
1
Share your stream monitoring data at www.cleanwaterhub.org.
I Z A A K W A L T O N L E A G U E O F A M E R I C A
Chemical Monitoring Instructions for Stream Monitors
As you explore your stream’s water chemistry, it is important to understand that water chemistry is very complex. While
some chemicals are absolutely necessary for life (such as nutrients) others can be harmful (such as pesticides) or harmful
in large amounts (back to nutrients). Some chemicals may not directly affect human health, while others (such as nitrate)
can have harmful effects in our drinking water.
The following are a few examples of how environmental
conditions can influence water chemistry:
Time of day: Dissolved oxygen levels rise during sunlight
hours due to increased photosynthesis in aquatic plants
and algae. Levels decrease overnight when
photosynthesis is not occurring and plants and animals
are using up dissolved oxygen.
Weather: Runoff from heavy rains can transport
pollutants into streams (which is called nonpoint source
pollution). Very dry conditions over a long period of time
(drought) lower stream flow, raising the concentration of
chemicals in the stream.
Physical influences: Decreased canopy cover causes
sunlight to warm the water, which can decrease
dissolved oxygen levels.
Land use: What we do on the land greatly affects the
volume of stream water flow and quality of our streams’
water, including:
o Development (filling in of wetlands; runoff from paved
areas, roofs, and lawns with their many applied
chemicals)
o Agriculture (runoff containing crop chemicals and
animal waste, tilling of the land, and filling in of
wetlands
o Recreation (clearing of land, chemical applications,
introduction of invasive species)
Monitoring should be conducted at the same station
(location) each time. Carefully record the location of your
monitoring station on your Chemical Monitoring Data Form.
Include roads, bridges, and significant landmarks. Use your
smart phone’s GPS functionality to determine your
longitude and latitude.
USE AND STORAGE OF TESTING MATERIALS
Chemicals in test kits, though not dangerous, can cause
mild skin and eye irritation and should be handled with
care. Ampules are made of glass and can be very sharp.
Test strips along with waste materials can be disposed of
as you would any household item.
Store all chemical testing materials at room temperature.
Dissolved oxygen and phosphate testing kits must be
stored in the dark. Check expiration dates and avoid using
expired materials (which could provide inaccurate results).
You can find links to purchase stream monitoring test
kits and equipment on the Izaak Walton League website at
iwla.org/water/resources-for-monitors.
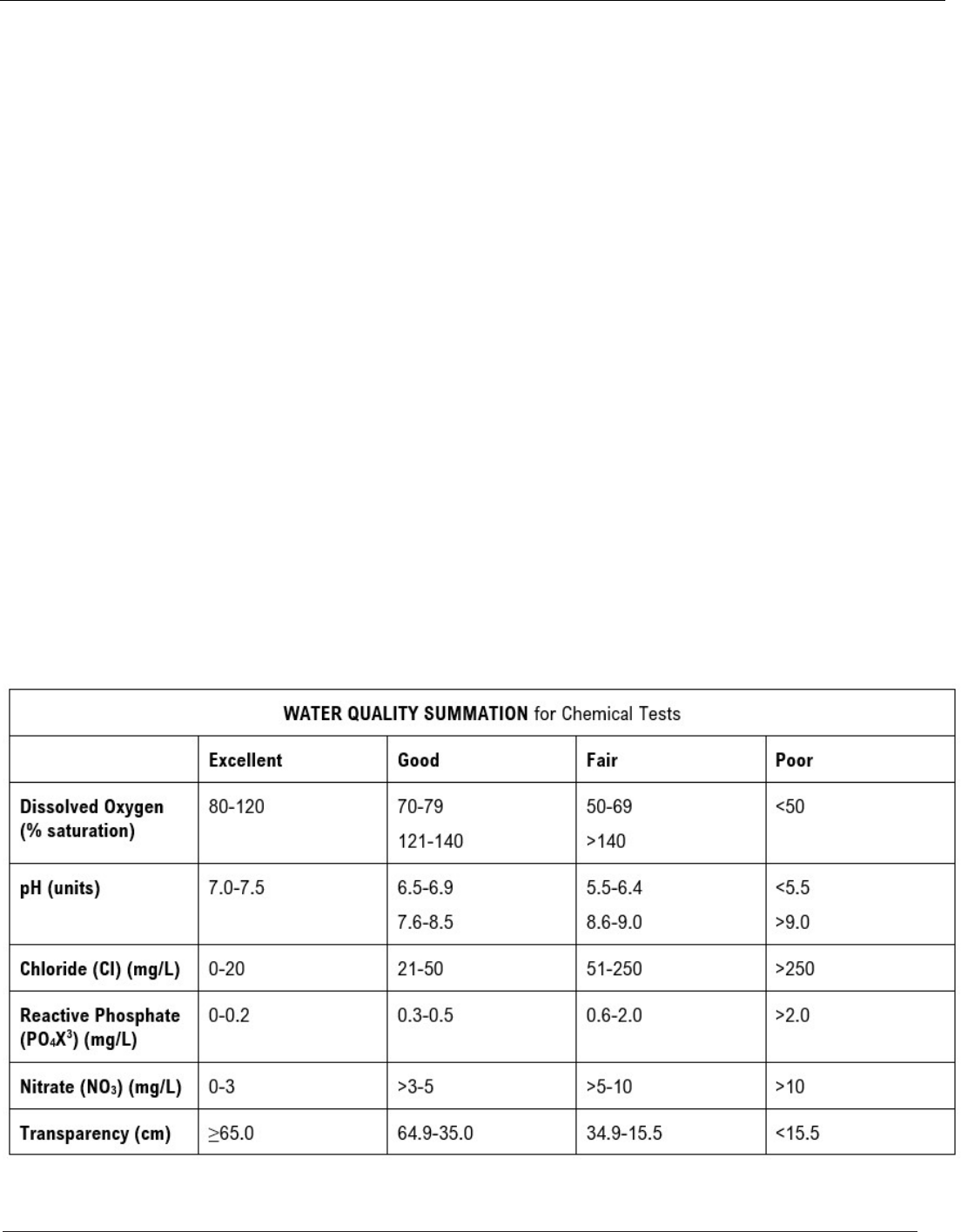
DISSOLVED OXYGEN
Dissolved oxygen (DO) is necessary for nearly all aquatic
life to survive. Certain processes add oxygen to a stream,
while others remove or consume oxygen. Oxygen is added
to a stream from the atmosphere through mixing in
turbulent areas. Plants also contribute oxygen through
photosynthesis. DO in streams can be affected by:
Water Temperature: Cold water holds more oxygen than
warm water.
Time of Day: On a sunny day, DO levels rise from morning
through the afternoon as a result of photosynthesis, reach
a maximum in late afternoon, and steadily fall during the
night, reaching their lowest point before dawn.
Stream Flow: DO will vary with the volume and velocity
of water in a stream. Faster moving water mixes readily
with atmospheric oxygen, thus increasing DO.
Aquatic Plants: Plant and algae growth in a stream will
affect the oxygen contributed by photosynthesis during
the day and depleted by plant respiration at night.
Dissolved or Suspended Solids: Oxygen dissolves more
readily in water that does not contain high amounts of
salts, minerals, or other solids.
Human Impacts: Lower DO levels may result from
human impacts including organic enrichment, urban
stormwater runoff, riparian corridor removal, stream
channelization, and dams.

CHEMICAL MONITORING INSTRUCTIONS FOR STREAM MONITORS
2
Share your stream monitoring data at www.cleanwaterhub.org.
Typical range for dissolved oxygen = 8.7 to 12.9
mg/L (rivers); 7.4 to 10.4 mg/L (lakes)
Reporting Technique: For use with the CHEMetrics
dissolved oxygen test kit
1. Check the expiration dates on all materials in the kit. If
materials have expired, DO NOT USE them.
2. Remove the 25 ml sample cup from the kit and rinse it
three times with stream water.
3. From your monitoring station, wade straight out to the
spot with the greatest flow of water and, facing
upstream, fill the sample cup to the 25 ml mark, mixing
the water and air as little as possible.
4. Lower the sample cup down to wrist depth while
holding it upside down. Turn the opening downstream
so that the cup backfills with water, then turn the cup
upstream and carefully remove cup and water sample
from stream.
5. Gently tip the sample cup to pour off excess water.
6. Place the ampoule in the sample cup, tilting it so the tip
is wedged in one of the spaces along the side of the
sample cup.
7. Snap off the tip of the ampoule by pressing it against
the side of the cup, allowing it to fill with water.
8. Remove the ampoule from the cup and mix the water
by inverting the ampoule several times. Be careful not
to touch the broken end, as it will be sharp.
9. Two minutes after you break off the ampoule tip,
compare the ampoule to the color standards provided in
the kit. Remove sunglasses before making a color
determination. NOTE: It’s important to read the ampoule
exactly at two minutes – it will continue to change color.
10. Hold the comparator nearly flat while standing directly
beneath a bright source of light. Place your ampoule
between the color standards moving it from left to right
until the best color match is found. Record your result
on the Chemical Data Form.
Avoid breaking the ampoule open, as the contents may be
mild skin and/or eye irritants.
pH
pH is a measure of a water’s acid/base content and is
measured in pH units on a scale of 0 to 14. A pH of seven
(7) is neutral (distilled water), while a pH greater than
seven is basic/alkaline and a pH less than seven is acidic.
The pH level of stream water is influenced by the
concentration of acids in rain and the types of soils and
bedrock in the state. The typical pH of rainfall in the United
States is slightly acidic, ranging from 5.0 to 5.6.
Rainwater pH is determined by natural atmospheric
processes and human activities (e.g., industry emissions
causing acid rain). Low pH levels (acidic) can have a
harmful impact on the health of aquatic communities.
Most aquatic organisms require habitats with a pH
of 6.5 to 9.0.
Reporting Technique: For use with Hach® pH test strips
1. Check the expiration date on the bottom of the bottle. If
the test strips have expired, DO NOT USE them.
2. From your monitoring station, wade straight out to the
spot with the greatest flow of water and, facing
upstream, dip the test strip in the water and remove it
immediately.
3. Hold the test strip level for 15 seconds. DO NOT SHAKE
excess water from the test strip.
4. Estimate pH by comparing the test strip to the color
chart on the test strip bottle. Remove sunglasses before
reading the strip. The strip will continue to change
color, so it is important to make a color determination
immediately after 15 seconds.
5. Record results on the Chemical Data Form.
CHLORIDE
Chloride is a chemical found in salts, which tend to
dissolve easily in water. Elevated levels of chloride in a
stream may indicate inputs of human/animal waste or from
fertilizers, many of which contain salts. During winter
months, elevated chloride levels may occur as a result of
road salt runoff into nearby streams. Chloride can be used
as a conservative measure of water contamination since
other natural processes, such as breakdown by bacteria,
do not affect it.
Typical range for chloride = 16 to 29 mg/L (rivers)
Reporting Technique: For use with Hach Chloride
QuanTab® titration strips and a sample cup from one of
the CHEMetrics test kits.
1. Check the expiration date on the side of the bottle. If the
test strips have expired, DO NOT USE them.
2. Rinse the sample cup three times with stream water.
3. From your monitoring station, wade straight out to the
spot with the greatest flow of water and, facing
upstream, fill the sample cup with approximately 1 inch
of water.

CHEMICAL MONITORING INSTRUCTIONS FOR STREAM MONITORS
3
Share your stream monitoring data at www.cleanwaterhub.org.
4. Remove a test strip from bottle and replace the cap
immediately.
5. Insert the lower end of the test strip into the sample
cup filled with water. Do not submerge past the yellow
line at the top of the titrator.
6. Allow the sample water to completely saturate the wick
of the titrator. There is no time limit for this test – the
reaction is complete when the yellow line turns dark
(this will take a few minutes).
7. Note where the tip of the white chloride peak falls on
the numbered QuanTab scale. This represents the
QuanTab unit value.
8. Refer to the table on the QuanTab test strip bottle to
convert the QuanTab units into a chloride concentration
(units of ppm or mg/L). Record the result on the
Chemical Data Form.
9. If the QuanTab unit value is below the smallest value on
your test strip bottle, report the chloride concentration
as the lowest concentration listed on the test strip bottle
and make a note in the comments section.
PHOSPHATE
Phosphorus is an essential nutrient for plants and animals
and is usually present in natural waters as dissolved
orthophosphate. Plant growth in surface waters is generally
limited by the amount of orthophosphate present. It is the
simplest form of phosphorus found in natural waters and is
most available for plants to use. In most waters,
orthophosphate is present in very low concentrations. For
our purposes, we will refer to orthophosphate as simply
“phosphate.”
There are natural sources of phosphorus, such as certain
soils and rocks, but most elevated levels of phosphorus
are caused by human activities. These include human,
animal, and industrial wastes as well as runoff from
fertilized lawns and cropland. Excess phosphorus in water
speeds up plant growth, causes algal blooms, and can
result in low dissolved oxygen (“hypoxic”) conditions that
can lead to the death of certain fish, invertebrates, and
other aquatic animals.
Typical range for total phosphorus = 0.11 to 0.34
mg/L (rivers); 0.05 to 0.13 mg/L (lakes)
Reporting Technique: For use with CHEMetrics phosphate
test kit
1. Check the expiration dates on all materials in the kit. If
materials have expired, DO NOT USE them.
2. Remove the 25 ml sample cup and black lid from the kit
and rinse them three times with stream water.
3. From your monitoring station, wade straight out to the
spot with the greatest flow of water and, facing
upstream, fill the sample cup to the 25 ml mark.
4. Gently tip the sample cup to pour off excess water.
5. Add 2 drops of A-8500 Activator Solution, place the
black cap on the sample cup, and shake to mix the
contents.
6. Place the ampoule in the sample cup, tilting it so the tip
is wedged in one of the spaces along the side of the
sample cup.
7. Snap off the tip of the ampoule by pressing it against
the side of the cup, allowing it to fill with water.
8. Remove the ampoule from the cup and mix the water in
the ampoule by inverting it slowly several times. Be
careful not to touch the broken end, as it will be sharp.
9. Two minutes after you break off the ampoule tip,
compare the ampoule to the color standards provided in
the kit. Remove your sunglasses before making a color
determination. NOTE: It’s important to read the ampoule
exactly at two minutes – it will continue to change color.
10. Based on the color of your ampoule, use the
appropriate color comparator to estimate the
orthophosphate concentration.
a. The low-range circular comparator measures
concentrations ranging from 0 to 1 mg/L. To use the
circular comparator, place your ampoule, flat end
downward, into the center tube. Direct the top of the
comparator up toward a good light source while
viewing from the bottom. Rotate the comparator to
match your ampoule to the standards and record
your results on the Chemical Data Form.
b. The high-range comparator in the lid of the kit
measures concentrations ranging from 1 to 10 mg/L.
Hold the high range comparator nearly flat while
standing directly beneath a bright source of light.
Place your ampoule between the color standards
moving it from left to right until the best color match
is found. Record result on the Chemical Data Form.

CHEMICAL MONITORING INSTRUCTIONS FOR STREAM MONITORS
4
Share your stream monitoring data at www.cleanwaterhub.org.
NITRATE-N/NITRITE-N
Nitrogen is an essential plant nutrient, but excess nitrogen
can cause water quality problems. Too much nitrogen and
phosphorus in surface waters cause nutrient enrichment,
increasing aquatic plant growth and changing the types of
plants and animals that live in a stream. This process,
called eutrophication, can also affect other water quality
parameters such as temperature and dissolved oxygen.
Nitrate and nitrite are two forms of nitrogen.
Nitrate is very easily dissolved in water and is more
common in streams. Sources of nitrate include soil
organic matter, animal waste, decomposing plants,
sewage, and fertilizers. Nitrate is more soluble in water
than phosphorus and can move more readily into
streams.
Nitrite is another form of nitrogen that is rare because it
is quickly converted to nitrate or returned back to the
atmosphere as nitrogen gas. Due to its instability,
detectable levels of nitrite in streams and lakes are
uncommon. Detectable nitrite levels in streams may
indicate a relatively fresh source of ammonia.
The amount of nitrate or nitrite dissolved in water is
reported as nitrate-N (nitrate expressed as the element
nitrogen) or nitrite-N in milligrams per liter of water (mg/L).
Stream water nitrate rates may vary greatly depending on
season and rainfall, fertilizer application rates, tillage
methods, land use practices, soil types, and drainage
systems. Consistently high nitrate readings (over 10 mg/L)
may be cause for concern and warrant further
investigation.
Typical range for Nitrate + Nitrite-N = 3 to 8.5 mg/L
(rivers); 0.05 to 0.94 mg/L (lakes)
Reporting Technique: For use with Hach® nitrate-
N/nitrite-N test strips
1. Check the expiration date on the bottom of the bottle. If
the test strips have expired, DO NOT USE them.
2. Dip the test strip into the water for one second and
remove. DO NOT SHAKE excess water from the test
strip.
3. Hold the strip level, with pad side up, for 30 seconds.
4. At exactly 30 seconds, compare the NITRATE (upper)
test pad to the nitrate-nitrogen color chart on test strip
bottle, estimate the nitrate concentration in mg/L, and
record your reading on the Chemical Data Form.
(Remove sunglasses before reading the strip.) The pad
will continue to change color, so make a determination
immediately after 30 seconds.
Note: Each nitrate-N/nitrite-N test strip also has a second
tab for measuring nitrite-N. Save Our Streams chemical
monitoring does not collect nitrite-N data, so you may
disregard this test pad.
TRANSPARENCY
Transparency is a measure of water clarity and is affected
by the amount of material suspended in water. As more
material is suspended, less light can pass through, making
it less transparent. Suspended materials may include soil,
algae, plankton, and microbes. Transparency is measured
using a transparency tube and is measured in centimeters.
It is important to note that transparency is different from
turbidity; transparency is a measure of water clarity
measured in centimeters, while turbidity measures how
much light is scattered by suspended particles using NTUs
(Nephelometric Turbidity Units).
Low transparency (or a high number of suspended
particles) is a condition that is rarely toxic to aquatic
animals, but it indirectly harms them when solids settle out
of the water, which can clog gills, destroy habitat, and
reduce the availability of food. Furthermore, suspended
materials in streams promote solar heating, which can
increase water temperatures (see Water Temperature) and
reduce light penetration (which reduces photosynthesis) –
both of which contribute to lower dissolved oxygen levels.
Sediment also can carry chemicals attached to the
particles, which can have harmful environmental effects.
Sources of suspended particles include soil erosion,
waste discharge, urban runoff, eroding stream banks,
disturbance of bottom sediments by bottom-feeding fish
(such as carp), and excess algal growth.
Reporting Technique: We measure transparency with a
transparency tube that shows how many centimeters down
into the tube you can see the black and white pattern at the
bottom.
1. Make sure the finger clamp on the hose is closed.
2. From your monitoring station, wade straight out to the
spot with the greatest flow of water and, facing
upstream, fill the transparency tube.
3. Hold the tube upright and in the shade. Use your body
to shade the tube if nothing else is available.
4. With your back to the sun, look directly into the tube
from the open top and release water through the small
hose, regulating the flow with the finger clamp until you
are able to distinguish the black and white pattern
(Secchi pattern) on the bottom of the tube. Close the
finger clamp.
CHEMICAL MONITORING INSTRUCTIONS FOR STREAM MONITORS
5
Share your stream monitoring data at www.cleanwaterhub.org.
5. Read the number on the outside of the tube that is
closest to the water line. Record your reading in
centimeters (cm).
6. If the Secchi pattern is visible when the transparency
tube is completely full of water, record a transparency
reading of 65.0 cm and make a note in the comments
section.
7. Rinse the tube after each use so that the bottom Secchi
pattern does not become dirty and clouded.
WATER TEMPERATURE
Many of the chemical, physical, and biological
characteristics of a stream are directly affected by water
temperature. Some species, such as trout, are quite
sensitive to temperature changes. Water temperatures can
fluctuate seasonally, daily, and even hourly.
Human activities can adversely raise stream temperatures
in a variety of ways., including by:
Releasing warmed water into a stream from industry
discharges or runoff from paved surfaces
Removing riparian corridors, which increases solar
heating
Causing soil erosion, which results in darker water that
absorbs more sunlight
Changes in water temperature affect water quality:
Cool water holds more oxygen than warm water, so the
amount of dissolved oxygen decreases when
temperatures rise
The rate of photosynthesis by algae and aquatic plants
increases with higher temperatures
The metabolic rates of aquatic animals increase with
higher temperatures
The sensitivity of organisms to diseases, parasites, and
toxic wastes increases with rising water temperatures
Human impacts are most critical during the summer, when
low flows and higher temperatures can cause greater
stress on aquatic life. It is important to note that the
temperature of some streams is normally higher than
others, depending on groundwater flow into the stream,
weather, and other factors.
Reporting Technique: Use a non-mercury thermometer or
meter probe to measure stream temperature. From your
monitoring station, wade straight out to the spot with the
greatest flow of water and place the thermometer or probe
directly into the stream, holding it underwater for at least
two minutes so the reading can stabilize. Record the
temperature on your Chemical Data Form in degrees
Celsius (°C).
