September 2012
Readmissions Due to Hospital-Acquired
Conditions (HACs):
Multivariate Modeling and Under-coding Analyses
Final Report
Prepared for
Susannah G. Cafardi, MSW, LCSW, MPH
Centers for Medicare & Medicaid Services
Rapid-cycle Evaluation Group
Innovation Center Mail Stop C3-19-26
7500 Security Boulevard
Baltimore, MD 21244-1850
Prepared by
Richard D. Miller, Jr., PhD
Terry Eng, RN, PhD (c)
Amy M.G. Kandilov, PhD
Jerry Cromwell, PhD
Nancy McCall, ScD
RTI International
3040 Cornwallis Road
Research Triangle Park, NC 27709
RTI Project Number 0209853.231.002.128
_________________________________
Readmissions Due to Hospital-Acquired Conditions (HACs):
Multivariate Modeling and Timing of Clinical Presentation Analyses
Draft Final Report
by Richard D. Miller, Jr., PhD
Terry Eng, RN, PhD (c)
Amy M.G. Kandilov, PhD
Jerry Cromwell, PhD
Nancy T. McCall, ScD, Project Director
CMS Project Officer: Susannah G. Cafardi, MSW, LCSW, MPH
RTI International
CMS Contract No. HHSM-500-2005-00029I
August 2012
This project was funded by the Centers for Medicare & Medicaid Services under Contract No.
HHSM-500-2005-00029I. The statements contained in this report are solely those of the authors
and do not necessarily reflect the views or policies of the Centers for Medicare & Medicaid
Services. RTI assumes responsibility for the accuracy and completeness of the information
contained in this report.
RTI International is a trade name of Research Triangle Institute.
ACKNOWLEDGMENTS
We would like to acknowledge assistance we received in conducting analyses related to
hospital readmissions and preparing this report for submission. First, we would like to thank
Merry Rabb, Matt Urato, and Arnold Bragg who provided valuable assistance in the construction
of the episode-of-care linked file that made this analysis possible and in programming assistance
through the analysis phase. Lastly, we would like to thank Loretta Bohn and Norma DiVito for
assistance with preparation of this report.
This page intentionally left blank.
v
CONTENTS
SECTION 1 INTRODUCTION AND OVERVIEW OF RESEARCH APPROACH ....................1
1.1 Introduction and Findings from Previous Research.......................................................1
1.2 Summary of Phase II Findings on Readmissions and Potential Estimation Bias ..........2
1.3 Overview of Phase III Research Questions and Analytic Approach .............................4
1.4 Organization of Report ..................................................................................................5
SECTION 2 TECHNICAL APPROACH ........................................................................................7
2.1 Study Sample and Data ..................................................................................................7
2.2 Defining Readmissions ..................................................................................................7
2.3 Defining Discharges to PAC Settings ............................................................................8
2.4 Selection of the Three Study HACs ...............................................................................8
2.5 Comparison Group Matching Criteria ...........................................................................8
2.6 Multivariate Analyses ....................................................................................................9
2.7 Co-morbid Condition Measures ...................................................................................11
SECTION 3 MULTIVARIATE RESULTS FOR LIKELIHOOD OF READMISSION
WITHIN 30 DAYS OF DISCHARGE .................................................................................15
3.1 Choosing among Potential Model Specifications ........................................................15
3.2 Logistic Model Results ................................................................................................15
3.3 The Excess Likelihood of Readmission Attributable to Three Hospital-
Acquired Conditions ....................................................................................................18
SECTION 4 DISCHARGE TO POST-ACUTE CARE SETTINGS ............................................21
4.1 Descriptive Statistics on Discharge Destination ..........................................................21
4.2 Relationship between Discharge Destination and the Likelihood of
Readmission .................................................................................................................22
4.3 Probability of Discharge to a Post-Acute Care Setting ................................................22
SECTION 5 SPECIAL STUDIES .................................................................................................27
5.1 Potential Under-Coding of Hospital-Acquired Conditions..........................................27
5.1.1 Introduction .........................................................................................................27
5.1.2 Data and Methods ...............................................................................................28
5.1.3 Correlation Between Hospital-Acquired Conditions Coded on Hospital
Claims and Hospital-Acquired-Related Conditions Coded on Physician
Claims .................................................................................................................30
5.1.4 Under-Coding of Hospital-Acquired Conditions and the Effect on
Readmission Rates ..............................................................................................35
5.2 Timing to Clinical Presentation of Selected Conditions ..............................................38
5.2.1 Time to Physician Diagnosis of Conditions During Hospitalization ..................38
5.2.2 Post-Discharge Diagnosis of Conditions in Inpatient Settings ...........................42
5.3 Post-Discharge Use of Outpatient Department Drugs for Infection Treatment ..........44
SECTION 6 SUMMARY AND CONCLUSIONS .......................................................................49
BIBLIOGRAPHY .........................................................................................................................51
vi
List of Tables
Table 2-1 The Yale co-morbid condition measures ................................................................. 11
Table 3-1 Multivariate regression estimates of the likelihood of a 30-day readmission for
selected hospital-acquired conditions (HAC) .......................................................... 17
Table 3-2 Excess likelihood of readmission for selected hospital-acquired conditions ........... 19
Table 4-1 Discharge destination for selected hospital-acquired conditions ............................. 21
Table 4-2 Relationship between discharge to a post-acute care (PAC) setting and the
likelihood of readmission for beneficiaries in the hospital-acquired condition and
comparison groups ................................................................................................... 22
Table 4-3 Multivariate regression models for the likelihood of discharge to a post-acute care
setting ....................................................................................................................... 23
Table 5-1 Frequency of hospital and physician coding of selected hospital-acquired
conditions ................................................................................................................. 32
Table 5-2a Readmission rates per 100 discharges for 7-day readmission window for selected
hospital-acquired conditions (HACs) identified from hospital and or physician
claims and for comparisons with discharges with no reported HAC ....................... 36
Table 5-2b Readmission rates per 100 discharges for 15-day readmission window for selected
hospital-acquired conditions (HACs) identified from hospital and or physician
claims and for comparisons with discharges with no reported HAC ....................... 36
Table 5-2c Readmission rates per 100 discharges for 30-day readmission window for selected
hospital-acquired conditions (HACs) identified from hospital and or physician
claims and for comparisons with discharges with no reported HAC ....................... 37
Table 5-3 Time to presentation of selected conditions from physician claims during initial
hospitalization, all claims with a physician diagnosis of selected hospital-acquired
conditions ................................................................................................................. 39
Table 5-4 Physician specialty from the first diagnosis of HAC-related condition, among all
hospital claims linked to a physician claim with a HAC-related diagnosis code .... 41
Table 5-5 Time to presentation of selected conditions on inpatient claims following index
hospital discharge with no hospital or physician diagnosis of selected hospital-
acquired conditions .................................................................................................. 43
Table 5-6 Outpatient department (OPD) drug claims within 30 days of hospital discharge, for
patients with and without a HAC ............................................................................. 46
1
SECTION 1
INTRODUCTION AND OVERVIEW OF RESEARCH APPROACH
1.1 Introduction and Findings from Previous Research
This report continues RTI’s analysis of the effects of the hospital-acquired conditions-
present on admission (HAC-POA) program on utilization, with a specific focus on readmissions.
The HAC-POA program was designed to improve the quality of inpatient care to Medicare
beneficiaries by providing a negative financial incentive, in which inpatient prospective payment
system (IPPS) cases can no longer be assigned to higher-paying MS-DRGs on the basis of
reasonably preventable complications or co-morbid (CC) conditions or major complications or
co-morbid (MCC) conditions that are acquired during the hospital stay. The reimbursement
effects are limited to the initial or index admission only. Thus, even though the hospital may not
receive a higher rate of payment for index admissions in which a HAC occurred under the HAC-
POA program, hospitals could receive additional payments from the Medicare program for care
provided during readmissions related to the hospital-acquired condition. A recent study has
found that an additional $103 million in payment would be withheld if Medicare expanded the
non-payment policy to HAC-related readmissions (see McNair and Luft, 2012).
Previous research has shown to varying degrees that the likelihood of readmission is
greater for patients who experience adverse events than for similar patients who have no such
adverse events (Ashton et al., 1997; Herwaldt et al., 2006; Encinosa and Hellinger, 2008;
Friedman et al., 2009; Friedman and Basu, 2004). Ashton and colleagues (1997) conducted a
meta-analysis of the relationship between early readmission rates (31 days) and inpatient
processes of care and concluded that substandard care was correlated with higher readmission
rates; patients who were readmitted for unplanned reasons were 55% more likely to have had
poor quality of care.
Encinosa and Hellinger (2008) studied the occurrence of seven categories of Agency for
Healthcare Research and Quality patient safety indicators (PSIs) among 161,004 privately
insured patients undergoing surgery. These seven groups of PSIs span the 10 HAC categories
included in the HAC-POA program. Excessive 90-day readmission rates, calculated as the
difference between the readmission rate estimated if all patients had the PSI and the readmission
rate estimated if none of the patients had the PSI, were found for four of the seven PSI groups:
infections (7.7%), pulmonary and vascular problems (3.4%), acute respiratory failure (4.3%),
and metabolic problems (6.3%).
Infections after surgical procedures are an important reason for early readmissions and
have been the focus of a number of recent studies. Herwaldt and colleagues (2006) studied
postoperative nosocomial infections associated with general, cardiothoracic, and neurosurgical
procedures in a large tertiary care medical center and associated VA hospital. They found that
the risk adjusted odds ratio of being readmitted within 30 days of surgery ranged across the three
surgical services from 2.15 to 5.62 for patients with a SSI compared with patients with no SSI.
Friedman and colleagues (2009) used an all payer data set of hospitalizations for surgical
procedures from seven states and found that the relative risk of readmission was higher for
patients experiencing at least one of nine PSIs. The unadjusted rate of 3-month readmission was
2
25% among patients with a positive PSI compared with 17% among those without a positive PSI.
Risk adjustment reduced the 3-month readmission rate differences yet the rates remained
statistically higher for patients with each of the nine PSIs.
The most recent literature points to a similar relationship between hospital-acquired
conditions and readmissions. Morris et al. (2011) considered unplanned 30-day same-hospital
readmissions among 1,808 surgical patients in an urban, tertiary hospital in FY 2009 and found
that deep vein thrombosis significantly increased the probability of a readmission, with an odds
ratio of 4.7. The reasons for readmission among these patients, however, did not seem to be
related to the deep vein thrombosis.
1.2 Summary of Phase II Findings on Readmissions and Potential Estimation Bias
RTI completed a descriptive analysis of the relationship between the Medicare hospital-
acquired conditions and readmissions earlier this year (see Kandilov et al., 2012). In that study,
we examined the rates and reasons for all-cause readmissions among all discharges in FY 2009
and the first 10 months of FY 2010 in which a HAC was coded by the hospital and the patient
was discharged alive. The rates of readmission varied considerably across the different HACs,
with the lowest readmission rate for deep vein thrombosis or pulmonary embolism (DVT/PE)
following certain orthopedic procedures and the highest readmission rate for blood
incompatibility and surgical site infection (SSI) of mediastinitis following a coronary artery
bypass graft (CABG) procedure. Readmission rates increased as the readmission window
expanded from 7 days to 60 days.
Between FY 2009 and FY 2010, we did not discover any large changes in the
readmission rates for any of the HACs, except for among the low-volume surgical site infections,
where fluctuations in the readmission rate from year to year likely have more to do with small
sample sizes than with actual changes in readmissions for this patient population. Septicemia
and pneumonia were among the most common primary diagnoses for readmission across many
of the HACs, and for the surgical site infections, post-operative infections were a common
reason for readmission. Comparing FY 2009 and FY 2010 data, we did not detect any
substantive changes in the reasons for readmission following the development of a HAC during
the initial hospitalization.
To address the incremental effect of a HAC on readmissions for falls and trauma,
vascular catheter-associated infections, and DVT/PE following certain orthopedic procedures,
we developed comparison groups for each of the three HACs using a random sample of
discharges matched to the HAC cases by key clinical and demographic characteristics. For all
three HACs, we found large and statistically significant differences in the readmission rates
between the HAC cases and the matched comparison groups. FY 2009 and FY 2010,
readmission rates were 3 to 6 percentage points higher for discharges with falls and trauma, 6 to
7 percentage points higher for discharges with a vascular catheter-associated infection, and 2 to 3
percentage points higher for discharges with a DVT/PE following certain orthopedic procedures.
Although we found that readmission rates vary by key patient criteria, such as age,
Medicaid status, disability status, and HCC scores, differences in readmission rates between
discharges with the HAC and its respective comparison group persisted across most of these
3
stratifications. For patients with a fall or trauma, readmissions remained significantly higher
than among the comparison group within all age groups, those with and without Medicaid, all
eligibility groups (aged, disabled, ESRD), both genders, within two racial groups (white and
other), within all levels of HCC score (low, medium, or high), and within those who were not
institutionalized. Significant differences in readmissions also remained when we stratified the
vascular catheter-associated infection patients and comparisons by these same categories, and
additionally there was a significant difference in readmissions among the black patients. For
HACs and comparisons in the DVT/PE group, the only patient characteristics where a significant
difference in readmissions did not persist were within patients over 85, those enrolled in
Medicaid, those with ESRD, those whose race was other, those who had medium or high HCC
scores, and those who were institutionalized.
The significant differences in readmission rates also persisted when we stratify by
important hospital characteristics such as Census division, urban location, teaching status, and
bed size. For falls and trauma, the rate of readmissions was significantly higher for the HAC
cases than for the control cases across all of these stratifications of hospital characteristics. For
vascular catheter-associated infections, readmission rates for HAC cases were also significantly
higher within all Census divisions, urban and rural hospitals, teaching and non-teaching
hospitals, and for hospitals with 100-299 beds and hospitals with 300 or more beds.
While the rates of readmission for the beneficiaries who acquired one of the three
conditions during their hospitalization were much higher than for comparison beneficiaries, we
found many of the same reasons for readmission for these two groups across our two years of
data. The primary exception was the “infection of a central venous catheter,” which was one of
the top five reasons for readmission among those with a hospital-acquired vascular catheter-
associated infection, while it was not among the top reasons for readmission among the
comparison group beneficiaries.
Finally, we created a separate study sample to conduct further investigations of
mediastinitis following coronary artery bypass graft (CABG) surgery. The number of
mediastinitis cases that were HACs is very small so we undertook this study to examine the
possible degree of under-reporting of mediastinitis during the hospital period or clinical
presentation of mediastinitis after discharge. The primary motivation for this study was to
examine the degree to which readmission estimation bias may exist due to identification errors in
the dependent variable because of either under-reporting of the HAC by the hospital or a delay in
clinical presentation until after discharge.
The mediastinitis study sample included all discharges with a CABG procedure in either
FY 2009 or FY 2010. We linked the MedPAR records for these discharges with all physician
claims billed during the admission and all physician and hospital outpatient department claims
for a 60-day follow-up period and explored the reporting of mediastinitis by physicians during
the hospitalization and follow-up periods.
Of the 195 cases of mediastinitis identified during the index hospitalization using a
hospital or physician diagnosis, 65% were coded only on the physician claims, and 21% were
coded only on the hospital claims. The rate of agreement between hospital and physician coding
of mediastinitis was poor, with only 14% of all mediastinitis cases identified in the hospital
4
coded on both hospital and physician claims. Most physician-reported diagnoses of mediastinitis
occurred between day 9 and day 23 following the CABG surgery. The first physician diagnosis
of mediastinitis was most likely to be made by an infectious disease specialist.
Overall, we found low rates of reporting of mediastinitis by physicians after discharge
from the hospital. However, the rate of observed interactions between the patient and their
primary surgeon post-discharge was extremely low; only 14 out of 149,395 Medicare
beneficiaries had a follow-up appointment within 30 days. This low number is likely a reflection
of the global billing payment policy. Thus, it would appear that the use of Medicare claims with
the global billing convention may not be an adequate source of information to conduct post-
discharge analyses for beneficiaries having major surgical procedures subject to the global
surgical payment policy.
1.3 Overview of Phase III Research Questions and Analytic Approach
The readmission multivariate analyses presented in this report represent an extension of
the descriptive analysis of readmissions that we completed in Phase II of the study. The Phase
III research questions that we address in this study are:
• Does the likelihood of readmission differ between cases that report HACs and similar
cases that do not report HACs?
• Does the likelihood of use of post-acute care (PAC) services differ between cases that
report HACs and similar cases that do not report HACs?
We use a two stage estimation strategy for readmissions. The first stage is the estimation
of a single period, mixed-effects level model where logistic regression is used to estimate the
likelihood of having a readmission within 30 days of discharge. A dichotomous variable is
included for the presence or absence of a HAC (1 = HAC; 0 = no HAC). We report the odds
ratio (OR) on the presence of a HAC from the logistic regressions along with an indication of the
level of statistical significance of the effect. Odds ratios greater than 1.0 indicate an increased
likelihood of a readmission; odds ratios less than 1.0 indicate a decreased likelihood of a
readmission.
In the second stage, we replicate the method of Encinosa and Hellinger (2008) and report
the excess likelihood of readmission associated with HAC status and its associated standard error
using the multivariate logistic regression models developed in the first stage. In general, the
strategy is to use the results of the logistic regression models to estimate the likelihood of
readmission assuming that all hospitalizations had an adverse event (HAC) and then estimate the
likelihood of readmission assuming that all hospitalizations had no adverse event (no HAC).
The difference between the two sets of predictions is the “excess” likelihood of readmission that
can be attributed to the HAC after controlling for hospital- and patient-level characteristics,
including co-morbidities.
To better understand the role of post-acute care services in observed readmission rates,
we also estimate the single-period, multilevel model on the likelihood of any post-acute care
services following the general estimation strategy for readmissions. We do not generate
5
estimates of the “excess” likelihood for PAC admissions, but do report the odds ratios from the
logistic regressions. We also provide descriptive statistics regarding the relationship between
two discharge destinations (PAC and home) and the likelihood of readmission. These analyses
consider this relationship for patients both with and without HACs present.
We also conduct a special study which is an extension of last year’s work to examine the
degree to which readmission estimation bias may exist due to identification errors in the
dependent variable because of either under-reporting of the HAC by the hospital or a delay in
clinical presentation until after discharge. The Phase III research questions that we address in
this special study are:
• What proportion of HAC cases are identified during the index hospitalization period
from physician claims and what is the degree of concordance with the MedPAR claim
diagnosis for these HACs?
• What is the typical timing to a physician diagnosis of a HAC-related condition during
a hospitalization? And what proportions of patients with a HAC and without a HAC-
related condition are diagnosed on a subsequent inpatient claim within 7, 15, or 30
days following hospital discharge?
• What evidence do we find of treatment for an infection among the outpatient
department drug claims for patients at-risk of developing a HAC-related infection
within 30 days following discharge?
To conduct further investigation of potential under-coding or post-discharge presentation
of the more frequent HACs, we conducted a series of descriptive analyses. To analyze the
potential degree of under-coding during the hospitalization, we compare the degree of
concordance in coding a HAC between hospital claims and linked physician claims for the
hospitalization period. We examined the timing between procedures that put patients at risk of
developing a HAC and physician diagnosis of the HAC-related conditions in the hospital, and we
also analyzed post-discharge presentation of the HAC-related conditions by linking hospital,
SNF, IRF, and LTCH claims in the 30-days following the index hospitalization to the index
hospital claim and reporting the 7-, 15-, and 30-day readmission rates for patients at risk of
developing the HAC during the hospitalization and for those that have a HAC reported. We do
not include physician or hospital outpatient department (OPD) claims in this analysis as the rate
of follow-up for patients with a major surgical procedure that puts them at risk of developing a
HAC is extremely low during the post-discharge period due to the Medicare global surgical
payment policy that does not allow physicians to routinely bill for services within 90 days of the
procedure that are related to follow-up care for the procedure. For the infection-related HACs,
we also linked outpatient department drug claims within a 30-day period of discharge and report
the percentage of beneficiaries who received antibiotics that would be appropriate for treatment
of a HAC-related infection.
1.4 Organization of Report
Section 2 of this report describes the study sample, data, and methods to answer the first
two research questions related to likelihood of readmission and likelihood of PAC transfer for
6
patients with a HAC and those at risk for development of a HAC but for whom no HAC is
recorded during the hospital stay. Section 3 provides results of the multivariate modeling of
readmission. Section 4 provides descriptive and multivariate analyses of likelihood of using
PAC services. Section 5 describes the study sample, data, methods, and presents the results of
the special study of potential under-coding and time to clinical presentation for selected HACs.
Section 6 presents a summary of the findings.
7
SECTION 2
TECHNICAL APPROACH
2.1 Study Sample and Data
For the analysis of readmissions among patients with hospital-acquired conditions
(HACs), we created our study sample by linking Medicare claims data to “index” HAC inpatient
prospective payment system (IPPS) hospital claims. These “index” claims were defined as
claims with the HAC-associated diagnoses coded as not present on admission (POA indicator =
“N” or “U”). The index HAC claims were taken from MedPAR files for FY 2009 and the first
ten months of FY 2010, to allow for a 60-day look-forward period. From these index HAC
claims, we used a cross-referenced beneficiary identifier (HIC number) to look back 180 days
prior to the index admission date in order to identify any Medicare claims (inpatient, outpatient,
home health, and physician claims) for that patient within that period. The claims data for the
look-backs came from FY 2008, FY 2009, and FY 2010, as needed. These look-back claims
were used to calculate concurrent Hierarchical Condition Category (HCC) indicators for these
patients, which were then used to generate indicators of pre-existing medical conditions as
described in Section 2.7. We then used the HIC number to look forward 30 days from the index
discharge date for additional hospital admissions. If a patient was discharged from their index
HAC hospitalization and admitted to another IPPS hospital within a day (with a discharge
designation of an acute care transfer), then the 60-day follow up period began with the discharge
date from that second transfer hospitalization.
The study sample was limited to beneficiaries who were residents of the U.S., who were
enrolled in Medicare Parts A & B, who did not have Medicare as a secondary payer, and who
were not enrolled in managed care during their HAC index claim, during the 180 days prior to
the index admission, and during the 30-day period following the index discharge. The sample
was also limited to patients who were discharged alive from their index hospitalization. These
exclusions allowed us to focus on Medicare patients with HACs who could possibly have a
readmission and whose readmission claims we would likely find using MedPAR claims data.
For example, if a Medicare beneficiary with an index HAC admission switched to Medicare
managed care during the 60-day follow-up period, any hospital readmissions they might have
had would not be present in the MedPAR claims data. Including these beneficiaries in the
sample could lead to an under-estimation of the readmission rates.
2.2 Defining Readmissions
For the statistics presented in this report, we use a measure of hospital all-cause
readmissions and include all admissions to acute care hospitals that occur within 30 days of the
index claim discharge date, regardless of the clinical reason for the admission. In addition to
IPPS hospitals, an admission to a critical access hospital (CAH) or to another non-IPPS hospital
that is paid under Medicare Part A (such as a Cancer hospital or a Children’s hospital) following
an index IPPS hospital discharge is considered a readmission. This measure of readmissions
does not include admissions to an inpatient rehabilitation facility (IRF) or to a long-term care
hospital (LTCH), which are included among our measure of post-acute care. Discharges from
the index hospitalization to another acute care IPPS hospital, where the index discharge date is
within one day of the next admission date and the discharge destination is a transfer, are treated
8
as transfer cases and so are not included as readmissions. The 30-day look-forward period
begins with the discharge date of the transfer hospitalization, if there is one.
2.3 Defining Discharges to PAC Settings
For the analyses of post-acute care utilization, we created a measure based on the
discharge destination variable in the MedPAR data. The following settings were included in our
PAC definition: skilled nursing facilities, organized home health service organizations,
intermediate care facilities, inpatient rehabilitation facilities, and long-term care hospitals.
2.4 Selection of the Three Study HACs
Based on our initial descriptive statistics produced for the Strategic Memo: Strategy to
Estimate Readmissions Due to Hospital-Acquired Conditions (HACs), we selected three HACs
from the current set of HACs for further analysis in this report. The primary criterion for our
selection was that the chosen HACs have a sufficient volume to estimate statistically reliable
descriptive statistics, allowing us to examine variation in readmission rates across beneficiary
characteristics. Using this criterion, we selected the following three HACs for the Phase II
report:
• Falls and trauma, with 7,954 HAC-associated live discharges in FY 2009 and the
first 10 months of FY 2010.
• Deep vein thrombosis or pulmonary embolism (DVT/PE) following certain
orthopedic procedures, with 4,195 HAC-associated live discharges in FY 2009 and
the first 10 months of FY 2010.
• Vascular catheter-associated infection with 5,167 HAC-associated live discharges in
FY 2009 and the first 10 months of FY 2010.
We continue to analyze these three HACs in this Phase III report.
2.5 Comparison Group Matching Criteria
To develop a valid comparison group we selected discharges based on a small set of
clinical or demographic characteristics held in common with the specific HAC cases, and then
used a larger set of covariates in the outcome regressions. Matching is a common technique
found among empirical studies on this topic. For the descriptive analysis in this report, we took
a multivariable matching approach. Multivariable matching uses a limited number of specific
characteristics and identifies controls that match on all of the variables.
To construct appropriate comparison groups for the three selected study HACs, we
matched each index claim identified with a HAC to 10 IPPS claims without a HAC but with the
same MS-DRG and demographic characteristics (sex, race, and age) as the HAC claim. In the
cases where a 10:1 match was not obtainable, we reweighted the matches that were made to
simulate a 10:1 match. Any claims with the HAC-associated diagnosis codes identified as
present on admission (POA indicator equal to “Y” or “W”) were excluded from the comparison
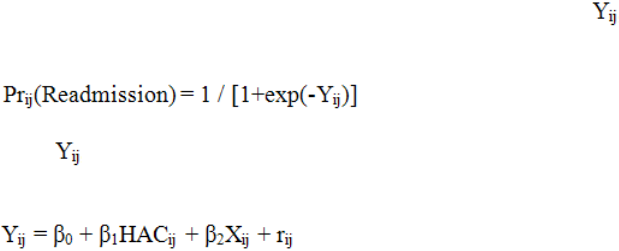
9
group, since conditions coded as present on admission could potentially be true HACs that were
miscoded. Including true HACs in the comparison group could introduce bias in our results.
Thus, the comparison group for each of the three HACs contained no index claims with the
specified HAC-associated diagnoses.
No additional restrictions were placed on the comparison group for the falls and trauma
HACs. For the DVT/PE following certain orthopedic procedures, the set of claims from which
the comparison group was drawn was further limited to those claims containing the orthopedic
procedure associated with this HAC. To better target the population who would be at risk for a
vascular catheter-associated infection, we limited this comparison group to index claims that had
one of two vascular catheter procedure codes (38.93 or 38.95). Note that among patients with
the vascular catheter-associated infection HAC, 38% did not have a vascular catheter procedure
code on their claims. The vascular catheter codes may have been coded after the fifth surgical
procedure code, and thus not picked up by the MedPAR data, or may have been left off of the
claim completely. Readmission rates were similar between the HAC claims that included the
vascular catheter procedure codes and those that did not include the codes.
From these index comparison claims, we linked additional claims data both before and
after the index comparison claim, as described in Section 2.1, in order to calculate readmission
rates and co-morbid conditions. The same sample exclusions – residents of the U.S., enrolled in
Medicare Parts A & B, Medicare not the secondary payer, and not enrolled in managed care –
were applied to the identified comparison groups to ensure analogous samples.
2.6 Multivariate Analyses
To estimate the impacts of each of the three study HACs on the likelihood of readmission
within 30 days, we estimated mixed effects (or multi-level) logistic models. The mixed effects
models are necessary due to the multi-level nature of the data being analyzed. The idea is to
control for both patient- or discharge-level covariates such as co-morbid conditions and age as
well as hospital-level covariates such as size (number of beds). Also, the discharges are
clustered within hospitals, so it is necessary to model this clustering.
The mixed effects logistic model is derived through using the logistic function to model
the probability of readmission based on the value of a latent variable , where i indexes
discharges within hospitals and j indexes hospitals.
The variable can be thought of as a function of HAC status as well as other patient- or
discharge-level characteristics (X) and an individual-level error term (r) as follows:
The mixed effects model is implemented by allowing the βs to vary across hospitals,
which constitute a second level of data. We considered three different specifications for
modeling the likelihood of readmission for each of the three HACs and the likelihood of
discharge to a PAC setting. The first was a random intercept model. The second was a random
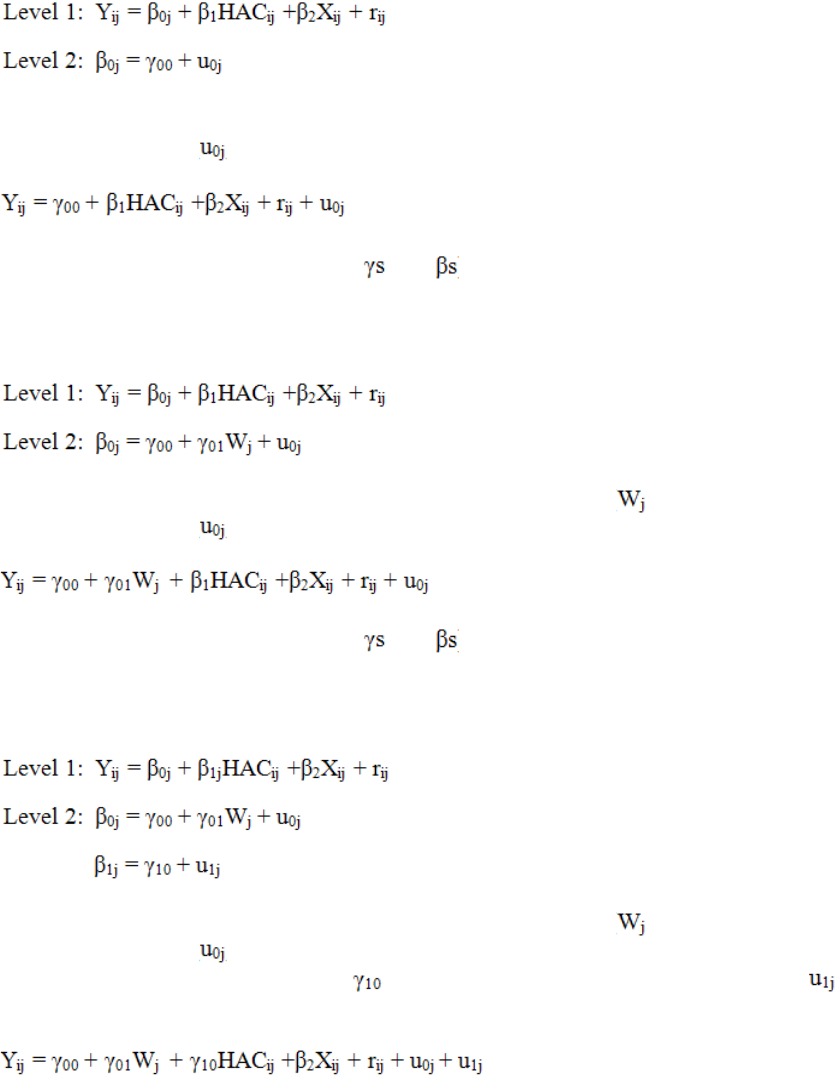
10
intercept model where we allowed the intercept to be a function of hospital-level covariates. The
third was a model, which built upon the second model by estimating a random effect for the
HAC indicator variable.
Model I
In this case the intercept term is equal to an average intercept across all hospitals and a hospital-
specific random error term ( ). The model may be re-written as one equation as follows:
This model is composed of fixed effects (the and ) and random effects (the random error
terms).
Model II
In this case the intercept term is a function of hospital-level covariates ( ) and a hospital-
specific random error term ( ). The model may be re-written as one equation as follows:
This model is composed of fixed effects (the and ) and random effects (the random error
terms).
Model III
In this case the intercept term is a function of hospital-level covariates ( ) and a hospital-
specific random error term ( ). The coefficient on the HAC indicator is equal to the average
value of the coefficient across all hospitals ( ) and a hospital-specific random error term ( ).
The model may be re-written as one equation as follows:
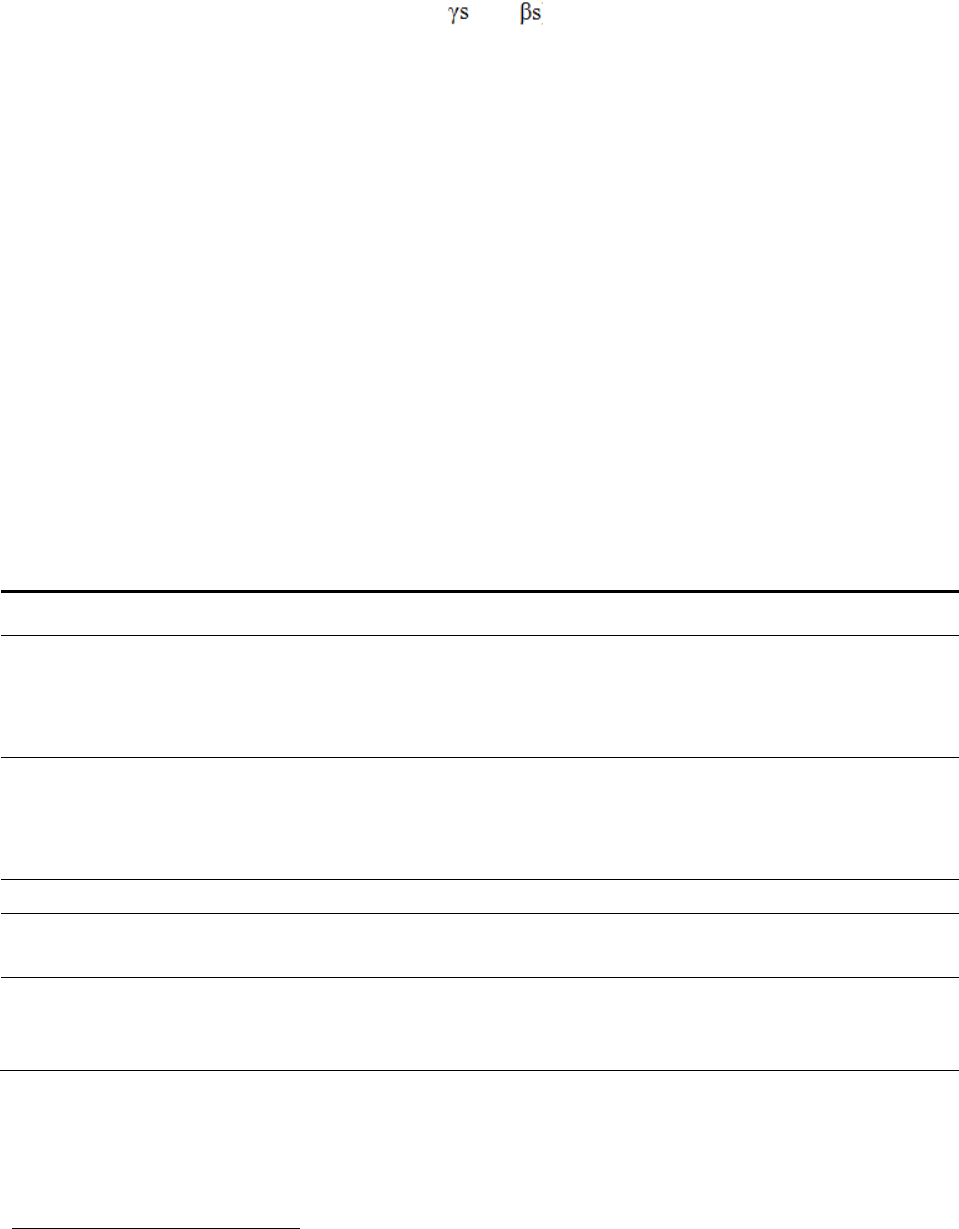
11
This model is composed of fixed effects (the and ) and random effects (the random error
terms).
In each of the specifications, we control for the following discharge- or patient-level
characteristics (the Xs): age, Medicaid enrollment, original eligibility status, gender, race,
institutional status, and several co-morbid conditions (we discuss the measures for co-morbid
conditions in Section 2.7). In the Models II and III, we control for the following hospital-level
characteristics (the Ws): whether the hospital is located in an urban area, number of beds, and
whether the hospital is an academic medical center (teaching hospital).
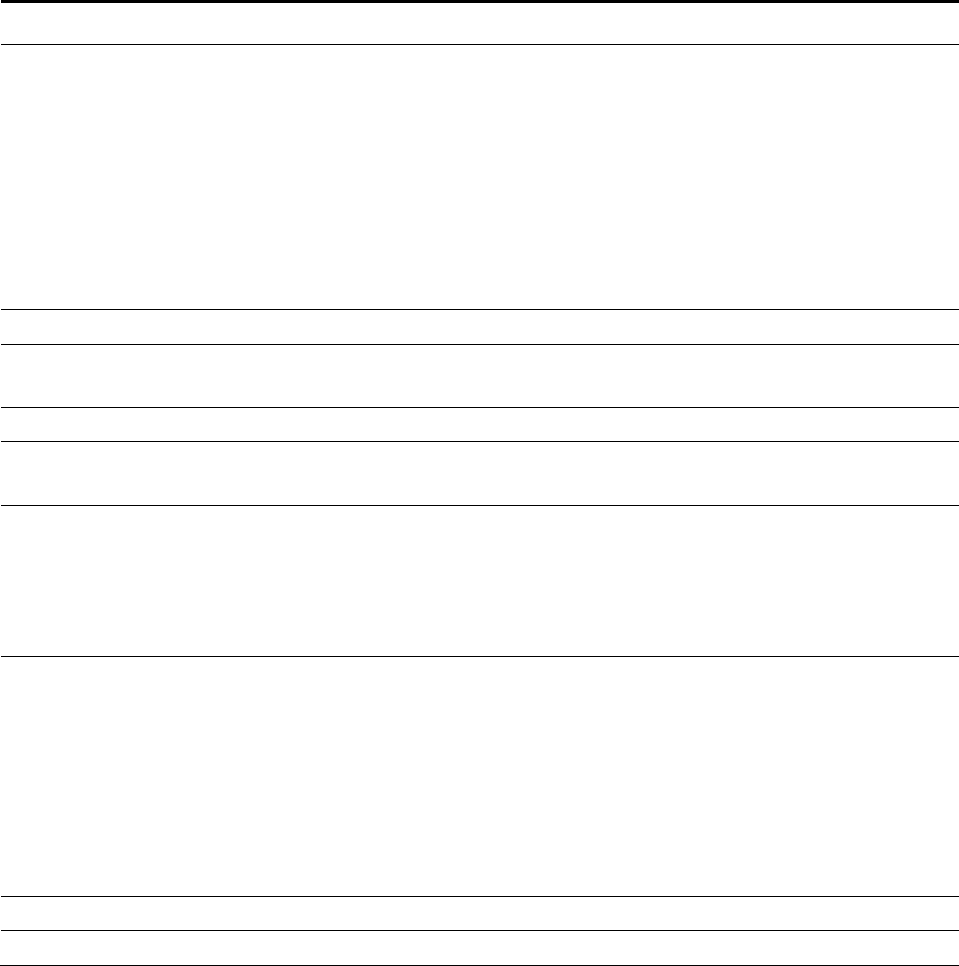
2.7 Co-morbid Condition Measures
To control for co-morbid conditions in our models, we included a series of indicator
variables suggested in a report to CMS by the Yale New Haven Health Services
Corporation/Center for Outcomes Research and Evaluation (referred to as Yale for the remainder
of this report). Based on several factors, the Yale team grouped CMS condition categories
(CMS-CCs) into a series of 31 co-morbid risk variables.
1
The grouping of the CMS-CCs into the
risk variables is presented in Table 2-1.
Table 2-1
The Yale co-morbid condition measures
Co-morbid condition measure CMS Co-morbid conditions included
Severe infection
1 HIV/AIDS
3 Central nervous system infection
4 Tuberculosis
5 Opportunistic infections
Other infectious disease
6 Other infectious disease
111 Aspiration and specified bacterial pneumonias
112 Pneumococcal pneumonia, emphysema, lung abscess
113 Viral and unspecified pneumonia, pleurisy
Metastatic cancer/acute leukemia
7 Metastatic cancer/acute leukemia
Severe cancer
8 Lung, upper digestive tract, and other severe cancers
9 Other major cancers
Other major cancers
10 Breast, prostate, colorectal and other cancers and tumors
11 Other respiratory and heart neoplasms
12 Other digestive and urinary neoplasms
(continued)
1
See pages 29-30 of Horwitz et al. (2011) for a fuller description of the rationale for the creation of the comorbid
risk variables.
12
Table 2-1 (continued)
The Yale co-morbid condition measures
Co-morbid condition measure CMS Co-morbid conditions included
Diabetes mellitus
15 Diabetes with renal manifestation
16 Diabetes with neurologic or peripheral circulatory
manifestation
17 Diabetes with acute complications x
18 Diabetes with ophthalmologic manifestation
19 Diabetes with no or unspecified complications
20 Type I diabetes mellitus
119 Proliferative diabetic retinopathy and vitreous hemorrhage
120 Diabetic and other vascular retinopathies
Protein-calorie malnutrition
21 Protein-calorie malnutrition
End-stage liver disease
25 End-Stage Liver Disease
26 Cirrhosis of Liver
Other hematological disorders
44 Other hematological disorders
Drug and alcohol disorders
51 Drug/alcohol psychosis
52 Drug/alcohol dependence
Psychiatric comorbidity
54 Schizophrenia
55 Major depressive, bipolar, and paranoid disorders
56 Reactive and unspecified psychosis
58 Depression
60 Other psychiatric disorders
Hemiplegia, paraplegia, paralysis
and functional disability
67 Quadriplegia, other extensive paralysis
68 Paraplegia
69 Spinal Cord Disorders/Injuries
100 Hemiplegia/hemiparesis
101 Diplegia (upper), monoplegia, and other paralytic syndromes
102 Speech, language, cognitive, perceptual
177 Amputation status, lower limb/amputation
178 Amputation status, upper limb
Seizure disorders and convulsions 74 Seizure disorders and convulsions
Congestive heart failure
80 Congestive heart failure
(continued)
13
Table 2-1 (continued)
The Yale co-morbid condition measures
Co-morbid condition measure CMS Co-morbid conditions included
Coronary atherosclerosis or angina,
cerebrovascular disease
81 Acute myocardial infarction
82 Unstable angina and other acute ischemic heart disease
83 Angina pectoris/old myocardial infarction
84 Coronary atherosclerosis/other chronic ischemic heart disease
89 Hypertensive heart and renal disease or encephalopathy
98 Cerebral atherosclerosis and aneurysm
99 Cerebrovascular disease, unspecified
103 Cerebrovascular disease late effects, unspecified
104 Vascular disease with complications
105 Vascular disease
106 Other circulatory disease
Specified arrhythmias
92 Specified heart arrhythmias
93 Other heart rhythm and conduction disorders
COPD
108 Chronic Obstructive Pulmonary Disease
Dialysis status
130 Dialysis status
Ulcers
148 Decubitus ulcer
149 Decubitus ulcer or chronic skin ulcer
Septicemia/shock
2 Septicemia/shock
Cardio-respiratory failure and shock 79 Cardio-respiratory failure and cardio-respiratory shock
Acute renal failure
131 Acute renal failure
Pancreatic disease
32 Pancreatic disease
Rheumatoid arthritis and
inflammatory connective tissue
disease
38 Rheumatoid arthritis and inflammatory connective tissue
disease
Respirator dependence
77 Respirator dependence/tracheostomy status
Transplants
128 Kidney transplant status
174 Major organ transplant status
Hip fracture/dislocation
158 Hip fracture/dislocation
SOURCE: Horwitz et al. (2011)
14
This page intentionally left blank.
15
SECTION 3
MULTIVARIATE RESULTS FOR LIKELIHOOD OF READMISSION WITHIN 30
DAYS OF DISCHARGE
3.1 Choosing among Potential Model Specifications
We estimated three separate specifications or models to estimate the impact of HAC
status on the likelihood of readmission, while controlling for patient and hospital characteristics.
In this section, we provide a rationale for the choice of final specification that we made for each
of the three HACs considered in this study. The results for the three specifications for each HAC
can be found in Appendix Tables 1 through 3.
Falls and Trauma. The result regarding the effect of having a HAC on the likelihood of
readmission within 30 days is quite consistent across all three specifications. This is also true for
the effect of the control variables. Including the hospital-level covariates has very little impact
on the coefficients for the patient- or discharge-level covariates, although at least some of the
hospital-level covariates are significant.
In all three models, the random effect on the intercept is significant. At the same time,
only roughly 7% of the total variance is determined to be due to differences in readmission rates
across hospitals. In Model III, the random effect on the HAC indicator is not significant and
neither is the covariance between the intercept and the HAC indicator. Allowing for the random
effect on the HAC indicator has no significant impact on the results. Based on these results, we
report the results from Model II for falls and trauma.
Vascular Catheter-Associated Infection. Again, the result regarding the effect of having
a HAC on the likelihood of readmission within 30 days is quite consistent across all three
specifications. This is also true for the effect on the control variables. Including the hospital-
level covariates has very little impact on the coefficients for the patient- or discharge-level
covariates, although at least some of the hospital-level covariates are significant.
In all three models, the random effect on the intercept is significant. At the same time,
only roughly 5% of the total variance is determined to be due to differences in readmission rates
across hospitals. In Model III, the random effect on the HAC indicator is not significant and
neither is the covariance between the intercept and the HAC indicator. Allowing for the random
effect on the HAC indicator has no significant impact on the results. Based on these results, we
report the results from Model II for vascular catheter-associated infection.
DVT/PE Following Certain Orthopedic Procedures. In this case, the result on the effect
of having a HAC is quite different when allowing for a random effect on the HAC indicator in
Model III. In addition, the random effect on the HAC indicator is quite significant. Based on the
significance of the random effect on the HAC indicator, we report the results from Model III for
DVT/PE.
3.2 Logistic Model Results
The results of the chosen mixed effects logistic model for each HAC are presented in
Table 3-1. The main finding is that the presence of each HAC has a significant positive impact
16
on the likelihood of readmission within 30 days. For the falls and trauma HAC and the DVT/PE
HAC, the presence of the HAC is associated with a 21 to 23% increase in the odds of being
readmitted within 30 days, respectively. The presence of the vascular catheter-associated
infection HAC has an even greater impact on the likelihood of readmission. It is associated with
a 33% increase in the odds of being readmitted within 30 days.
As far as the hospital-level covariates are concerned, larger hospitals tend to have higher
readmission rates. For instance, in the falls and trauma sample, the odds ratio for the largest
hospitals (those with 300 or more beds) is equal to 1.139, which indicates that the odds of a
readmission are 14% higher for patients from these larger hospitals than for patients from
hospital with fewer than 100 beds. The effect of hospital size is largest for patients in the
DVT/PE sample, or almost 40% higher. There is no association between bed size and likelihood
of readmission for patients at risk to develop a DVT or PE. Discharges from academic medical
centers are also associated with a higher likelihood of readmission for patients at risk of a fall or
trauma, 18%, and vascular catheter-associated infection, 22%. The results of the influence of
level of urbanicity are mixed. Urbanicity has no correlation with the likelihood of readmission in
the falls and trauma sample. At the same time, discharges from urban hospitals are associated
with a higher likelihood of readmission in the vascular catheter-associated infection sample,
10%, and with a lower likelihood of readmission in the DVT/PE sample, 12%.
The results on patient age are also mixed. In the falls and trauma and DVT/PE samples,
there is a positive relationship between age and the likelihood of readmission after controlling for
other factors, including co-morbidities. In the vascular catheter-associated infection sample,
there is to be a negative relationship. The effect of Medicaid enrollment is more consistent
across the three samples. In each case, Medicaid enrollment is associated with a greater
likelihood of readmission. In the DVT/PE sample, the odds of readmission is 35% higher for
Medicaid enrollees than for non-enrollees, while for the falls and trauma and vascular catheter-
associated infection samples, the odds of readmission among Medicaid enrollees are 20 and 15%
higher, respectively.
Among the discharges in the falls and trauma and DVT/PE samples, original Medicare
eligibility status and gender are important determinants of the likelihood of readmission.
Patients who initially became eligible for Medicare due either to disability or ESRD status have a
greater likelihood of readmission than patients who initially became eligible due to age. Women
generally have a lower likelihood of readmission than men. In the falls and trauma sample, the
odds of readmission were 9% lower for women than for men and in the DVT/PE sample, the
odds of readmission were more than 20% lower. Generally, race and institutional status have no
effect on the likelihood of readmission. Co-morbidities are very important determinants of the
likelihood of readmission. In each sample, at least one-half of the Yale co-morbidity measures
have significant odds ratios. In all cases where the odds ratios are significant, the odds ratio is
greater than one, indicating that the co-morbidities are associated with a greater likelihood of
readmission.

17
Table 3-1
Multivariate regression estimates of the likelihood of a 30-day readmission for selected
hospital-acquired conditions (HAC)
Variable
Falls and
trauma
(n=78,827)
Vascular
catheter-
associated
infection
(n=44,981)
DVT/PE
following
certain
orthopedic
procedures
(n=41,432)
HAC Indicator
1.214**
1.330**
1.229**
Hospital-Level Covariates
Urban
0.998
1.096*
0.875*
Number of beds (reference is “fewer than 100”
100-299
1.112**
1.028
1.376**
300 or more
1.139**
1.097
1.393**
Academic medical center
1.175**
1.218**
1.109
Discharge-Level Covariates
Age (reference is “less than 65”)
65-74
0.989
0.950
1.078
75-84
1.106*
0.893*
1.682**
85 and older
1.225**
0.779**
2.371**
Enrolled in Medicaid
1.197**
1.145**
1.346**
Original eligibility (reference is “aged”)
Disabled
1.092**
1.020
1.348**
ESRD
1.558**
1.080
2.091**
Gender: Female
0.910**
1.033
0.771**
Race (reference is “white”)
Black
0.960
0.960
0.984
Asian
0.891
1.018
0.876
Other
0.915
0.952
0.915
Institutionalized
0.778
0.823
0.400
Yale Comorbidity Measures
Severe infection
1.089
1.222*
0.510
Other infectious disease
1.142*
1.059
1.122
Metastatic cancer/acute leukemia
1.286**
1.260**
1.662*
Severe cancer
1.235**
0.993
1.226
Other major cancers
1.039
0.967
0.876
Diabetes mellitus
1.182**
1.134**
1.132*
Protein-calorie malnutrition
1.152**
0.987
1.503**
End-stage liver disease
1.368**
0.953
2.346**
Other hematological disorders
1.404**
1.160**
1.099
Drug and alcohol disorders
1.410**
1.116
1.789**
Psychiatric comorbidity
1.121*
1.185**
1.148
Hemiplegia, paraplegia, paralysis and functional disability
1.175**
1.027
1.124
Seizure disorders and convulsions
1.100
1.121*
1.674**
Congestive heart failure
1.331**
1.113**
1.353**
Coronary atherosclerosis or angina, cerebrovascular Disease
1.147**
1.076*
1.222**
Specified arrhythmias
1.120**
1.053
1.101
COPD
1.328**
1.072*
1.381**
(continued)
18
Table 3-1 (continued)
Multivariate regression estimates of the likelihood of a 30-day readmission for selected
hospital-acquired conditions (HAC)
Variable
Falls and
trauma
(n=78,827)
Vascular
catheter-
associated
infection
(n=44,981)
DVT/PE
following
certain
orthopedic
procedures
(n=41,432)
Dialysis status
1.242**
1.181**
0.997
Ulcers
1.138**
0.973
1.389*
Septicemia/shock
1.127**
1.147**
1.282
Cardio-respiratory failure and shock
1.057
1.068
1.017
Acute renal failure
1.342**
1.230**
1.657**
Pancreatic disease
1.254**
1.217**
1.427
Rheumatoid arthritis and inflammatory connective tissue disease
1.178**
1.127**
1.314**
Respirator dependence
1.497**
1.074
5.516**
Transplants
1.560**
1.152
0.794
Hip fracture/dislocation
0.938
0.915
1.330**
NOTES:
* indicates statistically significant difference using negative binomial regression with p<0.05.
**indicates statistically significant difference using negative binomial regression with p<0.01.
SOURCE: falls_re_readmt_models.log, vcath_centered_xtmelogitJun20_2012.log,
dvt_centered_xtmelogitJun20_2012.log
3.3 The Excess Likelihood of Readmission Attributable to Three Hospital-Acquired
Conditions
In Table 3-2, we present our multivariate regression results on the excess likelihood of
readmission attributable to three selected HACs. We generated the excess likelihood by using
the results of the logistic models to estimate the likelihood of readmission assuming that all
hospitalizations had an adverse event (HAC) and then to estimate the likelihood of readmission
assuming that all hospitalizations had no adverse event (no HAC). The difference between the
two sets of predictions is the “excess” likelihood of readmission that can be attributed to the
HAC after controlling for patient and hospital characteristics. We find that the falls and trauma
HAC leads to an excess likelihood of readmission of 2.9 percentage points while the vascular
catheter-associated infection HAC leads to an excess likelihood of readmission of 5.6 percentage
points and the DVT/PE HAC leads to an excess likelihood of readmission of 1.8 percentage
points. All of these results are statistically significant.
19
Table 3-2
Excess likelihood of readmission for selected hospital-acquired conditions
Hospital-acquired condition Excess likelihood Standard error
Falls and trauma
2.9%
0.5%
Vascular catheter-associated infection
5.6%
0.7%
DVT/PE following certain orthopedic procedures
1.8%
0.7%
NOTES: DVT/PE = Deep vein thrombosis or pulmonary embolism
SOURCE: DVT_predicted.xlsx, Vcath_predicted.xlsx, fall_readmt_predict.log
20
This page intentionally left blank.
21
SECTION 4
DISCHARGE TO POST-ACUTE CARE SETTINGS
4.1 Descriptive Statistics on Discharge Destination
Table 4-1 presents descriptive statistics on the discharge destinations of patients in the
HAC group and the comparison group. In the falls and trauma sample, 76.9% of the patients
with the HAC were discharged to one of the PAC settings, while 57.4% of the patients in the
control group were discharged to a PAC setting (statistically significant difference). On the
other hand, patients with the HAC were significantly less likely to be discharged to home—
13.7% compared to 33.7% among the controls. There is no significant difference in the
likelihood of discharge to other settings between those patients with the falls and trauma HAC
and the comparison group.
Table 4-1
Discharge destination for selected hospital-acquired conditions
Hospital-acquired condition HAC group
Comparison
group
Difference
Falls and trauma
Discharged to PAC setting
76.9%
57.4% 19.5%**
Discharged home 13.7%
33.7%
-20.0%**
Other 9.4%
8.9%
0.5%
Vascular catheter-associated infection
Discharged to PAC setting 67.9% 53.2% 14.7%**
Discharged home 25.0%
24.9%
0.1%
Other 7.1%
21.9%
-14.8%**
DVT/PE following certain orthopedic procedures
Discharged to PAC setting
88.0%
81.8% 6.2%**
Discharge home 8.5%
13.9%
-5.4%**
Other 3.5%
4.3%
-0.8%**
NOTES: HAC = Hospital-acquired condition; PAC = Post-acute care; DVT/PE = Deep vein thrombosis
or pulmonary embolism
**indicates statistically significant difference using negative binomial regression with p<0.01.
SOURCE: falls_pac_descriptive.log, vcath_pac_descriptive.log, dvt_pac_descriptive.log
In the vascular catheter-associated infection sample, 67.9% of the patients with the HAC
were discharged to a PAC setting, compared with 53.2% of the patients in the control group
(statistically significant difference) Patients with the HAC were discharged home at the same
rate as patients in the control group, leaving a significant difference in the rate of discharge to
other settings.
In the DVT/PE sample, there were significant differences between the HAC group and
the comparison group for all discharge destinations, with the HAC group more likely to be

22
discharged to a PAC setting (88.0% vs. 81.8%) and less likely to be discharged home (8.5% vs.
13.9%).
4.2 Relationship between Discharge Destination and the Likelihood of Readmission
Table 4-2 illustrates the relationship between the likelihood of readmission and whether
the patient was discharged to a PAC setting for patients in the HAC and comparison groups. The
results indicate that patients discharged to PAC settings are more likely to be readmitted within
30 days than those patients who were not discharged to PAC settings.
Table 4-2
Relationship between discharge to a post-acute care (PAC) setting and the likelihood of
readmission for beneficiaries in the hospital-acquired condition and comparison groups
Hospital-acquired condition
Likelihood of
readmission
:
HAC group
Likelihood of
readmission
:
Comparison group
Falls and trauma
Discharged to PAC setting 23.7%
19.8%
Not discharged to PAC 19.7%
14.5%
Vascular catheter-associated infection
Discharged to PAC setting 32.0% 30.0%
Not discharged to PAC 28.0%
16.6%
DVT/PE following certain orthopedic procedures
Discharged to PAC setting 12.8%
9.8%
Not discharged to PAC 6.8%
5.2%
NOTES: HAC = Hospital-acquired condition; PAC = Post-acute care; DVT/PE = Deep vein thrombosis
or pulmonary embolism
**indicates statistically significant difference using negative binomial regression with p<0.01.
SOURCE: falls_pac_descriptive.log, vcath_pac_descriptive.log, dvt_pac_descriptive.log
4.3 Probability of Discharge to a Post-Acute Care Setting
We estimated a series of mixed effect logistic models to predict the probability of
discharge to a PAC setting. As we did for readmissions, we estimated each of the three models
described in Section 2.6 for each of the HACs. Based on the results of these models, we present
the results of Model II for falls and trauma and vascular catheter-associated infection. We do
this, because the random effect on the HAC indicator is insignificant for each of these HACs.
We present the results of Model III for the DVT/PE sample, due to the fact that the random
effect on the HAC indicator is significant for this HAC. The model results are reported in
Table 4-3.

23
Table 4-3
Multivariate regression models for the likelihood of discharge to a post-acute care setting
Variable
Falls and
trauma
(n=78,827)
Vascular
catheter-
associated
infection
(n=44,981)
DVT/PE
following
certain
orthopedic
procedures
(n=41,432)
HAC Indicator
2.668**
1.943**
1.393**
Hospital-Level Covariates
Urban
1.311**
1.390**
1.891**
Number of beds (reference is “fewer than 100”
100-299
1.320**
1.093
2.226**
300 or more
1.297**
1.042
2.585**
Academic medical center
0.858**
0.894*
1.110
Discharge-Level Covariates
Age (reference is “less than 65”)
65-74
1.570**
1.453**
1.363**
75-84
2.074**
1.925**
2.550**
85 and older
2.630**
1.968**
3.009**
Enrolled in Medicaid
1.175**
1.180**
1.199**
Original eligibility (reference is “aged”)
Disabled
1.107**
1.144**
1.351**
ESRD
0.711**
0.740**
1.625
Gender: Female
1.368**
1.145**
1.533**
Race (reference is “white”)
Black
1.229**
0.948
1.321**
Asian
0.584**
0.925
1.249
Other
1.011
0.809**
1.032
Institutionalized
1.333
0.881
0.293
Yale Comorbidity Measures
Severe infection
0.907
0.962
1.141
Other infectious disease
1.220**
1.081
0.810
Metastatic cancer/acute leukemia
0.701**
0.618**
0.833
Severe cancer
0.702**
0.835**
0.840
Other major cancers
0.912**
1.102*
1.074
Diabetes mellitus
1.105**
1.098**
1.149**
Protein-calorie malnutrition
1.276**
1.173**
1.512
End-stage liver disease
0.932
0.844*
1.068
Other hematological disorders
0.825**
0.537**
2.084*
Drug and alcohol disorders
0.852*
0.870*
1.616
Psychiatric comorbidity
0.938
1.061
1.405*
Hemiplegia, paraplegia, paralysis and functional disability
1.310**
1.564**
1.093
Seizure disorders and convulsions
1.055
1.070
1.303
Congestive heart failure
0.913**
0.944*
0.907
Coronary atherosclerosis or angina, cerebrovascular disease
0.956*
1.022
0.904
Specified arrhythmias
1.023
1.029
1.044
COPD
0.924**
1.026
1.237**
(continued)
24
Table 4-3 (continued)
Multivariate regression models for the likelihood of discharge to a post-acute care setting
Variable
Falls and
trauma
(n=78,827)
Vascular
catheter-
associated
infection
(n=44,981)
DVT/PE
following
certain
orthopedic
procedures
(n=41,432)
Dialysis status
0.796**
0.740**
0.756
Ulcers
1.682**
1.360**
1.039
Septicemia/shock
1.328**
1.227**
1.182
Cardio-respiratory failure and shock
1.064
1.173**
0.856
Acute renal failure
1.018
0.976
1.142
Pancreatic disease
0.751**
0.796**
0.947
Rheumatoid arthritis and inflammatory connective tissue disease
1.137**
0.961
1.230*
Respirator dependence
1.585**
1.333**
1.369
Transplants
0.916
0.740**
1.047
Hip fracture/dislocation
2.395**
1.527**
1.299*
NOTES:
* indicates statistically significant difference using negative binomial regression with p<0.05.
**indicates statistically significant difference using negative binomial regression with p<0.01.
SOURCE: falls_pac_re_models.log, vcath_pac_re_models.log, DVT melogit Jun2012 Req2.log
The main finding is that the presence of each HAC is associated with a greater likelihood
of discharge to a PAC setting. The odds of being discharge to a PAC setting is 2.7 times greater
for patients with the falls and trauma HAC, two times greater for patients with the vascular
catheter-associated infection HAC, and 40% greater for patients with the DVT/PE HAC than for
similar patients without the HACs.
Among the hospital-level covariates, we find that patients discharged from teaching
hospitals are generally less likely to be discharged to a PAC setting, while patients from medium
and large hospitals (those with at least 100 beds) are more likely to be discharged to a PAC
setting than patients from smaller hospitals (those with fewer than 100 beds). Patients from
urban hospitals are more likely to be discharged to a PAC setting than patients from rural
hospitals.
Among the discharge-level covariates, we find that women are more likely to be
discharged to a PAC setting than men and that there is a positive relationship between age and
the likelihood of being discharged to a PAC setting. Medicaid enrollees are more likely to be
discharged to PAC settings than non-enrollees, while patients who were initially eligible for
Medicare due to disability are more likely to be discharged to a PAC setting than patients who
became eligible due to age. Interestingly, patients with a history of ESRD are less likely to be
discharged to a PAC setting, at least in two of the samples (falls and trauma and vascular
catheter-associated infection), although these sample sizes are quite small, 126 and 316 in the
HAC groups, respectively.
25
The results on the co-morbidity measures are mixed. Several of the measures that are
significant are associated with a greater likelihood of PAC admission, but about half are
associated with a smaller likelihood of PAC admission. It is likely that these co-morbidities are
related to discharge to other inpatient settings that are not included in our PAC measure.
26
This page intentionally left blank.

27
SECTION 5
SPECIAL STUDIES
5.1 Potential Under-Coding of Hospital-Acquired Conditions
5.1.1 Introduction
In the Phase II report for this task, “Readmissions Due to Hospital-acquired Conditions
(HACs),” we developed a mathematical model of readmission estimation bias that can occur
when there is error in the measurement of hospital-acquired conditions, particularly when HACs
are not reported. This can occur when clinical manifestation of the HAC occurs after the initial
hospital discharge, such as for a SSI, or under-reporting by hospital staff. In this section, we
focus on under-reporting of HACs on hospital claims, and in Section 5.2, we look more closely
at post-discharge presentation of HAC-related conditions and post-discharge treatment that could
be an indicator of a HAC-related condition.
The model demonstrates how one hospital can have a higher reported HAC rate if (a) it
has more infections in general than average, and/or (b) if it has a higher likelihood of reporting
its HACs. Conversely, a hospital with a lower-than-average HAC rate may truly have fewer
HACs than other hospitals, or it may be under-reporting the incidence of HACs. Thus, two
hospitals may have the same reported HAC rates but different readmission rates per admission
leading to little correlation between the presence of a hospital-acquired condition and the
likelihood of a readmission. It is also possible that one hospital has a lower reported HAC rate
yet has a higher true infection readmission rate. The paradox is explained by the fact that the
HAC rate calculated from claims data reflects two factors: the hospital’s true, overall, HAC rate
(once unreported, post-discharge, infections are accounted for) as well as the hospital’s rate at
which it reports HACs. The latter term may be both positive and negative; thus, an ambiguous
net effect on the overall readmission rate. The reported or coded HAC rate can also vary
positively or negatively with hospitals’ overall infection rate. Thus, it is possible that a hospital
has a high reported infection rate of all infections but a low readmission rate, thereby producing
a zero correlation of reported HAC rates with readmission rates.
Model Implications. Conceptually, we would expect that the relationship between HAC
rates and readmission rates to be positive; a HAC worsens a patient’s health and could require
multiple hospitalizations to treat. However, the “observed HAC” measure is imperfectly
sensitive by failing to capture all true HACs. As a result, the observed relationship between HAC
rates and readmission rates will not match the true relationship.
If the sensitivity is unrelated to the readmission rate and does not vary across providers,
then this situation is analogous to the classic errors-in-variables regression problem, and the
correlation between observed HAC rates and readmission rates will be lower than the true
correlation. This biases the reported HAC coefficient in any readmission model towards zero,
producing an under-estimate of the effect of true HACs on readmissions.
However, it is quite likely that the sensitivity of the observed HAC measure does vary
systematically across providers (and type of HAC). To see this, consider two hospitals which
differ only in their length of stay. One hospital tends to discharge patients as quickly as possible,
whereas the second hospital tends to permit patients to stay in the hospital longer. In this
28
hypothetical situation, we assume that the procedure infection rates and other aspects of
underlying quality are identical but only the lengths of stay differ. In the early-discharge
hospital, the infection may not be identified until after the patient is discharged. The inpatient
HAC rate for this hospital will be low, but the readmission rate will be high. In contrast, in the
second hospital, since the underlying length of stay is longer, the HAC may be identified and
treated in the hospital prior to discharge (even further lengthening that patient’s stay length).
Assuming the patient is discharged with the HAC fully treated, no readmission would be
necessary. Thus, the second hospital’s reported inpatient HAC rate will be high, but its
readmission rate will be low.
This confounding relationship between observed inpatient HAC rates and readmission
rates is due to the fact that hospitals vary on two dimensions. First, hospitals vary in their true
HAC rates because of differences in their quality of care. Second, hospitals will vary in their
lengths of stay (or any other factor that would impair the sensitivity of the HAC measure). To
counteract the confounding length of stay effect, one option must be to extend the time window
for measuring (recording) HACs into the post-discharge period. Using readmissions to enhance
the measure of true HAC rates can significantly improve the sensitivity of the initial HAC
measure and produce a higher, more accurate estimate of the HAC-readmission link. Care must
be taken, however, in inferring a HAC when using readmission data. Infections not acquired
during the earlier admission will likely be picked up in using readmission data and make the
measure somewhat less specific. Readmission data will also be imperfect to the extent that
infections and other late-appearing HACs are treated in an ambulatory setting without a
subsequent readmission. The modeling suggests taking a careful look at the complex
relationship between a very imperfectly measured estimate of hospital-acquired conditions and
any subsequent readmission rates. The shorter the window, the greater the likelihood that a HAC
had gone unreported during the earlier hospitalization. It also calls for using non-readmission
claims to track ambulatory follow-up of HACs (e.g., physician and outpatient department bills).
5.1.2 Data and Methods
From the twelve hospital-acquired conditions included in the HAC-POA payment policy,
we selected seven for this analysis. Foreign object retained after surgery, air embolism, and blood
incompatibility were excluded due to their relative infrequency in the hospital claims data. For the
remaining HACs – pressure ulcer stage III and IV, falls and trauma, catheter-associated urinary
tract infection, vascular catheter-associated infection, manifestations of poor glycemic control,
surgical site infections (included mediastinitis following CABG, SSI following certain orthopedic
procedures, and SSI following bariatric surgery for obesity), and DVT/PE following certain
orthopedic procedures – we constructed an episode of care file containing all IPPS hospitalizations
in FY 2009 and FY 2010 with at least one of these conditions coded as hospital-acquired (POA
indicator equal to “N” or “U”). In order to allow for a 30-day follow-up period after the initial (or
“index”) hospitalization, we excluded IPPS discharges that occurred on or after September 1, 2010
(approximately 4% of the initial sample).
For each of these HACs, we selected a comparison sample of IPPS hospitalizations that 1)
did not have any of these HACs coded on the hospital claim, 2) did not have any of these HAC-
related conditions coded as present on admission (POA indicators equal to “Y” or “W”), and 3) did
not have any of these HAC-related diagnosis codes as the primary diagnosis on the claim. Since
29
all hospital patients are potentially at risk for hospital-acquired pressure ulcer or falls and trauma,
we selected a 5% sample of all of the claims that met the above criteria. To look for evidence of
the manifestations of poor glycemic control HAC, we used a comparison group of 5% of IPPS
claims that had a principal diagnosis of diabetes (ICD-9_CM diagnosis codes 250.00 – 250.99),
since diabetic patients would be those at most at risk for poor glycemic control.
The remaining HACs, being specific to particular procedures or surgeries, had comparison
groups selected based on the presence of ICD-9_CM procedure codes on the hospital claim. To
check for under-coding of catheter-associated UTI, we selected all IPPS claims with a urinary
catheter procedure code (57.94 or 57.95); note that this procedure code greatly under-estimates the
actual rate of urinary catheters among hospital patients, as evidenced by the fact that only 5% of
IPPS claims with a catheter-associated UTI have one of the urinary catheter procedure codes. We
looked for evidence of vascular catheter-associated infections among a 25% sample of IPPS index
claims that had a vascular catheter ICD-9_CM procedure code (38.93 or 38.95). For the three SSIs
considered, we used the entire set of claims with the specified surgical procedures as our
comparison sample, and for DVT/PE following certain orthopedic procedures, we took a 50%
sample of all total hip replacement and total knee replacement surgeries to use for the population at
risk for a DVT/PE. The sampling that we chose allowed us to significantly reduce computational
time of these analyses while maintaining large enough comparison samples to find evidence of
HAC under-coding. When presented in the following tables and descriptions, all samples have
been adjusted to reflect 100% of the population; for example, any frequencies for the comparison
sample of diabetic patients have been multiplied by 20 to reflect their size in the entire at-risk
population in the FY 2009 and FY 2010 MedPAR files.
After selecting the IPPS index claims with HACs and the at-risk comparison samples for
each of the HACs, we then used the unique beneficiary identifiers and admission and discharge
dates on the index hospital claims to link physician claims that occurred during the index
hospitalization to the hospital claim. Approximately 2% of index hospital claims had no linked
physician claims. For index hospital claims that were linked to physician claims, we examined the
reported diagnosis codes on both types of claims to look for evidence of HAC-related conditions.
We also looked forward 30 days from the hospital discharge date to identify any hospital
readmissions that occurred for the beneficiaries in our sample, in order to examine readmission
rates for those with and without hospital- and physician-identified HAC-related conditions. This
analysis was limited to the hospital-acquired infections (catheter-associated UTI, vascular
associated-catheter infections, and SSIs) and DVT/PE following certain orthopedic procedures.
For our analysis of post-discharge clinical presentation of infections and DVT/PE, we
linked inpatient and outpatient Medicare claims – hospital, SNF, IRF, LTCH, outpatient, and
physician claims – that occurred within 30 days of the index hospital discharge—and report
frequency of post-discharge reporting of the HAC-related conditions and rates of readmission
within 30 days. We restrict the reporting to only institutional claims. Using the outpatient claims,
we further examined claims with HCPCS codes for antibiotic administration, to examine post-
discharge treatment consistent with HAC-related infections.

30
5.1.3 Correlation Between Hospital-Acquired Conditions Coded on Hospital
Claims and Hospital-Acquired-Related Conditions Coded on Physician
Claims
One way in which to determine the potential extent of under-coding of HACs on hospital
claims is to compare the diagnosis codes on the hospital claims with the diagnosis codes on
physician claims that were billed for the same patient during the hospitalization. In Table 5-1,
we show the frequencies of the HAC diagnosis codes on the hospital and the physician claims,
for both the HAC populations and the at-risk comparison samples. In the first row, we present
the number of index claims that had neither a hospital nor a physician diagnosis code of a HAC-
related condition. The vast majority of claims in our analysis fall into this category. The second
row shows, for each of the HACs in the analysis, the number of claims where the HAC is not
coded on the index MedPAR hospital claim, but at least one physician claim linked to the index
hospital claim has one of the HAC-related diagnosis codes. This is our set of potentially under-
reported HACs.
Some of these HAC-related conditions identified on the physician claims but not on the
hospital claim may have actually been coded on the hospital claim, but not picked up by the
MedPAR file and used for determining payment. Prior to January 2012, hospitals were not
required to submit claims using the 5010 electronic format, which captures up to 24 secondary
diagnosis codes. The older system captured only 8 secondary diagnosis codes. So while the
HAC-related diagnosis code may have actually been reported on the hospital claim, for the
purposes of analysis using pre-2012 hospital claims data, these HACs go unreported.
Appendix Table 4 provides some evidence that HAC diagnosis codes were reported after
the eighth secondary diagnosis code on the hospital claims. In this table, we show the rates of
HAC coding in FY 2010, when only the first 8 secondary diagnosis codes on the claim were
recorded, compared to the rates of HAC coding in FY 2011 for those hospitals that began using
the 5010 electronic format earlier than January 2012 and could report up to 24 secondary
diagnosis codes. We found that hospitals using the 5010 electronic format represented about
94% of claims in FY 2011. As we would expect, for almost all of the HACs,
2
pressure ulcer
stages III and IV, catheter associated urinary tract infection(CAUTI), vascular catheter
associated infection(CLABSI), surgical site infections(SSIs), mediastinitis, following coronary
artery bypass graft surgery, following certain orthopedic procedures, following bariatric surgery
for obesity, and manifestations of poor glycemic control, the HAC rates increase when more
diagnosis codes are used in the FY 2011 data. For example, see the Number of Discharges
Identified as a HAC per Thousand for pressure ulcers stage III and IV. In FY 2010, there were
0.14 discharges per thousand with a pressure ulcer HAC, while in FY 2011, among the claims
where up to 24 secondary diagnosis codes could be reported, the rate increased to 0.20.
2
Interestingly, HAC rates fall for both falls and trauma and DVT/PE following certain orthopedic procedures
between FY 2010 and FY 2011, despite the fact that more diagnosis codes are used in FY 2011. This could
reflect a significant, system-wide reduction in these HACs that is larger than the effect of the increased number
of diagnosis codes. It could also reflect that these HACs have a greater likelihood of facing a payment penalty,
and thus there is a greater incentive for under-reporting of these HACs. From Appendix 4, we see that more than
one quarter of claims with a falls and trauma HAC face a payment penalty, and more than 40% of DVT/PE HAC
claims face a payment penalty.
31
Therefore, some of the under-reporting we see in Table 5-1 is likely due to the limitation of 8
secondary diagnosis codes.

32
Table 5-1
Frequency of hospital and physician coding of selected hospital-acquired conditions
Hospital
Diagnosis
of HAC
Physician
Diagnosis
of HAC
Pressure
Ulcer –
Stages III &
IV
1
Falls and
Trauma
2
Catheter-
Associated
Urinary
Tract
Infection
(CAUTI)
3
Vascular
Catheter -
Associated
Infection
(CLABSI)
4
Manifestation
of Poor
Glycemic
Control
5
Surgical Site
Infection
(SSI),
Mediastinitis
following
Coronary
Artery
Bypass Graft
(CABG)
6
Surgical
Site
Infection
(SSI)
following
Certain
Orthopedic
Procedures
7
Surgical
Site
Infection
Following
Bariatric
Surgery
for
Obesity
8
Deep Vein
Thrombosis
or
Pulmonary
Embolism
(DVT/PE)
Following
Certain
Orthopedic
Procedures
9
No No 17,742
,320 17,392,660 151,795 1,365,324 4,683,940 156,114 203,780 29,679 673,928
No Yes 1,720 3
5,380 117 4,148 14,340 126 849 163 3,622
Yes No 2,508 3,
103 6,175 6,534 655 41 227 24 1,511
Yes Yes 60 7,064 81 4
88 226 28 151 8 3,740
— Tota
l 17,746,608 17,754,207 158,168 1,376,494 4,699,161 156,309 205,007 29,874 682,801
NOTES:
1
2.6% of MedPAR index admissions with no hospital diagnosis of pressure ulcer, and 2.2% of admissions with a pressure ulcer HAC, were excluded because
they had no linked physician claims during the hospitalization period. 5% sample of hospital claims without the HAC multiplied by 20 to estimate the full
population.
2
Includes fracture, dislocation, intracranial injury, crushing injury, burn, and other injuries. 2.6% of MedPAR index admissions with no hospital diagnosis of
falls and trauma, and 2.0% of admissions with a falls and trauma HAC, were excluded because they had no linked physician claims during the hospitalization
period. 5% sample of hospital claims without the HAC multiplied by 20 to estimate the full population.
3
2.4% of MedPAR index admissions with no hospital diagnosis of CAUTI, and 2.0% of admissions with a CAUTI HAC, were excluded because they had no
linked physician claims during the hospitalization period.
4
2.0% of MedPAR index admissions with no hospital diagnosis of CLABSI, and 2.4% of admissions with a CLABSI HAC, were excluded because they had no
linked physician claims during the hospitalization period. 25% sample of hospital claims with vascular catheter procedure without the HAC multiplied by 4 to
estimate the full population.
(continued)
33
5
Includes diabetic ketoacidosis, nonketotic hyperosmolar coma, hypoglycemic coma, secondary diabetes with ketoacidosis, and secondary diabetes with
hyperosmolarity. 2.6% of MedPAR index admissions with no hospital diagnosis of poor glycemic control, and 2.8% of admissions with a poor glycemic
control HAC, were excluded because they had no linked physician claims during the hospitalization period. 5% sample of hospital claims with diabetes without
the HAC multiplied by 20 to estimate the full population.
6
0.2% of MedPAR index admissions with no hospital diagnosis of mediastinitis, and 0.2% of admissions with a mediastinitis HAC, were excluded because they
had no linked physician claims during the hospitalization period.
7
Includes spine, neck, shoulder, and elbow surgeries. 2.4% of MedPAR index admissions with no hospital diagnosis of SSI, and 4.5% of admissions with an
SSI following certain orthopedic procedures HAC, were excluded because they had no linked physician claims during the hospitalization period.
8
Includes laparoscopic gastric bypass, gastroenterostomy, and laparoscopic gastric restrictive surgery. 2.4% of MedPAR index admissions with no hospital
diagnosis of SSI, and 5.9% of admissions with an SSI following bariatric surgery HAC, were excluded because they had no linked physician claims during the
hospitalization period.
9
Includes total hip replacement and total knee replacement. 2.2% of MedPAR index admissions with no hospital diagnosis of DVT/PE, and 2.3% of admissions
with a DVT/PE HAC, were excluded because they had no linked physician claims during the hospitalization period. 50% sample of orthopedic procedures
without the DVT HAC multiplied by 2 to estimate the full population.
SOURCE: MedPAR hospital claims from FY 2009 and the first eleven months of FY 2010 linked to Medicare Part B physician claims during the
hospitalization.

34
Additionally, some of these HAC-related conditions from the physician claims could
have been present on admission; the large numbers of physician-identified falls and trauma and
manifestations of poor glycemic control would seem to be more likely to reflect conditions that
were present on admission rather than hospital-acquired. However, it seems less likely that the
infections related to specific procedures or surgeries performed during the index hospitalization
would be present on the admission before the procedure or surgery occurred, meaning that some
of these physician-identified diagnoses could be truly un-reported HACs. For conditions such as
mediastinitis following CABG, the 126 cases of mediastinitis identified only on the physician
claims are likely to be true HACs. Regardless of the source of the under-reporting, the presence
of true HACs among the hospital claims identified as not having HACs has the potential to bias
our analysis of readmissions.
While the numbers of physician-identified HAC-related conditions are generally small
relative to the entire sample examined, they are often large relative to the number of HACs
identified on the hospital claims. For example, from the set of orthopedic surgery claims that did
not have a DVT/PE coded on the hospital claim (the first two rows in the table), only about 0.5%
had a DVT/PE coded on a linked physician claim (3,622 out of 677,550). But when we compare
that to the total number of DVT/PE HAC claims (the last two rows of the table) – 5,251 – it
seems to be a much more significant number. If all of the physician-identified DVT/PE claims
are true HACs, then the total count of DVT/PE HACs across the two years of data would be
about 70% higher, 8,873 instead of 5,251.
The physician claims for mediastinitis also point to potential under-reporting of HACs.
From the MedPAR data, a total of 156,309 Medicare claims for CABG surgery were identified
and linked to at least on physician claim during the period of hospitalization. Of those claims, 69
(.04%) had a diagnosis of mediastinitis on the hospital claim. In addition to these 69 hospital-
reported mediastinitis HACs, there were 126 physician claims linked to hospital claims for
CABG surgeries with a physician diagnosis code for mediastinitis. If all of these 126 physician-
identified cases of mediastinitis were true HACs, then this data suggests that approximately 65%
of mediastinitis HACs were unreported.
3
The third row of the table counts the claims where the HAC diagnosis code was on the
hospital claim but not on the physician claim, and the fourth row shows the claims where both
hospital and physician claims report the HAC. The rate of agreement between hospital and
physician coding of these HACs is poor. Except for falls and trauma and DVT/PE following
certain orthopedic procedures, there are more hospital-identified HACs without an
accompanying physician diagnosis of the HAC than there are claims with both hospital and
physician claims report the HAC. It would appear that neither hospital claims nor physician
claims are fully coding all of the HACs that are occurring in the hospital.
3
126 physician-identified mediastinitis cases + 69 mediastinitis HACs = 195 cases of mediastinitis. 126
physician-identified mediastinitis cases ÷ 195 mediastinitis cases = .646.
35
5.1.4 Under-Coding of Hospital-Acquired Conditions and the Effect on
Readmission Rates
Having identified a set of potentially under-coded HACs, we now examine how these
under-coded HACs could affect measures of readmission rates. In Tables 5-2a through 5-2c,
we present the 7-day, 15-day, and 30-day all-cause readmission rates for hospital claims with and
without HACs, linked to physician claims with and without the HAC-related diagnosis codes.
The rates presented calculate the number of discharges per 100 discharges that had at least one
hospital readmission within the specified window. We limit this analysis of readmission rates to
catheter-associated urinary tract infections, vascular catheter-associated infections, the three
subsets of SSIs, and DVT/PE following certain orthopedic procedures.
For four of the HACs in the 7-day readmission window, and for all of the HACs in the
15-day and 30-day readmission windows, the lowest readmission rates are seen for the claims
with neither a hospital- nor a physician-identified a HAC-related condition. When considering
discharges with a physician-identified (but no hospital-identified) HAC-related condition, the
second row in each series of 4 rows, the readmission rates are typically more similar to those of
the hospital-identified HACs than to those with no HAC-related diagnoses. For example,
consider the final column where we report the readmission rates for claims with certain
orthopedic procedures (total hip replacement and total knee replacement), with and without a
diagnosis of a DVT/PE. The readmission rate within 7 days is 3.3 per 100 discharges for
surgical patients with no DVT/PE-related diagnosis code, 9.2 per 100 discharges for those with
DVT/PE diagnosis code reported on the physician claim only, 7.2 per 100 discharges for those
with DVT/PE diagnosis code reported on the hospital claim only, and 7.1 per 100 discharges for
those with both a hospital and physician reported DVT/PE. The readmission rate for orthopedic
surgery patients with no DVT/PE diagnosis is half or less than half of the readmission rate for
patients with a DVT/PE diagnosis, regardless of whether the DVT/PE is coded on the physician
claim or the hospital claim or both.
36
Table 5-2a
Readmission rates per 100 discharges for 7-day readmission window for selected hospital-acquired conditions (HACs)
identified from hospital and or physician claims and for comparisons with discharges with no reported HAC
Hospital
Diagnosis of
HAC
Physician
Diagnosis of
HAC
Catheter-
Associated
Urinary Tract
Infection
(CAUTI)
3
Vascular Catheter
-Associated
Infection
(CLABSI)
4
Surgical Site
Infection (SSI),
Mediastinitis
following
Coronary Artery
Bypass Graft
(CABG)
6
Surgical Site
Infection (SSI)
following Certain
Orthopedic
Procedures
7
Surgical Site
Infection
Following
Bariatric Surgery
for Obesity
8
Deep Vein
Thrombosis and
Pulmonary
Embolism
(DVT/PE)
Following Certain
Orthopedic
Procedures
9
No No 9.0 1
0.3 8.5 4.0 5.3 3.3
No Yes 7.7 1
2.4 15.9 9.9 14.7 9.2
Yes No 9.1 1
2.4 14.6 9.3 4.2 7.2
Yes Yes 11.1
13.5 11.1 7.3 12.5 7.1
Table 5-2b
Readmission rates per 100 discharges for 15-day readmission window for selected hospital-acquired conditions (HACs)
identified from hospital and or physician claims and for comparisons with discharges with no reported HAC
Hospital
Diagnosis of
HAC
Physician
Diagnosis of
HAC
Catheter-
Associated
Urinary Tract
Infection
(CAUTI)
3
Vascular Catheter
-Associated
Infection
(CLABSI)
4
Surgical Site
Infection (SSI),
Mediastinitis
following
Coronary Artery
Bypass Graft
(CABG)
6
Surgical Site
Infection (SSI)
following Certain
Orthopedic
Procedures
7
Surgical Site
Infection
Following
Bariatric Surgery
for Obesity
8
Deep Vein
Thrombosis and
Pulmonary
Embolism
(DVT/PE)
Following Certain
Orthopedic
Procedures
9
No No 14.7
16.8 13.6 6.7 8.2 4.9
No Yes 17.1
22.5 22.2 17.7 21.5 12.3
Yes No 15.8
20.8 22.0 13.7 12.5 9.9
Yes Yes 16.0
22.5 18.5 11.9 25.0 10.6
37
Table 5-2c
Readmission rates per 100 discharges for 30-day readmission window for selected hospital-acquired conditions (HACs)
identified from hospital and or physician claims and for comparisons with discharges with no reported HAC
Hospital
Diagnosis of
HAC
Physician
Diagnosis of
HAC
Catheter-
Associated
Urinary Tract
Infection
(CAUTI)
3
Vascular Catheter
-Associated
Infection
(CLABSI)
4
Surgical Site
Infection (SSI),
Mediastinitis
following
Coronary Artery
Bypass Graft
(CABG)
6
Surgical Site
Infection (SSI)
following Certain
Orthopedic
Procedures
7
Surgical Site
Infection
Following
Bariatric Surgery
for Obesity
8
Deep Vein
Thrombosis and
Pulmonary
Embolism
(DVT/PE)
Following Certain
Orthopedic
Procedures
9
No No 21.7
24.8 18.9 9.6 11.6 6.9
No Yes 28.2
33.2 28.6 24.7 30.1 15.6
Yes No 24.2
30.0 34.1 22.0 20.8 13.2
Yes Yes 18.5
34.4 29.6 22.5 37.5 14.5
NOTES:
See Ta
ble 5-1 for frequencies for each cell, and see notes on Table 5-1 for further details on the sample
SOURCE: MedPAR hospital claims from FY 2009 and the first eleven months of FY 2010 linked to Medicare Part B physician claims during the hospitalization
and linked to MedPAR inpatient claims up to 30 days after the hospital discharge.
38
5.2 Timing to Clinical Presentation of Selected Conditions
5.2.1 Time to Physician Diagnosis of Conditions During Hospitalization
For catheter-associated urinary tract infections, vascular catheter-associated infections,
SSIs, and DVT/PE following certain orthopedic procedures that were identified on physician
claims linked to the hospital claim, we looked at the dates of the relevant procedures on the
hospital claim and compared them to the dates on the first physician claim with a HAC-related
diagnosis, to show the amount of time that passes between the procedure and the HAC-related
condition. We combined data from the physician-identified HAC-related conditions that were
identified as HACs on the hospital claim with those who were not identified as HACs on the
hospital claim, such that the sample sums the second and fourth rows of Table 5-1. In Table 5-3,
we report the time to a physician diagnosis of a HAC-related condition.
In the second row, we report the number and percent of hospitalizations (for HACs and
those at risk for HACs who had a physician diagnosis of the HAC-related condition) where the
physician diagnosis code of the HAC-related conditions occurred prior to the catheter insertion
or surgery data. These diagnoses occurring prior to procedure could be error in coding, either of
the diagnosis or procedure dates, or they could also represent conditions that were POA. CAUTI
and CLABSI are more likely to occur before the procedure date, with 22.5% of physician-
identified CAUTI diagnoses, and 45.9% of physician-identified CLABSI diagnoses, occurring
prior to the hospital procedure date for the catheter insertion. This could be caused by a catheter
being replaced (and coded) after the discovery of the infection.
Time to physician diagnosis of CAUTI and DVT/PE following certain orthopedic
procedures is typically much faster than the timing to the other HAC-related infections. For
CAUTI, 60% of physician-identified cases occur within 3 days of the catheter insertion
procedure, and 77.5% of DVT/PE cases diagnosed by a physician occur within 3 days of the
orthopedic surgery. In contrast, more than half of two of the SSIs are first diagnosed more than a
week after the relevant surgical procedures. For example, for all cases of mediastinitis following
CABG surgery identified on a physician claim, 35.7 % were first diagnosed between 8 and 15
days after the surgery and 44.8 % were first diagnosed 16 or more days after the surgery.
We also examined the physician specialty type for patients who had a physician-
identified HAC-related condition, whether or not the HAC was coded on the hospital claims, to
see what physician specialties might be potential indicators for the HAC-related conditions. In
Table 5-4, we present the top five physician specialties for the first physician claim linked to the
hospital claim with a HAC-related diagnosis code. Infectious disease specialists were the most
likely type of physician to make the initial diagnosis of the HAC-related condition for four of the
five infection conditions (CAUTI, CLABSI, mediastinitis following CABG, and SSI following
certain orthopedic procedures), accounting for more than 20% of initial diagnoses for these
conditions. For SSI following bariatric procedures, infectious disease specialists first diagnosed
18% of HAC-related conditions.
39
Table 5-3
Time to presentation of selected conditions from physician claims during initial hospitalization, all claims with a physician
diagnosis of selected hospital-acquired conditions
Time to Presentation
Catheter-
Associated
Urinary Tract
Infection
(CAUTI)
Vascular
Catheter-
Associated
Infection
(CLABSI)
Surgical Site Infection
(SSI), Mediastinitis
following Coronary
Artery Bypass Graft
(CABG)
Surgical Site
Infection (SSI)
following Certain
Orthopedic
Procedures
Surgical Site
Infection
Following
Bariatric Surgery
for Obesity
Deep Vein Thrombosis
or Pulmonary Embolism
(DVT/PE) Following
Certain Orthopedic
Procedures
Hospital claim with procedure
lin
ked to physician claim with
HAC diagnosis codes
Freq.
Percent
120
100.0%
4,442
100.0%
154
100.0%
1,000
100.0%
171
100.0%
7,362
100.0%
Physician diagnosis of HAC-
rela
ted condition prior to date
of procedure
Freq.
Percent
27
22.5%
2,039
45.9%
12
7.8%
72
7.2%
21
12.3%
292
4.0%
Physician diagnosis of HAC-
rel
ated condition 0 to 3 days
after procedure
Freq.
Percent
72
60.0%
1,469
33.1%
8
5.2%
400
40.0%
33
19.3%
5,708
77.5%
Physician diagnosis of HAC-
related
condition between 4 and
7 days after procedure
Freq.
Percent
9
7.5%
334
7.5%
10
6.5%
219
21.9%
24
13.8%
1,160
15.8%
Physician diagnosis of HAC-
related
condition between 8 and
15 days after procedure
Freq.
Percent
10
8.3%
332
7.5%
55
35.7%
207
20.7%
63
36.8%
168
2.3%
(continued)
40
Table 5-3 (continued)
Time to presentation of selected conditions from physician claims during initial hospitalization, all claims with a physician
diagnosis of selected hospital-acquired conditions
Time to Presentation
Catheter-
Associated
Urinary Tract
Infection
(CAUTI)
Vascular
Catheter-
Associated
Infection
(CLABSI)
Surgical Site Infection
(SSI), Mediastinitis
following Coronary
Artery Bypass Graft
(CABG)
Surgical Site
Infection (SSI)
following Certain
Orthopedic
Procedures
Surgical Site
Infection
Following
Bariatric Surgery
for Obesity
Deep Vein Thrombosis
or Pulmonary Embolism
(DVT/PE) Following
Certain Orthopedic
Procedures
Physician diagnosis of HAC-
related
condition between 16
days or more after procedure
Freq.
Percent
2
1.7%
268
6.0%
69
44.8%
102
10.2%
30
17.5%
34
0.5%
NOTES:
1. Ex
cludes 78 CAUTI HAC claims that did not have a urinary catheter procedure coded.
2. Excludes 194 CLABSI HAC claims that did not have a vascular catheter procedure coded.
See notes on Table 5-1 for further details on the sample
SOURCE: MedPAR hospital claims from FY 2009 and the first eleven months of FY 2010 linked to Medicare Part B physician claims during the
hospitalization.
41
Table 5-4
Physician specialty from the first diagnosis of HAC-related condition, among all hospital claims linked to a physician claim
with a HAC-related diagnosis code
Physician Specialty
Catheter-
Associated
Urinary Tract
Infection (CAUTI)
Vascular
Catheter-
Associated
Infection
(CLABSI)
Surgical Site
Infection (SSI),
Mediastinitis
following Coronary
Artery Bypass Graft
(CABG)
Surgical Site
Infection (SSI)
following Certain
Orthopedic
Procedures
Surgical Site
Infection
Following
Bariatric
Surgery for
Obesity
Deep Vein
Thrombosis or
Pulmonary
Embolism
(DVT/PE)
Following Certain
Orthopedic
Procedures
Number of phys
ician-identified
HAC-related diagnosis linked to
hospital claim
198 4,636 154 1,000 171 7,362
Most common physician
special
ty (Percent)
Infectious disease
specialist (31%)
Infectious disease
specialist (21%)
Infection disease
specialist (24%)
Infectious disease
specialist (24%)
General surgery
(21%)
Diagnostic
radiology (46%)
Second most common physician
special
ty (Percent)
Ambulance
service supplier
(22%)
Diagnostic
radiology (15%)
Plastic surgeons
(14%)
Anesthesiology
(22%)
Diagnostic
radiology (19%)
Internal medicine
(19%)
Third most common physician
special
ty (Percent)
Internal medicine
(17%)
Internal medicine
(14%)
Internal medicine
specialists (12%)
Orthopedic
surgery (15%)
Infectious
disease
specialist (18%)
Vascular surgery
(6%)
Fourth most common physician
special
ty (Percent)
Urology (7%)
General surgery
(13%)
Cardiac surgeons
(9%)
Neurosurgery
(9%)
Anesthesiology
(11%)
Pulmonary disease
(5%)
Fifth most common physician
special
ty (Percent)
Diagnostic
radiology (5%)
Anesthesiology
(8%)
Thoracic surgeons
(8%)
Internal medicine
(6%)
CRNA (7%) Cardiology (5%)
NOTES:
See no
tes on Table 5-1 for further details on the sample
SOURCE: MedPAR hospital claims from FY 2009 and the first eleven months of FY 2010 linked to Medicare Part B physician claims during the
hospitalization.
42
Internal medicine physician are often the first to diagnose many of the HAC-related
conditions. Urologists are among the top 5 physician types for diagnosing CAUTI, and
orthopedic surgeons are the third common physician specialty to diagnose an SSI following
certain orthopedic procedures. Interestingly, 22% of CAUTI conditions are diagnosed by
ambulance service suppliers. Recall from Table 5-3 that just over 22% of CAUTI diagnoses on
the physician claims occur before the date of the insertion of a urinary catheter on the hospital
claim. It is likely, then, that these infections identified by ambulance service suppliers occurred
in another healthcare setting (or at home) and are coded during ambulance transfer to the
hospital.
For those beneficiaries who had a DVT/PE coded on the physician claims linked to a
hospital claim for certain orthopedic surgeries, the most common first physician specialty type
coded was diagnostic radiology (46%), followed by internal medicine (19%), vascular surgery
(6%), pulmonary disease (5%), and cardiology (5%). It is not surprising that diagnostic
radiology was the most frequently coded first physician specialty and internal medicine was the
next most common physician specialty as patients are referred to diagnostic radiology for testing
to confirm the presence or absence of a DVT or PE, often by an internal medicine physician’s
assessment of a possible DVT or PE.
5.2.2 Post-Discharge Diagnosis of Conditions in Inpatient Settings
In Table 5-5, we consider the sample of index claims that had neither a hospital- nor a
physician-identified hospital-acquired condition, and we look for subsequent inpatient claims
with diagnosis codes that are used to identify hospital-acquired conditions. We do not include
any of the follow-up hospital claims where the relevant diagnosis codes are identified as being
hospital-acquired (POA indicator equal to “N” or “U”), since we are looking specifically for
evidence of the conditions that stem from the procedure or surgery in the index hospital claim.
We also do not include any of the conditions that can occur due to poor care in any inpatient
setting (pressure ulcer stages III or IV, falls and trauma, and manifestations of poor glycemic
control).
The number and percent of inpatient claims (hospital, SNF, LTCH, IRF, and other
inpatient claims) within the 7-day, 15-day, and 30-day window following the index hospital
discharge that have the relevant diagnosis codes for each group are presented. Note that we do
not refer to these as hospital-acquired conditions, because the first evidence from the claims data
that these conditions occurred comes after the index hospital discharge, and thus we cannot
determine if the conditions were actually acquired in the index hospitalization. Instead, we use
the term “HAC-related” to refer to diagnosis codes/conditions that are considered HACs when
they are coded as not present on admission (POA indicator equal to “N” or “U”) in an IPPS
hospital, but can present themselves in any health care setting. Some of these may have been
uncoded HACs in the index hospital setting, but many may be HAC-related conditions whose
clinical presentation did not occur until after the index discharge. As we saw in Table 5-3, the
time to presentation for many of these HAC-related conditions, particularly the surgical site
infections, can be more than 1 or 2 weeks. Short hospital stays increase the likelihood that an
infection will not clinically present and be reported until after the initial hospital discharge.

43
Table 5-5
Time to presentation of selected conditions on inpatient claims following index hospital discharge with no hospital or physician
diagnosis of selected hospital-acquired conditions
Time to Presentation
Catheter-
Associated
Urinary Tract
Infection
(CAUTI)
Vascular
Catheter-
Associated
Infection
(CLABSI)
Surgical Site Infection
(SSI), Mediastinitis
following Coronary
Artery Bypass Graft
(CABG)
Surgical Site
Infection (SSI)
following Certain
Orthopedic
Procedures
Surgical Site
Infection
Following
Bariatric Surgery
for Obesity
Deep Vein Thrombosis
or Pulmonary Embolism
(DVT/PE) Following
Certain Orthopedic
Procedures
Index hospital claim with
procedu
re code, without HAC
diagnosis codes, and linked to
physician claims without HAC
diagnosis codes
Freq.
Percent
151,795
100%
1,365,324
100%
156,114
100%
203,780
100%
29,679
100%
673,928
100%
7-day window:
any inpatient
claim with HAC-related
diagnosis codes
Freq.
Percent
230
0.2%
5,728
0.4%
132
0.1%
1,620
0.8%
243
0.8%
4,314
0.6%
15-day window: a
ny inpatient
claim with HAC-related
diagnosis codes
Freq.
Percent
436
0.3%
8,088
0.6%
238
0.2%
2,942
1.4%
364
1.2%
5,404
0.8%
30-day window: a
ny inpatient
claim with HAC-related
diagnosis codes
Freq.
Percent
715
0.5%
11,504
0.8%
329
0.2%
4,062
2.0%
465
1.6%
6,718
1.0%
NOTES:
See no
tes on Table 5-1 for further details on the sample
SOURCE: MedPAR hospital claims from FY 2009 and the first eleven months of FY 2010 linked to Medicare Part B physician claims during the hospitalization
and linked to MedPAR inpatient claims up to 30 days after the hospital discharge.
44
Within the 7-day follow-up window, about half of the claims identified with a HAC-
related condition are readmissions to a hospital, which illustrates the strong relationship between
post-discharge presentation of these conditions and hospital readmissions. Although a
readmission to a hospital is the primary source of post-discharge diagnosis of these HAC-related
conditions overall, DVT/PE is more likely to be found on a SNF claim and vascular catheter-
associated infection is more likely to be found on an LTCH claim.
Even though the rates of these conditions are fairly low within our samples, the
frequencies are often sizable relative to the number of hospital- and physician-identified
conditions. For example, among SSI following certain orthopedic procedures, there were 1,620
cases of surgical site infections identified on inpatient claims that occurred within 7 days of the
discharge from the index hospital claim where the surgery was performed, compared to 378
infections identified on the hospital claims and 849 infections identified on the physician claims
only (see Table 5-1).
As these are cumulative frequencies and rates, the numbers of these conditions identified
on inpatient claims increases as the follow-up window increases. Hospital readmissions are
responsible for an even larger percentage of these HAC-related diagnoses in the 15-day window,
around two thirds of the total, with other post-acute settings playing a less prominent role. After
hospitals, vascular catheter-associated infections, mediastinitis following CABG, and SSI
following bariatric surgery are most likely to be diagnosed in an LTCH, while SSI following
orthopedic surgery is most likely to be diagnosed in an IRF and DVT/PE following certain
orthopedic procedures is most likely to be diagnosed in a SNF.
At the 30-day window, there are more of HAC-related conditions diagnosed on the
follow-up inpatient claims than on the index hospital claims alone, except for catheter-associated
UTI; with the further exception of DVT/PE following certain orthopedic procedures, there are
more of these conditions diagnosed on the follow-up claims than on index hospital and physician
claims combined. While these may or may not have been true HACs, the presence of these
conditions post-discharge is leading to more readmissions and resource use.
5.3 Post-Discharge Use of Outpatient Department Drugs for Infection Treatment
In this portion of the analysis, we used outpatient department (OPD) drug claims in the
30 days after a hospital discharge to examine post-discharge treatment consistent with HAC-
related infections. We looked at four HAC-related conditions – CAUTI, SSI-mediastinitis after
CABG surgery, SSI following bariatric surgery, and SSI following certain orthopedic procedures
– to identify potential under-reporting of these conditions during the hospital period or clinical
presentation of infections after discharge. As with the previous analyses, our initial sample
contained all IPPS claims with one of these four HACs, and also those beneficiaries who did not
have a reported HAC on their hospital claims, but who were identified as being at risk for a HAC
due to the surgery (CABG, bariatric surgery, or orthopedic surgery) or procedure (placement of
urinary catheter) that was performed during the index hospitalization. Our population of interest
was the sample of beneficiaries who had at least one OPD drug claim that contained one of the
Healthcare Common Procedure Coding System (HCPCS) procedure codes for antibiotics known
to treat these infections.
45
We identified specific medications used to treat the HAC-related infections a priori
through peer-reviewed literature, drug-related websites, drug reference books, and limited
validation with physicians. We also identified the drugs that were used in the OPD data to treat
beneficiaries with a reported HAC and compared them to the a priori list of antibiotics to ensure
that these medications were included in our analysis. It should be noted that the HCPCS
antibiotic codes included in this analysis are specific to antibiotics that are administered
intramuscularly or intravenously. The OPD file provides a count of the number of occurrences
of the antibiotic HCPCS codes at the revenue center on a claim. This means that an individual
beneficiary may have multiple OPD drug claims for a specific antibiotic treatment plan that
includes one or more administrations within the 30 day period. The outcome of interest in our
analysis is the presence of at least one OPD drug claim for one of the specified antibiotics. In
this section of the report, we present a summary of our findings for all of the four studied HACs.
In Table 5-6, we present a summary of OPD drug claims 30 days after hospital
discharge, for beneficiaries with and without one of the studied HACs. The first row gives the
total number of hospital discharges with the HAC or at risk for the HAC. The second and third
rows report the number and percent of hospital discharges with a 30-day post-discharge OPD
drug claim for antibiotic treatment. The fourth row reports the most frequently administered
OPD antibiotics and the percent of the total among those with OPD antibiotics.
Using mediastinitis following CABG as an example, there are a total of 156,612
discharges with CABG procedures (ICD-9_CM procedure codes 36.10-36.19) without a
diagnosis code for mediastinitis on the hospital claim, and 71 discharges with CABG procedures
and a hospital-acquired diagnosis of mediastinitis. Among the 156,612 discharges without a
reported HAC that were at risk for mediastinitis, there are 2,401 discharges (1.5%) with OPD
antibiotic drug claims appropriate for the treatment of mediastinitis. The ratio of at-risk
discharges with an OPD antibiotic drug claim to reported hospital-based mediastinitis discharges
is 34-to-1.
There are at least five potential reasons 2,401 CABG patients are reporting anti-infective
drug use within 30 days after discharge. First, some patients may have already had an infection
of another origin when admitted for the procedure and so are continuing antibiotic treatment after
discharge. Second, some patients may have been infected after discharge from poor adherence to
post-discharge instructions. Third, some patients could have been treated for a post-discharge
infection unrelated to the surgery. Fourth, patients may have contracted a sternal wound
infection that advanced to mediastinitis post-discharge. And fifth, the hospital might not have
reported a mediastinitis infection during the CABG hospitalization.
46
Table 5-6
Outpatient department (OPD) drug claims within 30 days of hospital discharge, for patients with and without a HAC
OPD Drug Claims
Catheter-
Associated
Urinary Tract
Infection
(CAUTI):
HAC group
Catheter-
Associated
Urinary Tract
Infection
(CAUTI):
At-risk group
Surgical Site
Infection
(SSI),
Mediastinitis
following
Coronary
Artery
Bypass Graft
(CABG):
HAC group
Surgical Site
Infection
(SSI),
Mediastinitis
following
Coronary
Artery
Bypass Graft
(CABG):
At-risk group
Surgical Site
Infection
(SSI)
following
Certain
Orthopedic
Procedures:
HAC group
Surgical Site
Infection
(SSI)
following
Certain
Orthopedic
Procedures:
At-risk group
Surgical Site
Infection
Following
Bariatric
Surgery for
Obesity:
HAC group
Surgical Site
Infection
Following
Bariatric
Surgery for
Obesity:
At-risk group
Total of hospital claims 6,382 155,
726 71 156,612 396 209,569 34 30,573
Number with 30-day pos
t-
discharge OPD drug claim
for antibiotic treatment
124 3,500 2 2,401 16 2,143 0 513
Percent with 30-day pos
t-
discharge OPD drug claim
for antibiotic treatment 1.9% 2.2% 2.8% 1.5% 4.0% 1.0% 0% 1.7%
Most frequently observed
OPD dr
ugs/ Percent of
most frequently observed
OPD drugs
Cefazolin/
28.6%
Vancomycin/
25%
Levofloxacin/
21.4%
Unclassified
drugs/10.7%
Ceftriaxone/
3.6%
Vancomycin/
26.1%
Ceftriaxone/
16.2%
Cefazolin/
12.5%
Gentamicin/
8.0%
Levofloxacin/
7.9%
Unclassified
Drugs/ 7.2%
Daptomycin/
93.3%
Cefazolin/
6.7%
Vancomycin/
37.4%
Daptomycin/
10.7%
Ceftriaxone/
10.1%
Cefazolin/
8.9%
Daptomycin/
41.4%
Vancomycin/
26.7%
Ertapenem/
15.2%
Vancomycin/
31.5%
Daptomycin/
21.3%
Ceftriaxone/
16.5%
Cefazolin/
8.2%
Ertapenem/
4.7%
N/A
Cefazolin/
19.63%
Vancomycin/
15.5%
Unclassified
Drugs/ 13.9%
Ertapenem/
10.7%
Ceftriaxone/
10.3%
SOURCE: Me
dPAR hospital claims from FY 2009 and the first eleven months of FY 2010 linked to Medicare OPD drug claims up to 30 days after the hospital
discharge.
47
The most frequently observed OPD antibiotic drugs across these four HACs are similar
between both the reported and at-risk groups for each of the conditions as well as across all four
HACs. This is most likely attributed to the bacterial origin of the infection and the specific
antimicrobial therapy as all of these HACs have either a surgical or invasive procedure
component that places patients at higher risk for an infection. For example, vancomycin, a
glycopeptides antibiotic used for prophylaxis as well as treatment of infections caused by Gram-
positive bacteria, is observed in all four at-risk groups and two of the three HAC groups with
OPD antibiotic drug claims. (There are no observed OPD antibiotic drug claims for the 34
reported SSIs following bariatric surgery.) Gram-positive bacteria include such organisms as
Staphylococcus aureus and Staphylococcus epidermis. Vancomycin is also used in the treatment
of methicillin-resistant S. aureus (MRSA) infections that are resistant to other antibiotics. The
use of vancomycin across all HACs is highest among the CABG patients who are at risk for
mediastinitis (37.4% of those with OPD antibiotics received vancomycin) and lowest in the at-
risk group for SSIs following bariatric surgery (15.5%). Daptomycin, a newer Gram-positive
antibiotic, is used to treat skin and soft tissue infections as well as MRSA. Daptomycin claims
are present in both the reported and at-risk groups for mediastinitis and SSIs following
orthopedic surgery. Prescriptions for Daptomycin were not observed in OPD drug claims for
SSI following bariatric surgery nor CAUTI. Clinical trials are ongoing to test the efficacy of
Daptomycin in treating urinary tract infections.
When combined there are a total of 553,319 discharges used in this analysis, with only
6,883 reported HACs. Among the 552,480 discharges that are at risk but without a reported
HAC, there are 8,557 discharges with one or more OPD drug claims for antibiotics appropriate
for treatment of one or more of the hospital-acquired conditions. Some of these 8,557 discharges
could potentially have been true HACs that were not reported by the hospital.
We observed diverse rates of potentially unreported HACs based on outpatient drug
claims for CAUTI and SSIs for orthopedic, bariatric surgery, and CABG. The ratio of at-risk
patient with outpatient antibiotic treatment to reported HACs ranged from .55-to-1 for CAUTI to
34-to-1 for mediastinitis following CABG. Among the studied conditions, CAUTI had the
highest number of discharges with the HAC (6,382) as compared to the other three HACs. The
at-risk group for CAUTI had 3,500 discharges with an OPD antibiotic claim, which was higher
both in frequency terms and in percentage terms than the other three HACs. This difference may
be attributed to the fact that patients with urinary catheters are at higher risk for infection due to
the hospital environment and their own co-morbidities. Another explanation may be that CAUTI
infections are more readily identified when they do occur in the hospital due to the recent
national attention and success in implementing evidence –based procedures to prevent urinary
catheter infections. Or, they manifest clinically earlier than other infections, as we saw in
Table 5-3.
There are a number of limitations to using drug data to identify potentially unreported
healthcare-acquired infections. One is that many of the drugs used to treat a specific HAC, such
as mediastinitis, are also used to treat other infections. For instance Cefazolin may be used to
treat infections related to CABG surgery, but it is also used to treat the other three HACs, as
observed in our analysis. Antibiotics are prescribed based on the presenting bacterial pathogen
and the results of bacterial blood cultures and drug sensitivity testing rather than the specific
condition. A beneficiary with mediastinitis may present with the pathogen staphylococcus
48
aureus and be treated with a specific antibiotic for that pathogen and a different drug, like
daptomycin for a more serious infection like MRSA. Third, beneficiaries may have more than
one site at risk for infection. For example, a beneficiary undergoing a CABG procedure is at risk
for both a sternal infection, mediastinitis, as well as an infection at the surgical site where the
vein was harvested for the bypass graft (saphenous or internal mammary artery); these infections
may be treated with different antibiotics depending on the presenting pathogen.
OPD drug claims for administered antibiotics for patients without a reported HAC
provide some additional evidence that HACs related to infections are potentially unreported.
Limiting the observation period for a hospital-acquired condition to just the hospitalization
period may be too narrow, given the presence of these conditions in later inpatient claims and
evidence of antibiotic treatment post-discharge.
49
SECTION 6
SUMMARY AND CONCLUSIONS
In this report, we have investigated the impact of three different HACs on the likelihood
of readmission within 30 days and on the likelihood of discharge to a PAC setting. We used
mixed effect logistic modeling to control for various other characteristics that may explain the
likelihood of readmission and to account for the clustering of discharges within hospitals. The
results suggest a very strong relationship between the presence of a HAC and the likelihood of
both readmission and discharge to a PAC setting.
The relationship between the presence of a HAC and the likelihood of readmission within
30 days varied across the three HACs included in our analyses. For the falls and trauma HAC
and the DVT/PE HAC, the presence of the HAC is associated with a 21 to 23% increase in the
odds of being readmitted within 30 days. The presence of the vascular catheter-associated
infection HAC has an even greater impact on the likelihood of readmission. It is associated with
a 33% increase in the odds of being readmitted within 30 days.
The relationship between the presence of a HAC and the likelihood of discharge to a
PAC setting also varied across the three HACs. The odds of being discharged to a PAC setting
are 2.7 times greater for patients with the falls and trauma HAC, two times greater for patients
with the catheter-associated infection HAC, and 40% greater for patients with the DVT/PE HAC
compared to similar patients without the HACs. Discharge to a PAC setting does not appear to
be mutually exclusive with a hospital readmission within 30 days. In fact, patients who were
discharged to PAC settings were more likely to be readmitted within 30 days. This was true for
patients with each of the HACs as well as for patients in our control groups.
And lastly, we examined the degree to which readmission estimation bias may be present
in the Medicare claims data due to the presence of unreported HACs in the claims, using both
physician claims linked to the hospital claim, and also 30 days of follow-up claims. We found
that significant numbers of HAC-related conditions were reported on physician claims linked to
hospital discharges where no HAC (or POA) was coded. These potentially unreported HACs
could create bias in readmission estimations. There was fairly poor correspondence between
HACs coded on the hospital claims and HAC-related conditions coded on the linked physician
claims.
We examined physician claims linked to the hospital claims for the infection HACs and
DVT/PE following certain orthopedic procedures, and found that, particularly for the SSIs, the
time to clinical presentation of the infection can be more than a week, longer than the typical
hospital stay for some of these surgical procedures. However, physician claims from infectious
disease specialists identifying HAC-related conditions are common across many of the
conditions studied during the hospitalization and could signal the presence of the infection where
it is not otherwise identified on the hospital claim.
Significant numbers of HAC-related conditions are identified on inpatient claims within
the 30 days following a hospitalization with the relevant HAC-related procedure or surgery.
Outpatient claims data for administered antibiotics also points to potentially unreported HACs,
or HAC-related conditions that manifest after the initial hospitalization.
50
This page intentionally left blank.

51
BIBLIOGRAPHY
Ashton, C.M., DelJunco, D.J., Souchek, J., et al.: The association between the quality of
inpatient care and early readmission: a meta-analysis of the evidence. Med. Care 35(10):1044-
1059, October 1997.
Averill, R.F., McCullough, E.C., Hughes, J.S., et al.: Redesigning the Medicare Inpatient PPS to
Reduce Payments to Hospitals with High Readmission Rates. Health Care Financ. Rev.
30(4):1-15, Summer 2009.
Baser, O., Supina, D., Sengupta, N., & Kwong, L. (2010). Impact of postoperative venous
thromboembolism on Medicare recipients undergoing total hip replacement or total knee
replacement surgery. American Journal of Health-Systems Pharmacy, 67, 1438-1445.
Beckman, M., Hooper, C., Critichley, S., & Ortel, T. (2010). Venous thromboembolism a
public health concern. American Journal of Preventive Medicine, 38, S495-S501.
Carter, K., (2010). Identifying and managing deep vein thrombosis. Primary Health Care, 20,
30-39.
Encinosa, W.E., and Hellinger, F.J.: The impact of medical errors on ninety-day costs and
outcomes: an examination of surgical patients. HSR: Health Serv. Res. 43(6):2067-2085,
December 2008.
Friedman, B., and Basu, J.: The rate and cost of hospital readmissions for preventable
conditions. Med. Care Res. Rev. 61(2):225-240, June 2004.
Friedman, B., Encinosa, W., Jiang, J.H., et al.: Do patient safety events increase readmissions?
Med. Care 47(5):583-590, May 2009.
Fuji, T., Takahiro, O., Shigeo, N., et al. (2008). Prevention of postoperative venous
thromboembolism in Japanese patients undergoing total hip or knee arthroplasty: two
randomized, double-blind-placebo-controlled studies with three dosage regimens of enoxaparin.
Journal of Orthopaedic Science, 13, 442-451.
Geerts, W., Bergqvist, D., Pineo, G. et al. (2008). Prevention of venous thromboembolism:
American College of Chest Physicians evidence-based clinical practice guidelines (8
th
edition).Chest, 133 (6), (suppl): 381S-453S.
Goldfield, N.I., McCullough, E.C., Hughes, J.S., et al.: Identifying potentially preventable
readmissions. Health Care Financ. Rev. 30(1):75-91, Fall 2008.
Haines, S. (2010). Improving the quality of care for patients at risk for venous
thromboembolism. American Journal of Health-System Pharmacy, 67, S3-S8.
Herwaldt, L.A., Cullen, J.J., Scholz, D., et al.: A prospective study of outcomes, healthcare
resource utilization, and costs associated with postoperative nosocomial infections. Infect.
Control Hosp. Epidemiol. 27(12):1291-1298, December 2006.
52
Horwitz, L., Partovian, C., Lin, Z., et al.: Hospital-Wide (All Condition) 30-Day Risk-
Standardized Readmission Measure: Draft Measure Methodology Report. Contract no. HHSM-
500-2008-0025I/HHSM-500-T0001, Modification No. 000005. New Haven, Conn.Yale New
Haven Health Service Corporation/Center for Outcomes Research & Evaluation
(YNHHSC/CORE), August 2011.
Huang S.S., Placzek H., Livingston J., et al. Use of Medicare claims to rank hospitals by
surgical site infection risk following coronary artery bypass graft surgery. Infect. Control Hosp.
Epidemiol. 2011 Aug;32(8):775-83.
Jencks, S.F., Williams, M.V., and Coleman, E.A.: Rehospitalizations among patients in the
Medicare fee-for-service program. N. Engl. J. Med. 360(14):1418-1428, April 2009.
Kandilov, A., McCall, N., Dalton, K., and Miller, R.D. February 2012. Readmissions Due to
Hospital-Acquired Conditions (HACs). Report prepared for Centers for Medicare and Medicaid
Services Office of Research, Development, and Information under Contract no. HHSM-500-
2005-000291. Research Triangle Park, NC: RTI International.
Kim, E., & Bartholomew, J. Venous Thromboembolism. Retrieved November 5, 2010 from
http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/
Kohut, K. Guide for the Prevention of Mediastinitis Surgical Site Infections Following Cardiac
Surgery. Association for Professionals in Infection Control and Epidemiology. 2008.
Lip, G., & Tse H. (2007). Management of atrial fibrillation. Lancet, 370, 604-618.
McNair, P. D., & Luft, H. S.: Enhancing Medicare’s hospital-acquired conditions policy to
encompass readmissions. Medicare Care & Medicaid Res. Rev. 2012; 2(2), E1-E15.
Morris, D.S., Rohrbach, J., Rogers, M., Sundaram, L.M.T., Sonnad, S., Pascual, J., Sarani, B.,
Reilly, P., and Sims, C.: The Surgical Revolving Door: Risk Factors for Hospital Readmission.
Journal of Surgical Research, January 2011.
National Nosocomial Infections Surveillance (NNIS) System Report, data summary from
January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 2004; 32:470-85.
Rosenbaum, P.R., and Rubin, D.B.: The central role of the propensity score in observational
studies for causal effects. Biometrika 70:41-55, 1983.
Peterson, E., Coombs, L., Ferguson, T., Shroyer, A., DeLong, E., Grover, F.,& Edwards, F.,
(2002). Hospital variability in length of stay after coronary artery bypass surgery: results from
the Society of Thoracic Surgeons’ National Cardiac Database. Annals of Thoracic Surgery, 74,
464-473.
Swenne, C.L., Linholm, C., Borowiec, J., Carlsson, M.: Surgical-site infections within 60 days
of CABG, J. Hosp. Infect. (2004); 57(1):14-24 - Reported .5 - 5% within 60 days post discharge.

53
Wells, P., Borah, B., Sengupta, N., Supina, S., McDonald, H., & Kwong, L. (2010). Analysis of
venous thromboprophylaxis duration and outcomes in orthopedic patients. The American
Journal of Managed Care, 16, 857-866.
Zierler, B., Wittkowsky, A., Peterson, G., Lee, J., Jacobsen, C., Glenny, R., Wolf, F., Robins, L.,
Mitchell, P., Wolpin, S., Payne, T., Hendrie, P., Han, G., & Oh, H. (2008). Venous
thromboembolism safety toolkit: a systems approach to patient safety. Retrieved June 26, 2012
from http://www.ahrq.gov/downloads/pub/advances2/vol3/Advances-Zierler_81.pdf.
