
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Nov. 1996, p. 4195–4198 Vol. 62, No. 11
0099-2240/96/$04.0010
Copyright q 1996, American Society for Microbiology
Tellurite Resistance and Reduction by Obligately Aerobic
Photosynthetic Bacteria
VLADIMIR YURKOV,† JOCELYNE JAPPE
´
, AND ANDRE
´
VERME
´
GLIO*
De´partement d’E
´
cophysiologie Ve´ge´tale et Microbiologie, Laboratoire de BioEnerge´tique Cellulaire,
Centre d’Etudes Nucleaires de Cadarache, 13108 St. Paul lez Durance, France
Received 15 April 1996/Accepted 13 August 1996
Seven species of obligately aerobic photosynthetic bacteria of the genera Erythromicrobium, Erythrobacter,
and Roseococcus demonstrated high-level resistance to tellurite and accumulation of metallic tellurium crystals.
High-level resistance without tellurite reduction was observed for Roseococcus thiosulfatophilus and Erythromi-
crobium ezovicum grown with certain organic carbon sources, implying that tellurite reduction is not essential
to confer tellurite resistance.
Tellurium compounds can be found in high concentrations
in land and water near sites of waste discharge of industrial
manufacturing processes (5). Potassium tellurite (K
2
TeO
3
)is
toxic to many microorganisms at concentrations as low as 1
mg/ml (13, 14, 19). Intrinsic low-level resistance to TeO
3
22
has
been reported for a few gram-positive organisms (e.g., Coryne-
bacterium diphtheriae, Streptococcus faecalis, and some Staph-
ylococcus aureus strains), but little is known about the mecha-
nisms responsible for this resistance (14). Resistance to
tellurite of some gram-negative bacteria is associated with the
presence of plasmids and has been studied extensively (2, 5, 13,
16, 18–20).
A constitutive high-level resistance (HLR) to rare earth
oxides and oxyanions at concentrations approaching 1,000
mg/ml was recently described for photosynthetic purple non-
sulfur bacteria of the alpha subclass of Proteobacteria (9, 10).
Obligate aerobic anoxygenic phototrophs, a new physiological
group of the same subclass (4, 6, 12, 21, 28), produce bacteri-
ochlorophyll a but are unable to grow photosynthetically under
anaerobic conditions.
In this study we describe HLR to tellurite among freshwater
species of obligate aerobic phototrophs and their ability to
reduce TeO
3
22
to metallic Te in very large amounts.
Seven species, Erythrobacter litoralis T4, Roseococcus thio-
sulfatophilus RB3, Erythromicrobium ramosum E5 (28), Eryth-
romicrobium sibiricum RB16-17, Erythromicrobium ursincola
KR99, Erythromicrobium ezovicum E1, and Erythromicrobium
hydrolyticum E4(1) (23–27) (personal collection of Vladimir
Yurkov), have been studied.
Figure 1 shows examples of growth of Erythromicrobium
ezovicum (A), Erythromicrobium ramosum (B), Erythrobacter
litoralis (C) and Roseococcus thiosulfatophilus (D) supple-
mented with different K
2
TeO
3
concentrations in rich organic
(RO) medium (6, 28). With the exception of the highly tellu-
rite-sensitive Erythromicrobium ezovicum, for which no growth
occurred at 5.0 mgofK
2
TeO
3
per ml, six other species pre-
sented HLR (with MICs between 500 and 2,000 mgofK
2
TeO
3
per ml) in RO medium or minimal salts (MS) medium con-
taining yeast extract (3) (Table 1). However, these MICs de-
pend upon the carbon source. This is summarized in Table 1
for the different species studied, grown in MS medium in the
presence of trace elements (6), supplemented with different
TABLE 1. Reduction of K
2
TeO
3
by different species of obligate aerobic phototrophs depending on organic carbon source
C
source
a
Erythrobacter
litoralis
Erythromicrobium
hydrolyticum
Erythromicrobium
ursincola
Erythromicrobium
ramosum
Erythromicrobium
sibiricum
Roseococcus
thiosulfatophilus
Erythromicrobium
ezovicum
Grow-
th
b
Te
concn
c
MIC
d
Grow-
th
Te
concn
MIC
Grow-
th
Te
concn
MIC
Grow-
th
Te
concn
MIC
Grow-
th
Te
concn
MIC
Grow-
th
Te
concn
MIC
Grow-
th
Te
concn
MIC
RO 1 500 1,200 1 500 1,200 1 500 1,000 1 50 750 1 50 750 1 100 500 1 NR 5
Yeast
extract
1 250 1,200 1 500 2,000 1 500 2,000 1 250 1,500 1 500 1,200 1 250 1,000 1 NR 5
L-Glutamine 1 500 1,500 1 750 1,200 1 1,000 2,000 1 250 1,200 1 100 1,000 1 NR 1,200 1 1,000 2,000
Succinate 1 750 1,200 1 750 1,200 1 750 1,200 1 500 1,200 21NR 1,200 1 750 2,000
Malate 21750 1,200 1 500 1,200 1 250 1,200 21NR 1,200 1 500 1,200
Tartrate 26NR 5 26100 500 21NR 1,200 6 NR 5
Acetate 1 1,000 1,200 1 1,000 2,500 1 250 2,700 1 1,000 2,300 1 500 1,200 1 NR 1,200 1 NR 500
Ethanol 6 250 750 1 100 250 21250 1,000 221NR 5
a
The bacteria were grown on RO medium, MS medium with yeast extract, or MS medium with the indicated organic carbon source.
b
Possibility to grow without tellurite addition. Symbols: 1, good growth; 6, weak growth; 2, substrate cannot be utilized.
c
Concentration of tellurite (in micrograms per milliliter) with the highest amount of reduction observed (the tellurite reduction has been estimated by the level of
A
950
).
d
In micrograms per milliliter. NR, not reduced.
* Corresponding author. Phone: 33 04 42 25 46 30. Fax: 33 04 42 25
† Present address: Department of Microbiology and Immunology,
The University of British Columbia, Vancouver, British Columbia V6T
1Z3, Canada.
4195

4196
carbon sources (1 g/liter). The highest level of resistance to
tellurite (MICs between 2,300 and 2,700 mgofK
2
TeO
3
per ml)
was observed for cells of Erythromicrobium hydrolyticum, Eryth-
romicrobium ursincola,orErythromicrobium ramosum grown in
the presence of acetate. This is 2 to 3 times higher than the
highest MICs of tellurite (800 and 1,200 mg/ml) described by
Moore and Kaplan for Rhodobacter capsulatus and Rhodo-
bacter sphaeroides (9, 10).
In most cases, a black coloration appears during the growth
in the presence of tellurite (Fig. 1). This is due to the reduction
of tellurite in metallic tellurium with its intracellular accumu-
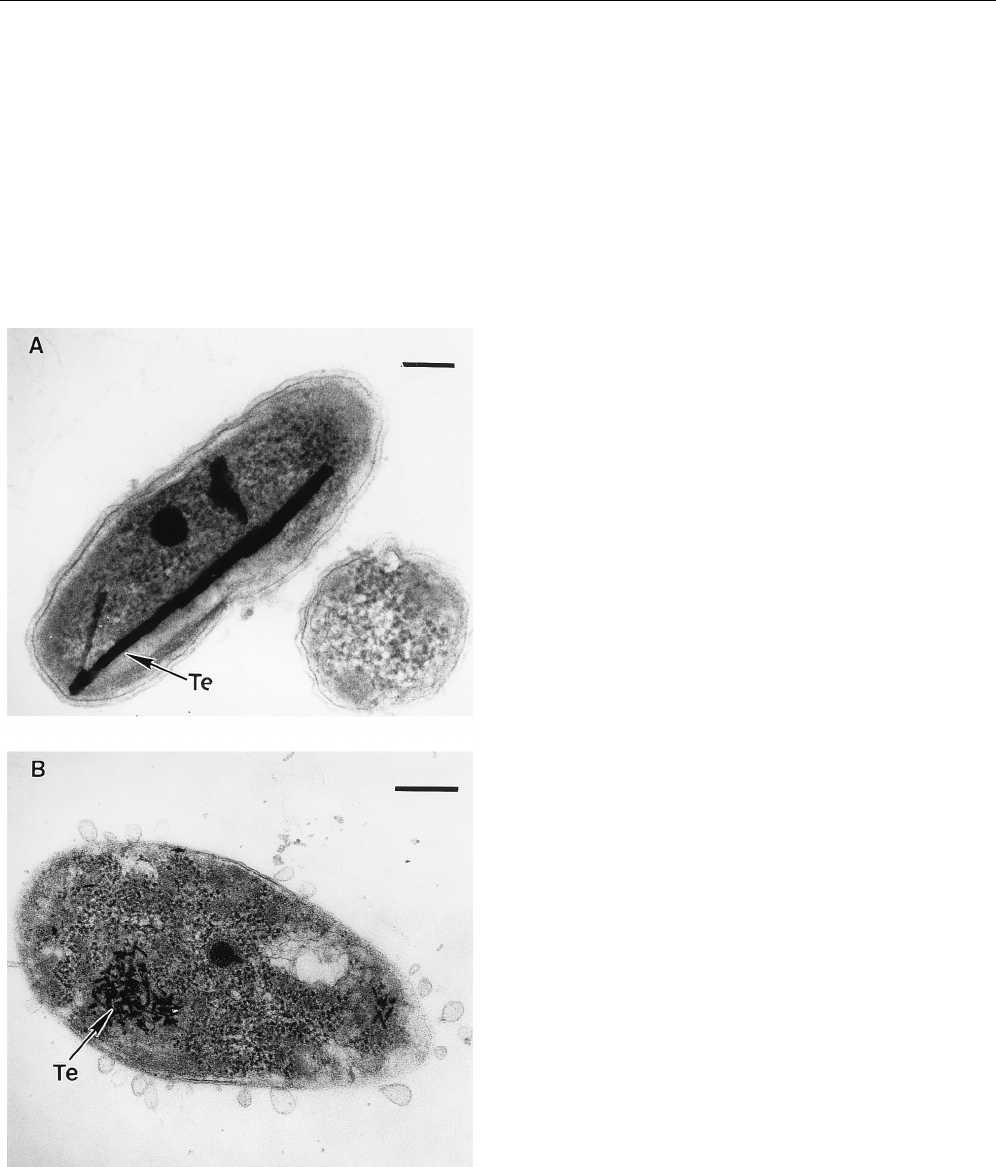
lation (8, 9, 17). Intracellular deposits appear as electron-dense
crystals in electron microscopy (Fig. 2) and have been shown to
consist of elemental tellurium in several strains of bacteria (1,
7–10, 15, 17). Excluding Roseococcus thiosulfatophilus grown in
the presence of
L-glutamine, succinate, malate, tartrate, or
acetate, and Erythromicrobium ezovicum grown with acetate,
all species examined in the present work were able to reduce
TeO
3
22
to elemental Te in combination with HLR to this
compound (Table 1 and Fig. 1). Most of the tellurite was
biotransformed to metallic Te in 24 h, with the exception of
Roseococcus thiosulfatophilus for which the tellurite reduction
occurred only after 72 to 96 h of growth. The amount of
reduced tellurite depended upon the species and the carbon
source (Table 1). Transmission electron microscopy pictures,
obtained as previously described (11) show very abundant and
large black crystals which sometimes occupied about 20 to 30%
of the cell volume (see, as an example, the case of Erythromi-
crobium ursincola in Fig. 2A). The size and total amount of the
Te crystals in the species studied in this study are usually
greater than those observed in Escherichia coli,orPseudomo-
nas or Rhodobacter species. The only exception was Roseococ-
cus thiosulfatophilus, which formed and accumulated relatively
small deposits (Fig. 2B).
The strong oxidant property of tellurite confers its toxic
character for microorganisms (13). In most cases analyzed in
this study, HLR to tellurite is correlated to its reduction into
Te. However, HLR without tellurite reduction was observed
for Roseococcus thiosulfatophilus grown with
L-glutamine, suc-
cinate, malate, tartrate, or acetate, and Erythromicrobium ezo-
vicum grown with acetate as the organic carbon source. These
results imply that tellurite reduction is not essential to confer
tellurite resistance and some other mechanisms, such as con-
tinuous tellurite efflux or tellurite complexing or methylation
could play an important role in the resistance character.
Previous work has demonstrated that the extent of tellurite
reduction in Rhodobacter sphaeroides was inversely related to
the oxidation state of the carbon source present in the growth
medium (9, 10). In addition to its detoxification effect, reduc-
tion of metal oxide could be a way to dispose of excess elec-
trons by the reoxidation of NADH, FADH
2
, or quinones and,
therefore, for maintenance of an optimal redox poise in vivo
(9, 10). A clear correlation between the tellurite reduction and
the oxidation state of the carbon source is not evident in the
case of obligate aerobic photosynthetic bacteria (Table 1) with
the exception of Roseococcus thiosulfatophilus. Indeed this spe-
cies can reduce Te(IV) to Te(0) in MS medium with yeast
extract and RO medium but not in MS medium containing
L-glutamine, succinate, malate, tartrate, or acetate as the sole
organic carbon source. These results are consistent with the
idea that during growth in RO medium Roseococcus thiosulfa-
tophilus cells presented with an excess of reducing power, and
electron carriers could be oxidized by giving electrons to tel-
lurite, thus equilibrating the redox poise in vivo.
Industrial activity has an enormous influence on the geo-
chemical migration processes of some toxic heavy metals by
their dispersion in water, soil, and atmosphere, or their con-
centration in specific areas. These processes contribute to se-
rious pollution problems (22). The reduction of soluble Te(IV)
to solid Te(0) could be an important mechanism for the re-
moval of this element from polluted places. In this context, the
FIG. 2. Intracellular localization of accumulated tellurium (A) Erythromicro-
bium ursincola; (B) Roseococcus thiosulfatophilus. Bar in panel A, 100 nm; bar in
panel B, 200 nm.
FIG. 1. Appearance of cultures of obligately aerobic photosynthetic bacteria growing in RO medium supplemented with K
2
TeO
3
. (A) Erythromicrobium ezovicum;
(B) Erythromicrobium ramosum; (C) Erythrobacter litoralis; (D) Roseococcus thiosulfatophilus. The amounts of K
2
TeO
3
(in micrograms per milliliter) in the flasks are
as follows: no tellurite addition (flasks 1), 1,000 (cell-free control) (flasks 2), 50 (flasks 3), 100 (flasks 4), 500 (flasks 5), 750 (flasks 6), and 1,000 (flasks 7). In control
experiments with 1,000 mg/ml K
2
TeO
3
added to growth medium in the absence of cells (flasks 2), no darkening was observed, indicating that tellurite reduction was
caused by bacterial activities and not by the direct chemical reaction with the compounds in the medium.
VOL. 62, 1996 NOTES 4197
development of microbiological methods for environmental
cleaning systems for tellurium oxides is of interest. Obligate
aerobic photosynthetic bacteria, being able to transform
Te(IV) to Te(0) in very high concentrations, are promising
candidates for such a process.
This work was supported by the Commissariat a` l’Energie Atom-
ique.
We thank Tom Beatty (UBC, Vancouver, British Columbia, Can-
ada) for suggestions. V. Yurkov thanks Natalie Yurkova and Ce´cile
Avaze´ri for helpful discussions.
REFERENCES
1. Bradley, D. E., K. K. Grewal, D. E. Taylor, and J. Whelan. 1988. Charac-
teristics of RP4 tellurite-resistance transposon Tn521. J. Gen. Microbiol.
134:2009–2018.
2. Bradley, D. E., and D. E. Taylor. 1987. Transposition from RP4 to other
replicons of a tellurite resistance determinant not normally expressed by Inc
Pa plasmids. FEMS Microbiol. Lett. 41:187–240.
3. Drews, G. 1983. Mikrobiologisches Praktikum, p. 11. Springer-Verlag, Berlin.
4. Fuerst, J. A., J. A. Hawkins, A. Holmes, L. I. Sly, C. I. Moore, and E.
Stackebrandt. 1993. Porphyrobacter neustonensis gen. nov., sp. nov., an aer-
obic bacteriochlorophyll-synthesizing budding bacterium from freshwater.
Int. J. Syst. Bacteriol. 43:125–134.
5. Jobling, M. G., and D. A. Ritchie. 1987. Genetic and physiological analysis of
plasmid genes expressing inducible resistance of tellurite in Escherichia coli.
Mol. Gen. Genet. 208:288–293.
6. Komagata, K. 1989. Taxonomy of facultative methylotrophs, p. 25–38. In K.
Harashima, T. Shiba, and N. Murata (ed.), Aerobic photosynthetic bacte-
ria—1989. Springer-Verlag, Berlin.
7. Lloyd-Jones, G., D. A. Ritchie, D. A. Ritchie, P. Strike, J. L. Hobman, N. L.
Brown, and D. A. Rouch. 1994. Accumulation and intracellular fate of tel-
lurite in tellurite-resistant Escherichia coli: a model for the mechanism of
resistance. FEMS Microbiol. Lett. 118:113–120.
8. Lloyd-Jones, G., D. A. Ritchie, and P. Strike. 1991. Biochemical and bio-
physical analysis of plasmid pMJ600-encoded tellurite (TeO
3
22
) resistance.
FEMS Microbiol. Lett. 81:19–24.
9. Moore, M. D., and S. Kaplan. 1992. Identification of intrinsic high-level
resistance to rare-earth oxides and oxyanions in members of the class Pro-
teobacteria: characterization of tellurite, selenite, and rhodium sesquioxide
reduction in Rhodobacter sphaeroides. J. Bacteriol. 174:1505–1514.
10. Moore, M. D., and S. Kaplan. 1994. Members of the family Rhodospirillaceae
reduce heavy-metal oxyanions to maintain redox poise during photosynthetic
growth. ASM News 60:17–24.
11. Sabaty, M., J. Jappe´, J. Olive, and A. Verme´glio. 1994. Organization of
electron transfer components in Rhodobacter sphaeroides forma sp.
denitrificans whole cells. Biochim. Biophys. Acta 1187:313–323.
12. Shimada, K. 1995. Aerobic anoxygenic phototrophs, p. 105–122. In R. E.
Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosyn-
thetic bacteria—1995. Kluwer Academic Publishers, Dordrecht, The Neth-
erlands.
13. Summers, A. O., and G. A. Jacoby. 1977. Plasmid-determined resistance to
tellurium compounds. J. Bacteriol. 129:276–281.
14. Summers, A. O., and S. Silver. 1978. Microbial transformation of metals.
Annu. Rev. Microbiol. 32:637–672.
15. Suzina, N. E., V. I. Duda, L. A. Anisimova, V. V. Dmitriev, and A. M.
Borovin. 1995. Cytological aspects of resistance to potassium tellurite con-
firmed on Pseudomonas cells by plasmids. Arch. Microbiol. 163:282–285.
16. Taylor, D. E., and D. E. Bradley. 1987. Location on RP4 of a tellurite
resistance determinant not normally expressed in IncPa plasmids. Antimi-
crob. Agents Chemother. 31:823–825.
17. Taylor, D. E., E. G. Walter, R. Sherburne, and D. P. Bazett-Jones. 1988.
Structure and location of tellurium deposited in Escherichia coli cells har-
boring tellurite resistance plasmids. J. Ultrastruct. Mol. Struct. Res. 99:18–
26.
18. Walter, E. G., and D. E. Taylor. 1989. Comparison of tellurite resistance
determinants from the IncPa plasmid RP4Te
r
and the IncHII plasmid
pHH1508a. J. Bacteriol. 171:2160–2165.
19. Walter, E. G., and D. E. Taylor. 1992. Plasmid-mediated resistance to tellu-
rite: expressed and cryptic. Plasmid 27:52–64.
20. Walter, E. G., C. M. Thomas, J. P. Ibbotson, and D. E. Taylor. 1991.
Transcriptional analysis, translational analysis, and sequence of the kilA-
tellurite resistance region of plasmid RK2Te
r
. J. Bacteriol. 173:1111–1119.
21. Woese, C. R., E. Stackebrandt, W. G. Weisburg, B. J. Paster, M. T. Madigan,
V. J. Fowler, C. M. Hanh, P. Blanz, R. Gupta, K. H. Nealson, and G. E. Fox.
1984. The phylogeny of purple bacteria. The alpha subdivision. Syst. Appl.
Microbiol. 5:315–326.
22. Wood, J. M. 1974. Biological cycles for toxic elements in the environment.
Science 4129:1049–1052.
23. Yurkov, V., and V. M. Gorlenko. 1990. Erythrobacter sibiricus sp. nov., a new
freshwater aerobic bacterial species containing bacteriochlorophyll a. Micro-
biology (New York) 59:85–89.
24. Yurkov, V., and V. M. Gorlenko. 1992. A new genus of freshwater aerobic
bacteriochlorophyll a-containing bacteria, Roseococcus gen. nov. Microbiol-
ogy (New York) 60:628–632.
25. Yurkov, V., and V. M. Gorlenko. 1993. New species of aerobic bacteria from
the genus Erythromicrobium containing bacteriochlorophyll a. Microbiology
(New York) 61:163–168.
26. Yurkov, V., V. M. Gorlenko, and E. I. Kompantseva. 1992. A new type of
freshwater aerobic orange-colored bacterium, Erythromicrobium gen. nov.,
containing bacteriochlorophyll a. Microbiology (New York) 61:169–172.
27. Yurkov, V., A. M. Lysenko, and V. M. Gorlenko. 1991. Hybridization analysis
of the classification of bacteriochlorophyll a-containing freshwater aerobic
bacteria. Microbiology (New York) 60:362–366.
28. Yurkov, V., E. Stackebrandt, A. Holmes, J. A. Fuerst, P. Hugenholtz, J.
Golecki, N. Gad*on, V. M. Gorlenko, E. I. Kompantseva, and G. Drews. 1994.
Phylogenetic positions of novel aerobic bacteriochlorophyll a-containing
bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov.,
Erythromicrobium ramosum gen. nov., sp. nov., and Erythrobacter litoralis sp.
nov. Int. J. Syst. Bacteriol. 44:427–434.
4198 NOTES APPL.ENVIRON.MICROBIOL.
