 hours, and total body clearance
has ranged from 11.6 to 24.0 L/h/m
2
. Mean steady state volume of distribution has ranged
from 198 to 688 L/m
2
, indicating extensive extravascular distribution and/or tissue binding.
Following 3 hour infusions of 175 mg/m
2
, mean terminal half-life was estimated to be 9.9 hours;
mean total body clearance was 12.4 L/h/m
2
.
Variability in systemic paclitaxel exposure, as measured by AUC
0-
∝
for successive treatment
courses was minimal; there was no evidence of accumulation of paclitaxel with multiple
treatment courses.

2
The pharmacokinetics of paclitaxel have been shown to be non-linear. There is a
disproportionately large increase in C
max
and AUC with increasing dose, accompanied by an
apparent dose-related decrease in total body clearance. These findings are most readily
observed in patients in whom high plasma concentrations of paclitaxel are achieved. Saturable
processes in distribution and elimination/metabolism may account for these findings.
In vitro studies of binding to human serum proteins, using paclitaxel concentrations ranging from
0.1 to 50 μg/mL, indicated that on average 89% of drug is bound; the presence of cimetidine,
ranitidine, dexamethasone, or diphenhydramine did not affect protein binding of paclitaxel.
In vitro studies with human liver microsomes and tissue slices showed that paclitaxel was
metabolized primarily to 6α-hydroxypaclitaxel by the cytochrome P450 isozyme CYP2C8; and to
two minor metabolites, 3-p-hydroxypaclitaxel and 6α, 3'-p-dihydroxypaclitaxel by CYP3A4. In
vitro, the metabolism of paclitaxel to 6α-hydroxypaclitaxel was inhibited by a number of agents
(see PRECAUTIONS: Drug Interactions). The effect of renal or hepatic dysfunction on the
disposition of paclitaxel has not been investigated.
The disposition of paclitaxel has not been fully elucidated in humans. After intravenous
administration of TAXOL, mean values for cumulative urinary recovery of unchanged drug
ranged from 1.3 to 12.7% of the dose, indicating extensive non-renal clearance. In five
patients administered a 225 or 250 mg/m
2
dose of radiolabeled TAXOL as a 3-hour infusion,
14% of the radioactivity was recovered in the urine and 71% was excreted in the feces in 120
hours. Total recovery of radioactivity ranged from 56% to 101% of the dose. Paclitaxel
represented a mean of 5% of the administered radioactivity recovered in the feces while
metabolites, primarily 6α-hydroxypaclitaxel, accounted for the balance.
INDICATIONS AND CLINICAL USE
TAXOL (paclitaxel) is indicated, alone or in combination, for the treatment of carcinoma of the
ovary, breast, lung, or AIDS-related Kaposi’s Sarcoma.
Ovarian Carcinoma
- First-line treatment in combination with other chemotherapeutic agents.
- Second-line treatment of metastatic carcinoma of the ovary after failure of standard therapy.
Breast Carcinoma
- Adjuvant treatment of node-positive breast cancer administered sequentially to standard
combination therapy. In the clinical trial, there was an overall favorable effect on
disease-free and overall survival in the total population of patients with receptor-positive and
receptor-negative tumors, but the benefit has been specifically demonstrated by available
data (median follow-up 30 months) only in the patients with estrogen and progesterone
receptor-negative tumors. (See PHARMACOLOGY - Clinical Trials).
- Second-line treatment of metastatic carcinoma of the breast after failure of standard therapy.

3
Lung Carcinoma
- First-line treatment of advanced non-small cell lung cancer.
Kaposi's Sarcoma
- Treatment of advanced, liposomal anthracycline-refractory AIDS-related Kaposi's Sarcoma.
CONTRAINDICATIONS
TAXOL (paclitaxel) is contraindicated in patients who have a history of severe hypersensitivity
reactions to paclitaxel or other drugs formulated in Cremophor
H
EL (polyethoxylated castor oil).
TAXOL should not be used in patients with severe baseline neutropenia (<1,500 cells/mm
3
) nor
in patients with AIDS-related Kaposi's Sarcoma with baseline or subsequent neutrophil counts
of <1,000 cells/mm
3
.
WARNINGS
TAXOL (paclitaxel) should be administered under the supervision of a physician experienced in
the use of cancer chemotherapeutic agents.
TAXOL should be administered as a diluted infusion. Patients receiving TAXOL should be
pretreated with corticosteroids, antihistamines, and H
2
antagonists (such as dexamethasone,
diphenhydramine and cimetidine or ranitidine) to minimize hypersensitivity reactions (see
DOSAGE AND ADMINISTRATION). Anaphylaxis and severe hypersensitivity reactions
characterized by dyspnea and hypotension requiring treatment, angioedema, or generalized
urticaria have occurred in approximately 2% of patients receiving TAXOL. These reactions are
probably histamine-mediated. Rare fatal reactions have occurred in patients despite
pre-treatment. In case of a severe hypersensitivity reaction, TAXOL infusion should be
discontinued immediately and the patient should not be rechallenged with the drug (see
ADVERSE REACTIONS).
TAXOL should not be administered to patients with baseline neutrophil counts of less than
1,500 cells/mm
3
(<1,000 cells/mm
3
for patients with Kaposi's Sarcoma). Bone marrow
suppression (primarily neutropenia) is dose and schedule dependent and is the dose-limiting
toxicity within a regimen. Neutrophil nadirs occurred at a median of 11 days. Frequent
monitoring of blood counts should be instituted during TAXOL treatment. Patients should not
be retreated with subsequent cycles of TAXOL until neutrophils recover to a level
>1,500 cells/mm
3
(>1,000 cells/mm
3
for patients with Kaposi's Sarcoma) and platelets recover to
a level >100,000 cells/mm
3
(see DOSAGE AND ADMINISTRATION).
Severe cardiac conduction abnormalities have been reported in < 1% of patients during TAXOL
therapy. If patients develop significant conduction abnormalities during administration,
appropriate therapy should be administered and continuous electrocardiographic monitoring
should be performed during subsequent therapy with TAXOL (see ADVERSE REACTIONS).
H
T.M. of B.A.S.F.

4
Use in Pregnancy
TAXOL may cause fetal harm when administered to a pregnant woman. TAXOL has been
shown to be embryotoxic and fetotoxic in rabbits and to decrease fertility in rats. There are no
studies in pregnant women. Women of childbearing potential should be advised to avoid
becoming pregnant during therapy with TAXOL. If TAXOL is used during pregnancy, or if the
patient becomes pregnant while receiving this drug, the patient should be apprised of the
potential hazard.
Nursing Mothers
It is not known whether TAXOL is excreted in human milk. Breast feeding should be
discontinued for the duration of TAXOL therapy.
Use in Children
The safety and effectiveness of TAXOL in pediatric patients have not been established. There
have been reports of central nervous system (CNS) toxicity (rarely associated with death) in a
clinical trial in pediatric patients in which TAXOL was infused intravenously over 3 hours at
doses ranging from 350 mg/m
2
to 420 mg/m
2
. The toxicity is most likely attributable to the high
dose of the ethanol component of the TAXOL vehicle given over a short infusion time. The use
of concomitant antihistamines may intensify this effect. Although a direct effect of the
paclitaxel itself cannot be discounted, the high doses used in this study (over twice the
recommended adult dosage) must be considered in assessing the safety of TAXOL for use in
this population.
PRECAUTIONS
Contact of the undiluted concentrate with plasticized polyvinyl chloride (PVC) equipment or
devices used to prepare solutions for infusion is not recommended. In order to minimize
patient exposure to the plasticizer DEHP [di-(2-ethylhexyl)phthalate], which may be leached
from PVC infusion bags or sets, diluted TAXOL (paclitaxel) solutions should preferably be
stored in bottles (glass, polypropylene) or plastic bags (polypropylene, polyolefin) and
administered through polyethylene-lined administration sets.
Drug Interactions
Cisplatin
In a Phase I trial in which TAXOL was administered as a 24-hour infusion and cisplatin was
administered as a 1 mg/min infusion, myelosuppression was more profound when TAXOL was
given after cisplatin than with the alternate sequence (i.e. TAXOL before cisplatin). When
TAXOL is given before cisplatin, the safety profile of TAXOL is consistent with that reported for
single-agent use. Pharmacokinetic data from these patients demonstrated a decrease in
paclitaxel clearance of approximately 33% when TAXOL was administered following cisplatin.
Therefore, TAXOL should be given before cisplatin when used in combination. Patients treated
with TAXOL and cisplatin may have an increased risk of renal failure during the combination
therapy of paclitaxel and cisplatin in gynecological cancers as compared to cisplatin alone.
Cimetidine

5
The effect of cimetidine premedication on the metabolism of paclitaxel has been investigated;
the clearance of paclitaxel was not affected by cimetidine pretreatment.
Substrates, Inducers, Inhibitors of Cytochrome P450 2C8 and 3A4
The metabolism of TAXOL is catalyzed by cytochrome P450 isoenzymes CYP2C8 and CYP3A4.
Caution should be exercised when administering TAXOL concomitantly with known substrates,
inducers or inhibitors of the cytochrome P450 isoenzymes CYP2C8 and CYP3A4. In vitro, the
metabolism of paclitaxel to 6α-hydroxypaclitaxel was inhibited by a number of agents
(ketoconazole, verapamil, diazepam, quinidine, dexamethasone, cyclosporine, teniposide,
etoposide, and vincristine), but the concentrations used exceeded those found in vivo following
normal therapeutic doses. Testosterone, 17α-ethinyl estradiol, retinoic acid, montelukast and
quercetin, a specific inhibitor of CYP2C8, also inhibited the formation of 6α-hydroxypaclitaxel in
vitro. The pharmacokinetics of paclitaxel may also be altered in vivo as a result of interactions
with compounds that are substrates, inducers, or inhibitors of CYP2C8 and/or CYP3A4.
Potential interactions between TAXOL, a substrate of CYP3A4, and protease inhibitors (ritonavir,
saquinavir, indinavir, and nelfinavir), which are substrates and/or inhibitors of CYP3A4, have not
been evaluated in clinical trials. Caution and close monitoring of liver function is required;
further, no unapproved (e.g., investigational) protease inhibitor should be administered with
TAXOL.
Doxorubicin
Sequence effects characterized by more profound neutropenic and stomatitis episodes, have
been observed with combination use of TAXOL and doxorubicin when TAXOL was administered
BEFORE doxorubicin and using longer than recommended infusion times (TAXOL administered
over 24 hours; doxorubicin administered over 48 hours). Plasma levels of doxorubicin (and its
active metabolite doxorubicinol) may be increased when TAXOL and doxorubicin are used in
combination. However, data from a trial using bolus doxorubicin and 3-hour TAXOL infusion
found no sequence effects on the pattern of toxicity.
Hematology
TAXOL should not be administered to patients with baseline neutrophil counts of less than
1,500 cells/mm
3
(see WARNINGS, CONTRAINDICATIONS). In order to monitor the
occurrence of myelotoxicity, it is recommended that frequent peripheral blood cell counts be
performed on all patients receiving TAXOL. Patients should not be retreated with subsequent
cycles of TAXOL until neutrophils recover to a level > 1,500 cells/mm
3
and platelets recover to a
level >100,000 cells/mm
3
. In the case of severe neutropenia (< 500 cells/mm
3
) during a course
of TAXOL therapy, a 20% reduction in dose for subsequent courses of therapy is recommended.
For patients with advanced HIV disease and poor-risk AIDS-related Kaposi’s sarcoma, TAXOL,
at the recommended dose for this disease, can be initiated and repeated if the neutrophil count
is at least 1,000 cells/mm
3
. (See DOSAGE AND ADMINISTRATION).
Hypersensitivity Reactions
Patients with a history of severe hypersensitivity reactions to products containing Cremophor
†
EL should not be treated with TAXOL (see WARNINGS, CONTRAINDICATIONS). Minor

6
symptoms such as flushing, skin reactions, dyspnea, hypotension or tachycardia do not require
interruption of therapy. However, severe reactions, such as hypotension requiring treatment,
dyspnea requiring bronchodilators, angioedema or generalized urticaria require immediate
discontinuation of TAXOL and aggressive symptomatic therapy. Patients who have developed
severe hypersensitivity reactions should not be rechallenged with TAXOL.
Cardiovascular
Hypotension, hypertension and bradycardia have been observed during TAXOL administration;
patients are usually asymptomatic and generally do not require treatment. In severe cases,
TAXOL infusions may need to be interrupted or discontinued at the discretion of the treating
physician. Frequent monitoring of vital signs, particularly during the first hour of TAXOL
infusion, is recommended. Continuous cardiac monitoring is not required except for patients
who develop serious conduction abnormalities (see WARNINGS, ADVERSE REACTIONS).
When TAXOL is used in combination with doxorubicin for treatment of metastatic breast cancer,
monitoring of cardiac function is recommended.
Nervous System
Although the occurrence of peripheral neuropathy is frequent, the development of severe
symptomatology is unusual. A dose reduction of 20% is recommended for all subsequent
courses of TAXOL for severe neuropathy (see ADVERSE REACTIONS, DOSAGE AND
ADMINISTRATION).
TAXOL contains dehydrated ethanol, 396 mg/mL; consideration should be given to possible
CNS and other effects of ethanol. Children may be more sensitive than the adults to the
effects of ethanol (see WARNINGS; Use in Children).
Hepatic
There is evidence that the toxicity of TAXOL is enhanced in patients with elevated liver enzymes.
Patients with hepatic impairment may be at increased risk of toxicity, particularly grade III-IV
myelosuppression Caution should be exercised when administering TAXOL to patients with
moderate to severe hepatic impairment. Patients should be monitored closely for the
development of profound myelosuppression. (see ADVERSE REACTIONS)
Injection Site Reaction
Injection site reactions, including reactions secondary to extravasation, were usually mild and
consisted of pain, erythema, tenderness, skin discoloration, or swelling at the injection site.
These reactions have been observed more frequently with the 24-hour infusion than with the
3-hour infusion. Recurrence of skin reactions at a site of previous extravasation following
administration of TAXOL at a different site, i.e., “recall”, has been reported rarely.
Rare reports of more severe events such as phlebitis, cellulitis, induration, skin exfoliation,
necrosis and fibrosis have been received as part of the continuing surveillance of TAXOL safety.
In some cases the onset of the injection site reaction either occurred during a prolonged
infusion or was delayed by a week to ten days.
A specific treatment for extravasation reactions is unknown at this time. Given the possibility of
extravasation, it is advisable to closely monitor the infusion site for possible infiltration during
7
drug administration.
Driving/Operating Machinery
Since TAXOL contains ethanol, consideration should be given to the possibility of CNS and
other effects.
ADVERSE REACTIONS
The frequency and severity of adverse events are generally similar between patients receiving
TAXOL for the treatment of ovarian, breast non-small cell lung carcinoma, or Kaposi’s Sarcoma,
but patients with AIDS-related Kaposi's sarcoma may have more frequent and severe
hematologic toxicity, infections, and febrile neutropenia. These patients require a lower dose
intensity and supportive care. (See CLINICAL TRIALS: AIDS-Related Kaposi's Sarcoma).
The incidences of adverse reactions in the table that follows are derived from ten clinical trials in
carcinoma of the ovary and of the breast involving 812 patients treated with single-agent TAXOL
(paclitaxel) at doses ranging from 135-300 mg/m
2
/day and schedules of 3 or 24 hours. Data
from a subset of 181 patients treated at the recommended dose of 175 mg/m
2
and a 3-hour
infusion schedule is also included in the table.
135-300 mg/m
2
% of Patients
N=812
175 mg/m
2
% of Patients
N=181
Bone Marrow
Neutropenia < 2,000/mm
3
< 500/mm
3
Leukopenia < 4,000/mm
3
< 1,000/mm
3
Thrombocytopenia < 100,000/mm
3
< 50,000/mm
3
Anemia < 11 g/dL
< 8 g/dL
Infections
Bleeding
Red Cell Transfusions
Red Cell Transfusions (normal baseline)
Platelet Transfusions
90
52
90
17
20
7
78
16
30
14
25
12
2
87
27
86
4
6
1
62
6
18
9
13
6
0
Hypersensitivity Reactions
All
Severe
41
2
40
1
Cardiovascular
Bradycardia (first 3 hours of infusion)
Hypotension (first 3 hours of infusion)
Severe events
3
12
1
3
11
2

8
135-300 mg/m
2
% of Patients
N=812
175 mg/m
2
% of Patients
N=181
Abnormal ECG
All Patients
Patients with normal baseline
23
14
13
8
Peripheral Neuropathy
Any symptoms
Severe symptoms
60
3
64
4
Myalgia/Arthralgia
Any symptoms
Severe symptoms
60
8
54
12
Gastrointestinal
Nausea and vomiting
Diarrhea
Mucositis
52
38
31
44
25
20
Alopecia 87 93
Hepatic (Patients with normal baseline)
Bilirubin elevations
Alkaline phosphatase elevations
AST elevations
7
22
19
4
18
18
Injection site reactions 13 4
Safety referring to a large randomized trial of TAXOL (135 mg/m
2
over 24 hours) / cisplatin
(75 mg/m
2
) versus cyclophosphamide/cisplatin, including 410 patients (196 receiving TAXOL),
has been evaluated. The combination of TAXOL with platinum agents has not resulted in any
clinically relevant changes to the safety profile of the drug when used at the recommended
dosage.
Safety data were collected for 3,121 patients in the Phase III adjuvant breast carcinoma study.
The adverse event profile for the patients who received TAXOL subsequent to
cyclophosphamide and doxorubicin was consistent with that seen in the pooled analysis of data
from 812 patients treated with single-agent TAXOL in 10 clinical studies.
SUMMARY OF 3-HOUR INFUSION DATA AT A DOSE OF 175 mg/m
2
Unless otherwise stated, the following safety data relate to 62 patients with ovarian cancer and
119 patients with breast cancer treated at a dose of 175 mg/m
2
and a 3-hour infusion schedule,
in phase III clinical trials. All patients were premedicated to minimize hypersensitivity reactions.
Data from these clinical trials demonstrate that TAXOL given at this dose and schedule is well
tolerated. Bone marrow suppression and peripheral neuropathy were the principle
dose-related adverse effects associated with TAXOL. Compared to 24-hour infusion
schedules, neutropenia was less common when TAXOL was given as a 3-hour infusion.
Neutropenia was generally rapidly reversible and did not worsen with cumulative exposure.
The frequency of neurologic symptoms increases with repeated exposure.
9
None of the observed toxicities were influenced by age.
AIDS-related KAPOSI’S SARCOMA
The following table shows the frequency of important adverse events in the 85 patients with KS
treated with two different single-agent TAXOL regimens.
Frequency
a
of Important* Adverse Events in the AIDS-Related Kaposi’s Sarcoma Studies
Percent of Patients
Study CA139-174
135/3
b
/3 wk
(n = 29)
Study CA139-281
100/3
b
/2 wk
(n = 56)
Bone Marrow
Neutropenia < 2,000/mm
3
100 95
< 500/mm
3
76 35
Thrombocytopenia < 100,000/mm
3
52 27
< 50,000/mm
3
17 5
Anemia < 11 g/dL 86 73
< 8 g/dL 34 25
Febrile Neutropenia 55 9
Opportunistic Infections
Any 76 54
Cytomegalovirus 45 27
Herpes Simplex 38 11
Pneumocystis carinii 14 21
M. avium intracellulare 24 4
Candidiasis, esophageal 7 9
Cryptosporidiosis 7 7
Cryptococcal meningitis 3 2
Leukoencephalopathy — 2
Hypersensitivity Reaction
c
All 14 9
Cardiovascular
Hypotension 17 9
Bradycardia 3 —
Peripheral Neuropathy
Any 79 46
Severe** 14 16
Myalgia/Arthralgia
10
Percent of Patients
Study CA139-174
135/3
b
/3 wk
(n = 29)
Study CA139-281
100/3
b
/2 wk
(n = 56)
Any 93 48
Severe** 14 16
Gastrointestinal
Nausea and vomiting 69 70
Diarrhea 90 73
Mucositis 45 20
Renal (Creatinine elevation)
Any 34 18
Severe** 7 5
Discontinuation for drug toxicity
7 16
a
Based on worst course analysis.
b
TAXOL dose in mg/m
2
/infusion duration in hours.
c
All patients received premedication.
* Clinically relevant and/or possibly related.
** Severe events are defined as at least Grade III toxicity.
As demonstrated in the above table, toxicity was more pronounced in the study utilizing TAXOL
at a dose of 135 mg/m
2
every 3 weeks than in the study utilizing TAXOL at a dose of 100 mg/m
2
every 2 weeks. Notably, severe neutropenia (76% versus 35%), febrile neutropenia (55%
versus 9%), and opportunistic infections (76% versus 54%) were more common with the former
dose and schedule. The differences between the two studies with respect to dose escalation
and use of hematopoietic growth factors, as described below, should be taken into account.
(See CLINICAL TRIALS: AIDS-Related Kaposi's Sarcoma).
Adverse Experiences by Body System
Unless otherwise noted, the following discussion refers to the overall safety database of 812
patients with solid tumors treated with single-agent TAXOL in 10 clinical studies. Toxicities that
occurred with greater severity or frequency in previously untreated patients with ovarian
carcinoma or NSCLC who received TAXOL in combination with cisplatin or in patients with
breast cancer who received TAXOL after doxorubicin/cyclophosphamide in the adjuvant setting,
or in patients with AIDS-related Kaposi’s sarcoma, and that occurred with a difference that was
clinically significant in these populations are also described. In addition, rare events have been
reported from postmarketing experience or from other clinical studies.

11
The frequency and severity of adverse events have been generally similar for all patients
receiving TAXOL. However, patients with AIDS-related Kaposi's sarcoma may have more
frequent and severe hematologic toxicity, infections, and febrile neutropenia. These patients
require a lower dose intensity and supportive care. Toxicities that were observed only in or
were noted to have occurred with greater severity in the population with Kaposi's sarcoma and
that occurred with a difference that was clinically significant in this population are described.
Hematologic
The most frequent significant undesirable effect of TAXOL was bone marrow suppression.
Neutropenia was dose and schedule dependent and was generally rapidly reversible. Severe
neutropenia (<500 cells/mm
3
) occurred in 27% of patients treated at a dose of 175 mg/m
2
, but
was not associated with febrile episodes. Only 1% of patients experienced severe neutropenia
for 7 days or more. Neutropenia was not more frequent or severe in patients who received
prior radiation therapy, nor did it appear to be affected by treatment duration or cumulative
exposure.
When TAXOL was administered to patients with ovarian carcinoma at a dose of 175 mg/m
2
/3
hours in combination with cisplatin versus the control arm of cyclophosphamide plus cisplatin,
the incidences of severe neutropenia and of febrile neutropenia were similar in the TAXOL plus
cisplatin arm and in the control arm.
When TAXOL was administered in combination with cisplatin to patients with advanced NSCLC
in the Eastern Cooperative Oncology Group (ECOG) study, the incidence of neutropenia (Grade
IV) was 74% (TAXOL 135 mg/m
2
/24 hours plus cisplatin) and 65% (TAXOL 250 mg/m
2
/24 hours
plus cisplatin and G-CSF) compared with 55% in patients who received cisplatin/etoposide.
Considerably less Grade IV neutropenia was observed in the European Organization for
Research and Treatment of Cancer (EORTC) (28%) and CA139-208 (45%) studies for TAXOL
175 mg/m
2
/3 hours plus cisplatin (without G-CSF).
Fever was frequent (12% of all treatment courses). Infectious episodes occurred in 30% of all
patients and 9% of all courses; these episodes were fatal in 1% of all patients, and included
sepsis, pneumonia and peritonitis. In the Phase 3 second-line ovarian study, infectious
episodes were reported in 20% of the patients given 135 mg/m
2
and 26% of the patients given
175 mg/m
2
by a 3-hour infusion. Urinary tract infections and upper respiratory tract infections
were the most frequently reported infectious complications. In the immunosuppressed patient
population with advanced HIV disease and poor-risk AIDS-related Kaposi's sarcoma, 61% of
the patients reported at least one opportunistic infection. The use of supportive therapy,
including G-CSF, is recommended for patients who have experienced severe neutropenia.
(See DOSAGE AND ADMINISTRATION).
Twenty percent of the patients experienced a drop in their platelet count below
100,000 cells/mm
3
at least once while on treatment; 7% had a platelet count < 50,000 cells/mm
3
at the time of their worst nadir. Bleeding episodes were reported in 4% of all courses and by
14% of all patients, but most of the hemorrhagic episodes were localized and the frequency of
these events was unrelated to the TAXOL dose and schedule. In the Phase III second-line
ovarian cancer study, bleeding episodes were reported in 10% of the patients who received
study medication; however, none of the patients treated with the 3-hour infusion received
platelet transfusions. In the adjuvant breast carcinoma trial, the incidence of severe
thrombocytopenia and platelet transfusions increased with higher doses of doxorubicin.

12
Anemia (Hb<11 g/dL) was observed in 78% of all patients and was severe (Hb<8 g/dL) in 16%
of the cases. No consistent relationship between dose or schedule and the frequency of
anemia was observed. Among all patients with normal baseline hemoglobin, 69% became
anemic on study but only 7% had severe anemia. Red cell transfusions were required in 25%
of all patients and in 12% of those with normal baseline hemoglobin levels.
Hypersensitivity Reactions (HSR)
All patients received premedication prior to TAXOL (see WARNINGS section). The frequency
and severity of HSR were not affected by the dose or schedule of TAXOL administration. In
the Phase III second-line ovarian study, the 3-hour infusion was not associated with a greater
increase in HSR when compared to the 24-hour infusion. Hypersensitivity reactions were
observed in 20% of all courses and in 41% of all patients. These reactions were severe in less
than 2% of the patients and 1% of the courses. No severe reactions were observed after
course 3 and severe symptoms occurred generally within the first hour of TAXOL infusion. The
most frequent symptoms observed during these severe reactions were dyspnea, flushing, chest
pain and tachycardia. Abdominal pain, pain in the extremities, diaphoresis, and hypertension
are also noted.
The minor hypersensitivity reactions consisted mostly of flushing (28%), rash (12%),
hypotension (4%), dyspnea (2%), tachycardia (2%) and hypertension (1%). The frequency of
hypersensitivity reactions remained relatively stable during the entire treatment period.
Rare reports of chills and reports of back pain in association with hypersensitivity reactions have
been received as part of the continuing surveillance of TAXOL safety.
Cardiovascular
Hypotension, during the first 3 hours of infusion, occurred in 12% of all patients and 3% of all
courses administered. Bradycardia, during the first 3 hours of infusion, occurred in 3% of all
patients and 1% of all courses. In the Phase III second-line ovarian study, neither dose nor
schedule had an effect on the frequency of hypotension and bradycardia. These vital sign
changes most often caused no symptoms and required neither specific therapy nor treatment
discontinuation. The frequency of hypotension and bradycardia were not influenced by prior
anthracycline therapy.
Significant cardiovascular events possibly related to single-agent TAXOL occurred in
approximately 1% of all patients. These events included syncope, rhythm abnormalities,
hypertension and venous thrombosis. One of the patients with syncope treated with TAXOL at
175 mg/m
2
over 24 hours had progressive hypotension and died. The arrhythmias included
asymptomatic ventricular tachycardia, bigeminy and complete AV block requiring pacemaker
placement. The incidence of Grade III or greater cardiovascular events was 13% (TAXOL
135 mg/m
2
/24 hours plus cisplatin), 12% (TAXOL 250 mg/m
2
/24 hours plus cisplatin and G--
CSF), and 6% (TAXOL 175 mg/m
2
/3 hours plus cisplatin) when TAXOL followed by cisplatin
was administered to patients with advanced NSCLC; there was a similar incidence in the
non-TAXOL control arms. The apparent increase in these cardiovascular events in patients
with NSCLC compared to patients with breast or ovarian cancer is possibly related to the
difference in cardiovascular risk factors among patients with lung cancer.

13
Electrocardiogram (ECG) abnormalities were common among patients at baseline. ECG
abnormalities on study did not usually result in symptoms, were not dose-limiting, and required
no intervention. ECG abnormalities were noted in 23% of all patients. Among patients with a
normal ECG prior to study entry, 14% of all patients developed an abnormal tracing while on
study. The most frequently reported ECG modifications were non-specific repolarization
abnormalities, sinus bradycardia, sinus tachycardia and premature beats. Among patients with
normal ECG at baseline, prior therapy with anthracyclines did not influence the frequency of
ECG abnormalities.
Cases of myocardial infarction have been reported rarely. Congestive heart failure (cardiac
dysfunction and reduction of left ventricular ejection fraction or ventricular failure) has been
reported typically in patients who have received other chemotherapy, notably anthracyclines.
(See PRECAUTIONS: Drug Interactions)
Rare reports of atrial fibrillation and supraventricular tachycardia have been received as part of
the continuing surveillance of TAXOL safety.
Respiratory
Rare reports of interstitial pneumonia, lung fibrosis and pulmonary embolism, have been
received as part of the continuing surveillance of TAXOL safety. Rare reports of radiation
pneumonitis have been received in patients receiving concurrent radiotherapy.
Neurologic
The frequency and severity of neurologic manifestations were influenced by prior and
concomitant therapy with cisplatin. In general, the frequency and severity of neurologic
manifestations were dose-dependent in patients receiving single-agent TAXOL. Paresthesia
commonly occurs in the form of hyperesthesia. Peripheral neuropathy was observed in 60% of
all patients (3% severe) and in 52% (2% severe) of the patients without pre-existing neuropathy.
The frequency of peripheral neuropathy increased with cumulative dose. Neurologic
symptoms were observed in 27% of the patients after the first course of treatment and in
34-51% from course 2 to 10. Peripheral neuropathy was the cause of TAXOL discontinuation in
1% of all patients. Sensory symptoms have usually improved or resolved within several
months of TAXOL discontinuation. The incidence of neurologic symptoms did not increase in
the subset of patients previously treated with cisplatin. Pre-existing neuropathies resulting from
prior therapies are not a contraindication for TAXOL therapy. In the Intergroup first-line ovarian
carcinoma study, the regimen with TAXOL 175 mg/m
2
by 3-hour infusion followed by cisplatin
75 mg/m
2
resulted in greater incidence and severity of neurotoxicity (reported as neuromotor or
neurosensory events) than the regimen containing cyclophosphamide 750 mg/m
2
followed by
cisplatin 75 mg/m
2
, 87% (21% severe) versus 52% (2% severe), respectively. In the GOG
first-line ovarian carcinoma study, the regimen with TAXOL (135 mg/m
2
over 24 hours) followed
by cisplatin (75 mg/m
2
) resulted in an incidence of neurotoxicity (reported as peripheral
neuropathy) that was similar to the regimen containing cyclophosphamide 750 mg/m
2
followed
by cisplatin 75 mg/m
2
, 25% (3% severe) versus 20% (0% severe), respectively. Cross-study
comparison of neurotoxicity in Intergroup and GOG trials suggests that when TAXOL is given in
combinations with cisplatin 75 mg/m
2
, the incidence of severe neurotoxicity is more common at
a TAXOL dose of 175 mg/m
2
given by 3-hour infusion (21%) than at a dose of 135 mg/m
2
given
by 24-hour infusion (3%). In patients with NSCLC, administration of TAXOL followed by

14
cisplatin resulted in greater incidence of severe neurotoxicity compared to the incidence in
patients with ovarian or breast cancer treated with single-agent TAXOL. Severe neurosensory
symptoms were noted in 13% of NSCLC patients receiving TAXOL 135 mg/m
2
by 24-hour
infusion followed by cisplatin 75 mg/m
2
and 8% of NSCLC patients receiving cisplatin/etoposide.
Other than peripheral neuropathy, serious neurologic events following TAXOL administration
have been rare (<1%) and have included grand mal seizures, ataxia and encephalopathy.
Rare reports of autonomic neuropathy resulting in paralytic ileus and motor neuropathy with
resultant minor distal weakness have been received as part of the continuing surveillance of
TAXOL safety. Optic nerve and/or visual disturbances (scintillating scotoma) have also been
reported, particularly in patients who have received higher doses than those recommended.
These effects generally have been reversible. However, rare reports in the literature of
abnormal visual evoked potentials in patients have suggested persistent optic nerve damage.
Postmarketing reports of ototoxicity (hearing loss and tinnitus) have been received.
Arthralgia/myalgia
There was no consistent relationship between dose or schedule of TAXOL and the frequency or
severity of arthralgia/myalgia. Sixty percent of all patients treated in single-agent trials
experienced arthralgia/myalgia; 8% experienced severe symptoms. The symptoms were
usually transient, occurred two or three days after TAXOL administration, and resolved within a
few days. The frequency and severity of musculoskeletal symptoms remained unchanged
throughout the treatment period.
Alopecia
Alopecia was observed in almost all patients.
Gastrointestinal
Nausea/vomiting, diarrhea and mucositis were reported by 52%, 38% and 31% of all patients,
respectively. These manifestations were usually mild to moderate. Mucositis was schedule
dependent and occurred more frequently with the 24-hour than with the 3-hour infusion.
In the first-line Phase III ovarian carcinoma study, the incidence of nausea and vomiting when
TAXOL was administered in combination with cisplatin appeared to be greater compared with
the database for single-agent TAXOL in ovarian and breast carcinoma. In the same study,
diarrhea of any grade was reported more frequently (16%) compared to the control arm (8%)
(p=0.008), but there was no difference for severe diarrhea.
Rare reports of intestinal obstruction, intestinal perforation, pancreatitis, ischemic colitis, and
dehydration have been received as part of the continuing surveillance of TAXOL safety.
Rare
reports of neutropenic enterocolitis (typhlitis), despite the coadministration of G-CSF, were
observed in patients treated with TAXOL alone and in combination with other chemotherapeutic
agents.
In patients with poor-risk AIDS-related Kaposi's sarcoma, nausea/vomiting, diarrhea, and
mucositis were reported by 69%, 79% and 28% of patients, respectively. One third of patients
with Kaposi's sarcoma complained of diarrhea prior to study start.
15
Hepatic
No relationship was observed between liver function abnormalities and either dose or schedule
of TAXOL administration. Among patients with normal baseline liver function 7%, 22% and
19% had elevations in bilirubin, alkaline phosphatase and AST (SGOT), respectively. There is
no evidence that TAXOL when given as a 3-hour infusion to patients with mildly abnormal liver
function causes exacerbation of abnormal liver function. Prolonged exposure to TAXOL was
not associated with cumulative hepatic toxicity.
Rare reports of hepatic necrosis and hepatic encephalopathy leading to death have been
received as part of the continuing surveillance of TAXOL safety.
Renal
Among the patients treated for Kaposi's sarcoma with TAXOL, five patients had renal toxicity of
grade III or IV severity. One patient with suspected HIV nephropathy of grade IV severity had
to discontinue therapy. The other four patients had renal insufficiency with reversible
elevations of serum creatinine.
Injection Site Reactions
Injection site reactions, including reactions secondary to extravasation, were usually mild and
consisted of pain, erythema, tenderness, skin discoloration, or swelling at the injection site.
These reactions have been observed more frequently with the 24-hour infusion than with the
3-hour infusion. Recurrence of skin reactions at a site of previous extravasation following
administration of TAXOL at a different site, i.e., “recall”, has been reported rarely.
Rare reports of more severe events such as phlebitis, cellulitis, induration, skin exfoliation,
necrosis and fibrosis have been received as part of the continuing surveillance of TAXOL safety.
In some cases the onset of the injection site reaction either occurred during a prolonged
infusion or was delayed by a week to ten days.
A specific treatment for extravasation reactions is unknown at this time. Given the possibility of
extravasation, it is advisable to closely monitor the infusion site for possible infiltration during
drug administration.
Other
Transient skin changes due to TAXOL-related hypersensitivity reactions have been observed,
but no other skin toxicities were significantly associated with TAXOL administration. Nail
changes (changes in pigmentation or discoloration of nail bed) were uncommon (2%). Edema
was reported in 21% of all patients (17% of those without baseline edema); only 1% had severe
edema and none of these patients required treatment discontinuation. Edema was most
commonly focal and disease-related. Edema was observed in 5% of all courses for patients
with normal baseline and did not increase with time on study.
Rare reports of skin abnormalities related to radiation recall as well as reports of maculopapular
rash, pruritus, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been received
as part of the continuing surveillance of TAXOL safety.
16
Reports of asthenia and malaise have been received as part of the continuing surveillance of
TAXOL safety. In the Phase III trial of TAXOL 135 mg/m
2
over 24 hours in combination with
cisplatin as first-line therapy of ovarian cancer, asthenia was reported in 17% of the patients,
significantly greater than the 10% incidence observed in the control arm of cyclophosphamide/
cisplatin.
Post-Marketing Adverse Drug Events
Unless otherwise indicated, the table below lists undesirable effects regardless of severity
associated with the administration of single agent TAXOL (812 patients treated in clinical trials)
or as reported in the postmarketing surveillance* of TAXOL.
The frequency of undesirable effects listed below is defined using the following convention: very
common (≥ 1/10); common (≥ 1/100, < 1/10); uncommon (≥ 1/1,000, < 1/100); rare (≥ 1/10,000,
< 1/1,000); very rare (< 1/10,000).
Infections and infestations:
Very common: infection
Uncommon: septic shock
Rare*: pneumonia, sepsis
Blood and the lymphatic
system disorders:
Very common: myelosuppression, neutropenia,
anemia, thrombocytopenia, leukopenia, fever, bleeding
Rare: febrile neutropenia
Very rare*: acute myeloid leukemia, myelodysplastic
syndrome
Immune system disorders:
Very common: minor hypersensitivity reactions (mainly
flushing and rash)
Uncommon: significant hypersensitivity reactions
requiring therapy (eg, hypotension, angioneurotic
edema, respiratory distress, generalised urticaria,
edema, back pain, chills)
Rare*: anaphylactic reactions (with fatal outcome)
Very rare*: anaphylactic shock
Metabolism and nutrition
disorders:
Very rare*: anorexia
Psychiatric disorders:
Very rare*: confusional state
Nervous system disorders:
Very common: neurotoxicity (mainly: peripheral
neuropathy)
Rare*: motor neuropathy (with resultant minor distal
weakness)
Very rare*: autonomic neuropathy (resulting in
paralytic ileus and orthostatic hypotension), grand mal
seizures, convulsions, encephalopathy, dizziness,
headache, ataxia
Eye disorders:
Very rare*: reversible optic nerve and/or visual
17
disturbances (scintillating scotomata), particularly in
patients who have received higher doses than
recommended, photopsia, visual floaters
Ear and labyrinth disorders:
Very rare*: hearing loss, tinnitus, vertigo, ototoxicity
Cardiac disorders:
Very common: abnormal ECG
Common: bradycardia
Uncommon: cardiomyopathy, asymptomatic ventricular
tachycardia, tachycardia with bigeminy, AV block and
syncope, myocardial infarction.
Very rare*: atrial fibrillation, supraventricular
tachycardia
Vascular disorders:
Very common: hypotension
Uncommon: hypertension, thrombosis,
thrombophlebitis
Very rare*: shock
Respiratory, thoracic and
mediastinal disorders:
Rare*: dyspnea, pleural effusion, respiratory failure,
interstitial pneumonia, lung fibrosis, pulmonary
embolism
Very rare*: cough
Gastrointestinal disorders:
Very common: nausea, vomiting, diarrhea, mucosal
inflammation
Rare*: bowel obstruction, bowel perforation, ischemic
colitis, pancreatitis
Very rare*: mesenteric thrombosis,
pseudomembranous colitis, esophagitis, constipation,
ascites
Hepato-biliary disorders:
Very rare*: hepatic necrosis (with fatal outcome),
hepatic encephalopathy (with fatal outcome)
Renal disorders:
Unknown*: renal failure
Skin and subcutaneous tissue
disorders:
Very common: alopecia
Common: transient and mild nail and skin changes
Rare*: pruritus, rash, erythema, phlebitis, cellulitis, skin
exfoliation, necrosis and fibrosis, radiation recall
Very rare*: Stevens-Johnson syndrome, epidermal
necrolysis, erythema multiforme, exfoliative dermatitis,
urticaria, onycholysis (patients on therapy should wear
sun protection on hands and feet), scleroderma-like
changes preceded by chronic edema
Musculoskeletal, connective
tissue
and bone disorders:
Very common: arthralgia, myalgia
18
General disorders and
administration site conditions:
Common: injection site reactions (including localised
edema, pain, erythema, induration, on occasion
extravasation can result in cellulitis)
Rare*: asthenia, malaise, pyrexia, dehydration, edema
Investigations:
Common: severe elevation in AST (SGOT), severe
elevation in alkaline phosphatase
Uncommon: severe elevation in bilirubin
Rare*: Increase in blood creatinine
SYMPTOMS AND TREATMENT OF OVERDOSAGE
There is no known antidote for TAXOL (paclitaxel) overdosage. The primary anticipated
complications of overdosage would consist of bone marrow suppression, peripheral
neurotoxicity and mucositis. Overdoses in pediatric patients may be associated with acute
ethanol toxicity (see PRECAUTIONS, Pediatric Use section WARNINGS, Use in Children).
DOSAGE AND ADMINISTRATION
Note: Undiluted concentrate should not come in contact with plasticized PVC
equipment. In order to minimize patients exposure to the plasticizer DEHP
[di-(2-ethylhexyl)phthalate], which may be leached from PVC infusion bags or sets,
diluted TAXOL (paclitaxel) solutions should preferably be stored in bottles (glass,
polypropylene) or plastic bags (polypropylene, polyolefin) and administered through
polyethylene-lined administration sets.
TAXOL should be administered through an in-line filter with a microporous membrane
not greater than 0.22 microns. Use of filter devices such as IVEX-2® filters which
incorporate short inlet and outlet PVC-coated tubing has not resulted in significant
leaching of DEHP.
All patients should be premedicated prior to TAXOL administration in order to reduce the
risk of severe hypersensitivity reactions. Such premedication may consist of
dexamethasone 20 mg orally (or its equivalent) approximately 12 and 6 hours before TAXOL,
diphenhydramine 50 mg I.V. (or its equivalent), 30 to 60 minutes prior to TAXOL, and cimetidine
(300 mg) or ranitidine (50 mg) I.V. 30 to 60 minutes before TAXOL.

19
Metastatic carcinoma of the ovary
The administration of TAXOL at a dose of 175 mg/m
2
over 3 hours in combination with cisplatin
75 mg/m
2
every 3 weeks is recommended for the primary treatment of patients with advanced
carcinoma of the ovary. TAXOL should be given before cisplatin when used in combination.
In patients previously treated with chemotherapy, the recommended regimen is 175 mg/m
2
administered intravenously over 3 hours every 3 weeks.
Carcinoma of the breast
For the adjuvant treatment of node-positive breast cancer, the recommended regimen is TAXOL,
at a dose of 175 mg/m
2
intravenously over 3 hours every 3 weeks for four courses administered
sequentially to standard combination therapy.
After failure of initial chemotherapy for metastatic disease or relapse within 6 months of adjuvant
chemotherapy, TAXOL at a dose of 175 mg/m
2
administered intravenously over 3 hours every 3
weeks has been shown to be effective.
Non-small cell lung carcinoma
The recommended regimen, given every 3 weeks, is TAXOL administered intravenously over 3
hours at a dose of 175 mg/m
2
followed by cisplatin.
Single courses of TAXOL should not be repeated until the neutrophil count is at least
1,500 cells/mm
3
and the platelet count is at least 100 000 cells/mm
3
. Patients who experience
severe neutropenia (neutrophil < 500 cells/mm
3
) or severe peripheral neuropathy during TAXOL
therapy should have the dosage reduced by 20% for subsequent courses of TAXOL.
AIDS-related Kaposi's Sarcoma
TAXOL 135 mg/m
2
administered intravenously over 3 hours with a 3 week interval between
courses or 100 mg/m
2
administered intravenously over 3 hours with a 2 week interval between
courses (dose intensity 45-50 mg/m
2
/week). In the two clinical trials evaluating these
schedules (see CLINICAL TRIALS: AIDS-Related Kaposi's Sarcoma), the former schedule
(135 mg/m
2
every 3 weeks) was more toxic than the latter. In addition, all patients with low
performance status were treated with the latter schedule (100 mg/m
2
every 2 weeks).
Based upon the immunosuppression observed in patients with advanced HIV disease, the
following modifications are recommended in these patients.
1. the dose of dexamethasone as one of the three premedication drugs should be reduced to
10 mg orally.
2. treatment with TAXOL should be initiated or repeated only if the neutrophil count is at least
1,000 cells/mm
3
.
3. the dose of subsequent courses of TAXOL should be reduced by 20% for those
patients who experience severe neutropenia (<500 cell/mm
3
for a week or longer).
4. concomitant hematopoietic growth factor (G-CSF), should be initiated as clinically indicated.
Preparation and Administration Precautions

20
TAXOL is a cytotoxic anticancer drug and, as with other potentially toxic compounds, caution
should be exercised in handling TAXOL. The use of gloves is recommended. Following
topical exposure, tingling, burning, redness have been observed. If TAXOL solution contacts
the skin, wash the skin immediately and thoroughly with soap and water.
If TAXOL contacts mucous membranes, the membranes should be flushed thoroughly with
water. Upon inhalation, dyspnea, chest pain, burning eyes, sore throat and nausea have been
reported. Given the possibility of extravasation, it is advisable to closely monitor the infusion
site for possible infiltration during drug administration (see PRECAUTIONS and ADVERSE
REACTIONS; Injection Site Reaction).
Preparation for Intravenous Administration
TAXOL for Injection must be diluted prior to infusion. TAXOL should be diluted in 0.9%
Sodium Chloride Injection, 5% Dextrose Injection, 5% Dextrose and 0.9% Sodium Chloride
Injection, or 5% Dextrose in Ringer's Injection to a final concentration of 0.3 to 1.2 mg/mL. The
solutions are physically and chemically stable for up to 27 hours at ambient temperature
(15-30°C) and room lighting conditions; infusions should be completed within this timeframe.
There have been rare reports of precipitation with longer than the recommended 3-hour infusion
schedules. Excessive agitation, vibration or shaking may induce precipitation and should be
avoided. Infusion sets should be flushed thoroughly with a compatible diluent before use.
Upon preparation, solutions may show haziness, which is attributed to the formulation vehicle.
No significant loss in potency has been noted following simulated delivery of the solution
through i.v. tubing containing an in-line (0.22 micron) filter.
Data collected for the presence of the extractable plasticizer DEHP [di-(2-ethylhexyl)phthalate]
show that levels increase with time and concentration when dilutions are prepared in PVC
containers. Consequently, the use of plasticized PVC containers and administration sets is not
recommended. TAXOL solutions should be prepared and stored in glass, polypropylene, or
polyolefin containers. Non-PVC containing administration sets, such as those which are
polyethylene-lined, should be used.
Devices with spikes should not be used with vials of TAXOL since they can cause the stopper to
collapse resulting in loss of sterile integrity of TAXOL solution.
21
PHARMACEUTICAL INFORMATION
I. DRUG SUBSTANCE
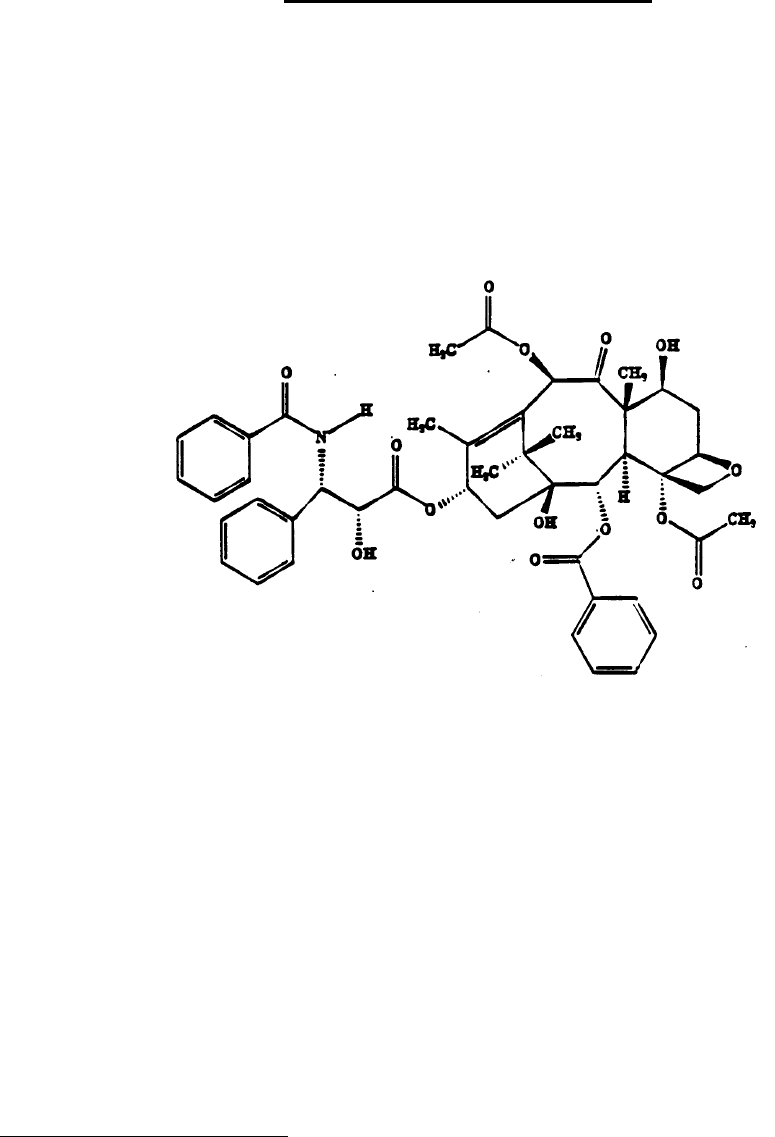
Proper Name: Paclitaxel
Chemical Name: 5ϐ,20-Epoxy-1,2α,4,7ϐ,10ϐ,13α-hexahydroxytax-11-en-9-one
4,10-diacetate 2-benzoate 13-ester with (2R,
3S)-N-benzoyl-3-phenylisoserine
Structural Formula:
Molecular Formula: C
47
H
51
NO
14
Molecular Weight: 853.9
Description: Paclitaxel is a white to off-white crystalline powder with a
melting point of 213.5-223°C. It is highly lipophilic and
insoluble in water.
II. COMPOSITION
Each mL of TAXOL (paclitaxel) Injection contains paclitaxel 6 mg, purified Cremophor
HH
EL (polyethoxylated castor oil) 527 mg and dehydrated ethanol 49.7% v/v.
III. STABILITY AND STORAGE RECOMMENDATIONS
TAXOL for Injection should be stored at room temperature (15-30°C). Retain in the
HH
T.M. of B.A.S.F.

22
original package and protect from light. Once punctured, the 5 and 16.7 mL vials of
TAXOL are stable for 28 days at room temperature. The 50 mL pharmacy bulk vial
should be used within 24 hours after initial entry.
Solutions for infusion prepared as recommended may be stored at room temperature
(15-30°C) only if necessary. However, the infusion should be initiated within 24 hours
of reconstitution.
If unopened vials are refrigerated, a precipitate may form which redissolves with little or
no agitation upon reaching room temperature. Product quality is not affected. If the
solution remains cloudy or if an insoluble precipitate is noted, the vial should be
discarded.
IV. PREPARATION FOR INTRAVENOUS ADMINISTRATION
Contact of undiluted TAXOL with plasticized PVC equipment or devices used to prepare
solutions for infusion is not recommended (see DOSAGE AND ADMINISTRATION).
Prior to infusion, TAXOL should be diluted in 0.9% Sodium Chloride Injection, 5%
Dextrose Injection, 5% Dextrose and 0.9% Sodium Chloride Injection or 5% Dextrose in
Ringer's Injection to a final concentration of 0.3 to 1.2 mg/mL.
As with all parenteral drug products, intravenous admixtures should be inspected
visually for clarity, particulate matter, precipitate, discoloration and leakage prior to
administration, whenever solution and container permit.
TAXOL should be administered through an in-line filter with a microporous membrane
not greater than 0.22 microns.
V. SPECIAL INSTRUCTIONS
1. Preparation of TAXOL should be done in a vertical laminar flow hood (Biological
Safety Cabinet - Class II).
2. Personnel preparing TAXOL should wear PVC gloves, safety glasses, disposable
gowns and masks.
3. All needles, syringes, vials and other materials which have come in contact with
TAXOL should be segregated and incinerated at 1000°C or more. Sealed
containers may explode. Intact vials should be returned to the Manufacturer for
destruction. Proper precautions should be taken in packaging these materials for
transport.
4. Personnel regularly involved in the preparation and handling of TAXOL should have
bi-annual blood examinations.
5. Directions for Dispensing from Pharmacy Bulk Vial
The use of Pharmacy Bulk Vial is restricted to hospitals with a recognized
intravenous admixture program. The Pharmacy Bulk Vial is intended for single
puncture, multiple dispensing and for intravenous use only. Dispensing from the

23
Pharmacy Bulk Vial should be completed within 24 hours after initial entry.
AVAILABILITY OF DOSAGE FORMS
TAXOL (paclitaxel) Injection is available in multidose vials of 5 mL and 16.7 mL and pharmacy
bulk vial of 50 mL containing respectively 30 mg, 100 mg and 300 mg paclitaxel at a
concentration of 6 mg/mL
PHARMACOLOGY
In vitro
TAXOL (paclitaxel) exhibits cytotoxic activity against a wide variety of both human and rodent
tumor cell lines in vitro including leukemia, non-small cell lung carcinoma, small cell lung
carcinoma, colon carcinoma, CNS carcinoma, melanoma, renal carcinoma, ovarian carcinoma
and breast carcinoma at IC
50
concentration (defined as the concentration required to inhibit cell
proliferation to 50% of that of untreated control cells) in the nM range. TAXOL blocks cell
replication in the late G2 and/or M phases of the cell cycle. Additionally, TAXOL produces
unusual cytoskeletons characterized by discrete bundles or microtubules and the formation of
abnormal spindle asters during mitosis. As a consequence of the disruption of the microtubule
cytoskeleton, TAXOL inhibits a variety of cell functions including chemotaxis, migration, cell
spreading, polarization, generation of hydrogen peroxide and killing of phagocytosed
microorganisms.
In addition to its ability to induce microtubule polymerization, exposure of murine macrophages
to TAXOL results in the release of tumor necrosis factor-α (TNF-α) accompanied by down
regulation of the receptor.
In Vivo
TAXOL has shown antitumor activity against many tumor models including leukemias and solid
tumors and human solid xenografts. The table that follows summarizes TAXOL's activity.
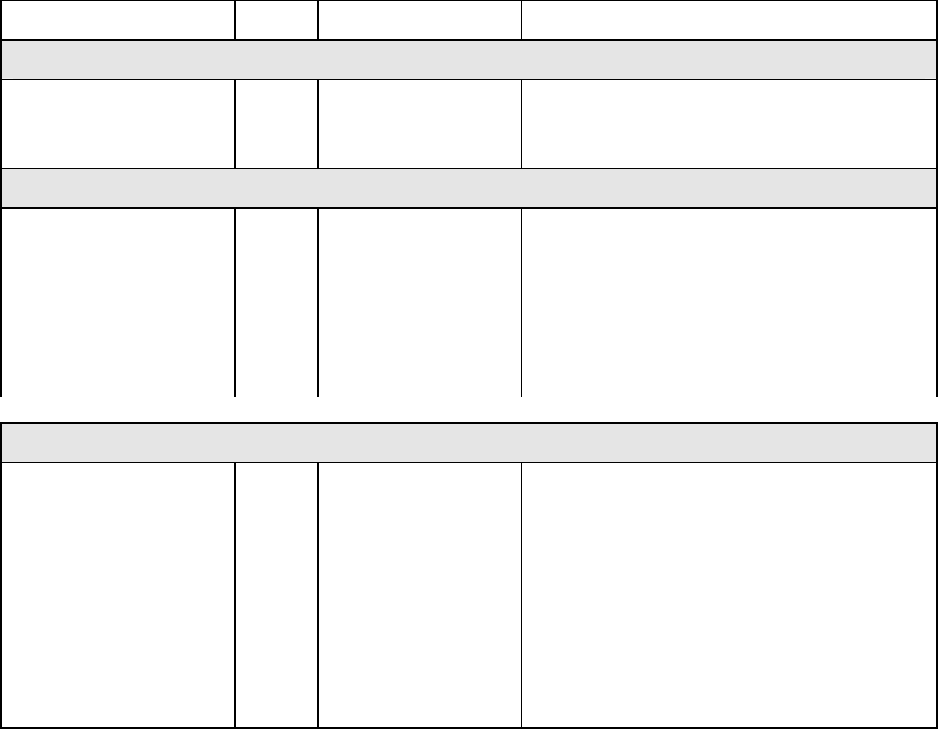
24
Tumor, Site Form Route Activity
MURINE LEUKEMIAS
L1210, ip
P388, ip
P1534, ip
*
*
*
ip
ip
ip
Borderline → modest
Mild
Mild → substantial
MURINE SOLID TUMORS
ADJ/PC 6, ip
C26,ip
B16, ip
M109, ip
M109, ip (staged)
M109, sc
M109 src
*
*
*
*
**
**
**
ip
ip
ip
ip
ip
sc
sc
Mild
Mild
Moderate → potentially curative
Moderate → potentially curative
Moderate → substantial
Moderate
Moderate
HUMAN TUMOR XENOGRAFTS
CX-1, src
LOX, ip
MX-1, src
A431, src
A2780, src
A2780, sc
H2981, src
HCT-116
L2987, src
LX-1, src
*
*
*
**
**
**
**
**
**
**
sc
ip
sc
iv
iv
iv
iv
iv
iv
iv
Mild → substantial
Moderate → potentially curative
Potentially curative
Substantial
Substantial
Moderate
Substantial
Moderate
Moderate
Moderate
* Suspension in hydroxypropylcellulose
** TAXOL in ethanol/cremophor diluted with saline
25
Clinical trials
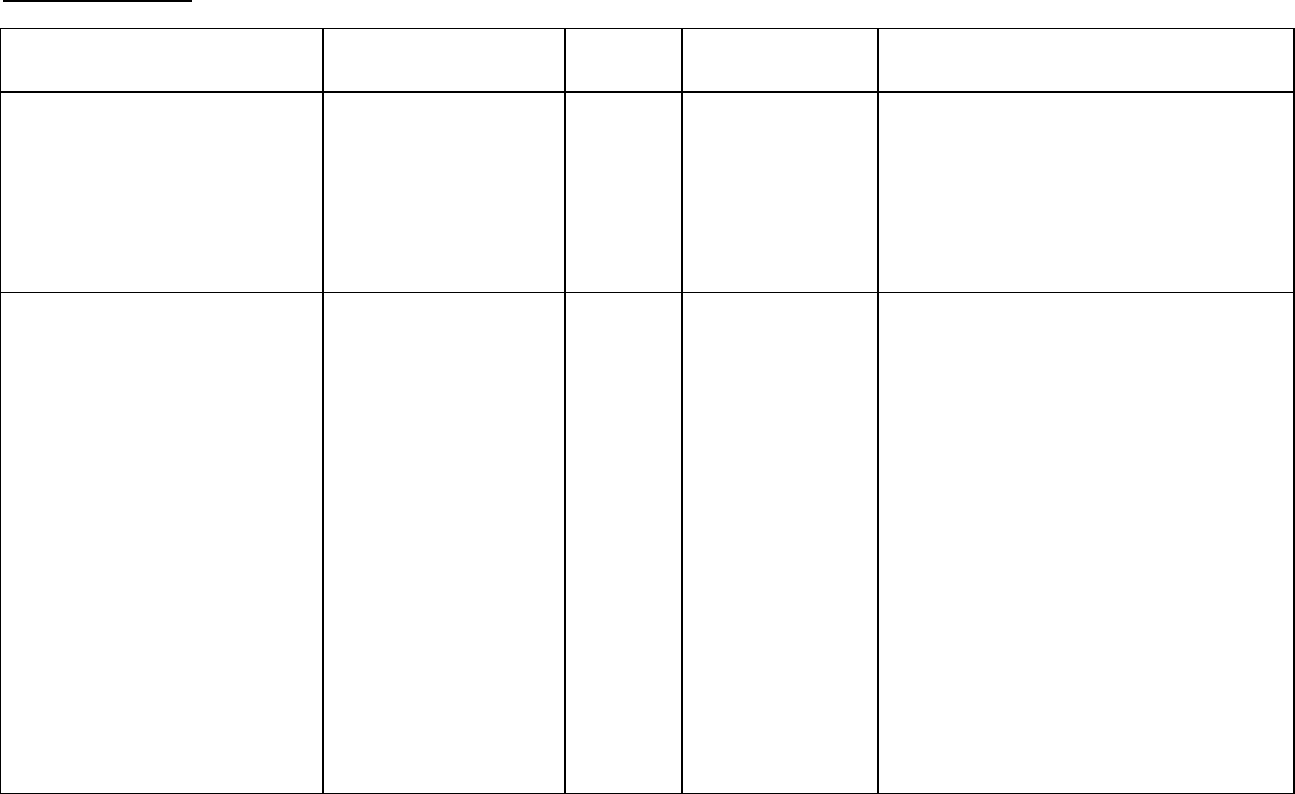
Ovarian Carcinoma
Study Design
Treatments / Doses
No. of
Patients
Population Endpoints/Conclusion
First-Line data:
Phase 3 multicenter, randomized,
controlled trial conducted by
GOG, comparing therapy with
Taxol (T) in combination with
cisplatin (c) to cyclophosphamide
(AC) in combination with cisplatin
(c)
- 135 mg/m
2
of T over
24 hrs + 75 mg/m
2
of c
- 750 mg/m
2
of AC +
75 mg/m
2
of c
410 Stage III or IV
disease (> 1 cm
residual disease
after staging
laparotomy or
distant metastases)
with no prior
chemotherapy
Patients treated with T in combination with
cisplatin has significantly longer time to
progression (median 16.6 vs. 13.0 months,
p = 0.0008) and nearly a year longer median
survival time (p = 0.0002) compared with
standard therapy.
Second-Line data:
Phase 3 multicenter , bifactorial,
randomized trial comparing two
dosage regimens of Taxol (T)
irrespective of the schedules and
two schedules irrespective of
dose.
- 175 mg/m
2
of T over 24
hrs
- 175 mg/m
2
of T over 3
hrs
- 135 mg/m
2
of T over 24
hrs
- 135 mg/m
2
of T over 3
hrs
407 Patients (pts) who
have failed initial or
subsequent
chemotherapy for
metastatic
carcinoma of the
ovary.
Pts receiving the 175 mg/m
2
dose had a
response rate (RR) similar to that for those
receiving the 135 mg/m
2
dose: 18% vs. 14%
(p=0.28). No difference in RR was
detected when comparing the 3-hr with the
24-hr infusion: 15% vs. 17% (p=0.50).
Pts receiving the 175 mg/m
2
dose of T had a
longer time to progression (TTP) than those
receiving the 135 mg/m
2
dose: median 4.2
vs. 3.1 months (p=0.03). The median TTP
for pts receiving the 3-hour vs. the 24-hr
infusion were 4.0 months vs. 3.7 months,
respectively.
Median survival was 11.6 months in pts
receiving the 175 mg/m
2
dose of T and 11.0
months in pts receiving the 135 mg/m
2
dose
(p=0.92).
Median survival was 11.7 months for pts
receiving the 3-hr infusion of T and 11.2
months for pts receiving the 24-hr infusion
(p=0.91).
26
First-Line data: The adverse event profile for patients receiving TAXOL in combination with
cisplatin was consistent with that seen in previous clinical studies (see ADVERSE
REACTIONS).
Second -Line data: In addition to the Phase 3 trial described above, data from five Phase 1 and
2 clinical studies as well as an interim analysis of data from more than 300 patients enrolled in a
treatment referral center program were used in support of the use of Taxol in patients who have
failed initial or subsequent chemotherapy for metastatic carcinoma of the ovary. TAXOL
remained active in patients who had developed resistance to platinum-containing therapy
(defined as tumor progression while on, or tumor relapse within 6 months from completion of, a
platinum containing regimen) with response rates of 14% in the Phase 3 study and 31% in the
Phase 1 & 2 clinical studies. The adverse event profile in this Phase 3 study was consistent
with that seen in previous clinical studies (see ADVERSE REACTIONS).
The results of this randomized study support the use of TAXOL at doses of 135 to 175 mg/m
2
,
administered by a 3-hour intravenous infusion. The same doses administered by 24-hour
infusion were more toxic.
27
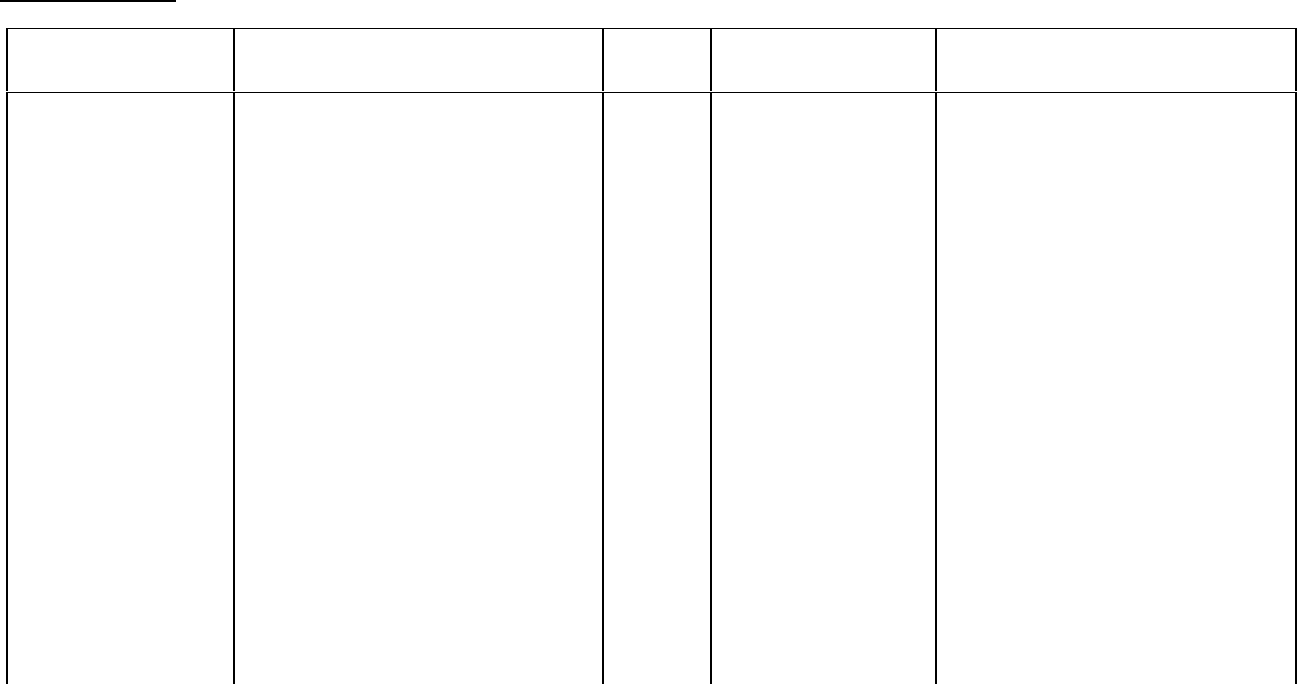
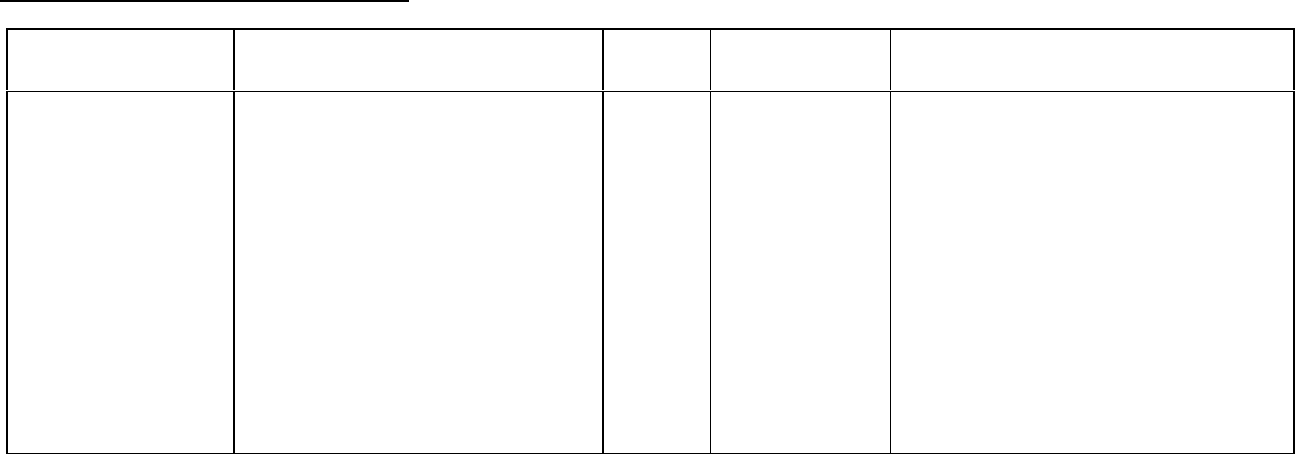
Breast Carcinoma
Study Design Treatments / Doses
No. of
Patients
Population Endpoints/Conclusion
Adjuvant Breast
Carcinoma Study:
Phase 3 multicenter,
3X2 factorial,
randomized trial,
conducted by CALGB,
ECOG, NCCTG and
SWOG, comparing
adjuvant therapy with
Taxol (T) to no further
chemotherapy
following four courses
of doxorubicin (A) and
cyclophosphamide (C)
600 mg/m
2
of C + A at doses of either
- 60 mg/m
2
(on day 1),
- 75 mg/m
2
(in two divided doses on
days 1 and 2), or
- 90 mg/m
2
(in two divided doses on
days 1 and 2 with prophylactic G-CSF
support and ciprofloxacin)
every 3 weeks for four courses and
either
- 175 mg/m
2
of T over 3 hrs every 3
weeks for four additional courses or
- no additional chemotherapy.
Patients (pts) whose tumors were +ve
were to receive subsequent tamoxifen
(20 mg daily for 5 years); patients who
received segmental mastectomies prior
to study were to receive breast
irradiation after recovery from
treatment-related toxicities.
3170 Node-positive breast
carcinoma following
either mastectomy or
segmental
mastectomy and nodal
dissections.
Median follow-up was 30 .1 months.
Of 2066 pts who were hormone
receptor positive, 93% received
tamoxifen. Based on a multivariate
Cox model for disease- free survival,
pts on AC+T had 22% risk reduction
of disease recurrence compared to
pts on AC (Hazard Ratio [HR] = 0.78,
95% CI 0.67-0.91, p = 0.0022) and
26% reduction in the risk of death (HR
= 0.74, 95% CI 0.60-0.92, p =
0.0065). Increasing the dose of A
higher than 60 mg/m
2
had no effect on
either disease-free survival or overall
survival. Subset analyses including
number of positive lymph nodes,
tumor size, hormone receptor status,
and menopausal status showed a
reduction in hazard similar to above
for disease-free and overall survival in
all larger subsets with one exception;
pts with receptor-positive tumors had
a smaller reduction in hazard (HR =
0.92) for disease-free survival with T
than other groups.
28
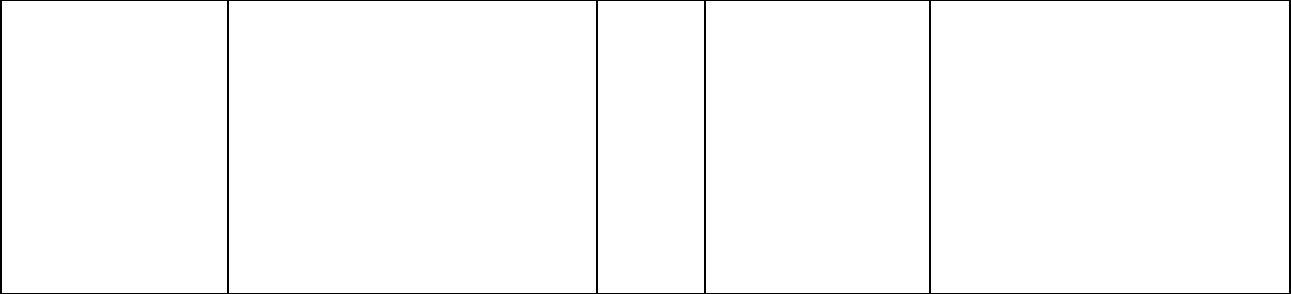
After Failure of Initial
Chemotherapy:
Phase 3 multicenter,
randomized trial
comparing two dosage
regimens of Taxol (T).
- 175 mg/m
2
of T over 3 hrs
- 135 mg/m
2
of T over 3 hrs
471 Patients (pts) who
failed chemotherapy
either in the adjuvant
(30%) or metastatic
(39%) setting or both
(31%). At study
entry, 60% had
symptomatic disease
with impaired
performance status
and 73% had visceral
metastases.
The overall response rate was 26%
(95% Cl: 22 to 30%), with 17
complete and 99 partial responses.
The median duration of response,
measured from the first day of
treatment, was 8.1 months (range:
3.4-18.1 + months). Overall, the
median time to progression was 3.5
months (range: 0.03-17.1 months).
Median survival was 11.7 months
(range: 0-18.9 months).
Adjuvant Breast Carcinoma Study: The adverse event profile for patients receiving TAXOL subsequent to AC was consistent with
that seen in previous clinical studies (see ADVERSE REACTIONS).
After Failure of Initial Chemotherapy: In addition to the Phase 3 trial described above, data from three Phase 2 clinical studies were
used in support of the use of Taxol in patients with metastatic breast carcinoma. The adverse event profile for patients receiving
TAXOL subsequent to AC was consistent with that seen in previous clinical studies (see ADVERSE REACTIONS).
29
Non-Small Cell Lung Carcinoma (NSCLC)
Study Design Treatments / Doses
No. of
Patients
Population Endpoints/Conclusion
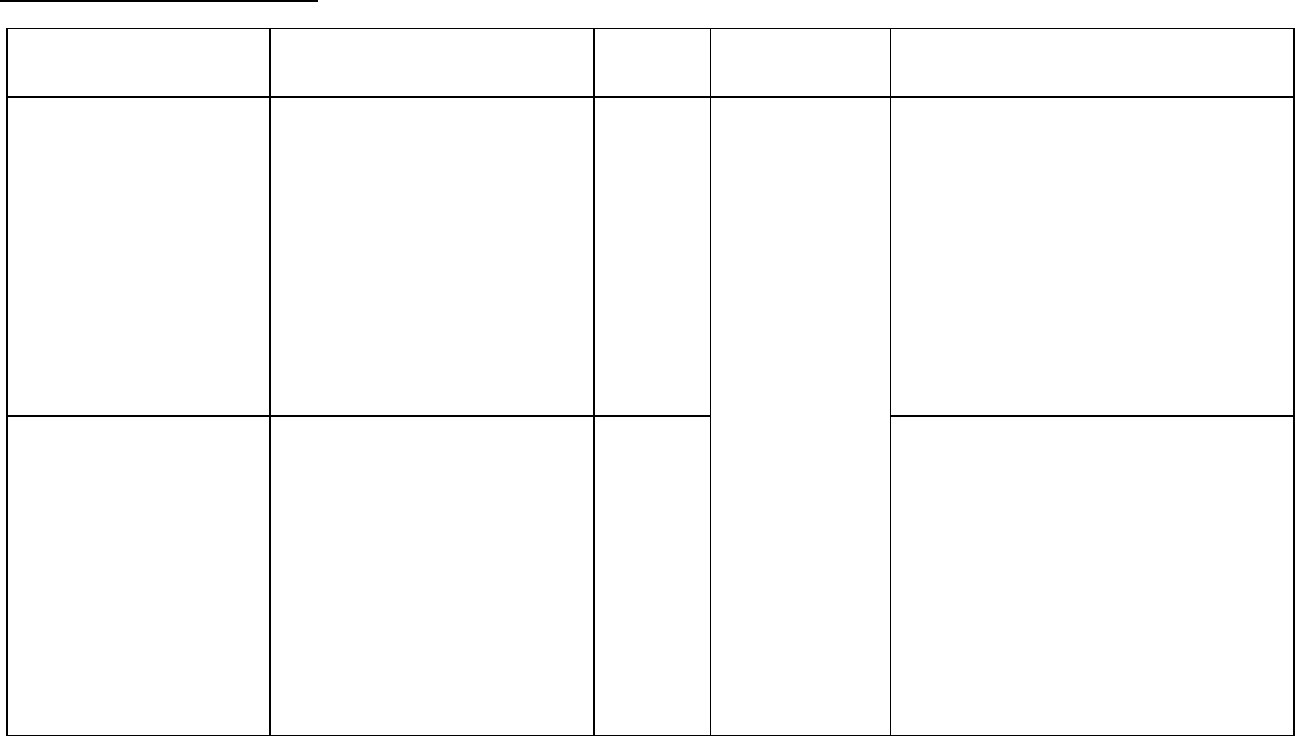
Phase 3 multicenter,
open label,
randomized trial
conducted by ECOG,
comparing two dosage
regimens of Taxol (T)
in combination with
cisplatin (c) to cisplatin
(c) followed by
etoposide (VP)
- 135 mg/m
2
of T over 24 hrs + 75
mg/m
2
of c
- 250 mg/m
2
of T over 24 hrs + 75
mg/m
2
of c with G-CSF support
- 75 mg/m
2
of c on day 1 followed by
100 mg/m
2
of VP on days 1, 2 and 3
(control)
599 Non-Small Cell
Lung Cancer
There were statistically significant
differences favoring each of the T plus c
arms for response rate and time to tumor
progression. There was no statistically
significant difference in survival between
either T plus c arm and the c plus VP arm.
In this study, the Functional Assessment of
Cancer Therapy-Lung (FACT-L)
questionnaire had seven subscales that
measured subjective assessment of
treatment. Of the seven, the Lung Cancer
Specific Symptoms subscale favored T at
135 mg/m
2
of T as a 24-hr infusion + 75
mg/m
2
of c. For all other factors, there
was no difference in the treatment groups.
The adverse event profile for patients who received TAXOL in combination with cisplatin was consistent with that seen in previous
clinical studies (see ADVERSE REACTIONS).
30
AIDS-Related Kaposi’s Sarcoma
Study Design Treatments / Doses
No. of
Patients
Population Endpoints/Conclusion
CA139-174: Phase 2
single-centre,
open-label,
non-randomized study to
assess the activity of
Taxol (T) against
AIDS-related Kaposi’s
Sarcoma.
135 mg/m
2
of T over 3 hrs
every 3 weeks (intended dose
intensity 45 mg/m
2
/wk). If no
dose-limiting toxicity was
observed, subjects were to
receive 155 mg/m
2
/ and
175 mg/m
2
in subsequent
courses. Hematopoietic
growth factors were not to be
used initially.
29 Objective response rate was 69%,
including two complete responses (CR)
and 18 partial responses (PR). An
additional 28% of patients achieved
stabilization of disease. Response rate
for patients receiving prior systemic
therapy was 79% (including 2 CRs and
13 Prs). Median time to response was
11.9 wks (range: 2.9 to 19.0 wks).
Median duration of response was 7.0
months (range 3.5 to 29.2 months).
CA139-281: Phase 2,
two-centre, open-label,
non-randomized study to
assess the efficacy and
safety of Taxol (T) in
patients with advanced
AIDS-related Kaposi’s
Sarcoma.
100 mg/m
2
of T over 3 hrs
every 2 weeks (intended dose
intensity 50 mg/m
2
/wk).
Patients could be receiving
hematopoietic growth factors
before the start of TAXOL
therapy or this support was to
be initiated as indicated; the
dose of TAXOL was not
increased.
56
AIDS-related
Kaposi's
sarcoma for
which systemic
chemotherapy
was warranted
Objective response rate was 59%
(95% C.I.: 45% to 77%), including one
complete response (CR) and 32 partial
responses (PR). An additional 25% of
patients achieved stabilization of
disease. Response rate for patients
receiving prior systemic therapy was
55% (22 PRs). Median time to
response was 6.1 wks (range: 4.0 to
36.0 wks). Median duration of
response was 10.4 months (range 2.8
to 18+ months).
31
All patients had widespread and poor-risk disease. Applying the ACTG staging criteria to
patients with prior systemic therapy, 93% were poor risk for extent of disease (T1), 88% had a
CD4 count <200 cells/mm
3
(I1), and 97% had poor risk considering their systemic illness (S1).
All patients in Study CA139-174 had a Karnofsky performance status of 80 or 90 at baseline; in
Study CA139-281, there were 26 (46%) patients with a Karnofsky performance status of 70 or
worse at baseline.
Although the planned dose intensity in the two studies was slightly different (45 mg/m
2
/week in
Study CA139-174 and 50 mg/m
2
/week in Study CA139-281), delivered dose intensity was
38-39 mg/m
2
/week in both studies, with a similar range (20-24 to 51-61).
Efficacy: The efficacy of TAXOL (paclitaxel) Injection was evaluated by assessing cutaneous
tumor response according to the amended ACTG criteria and by seeking evidence of clinical
benefit in patients in six domains of symptoms and/or conditions that are commonly related to
AIDS-related Kaposi's sarcoma.
Cutaneous Tumor Response (Amended ACTG Criteria): The objective response rate was
63% (95% CI: 49% to 75%) (37 of 59 patients) in patients with prior systemic therapy.
Cutaneous responses were primarily defined as flattening of more than 50% of previously raised
lesions.
The median time to response was 8.1 weeks and the median duration of response measured
from the first day of treatment was 9.1 months (95% CI: 6.9 - 11.0 months) for the patients who
had previously received systemic therapy. The median time to progression was 6.2 months
(95% CI: 4.6 to 8.7 months).
Additional Clinical Benefit: Most data on patient benefit were assessed retrospectively
(plans for such analyses were not included in the study protocols). Nonetheless, clinical
descriptions and photographs indicated clear benefit in some patients, including instances of
improved pulmonary function in patients with pulmonary involvement, improved ambulation,
resolution of ulcers, and decreased analgesic requirements in patients with KS involving the feet
and resolution of facial lesions and edema in patients with KS involving the face, extremities,
and genitalia.
Safety: The adverse event profile of TAXOL administered to patients with advanced HIV
disease and poor-risk AIDS-related Kaposi's sarcoma was generally similar to that seen in a
pooled analysis of data from 812 patients with solid tumors (See ADVERSE REACTIONS).
In this immunosuppressed patient population, however, a lower dose intensity of TAXOL and
supportive therapy including hematopoietic growth factors in patients with severe neutropenia
are recommended. (See DOSAGE AND ADMINISTRATION). Patients with AIDS-related
Kaposi's sarcoma may have more severe hematologic toxicities than patients with solid tumors.
(See ADVERSE REACTIONS).
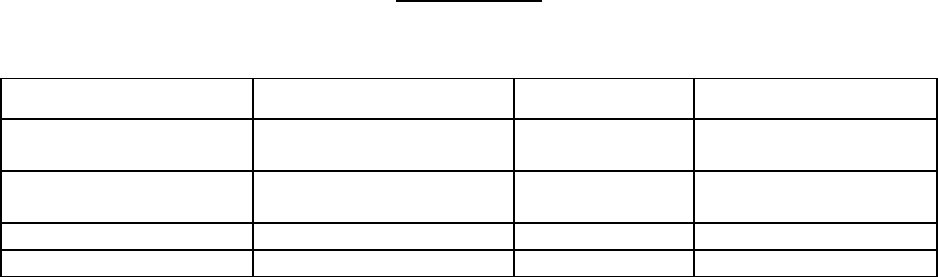
32
TOXICOLOGY
ACUTE TOXICITY
Species / Strain No. / Sex / Group Route LD
50
(mg/kg)
Rat/Sprague-Dawley
5 M/F (RF)a
10 M/F (L)b
ip
ip
34 (combined)
Rat/Sprague-Dawley
10 M/F
ip M: 32
F: 36
Rat/Sprague-Dawley 5 M/F iv >85
Dog/Beagle 1 M/F iv >9
a Range-Finding phase
b Lethality phase
Signs of toxicity in rats were lethargy, rough coat, thinness, hunched posture, neck abscesses,
soft stool, decreased body weight, squinted eyes, alopecia.
Signs of toxicity in dogs were decreased body weight.
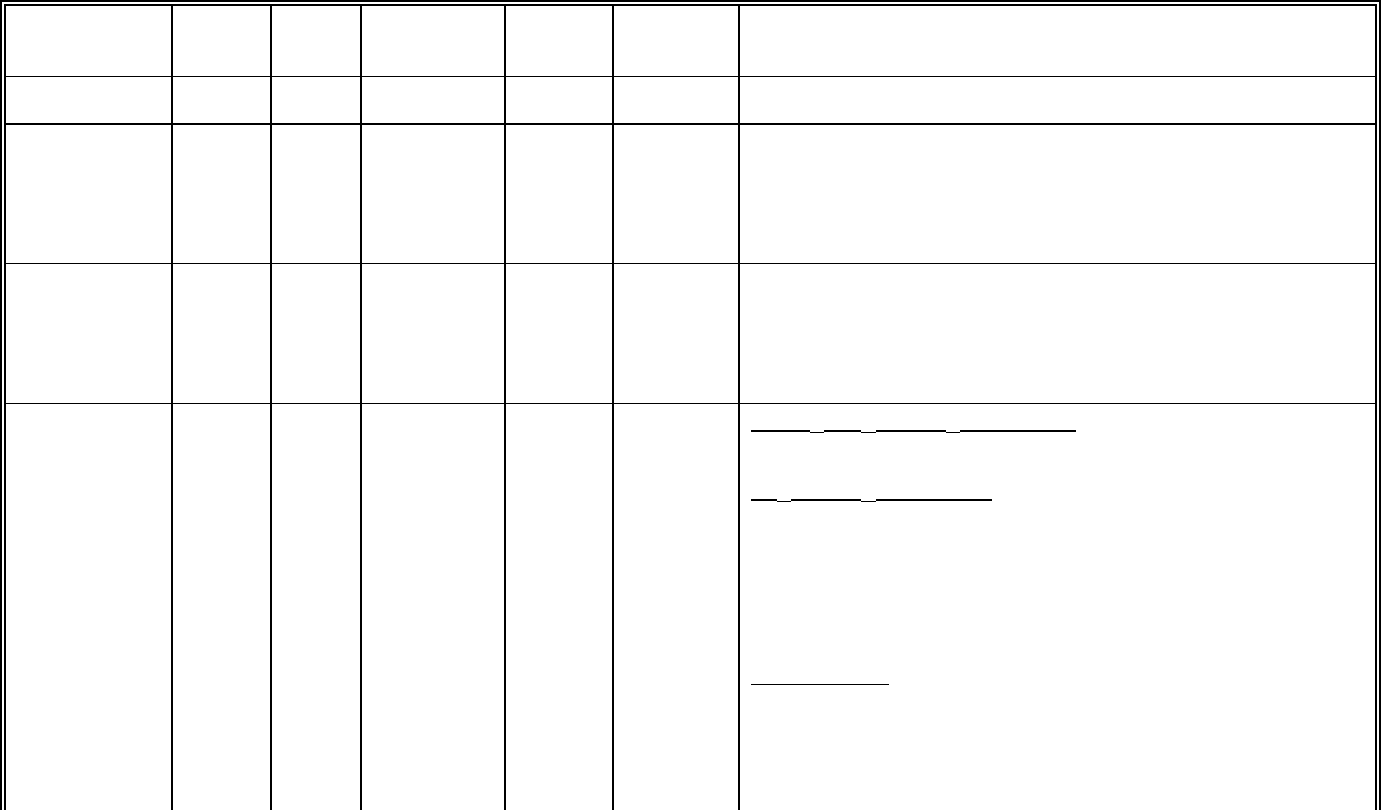
33
SUBACUTE TOXICITY
Species/Strain
No./
Group
Sex
Dose
Rangea
mg/kg/day
Route Duration Drug Related Findings
Mouse/CD2F
1
5
5
M
F
0, 1-15 iv 5 Days No drug related toxicities.
Mouse/CD2F
1
5
5
15
15
M
F
M
F
0, 1-15*
0, 21-43**
ip
ip
5 Days
5 Days
20 and 45 mg/kg/day: Decreased body weight >10%
45 mg/kg/day: Rough coat, thin/hunched posture. All died.
≥24 mg/kg/day: Dose-related decreased body weight, rough coat,
thin/hunched posture, ataxia, hypothermia, squinted eyes and
dyspnea, deaths (74/88 M, 56/90 F).
Rat/Sprague-
Dawley
5
5
10
10
M
F
M
F
0, 5-45*
0, 5.3-14.2**
ip
ip
5 Days
5 Days
≥8.66 mg/kg/day: Dose-related decreased body weight, rough coat,
thin/hunched posture, stool changes, soiling, hypothermia, eye
tearing and squinting, abscesses, deaths [(19/20 M, 18/20 F)*; (44/70
M at all doses, 26/40 F)**].
Mouse/CD2F
1
10
10
10
10
10
10
10
10
10
10
M
F
M
F
M
F
M
F
M
F
Negative
b
Control
Vehicle
Control
1/2 LD
10
10.79
13.05
LD
10
21.57
26.09
LD
50
25.50
29.52
ip 5 Days 1/2 LD
10
, LD
10
and LD
50
dose groups: Necrosis of developing
spermatocytes. Giant cell formation.
LD
10
and LD
50
dose groups: Decrease in reticulocyte and neutrophil
values. Lower liver and testicular weights. Moderate to severe
thymic cortical lymphoid depletion. Necrosis or atrophy of small
intestinal mucosa and crypt cell hypoplasia. Neurophilic
hyperplasia, eosinopenia, lymphoid hypoplasia and atypical
megakaryocytes, deaths (2/10 M, 8/10 F at LD
10
; 8/10 M, 9/9 F at
LD
50
).
All dose groups
: Dose-related decreased body weight, lethargy,
rapid respiration, rough coat, thin/hunched posture, hypothermia,
squinted eyes with exudate.
34
Species/Strain
No./
Group
Sex
Dose
Rangea
mg/kg/day
Route Duration Drug Related Findings
Rat/Sprague-
Dawley
10
10
10
10
10
10
10
10
10
10
M
F
M
F
M
F
M
F
M
F
Negative
b
control
Vehicle
Control
1/2 LD
10
2.55
4.29
LD
10
5.11
8.58
LD
50
7.47
9.99
ip 5 Days LD
50
dose group: Testicular necrosis, visceral peritoneum
inflammation (F only), deaths (3/10 M, 3/10 F).
LD
10
and LD
50
dose groups: Markedly decreased leukocyte and
platelet counts. Weight loss, bone marrow hypoplasia, deaths (1/10
M, 3/10 F at LD
10
).
All dose groups
: Dose related thymic and splenic lymphoid
depletion, rough coat, thin/hunched posture, lethargy, soft stool, neck
abscesses. Decreased reticulocycte counts, white foci in
submandibular lymph nodes and/or salivary glands.
Dog/Beagle 1
1
M
F
0, 0.375,
0.75, 1.5,
3.0, 6.0
iv 5 Days All doses: Decreased body weight. Increased ALT, cholesterol,
triglycerides and total lipids. Intestinal hemorrhage or agonal
changes. Lymphoid depletion of tonsils and/or bronchial lymph
node.
≥1.5 mg/kg/day
: Marked decreases in leukocyte, reticulocyte,
platelet, and erythrocyte counts.
≤1.5 mg/kg/day
: Moderate to severe bone marrow hematopoietic
hypoplasia.
3.0 to 6.0 mg/kg/day
: Deaths (All)
* Range Finding phase
** Lethality phase
a
Taxol dissolved in Cremophor
†
EL (50%): ethanol (50%) and then diluted with saline to provide dosing solutions
b
Untreated
35
CHRONIC TOXICITY
Species/ Strain
No./
Group
Sex
Dose*
(mg/kg/day)
Route Duration Drug Related Findings
Rat/Sprague-
Dawley
10
10
10
10
10
10
M
F
M
F
M
F
Neg. Cont.,
saline
Vehicle
Control
1, 3.3, 10
iv 1 Month 3.3 mg/kg/day: Slight decreases in erythrocyte, neutrophil and
platelet counts and hemoglobin and hematocrit values; moderate
decreases in leukocyte counts. Increased splenic extramedullary
hematopoiesis and bone marrow hypoplasia. Moderate to severe
decrease in reticulocyte counts. Minimal increase in lymphocyte
counts.
10 mg/kg/day
: Rough coat, alopecia, decreased body weight/weight
gain and food and water intakes. Slight decreases in erythrocyte and
neutrophil counts, hemoglobin and hemocrit values; moderate to
severe decreases in reticulocyte count and slight increases in platelet
and relative lymphocyte counts. Decreased weight of thymus, testes
and seminal vesicles. Lower weights of testes and epididymides
present at end of observation period.
Microspopically, increased splenic extra medullary hematopoiesis and
lymphoid depletion, thymic atrophy and lymphoid depletion,
mandibular lymph node atrophy of lymph follicle, and lymphadenitis;
bone marrow hypoplasia; hypospermatogenesis and atrophy of
seminiferous tubules; glandular atrophy in seminal vesicle and
prostate and giant cell formation in the epididymides.
36
Species/ Strain
No./
Group
Sex
Dose*
(mg/kg/day)
Route Duration Drug Related Findings
Dog/Beagle 5
5
3
3
3
3
5
5
M
F
M
F
M
F
M
F
Neg. Cont.,
saline
Vehicle
Control
0.3, 1
3
iv 1 Month 0.3 and 1 mg/kg/day: Reversible minimal decreases in bone marrow
cellularity.
3 mg/kg/day
: Interdigital cysts, swollen infusion sites, and transient
decreased weight gain and food intake. Decreased erythrocyte
numbers, hemoglobin concentration and hemocrit (M/F) and
decreased leucocyte (severe neutropenia) counts in individual
females. Lymphoid depletion of spleen or lymph nodes, duodenal
inflammation and crypt dilation, decreased bone marrow cellularity,
skin lesions and giant cell formation in the testes and epididymides.
Residual drug-effects present in some lymphoid organs, duodenum,
testes and skin at the end of recovery period.
* Taxol in Cremophor EL: ethanol (50/50) diluted with saline for dosing solutions
37
REPRODUCTION AND TERATOLOGY
Species/
Strain
No./
Group
Sex Route Dose* and Frequency Drug Related Findings
SEGMENT I
Rat/Sprague-
Dawley
20
20
20
M
F
F
iv
0 (vehicle), 0 (saline)
0.1, 0.3, 1.0 mg/kg
M: 63 days prior to mating
and during mating
F: During mating and
through day 7 of gestation
0 (Non-treated)
Body weight gain and food intake were lower in F
0
males and females Days
25-63 and Days 28-62, respectively, of premating period. Body weight
gain and food intake were lower in F
0
females during Days 2-20 of
gestation at the high dose level. Fertility indices in the F
0
generation were
lower at 1 mg/kg/day compared to saline and vehicle control groups.
Copulation indices were similar to control.
Adrenal, uterine and ovarian weights lower in F
0
dams compared to
controls.
Numbers of corpora lutea, implantations and live fetuses were decreased,
and numbers of empty implantation sites and fetal deaths were increased
at 1 mg/kg/day. The no-effect dose was 0.3 mg/kg/day in both F
0
and F
1
generations.
38
Species/
Strain
No./
Group
Sex Route Dose* and Frequency Drug Related Findings
SEGMENT II
Rabbit/New
Zealand
White
20
F
iv
O (saline), 0 (vehicle), 0.3, 1,
3 mg/kg, Days 6-18 of
presumed gestation.
Twelve of 20 does given the high dose died or were sacrificed as
moribund. Clinical signs of toxicity in the does that died included red
excreta, stool consistency changes, decreased activity, food intake
decreases and body weight loss.
Liver and kidney weights were increased and ovary weights were
decreased in the does given the high dose.
Litter group mean values for corpora lutea, litter size, live fetuses and the
number of does with viable fetuses in the high dose group were reduced.
Litter group mean values for resorption (total or early), percentage of dead
or resorbed conceptuses and the number of does with all conceptuses
dead or resorbed were increased in the high dose group.
In summary, taxol at 3 mg/kg/day caused severe maternal toxicity
(mortality, abortions, clinical signs and reduced organ weights, body
weights and food consumption) and severe developmental toxicity
(reduced corpora lutea, litter size and live fetuses and increased
resorption). Taxol doses as high as 1 mg/kg/day did not cause any
maternal or fetal toxicity.
* Taxol in Cremophor
†
EL: ethanol 50/50 diluted with saline for dosing solutions.
39
MUTAGENECITY AND GENOTOXICITY
TAXOL (paclitaxel) was not mutagenic in the Ames/Salmonella and Escherichia Coli WP2
reverse mutation assays but was found to be clastogenic, in the in vitro cytogenetics assay in
primary human lymphocytes.
TAXOL was genotoxic in vivo on the mouse erythropoietic system in the mouse bone marrow
erythrocyte micronucleus assay.

40
REFERENCES
1. Berg S.L., Cowan K.H., Balis F.M., et al
Pharmacokinetics of Taxol and doxorubicin
administered alone and in combination by continuous 72-hour infusion. J Nat Can Inst 1994;
86
:143-145
2. Brown T., Havlin K., Weiss G., Cagnola J., Kuhn J., Rizzo J., Craig J., Phillips J., and Van
Hoff D. A phase I trial of taxol given by a 6-hour intravenous infusion. J Clin Oncol 1991; 9
:
1261-1267.
3. Cabral F.R., Wible L., Brenner S., and Brinkley B.R. Taxol-requiring mutant of Chinese
hamster ovary cells with impaired mitotic spindle assembly. J Cell Biol 1983; 97
: 30-39.
4. Capri G., Munzone E., Tarenzi E., et al
Optic nerve disturbances: A new form of paclitaxel
neurotoxicity. J Nat Cancer Inst. 1994; 86
: 1099-1101
5. DeBrabander M.. A model for the microtubule organizing activity of the centrosomes and
kinetochores in mammalian cells. Cell Biol Int Rep 1982; 6
: 901-915.
6. Donehower R.C., Rowinsky E.K., Grochow L.B., et al
. Phase I trial of taxol in patients with
advanced cancer. Cancer Treat Reports 1987; 71
(12): 1171-1177.
7. Dorr R.T., Snead K., Liddil, J.D.. Skin Ulceration Potential of Paclitaxel in a Mouse Skin
Model In Vivo. Cancer 1996; 78
(1): 152-156.
8. Einzig A.I., Wiernik P.H., and Schwartz E.L. Taxol: A new agent active in melanoma and
ovarian cancer. In New Drugs, Concepts and Results in Cancer Chemotherapy
, FM Muggia
(ed.), pp. 89-100. Kluwer Academic Publishers, Inc. (1992).
9. Gianni L., Kearns C.M., Giani A., et al.
Nonlinear pharmacokinetics and metabolism of
paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol
1995; 13
: 180-190
10. Grem T.L., Tutsch K.D., Simon K.J., Alberti D.B., Willson J.K.V., Tormey D.C., Swaminathan
S., Trump D.L. Phase I study of taxol administered as a short iv infusion daily for 5 days.
Cancer Treat Reports 1987; 71
(12): 1179-1184.
11. Harris J.W., Rahman A., Kim B.-R., et al.
Metabolism of TAXOL by human hepatic
microsomes and liver slices: Participation of cytochrome P450 3A4 and an unknown P450
enzyme. Cancer Res 1994; 54
: 4026-4035
12. Kecker R.W., Jamis-Dow C.A., Egorin M.J., et al.
Effect of cimetidine, probenecid, and
ketoconazole on the distribution, biliary secretion, and metabolism of [
3
H] TAXOL in the
Sprague-Dawley rat. Drug Metab Disposit 1994; 22
; 254-258
13. Legha S.S., Tenney D.M., Krakoff I.R. Phase I study of taxol using a 5-day intermittent
schedule. J Clin Oncol 1986; 4
(5): 762-766.
14. Manfredi J.J. and Horwitz S.B. An antimitotic agent with a new mechanism of action.
Pharmacol Ther 1984; 25
: 83-125.
41
15. Manfredi J.J., Parness J., and Horwitz S.B. Taxol binds to cellular microtubules. J Cell Biol
1982; 94
: 688-696.
16. McGuire W.P., Rowinsky E.K., Rosenshein N.B., Grumbine F.C., Ettinger D.S., Armstrong
D.K., and Donehower R.C. Taxol: A unigue antineoplastic agent with significant activity in
advanced ovarian epithelial neoplasms. Ann Int Med 1989; 111
: 273-279.
17. McGuire W.P., Hoskins W.J., Brady M.F., Kugera P.R., Partridge E.E., Look K.Y.,
Clarke-Pearson D.L. and Davidson M. Cyclophosphamide and cisplatin compared with
paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New Eng J
Med 1996; 334
: 1-6.
18. Mole-Bajer J. and Bajer A.S. Action of taxol on mitosis: modification of microtubule
arrangements and function of the mitotic spindle in Haemanthus
endosperm. J Cell Biol
1983; 96
: 527-540.
19. O’Shaghnessy J.A., Fisherman J.S., Cowan K.H. Combination paclitaxel (TAXOL) and
doxorubicin therapy for metastatic breast cancer. Sem Oncol 1994; 21
(suppl 8): 19-23
20. Roberts L.P., Nath J., Friedman M.M. and Gallin J.I. Effects of taxol on human neutrophils. J
Immunol 1982; 129
: 2134-2141.
21. Rowinsky E.K., Burke P.J., Karp J.E., Tucker R.W. Ettinger D.S., and Donehower R.C.
Phase I and pharmacodynamic study of taxol in refractory acute leukemias. Cancer res
1989 49
: 4640-4647.
22. Rowinsky E.K., Cazenave L.A., and Donehower R.C. Taxol: a novel investigational
antimicrotubule agent. J Natl Canc Inst 1990; 82
: 1247-1259.
23. Rowinsky E.K., Gilbert M.R., McGuire W.P., Noe D.A., Grochow L.B., Forastiere A.A.,
Erringer D.S., Lubejko B.G. Clark B., Sartorius S.E., Cornblath D.R., Hendricks C.B. and
Donehower R.C. Sequences of taxol and cisplatin: A Phase I and pharmacologic study. J
Clin Oncol 1991; 9
(9): 1692-1703.
24. Sarosy G., Kohn E., Stone D.A., Rothenberg M., Jacob J., Adamo D.O., Ognibene F.P.,
Cunnoin R.E. and Reed E. Phase I study of taxol and granulocyte colony-stimulating factor
in patients with refractory ovarian cancer. J Clin Oncol 1992; 10
(7): 1165-1170
25. Schiff P.B. and Horwitz S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc
Natl Acad Sci, USA 1980; 77
: 1561-1565.
26. Seidman A.D., Barrett S., Canezo S. Photopsia during 3-hour paclitaxel administration at
doses ≥ 250 mg/m
2.
J Clin Oncol 1994; 12: 1741-1742
27. Slichenmyer W.J., and Von Hoff D.D. Taxol: A new and effective anti-cancer drug.
Anti-Cancer Drugs 1991; 2
: 519-530.
28. Turner P.F. and Margolis R.L. Taxol-induced bundling of brain-derived microtubules. J Cell
Biol 1984; 99
: 940-946.
29. Venook A.P., Egorin M., Brown T.D., et al.
Paclitaxel (Taxol) in patients with liver
dysfunction (CALGB 9264). pROC asco 1994; 13
: 139 (Abstract #350)
42
30. Waugh W.N., Trissel L.A., and Stella V.J. Stability, compatibility and plasticizer extraction of
taxol injection diluted in infusion solutions and stored in various containers. Am J Hosp
Pharm 1991; 48
: 1520-1524.
31. Weiss R.B., Donehower R.C., Wiernik P.H. et al
. Hypersensitivity reactions from taxol. J Clin
Oncol 1990; 8
: 1263-1268.
32. Wiernik P.H., Schwartz E.L., Strauman J.J., Dutcher J.P., Lipton R.B., and Einzig A. Phase I
trial of taxol given as a 24-hour infusion every 21 days: Responses observed in metastatic
melanoma. J Clin Oncol 1987; 5
(8): 1232-1239.
33. Wiernik P.H., Schwartz E.L., Strauman J.J., Dutcher J.P., Lipton R.B., and Paietta E. Phase
I clinical and pharmacokinetic study of taxol. Cancer Res 1987; 47
: 2486-2493.
34. Wright M., Monsarrat B., Alvinerie P., et al.
Hepatic metabolism and biliary excretion of taxol.
Second National Cancer Institute Workshop on Taxol and Toxus. Alexandria, Virginia
(1992)
35. Kelly K., Crowley J., Bunn P.A., et al.
A Randomized Phase III Trial of Paclitaxel Plus
Carboplatin (PC) Versus Vinorelbine Plus Cisplatin (VC) in Untreated Advanced Non-Small
Cell Lung Cancer (NSCLC): A Southwest Oncology Group (SWOG) Trial
36. Norton L., Slamon D., Leyland-Jones B., et al.
Overall Survival (OS) Advantage to
Simultaneous Chemotherapy (Crx) Plus the Humanized Anti-HER2 Monoclonal Antibody
Herceptin (H) in HER2-Overexpressing (HER2+) Metastatic Breast Cancer (MBC)
37. Walsky RL, Obach RS, Gaman EA, et al.
Selective Inhibition of Human Cytochrome
P4502C8 by Montelukast. Drug Metabolsim and Disposition 2005; 33
(3): 413-418.
38. Walsky RL, Gaman EA and Obach RS. Examination of 209 drugs for inhibition of
cytochrome P450 2C8. J Clin Pharmacol 2005; 45
: 68-78.
