
Faculty & Staff Scholarship
2005
Size-Dependent Properties Of Cdse Quantum Dots Size-Dependent Properties Of Cdse Quantum Dots
S. Neeleshwar
C. L. Chen
C. B. Tsai
Y. Y. Chen
C. C. Chen
See next page for additional authors
Follow this and additional works at: https://researchrepository.wvu.edu/faculty_publications
Digital Commons Citation Digital Commons Citation
Neeleshwar, S.; Chen, C. L.; Tsai, C. B.; Chen, Y. Y.; Chen, C. C.; Shyu, S. G.; and Seehra, M. S., "Size-
Dependent Properties Of Cdse Quantum Dots" (2005).
Faculty & Staff Scholarship
. 676.
https://researchrepository.wvu.edu/faculty_publications/676
This Article is brought to you for free and open access by The Research Repository @ WVU. It has been accepted
for inclusion in Faculty & Staff Scholarship by an authorized administrator of The Research Repository @ WVU. For
more information, please contact [email protected].

Size-dependent properties of CdSe quantum dots
S. Neeleshwar, C. L. Chen, C. B. Tsai, and Y. Y. Chen
*
Institute of Physics, Academia Sinica, Taipei, Taiwan, Republic of China
C. C. Chen
Department of Chemistry, National Taiwan Normal University, and Institute of Atomic and Molecular Sciences,
Academia Sinica, Taipei, Taiwan, Republic of China
S. G. Shyu
Institute of Chemistry, Academia Sinica, Taipei, Taiwan, Republic of China
M. S. Seehra
Physics Department, West Virginia University, Morgantown, West Virginia 26506, USA
共Received 16 March 2005; revised manuscript received 27 April 2005; published 23 May 2005
兲
Temperature dependences of the magnetic susceptibility
and heat capacity C
p
of CdSe quantum dots with
size d=2.8, 4.1, and 5.6 nm are compared to those of bulk CdSe to determine the size-dependent effects. With
decreasing size d, the following effects are observed: 共i兲 room temperature optical absorption shows a blueshift
of the band gap; 共ii兲 room temperature x-ray diffraction show wurtzite structure but with smaller lattice
constants; 共iii兲 magnetic susceptibility changes from negative 共diamagnetic兲 for the bulk to positive
with
magnitude increasing with decreasing d; and 共iv兲 the Sommerfeld constant
␥
determined from the C
p
/T vs T
2
data increases. Possible explanations for these size-dependent properties are presented.
DOI: 10.1103/PhysRevB.71.201307 PACS number共s兲: 75.75.⫹a, 65.80.⫹n
I. INTRODUCTION
In recent years, properties of nanosized materials have
generated a great deal of interest because of the science in-
volved in these studies and technological applications of the
quantum dots 共QDs兲. As the physical dimensions of the par-
ticle approach to the nanometer scales, quantization and sur-
face effects begin to play an important role, leading to drastic
changes in measured properties.
1
Among the semiconductor
QD, studies have been reported for the II-IV 共Ref. 2兲 and
III-V 共Ref. 3兲 materials, where a shift in the electronic tran-
sitions to higher energies accompanied by an increase of the
oscillator strength with the decrease in the particles size were
reported. Applications of the semiconductor QD have been
reported for photovoltaics,
4
light emitting diodes,
5
lasers,
6
and biological imagings.
7
Other reports studied include opti-
cal spectroscopy,
8
photoconductivity,
9
and LO-phonon
coupling.
10
None of the studies listed above in semiconductor QD
have focused on the effect of size on thermodynamic prop-
erties such as magnetic susceptibility
and heat capacity C
p
.
Consequently in this paper we report detailed studies of the
temperature dependence of
and C
p
for CdSe quantum dots
with size d=2.8, 4.1, and 5.6 nm vis-a-vis bulk CdSe. Im-
portant size-dependent effects are observed, whose discus-
sion and analysis are presented below.
II. EXPERIMENTAL
CdSe semiconductor quantum dots were prepared from
the pyrolysis of dimethylcadmium and tri-n-octylphosphine
selenide 共TOPSe兲 in a hot coordinating solvent of tri-
n-octylphosphine oxide 共TOPO兲 using the procedure de-
scribed previously.
11
In this method, the surface of the CdSe
quantum dot was passivated with TOPO molecules to avoid
surface oxidation and aggregation. Different sizes of quan-
tum dots were obtained by controlling its nucleation and
growth process. For further size selection, size-selective pre-
cipitation can be carried out in a chloroform-methanol sol-
vent system. Three sizes of quantum dots were prepared with
d=2.8, 4.1, and 5.6 nm with a standard deviation of ⬃10%
as determined by the high-resolution transmission electron
microscopy 共HRTEM兲; see inset of Fig. 1 for the 5.6-nm QD.
Optical absorption spectra of CdSe quantum dots were ob-
tained by a HP 8452 diode array spectrophotometer using
FIG. 1. Optical absorption spectra for d=2.8-, 4.1-, and 5.6-nm
CdSe quantum dots dispersed in chloroform were taken at 300 K.
Inset: The HRTEM image of 5.6-nm CdSe quantum dots; an ex-
ample particle marked by a circle is shown.
PHYSICAL REVIEW B 71, 201307共R兲共2005兲
RAPID COMMUNICATIONS
1098-0121/2005/71共20兲/201307共4兲/$23.00 ©2005 The American Physical Society201307-1
1-cm quartz cuvettes at room temperature as shown in Fig. 1.
The blueshift of the absorption edge with the decreasing d of
quantum dots is consistent with an earlier report.
12
X-ray
diffraction 共XRD兲 of the quantum dots 共carried out with a
3-KW Philips diffractometer equipped with an array detector
based on a real-time multiple strip兲 showed the wurtzite
structure of the bulk CdSe but with the expected line broad-
ening with decreasing d 共Fig. 2兲. In addition, there is a
shrinkage of the lattice constants 共the inset of Fig. 2兲, due to
size effect, somewhat similar to that reported in the
literature.
13
No additional lines due to any impurity phase
could be detected in the XRD spectra.
A calorimetric study was made in the range of 0.4 to 10
K, using a thermal-relaxation microcalorimeter in a
3
He
cryostat.
14
Each milligram-sized sample was prepared by
lightly pressing fine powders together. It was then attached
with thermal-conducting N grease to a sapphire disk, having
two deposited thin films serving as heater and thermometer,
respectively. The heat capacity of the sapphire disk and
grease were measured separately, and used as addenda cor-
rection in data analysis. The relative precision and the abso-
lute accuracy of the calorimeter were confirmed to be within
3% by measuring the copper standard. Magnetization mea-
surements were performed as a function of temperature using
the Quantum Design superconducting quantum interference
device 共SQUID兲 magnetometer in the range 2 to 300 K. The
magnetic susceptibility of straw and capsule were measured
separately and subtracted from the data.
III. RESULTS AND DISCUSSION
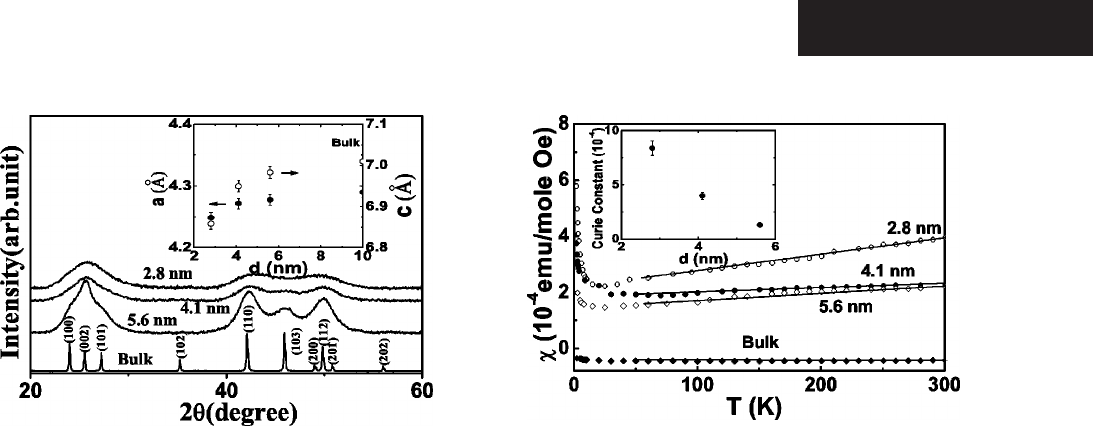
The temperature dependence of magnetic susceptibility
for CdSe QD with size d=2.8, 4.1, and 5.6 nm and bulk
CdSe is shown in Fig. 3. For the bulk CdSe,
is diamagnetic
and temperature independent with the magnitude
⬇−43⫻10
−6
emu/mole in good agreement with the earlier
results.
15
We report that for QD,
is positive and it has a
strong temperature dependence, especially below 30 K. Also
the magnitude of
is larger for the smaller particles at all
temperatures, showing the effect of size on magnetism.
In general for pure semiconductors,
=
l
+
f
+
i
, where
l
is the temperature-independent lattice contribution,
f
is
the free charge carrier 共electrons and holes兲 contribution and
i
is the contribution from bounded carriers and dangling
bonds. For bulk CdSe,
f
,
i
Ⰶ
l
, leading to magnetic sus-
ceptibility determined by
l
, which is usually negative
共Fig. 3兲.
15
Shaldin et al.
16
have shown that in II-IV semicon-
ductors, vacancies and interstitial can occur during the
growth. For QD, such defects will be more prevalent as com-
pared to bulk materials because of the increase in the relative
surface area. Specific magnetic clusters created by the donor-
acceptor pairs can exhibit paramagnetic behavior.
17
On the
surfaces of semiconductors, the free dangling bond bears an
electron spin by nature and can make semiconductor surfaces
magnetic. These phenomena are expected to be more signifi-
cant in QD.
18
With these considerations in mind, we suggest that the
low-temperature Curie tail in
is most likely due to surface
dangling bonds. These surface dangling bonds result from
decreased coordination of the surface atoms of the QD. We
have fitted the low-temperature data for T⬍ 30 K to the
modified Curie law:
=
o
+C/T, where
o
is temperature
independent contribution mainly from Pauli paramagnetism
of
f
mentioned above. The details will be discussed later.
The fits are excellent with C=1.33, 4.0, and 8.38 共in units of
10
−4
emu K/mol兲 for d=5.6, 4.1, and 2.8 nm, respectively
共inset to Fig. 3兲. This rapid increase in C with a decrease in
d is due to increase in surface/volume ratio as d decreases.
Note that C=N
2
/3k
B
where N is the number of dangling
bonds/mol, each with effective magnetic moment
and
k
B
is the Boltzmann constant. If we assume spin S=1/2
with each dangling bond, leading to
=1.73
B
, then
N=13.5⫻ 10
20
/mol for d=2.8 nm, thus yielding the concen-
tration of the dangling bonds ⬇2000 ppm. For d=4.1 and
5.6 nm, a similar calculation yields the concentration ⬇1000
and 300 ppm, respectively. It is noted that in amorphous Si
and Ge, low-temperature magnetic susceptibility studies
yielded similar concentration of spin density due to dangling
bonds.
19
The increase in
with increasing temperature above 30 K
seen for the QD in Fig. 3 is another interesting feature of our
FIG. 2. X-ray diffraction patterns for the bulk and d=2.8-, 4.1-,
and 5.6-nm quantum dots. Inset: The size dependence of lattice
constants of a and c axes.
FIG. 3. The magnetic susceptibility as function of temperature
for the bulk and d=2.8-, 4.1-, and 5.6-nm quantum dots; the lines
are for eye’s guide. Inset: The Curie constant vs d.
NEELESHWAR et al. PHYSICAL REVIEW B 71, 201307共R兲共2005兲
RAPID COMMUNICATIONS
201307-2
results. At the outset we note that a similar increase was
reported by Burgardt and Seehra in semiconductor FeS
2
.
20
In
Fig. 3, both the magnitude and the slope increase with de-
crease in d. For FeS
2
共Ref. 20兲 and amorphous Si and Ge,
21
the positive
and its temperature dependence at higher tem-
perature were explained by the Van Vleck susceptibility
vv
=2N
A
B
2
兺
k
兩具l兩L
Z
兩k典兩
2
E
k
− E
l
, 共1兲
where N
A
is the Avogadro’s number and L
Z
is the z compo-
nent of the orbital angular momentum coupling the excited
state 兩k典 with energy E
k
with the ground state 兩l典 with energy
E
l
. For semiconductors, E
k
−E
l
艌E
g
共energy gap兲. Note that
E
g
usually decreases with increase in temperature
2,22
and in
CdS nanoclusters, a much steeper temperature dependence
with decreasing particle size is observed. Assuming similar
results are valid for CdSe QD, it then explains why
in-
creases with increasing temperature, and increasing slope
with decreasing d, as observed in Fig. 3. To estimate
vv
from Eq. 共1兲, if we approximate the sum over all the states
by 1/E
g
assuming the matrix elements to be unity,
vv
=0.3⫻ 10
−4
emu/mol Oe is obtained for E
g
⯝1.75 eV
valid for CdSe QD. This estimate of
vv
is about a factor of
three times smaller than the enhancement of
observed for
QD. This confirms that
o
is mainly contributed by
f
as
proposed earlier. This issue requires further investigation.
Since the surface free charge carriers 共which gives
f
兲 are
easily formed in QDs,
13
the increase in the number of free
charge carriers with surface for smaller particles is expected
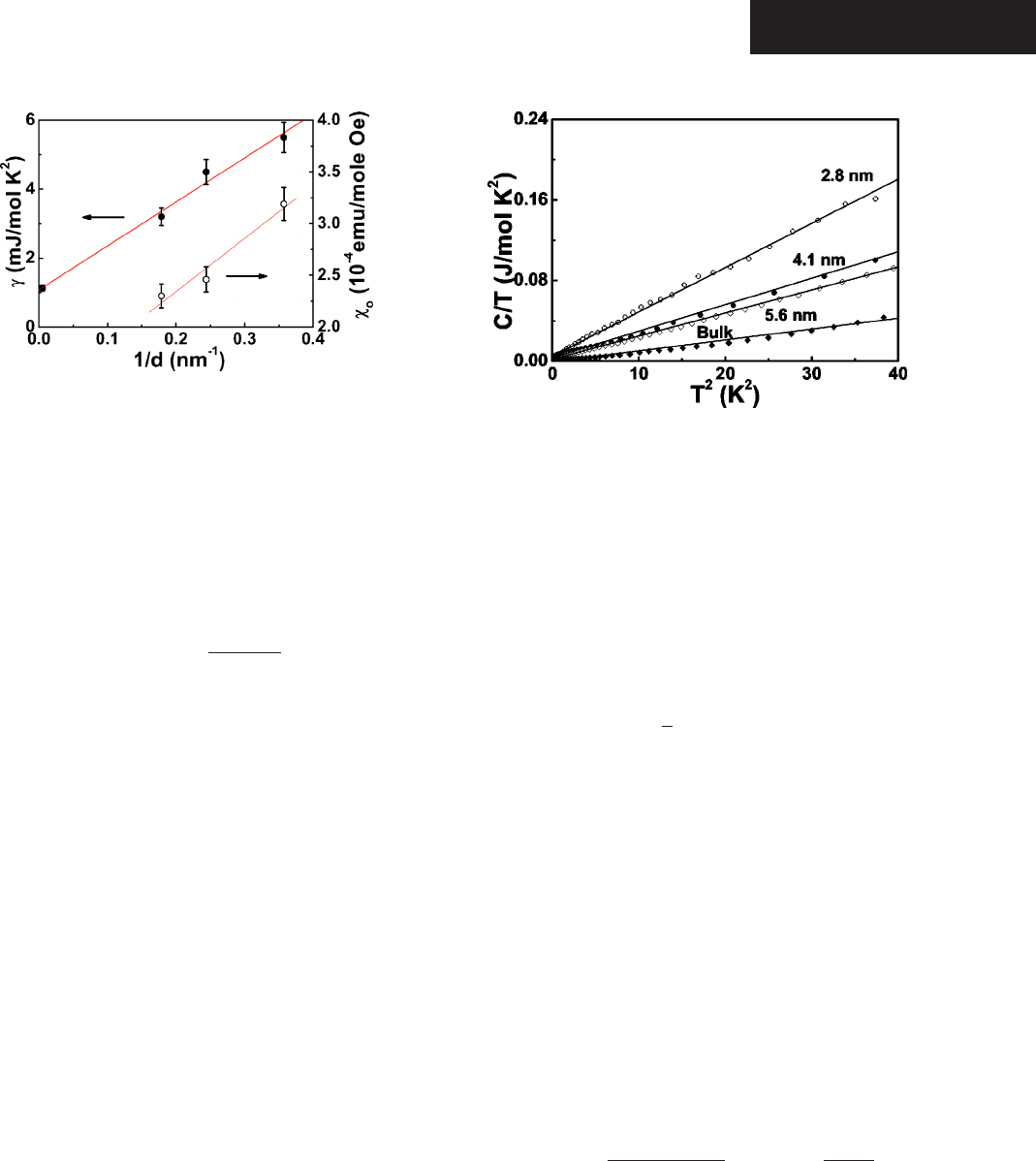
to vary as 1/d. In Fig. 4
o
vs 1/d shows linear dependence.
The fact that there are systematic changes, in both the mag-
nitudes and temperature dependence of
, with the particle
size d suggests that
is dominated by the size effect and
surface effects rather than any impurity.
To further examine the consequences of the size effect,
measurements of specific heat of bulk CdSe and quantum
dots with size d=2.8, 4.1, and 5.6 nm were carried out for
T=0.3–40 K. The temperature dependence of specific heat
for the bulk CdSe and quantum dots, plotted as C/T vs T
2
is
shown in Fig. 5. The heat capacity of the bulk is in good
agreement with earlier report.
23
The enhancement of specific
heat of quantum dots as the evolution of size is clearly re-
vealed. In general, the specific heat of a material can be
represented by the summation of contributions of conduction
electrons C
el
=
␥
T, lattice phonon C
ph
and magnetic correla-
tions C
mag
. The value of the Sommerfeld constant
␥
obtained
from the intercept of the linear fits gradually increases from
1.12 mJ/mole K
2
for the bulk to 5.50 mJ/mole K
2
for 2.8
nm with decreasing d. The relation of
␥
and the density of
states of conduction electrons N共
F
兲 can be represented by
␥
=
1
3
2
k
B
2
N共
F
兲, 共2兲
where k
B
is Boltzmann constant. The value of
␥
increases
with decreasing d and indicates an enhancement of density of
states of conduction electrons N共
F
兲 in quantum dots. The
value of
␥
is approximately linear proportional to 1/d, im-
plying the correlation of the density of states of conduction
electrons N共
F
兲 with the surface of quantum dot 共Fig. 4兲.
Since
f
is also proportional to N共
F
兲, the similar variations
of
␥
and
o
are understandable. It is noted that quantum dots
have an enormous surface-to-volume ratio; consequently, the
free charge from delocalized electrons of dangling bonds and
defects on surface will have more contribution to magnetic
susceptibility
o
and heat capacity
␥
as well. For quantum
dots, the lattice phonons C
ph
can be calculated by the theo-
retical model for a small particle represented by the follow-
ing equation:
24
C
ph
= V
m
兺
l,s
3共2l +1兲k
B
x
2
e
x
4
R
3
共e
x
−1兲
2
, with x =
បca
⬘
l,s
RT
. 共3兲
Here V
m
is the molar volume, R denotes the particle ra-
dius, a
⬘
l,s
,isthesth zero of the derivative of the lth spherical
Bessel function, and c is the effective sound velocity. The
number of atoms N
o
in quantum dots with d=2.8, 4.1, and
5.6 nm is estimated to be about 500, 1200, and 2200, respec-
tively. We use the constraint 兺
l
l max
共2l+1兲=N
o
and subtract
the contribution to the heat capacity from free charge carriers
C
el
=
␥
T. The remaining heat capacity yields C
ph
from which
c=795, 895, 915 m/s with Debye temperature ⍜ =61, 68, 70
K are obtained for d=2.8, 4.1, and 5.6 nm, respectively.
FIG. 4. The Sommerfield constant and
o
共mainly from the con-
tribution of free charge carrier兲 vs 1/d 共⬃surface/volume ratio兲,
the lines are linear fits.
FIG. 5. The specific heat, plotted as C/T vs T
2
for the bulk and
d=2.8-, 4.1-, and 5.6-nm quantum dots; the lines are linear fits.
SIZE-DEPENDENT PROPERTIES OF CdSe QUANTUM DOTS PHYSICAL REVIEW B 71, 201307共R兲共2005兲
RAPID COMMUNICATIONS
201307-3

Compared to ⍜ =139 K for bulk CdSe, ⍜ for the quantum
dots are really half, an anticipated result from lattice soften-
ing with decreasing d.
24
IV. CONCLUSION
The optical absorption spectra show a blueshift in the
CdSe quantum dot. X-ray diffraction confirmed that QDs
have the same wurtzite crystal structure as the bulk but with
smaller lattice constants. The low-temperature magnetic sus-
ceptibility studies reveal the increase of spin density of dan-
gling bonds with decreasing size. The magnetic susceptibility
o
and Sommerfeld constant
␥
increases linearly with
surface-to-volume ratio, giving the evidence of free charge
carriers on the surface of CdSe quantum dot. The systematic
changes in the magnitudes of
and
␥
with the size d suggest
the role of quantum size effect and surface effects rather than
any impurity in CdSe quantum dots.
ACKNOWLEDGMENT
This work was supported by the National Research Coun-
cil of the Republic of China under Grant No. NSC 93-2112-
M-001-022.
*
1
A. P. Alivisatos, Science 271, 933 共1996兲.
2
T. Vossmeyer, L. Katsikas, M. Giersig, I. G. Popovic, K. Diesner,
A. Chemseddine, A. Eychmüller, and H. Weller, J. Phys. Chem.
98, 7665 共1994兲.
3
A. A. Guzelian, U. Banin, A. V. Kadavanich, X. Peng, and A. P.
Alivisatos, Appl. Phys. Lett. 69, 1432 共1996兲.
4
R. P. Raffaelle, S. L. Castro, A. F. Hepp, and S. G. Bailey, Prog.
Photovoltaics 10, 433 共2002兲.
5
S. Coe, W. K. Woo, M. G. Bawendi, and V. Bulovic, Nature
共London兲 420, 800 共2002兲.
6
V. I. Klimov, A. A. Mikhailovsky, S. Xu, A. Malko, J. A. Holl-
ingsworth, C. A. Leatherdale, H. J. Eisler, and M. G. Bawendi,
Science 290, 314 共2000兲.
7
M. Bruchez, Jr., M. Moronne, P. Gin, S. Weiss, and A. P. Alivi-
satos, Science 281, 2013 共1998兲.
8
M. Nirmal, C. B. Murray, and M. G. Bawendi, Phys. Rev. B 50,
2293 共1994兲.
9
M. C. Beard, G. M. Turner, and C. A. Schmuttenmaer, Nano Lett.
2, 983 共2002兲.
10
M. Nirmal, C. B. Murray, D. J. Norris, and M. G. Bawendi, Z.
Phys. D: At., Mol. Clusters 26, 361 共1993兲.
11
C. B. Murray, D. J. Norris, and M. G. Bawendi, J. Am. Chem.
Soc. 115, 8706 共1993兲.
12
C. D. Dushkin, S. Saita, K. Yoshie, and Y. Yamaguchi, Adv. Col-
loid Interface Sci. 88,37共2000兲.
13
C. B. Murray, C. R. Kagan, and M. G. Bawendi, Annu. Rev.
Mater. Sci. 30, 545 共2000兲.
14
Y. Y. Chen, Y. D. Yao, S. S. Hsiao, S. U. Jen, B. T. Lin, H. M.
Lin, and C. Y. Tung, Phys. Rev. B 52, 9364 共1995兲.
15
D. J. Chadi, R. M. White, and W. A. Harrison, Phys. Rev. Lett.
35, 1372 共1975兲.
16
Y. V. Shaldin, I. Warchulska, M. K. Rabadanov, and V. K. Komar,
Semiconductors 38, 288 共2004兲.
17
J. van Wieringen, Philips Tech. Rev. 19, 301 共1957/1958兲.
18
Takanori Suzuki, V. Venkataramanan, and Masakazu Aono,
RIKEN Rev. 37,9共2001兲.
19
S. J. Hudgens, Phys. Rev. B 14, 1547 共1976兲.
20
P. Burgardt and M. S. Seehra, Solid State Commun. 22, 153
共1977兲.
21
S. J. Hudgens, Phys. Rev. B 7, 2481 共1973兲.
22
M. S. Seehra and S. S. Seehra, Phys. Rev. B 19, 6620 共1979兲.
23
A. Twardowski, H. J. M. Swagten, and W. J. M. de Jonge, Phys.
Rev. B 42, 2455 共1990兲.
24
G. H. Comsa, D. Heitkamp, and H. S. Räde, Solid State Commun.
24, 547 共1977兲.
NEELESHWAR et al. PHYSICAL REVIEW B 71, 201307共R兲共2005兲
RAPID COMMUNICATIONS
201307-4

