
Advanced Higher Chemistry
Course code:
C813 77
Course assessment code:
X813 77
SCQF:
level 7 (32 SCQF credit points)
Valid from:
session 2019–20
This document provides detailed information about the course and course assessment to
ensure consistent and transparent assessment year on year. It describes the structure of
the course and the course assessment in terms of the skills, knowledge and understanding
that are assessed.
This document is for teachers and lecturers and contains all the mandatory information
required to deliver the course.
The information in this document may be reproduced in support of SQA qualifications only on
a non-commercial basis. If it is reproduced, SQA must be clearly acknowledged as the
source. If it is to be reproduced for any other purpose, written permission must be obtained
from permissions@sqa.org.uk
.
This edition: April 2021 (version 3.1)
© Scottish Qualifications Authority 2014, 2019, 2020, 2021
Contents
Course overview 1
Course rationale 2
Purpose and aims 2
Who is this course for? 3
Course content 4
Skills, knowledge and understanding 5
Skills for learning, skills for life and skills for work 38
Course assessment 39
Course assessment structure: question paper 39
Course assessment structure: project 40
Grading 46
Equality and inclusion 47
Further information 48
Appendix: course support notes 49
Introduction 49
Approaches to learning and teaching 49
Preparing for course assessment 126
Developing skills for learning, skills for life and skills for work 126
Version 3.1 1
Course overview
This course consists of 32 SCQF credit points, which includes time for preparation for course
assessment. The notional length of time for candidates to complete the course is 160 hours.
The course assessment has two components.
Component Marks Scaled mark Duration
Question paper
110
120
3 hours
Project
25
40
see ‘Course
assessment’ section
Recommended entry Progression
Entry to this course is at the discretion of the
centre.
Candidates should have achieved the Higher
Chemistry course or equivalent qualifications
and/or experience prior to starting this course.
♦ an Higher National Diploma (HND), or
degree in Chemistry or a related area,
such as medicine, law, dentistry, veterinary
medicine, engineering, environmental and
health sciences
♦ a career in a Chemistry-based discipline or
related area such as renewable energy
development, engineering, technology,
pharmaceuticals, environmental
monitoring, forensics, research and
development, oil and gas exploration,
management, civil service and education,
or in a wide range of other areas
♦ further study, employment and/or training
Conditions of award
The grade awarded is based on the total marks achieved across both course assessment
components.
Version 3.1 2
Course rationale
National Courses reflect Curriculum for Excellence values, purposes and principles. They offer
flexibility, provide time for learning, focus on skills and applying learning, and provide scope for
personalisation and choice.
Every course provides opportunities for candidates to develop breadth, challenge and application.
The focus and balance of assessment is tailored to each subject area.
Chemistry, the study of matter and its interactions, plays an increasingly important role in most
aspects of modern life. This course allows candidates to develop a deep understanding of the
nature of matter, from its most fundamental level to the macroscopic interactions driving chemical
change.
Candidates develop their abilities to think analytically, creatively, and independently to make
reasoned evaluations, and to apply critical thinking in new and unfamiliar contexts to solve
problems. The course offers candidates’ flexibility and personalisation as they decide the choice of
topic for their project.
Purpose and aims
The course builds on the knowledge and skills developed by candidates in the Higher Chemistry
course and continues to develop their curiosity, interest and enthusiasm for chemistry in a range of
contexts. Skills of scientific inquiry and investigation are developed throughout the course.
The course offers opportunities for collaborative and independent learning set within familiar and
unfamiliar contexts, and seeks to illustrate and emphasise situations where the principles of
chemistry are used and applied in everyday life.
Candidates develop important skills relating to chemistry, including developing scientific and
analytical thinking skills and making reasoned evaluations.
The course aims to:
♦ develop a critical understanding of the role of chemistry in scientific issues and relevant
applications, including the impact these could make in society and the environment
♦ extend and apply skills, knowledge and understanding of chemistry
♦ develop and apply the skills to carry out complex practical scientific activities, including the use
of risk assessments, technology, equipment and materials
♦ develop and apply scientific inquiry and investigative skills, including planning and experimental
design
♦ develop and apply analytical thinking skills, including critical evaluation of experimental
procedures in a chemistry context
♦ extend and apply problem-solving skills in a chemistry context
♦ further develop an understanding of scientific literacy, using a wide range of resources, in order to
communicate complex ideas and issues and to make scientifically informed choices
♦ extend and apply skills of autonomous working in chemistry
Version 3.1 3
Who is this course for?
The course is suitable for candidates who are secure in their attainment of Higher Chemistry or
equivalent qualifications. It is designed for candidates who can respond to a level of challenge,
especially those considering further study or a career in chemistry and related disciplines.
The course emphasises practical and experiential learning opportunities, with a strong skills-based
approach to learning. It takes account of the needs of all candidates, and provides sufficient
flexibility to enable candidates to achieve in different ways.
Version 3.1 4
Course content
The course content includes the following areas of chemistry:
Inorganic chemistry
The topics covered are:
♦ electromagnetic radiation and atomic spectra
♦ atomic orbitals, electronic configurations and the periodic table
♦ transition metals
Physical chemistry
The topics covered are:
♦ chemical equilibrium
♦ reaction feasibility
♦ kinetics
Organic chemistry and instrumental analysis
The topics covered are:
♦ molecular orbitals
♦ synthesis
♦ stereo chemistry
♦ experimental determination of structure
♦ pharmaceutical chemistry
Researching chemistry
The topics covered are:
♦ common chemical apparatus
♦ skills involved in experimental work
♦ stoichiometric calculations
♦ gravimetric analysis
♦ volumetric analysis
♦ practical skills and techniques
Version 3.1 5
Skills, knowledge and understanding
Skills, knowledge and understanding for the course
The following provides a broad overview of the subject skills, knowledge and understanding
developed in the course:
♦ extending and applying knowledge of chemistry to new situations, interpreting and analysing
information to solve complex problems
♦ planning and designing chemical experiments/investigations, including risk assessments, to
make a discovery, demonstrate a known fact, illustrate particular effects or test a hypothesis
♦ carrying out complex experiments in chemistry safely, recording systematic detailed
observations and collecting data
♦ selecting information from a variety of sources and presenting detailed information
appropriately, in a variety of forms
♦ processing and analysing chemical information and data (using calculations, significant figures
and units, where appropriate)
♦ making reasoned predictions and generalisations from a range of evidence and/or information
♦ drawing valid conclusions and giving explanations supported by evidence and/or justification
♦ critically evaluating experimental procedures by identifying sources of uncertainty and
suggesting and implementing improvements
♦ drawing on knowledge and understanding of chemistry to make accurate statements, describe
complex information, provide detailed explanations and integrate knowledge
♦ communicating chemical findings and information fully and effectively
♦ analysing and evaluating scientific publications and media reports

Version 3.1 6
Skills, knowledge and understanding for the course assessment
The following provides details of skills, knowledge and understanding sampled in the course
assessment:
Inorganic chemistry
(a) Electromagnetic radiation and atomic spectra
Electromagnetic radiation can be described in terms of waves and characterised in terms
of wavelength and/or frequency.
The relationship between these quantities is given by
cf
λ
=
.
The different types of radiation arranged in order of wavelength is known as the
electromagnetic spectrum.
Wavelengths of visible light are normally expressed in nanometres (nm).
Electromagnetic radiation can be described as a wave (has a wavelength and frequency),
and as a particle, and is said to have a dual nature.
When electromagnetic radiation is absorbed or emitted by matter it behaves like a stream
of particles. These particles are known as photons.
A photon carries quantised energy proportional to the frequency of radiation.
When a photon is absorbed or emitted, energy is gained or lost by electrons within the
substance.
The photons in high frequency radiation can transfer greater amounts of energy than
photons in low frequency radiation.
The energy associated with a single photon is given by:
or
hc
E hf E
λ
= =
The energy associated with one mole of photons is given by:
or
Lhc
E Lhf E
λ
= =
Energy is often in units of kJ mol
-1
.
When energy is transferred to atoms, electrons within the atoms may be promoted to
higher energy levels.
An atom emits a photon of light energy when an excited electron moves from a higher
energy level to a lower energy level.
The light energy emitted by an atom produces a spectrum that is made up of a series of
lines at discrete (quantised) energy levels. This provides direct evidence for the existence
of these energy levels.

Version 3.1 7
Inorganic chemistry (continued)
(a) Electromagnetic radiation and atomic spectra (continued)
Each element in a sample produces characteristic absorption and emission spectra. These
spectra can be used to identify and quantify the element.
In absorption spectroscopy, electromagnetic radiation is directed at an atomised sample.
Radiation is absorbed as electrons are promoted to higher energy levels.
An absorption spectrum is produced by measuring how the intensity of absorbed light
varies with wavelength.
In emission spectroscopy, high temperatures are used to excite the electrons within atoms.
As the electrons drop to lower energy levels, photons are emitted.
An emission spectrum of a sample is produced by measuring the intensity of light emitted
at different wavelengths.
In atomic spectroscopy, the concentration of an element within a sample is related to the
intensity of light emitted or absorbed.
(b) Atomic orbitals, electronic configurations and the periodic table
The discrete lines observed in atomic spectra can be explained if electrons, like photons,
also display the properties of both particles and waves.
Electrons behave as standing (stationary) waves in an atom. These are waves that vibrate
in time but do not move in space. There are different sizes and shapes of standing wave
possible around the nucleus, known as orbitals. Orbitals can hold a maximum of two
electrons.
The different shapes of orbitals are identified as s, p, d and f (knowledge of the shape of f
orbitals is not required).
Electrons within atoms have fixed amounts of energy called quanta.
It is possible to describe any electron in an atom using four quantum numbers:
♦ the principal quantum number
n
indicates the main energy level for an electron and is
related to the size of the orbital
♦ the angular momentum quantum number
l
determines the shape of the subshell and
can have values from zero to
1n −
♦ the magnetic quantum number
l
m
determines the orientation of the orbital and can
have values between
and ll−+
♦ the spin magnetic quantum number
s
m
determines the direction of spin and can have
values of
11
or
22
+−

Version 3.1 8
Inorganic chemistry (continued)
(b) Atomic orbitals, electronic configurations and the periodic table (continued)
Electrons within atoms are arranged according to:
♦ the aufbau principle — electrons fill orbitals in order of increasing energy (‘aufbau’
means ‘building up’ in German)
♦ Hund’s rule — when degenerate orbitals are available, electrons fill each singly,
keeping their spins parallel before spin pairing starts
♦ the Pauli exclusion principle — no two electrons in one atom can have the same set of
four quantum numbers, therefore, no orbital can hold more than two electrons and
these two electrons must have opposite spins
In an isolated atom the orbitals within each subshell are degenerate.
The relative energies corresponding to each orbital can be represented diagrammatically
using orbital box notation for the first four shells of a multi-electron atom.
Electronic configurations using spectroscopic notation and orbital box notation can be
written for elements of atomic numbers 1 to 36.
The periodic table is subdivided into four blocks (s, p, d and f) corresponding to the outer
electronic configurations of the elements within these blocks.
The variation in first, second and subsequent ionisation energies with increasing atomic
number for the first 36 elements can be explained in terms of the relative stability of
different subshell electronic configurations. This provides evidence for these electronic
configurations. Anomalies in the trends of ionisation energies can be explained by
considering the electronic configurations.
There is a special stability associated with half-filled and full subshells. The more stable
the electronic configuration, the higher the ionisation energy.
VSEPR (valence shell electron pair repulsion) theory can be used to predict the shapes of
molecules and polyatomic ions.
The number of electron pairs surrounding a central atom can be found by:
♦ taking the total number of valence (outer) electrons on the central atom and adding
one for each atom attached
♦ adding an electron for every negative charge
♦ removing an electron for every positive charge
♦ dividing the total number of electrons by two to give the number of electron pairs
Electron pairs are negatively charged and repel each other. They are arranged to minimise
repulsion and maximise separation.

Version 3.1 9
Inorganic chemistry (continued)
(b) Atomic orbitals, electronic configurations and the periodic table (continued)
The arrangement of electron pairs around a central atom is:
♦ linear for two electron pairs
♦ trigonal planar for three electron pairs
♦ tetrahedral for four electron pairs
♦ trigonal bipyramidal for five electron pairs
♦ octahedral for six electron pairs
Shapes of molecules or polyatomic ions are determined by the shapes adopted by the
atoms present based on the arrangement of electron pairs. Electron dot diagrams can be
used to show these arrangements.
Electron pair repulsions decrease in strength in the order:
non-bonding pair/non-bonding pair
>
non-bonding pair/bonding pair
>
bonding
pair/bonding pair
(c) Transition metals
The d-block transition metals are metals with an incomplete d subshell in at least one of
their ions.
The filling of the d orbitals follows the aufbau principle, with the exception of chromium and
copper atoms.
These exceptions are due to the special stability associated with the d subshell being half-
filled or completely filled.
When atoms from the first row of the transition elements form ions, it is the 4s electrons
that are lost first rather than the 3d electrons.
An element is said to be in a particular oxidation state when it has a specific oxidation
number.
The oxidation number can be determined by the following:
♦ uncombined elements have an oxidation number of 0
♦ ions containing single atoms have an oxidation number that is the same as the charge
on the ion
♦ in most of its compounds, oxygen has an oxidation number of
2−
♦ in most of its compounds, hydrogen has an oxidation number of
1+
♦ the sum of all the oxidation numbers of all the atoms in a neutral compound must add
up to zero
♦ the sum of all the oxidation numbers of all the atoms in a polyatomic ion must be equal
to the charge on the ion

Version 3.1 10
Inorganic chemistry (continued)
(c) Transition metals (continued)
A transition metal can have different oxidation states in its compounds
.
Compounds of the same transition metal in different oxidation states may have different
colours.
Oxidation can be defined as an increase in oxidation number. Reduction can be
considered as a decrease in oxidation number.
Changes in oxidation number of transition metal ions can be used to determine whether
oxidation or reduction has occurred.
Compounds containing metals in high oxidation states are often oxidising agents, whereas
compounds with metals in low oxidation states are often reducing agents.
Ligands may be negative ions or molecules with non-bonding pairs of electrons that they
donate to the central metal atom or ion, forming dative covalent bonds.
Ligands can be classified as monodentate, bidentate, up to hexadentate.
It is possible to deduce the ligand classification from a formula or structure of the ligand or
complex.
The total number of bonds from the ligands to the central transition metal is known as the
coordination number.
Names and formulae can be written according to IUPAC rules for complexes containing:
♦ central metals that obey the normal IUPAC rules
♦ copper (cuprate) and iron (ferrate)
♦ ligands, including water, ammonia, halogens, cyanide, hydroxide, and oxalate
In a complex of a transition metal, the d orbitals are no longer degenerate.
Splitting of d orbitals to higher and lower energies occurs when the electrons present in
approaching ligands cause the electrons in the orbitals lying along the axes to be repelled.
Ligands that cause a large difference in energy between subsets of d orbitals are strong
field ligands. Weak field ligands cause a small energy difference.
Ligands can be placed in an order of their ability to split d orbitals. This is called the
spectrochemical series.
Colours of many transition metal complexes can be explained in terms of d-d transitions.
Light is absorbed when electrons in a lower energy d orbital are promoted to a d orbital of
higher energy.
Version 3.1 11
Inorganic chemistry (continued)
(c) Transition metals (continued)
If light of one colour is absorbed, then the complementary colour will be observed.
Electrons transition to higher energy levels when energy corresponding to the ultraviolet or
visible regions of the electromagnetic spectrum is absorbed.
Transition metals and their compounds can act as catalysts.
Heterogeneous catalysts are in a different state to the reactants.
Heterogeneous catalysis can be explained in terms of the formation of activated
complexes and the adsorption of reactive molecules onto active sites. The presence of
unpaired d electrons or unfilled d orbitals is thought to allow activated complexes to form.
This can provide reaction pathways with lower activation energies compared to the
uncatalysed reaction.
Homogeneous catalysts are in the same state as the reactants.
Homogeneous catalysis can be explained in terms of changing oxidation states with the
formation of intermediate complexes.

Version 3.1 12
Physical chemistry
(a) Chemical equilibrium
A chemical reaction is in equilibrium when the composition of the reactants and products
remains constant indefinitely.
The equilibrium constant (
K
) characterises the equilibrium composition of the reaction
mixture.
For the general reaction
aA bB cC dD
++
the equilibrium expression is:
[ ] [ ]
[ ] [ ]
cd
ab
CD
K
AB
=
[
] [
]
[ ]
[
]
, , and ABC D
are the equilibrium concentrations of
, , and
ABC D
and
, , and
abc d
are the stoichiometric coefficients in the balanced reaction equation.
The value of equilibrium constants can be calculated.
The value of an equilibrium constant indicates the position of equilibrium.
Equilibrium constants have no units.
The concentrations of pure solids and pure liquids at equilibrium are taken as constant and
given a value of 1 in the equilibrium expression.
The numerical value of the equilibrium constant depends on the reaction temperature and
is independent of concentration and/or pressure.
For endothermic reactions, a rise in temperature causes an increase in
K
and the yield of
the product is increased.
For exothermic reactions, a rise in temperature causes a decrease in
K
and the yield of
the product is decreased.
The presence of a catalyst does not affect the value of the equilibrium constant.
In water and aqueous solutions there is an equilibrium between the water molecules and
hydronium (hydrogen) and hydroxide ions.
This ionisation of water can be represented by:
( ) ( )
() ()
+
22 3
HO HO HO aq OH aq
−
++
()
3
H O aq
+
represents a hydronium ion, a hydrated proton. A shorthand representation of
()
3
H O aq
+
is
)
+
H (aq
.

Version 3.1 13
Physical chemistry (continued)
(a) Chemical equilibrium (continued)
Water is amphoteric (can react as an acid and a base).
The dissociation constant for the ionisation of water is known as the ionic product and is
represented by
w
K
:
–
3
H O OH
w
K
+
=
The value of the ionic product varies with temperature.
At 25°C the value of
w
K
is approximately
-14
1 × 10
.
The relationship between pH and the hydrogen ion concentration is given by:
log
pH
10 3 3
pH H O and H O 10
+ +−
=−=
In water and aqueous solutions with a pH value of 7 the concentrations of
()
3
H O aq
+
and
OH (aq)
−
are both
-7
10
mol l
-1
at 25°C.
If the concentration of
()
3
H O aq
+
or the concentration of
OH (aq)
−
is known, the
concentration of the other ion can be calculated using
w
K
or by using
pH pOH 14+=
.
The Brønsted-Lowry definitions of acids and bases state that an acid is a proton donor and
a base is a proton acceptor.
For every acid there is a conjugate base, formed by the loss of a proton.
For every base there is a conjugate acid, formed by the gain of a proton.
Strong acids and strong bases are completely dissociated into ions in aqueous solution.
Weak acids and weak bases are only partially dissociated into ions in aqueous solution.
Examples of strong acids include hydrochloric acid, sulfuric acid and nitric acid.
Ethanoic acid, carbonic acid and sulfurous acid are examples of weak acids.
Solutions of metal hydroxides are strong bases.
Ammonia and amines are examples of weak bases.
The weakly acidic nature of solutions of carboxylic acids, sulfur dioxide and carbon dioxide
can be explained by reference to equations showing the equilibria.
The weakly alkaline nature of a solution of ammonia or amines can be explained by
reference to an equation showing the equilibrium.

Version 3.1 14
Physical chemistry (continued)
(a) Chemical equilibrium (continued)
Equimolar solutions of weak and strong acids (or bases) have different pH values,
conductivity, and reaction rates, but the stoichiometry of reactions are the same.
The acid dissociation constant is represented by
a
K
:
[ ]
3
HO A
HA
a
K
+−
=
or by:
log
10
p where p
a aa
KKK= −
The approximate pH of a weak acid can be calculated using:
log
10
11
22
pH p c
a
K= −
.
A soluble salt of a strong acid and a strong base dissolves in water to produce a neutral
solution.
A soluble salt of a weak acid and a strong base dissolves in water to produce an alkaline
solution.
A soluble salt of a strong acid and a weak base dissolves in water to produce an acidic
solution.
The name of the salt produced depends on the acid and base used.
Using the appropriate equilibria, the changes in concentrations of
3
HO
+
and
OH
−
ions of
salt solutions can be explained.
A buffer solution is one in which the pH remains approximately constant when small
amounts of acid, base or water are added.
An acid buffer consists of a solution of a weak acid and one of its salts made from a strong
base.
In an acid buffer solution the weak acid provides hydrogen ions when these are removed
by the addition of a small amount of base. The salt of the weak acid provides the conjugate
base, which can absorb excess hydrogen ions produced by the addition of a small amount
of acid.
A basic buffer consists of a solution of a weak base and one of its salts.
In a basic buffer solution the weak base removes excess hydrogen ions, and the conjugate
acid provided by the salt supplies hydrogen ions when these are removed.

Version 3.1 15
Physical chemistry (continued)
(a) Chemical equilibrium (continued)
An approximate pH of an acid buffer solution can be calculated from its composition and
from the acid dissociation constant:
[ ]
[
]
log
10
acid
pH p
salt
a
K= −
Indicators are weak acids for which the dissociation can be represented as:
In ( ) In ( )
23
H (aq) H O H O (aq) aq
+−
++
The acid indicator dissociation constant is represented as
In
K
and is given by the following
expression:
[ ]
In
In
In
+
3
HO
H
K
−
=
In aqueous solution the colour of an acid indicator is distinctly different from that of its
conjugate base.
The colour of the indicator is determined by the ratio of
[ ]
In In
H to
−
.
The theoretical point at which colour change occurs is when
In3
HO K
+
=
.
The colour change is assumed to be distinguishable when
[ ]
In InH and
−
differ by a
factor of 10.
The pH range over which a colour change occurs can be estimated by the expression:
In
pH p 1K= ±
Suitable indicators can be selected from pH data, including titration curves.
(b) Reaction feasibility
The standard enthalpy of formation,
f
H∆°
, is the enthalpy change when one mole of a
substance is formed from its elements in their standard states.
The standard state of a substance is its most stable state at a pressure of 1 atmosphere
and at a specified temperature, usually taken as 298 K.
The standard enthalpy of a reaction can be calculated from the standard enthalpies of
formation of the reactants and products:
()
ff
(products) reactantsHH H
∆°=Σ∆° −Σ∆°

Version 3.1 16
Physical chemistry (continued)
(b) Reaction feasibility (continued)
The entropy (S) of a system is a measure of the degree of disorder of the system.
The greater the degree of disorder, the greater the entropy.
Solids have low disorder and gases have high disorder.
Entropy increases as temperature increases.
There is a rapid increase in entropy at the melting point of a substance and an even more
rapid and larger change in entropy at the boiling point.
The second law of thermodynamics states that the total entropy of a reaction system and
its surroundings always increases for a spontaneous process.
Heat energy released by the reaction system into the surroundings increases the entropy
of the surroundings.
Heat energy absorbed by the reaction system from the surroundings decreases the
entropy of the surroundings.
The third law of thermodynamics states that the entropy of a perfect crystal at 0 K is zero.
The standard entropy of a substance is the entropy value for the substance in its standard
state.
The change in standard entropy for a reaction system can be calculated from the standard
entropies of the reactants and products:
()SS S∆°=Σ° −Σ°(products) reactants
The change in free energy for a reaction is related to the enthalpy and entropy changes:
G H TS∆ °=∆ °− ∆ °
If the change in free energy (
G∆°
) between reactants and products is negative, a reaction
may occur and the reaction is said to be feasible. A feasible reaction is one that tends
towards the products rather than the reactants. This does not give any indication of the
rate of the reaction.
The standard free energy change for a reaction can be calculated from the standard free
energies of formation of the reactants and products using the relationship:
()(products) reactantsGG G∆°=Σ∆° −Σ∆°
The feasibility of a chemical reaction under standard conditions can be predicted from the
calculated value of the change in standard free energy (
G∆°
).
The temperatures at which a reaction may be feasible can be estimated by considering the
range of values of
T
for which
0G∆ °<
.

Version 3.1 17
Physical chemistry (continued)
(b) Reaction feasibility (continued)
Under non-standard conditions any reaction is feasible if
G∆
is negative.
At equilibrium,
0G∆=
.
A reversible reaction will proceed spontaneously until the composition is reached where
0G∆=
.
(c) Kinetics
The rate of a chemical reaction normally depends on the concentrations of the reactants.
Orders of reaction are used to relate the rate of a reaction to the reacting species.
If changing the concentration of a reactant
A
has no effect on the rate of the reaction, then
the reaction is zero order with respect to
A
.
If doubling the concentration of a reactant
A
doubles the rate of the reaction, then the
reaction is first order with respect to
A
. The rate can be expressed as:
[ ]
rate kA=
where
k
is the rate constant and
[ ]
A
is the concentration of reactant
A
in
mol
l
-1
If doubling the concentration of a reactant
A
increases the rate of the reaction fourfold,
then the reaction is second order with respect to
A
. The rate can be expressed as:
[ ]
2
rate kA=
The order of a reaction with respect to any one reactant is the power to which the
concentration of that reactant is raised in the rate equation.
The overall order of a reaction is the sum of the powers to which the concentrations of the
reactants are raised in the rate equation.
The order of a reaction can only be determined from experimental data.
The rate equation and the rate constant, including units, can be determined from initial rate
data for a series of reactions in which the initial concentrations of reactants are varied.
These can be zero, first, second or third order.
Reactions usually occur by a series of steps called a reaction mechanism.
The rate of reaction is dependent on the slowest step, which is called the ‘rate determining
step’.
Experimentally determined rate equations can be used to determine possible reaction
mechanisms.

Version 3.1 18
Organic chemistry and instrumental analysis
(a) Molecular orbitals
VSEPR cannot explain the bonding in all compounds. Molecular orbital theory can provide
an explanation for more complex molecules.
Molecular orbitals form when atomic orbitals combine. The number of molecular orbitals
formed is equal to the number of atomic orbitals that combine. The combination of two
atomic orbitals results in the formation of a bonding molecular orbital and an antibonding
orbital. The bonding molecular orbital encompasses both nuclei. The attraction of the
positively charged nuclei and the negatively charged electrons in the bonding molecular
orbital is the basis of bonding between atoms. Each molecular orbital can hold a maximum
of two electrons.
In a non-polar covalent bond, the bonding molecular orbital is symmetrical about the
midpoint between two atoms. Polar covalent bonds result from bonding molecular orbitals
that are asymmetric about the midpoint between two atoms. The atom with the greater
value for electronegativity has the greater share of the bonding electrons. Ionic compounds
are an extreme case of asymmetry, with the bonding molecular orbitals being almost
entirely located around just one atom, resulting in the formation of ions.
Molecular orbitals that form by end-on overlap of atomic orbitals along the axis of the
covalent bond are called sigma (
σ
) molecular orbitals or sigma bonds.
Molecular orbitals that form by side-on overlap of parallel atomic orbitals that lie
perpendicular to the axis of the covalent bond are called pi (
π
) molecular orbitals or
pi bonds.
The electronic configuration of an isolated carbon atom cannot explain the number of
bonds formed by carbon atoms in molecules. The bonding and shape of molecules of
carbon can be explained by hybridisation.
Hybridisation is the process of mixing atomic orbitals within an atom to generate a set of
new atomic orbitals called hybrid orbitals. These hybrid orbitals are degenerate.
In alkanes, the 2s orbital and the three 2p orbitals of carbon hybridise to form four
degenerate sp
3
hybrid orbitals. These adopt a tetrahedral arrangement. The sp
3
hybrid
orbitals overlap end-on with other atomic orbitals to form
σ
bonds.
The bonding in alkenes can be described in terms of sp
2
hybridisation. The 2s orbital and
two of the 2p orbitals hybridise to form three degenerate sp
2
hybrid orbitals. These adopt a
trigonal planar arrangement. The hybrid sp
2
orbitals overlap end-on to form
σ
bonds. The
remaining 2p orbital on each carbon atom of the double bond is unhybridised and lies
perpendicular to the axis of the
σ
bond. The unhybridised p orbitals overlap side-on to
form
π
bonds.

Version 3.1 19
Organic chemistry and instrumental analysis (continued)
(a) Molecular orbitals (continued)
The bonding in benzene and other aromatic systems can be described in terms of sp
2
hybridisation. The six carbon atoms in benzene are arranged in a cyclic structure with
σ
bonds between the carbon atoms. The unhybridised p orbitals on each carbon atom
overlap side-on to form a
π
molecular system, perpendicular to the plane of the
σ
bonds.
This
π
molecular system extends across all six carbon atoms. The electrons in this
system are delocalised.
The bonding in alkynes can be described in terms of sp hybridisation. The 2s orbital and
one 2p orbital of carbon hybridise to form two degenerate hybrid orbitals. These adopt a
linear arrangement. The hybrid sp orbitals overlap end-on to form
σ
bonds. The remaining
two 2p orbitals on each carbon atom lie perpendicular to each other and to the axis of the
σ
bond. The unhybridised p orbitals overlap side-on to form two
π
bonds.
Molecular orbital theory can be used to explain why organic molecules are colourless or
coloured. Electrons fill bonding molecular orbitals, leaving higher energy antibonding
orbitals unfilled. The highest bonding molecular orbital containing electrons is called the
highest occupied molecular orbital (HOMO). The lowest antibonding molecular orbital is
called the lowest unoccupied molecular orbital (LUMO).
Absorption of electromagnetic energy can cause electrons to be promoted from HOMO to
LUMO.
Most organic molecules appear colourless because the energy difference between HOMO
and LUMO is relatively large. This results in absorption of light from the ultraviolet region of
the spectrum.
Some organic molecules contain chromophores. A chromophore is a group of atoms within
a molecule that is responsible for absorption of light in the visible region of the spectrum.
Light can be absorbed when electrons in a chromophore are promoted from the HOMO to
the LUMO.
Chromophores exist in molecules containing a conjugated system — a system of adjacent
unhybridised p orbitals that overlap side-on to form a molecular orbital across a number of
carbon atoms. Electrons within this conjugated system are delocalised. Molecules with
alternating single and double bonds, and aromatic molecules have conjugated systems.
The more atoms in the conjugated system the smaller the energy gap between HOMO and
LUMO. A lower frequency of light (longer wavelength, lower energy) is absorbed by the
compound. When the wavelength of light absorbed is in the visible region, the compound
will exhibit the complementary colour.

Version 3.1 20
Organic chemistry and instrumental analysis (continued)
(b) Synthesis
When an organic reaction takes place, bonds in the reactant molecules are broken and
bonds in the product molecules are made. The process of bond breaking is known as bond
fission.
There are two types of bond fission, homolytic and heterolytic.
Homolytic fission:
♦ results in the formation of two neutral radicals
♦ occurs when each atom retains one electron from the
σ
covalent bond and the bond
breaks evenly
♦ normally occurs when non-polar covalent bonds are broken
Reactions involving homolytic fission tend to result in the formation of very complex
mixtures of products, making them unsuitable for organic synthesis.
Heterolytic fission:
♦ results in the formation of two oppositely charged ions
♦ occurs when one atom retains both electrons from the
σ
covalent bond and the bond
breaks unevenly
♦ normally occurs when polar covalent bonds are broken
Reactions involving heterolytic fission tend to result in far fewer products than reactions
involving homolytic fission, and so are better suited for organic synthesis.
The movement of electrons during bond fission and bond making can be represented
using curly arrow notation where:
♦ a single-headed arrow indicates the movement of a single electron
♦ a double-headed arrow indicates the movement of an electron pair
♦ the tail of the arrow shows the source of the electron(s)
♦ the head of the arrow indicates the destination of the electron(s)
♦ two single-headed arrows starting at the middle of a covalent bond indicate homolytic
bond fission is occurring
♦ a double-headed arrow starting at the middle of a covalent bond indicates heterolytic
bond fission is occurring
♦ an arrow drawn with the head pointing to the space between two atoms indicates that a
covalent bond will be formed between those two atoms

Version 3.1 21
Organic chemistry and instrumental analysis (continued)
(b) Synthesis (continued)
In reactions involving heterolytic bond fission, attacking groups are classified as
nucleophiles or electrophiles.
Nucleophiles are:
♦ negatively charged ions or neutral molecules that are electron rich, such as
Cl , Br , OH , CN
−− − −
,
32
NH and H O
♦ attracted towards atoms bearing a partial
()
δ
+
or full positive charge
♦ capable of donating an electron pair to form a new covalent bond
Electrophiles are:
♦ positively charged ions or neutral molecules that are electron deficient, such as
H
+
,
2
NO
+
3
and SO
♦ attracted towards atoms bearing a partial
()
δ
−
or full negative charge
♦ capable of accepting an electron pair to form a new covalent bond
The following reaction types can be identified from a chemical equation:
♦ substitution
♦ addition
♦ elimination
♦ condensation
♦ hydrolysis
♦ oxidation
♦ reduction
♦ neutralisation
Synthetic routes can be devised, with no more than three steps, from a given reactant to a
final product.
The possible reactions of a particular molecule can be deduced by looking at the structural
formula.
The structure of any molecule can be drawn as a full, shortened or skeletal structural
formula.
In a skeletal structural formula, neither the carbon atoms, nor any hydrogens attached to
the carbon atoms, are shown. The presence of a carbon atom is implied by a ‘kink’ in the
carbon backbone, and at the end of a line.
Given a full or shortened structural formula for a compound, the skeletal structural formula
can be drawn.
Given a skeletal structural formula for a compound, the full or shortened structural formula
can be drawn.
Molecular formulae can be written from a full, shortened or skeletal structural formula.

Version 3.1 22
Organic chemistry and instrumental analysis (continued)
(b) Synthesis (continued)
Straight and branched chain alkanes; alkenes; alcohols; carboxylic acids; aldehydes and
ketones; haloalkanes; and ethers can be systematically named, indicating the position of
the functional group where appropriate, from structural formulae containing no more than
eight carbon atoms in their longest chain. Straight chain esters can be systematically
named from the names of their parent alcohol and carboxylic acid or their structural
formula.
Molecular formulae can be written and structural formulae drawn from systematic names of
straight and branched chain alkanes; alkenes; alcohols; carboxylic acids; aldehydes and
ketones; haloalkanes; and ethers containing no more than eight carbon atoms in their
longest chain. Molecular formulae can be written and structural formulae drawn for esters
from the systematic name or the structural formulae of their parent alcohol and carboxylic
acid.
Haloalkanes (alkyl halides) are substituted alkanes in which one or more of the hydrogen
atoms is replaced with a halogen atom.
Monohaloalkanes:
♦ contain only one halogen atom
♦ can be classified as primary, secondary or tertiary according to the number of alkyl
groups attached to the carbon atom containing the halogen atom
♦ take part in elimination reactions to form alkenes using a strong base, such as
potassium or sodium hydroxide in ethanol
♦ take part in nucleophilic substitution reactions with:
— aqueous alkalis to form alcohols
— alcoholic alkoxides to form ethers
— ethanolic cyanide to form nitriles (chain length increased by one carbon atom)
that can be hydrolysed to carboxylic acids
A monohaloalkane can take part in nucleophilic substitution reactions by one of two
different mechanisms.
S
N
1 is a nucleophilic substitution reaction with one species in the rate determining step
and occurs in a minimum of two steps via a trigonal planar carbocation intermediate.
S
N
2 is a nucleophilic substitution reaction with two species in the rate determining step and
occurs in a single step via a single five-centred, trigonal bipyramidal transition state.
The reaction mechanisms for S
N
1 and S
N
2 reactions can be represented using curly
arrows. Steric hindrance and the inductive stabilisation of the carbocation intermediate can
be used to explain which mechanism will be preferred for a given haloalkane.
Version 3.1 23
Organic chemistry and instrumental analysis (continued)
(b) Synthesis (continued)
Alcohols are substituted alkanes in which one or more of the hydrogen atoms is replaced
with a hydroxyl functional group, –OH group.
Alcohols can be prepared from:
♦ haloalkanes by substitution
♦ alkenes by acid-catalysed hydration (addition)
♦ aldehydes and ketones by reduction using a reducing agent such as lithium aluminium
hydride
Reactions of alcohols include:
♦ dehydration to form alkenes using aluminium oxide, concentrated sulfuric acid or
concentrated phosphoric acid
♦ oxidation of primary alcohols to form aldehydes and then carboxylic acids and
secondary alcohols to form ketones, using acidified permanganate, acidified
dichromate or hot copper(II) oxide
♦ formation of alcoholic alkoxides by reaction with some reactive metals such as
potassium or sodium, which can then be reacted with monohaloalkanes to form ethers
♦ formation of esters by reaction with carboxylic acids using concentrated sulfuric acid or
concentrated phosphoric acid as a catalyst
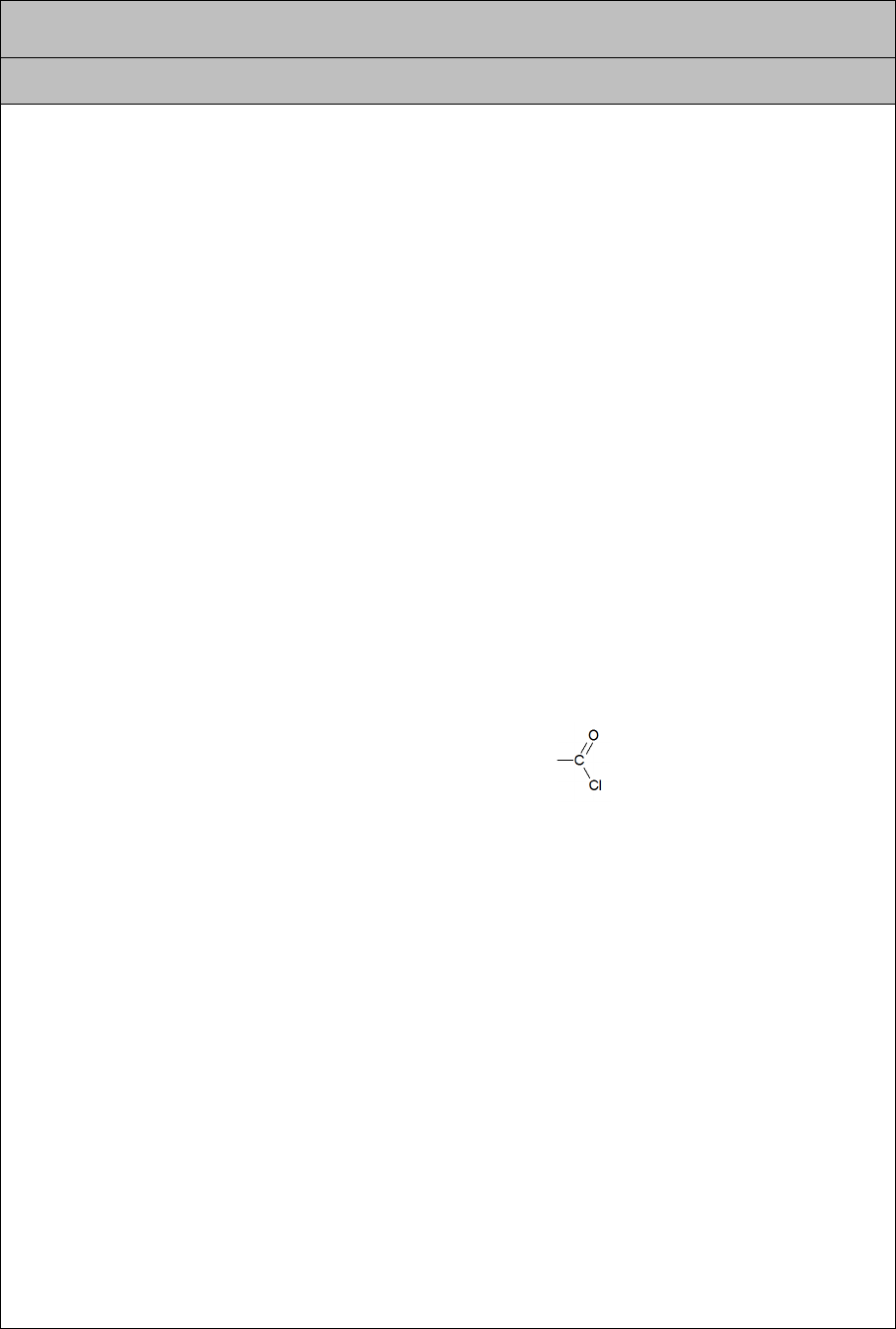
♦ formation of esters by reaction with acid chlorides ( ) — this gives a faster
reaction than reaction with carboxylic acids, and no catalyst is needed
Hydroxyl groups make alcohols polar, which gives rise to hydrogen bonding. Hydrogen
bonding can be used to explain the properties of alcohols including boiling points, melting
points, viscosity and solubility or miscibility in water.
Ethers can be regarded as substituted alkanes in which a hydrogen atom is replaced with
an alkoxy functional group, –OR, and have the general structure R' – O – R'', where R' and
R'' are alkyl groups.
Ethers are named as substituted alkanes. The alkoxy group is named by adding the
ending ‘oxy’ to the alkyl substituent, and this prefixes the name of the longest carbon
chain.
Ethers can be prepared in a nucleophilic substitution reaction by reacting a
monohaloalkane with an alkoxide.
Due to the lack of hydrogen bonding between ether molecules, they have lower boiling
points than the corresponding isomeric alcohols.

Version 3.1 24
Organic chemistry and instrumental analysis (continued)
(b) Synthesis (continued)
Methoxymethane and methoxyethane are soluble in water. Larger ethers are insoluble in
water due to their increased molecular size.
Ethers are commonly used as solvents since they are relatively inert chemically and will
dissolve many organic compounds.
Alkenes can be prepared by:
♦ dehydration of alcohols using aluminium oxide, concentrated sulfuric acid or
concentrated phosphoric acid
♦ base-induced elimination of hydrogen halides from monohaloalkanes
Alkenes take part in electrophilic addition reactions with:
♦ hydrogen to form alkanes in the presence of a catalyst
♦ halogens to form dihaloalkanes
♦ hydrogen halides to form monohaloalkanes
♦ water using an acid catalyst to form alcohols
Markovnikov’s rule states that when a hydrogen halide or water is added to an
unsymmetrical alkene, the hydrogen atom becomes attached to the carbon with the most
hydrogen atoms attached to it already. Markovnikov’s rule can be used to predict major
and minor products formed during the reaction of a hydrogen halide or water with alkenes.
The reaction mechanisms for the addition of a hydrogen halide and the acid-catalysed
addition of water can be represented using curly arrows and showing the intermediate
carbocation. The inductive stabilisation of intermediate carbocations formed during these
reactions can be used to explain the products formed.
The reaction mechanism for the addition of a halogen can be represented using curly
arrows and showing the cyclic ion intermediate.
Carboxylic acids can be prepared by:
♦ oxidising primary alcohols using acidified permanganate, acidified dichromate and hot
copper(II) oxide
♦ oxidising aldehydes using acidified permanganate, acidified dichromate, Fehling’s
solution and Tollens’ reagent
♦ hydrolysing nitriles, esters or amides

Version 3.1 25
Organic chemistry and instrumental analysis (continued)
(b) Synthesis (continued)
Reactions of carboxylic acids include:
♦ formation of salts by reactions with metals or bases
♦ condensation reactions with alcohols to form esters in the presence of concentrated
sulfuric or concentrated phosphoric acid
♦ reaction with amines to form alkylammonium salts that form amides when heated
♦ reduction with lithium aluminium hydride to form primary alcohols
Amines are organic derivatives of ammonia in which one or more hydrogen atoms of
ammonia has been replaced by an alkyl group.
Amines can be classified as primary, secondary or tertiary according to the number of alkyl
groups attached to the nitrogen atom.
Amines react with acids to form salts.
Primary and secondary amines, but not tertiary amines, display hydrogen bonding. As a
result, primary and secondary amines have higher boiling points than isomeric tertiary
amines.
Primary, secondary and tertiary amine molecules can hydrogen-bond with water molecules,
thus explaining the appreciable solubility of the shorter chain length amines in water.
Amines like ammonia are weak bases and dissociate to a slight extent in aqueous solution.
The nitrogen atom has a lone pair of electrons which can accept a proton from water,
producing hydroxide ions.
Benzene (C
6
H
6
) is the simplest member of the class of aromatic hydrocarbons.
The benzene ring has a distinctive structural formula. The stability of the benzene ring is due
to the delocalisation of electrons in the conjugated system. The presence of delocalised
electrons explains why the benzene ring does not take part in addition reactions.
Bonding in benzene can be described in terms of sp
2
hybridisation, sigma and pi bonds, and
electron delocalisation.
A benzene ring in which one hydrogen atom has been substituted by another group is
known as the phenyl group. The phenyl group has the formula –C
6
H
5
.
Benzene rings can take part in electrophilic substitution reactions. Reactions at benzene
rings include:
♦ halogenation by reaction of a halogen using aluminium chloride or iron(III) chloride for
chlorination and aluminium bromide or iron(III) bromide for bromination
♦ alkylation by reaction of a haloalkane using aluminium chloride
♦ nitration using concentrated sulfuric acid and concentrated nitric acid
♦ sulfonation using concentrated sulfuric acid

Version 3.1 26
Organic chemistry and instrumental analysis (continued)
(c) Stereo chemistry
Molecules that have the same molecular formula but different structural formulae are
called isomers.
Structural isomers occur when the atoms are bonded together in a different order in each
isomer.
Stereoisomers occur when the order of the bonding in the atoms is the same but the
spatial arrangement of the atoms is different in each isomer. There are two types of
stereoisomer, geometric and optical.
Geometric isomers:
♦ can occur when there is restricted rotation around a carbon-carbon double bond or a
carbon-carbon single bond in a cyclic compound
♦ must have two different groups attached to each of the carbon atoms that make up the
bond with restricted rotation
♦ can be labelled cis or trans according to whether the substituent groups are on the
same side (cis) or on different sides (trans) of the bond with restricted rotation
♦ have differences in physical properties, such as melting point and boiling point
♦ can have differences in chemical properties
Optical isomers:
♦ occur in compounds in which four different groups are arranged tetrahedrally around a
central carbon atom (chiral carbon or chiral centre)
♦ are asymmetric
♦ are non-superimposable mirror images of each other
♦ can be described as enantiomers
♦ have identical physical properties, except for their effect on plane-polarised light
♦ have identical chemical properties, except when in a chiral environment such as that
found in biological systems (only one optical isomer is usually present)
♦ rotate plane-polarised light by the same amount but in opposite directions and so are
optically active
♦ when mixed in equal amounts are optically inactive because the rotational effect of the
plane-polarised light cancels out — this is called a racemic mixture

Version 3.1 27
Organic chemistry and instrumental analysis (continued)
(d) Experimental determination of structure
In organic chemistry a number of experimental techniques are carried out to verify the
chemical structure of a substance.
Elemental microanalysis is used to determine the masses of C, H, O, S and N in a sample
of an organic compound in order to determine its empirical formula.
An empirical formula shows the simplest ratio of the elements in a molecule.
Elemental microanalysis can be determined from:
♦ combustion product masses
♦ percentage product by mass
Mass spectrometry can be used to determine the accurate gram formula mass (GFM) and
structural features of an organic compound.
In mass spectrometry, a small sample of an organic compound is bombarded by
high-energy electrons. This removes electrons from the organic molecule generating
positively charged molecular ions known as parent ions. These molecular ions then break
into smaller positively charged ion fragments. A mass spectrum is obtained showing a plot
of the relative abundance of the ions detected against the mass-to-charge (m/z) ratio.
The mass-to-charge ratio of the parent ion can be used to determine the GFM of the
molecular ion, and so a molecular formula can be determined using the empirical formula.
The fragmentation data can be interpreted to gain structural information.
Infrared spectroscopy is used to identify certain functional groups in an organic compound.
When infrared radiation is absorbed by organic compounds, bonds within the molecule
vibrate (stretch and bend). The wavelengths of infrared radiation that are absorbed depend
on the type of atoms that make up the bond and the strength of the bond.
In infrared spectroscopy, infrared radiation is passed through a sample of the organic
compound and then into a detector that measures the intensity of the transmitted radiation
at different wavelengths. The absorbance of infrared radiation is measured in
wavenumbers, the reciprocal of wavelength, in units of cm
-1
.
Characteristic absorptions by particular vibrations are given in the data booklet.

Version 3.1 28
Organic chemistry and instrumental analysis (continued)
(d) Experimental determination of structure (continued)
Proton nuclear magnetic resonance spectroscopy (proton NMR or
1
H NMR) can give
information about the different chemical environments of hydrogen atoms (protons or
1
H)
in an organic molecule, and about how many hydrogen atoms there are in each of these
environments.
1
H nuclei behave like tiny magnets and in a strong magnetic field some align with the field
(lower energy), whilst the rest align against it (higher energy). Absorption of radiation in the
radio frequency region of the electromagnetic spectrum causes the
1
H nuclei to ‘flip’ from
the lower to the higher energy alignment. As they fall back from the higher to the lower
energy alignment the emitted radiation is detected and plotted on a spectrum.
In a
1
H NMR spectrum the chemical shift,
δ
, (peak position) is related to the environment
of the
1
H atom and is measured in parts per million (ppm).
Chemical shift values for
1
H in different chemical environments are given in the data
booklet.
The area under the peak is related to the number of
1
H atoms in that environment and is
often given by an integration curve on a spectrum. The height of an integration curve is
proportional to the number of
1
H atoms in that environment, and so a ratio of
1
H atoms in
each environment can be determined.
The standard reference substance used in
1
H NMR spectroscopy is tetramethylsilane
(TMS), which is assigned a chemical shift value equal to zero.
1
H NMR spectra can be obtained using low-resolution or high-resolution NMR.
High-resolution
1
H NMR uses higher radio frequencies than those used in low-resolution
1
H NMR and provides more detailed spectra.
In a high-resolution
1
H NMR an interaction with
1
H atoms on neighbouring carbon atoms
can result in the splitting of peaks into multiplets. The number of
1
H atoms on neighbouring
carbon atoms will determine the number of peaks within a multiplet and can be determined
using the n+1 rule, where n is the number of
1
H atoms on the neighbouring carbon atom.
Low- and high-resolution
1
H NMR spectra can be analysed, and low-resolution
1
H NMR
spectra can be sketched for any given compound.
Version 3.1 29
Organic chemistry and instrumental analysis (continued)
(e) Pharmaceutical chemistry
Drugs are substances that alter the biochemical processes in the body.
Drugs that have beneficial effects are used in medicines.
A medicine usually contains the drug plus other ingredients such as fillers to add bulk or
sweeteners to improve the taste.
Drugs generally work by binding to specific protein molecules. These protein molecules
can be found on the surface of a cell (receptor) or can be specific enzyme molecules within
a cell.
Drugs that act on receptors can be classified as agonists or antagonists.
♦ An agonist mimics the natural compound and binds to the receptor molecules to
produce a response similar to the natural active compound.
♦ An antagonist prevents the natural compound from binding to the receptor, and so
blocks the natural response from occurring.
Many drugs that act on enzymes are classified as enzyme inhibitors and act by binding to
the active site of the enzyme and blocking the reaction normally catalysed there.
The overall shape and size of a drug is such that it interacts with a receptor binding site or
to the active site of an enzyme. The types of interactions formed can include van der
Waals forces and/or ionic bonds.
The structural fragment of a drug molecule that allows it to form interactions with a
receptor binding site or to an enzyme active site normally consists of different functional
groups correctly orientated with respect to each other.
By comparing the structures of drugs that have similar effects on the body, the structural
fragment that is involved in the drug action can be identified.

Version 3.1 30
Researching chemistry
(a) Common chemical apparatus
Candidates must be familiar with the use(s) of the following types of apparatus:
♦ conical flask
♦ digital balance
♦ pipette with safety filler
♦ burette
♦ volumetric (standard) flask
♦ distillation (round-bottomed) flask
♦ condenser
♦ thermometer
♦ Buchner or Hirsch or sintered glass funnel
♦ glassware with ground glass joints (‘Quickfit’ or similar)
♦ thin-layer chromatography apparatus
♦ colorimeter
♦ melting point
♦ separating funnel
(b) Skills involved in experimental work
Candidates must be able to:
♦ tabulate data using appropriate headings and units of measurement
♦ represent data as a scatter graph with suitable scales and labels
♦ sketch a line of best fit (straight or curved) to represent the trend observed in the data
♦ calculate average (mean) values
♦ identify and eliminate rogue points
♦ qualitatively appreciate the relative accuracy of apparatus used to measure the volume
of liquids
♦ comment on the reproducibility of results where measurements have been repeated
♦ carry out quantitative stoichiometric calculations
♦ interpret spectral data
♦ appropriately use a positive control, for example a known substance, to validate a
technique or procedure
(c) Stoichiometric calculations
Stoichiometry is the study of mole relationships involved in chemical reactions.
Chemical equations, using formulae and state symbols, can be written and balanced to
show the mole ratio(s) of reactants and products, including multi-step reactions.
The mass of a mole of any substance, in grams (g), is equal to the gram formula mass
(GFM) and can be calculated using relative atomic masses.

Version 3.1 31
Researching chemistry (continued)
(c) Stoichiometric calculations (continued)
Calculations can be performed using the relationship between the mass and the number of
moles of a substance.
For solutions, the mass of solute (grams or g), the number of moles of solute (moles or
mol), the volume of solution (litres or l), or the concentration of the solution (moles per litre
or mol l
-1
), can be calculated from data provided.
Percentage by mass is the mass of solute made up to 100 cm
3
of solution.
Percentage by volume is the number of cm
3
of solute made up to 100 cm
3
of solution.
The unit ppm stands for parts per million and refers to 1 mg per kg or 1 mg per litre.
Calculations can be performed using data, including:
♦ GFM
♦ masses
♦ number of moles
♦ concentrations and volumes of solutions
♦ volumes of gases
♦ reactant excess
♦ theoretical and percentage yield
♦ empirical formulae
Theoretical yields can be calculated and compared with actual yields, leading to
determining the percentage yield. The percentage yield is reduced by:
♦ mass transfer or mechanical losses
♦ purification of product
♦ side reactions
♦ equilibrium position
Candidates must be able to carry out stoichiometric calculations for all of the skills and
techniques in the course where appropriate.

Version 3.1 32
Researching chemistry (continued)
(d) Gravimetric analysis
Candidates must be familiar with the technique of gravimetric analysis, including use of:
♦ an accurate electronic balance, including the tare function
♦ a weighing boat
♦ weighing by difference
♦ the term ‘weighing accurately approximately’
♦ heating to constant mass:
— heating a substance
— allowing to cool in a desiccator to prevent absorption of water
— weighing
— repeating the steps of heating, cooling and weighing until no further changes in
mass are observed
Gravimetric analysis is used to determine the mass of an element or compound in a
substance.
The substance is converted into another substance of known chemical composition, which
can be readily isolated and purified.
The conversion can occur either through precipitation or volatilisation.
In precipitation conversion the substance undergoes a precipitation reaction. The
precipitate is separated from the filtrate and the filtrate tested to ensure the reaction has
gone to completion. The precipitate is washed, dried to constant mass and then weighed.
In volatilisation conversion the substance is heated and any volatile products (often water)
are evaporated. The substance is heated to constant mass and the final mass recorded.
(e) Volumetric analysis
Candidates must be familiar with use of the technique of volumetric analysis, including:
♦ preparing a standard solution
♦ accurate dilution
♦ standardising solutions to determine accurate concentration
♦ titrating to obtain concordancy using burettes, pipettes and volumetric flasks
♦ choosing an appropriate indicator

Version 3.1 33
Researching chemistry (continued)
(e) Volumetric analysis (continued)
A solution of accurately known concentration is known as a standard solution.
A standard solution can be prepared by:
♦ weighing a primary standard accurately
♦ dissolving in a small volume of solvent (usually deionised or distilled water) in a beaker
♦ transferring the solution and rinsings into a volumetric flask
♦ making up to the graduation mark with solvent
♦ stoppering and inverting
Standard solutions can also be prepared by accurate dilution by pipetting an appropriate
volume of a standard solution into a volumetric flask, making up to the graduation mark
with solvent, stoppering and inverting.
A primary standard must:
♦ be available in a high state of purity
♦ be stable when solid and in solution
♦ be soluble
♦ have a reasonably high GFM
Examples of primary standards include:
♦
23
sodium carbonate, Na CO
♦
22 4 2
hydrated oxalic acid, H C O ·2H O
♦
84 4
potassium hydrogen phthalate, KH(C H O )
♦
3
silver nitrate, AgNO
♦
3
potassium iodate, KIO
♦
2 27
potassium dichromate, K Cr O
Sodium hydroxide is not a primary standard as it has a relatively low GFM, is unstable as a
solid (absorbs moisture) and unstable as a solution. Sodium hydroxide solution must be
standardised before being used in volumetric analysis.

Version 3.1 34
Researching chemistry (continued)
(e) Volumetric analysis (continued)
Candidates must be familiar with use of the following types of volumetric analysis:
♦ acid-base titrations
♦ redox titrations based on reactions between oxidising and reducing agents
♦ complexometric titrations based on reactions in which complexes are formed — EDTA
is an important complexometric reagent and can be used to determine the
concentration of metal ions in solution
♦ back titrations used to find the number of moles of a substance by reacting it with an
excess volume of a reactant of known concentration. The resulting mixture is then
titrated to work out the number of moles of the reactant in excess. From the initial
number of moles of that reactant, the number of moles used in the reaction can be
determined. The initial number of moles of the substance being analysed can then be
calculated. A back titration is useful when trying to work out the quantity of substance
in a solid with a low solubility.
(f) Practical skills and techniques
Candidates must be familiar with use of the technique of colorimetry, including:
♦ preparing a series of standard solutions of appropriate concentration
♦ choosing an appropriate colour or wavelength of filter complementary to the colour of
the species being tested
♦ using a blank
♦ preparing a calibration graph
Colorimetry uses the relationship between colour intensity of a solution and the
concentration of the coloured species present.
A colorimeter or a spectrophotometer is used to measure the absorbance of light of a
series of standard solutions, and this data is used to plot a calibration graph.
The concentration of the solution being tested is determined from its absorbance and by
referring to the calibration curve.
The concentration of coloured species in the solution being tested must lie in the straight
line section of the calibration graph.
Candidates must be familiar with use of the technique of distillation. Distillation is used for
identification and purification of organic compounds.
The boiling point of a compound, determined by distillation, is one of the physical
properties that can be used to confirm its identity.
Distillation can be used to purify a compound by separating it from less volatile substances
in the mixture.

Version 3.1 35
Researching chemistry (continued)
(f) Practical skills and techniques (continued)
Candidates must be familiar with use of the technique of heating under reflux. Heating
under reflux allows heat energy to be applied to a chemical reaction mixture over an
extended period of time without volatile substances escaping.
When carrying out heating under reflux, the reaction mixture is placed in a round-bottomed
flask with anti-bumping granules and the flask is fitted with a condenser. The flask is then
heated using an appropriate source of heat.
Candidates must be familiar with use of the technique of vacuum filtration. Vacuum
filtration involves carrying out a filtration under reduced pressure and provides a faster
means of separating a precipitate from a filtrate. A Büchner, Hirsch or sintered glass funnel
can be used during vacuum filtration.
Candidates must be familiar with use of the technique of recrystallisation to purify an
impure solid involving:
♦ dissolving an impure solid gently in a minimum volume of a hot solvent
♦ hot filtration of the resulting mixture to remove any insoluble impurities
♦ cooling the filtrate slowly to allow crystals of the pure compound to form, leaving
soluble impurities dissolved in the solvent
♦ filtering, washing and drying the pure crystals
The solvent for recrystallisation is chosen so that the compound being purified is
completely soluble at high temperatures and only sparingly soluble at lower temperatures.
Candidates must be familiar with use of the technique of solvent extraction. Solvent
extraction involves isolating a solute from a liquid mixture or solution by extraction using an
immiscible solvent in which the solute is soluble.
When carrying out a solvent extraction, the two immiscible solvents form two layers in the
separating funnel. The solute dissolves in both solvents and an equilibrium establishes
between the two layers. The ratio of solute dissolved in each layer is determined by the
equilibrium constant,
K
. The lower layer is run off into a container and the upper layer is
poured into a second container. This process is repeated to maximise the quantity of
solute extracted.

Version 3.1 36
Researching chemistry (continued)
(f) Practical skills and techniques (continued)
The quantity of solute extracted is greater if a number of extractions using smaller volumes
of solvent are carried out rather than a single extraction using a large volume of solvent.
The solvent used should be:
♦ immiscible with the liquid mixture or solution (usually water)
♦ one in which the solute is more soluble in than the liquid mixture or solution
(usually water)
♦ volatile to allow the solute to be obtained by evaporation of the solvent
♦ unreactive with the solute
Candidates must be familiar with use of the techniques of melting point and mixed melting
point determination. The melting point of a substance is the temperature range over which
the solid first starts to melt, to when all of the solid has melted.
The identity of a pure compound can be confirmed by melting point analysis and a
comparison of the experimentally determined melting point with a literature or known
melting point value.
Determination of the melting point of a compound can give an indication of the purity of a
compound. The presence of impurities in the compound lowers the melting point and
broadens its melting temperature range due to the disruption in intermolecular bonding in
the crystal lattice.
Determination of a mixed melting point involves mixing a small quantity of the product with
some of the pure compound and determining the melting point. The melting point value
and the range of the melting temperature can be used to determine if the product and the
pure compound are the same substance.
Candidates must be familiar with use of the technique of thin-layer chromatography.
Chromatography is a technique used to separate the components present within a mixture.
Chromatography separates substances by making use of differences in their polarity or
molecular size.
Thin-layer chromatography (TLC) uses a fine film of silica or aluminium oxide spread over
glass, aluminium foil or plastic. A small sample of the mixture being tested is spotted onto
the base (pencil) line of the chromatogram. A solvent dissolves the compounds in the spot
and carries the compounds up the chromatogram. How far the compounds are carried
depends on how soluble the compounds are in the chosen solvent and how well they
adhere to the plate. A developing agent or ultraviolet light is normally required to visualise
the spots on the chromatogram.
Version 3.1 37
Researching chemistry (continued)
(f) Practical skills and techniques (continued)
f
R
values can be calculated:
f
distance travelled by the sample
R
distance travelled by the solvent
=
Under the same conditions (temperature, solvent, and saturation levels) a compound
always has the same
f
R
value (within experimental error).
The identity of a compound can be confirmed by:
♦ comparing the experimentally determined
f
R
values with a literature or known value
determined under the same conditions
♦ making a direct comparison on a TLC plate between the compound being tested and
the pure substance — a co-spot could be used
TLC is used to assess the purity of substances. A pure substance, when spotted and
developed on a TLC plate, should appear as a single spot (some impurities may not be
visible by TLC analysis). The presence of more than one spot shows that impurities are
present.
Skills, knowledge and understanding included in the course are appropriate to the SCQF level of
the course. The SCQF level descriptors give further information on characteristics and expected
performance at each SCQF level, and are available on the SCQF website.

Version 3.1 38
Skills for learning, skills for life and skills for work
This course helps candidates to develop broad, generic skills. These skills are based on SQA’s
Skills Framework: Skills for Learning, Skills for Life and Skills for Work and draw from the following
main skills areas:
1
Literacy
1.1
Reading
1.2
Writing
2
Numeracy
2.1
Number processes
2.2
Money, time and measurement
2.3
Information handling
5
Thinking skills
5.3
Applying
5.4
Analysing and evaluating
5.5
Creating
Teachers and/or lecturers must build these skills into the course at an appropriate level, where
there are suitable opportunities.
Version 3.1 39
Course assessment
Course assessment is based on the information in this course specification.
The course assessment meets the purposes and aims of the course by addressing:
♦ breadth — drawing on knowledge and skills from across the course
♦ challenge — requiring greater depth or extension of knowledge and/or skills
♦ application — requiring application of knowledge and/or skills in practical or theoretical contexts
as appropriate
This enables candidates to apply:
♦ breadth and depth of skills, knowledge and understanding from across the course to answer
questions in chemistry
♦ skills of scientific inquiry, using related knowledge, to carry out a meaningful and appropriately
challenging project in chemistry and communicate findings
Course assessment structure: question paper
Question paper 110 marks
The question paper has 110 marks. This is scaled by SQA to represent 75% of the overall marks
for the course assessment.
The question paper has 2 sections.
Section 1 contains multiple-choice questions and has 25 marks.
Section 2 contains restricted-response and extended-response questions and has 85 marks.
A data booklet is provided.
The majority of the marks are awarded for applying knowledge and understanding. The other
marks are awarded for applying skills of scientific inquiry, scientific analytical thinking and problem
solving.
The question paper gives candidates an opportunity to demonstrate the following skills, knowledge
and understanding:
♦ making accurate statements
♦ describing information, providing explanations and integrating knowledge
♦ applying knowledge of chemistry to new situations, interpreting information and solving
problems
♦ planning and designing chemical experiments/investigations, including safety measures to
make a discovery, demonstrate a known fact, illustrate particular effects or test a hypothesis
♦ selecting information from a variety of sources
♦ presenting information appropriately in a variety of forms
Version 3.1 40
♦ processing information and data (using calculations, significant figures and units, where
appropriate)
♦ making predictions and generalisations based on evidence and/or information
♦ drawing valid conclusions and giving explanations supported by evidence and/or justification
♦ evaluating experiments and suggesting improvements
Setting, conducting and marking the question paper
The question paper is set and marked by SQA, and conducted in centres under conditions
specified for external examinations by SQA.
Candidates have 3 hours to complete the question paper.
Specimen question papers for Advanced Higher courses are published on SQA’s website. These
illustrate the standard, structure and requirements of the question papers. The specimen papers
also include marking instructions.
Course assessment structure: project
Project 25 marks
The project has 25 marks. This is scaled by SQA to represent 25% of the overall marks for the
course assessment.
The project allows candidates to carry out an in-depth investigation of a chemistry topic and
produce a project report. Candidates are required to individually plan and carry out a chemistry
investigation.
Candidates should keep a record of their work (lab book) as this will form the basis of their project
report. This record should include details of their research, experiments and recorded data.
The project assesses the application of skills of scientific inquiry and related chemistry knowledge
and understanding. It gives candidates an opportunity to demonstrate the following skills,
knowledge and understanding:
♦ extending and applying knowledge of chemistry to new situations, interpreting and analysing
information to solve more complex problems
♦ planning, designing and safely carrying out chemical experiments/investigations, including risk
assessments to make a discovery, demonstrate a known fact, illustrate particular effects or test
a hypothesis
♦ recording systematic detailed observations and collecting data
♦ selecting information from a variety of sources
♦ presenting detailed information appropriately in a variety of forms
♦ processing and analysing chemical information and data (using calculations, significant figures
and units, where appropriate)
♦ making reasoned predictions and generalisations from a range of evidence and/or information
♦ drawing valid conclusions and giving explanations supported by evidence and/or justification
♦ critically evaluating experimental procedures by identifying sources of uncertainty and
suggesting and implementing improvements
Version 3.1 41
♦ drawing on knowledge and understanding of chemistry to make accurate statements, describe
complex information, provide detailed explanations and integrate knowledge
♦ communicating chemical findings and information fully and effectively
♦ analysing and evaluating scientific publications and media reports
Project overview
Candidates carry out an in-depth investigation of a chemistry topic. Candidates choose their topic
and individually investigate/research its underlying chemistry. Candidates must discuss potential
topics with their teacher or lecturer to ensure that they do not waste time researching unsuitable
topics. This is an open-ended task that may involve candidates carrying out a significant part of the
work without close supervision.
Throughout the project candidates work autonomously, making independent and rational decisions
based on evidence and interpretation of scientific information, which involves analysing and
evaluating results. Through this, candidates further develop and enhance their scientific literacy
skills.
The project offers challenge by requiring candidates to apply skills, knowledge and understanding
in a context that is one or more of the following:
♦ unfamiliar
♦ familiar but investigated in greater depth
♦ integrating a number of familiar contexts
Candidates will produce a project report that has a logical structure.
Refer to the Advanced Higher Chemistry Project Assessment Task for detailed advice on the
content of the project report.
Version 3.1 42
Setting, conducting and marking the project
Setting
The project is set:
♦ by centres within SQA guidelines
♦ at a time appropriate to the candidate’s needs
♦ within teaching and learning and includes experimental work at a level appropriate to Advanced
Higher
Conducting
The project is conducted:
♦ under some supervision and control
♦ in time to meet a submission date set by SQA
♦ independently by the candidate
Marking
The project has 25 marks.
The table below gives the mark allocation for each assessment category of the project report.
Section Expected response
Mark
allocation
Abstract
A brief abstract (summary) stating the overall aim and
conclusion of the project
1
Underlying
chemistry
A description of the underlying chemistry that:
♦ is relevant to the project
♦ demonstrates an understanding of the chemistry theory
underpinning the project
♦ is of a level of demand commensurate with Advanced
Higher Chemistry
3
Data collection and
handling
Procedure(s) clearly described in the past tense and use
the impersonal voice
2
Statement of appropriate safety measure(s) with
justification(s)
1
Data collected using methods of appropriate complexity,
including one procedure and at least one of the following:
♦ a second procedure
♦ a modification in light of experience
♦ a control experiment
♦ standardisation of any solution where the accuracy of
the concentration is crucial in an analysis
1
Experimental results providing evidence that the
procedure(s) has (have) been carried out in duplicate
1
Version 3.1 43
Section Expected response
Mark
allocation
A description of the correct use of the appropriate
apparatus, chemicals and any other substances to achieve
the required levels of precision and/or accuracy
1
All relevant raw data is recorded
1
Numerical data is appropriately presented
1
Citations and references for three sources of
internet/literature data, using any relevant referencing
system
1
Data analysis
Analysis of data of a level of demand commensurate with
Advanced Higher Chemistry. Analysis to include, as
appropriate:
♦ values calculated correctly using a chemical
relationship
♦ scatter, line or bar graph
♦ chromatograms
♦ spectra
4
Calculated values stated to an appropriate number of
significant figures
1
Conclusion
A valid conclusion that relates to the aim and is supported
by all the data in the report
1
Analysis
A valid comparison of the experimental data with data from
the internet/literature source(s), or a comparison of
duplicate experimental data if no internet/literature source
is available
1
Evaluation
Evaluation of the investigation including, as appropriate:
♦ evaluation of data from the internet/literature
♦ evaluative statements supported by justification
♦ quantitative treatment of uncertainties
4
Structure
A clear and concise report with an informative title,
contents page and page numbers
1
Total 25
The project report is submitted to SQA for external marking.
All marking is quality assured by SQA.
Version 3.1 44
Assessment conditions
Time
Candidates should start their project at an appropriate point in the course. SQA does not prescribe
a maximum time allocation for the project but it is expected that candidates will spend 10-15 hours
on experimental work. Candidates may choose to spend additional time on experimental work.
Supervision, control and authentication
The project is conducted under some supervision and control. This means that candidates may
complete part of the work outwith the learning and teaching setting.
Teachers and lecturers must make sure candidates understand the requirements of the project
from the outset.
Teachers and lecturers must ensure that the project is the work of the individual candidate, for
example by:
♦ having regular progress meetings with candidates
♦ conducting spot-check interviews with candidates
♦ regularly reviewing candidates’ lab books
♦ completing checklists to record candidates’ progress
Teachers and lecturers must exercise their professional responsibility to ensure that the project
report submitted by a candidate is the candidate’s own work.
Resources
There are no restrictions on the resources to which candidates may have access.
Reasonable assistance
The term ‘reasonable assistance’ is used to try to balance the need for support with the need to
avoid giving too much assistance, for example, drawing out or teasing out points without leading
candidates. Candidates sometimes get stuck at a particular part of a task. In such cases, a teacher
or lecturer could assist by raising other questions that make the candidate think about the original
problem, therefore giving them the opportunity to answer their own questions without supplying the
actual answers.
Teachers and lecturers must be careful that the integrity of the assessment is not compromised.
Centres must not provide model answers.
Version 3.1 45
Evidence to be gathered
The following candidate evidence is required for this assessment:
♦ a project report
The project report is submitted to SQA, within a given timeframe, for marking.
The same project report cannot be submitted for more than one subject.
Volume
The project report should be between 2500 and 4500 words in length, excluding the title page,
contents page, tables of data, graphs, diagrams, calculations, references and acknowledgements.
Candidates must include their word count on the project report flyleaf.
If the word count exceeds the maximum by more than 10%, a penalty is applied.
Version 3.1 46
Grading
Candidates’ overall grades are determined by their performance across the course assessment.
The course assessment is graded A–D on the basis of the total mark for both course assessment
components.
Grade description for C
For the award of grade C, candidates will typically have demonstrated successful performance in
relation to the skills, knowledge and understanding for the course by:
♦ retaining knowledge and scientific skills over an extended period of time
♦ integrating knowledge and understanding and scientific skills acquired throughout the course
♦ applying knowledge and understanding and scientific skills in a variety of contexts
♦ applying knowledge and understanding and scientific skills to solve problems
♦ selecting, analysing and presenting relevant information collected through experimental,
observational or research work
♦ reporting in a scientific manner that communicates the chemistry
Grade description for A
For the award of grade A, candidates will typically have demonstrated a consistently high level of
performance in relation to the skills, knowledge and understanding for the course by:
♦ retaining an extensive range of knowledge and scientific skills over an extended period of time
♦ integrating an extensive range of knowledge and understanding and scientific skills acquired
throughout the course
♦ applying knowledge and understanding and scientific skills in a variety of complex contexts
♦ integrating knowledge and understanding and scientific skills to solve problems in a variety of
complex contexts
♦ showing proficiency in selecting, analysing and presenting relevant information, collected
through experimental, observational or research work
♦ showing proficiency in reporting in a scientific manner that communicates the chemistry by
analysing and interpreting information in a critical and scientific manner, and demonstrating
depth of knowledge and understanding

Version 3.1 47
Equality and inclusion
This course is designed to be as fair and as accessible as possible with no unnecessary barriers to
learning or assessment.
Guidance on assessment arrangements for disabled candidates and/or those with additional
support needs is available on the assessment arrangements web page:
www.sqa.org.uk/assessmentarrangements
.
Version 3.1 48
Further information
♦ Advanced Higher Chemistry subject page
♦ Assessment arrangements web page
♦ Building the Curriculum 3–5
♦ Guide to Assessment
♦ Guidance on conditions of assessment for coursework
♦ SQA Skills Framework: Skills for Learning, Skills for Life and Skills for Work
♦ Coursework Authenticity: A Guide for Teachers and Lecturers
♦ Educational Research Reports
♦ SQA Guidelines on e-assessment for Schools
♦ SQA e-assessment web page
The SCQF Framework, level descriptors and handbook are available on the SCQF website.
Version 3.1 49
Appendix: course support notes
Introduction
These support notes are not mandatory. They provide advice and guidance to teachers and
lecturers on approaches to delivering the course. Please read these course support notes in
conjunction with the course specification, the specimen question paper and the project assessment
task.
Approaches to learning and teaching
This section provides you with advice and guidance on learning and teaching. You should use a
variety of learning and teaching approaches to allow candidates with different needs and prior
attainment to demonstrate achievement. You have considerable flexibility to select contexts that
stimulate and challenge candidates, offering both breadth and depth.
Discussion and questioning are effective ways of developing candidates’ knowledge and
understanding of chemical concepts. Teachers, lecturers and candidates should make full use of
models to develop the understanding of concepts in chemistry, and use information communication
technology to support learning and to process data. As well as using computers as a learning tool,
computer animations and simulations can help candidates understand chemical concepts.
Computer-interfacing equipment can detect changes in variables, allowing experimental results to
be recorded and processed. Results can also be displayed in real time, helping to improve
understanding.
Advanced Higher courses encourage independent study. Candidates should be given opportunities
to work independently, collaboratively, co-operatively and as a whole class.
You should adopt a holistic approach to encourage the simultaneous development of candidates’
conceptual understanding and skills. Practical and investigative skills are strongly recommended to
be progressively developed throughout the course. This allows practical techniques to be
introduced and practised within real-life contexts, and for candidates to make the connections
between theory and practical applications. You should encourage candidates to see risk
assessment as part of the planning process for any practical activity. Throughout the course,
candidates should have the opportunity to assess risks and make informed decisions regarding the
use of appropriate control measures. During the project, candidates must identify safety measures
taken to minimise risk during their experimental work.
Although the mandatory knowledge and skills may be similar in Higher and Advanced Higher
courses, there are differences in the:
♦ depth of underpinning knowledge and understanding
♦ complexity and sophistication of the applied skills
♦ ways that candidates learn — they take more responsibility for their learning at Advanced
Higher, and work more autonomously
The mandatory content is described in four areas: inorganic chemistry; physical chemistry; organic
chemistry and instrumental analysis; and researching chemistry. Centres can deliver the course
content in whichever order best meets the needs of their candidates.
Version 3.1 50
Partnership working can enhance the learning experience. You could invite guest speakers from,
industry, further education and higher education to share their knowledge of particular aspects of
chemistry.
Assessment should be integral to and improve learning and teaching. The approach should involve
candidates and provide supportive feedback. Self- and peer-assessment techniques are
encouraged, wherever appropriate. Assessment information should be used to set learning targets
and next steps and provide supportive feedback.
Examples of possible learning and teaching activities can be found in the following table.
The first column matches the ‘Skills, knowledge and understanding for the course assessment’
section in the course specification. The second column offers suggestions for activities that you
could use to enhance teaching and learning.
All resources named were correct at the time of publication and may be subject to change.
The Strathclyde resource, and the RSC site, require you to register, but registration is free.
Version 3.1 51
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Electromagnetic radiation and atomic spectra
Electromagnetic radiation can be described in terms of waves and
characterised in terms of wavelength and/or frequency.
The relationship between these quantities is given by
cf
λ
=
.
Frequency is often quoted in Hz, which is the same
as s
-1
.
The different types of radiation arranged in order of wavelength is
known as the electromagnetic spectrum.
Wavelengths of visible light are normally expressed in nanometres
(nm).
Electromagnetic radiation can be described as a wave (has a
wavelength and frequency), and as a particle, and is said to have a
dual nature.
A variety of different resources on this topic are available from RSC
education resources, including:
♦ a printable handout containing a chart of the electromagnetic
spectrum
♦ a vignette showing the quantisation of energy levels within an
atom
When electromagnetic radiation is absorbed or emitted by matter it
behaves like a stream of particles. These particles are known as
photons.
A photon carries quantised energy proportional to the frequency of
radiation.
When a photon is absorbed or emitted, energy is gained or lost by
electrons within the substance.
Video tutorials on light and the electromagnetic spectrum are
available from Khan Academy.
Version 3.1 52
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Electromagnetic radiation and atomic spectra (continued)
The photons in high frequency radiation can transfer greater amounts
of energy than photons in low frequency radiation.
The energy associated with a single photon is given by:
or
hc
E hf E
λ
= =
The values for the constants are h = 6·63 × 10
–34
J
s and
L = 6·02 × 10
23
mol
–1
and are given in the data booklet.
L is Avogadro’s constant and is the number of formula units in one
mole of the substance. (Formula units can be atoms, molecules or
groups of ions, depending on the type of bonding present.)
The energy associated with one mole of photons is given by:
or
Lhc
E Lhf E
λ
= =
Energy is often in units of kJ mol
-1
.
When energy is transferred to atoms, electrons within the atoms may
be promoted to higher energy levels.
An atom emits a photon of light energy when an excited electron
moves from a higher energy level to a lower energy level.
To calculate the energy of one mole of photons in kJ mol
-1
, it may be
more convenient to use:
or
1000 1000
Lhf Lhc
EE
λ
= =
The light energy emitted by an atom produces a spectrum that is
made up of a series of lines at discrete (quantised) energy levels.
This provides direct evidence for the existence of these energy
levels.
Chemguide explains the atomic emission spectrum of hydrogen.
Version 3.1 53
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Electromagnetic radiation and atomic spectra (continued)
A number of resources detail activities relating to atomic emission
spectroscopy, including:
♦ RSC education resources:
— spray bottle flame test demonstration
— flame test class experiment
♦ SSERC:
— flame test demonstration
— instructions for making a spectroscope from a CD, a DVD, or
using a smart phone
♦ chemistry hypermedia project — provides information about
atomic emission instrumentation
Each element in a sample produces characteristic absorption and
emission spectra. These spectra can be used to identify and quantify
the element.
Khan Academy provides a video tutorial explaining the difference
between atomic absorption and emission spectroscopy.
Version 3.1 54
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Electromagnetic radiation and atomic spectra (continued)
In absorption spectroscopy, electromagnetic radiation is directed at
an atomised sample. Radiation is absorbed as electrons are
promoted to higher energy levels.
An absorption spectrum is produced by measuring how the intensity
of absorbed light varies with wavelength.
In emission spectroscopy, high temperatures are used to excite the
electrons within atoms.
As the electrons drop to lower energy levels, photons are emitted.
An emission spectrum of a sample is produced by measuring the
intensity of light emitted at different wavelengths.
In atomic spectroscopy, the concentration of an element within a
sample is related to the intensity of light emitted or absorbed.
A number of activities demonstrate atomic absorption spectroscopy,
including:
♦ SSERC — an activity with filter paper soaked in brine
used to
observe the sodium absorption spectrum
♦ vapour discharge lamps or fluorescent tube lamps used to
observe the emission spectrum of mercury (a series of purple
lines) when viewed through a spectroscope
RSC education resources has an interesting anecdote describing a
forensic use of atomic absorption spectroscopy in an
investigation of
lead in hair treated with products to reduce greyness. An applet from
the University of Oregon shows the absorption and emission spectra
of elements by clicking on the appropriate element on a periodic
table.
(b) Atomic orbitals, electronic configurations and the periodic table
The discrete lines observed in atomic spectra can be explained if
electrons, like photons, also display the properties of both particles
and waves.
Animated videos explaining the behaviour of electrons as waves and
particles are available online.
Version 3.1 55
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Atomic orbitals, electronic configurations and the periodic table (continued)
Electrons behave as standing (stationary) waves in an atom. These
are waves that vibrate in time but do not move in space. There are
different sizes and shapes of standing wave possible around the
nucleus, known as orbitals. Orbitals can hold a maximum of two
electrons.
The different shapes of orbitals are identified as s, p, d and f
(knowledge of the shape of f orbitals is not required).
Electrons within atoms have fixed amounts of energy called quanta.
It is possible to describe any electron in an atom using four quantum
numbers:
♦ the principal quantum number
n
indicates the main energy level
for an electron and is related to the size of the orbital
♦ the angular momentum quantum number
l
determines the shape
of the subshell and can have values from zero to
1n −
♦ the magnetic quantum number
l
m
determines the orientation of
the orbital and can have values between
and ll−+
♦ the spin magnetic quantum number
s
m
determines the direction of
spin and can have values of
11
or
22
+−
A number of resources provide details of activities relating to atomic
orbitals and the quantum mechanical model of the atom:
♦ ChemTube3D, available through RSC education resources,
has
an illustration of the shapes of atomic orbitals
♦ Chemguide has information about atomic orbitals, including
electronic configurations
♦ Khan Academy has videos and tutorials giving information on:
— the quantum mechanical model of the atom
— electronic configurations
— quantum numbers
♦ RSC education resources has an interactive gridlocks game on
subshells
Version 3.1 56
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Atomic orbitals, electronic configurations and the periodic table (continued)
Electrons within atoms are arranged according to:
♦ the aufbau principle — electrons fill orbitals in order of increasing
energy (‘aufbau’ means ‘building up’ in German)
♦ Hund’s rule — when degenerate orbitals are available, electrons
fill each singly, keeping their spins parallel before spin pairing
starts
♦ the Pauli exclusion principle — no two electrons in one atom can
have the same set of four quantum numbers, therefore, no orbital
can hold more than two electrons and these two electrons must
have opposite spins
An interesting audio discussion of Pauli’s exclusion principle and the
life of Wolfgang Pauli is available for download from BBC Radio 4 —
In Our Time.
In an isolated atom the orbitals within each subshell are degenerate.
The relative energies corresponding to each orbital can be
represented diagrammatically using orbital box notation for the first
four shells of a multi-electron atom.
Electronic configurations using spectroscopic notation and orbital box
notation can be written for elements of atomic numbers 1 to 36.
The periodic table is subdivided into four blocks (s, p, d and f)
corresponding to the outer electronic configurations of the elements
within these blocks.
There are a number of online resources providing tutorial notes
covering electronic configuration, spectroscopic notation and orbital
box notation, including
Chemistry Libretexts and Chemguide.
Version 3.1 57
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Atomic orbitals, electronic configurations and the periodic table (continued)
The variation in first, second and subsequent ionisation energies with
increasing atomic number for the first 36 elements can be explained
in terms of the relative stability of different subshell electronic
configurations. This provides evidence for these electronic
configurations. Anomalies in the trends of ionisation energies can be
explained by considering the electronic configurations.
A graph of first ionisation energies against atomic number shows
anomalies, which provides good evidence of s and p orbitals being
filled. Chemguide
explains these anomalies.
There is a special stability associated with half-filled and full
subshells. The more stable the electronic configuration, the higher
the ionisation energy.
VSEPR (valence shell electron pair repulsion) theory can be used to
predict the shapes of molecules and polyatomic ions.
The number of electron pairs surrounding a central atom can be
found by:
♦ taking the total number of valence (outer) electrons on the central
atom and adding one for each atom attached
♦ adding an electron for every negative charge
♦ removing an electron for every positive charge
♦ dividing the total number of electrons by two to give the number
of electron pairs
Although valence shell electron pair repulsion (VSEPR) theory does
not describe the actual molecular orbitals in a molecule, the shapes
predicted are usually quite accurate. Bristol University ChemLabS
has
instructions for working out electron pairs with worked examples.
RSC education resources has a number of vignettes on
bonding
theory and VSEPR.
Version 3.1 58
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Atomic orbitals, electronic configurations and the periodic table (continued)
Electron pairs are negatively charged and repel each other. They are
arranged to minimise repulsion and maximise separation.
A fun practical using soap bubbles to demonstrate the concept of
VSEPR is available from Boise State University.
The arrangement of electron pairs around a central atom is:
♦ linear for two electron pairs
♦ trigonal planar for three electron pairs
♦ tetrahedral for four electron pairs
♦ trigonal bipyramidal for five electron pairs
♦ octahedral for six electron pairs
Shapes of molecules or polyatomic ions are determined by the
shapes adopted by the atoms present based on the arrangement of
electron pairs. Electron dot diagrams can be used to show these
arrangements.
Electron pair repulsions decrease in strength in the order:
non-bonding pair/non-bonding pair
>
non-bonding pair/bonding pair
>
bonding pair/bonding pair
Version 3.1 59
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Transition metals
The d-block transition metals are metals with an incomplete d
subshell in at least one of their ions.
The filling of the d orbitals follows the aufbau principle, with the
exception of chromium and copper atoms.
These exceptions are due to the special stability associated with the
d subshell being half-filled or completely filled.
When atoms from the first row of the transition elements form ions, it
is the 4s electrons that are lost first rather than the 3d electrons.
An element is said to be in a particular oxidation state when it has a
specific oxidation number.
The oxidation number can be determined by the following:
♦ uncombined elements have an oxidation number of 0
♦ ions containing single atoms have an oxidation number that is the
same as the charge on the ion
♦ in most of its compounds, oxygen has an oxidation number of
2−
♦ in most of its compounds, hydrogen has an oxidation number of
1+
♦ the sum of all the oxidation numbers of all the atoms in a neutral
compound must add up to zero
♦ the sum of all the oxidation numbers of all the atoms in a
polyatomic ion must be equal to the charge on the ion
A display of sample bottles containing salts or compounds of the first
30 elements arranged on a large (A1 or A2 size) periodic table show
that only the d-block compounds are coloured.
Candidates may also
notice that zinc compounds are white, indicating that, although lying
in the central region of the periodic table, zinc is different from the
transition metals. Scandium is also different since it forms only the 3
+
ion, which has no d electrons.
Khan Academy has a number of video tutorials on transition metals
and electronic configurations of d-block elements.
Version 3.1 60
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Transition metals (continued)
A transition metal can have different oxidation states in its
compounds.
Compounds of the same transition metal in different oxidation states
may have different colours.
Oxidation can be defined as an increase in oxidation number.
Reduction can be considered as a decrease in oxidation number.
Changes in oxidation number of transition metal ions can be used to
determine whether oxidation or reduction has occurred.
Compounds containing metals in high oxidation states are often
oxidising agents, whereas compounds with metals in low oxidation
states are often reducing agents.
Ligands may be negative ions or molecules with non-bonding pairs of
electrons that they donate to the central metal atom or ion, forming
dative covalent bonds.
Ligands can be classified as monodentate, bidentate, up to
hexadentate.
It is possible to deduce the ligand classification from a formula or
structure of the ligand or complex.
A number of resources provide instructions for experiments involving
oxidation states of transition metals and transition metal complexes,
including:
♦ RSC education resources:
— oxidation states of vanadium
— transition elements — a microscale investigation
— preparation of nickel complexes (Skills’ block 2, page 10)
— complexes of cobalt (Discovery block 4, page 20)
♦ SSERC:
— oxidation states of vanadium
— oxidation states of manganese
— colour change chameleon
— ligands of copper complexes
— copper amino complexes
— microscale iron drops practical
♦ Science in School:
— a redox reaction using lollipops
Version 3.1 61
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Transition metals (continued)
The total number of bonds from the ligands to the central transition
metal is known as the coordination number.
RSC education resources has a gridlocks game to aid revision of
shapes of complex ions and coordination numbers.
Names and formulae can be written according to IUPAC rules for
complexes containing:
♦ central metals that obey the normal IUPAC rules
♦ copper (cuprate) and iron (ferrate)
♦ ligands, including water, ammonia, halogens, cyanide, hydroxide,
and oxalate
In a complex of a transition metal, the d orbitals are no longer
degenerate.
Nomenclature of Inorganic Chemistry (Red Book) has information
about IUPAC rules for naming complexes.
Splitting of d orbitals to higher and lower energies occurs when the
electrons present in approaching ligands cause the electrons in the
orbitals lying along the axes to be repelled.
Ligands that cause a large difference in energy between subsets of d
orbitals are strong field ligands. Weak field ligands cause a small
energy difference.
Ligands can be placed in an order of their ability to split d orbitals.
This is called the spectrochemical series.
Candidates can investigate the spectrochemical series and discover
how the position of a ligand in the series may affect the colour of the
complex. RSC Education in Chemistry details an experiment that can
be used to demonstrate the spectrochemical
series.
Version 3.1 62
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Transition metals (continued)
Colours of many transition metal complexes can be explained in
terms of d-d transitions.
Light is absorbed when electrons in a lower energy d orbital are
promoted to a d orbital of higher energy.
If light of one colour is absorbed, then the complementary colour will
be observed.
Electrons transition to higher energy levels when energy
corresponding to the ultraviolet or visible regions of the
electromagnetic spectrum is absorbed.
Chemguide explains colours of transition metal complexes.
RSC education resources hosts an exciting Nuffield Foundation
experiment involving transition metal ions in coloured glass
as an
interesting introduction, and looks at an everyday life application of
the use of transition metal chemistry.
STEM Learning details the RSC Classic Chemistry Demonstrations
No. 93, page 261, that shows different colours of nickel complexes
with water and ethylenediamine as ligands in different ratios.
UV-visible spectrometers and colorimeters measure the intensity of
radiation transmitted through a sample, and compares this with the
intensity of incident radiation.
Chemistry Practical Guide — Support
Materials (2012), produced by Education Scotland and available
through SSERC, details an experiment to determine the manganese
content in steel using the practical technique of colorimetry.
The wavelength ranges are approximately 200–400 nm for ultraviolet
and 400–700 nm for visible light.
Version 3.1 63
Inorganic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Transition metals (continued)
Transition metals and their compounds can act as catalysts.
Heterogeneous catalysts are in a different state to the reactants.
Heterogeneous catalysis can be explained in terms of the formation
of activated complexes and the adsorption of reactive molecules onto
active sites. The presence of unpaired d electrons or unfilled d
orbitals is thought to allow activated complexes to form. This can
provide reaction pathways with lower activation energies compared to
the uncatalysed reaction.
Chemguide has a range of information on catalysts starting with an
introduction to catalysts.
Homogeneous catalysts are in the same state as the reactants.
Homogeneous catalysis can be explained in terms of changing
oxidation states with the formation of intermediate complexes.
The demonstration of cobalt(II) chloride as a homogeneous transition
metal catalyst in the oxidation of Rochelle salt may have been carried
out at Higher, but can now be discussed in terms of oxidation states.
There are a number of online resources detailing the instructions for
this, including:
♦ RSC education resources:
— a visible activated complex
— catalysts in reactions
♦ SSERC:
— catalysts at work
Version 3.1 64
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium
A chemical reaction is in equilibrium when the composition of the
reactants and products remains constant indefinitely.
The equilibrium constant (
K
) characterises the equilibrium
composition of the reaction mixture.
For the general reaction
aA bB cC dD++
the equilibrium
expression is:
[ ] [ ]
[ ] [ ]
cd
ab
CD
K
AB
=
You should reinforce the links between equilibrium where applicable
in the ‘Organic chemistry and instrumental analysis’, and
‘Researching Chemistry areas.
Chemguide’s An introduction to equilibrium
has a useful recap of
prior knowledge of equilibrium.
RSC education resources,
Advanced starters for ten: section 2
‘Equilibria’, has a selection of easily editable short quizzes and
activities.
[ ] [ ] [ ] [ ]
, , and ABC D
are the equilibrium concentrations of
, , and
ABC D
and
, , and abc d
are the stoichiometric coefficients in
the balanced reaction equation.
The value of equilibrium constants can be calculated.
A number of tutorials on equilibrium constant are available online, for
example:
♦ Khan Academy — The equilibrium constant K
♦ Chemguide — Equilibrium constants: K
c
The value of an equilibrium constant indicates the position of
equilibrium.
Equilibrium constants have no units.
Partition coefficients could be included as a specific example of an
equilibrium constant. RSC education resources has details of a
practical on the partition of iodine across two immiscible liquids
.
Version 3.1 65
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
The concentrations of pure solids and pure liquids at equilibrium are
taken as constant and given a value of 1 in the equilibrium
expression.
Introduce thin-layer chromatography (TLC) and solvent extraction
activities to reinforce the concept of equilibria through practical
applications and to reinforce links to the ‘Researching chemistry’
area.
The numerical value of the equilibrium constant depends on the
reaction temperature and is independent of concentration and/or
pressure.
For endothermic reactions, a rise in temperature causes an increase
in
K
and the yield of the product is increased.
For exothermic reactions, a rise in temperature causes a decrease in
K
and the yield of the product is decreased.
The presence of a catalyst does not affect the value of the equilibrium
constant.
Chemguide explains the relationship between equilibrium constant
and Le Chatelier’s principle.
In water and aqueous solutions there is an equilibrium between the
water molecules and hydronium (hydrogen) and hydroxide ions.
This ionisation of water can be represented by:
( ) ( )
() ()
+
22 3
HO HO HO aq OH aq
−
++
Chemguide has a good explanation about the ionic product of water.
RSC education resources, Advanced starters for ten: section 3,
‘Acids and bases’, has a selection of easily editable short quizzes
and activities relating to the ionic product of water.
Version 3.1 66
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
()
3
H O aq
+
represents a hydronium ion, a hydrated proton. A
shorthand representation of
()
3
H O aq
+
is
)
+
H (aq
.
Water is amphoteric (can react as an acid and a base).
The dissociation constant for the ionisation of water is known as the
ionic product and is represented by
w
K
:
–
3
H O OH
w
K
+
=
The value of the ionic product varies with temperature.
At 25°C the value of
w
K
is approximately
-14
1 × 10
.
The relationship between pH and the hydrogen ion concentration is
given by:
log
pH
10 3 3
pH H O and H O 10
+ +−
=−=
There are a number of virtual lab simulations on acids and bases
available, including:
♦ Chemcollective — acid-base chemistry
♦ RSC education resources — pH scale simulation
In water and aqueous solutions with a pH value of 7 the
concentrations of
()
3
H O aq
+
and
OH (aq)
−
are both
-7
10
mol l
-1
at
25°C.
Version 3.1 67
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
If the concentration of
()
3
H O aq
+
or the concentration of
OH (aq)
−
is
known, the concentration of the other ion can be calculated using
w
K
or by using
pH pOH 14
+=
.
The Brønsted-Lowry definitions of acids and bases state that an acid
is a proton donor and a base is a proton acceptor.
For every acid there is a conjugate base, formed by the loss of a
proton.
For every base there is a conjugate acid, formed by the gain of a
proton.
Online tutorials available include:
♦ Khan Academy:
— pH, pOH and the pH scale
— Brønsted-Lowry acid-base theory
♦ Chemguide:
— theories of acids and bases
, which provides information on
Brønsted Lowry acids and bases, conjugate acids and bases,
and amphoteric substances
Strong acids and strong bases are completely dissociated into ions in
aqueous solution.
Weak acids and weak bases are only partially dissociated into ions in
aqueous solution.
Examples of strong acids include hydrochloric acid, sulfuric acid and
nitric acid.
Ethanoic acid, carbonic acid and sulfurous acid are examples of
weak acids.
Investigate the pH of strong and weak acids and bases and different
metal and non-metal hydroxide solutions. Ideas for investigations
include:
♦ Salters’ archive — investigating acid-base reactions
♦ STEM learning — strong and weak acids — the common ion effect
Version 3.1 68
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
Solutions of metal hydroxides are strong bases.
Ammonia and amines are examples of weak bases.
The weakly acidic nature of solutions of carboxylic acids, sulfur
dioxide and carbon dioxide can be explained by reference to
equations showing the equilibria.
The weakly alkaline nature of a solution of ammonia or amines can
be explained by reference to an equation showing the equilibrium.
Equimolar solutions of weak and strong acids (or bases) have
different pH values, conductivity, and reaction rates, but the
stoichiometry of reactions are the same.
The acid dissociation constant is represented by
a
K
:
[ ]
3
HO A
HA
a
K
+−
=
Chemguide has tutorial notes available on strong and weak acids and
bases.
RSC education resources
Advanced starters for ten: section 3, ‘Acids
and bases’, offers easily editable short quizzes and activities covering
acids and bases.
To highlight the difference between strong and weak acids, one
possible activity involves calculating the pH of a 0·1 mol l
-1
solution of
a weak acid, and confirming the result by measurement. The solution
can then be diluted tenfold to show that the pH rises by 0·5 rather
than by 1, as it would when diluting a strong acid such as 0·1 mol l
-1
HCl. This is also a good opportunity for candidates to practise the
technique of accurate dilution.
or by:
log
10
p where p
a aa
KKK= −
The approximate pH of a weak acid can be calculated using:
log
10
11
22
pH p c
a
K= −
.
Version 3.1 69
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
A soluble salt of a strong acid and a strong base dissolves in water to
produce a neutral solution.
A soluble salt of a weak acid and a strong base dissolves in water to
produce an alkaline solution.
A soluble salt of a strong acid and a weak base dissolves in water to
produce an acidic solution.
The name of the salt produced depends on the acid and base used.
Using the appropriate equilibria, the changes in concentrations of
3
HO
+
and
OH
−
ions of salt solutions can be explained.
RSC education resources provides resources relating to the pH of
salt solutions:
♦ Titration screen experiment: Titration 2 and titration 3
provide a
good explanation of pH curves
♦ Problem-based practical activities ‘Acid erosion’, problem 6,
contains practice calculations and a practical activity relating to pH,
pH curves, strong and weak acids, and
p
a
K
values.
Further information about
pH (titration) curves, pH of salts and choice
of indicator is available on Chemguide.
ChemCollective has a concept test on acids and bases.
Candidates can calculate the pH of a given salt solution and confirm
the value obtained by measuring the pH (examples of salt solutions
include: sodium carbonate; sodium sulphite; sodium stearate;
ammonium chloride; ammonium nitrate). Candidates can carry out
titration experiments measuring the pH after each small addition of an
acid or base, and plotting the results on a graph to create pH curves.
Data logging equipment can be used and the pH curves plotted can
be used to introduce indicator solutions.
Version 3.1 70
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
A buffer solution is one in which the pH remains approximately
constant when small amounts of acid, base or water are added.
An acid buffer consists of a solution of a weak acid and one of its salts
made from a strong base.
In an acid buffer solution the weak acid provides hydrogen ions when
these are removed by the addition of a small amount of base. The
salt of the weak acid provides the conjugate base, which can absorb
excess hydrogen ions produced by the addition of a small amount of
acid.
A basic buffer consists of a solution of a weak base and one of its
salts.
In a basic buffer solution the weak base removes excess hydrogen
ions, and the conjugate acid provided by the salt supplies hydrogen
ions when these are removed.
Candidates can prepare buffer solutions, calculate their pH and
confirm the value obtained by measuring the pH. A pH meter could
be first calibrated using a buffer solution, and then used to measure
the pH, and so provide a practical application of the use of a buffer
solution.
Khan Academy has a range of resources including:
♦ pH and pKa relationship in buffers
♦ buffer solution pH calculations
♦ introduction to buffer systems, which regulate pH in blood
Version 3.1 71
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
An approximate pH of an acid buffer solution can be calculated from
its composition and from the acid dissociation constant:
[ ]
[ ]
log
10
acid
pH p
salt
a
K= −
Indicators are weak acids for which the dissociation can be
represented as:
In ( ) In ( )
23
H (aq) H O H O (aq) aq
+−
++
Candidates can determine the pH range over which an indicator
changes colour. Natural indicators extracted from plants could be
used.
Use pH curves to explain how appropriate indicators for titration
reactions are selected. Khan Academy has a video explaining
titration curves and acid-base indicators
.
The acid indicator dissociation constant is represented as
In
K
and is
given by the following expression:
[ ]
In
In
In
+
3
HO
H
K
−
=
In aqueous solution the colour of an acid indicator is distinctly
different from that of its conjugate base.
Demonstrate the colour changes of the various indicators present in
Universal Indicator with an effervescent rainbow. Instructions are
available through the
RSC education resources.
Version 3.1 72
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Chemical equilibrium (continued)
The colour of the indicator is determined by the ratio of
[ ]
In In
H to
−
.
The theoretical point at which colour change occurs is when
In3
HO K
+
=
.
The colour change is assumed to be distinguishable when
[ ]
In InH and
−
differ by a factor of 10.
The pH range over which a colour change occurs can be estimated
by the expression:
In
pH p 1K= ±
Suitable indicators can be selected from pH data, including titration
curves.
Version 3.1 73
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Reaction feasibility
The standard enthalpy of formation,
f
H∆°
, is the enthalpy change
when one mole of a substance is formed from its elements in their
standard states.
The standard state of a substance is its most stable state at a
pressure of 1 atmosphere and at a specified temperature, usually
taken as 298 K.
The standard enthalpy of a reaction can be calculated from the
standard enthalpies of formation of the reactants and products:
()
ff
()products reactants
HH H∆°=Σ∆° −Σ∆°
RSC education resources, Advanced starters for ten: section 10,
offers editable lesson resources on thermodynamic definitions,
enthalpy, entropy and free energy.
The entropy (S) of a system is a measure of the degree of disorder of
the system.
The greater the degree of disorder, the greater the entropy.
Solids have low disorder and gases have high disorder.
Entropy increases as temperature increases.
There is a rapid increase in entropy at the melting point of a
substance and an even more rapid and larger change in entropy at
the boiling point.
The second law of thermodynamics states that the total entropy of a
reaction system and its surroundings always increases for a
spontaneous process.
Khan Academy has a video that introduces entropy.
Chemguide has useful information relating to entropy:
♦ an introduction to entropy
♦ taking entropy changes further, which links to free energy
A fun flash animation of entropy using an Einstein quote
is available
from the University of Toronto.
An audio discussion of the second law of thermodynamics is
available for download from BBC Radio 4 — In Our Time
.
Version 3.1 74
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Reaction feasibility (continued)
Heat energy released by the reaction system into the surroundings
increases the entropy of the surroundings.
Heat energy absorbed by the reaction system from the surroundings
decreases the entropy of the surroundings.
The third law of thermodynamics states that the entropy of a perfect
crystal at 0 K is zero.
The standard entropy of a substance is the entropy value for the
substance in its standard state.
The change in standard entropy for a reaction system can be
calculated from the standard entropies of the reactants and products:
()SS S∆°=Σ° −Σ°(products) reactants
RSC education resources has details for the endothermic solid-solid
reaction between barium hydroxide and ammonium chloride.
Chemicool has ideas and examples of other spontaneous
endothermic reactions.
The change in free energy for a reaction is related to the enthalpy
and entropy changes:
G H TS∆ °=∆ °− ∆ °
If the change in free energy (
G∆°
) between reactants and products is
negative, a reaction may occur and the reaction is said to be feasible.
A feasible reaction is one that tends towards the products rather than
the reactants. This does not give any indication of the rate of the
reaction.
Education in Chemistry magazine provides an article outlining an
experiment involving exploding soap bubbles that can be used to link
between entropy changes and free energy by calculating the entropy
change for the reaction of methane and oxygen.
Chemguide provides an introduction to Gibbs free energy.
RSC education resources has two problem-solving activities:
♦ Thermodynamics
♦ What makes it go?
Version 3.1 75
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Reaction feasibility (continued)
The standard free energy change for a reaction can be calculated
from the standard free energies of formation of the reactants and
products using the relationship:
()
(products) reactantsGG G∆°=Σ∆° −Σ∆°
The feasibility of a chemical reaction under standard conditions can
be predicted from the calculated value of the change in standard free
energy (
G∆°
).
The temperatures at which a reaction may be feasible can be
estimated by considering the range of values of
T
for which
0.G∆ °<
Under non-standard conditions any reaction is feasible if
G∆
is
negative.
At equilibrium,
0G∆=
.
A reversible reaction will proceed spontaneously until the composition
is reached where
0G∆=
.
Carry out an experiment to verify a thermodynamic prediction using,
for example, NaHCO
3
(s).
(c) Kinetics
The rate of a chemical reaction normally depends on the
concentrations of the reactants.
RSC education resources, Advanced starters for ten: section 1, offers
editable lesson resources on kinetics.
Version 3.1 76
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Kinetics (continued)
Orders of reaction are used to relate the rate of a reaction to the
reacting species.
If changing the concentration of a reactant
A
has no effect on the
rate of the reaction, then the reaction is zero order with respect to
A
.
If doubling the concentration of a reactant
A
doubles the rate of the
reaction, then the reaction is first order with respect to
A
. The rate
can be expressed as:
[ ]
rate kA=
where
k
is the rate constant and
[ ]
A
is the
concentration of reactant
A
in mol l
-1
Chemguide has information on orders of reaction and rate equations
and order of reaction and organic mechanisms.
A video tutorial is also available on Khan Academy.
If doubling the concentration of a reactant
A
increases the rate of the
reaction fourfold, then the reaction is second order with respect to
A
.
The rate can be expressed as:
[
]
2
rate kA=
The order of a reaction with respect to any one reactant is the power
to which the concentration of that reactant is raised in the rate
equation.
The overall order of a reaction is the sum of the powers to which the
concentrations of the reactants are raised in the rate equation.
Version 3.1 77
Physical chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Kinetics (continued)
The order of a reaction can only be determined from experimental
data.
The rate equation and the rate constant, including units, can be
determined from initial rate data for a series of reactions in which the
initial concentrations of reactants are varied. These can be zero, first,
second or third order.
Reactions usually occur by a series of steps called a reaction
mechanism.
The rate of reaction is dependent on the slowest step, which is called
the ‘rate determining step’.
Experimentally determined rate equations can be used to determine
possible reaction mechanisms.
A number of instructions for practical activities are available:
♦ RSC education resources problem-based practical activities
provides problem-solving activities and experimental details for
the propanone and iodine reaction
♦ SSERC describes an experiment to determine the rate constant
and order of reaction using bleach and blue food dye
, and also
provides an opportunity to introduce the practical technique of
colorimetry
♦ the University of Strathclyde has details of an experiment,
determination of the rate of a reaction that includes detailed
kinetics information, as well as the experimental procedure
Version 3.1 78
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Molecular orbitals
VSEPR cannot explain the bonding in all compounds. Molecular
orbital theory can provide an explanation for more complex
molecules.
There are websites available with information and animations
showing sigma bonds, pi bonds and hybridisation.
Molecular orbitals form when atomic orbitals combine. The number of
molecular orbitals formed is equal to the number of atomic orbitals
that combine. The combination of two atomic orbitals results in the
formation of a bonding molecular orbital and an antibonding orbital.
The bonding molecular orbital encompasses both nuclei. The
attraction of the positively charged nuclei and the negatively charged
electrons in the bonding molecular orbital is the basis of bonding
between atoms. Each molecular orbital can hold a maximum of two
electrons.
In a non-polar covalent bond, the bonding molecular orbital is
symmetrical about the midpoint between two atoms. Polar covalent
bonds result from bonding molecular orbitals that are asymmetric
about the midpoint between two atoms. The atom with the greater
value for electronegativity has the greater share of the bonding
electrons. Ionic compounds are an extreme case of asymmetry, with
the bonding molecular orbitals being almost entirely located around
just one atom, resulting in the formation of ions.
Molecular orbitals that form by end-on overlap of atomic orbitals
along the axis of the covalent bond are called sigma (
σ
) molecular
orbitals or sigma bonds.
RSC education resources has a series of vignettes covering
molecular orbitals, hybridisation, aromaticity and conjugation.
Version 3.1 79
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Molecular orbitals (continued)
Molecular orbitals that form by side-on overlap of parallel atomic
orbitals that lie perpendicular to the axis of the covalent bond are
called pi (
π
) molecular orbitals or pi bonds.
The electronic configuration of an isolated carbon atom cannot
explain the number of bonds formed by carbon atoms in molecules.
The bonding and shape of molecules of carbon can be explained by
hybridisation.
Hybridisation is the process of mixing atomic orbitals within an atom
to generate a set of new atomic orbitals called hybrid orbitals. These
hybrid orbitals are degenerate.
In alkanes, the 2s orbital and the three 2p orbitals of carbon hybridise
to form four degenerate sp
3
hybrid orbitals. These adopt a tetrahedral
arrangement. The sp
3
hybrid orbitals overlap end-on with other
atomic orbitals to form
σ
bonds.
The bonding in alkenes can be described in terms of sp2
hybridisation. The 2s orbital and two of the 2p orbitals hybridise to
form three degenerate sp2 hybrid orbitals. These adopt a trigonal
planar arrangement. The hybrid sp2 orbitals overlap end-on to form
σ
bonds. The remaining 2p orbital on each carbon atom of the
double bond is unhybridised and lies perpendicular to the axis of the
σ
bond. The unhybridised p orbitals overlap side-on to form
π
bonds.
ChemTube3D, available through RSC education resources, contains
interactive 3D models for some important organic molecules including
methane, ethane, ethyne and benzene. The model view can be
altered to show the hybrid orbitals.
Version 3.1 80
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Molecular orbitals (continued)
The bonding in benzene and other aromatic systems can be
described in terms of sp
2
hybridisation. The six carbon atoms in
benzene are arranged in a cyclic structure with
σ
bonds between the
carbon atoms. The unhybridised p orbitals on each carbon atom
overlap side-on to form a
π
molecular system, perpendicular to the
plane of the
σ
bonds. This
π
molecular system extends across all
six carbon atoms. The electrons in this system are delocalised.
The bonding in alkynes can be described in terms of sp hybridisation.
The 2s orbital and one 2p orbital of carbon hybridise to form two
degenerate hybrid orbitals. These adopt a linear arrangement. The
hybrid sp orbitals overlap end-on to form
σ
bonds. The remaining
two 2p orbitals on each carbon atom lie perpendicular to each other
and to the axis of the
σ
bond. The unhybridised p orbitals overlap
side-on to form two
π
bonds.
Molecular orbital theory can be used to explain why organic
molecules are colourless or coloured. Electrons fill bonding molecular
orbitals, leaving higher energy antibonding orbitals unfilled. The
highest bonding molecular orbital containing electrons is called the
highest occupied molecular orbital (HOMO). The lowest antibonding
molecular orbital is called the lowest unoccupied molecular orbital
(LUMO).
Absorption of electromagnetic energy can cause electrons to be
promoted from HOMO to LUMO.
Most organic molecules appear colourless because the energy
difference between HOMO and LUMO is relatively large. This results
in absorption of light from the ultraviolet region of the spectrum.
Khan Academy has a series of videos covering molecular orbitals,
HOMO and LUMO, and UV/Vis spectroscopy in organic molecules as
well as explaining the link between conjugation and colour in organic
molecules. These videos compare the absorptions of molecules with
different degrees of conjugated systems.
ChemTube3D, available through RSC education resources, has
many useful resources including a graphic showing the difference in
energy between HOMO and LUMO in linear polyenes as well as
3D
models showing the conjugation in a number of dyes.
A PowerPoint presentation introducing molecules and colour is a
resource produced by Education Scotland, and available on the
Science NQ GLOW portal.
Version 3.1 81
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Molecular orbitals (continued)
Some organic molecules contain chromophores. A chromophore is a
group of atoms within a molecule that is responsible for absorption of
light in the visible region of the spectrum. Light can be absorbed
when electrons in a chromophore are promoted from the HOMO to
the LUMO.
Chemistry in your cupboard is a resource available on RSC
education resources, and links the action of the stain remover Vanish
to the structure of coloured organic molecules, providing a real-life
example of the importance of understanding the chemistry of colour.
Chromophores exist in molecules containing a conjugated system —
a system of adjacent unhybridised p orbitals that overlap side-on to
form a molecular orbital across a number of carbon atoms. Electrons
within this conjugated system are delocalised. Molecules with
alternating single and double bonds, and aromatic molecules have
conjugated systems.
The more atoms in the conjugated system the smaller the energy gap
between HOMO and LUMO. A lower frequency of light (longer
wavelength, lower energy) is absorbed by the compound. When the
wavelength of light absorbed is in the visible region, the compound
will exhibit the complementary colour.
Colourful Chemistry infographics from RSC education resources has
a visually stimulating and informative infographic about colours of
organic molecules including the colours of autumn leaves, poinsettia
plants and glow sticks.
The Science of Sunscreen infographic from RSC
education
resources has information about organic molecules with conjugated
systems that are used to absorb UV light.
Compounds highlighting the effect of increasing the length of the
conjugated system on the colour can be compared. For example,
vitamin A (yellow) can be compared with β-carotene (orange).
Ninhydrin reacts with amino acids and forms a highly conjugated
product that absorbs light in the visible region, and an intense purple
colour (λ
max
750 nm) is observed. This is used in the detection of
amino acids both as a locating agent in chromatography and in
detecting latent fingerprints in crime scenes.
Molecule of the Month:
April 2018 outlines the steps involved in formation of one of these
conjugated products.
Version 3.1 82
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Molecular orbitals (continued)
Explain the colours observed for the indicator phenolphthalein by
looking at the degree of conjugation in the molecule in both acid and
in base conditions. Structures of the two forms of phenolphthalein
can be seen on the
Elmhurst College website.
An experiment to synthesise phenolphthalein and its derivatives
is
available through the University of Strathclyde website.
Prepare a variety of dyes and examine their structures to identify the
chromophore. RSC education resources has instructions for the
microscale synthesis of an azo dye and the
microscale synthesis of
indigo-dye. The University of Strathclyde has instructions for the
synthesis of methyl orange.
A number of online resources allow complementary colours to be
explained by demonstrating colour mixing. One example is hosted by
Stanford University
and has sliders to change the colour of light being
transmitted or absorbed.
Candidates can use simple spectroscopes made from DVDs or using
a smart phone — available from SSERC —to view light transmitted or
reflected by coloured compounds. SSERC has
instructions for
making spectroscopes.
Version 3.1 83
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis
When an organic reaction takes place, bonds in the reactant
molecules are broken and bonds in the product molecules are made.
The process of bond breaking is known as bond fission.
There are two types of bond fission, homolytic and heterolytic.
Homolytic fission:
♦ results in the formation of two neutral radicals
♦ occurs when each atom retains one electron from the
σ
covalent
bond and the bond breaks evenly
♦ normally occurs when non-polar covalent bonds are broken
Reactions involving homolytic fission tend to result in the formation of
very complex mixtures of products, making them unsuitable for
organic synthesis.
Heterolytic fission:
♦ results in the formation of two oppositely charged ions
♦ occurs when one atom retains both electrons from the
σ
covalent bond and the bond breaks unevenly
♦ normally occurs when polar covalent bonds are broken
Reactions involving heterolytic fission tend to result in far fewer
products than reactions involving homolytic fission, and so are better
suited for organic synthesis.
The University of Bath offers a PowerPoint presentation
that covers
homolytic and heterolytic fission, along with curly arrow notation, and
definitions of nucleophiles and electrophiles.
Version 3.1 84
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
The movement of electrons during bond fission and bond making can
be represented using curly arrow notation where:
♦ a single-headed arrow indicates the movement of a single
electron
♦ a double-headed arrow indicates the movement of an electron
pair
♦ the tail of the arrow shows the source of the electron(s)
♦ the head of the arrow indicates the destination of the electron(s)
♦ two single-headed arrows starting at the middle of a covalent
bond indicate homolytic bond fission is occurring
♦ a double-headed arrow starting at the middle of a covalent bond
indicates heterolytic bond fission is occurring
♦ an arrow drawn with the head pointing to the space between two
atoms indicates that a covalent bond will be formed between
those two atoms
Chemguide has information on use of curly arrows.
The University of Edinburgh has a curly arrow resource
that provides
activities that test understanding of curly arrows in a number of
different reaction types.
RSC Mechanism Inspector has information and interactive activities
relating to single and double-headed
curly arrows, electrophiles and
nucleophiles.
Education in Chemistry magazine provides an article, ‘
End curly
arrow anxiety’, which has a downloadable exercise to practice curly
arrow mechanisms.
RSC education resources has a set of activity sheets
to aid
understanding of curly arrows and reaction mechanisms.
The University of Southampton has created a set of exam-style self-
assessment questions covering various aspects of
organic reactions
and mechanisms.
In reactions involving heterolytic bond fission, attacking groups are
classified as nucleophiles or electrophiles.
RSC Mechanism Inspector has information and interactive activities
relating to electrophiles and nucleophiles.
RSC education resources has a series of vignettes
covering
mechanism theory including electrophiles, nucleophiles and curly
arrow notation.
Version 3.1 85
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Nucleophiles are:
♦ negatively charged ions or neutral molecules that are electron
rich, such as
Cl , Br , OH , CN
−− − −
,
32
NH and H O
♦ attracted towards atoms bearing a partial
()
δ
+
or full positive
charge
♦ capable of donating an electron pair to form a new covalent bond
Electrophiles are:
♦ positively charged ions or neutral molecules that are electron
deficient, such as
H
+
,
2
NO
+
3
and SO
♦ attracted towards atoms bearing a partial
()
δ
−
or full negative
charge
♦ capable of accepting an electron pair to form a new covalent bond
RSC education resources, Starters for ten: chapters 1-11, section 5
‘Organic Chemistry’, offers a selection of easily editable short quizzes
and activities covering curly arrows and electrophiles and
nucleophiles.
The following reaction types can be identified from a chemical
equation:
♦ substitution
♦ addition
♦ elimination
♦ condensation
♦ hydrolysis
♦ oxidation
♦ reduction
♦ neutralisation
National 5 and Higher courses cover some of the reaction types.
Candidates should revise these in the context of this course.
Organic Chemistry
infographics from RSC education resources has a
visually stimulating and informative infographic relating different
reaction types in organic chemistry.
It is important that you give candidates many varied, real-life contexts
for these reactions. Similarities between the different reaction types
should be reinforced and opportunities given to make connections
between reaction types and to develop skills that enable synthetic
routes to be devised for given products.
Version 3.1 86
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Synthetic routes can be devised, with no more than three steps, from
a given reactant to a final product.
The possible reactions of a particular molecule can be deduced by
looking at the structural formula.
Which Compound? Which Route?
is available on RSC education
resources, and has an activity to plan a synthetic route for a drug.
RSC education resources has details on Synthesis Explorer
that
allow synthetic routes to be devised, and shows details of reagents
involved. A help sheet for this is
available.
The structure of any molecule can be drawn as a full, shortened or
skeletal structural formula.
In a skeletal structural formula, neither the carbon atoms, nor any
hydrogens attached to the carbon atoms, are shown. The presence
of a carbon atom is implied by a ‘kink’ in the carbon backbone, and at
the end of a line.
Given a full or shortened structural formula for a compound, the
skeletal structural formula can be drawn.
Given a skeletal structural formula for a compound, the full or
shortened structural formula can be drawn.
Molecular formulae can be written from a full, shortened or skeletal
structural formula.
Chemguide provides information about skeletal formula.
Organic chemistry infographics
from RSC education resources has a
visually stimulating and informative infographic showing the different
types of organic formulae.
Molecular drawing packages such as ChemSketch
(a free
downloadable application) can be set to display structures in skeletal
representation if required. Wireframe, stick, ball and stick, and space-
filling representations should all be familiar. Molecules can be rotated
around the x, y and z axes to align any chosen bond horizontally or
vertically; to align any three atoms in a given plane; to zoom in and
out; and to switch on and off atom labels. Molecules sketched in 2D
mode can be converted into 3D representations in ChemSketch.
Candidates can create and manipulate 3D representations of
relatively small molecules (fewer than 10 carbon atoms) containing
common functional groups using Molymods or other molecular model
kits.
Version 3.1 87
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
ChemSpider has a searchable library of molecules that can be
viewed as skeletal formula or as 3D molecules.
Straight and branched chain alkanes; alkenes; alcohols; carboxylic
acids; aldehydes and ketones; haloalkanes; and ethers can be
systematically named, indicating the position of the functional group
where appropriate, from structural formulae containing no more than
eight carbon atoms in their longest chain. Straight chain esters can
be systematically named from the names of their parent alcohol and
carboxylic acid or their structural formula.
Molecular formulae can be written and structural formulae drawn from
systematic names of straight and branched chain alkanes; alkenes;
alcohols; carboxylic acids; aldehydes and ketones; haloalkanes; and
ethers containing no more than eight carbon atoms in their longest
chain. Molecular formulae can be written and structural formulae
drawn for esters from the systematic name or the structural formulae
of their parent alcohol and carboxylic acid.
The RSC education resource, Gridlocks, has some activities that may
help to revise naming rules from previous courses.
Chemguide has useful information that explains the
naming of all
types of organic compound.
Orgchem101, produced by the University of Ottawa, has an
interactive organic nomenclature quiz
.
Haloalkanes (alkyl halides) are substituted alkanes in which one or
more of the hydrogen atoms is replaced with a halogen atom.
A podcast on the environmental significance and the chemistry of
haloalkanes is available on RSC education resources.
RSC education resources details a Nuffield Foundation experiment to
synthesise bromoethane from ethanol in a test tube.
Version 3.1 88
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Monohaloalkanes:
♦ contain only one halogen atom
♦ can be classified as primary, secondary or tertiary according to
the number of alkyl groups attached to the carbon atom
containing the halogen atom
♦ take part in elimination reactions to form alkenes using a strong
base, such as potassium or sodium hydroxide in ethanol
RSC education resources, Starters for ten: chapters 1-11, section 5
‘Organic Chemistry’, offers a selection of easily editable short quizzes
and activities relating to haloalkanes.
Chemguide has a description of the different kinds of haloalkanes
as
well as elimination and nucleophilic reactions.
♦ take part in nucleophilic substitution reactions with:
— aqueous alkalis to form alcohols
— alcoholic alkoxides to form ethers
— ethanolic cyanide to form nitriles (chain length increased by
one carbon atom) that can be hydrolysed to carboxylic acids
The University of York has instructions for the reaction involving a
haloalkane and water.
A monohaloalkane can take part in nucleophilic substitution reactions
by one of two different mechanisms.
Education in Chemistry magazine provides an article outlining an
investigation into the mechanism of the
hydrolysis of 2-bromo-2-methylpropane.
S
N
1 is a nucleophilic substitution reaction with one species in the rate
determining step and occurs in a minimum of two steps via a trigonal
planar carbocation intermediate.
RSC Mechanism Inspector has information and interactive activities
about nucleophilic substitution reactions.
Version 3.1 89
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
S
N
2 is a nucleophilic substitution reaction with two species in the rate
determining step and occurs in a single step via a single five-centred,
trigonal bipyramidal transition state.
The reaction mechanisms for S
N
1 and S
N
2 reactions can be
represented using curly arrows. Steric hindrance and the inductive
stabilisation of the carbocation intermediate can be used to explain
which mechanism will be preferred for a given haloalkane.
The University of Edinburgh has a curly arrow resource that provides
activities that test understanding of the mechanism of nucleophilic
substitution reactions.
The University of Oxford has an interactive quiz to test knowledge of
nucleophilic substitution reactions
and curly arrow mechanisms.
ChemTube3D, available through RSC education resources, has
simple
animated mechanisms and 3D models showing nucleophilic
substitution reactions as well as showing more complex examples.
Khan Academy has videos showing the mechanism of both S
N
1 and
S
N
2 reactions.
RSC education resources, Starters for ten: chapters 1-11, section 5
‘Organic Chemistry’, offers a selection of easily editable short quizzes
and activities relating to substitution reactions and elimination
reactions of haloalkanes.
Version 3.1 90
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Alcohols are substituted alkanes in which one or more of the
hydrogen atoms is replaced with a hydroxyl functional group, –OH
group.
RSC education resources, Starters for ten: chapters 1-11, section 5
‘Organic Chemistry’, offers a selection of easily editable short quizzes
and activities testing knowledge of alcohols and their reactions.
Alcohols can be prepared from:
♦ haloalkanes by substitution
♦ alkenes by acid-catalysed hydration (addition)
♦ aldehydes and ketones by reduction using a reducing agent such
as lithium aluminium hydride
Reactions of alcohols include:
♦ dehydration to form alkenes using aluminium oxide, concentrated
sulfuric acid or concentrated phosphoric acid
♦ oxidation of primary alcohols to form aldehydes and then
carboxylic acids and secondary alcohols to form ketones, using
acidified permanganate, acidified dichromate or hot copper(II)
oxide
♦ formation of alcoholic alkoxides by reaction with some reactive
metals such as potassium or sodium, which can then be reacted
with monohaloalkanes to form ethers
♦ formation of esters by reaction with carboxylic acids using
concentrated sulfuric acid or concentrated phosphoric acid as a
catalyst
RSC education resources provides a range of activities on reactions
of alcohols:
♦ dehydration of ethanol to ethene
using porcelain chips as a
catalyst
♦ preparation of cyclohexene from cyclohexanol (Skills – Block 1,
page 39) with purification by distillation and solvent extraction and
testing the product for unsaturation
♦ oxidation of ethanol – the alcohol is oxidised to ethanal and, with
further oxidation, to ethanoic acid
♦ the ‘breathalyser’ reaction is a quick demonstration of the reaction
used in early forms of breathalysers
♦ a microscale oxidation of alcohols allows the difference in the
oxidation reactions of primary, secondary and tertiary alcohols to
be observed by the addition of acidified dichromate(VI)
♦ properties of alcohols details the reaction of sodium with ethanol
♦ Alcohols (16-19) is a game and resource based on naming,
classifying and identifying the products of oxidation
♦ making esters from alcohols and acids
♦ microscale synthesis of ethyl benzoate
♦ Chemguide, which has an explanation of the properties of
alcohols in relation to hydrogen bonding
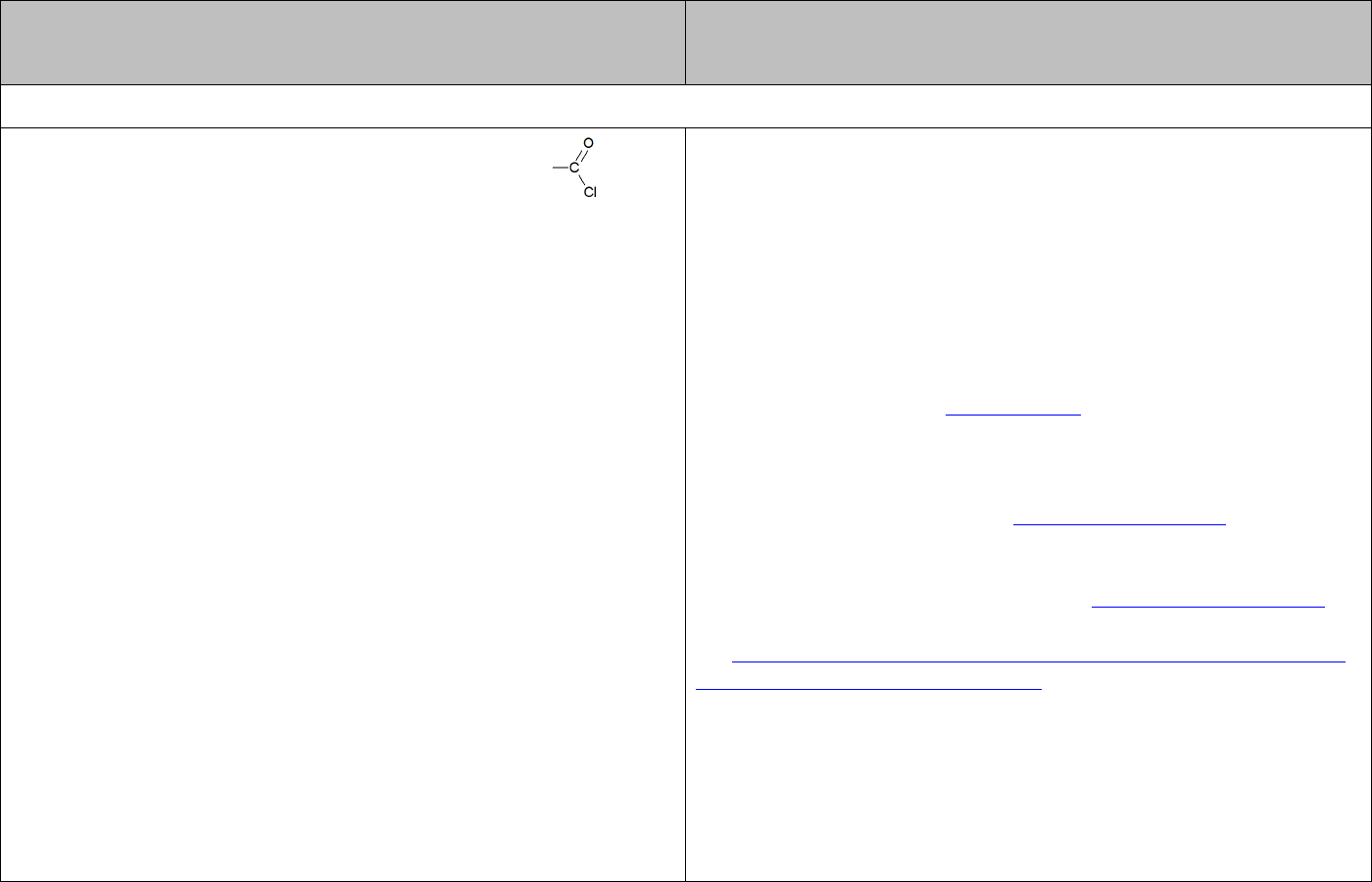
Version 3.1 91
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
♦ formation of esters by reaction with acid chlorides ( ) —
this gives a faster reaction than reaction with carboxylic acids,
and no catalyst is needed
Hydroxyl groups make alcohols polar, which gives rise to hydrogen
bonding. Hydrogen bonding can be used to explain the properties of
alcohols including boiling points, melting points, viscosity and
solubility or miscibility in water.
Ethers can be regarded as substituted alkanes in which a hydrogen
atom is replaced with an alkoxy functional group, –OR, and have the
general structure R' – O – R'', where R' and R'' are alkyl groups.
Ethers are named as substituted alkanes. The alkoxy group is named
by adding the ending ‘oxy’ to the alkyl substituent, and this prefixes
the name of the longest carbon chain.
Ethers can be prepared in a nucleophilic substitution reaction by
reacting a monohaloalkane with an alkoxide.
Due to the lack of hydrogen bonding between ether molecules, they
have lower boiling points than the corresponding isomeric alcohols.
Methoxymethane and methoxyethane are soluble in water. Larger
ethers are insoluble in water due to their increased molecular size.
Ethers are commonly used as solvents since they are relatively inert
chemically and will dissolve many organic compounds.
SSERC has details of the Ether Runway demonstration, which
provides an interesting introduction to ethers, illustrating their
flammability.
ChemistryWorld magazine has a podcast and transcript
providing
some of the history of diethyl ether.
Khan Academy has a video that explains IUPAC naming of ethers
as
well as describing some of their properties. Another video describes
the
Williamson ether synthesis of an alcohol and alkyl halide using a
strong base such as sodium hydride.
Version 3.1 92
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Alkenes can be prepared by:
♦ dehydration of alcohols using aluminium oxide, concentrated
sulfuric acid or concentrated phosphoric acid
♦ base-induced elimination of hydrogen halides from
monohaloalkanes
Chemistry Practical Guide — Support Materials (2012),produced by
Education Scotland and available through SSERC, has details of the
preparation of cyclohexene from cyclohexane using concentrated
phosphoric acid and provides an opportunity to introduce the practical
techniques or distillation and solvent extraction (only one extraction is
carried out in this procedure).
Alkenes take part in electrophilic addition reactions with:
♦ hydrogen to form alkanes in the presence of a catalyst
♦ halogens to form dihaloalkanes
♦ hydrogen halides to form monohaloalkanes
♦ water using an acid catalyst to form alcohols
RSC education resources has a number of experiments and activities
relating to alkenes including:
♦ dehydration of ethanol to ethene
using porcelain chips as a
catalyst
♦ preparation of cyclohexene from cyclohexanol with purification by
distillation and solvent extraction
♦ Starters for ten: chapters 1-11, section 5 ‘Organic Chemistry’
offers a selection of easily editable short quizzes and activities
testing knowledge of electrophilic addition reactions of alkenes,
including reaction mechanisms
RSC Mechanism Inspector has information and interactive activities
about electrophilic addition reactions.
Version 3.1 93
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Markovnikov’s rule states that when a hydrogen halide or water is
added to an unsymmetrical alkene, the hydrogen atom becomes
attached to the carbon with the most hydrogen atoms attached to it
already. Markovnikov’s rule can be used to predict major and minor
products formed during the reaction of a hydrogen halide or water
with alkenes.
The reaction mechanisms for the addition of a hydrogen halide and
the acid-catalysed addition of water can be represented using curly
arrows and showing the intermediate carbocation. The inductive
stabilisation of intermediate carbocations formed during these
reactions can be used to explain the products formed.
The reaction mechanism for the addition of a halogen can be
represented using curly arrows and showing the cyclic ion
intermediate.
Carboxylic acids can be prepared by:
♦ oxidising primary alcohols using acidified permanganate, acidified
dichromate and hot copper(II) oxide
♦ oxidising aldehydes using acidified permanganate, acidified
dichromate, Fehling’s solution and Tollens’ reagent
♦ hydrolysing nitriles, esters or amides
Khan Academy has a video that explains Markovnikov’s rule, using
curly arrows and inductive stabilisation of carbocation intermediates.
Chemguide has mechanisms for electrophilic addition reactions
of
alkenes with hydrogen halides and halogens.
The Khan Academy video hydration of an alkene
shows the
mechanism for the reaction of an alkene with water and a sulphuric
acid catalyst.
The University of Edinburgh has a curly arrow resource
that provides
activities that test understanding of the mechanism of electrophilic
addition reactions of alkenes.
Version 3.1 94
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Reactions of carboxylic acids include:
♦ formation of salts by reactions with metals or bases
♦ condensation reactions with alcohols to form esters in the presence
of concentrated sulfuric or concentrated phosphoric acid
♦ reaction with amines to form alkylammonium salts that form
amides when heated
♦ reduction with lithium aluminium hydride to form primary alcohols
RSC education resources provides a range of resources relating to
carboxylic acids:
♦ Advanced starters for ten: section 4, ‘Carbonyl Chemistry’
has
easily editable short quizzes on reactions of carboxylic acids
♦ oxidation of ethanol experiment
♦ salicylic acid infographic provides information relating to the wide-
ranging use of this carboxylic acid
♦ reactions of ethanoic acid compares the pH of ethanoic acid and
hydrochloric acid as well as their reactions with magnesium and
sodium carbonate
♦ microscale preparation of ethyl benzoate
♦ microscale synthesis of aspirin
♦ synthesis of aspirin, which also provides an opportunity to
introduce important practical techniques including reflux,
recrystallisation, melting point analysis, thin layer chromatography
and % yield calculations. A similar procedure can also be found in
Chemistry Practical Guide — Support Materials (2012), produced
by Education Scotland and available through SSERC
♦ The First Year Undergraduate Chemistry Laboratory Course
Manual 2011-2012 provides experimental details that include use
of important practical techniques (including reflux, vacuum
filtration, recrystallisation, melting point analysis and % yield
calculations) for preparation of the ester propyl ethanoate as well
as the preparation of benzoic acid from methyl benzoate
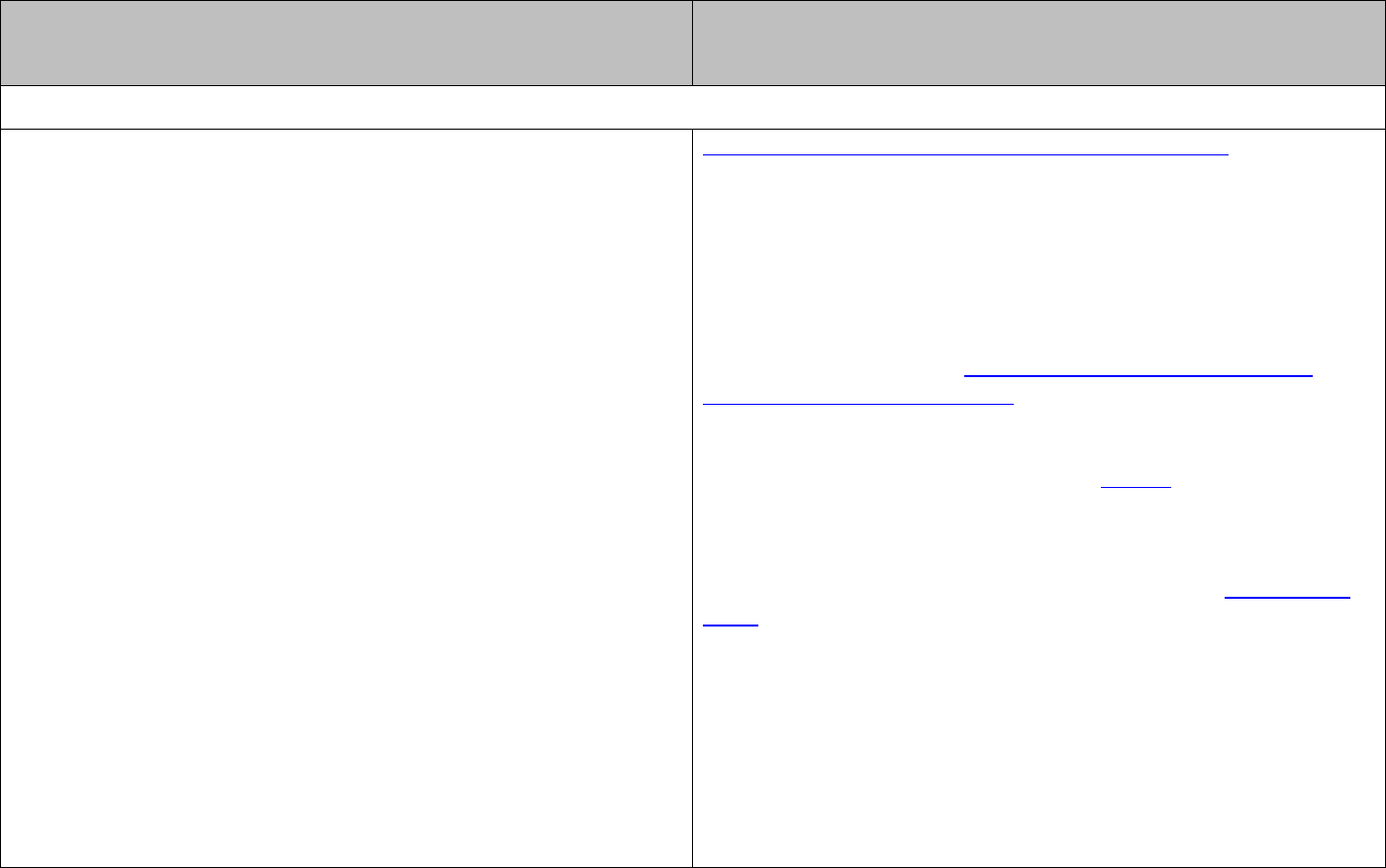
Version 3.1 95
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Chemistry Practical Guide — Support Materials (2012), produced by
Education Scotland and available through SSERC, has details of an
experiment to hydrolyse ethyl benzoate to prepare benzoic acid, and
provides an opportunity to introduce important practical techniques
including reflux, recrystallisation, melting point analysis, thin layer
chromatography and % yield calculations. This guide also contains
experimental details to prepare ethyl ethanoate.
Amines are organic derivatives of ammonia in which one or more
hydrogen atoms of ammonia has been replaced by an alkyl group.
Amines can be classified as primary, secondary or tertiary according to
the number of alkyl groups attached to the nitrogen atom.
Amines react with acids to form salts.
Primary and secondary amines, but not tertiary amines, display
hydrogen bonding. As a result, primary and secondary amines have
higher boiling points than isomeric tertiary amines.
Primary, secondary and tertiary amine molecules can hydrogen-bond
with water molecules, thus explaining the appreciable solubility of the
shorter chain length amines in water.
Amines like ammonia are weak bases and dissociate to a slight extent
in aqueous solution. The nitrogen atom has a lone pair of electrons
which can accept a proton from water, producing hydroxide ions.
RSC education resources, Advanced starters for ten: section 6,
‘Compounds with amine groups’, has easily editable short quizzes on
reactions and properties of amines.
Chemguide has useful information about amines
including
descriptions of the different classifications and their properties and
reactions.
The University of Purdue has some information about
amine-based
drugs and discusses the solubility of some of the available forms.
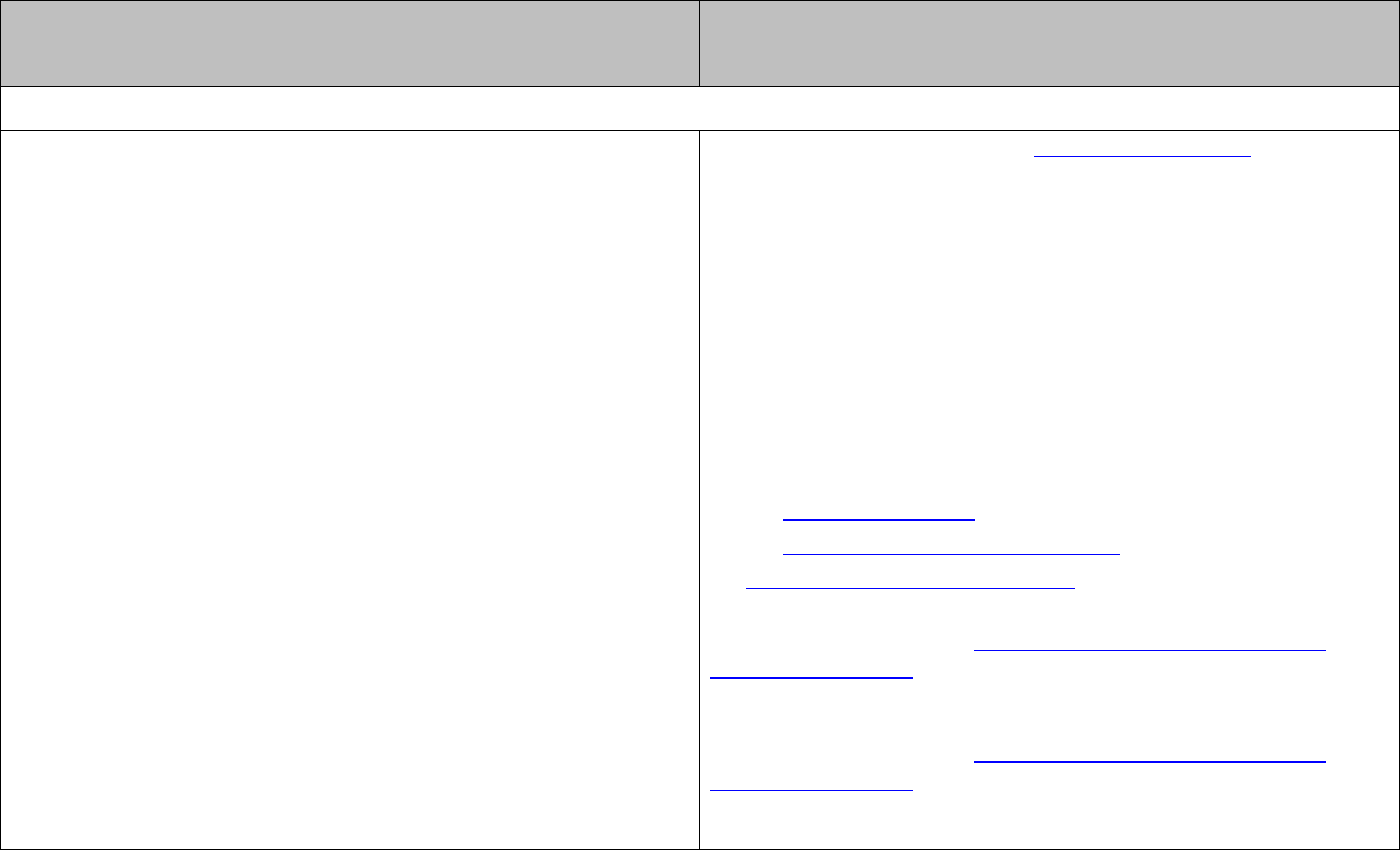
Version 3.1 96
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
Benzene (C
6
H
6
) is the simplest member of the class of aromatic
hydrocarbons.
The benzene ring has a distinctive structural formula. The stability of
the benzene ring is due to the delocalisation of electrons in the
conjugated system. The presence of delocalised electrons explains
why the benzene ring does not take part in addition reactions.
Bonding in benzene can be described in terms of sp
2
hybridisation,
sigma and pi bonds, and electron delocalisation.
A benzene ring in which one hydrogen atom has been substituted by
another group is known as the phenyl group. The phenyl group has the
formula –C
6
H
5
.
Benzene rings can take part in electrophilic substitution reactions.
Reactions at benzene rings include:
♦ halogenation by reaction of a halogen using aluminium chloride or
iron(III) chloride for chlorination and aluminium bromide or iron(III)
bromide for bromination
♦ alkylation by reaction of a haloalkane using aluminium chloride
♦ nitration using concentrated sulfuric acid and concentrated nitric
acid
♦ sulfonation using concentrated sulfuric acid
ChemistryWorld magazine has a podcast and transcript providing
some of the history of benzene.
Many everyday consumer products have very distinctive smells as a
result of the presence of key aromatic compounds. Create a display
of household products containing these aromatic compounds to
capture interest. Examples could include well known antiseptics and
disinfectants containing tricholorophenol or
4-chloro-3,5-dimethylphenol, or permanent markers containing xylene
or toluene.
ChemGuide provides a good explanation about:
♦ the bonding in benzene
♦ the modern representation of benzene
♦ electrophilic substitution reactions
RSC education resources,
Advanced starters for ten: section 5,
‘Aromatic Chemistry’, has easily editable short quizzes and activities
on reactions and properties of aromatic compounds.
RSC education resources,
Advanced starters for ten: section 5,
‘Aromatic Chemistry’, has easily editable short quizzes and activities
on reactions and properties of aromatic compounds.
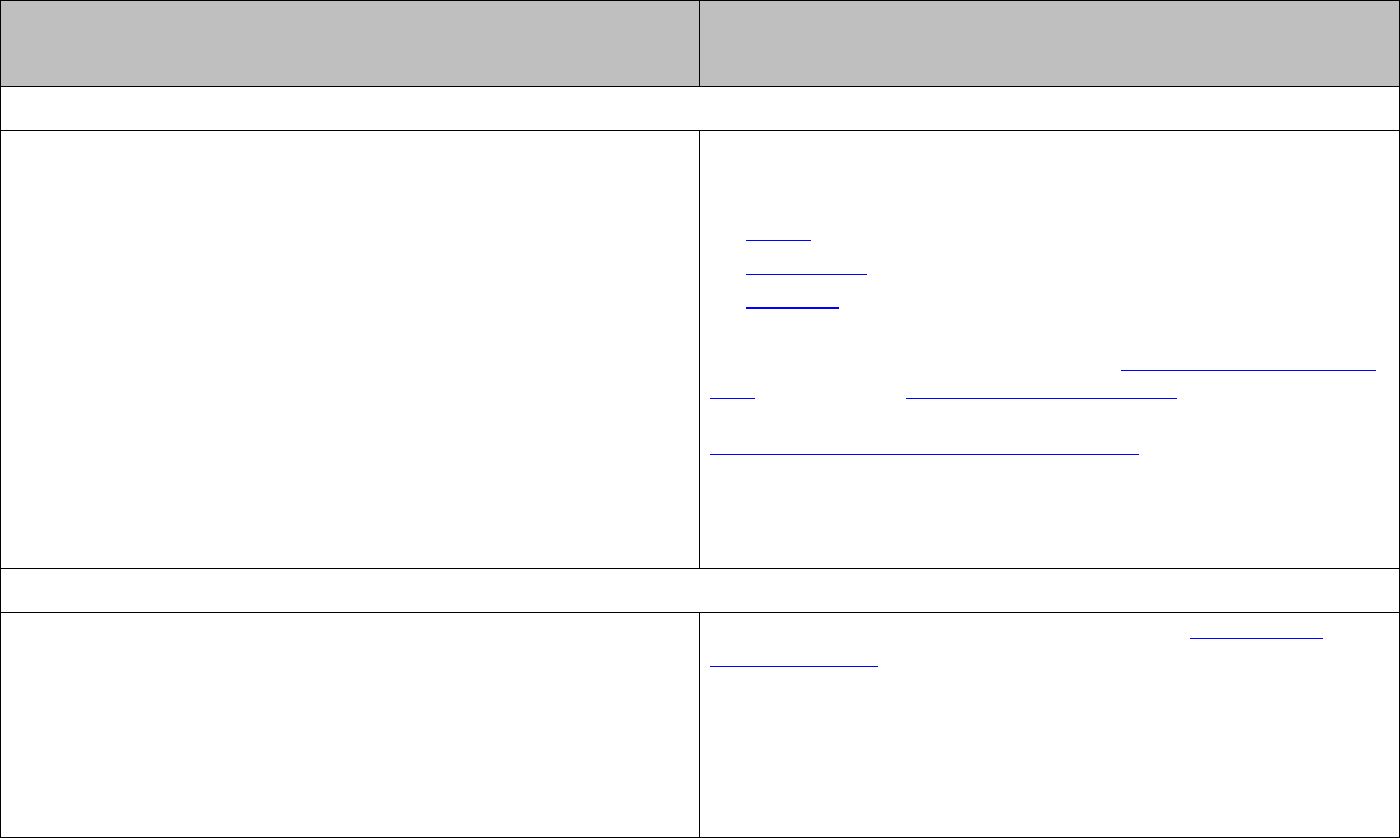
Version 3.1 97
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Synthesis (continued)
There are many important compounds that contain benzene rings.
The RSC education resources provides details about:
♦ aspirin
♦ paracetamol
♦ ibuprofen (Nurofen)
RSC education resources has details of a
micro-scale preparation of
TCP, as well as the nitration of methyl benzoate.
Electrophilic aromatic substitution reactions
, available through the
University of Strathclyde, provides details for carrying out some
electrophilic aromatic substitution reactions and also provides an
opportunity to introduce the practical techniques of recrystallisation,
melting point analysis and thin layer chromatography.
(c) Stereo chemistry
Molecules that have the same molecular formula but different
structural formulae are called isomers.
Khan Academy has a short video that introduces structural and
stereo isomerism.
Structural isomers occur when the atoms are bonded together in a
different order in each isomer.
Candidates can create and manipulate 3D representations of
relatively small molecules (fewer than 10 carbon atoms) using
Molymods or other molecular model kits, to show the difference in
structures of geometric and optical isomers.
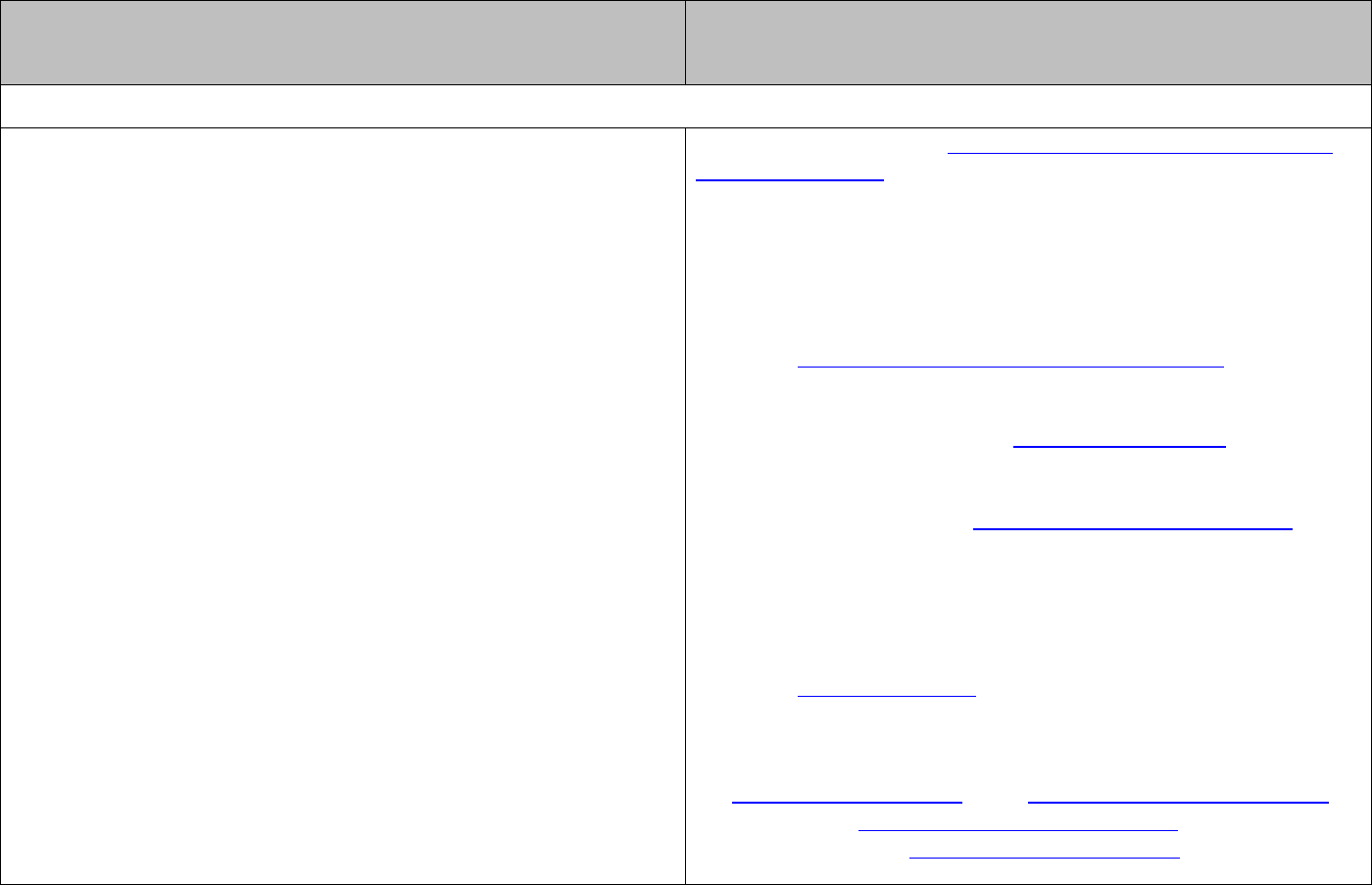
Version 3.1 98
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Stereo chemistry (continued)
Stereoisomers occur when the order of the bonding in the atoms is
the same but the spatial arrangement of the atoms is different in each
isomer. There are two types of stereoisomer, geometric and optical.
Geometric isomers:
♦ can occur when there is restricted rotation around a carbon-
carbon double bond or a carbon-carbon single bond in a cyclic
compound
♦ must have two different groups attached to each of the carbon
atoms that make up the bond with restricted rotation
♦ can be labelled cis or trans according to whether the substituent
groups are on the same side (cis) or on different sides (trans) of
the bond with restricted rotation
♦ have differences in physical properties, such as melting point and
boiling point
♦ can have differences in chemical properties
RSC education resources Starters for ten: chapters 1-11, section 5
‘Organic Chemistry’ offers a selection of easily editable short quizzes
and activities testing knowledge of structural and geometric isomers.
Videos explaining the difference in physical and chemical properties
of geometric isomers are available online.
A useful article produced by the American Chemical Society
discusses saturated, unsaturated and cis and trans fats
, and some of
the health concerns relating to trans fats.
ChemistryWorld magazine has a podcast and transcript
about the
anticancer drug cis-platin.
An experiment to synthesise cis and trans complexes of cobalt
is
available through the University of Strathclyde.
Optical isomers:
♦ occur in compounds in which four different groups are arranged
tetrahedrally around a central carbon atom (chiral carbon or chiral
centre)
♦ are asymmetric
♦ are non-superimposable mirror images of each other
♦ can be described as enantiomers
The Khan Academy video available through RSC education
resources introduces chirality and provides worked examples of
molecules with and without chiral carbon atoms.
RSC education resources has details of two short activities to look at
the differences in properties of the two optical isomers of limonene as
well as those for carvone (caraway and spearmint). There is also an
experiment to explore the optical rotation of sugars.
Version 3.1 99
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Stereo chemistry (continued)
♦ have identical physical properties, except for their effect on plane-
polarised light
♦ have identical chemical properties, except when in a chiral
environment such as that found in biological systems (only one
optical isomer is usually present)
♦ rotate plane-polarised light by the same amount but in opposite
directions and so are optically active
♦ when mixed in equal amounts are optically inactive because the
rotational effect of the plane-polarised light cancels out — this is
called a racemic mixture
Candidates can build their own polarimeters. The University of
Strathclyde has instructions for a cardboard box and a coffee cup
polarimeter, as well as a zero cost, technology enabled polarimeter
using a smart phone. Stem Learning has details of the RSC Classic
Chemistry Demonstrations No.13, page 26,
The Optical Activity of
Sucrose, which also has instructions for constructing a polarimeter.
ChemTube3D, available through RSC education resources, has a
tutorial that discusses the differences between
chiral and non-chiral
molecules, including an activity in which the molecules can be rotated
to show they are non-superimposable.
The University of Bristol’s, Molecule of the Month, July 2000
, features
thalidomide and discusses the uses of the drug, the enantiomeric
forms and the associated consequences of the use of thalidomide in
pregnant women. Limonene features as
Molecule of the Month:
March 2008.
RSC education resources features
Chemistry in your Cupboard:
Nurofen, which is normally sold as a mixture of two optical isomers,
one of which is an effective pain-killing drug and the other of which is
inactive.
One enantiomer of the drug naproxen is a pain reliever and the other
enantiomer is a liver toxin. Information about the structure and optical
activity of these enantiomers is available online.
Version 3.1 100
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(d) Experimental determination of structure
In organic chemistry a number of experimental techniques are carried
out to verify the chemical structure of a substance.
The RSC website SpectraSchool provides many resources relating to
spectroscopy techniques, with animations, videos and interactive
spectra.
Spectroscopy in a suitcase
is an RSC outreach activity giving
candidates the opportunity to learn about spectroscopy through
hands-on experience. As well as covering the principles of
spectroscopic techniques, the activities use real-life contexts to
demonstrate the applications of the techniques. There is also a
student resource that introduces spectroscopy, as well as providing
background information on mass spectrometry, infrared spectroscopy
and
1
H NMR spectroscopy.
RSC education resources,
Advanced starters for ten: section 8,
‘Structure Determination’, has easily editable short quizzes testing
knowledge of functional groups, mass spectrometry and NMR
spectroscopy. Problem-based practical activities —
Compound
confusion, problem 8 offers a problem-solving activity using various
spectroscopic and analytical techniques.
RSC education resources Following a synthetic route
exemplifies the
use of spectroscopy techniques to monitor reactions.
Elemental microanalysis is used to determine the masses of C, H, O,
S and N in a sample of an organic compound in order to determine its
empirical formula.
RSC education resources, Starters for ten: chapters 1-11, section 5
‘Organic Chemistry’, offers a selection of easily editable short quizzes
and activities providing the opportunity to practise empirical formula
calculations.
Version 3.1 101
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(d) Experimental determination of structure (continued)
An empirical formula shows the simplest ratio of the elements in a
molecule.
ChemCollective, produced by the National Science Foundations, has
a video tutorial: determining the empirical formula from an elemental
analysis that shows a worked example of an empirical formula
calculation from combustion product masses.
Elemental microanalysis can be determined from:
♦ combustion product masses
♦ percentage product by mass
Khan Academy has two videos, empirical formula from mass
composition and another mass composition problem, that show
worked examples of empirical formulae calculations from percentage
product by mass.
Mass spectrometry can be used to determine the accurate gram
formula mass (GFM) and structural features of an organic compound.
In mass spectrometry, a small sample of an organic compound is
bombarded by high-energy electrons. This removes electrons from
the organic molecule generating positively charged molecular ions
known as parent ions. These molecular ions then break into smaller
positively charged ion fragments. A mass spectrum is obtained
showing a plot of the relative abundance of the ions detected against
the mass-to-charge (m/z) ratio.
The mass-to-charge ratio of the parent ion can be used to determine
the GFM of the molecular ion, and so a molecular formula can be
determined using the empirical formula.
The fragmentation data can be interpreted to gain structural
information.
Chemguide has useful information about mass spectrometry
including an outline of the process of obtaining a mass spectrum of a
sample, fragmentation patterns and molecular ions.
A PowerPoint presentation introducing mass spectroscopy is a
resource produced by Education Scotland and is available on the
Science NQ GLOW portal.
Spectroscopy in a suitcase, available through the RSC education
resources website, has a Students mass spectrometry exercise
providing a forensic basis to analysing mass spectra.
Version 3.1 102
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(d) Experimental determination of structure (continued)
Infrared spectroscopy is used to identify certain functional groups in
an organic compound.
When infrared radiation is absorbed by organic compounds, bonds
within the molecule vibrate (stretch and bend). The wavelengths of
infrared radiation that are absorbed depend on the type of atoms that
make up the bond and the strength of the bond.
A PowerPoint presentation introducing Infrared Spectroscopy is a
resource produced by Education Scotland and available on the
Science NQ GLOW portal.
Chemguide has useful information about infrared spectroscopy
including an outline of the process of obtaining an infrared spectrum,
fingerprint regions and functional group analysis.
In infrared spectroscopy, infrared radiation is passed through a
sample of the organic compound and then into a detector that
measures the intensity of the transmitted radiation at different
wavelengths. The absorbance of infrared radiation is measured in
wavenumbers, the reciprocal of wavelength, in units of cm
-1
.
Characteristic absorptions by particular vibrations are given in the
data booklet.
RSC education resources has a handout, Modern chemical
techniques — infrared, that contains some spectra that candidates
can analyse.
Khan Academy has a number of videos explaining
infrared
spectroscopy, including practice examples.
Proton nuclear magnetic resonance spectroscopy (proton NMR or
1
H
NMR) can give information about the different chemical environments
of hydrogen atoms (protons or
1
H) in an organic molecule, and about
how many hydrogen atoms there are in each of these environments.
RSC education resources has a handout, Modern chemical
techniques — nuclear magnetic resonance spectroscopy, that
contains some spectra that candidates can analyse.
Khan Academy has a number of videos
explaining
1
H
NMR
spectroscopy, including practice examples.
Version 3.1 103
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(d) Experimental determination of structure (continued)
1
H nuclei behave like tiny magnets and in a strong magnetic field
some align with the field (lower energy), whilst the rest align against it
(higher energy). Absorption of radiation in the radio frequency region
of the electromagnetic spectrum causes the
1
H nuclei to ‘flip’ from the
lower to the higher energy alignment. As they fall back from the
higher to the lower energy alignment the emitted radiation is detected
and plotted on a spectrum.
In a
1
H NMR spectrum the chemical shift,
δ
, (peak position) is related
to the environment of the
1
H atom and is measured in parts per
million (ppm).
Chemical shift values for
1
H in different chemical environments are
given in the data booklet.
The area under the peak is related to the number of
1
H atoms in that
environment and is often given by an integration curve on a
spectrum. The height of an integration curve is proportional to the
number of
1
H atoms in that environment, and so a ratio of
1
H atoms in
each environment can be determined.
The standard reference substance used in
1
H NMR spectroscopy is
tetramethylsilane (TMS), which is assigned a chemical shift value
equal to zero.
1
H NMR spectra can be obtained using low-resolution or high-
resolution NMR.
RSC education resources has a handout, Modern chemical
techniques — nuclear magnetic resonance spectroscopy, that
contains some spectra that candidates can analyse.
Khan Academy has a number of videos
explaining
1
H
NMR
spectroscopy, including practice examples.
Chemguide has useful information about
1
H NMR spectroscopy,
including an outline of the process of obtaining a spectrum of a
sample, low- and high-resolution spectroscopy and integration of
peaks.
Education Scotland has a number of resources available on the
Science NQ GLOW portal including:
♦ PowerPoint presentations introducing Proton NMR Spectroscopy
♦ learners’ workbooks and answers
♦ an organic spectroscopy structural determination workshop that
provides an opportunity for candidates to use mass, IR and
1
H
NMR spectra to determine the structure of a molecule
Version 3.1 104
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(d) Experimental determination of structure (continued)
High-resolution
1
H NMR uses higher radio frequencies than those
used in low-resolution
1
H NMR and provides more detailed spectra.
In a high-resolution
1
H NMR an interaction with
1
H atoms on
neighbouring carbon atoms can result in the splitting of peaks into
multiplets. The number of
1
H atoms on neighbouring carbon atoms
will determine the number of peaks within a multiplet and can be
determined using the n+1 rule, where n is the number of
1
H atoms on
the neighbouring carbon atom.
Low- and high-resolution
1
H NMR spectra can be analysed, and low-
resolution
1
H NMR spectra can be sketched for any given compound.
(e) Pharmaceutical chemistry
Drugs are substances that alter the biochemical processes in the
body.
Drugs that have beneficial effects are used in medicines.
A medicine usually contains the drug plus other ingredients such as
fillers to add bulk or sweeteners to improve the taste.
RSC education resources, Making medicines video, provides a useful
introduction to drug development.
Encyclopaedia Britannica has a good introduction to different types of
drug action
.
The articles ‘Big picture on drug development’ and ‘
Drug formulation’,
available through Stem Learning, introduces the process of drug
development and formulation.
The Association of the British Pharmaceutical Industry has the
resource making medicines that gives background information about
the drug development process and also has interesting information
about the history of medicine through to development of modern
medicines.
Version 3.1 105
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(e) Pharmaceutical chemistry (continued)
The RSC Education in Chemistry article ‘Pain relief: from coal tar to
paracetamol’ discusses the development of paracetamol, its mode of
action and the importance of correct doses. The Guardian article,
‘
Does paracetamol do you more harm than good?’, also provides
some useful information.
Drugs generally work by binding to specific protein molecules. These
protein molecules can be found on the surface of a cell (receptor) or
can be specific enzyme molecules within a cell.
Drugs that act on receptors can be classified as agonists or
antagonists.
♦ An agonist mimics the natural compound and binds to the
receptor molecules to produce a response similar to the natural
active compound.
♦ An antagonist prevents the natural compound from binding to the
receptor, and so blocks the natural response from occurring.
Many drugs that act on enzymes are classified as enzyme inhibitors
and act by binding to the active site of the enzyme and blocking the
reaction normally catalysed there.
The Conversation article, ‘Explainer: how do drugs work’, provides a
condensed explanation about agonist and antagonist drugs.
Edinformatics has an article that discusses how drugs work
and
provides examples of enzyme inhibitors, agonist and antagonist
drugs, as well as having 3D models of a number of drugs bound to
active sites.
The British Heart Foundation has short videos with a brief
explanation of how aspirin
prevents blood clots (enzyme inhibition)
forming and how beta blockers work (receptor antagonists).
RSC education resources has details about aspirin
, including a short
explanation of its action as an enzyme inhibitor and the history of its
development. There are also details of experiments to synthesise,
purify, and characterise aspirin that include important practical
techniques such as recrystallisation, melting point and thin layer
chromatography analysis.
The overall shape and size of a drug is such that it interacts with a
receptor binding site or to the active site of an enzyme. The types of
interactions formed can include van der Waals forces and/or ionic
bonds.
Khan Academy has a video, Beta-lactam antibiotics, which discusses
the chemistry of beta-lactam derivatives and how they act as enzyme
inhibitors.
Version 3.1 106
Organic chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(e) Pharmaceutical chemistry (continued)
The structural fragment of a drug molecule that allows it to form
interactions with a receptor binding site or to an enzyme active site
normally consists of different functional groups correctly orientated
with respect to each other.
By comparing the structures of drugs that have similar effects on the
body, the structural fragment that is involved in the drug action can
be identified.
The article ‘Caffeine: The chemistry behind the world’s most popular
drug’ discusses the antagonist nature of caffeine and compares the
structure of caffeine to the natural molecule adenosine.
RSC education resources Nurofen worksheet
provides some
information about how Nurofen acts.
The website Protein Data Bank has a large number of protein
structures to view. For example, the structure of the enzyme
neurominidase
, involved in influenza, is shown with its natural
substrate as well as with two active drugs.
An interactive resource from the RSC education resources is
masterminding molecules. This resource combines learning with
game-play and involves cracking a code to reveal hidden chemical
concepts involved in the design of drugs and medicines.
A PowerPoint presentation, Medicinal Chemistry, is a resource
produced by Education Scotland and available on the Science NQ
GLOW portal. It provides some examples of drugs, their active
structural fragments, and their interactions with the active sites.
World of Molecules
has a selection of 3D drug molecules that can be
viewed, as well as an explanation about the mode of action and the
history of their development. Another resource available gives the
structure of some drug molecules and allows for comparison of the
key functional groups necessary for biological activity.
Explain it with
molecules has information about caffeine.
Version 3.1 107
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(a) Common chemical apparatus
Candidates must be familiar with the use(s) of the following types of
apparatus:
♦ conical flask
♦ digital balance
♦ pipette with safety filler
♦ burette
♦ volumetric (standard) flask
♦ distillation (round-bottomed) flask
♦ condenser
♦ thermometer
♦ Buchner or Hirsch or sintered glass funnel
♦ glassware with ground glass joints (‘Quickfit’ or similar)
♦ thin-layer chromatography apparatus
♦ colorimeter
♦ melting point
♦ separating funnel
RSC education resources provide details of standard pieces of
laboratory equipment in The interactive lab primer — lab apparatus.
The interactive lab primer — weighing compounds using a balance
,
available through RSC education resources, contains a video and an
online simulation that allows candidates to become familiar with the
correct use of chemical balances.
Version 3.1 108
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(b) Skills involved in experimental work
Candidates must be able to:
♦ tabulate data using appropriate headings and units of
measurement
♦ represent data as a scatter graph with suitable scales and labels
♦ sketch a line of best fit (straight or curved) to represent the trend
observed in the data
♦ calculate average (mean) values
♦ identify and eliminate rogue points
♦ qualitatively appreciate the relative accuracy of apparatus used to
measure the volume of liquids
♦ comment on the reproducibility of results where measurements
have been repeated
♦ carry out quantitative stoichiometric calculations
♦ interpret spectral data
♦ appropriately use a positive control, for example a known
substance, to validate a technique or procedure
RSC education resources offer a number of activities related to
experimental work:
♦ a guide to keeping a lab book
gives useful advice to candidates
about how to keep a good lab book to help with planning their
projects
♦ The nature of science: measurement, accuracy and precision
supports the teaching of reproducibility, and identifying rogue
points and uncertainties
A Guide to Practical Work (2012)
, produced by Education Scotland,
is available through SSERC and contains useful information about
errors and uncertainty calculations (that may be useful for candidates
carrying out data analysis as part of their project work).
Why do scientists do what scientist do
describes and explains how to
use a positive control in an experiment. It also gives an introduction
to and video on accuracy, precision, errors and statistics.
Many of the suggested experiments show the appropriate use of a
positive control to validate a technique or procedure.
Chemistry
Practical Guide — Support Materials (2012), produced by Education
Scotland and available through SSERC, has details of the
determination of vitamin C by titration with iodine using a sample of
pure ascorbic as a positive control.

Version 3.1 109
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Stoichiometric calculations
Stoichiometry is the study of mole relationships involved in chemical
reactions.
Chemical equations, using formulae and state symbols, can be
written and balanced to show the mole ratio(s) of reactants and
products, including multi-step reactions.
The mass of a mole of any substance, in grams (g), is equal to the
gram formula mass (GFM) and can be calculated using relative
atomic masses.
Calculations can be performed using the relationship between the
mass and the number of moles of a substance.
For solutions, the mass of solute (grams or g), the number of moles
of solute (moles or mol), the volume of solution (litres or l), or the
concentration of the solution (moles per litre or mol l
-1
), can be
calculated from data provided.
Percentage by mass is the mass of solute made up to 100 cm
3
of
solution.
Percentage by volume is the number of cm
3
of solute made up to 100
cm
3
of solution.
The unit ppm stands for parts per million and refers to 1 mg per kg or
1 mg per litre.
Integrating stoichiometric calculations throughout the course and
practical work offers the opportunity to link with real-life examples,
leading to deeper understanding.
RSC education resources,
Starters for ten: chapters 1-11, section 1
‘Quantitative Chemistry’, offers a selection of easily editable short
quizzes and activities testing stoichiometric calculations.
Version 3.1 110
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c)Stoichiometric calculations (continued)
Calculations can be performed using data, including:
♦ GFM
♦ masses
♦ number of moles
♦ concentrations and volumes of solutions
♦ volumes of gases
♦ reactant excess
♦ theoretical and percentage yield
♦ empirical formulae
Theoretical yields can be calculated and compared with actual yields,
leading to determining the percentage yield. The percentage yield is
reduced by:
♦ mass transfer or mechanical losses
♦ purification of product
♦ side reactions
♦ equilibrium position
Candidates must be able to carry out stoichiometric calculations for
all of the skills and techniques in the course where appropriate.
Possible experiments to determine number of moles, reactant excess
and theoretical and percentage yields include:
♦ preparation of aspirin — the RSC offers two aspirin preparations:
— the Aspirin book
contains details of a number of experiments
relating to aspirin, including the synthesis, recrystallisation,
melting point and TLC analysis of aspirin
— The microscale synthesis of aspirin
provides instructions for
producing small quantities of aspirin that could be used to
determine the percentage yield of aspirin produced
♦ Chemistry Practical Guide — Support Materials (2012)
, produced
by Education Scotland and available through SSERC, has details
of the theory of percentage yield and also has instructions for
experiments that can be used to practise percentage yield
calculations and evaluations:
— preparation of potassium trioxalatoferrate(III) also provides an
opportunity to introduce the practical technique of hot filtration
— preparation of benzoic acid by hydrolysis of ethyl benzoate
also provides an opportunity to introduce the practical
techniques of reflux, recrystallisation, melting point analysis
and thin layer chromatography
Version 3.1 111
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(c) Stoichiometric calculations (continued)
♦ the University of Strathclyde
provides details of experiments that
can be used in practising percentage yield calculations and also
involve other practical techniques such as recrystallisation,
melting point and thin layer chromatography:
— synthesis and analysis of aluminium compounds prepared
from Coke cans
— electrophilic aromatic substitution organic photosynthesis
reactions
— preparation of cis- and trans- complexes of cobalt
RSC education resources,
Starters for ten: chapters 1-11, section 1
‘Quantitative Chemistry’, offers a selection of easily editable short
quizzes and activities that includes percentage yield calculations.
(d) Gravimetric analysis
Candidates must be familiar with the technique of gravimetric
analysis, including use of:
♦ an accurate electronic balance, including the tare function
♦ a weighing boat
♦ weighing by difference
♦ the term ‘weighing accurately approximately’
♦ heating to constant mass:
— heating a substance
— allowing to cool in a desiccator to prevent absorption of water
— weighing
Chemistry Practical Guide — Support Materials (2012), produced by
Education Scotland and available through SSERC, contains useful
theory about gravimetric analysis as well as details of the following
experiments:
♦ gravimetric determination of water in hydrated barium chloride by
volatilisation
♦ gravimetric determination of Ni using dimethylglyoxime by
precipitation
Version 3.1 112
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(d) Gravimetric analysis (continued)
— repeating the steps of heating, cooling and weighing until no
further changes in mass are observed
Gravimetric analysis is used to determine the mass of an element or
compound in a substance.
The substance is converted into another substance of known
chemical composition, which can be readily isolated and purified.
The conversion can occur either through precipitation or volatilisation.
In precipitation conversion the substance undergoes a precipitation
reaction. The precipitate is separated from the filtrate and the filtrate
tested to ensure the reaction has gone to completion. The precipitate
is washed, dried to constant mass and then weighed.
In volatilisation conversion the substance is heated and any volatile
products (often water) are evaporated. The substance is heated to
constant mass and the final mass recorded.
Preparation and characterisation of transition metal-oxalate ligand
complexes, available through the University of Strathclyde, details the
preparation of potassium trioxalatoferrate(III), followed by gravimetric
analysis to determine the water of hydration as well as volumetric
analysis of oxalate and iron(III) content.
Version 3.1 113
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(e) Volumetric analysis
Candidates must be familiar with use of the technique of volumetric
analysis, including:
♦ preparing a standard solution
♦ accurate dilution
♦ standardising solutions to determine accurate concentration
♦ titrating to obtain concordancy using burettes, pipettes and
volumetric flasks
♦ choosing an appropriate indicator
A solution of accurately known concentration is known as a standard
solution.
A standard solution can be prepared by:
♦ weighing a primary standard accurately
♦ dissolving in a small volume of solvent (usually deionised or
distilled water) in a beaker
♦ transferring the solution and rinsings into a volumetric flask
♦ making up to the graduation mark with solvent
♦ stoppering and inverting
Standard solutions can also be prepared by accurate dilution by
pipetting an appropriate volume of a standard solution into a
volumetric flask, making up to the graduation mark with solvent,
stoppering and inverting.
Chemistry — a practical guide, support materials (2012), produced by
Education Scotland and available through SSERC, contains useful
theory about volumetric analysis as well as details of possible
experiments including:
♦ preparation of a standard solution of 0·1 mol l
-1
oxalic acid
♦ standardisation of a solution of sodium hydroxide using oxalic
acid
♦ determination of the ethanoic acid content of vinegar
♦ preparation of a standard solution of 0·1 mol l
-1
sodium carbonate
♦ standardisation of a solution of hydrochloric acid using sodium
carbonate solution
♦ determination of the purity of marble by back titration
♦ determination of nickel in a nickel(II) salt using EDTA
♦ determination of vitamin C by titration with iodine
The water testing
resources produced by SSERC contain details of
complexometric titrations using EDTA. The Cobalt complexes booklet
has details on the preparation and analysis by titration of a cobalt
complex and also involves vacuum filtration.
Version 3.1 114
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(e) Volumetric analysis (continued)
A primary standard must:
♦ be available in a high state of purity
♦ be stable when solid and in solution
♦ be soluble
♦ have a reasonably high GFM
Examples of primary standards include:
♦
23
, Na COsodium carbonate
♦
22 4 2
hydrated oxalic acid, H C O ·2H O
♦
84 4
potassium hydrogen phthalate, KH(C H O )
♦
3
silver nitrate, AgNO
♦
3
potassium iodate, KIO
♦
2 27
potassium dichromate, K Cr O
Sodium hydroxide is not a primary standard as it has a relatively low
GFM, is unstable as a solid (absorbs moisture) and unstable as a
solution. Sodium hydroxide solution must be standardised before
being used in volumetric analysis.
RSC education resources has a number of resources relating to
volumetric analysis:
♦ a video with instructions on making a standard solution
♦ the interactive lab primer — standard solution, apparatus guide,
and mass and concentration calculators
♦ the interactive lab primer — titration has a video, apparatus guide
and information relating to pH curves and indicators as well as
instructions for correct use of pipettes and burettes
♦ titration screen experiment provides videos, animations and
interactive quizzes relating to titration, including acid base and
redox titrations
♦ a redox titration using wine video and experiment instructions
The University of Strathclyde details a number of experiments
involving volumetric analysis, including:
♦ preparation and characterisation of transition metal-oxalate ligand
complexes — preparation of potassium trioxaatoferrate(III)
followed by gravimetric analysis to determine the water of
hydration, as well as volumetric analysis of oxalate and iron(III)
content
♦ Build-a-stomach: antacid study — direct and back titration
experiment with antacid treatments
Version 3.1 115
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(e) Volumetric analysis (continued)
Candidates must be familiar with use of the following types of
volumetric analysis:
♦ acid-base titrations
♦ redox titrations based on reactions between oxidising and
reducing agents
♦ complexometric titrations based on reactions in which complexes
are formed — EDTA is an important complexometric reagent and
can be used to determine the concentration of metal ions in
solution
♦ back titrations used to find the number of moles of a substance by
reacting it with an excess volume of a reactant of known
concentration. The resulting mixture is then titrated to work out
the number of moles of the reactant in excess. From the initial
number of moles of that reactant, the number of moles used in
the reaction can be determined. The initial number of moles of the
substance being analysed can then be calculated. A back titration
is useful when trying to work out the quantity of substance in a
solid with a low solubility
Version 3.1 116
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques
Candidates can carry out practical skills and techniques as individual
activities or combined together during single experiments. Some of
the suggestions listed below allow candidates to use a number of
practical skills and techniques in a single experiment.
The RSC website ‘SpectraSchool
’ provides many resources relating
to spectroscopy techniques with animations, videos and interactive
spectra.
‘Spectroscopy in a suitcase’ is an RSC outreach activity
giving candidates the opportunity to learn about spectroscopy
through hands-on experience. As well as covering the principles of
spectroscopic techniques, the activities use real-life contexts to
demonstrate the applications of the techniques. You can use this to
teach colorimetry. There is also a
student resource that introduces
spectroscopy as well as providing background information the
technique of colorimetry.
Candidates must be familiar with use of the technique of colorimetry,
including:
♦ preparing a series of standard solutions of appropriate
concentration
♦ choosing an appropriate colour or wavelength of filter
complementary to the colour of the species being tested
♦ using a blank
♦ preparing a calibration graph
Challenging medicines resource, available through RSC education
resources, details colorimetric analysis of 2-hydroxybenzoic acid,
aspirin and paracetamol. Also available through RSC
education
resources is challenging plants
that details colorimetric analysis of
soil samples.
Chemistry Practical Guide — Support Materials (2012)
, produced by
Education Scotland and available through SSERC, contains useful
theory about colorimetric analysis as well as instructions for the
determination of manganese in steel experiment.
Version 3.1 117
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
Colorimetry uses the relationship between colour intensity of a
solution and the concentration of the coloured species present.
A colorimeter or a spectrophotometer is used to measure the
absorbance of light of a series of standard solutions, and this data is
used to plot a calibration graph.
The concentration of the solution being tested is determined from its
absorbance and by referring to the calibration curve.
The concentration of coloured species in the solution being tested
must lie in the straight line section of the calibration graph.
SSERC provides a method for determination of iron and manganese
in tea using colorimetry.
Colorimetric determination of iron content
, available through the
University of Strathclyde, provides theory as well as instructions for
determination of iron in Irn Bru, iron tablets and spinach.
Candidates must be familiar with use of the technique of distillation.
Distillation is used for identification and purification of organic
compounds.
The boiling point of a compound, determined by distillation, is one of
the physical properties that can be used to confirm its identity.
Distillation can be used to purify a compound by separating it from
less volatile substances in the mixture.
Theory of the organic techniques distillation, heating under reflux,
vacuum filtration, solvent extraction, recrystallisation, melting point
and mixed melting point determination and thin layer chromatography
are available in
Chemistry Practical Guide — Support Materials
(2012), produced by Education Scotland and available through
SSERC.
RSC education resources provides a number of resources for
distillation:
♦ interactive lab primer — distillation
contains a video, animation
and apparatus guide to distillation
♦ extracting limonene from oranges by steam distillation
Version 3.1 118
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
Chemistry Practical Guide — Support Materials (2012), produced by
Education Scotland and available through SSERC, contains details of
two experiments that involve distillation:
♦ preparation of ethyl ethanoate
♦ preparation of cyclohexene from cyclohexanol
Oils and spices
, available through the University of Strathclyde,
details the extraction of essential oils by steam distillation followed by
solvent extraction.
Candidates must be familiar with use of the technique of heating
under reflux. Heating under reflux allows heat energy to be applied to
a chemical reaction mixture over an extended period of time without
volatile substances escaping.
When carrying out heating under reflux, the reaction mixture is placed
in a round-bottomed flask with anti-bumping granules and the flask is
fitted with a condenser. The flask is then heated using an appropriate
source of heat.
RSC education resources provides a number of resources for heating
under reflux:
♦ interactive lab primer — heating under reflux
has a video,
animation and apparatus guide
♦ the synthesis of aspirin also provides an opportunity to introduce
other important practical techniques including recrystallisation,
vacuum filtration, melting point analysis, thin layer
chromatography and % yield calculations. A similar procedure is
available in
Chemistry Practical Guide — Support Materials
(2012)
♦ the RSC aspirin screen experiment gives an opportunity to learn
about the synthesis of aspirin before beginning practical work. It
provides videos, animations and interactive quizzes relating to the
synthesis of aspirin, purification by recrystallisation and analysis
by TLC and relates these experiments to the functional groups
and properties of aspirin
Version 3.1 119
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
♦ The First Year Undergraduate Chemistry Laboratory Course
Manual 2011-2012 provides experimental details for the
preparation of ester propyl ethanoate and the preparation of
benzoic acid from methyl benzoate, and also provides an
opportunity to include other important practical techniques
(vacuum filtration, recrystallisation, melting point analysis and %
yield calculations)
Chemistry Practical Guide — Support Materials (2012)
, produced by
Education Scotland and available through SSERC, contains details of
an experiment to hydrolyse ethyl benzoate, by heating under reflux,
to prepare benzoic acid. It also provides an opportunity to include
other important practical techniques (recrystallisation, vacuum
filtration, melting point analysis, thin layer chromatography and %
yield calculations). This guide also contains experimental details for
preparation of ethyl ethanoate.
The University of Strathclyde details a number of experiments
involving heating under reflux
:
♦ preparation and characterisation of methyl esters details
experimental procedures to make two solid methyl esters, and
involves the practical techniques of solvent extraction and melting
point determination as well as providing some spectral data
♦ using an inorganic complex to catalyse an organic molecular
reaction provides an opportunity to carry out vacuum filtration,
solvent extraction and thin layer chromatography
Version 3.1 120
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
Candidates must be familiar with use of the technique of vacuum
filtration. Vacuum filtration involves carrying out a filtration under
reduced pressure and provides a faster means of separating a
precipitate from a filtrate. A Büchner, Hirsch or sintered glass funnel
can be used during vacuum filtration.
RSC education resources interactive lab primer — vacuum filtration
contains a video and an apparatus guide.
There are many experiments that could include vacuum filtration. A
selection provided in
Chemistry Practical Guide — Support Materials
(2012) includes:
♦ preparation of potassium trioxalatoferrate(III)
♦ preparation of aspirin
♦ preparation of benzoic acid by hydrolysis of ethyl benzoate
The University of Strathclyde also details the following experiments
that use vacuum filtration
:
♦ cis- and trans-complexes of cobalt
♦ methyl orange synthesis
♦ electrophilic aromatic substitution reactions provides an
opportunity to introduce the practical techniques of
recrystallisation, melting point analysis and thin layer
chromatography
Version 3.1 121
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
Candidates must be familiar with use of the technique of
recrystallisation to purify an impure solid involving:
♦ dissolving an impure solid gently in a minimum volume of a hot
solvent
♦ hot filtration of the resulting mixture to remove any insoluble
impurities
♦ cooling the filtrate slowly to allow crystals of the pure compound
to form, leaving soluble impurities dissolved in the solvent
♦ filtering, washing and drying the pure crystals
The solvent for recrystallisation is chosen so that the compound
being purified is completely soluble at high temperatures and only
sparingly soluble at lower temperatures.
In addition to the previous suggested experiments that could include
recrystallisation, RSC education resources also provides:
♦ interactive lab primer – recrystallisation
, including a video,
animation and an apparatus guide as well as details about hot
filtration
♦ preparation of paracetamol
There are many experiments that could include recrystallisation. A
selection provided in
Chemistry Practical Guide — Support Materials
(2012) includes:
♦ preparation of aspirin
♦ preparation of benzoic acid by hydrolysis of ethyl benzoate
Candidates must be familiar with use of the technique of solvent
extraction. Solvent extraction involves isolating a solute from a liquid
mixture or solution by extraction using an immiscible solvent in which
the solute is soluble.
RSC education resources provides a number of resources for solvent
extraction including:
♦ interactive lab primer — solvent extraction
, which has a video,
animation and apparatus guide
Version 3.1 122
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
When carrying out a solvent extraction, the two immiscible solvents
form two layers in the separating funnel. The solute dissolves in both
solvents and an equilibrium establishes between the two layers. The
ratio of solute dissolved in each layer is determined by the equilibrium
constant,
K
. The lower layer is run off into a container and the upper
layer is poured into a second container. This process is repeated to
maximise the quantity of solute extracted.
The quantity of solute extracted is greater if a number of extractions
using smaller volumes of solvent are carried out rather than a single
extraction using a large volume of solvent.
The solvent used should be:
♦ immiscible with the liquid mixture or solution (usually water)
♦ one in which the solute is more soluble in than the liquid mixture
or solution (usually water)
♦ volatile to allow the solute to be obtained by evaporation of the
solvent
♦ unreactive with the solute
♦ extracting iodine from seaweed by solvent extraction (only one
extraction is carried out in this procedure)
♦ preparation of cyclohexene from cyclohexanol with purification by
distillation and solvent extraction
Chemistry Practical Guide — Support Materials (2012)
, produced by
Education Scotland and available through SSERC, details
experiments that provide an opportunity to introduce the practical
techniques of distillation and solvent extraction (only one extraction is
carried out in both of these experiments):
♦ preparation of cyclohexene from cyclohexane using concentrated
phosphoric acid
♦ preparation of ethyl ethanoate
The University of Strathclyde also details the following experiments
that use solvent extraction
:
♦ extraction of caffeine from tea provides an opportunity to perform
melting point analysis and percentage yield calculations
♦ plant pigments and pH indicators provides an opportunity to carry
out thin layer chromatography
♦ selective reductions with sodium borohydride provides an
opportunity to monitor reactions using thin layer chromatography
Version 3.1 123
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
Candidates must be familiar with use of the techniques of melting
point and mixed melting point determination. The melting point of a
substance is the temperature range over which the solid first starts to
melt, to when all of the solid has melted.
The identity of a pure compound can be confirmed by melting point
analysis and a comparison of the experimentally determined melting
point with a literature or known melting point value.
Determination of the melting point of a compound can give an
indication of the purity of a compound. The presence of impurities in
the compound lowers the melting point and broadens its melting
temperature range due to the disruption in intermolecular bonding in
the crystal lattice.
Determination of a mixed melting point involves mixing a small
quantity of the product with some of the pure compound and
determining the melting point. The melting point value and the range
of the melting temperature can be used to determine if the product
and the pure compound are the same substance.
Candidates must be familiar with use of the technique of thin-layer
chromatography. Chromatography is a technique used to separate
the components present within a mixture. Chromatography separates
substances by making use of differences in their polarity or molecular
size.
In addition to the previous suggested experiments that could include
melting point determination, RSC education resources interactive lab
primer has a video and apparatus guide to
melting point
determination.
As well as learning about the practical technique of melting point
analysis, candidates could explain the effect impurities have on the
melting point of a solid in terms of the strengths of intermolecular
forces of attraction. The University of Rhode Island
explains the effect
impurities have on melting point, in relation to strength of
intermolecular forces.
Version 3.1 124
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
Thin-layer chromatography (TLC) uses a fine film of silica or
aluminium oxide spread over glass, aluminium foil or plastic. A small
sample of the mixture being tested is spotted onto the base (pencil)
line of the chromatogram. A solvent dissolves the compounds in the
spot and carries the compounds up the chromatogram. How far the
compounds are carried depends on how soluble the compounds are
in the chosen solvent and how well they adhere to the plate. A
developing agent or ultraviolet light is normally required to visualise
the spots on the chromatogram.
f
R
values can be calculated:
f
distance travelled by the sample
R
distance travelled by the solvent
=
Under the same conditions (temperature, solvent, and saturation
levels) a compound always has the same
f
R
value (within
experimental error).
The identity of a compound can be confirmed by:
♦ comparing the experimentally determined
f
R
values with a
literature or known value determined under the same conditions
♦ making a direct comparison on a TLC plate between the
compound being tested and the pure substance — a co-spot
could be used
In addition to the previous suggested experiments that could include
TLC, RSC education resources interactive lab primer has a video,
animation and apparatus guide to thin layer chromatography
.
The University of Strathclyde details
electrophilic aromatic
substitution reactions that include instructions for carrying out TLC,
as well as providing IR spectra for analysis.
Version 3.1 125
Researching chemistry
Mandatory knowledge
Suggested learning activities and resources, and/or further
guidance on mandatory knowledge
(f) Practical skills and techniques (continued)
TLC is used to assess the purity of substances. A pure substance,
when spotted and developed on a TLC plate, should appear as a
single spot (some impurities may not be visible by TLC analysis). The
presence of more than one spot shows that impurities are present.
Version 3.1 126
Preparing for course assessment
The course is assessed by a question paper and a project. To help candidates prepare for
the course assessment, you should give them opportunities to practise activities similar to
those expected in the course assessment, for example:
♦ practising question paper techniques and revising for the question paper. To support this
learning, you may find it helpful to refer to: Advanced Higher Chemistry Specimen
Question Paper and Guidance on the use of past papers for Advanced Higher Chemistry
♦ preparing for the project: selecting topics; gathering and researching information/data;
evaluating and analysing findings; developing and justifying conclusions; and presenting
the information/data (as appropriate). You may find it helpful to refer to the Advanced
Higher Chemistry coursework assessment task
Developing skills for learning, skills for life and skills
for work
You should identify opportunities throughout the course for candidates to develop skills for
learning, skills for life and skills for work.
Candidates should be aware of the skills they are developing and you can provide advice on
opportunities to practise and improve them.
SQA does not formally assess skills for learning, skills for life and skills for work.
There may also be opportunities to develop additional skills depending on the approach
centres use to deliver the course. This is for individual teachers and lecturers to manage.
Some examples of potential opportunities to practise or improve these skills are provided
below.
Literacy
Writing means the ability to create texts which communicate ideas, opinions and information,
to meet a purpose and within a context. In this context, ‘texts’ are defined as word-based
materials (sometimes with supporting images) which are written, printed, Braille or displayed
on screen. These are technically accurate for the purpose, audience and context.

Version 3.1 127
1.1 Reading
Candidates understand and interpret a variety of scientific texts.
1.2 Writing
Candidates use skills to effectively communicate key areas of chemistry, make informed
decisions and explain, clearly, chemistry issues in various media forms. Candidates have the
opportunity to communicate applied knowledge and understanding throughout the course.
There are opportunities to develop the literacy skills of listening and reading when gathering
and processing information in chemistry.
Numeracy
This is the ability to use numbers in order to solve problems by counting, doing calculations,
measuring, and understanding graphs and charts. This is also the ability to understand the
results.
Candidates extract, process and interpret information presented in numerous formats
including tabular and graphical. Practical work provides opportunities to develop time
management and measurement skills.
2.1 Number processes
Number processes means solving problems through: carrying out calculations, when dealing
with data and results from experiments/investigations and class work; making informed
decisions based on the results of these calculations, and understanding these results.
2.2 Money, time and measurement
The accuracy of measurements is important when handling data in a variety of chemistry
contexts, including practical and investigative. Candidates should consider uncertainties.
2.3 Information handling
Candidates experience information handling opportunities when dealing with data in tables,
charts and other graphical displays, to draw conclusions with justifications throughout the
course. This involves interpreting the data, and considering its reliability in making reasoned
deductions and informed decisions with justifications.
Thinking skills
This is the ability to develop the cognitive skills of remembering and identifying,
understanding and applying.
The course allows candidates to develop skills of applying, analysing and evaluating.
Candidates can analyse and evaluate practical work and data by reviewing the process,
identifying issues and forming valid conclusions. They can demonstrate understanding and
application of concepts and explain and interpret information and data.
Version 3.1 128
5.3 Applying
Candidates plan experiments and investigations throughout the course and use existing
information to solve problems in different contexts.
5.4 Analysing and evaluating
During practical work, candidates review and evaluate experimental procedure and identify
improvements. Candidates use their judgement when drawing conclusions from experiments.
Analysis is the ability to solve problems in chemistry and make decisions that are based on
available information.
It may involve reviewing and evaluating relevant information and/or prior knowledge to
provide an explanation.
It may build on selecting and/or processing information, so is a higher skill.
5.5 Creating
This is the ability to design something innovative or to further develop an existing thing by
adding new dimensions or approaches. Candidates can demonstrate creativity, in particular,
when planning and designing experiments/investigations. They have the opportunity to be
innovative in their approach, and to make, write, say or do something new.
Candidates also have opportunities to develop the skills of working with others, creating, and
citizenship.
Working with others
Learning activities provide many opportunities, in all areas of the course, for candidates to
work with others. Practical activities and investigations, in particular, offer opportunities for
group work, which is an important aspect of chemistry.
Citizenship
Candidates develop citizenship skills, when considering the applications of chemistry on our
lives, as well as the implications for the environment and society.
Version 3.1 129
Administrative information
Published: April 2021 (version 3.1)
History of changes
Version Description of change Date
2.0
Amendments made in ‘Skills knowledge and understanding’
section:
♦ ‘
Inorganic Chemistry’ section, sub-section ‘(c) transitiona
l
m
etals’: information about heterogeneous catalysts,
heterogeneous catalysis and homogeneous catalysis clarified.
♦ ‘
Physical Chemistry’ section, sub-section ‘(b) reacti
on
f
easibility’: error in entropy equation corrected.
♦ ‘Organic Chemistry and instrumental analysis’ section, sub-
section ‘(b) synthesis’: reference to Markonikov’s rule clarified.
Course support notes added as appendix.
September
2019
3.0
Error in terminology corrected on p9 and p59 — ‘…in a molecule
or neutral ion’ changed to ‘…in a neutral compound’.
October
2020
3.1
Fixed software formatting error that occurred in version 3.0 on p12
and p15.
April 2021
N
ote: please check SQA’s website to ensure you are using the most up-to-date version of
this document.
©
Scottish Qualifications Authority 2014, 2019, 2020, 2021
