Physical Properties of Minerals
Color and Streak
Color is among the more obvious qualities of a mineral, yet the color of a mineral may vary
considerably depending on slight variations in chemical composition. Some chemical elements
can create strong color effects, even when they are present only as trace impurities. For
example, the mineral corundum is commonly white or grayish, but when small amounts of
chromium are present, corundum is deep red and given the gem name
ruby
. Similarly, when
small amounts of iron and titanium are present, corundum is deep blue, producing the gem
sapphire
.
Streak is the color of the fine powder of a mineral. Streak is observed by rubbing the mineral
across a piece of unglazed porcelain known as a streak plate. Many minerals leave a streak of
powder with a diagnostic color. Thus, streak is commonly more reliable than the color of the
mineral itself.
Luster
Luster is a property that describes the way light reflects from a fresh surface of the mineral.
Minerals that have the appearance of metals, regardless of color, are said to have a
metallic
luster
. Minerals with a
nonmetallic luster
are further described by various adjectives such as
glassy (vitreous), silky, pearly, milky, or earthy (dull).
Hardness
Hardness is the resistance of a mineral to scratching. The physical property of hardness is
determined by crystal structure and strength of the bonds between atoms. Generally, the
stronger the chemical bonds, the harder the mineral.
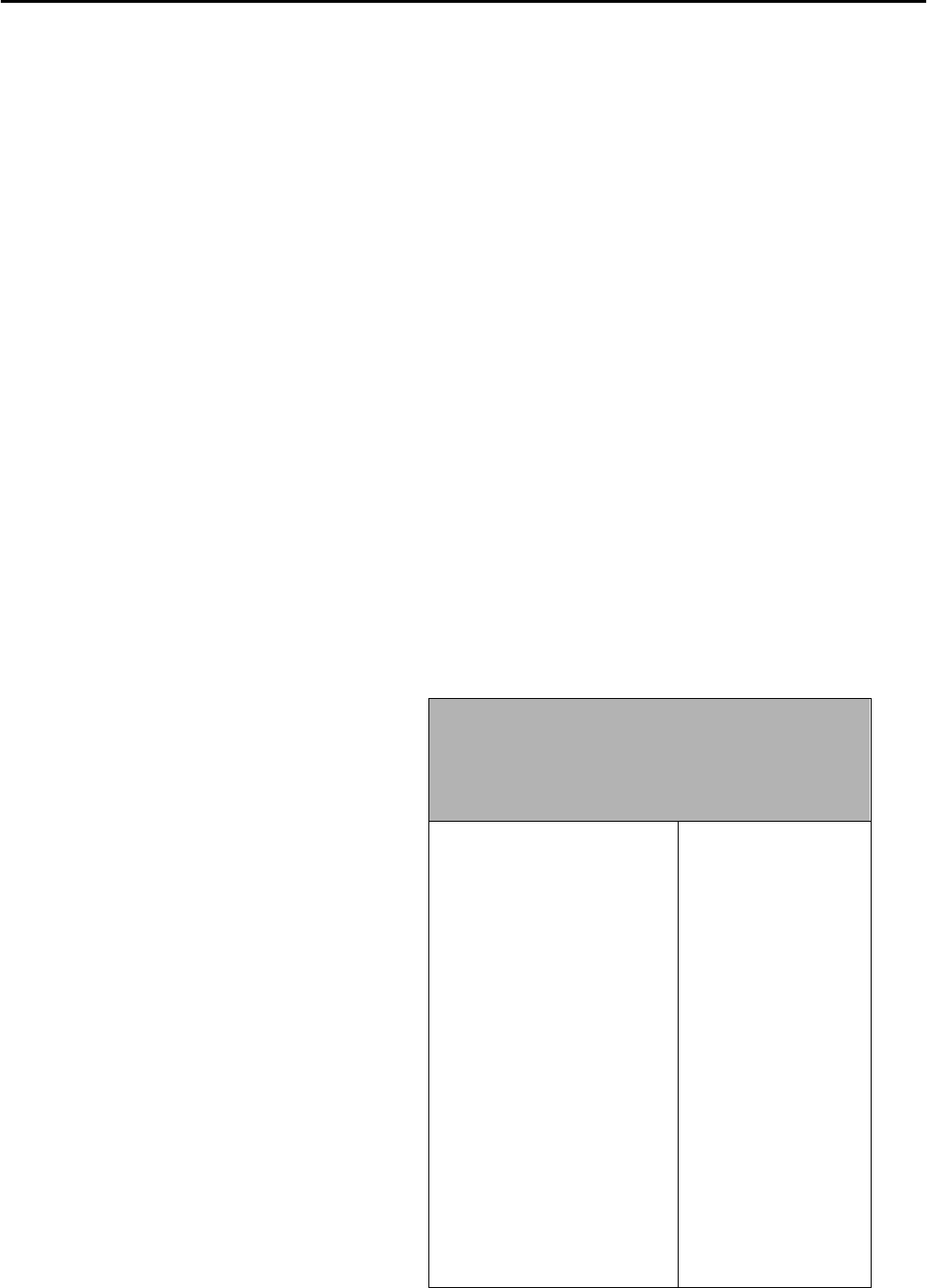
Minerals come in a wide range of
hardness. To compare them, geologists
use the Mohs Hardness Scale (Fig. 3.1
at right). On this
relative scale
, each
mineral is harder compared to all those
with lower numbers on the scale. For
example, 10 (diamond) is the hardest
and so will scratch every mineral listed
down to 1 (talc), which is the softest.
Using this information, a
range of
hardness
is determined. For example, a
mineral that can be scratched by quartz
but not by potassium feldspar has a
hardness range
between 6 and 7 on
Mohs.
Because minerals of the Mohs scale are
not always handy, it is useful to also
know the hardness of common objects
like a fingernail or knife blade (Fig. 3.1)
for comparison testing.
Figure 3.1 - MINERALS OF THE MOHS
HARDNESS SCALE
Minerals of Mohs
Hardness Scale
Mohs Hardness of
Common Objects
1. Talc
2. Gypsum
3. Calcite
4. Fluorite
5. Apatite
6. Potassium Feldspar
7. Quartz
8. Topaz
9. Corundum
10. Diamond
Fingernail (2.5)
Copper Penny (3.5)
Knife blade
Glass plate (5.5)
Steel file
Streak plate (6.5)

The more easily one mineral scratches another, the greater their difference in hardness. If two
minerals have same hardness, they will scratch each other but it will take some effort. A softer
mineral will not scratch a harder mineral, regardless of the amount of force you put into the
attempt. Take care that you are getting accurate test results. Sometimes it may appear that the
softer mineral has scratched the harder mineral because there is a ‘line’ left behind on the harder
mineral. Close inspection shows that the ‘line’ can be wiped away and there is no scratch under
it, similar to chalk on a chalkboard. In these cases, the line was left by the softer mineral
because the harder one was actually scratching it.
To perform a hardness test, first see if you can scratch the mineral with your fingernail. If you
can, the mineral is the same hardness or softer than your fingernail depending on how much
force is required. If you cannot, see if the mineral can scratch a copper penny, then a glass plate,
and so on up the scale. This will give you a narrow
range
of the hardness. For example, if it
scratches a penny but not a glass plate, the mineral has a
hardness range
of between 3 and 5. If
it scratches glass, the mineral is harder than 5.5. Remember that the easier it is to scratch the
glass, the greater the difference between the hardness of the mineral and the glass, but you
must record ONLY the results of your tests (e.g., you can record ≥5.5, >5.5 or >>5.5 based on
how easy it is to scratch the glass but NOT 8 or 9).
Cleavage and Fracture
Cleavage and fracture refer to the way minerals break. Cleavage is the tendency of some
minerals to break along plane of weakness in the mineral’s crystalline structure. If breaking a
mineral leaves behind relatively flat surfaces that give off flashes of reflected light when the hand
sample is rotated, the mineral has cleavage. These cleavage surfaces are the planes of
weakness. Some minerals, such as mica, have one set cleavage planes that are all parallel. Such
minerals will repeatedly break into smaller and smaller pieces along that one cleavage plane.
Others minerals have two, three, or even four cleavage planes. The quality of the cleavage for
each plane varies. Some minerals have excellent cleavage. For instance, you can peel sheet after
sheet from a mica crystal to see layer after layer of very reflective (shiny) planes. Others have
poor cleavage and only produce relatively flat surfaces with a dull shine. Minerals with no
cleavage are said to fracture.
Figure 3.2 on the next page shows the common cleavage patterns that cause minerals to break
along the planes of weakness, forming certain preferred shapes. Use this figure to help you
evaluate the cleavage of mineral samples. There are three observations you must make:
1. The number of different (non-parallel) cleavage planes.
2. The angle at which different planes intersect.
3. The quality of cleavage: excellent, perfect, good, fair, poor
Fracture occurs when a mineral breaks but not along cleavage planes. Many minerals fracture
because they have no planes of weak bonds in their atomic structure. In these cases, fractures
still can form characteristic shapes or patterns. For example,
conchoidal
fracture creates smooth
and curved surfaces, similar to a clam shell. Conchoidal fracture is commonly seen in the mineral
quartz. Some minerals break into splintery or fibrous fragments. Others fracture into irregular
shapes.
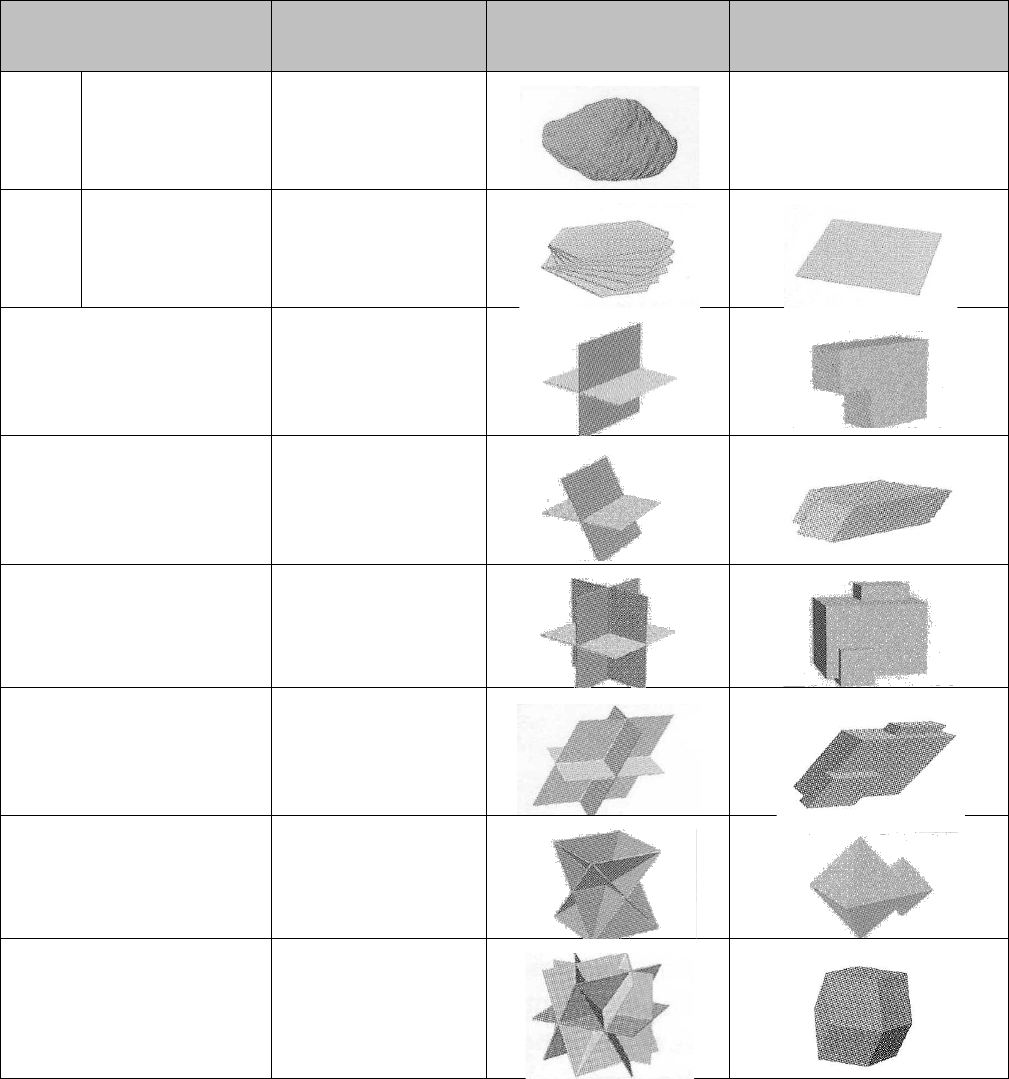
Figure 3.2
Common cleavage patterns of minerals. (From:
Laboratory Manual in Physical
Geology
, 4/E by Busch, © 1997. Reprinted by permission of Prentice-Hall,
Inc., Upper Saddle River, NJ.)
Number of Cleavage
Directions
Shape
Sketch of
Cleavage Planes
Directions of
cleavage
0
Fracture only,
No cleavage
1
Planar
Flat Sheets
2 at 90°
Elongated form
with rectangular
cross-section
2 not at 90°
Elongated form
with
parallelogram
cross-section
3 at 90°
Cube
3 not at 90°
Rhombohedron
4
Octahedron
6
Dodecahedron

Density
An important physical property of a mineral is how light or heavy it feels relative to the size of
the sample; its heft. The property that causes this observed difference is density, which is mass
per unit volume. Minerals with a high density, such as gold, have closely packed atoms. Minerals
with a low density, such as ice, have loosely packed atoms. The density of minerals is often
reported as specific gravity (S.G.), the density of a substance relative to that of an equal
volume of water. The most common silicate minerals have densities in the range of 2.5-3.0 g/cm
3
or 2.5-3 times that of water. Minerals made of metallic elements have higher densities. For
example, gold (Au) has a density of 19.3 g/cm
3
; galena (PbS) about 7.5 g/cm
3
, silver (Ag) about
10.5 g/cm
3
and copper (Cu) is 8.9 g/cm
3
. Density can be judged by holding (
hefting
) different
minerals of similar size and comparing their weights qualitatively. Heavier minerals have higher
than average densities, and will seem heavy for their size.
Magnetism
Most mineral are not magnetic at all, but iron-bearing minerals often exhibit the property of
magnetism. Some are strongly magnetic and some weakly magnetic. Magnetite is strongly
attracted to a magnet but ilmenite, and sometimes hematite, exhibits only a weak attraction.
To test for magnetism, use a magnet suspended on a string and slowly bring the magnet in the
vicinity of the mineral. You should be able to feel the suspended magnet’s attraction (or not) to
the mineral.
Reaction to Acid
Carbonate minerals (those containing the anion (CO
3
)
2-
) will effervesce (fizz / form bubbles)
when a drop of dilute hydrochloric acid (HCl) is applied to a freshly exposed surface. The fizzing
is the release of CO
2
gas, the same gas that is released when you pop the top of a soda bottle.
Some minerals, like calcite, react quickly and effervesce vigorously. Others, like dolomite,
effervesce slowly in dilute acid and the reaction is more easily seen only if the mineral is first
made into a powder (i.e., powder effervescence).
You can quickly perform an acid test by applying a small drop of dilute HCl to the mineral
surface. If you get no reaction (liquid stays clear) or a very slow reaction (liquid turns ‘cloudy’),
scratch the mineral surface with a wire probe to form a powder and then reapply the acid. If you
get a faster reaction, that is powdered effervescence and a diagnostic result for dolomite.
Otherwise, record that there is no reaction.
Please use a paper towel to wipe the mineral dry after your test, so that the next person doesn’t
get acid all over their hands!
