June 30, 2012
Accuracy of Coding in the Hospital-
Acquired Conditions–Present on
Admission Program
Final Report
Prepared for
Susannah G. Cafardi
Centers for Medicare & Medicaid Services
Rapid Cycle Evaluation Group
Division of Research on Traditional Medicare
Mail Stop WB-06-05
7500 Security Boulevard
Baltimore, MD 21244-1850
Prepared by
Catherine L. Snow, MPH
Linda Holtzman, MHA
Hugh Waters, PhD
Nancy T. McCall, ScD
Michael Halpern, MD
Lisa Newman, MSPH
John Langer, PhD
Terry Eng, MS
Carolyn Reyes Guzman, MPH
RTI International
3040 Cornwallis Road
Research Triangle Park, NC 27709
RTI Project Number 0209853.230.001.085
_________________________________
RTI International is a trade name of Research Triangle Institute.
Accuracy of Coding in the Hospital-Acquired Conditions—Present on Admission Program
Final Report
by Catherine L. Snow, MPH; Linda Holtzman, MHA; Hugh Waters, PhD;
Nancy T. McCall, ScD; Michael Halpern, MD; Lisa Newman, MSPH;
John Langer, PhD; Terry Eng, MS; Carolyn Reyes Guzman, MPH
Federal Project Officer: Susannah G. Cafardi
RTI International
CMS Contract No. HHSM-500-2005-00029I
June 30, 2012
This project was funded by the Centers for Medicare & Medicaid Services under contract no.
HHSM-500-2005-00029I. The statements contained in this report are solely those of the authors
and do not necessarily reflect the views or policies of the Centers for Medicare & Medicaid
Services. RTI assumes responsibility for the accuracy and completeness of the information
contained in this report.
iii
CONTENTS
EXECUTIVE SUMMARY .............................................................................................................1
ES.1 Methods..........................................................................................................................1
ES.2 Data ...............................................................................................................................2
ES.3 Results ............................................................................................................................3
SECTION 1 INTRODUCTION AND BACKGROUND ON ACCURACY OF CODING ...........7
1.1 Objectives ......................................................................................................................7
1.2 Selected Phase II Conditions for Medical Record Validation .......................................7
1.3 Conceptual, Operational, and Clinical Definitions of Accuracy of Coding ..................8
1.3.1 Conceptual Definition of Accuracy of Coding .....................................................8
1.3.2 Operational Definitions of Accuracy of Coding ...................................................9
1.3.3 RTI Physician Review for Accuracy of Coding .................................................10
SECTION 2 SAMPLING DESIGN...............................................................................................11
2.1 Sampling Plan ..............................................................................................................11
2.1.1 Measures of Interest ............................................................................................11
2.1.2 Estimated Magnitude of the Parameters .............................................................11
2.1.3 Margin of Error and Confidence Intervals ..........................................................12
2.1.4 Sample Size Requirements for Medical Record Abstraction .............................12
2.2 Sampling Frame ...........................................................................................................13
2.3 Medical Record Sampling Plan ...................................................................................13
2.3.1 Linking CERT Medical Records to MedPAR Data ............................................13
2.3.2 Sampling Plan for Assessing Accuracy of POA Coding ....................................14
2.3.3 Sampling Plan for Assessing Accuracy of HAC Coding....................................14
SECTION 3 MEDICAL RECORD VALIDATION PLAN ..........................................................17
3.1 Medical Record Validation Flow Diagrams ................................................................17
3.2 Medical Record Abstraction Tools ..............................................................................19
3.2.1 Type of Review ...................................................................................................20
3.2.2 Preliminary Evidence ..........................................................................................20
3.2.3 Part I—Should the Listed Condition Be Coded? ................................................20
3.2.4 Part II—Was the Listed Condition Present on Admission? ...............................21
3.2.5 Disposition ..........................................................................................................21
3.3 Medical Record Validation Process .............................................................................21
3.4 RTI Institutional Review Board ...................................................................................22
iv
SECTION 4 DATA ANALYSIS ...................................................................................................23
4.1 Unreported HAC Observations ....................................................................................23
4.2 Over-Reported POA Observations ..............................................................................25
4.3 Strength of Evidence for POA .....................................................................................27
4.4 Abstraction Observations .............................................................................................28
4.4.1 Physician Queries Related to Potential HACs ..................................................28
4.4.2 Pressure Ulcer Coding ......................................................................................30
SECTION 5 DISCUSSION ...........................................................................................................31
REFERENCES ..............................................................................................................................35
APPENDIX A: ASSESSMENT OF THE REPRESENTATIVENESS OF THE CERT
RECORDS ..........................................................................................................36
APPENDIX B: APPENDIX OF TOOLS .....................................................................................45
APPENDIX C: CLINICAL CODING GUIDELINES .................................................................63
LIST OF FIGURES
Figure 1-1 Conceptual definition of the accuracy of coding ....................................................... 8
Figure 3-1 Unreported HACs: Medical record validation flow diagram .................................. 18
Figure 3-2 Over-reported POA: Medical record validation flow diagram ................................ 19
LIST OF TABLES
Table ES-1 Summary of HAC coding accuracy: CAUTI, VCAI, DVT/PE ................................. 3
Table ES-2 Summary of POA coding accuracy: All five POA conditions combined .................. 4
Table 2-1 Sample sizes for each condition ............................................................................... 12
Table 4-1 Summary of HAC coding accuracy: CAUTI, VCAI, DVT/PE ............................... 23
Table 4-2 Number of unreported HAC cases determined to be present on admission for
three clinical conditions: CAUTI, VCAI, DVT/PE ................................................. 24
Table 4-3 Number of cases where condition was present and how it affected patient care
or three clinical conditions: CAUTI, VCAI, DVT/PE ............................................. 24
Table 4-4 Number of cases stratified by level of evidence that the unreported HAC cases
were present for three clinical conditions: CAUTI, VCAI, DVT/PE ...................... 25
Table 4-5 Summary of POA coding accuracy: All five POA conditions combined ................ 25
Table 4-6 Over-reported POA: number of cases where the condition was present, but not
on admission, or was not present at all .................................................................... 26
Table 4-7 Over-reported POA: POA summary of evidence .................................................... 27
Table 4-8 Over-reported POA: POA pressure ulcer evidence ................................................. 28
1
EXECUTIVE SUMMARY
Under the Hospital-Acquired Conditions—Present on Admission (HAC-POA) program,
accurate coding of hospital-acquired conditions (HACs) and present on admission (POA)
conditions is critical for correct payment. The purpose of the HAC-POA program, funded by the
Centers for Medicare & Medicaid Services (CMS), is to evaluate the HAC-POA payment policy
related to preventable HACs. The principal objective of the Accuracy of Coding component,
which is the subject of this report, is to determine the level of accuracy of coding for HACs and
for POA conditions. This study was conducted jointly by RTI International and Clarity Coding.
For payment purposes, for each condition there are two questions that are key to
assessing the accuracy of coding:
1. Is there documented clinical evidence that a condition was present during the
hospitalization? We identified unreported cases, where a HAC-associated
condition existed but was not reported by the hospital.
2. If yes, was the condition POA?
We identified over-reported POA cases, where a HAC-associated secondary
diagnosis code was reported as POA when it was not in fact POA.
After considering a wide range of data sources and discussing priorities with the projects’
funders, we focused on three types of POA s for examining under-reporting:
1. Catheter-associated urinary tract infections (CAUTI),
2. Vascular catheter-associated infections (VCAI),
3. Deep vein thrombosis/pulmonary embolisms (DVT/PE); and
five types of HACs for examining POA over-reporting:
1. CAUTI ,
2. VCAI,
3. Falls and trauma,
4. Stage III and IV pressure ulcers, and
5. Extreme manifestations of poor glycemic control.
ES.1 Methods
Clarity Coding, under subcontract to RTI, abstracted medical records to confirm if cases
were correctly coded for both HAC and POA status. Our operational definition for accuracy of
coding is based on diagnostic and procedural information about the patient as coded and reported
2
by the hospital on the claim form, matched against the documentation in the patient’s medical
record, while factoring in relevant coding guidelines from definitive sources.
We selected claims included in the Fiscal Year (FY) 2009 and FY 2010 Medicare
Provider Analysis and Review (MedPAR) file that had one or more of the five HAC-associated
diagnoses coded as POA. We also included records of patients that did not have a HAC coded,
but were at risk of developing the condition (e.g., had an indwelling urinary catheter, central
catheter, or certain orthopedic procedures). We merged these MedPAR data with medical
records—obtained by CMS through its Comprehensive Error Rate Testing (CERT) program.
To assess accuracy of HAC and POA coding, a coding expert reviewed the full medical
record for each case. The Clarity Coding coder would make a technical coding change without
physician review if the medical record clearly stated that the condition presented during the
hospitalization but the wrong diagnosis code was used by the hospital. If the coders were unable
to make a decision due to clinical ambiguity, they referred the case to an RTI physician for
review and decision. Linda Holtzman, MHA, was the Clarity Coding Project Director for this
activity, supervising a team of four coders.
ES.2 Data
Through a combination of training, monitoring, and inspection, RTI and Clarity Coding
provided a high level of data protection and quality control. CMS staff provided us with the FY
2009 and FY 2010 medical records received by the CERT program, including the information
necessary to link these medical records to Medicare claims data. After excluding those hospitals
not subject to the Medicare Inpatient Prospective Payment System (IPPS)—and therefore not
subject to the HAC-POA policy—our sampling frame was 10,465 unique CERT medical
records. Using beneficiary and hospital identification information in the medical records, we
linked the records to the MedPAR claims data for FY 2009 and FY 2010. This step allowed us
to create an electronic database linking claims data to the medical records, and populating the
database with information from the MedPAR claims, including diagnosis codes, procedure
codes, and other data elements that might be useful for our analyses.
To ensure that the CERT records were representative of all Medicare IPPS discharges,
we compared characteristics of the CERT medical records to the characteristics of all discharges
from IPPS hospitals in the FY 2009 and FY 2010 MedPAR database. We verified that the
distributional properties of the CERT records are consistently similar to the MedPAR records for
patient and hospital characteristics such as patient age, gender, race, principal diagnoses, and
hospital size and location. (For a more detailed analysis of the representativeness of the CERT
records, please refer to Appendix A.)
We sought to have 264 CERT records for assessing accuracy of reporting HACs for each
of the three selected conditions. To identify VCAI records for validation, we screened MedPAR
records with a central line or venous catheterization coded as having been inserted during the
hospitalization—excluding records where VCAI was coded as hospital acquired (POA = N or U)
or present on admission (POA = Y or W). This yielded 881 CERT records; a random sample of
264 was selected.
3
CERT medical records for DVT/PE validation were selected by identifying MedPAR
records with corresponding claims that did not have DVT/PE coded as hospital acquired or POA,
but did have certain orthopedic procedures with a high risk for developing DVT/PE (hip
resurfacing, hip replacement, and knee replacement)—for a total of 222 CERT records. While
this was less than the 264 desired for coding review, there were no additional discharges in
which the patient had undergone one of these orthopedic procedures.
For CAUTI cases, we first identified MedPAR records that linked to CERT records and
had the presence of an indwelling urinary catheter coded, excluding any MedPAR cases where
CAUTI was coded as hospital acquired (POA = N or U) or present on admission (POA = Y or
W). This produced 90 CERT records for review. Next, MedPAR records were linked with their
corresponding physician claims to identify cases where a physician billed for insertion of
indwelling urinary catheter during the inpatient stay. This yielded an additional 35 CERT
records for review. And, third, RTI staff manually screened the CERT records for the presence
of an indwelling urinary catheter. Of 308 cases screened, 139 had evidence that the beneficiary
had an indwelling catheter inserted at some time during the hospital admission. Of these, one
record could not be read, leaving a total of 263 CERT records.
To test for POA status, we selected all CERT records that had one of the 12 HACs coded
as POA on its linked MedPAR claim. This process yielded a total sample of 318 cases across
five conditions: CAUTI (13), VCAI (5), Stage III or IV pressure ulcers (105), falls and trauma
(181), and extreme manifestations of poor glycemic control (14).
ES.3 Results
We did not find patterns of widespread under-reporting of HACs or over-reporting of
POA status. In just 23 out of a total of 749 HAC cases (3%), the condition was determined to be
present but not reported. Of the disagreements that were observed, the most frequent were for
CAUTI cases, 6% of which were inaccurately coded (i.e., the condition was present but not
coded by the hospital). The least frequent disagreement was for DVT/PE cases, with no
inaccurately coded HACs (Table ES-1). For 17 of 23 HAC cases, the condition was POA. This
leaves just 6 of the 749 cases that were both hospital acquired and inaccurately coded.
Table ES-1
Summary of HAC coding accuracy: CAUTI, VCAI, DVT/PE
Disposition
CAUTI
n
CAUTI
%
VCAI
n
VCAI
%
DVT/PE
n
DVT/PE
%
Hospital coded/reported accurately
247
94%
257
97%
222
100%
Hospital did not code/report
accurately
16 6% 7 3% 0 0%
Total 263 100% 264 100% 222 100%
NOTE: CAUTI, catheter-associated urinary tract infection; DVT/PE, deep vein
thrombosis/pulmonary embolism; HAC, hospital-acquired condition; VCAI, vascular catheter-
associated infections.
4
The results for over-reported POA cases are similar in magnitude. Of all the cases coded
POA, 91% were coded accurately (Table ES-2). However, the level of uncertainty around this
estimate is large, given the small number of CERT medical records available for abstraction. Of
the 28 POA cases coded inaccurately, the highest percentages are attributable to Stage III and IV
pressure ulcers, with 9% (9 out of 105 cases) being incorrectly reported as POA; and falls and
trauma, with 8% (14 out of 181 cases) incorrectly reported as POA.
Table ES-2
Summary of POA coding accuracy: All five POA conditions combined
Disposition N %
Hospital coded/reported accurately 290 91%
Hospital did not code/report accurately 28 8%
Total
318
100%
NOTE: POA, present on admission.
When evaluating medical records coded by the hospital as the condition being POA, the
coders looked for two things: (1) whether the condition existed during the stay, and (2) whether
the condition was POA. This approach allowed for two ways in which the Clarity Coding coder
could disagree with the hospital coder. From the cases reviewed in this study, the former seemed
to be the main reason for coder disagreement; 23 out of 28 inaccurately coded POA cases were
inaccurate because the condition was not present at the time of admission or at any time during
the hospitalization.
Clarity Coding was asked to provide RTI with their observations from the detailed
medical record reviews they performed. They noted that two specific types of cases were
particularly challenging: unreported CAUTI and over-reported POA pressure ulcers. They
provided specific cases illustrating that two coding issues identified may affect interpretation of
the validation results: a lack of physician queries in the medical records, and the requirement to
code progression of pressure ulcers to Stage III or IV during the hospitalization as POA. The
coders found numerous instances in which the hospital coding was in accordance with coding
guidelines, but the conditions might have been perceived as hospital acquired by clinicians
unfamiliar with coding practices. Using exclusively clinical validation criteria not requiring
conformance with official coding guidelines, more instances of under-reporting of HACs or
over-reporting of POA may have been found. However, from a coding perspective the
conditions could not be determined to be hospital acquired. Coding is fundamental to
administration of the HAC-POA program, and its requisites must be observed.
With respect to progression of pressure ulcers to Stage III or IV during the
hospitalization, coding guidelines direct that the Stage III or IV pressure ulcer be confirmed as
POA if a lower stage ulcer was recognized on admission and progressed to a higher stage ulcer
during the admission. CMS may wish to discuss the unintended consequences of coding
guidelines on the HAC-POA payment policy with the other Cooperating Parties.
5
The inconsistency in how hospitals store queries creates issues with accessing them. This
can impede any type of external coding review and inadvertently skew its findings. If possible,
hospitals should be urged to uniformly include all queries and their responses as part of the
permanent medical record. This would ensure that a complete clinical picture is available to
reviewers and can be reflected in their findings.
6
[This page left intentionally blank]
7
SECTION 1
INTRODUCTION AND BACKGROUND ON ACCURACY OF CODING
1.1 Objectives
The purpose of this project, funded by the Centers for Medicare & Medicaid Services
(CMS), is to evaluate the Hospital-Acquired Conditions–Present on Admission (HAC-POA)
payment policy related to preventable hospital-acquired conditions (HACs). This policy is one
of several recent CMS value-based purchasing initiatives designed to improve the structure of
payment incentives aimed at improving health care performance. Accurate coding of HACs is
essential for these payment incentives to be effective—as is correctly identifying whether HAC-
associated conditions are present on admission (POA) rather than acquired in the health care
setting.
The principal objective of the Accuracy of Coding component of the HAC-POA Project
is to determine the level of coding accuracy for HACs and for POA conditions. Through
medical record abstraction, we have evaluated the degree to which independent coders validated
hospitals’ coding of conditions that are hospital acquired and those that are POA. This study was
conducted jointly by RTI and Clarity Coding.
1.2 Selected Phase II Conditions for Medical Record Validation
RTI worked with the project’s funders to identify the Phase II conditions for the study.
The funders provided key inputs for this process, including information and recommendations
regarding the selection of HACs, examples of algorithms for assessing the accuracy of coding,
feasibility of case identification, relevant literature, and, when physician reviews were necessary,
which data elements to consider including in these reviews. For Phase II, we focused on
assessing the accuracy of coding of three types of HACs: (1) catheter-associated urinary tract
infections (CAUTI); (2) vascular catheter-associated infections (VCAI), also described as central
line-associated bloodstream infections (CLABSI); and (3) deep vein thrombosis/pulmonary
embolisms (DVT/PE). CAUTI was identified by the Office of the Inspector General (OIG) as a
priority condition in terms of incorrect coding and reporting. The CDC suggested VCAI for
consideration—and provided a computer algorithm to use as a template in developing our
abstraction tool (Trick et al., 2004). DVT/PE was selected as a clinical condition for the study
because of strong, objective confirmatory diagnostic testing readily available for patients
presenting with symptoms of either DVT or a PE.
As discussed in more detail below, we identified 10,465 medical records—obtained by
CMS from its Comprehensive Error Rate Testing (CERT) program. However, when we merged
these CERT records with the Fiscal Year (FY) 2009 and FY 2010 Medicare Provider Analysis
and Review (MedPAR) records that had one or more of the HAC-associated diagnoses coded as
POA, we obtained only 318 matches. The project’s funders therefore determined that all CERT
records that matched a MedPAR record with a relevant POA code would be abstracted. The
following five conditions had matching CERT records and so are the subject of our assessment
of accuracy of POA coding: (1) CAUTI, (2) VCAI, (3) falls and trauma, (4) Stage III and IV
pressure ulcers, and (5) extreme manifestations of poor glycemic control.
8
1.3 Conceptual, Operational, and Clinical Definitions of Accuracy of Coding
1.3.1 Conceptual Definition of Accuracy of Coding
Under CMS’s HAC-POA program, hospitals have financial incentives to record clinical
conditions as POA—for example, a history of a DVT following a prior orthopedic procedure.
Hospitals also potentially have incentives to mischaracterize or under-report clinical conditions
that are acquired during the hospitalization. For example, a hospital could label a Stage III
pressure ulcer as a Stage II instead if the ulcer was acquired during the hospitalization, or label a
pressure ulcer as Stage III rather than Stage II if it is POA.
Accurately coding HACs and POA conditions is critical for correct payment under the
HAC-POA program. For payment purposes, for each condition, two questions are key to
assessing the accuracy of coding:
1. Is there documented clinical evidence that the condition was present during the
hospitalization?
2. If yes, was the condition POA?
This task looks at how accurately hospital coders reported the answers to these two
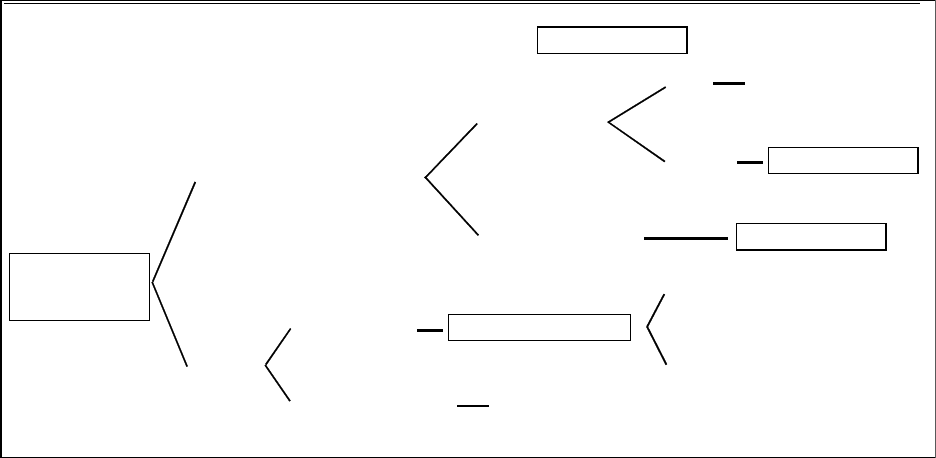
questions on claims submissions. Figure 1-1, below, provides a conceptual framework.
Figure 1-1
Conceptual definition of the accuracy of coding
POA
Accurately Coded
Condition Existed
Not POA
Coded
Coded as POA
Condition did not Exist
POA
Condition Existed
Not Coded
Not POA
Condition did not Exist
Accurately Coded
HAC
-
Diagnosis Code
POA
Inaccurately Coded
Inaccurately Coded
Inaccurately Coded
Associated
For each condition, the first question is whether a HAC-associated diagnosis was coded
on the claim, as shown in the far left box of Figure1-1. Following the path in the lower section
9
of the figure, a “Not Coded” leads to the possibility of either incorrect coding (coded that the
condition did exist) or correct coding (coded that the condition did not exist). This study focuses
on whether a condition not coded by the hospital did exist, resulting in an inaccurately coded
case as illustrated in the diagram. To further understand what this kind of inaccurate coding
represents, we looked at these cases to determine if they were POA or hospital acquired. We
first identified the total number of cases for which the coding was inaccurate—in other words,
evidence that the condition existed during the hospitalization but was not coded. Secondly, we
identified which of these unreported cases should have been coded POA.
At the top of Figure1-1, the HAC-associated diagnosis is correctly coded, and the
concern is the accuracy of POA coding. POA can be over-reported in two ways. The first is
when the condition is coded as POA, but was in fact hospital acquired. The second type of over-
reporting occurs when the condition is coded and reported as POA, but the condition itself did
not actually exist during the stay. Both conditions are presented in the top half of Figure 1-1, and
we analyze both when we assess the accuracy of POA coding.
To summarize, this study focuses on two types of coding inaccuracies:
• HAC-associated secondary diagnosis code is not coded but the condition existed
during the hospital stay; and
• HAC-associated secondary diagnosis code is coded and reported as POA when it was
not POA.
1.3.2 Operational Definitions of Accuracy of Coding
RTI explored several alternatives for defining and measuring accuracy of coding in Phase
I, including literature reviews, discussions with the project funders, and conversations with
coding and other experts. Our operational definition of accuracy of coding is based on
diagnostic and procedural information about the patient as coded and reported by the hospital on
the claim form, matched against the documentation in the patient’s medical record, while
factoring in relevant coding guidelines from definitive sources.
We abstracted medical records to confirm if cases were correctly coded—based on
clinical information documented by the responsible physicians and other qualified health care
providers. Cases were referred for review by an RTI physician whenever medical judgment was
needed. If the Clarity Coding coder did not agree with the diagnosis code based on clinical
documentation and coding guidelines and felt confident that a diagnosis change was appropriate,
the coder reported the case as coded incorrectly by the hospital. For example, if the physician
narrative description states, “UTI due to indwelling catheter,” yet the diagnosis code reported by
the hospital was for a simple UTI, the Clarity Coding coder would make a technical coding
change without physician review.
If the coders were unable to make a decision due to clinical ambiguity, they referred the
case to an RTI physician for review and comment. These referral cases included clinical or
diagnostic uncertainty as well as unclear or incomplete documentation. This type of HAC under-
reporting was identified by the 2010 report from the Inspector General of Health and Human
10
Services on the topic of adverse events in hospitals—for cases in which “… physician reviews
determined that the beneficiary experienced a ‘catheter-associated urinary tract infection,’ yet the
billing data included a more general diagnosis code for ‘urinary tract infections, not otherwise
specified’” (Levinson, 2010).
To assess accuracy of HAC and POA coding, the full medical records for these cases
were reviewed by a coder. While validating the coding, the coders also abstracted information
from the medical record, allowing us to develop categories of explanations, where possible, for
over-reporting of POA cases and under-reporting of HAC cases.
There may be instances of “false disagreement” between the hospital and the Clarity
Coding coder that arose due to incompleteness of the medical record. The medical records
obtained through the CERT program did not always include the physician query forms. These
forms allow the physician to augment or clarify ambiguous documentation in the medical record.
Although physician query forms are the approved means for clarifying documentation, these
forms are not consistently included in the formal medical record. Depending on the hospital, and
sometimes on the physician, queries may be made in a nonpermanent form—for example, with a
sticky note—and responses may be documented in a query database and not included in the
medical record. As a result, we may not have had complete physician query information
consistently available as part of the medical record requested by the CERT program.
Linda Holtzman, MHA, was the Clarity Coding Project Director for this activity; she
supervised a team of four coders. Ms. Holtzman and each of the coders are credentialed as
Certified Coding Specialists (CCS), Registered Health Information Technicians (RHIT), or
Registered Health Information Administrators (RHIA). Ms. Holtzman is a CCS-P (specialized in
physician coding) and Certified Professional Coder specialized in hospital coding (CPC-H), as
well as an RHIA. She personally conducted validation coding and provided guidance on the
development of the final validation tools and insights into validation findings.
1.3.3 RTI Physician Review for Accuracy of Coding
Two kinds of physician assistance were made available to the Clarity Coding coders to
help determine the accuracy of a given case. If only minimal clinical clarification on a case was
needed, the Clarity Coding coder was able to query an RTI physician directly by phone. If more
formal physician review was needed, the coder was able to submit the case to the RTI physician
in writing, including a brief reason for the review request. RTI physicians made a diagnostic
assessment concerning the potential presence of an identified condition and made the final
determination on coding accuracy of the record on all formally reviewed cases.
11
SECTION 2
SAMPLING DESIGN
2.1 Sampling Plan
There were several key components of the sampling plan for this study:
1. Assumptions about the rates of coding accuracy (error) to be estimated through
medical record abstraction and review;
2. Descriptions of the parameters to be estimated;
3. Desired margins of error of the parameter estimates;
4. The sample sizes required to achieve the objective of estimating the coding
accuracy rates; and
5. The medical record sampling plan.
As described below, we discussed these components of the sampling plan with the
funders, and drew upon evidence from the literature and other sources to develop our sample size
estimates.
2.1.1 Measures of Interest
As described above in Section 1.3, the statistical measure of interest for HACs is the rate
at which a condition is not coded by the hospital as a HAC, but where our validation process
determines the condition was hospital acquired. Similarly, the statistical measure of interest for
POA coding is the rate at which a condition is reported by the hospital as POA, but our
validation process determined the condition was not POA.
2.1.2 Estimated Magnitude of the Parameters
For the reasons described below in Sections 2.3.2 and 2.3.3, we employed a single-stage
sampling process to identify medical records for POA validation, and a multistage process to
identify medical records for HAC validation. This necessitated drawing two independent
samples for the HAC under-reporting sample and the POA over-reporting sample.
To calculate the sample size for this study, we assumed a 20% error rate in reporting
HACs. There is little empirical evidence as to the likely error rate in coding HACs, so the results
of a small study that reviewed medical records for 80 patients at a single academic medical
center were used as a guide. That study found that 35% of patients with a secondary diagnosis of
a urinary tract infection (UTI) actually had a CAUTI (Meddings et al., 2009). Given that RTI is
using a broader sample of patients, and not just those with a secondary diagnosis of a UTI, we
used a 20% error rate.
We assumed a 12.5% error rate for reporting HACs as POA. Studies in California
involving pneumonia and lung cancer cases indicated that POA coding agreed with two widely
used comorbidity algorithms—the Deyo/Charlson and the Elixhauser algorithms, respectively

12
(Southern, Quan, and Ghali, 2004)—86% to 95% of the time, respectively (Stukenborg et al.,
2007). Therefore, as the starting point for our sample size calculation, we took the midpoint of
the 86–95% range (90.5%) and subtracted from 100% to obtain 9.5%. Because most other states
have not had as much experience with POA coding as California has, we increased the estimate
of the error rate by 3 percentage points to 12.5%.
2.1.3 Margin of Error and Confidence Intervals
For quality improvement studies and reporting, many of the Healthcare Effectiveness
Data and Information Set (HEDIS) measure samples developed by the National Committee for
Quality Assurance (NCQA) are designed to yield a probability of Type I error not greater than
±5%. Type I error is equivalent to an unreported HAC—the failure to identify a true null
hypothesis that a condition exists. For this task, we agreed upon a ±5% margin of error for the
HAC under-reporting sample, and ±3% margin of error for the POA over-reporting sample.
2.1.4 Sample Size Requirements for Medical Record Abstraction
Based on the above assumptions, the base sample size calculated for under-reporting for
each HAC was 264 records, and the sample size for estimating POA over-reporting was 499
records. As the project progressed, it became clear not enough records would be eligible for the
POA accuracy of coding review. Table 2-1, below, shows the actual number of records eligible
for review by condition. For CAUTI and VCAI under-reporting, 264 records were selected. For
DVT/PE, only 222 cases were identified as eligible for review. We selected all CERT records
that matched MedPAR records containing one of the orthopedic procedure codes that define the
denominator for the DVT/PE HAC measure.
The sample size issue was more acute for the POA cases, rendering RTI unable to
conduct POA coding accuracy by individual condition. We therefore developed abstraction tools
for all conditions coded as POA that had five or more eligible medical records, and conducted
medical record abstraction for all such cases. We conducted our analyses across the full set of
cases.
Table 2-1
Sample sizes for each condition
Hospital-acquired condition
HAC unreported
sample
POA over-
reported sample
Vascular catheter-associated infection 264 13
Catheter-associated urinary tract infection
264
5
Deep vein thrombosis/pulmonary embolism
222
—
Pressure ulcers — 105
Falls and trauma — 181
Manifestations of poor glycemic control — 14
NOTE: HAC, hospital-acquired condition; POA, present on admission.
13
2.2 Sampling Frame
A sampling frame represents the population from which the sample is to be drawn. To
comply with the Improper Payments Information Act (IPIA) of 2002 and support Medicare Fee
for Service (FFS) contractors in targeting review and education, CMS runs the CERT program
(CMS, 2012). This initiative selects a sample of Medicare claims and reviews them for accuracy
of payment, including the medical necessity of the hospitalization. Each year, the CERT
program samples discharges for all clinical conditions from Medicare claims. The component of
the CERT process relevant to this task is discharges from acute care hospitals subject to the
Medicare Inpatient Prospective Payment System (IPPS). There were approximately 20 million
such discharges in FY 2009 and FY 2010. Note that the IPPS excludes many types of hospitals
and other providers, such as comprehensive cancer centers, psychiatric hospitals, and
rehabilitation facilities.
CMS staff provided us with the FY 2009 and FY 2010 medical records received by the
CERT program for use in this task, including the information necessary to link the medical
records to Medicare claims data. After excluding those hospitals not subject to the IPPS (and
therefore not subject to the HAC-POA policy), our sampling frame was 10,465 unique CERT
medical records. Using beneficiary and hospital identification information in the medical
records, we linked the records to the MedPAR claims data for FY 2009 and FY 2010.
The CERT program uses random sampling to choose records for review; however, the
randomization process is not publicly available. It should be noted that RTI was not provided
with complete information about the degree of randomness used in the chart selection process.
To ensure that the CERT records were representative of all Medicare IPPS discharges, we
compared characteristics of the CERT medical records to the characteristics of all discharges
from IPPS hospitals in the FY 2009 and FY 2010 MedPAR database. The distributional
properties of the CERT records are consistently similar to the MedPAR records across patient
and hospital characteristics such as patient age, gender, race, principal diagnoses, and hospital
size and location. Given the large sample sizes of approximately 20 million MedPAR records
and 11,000 CERT records, further analysis of this question should not be necessary. The CERT
records are broadly representative of the MedPAR records and therefore are representative of the
population of IPPS-eligible Medicare discharges. For our analysis, it should therefore not be
necessary to apply weighting to the CERT records when conducting analysis applicable to claims
data from the entire IPPS-eligible Medicare population. For a more detailed analysis of the
representativeness of the CERT records, please refer to Appendix A.
2.3 Medical Record Sampling Plan
The key issues encountered in the medical record sampling included linking the CERT
medical records to MedPAR claims, and identifying medical records for coding validation.
2.3.1 Linking CERT Medical Records to MedPAR Data
The CERT program’s medical records are electronic images that are linkable to the FY
2009 and FY 2010 MedPAR claims data. We linked 10,465 unique CERT medical records to
their respective IPPS-eligible MedPAR claims, using beneficiary and hospital identification
information in the medical record. This step allowed us to create an electronic database linking
14
claims data to the medical records, and populating the database with information from the
MedPAR claims—including diagnosis code(s), procedure code(s), and other data elements that
might be useful in our analyses. This database enabled us to identify which of the linked medical
records were eligible for inclusion in the validation samples.
2.3.2 Sampling Plan for Assessing Accuracy of POA Coding
Identification of medical records for validating POA coding was straightforward. Using
the linked MedPAR–medical record database, claims with at least one of the 10 HAC-associated
diagnosis codes reported as POA for coding validation were selected. The following five
conditions were selected for medical record abstraction, with the number of CERT records in
parentheses: CAUTI (13); VCAI (5); Stage III or IV pressure ulcers (105); falls and trauma
(181); and extreme manifestations of poor glycemic control (14). This process yielded a total
sample of 318 cases across all five conditions. This was in contrast to the desired 499 records
per condition. We developed abstraction tools for these five conditions.
2.3.3 Sampling Plan for Assessing Accuracy of HAC Coding
A multistage process was used to identify medical records for validation of appropriate
reporting for CAUTI. DVT/PE and VCAI record identification was more straightforward. The
sampling plans for each of these conditions are outlined below.
2.3.3.1 Sampling Plan for CAUTI
To identify CAUTI cases for review, we first identified MedPAR records that linked to
CERT records and had the presence of an indwelling urinary catheter coded on the MedPAR
record (ICD-9 code 57.94 or 57.95), excluding any cases where CAUTI was coded as hospital
acquired or POA. This produced 90 CERT records for review.
To produce more cases, MedPAR records were linked with their corresponding physician
claims (Medicare Part B claims) to identify cases where a urology consult was provided
(identified using CMS specialty code 34) or a physician billed for insertion of indwelling urinary
catheter during the inpatient stay (CPT code 51702 or 51703). This yielded an additional 35
CERT records for review.
We then explored the use of proxies to identify MedPAR records that showed a high
likelihood of the patient having had an indwelling catheter inserted. Of the MedPAR records
with an indwelling urinary catheter coded, 35% had a general infection reported and 37% had
stays in an intensive care unit (ICU) or critical care unit (CCU). To obtain more CAUTI cases
for review, we identified 5,236 cases that did not have an indwelling urinary catheter coded, but
did have an ICU or CCU stay, as well as one of the following infections coded as a secondary
diagnosis:
112.2 Candidiasis of other urogenital sites
590.10 Acute pyelonephritis without lesion of renal medullary necrosis.
590.11 Acute pyelonephritis with lesion of renal medullary necrosis
15
590.2 Renal and perinephric abscess
590.3 Pyeloureteritis cystica
590.8 Pyelonephritis unspecified-inflammation of the kidney and its pelvis due to
infection
590.81 Pyelitis or pyelonephritis in diseases classified elsewhere
595.0 Acute cystitis
597.0 Urethral abscess
599.0 Urinary tract infection site not specified
RTI’s staff screened these CERT records for the presence of an indwelling urinary
catheter. Of the 308 such cases screened, 139 had evidence that the beneficiary had an
indwelling catheter inserted at some time during the hospital admission. These cases, in addition
to the 125 cases previously identified, provided the cases needed to obtain a sample of 264
CERT medical records.
2.3.3.2 Sampling Plan for VCAI
To identify VCAI records for validation, we screened MedPAR records with a central
line or venous catheterization coded as having been inserted during the hospitalization, excluding
records where VCAI was coded as hospital acquired or POA. This yielded 881 CERT records; a
random sample of 264 was selected.
2.3.3.3 Sampling Plan for DVT/PE
CERT medical records for DVT/PE validation were selected by identifying MedPAR
records with corresponding claims that did not have DVT/PE coded as hospital acquired or POA,
but did have one of the following orthopedic procedures coded:
00.85—00.87 Hip resurfacing, total or partial
81.51—81.52 Hip replacement, total or partial (not revision)
81.54 Knee replacement, total or partial
This yielded a total of 222 CERT records. While this was less than the 264 desired for
the coding review, there were no additional discharges available in which the patient had
undergone one of these orthopedic procedures.
16
[This page left intentionally blank]
17
SECTION 3
MEDICAL RECORD VALIDATION PLAN
To facilitate the HAC and POA coding validation, we developed criteria for each
condition based on materials provided by the funding partners, the published literature, and other
key informant sources. We incorporated these criteria into an abstraction tool for each condition.
Clarity Coding used the abstraction tool to gather information from the medical records
concerning the characteristics of the cases where there was disagreement between the coders and
the hospital coding.
While there are common elements among the conditions being examined, each
abstraction tool is tailored for the validation needs of the specific condition. Since the general
flow of coding validation is the same across the conditions, in this section we describe the
general process for validating unreported HAC cases and over-reported POA cases. A general
description of the abstraction tool follows. A copy of each abstraction tool, with condition-
specific criteria, is available in Appendix B.
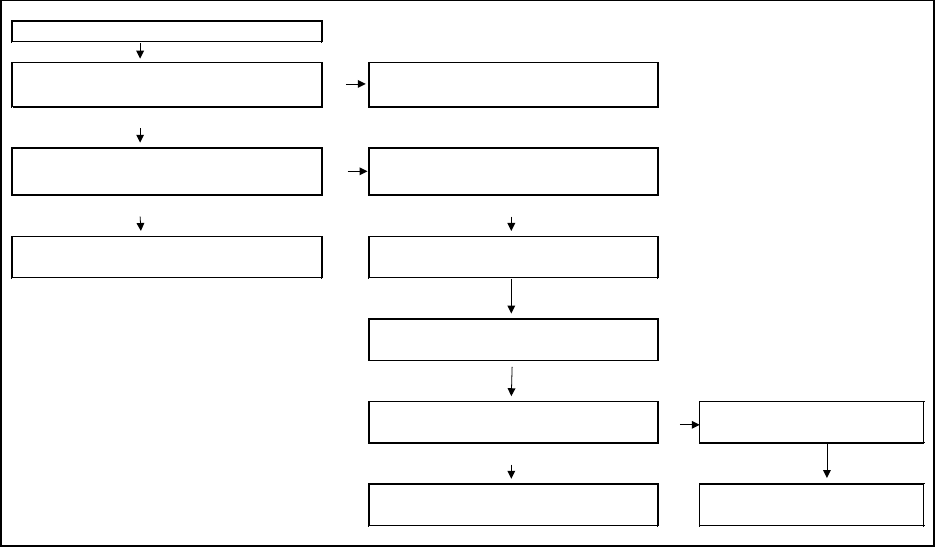
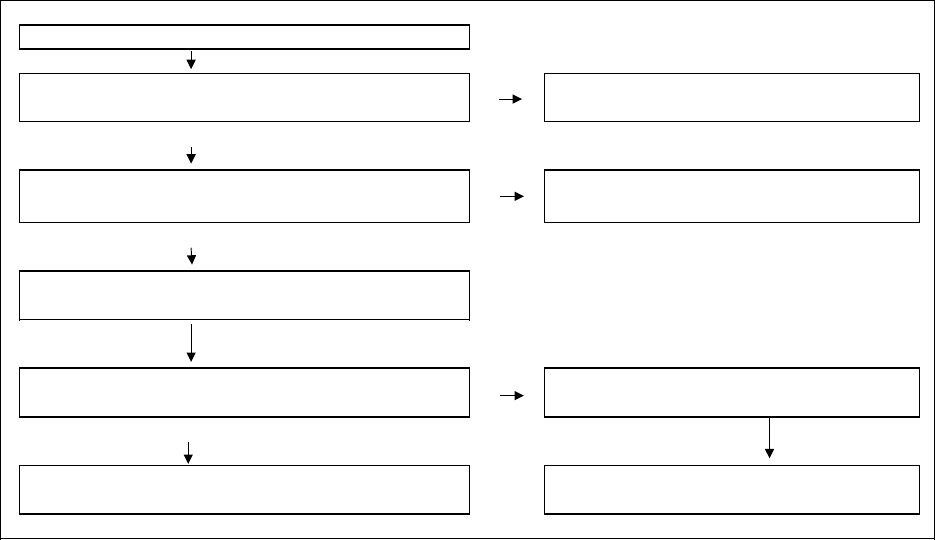
3.1 Medical Record Validation Flow Diagrams
Figures 3-1 and 3-2, below, show the medical record validation flow diagrams. The
process starts with the linking of the FY 2009 or FY 2010 MedPAR data to the CERT program
medical records, and ends with the outputs of the data analysis. Figure 3-1 displays the medical
record validation flow diagram for assessing under-reporting of hospital-acquired CAUTI,
VCAI, and DVT/PE. CERT records without a corresponding MedPAR record were excluded
from the study. Of those CERT records that did have a corresponding MedPAR file, those with
a CAUTI, VCAI, or DVT/PE diagnosis were excluded—since they by definition do not represent
an unreported HAC case.
The linked MedPAR and CERT data were then screened for the presence of clinical
proxies and procedures that might indicate the presence of an unreported HAC. For those
records that did show one or more of these proxies and procedures, a sample was generated if
there were more than enough cases of that HAC type (see Section 2.1.4—Sample Size
Requirements for Medical Record Abstraction). The record was then sent for coding validation,
as shown in Figure 3-1. Records containing complexities or ambiguities that required additional
physician review were set aside by the coders and sent to one of the study physicians. Once
coded, all the records were reassembled for data entry.
18
Figure 3-1
Unreported HACs: Medical record validation flow diagram
Yes
Yes Yes
Yes
Excluded from Under Reporting Study
Record Sent for Coding Validation
Corresponding CERT Record
Linked to Claims Data
No
FY2009 & FY2010 CERT Records
CAUTI, VCAI, or DVT/PE Coded on claim as
Hospital Acquired or Present on Admission?
Excluded from Under Reporting Study
No
Output for Data Analysis
Output for Data Analysis
Presence of Proxy or Procedure?
Sample Generated (if applicable)
Abstraction Tool Completed by Coder?
No
Reviewed by RTI Physican
Figure 3-2, below, displays the medical record validation flow diagram for assessing
over-reported POA coding. As with the potential HAC cases, for each case it was essential to
have a CERT record that corresponded with the MedPAR claim(s) available for validation
purposes. If this condition was not met, the record in question was excluded.
In cases where a CERT record was sent for validation, the Clarity Coding coder
abstracted the information required to complete the validation tool. If the Clarity Coding coder
agreed with the hospital coder or made a technical coding change, the record was then sent to be
entered into the analytic database. If the Clarity Coding coder believed that medical judgment
was necessary, the coder referred the case to an RTI physician reviewer. Prior to doing so, the
coder populated the validation tool with information from the record—including specific
comments as to why the case was ambiguous. A similar flow occurred for the validation of POA
coding.
19
Figure 3-2
Over-reported POA: Medical record validation flow diagram
Yes
Yes
Yes
FY2009 & FY2010 CERT Records
Corresponding CERT Record Linked to Claims Data
CAUTI, VCAI, Stage II or IV Pressure Ulcer, Fall/Trama,
or Poor Glycemic Control Coded as Present on Admission?
Chart Sent for Coding Validation
Abstraction Tool Completed by Coder?
Output for Data Analysis
Excluded from Over-Reported Study
Excluded from Over-Reported Study
Output for Data Analysis
No
Reviewed by RTI Physican
No
No
If one of the potentially over-reported conditions—CAUTI, VCAI, Stage III or IV
pressure ulcers, falls and trauma, or poor glycemic control—was not coded as POA, then the
record was excluded. If one or more of these conditions was included, then the record was sent
for review by a coder, who completed the appropriate abstraction tool based on the type of
suspected over-reported condition (see Section 3.2, below). If coders had difficulties interpreting
the record or noted ambiguities, then the record was sent for review by an RTI physician.
Finally, the completed abstraction records were collected and entered into a database.
3.2 Medical Record Abstraction Tools
RTI developed abstraction tools for each condition of interest. These tools contain a list
of data elements for coders to collect from the medical record when conducting the validation.
The tools document evidence abstracted by the coders that supports their agreement or lack of
agreement with the hospital’s coding. The abstraction tools also provide the means by which
coders submitted cases for formal physician review. For CAUTI and VCAI—the only
conditions evaluated for both under-reporting and over-reporting—a single tool was developed
that could be used for either kind of review. The following sections describe the five main
components of the tools: Type of Review, Preliminary Evidence, Part I—Should the Listed
Condition be Coded?, Part II—Was the Listed Condition Present on Admission?, and
Disposition. Copies of the abstraction tools, including details of their clinical content, are
included in Appendix B.
20
3.2.1 Type of Review
Each tool has a similar format. At the top of each page is a box with checkboxes to
identify the type of record being abstracted. There are two record types: (1) over-reported, when
the listed condition is coded and indicated as POA; and (2) unreported, when the listed condition
was not coded. The applicable review type is designated in this section for each record.
Additional record-specific information is also displayed here. This includes the CERT record
identification number (CID#), admission date, discharge date, principal diagnosis code,
secondary diagnosis code(s), and procedure code(s). All of this information was prepopulated by
RTI from the linked MedPAR/CERT database. There are also fields for the coder abstracting the
record to self-identify and document the date of the abstraction. These are the only two fields in
this section that must be entered directly by the abstractor.
3.2.2 Preliminary Evidence
The conditions being reviewed for an unreported HAC have a “Preliminary” section in
the beginning of the abstraction tool. This section confirms that the condition exists, using
appropriate criteria such as presence of an indwelling urinary catheter. On each abstraction tool,
either Y or N is circled to indicate yes or no in response to the principal questions. More specific
information is entered as appropriate—for example, the date of insertion of an indwelling urinary
catheter.
3.2.3 Part I—Should the Listed Condition Be Coded?
Part I—Should the Condition be Coded—establishes whether the condition was
genuinely present during the stay and actually affected patient care. The response to the title
question is Yes, No, or Refer to Physician Advisor (PA). The response marked here is
dependent on the Yes or No responses to the questions in subparts A and B, described below.
Part I is separated into two subparts: (A) Did the Listed Condition Exist during the
Stay?, and (B) Did the Listed Condition Affect Patient Care? In cases where the listed condition
is being reviewed for an unreported HAC, subpart A asks if there is physician documentation of
the diagnosis. It also asks whether the listed condition was listed in the discharge diagnoses, and
if so, which position in the list it occupied. This is necessary because the discharge forms do not
identify specific conditions beyond the eighth secondary diagnosis.
Subpart A continues with two levels of evidence that vary in terms of how strongly the
clinical information supports the presence of a condition. Both levels include one or more yes-
or-no questions. These responses must be supported by documented findings in the medical
record. Level I evidence is sufficient to conclusively determine a condition was hospital
acquired. Further abstraction in this subpart, including looking for Level II evidence, was not
necessary after Level I evidence was identified.
All of the abstraction tools include Subpart A, but conditions reviewed exclusively for
POA do not have the initial questions about relevant physician documentation and discharge
diagnoses and are not divided into the Levels of Evidence. In other respects, Subpart A is the
same in all abstraction tools regardless of review type.
21
Subpart B is also the same on all of the tools regardless of review type. This component
assesses if the condition, once it is confirmed as present, affected patient care. This was assessed
by responses to a series of yes-or-no questions related to the treatments received and their impact
on patient care. The responses to these questions must be supported by evidence from the
medical record by checking box(es) from the provided list of appropriate evidence.
If the answer to either “should the listed condition be coded” or “did the listed condition
affect patient care” differed from the medical record, then the coder used his or her judgment to
make a yes-or-no determination. If the coder could not confidently make a decision, or if
medical judgment was necessary (or both), then the coder referred the case to a physician.
3.2.4 Part II—Was the Listed Condition Present on Admission?
The Part II title question was answered by checking one of the following options: (1)
Yes, present on admission; (2) No, developed after admission; or (3) Refer to physician. A
subsequent question asks for medical record documentation. As above, if the coder had any
doubts regarding the answers and believed that medical judgment was necessary, Refer to
physician was the response chosen.
3.2.5 Disposition
Each abstraction tool concluded with a disposition box. Each review type has a
corresponding series of options that are checked as appropriate. For the over-reported POA
review type, the hospital either correctly coded the condition as POA (the condition was
correctly coded as both existing and being POA) or did not correctly code the condition.
Incorrect coding includes records that were coded as POA when the condition was in fact
hospital acquired, or a condition that was coded as existing when it did not exist or did not affect
patient care.
In the unreported HAC review type, the hospital either correctly coded the absence of
condition or did not correctly code a condition that was in fact present. If it was determined that
the hospital is not correct—the condition should have been coded but was not—then the coder
proceeded to determine if the unreported condition was POA or hospital acquired.
3.3 Medical Record Validation Process
For a task designed to estimate accuracy of coding, uncompromising quality in data
collection, transmission, storage, and analysis is essential. Through a combination of training,
monitoring, and inspection, RTI has provided a high level of quality control, based on a quality
assurance plan developed collaboratively with Clarity Coding.
RTI obtained a selected group of acute care medical records from the CERT program for
FY 2009 and FY 2010. For tracking purposes, these medical records were delivered to RTI via
overnight courier by the CERT program contractor. The records were kept on an encrypted hard
drive, and a confidential process was used for obtaining, analyzing, and storing the medical
records. At the outset of this task, RTI also created an electronic data management system.
After documenting the receipt of incoming records, RTI entered the information into a dataset
22
containing the key data elements—including those needed to link the CERT records to the
MedPAR claims.
RTI worked with Clarity Coding to develop a data exchange protocol that ensured a
secure exchange of the medical records, similar to the data exchange with the CERT program
contractor. An encrypted hard drive containing the medical records was sent by courier to
Clarity Coding, with a signature receipt required. RTI also tracked the transmission of all of the
abstraction forms sent to and received from Clarity Coding—again using signed receipts for
confirmation.
To identify errors in screening or data collection early on, RTI reviewed the abstraction
tools with the abstraction task leader, Linda Holtzman, on multiple occasions to ensure that these
tools were clear and consistent. Ms. Holtzman also abstracted an initial batch of cases to confirm
that they had been appropriately identified for abstraction. The coders received an abstraction
training manual detailing the abstraction process, the use of each abstraction tool, and the key
procedures relative to the abstraction.
Each completed abstraction form was keyed into a form-based data entry tool that RTI
created and programmed for MS Excel, using VBA code for greater functionality. Checkboxes
and yes-or-no answers allowed only valid responses to be entered, while any free text sections of
the form allowed free form data entry so data could be keyed exactly as it appeared on the form.
All keying was completed by RTI staff, with independent rekeying by a different staff member.
Any differences were reconciled and recorded by the task leader.
3.4 RTI Institutional Review Board
In December 2010, the project received an exemption from the RTI Institutional Review
Board (IRB). We completed and submitted the request for exemption to the IRB, describing the
study procedures, participant population, risks to participants, methods of receipt and storage of
the medical records, and the measures taken to protect patient confidentiality. The medical
record validation activities began only after receiving written IRB approval. After IRB approval,
we modified our Data Use Agreement (DUA) with CMS to include the analysis of the CERT
program’s medical records. All individuals with direct access to the records—including the
coders at Clarity Coding, the RTI physicians, and all other RTI staff with access to the data—
were added to the DUA.
23
SECTION 4
DATA ANALYSIS
In this section, we describe the analyses carried out to assess the accuracy of coding and
the level of agreement or disagreement between the hospital-coded MedPAR records and the
RTI/Clarity Coding assessment of the same medical records. The initial strategy for this study
included analyses using the Kappa statistic and the Prevalence-Adjusted Bias-Adjusted Kappa
(PABAK) statistic. However, given the small sample sizes for assessing accuracy of POA
coding and the lack of discordant results (see discussion below), we concluded that summarizing
the level of agreement between hospital and Clarity Coding coders using the Kappa statistic and
the PABAK, which is used to interpret the Kappa, would not have yielded meaningful results
(Cunningham, 2009).
4.1 Unreported HAC Observations
When evaluating medical records for unreported HACs, the initial question is whether the
condition—CAUTI, VCAI, or DVT/PE—existed and should have been coded. If the condition
did indeed exist, the second question then is whether it was hospital acquired or POA. Table 4-1
shows that in 23 out of 749 total HAC cases, the condition was present but not reported.
In aggregate, we found an unreported HAC in just 3% (23 of 749) of the medical records
with final dispositions that we evaluated for under-reporting of CAUTI, VCAI, and DVT/PE. Of
the disagreements that were observed, the most frequent were for CAUTI cases, of which 6% (16
of 263) were inaccurately coded (i.e. the condition was present but not coded by the hospital).
The least frequent disagreement was for DVT/PE cases, with zero unreported HACs.
Table 4-1
Summary of HAC coding accuracy: CAUTI, VCAI, DVT/PE
Disposition
CAUTI
n
CAUTI
%
VCAI
n
VCAI
%
DVT/PE
n
DVT/PE
%
Hospital coded/reported
accurately
247
94%
257
97%
222
100%
Hospital did not code/report
accurately
16 6% 7 3% 0 0%
Total 263* 100% 264 100% 222 100%
NOTE: CAUTI, catheter-associated urinary tract infection; DVT/PE, deep vein
thrombosis/pulmonary embolism; HAC, hospital-acquired condition; VCAI, vascular catheter-
associated infection.
* There were originally 264 CAUTI cases, but one record was damaged and could not be opened
to be read.
However, in 17 of these 23 cases, the condition was POA. This leaves just 6 of the 749
cases as unreported HACs that were not POA (Table 4-2).
24
Table 4-2
Number of unreported HAC cases determined to be present on admission for three clinical
conditions: CAUTI, VCAI, DVT/PE
Disposition CAUTI VCAI DVT/PE
Unreported HAC was present on admission
10
7
0
Unreported HAC was not present on admission 6 0 0
Total 16 7 0
NOTE: CAUTI, catheter-associated urinary tract infection; DVT/PE, deep vein
thrombosis/pulmonary embolism; HAC, hospital-acquired condition; VCAI, vascular catheter-
associated infection.
For a coder to mark a condition as an unreported HAC, it must have both existed during
the hospital stay and affected patient care. Further information on this particular guideline,
which has consistently defined “other diagnoses” for more than 20 years, can be found in
Appendix C. For detailed information on how patient care could be affected by each condition,
please refer to Part I.B within each of the abstraction tools, in Appendix B. Table 4-3
summarizes how these distinctions influenced medical record validation. Overall, the coding
guideline requiring that the condition affect patient care appears to have had very little influence
on the final outcome of the cases, as evidenced by the fact that in only one case a present
condition was judged to have not affected patient care. That is the only case where a condition
was identified but not considered to be an unreported HAC.
Table 4-3
Number of cases where condition was present and how it affected patient care for three
clinical conditions: CAUTI, VCAI, DVT/PE
Disposition CAUTI VCAI DVT/PE
Condition was present and did affect patient care 16 7 0
Condition was present and did not affect patient care 1 0 0
Total
1
7
7 0
NOTE: CAUTI, catheter-associated urinary tract infection; DVT/PE, deep vein
thrombosis/pulmonary embolism; VCAI, vascular catheter-associated infection.
The strength of evidence supporting unreported classifications varies considerably. Each
of the unreported HAC abstraction tools includes two levels of evidence. Level I is clear and
objective evidence that the condition was present, such as specific laboratory results. Level II
evidence, while sufficient to confirm that a HAC was present, is more subjective. General signs
of infection counted as Level II for some conditions. Condition-specific details for both levels of
evidence are available in the abstraction tools, in Appendix B.
As evidenced in Table 4-4, the majority of the cases where the Clarity Coding coders
determined that the condition existed did in fact include Level I evidence. There are only two
cases where only Level II evidence was present and one case where both Level I evidence and
25
Level II evidence are present. This is partly due to the fact that the Clarity coders were
instructed to not continue abstracting for Level II evidence once Level I evidence was confirmed,
since Level I was sufficient to determine the existence of the condition. The small number of
cases with both levels of evidence does not mean that there might not be more such cases.
Table 4-4
Number of cases stratified by level of evidence that the unreported HAC cases were present
for three clinical conditions: CAUTI, VCAI, DVT/PE
Level of evidence
CAUTI
VCAI
DVT/PE
Level I evidence present 15 5 0
Level II evidence present
0
2
0
Level I & Level II evidence present
1
0
0
Total 16 7 0
NOTE: Coders may not have looked for Level II evidence after identifying Level I evidence,
acting consistently with the abstraction instructions provided to them. CAUTI, catheter-
associated urinary tract infection; DVT/PE, deep vein thrombosis/pulmonary embolism; HAC,
hospital-acquired condition; VCAI, vascular catheter-associated infection.
4.2 Over-Reported POA Observations
The results for over-reported POA cases are similar in magnitude. As shown in
Table 4-5, 91% of all cases coded POA were coded accurately. However, the level of
uncertainty around this estimate is large, given the small number of CERT medical records
available for abstraction. Of the 28 POA cases coded inaccurately, the highest percentages are
attributable to Stage III and IV pressure ulcers, with 10% (10 out of 105 cases), and falls and
trauma, with 8% (14 out of 181 cases) being incorrectly reported as POA, respectively. VCAI
and poor glycemic control each had only one inaccurately coded CERT record, while CAUTI
had two inaccurately coded CERT records.
Table 4-5
Summary of POA coding accuracy: All five POA conditions combined
Disposition N %
Hospital coded/reported accurately
290
91%
Hospital did not code/report accurately 28 9%
Total 318 100%
NOTE: POA, present on admission.
When evaluating medical records coded by the hospital with the condition being POA,
the coders looked for two things: (1) whether the condition existed during the stay, and (2)
whether the condition was POA. This approach allowed for two ways in which the Clarity
Coding coder could disagree with the hospital coder. From the cases reviewed in this study, the
former seemed to be the main reason for coder disagreement; 23 out of 28 inaccurately coded

26
POA cases were inaccurate because the condition did not exist at the time of admission or at any
time during the hospitalization (Table 4-6).
Table 4-6
Over-reported POA: number of cases where the condition was present, but not on
admission, or was not present at all
Disposition N
Condition was present but not on admission 5
Condition was not present at all 23
Total 28
NOTE: POA, present on admission.
Of the 23 cases incorrectly coded with respect to the presence of the condition, falls and
trauma accounted for 13 cases and Stage III and IV pressure ulcers accounted for 8 cases.
A single case each was attributable to CAUTI and poor glycemic control. VCAI did not account
for any such cases. Clarity Coding provided us with two concrete examples of records they
abstracted where this was true. The first is a pressure ulcer case, summarized as follows:
73-year-old nursing facility resident was admitted through the emergency
department with change in mental status, uncontrolled diabetes, and dehydration
on April 19 (discharged on May 2). The emergency department nurse
documented a pressure ulcer present on admission and formally notified the
emergency department physician. A wound care consult was ordered on
admission. Multiple wound care notes documented the skin breakdown variously
as “denuded areas,” “partial to full thickness skin loss,” and “partial thickness
skin loss.” However, the wound care notes did not use the term “decubitus” or
“pressure ulcer” and never documented the stage. Nurse’s notes variously
documented Stage I and II pressure ulcers and excoriations. Unfortunately, all
physician progress notes were too faint to read and the Discharge Summary,
while documenting decubitus ulcer of the buttocks, did not document the stage.
The hospital coded 707.23 for stage III pressure ulcer of the buttocks, present on
admission. The Clarity Coding coder disallowed Stage III because the stage
could not be confirmed with the existing documentation.
Here is another example of how this concept applied to a CAUTI case:
An 82-year-old male was admitted on March 15 (discharged on March 16) for
right lower quadrant pain ascribed to an incarcerated inguinal hernia with
partial bowel obstruction. The patient had a chronic indwelling Foley catheter
with a history of recurrent urinary tract infections with MRSA. Urinalysis in the
emergency department was positive for more than 50 WBCs and the patient was
put on Vancomycin. A urology consultation documented the impression as
“chronically colonized bladder due to catheter dependent status” and stated that
27
“regardless of what the culture shows, the patient does not appear to be overtly
septic.” The urologist recommended stopping antibiotics “unless overt infection
apparent.” The urine culture showed a colony count >100,000 for MRSA and
>100,000 for Corynebacterium. Vancomycin was continued through the stay and
the patient was sent home on Bactrim. The Discharge Summary gave the
diagnosis as “recurrent urinary tract infection versus bacterial colonization with
chronic indwelling Foley catheter.”
The hospital coded 996.64 and 599.0 for CAUTI, present on admission. In the
absence of coding guidelines on chronic bacterial colonization associated with
indwelling urinary catheters, and after discussion with an RTI physician reviewer
centering on continuation of antibiotics, the Clarity Coding coder ultimately
allowed this.
4.3 Strength of Evidence for POA
The type of evidence supporting a condition as being POA varies. The first main
category is documentation that the condition was either established or evolving upon admission,
as evidenced by one of the following being documented upon admission: (1) a diagnosis of the
condition, with documentation by a physician that the condition existed or that it cannot be
clinically determined, or (2) the possibility or suspicion that the condition is present on
admission..
The other main category is documentation of definitive treatment for the condition upon
admission; this documentation is by definition condition-specific. The specific types of evidence
cited to support a condition as being POA for each case are presented in Table 4-7, below. The
summary of the evidence presented in the table shows that in nearly all cases there was
documentation of the condition having been established or evolving at admission. In more than
half of the correctly coded cases, both types of evidence were present to support the hospital’s
coding.
Table 4-7
Over-reported POA: POA summary of evidence
Level of evidence
CAUTI
VCAI
Poor
glycemic
control
Falls
&
trauma
Pressure
ulcer
Condition was established or evolving
upon admission
9 3 6 73 36
Definitive treatment was ordered upon
admission
0 0 0 0 3
Both types of evidence were present
2
1
7
94
56
Total
11
4
13
167
95
NOTE: CAUTI, catheter-associated urinary tract infection; POA, present on admission; VCAI,
vascular catheter-associated infection.

28
Stage III and IV pressure ulcers have some unique criteria to support the condition being
POA. Pressure ulcers also had the highest degree of coder disagreement among the POA
conditions. Table 4-8, below, presents evidence for a POA determination, specifically for the
pressure ulcer cases. Only 32 of the 95 correctly coded cases had a single piece of evidence in
support of the POA coding; 58 of the cases had two pieces of evidence, and 5 cases had three.
Documentation of a current or healing pressure ulcer was cited in 83% of the cases, followed by
treatment of other measures ordered within 24 hours of admission, which was cited 62% of the
time. A pressure ulcer was never documented as a possible, suspected, or differential diagnosis
in our review. While cited only four times, cases where a Stage I or II pressure ulcer POA
progressed to a Stage III or IV during the stay are of particular interest. A specific case that
exemplifies this issue is discussed in detail in Section 4.4.2.
Table 4-8
Over-reported POA: POA pressure ulcer evidence
Level of evidence N %
Documentation of current or healing pressure ulcer upon admit 79 83%
Pressure ulcer possible, suspected, or differential diagnosis within 24
hours of admission
0 0%
Localized skin or underlying tissue injury, sore, ulcer, or wound over
bony prominence documented on admission at site later diagnosed
with pressure ulcer
7 7%
Treatment or other measures, including consultation, ordered within
24 hours of admission
59 62%
Stage I or II pressure ulcer present on admission that progressed to
Stage III or IV during the stay
4 4%
Primary source physician documentation of present on admission, or
inability to clinically determine
14 15%
NOTE: POA, present on admission.
4.4 Abstraction Observations
Clarity Coding was asked to provide RTI with their observations from the detailed
medical record reviews they performed that may have implications for interpreting the findings.
They provided specific cases that illustrate two coding issues identified that may affect
interpretation of the validation results: lack of physician queries in the medical records with
respect to CAUTI, and the requirement to code progression of pressure ulcers to Stage III or IV
during the hospitalization as POA.
4.4.1 Physician Queries Related to Potential HACs
Clinical coders are not clinicians and therefore cannot make clinical inferences about a
case. In the hospital, clinical ambiguities may be resolved by querying the physician. Clarity
Coding did not have such an option, and often when the Clarity Coding coder felt such a query
was necessary it could not be found in the medical record.
29
RTI physicians were asked to review a total of nine records to clarify confusion about
diagnoses and recommend a final determination. Six were potential VCAI cases; two were
questions about pressure ulcer cases; and one was for a possible CAUTI. The VCAI questions
all concerned possible under-reporting of a HAC and were referred because of poor
documentation. In five of the six cases, the physician recommended not classifying the record as
a HAC. The two pressure ulcer records that were referred for physician adjudication concerned
possible over-reporting as a HAC. The physician recommended accepting one of the HACs and
disallowing the other. Finally, the single CAUTI record referred questioned whether it was in
fact a CAUTI and a HAC. The RTI physician determined that it was both.
In addition, in the unreported CAUTI medical record review were cases in which the
coder identified both an indwelling urinary catheter and a proximal UTI, but without a physician
clinically connecting the two events the record could not be confirmed as miscoded. The
following is a summary of an abstracted case that exemplifies this:
A 68-year-old man was seen in the emergency department with chest pain and
hypotension, and was admitted to the intensive care unit with concern for septic
shock on April 25 (discharged May 8). The patient had history of a recent
urinary tract infection and had a chronic indwelling Foley catheter for urinary
retention. Urinalysis taken in the emergency department was positive for more
than 50 white blood cells. Recorded diagnoses for this admission include
urosepsis, UTI, and early sepsis. The physician documented that “cultures would
not grow as he was on antibiotics prophylactically prior to coming to the
hospital.” The patient remained on multiple antibiotics during the stay and was
discharged with the Foley catheter in place.
The hospital coded only 599.0 for UTI, and did not code 996.64 for infection due
to indwelling urinary catheter. No physicians, including a urology consultant,
specifically linked the UTI to the indwelling catheter and coding guidelines do not
allow coders to assume a relationship.
Lack of physician query influencing the outcome of an abstraction was also evident in a
pressure ulcer case concerning a record reviewed for POA validation:
An 87-year-old female was admitted on August 7 with severe abdominal pain
following an outpatient ERCP (discharged September 10). The Nursing
Admission History indicated erythema of the buttocks, while not checking
pressure ulcer. On the physician History and Physical, the Physical Exam
documented “
∅
” skin problems. Another physician Physical Exam three days
later (8-11) documented “
∅
” problems for skin, buttocks, and back. Although
nursing notes intermittently documented erythema of the buttocks, the first
mention of a skin tear in the nurse’s notes was twelve days after admission (8-19).
Four days after this (8-23), nurse’s notes documented a Stage II ulcer of the
sacrum and the physician ordered a wound care consult. The following day (8-
24), the wound care physician documented a Stage III pressure ulcer of the
sacrum.
30
The hospital coded 707.23 for Stage III pressure ulcer, present on admission. The
Clarity Coding coder confirmed the Stage III ulcer but disallowed the designation
of present on admission as insufficiently supported. Nursing and physician
documentation were inconsistent regarding erythema of the buttock on admission
and it is also not clear if this could be linked to the eventual pressure ulcer of the
sacrum. A physician query would have been helpful but no query was present in
the record.
These anecdotal cases demonstrate cases in which it appears that physician queries were
not requested to clarify ambiguous cases. While not technically miscoded, these cases do not
seem to accurately reflect the true condition of the patient and its relationship to the
hospitalization.
4.4.2 Pressure Ulcer Coding
This last example illustrates a coding guideline specific to pressure ulcers that also serves
to potentially misrepresent a patient’s condition and its relationship to the hospitalization:
An 85-year-old female was admitted through the emergency department on July 4
(discharged on August 4) for respiratory failure. Documentation in the
emergency department did not identify any skin issues and examination of the
back was deferred on the history and physical However, on the day of admission,
a stage II sacral decubitus ulcer was documented in the physician progress notes.
Ten days after admission (July 14), the ulcer was documented in the physician
progress notes as stage III and an order was written for a wound care consult.
The wound care documentation stated the patient had been admitted with a stage
II pressure ulcer which had then progressed to stage III. Sixteen days later (July
30), the wound care documentation stated that the ulcer was sloughing, and the
following day (July 31), the physician documented the ulcer as unstageable.
The hospital coded 707.23 for stage III pressure ulcer, present on admission, and
the Clarity Coding coder ultimately confirmed the hospital coding. In addition to
the clinical issues, this case involved two coding principles. First, although the
pressure ulcer eventually became unstageable, coding guidelines do not state that
a pressure ulcer is coded at its final stage, but rather at its highest stage. Second,
coding guidelines direct that the stage III pressure ulcer be confirmed as present
on admission, because a lower stage ulcer was recognized on admission and
progressed to a higher stage ulcer during the admission.
31
SECTION 5
DISCUSSION
The principal objective of the Accuracy of Coding component of the HAC-POA payment
policy project is to calculate the accuracy of hospitals’ coding of HACs and POA conditions.
Accurate coding of HACs and whether HAC-associated conditions are POA is essential—both
for the program’s payment incentives to be effective and also to enable effective evaluation of
the program’s effects. We have evaluated the degree to which independent coders validated
hospital coding of HACs and the presence of these conditions on admission. To carry out this
project, RTI International has collaborated with Clarity Coding, which conducted the medical
record review necessary to provide the data for this evaluation.
In summary, we did not find patterns of widespread under-reporting of HACs or over-
reporting of POA status. In just 23 out of a total of 749 HAC cases (3%), the condition was
determined to be present but not reported. Of the disagreements that were observed, the most
frequent were for CAUTI cases, 6% of which were miscoded. The least frequent disagreement
was for DVT/PE cases, with no unreported HACs (Table ES-1). For 17 of 23 HAC cases, the
condition was POA. This leaves just 6 of the 749 cases that were both hospital acquired and
unreported.
The results for over-reported POA cases are similar in magnitude. Of all the cases coded
POA, 91% were coded accurately. The highest percentages are attributable to Stage III and IV
pressure ulcers, with 9% (9 out of 105 cases), and falls and trauma, with 8% (14 out of 181
cases) being incorrectly reported as POA.
There is considerable variation in the strength of evidence supporting the confirmation of
conditions as unreported. Each of the unreported HAC abstraction tools was divided into two
levels of evidence. Level I was clear and objective evidence that the condition was present, such
as specific laboratory results. Level II evidence, while sufficient to confirm a HAC was present,
was more subjective. General signs of infection counted as Level II for some conditions. Of all
the cases where the Clarity Coding coders determined that the condition existed, the majority of
cases contained Level I Evidence.
Clarity Coding was asked to provide RTI with their observations from the detailed
medical record reviews they performed. They noted that two specific types of cases were
particularly challenging: unreported CAUTI and over-reported POA pressure ulcers. They
provided specific cases illustrating that two coding issues identified may affect interpretation of
the validation results: a lack of physician queries in the medical records, and the requirement to
code progression of pressure ulcers to Stage III or IV during the hospitalization as POA. The
coders found numerous instances in which the hospital coding was in accordance with coding
guidelines, but the conditions might have been perceived as hospital acquired by clinicians
unfamiliar with coding practices. Using exclusively clinical validation criteria not requiring
conformance with official coding guidelines, more instances of under-reporting of HACs or
over-reporting of POA may have been found. However, from a coding perspective, the
conditions could not be determined to be hospital acquired. Coding is fundamental to
administration of the HAC-POA program, and its requisites must be observed.
32
The coding guidelines themselves are fundamental to successful execution of the HAC-
POA program. These guidelines are found in either ICD-9-CM Official Guidelines for Coding
and Reporting or Coding Clinic for ICD-9-CM. Guidelines from these sources are approved by
the Cooperating Parties, consisting of the American Hospital Association (AHA), the American
Health Information Management Association (AHIMA), the National Center for Health Statistics
(NCHS), and CMS. All clinical coders must comply with these guidelines. Further information
on clinical coding guidelines—as well as the specific guidelines referenced in this report—is
presented in Appendix C.
Clinical coders are not clinicians, and therefore cannot make clinical inferences about a
case. In the hospital, clinical ambiguities may be resolved by querying the physician. Clarity
Coding did not have such an option, and often when the Clarity Coding coder felt such a query
was necessary, it could not be found in the medical record. With unreported CAUTI medical
record review, there were cases in which the coder identified both an indwelling urinary catheter
and a proximal UTI, but without a physician clinically connecting the two events, the record
could not be confirmed as miscoded. Anecdotal cases serve to show that physician queries are
potentially not being requested to clarify ambiguous cases. While not technically miscoded,
these cases do not seem to accurately reflect the true condition of the patient and its relationship
to the hospitalization. However, the lack of physician queries may, in fact, be a reflection of
guidance. Coding guidelines instruct coders to not make an assumption that a UTI and an
indwelling catheter are related (refer to Appendix C). It may be desirable for purposes of coding
accuracy to revise the guidelines to encourage or require physician queries about the relationship
and to provide greater education to physicians about carefully documenting whether a UTI is
associated with an indwelling urinary catheter.
In addition, the coders also encountered ambiguity related to chronic bacterial
colonization associated with long-term indwelling urinary catheterization. In several cases, the
coders reviewed physician documentation stating that colonization is an expected state and
patients should not be assumed to have a UTI in the absence of other findings of active infection
(e.g., fever), regardless of a positive urine culture. In addition, it was not clear if antibiotic use in
these cases was directly therapeutic or prophylactic. At this time, no official guideline
specifically addresses coding bacterial colonization versus urinary tract infection in this context.
Coding Clinic may wish to consider publishing specific guidance on this useful and practical
topic.
In looking at the pressure ulcer cases, the coders noted a number of cases in which
pressure ulcers were documented as “Stage II–III.” In all cases the hospital coded the higher
stage. The difference between a Stage II and a Stage III is significant for the HAC-POA
program. While some pressure ulcers may clinically be between stages, lack of a coding
guideline for this scenario inadvertently provides incentives to be imprecise in the determination
and documentation of pressure ulcers. It may be worthwhile for Coding Clinic to address this
topic and provide guidance on, for example, which stage to code and whether a query is
necessary.
With respect to progression of pressure ulcers to Stage III or IV during the
hospitalization, coding guidelines direct that the Stage III or IV pressure ulcer be confirmed as
POA if a lower stage ulcer was recognized on admission and progressed to a higher stage ulcer
33
during the admission (refer to Appendix C). CMS may wish to discuss the unintended
consequences of coding guidelines on the HAC-POA payment policy with the other Cooperating
Parties.
Finally, the inconsistency in how hospitals store queries creates issues with accessing
them. This can impede any type of external coding review and inadvertently skew its findings.
If possible, hospitals should be urged to uniformly include all queries and their responses as part
of the permanent medical record. This would ensure that a complete clinical picture is available
to reviewers and can be reflected in their findings.
34
[This page left intentionally blank]
35
REFERENCES
CMS. Comprehensive Error Rate Testing (CERT). Available at http://www.cms.gov/Research-
Statistics-Data-and-Systems/Monitoring-Programs/CERT/index.html?redirect=/CERT/
Cunningham, M.: More than Just the Kappa Coefficient: A Program to Fully Characterize Inter-
Rater Reliability between Two Raters. Paper 242-2009, SAS Global Forum, Washington, DC,
2009. Available at http://support.sas.com/resources/papers/proceedings09/242-2009.pdf
Levinson, R. D.: Adverse Events in Hospitals: Methods for Identifying Events. Department of
Health and Human Services. OEI-06-08-00221. March, 2010. Available at
http://oig.hhs.gov/oei/reports/oei-06-08-00221.pdf
Meddings, J., Saint, S., and McMahon, L.: Are Urinary Tract Infections Coded Accurately to
Trigger Non-Payment by the CMS Hospital-Acquired Conditions Initiative? Presentation at
AcademyHealth, Chicago, 2009.
Southern, D.A., Quan, H., and Ghali, W.A.: Comparison of the Elixhauser and Charlson/Deyo
methods of comorbidity measurement in administrative data. Medical Care 42(4):355-360,
2004.
Stukenborg, G.J., Wagner, D.P., Harrell, F.E., et al.: Which hospitals have significantly better or
worse than expected mortality rates for acute myocardial infarction patients? Improved risk
adjustment with present-at admission diagnoses. Circulation 116(25): 2960-2968, 2007.
Trick, W., Zagorski, B., Tokars, J., et al.: Computer Algorithms to Detect Bloodstream
Infections. Emerging Infectious Disease 10(9), 1612-1620, 2004.
36
[This page left intentionally blank]
37
APPENDIX A
ASSESSMENT OF THE REPRESENTATIVENESS OF THE CERT RECORDS
This section summarizes our findings with respect to the similarities and differences
between Medicare claims data and Comprehensive Error Rate Testing (CERT) records. Only a
sample of Medicare claims have corresponding CERT records, which allow access to detailed
records of medical treatment.
Background
For the Accuracy of Coding project, we are using the FY 2009 and FY 2010 Medicare
Provider Analysis and Review (MedPAR) files, representing the entire population of discharges
eligible for the Inpatient Prospective Payment System (IPPS). The MedPAR data include
diagnosis and claims records for all Medicare beneficiaries with acute care hospital stays. We are
using CERT records from the same time frame, and have linked these two datasets. There are a
total of approximately 50,000 CERT records available per year. We requested those that are from
acute care hospitals; had dates of discharge in FY 2009 or FY 2010; and are subject to IPPS—
and therefore also subject to the HAC-POA program. This request resulted in approximately
11,000 CERT records.
From these 11,000, we took all discharges with a condition reported as POA, and all
cases meeting the criteria for DVT/PE. We took a sample of records meeting the criteria for
CLABSI and CAUTI—as there are more of these records than needed, based on our sample size
calculations described above in Section 2.1.4.
To broadly apply our findings to Medicare participating hospitals, it is important to
determine whether or not the MedPAR claims that do have corresponding CERT records are
representative of the full MedPAR dataset. These findings will guide as to whether weighting our
data will be necessary to reflect the broader Medicare population—as discussed in Section 2.3.4
of the Accuracy of Coding Strategy Memo. The CERT program uses random sampling to choose
records for review. However, the randomization process is not publicly available.
To determine if the CERT records are representative of the MedPAR files, we examined
several key characteristics for patients, hospitals, and diagnoses. We created frequency
distributions for each of these characteristics for both the MedPAR files and the CERT datasets,
and then compared these distributions. The following sections summarize this analysis. For each
key characteristic, we have provided tables showing the distributions and sample sizes being
compared.
We have focused on the absolute differences across the two datasets in the percentages of
key characteristics. We have not applied a test of statistical significance—since tests based on
statistical inference, such as the T-test, show high levels of statistical significance simply
because of the very large sample size of the MedPAR dataset. Because of the sample size, the
variances and standard errors for the variables are very small. Since the standard error of the X’s
is in the denominator for the calculation of the t-statistic, this statistic will be large and highly
statistically significant simply as a result of the sample size. To get around this problem, we have
used the absolute difference in the frequency of a given variable in the two datasets. For a given
38
variable, if the prevalence in one dataset is more than two percentage points higher or lower than
in the other dataset, we have considered this as a difference meriting further exploration and
discussion.
Comparisons by Beneficiaries’ Characteristics
We compared the two distributions by the age, gender, and race of the beneficiary. The
two distributions are remarkably consistent across these analyses (Table A1 to Table A3). None
of the differences in outcomes is greater than two percentage points.
Table A1
Comparison by age
Age
MedPAR
N
MedPAR
%
CERT
N
CERT
%
<65 4,196,287 19.5 2,056 19.7
65-74 6,454,631 30.0 3,046 29.1
75-84 6,449,584 30.0 3,215 30.7
85+ 4,420,927 20.5 2,144 20.5
Total 21,521,429 100.0 10,461 100.0
NOTE: CERT, Comprehensive Error Rate Testing; MedPAR, Medicare Provider Analysis and
Review.
Table A2
Comparison by gender
Gender
MedPAR
N
MedPAR
%
CERT
N
CERT
%
Male
9,518,624
44.2
4,826
46.1
Female 12,002,783 55.8 5,635 53.9
Total 21,521,407 100.0 10,461 100.0
NOTE: CERT, Comprehensive Error Rate Testing; MedPAR, Medicare Provider Analysis and
Review.
39
Table A3
Comparison by race
Race
MedPAR
N
MedPAR
%
CERT
N
CERT
%
White 17,624,189 81.9 8,499 81.2
Black 2,713,535 12.6 1,389 13.3
Asian 239,371 1.1 113 1.1
Hispanic
483,599
2.3
225
2.2
Native American
128,750
0.6
81
0.8
Other 275,359 1.3 145 1.4
Unknown 56,626 0.3 9 0.1
Total 21,521,429 100.1 10,461 100.1
NOTE: Some totals are greater than 100% due to rounding. CERT, Comprehensive Error Rate
Testing; MedPAR, Medicare Provider Analysis and Review.
Comparisons by Hospital Characteristics
We considered hospital size in terms of numbers of beds (Table A4); whether a hospital
is an academic medical center (Table A5); type of hospital ownership (Table A6); and whether
the hospital is located in an urban or rural area (Table A7). Only one of the differences is greater
than two percentage points—MedPAR records are more likely (48.2%) than CERT records
(45.1%)to be from hospitals in large urban areas. Since all of these hospitals are in urban areas,
this difference is not important.
The rest of the differences are all less than two percentage points. MedPAR records are
also somewhat more likely to be from private hospitals than CERT records are (14.9% vs.
13.9%—Table A6), but this difference is not large. Likewise, CERT records more commonly
show that patients received care in larger hospitals—54.8% were treated in a hospital of 300 or
more beds, compared with 53.6% for MedPAR records (Table A4).
Table A4
Comparison by bed size
Beds
MedPAR
N
MedPAR
%
CERT
N
CERT
%
<100 2,077,582 9.7 936 9.0
100-299 7,913,689 36.8 3,789 36.2
300+ 11,523,285 53.6 5,736 54.8
Total
21,514,556
100.1
10,461
100.0
NOTE: Some totals are greater than 100% due to rounding. CERT, Comprehensive Error Rate
Testing; MedPAR, Medicare Provider Analysis and Review.

40
Table A5
Comparison by whether an academic medical center
Academic medical
center
MedPAR
N
MedPAR
%
CERT
N
CERT
%
No 19,784,279 92.0 9,544 91.2
Yes 1,730,277 8.0 917 8.8
Total 21,514,556 100.0 10,461 100.0
NOTE: CERT, Comprehensive Error Rate Testing; MedPAR, Medicare Provider Analysis and
Review.
Table A6
Comparison by ownership
Ownership
MedPAR
N
MedPAR
%
CERT
N
CERT
%
Private for-profit
3,203,482
14.9
1,456
13.9
Private nonprofit
15,345,814
71.3
7,484
71.5
State or local 1,248,429 5.8 616 5.9
Other government 1,716,831 8.0 905 8.7
Total 21,514,556 100.0 10,461 100.0
NOTE: CERT, Comprehensive Error Rate Testing; MedPAR, Medicare Provider Analysis and
Review.
Table A7
Comparison by urban vs. rural status
Urbanicity
MedPAR
N
MedPAR
%
CERT
N
CERT
%
Large urban
10,369,468
48.2
4,713
45.1
Small urban 7,881,090 36.6 4,155 39.7
Rural 3,263,998 15.2 1,593 15.2
Total 21,514,556 100.0 10,461 100.0
NOTE: CERT, Comprehensive Error Rate Testing; MedPAR, Medicare Provider Analysis and
Review.
Comparisons by Case Mix
We also compared distributions of both principal and secondary diagnoses, grouped by
ICD-9 code (Table A8 and Table A9, below). One principal diagnosis code, and up to eight
secondary diagnosis codes, can be entered for a given discharge. Among the principal diagnoses,
41
there is a potentially important difference between the MedPAR and CERT records for diseases
of the circulatory system (25.0% and 30.6%, respectively), and diseases of the respiratory system
(12.6% and 10.4%, respectively). In the secondary diagnoses, diseases of the circulatory system
again show a difference—24.4% in the MedPAR records compared with 27.5% in CERT. It is
not clear why these differences exist; however, they should not be important factors for our
analysis of the accuracy of coding for HACs and their presence on admission. Beyond this
difference, none of the other comparisons by conditions stand out as indicative of discrepancies
in the two distributions, for either principal diagnoses or secondary diagnoses.

42
Table A8
Comparison by principal diagnosis
Primary diagnosis
MedPAR
N
MedPAR
%
CERT
N
CERT
%
Infectious and parasitic
diseases 1,177,318 5.5 643 6.2
Neoplasms 992,010 4.6 310 3.0
Endocrine, nutritional, and
metabolic diseases 938,905 4.4 540 5.2
Diseases of blood and blood
forming organ 334,270 1.6 111 1.1
Mental disorders 367,358 1.7 66 0.6
Diseases of nervous system
and sense organs 462,520 2.2 110 1.1
Diseases of circulatory system 5,390,627 25.1 3,203 30.6
Diseases of respiratory system 2,715,882 12.6 1,086 10.4
Diseases of digestive system 2,200,583 10.2 927 8.9
Diseases of genitourinary
system 1,347,986 6.3 792 7.6
Complications of pregnancy,
childbirth, and puerperium
38,874 0.2 11 0.1
Diseases of skin and
subcutaneous tissue 438,178 2.0 118 1.1
Diseases of musculoskeletal
and connective tissue 1,601,007 7.4 727 7.0
Congenital anomalies 23,424 0.1 12 0.1
Newborn guidelines 13 0.0 0 0.0
Signs, symptoms and ill-
defined conditions 1,243,209 5.8 645 6.2
Injury and poisoning 2,122,777 9.9 1,046 10.0
Factors influencing health
status or use of services
126,488 0.6 114 1.1
Total
21,521,429
100.2
10,461
100.3
NOTE: Some totals are greater than 100% due to rounding. CERT, Comprehensive Error Rate
Testing; MedPAR, Medicare Provider Analysis and Review.

43
Table A9
Comparison by secondary diagnosis
Primary diagnosis
MedPAR
N
MedPAR
%
CERT
N
CERT
%
Infectious and parasitic
diseases 2,921,828 1.9 1,249 1.6
Neoplasms 2,803,018 1.8 1,177 1.6
Endocrine, nutritional, and
metabolic diseases 23,456,057 15.2 11,534 15.2
Diseases of blood and blood
forming organ 5,740,870 3.7 2,457 3.2
Mental disorders 7,773,083 5.0 3,366 4.4
Diseases of nervous system
and sense organs 6,182,367 4.0 2,711 3.6
Diseases of circulatory system 37,777,076 24.4 20,897 27.5
Diseases of respiratory system 9,864,769 6.4 4,752 6.3
Diseases of digestive system 8,400,185 5.4 3,928 5.2
Diseases of genitourinary
system 11,105,962 7.2 5,835 7.7
Complications of pregnancy,
childbirth, and puerperium
88,920 0.1 31 0.0
Diseases of skin and
subcutaneous tissue 2,308,961 1.5 1,029 1.4
Diseases of musculoskeletal
and connective tissue 6,206,485 4.0 2,924 3.9
Congenital anomalies 325,128 0.2 145 0.2
Newborn guidelines 729 0.0 0 0.0
Signs, symptoms and ill-
defined conditions 8,465,396 5.5 3,798 5.0
Injury and poisoning 3,746,033 2.4 1,870 2.5
Supplemental external causes
of injury and poisoning
3,862,118 2.5 1,805 2.4
Factors influencing health
status or use of services 13,600,946 8.8 6,436 8.5
Total 154,629,931 100.0 75,944 100.2
NOTE: Some totals are greater than 100% due to rounding. CERT, Comprehensive Error Rate
Testing; MedPAR, Medicare Provider Analysis and Review.
44
Conclusions
As evidenced in the tables and analysis presented above, the distribution of the CERT
records is consistently similar to the MedPAR records, whether the distribution is calculated by
age, gender, race, hospital size and location, or patient diagnoses. Given the large sample sizes—
approximately 20 million MedPAR records and 11,000 CERT records—further analysis of this
question should not be necessary. The CERT records are broadly representative of the MedPAR
records, and therefore are representative of the population of IPPS-eligible Medicare discharges.
For our analysis, it should therefore not be necessary to apply weighting to the CERT records
when conducting analysis applicable to claims data from the entire IPPS-eligible Medicare
population.

45
APPENDIX B
APPENDIX OF TOOLS
Type of Review:
□
Over-reported
(Listed condition was
coded, POA = Y)
□
Unreported
(Listed condition was
not coded)
CID#:__________________
Primary Diagnosis
Code
Secondary Diagnosis
Code(s)
Procedure Code(s)
Coder ID: _______________
_________________
__________
__________
_________
_________
Date Coded: _____________
_________________
__________
__________
_________
_________
Admission Date: __________
_________________
__________
__________
_________
_________
Discharge Date:___________
Catheter Associated Urinary Tract Infection (CAUTI)
PRELIMINARY
Was an indwelling urinary catheter present during the admission
Y
N
□
Present when patient was admitted
□
Inserted during the hospitalization
Date Inserted:______________________ Date Removed:______________________
PART I—SHOULD THE CONDITION BE CODED
A. Did the Condition Exist During the Stay □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Physician documentation of a diagnosis of CAUTI in primary source document
Y
N
Was a diagnosis of CAUTI documented in the discharge list of diagnoses
Y
N
If yes, specify list position:_______________________________________________________

46
Level I Evidence
If any of the following two criteria are met, it is sufficient evidence of presence of the condition and no
further evidence needs to needs to be collected for Part A.
1. Physician documentation of diagnosis of any type of UTI > 48 hours after insertion
of indwelling catheter in primary source document
Y N
2. Urinary catheter inserted at >48 hours prior to collection of specimen with positive
initial laboratory findings of any of the below:
Y N
□ Positive urine culture (non-contaminant)
Culture Source Date Positive Sample Collected Culture Result (CC, organism)
___________________ ___________________ ___________________
___________________ ___________________ ___________________
Note:
• Positive is defined as CC ≥ 100,000 (or 10,000 < 99,000 with physician confirmation)
• If the culture has 3 or more organisms, then not CAUTI
• If catheter removed > 48 hours before urine was collected for culture, then not CAUTI
□ Urinalysis positive for WBC
Level II Evidence
Continue in this section of Part A only if no Level I Evidence has been indicated above.
3.
Urinary catheter inserted
>
48 hours prior to appearance of any of the below
signs/symptoms:
Y
N
□
Temperature above 101
o
(not ascribed to another condition)
□
Suprapubic or flank pain/tenderness
□
Dysuria or burning on urination
□
Urinary urgency or frequency after catheter is removed
B. Did the Condition Affect Patient Care Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Meeting any of the following criteria indicates an impact on patient care
1.
Was there at least one definitive treatment:
Y
N
□
Directed use of organism-sensitive/UTI-specific antibiotic
□
Altering the type or dosage of antibiotic concurrent with culture results
□
Removing indwelling catheter concurrent with culture results, urinalysis
findings, or patient symptoms
2.
Documentation of any of the following impacts on patient care:
Y
N
□
Delay in discharge
□
Increased monitoring (e.g. directed repetition of diagnostic tests)
□
Increased nursing care
Specify:________________________________________________________
□
47
Part II—Was the Condition Present on Admission
□ Yes, present on admission □ No, developed after admission □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Documentation of indwelling catheter being in place upon admission, or within prior
48 hours, and any of the following:
Y
N
□
Documentation of infection evolving or established upon admission as
evidenced by documentation (in ER report, H&P, admission note, transfer
records from outside facility, etc.) of any of the following:
□
Documentation by physician in primary source document that condition was
present on admission or it cannot be clinically determined.
□
Signs/symptoms of UTI (as in A.5 above) documented on admission or
within
24 hours
□
Orders for urinalysis or urine culture on admission or within 24 hours
□
UTI documented as possible, suspected, or differential diagnosis on
admission or within 24 hours
□
Documentation on admission of orders for definitive treatment (as in B.1 above)
Disposition:
Over-reported [POA]
Case
□
Hospital Correct (the listed condition should be coded and POA was Y)
□
Hospital Not Correct [Answer the following]:
□
POA was Y but should be N
□
Listed condition was coded but should not be
Unreported [HAC] Case
□
Hospital correct (no listed condition was or should be coded)
□
Hospital not correct (a listed condition was not coded but should be)
[Answer the following]:
□
POA should be Y
□
POA should be N
□
Refer to Physician Advisor:______________________________________________________________
Physician Advisor ID:__________________________________________________________________
Comments: ____________________________________________________________________________

48
Type of Review:
□
Over-reported
(Listed condition was
coded, POA = Y)
□
Unreported
(Listed condition was
not coded)
CID#:_________________
Primary Diagnosis Code
Secondary Diagnosis
Code(s)
Procedure Code(s)
Coder ID:______________
____________________
__________
__________
__________
__________
Date Coded: ___________
____________________
__________
__________
__________
__________
Admission Date: ________
____________________
__________
__________
__________
__________
Discharge Date:_________
Vascular Catheter Associated Infection including Central Line Associated Blood Stream Infection
PRELIMINARY
Was a central line (e.g. CVC, PICC, port-a-cath) present during the admission
Y
N
□
Present when patient was admitted
□
Inserted during the hospitalization
Date Inserted:_________________ Date Removed:_________________
PART I—SHOULD THE CONDITION BE CODED
A. Did the Condition Exist During the Stay □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Physician documentation of a diagnosis of local infection (port, reservoir, pump pocket,
tunnel, catheter insertion or exit site) associated with a central venous catheter in
primary source document
Y
N
Physician documentation of both of the following in a primary source document
Y
N
□
Diagnosis of blood stream infection (sepsis, septicemia, bacteremia, fungemia)
□
Blood stream infection is associated with central line
Was a diagnosis of a VCAI documented in the discharge list of diagnoses
Y
N
If yes, specify list position:_______________________________________________
Level I Evidence
If the following criterion is met, it is sufficient evidence of presence of the condition and no further
evidence needs to be collected for Part A.
1. Central line inserted at least two days prior to collection of specimen for any of the
following diagnostic tests:
Y
N
□ Positive culture of catheter tip (non-contaminant)
□ Positive blood culture (non-contaminant)
□ Positive culture from associated skin site (non-contaminant)
Culture Source Date Positive Sample Collected Culture Result (CC, organism)
___________________ ___________________ ___________________
___________________ ___________________ ___________________
___________________ ___________________ ___________________
49
Level II Evidence
Continue in this section of Part A only if no Level I Evidence has been indicated above.
2.
Central line inserted at least two days prior to any two of the following
sign/symptoms
Y
N
□
Temperature above 101
o
(not ascribed to another condition) after placement of
central line
□
Erythema, induration, pus, or tenderness at catheter site
□
Rigors or hypotension when central line is flushed
□
Removal of central line or initiation of antibiotics with improvement of symptoms
B. Did the Condition Affect Patient Care □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Meeting any of the following criteria indicates an impact on patient care
1.
Were there any of the following definitive treatments:
Y
N
□
Directed use of antibiotics
□
Alteration of the type or dosage of antibiotic concurrent with culture results
□
Removal of central line
2.
Documentation of any of the following impacts on patient care:
Y
N
□
Delay in discharge
□
Increased monitoring (e.g. directed repetition of diagnostic tests)
□
Increased nursing care
Specify:________________________________________________________
PART II—WAS THE CONDITION PRESENT ON ADMISSION
□ Yes, present on admission □ No, developed after admission □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Documentation of central line being in place upon admission or within prior 72
hours, and any of the following:
Y
N
□
Indication of catheter-site or blood stream infection evolving or established upon
admission as evidenced by documentation (e.g. ER reports, H&P, admission
note, transfer records for outside facility, etc.) of any of the following:
□
Documentation by physician in primary source document that condition was
present on admission or it cannot be clinically determined.
□
Signs/symptoms of infection (as in A.3above) documented on admission
□
Orders for diagnostic tests (as in A.2 above) for infection on admission
□
Infection documented as possible, suspected, or differential diagnosis on
admission
□
Definitive treatment (as in B.1 above) documented on admission
50
Disposition:
Over-reported [POA]
Case
□
Hospital Correct (the listed condition should be coded and POA was Y)
□
Hospital Not Correct [Answer the following]:
□
POA was Y but should be N
□
Listed condition was coded but should not be
Unreported [HAC] Case
□
Hospital correct (no listed condition was or should be coded)
□
Hospital not correct (a listed condition was not coded but should be)
[Answer the following]:
□
POA should be Y
□
POA should be N
□
Refer to Physician Advisor:_____________________________________________________________
Physician Advisor ID:_________________________________________________________________
Comments: ___________________________________________________________________________
51
Type of Review:
□
Over-reported
(Listed condition was
coded, POA = Y)
□
Unreported
(Listed condition was
not coded)
CID#:_______________
Primary Diagnosis Code
Secondary Diagnosis
Code(s)
Procedure Code(s)
Coder ID:____________
____________________
___________
__________
__________
__________
Date Coded:_________
_____________________
__________
__________
__________
__________
Admission Date: ______
_____________________
__________
__________
__________
__________
Discharge Date:_______
Deep Vein Thrombosis/Pulmonary Embolism Following Certain Orthopedic Procedures
PRELIMINARY
Confirm one of the following procedures took place during the current admission
□
Hip resurfacing, partial or total (00.85-00.87)
□
Hip replacement, total or partial (not revision) (81.51-81.52)
□
Knee replacement, total or partial (81.54)
If none of the above conditions are present, do not continue with chart review
PART I—SHOULD THE CONDITION BE CODED
A. Did the Condition Exist During the Stay □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Physician documentation of a diagnosis of DVT or PE in primary source document
Y
N
Was a diagnosis of DVT/PE documented in the discharge list of diagnoses
Y
N
If yes, specify list position:_______________________________________________
Level I Evidence
If the following criterion is met, it is sufficient evidence of presence of the condition and no further
evidence needs to be collected for Part A.
1.
Documentation of any of the following positive diagnostic tests:
Y
N
□
V/Q scan indicating normal ventilation associated with perfusion defects
□
Pulmonary angiography, CTA, or MRA indicating thrombi or filling defect
□
EKG with McConnell sign (RV free wall akinesia/dyskinesia with normal apex
contractility)
□
US, CT, MRI of lower extremity indicating venous thrombus
□
Venography of lower extremity indicating venous thrombus
52
Level II Evidence
Continue in this section of Part A only if no Level I Evidence has been indicated above.
2.
Documentation of at least two of the following signs/symptoms:
Y
N
□
EKG indicating dilated right ventricle RV or dilated pulmonary artery
□
D-dimer > 500 ug/L
□
New onset shortness of breath, tachypnea, chest pain
□
Unilateral swollen, edematous, painful or erythematous limb
□
Increase in systolic pulmonary artery pressure
□
Persistent hypotension
B. Did the Listed Condition Affect Patient Care □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
M
eeting any of the following criteria indicates an impact on patient care
1. Were there any of the following definitive treatments:
Y
N
□ Embolectomy or thrombectomy
□ Insertion of IVC filter
□ Directed therapeutic use of thrombolytic (streptokinase, urokinase, TPA)
2. Were there any of the following secondary treatments:
Y
N
□ Directed use of anticoagulants (heparin, Enoxaparim, Tinzaparin,
Fondaparinux)
□ Directed therapeutic use of compression stockings
3. Documentation of any of the following other impacts on patient care:
Y
N
□ Delay in discharge
□ Increased monitoring (e.g. directed repetition of diagnostic tests)
□ Increased nursing care
Specify:________________________________________________________
P
ART II—WAS THE CONDITION PRESENT ON ADMISSION
□ Yes, present on admission □ No, developed after admission □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Were any of the following present:
Y
N
□
Documentation that condition was evolving or established upon admission as
by documentation (e.g. ER report, H&P, admission note, transfer records from
outside facility, etc.) of any of the following:
□
Documentation by physician that condition was present on admission or that
it cannot be clinically determined
□
Signs/symptoms of condition (as in A.2 above) documented on admission
□
Orders for diagnostic tests (as in A.1 above) for condition on admission
□
Condition documented as possible, suspected, or differential diagnosis on
admission
□
Documentation on admission of orders for definitive treatment (as in B.1 above)
53
Disposition:
Over-reported [POA]
Case
□
Hospital Correct (the listed condition should be coded and POA was Y)
□
Hospital Not Correct [Answer the following]:
□
POA was Y but should be N
□
Listed condition was coded but should not be
Unreported [HAC] Case
□
Hospital correct (no listed condition was or should be coded)
□
Hospital not correct (a listed condition was not coded but should be)
[Answer the following]:
□
POA should be Y
□
POA should be N
□
Refer to Physician Advisor: ____________________________________________________________
Physician Advisor ID:_________________________________________________________________
Comments: ___________________________________________________________________________

54
Type of Review:
□
Over-reported
(Listed condition was
coded, POA = Y)
□
Unreported
(Listed condition was
not coded)
CID#:_________________
Primary Diagnosis Code
Secondary Diagnosis
Code(s)
Procedure Code(s)
Coder ID:_____________
____________________
___________
__________
__________
__________
Date Coded:___________
____________________
__________
__________
__________
__________
Admission Date:________
____________________
__________
__________
__________
__________
Discharge Date:________
Falls and Trauma
In the case of multiple traumas, all questions relate to the same injury at the same site.
PART I—SHOULD THE CONDITION BE CODED
A. Did the Condition Exist During the Stay □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Does the record contain both of the following:
Y
N
□
Diagnosis documented by a physician of a fall or trauma in a primary source
document
“Fall/Trauma” is any diagnosis with a code on the CC/MCC list within these categories:
Fracture 800-829
Crush Injury 925-929
Dislocation 830-839
Effects of External Causes (frostbite, heat
stroke, submersion, suffocation)
991-994
Intracranial Injury 850-854
□
Documentation by a physician or nurse of corresponding physical indicators of
injury
Specify nature of injury: _____________________________________________
Specify anatomic site if injury:________________________________________
B. Did the Condition Affect Patient Care □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Meeting any of the following criteria indicates an impact on patient care
1.
Were there any of the following treatments:
Y
N
□
Reduction of fracture or dislocation
□
Casting or splinting
□
Other related treatment (specify):______________________________________
2.
Were there any of the following work-ups for injury
Y
N
□
Imaging (e.g. X-ray, CT, MRI) to assess injury
□
Summoning attending or on-call physician to assess injury
□
Physician consultation specifically for injury
□
Other related work-up (specify):_______________________________________
55
3.
Is there documentation of any of the following impacts on patient care:
Y
N
□
Delay in discharge
□
Increased monitoring (e.g. repeated imaging)
□
Increased nursing care (e.g. repeated neurological checks)
Specify:__________________________________________________________
PART II—WAS THE CONDITION PRESENT ON ADMISSION
□ Yes, present on admission □ No, developed after admission □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Documentation of any of the following:
Y
N
□
Documentation on admission (e.g. ER report, admission note, transfer records
from outside facility) of existing trauma or injury
□
Trauma or injury documented as possible, suspected, differential diagnosis at
time of admission
□
Treatment or work-up (as in B.1 and B.2 above) ordered on admission
□
Documentation by physician in primary source document that condition was
present on admission , or that it cannot be clinically determined
Specify circumstances and timing of trauma or injury as documented:
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
Disposition:
Over-reported [POA]
Case
□
Hospital Correct (the listed condition should be coded and POA was Y)
□
Hospital Not Correct [Answer the following]:
□
POA was Y but should be N
□
Listed condition was coded but should not be
Unreported [HAC] Case
□
Hospital correct (no listed condition was or should be coded)
□
Hospital not correct (a listed condition was not coded but should be)
[Answer the following]:
□
POA should be Y
□
POA should be N
□
Refer to Physician Advisor: ____________________________________________________________
Physician Advisor ID:_________________________________________________________________
Comments: ___________________________________________________________________________

56
Type of Review:
□
Over-reported
(Listed condition was
coded, POA = Y)
□
Unreported
(Listed condition was not
coded)
CID#:________________
Primary Diagnosis Code
Secondary Diagnosis
Code(s)
Procedure Code(s)
Coder ID:_____________
_____________________
__________
__________
__________
__________
Date Coded:___________
_____________________
__________
__________
__________
__________
Admission Date:________
_____________________
__________
__________
__________
__________
Discharge Date:________
Extreme Manifestations of Poor Glycemic Control
(*Hyperglycemic Only)
PART I—SHOULD THE CONDITION BE CODED
A. Did the Condition Exist During the Stay □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Physician documentation in primary source document that patients is diabetic
(primary of secondary, insulin or non-insulin dependent) and has any of the
following:
Y
N
□
Ketoacidosis/DKA, as evidenced by any of the following positive lab results or
signs/symptoms:
□
Blood glucose level
≥
300 mg/dL
□
Serum bicarbonate (HCO3)
≤
15 mEq/L
□
Blood pH < 7.30
□
Positive ketones in blood and/or urine
□
Dry mucus membranes and skin (dehydration)
□
Polydipsia and/or polyuria
□
Alteration in consciousness
□
Abdominal pain or tenderness
□
Ketotic or acetone breath
□
Hyperosmolarity/HHS, as evidenced by any of the following positive lab results or
signs/symptoms:
□
Blood glucose level
≥
600 mg/dL
□
Serum osmolality
≥
320 mOsm/kg
□
Dry mucous membranes and skin (dehydration)
□
Polydipsia and/or polyuria
□
Alteration in consciousness
Note: In HHS, HCO
3
and blood pH may be closer to normal and ketones
may not be present.
57
B. Did the Listed Condition Affect Patient Care □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Meeting any of the following criteria indicates an impact on patient care
1.
Were any of the following corrective measures taken:
Y
N
□
Directed IV infusion (e.g. NaCl, Ringer’s) to correct fluid loss and dehydration
□
Use of short-acting insulin (e.g. Novolog, Humalog, Humulin) to correct
hyperglycemia
□
Administration of electrolytes (e.g. KCl ) to correct imbalances
Note: Patients with HHS may respond to fluids alone and may not require insulin
2.
Documentation of any of the following impacts on patient care:
Y
N
□
Transfer to ICU specifically for glycemic management
□
Physician consultation (e.g. endocrinologist) specifically for glycemic
management
□
Delay in discharge
□
Increased monitoring (e.g. repeated blood glucose levels, ABGs, electrolytes)
□
Increased nursing care (e.g. intensive diabetic teaching)
□
Specify: _______________________________________
PART II—WAS THE LISTED CONDITION PRESENT ON ADMISSION
□ Yes, present on admission □ No, developed after admission □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Were any of the following present:
Y
N
□
Indication of DKA or HHS evolving or established upon admission as evidenced
by documentation (e.g. ER report, H&P, admission note, transfer records from
outside facility, etc.) of any of the following:
□
Documentation by physician in primary source document that condition was
present on admission, or that it cannot be clinically determined.
□
Orders for laboratory tests (as in A.1.i and ii above) for condition on
admission
□
Signs/symptoms of condition (as in A.1.i and ii above) documented on
admission
□
Condition documented as possible, suspected, or differential diagnosis on
admission
□
Corrective measures (as in B.1 above) ordered on admission
58
Disposition:
Over-reported [POA]
Case
□
Hospital Correct (the listed condition should be coded and POA was Y)
□
Hospital Not Correct [Answer the following]:
□
POA was Y but should be N
□
Listed condition was coded but should not be
Unreported [HAC] Case
□
Hospital correct (no listed condition was or should be coded)
□
Hospital not correct (a listed condition was not coded but should be)
[Answer the following]:
□
POA should be Y
□
POA should be N
□
Refer to Physician Advisor: ____________________________________________________________
Physician Advisor ID:_________________________________________________________________
Comments: ___________________________________________________________________________

59
Type of Review:
□
Over-reported
(Listed condition was
coded, POA = Y)
□
Unreported
(Listed condition was not
coded)
CID#:__________________
Primary Diagnosis Code
Secondary Diagnosis
Code(s)
Procedure Code(s)
Coder ID:_______________
____________________
__________
__________
__________
__________
Date Coded:____________
____________________
__________
__________
__________
__________
Admission Date: _________
____________________
__________
__________
__________
__________
Discharge Date:__________
Pressure Ulcer Stage III or Stage IV
In the case of multiple pressure ulcers, all questions relate to the same site and to the ulcer designated as
stage III or stage IV.
PART I—SHOULD THE CONDITION BE CODED
A. Did the Condition Exist During the Stay □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Physician documentation of a diagnosis of stage III or stage IV pressure ulcer
(decubitus ulcer, bedsore) in primary source document and all of the following:
Y
N
□
Documentation of the final stage as stage III or stage IV by any of the following:
□
Physician
□
Nurse
□
Documentation of applicable corresponding physical indicators of stage
□
Stage III: Full thickness skin loss with visible, damaged, or necrotic
subcutaneous tissue
□
Stage IV: Full thickness skin loss with exposed muscle, tendon, or bone
Ulcer diagnosis must be documented by a physician, but stage may be
documented by a nurse.
Note:
• Pressure ulcers with intact skin and non-blanchable erythema are Stage I
• Pressure ulcers with partial thickness skin loss (epidermis or dermis) with
blanchable erythema (red-pink wound bed) without slough or with blistered
appearance are Stage II
• Pressure ulcers with depth completely obscured by slough, eschar, or graft,
or documented only as a deep tissue injury without depth, are unstageable.
60
B. Did the Listed Condition Affect Patient Care □ Yes □ No □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
Meeting any of the following criteria indicates an impact on patient care
1. Documentation of any of the following surgical treatments:
Y
N
□ Excision with skin grafting
□ Excisional debridement in the operating room, during a consultation, or bedside
2. Documentation of any of the following non-surgical treatments
Y
N
□ Use of foam, water, gel, air, alternating pressure mattress or overlay
□ Use of foam wedges
□ Use of low air loss bed, air fluidized bed, or air flotation bed
□ Non-surgical mechanical debridement (e.g. whirlpool, Versajet)
□ Ulcer irrigation (e.g. pulsed), cleansing, packing and/or dressing
□ Negative pressure wound therapy (vacuum)
3. Documentation of any of the following other impacts on patient care
Y
N
□ Physician consultation specifically for pressure ulcer
□ Nutrition consultation for pressure ulcer
□ Delay in discharge
□ Increased Monitoring (e.g. repeated assessment via PUSH, BWAT, PSST)
□ Increased Nursing Care (e.g. frequent repositioning)
Specify:__________________________________________________________
PART II—WAS THE LISTED CONDITION PRESENT ON ADMISSION
□ Yes, present on admission □ No, developed after admission □ Refer to PA
If medical judgment is necessary to answer the above question, check Refer to PA
1.
Documentation of any of the following:
Y
N
□
Diagnosis of current or healing pressure ulcer documented on admission (e.g. ER
report, H&P, Admission Note, transfer records from outside facility)
□
Pressure ulcer possible, suspected, or differential diagnosis at time of admission
or within 24 hours
□
Localized injury to skin or underlying tissue, ulcer, sore, or wound over bony
prominence (e.g. sacrum, coccyx, heel, hip, ankle, elbow) documented on
admission (including in nursing admission note) at site later diagnosed with
pressure ulcer
□
Surgical treatment, non-surgical measures, or consultation (as in B.1 and B.2
above) ordered on admission or within 24 hours
□
Stage I or II ulcer present on admission progressed to stage III or IV during
hospital stay
□
Documentation by physician that condition was present on admission or that it
cannot be clinically determined
61
Disposition:
Over-reported [POA]
Case
□
Hospital Correct (the listed condition should be coded and POA was Y)
□
Hospital Not Correct [Answer the following]:
□
POA was Y but should be N
□
Listed condition was coded but should not be
Unreported [HAC] Case
□
Hospital correct (no listed condition was or should be coded)
□
Hospital not correct (a listed condition was not coded but should be)
[Answer the following]:
□
POA should be Y
□
POA should be N
□
Refer to Physician Advisor: ____________________________________________________________
Physician Advisor ID:_________________________________________________________________
Comments: ___________________________________________________________________________
62
[This page left intentionally blank]
63
APPENDIX C
CLINICAL CODING GUIDELINES
Like all specialized fields, coding has its own internal logic that may not be familiar, or
perhaps intelligible, to non-coders. In addition to accurately reflecting the clinical
documentation, ICD-9-CM code assignment and sequencing must comply with specific coding
guidelines to be considered correct. Although informal coding advice may be available from
multiple areas, official coding guidelines are published in just two sources.
The first source is the ICD-9-CM Official Guidelines for Coding and Reporting. The
Official Guidelines form the basic rules for correct coding and are updated annually. All material
within the Official Guidelines is approved by the Cooperating Parties for ICD-9-CM: the
American Hospital Association (AHA), the American Health Information Management
Association (AHIMA, a professional association for coders and others involved in the
administration of medical records), the National Center for Health Statistics (NCHS, an agency
within the Centers for Disease Control and Prevention) and CMS. Compliance with the Official
Guidelines is mandatory under HIPAA and adherence is compulsory when ICD-9-CM codes are
assigned (Federal Register, August 17, 2000, p. 50323)
The second source is Coding Clinic for ICD-9-CM, a quarterly journal for coding
guidelines and advice. Although published by the AHA, all material in Coding Clinic is
approved by the Cooperating Parties. For this reason, Coding Clinic is an authoritative source for
ICD-9-CM coding and its guidance must be followed.
It should be noted that there are a few other sources which also have the weight of
definitive advice, primarily those issued by one or more of the Cooperating Parties through a
separate avenue. For example, minutes of the biannual meetings of the ICD-9-CM Coordination
and Maintenance Committee sometimes contain instructions on the current code to assign for a
particular diagnosis or procedure prior to creation of a new code. Because the Committee is co-
chaired by NCHS and CMS, these instructions are considered definitive and must be followed.
Specific guidelines which are referenced in the report are quoted below.
III. Reporting Additional Diagnoses
General Rules for Other (Additional) Diagnoses
For reporting purposes the definition for “other diagnoses” is interpreted as
additional conditions that affect patient care in terms of requiring:
• clinical evaluation; or
• therapeutic treatment; or
• diagnostic procedures; or
• extended length of hospital stay; or
• increased nursing care and/or monitoring.
64
The UHDDS [Uniform Hospital Discharge Data Set] item #11-b defines Other
Diagnoses as “all conditions that coexist at the time of admission, that develop
subsequently, or that affect the treatment received and/or the length of stay.
Diagnoses that relate to an earlier episode which have no bearing on the current
hospital stay are to be excluded.” UHDDS definitions apply to inpatients in acute
care, short-term, long term care and psychiatric hospital setting. The UHDDS
definitions are used by acute care short-term hospitals to report inpatient data
elements in a standardized manner. These data elements and their definitions can
be found in the July 31, 1985, Federal Register (Vol. 50, No. 147), pp. 31038-40.
Source: ICD-9-CM Official Guidelines for Coding and Reporting, effective
October 1, 2008, p. 98.
Progressive Pressure Ulcer
A. Frequently Asked POA Questions
Clarification: Stage II Pressure Progressing to Stage III
QUESTION:
Coding Clinic Fourth Quarter 2008, page 194 stated that a stage II
pressure ulcer, which was present on admission, and progresses to become a stage
III pressure ulcer during the stay is reported as “Yes” for the present on admission
(POA) indicator. However, the POA indicator is reported for conditions present at
the time of inpatient admission. It appears inconsistent to report a Stage III
pressure ulcer as present on admission since the pressure ulcer gradually
deteriorated during the hospital stay. Could Coding Clinic please clarify this issue
for coders and clinical teams that rely on this guidance?
ANSWER:
In terms of coding and POA reporting, a pressure ulcer is only coded and
reported once at the highest stage. The information published in Coding Clinic
Fourth Quarter 2008, page 194, instructing to report a Stage II pressure ulcer that
progresses to a Stage III as present on admission is correct. The pressure ulcer
was present on admission; therefore, the POA should be yes. This advice is
consistent with the National Quality Forum (NQF) endorsed measures. The NQF
established a standardized set of serious reportable events also called never
events. The list of serious reportable events excludes the progression of a pressure
ulcer from stage II to Stage III, if stage II was recognized upon admission.
The NQF is an organization created to develop and implement a national
strategy for health care quality measurement and reporting. Please refer to the
NQF website for additional information about “Serious Reportable Events in
Healthcare”: http://www.qualityforum.org/projects/hacs_and_sres.aspx
Source: Coding Clinic, First Quarter 2009, p. 19
65
B. Frequently Asked POA Questions
QUESTION:
A patient is admitted to the hospital with a stage II pressure ulcer of the
heel. During the hospitalization, the pressure ulcer worsens and becomes a stage
III. Based on the new Official Coding Guidelines, we would be assigning the code
for the highest stage for that site. What would be the correct POA indicator
assignment for the stage III code?
ANSWER:
Assign “Y” to the pressure ulcer stage III code since this code is referring
to a pressure ulcer that was present on admission rather than a new ulcer.
Source: Coding Clinic, Fourth Quarter 2008, p. 194
Indwelling Urinary Catheter and Urinary Tract Infection
QUESTION:
When a patient who has an indwelling urinary catheter such as a Foley
catheter is diagnosed with a urinary tract infection (UTI), is the provider required
to document that the UTI is due to the catheter in order to assign code 996.64,
Infection and inflammatory reaction due to indwelling urinary catheter? Can the
coder assign 996.64 based on the presence of the catheter and the fact that the
provider diagnosed UTI?
ANSWER:
The provider must clearly document the causal relationship. If the
provider states that the UTI is secondary to the indwelling urinary catheter, assign
code 996.64, Infection and inflammatory reaction due to indwelling urinary
catheter, and code 599.0, Urinary tract infection, site not specified. If the provider
does not state that the urinary tract infection is due to the catheter, assign only
code 599.0.
The Official Guidelines for Coding and Reporting state, “As with all
procedural or post procedural complications, code assignment is based on the
provider’s documentation of the relationship between the condition and the
procedure.”
Source: Coding Clinic, Third Quarter 2009, p. 10-11
66
[This page left intentionally blank]
