
PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 1
March 13, 2024
This checklist is intended as an aid to support clinical decision making for prescribers.
However, use of this checklist is not required to prescribe PAXLOVID.
Medical History
❑ Has mild to moderate COVID-19 (symptomatic SARS-CoV-2 infection not requiring
hospitalization).
❑ Age ≥ 18 years OR > 12 years of age and weighing at least 40 kg
❑ Has one or more risk factors for progression to severe COVID-19
1
❑ Not requiring hospitalization due to severe or critical COVID-19 at treatment initiation
❑ No known or suspected severe renal impairment (eGFR < 30 mL/min)
• Note that a dose reduction is required for patients with moderate renal impairment
(eGFR ≥30-<60 mL/min); see the Fact Sheet for Healthcare Providers.
• To assess renal function:
o Physicians, advanced practice registered nurses, and physician assistants who are licensed
or authorized under state law to prescribe drugs may rely on patient history and access to
the patient’s health records to make an assessment regarding the likelihood of renal
impairment. Providers may consider ordering a serum creatinine or calculating the
estimated glomerular filtration rate (eGFR) for certain patients after assessment on a
case-by-case basis based on history or exam.
o State-licensed pharmacists must have sufficient information available, such as through
access to health records less than 12 months old or consultation with a health care
provider in an established provider-patient relationship with the individual patient; see
the Fact Sheet for Healthcare Providers.
❑ No known or suspected severe hepatic impairment (Child-Pugh Class C)
• To assess hepatic impairment:
o Physicians, advanced practice registered nurses, and physician assistants who are licensed
or authorized under state law to prescribe drugs may rely on patient history and access to
the patient’s health records to make an assessment regarding the likelihood of hepatic
impairment.
o State-licensed pharmacists must have sufficient information available, such as through
access to health records less than 12 months old or consultation with a health care
provider in an established provider-patient relationship with the individual patient; see
the Fact Sheet for Healthcare Providers.
❑ No history of clinically significant hypersensitivity reactions [e.g., toxic epidermal
necrolysis (TEN) or Stevens-Johnson syndrome] to the active ingredients (nirmatrelvir or
ritonavir) or other components of the product
NOTES:
1
Determining whether a patient is at high risk for progression to severe COVID-19, including hospitalization or death, is
based on the provider’s assessment of the individual patient being considered for treatment of COVID-19 and that
patient’s medical history. For information on medical conditions and factors associated with increased risk for progression
to severe COVID-19, see the Centers for Disease Control and Prevention (CDC) website:
https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html

PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 2
March 13, 2024
Concomitant Medications
NOTE: The state-licensed pharmacist should refer an individual patient for clinical evaluation
(e.g., telehealth, in-person visit) with a physician, advanced practice registered nurse, or physician
assistant licensed or authorized under state law to prescribe drugs, if:
• Sufficient information is not available to assess for a potential drug interaction
• Modification of other medications is needed due to a potential drug interaction.
• PAXLOVID is not an appropriate therapeutic option based on the authorized Fact Sheet for
Healthcare Providers or due to potential drug interactions for which recommended
monitoring would not be feasible.
See the Fact Sheet for Healthcare Providers and USPI for the full Limitations of Authorized Use.
❑ HMG-CoA reductase inhibitors (statins)
• If the patient is taking lovastatin or simvastatin, which are contraindicated with
PAXLOVID coadministration, PAXLOVID can be given if the statin can be held 12
hours prior to the first dose of PAXLOVID treatment, held during the 5 days of
treatment, and restarted 5 days after completing PAXLOVID.
• If the patient is taking atorvastatin or rosuvastatin, consider temporary
discontinuation of atorvastatin and rosuvastatin during treatment with
PAXLOVID. Atorvastatin and rosuvastatin do not need to be held prior to or after
completing PAXLOVID.
❑ Hormonal contraceptives containing ethinyl estradiol: If the patient is taking a hormonal
contraceptive containing ethinyl estradiol, consider an additional non-hormonal method
of contraception during the 5 days of PAXLOVID treatment and until one menstrual
cycle after stopping PAXLOVID.
❑ Medications for HIV-1 Treatment: If the patient is taking medications for the treatment
of HIV-1 infection, with the exception of maraviroc
2
, HIV antiretroviral medications can
be co-administered with PAXLOVID without dose adjustment, but arranging follow-up
by the HIV care provider to monitor for side effects is recommended.
3
,
4
,
5
2
Please see the maraviroc prescribing information here:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022128Orig1s019,208984Orig1s002lbl.pdf
3
Exposure of certain HIV medications may be altered with PAXLOVID co-administration.
4
Patients on ritonavir- or cobicistat-containing HIV or HCV regimens should continue their treatment as indicated.
5
PAXLOVID use may lead to a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with
uncontrolled or undiagnosed HIV-1 infection.

PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 3
March 13, 2024
❑ Other Drugs with Established and Other Potentially Significant Drug Interactions with
PAXLOVID
❑ Patient is not taking any other medications
❑ Patient is not taking any of the medications listed below.
• In addition, the patient’s other medications have been checked for
contraindications, the need for dose adjustment, or increased monitoring due
to drug interactions with a strong CYP3A inhibitor such as ritonavir based on
appropriate resources such as the prescribing information of these
medications.
❑ Patient is NOT taking any of the medications listed in RED but is taking one or
more of the medications listed below in YELLOW, and dose adjustment, holding
of medication, or increased monitoring is planned (additional resources which
include instructions for managing specific drug interactions are included at the
end of this document).
• In addition, the patient’s other medications not listed below have been
checked for contraindications, the need for dose adjustment, or increased
monitoring due to drug interactions with a strong CYP3A inhibitor such as
ritonavir based on appropriate resources such as the prescribing information of
these medications.
NOTES:
PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 4
March 13, 2024
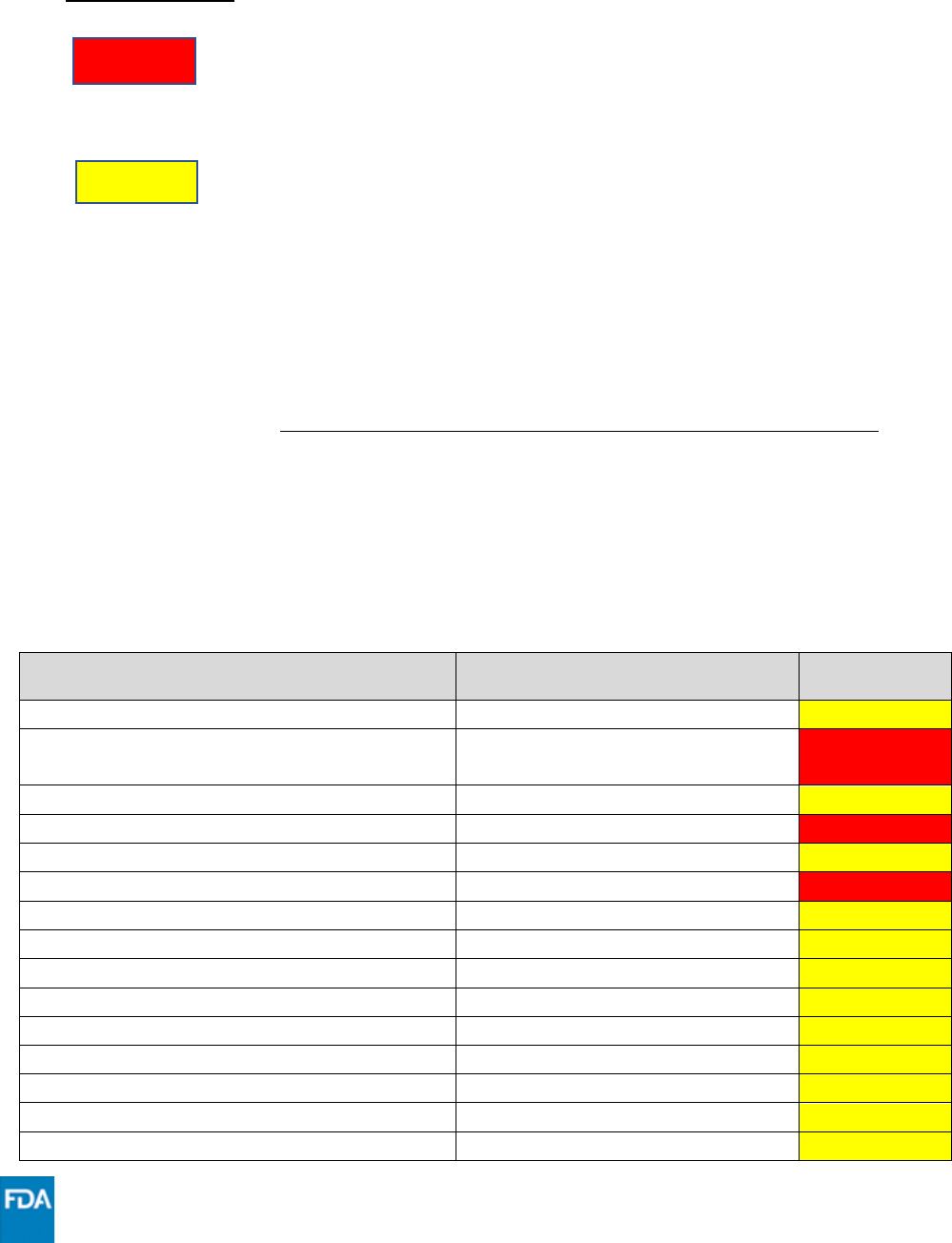
Other Drugs with Established and Other Potentially Significant Drug Interactions with PAXLOVID
(listed alphabetically by generic name)
Interaction Codes:
Coadministration of this drug with PAXLOVID is CONTRAINDICATED.
For further information, refer to the Fact Sheet for Healthcare
Providers and the individual Prescribing Information for the drug.
Coadministration of this drug with PAXLOVID should be avoided
and/or holding of this drug, dose adjustment of this drug, or special
monitoring is necessary. Consultation with the prescriber of the
potentially interacting drug is recommended. For further information,
refer to the Health Care Provider Fact Sheet, the USPI, and the
individual Prescribing Information for the drug.
The table below provides a listing of clinically significant drug interactions, including
contraindicated drugs, in addition to those listed under Concomitant Medications above
(HMG-CoA reductase inhibitors [statins], hormonal contraceptives containing ethinyl
estradiol, and medications for HIV-1 treatment). Drugs listed in this table are a guide and
are not considered a comprehensive list of all possible drugs that may interact with
PAXLOVID. The healthcare provider should consult other appropriate resources such as the
prescribing information for the interacting drug for comprehensive information on dosing
or monitoring with concomitant use of a strong CYP3A inhibitor such as ritonavir.
Drug
Drug Class
Interaction
Code
abemaciclib
Anticancer drug
***
alfuzosin
Alpha 1-adrenoreceptor
antagonist
XXX
aliskiren
Cardiovascular agent
***
amiodarone
Antiarrhythmic
XXX
amlodipine
Calcium channel blocker
***
apalutamide
Anticancer drug
XXX
apixaban
Anticoagulant
***
aripiprazole
Neuropsychiatric agent
***
avanafil
PDE5 inhibitor
***
bedaquiline
Antimycobacterial
***
betamethasone
Systemic corticosteroid
***
brexpiprazole
Neuropsychiatric agent
***
bosentan
Endothelin receptor antagonist
***
budesonide
Systemic corticosteroid
***
bupropion
Antidepressant
***
***
XXX
PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 5
March 13, 2024
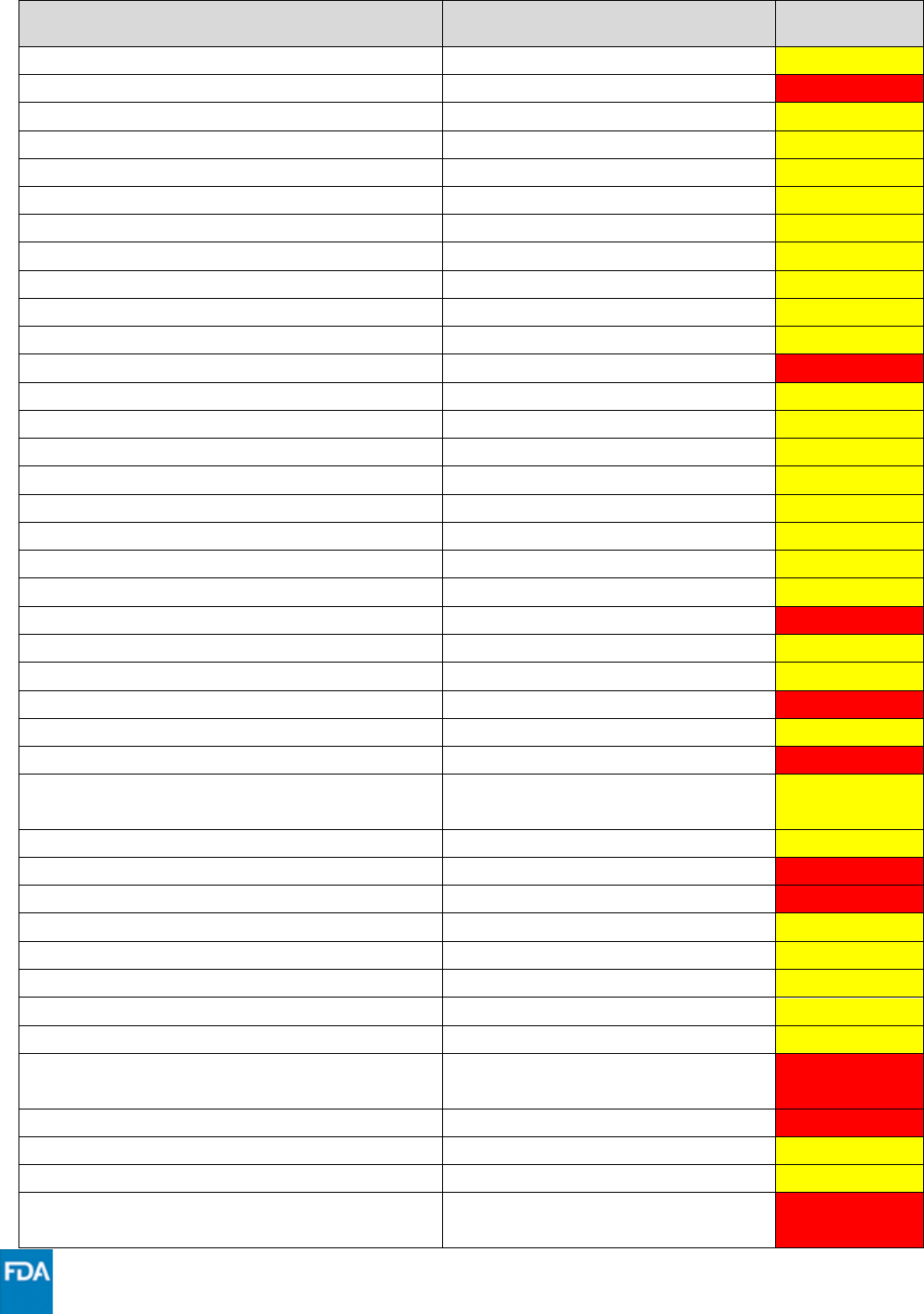
Drug
Drug Class
Interaction
Code
buspirone
Sedative/hypnotic
***
carbamazepine
Anticonvulsant
XXX
cariprazine
Neuropsychiatric agent
***
ceritinib
Anticancer drug
***
ciclesonide
Systemic corticosteroid
***
cilostazol
Cardiovascular agent
***
clarithromycin
Anti-infective
***
clonazepam
Anticonvulsant
***
clorazepate
Sedative/hypnotic
***
clopidogrel
Cardiovascular agent
***
clozapine
Antipsychotic
***
colchicine
Anti-gout
XXX
cyclosporine
Immunosuppressant
***
dabigatran
Anticoagulants
***
darifenacin
Muscarinic receptor antagonist
***
dasabuvir
Hepatitis C direct acting antiviral
***
dasatinib
Anticancer drug
***
dexamethasone
Systemic corticosteroid
***
diazepam
Sedative/hypnotic
***
digoxin
Cardiac glycoside
***
dihydroergotamine
Ergot derivative
XXX
diltiazem
Calcium channel blocker
***
disopyramide
Antiarrhythmic
***
dronedarone
Antiarrhythmic
XXX
elbasvir/grazoprevir
Hepatitis C direct acting antiviral
***
eletriptan
Migraine medication
XXX
elexacaftor/tezacaftor/ivacaftor
Cystic fibrosis transmembrane
conductance regulator potentiator
***
encorafenib
Anticancer drug
***
eplerenone
Cardiovascular agent
XXX
ergotamine
Ergot derivative
XXX
erythromycin
Anti-infective
***
estazolam
Sedative/hypnotic
***
everolimus
Immunosuppressant
***
felodipine
Calcium channel blocker
***
fentanyl
Narcotic analgesic
***
finerenone
Mineralocorticoid receptor
antagonist
XXX
flecainide
Antiarrhythmic
XXX
flurazepam
Sedative/hypnotic
***
fluticasone
Systemic corticosteroid
***
flibanserin
Serotonin receptor 1A agonist/
serotonin receptor 2A antagonist
XXX
PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 6
March 13, 2024
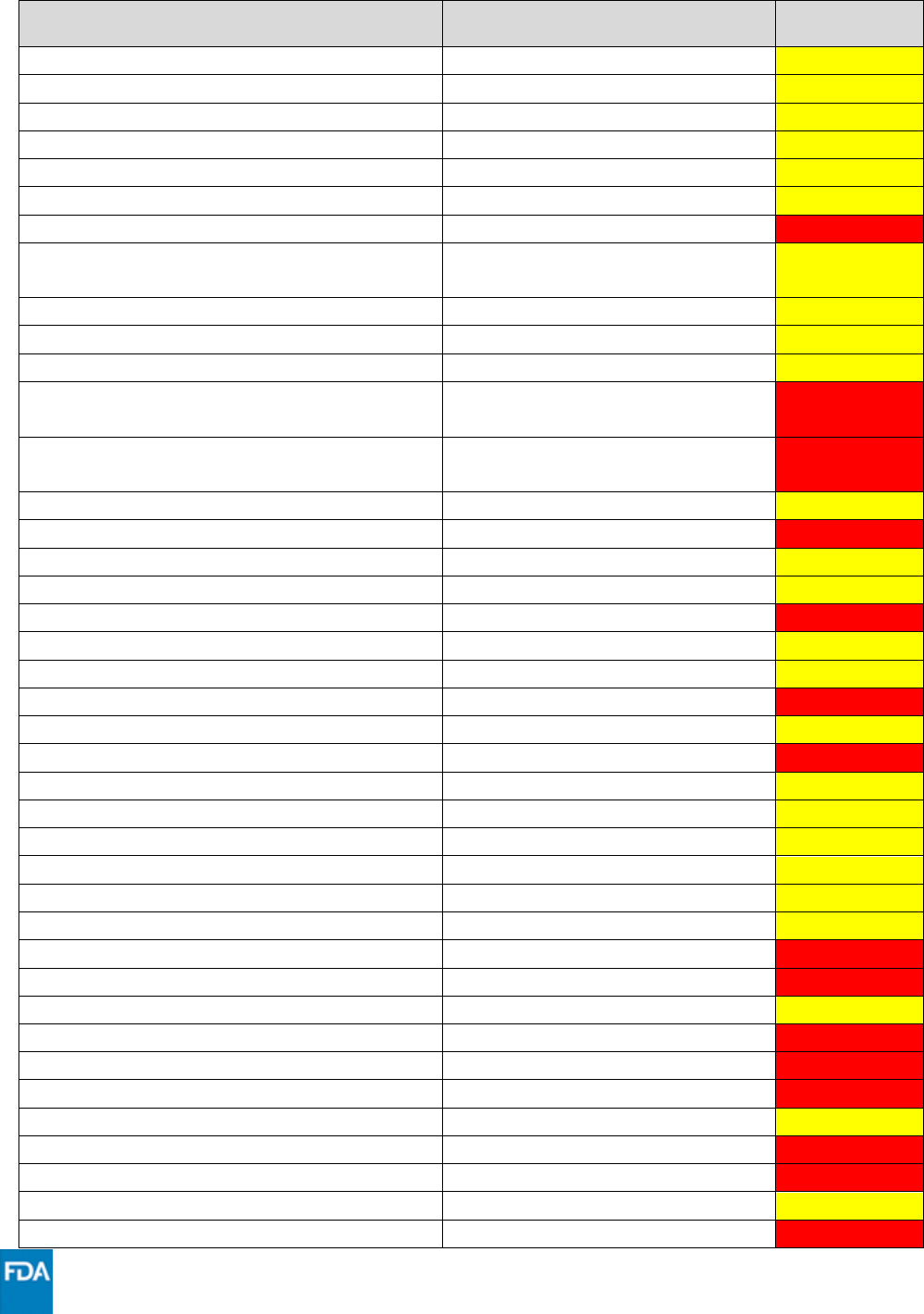
Drug
Drug Class
Interaction
Code
glecaprevir/pibrentasvir
Hepatitis C direct acting antiviral
***
hydrocodone
Narcotic analgesic
***
ibrutinib
Anticancer drug
***
iloperidone
Neuropsychiatric agent
***
isavuconazonium sulfate
Antifungal
***
itraconazole
Antifungal
***
ivabradine
Cardiovascular agent
XXX
ivacaftor
Cystic fibrosis transmembrane
conductance regulator potentiator
***
ivosidenib
Anticancer drug
***
ketoconazole
Antifungal
***
lidocaine (systemic)
Antiarrhythmic
***
lomitapide
Microsomal triglyceride transfer
protein (MTTP) inhibitor
XXX
lumacaftor/ivacaftor
Cystic fibrosis transmembrane
conductance regulator potentiator
XXX
lumateperone
Neuropsychiatric agent
***
lurasidone
Antipsychotic
XXX
meperidine
Narcotic analgesic
***
methadone
Narcotic analgesic
***
methylergonovine
Ergot derivative
XXX
methylprednisolone
Systemic corticosteroid
***
midazolam (administered parentally)
Sedative/hypnotic
***
midazolam (oral)
Sedative/hypnotic
XXX
mometasone
Systemic corticosteroid
***
naloxegol
Opioid antagonist
XXX
neratinib
Anticancer drug
***
nicardipine
Calcium channel blocker
***
nifedipine
Calcium channel blocker
***
nilotinib
Anticancer drug
***
ombitasvir/paritaprevir /ritonavir
Hepatitis C direct acting antiviral
***
oxycodone
Narcotic analgesic
***
phenobarbital
Anticonvulsant
XXX
phenytoin
Anticonvulsant
XXX
pimavanserin
Neuropsychiatric agent
***
pimozide
Antipsychotic
XXX
primidone
Anticonvulsant
XXX
propafenone
Antiarrhythmic
XXX
quetiapine
Antipsychotic
***
quinidine
Antiarrhythmic
XXX
ranolazine
Antianginal
XXX
rifabutin
Antimycobacterial
***
rifampin
Antimycobacterial
XXX
PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 7
March 13, 2024
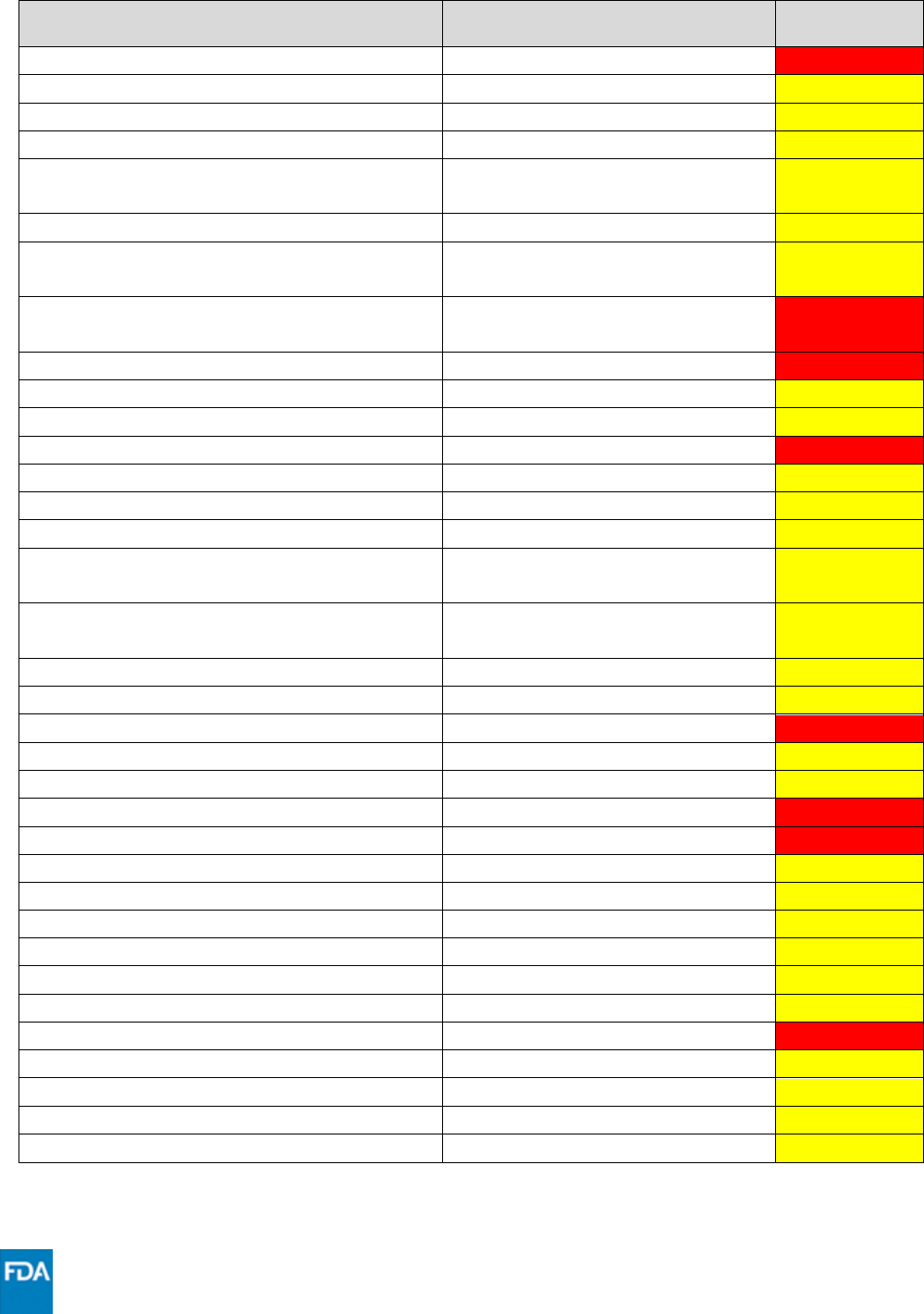
Drug
Drug Class
Interaction
Code
rifapentine
Antimycobacterial
XXX
rimegepant
Migraine medication
***
riociguat
sGC stimulator
***
rivaroxaban
Anticoagulant
***
salmeterol
Long-acting beta-adrenoceptor
agonist
***
saxagliptin
DPP4 inhibitor
***
Sildenafil (when used for erectile
dysfunction)
PDE5 inhibitor
***
sildenafil (Revatio®) when used for
pulmonary arterial hypertension
PDE5 inhibitor
XXX
silodosin
Benign prostatic hyperplasia agent
XXX
sirolimus
Immunosuppressant
***
sofosbuvir/velpatasvir/ voxilaprevir
Hepatitis C direct acting antiviral
***
St. John’s Wort (hypericum perforatum)
Herbal product
XXX
suvorexant
Neuropsychiatric agent
***
tacrolimus
Immunosuppressant
***
tadalafil
PDE5 inhibitor
***
tamsulosin
Alpha 1-adrenoreceptor
antagonist
***
tezacaftor/ivacaftor
Cystic fibrosis transmembrane
conductance regulator potentiator
***
ticagrelor
Cardiovascular agent
***
tofacitinib
JAK inhibitor
***
tolvaptan
Vasopressin receptor antagonist
XXX
trazodone
Antidepressant
***
triamcinolone
Systemic corticosteroid
***
triazolam
Sedative/hypnotic
XXX
ubrogepant
Migraine medication
XXX
upadacitinib
JAK inhibitor
***
vardenafil
PDE5 inhibitor
***
venetoclax
Anticancer drug
***
verapamil
Calcium channel blocker
***
vinblastine
Anticancer drug
***
vincristine
Anticancer drug
***
voclosporin
Immunosuppressant
XXX
vorapaxar
Cardiovascular agent
***
voriconazole
Antifungal
***
warfarin
Anticoagulant
***
zolpidem
Sedative/hypnotic
***

PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Page | 8
March 13, 2024
ADDITIONAL RESOURCES:
PAXLOVID - Fact Sheet for Healthcare Providers: https://www.fda.gov/drugs/emergency-
preparedness-drugs/emergency-use-authorizations-drugs-and-non-vaccine-biological-products
Prescribing Information (Label/Package Insert) for Individual Drugs and for PAXLOVID (Drugs@FDA):
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
University of Liverpool COVID-19 Drug Interactions:
https://www.covid19-druginteractions.org/checker
