
231127-paclitaxel-ebewe-ds-v1.0 Page 1 of 18
NEW ZEALAND DATA SHEET
1. PRODUCT NAME
Paclitaxel Ebewe
®
6 mg/mL concentrate for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
1 mL of Paclitaxel Ebewe® concentrated injection contains 6 mg of paclitaxel.
Excipient with known effect: Ethanol.
It is a white to off-white crystalline powder that is extremely highly lipophilic and practically
insoluble in water. Paclitaxel is partially soluble in ethanol and is therefore formulated with
PEG-35 castor oil and absolute ethanol.
For the full list of excipients, see Section 6.1 List of excipients.
3. PHARMACEUTICAL FORM
Injection, concentrated.
4. CLINICAL PARTICULARS
4.1. THERAPEUTIC INDICATIONS
Primary treatment of ovarian cancer in combination with a platinum agent.
Treatment of metastatic ovarian cancer and metastatic breast cancer, after failure of standard
therapy.
Treatment of non-small cell lung cancer (NSCLC).
Adjuvant treatment of node-positive breast cancer administered sequentially to doxorubicin
and cyclophosphamide.
Treatment of metastatic cancer of the breast, in combination with trastuzumab (Herceptin), in
patients who have tumours that overexpress HER-2 and who have not received previous
chemotherapy for their metastatic disease.
4.2. DOSE AND METHOD OF ADMINISTRATION
Dosage
Product is for single use in one patient only.
All patients must be premedicated before paclitaxel is administered to prevent severe
hypersensitivity effects (see Section 4.4 Special warnings and precautions for use). Such
premedication may consist of:
• dexamethasone 20 mg orally (or its equivalent), approximately 12 and 6 hours prior to
starting the paclitaxel infusion.
• promethazine 25 mg or 50 mg intravenously or other suitable H1-antagonist, 30 minutes
prior to starting the paclitaxel infusion
• cimetidine 300 mg or ranitidine 50 mg by intravenous infusion over 15 minutes, starting
30 minutes prior to the paclitaxel infusion.

231127-paclitaxel-ebewe-ds-v1.0 Page 2 of 18
Repeat courses of Paclitaxel Ebewe Injection Concentrate should not be administered to
patients with solid tumors until the neutrophil count is at least 1.5 x 10
9
cells/L and the platelet
count is at least 100 x 10
9
cells/L. Patients who experience severe neutropenia (< 0.5 x 10
9
cells/L) or severe peripheral neuropathy should receive a dosage reduction by 20% for
subsequent courses.
For primary treatment of ovarian cancer - it is recommended that paclitaxel be used at a dose
of 135 mg/m
2
, administered intravenously over three hours, followed by cisplatin 75 mg/m
2
.
The infusion should be repeated every three weeks.
For the treatment of metastatic ovarian cancer or metastatic breast cancer - it is recommended
that paclitaxel be used as a single agent at a dose of 175 mg/m
2
. Paclitaxel should be
administered as an intravenous infusion over three hours. The infusion should be repeated
every three weeks as tolerated. Patients have tolerated treatment with up to nine cycles of
paclitaxel therapy, but the optimal course of therapy remains to be established.
For primary or secondary treatment of NSCLC (non-small cell lung cancer) - the
recommended dose of paclitaxel is 175 mg/m
2
administered intravenously over three hours
with a three-week interval between courses.
For node-positive breast cancer - the recommended dose of paclitaxel is 175 mg/m
2
administered intravenously over three hours every three weeks for four courses following
doxorubicin and cyclophosphamide combination therapy.
For overexpression of HER-2 breast cancer - paclitaxel 175 mg/m
2
administered intravenously
over three hours with a three-week interval between courses for six cycles. Herceptin 2 mg/kg
administered intravenously once a week until progression of disease after an initial loading
dose of 4 mg/kg bodyweight.
Subsequent doses of paclitaxel should be administered according to individual patient
tolerance. Repetition of a course of paclitaxel is not recommended until the patient's neutrophil
count is at least 1.5 x 10
9
cells/L (1,500 cells/mm
3
) and the platelet count is at least
100 x 10
9
cells/L (100,000 cells/mm
3
). If there is severe neutropenia (neutrophil count less than
0.5 x 10
9
cells/L for a minimum of 7 days), severe peripheral neuropathy or severe mucositis
during paclitaxel therapy, the dose of paclitaxel in subsequent courses should be reduced by
20% (see Section 4.4 Special warnings and precautions for use). The incidence of neurotoxicity
and the severity of neutropenia increase with dose within a regimen.
Hepatic impairment
Inadequate data are available to recommend dosage alteration in patients with mild, moderate
and severe hepatic impairments (see Section 4.4 Special warnings and precautions for use).
Paediatric population
Paclitaxel is not recommended for use in children below 18 years due to lack of data on safety
and efficacy (see Section 4.4 Special warnings and precautions for use).
Method of administration
For intravenous use:
For instructions on reconstitution of the medicine before administration, see Section 6.6
Special precautions for disposal and other handling.

231127-paclitaxel-ebewe-ds-v1.0 Page 3 of 18
4.3. CONTRAINDICATIONS
Patients who have exhibited hypersensitivity reactions to paclitaxel, other taxanes or to any
excipient especially patients who have a history of hypersensitivity effects to PEG-35 castor
oil (Cremophor EL) or medicines formulated in PEG-35 castor oil (e.g. ciclosporin for injection
concentrate, teniposide for injection concentrate).
Paclitaxel should not be administered to patients who have baseline neutrophils counts of <
1.5x10
9
cells/L.
Paclitaxel is contraindicated during lactation (see Section 4.6 Fertility, pregnancy and lactation
– Use in Pregnancy and Use in Lactation).
4.4. SPECIAL WARNINGS AND PRECAUTIONS FOR USE
General
Paclitaxel should be administered under the supervision of medical staff experienced in the use
of cancer chemotherapeutic agents. Since significant hypersensitivity effects may occur,
appropriate supportive equipment should be available.
Paclitaxel should be given before a platinum compound when it is given in combination with
a platinum compound, e.g. cisplatin (see Section 4.5 Interactions with other medications and
other forms of interactions).
Premedication
In order to minimise the possibility of hypersensitivity effects due to histamine release, patients
must be premedicated before every treatment cycle of paclitaxel. Premedication should include
corticosteroids (e.g. dexamethasone), antihistamines (e.g. diphenhydramine or promethazine)
and an H
2
-receptor antagonist (e.g. cimetidine or ranitidine). (See Section 4.2 Dose and method
of administration.) The characteristic symptoms of hypersensitivity effects are dyspnoea and
hypotension (both requiring treatment), angioedema and widespread urticaria. In clinical trials,
2% of patients treated with paclitaxel experienced severe hypersensitivity. One of these effects
was fatal in a patient treated without premedication.
Paclitaxel Ebewe Injection Concentrate must not be used in patients who have exhibited
hypersensitivity effects to paclitaxel.
Haematologic Toxicity
Neutropenia (See Section 4.8 Undesirable effects).
Bone marrow suppression (primarily neutropenia) is the dose-limiting toxicity. Blood counts
should be frequently monitored during treatment with paclitaxel. Extreme care should be taken
when paclitaxel is given to patients with a pretreatment neutrophil count of less than 1.5 x 10
9
cells/L (1,500 cells/mm
3
). Pretreatment with paclitaxel should not be administered until the
patient’s neutrophil count is greater than 1.5 x 10
9
cells/L (1,500 cells/mm
3
) and the platelet
count is greater than 100 x 10
9
cells/L (100,000 cells/mm
3
).
If there is severe neutropenia during a course of paclitaxel (i.e. neutrophil count less than 0.5
x 10
9
cells/L (500 cells/mm
3
) for seven or more days), the dose of paclitaxel in subsequent
cycles should be reduced by 20%. Previous radiation therapy may induce more severe
myelosuppression. There is little information available from such patients at doses above 135
mg/m
2
.

231127-paclitaxel-ebewe-ds-v1.0 Page 4 of 18
Cardiovascular toxicity
Hypotension, hypertension and bradycardia have been observed during Paclitaxel Ebewe
Injection Concentrate administration, but generally do not require treatment. Frequent
monitoring of vital signs, particularly during the first hour of Paclitaxel Ebewe Injection
Concentrate infusion is recommended. (See also Section 4.8 Undesirable effects).
Electrocardiographic monitoring is recommended for patients with serious conduction
abnormalities, and should be commenced for patients who develop abnormal cardiovascular
symptoms or signs during monitoring of vital signs.
Severe cardiac conduction abnormalities have been reported rarely during paclitaxel therapy.
If patients develop significant conduction abnormalities during paclitaxel infusion, appropriate
therapy should be administered and continuous electrocardiographic monitoring should be
commenced and performed during subsequent therapy with paclitaxel. (See also Section 4.8
Undesirable effects). Severe cardiovascular events have been observed more frequently in
patients with non-small cell lung cancer (NSCLC) than breast or ovarian cancer.
When paclitaxel is used in combination with trastuzumab or doxorubicin for treatment of
metastatic breast cancer, monitoring of cardiac function is recommended. When patients are
candidates for treatment with paclitaxel in these combinations, they should undergo baseline
cardiac assessment including history, physical examinations, ECG, echocardiogram, and/or
multi-gated radionuclide angiography (MUGA) scan. Cardiac function should be further
monitored during treatment (e.g. every 3 months). Monitoring may help to identify patients
who develop cardiac dysfunction and treating physicians should carefully assess the cumulative
dose (mg/m
2
) of anthracycline administered when making decisions regarding frequency of
ventricular function assessment. When testing indicates deterioration in cardiac function, even
asymptomatic, treating physicians should carefully assess the clinical benefits of further
therapy against the potential for producing cardiac damage, including potentially irreversible
damage. If further treatment is administered, monitoring of cardiac function should be more
frequent (e.g. every 1-2 cycles).
Gastrointestinal toxicity
In patients receiving Paclitaxel who complain of abdominal pain with other signs and
symptoms, bowel perforation should be excluded.
Injection Site Reaction
A specific treatment for extravasation reactions is unknown at this time. Given the possibility
of extravasation, it is advisable to closely monitor the infusion site for possible infiltration
during drug administration.
Special care should be taken to avoid intra-arterial application of paclitaxel, since in animal
studies testing for local tolerance severe tissue reactions were observed after intra-arterial
application.
Anaphylaxis and Severe Hypersensitivity Reactions
Severe hypersensitivity (anaphylactoid) reactions characterised by dyspnoea and hypotension
requiring treatment, angioedema, and generalised urticaria have occurred rarely in
premedicated patients receiving paclitaxel.
Rare fatal reactions have occurred in patients despite pretreatment.

231127-paclitaxel-ebewe-ds-v1.0 Page 5 of 18
Cross-hypersensitivity between paclitaxel and other taxane products has been reported and may
include severe reactions such as anaphylaxis. Patients with a previous history of
hypersensitivity to other taxanes should be closely monitored during initiation of paclitaxel
therapy.
Since significant hypersensitivity reactions may occur, appropriate supportive equipment
should be available. Patients receiving paclitaxel should be under continuous observation for
at least the first 30 minutes following the start of the infusion and frequently thereafter. In case
of a severe hypersensitivity reaction, paclitaxel infusion should be discontinued immediately
and appropriate treatment given as indicated for anaphylaxis. The patient should not be
rechallenged with the drug. Minor hypersensitivity reactions such as flushing, skin reactions,
etc., do not require interruption of therapy (see also Section 4.8 Undesirable effects).
Hypotension and bradycardia
Patients may develop hypotension and bradycardia during paclitaxel treatment, but generally
not to a level requiring treatment. Vital signs should be monitored frequently, particularly
during the first hour of paclitaxel infusion. Only patients with serious conduction abnormalities
require continuous cardiac monitoring (see Conduction abnormalities (above) and Section 4.8
Undesirable effects).
Nervous system
Patients with pre-existing neuropathy should be carefully monitored. Peripheral neuropathy is
frequently reported in patients receiving paclitaxel and the severity is dose-dependent.
Although the occurrence of peripheral neuropathy is frequent, the development of severe
symptoms is rare. A 20% dose reduction in paclitaxel dose for all subsequent doses is
recommended for patients who develop severe peripheral neuropathy (See Section 4.8
Undesirable effects).
In NSCLC patients, the administration of paclitaxel in combination with cisplatin resulted in a
greater incidence of neurotoxicity than usually seen in patients receiving single-agent
paclitaxel.
Paclitaxel Ebewe Injection Concentrate contains absolute ethanol, 402 mg/mL and
consideration should be given to possible central nervous system and other effects of absolute
ethanol.
For instance, children may be more sensitive than adults to the effects of absolute ethanol.
Interstitial pneumonia
Paclitaxel in combination with radiation of the lung, irrespective of their chronological order,
may contribute to the development of interstitial pneumonia.
Pseudomembranous colitis
Pseudomembranous colitis has been reported in patients who have not been concomitantly
treated with antibiotics. This reaction should be considered in the differential diagnosis of cases
of severe or persistent diarrhoea occurring during or shortly after treatment with paclitaxel.
Mucositis
Severe mucositis has been reported which requires dose reduction (see Section 4.2 Dose and
method of administration).

231127-paclitaxel-ebewe-ds-v1.0 Page 6 of 18
Ophthalmology
There have been reports of reduced visual acuity due to cystoid macular oedema (CMO) during
treatment with paclitaxel as well as with other taxanes (see Section 4.8 Undesirable effects).
Patients with visual impairment during paclitaxel treatment should seek a prompt and complete
ophthalmologic examination. Paclitaxel should be discontinued if a CMO diagnosis is
confirmed.
Use in hepatic impairment
There is evidence that the toxicity of paclitaxel is enhanced in patients with elevated liver
enzymes. Caution should be exercised when administering paclitaxel to patients with moderate
impairment and dose adjustments should be considered. Patients with severe hepatic
impairment must not be treated with paclitaxel.
Patients with hepatic impairment may be at increased risk of toxicity, particularly Grade 3-4
myelosuppression. There is no evidence that the toxicity of paclitaxel is increased when given
as a 3 hour infusion to patients with mildly abnormal liver function. When paclitaxel is given
as a a greater than 3 hour infusion to patients with moderate to severe hepatic impairment,
increased myelosuppression may be seen as compared to patients with mildly elevated liver
function tests given 24-hour infusions. Patients should be monitored closely for the
development of profound myelosuppression (see Section 4.2 Dose and method of
administration). Inadequate data are available to recommend dosage alterations in patients with
mild to moderate hepatic impairments (see Section 5 Pharmacological properties).
No data are available for patients with severe baseline cholestasis.
Use in the elderly
Of 2228 patients who received paclitaxel in eight clinical studies evaluating its safety and
efficacy in the treatment of advanced ovarian cancer, breast carcinoma, or NSCLC, and 1570
patients who were randomised to receive paclitaxel in the adjuvant breast cancer study, 649
patients (17%) were 65 years or older, including 49 patients (1%) 75 years or older. In most
studies, severe myelosuppression was more frequent in elderly patients; in some studies, severe
neuropathy was more common in elderly patients. In two clinical studies in NSCLC, the elderly
patients treated with paclitaxel had a higher incidence of cardiovascular events. Estimates of
efficacy appeared similar in elderly patients and in younger patients; however, comparative
efficacy cannot be determined with confidence due to the small number of elderly patients
studied. In a study of first-line treatment of ovarian cancer, elderly patients had a lower medical
survival than younger patients, but no other efficacy parameters favoured the younger group.
Paediatric use
The safety and effectiveness of paclitaxel in children have not been established. There have
been reports of central nervous system (CNS) toxicity (rarely associated with death) in a
clinical trial in paediatric patients in which paclitaxel was infused over 3 hours at doses ranging
from 350 mg/m
2
to 420 mg/m
2
. The toxicity is most likely attributable to the high dose of the
ethanol component of paclitaxel vehicle given over a short infusion time. The use of
concomitant antihistamines may intensify this effect. Although a direct effect of the paclitaxel
itself cannot be discounted, the high doses used in this study (over twice the recommended
adult dosage) must be considered in assessing the safety of paclitaxel for use in this population.
Effects on laboratory tests
No data available.

231127-paclitaxel-ebewe-ds-v1.0 Page 7 of 18
4.5. INTERACTIONS WITH OTHER MEDICINES AND OTHER FORMS OF INTERACTIONS
Cisplatin
The recommended regimen of paclitaxel administration for the first-line chemotherapy of
ovarian carcinoma is for paclitaxel to be given before cisplatin. When paclitaxel is given before
cisplatin, the safety profile of paclitaxel is consistent with that reported for single-agent use. In
a dose-finding trial in which paclitaxel was administered as a 24-hour infusion and cisplatin
was administered as a 1 mg/min infusion, myelosuppression was more profound when
paclitaxel was given after cisplatin than when paclitaxel was given before cisplatin. In patients
receiving cisplatin prior to paclitaxel, there is about a 20% decrease in paclitaxel clearance.
Patients treated with paclitaxel and cisplatin may have an increased risk of renal failure as
compared to cisplatin alone in gynecological cancers.
Ketoconazole
As ketoconazole may inhibit the metabolism of paclitaxel, patients receiving paclitaxel and
ketoconazole should be closely monitored or the combination of these medicines should be
avoided.
Doxorubicin
Sequence effects characterised by more profound neutropenic and stomatitis episodes have
been observed with combination use of paclitaxel and doxorubicin when paclitaxel was
administered before doxorubicin and using longer than recommended infusion times
(paclitaxel administered over 24 hours; doxorubicin over 48 hours). Plasma levels of
doxorubicin (and its active metabolite doxorubicinol) may be increased when paclitaxel and
doxorubicin are used in combination and are given closer in time. Paclitaxel for initial treatment
of metastatic breast cancer should be administered 24 hours after doxorubicin (see Section 4.2
Dose and method of administration). However, data from a trial using bolus doxorubicin and
three-hour paclitaxel infusion found no sequence effects on the pattern of toxicity.
Cimetidine
Paclitaxel clearance is not affected by cimetidine premedication.
Medicines metabolised in the liver
The metabolism of paclitaxel is catalysed by cytochrome P450 isoenzymes CYP2C8 and
CYP3A4. Clinical studies have demonstrated that CYP2C8 mediated metabolism of paclitaxel,
to 6α-hydroxypaclitaxel, is the major metabolic pathway in humans. Concurrent administration
to ketoconazole, a known potent inhibitor of CYP3A4, does not inhibit the elimination of
paclitaxel in patients; thus, both medicinal products may be administered together without
dosage adjustment. Further data on the potential of drug interactions between paclitaxel and
CYP2C8 and 3A4 substrates/inhibitors are limited. Therefore, caution should be exercised
when administering paclitaxel concomitantly with medicinesknown to inhibit (e.g.
erythromycin, fluoxetine, gemfibrozil, deferasirox, trimethoprim) or induce (e.g. rifampicin,
carbamazepine, phenytoin, phenobarbital, efavirenz, nevirapine, St. John’s wort) either
CYP2C8 or 3A4.
In the clinical trial of paclitaxel in combination with trastuzumab (Herceptin), mean serum
trough concentration of trastuzumab were consistently elevated 1.5-fold as compared with
serum concentrations of trastuzumab in combination with anthracycline plus
cyclophosphamide (AC).

231127-paclitaxel-ebewe-ds-v1.0 Page 8 of 18
Arthralgia or myalgia adverse events of paclitaxel appear to be of a higher incidence in patients
being treated concurrently with filgrastim (granulocyte colony stimulating factor (G-CSF)).
4.6. FERTILITY, PREGNANCY AND LACTATION
Effects on fertility
Following treatment with intravenous paclitaxel at a dose of 1 mg/kg (6 mg/m
2
), rats showed
decreased fertility and toxicity in unborn offspring. Paclitaxel administered intravenously to
rabbits during organogenesis at a dose of 3 mg/kg (33 mg/m
2
) was toxic to both mother and
foetus.
Infertility in Females and Males
Based on findings in animal studies, paclitaxel may impair fertility in females and males of
reproductive potential. Male patients should seek advice regarding cryoconservation of sperm
prior to treatment with paclitaxel because of the possibility of infertility.
Use in pregnancy
Category D
Paclitaxel is a cytotoxic agent that can produce spontaneous abortion, fetal loss and birth
defects and may cause foetal harm when administered to a pregnant woman. Therefore,
paclitaxel should not be used during pregnancy unless clearly necessary.
Studies have shown paclitaxel to be embryotoxic and foetotoxic in rabbits at an intravenous
dose of 3 mg/kg (33 mg/m
2
) given during organogenesis. Paclitaxel is toxic to rat foetuses at a
dose of 1 mg/kg (6 mg/m
2
). Examination revealed that no gross external, soft tissue or skeletal
alterations occurred.
There are no studies in pregnant women. Women of children-bearing potential should have a
pregnancy test prior to starting treatment with paclitaxel. These women are strongly advised to
use contraception throughout therapy and for at least six months after the last dose of paclitaxel.
If Paclitaxel Ebewe Injection Concentrate is used during pregnancy, or if the patient becomes
pregnant while receiving this medicine, the patient should be apprised of the potential hazard.
Females and Males of Reproductive Potential
Males
Based on findings in genetic toxicity and animal reproduction studies, males should be advised
to use effective contraception in order to avoid fathering a child during treatment and for at
least three months after the last dose of paclitaxel.
Females
Women of childbearing potential should use effective contraception in order to avoid
becoming pregnant during treatment and for at least 6 months after the last dose of paclitaxel.
Use in lactation
Paclitaxel is contraindicated during lactation (see Section 4.3 Contraindications). It is not
known whether paclitaxel is excreted in human milk. The evidence from many medicines
would suggest that paclitaxel could be excreted in breast milk, though this has not been
established. Because of the potential for serious adverse effects in breastfeeding infants, it is
recommended that breastfeeding be discontinued when receiving paclitaxel therapy.

231127-paclitaxel-ebewe-ds-v1.0 Page 9 of 18
4.7. EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
It is not known whether paclitaxel interferes with the ability to drive and use machines;
however, Paclitaxel Ebewe does contain alcohol. Paclitaxel is likely to product minor or
moderate adverse effects, which may impair the patient’s ability to concentrate and react and
therefore constitute a risk in the ability to drive and use machines. Patients should refrain from
driving or using machines until they know that paclitaxel does not negatively affect these
abilities.
4.8. UNDESIRABLE EFFECTS
Unless otherwise noted, the following is based on the experience of 812 patients treated in
phase II and III clinical trials.
The frequency and severity of adverse effects are generally similar between patients receiving
paclitaxel for the treatment of ovarian, breast or lung cancer. None of the observed effects were
clearly influenced by age.
Unless stated otherwise percent figures, where given, are based on observed incidence when
using the recommended dosing regimen. If other regimens are used, the incidence of effect
may be higher.
Safety of the paclitaxel/ platinum combination has been investigated in a large randomised trial
in ovarian cancer and in two-phase III trials in NSCLC (non-small cell lung cancer). Unless
otherwise mentioned the combination of paclitaxel with platinum agents did not result in any
clinically relevant changes to the safety profile of single-agent paclitaxel.
Adverse effects reported were those occurring during or following the first course of therapy,
and have, where possible, been grouped by frequency according to the following criteria:
Very common: ≥1/10; common: ≥1/100 and < 1/10; uncommon: ≥1/1,000 and < 1/100; rare:
≥1/10,000 and < 1/1,000 and very rare: < 1/10,000.
Infections and Infestations
Very common: infection (mainly urinary tract and upper respiratory tract infections),
with reported cases of fatal outcome
Uncommon: septic shock
Rare: sepsis, peritonitis, pneumonia
Cardiovascular
Very common: hypotension
Common: bradycardia, ECG abnormalities (nonspecific repolarisation and sinus
tachycardia).
Uncommon: ECG abnormalities (premature beats), cardiomyopathy
Rare: myocardial infarction, congestive heart failure (typically in patients who
have received other chemotherapy, notably anthracyclines)
Very rare: atrial fibrillation, supraventricular tachycardia
Six severe cardiovascular events possibly related to paclitaxel administration occurred
including asymptomatic ventricular tachycardia, tachycardia with bigeminy, atrioventricular
block (two patients), and syncopal episodes (two patients, in one associated with severe

231127-paclitaxel-ebewe-ds-v1.0 Page 10 of 18
hypotension and coronary stenosis resulting in death). Severe hypotensive effects have been
associated with serious hypersensitivity effects and have required intervention.
Haematological
Very common: myelosuppression, thrombocytopenia, leucopenia, fever, bleeding,
anaemia, neutropenia (overall, 52% of the patients experienced severe
grade IV neutropenia and 56% had grade III/IV severe neutropenia on
their first course. Neutrophil nadirs occurred at a median of eleven days
after paclitaxel administration)
Common: febrile neutropenia (associated with an infectious episode, including
urinary tract infection (UTI) and upper respiratory tract infection
(URTI))
Rare: five septic episodes, which were associated with severe neutropenia
attributable to paclitaxel administration, had a fatal outcome
Very Rare: acute myeloid leukaemia, myelodysplastic syndrome
Patients who have received prior radiation or cisplatin therapy exhibit more frequent
myelosuppression, which is generally of greater severity (see Sections 4.4 Special warnings
and precautions for use and 4.5 Interactions with other medicines and other forms of
interactions).
Reports of thrombocytopenia after paclitaxel therapy are less frequent and less severe than
neutropenia, with platelet nadir (< 50 x 10
9
cells/L) observed eight or nine days after paclitaxel
administration in 5% of patients. Haemorrhage has been reported in patients receiving
paclitaxel but this does not appear to be related to thrombocytopenia. Patients (3%) may require
platelet transfusions.
Not known: Disseminated intravascular coagulation.
Hepatobiliary
Very common: elevated alkaline phosphatase; elevated AST; elevated ALT
Common: elevated bilirubin
Rare: hepatic necrosis (leading to death), hepatic encephalopathy (leading to
death)
Immune System Disorders
Very common: minor hypersensitivity reactions (mainly flushing and rash)
Common: dyspnoea, hypotension, chest pains, tachycardia
Uncommon: significant hypersensitivity reactions requiring therapy (e.g.
hypotension, angioneurotic oedema, bronchospasm, respiratory distress,
generalised urticaria, oedema, back pains, pain in extremities, chills,
diaphoresis). The most frequent symptoms observed during severe
reactions were dyspnoea, flushing, chest pain and tachycardia.
Abdominal pain, pain in the extremities, hyperhydrosis, and
hypertension were also noted.
Infections: Febrile neutropenia occurred in 5% of all courses and 30%
of all courses were associated with an infectious episode. The most
common infections involve the upper respiratory tract, urinary tract and
blood (sepsis). In phase II clinical trials, five septic episodes resulted in
death.
Rare: anaphylactic reactions
Very Rare: anaphylactic shock

231127-paclitaxel-ebewe-ds-v1.0 Page 11 of 18
Vascular Disorders
Very common: hypotension
Uncommon: hypertension, thrombosis, thrombophlebitis
Very Rare: shock
Not known: phlebitis
Metabolism and nutrition disorders
Very Rare: anorexia
Not Known: tumor lysis syndrome
Gastrointestinal
Very common: nausea, vomiting, diarrhoea, mucositis (these manifestations were
usually mild to moderate at the recommended dose)
Rare: bowel obstruction, ischaemic colitis, pancreatitis, bowel perforation
(there have been several cases of bowel perforation associated with
patients receiving paclitaxel. Patients receiving paclitaxel who complain
of abdominal pain with other signs and symptoms should have bowel
perforation excluded)
Neutropenic enterocolitis has been reported
Very rare: mesenteric thrombosis, pseudomembranous colitis, neutropenic colitis,
ascites, oesophagitis, constipation
Musculoskeletal, Connective Tissue and Bone Disorders
Very common: arthralgia; myalgia (the symptoms were usually transient occurring two
to three days after paclitaxel administration and resolving within a few
days)
Not Known: systemic lupus erythematosus
Psychiatric disorders
Very rare: confusional state
Neurological
Very common: neurotoxicity (mainly: peripheral neuropathy. Peripheral neuropathy
occurs and is dose-dependent with 60% of patients experiencing grade I
toxicity, 10% grade II and 2% grade III at the recommended doses.
Neuropathy was present in 87% of patients at higher doses. Severity of
symptoms also increased with dose; 4% of patients experienced severe
symptoms at the recommended dose versus 10% at higher doses.
Neurological symptoms may occur following the first course and
symptoms may worsen with increasing exposure to paclitaxel.
Peripheral neuropathy was the cause of paclitaxel discontinuation in 2%
of patients. Sensory symptoms have usually improved or resolved within
several months of paclitaxel discontinuation).
Rare: motor neuropathy (with resultant minor distal weakness).
Optic nerve and/or visual disturbances (scintillating scotomata)
particularly in patients who have received higher doses than
recommended; these effects generally have been reversible; motor

231127-paclitaxel-ebewe-ds-v1.0 Page 12 of 18
neuropathy with resultant minor distal weakness and autonomic
neuropathy resulting in paralytic ileus and orthostatic hypotension.
There is a report of a grand mal seizure in a patient receiving paclitaxel and the seizure recurred
after treatment with paclitaxel was recommenced. There is also a second report of a grand
mal seizure in a patient with significant hepatic impairment during infusion with paclitaxel.
Very rare: grand mal seizures, autonomic neuropathy (resulting in paralytic ileus
and orthostatic hypotension), encephalopathy, convulsions, dizziness,
ataxia, headache
Eye disorders
Very rare: Optic nerve and/or visual disturbances (scintillating scotomata),
particularly in patients who have received higher doses than
recommended.
Ear and labyrinth disorders
Very rare: hearing loss, ototoxicity, tinnitus, vertigo
Respiratory, thoracic and mediastinal disorders
Rare: respiratory failure, pulmonary embolism, lung fibrosis, interstitial
pneumonia, dyspnoea, pleural effusion
Very Rare: cough
Skin and appendages
Very common: alopecia
Common: transient and mild nail and skin changes
Rare: pruritus, rash, erythema, radiation recall dermatitis, recall dermatitis.
Local effects: phlebitis following intravenous administration has been
reported. Extravasation leading to oedema, pain, erythema and
induration has been reported. On occasions, extravasation can lead to
cellulitis. Skin discolouration may also occur.
Very Rare: Stevens-Johnson syndrome, epidermal necrolysis, erythema
multiforme, exfoliative dermatitis, urticaria, onycholysis (patients on
therapy should wear sun protection on hands and feet).
Not known: scleroderma
In some cases, the onset of the injection site reaction either occurred during a prolonged
infusion or was delayed by a week to 10 days.
General Disorders and Administration Site Conditions
Very common: mucosal inflammation
Common: injection site reactions (including localised oedema, pain, erythema,
induraton, on occasion extravasation can result in cellulitis, skin fibrosis
and skin necrosis).
Rare: pyrexia, dehydration, asthenia, oedema, malaise
Injection site reactions, including reactions secondary to extravasation, were usually mild and
consisted of erythema, tenderness, skin discolouration, or swelling at the injection site. These
reactions have been observed more frequently with the 24-hour infusion than with the 3-hour
infusion. Recurrence of skin reactions at a site of previous extravasation following
administration of paclitaxel at a different site, i.e. ‘recall’, has been reported rarely.

231127-paclitaxel-ebewe-ds-v1.0 Page 13 of 18
Rare reports of more severe events such as phlebitis, cellulitis, induration, skin exfoliation,
necrosis and fibrosis have been received as part of the continuing surveillance of paclitaxel
safety. In some cases, the onset of the injection site reaction either occurred during a prolonged
infusion or was delayed by a week to ten days.
A specific treatment for extravasation reactions is unknown at this time. Given the possibility
of extravasation, it is advisable to closely monitor the infusion site for possible infiltration
during frug administration.
Radiation pneumonitis has been reported in patients receiving concurrent radiotherapy.
Investigations
Common: severe elevation in AST (SGOT), severe elevation in alkaline
phosphatase
Uncommon: severe elevation in bilirubin
Rare: increase in blood creatinine
Combination treatment
When paclitaxel was administered as a 3-hour infusion in combination with trastuzumab for
the first-line treatment of patients with metastatic breast cancer, the following events
(regardless of relationship to paclitaxel or trastuzumab) were reported more frequently than
with single-agent paclitaxel: heart failure, infection, chills, fever, cough, rash, arthralgia,
tachycardia, diarrhoea, hypertonia, epistaxis, acne, herpes simplex, accidental injury, insomnia,
rhinitis, sinusitis, and injection site reaction. Some of these frequency differences may be due
to the increased number and duration of treatments with paclitaxel/trastuzumab combination
vs. single-agent paclitaxel. Severe events were reported at similar rates for
paclitaxel/trastuzumab and single-agent paclitaxel.
Postmarketing Experience
The following additional adverse effects have been identified during post-approval use of
Paclitaxel Ebewe Injection Concentrate. Because their reactions are reported voluntarily from
a population of uncertain size, it is not always possible to reliably estimate their frequency or
establish a causal relationship to medicine exposure.
Infections and infestations: pneumonia, sepsis
Cardiac Disorders: atrial fibrillation, supraventricular tachycardia, reduction of left
ventricular ejection fraction, ventricular failure
Hematological Disorders: acute myeloid leukaemia, myelodysplastic syndrome
Immune System Disorders: anaphylactic reactions (with fatal outcome); anaphylactic
shock, cross-hypersensitivity between paclitaxel and other
taxanes has been reported
Metabolic, and Nutrition
Disorders: anorexia, tumour lysis syndrome
Psychiatric Disorder: confusional state
Vascular Disorders: shock
Respiratory, Thoracic and

231127-paclitaxel-ebewe-ds-v1.0 Page 14 of 18
Mediastinal Disorders: dyspnoea, pleural effusion, respiratory failure, interstitial
pneumonia, lung fibrosis, pulmonary embolism, cough
Gastrointestinal Disorders: bowel obstruction, bowel perforation, ischemic colitis,
pancreatitis, mesenteric thrombosis, pseudoemembranous
colitis, oesophagitis, constipation, ascites
Neurological Disorders: autonomic neuropathy (resulting in paralytic ileus and
orthostatic hypotension), grand mal seizures, convulsions,
encephalopathy, dizziness, headache, ataxia, paresthesia,
hyperesthesia.
Eye Disorders: photopsia, visual floaters, cystoid macular oedema, macular
oedema.
Ear and Labyrinth Disorders: hearing loss, tinnitus, vertigo, ototoxicity
Skin and Subcutaneous
Tissue Disorders: Stevens- Johnson syndrome, epidermal necrolysis, erythema
multiforme, exfoliative dermatitis, urticaria, onycholysis
(patients on therapy should wear sun protection on hands and
feet), scleroderma, pruritus, rash, erythema, phlebitis, cellulitis,
skin exfoliation, necrosis, fibrosis, palmar-plantar
erythrodysesthesia syndrome and cutaneous lupus
erythematosus.
Musculoskeletal, Connective
Tissue and Bone Disorders: systemic lupus erythematosus, scleroderma.
Investigations: increase in blood creatine.
General Disorders and
Administration Site Conditions: asthenia, malaise, pyrexia, dehydration, oedema.
Reporting suspected adverse effects
Reporting suspected adverse reactions after authorisation of the medicine is important. It allows
continued monitoring of the benefit/risk balance of the medicine. Healthcare professionals are
asked to report any suspected adverse reactions https://nzphvc.otago.ac.nz/reporting/
4.9. OVERDOSE
There is no known antidote for paclitaxel overdose.
At present, there is no specific treatment for paclitaxel overdosage. In case of overdose, the
patient should be closely monitored. Probable consequences of an overdosage are mucositis,
severe bone marrow suppression and peripheral neurotoxicity and treatment should be
supportive.
Overdosage in paediatric patients may be associated with acute ethanol toxicity. Treatment is
symptomatic and supportive.
For advice on the management of overdose please contact the National Poisons Centre on 0800
POISON (0800 764766).
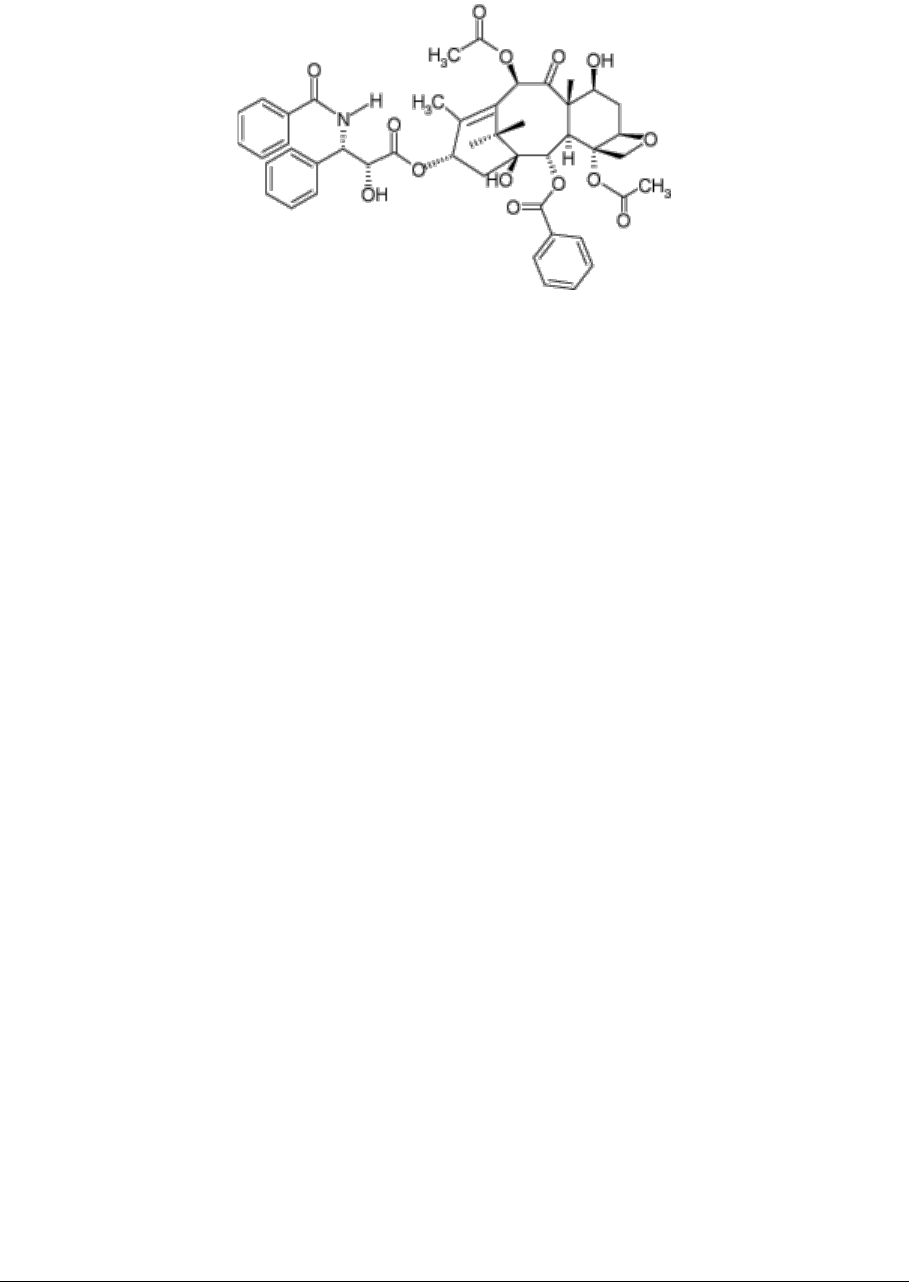
231127-paclitaxel-ebewe-ds-v1.0 Page 15 of 18
5. PHARMACOLOGICAL PROPERTIES
5.1. PHARMACODYNAMIC PROPERTIES
Paclitaxel is a natural product with antitumour activity. Its Molecular formula: C
47
H
51
NO
14
(Molecular weight: 853.9)
,
CAS: 33069-62-4 and its chemical structure is:
Mechanism of action
Paclitaxel is an antimicrotubule antineoplastic agent. It promotes microtubule assembly by
enhancing the polymerisation of tubulin, the protein subunit of spindle microtubules, even in
the absence of the mediators normally required for microtubule assembly (e.g. guanosine
triphosphate (GTP)), thereby inducing the formation of stable, non-functional microtubules.
While the precise mechanism of action of the medicine is not completely known, paclitaxel
disrupts the dynamic equilibrium within the microtubule system and blocks cells in the late G2
phase and M phase of the cell cycle, inhibiting cell replication and impairing function of
nervous tissue.
Clinical trials
No data available.
5.2. PHARMACOKINETIC PROPERTIES
Absorption
After paclitaxel is administered intravenously, its plasma concentration declines biphasically.
Distribution
The first phase shows rapid decline, representing distribution of paclitaxel to the peripheral
compartment and elimination. This initial phase is followed by a relatively slow elimination of
paclitaxel from the peripheral compartment.
The following ranges for the pharmacokinetic parameters have been determined in patients
given doses of 135 and 175 mg/m
2
as 3- and 24-hour infusions of paclitaxel. Mean terminal
half-life: 3.0 to 52.7 hours; total body clearance: 11.6 to 24.0 L/hour/m
2
; mean steady-state
volume of distribution: 198 to 688 L/m
2
. These indicate extensive distribution of paclitaxel
outside the vascular system and/or tissue binding. The volume of distribution is reduced in
female subjects. The following mean values for the pharmacokinetic parameters have been
reported following a three-hour infusion of 175 mg/m
2
paclitaxel. Mean terminal half-life: 9.9
hours, mean total body clearance: 12.4 L/hour/m
2
. The serum protein binding of paclitaxel is
89%. The presence of cimetidine, ranitidine, dexamethasone or diphenhydramine does not
affect protein binding of paclitaxel.

231127-paclitaxel-ebewe-ds-v1.0 Page 16 of 18
Metabolism
The liver is thought to be the primary site of metabolism for paclitaxel.
Excretion
The mean cumulative urinary recovery of unchanged paclitaxel has been reported to be 1.8 to
12.6% of the dose, indicating extensive non-renal clearance.
5.3. PRECLINICAL SAFETY DATA
Genotoxicity
In vitro studies (chromosome abnormalities in human lymphocytes) and in vivo (micronucleus
test using mice) mammalian test systems have shown paclitaxel to be mutagenic. When tested
using the Ames test or the CHO/HGPRT (Chinese hamster ovary/ hypoxanthine-guanine
phosphoribosyl transferase) gene mutation assay, paclitaxel did not induce mutagenicity.
Carcinogenicity
No studies have examined the carcinogenic potential of paclitaxel; however, medicines similar
to paclitaxel are carcinogens.
6. PHARMACEUTICAL PARTICULARS
6.1. LIST OF EXCIPIENTS
Each mL contains absolute ethanol 402 mg, PEG-35 castor oil 522 mg.
6.2. INCOMPATIBILITIES
Avoid contact of paclitaxel solutions with plasticised polyvinyl chloride (PVC) equipment,
infusion lines or devices used when preparing infusion solutions. Prepare and store diluted
paclitaxel solutions in glass or polyethylene containers. These precautions are to avoid leaching
of the plasticiser DEHP (di-[2-ethylhexyl] phthalate) from PVC infusion bags or sets.
Paclitaxel solutions should be administered through polyethylene lined administration sets (e.g.
Gemini 20 giving set) using an IMED pump.
This medicine must not be mixed with other medicinal products except those mentioned in
Section 6.6.
6.3. SHELF LIFE
3 years from date of manufacture.
To reduce microbiological hazard, use as soon as practicable after reconstitution/preparation.
If storage is necessary, hold at 2º - 8°C for not more than 24 hours after preparation.
A shelf life of 14 days at 2º to 8ºC (refrigerate; do not freeze) for facilities preparing paclitaxel
solutions reconstituted under controlled aseptic conditions for IV infusion.
6.4. SPECIAL PRECAUTIONS FOR STORAGE
Store below 25°C. Protect from light.
6.5. NATURE AND CONTENTS OF CONTAINER
30 mg in 5 mL glass vials: 1’s and 5’s

231127-paclitaxel-ebewe-ds-v1.0 Page 17 of 18
100 mg in 16.7 mL glass vials: 1’s
150 mg in 25 mL glass vials: 1’s
300 mg in 50 mL glass vials: 1’s
600 mg in 100 mL glass vials: 1’s
6.6. SPECIAL PRECAUTIONS FOR DISPOSAL AND OTHER HANDLING
Preparation for intravenous administration
Avoid contact of paclitaxel solutions with plasticised polyvinyl chloride (PVC) equipment,
infusion lines or devices used when preparing infusion solutions. Prepare and store diluted
paclitaxel solutions in glass or polyethylene containers. These precautions are to avoid leaching
of the plasticiser DEHP (di-[2-ethylhexyl] phthalate) from PVC infusion bags or sets.
Paclitaxel solutions should be administered through polyethylene lined administration sets (e.g.
Gemini 20 giving set) using an IMED pump.
Paclitaxel Ebewe Injection Concentrate must be diluted prior to intravenous infusion. It should
be diluted in glucose 5% or sodium chloride 0.9% intravenous infusion. Dilution should be
made to a final concentration of 0.3 to 1.2 mg/mL.
After the final dilution of Paclitaxel Ebewe Injection Concentrate, the bottle should be swirled
gently to disperse the paclitaxel. Do not shake.
To reduce microbiological hazard, use as soon as practicable after reconstitution/preparation.
If storage is necessary, hold at 2º - 8⁰C for not more than 24 hours after preparation.
Administration should be completed within 24 hours of preparation of the infusion and any
residue discarded according to the guidelines for the disposal of cytotoxic medicines (see
Handling and disposal, below).
Facilities preparing paclitaxel solutions reconstituted under controlled aseptic conditions for
IV infusion, may apply a shelf life of 14 days at 2º to 8ºC (refrigerate; do not freeze) when
diluted with glucose 5% or sodium chloride 0.9% for intravenous infusion and stored in glass
or polyethylene containers. Diluted solutions prepared this way have been shown to be
chemically stable for these periods. Administration should be completed within 24 hours of the
start of the infusion and any residue discarded according to the guidelines for the disposal of
cytotoxic medicines.
Filtration. A microporous membrane of 0.22 microns or less in size should be used as the in-
line filter for all infusions of paclitaxel. The IMED 0.2 micron add-on filter set composed of
polysulfone and the IVEX II 0.2 micron filter composed of cellulose have both been found to
be suitable for Paclitaxel Injection Concentrate.
Devices with spikes should not be used with vials of Paclitaxel Ebewe Injection Concentrate
since they can cause the stopper to collapse, resulting in a loss of sterile integrity of the
Paclitaxel Ebewe Injection Concentrate solution.
Preparation and administration precautions
Paclitaxel is a cytotoxic anti-cancer medicine and, as with other potentially toxic compounds,
caution should be exercised in handling Paclitaxel. The use of gloves is recommended.
Following topical exposure, tingling, burning and redness have been observed. If Paclitaxel
solution contacts the skin, wash the skin immediately and thoroughly with soap and water. If
231127-paclitaxel-ebewe-ds-v1.0 Page 18 of 18
Paclitaxel contacts mucous membranes, the membranes should be flushed thoroughly with
water. Upon inhalation, dyspnoea, chest pain, burning eyes, sore throat and nausea have been
reported. Given the possibility of extravasation, it is advisable to closely monitor the infusion
site for possible infiltration during medicine administration.
Handling and disposal
The published guidelines related to procedures for the proper handling and disposal of
cytotoxic medicines should be followed.
Care must be taken whenever handling cytotoxic products. Always take steps to prevent
exposure. This includes appropriate equipment, such as, wearing gloves, and washing hands
with soap and water after handling such products.
Any unused medicine or waste material should be disposed of in accordance with local
requirements.
7. MEDICINE SCHEDULE
Prescription Medicine.
8. SPONSOR
Sandoz New Zealand Limited
12 Madden Street
Auckland 1010
New Zealand
Telephone: 0800 726 369
9. DATE OF FIRST APPROVAL
14/10/2016
10. DATE OF REVISION OF THE TEXT
27/11/2023
SUMMARY TABLE OF CHANGES
Section
Changed
Summary of new information
All
Minor editorial changes
4.2
Clarification of definition of severe neutropenia.
4.3
Removed redundant text – ‘with solid tumours.’
4.4
Update information regarding myelosuppression toxicity in patients with hepatic
impairment.
Clarification of statement regarding the reduced paclitaxel dosing in patients with peripheral
neuropathy.
4.5
Updated paclitaxel clearance figure.
4.6
Addition of contraceptive use in males and females during treatment.
4.8
Addition of mucosal inflammation adverse reaction and palmar-plantar erythrodysesthesia
syndrome in post-marketing experience.
