
FORMS VERSION H SERIES
Released:August 5
th
, 2023
RESEARCH INSTRUCTIONS FOR NIH
AND OTHER PHS AGENCIES
SF424 (R&R) APPLICATION PACKAGES
Guidance developed and maintained by NIH for preparing and
submitting applications via Grants.gov to NIH and other PHS
agencies using the SF424 (R&R)

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
TABLE OF CONTENTS
TABLE OF CONTENTS
TABLE OF CONTENTS 2
R.100 - How to Use the Application Instructions 3
R.110 - Application Process 6
R.120 - Significant Changes 10
R.130 - Program Overview 13
R.200 - SF 424 (R&R) Form 16
R.210 - PHS 398 Cover Page Supplement Form 30
R.220 - R&R Other Project Information Form 35
R.230 - Project/Performance Site Location(s) Form 44
R.240 - R&RSenior/Key Person Profile (Expanded) Form 48
R.300 - R&R Budget Form 58
R.310 - R&R Subaward Budget Attachment(s) Form 73
R.320 - PHS 398 Modular Budget Form 76
R.400 - PHS 398 Research Plan Form 82
R.500 - PHS Human Subjects and Clinical Trials Information 98
R.600 - PHS Assignment Request Form 135
Form Screenshots i
R - 2

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.100 - How to Use the Application Instructions
R.100 - How to Use the Application
Instructions
Use these application instructions to fill out the forms that are posted in your funding
opportunity announcement.
View the How to Apply Video Tutorials.
Quick Links
Step 1. Become familiar with the application process
Step 2. Use these instructions, together with the forms and information in the funding oppor-
tunity announcement, to complete your application
Step 3. Choose an application instruction format
Step 4. Complete the appropriate forms
Step 5. Stay informed of policy changes and updates
Step 6. Understand what data NIH makes public
Helpful Links
The information on the following pages may be useful in the application process
l
NIH Grants & Funding Glossary
l
Grants Policy Statement
l
NIH Guide to Grants and Contracts
l
Frequently Asked Questions
Step 1. Become familiar with the application process.
Understanding the application process is critical to successfully submitting your application.
Use the R.110 - Application Process section of these instructions to learn the importance of
completing required registrations before submission, how to submit and track your application,
where to find page limits and formatting requirements, and more information about the
application process.
R - 3
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.100 - How to Use the Application Instructions
Step 2. Use these instructions, together with the forms and information
found in the funding opportunity announcement, to complete your
application.
The funding opportunity announcement (FOA)will include specific instructions and the forms
needed for your application submission.
Remember that the FOA instructions always supersede these application instructions.
Step 3. Choose an application instruction format.
Do you know your activity code, but don’t know which application instructions to use? Refer to
NIH’s table on Determine the Correct Application Instructions for Your Activity Code to identify the
set of application instructions applies to your grant program.
Comprehensive Instructions Program-Specific Instructions
Use the General (G) instructions, available in
both
HTML
and
PDF
format, to complete
the application forms for any type of grant
program.
Take advantage of the filtered PDFs to view
specific application instructions for:
l
Research (R)
l
Career Development (K)
l
Training (T)
l
Fellowship (F)
l
Multi-project (M)
l
SBIR/STTR (B)
Step 4. Complete the appropriate forms.
Unless otherwise specified in the FOA, follow the standard instruction, as well as any additional
program-specific instructions for each form in your application.
Program-specific instructions are presented in gray call-out boxes that are color coded throughout
the application instructions. Consult the R.130 - Program Overview section for context for program
specific instructions.
IMPORTANT: Do Not Include Personal Identifiable Information (PII) Or Protected
Health Information (PHI) In the Application
Sensitive PII (e.g., Social Security Number, personal financial information, Alien Registration
Number) and PHI (e.g., personal medical conditions) require strict handling due to the increased
risk to an individual if the data is compromised. Documents containing sensitive PII or PHI must
not be included in the application.
R - 4
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.100 - How to Use the Application Instructions
NIH GRANTS ONLY: Additional information on this topic can be found in this FAQ or by
contacting your IC Privacy Coordinator.
Step 5. Stay informed of policy changes and updates.
l
Refer to the R.120 - Significant Changes section for the most recent changes to these
application instructions.
l
Review Notices of NIH Policy Changes since the posting of the Application Guide.
Step 6. Understand what data NIH makes public.
Information submitted as part of the application will be used by reviewers to evaluate the scientific
merit of the application and by NIH staff to make the grant award and monitor the grant after
award. The exception to this is the R.600 - PHS Assignment Request Form, which is only seen by
staff in the Division of Receipt and Referral (DRR), Center for Scientific Review (CSR).
If the application is funded, the following fields will be made available to the public through the
NIH Research Portfolio Online Reporting Tool (RePORTER) and will become public information:
l
Name of Project Director/Principal Investigator (PD/PI), to also include Project Leaders on sub-
projects to multi-project projects
l
PD/PI title
l
PD/PI email address
l
Organizational name
l
Institutional address
l
Project summary/abstract
l
Public health relevance statement
In addition, key elements related to ongoing funded projects will be made available to the public,
including those listed in the data dictionary at ExPORTER. Additional elements may be made
available after announcements through the NIH Guide for Grants and Contracts, a weekly
electronic publication that is available on NIH’s Funding page, or additions to the NIH Grants
Policy Statement, as needed.
R - 5

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.110 - Application Process
R.110 - Application Process
Understanding the application process is critical to successfully submitting your application.
Use this section of this guide to learn the importance of completing required registrations
before submission; how to submit and track your application; where to find information about
page limits, formatting requirements, due dates, and submission policies; and more
information about the application process. This application process information is also
available on our How to Apply – Application Guide page.
Quick Links
Prepare to Apply and Register
Write Application
Submit
Related Resources
Prepare to Apply and Register
Systems and Roles
Learn about the main systems involved in application submission and the role you and your
colleagues play in the submission process. The main systems are Grants.gov, eRA Commons, and
ASSIST.
Register
Determine your registration status. Organizations, organizational representatives, investigators,
and others need to register in multiple federal systems in order to for you to submit a grant
application. Registration can take six weeks or more to complete. Start today! See NIH’s
Registration website.
Understand Funding Opportunities
Identify the right funding opportunity announcement (FOA) for your research and learn about key
information you will find in the FOA.
Types of Applications
Are you submitting a new, renewal, revision, or resubmission application? Learn about the
different types of applications and special submission requirements.
Submission Options
Determine which system is most convenient for your application submission: NIH’s ASSIST web-
based application submission system, Grants.gov Workspace, or, if applicable, your organization’s
own submission system.
R - 6
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.110 - Application Process
Obtain Software
Applicants must have the free Adobe Reader software, a PDF generator, and a web browser to
submit an application. Learn which versions are compatible with our systems.
Write Application
Write Your Application
Read tips for developing a strong application that helps reviewers evaluate its science and merit.
Develop Your Budget
Learn about the kinds of costs you may include in your budget submission, the difference between
modular and detailed budgets, and more about how to develop your budget.
Format Attachments
Follow these requirements for preparing the documents you attach to your application.
Requirements include criteria for the PDF files, fonts, margins, headers and footers, paper size,
citations, formatting pages, use of hyperlinks and URLs, etc.
Rules for Text Fields
Learn the rules for form text fields – allowable characters, cutting and pasting, character limits, and
formatting.
Page Limits
Follow the page limits specified in this table for your specific grant program, unless otherwise
specified in the FOA.
Data Tables
Find instructions, blank data tables, and samples to use with institutional research training
applications.
Reference Letters
Some types of programs, such as fellowships and some career development awards, require the
submission of reference letters by the referee. Learn about selecting a referee and find instructions
for submission.
Biosketches
Biosketches are required in both competing applications and progress reports. Find instructions,
blank format pages, limitation on use of hyperlinks and URLs, and sample biosketches.
Submit
Submit, Track and View
Learn how to submit your application, and about your responsibility for tracking your application
and viewing the application image in the eRA Commons before the application deadline. If you
can’t view your application in eRA Commons, we can’t review it.
R - 7

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.110 - Application Process
How We Check for Completeness
Your application will be checked at Grants.gov, by eRA systems, and by federal staff before it is
referred for review.
Changed/Corrected Applications
You will need to submit a changed/corrected application to correct issues that either you or our
systems find with your application. Learn how and when you may submit a changed/corrected
application.
Related Resources
Due Dates and Policies
Due Dates
View standard due dates for competing applications. The FOA will identify whether to follow
standard due dates or whether to follow an alternative due date.
Submission Policies
Learn the nuances of application submission policies, including when late applications might be
allowed, what to do if due dates fall on a weekend or holiday, whether we allow post-submission
materials, how to document system issues, the rules around resubmission applications, etc.
Dealing with System Issues
Are you experiencing system issues with ASSIST, Grants.gov, System for Award Management
(SAM), or the eRA Commons that you believe threaten your ability to submit on time? NIH will not
penalize applicants who experience confirmed issues with federal systems that are beyond their
control. You must report the problem before the submission deadline.
After Submission
Receipt and Referral
Understand how and when applications are given an application identification number and
assigned to a review group and an NIH Institute or Center (IC) for possible funding.
Peer Review
Learn about our two phase peer review process, including initial peer review, Council review,
review criteria, scoring, and summary statements.
Pre-award Process
Learn what happens between peer review and award for applications that have been deemed
highly meritorious in the scientific peer review process. Be ready: if you received a great score in
peer review, you’ll have to submit Just-in-Time information.
Post award Monitoring andReporting
If you receive a grant from the NIH, you will need a lot of information to be a successful steward of
federal funds. This page provides a brief overview of grantee monitoring and reporting
requirements.
R - 8
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.110 - Application Process
Resources
Annotated Form Sets
These handy documents are a great visual resource for understanding many of the validation
checks we will run against your submitted application.
Contacting NIH Staff
NIH staff is here to help. We strongly encourage NIH applicants and grantees to communicate
with us throughout the grant life cycle. Understanding the roles of NIH staff can help you contact
the right person at each phase of the application and award process.
Contacting Staff at Other PHS Agencies
Applicants are strongly encouraged to communicate with agency staff throughout the entire
application review and awards process.
Systems
ASSIST
eRA Commons
Grants.gov
Information Collection
Authorization
The PHS Act establishes the authority with which NIH and other PHS agencies award grants and
collect information related to grant awards.
Paperwork Burden
The paperwork burden provides the estimated time for completing a grant application.
Collection of Personal Demographic Data
NIH collects personal data through the eRA Commons Personal Profile. The data is confidential
and is maintained under the Privacy Act record system.
R - 9
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.120 - Significant Changes
R.120 - Significant Changes
The Application Instructions are updated and released 2-3 times per year as needed.
Additionally, minor revisions may be made outside of these releases.
This section details all significant changes and revisions made to the instructions since the last
major release.
Within the instructions, new instructions will be marked with this symbol.
In the web version, use your mouse to hover over the icon to read an explanation of the
change.
In a PDF version, this symbol will be visible but will not display hover text. For more
information, see the explanation in the Significant Changes section below.
Release Notes - August 5, 2023
Program Overview - Small Business Innovation Research (SBIR) and Small Business Technology
Transfer (STTR)
Added new “Disclosure Requirements Regarding Ties to Foreign Countries” and “Denial of
Awards” subsections outlining requirements related to the implementation of NIH SBIR and STTR
Foreign Disclosure Pre-award Requirements and the HHS Due Diligence Program, applicable for
competing applications submitted for due dates on or after September 5, 2023.
R&R Other Project Information Form
Updated Additional Instructions for SBIR/STTR for the content of 10. Facilities & Other Resources
for applications submitted for due dates on or after September 5, 2023.
R&R Budget and associated R&R Subaward Budget Attachment(s) Form
Updated special instructions for applicants submitting a Data Management and Sharing (DMS)
Plan within the following sections for applications submitted for due dates on or after October 5,
2023:
Under “Who should use the R&R Budget Form?” “Additional instructions for Multi-Project”
B. Other Personnel. Other Direct Costs “8-17 Other”
L. Budget Justification
PHS 398 Modular Budget Form
Updated instructions for the “Additional Narrative Justification” Budget Justification attachment
content and special instructions for applicants submitting Data Management and Sharing (DMS)
Plans.
R - 10
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.120 - Significant Changes
Release Notes - October 25, 2022
How to Use the Application Instructions
l
Added reminder to not include personal identifiable information (PII) or protected health
information (PHI) in the application.
SF-424 Research and Related (R&R)Form Changes
R&RBudget and associated R&RSubaward Budget Attachment(s) Form
l
Added special instructions for applicants submitting a Data Management and Sharing
(DMS)Plan within the following sections:
l
Under “Who should use the R&R Budget Form?” “Additional instructions for Multi-
Project”
l
B. Other Personnel. Other Direct Costs “8-17 Other”
l
L. Budget Justification
Forms-H Changes
FORMS-H application packages incorporate the latest versions of the PHS forms managed by
NIH (OMB Number: 0925-0001 and 0925-0770, Expiration Date: 01/31/2026).
PHS 398 Modular Budget Form
l
Added special instructions for applicants submitting Data Management and Sharing (DMS)
Plans.
PHS 398 Research Plan Form
l
Added new item 11. Other Plan(s) and added instructions for applicants submitting Data
Management and Sharing (DMS) Plans.
l
Renumbered form fields.
l
Updated instructions for section 10, Resource Sharing Plan(s) to remove instructions for the
Data Sharing Plan and Genomic Data Sharing (GDS). The DMS Plan will now be attached in the
new item 11, Other Plan(s).
l
Clarified instructions for renewal or resubmission applications involving changes between
single PD/PI to or from multiple PD/PIs.
PHS 398 Career Development Award Supplemental Form
l
Added new item 17. Other Plan(s) and added instructions for applicants submitting Data
Management and Sharing (DMS) Plans.
l
Renumbered form fields.
R - 11
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.120 - Significant Changes
l
Updated instructions for 16. Resource Sharing Plan(s) to remove instructions for the Data
Sharing Plan and Genomic Data Sharing (GDS). The DMS Plan will now be attached in the new
item 17. Other Plan(s).
PHS 398 Research Training Program Plan Form
l
Added new item 13. Other Plan(s) for potential future use with other plans.
l
Renumbered form fields.
l
Clarified instructions for renewal or resubmission applications involving changes between
single PD/PI to or from multiple PD/PIs.
PHS Fellowship Supplemental Form
l
Added new item 17. Other Plan(s) for potential future use with other plans.
l
Renumbered form fields.
l
Updated instructions for 16. Resource Sharing Plan(s) to remove instructions for the Data
Sharing Plan and Genomic Data Sharing (GDS).
Form Screenshots
l
Updated form screenshots.
R - 12

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.130 - Program Overview
R.130 - Program Overview
Quick Links
Research and Other ("R" Series).
Research and Other ("R" Series)
The purpose of research and other awards is to provide support for health-related research and
development based on the mission of the NIH. Some examples of support include pilot studies;
conferences and scientific meetings; small research projects; institutional training and director
program projects; resource programs; and new, exploratory, and developmental research projects.
Awards may be in the form of grants or cooperative agreements.
Additional Instructions for Research:
Additional research instructions will be denoted by a gray call-out box with yellow
color coding and with the heading “Additional Instructions for Research” throughout
these application instructions.
Before Applying:
1. Become familiar with Activity Code: Applicants should become familiar with the
activity code for which support is being requested. These include many “R” activity codes,
as well as some “DP,” “G," “S,” and “U” activity codes. A comprehensive list of all activity
codes, with their descriptions, is available on NIH’s Activity Codes Search Results website.
2. Refer to your specific FOA: Refer to your FOA for specific information associated with
the award mechanism, including the eligibility requirements, review criteria, award
provisions, any special application instructions, and names of individuals who may be
contacted for additional or clarifying information prior to application submission.
3. Contact Awarding Component: Applicants are encouraged to consult with the NIH
Scientific/Research contact of the appropriate awarding component prior to submitting
an application, as eligibility criteria, support levels, and availability of awards may vary
among NIH Institutes or Centers and other PHS agencies.
The following chart provides a summary of the existing research programs; however, the chart is
not a comprehensive list of activity codes. Since this information is subject to change, prospective
applicants are encouraged to review NIH’s Types of Grant Programs for the most current program
information.
R - 13

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.130 - Program Overview
Summary of Research Award Programs*
Activity
Code
Program Description
R01 Research Project
R03 NIHSmall Grant Program
R13 Conference
R15 Research Enhancement Awards
R21 NIH Exploratory / Developmental Research Grant Award
R25 Education Projects
R41 Small Business Technology Transfer (STTR) Grants - Phase I
R42 Small Business Technology Transfer (STTR) Grants - Phase II
R43 Small Business Innovation Research (SBIR) Grants - Phase I
R44 Small Business Innovation Research (SBIR) Grants - Phase II
U01 Research Project – Cooperative Agreements
U13 Conference - Cooperative Agreements
G07 Resources Improvement Grant
S10 Biomedical Research Support Shared Instrumentation Grants
DP1 NIHDirector's Pioneer Award (NDPA)
*This is not a comprehensive list of activity codes.
Disclosure Requirements Regarding Ties to Foreign Countries
Effective for competing applications submitted on or after September 5, 2023, applicants will be
required to disclose all funded and unfunded relationships with foreign countries, using the
Required Disclosures of Foreign Affiliations or Relationships to Foreign Countries form, (referred to
hereafter as the SBIR STTR Foreign Disclosure Form) for all owners and covered individuals.
Upon request, applicants will submit the completed SBIR STTR Foreign Disclosure Form via the
Just-In-Time (JIT) process described in the NIH GPS section 2.5.1 Just-in-Time Procedures. The
SBIR STTR Foreign Disclosure Form and any additional agency-specific information must be
submitted electronically using the Just-in-Time feature in the eRA Commons. Applicants must
continue to comply with NIH Other Support disclosure requirements as provided in Section 2.5.1.
SBC applicants applying to CDC and FDA will follow each agency’s policies for submitting
additional documents during the pre-award process. Applicants may be required to provide
similar information on the SBIR STTR Foreign Disclosure Form that is also submitted as a part of
the other support reporting for senior/key personnel identified in the application. Applicants that
do not submit the completed SBIR STTR Foreign Disclosure Form during the JIT process will not be
considered for funding.
R - 14

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.130 - Program Overview
Denial of Awards
Applicants are encouraged to consider whether their entity’s relationships with foreign countries
of concern will pose a security risk. Prior to issuing an award, NIH, CDC, and FDA will determine
whether the SBC submitting the application:
l
has an owner or covered individual that is party to a malign foreign talent recruitment
program;
l
has a business entity, parent company, or subsidiary located in the People’s Republic of China
or another foreign country of concern; or
l
has an owner or covered individual that has a foreign affiliation with a research institution
located in the People’s Republic of China or another foreign country of concern.
A finding of foreign involvement with countries of concern will not necessarily disqualify an
applicant. NIH, CDC, and FDA will provide SBC applicants the opportunity to address any identified
security risks prior to award. Final award determinations will be based on whether the applicant’s
involvement falls within any of the following risk criteria, per the Act:
• interfere with the capacity for activities supported by NIH, CDC, or FDA to be carried out;
• create duplication with activities supported by NIH, CDC, or FDA;
• present concerns about conflicts of interest;
• were not appropriately disclosed to NIH, CDC, or FDA;
• violate Federal law or terms and conditions of NIH, CDC, or FDA; or
• pose a risk to national security.
NIH, CDC, and FDA will not issue an award under the SBIR/STTR program if the covered
relationship with a foreign country of concern identified in this guidance is determined to fall
under any of the criteria provided above, and the risk cannot be resolved.
R - 15
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
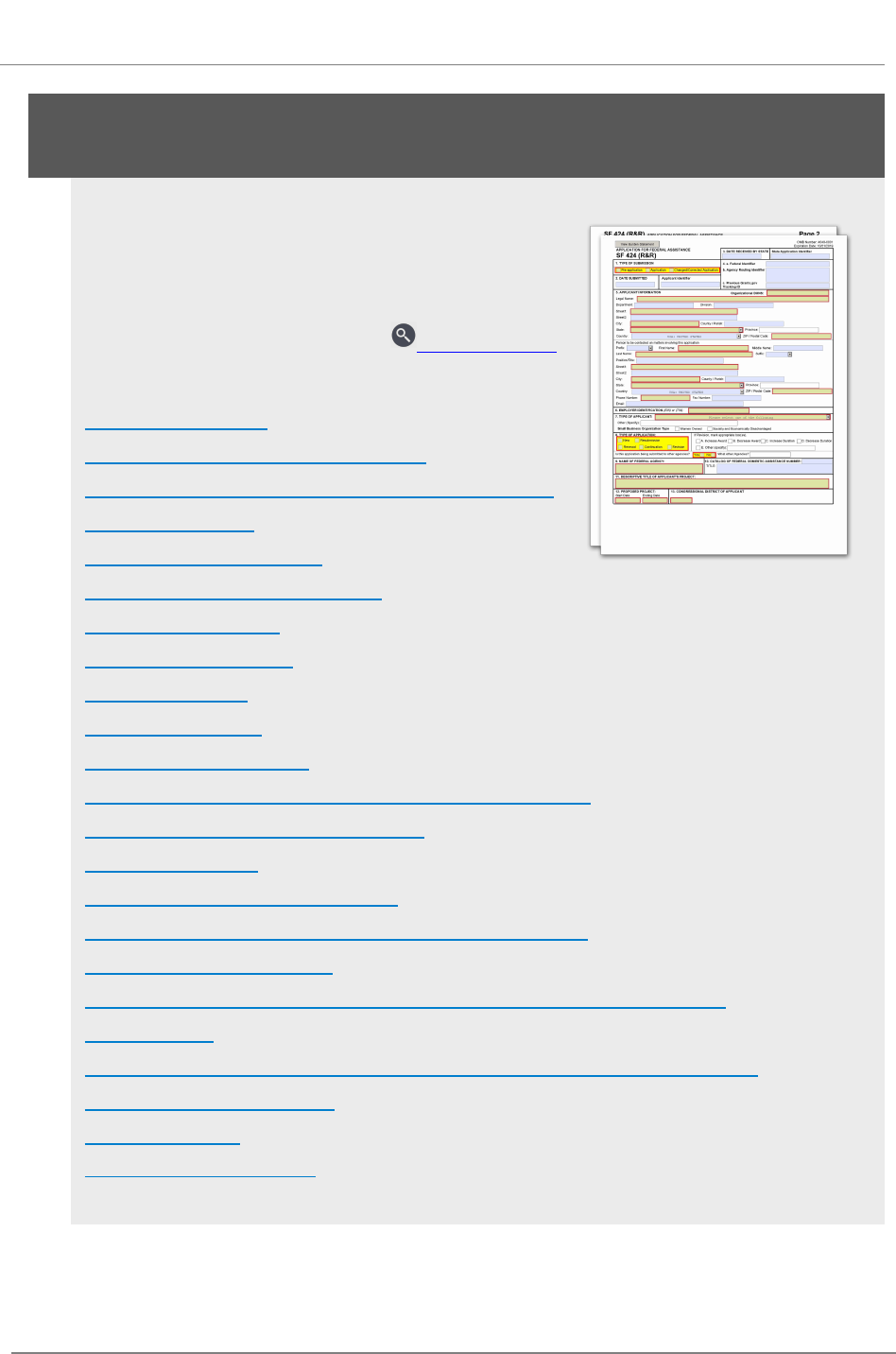
R.200 - SF 424 (R&R) Form
R.200 - SF 424 (R&R) Form
The SF 424 (R&R) Form is used in all grant applications.
This form collects information including type of
submission, applicant information, type of applicant, and
proposed project dates.
View larger image
Quick Links
1. Type of Submission
2. Date Submitted and Applicant Identifier
3. Date Received by State and State Application Identifier
4a. Federal Identifier
4b. Agency Routing Identifier
4c. Previous Grants.gov Tracking ID
5. Applicant Information
6. Employer Identification
7. Type of Applicant
8. Type of Application
9. Name of Federal Agency
10. Catalog of Federal Domestic Assistance Number and Title
11. Descriptive Title of Applicant's Project
12. Proposed Project
13. Congressional District of Applicant
14. Project Director/Principal Investigator Contact Information
15. Estimated Project Funding
16. Is Application Subject to Review by State Executive Order 12372 Process?
17. Certification
18. SFLLL (Disclosure of Lobbying Activities) or Other Explanatory Documentation
19. Authorized Representative
20. Pre-application
21. Cover Letter Attachment
R - 16

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
1. Type of Submission
This field is required. Check one of the "Type of Submission" boxes:
Pre-application:
The pre-application option is not used by NIH or other PHS agencies unless specifically noted in a
funding opportunity announcement (FOA).
Application:
An "Application" is a request for financial support of a project or activity submitted on specified
forms and in accordance with NIH instructions. (See NIH Types of Applications for an explanation
of the types of applications).
Changed/Corrected Application:
Check this box if you are correcting either system validation errors or application assembly
problems that occurred during the submission process. Changed/corrected applications must be
submitted before the application due date.
When you submit a changed/corrected application, follow these guidelines:
l
After submission of an application, there is a two-day application viewing window. Prior to the
due date, you may submit a changed/corrected application. Submitting a changed/corrected
application will replace the previous submission and remove the previous submission from
consideration.
l
If you check the “Changed/Corrected Application” box, then "Field 4.c Previous Grants.gov
Tracking ID” is required.
l
Do not use the “Changed/Corrected Application” box to denote a resubmission application.
Resubmission applications will be indicated in “Field 8. Type of Application.” See NIH Glossary
for the definition of Resubmission.
2. Date Submitted and Applicant Identifier
The “Date Submitted” field will auto-populate upon application submission.
Fill in the “Applicant Identifier” field, if applicable. The Applicant Identifier is reserved for applicant
use, not the federal agency to which the application is being submitted.
3. Date Received by State and State Application Identifier
Skip the “Date Received by State” and “State Application Identifier” fields.
4.a. Federal Identifier
New Applications without Pre-application: Leave this field blank.
New Applications following Pre-application: Enter the agency-assigned pre-application
number.
R - 17

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
Resubmission, Renewal, and Revision Applications: The Federal Identifier is required. Include
only the IC and serial number of the previously assigned application / award number (e.g., use
CA987654 from 1R01CA987654-01A1).
4.b. Agency Routing Identifier
Skip the “Agency Routing Identifier” field unless otherwise specified in the FOA or notice in the
NIH Guide for Grants & Contracts.
Applications in response to a NIH Notice of Special Interest require the notice number (e.g., NOT-
IC-FY-XXX) to be entered into this field in order to assign and track applications and awards for the
described initiative.
4.c. Previous Grants.gov Tracking ID
The “Previous Grants.gov Tracking ID” field is required if you checked the “Changed/Corrected
Application” box in “Field 1. Type of Submission.” A Tracking ID number is of the form, for
example, GRANT12345678.
5. Applicant Information
The "Applicant Information" fields reflect information for the applicant organization, not a specific
individual.
Unique Entity Identifier (UEI):
This field is required.
Enter the UEI of the applicant organization.
This UEI must match the number entered in the eRA Commons Institutional Profile (IPF) for the
applicant organization. The applicant’s Authorized Organization Representative (AOR) is
encouraged to confirm that a UEI has been entered into the eRA Commons IPF prior to application
submission. The same UEI should be used in the eRA Commons IPF, Grants.gov, System for Award
Management (SAM) registration, and in the UEI field in the application.
If your organization does not already have a UEI, you will need to go to the System for Award
Management (SAM.gov) to register and obtain a UEI.
Legal Name:
Enter the legal name of the organization.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level
within the organization.
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level
within the organization.
Street1:
This field is required. Enter the first line of the street address for the applicant organization.
R - 18
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
Street2:
Enter the second line of the street address for the applicant organization.
City:
This field is required. Enter the city for the address of the applicant organization.
County/Parish:
Enter the county/parish for the address of the applicant organization.
State:
This field is required if the applicant organization is located in the United States or its territories.
Enter the state or territory where the applicant organization is located.
Province:
If “Country” is Canada, enter the province of the applicant organization; otherwise, skip the
“Province” field.
Country:
This field is required. Select the country for the address of the applicant organization.
ZIP/Postal Code:
The ZIP+4 is required if the applicant organization is located in the United States. Otherwise, the
postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the applicant
organization.
Person to be contacted on matters involving this application
This information is for the administrative contact (e.g., AOR or business official), not the PD/PI. This
person is the individual to be notified if additional information is needed and/or if an award is
made.
Prefix:
Enter or select the prefix, if applicable, for the name of the person to contact on matters related to
this application.
First Name:
This field is required. Enter the first (given) name of the person to contact on matters related to this
application.
Middle Name:
Enter the middle name of the person to contact on matters related to this application.
Last Name:
This field is required. Enter the last (family) name of the person to contact on matters related to
this application.
Suffix:
Enter or select the suffix, if applicable, for the name of the person to contact on matters related to
this application.
Position/Title:
Enter the position/title for the person to contact on matters related to this application.
R - 19

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
Street1:
This field is required. Enter the first line of the street address for the person to contact on matters
related to this application.
Street2:
Enter the second line of the street address for the person to contact on matters related to this
application.
City:
This field is required. Enter the city for the address of the person to contact on matters related to
this application.
County/Parish:
Enter the county/parish for the address of the person to contact on matters related to this
application.
State:
This field is required if the person to contact on matters related to this application is located in the
United States or its Territories. Enter the state or territory where the person to contact on matters
related to this application is located.
Province:
If “Country” is Canada, enter the province for the person to contact on matters related to this
application; otherwise, skip the “Province” field.
Country:
Select the country for the address of the person to contact on matters related to this application.
ZIP/Postal Code:
The ZIP+4 is required if the person to contact on matters related to this application is in the United
States. Otherwise, the postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal
code of the person to contact on matters related to this application.
Phone Number:
This field is required. Enter the daytime phone number for the person to contact on matters
related to this application.
Fax Number:
Enter the fax number for the person to contact on matters related to this application.
E-mail:
Enter the e-mail address for the person to contact on matters related to this application. Only one
e-mail address is allowed, but it may be a distribution list.
6. Employer Identification
This field is required.
Enter either the organization’s Taxpayer Identification Number (TIN) or Employer Identification
Number (EIN) as assigned by the Internal Revenue Service. If your organization is not in the United
States, enter 44-4444444. Your EIN may be 12 digits (e.g., Payment Management System (PMS)
Entity Identification Number), and if this is the case, enter all 12 digits.
R - 20

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
7. Type of Applicant
This field is required.
In the first field under “7. Type of Applicant,” enter the appropriate applicant type. If your applicant
type is not specified (e.g., for eligible Agencies of the Federal Government), select “X: Other
(specify),” and indicate the name (e.g., the appropriate federal agency) in the space below.
Other (Specify):
Complete only if “X. Other (specify)” is selected as the "Type of Applicant."
Women Owned:
Do not use the "Women Owned" checkbox.
Socially and Economically Disadvantaged:
Do not use the "Socially and Economically Disadvantaged" checkbox.
Note: NIH, CDC, and FDA use the Business Type information provided in the System for Award
Management entity record for the applicant organization, rather than the “Woman Owned” and
“Socially and Economically Disadvantaged” checkboxes, to determine the small business
organization type. For more information, see the NIH Guide Notice on Small Business Organization
Type Information Pulled from System for Award Management Record Rather than Grant
Application Form.
8. Type of Application
This field is required.
Select the type of application. Check only one application type. Use the following list of existing
definitions to determine what application type you have. For more information, see NIH Types of
Applications.
l
New. Check this option when submitting an application for the first time or in accordance
with other submission policies. See the NIH Grants Policy Statement, Section 2.3.7.4:
Submission of Resubmission Application.
l
Resubmission. Check this option when submitting a revised (altered or corrected) or
amended application. See also the NIH Application Submission Policies. If your
application is both a “New/Revision/Renewal” and a “Resubmission,” check only the
“Resubmission” box.
l
Renewal. Check this option if you are requesting additional funding for a period
subsequent to that provided by a current award. A renewal application competes with all
other applications and must be developed as fully as if the applicant were applying for the
first time.
l
Continuation. The box for “Continuation” is used only for specific FOAs.
l
Revision. Check this option for competing revisions and non-competing administrative
supplements. For more information on competing revisions, see NIH Competing
Revisions. For more information on administrative supplements, see NIH Administrative
Supplements.
R - 21

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
If Revision, mark appropriate box(es).
You may select more than one.
A. Increase Award
B. Decrease Award
C. Increase Duration
D. Decrease Duration
E. Other (specify)
If “E. Other (specify)” is selected, specify in the space provided.
The boxes for options B, C, D, and E will generally not be used and should not be selected unless
specifically addressed in a particular FOA.
Is this application being submitted to other agencies? What Other Agencies?
In the field “Is this application being submitted to other agencies?” check “Yes” if one or more of
the specific aims submitted in your application is also contained in a similar, identical, or essentially
identical application submitted to another federal agency.
Otherwise, check "No."
If you checked "Yes," indicate the agency or agencies to which the application has been submitted.
9. Name of Federal Agency
The “Name of Federal Agency” field is pre-populated from the opportunity package and reflects
the agency from which assistance is being requested with this application.
10. Catalog of Federal Domestic Assistance Number and Title
This field is pre-populated from the opportunity package and reflects the Catalog of Federal
Domestic Assistance (CFDA) number of the program under which assistance is requested.
This field may be blank if you are applying to an opportunity that references multiple CFDA
numbers. When this field is blank, leave it blank. The appropriate CFDA number will be
automatically assigned by the agency once the application is assigned to the appropriate
awarding component.
11. Descriptive Title of Applicant’s Project
This field is required.
Enter a brief descriptive title of the project.
The descriptive title is limited to 200 characters, including spaces and punctuation.
New Applications: You must have a title different than any other NIH or other PHS Agency
project submitted for the same application due date with the same Project Director/Principal
Investigator (PD/PI).
Resubmission or Renewal Applications: You should normally have the same title as the previous
grant or application; however, if the specific aims of the project have significantly changed, choose
a new title.
R - 22
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
Revision Applications: You must have the same title as the currently funded grant.
12. Proposed Project
Start Date:
This field is required. Enter the proposed start date of the project. The start date is an estimate, and
is typically at least nine months after application submission. The project period should not exceed
what is allowed in the FOA.
Ending Date:
This field is required. Enter the proposed ending date of the project.
13. Congressional District of Applicant
Enter the Congressional District as follows: a 2-character state abbreviation, a hyphen, and a 3-
character district number. Examples: CA-005 for California’s 5th district, VA-008 for Virginia’s 8th
district.
If outside the United States, enter 00-000.
For States and U.S. Territories with only a single congressional district, enter “001” for the district
number.
For jurisdictions with no representative, enter “099.”
For jurisdictions with a nonvoting delegate, enter “098” for the district number. Example: DC-098
or PR-098.
If you do not know your Congressional District: Go to The United States House of
Representatives website and search for your Congressional District by entering your ZIP+4. If you
do not know your ZIP+4, look it up on the USPS Look Up Zip Code website.
14. Project Director/Principal Investigator Contact Information
This information is for the PD/PI. The PD/PI is the individual responsible for the overall scientific
and technical direction of the project.
In the eRA Commons profile, the person listed here in “14. Project Director/Principal Investigator
Contact Information” must be affiliated with the applicant organization entered in “5. Applicant
Information.” If you are proposing research at an institute other than the one you are currently at,
do not create a separate Commons account with the proposed applicant organization. For
additional information on creating affiliations for users in the eRA Commons, see eRA Account
Management System's Online Help.
If submitting an application reflecting multiple PD/PIs, the individual listed here as the Contact
PD/PI in “14. Project Director/Principal Investigator Contact Information” will be the first PD/PI
listed in R.240 - R&RSenior/Key Person Profile (Expanded) Form.
See R.240 - R&R Senior/Key Person Profile (Expanded) Form for additional instructions for multiple
PD/PIs. To avoid potential errors and delays in processing, ensure that the information provided in
this section is identical to the PD/PI profile information contained in the eRA Commons.
Prefix:
Enter or select the prefix, if applicable, for the name of the PD/PI.
R - 23

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
First Name:
This field is required. Enter the first (given) name of the PD/PI.
Middle Name:
Enter the middle name of the PD/PI.
Last Name:
This field is required. Enter the last (family) name of the PD/PI.
Suffix:
Enter or select the suffix, if applicable, for the PD/PI. Do not use this field to record degrees (e.g.,
Ph.D. or M.D.). Degrees for the PD/PI are requested separately in the R&R Senior/Key Person
Profile (Expanded) Form.
Position/Title:
Enter the position/title of the PD/PI.
Organization Name:
This field is required. This field may be pre-populated from the applicant information section in
this form.
Department:
Enter the name of primary organizational department, service, laboratory, or equivalent level
within the organization of the PD/PI.
Division:
Enter the name of primary organizational division, office, major subdivision, or equivalent level
within the organization of the PD/PI.
Street1:
This field is required. Enter first line of the street address for the PD/PI.
Street2:
Enter the second line of the street address for the PD/PI.
City:
This field is required. Enter the city for the address of the PD/PI.
County/Parish:
Enter the county/parish for the address of the PD/PI.
State:
This field is required if the PD/PI is located in the United States or its Territories. Enter the state or
territory where the PD/PI is located.
Province:
If “Country” is Canada, enter the province for the PD/PI; otherwise, skip the “Province” field.
Country:
Select the country for the PD/PI.
R - 24

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
ZIP/Postal Code:
The ZIP+4 is required if the PD/PI address is in the United States. Otherwise, the postal code is
optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the PD/PI.
Phone Number:
This field is required. Enter the daytime phone number for the PD/PI.
Fax Number:
Enter the fax number for the PD/PI.
E-mail:
This field is required. Enter the e-mail address for the PD/PI.
15. Estimated Project Funding
All four fields in “15. Estimated Project Funding” are required.
a. Total Federal Funds Requested
Enter the total federal funds, including Direct Costs and F&A Costs (Indirect Costs), requested for
the entire project period.
b. Total Non-Federal Funds
For applications to NIH and other PHS agencies, enter “0” in this field unless cost sharing is a
requirement for the specific FOA.
c. Total Federal & Non-Federal Funds
Enter the total federal and non-federal Funds requested. The amount in this field will be the same
as the amount in the “Total Federal Funds Requested” field unless the specific FOA indicates that
cost sharing is a requirement.
d. Estimated Program Income
Indicate any program income estimated for this project, if applicable.
16. Is Application Subject to Review by State Executive Order 12372 Process?
Applicants should check “No, Program is not covered by E.O. 12372.”
17. Certification
This field is required.
The list of NIH and other PHS agencies Certifications, Assurances, and other Policies is found in the
NIH Grants Policy Statement, Section 4: Public Policy Requirements and Objectives.
The applicant organization is responsible for verifying its eligibility and the accuracy, validity, and
conformity with the most current institutional guidelines of all the administrative, fiscal, and
scientific information in the application, including the Facilities and Administrative rate. Deliberate
withholding, falsification, or misrepresentation of information could result in administrative
actions, such as withdrawal of an application, suspension and/or termination of an award,
debarment of individuals, as well as possible criminal and/or civil penalties. The signer further
certifies that the applicant organization will be accountable both for the appropriate use of any
R - 25

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
funds awarded and for the performance of the grant-supported project or activities resulting from
this application. The grantee institution may be liable for the reimbursement of funds associated
with any inappropriate or fraudulent conduct of the project activity.
Check “I agree” to provide the required certifications and assurances.
18. SFLLL (Disclosure of Lobbying Activities) or Other Explanatory
Documentation
If applicable, attach the SFLLL or other explanatory document as per FOA instructions.
If unable to certify compliance with the Certification in the “17. Certification” section above, attach
an explanation. Additionally, as applicable, attach the SFLLL (Standard Form LLL, Disclosure of
Lobbying Activities) or other documents in this item.
For more information:
See the NIH Grants Policy Statement, Section 4.1.17: Lobbying Prohibition, and the NIH Lobbying
Guidance for Grantee Activities page.
19. Authorized Representative
The authorized representative is equivalent to the individual with the organizational authority to
sign for an application. This individual is otherwise known as the authorized organization
representative (AOR) in Grants.gov or the signing official (SO) in eRA Commons.
Prefix:
Enter or select the prefix, if applicable, for the name of the AOR/SO.
First Name:
This field is required. Enter the first (given) name of the AOR/SO
Middle Name:
Enter the middle name of the AOR/SO.
Last Name:
This field is required. Enter the last (family) name of the AOR/SO.
Suffix:
Enter or select the suffix, if applicable, for the AOR/SO.
Position/Title:
This field is required. Enter the position/title of the name of the AOR/SO.
Organization Name:
This field is required. Enter the name of the organization for the AOR/SO.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level
within the organization for the AOR/SO.
R - 26

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level
within the organization for the AOR/SO.
Street1:
This field is required. Enter the first line of the street address for the AOR/SO.
Street2:
Enter the second line of the street address for the AOR/SO.
City:
This field is required. Enter the city for the address of the AOR/SO.
County/Parish:
Enter the county/parish for the address of the AOR/SO.
State:
This field is required if the AOR/SO is located in the United States or its Territories. Enter the state
or territory where the AOR/SO is located.
Province:
If “Country” is Canada, enter the province for the AOR/SO; otherwise, skip the “Province” field.
Country:
Select the country for the address of the AOR/SO.
ZIP/Postal Code:
The ZIP+4 is required if the AOR/SO is in the United States. Otherwise, the postal code is optional
Enter the ZIP+4 (nine-digit postal code) or postal code of the AOR/SO.
Phone Number:
This field is required. Enter the daytime phone number for the AOR/SO.
Fax Number:
Enter the fax number for the AOR/SO.
Email:
This field is required. Enter the e-mail address for the AOR/SO.
Signature of Authorized Representative:
Grants.gov will record the electronic signature for the AOR/SO who submits the application.
It is the organization’s responsibility to assure that only properly authorized individuals sign in this
capacity and/or submit the application to Grants.gov.
Date Signed:
Grants.gov will generate this date upon application submission.
20. Pre-application
Unless specifically noted in a FOA, NIH and other PHS agencies do not use pre-applications. The
"Pre-application" attachment field should not be used for any other purpose.
R - 27
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
If permitted by your FOA, attach this information as a PDF.
21. Cover Letter Attachment
The cover letter is for internal use only and will not be shared with peer reviewers.
Who must complete the “Cover Letter Attachment”:
Refer to the “content” list below for items that are permitted, as well as for specific situations in
which a cover letter must be included.
A cover letter must not be included with post-award submissions, such as administrative
supplements, change of grantee institution, or successor-in-interest.
Format:
Attach the cover letter, addressed to the Division of Receipt and Referral, in accordance with the
FOA and/or these instructions.
Attach the cover letter in the correct location, specifically verifying that the cover letter has not
been uploaded to the “20. Pre-application” field which is directly above the “21. Cover
Letter Attachment” field. This will ensure the cover letter attachment is kept separate from the
assembled application in the eRA Commons and made available only to appropriate staff.
Content:
Do not use the cover letter to communicate application assignment preferences. The Assignment
Request Form is provided for that purpose.
The letter should contain any of the following information, as applicable:
1. Application title.
2. Title of FOA (PA or RFA).
3. For late applications (see Late Application policy on NIH's Application Submission
Policies) include specific information about the timing and nature of the delay.
4. For changed/corrected applications submitted after the due date, a cover letter is
required, and it must explain the reason for late submission of the changed/corrected
applications. If you already submitted a cover letter with a previous submission and are
now submitting a late change/corrected application, you must include all previous cover
letter text in the revised cover letter attachment. The system does not retain any
previously submitted cover letters; therefore, you must repeat all information previously
submitted in the cover letter as well as any additional information.
5. Explanation of any subaward budget components that are not active for all budget
periods of the proposed grant (see R.310 – R&R Subaward Budget Attachment(s) Form).
6. Statement that you have attached any required agency approval documentation for the
type of application submitted. This may include approval for applications that request
$500,000 or more, approval for Conference Grant or Cooperative Agreement (R13 or U13),
etc. It is recommended that you include the official communication from an NIH official as
part of your cover letter attachment.
7. When intending to submit a video as part of the application, the cover letter must include
information about the intent to submit it; if this is not done, the video will not be
R - 28

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.200 - SF 424 (R&R) Form
accepted. See NIH Grants Policy Statement, Section 2.3.7.7: Post Submission Grant
Application Materials for additional information.
8. Include a statement in the cover letter if the proposed studies will generate large-scale
human or non-human genomic data as detailed in the NIH Genomic Data Sharing Policy
(see the NIH Grants Policy Statement, Section 2.3.7.10: NIH Genomic Data Sharing and
Section 8.2.3.3: Genomic Data Sharing (GDS) Policy/Policy for Genome-Wide Association
Studies (GWAS)).
9. Include a statement in the cover letter if the proposed studies involve human fetal tissue
obtained from elective abortions (HFT), regardless of whether or not Human Subjects are
involved and/or there are costs associated with the HFT. For further information on
HFTpolicy refer to the NIHGrants Policy Statement, Section 2.3.7.11 Human Fetal Tissue
from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research and Section 4.1.14.2
Human Fetal Tissue from Elective Abortions.
R - 29

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.210 - PHS 398 Cover Page Supplement Form
R.210 - PHS 398 Cover Page Supplement
Form
The PHS 398 Cover Page Supplement Form is used for all
grant applications except fellowships. This form collects
information on human subjects, vertebrate animals,
program income, human embryonic stem cells, inventions
and patents, and changes of investigator/change of
institution.
View larger image
Quick Links
1. Vertebrate Animals Section
2. Program Income Section
3. Human Embryonic Stem Cell Section
4. Human Fetal Tissue Section.
5. Inventions and Patents Section (for Renewal applications)
6. Change of Investigator/Change of Institution Section
1. Vertebrate Animals Section
Are vertebrate animals euthanized?
You must answer this question if you answered “Yes” to the question “Are Vertebrate Animals
Used?” on the R.220 – R&R Other Project Information Form.
Check "Yes" or "No" to indicate whether vertebrate animals in the project are euthanized.
If “Yes” to euthanasia: Is method consistent with American Veterinary Medical Association
(AVMA) guidelines?
You must answer this question if you answered “Yes” to the “Are vertebrate animals euthanized?”
question above. Check “Yes” or “No” to indicate whether the method of euthanasia is consistent
with the AVMA Guidelines for the Euthanasia of Animals.
For more information: See AVMA Guidelines for the Euthanasia of Animals.
If “No” to AVMA guidelines, describe method and provide scientific justification:
If you answered “No” to the “Is method consistent with AVMA guidelines?” question, you must
describe (in 1000 characters or fewer) the method of euthanasia and provide a scientific
justification for its use. This justification will be reviewed by Office of Laboratory Animal Welfare
(OLAW).
If you answered “Yes” to the “Is method consistent with AVMA guidelines” question, skip this
question.
R - 30

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.210 - PHS 398 Cover Page Supplement Form
2. Program Income Section
Is program income anticipated during the periods for which the grant support is requested?
This field is required.
If program income is anticipated during the periods for which grant support is requested, check
“Yes,” and complete the rest of the “Program Income” section.
If no program income is anticipated, check “No” and skip the rest of the “Program Income” section.
Budget Period:
Enter the budget periods for which program income is anticipated. If the application is funded, the
Notice of Grant Award will provide specific instructions regarding the use of such income.
Anticipated Amount ($):
Enter the amount of anticipated program income for each budget period listed.
Source(s):
Enter the source of anticipated program income for each budget period listed.
3. Human Embryonic Stem Cells Section
Use the following instructions to complete the fields in this section.
For additional guidance, see the NIH Grants Policy Statement, Section 4.1.13: Human Stem Cell
Research.
Does the proposed project involve human embryonic stem cells?
This field is required.
If the proposed project involves human embryonic stem cells (hESC), check “Yes” and complete the
rest of the “Human Embryonic Stem Cells” section.
l
Use of the cell lines must be in accordance with the NIH Guidelines for Human Stem Cell
Research.
If the proposed project does not involve hESC, check “No” and skip the rest of the “Human
Embryonic Stem Cells” section.
Specific stem cell line cannot be referenced at this time. One from the registry will be used.
If you will use hESC but a specific line from the NIH hESC Registry cannot be chosen at the time of
application submission, check this box.
If you cannot specify which cell lines will be used at the time of application submission, specific cell
line information will be required as Just-in-Time information prior to award.
Additional Instructions for Research:
If you cannot choose an appropriate cell line from the registry at this time, provide a
justification in the R.400 - PHS 398 Research Plan Form, Research Strategy
attachment.
R - 31

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.210 - PHS 398 Cover Page Supplement Form
Cell Line(s):
List the 4-digit registration number of the specific cell line(s) from the NIH hESC Registry (e.g.
0123). Up to 200 lines can be added.
For more information:
See NIH’s Stem Cell Information page for additional information on stem cells, Federal policy
statements, and guidelines on federally funded stem cell research.
4. Human Fetal Tissue Section
Does the proposed project involve human fetal tissue from elective abortions?
This field is required.
If the proposed project involves the use of human fetal tissue obtained from elective abortions
(HFT), check “Yes” and complete the rest of the “Human Fetal Tissue” section.
If the proposed project does not involve the use of human fetal tissue obtained from elective
abortions (HFT), check “No” and skip the rest of the “Human Fetal Tissue” section.
If the answer is "yes" then provide the HFT Compliance Assurance:
If the proposed project involves the use of human fetal tissue obtained from elective abortions
(HFT), the applicant must provide a letter, signed by the PD/PI, assuring the HFT donating
organization or clinic adheres to the requirements of the informed consent process and
documenting that HFT was not obtained or acquired for valuable consideration. The PDF-
formatted letter must be named ‘HFTComplianceAssurance.pdf’.
If the answer is “yes” then provide the HFT Sample IRB Consent Form
If the proposed project involves the use of human fetal tissue obtained from elective abortions
(HFT), provide a blank sample of the IRB-approved consent form. The PDF-formatted form must be
a blank sample and named ‘HFTSampleIRBConsentForm.pdf’.
o The informed consent for use of HFT from elective abortions requires language that
acknowledges informed consent for donation of HFT was obtained by someone other than the
person who obtained the informed consent for abortion, that informed consent for donation of
HFT occurred after the informed consent for abortion was obtained will not affect the method of
abortion, and that no enticements, benefits, or financial incentives were used at any level of the
process to incentivize abortion or the donation of HFT. The form must be signed by both the
woman and the person who obtains the informed consent.
For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section 2.3.7.11
Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research and
Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
5. Inventions and Patents Section (for Renewal applications)
Who must complete the “Invention and Patents” section:
Complete the "Inventions and Patents" section only if you are submitting a renewal application or a
resubmission of a renewal application.
R - 32

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.210 - PHS 398 Cover Page Supplement Form
Inventions and Patents:
If no inventions were conceived or reduced to practice during the course of work under this
project, check “No” and skip the remainder of the “Inventions and Patents” section.
If any inventions were conceived or first actually reduced to practice during the previous period of
support, check “Yes.”
NIH recipient organizations must promptly report inventions to the Division of Extramural
Inventions and Technology Resources (DEITR) Branch of the Office of Policy for Extramural
Research Administration (OPERA), OER, NIH, 6705 Rockledge Drive, Bethesda, MD 20892-2750,
(301) 435-1986. You must report inventions in compliance with regulations at 37 CFR 401.14,
which are described at Interagency Edison (iEdison). The grantee is required to submit reports
electronically using iEdison. See the NIH Grants Policy Statement, Section 8.4.1.6: Invention
Reporting.
Previously Reported:
If you answered “Yes” to the “Inventions and Patents” question, indicate whether this information
has been reported previously to the NIH or PHS agency or to the applicant organization official
responsible for patent matters.
6. Change of Investigator/Change of Institution Section
Change of Project Director/Principal Investigator:
Check this box if your application reflects a change in project director/principal investigator
(PD/PI) from that indicated on your previous application or award. Note that this box not
applicable to a new application, nor is a change in PD/PI permitted for revision applications.
For a multiple PD/PI application, check this box if this application represents a change in the
contact PI.
If you check the box, fill in the rest of the “Change of PD/PI” section with the information for the
former PD/PI according to the instructions below.
Prefix:
Enter or select the prefix, if applicable, for the former PD/PI.
First Name:
Enter the first (given) name of the former PD/PI.
Middle Name:
Enter the middle name of the former PD/PI.
Last Name:
Enter the last (family) name of the former PD/PI.
Suffix:
Enter or select the suffix, if applicable, for the former PD/PI.
Change of Grantee Institution:
Check this box if your application reflects a change in grantee institution from that indicated
on your previous application or award. This question is not applicable to new applications.
R - 33

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.210 - PHS 398 Cover Page Supplement Form
Name of Former Institution:
Enter the name of the former institution if this application reflects a change in grantee
institution.
R - 34

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
R.220 - R&R Other Project Information
Form
The R&R Other Project Information Form is used for all
grant applications. This form includes questions on the
use of human subjects, vertebrate animals, and
environmental impact. This form also has fields to upload
an abstract, project narrative, references, information on
facilities, and equipment lists.
View larger image
Quick Links
1. Are Human Subjects Involved?
1a. If YES to Human Subjects
2. Are Vertebrate Animals Used?
2a. If YES to Vertebrate Animals
3. Is proprietary/privileged information included in the application?
4. Environmental Questions
5. Is the research performance site designated, or eligible to be designated, as a historic place?
6. Does this project involve activities outside of the United States or partnerships with inter-
national collaborators?
7. Project Summary/Abstract
8. Project Narrative
9. Bibliography & References Cited
10. Facilities & Other Resources
11. Equipment
12. Other Attachments
1. Are Human Subjects Involved?
This field is required.
If activities involving human subjects are planned at any time during the proposed project at any
performance site, check “Yes.” Check “Yes” even if the proposed project is exempt from regulations
for the Protection of Human Subjects, or if activities involving human subjects are anticipated
within the period of award but plans are indefinite, or if the proposed activities include public
health surveillance activities described in 45 CFR 46.102(l)(2).
R - 35

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
If activities involving human subjects are not planned at any time during the proposed project at
any performance site, select “No” and skip the rest of the "Are Human Subjects Involved" section.
Whether you answer “Yes” or “No” to the “Are Human Subjects Involved?” question here, your
answer will populate the relevant field in the R.500 – PHS Human Subjects and Clinical Trials
Information form (see exception for Training Applications in the Training-specific instructions).
Follow the R.500 – PHS Human Subjects and Clinical Trials Information form instructions to
complete the relevant questions in that form.
Need help determining whether your application includes human subjects? Check out the
NIH Research Involving Human Subjects website for information, including an Infopath
Questionnaire designed to walk applicants through the decision process.
Note on the use of human specimens or data: Applications involving the use of human
specimens or data may or may not be considered to be research involving human subjects,
depending on the details of the materials to be used. If you check “No” to “Are Human Subjects
Involved?” but your application proposes using human specimens or data, you will be required to
provide a clear justification about why this use does not constitute human subjects research.
Follow the R.500 – PHS Human Subjects and Clinical Trials Information form instructions.
For more information on human biospecimens or data: Refer to the NIH page on Frequently
Asked Questions on Human Specimens, Cell Lines, or Data and the Research Involving Private
Information or Biological Specimens flowchart.
1.a. If YES to Human Subjects
Your answers here in question “1.a. If YES to Human Subjects” will populate the corresponding
fields in the R.500 – PHS Human Subjects and Clinical Trials Information form.
Is the Project Exempt from Federal regulations? Yes/No
If the project is exempt from federal regulations, check “Yes” and check the appropriate exemption
number.
Human subjects research should only be designated as exempt if all of the proposed research
projects in an application meet the criteria for exemption.
If the project is not exempt from federal regulations, check “No.”
For more information, see the NIH’s Exempt Human Subjects Research infographic.
If yes, check appropriate exemption number 1, 2, 3, 4, 5, 6, 7, 8:
If you selected “Yes” to “Is the Project Exempt from Federal Regulations,” select the appropriate
exemption number.
The categories of research that qualify for exemption are defined in the Common Rule for the
Protection of Human Subjects. These regulations can be found at 45 CFR 46.
Need help determining the appropriate exemption number? Refer to NIH's Research
Involving Human Subjects Frequently Asked Questions.
The Office for Human Research Protections (OHRP) guidance states that appropriate use of
exemptions described in 45 CFR 46 should be determined by an authority independent from the
investigators (for more information, see OHRP's Frequently Asked Questions). Institutions often
designate their Institutional Review Board (IRB) to make this determination. Because NIH does not
require IRB approval at the time of application, the exemptions designated often represent the
opinion of the PD/PI, and the justification provided for the exemption by the PD/PI is evaluated
during peer review. See NIH Grants Policy Statement Section 4.1.15 for more information.
4. Human Fetal Tissue Section
R - 36
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
Notes on public health surveillance activities: Projects involving public health surveillance
activities described in 45 CFR 46.102(l)(2) must answer questions in Section 1.a. as if the exclusion
does not apply. In rare circumstances, applicants may request NIH approval for use of the
exclusion in accordance with Just-in-Time procedures.
If no, is the IRB review Pending? Yes/No
If IRB review is pending, check “Yes.”
Applicants should check “Yes” to the question “Is the IRB review Pending?” even if the IRB
review/approval process has not started by the time of submission.
If IRB review is not pending (e.g., if the review is complete), check “No.”
IRB Approval Date:
Enter the latest IRB approval date (if available). Leave blank if IRBapproval is pending.
An IRB approval date is not required at the time of submission when IRB review is pending. This
may be requested later in the pre-award cycle as a Just-In-Time requirement. See the NIH Grants
Policy Statement, Section 2.5.1: Just-in-Time Procedures for more information.
Human Subject Assurance Number:
Enter the approved Federalwide Assurance (FWA) number that the applicant has on file with
OHRP. Enter the 8-digit number. Do not enter “FWA” before the number.
Enter “None” if the applicant organization does not have an approved FWA on file with OHRP. In
this case, the applicant organization, by the signature in the Certification section on the R.200 -
SF424 (R&R) Form, is declaring that it will comply with 45 CFR 46 and proceed to obtain a FWA
(see Office for Human Research Protections website). Do not enter the FWA number of any
collaborating institution.
2. Are Vertebrate Animals Used?
This field is required.
If activities involving vertebrate animals are planned at any time during the proposed project at
any performance site, check “Yes.” Otherwise, check “No” and skip the rest of the “2. Are
Vertebrate Animals Used?” section.
Note that the generation of custom antibodies constitutes an activity involving vertebrate animals.
If animal involvement is anticipated within the period of award but plans are indefinite, check
"Yes."
Additional Instructions for Research:
If you have answered “Yes” to the “Are Vertebrate Animals Used?” question, you
must also provide an explanation and anticipated timing of animal use in R.400 -
PHS 398 Research Plan Form, Vertebrate Animals. This attachment must be
submitted and reviewed prior to the involvement of animals in any research studies.
2.a. If YES to Vertebrate Animals
Is the IACUC review Pending?
If an Institutional Animal Care and Use Committee (IACUC) review is pending, check "Yes."
R - 37

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
Applicants should check “Yes” to the "Is the IACUC review Pending?" question even if the IACUC
review/approval process has not started by the time of submission.
If IACUC review is not pending (e.g. if the review is complete), check “No."
IACUC Approval Date:
Enter the latest IACUC approval date (if available). Leave blank if IACUC approval is pending.
IACUC approval must have been granted within three years of the application submission date to
be valid.
An IACUC approval date is not required at the time of submission. NIH does not require
verification of review and approval of the proposed research by the IACUC before peer review of
the application. However, this information is required under the NIH Grants Policy Statement
Section 2.5.1: Just-in-Time Procedures.
Animal Welfare Assurance Number
Enter the federally approved assurance number, if available.
Enter “None” if the applicant organization does not have an Office of Laboratory Animal Welfare
(OLAW)-approved Animal Welfare Assurance.
To determine whether the applicant organization holds an Animal Welfare Assurance with an
associated number, see the lists of Domestic and Foreign Assured institutions. Do not enter the
Animal Welfare Assurance number for a Project/Performance Site of a collaborating
institution.
When an applicant organization does not have an Animal Welfare Assurance number, the
authorized organization representative’s signature on the application constitutes declaration that
the applicant organization will submit an Animal Welfare Assurance when requested by OLAW.
If the animal work will be conducted at an institution with an Animal Welfare Assurance and the
applicant organization does not have the following:
l
an animal care and use program;
l
facilities to house animals and conduct research on site; and
l
IACUC;
then, the applicant must obtain an Inter-institutional Assurance from OLAW prior to an award.
3. Is proprietary/privileged information included in the application?
This field is required.
Patentable ideas; trade secrets; or privileged, confidential commercial, or financial information
should be included in applications only when such information is necessary to convey an
understanding of the proposed project.
If the application includes such information, check “Yes” and clearly mark each line or paragraph
on the pages containing the proprietary/privileged information with a statement similar to: “The
following contains proprietary/privileged information that (name of applicant) requests not be
released to persons outside the government, except for purposes of review and evaluation.” This
statement can be included at the top of each page as applicable.
If a grant is awarded as a result of or in connection with the submission of this application, the
government shall have the right to use or disclose the information to the extent authorized by law.
Although the grantee institution and the PD/PI will be consulted about any such disclosure, the
R - 38

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
NIH and other PHS agencies will make the final determination. Any indication by the applicant that
the application contains proprietary or privileged information does not automatically shield the
information from release in response to a Freedom of Information Act (FOIA) request should the
application result in an award (see 45 CFR 5). Additionally, if an applicant fails to identify
proprietary information at the time of submission as instructed here, a significant substantive
justification will be required to withhold the information if requested under FOIA.
4. Environmental Questions
Question 4 pertains to the environmental impact of the proposed research.
4.a. Does this Project Have an Actual or Potential Impact - positive or negative - on the envir-
onment?
This field is required.
Indicate whether or not this project has an actual or potential impact on the environment.
Most NIH research grants are not expected to individually or cumulatively have a significant effect
on the environment, and NIH has established several categorical exclusions allowing most
applicants to answer “No” unless a specific FOA indicates that the National Environmental Policy
Act (NEPA) applies. However, if an applicant expects that the proposed project will have an actual
or potential impact on the environment, or if any part of the proposed research and/or project
includes one or more of the following scenarios, check “Yes.”
1. The potential environmental impacts of the proposed research may be of greater scope or
size than other actions included within a category.
2. The proposed research threatens to violate a federal, state, or local law established for the
protection of the environment or for public health and safety.
3. Potential effects of the proposed research are unique or highly uncertain.
4. Use of especially hazardous substances or processes is proposed for which adequate and
accepted controls and safeguards are unknown or not available.
5. The proposed research may overload existing waste treatment plants due to new loads
(volume, chemicals, toxicity, additional hazardous wasted, etc.).
6. The proposed research may have a possible impact on endangered or threatened species.
7. The proposed research may introduce new sources of hazardous/toxic wastes or require
storage of wastes pending new technology for safe disposal.
8. The proposed research may introduce new sources of radiation or radioactive materials.
9. Substantial and reasonable controversy exists about the environmental effects of the
proposed research.
4.b. If yes, please explain:
If you answered “Yes” to Question 4.a., you must provide an explanation here as to the actual or
potential impact of the proposed research on the environment. Your entry is limited to 55
characters.
R - 39

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
4.c. If this project has an actual or potential impact on the environment, has an exemption been
authorized or an environmental assessment (EA) or environmental impact statement (EIS) been
performed? Yes/No.
This field is required if you answered “Yes” to Question 4.a. Check “Yes” or “No.”
4.d. If yes, please explain:
Enter additional details about the EA or EIS here. Your entry is limited to 55 characters.
5. Is the research performance site designated, or eligible to be designated, as a
historic place?
This field is required.
If any research performance site is designated, or eligible to be designated, as a historic place,
check the “Yes” box. Otherwise, check “No.”
5.a. If yes, please explain:
If you checked “Yes” to indicate that any performance site is designated, or eligible to be
designated, as a historic place, provide the explanation here. Your entry is limited to 55 characters.
6. Does this project involve activities outside of the United States or partnerships
with international collaborators?
This field is required.
Indicate whether this project involves activities outside of the United States or partnerships with
international collaborators. Check “Yes” or “No.”
Applicants to NIH and other PHS agencies must check “Yes” if the applicant organization is a
foreign institution or if the project includes a foreign component. See NIH Glossary for a definition
of a foreign component.
If you have checked “Yes” to Question 6, you must include a “Foreign Justification” attachment in
Field 12, Other Attachments. Describe special resources or characteristics of the research project
(e.g., human subjects, animals, disease, equipment, and techniques), including the reasons why the
facilities or other aspects of the proposed project are more appropriate than a domestic setting. In
the body of the text, begin the section with a heading indicating “Foreign Justification” and name
the file “Foreign Justification.”
6.a. If yes, identify countries:
This field is required if you answered “Yes” to Question 6. Enter the countries with which
international cooperative activities are planned.
You may use abbreviations. Your entry is limited to 55 characters.
6.b. Optional Explanation:
This field is optional. Enter an explanation for involvement with outside entities. Your entry is
limited to 55 characters.
7. Project Summary/Abstract
The "Project Summary/Abstract" attachment is required.
R - 40

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
The project summary is a succinct and accurate description of the proposed work and should be
able to stand on its own (separate from the application). This section should be informative to
other persons working in the same or related fields and understandable to a scientifically literate
reader. Avoid both descriptions of past accomplishments and the use of the first person. Please be
concise.
Format:
This section is limited to 30 lines of text, and must follow the required font and margin
specifications. A summary that exceeds the 30-line limit will be flagged as an error by the Agency
upon submission. Use of hyperlinks and URLs in this section is not allowed unless specified in the
funding opportunity announcement.
Attach this information as a PDF file. See the Format Attachments page.
Content:
State the application's broad, long-term objectives and specific aims, making reference to the
health relatedness of the project (i.e., relevance to the mission of the agency). Describe the
research design and methods for achieving the stated goals. Be sure that the project summary
reflects the key focus of the proposed project so that the application can be appropriately
categorized.
Do not include proprietary, confidential information or trade secrets in the project summary. If the
application is funded, the project summary will be entered into an NIH database and made
available on the NIH Research Portfolio Online Reporting Tool (RePORT) and will become public
information.
Note that the "Project Summary/Abstract" attachment is not same as the "Research Strategy"
attachment.
8. Project Narrative
The "Project Narrative" attachment is required.
Content:
Describe the relevance of this research to public health in, at most, three sentences. For example,
NIH applicants can describe how, in the short or long term, the research would contribute to
fundamental knowledge about the nature and behavior of living systems and / or the application
of that knowledge to enhance health, lengthen life, and reduce illness and disability. Use of
hyperlinks and URLs in this section is not allowed unless specified in the funding opportunity
announcement. If the application is funded, this public health relevance statement will be
combined with the project summary (above) and will become public information.
9. Bibliography & References Cited
Who must complete the “Bibliography & References Cited” attachment:
The “Bibliography & References Cited” attachment is required unless otherwise noted in the FOA.
Format:
Attach this information as a PDF file. See the Format Attachments page. Use of hyperlinks and
URLs in this section is not allowed unless specified in the funding opportunity announcement.
R - 41

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
Content:
See the following instructions for which references to include in the “Bibliography and References
Cited” attachment.
Additional Instructions for Research:
The “Bibliography & References Cited” attachment should include any references
cited in R.400 - PHS 398 Research Plan Form and in the R.500 - PHS Human Subjects
and Clinical Trials Information form.
When citing articles that fall under the Public Access Policy, were authored or co-authored by the
applicant, and arose from NIH support, provide the NIH Manuscript Submission reference number
(e.g., NIHMS97531) or the PubMed Central (PMC) reference number (e.g., PMCID234567) for each
article. If the PMCID is not yet available because the Journal submits articles directly to PMC on
behalf of their authors, indicate “PMC Journal – In Process.” NIHmaintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free,
online format may include URLs or PubMed ID (PMID) numbers along with the full reference.
Active hyperlinks in this section are not allowed. The references should be limited to relevant and
current literature. While there is not a page limitation, it is important to be concise and to select
only those literature references pertinent to the proposed research.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions for more information.
10. Facilities & Other Resources
Format:
The “Facilities & Other Resources” attachment is required unless otherwise specified in the FOA.
Use of URLs and hyperlinks in this section is not allowed unless specified in the funding
opportunity announcement.
Content:
Describe how the scientific environment in which the research will be done contributes to the
probability of success (e.g., institutional support, physical resources, and intellectual rapport). In
describing the scientific environment in which the work will be done, discuss ways in which the
proposed studies will benefit from unique features of the scientific environment or from unique
subject populations or how studies will employ useful collaborative arrangements.
If there are multiple performance sites, describe the resources available at each site.
Describe any special facilities used for working with biohazards and any other potentially
dangerous substances. Note: Information about select agents must be described in the
Research Plan, Select Agent Research.
For early stage investigators (ESIs), describe institutional investment in the success of the
investigator. See NIH's Early Stage Investigator (ESI) Policies. Your description may include the
following elements:
l
resources for classes, travel, or training;
l
collegial support, such as career enrichment programs, assistance and guidance in the
supervision of trainees involved with the ESI’s project, and availability of organized peer
groups;
R - 42

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.220 - R&R Other Project Information Form
l
logistical support, such as administrative management and oversight and best practices
training;
l
financial support, such as protected time for research with salary support.
11. Equipment
The “Equipment” attachment is required.
Format:
Attach this information as a PDF file. Use of URLs and hyperlinks in this section is not allowed
unless specified by the funding opportunity announcement.
Content:
List major items of equipment already available for this project and, if appropriate, identify the
equipment's location and pertinent capabilities.
12. Other Attachments
Attach a file to provide additional information only in accordance with the FOA and/or agency-
specific instructions.
If applicable, attach a “Foreign Justification” here. (See Question 6 above).
R - 43

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.230 - Project/Performance Site Location(s) Form
R.230 - Project/Performance Site
Location(s) Form
The Project/Performance Site Location(s) Form is used
for all grant applications. It is used to report the primary
location and any other locations at which the project will
be performed.
View larger image
Quick Links
Project/Performance Site Primary Location
Project/Performance Site Location 1
Additional Location(s)
Using the Project/Performance Site Location(s) Form:
This form allows for the collection of multiple performance sites. If you need to add more
project/performance site locations than the form allows, enter the information in a separate file and add
it to the “Additional Locations” section.
Project/Performance Site Primary Location
Generally, the primary location should be that of the applicant organization or identified as off-site
in accordance with the conditions of the applicant organization’s negotiated Facilities and
Administrative (F&A) agreement. This information must agree with the F&A information on the
budget form of the application.
Provide an explanation of resources available from each project/performance site on the "Facilities
and Resources" attachment of the R.220 - R&R Other Project Information Form.
If the proposed project involves human subjects or live vertebrate animals, it is up to the applicant
organization to ensure that all sites meet certain criteria:
Human Subjects: If a project/performance site is engaged in research involving human subjects,
the applicant organization is responsible for ensuring that the project/performance site operates
under an appropriate Federal Wide Assurance for the protection of human subjects and complies
with 45 CFR 46 and other NIH human subject related policies described in the NIH Grants Policy
Statement, Section 4.1.15: Human Subjects Protections.
Vertebrate Animals: For research involving live vertebrate animals, the applicant organization
must ensure that all project/performance sites hold an Office of Laboratory Animal Welfare
(OLAW)-approved Animal Welfare Assurance. If the animal work will be conducted at an institution
with an Animal Welfare Assurance and the applicant organization does not have the following:
l
an animal care and use program;
l
facilities to house animals and conduct research on site; and
R - 44

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.230 - Project/Performance Site Location(s) Form
l
an IACUC;
then applicant must obtain an Inter-institutional Assurance from OLAW prior to an award.
Additional Instructions for Research:
Describe any consortium/contractual arrangements in the "Consortium/Contractual
Arrangements" attachment in R.400 – PHS 398 Research Plan Form.
“I am submitting an application as an individual, and not on behalf of a company, state, local or
tribal government, academia, or other type of organization”:
Do not check the box for “I am submitting an application as an individual, and not on behalf of a
company, state, local or tribal government, academia, or other type of organization” unless
otherwise specified by the FOA.
Organization Name:
This field is required. Enter the organization name of the primary site where the work will be
performed.
Unique Entity Identifier (UEI):
This field is required for the primary performance site.
Enter the UEI associated with the organization where the project will be performed.
Street1:
This field is required. Enter the first line of the street address of the primary performance site
location.
Street2:
Enter the second line of the street address of the primary performance site location.
City:
This field is required. Enter the city for the address of the primary performance site location.
County:
Enter the county of the primary performance site location.
State:
This field is required if the site is located in the United States or its Territories. Enter the state or
territory where the primary performance site is located.
Province:
If “Country" is Canada, enter the province for the primary performance site; otherwise, skip the
“Province” field.
Country:
This field is required. Select the country of the address for the primary performance site location.
ZIP/Postal Code:
The ZIP+4 is required if the primary performance site location is in the United States. Otherwise,
the postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the primary
performance site.
R - 45

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.230 - Project/Performance Site Location(s) Form
Project/Performance Site Congressional District:
Enter the Congressional District as follows: a 2-character state abbreviation, a hyphen, and a 3-
character district number. Examples: CA-005 for California’s 5th district, VA-008 for Virginia’s 8th
district.
It is likely this field will be identical to the “Congressional District of Applicant” field provided
elsewhere in the application.
If the program/project is outside the United States, enter 00-000.
For States and U.S. territories with only a single congressional district, enter “001” for the district
number.
For jurisdictions with no representative, enter “099.”
For jurisdictions with a nonvoting delegate, enter “098” for the district number. Example: DC-098
or PR-098.
If all districts in a state are affected, enter “all” for the district number. Example: "MD-all" for all
congressional districts in Maryland.
If nationwide (all districts in all states), enter "US-all."
If you do not know the Congressional District: Go to the United States House of
Representatives website and search for the Congressional District by entering the ZIP+4. If you do
not know the ZIP+4, look it up on the USPS Look Up Zip Code website.
Project/Performance Site Location 1
Use this “Project/Performance Site Location 1” block to provide information on performance sites
in addition to the Primary Performance Site listed above, if applicable. Include any VA facilities and
foreign sites.
Organization Name:
Enter the organization name of the performance site location.
Unique Entity Identifier (UEI):
Enter the UEI associated with the performance site.
Street1:
This field is required. Enter first line of the street address of the performance site location.
Street2:
Enter the second line of the street address of the performance site location.
City:
This field is required. Enter the city for the address of the performance site location.
County:
Enter the county of the performance site location.
State:
This field is required if the project performance site is located in the United States or its Territories.
Enter the state or territory where the performance site is located.
R - 46

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.230 - Project/Performance Site Location(s) Form
Province:
If “Country” is Canada, enter the province for the performance site; otherwise, skip the “Province”
field.
Country:
This field is required. Select the country of the performance site location.
ZIP/Postal Code:
The ZIP+4 is required if the performance site location is in the United States. Otherwise, the postal
code is optional. Enter the ZIP+4 (nine-digit postal code) of the performance site location.
Project/Performance Site Congressional District:
Enter the Congressional District as follows: a 2-character state abbreviation, a hyphen, and a 3-
character district number. Examples: CA-005 for California’s 5th district, VA-008 for Virginia’s 8th
district.
If the program/project is outside the United States, enter 00-000.
For States and U.S. territories with only a single congressional district enter “001” for the district
number.
For jurisdictions with no representative, enter “099.”
For jurisdictions with a nonvoting delegate, enter “098” for the district number. Example: DC-098
or PR-098.
If all districts in a state are affected, enter “all” for the district number. Example: "MD-all" (for all
congressional districts in Maryland).
If nationwide (all districts in all states), enter "US-all."
If you do not know the Congressional District: Go to the United States House of
Representatives website and search for your Congressional District by entering your ZIP+4. If you
do not know the ZIP+4 look it up on the USPS Look Up Zip Code website.
Additional Location(s)
If you need to add more project/performance site locations than the form allows, enter the
information in a separate file and add it to the “Additional Locations” section.
A format page for Additional Performance Sites can be found on NIH's Additional Performance
Site Format Page.
R - 47
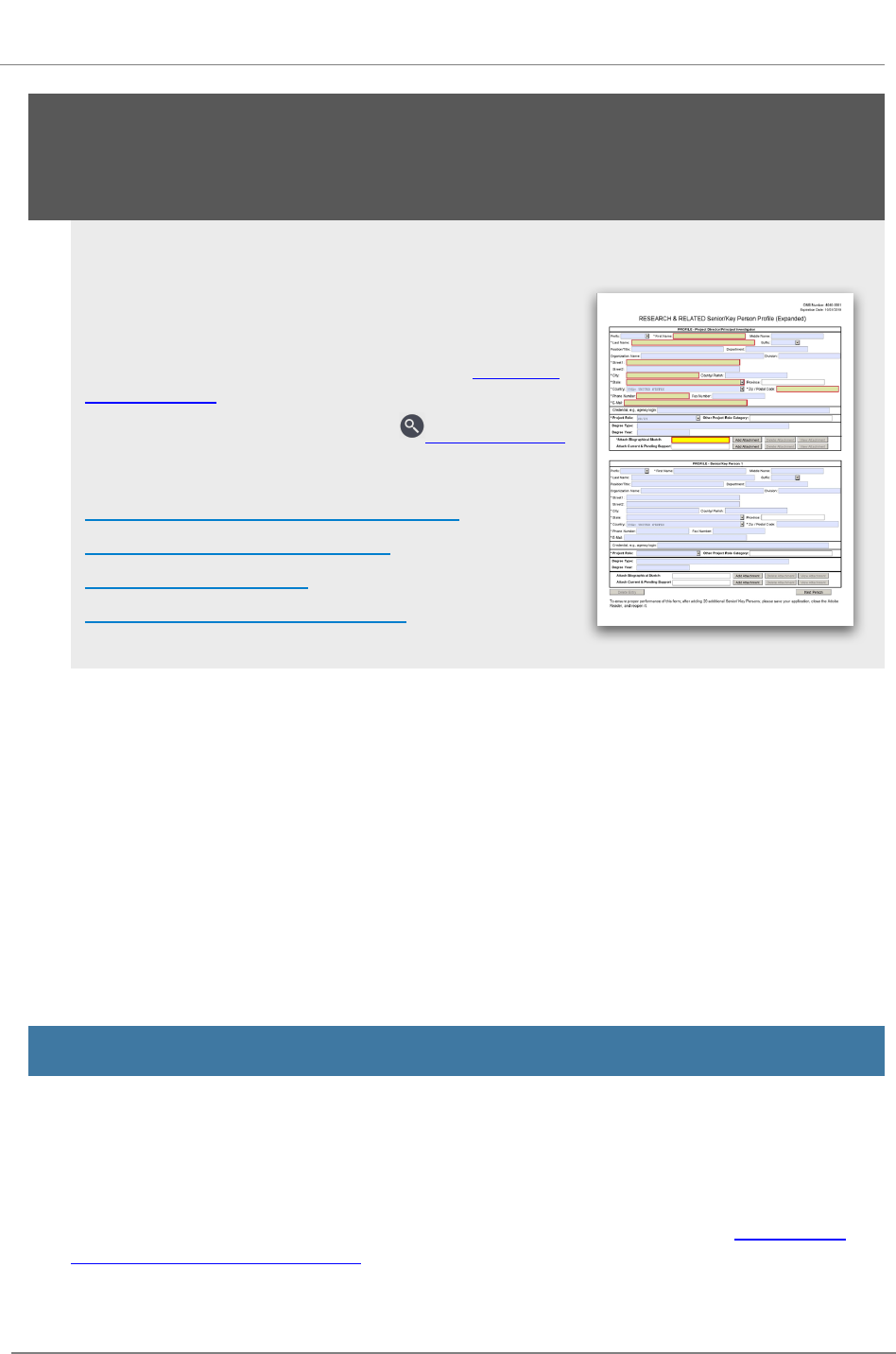
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
R.240 - R&R Senior/Key Person Profile
(Expanded) Form
The R&R Senior/Key Person Profile (Expanded) Form is
used for all grant applications, and allows the collection of
data for all senior/key persons associated with the project.
Some information for the PD/PI may be pre-populated
from the SF424 (R&R) form. See instructions in R.200 - SF
424 (R&R) Form if these fields are empty.
View larger image
Quick Links
Profile - Project Director/Principal Investigator
Instructions for a Biographical Sketch
Profile - Senior/Key Person
Additional Senior/Key Person Profile(s)
Using the R&R Senior/Key Person Profile (Expanded) Form
This form allows for the data collection for a PD/PI and up to 99 other senior/key individuals (including
any multi-PD/PIs). After the first 100 individuals have been entered, use the “Additional Senior/Key
Person Profiles Format Page” to attach any remaining data.
To ensure proper performance of this form, save your work frequently.
Who qualifies as a Senior/Key Person?
Unless otherwise specified in a FOA, senior/key personnel are defined as all individuals who contribute
in a substantive, meaningful way to the scientific development or execution of the project, whether or
not salaries are requested. Consultants should be included in this “Senior/Key Person Profile
(Expanded)” Form if they meet this definition.
List individuals that meet the definition of senior/key regardless of what organization they work for.
Profile - Project Director/Principal Investigator
Enter data in this “Profile – Project Director/Principal Investigator” section for the Project
Director/Principal Investigator (PD/PI).
The PD/PI must have an eRA Commons account with the PI role, and the account must be affiliated
with the applicant organization. If you are proposing research at an institute other than the one
you are currently at, do not create a separate Commons account with the proposed applicant
organization. For information on eRA Commons account administration, see the eRA Account
Management System’s Online Help.
R - 48

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
Special Instructions for Multiple PD/PIs: When submitting an application involving multiple
PD/PIs, list the “Contact” PD/PI in this field. List all additional PD/PIs in the Senior/Key Person
section(s) below.
Prefix:
This field may be pre-populated from the SF 424 (R&R) and reflects the prefix, if applicable, for the
name of the PD/PI.
First Name:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the first
(given) name of the PD/PI.
Middle Name:
This field may be pre-populated from the SF 424 (R&R) and reflects the middle name of the PD/PI.
Last Name:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the last
(family) name of the PD/PI.
Suffix:
This field may be pre-populated from the SF 424 (R&R) and reflects the suffix for the name of the
PD/PI.
Position/Title:
This field may be pre-populated from the SF 424 (R&R) and reflects the position/title of the PD/PI.
Department:
This field may be pre-populated from the SF 424 (R&R) and reflects the name of the primary
organizational department, service, laboratory, or equivalent level within the organization of the
PD/PI.
Organization Name:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the name
of the organization of the PD/PI.
Division:
This field may be pre-populated from the SF 424 (R&R) and reflects the name of the primary
organizational division, office, major subdivision, or equivalent level within the organization of the
PD/PI.
Street1:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the first
line of the street address for the PD/PI.
Street2:
This field may be pre-populated from the SF 424 (R&R) and reflects the second line of the street
address for the PD/PI.
City:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the city
for the address of the PD/PI.
R - 49

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
County/Parish:
This field may be pre-populated from the SF 424 (R&R) and reflects the county/parish for the
address of the PD/PI.
State:
This field is required if the PD/PI is located in the United States or its Territories. This field may be
pre-populated from the SF 424 (R&R) and reflects the state or territory in which the PD/PI is
located.
Province:
If “Country” is Canada, enter the province for the PD/PI; otherwise, skip the “Province” field. This
field may be pre-populated from the SF 424 (R&R) and reflects the province in which the PD/PI is
located.
Country:
This field may be pre-populated from the SF 424 (R&R) and reflects the country for the address of
the PD/PI.
ZIP/Postal Code:
The ZIP+4 is required if the PD/PI address is in the United States. Otherwise, the postal code is
optional. This field may be pre-populated from the SF 424 (R&R) and reflects the postal code of
the address of the PD/PI.
Phone Number:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the
daytime phone number for the PD/PI.
Fax Number:
This field may be pre-populated from the SF 424 (R&R) and reflects the fax number for the PD/PI.
E-mail:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the e-
mail address for the PD/PI.
Credential, e.g., agency login:
This field is required. Enter the assigned eRA Commons username for the project’s PD/PI. The eRA
Commons username must hold the PI role and be affiliated with the applicant organization.
Applications will not pass agency validation requirements without a valid eRA Commons
username.
Special Instructions for Multiple PD/PI: The Commons username must be provided for
all individuals assigned the Project Role of PD/PI on the application.
Project Role:
Enter "PD/PI" for the Project Role for the PD/PI.
Other Project Role Category:
Skip the “Other Project Role Category” field, as no other role can be added to the PD/PI role.
Degree Type:
Enter the highest academic or professional degree or other credentials (e.g., R.N.).
R - 50

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
Degree Year:
Enter the year the highest degree or other credential was obtained.
Attach Biographical Sketch
Provide a biographical sketch for each PD/PI. See instructions below on how to complete a
biographical sketch.
Attach Current & Pending Support:
Do not use this attachment upload for NIH and other PHS agency submissions unless otherwise
specified in the FOA.
While this information is not required at the time of application submission, it may be requested
later in the pre-award cycle. If and when this occurs, refer to the NIH Grants Policy Statement,
Section 2.5.1: Just-in-Time Procedures.
Instructions for a Biographical Sketch
These instructions apply to Research (R), Career Development (K), Training (T), Fellowship (F),
Multi-project (M), and SBIR/STTR (B). Hyperlinks and URLs are only allowed when specifically noted
in funding opportunity announcement (FOA) and form field instructions.
Who must complete the “Biographical Sketch” section:
All senior/key personnel and other significant contributors (OSCs) must include biographical
sketches (biosketches).
Format:
Use the sample format on the Biographical Sketch Format Page to prepare this section for all grant
applications.
Figures, tables (other than those included in the provided format pages), or graphics are not
allowed in the biosketch. Do not embed or attach files (e.g. video, graphics, sound, data).
The biosketch may not exceed five pages per person. This five-page limit includes the table at the
top of the first page.
Attach this information as a PDFfile. See the Format Attachments page.
Content:
Note that the instructions here follow the format of Biographical Sketch Format Page.
Name:
Fill in the name of the senior/key person or other significant contributor in the “Name” field of the
Biosketch Format Page.
eRA Commons User Name:
If the individual is registered in the eRA Commons, fill in the eRA Commons User Name in the “eRA
Commons User Name” field of the Biosketch Format Page.
The “eRA Commons User Name” field is required for the PD/PI (including career development and
fellowship applicants), primary sponsors of fellowship applicants, all mentors of candidates for
mentored career development awards, and candidates for diversity and reentry research
supplements.
The “eRA Commons User Name” field is optional for other project personnel.
R - 51

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
The eRA Commons User Name should match the information provided in the Credential
field of the R&R Senior/Key Person Profile (Expanded) Form in your grant application.
Position Title:
Fill in the position title of the senior/key person or other significant contributor in the “Position
Title” field of the Biosketch Format Page.
Education/Training
Complete the education block. Begin with the baccalaureate or other initial professional
education, such as nursing. Include postdoctoral, residency, and clinical fellowship training, as
applicable, listing each separately.
For each entry provide:
l
the name and location of the institution
l
the degree received (if applicable)
l
the month and year of end date (or expected end date). For fellowship applicants only,
also include the month and year of start date.
l
the field of study (for residency entries, the field of study should reflect the area of
residency training)
Following the education block, complete Sections A-D of the biographical sketch.
A. Personal Statement
Briefly describe why you are well-suited for your role(s) in this project. Relevant factors may
include: aspects of your training; your previous experimental work on this specific topic or related
topics; your technical expertise; your collaborators or scientific environment; and/or your past
performance in this or related fields, including ongoing and completed research projects from the
past three years that you want to draw attention to (previously captured under Section D. Research
Support).
You may cite up to four publications or research products that highlight your experience and
qualifications for this project. Research products can include, but are not limited to, audio or video
products; conference proceedings such as meeting abstracts, posters, or other presentations;
patents; data and research materials; databases; educational aids or curricula; instruments or
equipment; models; protocols; and software or netware. Use of hyperlinks and URLs to cite these
items is not allowed.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions for more information.
Note the following additional instructions for ALL applicants/candidates:
l
If you wish to explain factors that affected your past productivity, such as family care
responsibilities, illness, disability, or military service, you may address them in this “A.
Personal Statement” section.
l
Indicate whether you have published or created research products under another name.
l
You may mention specific contributions to science that are not included in Section C. Do
not present or expand on materials that should be described in other sections of this
Biosketch or application.
l
Figures, tables, or graphics are not allowed.
R - 52

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
Note the following instructions for specific subsets of applicants/candidates:
l
For institutional research training, institutional career development, or research education
grant applications, faculty who are not senior/key persons are encouraged, but not
required, to complete the "A. Personal Statement" section.
l
Applicants for dissertation research awards (e.g., R36) should, in addition to addressing
the points noted above, also include a description of their career goals, their intended
career trajectory, and their interest in the specific areas of research designated in the FOA.
l
Candidates for research supplements to promote diversity in health-related research
should, in addition to addressing the points noted above, also include a description of
their general scientific achievements and/or interests, specific research objectives, and
career goals. Indicate any current source(s) of educational funding.
B. Positions, Scientific Appointments and Honors
List in reverse chronological order all current positions and scientific appointments both domestic
and foreign, including affiliations with foreign entities or governments. This includes titled
academic, professional, or institutional appointments whether or not remuneration is received,
and whether full-time, part-time, or voluntary (including adjunct, visiting, or honorary). High
school students and undergraduates may include any previous positions. For individuals who are
not currently located at the applicant organization, include the expected position at the applicant
organization and the expected start date.
List any relevant academic and professional achievements and honors. In particular:
l
Students, postdoctorates, and junior faculty should include scholarships, traineeships,
fellowships, and development awards, as applicable.
l
Clinicians should include information on any clinical licensures and specialty board
certifications that they have achieved.
C. Contributions to Science
Who should complete the “Contributions to Science” section:
All senior/key persons should complete the “Contributions to Science” section except candidates
for research supplements to promote diversity in health-related research who are high school
students, undergraduates, and post-baccalaureates.
Format:
Briefly describe up to five of your most significant contributions to science. The description of each
contribution should be no longer than one half page, including citations.
While all applicants may describe up to five contributions, graduate students and postdoctorates
may wish to consider highlighting two or three they consider most significant.
Content:
For each contribution, indicate the following:
l
the historical background that frames the scientific problem;
l
the central finding(s);
l
the influence of the finding(s) on the progress of science or the application of those
finding(s) to health or technology; and
R - 53
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
l
your specific role in the described work.
l
Figures, tables, or graphics are not allowed.
For each contribution, you may cite up to four publications or research products that are relevant
to the contribution. If you are not the author of the product, indicate what your role or
contribution was. Note that while you may mention manuscripts that have not yet been accepted
for publication as part of your contribution, you may cite only published papers to support each
contribution. Research products can include audio or video products (see the NIH Grants Policy
Statement, Section 2.3.7.7: Post-Submission Grant Application Materials); conference proceedings
such as meeting abstracts, posters, or other presentations; patents; data and research materials;
databases; educational aids or curricula; instruments or equipment; models; protocols; and
software or netware. Use of hyperlinks and URLs to cite these items is not allowed.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions for more information.
You may provide a hyperlinked URL to a full list of your published work. This hyperlinked URL must
be to a Federal Government website (a .gov suffix). NIH recommends using My Bibliography.
Providing a URL to a list of published work is not required.
Descriptions of contributions may include a mention of research products under development,
such as manuscripts that have not yet been accepted for publication. These contributions do not
have to be related to the project proposed in this application.
D. Scholastic Performance
* Note that only the following types of applicants must complete this section:
o
applicants for predoctoral and postdoctoral fellowships
o
applicants to dissertation research grants (e.g., R36)
o
candidates for research supplements to promote diversity in health-related research
from the undergraduate through postdoctoral levels
Scholastic Performance
Predoctoral applicants/candidates (including undergraduates and post-baccalaureates): List
by institution and year all undergraduate and graduate courses, with grades. In addition, explain
any grading system used if it differs from a 1-100 scale; an A, B, C, D, F system; or a 0-4.0 scale.
Also indicate the levels required for a passing grade.
Postdoctoral applicants: List by institution and year all graduate scientific and/or professional
courses with grades. In addition, explain any grading system used if it differs from a 1-100 scale; an
A, B, C, D, F system; or a 0-4.0 scale. Also indicate the levels required for a passing grade.
Profile – Senior/Key Person 1
Enter data in this “Profile – Senior/Key Person 1” section to provide information on a senior/key
person (other than the PD/PI listed above), if applicable.
Format:
List all senior/key person profiles, followed by other significant contributors (OSC) profiles.
R - 54

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
Content – Who to include in the “Profile – Senior/Key Person” section:
Senior/Key Persons: Fill in a separate “Profile – Senior/Key Person” block for each senior/key
personnel. Those with a postdoctoral role should be included if they meet the NIH Glossary
definition of senior/key personnel. A biosketch is required for all senior/key persons.
Other Significant Contributors: Also use the “Profile – Senior/Key Person” section to list any
other significant contributors (OSCs). Consultants should be included if they meet the NIH
Glossary definition of OSC. OSCs should be listed after all other senior/key persons.
A biosketch is required for all OSCs. The biosketch should highlight the OSC’s accomplishments as
a scientist. Reviewers assess these pages during peer review. For more information on review
criteria, see the Review Criteria at a Glance document. Although Other Support information is
required as a just-in-time submission, Other Support information will NOT be required or accepted
for OSCs since considerations of overlap do not apply to these individuals.
Should the level of involvement increase for an individual listed as an OSC, thus requiring
measurable effort on the award, the individual must be redesignated as “senior/key personnel.”
This change must be made before any compensation is charged to the project.
For more information:
For more information, refer to these NIHSenior/Key Personnel Frequently Asked Questions.
Prefix:
Enter or select the prefix, if applicable, for the name of the senior/key person.
First Name:
This field is required. Enter the first (given) name of the senior/key person.
Middle Name:
Enter the middle name of the senior/key person.
Last Name:
This field is required. Enter the last (family) name of the senior/key person.
Suffix:
Enter or select the suffix, if applicable, for the senior/key person.
Position/Title:
Enter the position/title of the senior/key person.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level
within the organization of the senior/key person.
Organization Name:
This field is required. Enter the name of the organization of the senior/key person.
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level
within the organization of the senior/key person.
Street1:
This field is required. Enter the first line of the street address for the senior/key person.
R - 55
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
Street2:
Enter the second line of the street address for the senior/key person.
City:
This field is required. Enter the city for the address of the senior/key person.
County/Parish:
Enter the county/parish for the address of the senior/key person.
State:
This field is required if the Senior/Key person is located in the United States or its Territories. Enter
the state or territory where the senior/key person is located.
Province:
If “Country” is Canada, enter the province for the senior/key person; otherwise, skip the “Province”
field.
Country:
This field is required. Select the country for the address of the senior/key Person.
ZIP/Postal Code:
The ZIP+4 is required if the Senior/Key Person is in the United States. Otherwise, the postal code is
optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the senior/key person.
Phone Number:
This field is required. Enter the daytime phone number for the senior/key person.
Fax Number:
Enter the fax number for the senior/key person.
E-mail:
This field is required. Enter the e-mail address for the senior/key person.
Credential, e.g., agency login:
This field is required. Applies to Senior/Key Personnel as defined in the NIH Grants Policy
Statement (NIH GPS 1.2) as well as Other Significant Contributors (OSCs). Enter the assigned eRA
Commons username for the senior / key Person.
Additional Instructions for Research:
For Multiple PD/PI Applications: The eRA Commons username must be entered in
this field for any senior/key person with the PD/PI Project Role.
Candidates for diversity and reentry research supplement support must provide an
eRA Commons Username.
Project Role:
Select a project role. Use "Other (Specify)" if the desired category is not available.
Special Instructions for Multiple PD/PIs:All PD/PIs must be assigned the “PD/PI” role,
even those at organizations other than the applicant organization. The role of “Co-PD/PI”
R - 56

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.240 - R&RSenior/Key Person Profile (Expanded) Form
is not currently used by NIH or other PHS agencies to designate a multiple PD/PI
application. In order to avoid confusion, do not use the role of “Co-PD/PI.”
Note on OSCs: For OSCs, enter “Other (Specify)” for the “Project Role” field, and enter “Other
Significant Contributor” in the “Other Project Role Category” field.
Other Project Role Category:
Complete this field (e.g., Engineer, Chemist, Sponsor, Mentor) if you selected “Other Professional”
or “Other (Specify)” in the “Project Role” field.
Degree Type:
Enter the highest academic or professional degree or other credentials (e.g., R.N.).
Degree Year:
Enter the year the highest degree or other credential was obtained.
Attach Biographical Sketch:
Provide a biographical sketch for each senior/key person and each OSC. See instructions above on
how to complete a biographical sketch.
Attach Current & Pending Support:
Note: The terms “current and pending support,” “other support,” and “active and pending
support” are used interchangeably.
Do not use the "Current &Pending Support" attachment upload for NIH or other PHS agency
submissions unless otherwise specified in the FOA.
While this information is not required at the time of application submission, it may be requested
later in the pre-award cycle. If and when this occurs, refer to the NIH Grants Policy Statement,
Section 2.5.1: Just-in-Time Procedures for instructions and use the Current and Pending Support
Format Page.
Additional Senior / Key Person Profile(s)
If you need to add more Senior/Key Person Profiles than the form allows, enter the information in
a separate file and attach it as a PDF.
A format page for Additional Senior/Key Person Profiles can be found at NIH's Additional
Senior/Key Person Form page.
R - 57
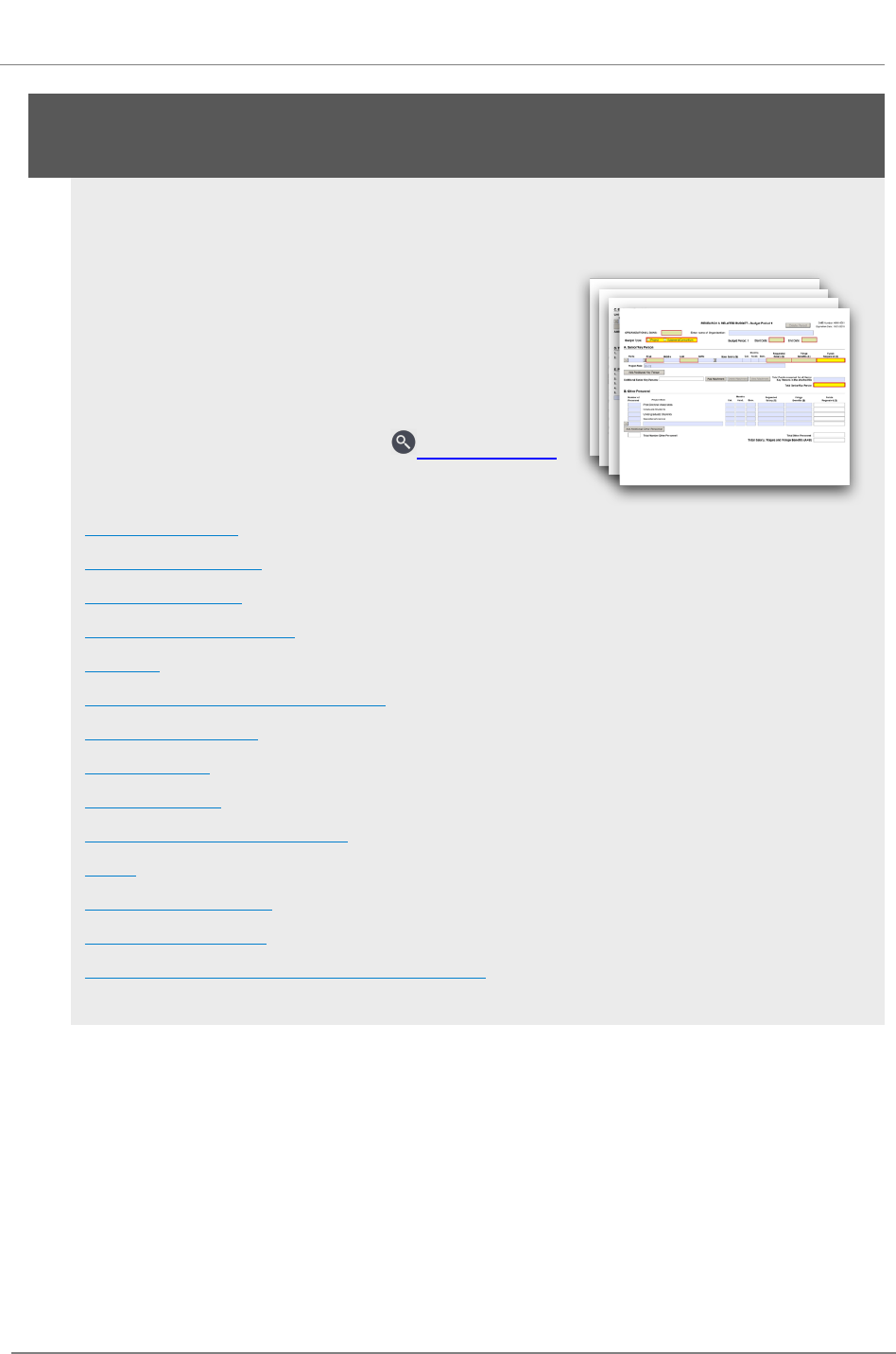
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
R.300 - R&R Budget Form
The R&R Budget Form is used in the majority of
applications; however, it is important to refer to your
specific FOA for guidance on which budget form(s) are
allowed for your application.
Some application forms packages include two optional
budget forms — (1) the R&R Budget Form and, (2) PHS
398 Modular Budget Form. Include only one of these
forms, but not both, in your application.
View larger image
Quick Links
Introductory Fields
A. Senior/Key Person
B. Other Personnel
C. Equipment Description
D. Travel
E. Participant/Trainee Support Costs
F. Other Direct Costs
G. Direct Costs
H. Indirect Costs
I. Total Direct and Indirect Costs
J. Fee
K. Total Costs and Fee
L. Budget Justification
Research & Related Budget - Cumulative Budget
Who should use the R&R Budget Form?
There are two primary types of Budget Forms: detailed R&R and PHS 398 modular. Generally, you must
use the R&R Budget Form if you are applying for more than $250,000 per budget period in direct costs,
and you must use the Modular Budget Form if you are applying for less than $250,000. However, some
grant mechanisms or programs (e.g., training grants) may require other budget forms to be used. Refer
to your FOA and to the following instructions for guidance on which Budget Form to use.
Note: The terms “detailed budget” and “R&R Budget” are used interchangeably.
If you are requesting a budget with $500,000 or more in direct costs for any budget period, contact the
awarding component to determine whether you must obtain prior approval before submitting the
R - 58
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
application. For more information on applications that request $500,000 or more in direct costs, see the
NIH Grants Policy Statement, Section 2.3.7.2: Acceptance for Review of Unsolicited Applications
Requesting $500,000 or More in Direct Costs.
Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities): All
competing (new, renewal, resubmission, and revision) grant applications from foreign (non-U.S.)
institutions must use the R&R Budget Form. Do not use the PHS 398 Modular Budget Form. For
additional information, see NIH Guide Notice on the Requirement for Detailed Budget Submissions
from Foreign Institutions and the NIH Grants Policy Statement, Section 13.3.1: Budget. Applications
from foreign organizations must request budgets in U.S. dollars.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application, you must use the R&R Budget Form and cannot
use the PHS Modular Budget Form, regardless of the activity code. Whether or not you incur costs to
obtain HFT, you will need to include a “Human Fetal Tissues Costs” line item (F.8-17) and a Budget
Justification attachment (L).
Note on Subawards/Consortiums: If you have a subaward/consortium, you must use the R&R
Subaward Budget Attachment(s) Form in conjunction with the R&R Budget Form. The prime must
extract the R&R Subaward Budget Attachment(s) from the R&R Subaward Budget Attachment(s) Form
and send the extracted file to the subaward/consortium. The consortium should complete the R&R
Subaward Budget Attachment, following the instructions here and in R.310 – R&R Subaward Budget
Attachment(s) Form.
For more information:
For more information on how to prepare your budget, see NIH's Develop Your Budget page.
Using the R&R Budget Form:
The location of the R&R Budget Form may vary with the type of submission (e.g., under an “Optional
Forms” tab).
You must complete a separate detailed budget for each budget period requested. The form will
generate a cumulative budget for the total project period. If no funds are requested for a required field,
enter “0.”
You must round to the nearest whole dollar amount in all dollar fields.
Competing Revision Applications: For a supplemental/revision application, complete fields for which
additional funds are requested in addition to all required fields. If the initial budget period of the
supplemental/revision application is less than 12 months, prorate the personnel costs and other
appropriate items of the detailed budget.
Introductory Fields
Unique Entity Identifier (UEI):
This field is required. This field may be pre-populated and should reflect the UEI of the applicant
organization (or of the lead organization for the component of a multi-project application).
Enter name of Organization:
This field may be pre-populated. Enter the name of the organization.
R - 59
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
Budget Type:
This field is required. Check the appropriate box for your budget type, following these guidelines:
l
Project: The budget being requested is for the primary applicant organization.
l
Subaward/Consortium: The budget being requested is for subaward/consortium
organization(s). Note, separate budgets are required only for subaward/consortium
organizations that perform a substantive portion of the project. For
subawards/consortiums that do not perform a substantive portion of the project, then
you must include their costs in Field F5. Subawards/Consortium/Contractual Costs and in
the prime’s Section L. Budget Justification.
If you are preparing an application that includes a subaward/consortium that performs a
substantive portion of the project, in addition to completing this form, see also the instructions for
R.310 - R&R Subaward Budget Attachment(s) Form.
Budget Period:
This field is required.
Identify the specific budget period (for example, 1, 2, 3, 4, 5).
Start Date:
This field is required and may be pre-populated from the SF 424 R&R Form. Enter the
requested/proposed start date of the budget period. For period 1, the start date is typically the
same date as the Proposed Project Start Date on the R.200 - SF 424 (R&R) Form.
End Date:
This field is required. Enter the requested/proposed end date of the budget period.
A. Senior/Key Person
Who to include in A. Senior/Key Person:
Include the names of senior/key persons at the applicant organization, (or organization leading
the component of a multi-project application), who are involved on the project in a particular
budget period. Include all collaborating investigators and other individuals who meet the
senior/key person definition if they are from the applicant organization.
Consultants designated as senior/key persons in the Senior/Key Person Profile Form can be
included in the "A. Senior/Key Person" section only if they are also employees of the applicant
organization. Otherwise, consultant costs should be included in Consultant Services in Question F
of this form.
Who not to include in A. Senior/Key Person:
Do not list details of collaborators at other institutions here, as they will be provided in the
Subaward Budget for each subaward/consortium organization.
Personnel listed as other significant contributors who are not committing any specific measurable
effort to the project should not be included in the Personnel section (sections "A. Senior/Key
Person" and "B. Other Personnel") since no associated salary and/or fringe benefits can be
requested for their contribution.
Prefix:
Enter the prefix (e.g., Mr., Mrs., Rev.), if applicable, for the name of the senior/key person.
R - 60
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
First Name:
This field is required. Enter the first (given) name of the senior/key person.
Middle Name:
Enter the middle name of the senior/key person.
Last Name:
This field is required. Enter the last (family) name of the senior/key person.
Suffix:
Enter the suffix (e.g., Jr., Sr., PhD), if applicable, of the senior/key person.
Base Salary ($):
Enter the annual compensation paid by the employer for the senior/key person. This includes all
activities such as research, teaching, patient care, and other. An applicant organization may choose
to leave this blank; however, NIH or other PHS Agency staff will request this information prior to
award.
Months (Cal./Acad./Sum.):
NIH and other PHS agencies use the concept of “person months” as a metric for determining
percent of effort. For more information about calculating person months, see NIH's information at
Frequently Asked Questions on Person Months.
Identify the number of months the senior/key person will devote to the project in the applicable
box (i.e., calendar, academic, summer).
Use either calendar months OR a combination of academic and summer months. Measurable
effort is required for every senior/key person entry.
For an explanation of "measurable effort," see the Frequently Asked Questions on Senior/Key
Personnel.
If effort does not change throughout the year, it is OK to use only the calendar months column.
However, you may use both the academic and summer months columns if your institutional
business process requires noting each separately even if effort remains constant. If effort varies
between academic and summer months, leave the calendar months column blank and use only
the academic and summer months columns.
If your institution does not use a 9-month academic year or a 3-month summer period, indicate
your institution’s definition of these in Section L. Budget Justification.
Requested Salary ($):
This field is required. Regardless of the number of months being devoted to the project, indicate
the salary being requested for this budget period for the senior/key person.
Salary limitations. Some PHS grant programs are currently subject to a legislatively imposed
salary limitation. Any adjustment for salary limits will be made at the time of award; therefore,
requested salary should be based on institutional base salary at the time the application is
submitted and not adjusted for any limitation. For guidance on current salary limitations, see the
NIH's Salary Cap Summary or contact your office of sponsored programs.
Graduate student compensation: NIH grants also limit compensation for graduate students.
Compensation includes salary or wages, fringe benefits, and tuition remission. While actual
R - 61

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
institutional-based compensation should be requested and justified, this may be adjusted at the
time of the award. For more guidance on this policy, see the NIH Grants Policy Statement, Section
2.3.7.9: Graduate Student Compensation.
Fringe Benefits ($):
Enter the amount of requested fringe benefits, if applicable, for the senior/key person.
Funds Requested ($):
This field is automatically calculated and will reflect the total requested salary and fringe benefits
for the senior/key person.
Project Role:
This field is required. Identify the project role of each senior/key person. Roles should correspond
to the roles included on the R.240 - R&R Senior/Key Person Profile (Expanded) Form. Note that
there must be at least one PD/PI per budget period.
Additional Senior/Key Persons:
If you are requesting funds for more senior/key persons than the form allows, you must include an
attachment listing the additional senior/key person(s) in this “Additional Senior/Key Persons” field.
Use the same format as the budget form and include all the information identified in this section.
Total Funds requested for all persons in the attached file:
If you have attached a file with additional senior/key persons, enter the total funds requested for
everyone listed in the attachment in the “Total Funds requested for all Senior/Key Persons in the
attached file” field.
Total Senior/Key Persons:
This total will be automatically calculated based on the sum of the “Funds Requested” column and
the “Total Funds requested for all Senior/Key Persons in the attached file” field.
Special Instructions for Joint University and Department of Veterans Affairs (V.A.)
Appointments: Individuals with joint university and V.A. appointments may request the university’s
share of their salary in proportion to the effort devoted to the research project. The individual’s salary
with the university determines the base for computing that request. The signature by the institutional
official on the application certifies that: (1) the individual is applying as part of a joint appointment
specified by a formal Memorandum of Understanding between the university and the V.A.; and (2)
there is no possibility of dual compensation for the same work, or of an actual or apparent conflict of
interest regarding such work. Additional information may be requested by the awarding
components.
B. Other Personnel
Number of Personnel:
For each project role category, identify the number of personnel proposed.
Administrative, Secretarial, and Clerical Support Salaries: In most circumstances, the salaries
of administrative, secretarial, or clerical staff at educational institutions and nonprofit
organizations are included as part of indirect costs (Section H. Indirect Costs). However, examples
of situations where direct charging of administrative or clerical staff salaries may be appropriate
may be found at: 45 CFR 75.403.
R - 62
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
Inclusion of such costs may be appropriate only if all of the following conditions are met:
1. Administrative or clerical services are integral to a project or activity;
2. Individuals involved can be specifically identified with the project or activity;
3. Such costs are explicitly included in the budget or have prior written approval of the
federal awarding agency; and
4. The costs are not also recovered as indirect costs.
Requests for direct charging for secretarial/clerical personnel (i.e., administrative and clerical staff)
must be appropriately justified in Section L. Budget Justification. For all individuals classified as
administrative/secretarial/clerical, provide a justification (in the Budget Justification) documenting
how they meet all four conditions. NIH ICs may request additional information for these positions
in order to assess allowability.
Postdoctoral and Graduate Students: For all postdoctoral associates and graduate students not
already named in "Section A. Senior/Key Person," individually list names, roles (e.g., postdoctoral
associates or graduate student), associated months, and requested salary and fringe benefits in
Section L. Budget Justification.
Project Role:
List any additional project role(s) (e.g., engineer, IT professionals, etc.) in the blank(s) provided.
Identify the number of each personnel proposed.
You may have up to six named roles. If you have more than six, you must combine project roles
here and add an explanation about the named roles in Section L. Budget Justification.
Do not include consultants in this section. Consultants are included below in Section F. Other
Direct Costs.
Months (Cal./Acad./Sum.):
NIH and other PHS agencies use the concept of “person months” as a metric for determining
percent of effort. For more information about calculating person months, see: NIH's Frequently
Asked Questions on Person Months.
Identify the number of months devoted to the project in the applicable box (i.e., calendar,
academic, summer) for each project role category.
Use either calendar months OR a combination of academic and summer months.
If effort does not change throughout the year, it is OK to use only the calendar months column.
However, you may use both academic and summer months columns if your institutional business
process requires noting each separately, even if effort remains constant. If effort varies between
academic and summer months, leave the calendar months column blank and use only the
academic and summer months columns.
If your institution does not use a 9-month academic year or a 3-month summer period, indicate
your institution’s definition of these in Section L. Budget Justification.
Requested Salary ($):
Regardless of the number of months being devoted to the project, indicate only the amount of
salary/wages being requested for this budget period for each project role. The amount entered
should reflect the total amount of funds requested for all personnel within a project role.
R - 63
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
Salary limitations: Some PHS grant programs are currently subject to a legislatively imposed
salary limitation. Any adjustment for salary limits will be made at the time of award; therefore,
requested salary should be based on institutional base salary at the time the application is
submitted and not adjusted for any limitation. For guidance on current salary limitations, see the
NIH's Salary Cap Summary or contact your office of sponsored programs.
Graduate student compensation: NIH grants also limit the compensation for graduate students.
Compensation includes salary or wages, fringe benefits, and tuition remission. While actual
institutional-based compensation should be requested and justified, this may be adjusted at the
time of the award. For more guidance on this policy, see the NIH Grants Policy Statement, Section
2.3.7.9: Graduate Student Compensation.
Fringe Benefits ($):
Enter the amount of requested fringe benefits, if applicable, for this project role category. The
amount entered should reflect the total amount of fringe benefits requested for all personnel
within a project role.
Funds Requested ($):
This field will be automatically calculated and will reflect the total requested salary and fringe
benefits for each project role category.
Total Number of Other Personnel:
This total will be automatically calculated based on the Number of Personnel for each project role
category.
Total Other Personnel:
This total will be automatically calculated based on the sum of the Funds Requested for all Other
Personnel.
Total Salary, Wages and Fringe Benefits (A+B):
This total will be automatically calculated and represents the total Funds Requested for all
Senior/Key persons and all Other Personnel
Special Instructions for Applications Submitted with a Data Management and Sharing Plan:
For applications submitted for due dates on or before October 4, 2023, if a Data
Management and Sharing Plan is required in the proposed application, personnel costs specific to
Data Management and Sharing activities must not be included here but listed as a specific line
item under Section F.8.-17 Other.
For applications submitted for due dates on or after October 5, 2023, DMS costs must be
requested in the appropriate costs category.
C. Equipment Description
The “C. Equipment Description” section is for you to list items and dollar amount for each item
exceeding $5,000 (unless the organization has established lower levels).
Equipment Item:
Equipment is defined as an item of property that has an acquisition cost of $5,000 or more (unless
the organization has established lower levels) and an expected service life of more than one year.
R - 64

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
List each item of equipment separately and justify each in Section L. Budget Justification. Allowable
items ordinarily will be limited to research equipment not already available for the conduct of the
work.
Funds Requested:
This information is required. List the estimated cost of each item, including shipping and any
maintenance costs and agreements.
Additional Equipment:
If you're requesting funds for more equipment than the form allows, you must include an
attachment listing the additional equipment items in this “Additional Equipment” field. Enter the
information in a separate file and attach it as a PDF. List each additional item and the funds
requested for each individual item. The dollar amount for each item should exceed $5,000 (unless
the organization has established lower levels).
Total funds requested for all equipment listed in the attached file:
If you have attached a file with additional equipment, enter the total funds requested for all the
equipment listed in the attachment.
Total Equipment:
This total will be automatically calculated based on the sum of the “Funds Requested” column and
the “Total funds requested for all equipment listed in the attached file” field.
D. Travel
1. Domestic Travel Costs (Incl. Canada, Mexico, and U.S. Possessions):
Enter the total funds requested for domestic travel. Domestic travel includes destinations in the
U.S., Canada, Mexico, and U.S. possessions. In Section L. Budget Justification, include the purpose,
destination, dates of travel (if known), and the number of individuals for each trip. If the dates of
travel are not known, specify the estimated length of trip (e.g., 3 days).
2. Foreign Travel Costs:
Identify the total funds requested for foreign travel. Foreign travel includes any destination outside
of the U.S., Canada, Mexico, or U.S. possessions. In Section L. Budget Justification, include the
purpose, destination, dates of travel (if known), and the number of individuals for each trip. If the
dates of travel are not known, specify the estimated length of trip (e.g., 3 days).
Total Travel Cost:
This total will be automatically calculated based on the sum of the Domestic and Foreign Funds
Requested fields.
E. Participant/Trainee Support Costs
Unless specifically stated otherwise in a FOA, NIH and other PHS agencies applicants should skip
Section E. Participant/Trainee Support Costs. Note: Tuition remission for graduate students should
be included in Section F. Other Direct Costs when applicable.
1. Tuition/Fees/Health Insurance:
List the total funds requested for Participant/Trainee Tuition/Fees/Health Insurance.
R - 65

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
2. Stipends:
List the total funds requested for Participant/Trainee stipends.
3. Travel:
List the total funds requested for Participant/Trainee travel.
4. Subsistence:
List the total funds requested for Participant/Trainee subsistence.
5. Other:
Describe any other Participant/Trainee support costs and list the total funds requested for all other
Participant/Trainee costs described.
Number of Participants/Trainees:
List the total number of proposed Participants/Trainees. Value cannot be greater than 999.
Total Participant/Trainee Support Costs:
This field is required if any data has been entered in “Section E. Participant/Trainee Support Costs.”
This total will be automatically calculated based on the sum of the Funds Requested column in
"Section E. Participant/Trainee Support Costs."
F. Other Direct Costs
1. Materials and Supplies:
List the total funds requested for materials and supplies. In Section L. Budget Justification, indicate
general categories such as glassware, chemicals, animal costs, etc., including an amount for each
category. Categories with amounts less than $1,000 are not required to be itemized.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If costs for
human fetal tissue obtained from elective abortions (HFT) as defined in the NIHGrants Policy
Statement are included in the proposed budget, they must not be included here but listed as a
specific line item under Section F.8-17 Other.
2. Publication Costs:
List the total funds requested for publication costs. The proposal budget may request funds for the
costs of documenting, preparing, publishing, or otherwise making available to others, the findings
and products of the work conducted under the award. Include supporting information in Section L.
Budget Justification.
3. Consultant Services:
List the total funds requested for all consultant services. Identify the following items in Section L.
Budget Justification, as applicable:
l
each consultant, the services he/she will perform, total number of days, travel costs, and
the total estimated costs;
l
the names and organizational affiliations of all consultants, other than those involved in
consortium/contractual arrangements;
l
consulting physicians in connection with patient care; and
R - 66

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
l
persons who are confirmed to serve on external monitoring boards or advisory
committees to the project. Describe the services to be performed.
4. Automatic Data Processing (ADP)/Computer Services:
List the total funds requested for ADP/computer services. The cost of computer services, including
computer-based retrieval of scientific, technical, and education information may be requested. In
Section L. Budget Justification, include the established computer service rates at the proposing
organization, if applicable.
5. Subawards/Consortium/Contractual Costs:
List the total funds requested for:
1. all subaward/consortium organization(s) proposed for the project and
2. any other contractual costs proposed for the project.
This line item should include both direct and indirect costs for all subaward/consortium
organizations.
Contractual costs for support services, such as laboratory testing of biological materials, clinical
services, or data processing, are occasionally sufficiently high to warrant a categorical breakdown
of costs. When this is the case, provide detailed information as part of Section L. Budget
Justification.
NIH policy provides for exclusion of consortium/contractual F&A costs when determining if an
applicant is in compliance with a direct cost limitation. However, you must include the full cost of
consortium/subawards in this field. See the NIH Grants Policy Statement, Section 2.3.7.1:
Applications that Include Consortium/Contractual F&A Costs for policy related to the exclusion of
consortium/subaward amounts in determining whether an applicant is in compliance with a direct
cost limitation.
6. Equipment or Facility Rental/User Fees:
List the total funds requested for equipment or facility rental/user fees. In Section L. Budget
Justification, identify and justify each rental user fee.
7. Alterations and Renovations:
List the total funds requested for alterations and renovations (A&R). In Section L. Budget
Justification, itemize by category and justify the costs of alterations and renovations, including
repairs, painting, and removal or installation of partitions, shielding, or air conditioning. Where
applicable, provide the square footage and costs.
Under certain circumstances the public policy requirements that apply to construction activities
may also apply to A&R activities. Refer to the NIH Grants Policy Statement, Section 10.10:
Construction Grants – Public Policy Requirements and Objectives for more information.
Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities): Minor
A&R costs (≤$500,000) are allowable on applications from foreign organizations and domestic
institutions with foreign components. When requesting minor A&R costs under this policy,
please provide detailed information on the planned A&R in the budget justification.
8-17 Other:
Add descriptions for any “other” direct costs not requested above. Use Section L. Budget
Justification to further itemize and justify.
R - 67
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
List funds requested for each of the items in lines "8-17 Other.” Use lines 8-17 for costs such as
patient care costs, tuition remission and SBIR/STTR "Technical Assistance"(TABA) costs. If
requesting patient care costs, request inpatient and outpatient costs separately.
Lines "8-17 Other" may also be used to request direct costs related to the use of single
Institutional Review Board (sIRB)for multi-site human subjects research.
For more information on charging direct and indirect costs for single IRBactivities, see the
Scenarios to Illustrate the Use of Direct and Indirect Costs for Single IRBReview under the
NIHPolicy on the Use of a Single IRBfor Multi-Site Research.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application, regardless of whether costs will be incurred, it
must be noted as a single line item here. The line item must be titled “Human Fetal Tissue Costs”
(without quotation marks, but following exact phrase and spacing). The line item must only be
used for HFT costs and cannot include or be combined with any “Other” costs. If no cost will be
incurred (e.g. if HFT will be donated), enter “0” in the “Funds Requested” column. Details regarding
HFT must be specified in the Budget Justification attachment (L), pursuant to the instructions.
Applications proposing HFT that do not address these requirements will be administratively
withdrawn. For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section
2.3.7.11 Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research
and Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
Special Instructions for Applications Submitted with a Data Management and Sharing
(DMS) Plan:
For applications submitted on or before October 4, 2023, NIH recognizes that making data
accessible and reusable for other researchers may incur costs. If a Data Management and Sharing
Plan is required in the proposed application (see instructions for the “Other Plan(s)” attachment on
the PHS 398 Research Plan Form and the PHS 398 Career Development Award Supplemental Form,
as applicable), costs to support these activities, including personnel costs (e.g., personnel who will
be curating data for the project) must be noted as a single line item. The line item must be titled
"Data Management and Sharing Costs" (without quotation marks, but following exact phrase and
spacing). The line item must only be used for Data Management and Sharing costs and cannot
include or be combined with any "Other" costs. If no cost will be incurred, enter "0" in the "Funds
Requested" column. Details regarding Data Management and Sharing costs must be specified in
the Budget Justification attachment (L), pursuant to the instructions.
For applications submitted for due dates on or after October 5, 2023, NIH recognizes that
making data accessible and reusable for other researchers may incur costs. If a Data Management
and Sharing Plan is required in the proposed application (see instructions for the “Other Plan(s)”
attachment on the PHS 398 Research Plan Form and the PHS 398 Career Development Award
Supplemental Form, as applicable), costs to support these activities, may be requested in the
appropriate cost category. Details regarding Data Management and Sharing costs must be
specified in the Budget Justification attachment (L), pursuant to the instructions.
Allowable and Unallowable Costs: Allowable costs submitted in budget requests must be
incurred during the performance period, even for scientific data and metadata preserved and
shared beyond the award period. Budget requests must NOT include: Infrastructure costs that are
included in institutional overhead (for instance, NIH Grants Policy Statement Section 7.3 Facilities
and Administrative costs); costs associated with the routine conduct of research, including costs
associated with collecting or gaining access to research data; or costs that are double charged or
R - 68
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
inconsistently charged as both direct and indirect costs. For more information, see Budgeting for
Data Management & Sharing on the NIH Scientific Data Sharing website and additional details to
help Develop Your Budget.
Additional Instructions for Research:
Special Instructions for Patient Care Costs: If inpatient and/or outpatient costs
are requested, provide the names of any hospitals and/or clinics and the amounts
requested for each in the Budget Justification.
State whether each hospital or clinic has a currently effective HHS-negotiated
research patient care rate agreement and, if not, what basis is used for calculating
costs. If an applicant does not have a HHS-negotiated rate, the PHS awarding
component can approve a provisional rate. Indicate, in detail, the basis for
estimating costs in this category, including the number of patient days, estimated
cost per day, and cost per test or treatment. If multiple sites are to be used, provide
detailed information by site.
Include information regarding projected patient accrual for the project/budget
periods and relate this information to the budget request for patient care costs. If
patient accrual is anticipated to be lower at the start or during the course of the
project, plan budget(s) accordingly.
Provide specific information regarding anticipated sources of Other Support for
patient care costs, e.g., third party recovery or pharmaceutical companies. Include
any potential or expected utilization of the Clinical and Translational Science Awards
(CTSA) program.
Total Other Direct Costs:
This total will be automatically calculated based on the sum of the Funds Requested column in
"Section F. Other Direct Cost."
G. Direct Costs
This total will be automatically calculated based on the sum of the Total funds requested for all
direct costs (sections A-F).
H. Indirect Costs
Indirect costs (Facilities & Administrative [F&A] costs) are defined as costs that are incurred by a
grantee for common or joint objectives and that, therefore, cannot be identified specifically with a
particular project or program. See the NIH Glossary's definition of Indirect Costs.
For more information:
You are encouraged to visit the following Division of Financial Advisory Services (DFAS) Websites
or call DFAS staff at 301-496-2444 for guidance: Main DFAS website, DFAS Frequently Asked
Questions. The following website has a listing of unallowable and unallocable costs and the related
Federal Acquisition Regulation (FAR) citation for each: NIH Office of Management's
Unallowable/Unallocable Costs.
Refer to the NIH Grants Policy Statement, Section 7.4: Reimbursement of Facilities and
Administrative Costs for more information.
R - 69

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities): Foreign
institutions and international organizations may request funds for limited F&A costs (8% of modified
total direct costs less equipment) to support the costs of compliance with HHS and NIH requirements
including, but not limited to, those related to the protection of human subjects, animal welfare,
invention reporting, financial conflict of interest, and research misconduct. Foreign organizations
may not include any charge-back of customs and import fees, such as consular fees, customs surtax,
value-added taxes (VAT), and other related charges.
Indirect Cost Type:
Enter the type of indirect cost (e.g., Salary & Wages, Modified Total Direct Costs, etc.) and whether
the cost is off-site. If more than one rate or base is involved for a given type of indirect cost, then
list them as separate entries. If you do not have a current indirect (F&A) rate(s) approved by a
federal agency, indicate “None--will negotiate” and include information for a proposed rate. Use
Section L. Budget Justification if additional space is needed.
Indirect Cost Rate (%):
Enter the most recent indirect cost rate(s) established with the cognizant federal office, or in the
case of for-profit organizations, the rate(s) established with the appropriate agency. If you have a
cognizant/oversight agency and are selected for an award, you must submit your indirect rate
proposal to the NIH awarding IC or to the PHS awarding office for approval. If you do not have a
cognizant/oversight agency, contact the awarding agency. This field should be entered using a
rate such as “55.5.”
Indirect Cost Base ($):
Enter the amount of the base for each indirect cost type.
Funds Requested ($):
Enter the funds requested for each indirect cost type.
Total Indirect Costs:
This total will be automatically calculated from the “Funds Requested” column in "Section H.
Indirect Cost."
Cognizant Federal Agency:
Enter the name of the cognizant Federal Agency and the name and phone number of the
individual responsible for negotiating your rate (your point of contact). If no cognizant agency is
known, enter “None.”
I. Total Direct and Indirect Costs
This total will be automatically populated from the sum of Total Direct Costs (from Section G.
Direct Cost) and the Total Indirect Costs (from Section H. Indirect Costs).
J. Fee
Do not include a fee in your budget, unless the FOA specifically allows inclusion of a “fee.” If a fee
is allowable, enter the requested fee.
R - 70

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
K. Total Costs and Fee
This total will be automatically calculated from the sum of Total Direct Costs and Fee (from
sections “I. Total Direct and Indirect Costs” and “J. Fee”).
L. Budget Justification
The “Budget Justification” attachment is required. Attach only one file.
Use the Budget Justification to provide the additional information requested in each budget
category identified above and any other information the applicant wishes to submit to support the
budget request. If you have a quote(s), you may include it here (information in the quote may be
not used to supplement information provided in page-limited sections of the application, such as
the Research Strategy). The following budget categories must be justified, where applicable:
equipment, travel, participant/trainee support, and other direct cost categories.
In addition to the justifications described in the above sections, also include a justification for any
significant increases or decreases from the initial budget period. Justify budgets with more than a
standard escalation from the initial to the future year(s) of support.
Also use the Budget Justification to explain any exclusions applied to the F&A base calculation.
If your application includes a subaward/consortium budget, a separate Budget Justification must
be submitted. See R.310 - R&R Subaward Budget Attachment(s) Form.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application include a detailed justification including the
quantity, type(s), and source(s) of the HFT, including the stage of fetal development. This
information must be included if costs for the HFT are assigned to the grant or if the HFT is
acquired under the grant at no costs. The HFT justification must be clearly labeled in the budget
justification attachment.
Special Instructions for Applications Submitted with a Data Management and Sharing
(DMS) Plan:
If a Data Management and Sharing Plan is required in the proposed application (see instructions
for the “Other Plan(s)” attachment on the PHS 398 Research Plan Form and the PHS 398 Career
Development Award Supplemental Form, as applicable), include a brief justification of the
proposed activities that will incur costs. The Data Management and Sharing justification must be
clearly labeled as “Data Management and Sharing Justification” within the budget justification
attachment followed by the estimated dollar amount (total direct costs). Provide a brief summary
of type and amount of scientific data to be preserved and shared and the name of the established
repository(ies) where they will be preserved and shared. Indicate general cost categories such as
curating data and developing supporting documentation, local data management activities,
preserving and sharing data through established repositories, etc., including an amount for each
category and a brief explanation. Specify in the justification if no costs will be incurred for Data
Management and Sharing, if applicable. The recommended length of the justification should be no
more than half a page. For more information, see Budgeting for Data Management & Sharing on
the NIH Scientific Data Sharing website and additional details to help Develop Your Budget.
R - 71

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.300 - R&R Budget Form
Research & Related Budget - Cumulative Budget
All values on this form are automatically calculated, and the fields are pre-populated. They present
the summations of the amounts you entered previously, under Sections A through K, for each of
the individual budget periods. Therefore, no data entry is allowed or required to complete this
“Cumulative Budget” section.
If any of the amounts displayed on this form appear to be incorrect, you may correct it by
adjusting one or more of the values that contribute to that total. To make any such corrections,
you will need to revisit the appropriate budget period form(s).
R - 72

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.310 - R&R Subaward Budget Attachment(s) Form
R.310 - R&R Subaward Budget
Attachment(s) Form
The R&R Subaward Budget Attachment(s) Form is used
for applications with a subaward or consortium.
This form is required only when the prime grantee is
submitting an R&R Budget Form and has
subaward/consortium budgets.
Applicants using the Modular Budget Form should see
R.320 - Modular Budget Form for instructions concerning
information on consortium budgets.
View larger image
Who should use the R&R Subaward Budget Attachment(s) Form?
The R&R Subaward Budget Attachment(s) Form is required if you have a subaward/consortium and are
using the R.300 - R&R Budget Form.
Do not use this form if you are using the PHS Modular Budget Form or if you do not have a
subaward/consortium.
Each consortium grantee organization that performs a substantive portion of the project must complete
an R&R Subaward Budget Attachment, including the Budget Justification section.
Consortium/Contractual F&A Costs:
NIH policy provides for the exclusion of consortium/contractual F&A costs when determining if an
applicant is in compliance with a direct cost limitation. However, you must include the full cost of
subaward/consortium in the Subawards/Consortium Costs field (R.300 - R&R Budget Form, Section F.
Other Direct Costs, Question 5). If a subaward/consortium is not performing a substantive portion of
the project, they do not need to complete an R&R Subaward Budget Form; however, their costs must be
included in the prime grantee’s R&R Budget Form. All F&A costs count toward the direct cost limit.
Refer to the NIH Grants Policy Statement, Section 2.3.7.1: Applications That Include
Consortium/Contractual F&A Costs for policy related to the exclusion of consortium/subaward amounts
in determining whether an applicant is in compliance with a direct cost limitation.
Applicants should document how their budget falls below the direct cost limit in their Budget
Justification on the R&R Subaward Budget Form.
Note on Project Roles for Consortium Lead Investigators:
It is appropriate and expected that someone may serve as the consortium lead investigator responsible
for ensuring proper conduct of the project or program at each subaward or consortium site.
R - 73
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.310 - R&R Subaward Budget Attachment(s) Form
Unless you are submitting your application under the multiple PD/PI policy, consortium lead
investigators are NOT considered PD/PIs for the “Project Role” field. This individual should be assigned
some other project role on the R.300 - R&R Budget Form and in the R.240 – R&R Senior/Key Person
Profile (Expanded) Form. However, the project role of “PD/PI” should be used for a consortium lead
investigator if they also serve as PD/PI for the entire application under the multiple PD/PI policy.
Using the R&R Subaward Budget Attachment(s) Form:
The location of the R&R Subaward Budget Attachment(s) Form may vary with the type of submission
(e.g., under an “Optional Forms” tab).
The steps needed to include a subaward budget in your application vary by submission method. If
submitting using the Grants.gov Workspace, the prime applicant can extract a copy of the R&R Budget
Form from the R&R Subaward Budget Attachment(s) Form and send the extracted file to the
consortium for completion. After the consortium completes the R&R Budget Form, following the
instructions here and in R.300 – R&R Budget Form, the prime grantee must then upload the R&R
Budget Form to the R&R Subaward Budget Attachment(s) Form.
For all submission methods, the R&R Budget Form with a "Budget Type" of Subaward/Consortium is
used to collect subaward budget data. However, ASSIST and other system-to-system solutions may
present a different interface than the R&R Subaward Budget Attachment Form shown here.
This form accommodates a set number of separate subaward budgets. If you need to add more
subaward budgets than the form allows, include the remaining budgets as part of Budget Justification
in R.300 – R&R Budget Form.
Regardless of how many subaward budgets you include, the sum of all subaward budgets (those
attached within the R&R Subaward Budget Attachment(s) Form and those provided as part of the
project budget’s Budget Justification), must be included in R.300 - R&R Budget Form, Section F. Other
Direct Costs, Question 5. Subawards/Consortium/Contractual Costs of the project budget.
Format:
All attachments, including all Subaward Budget Forms and Budget Justifications, must be PDF files. The
R&R Budget Forms are already PDFs when extracted. Do not alter the format. Use of hyperlinks and
URLs in this section is not allowed unless specified in the funding opportunity announcement.
Content:
On this R&R Subaward Budget Attachment(s) Form, you will attach the R&R Subaward Budget files for
your application. Each consortium should complete the Subaward Budget(s) in accordance with the
R.300 - R&R Budget Form instructions.
Submitting Subaward Budgets that are not Active for all Periods of the Prime Grant:
The R&R Budget Forms do not allow for “empty” budget periods.
Subaward/consortiums organizations should complete all budget periods in the R&R Subaward Budget
Form for their subaward budgets, aligning the budget period numbers, start dates, and end dates with
the budget periods of the prime grant.
Example: The prime fills out an R&R Budget Form with the following periods:
l
period 1 - Jan 1, 2017 – Dec 31, 2017
l
period 2 - Jan 1, 2018 – Dec 31, 2018
l
period 3 - Jan 1, 2019 – Dec 31, 2019
R - 74

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.310 - R&R Subaward Budget Attachment(s) Form
l
period 4 - Jan 1, 2020 – Dec 31, 2020
l
period 5 - Jan 1, 2021 – Dec 31, 2021
The budget period numbers and dates should be the same in all the R&R Subaward Budget Forms
included in the R&R Subaward Budget Attachment(s) Form.
The R&R Subaward Budget Forms include several required fields which must be completed (even for
inactive periods) in order to successfully submit the application. Provide the following information for
inactive budget periods in subaward/consortium budgets:
l
Unique Entity Identifier
l
Budget Type = Subaward/Consortium
l
Budget Period Start/End Dates (align with budget periods and dates of the prime budget)
l
In Question "A: Senior/Key Person," provide a single entry including the following:
o
PD/PI or subaward lead First and Last names
o
Project Role (may default to PD/PI; can be adjusted as needed)
o
Calendar Months = .01 (smallest amount effort allowed in the field)
o
Requested Salary = $0
o
Fringe Benefits = $0
l
Explanation of the inactive budget periods in the Budget Justification of the
subaward/consortium's R&R Subaward Budget Form
R - 75

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.320 - PHS 398 Modular Budget Form
R.320 - PHS 398 Modular Budget Form
Some application forms packages include two budget
forms — (1) the R&R Budget Form and (2) the PHS 398
Modular Budget Form. Include only one of these forms,
but not both, in your application.
Generally, the PHS 398 Modular Budget Form is
applicable only to research applications from domestic
organizations that are requesting $250,000 or less per
budget period in direct costs, but there are exceptions.
Refer to your specific FOA and these instructions for
guidance on which budget form(s) to use.
View larger image
Quick Links
Budget Period 1
A. Direct Costs
B. Indirect (F&A) Costs
C. Total Direct and Indirect (F&A) Costs (A+B)
Cumulative Budget Information
1. Total Costs, Entire Project Period
2. Budget Justifications
Who should use the PHS 398 Modular Budget Form?
There are two primary types of Budget Forms: the detailed R&R and PHS 398 modular. Generally, you
must use the PHS Modular Budget Form if you are submitting a research grant application from a
domestic organization and you are applying for $250,000 or less per budget period in direct costs. You
must use the R&R Budget Form if you are applying for more than $250,000 per budget period in direct
costs. However, there are exceptions and other distinctions. Refer to your FOA and to the following
instructions for guidance on which Budget Form to use.
Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities): Foreign
organizations must use the R&R Budget Form in R.300 - R&R Budget Form.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) isincluded in the proposed application, regardless of whether you will incur a cost for
HFT, you cannot use the PHS Modular Budget Form regardless of the activity code and must use the
R&R Budget Form in G.300 - R&R Budget Form.
Note: The terms “detailed budget” and “R&R Budget” are used interchangeably.
R - 76
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.320 - PHS 398 Modular Budget Form
For more information:
For more information on how to prepare your budget, see NIH's Develop Your Budget page.
Also see NIH's Modular Research Grant Applications page.
Modular Budget Guidelines:
Modular budgets are simplified; therefore, detailed categorical information is not to be submitted with
the application.
For all modular budgets, request total direct costs (in modules of $25,000), reflecting appropriate
support for the project. There will be no future year escalations. A typical modular grant application will
request the same number of modules in each budget period. Provide an additional narrative budget
justification (in the Additional Narrative Justification section) for any variation in the number of modules
requested.
Prior to award, NIH may request additional budget justification in exceptional circumstances.
Using the Modular Budget Form:
The Modular Budget Form provides budget fields for up to 5 periods of support (e.g., Budget Periods 1
- 5). A budget period is typically 1 year of support. If requesting fewer than 5 periods/years of support,
complete only the applicable budget periods and leave the others blank. The fields are the same for all
budget periods.
The form will generate information for the Cumulative Budget Information section, which reflects
information for the total project period.
The following instructions (under “Budget Period 1”) can be used for each Budget Period (1-5).
Budget Period 1
Start Date:
This field is required. Enter the requested/proposed start date of the budget period. Use the
following format: MM/DD/YYYY. For period 1, the start date is typically the same date as the
Proposed Project Start Date on the SF 424 (R&R) Form.
End Date:
This field is required. Enter the requested/proposed end date of the budget period. Use the
following format: MM/DD/YYYY.
A. Direct Costs
Direct Cost less Consortium Indirect (F&A):
This field is required.
Enter the amount of direct costs, but do not include actual consortium indirect (F&A) costs. This
figure must be in $25,000 increments, and it may not exceed $250,000 in a budget period. See the
NIH Glossary’s definitions of Direct Cost and Indirect Cost.
R - 77

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.320 - PHS 398 Modular Budget Form
Consortium Indirect (F&A):
If this project involves a subaward/consortium, enter the actual consortium indirect (F&A) costs for
the budget period. If this project does not involve a subaward/consortium, leave the field blank.
Total Direct Costs:
This field will be automatically calculated based on the sum of the “Direct Cost less Consortium
Indirect (F&A)” and “Consortium Indirect (F&A)” fields.
B. Indirect (F&A) Costs
Indirect costs (Facilities & Administrative [F&A] costs) are defined as costs that are incurred by a
grantee for common or joint objectives and that, therefore, cannot be identified specifically with a
particular project or program. See the NIH Glossary's definition of Indirect Costs.
For more information:
You are encouraged to visit the following Division of Financial Advisory Services (DFAS) Websites
or call DFAS staff at 301-496-2444 for guidance: Main DFAS website, DFAS Frequently Asked
Questions. The following website has a listing of unallowable and unallocable costs and the related
Federal Acquisition Regulation (FAR) citation for each: NIH Office of Management's Unallowable /
Unallocated costs.
Refer to the NIH Grants Policy Statement, Section 7.4: Reimbursement of Facilities and
Administrative Costs for more information.
Indirect (F&A) Type:
Enter the type/base of indirect cost (e.g., Salary & Wages, Modified Total Direct Costs, etc.) and
whether the cost is off-site. If more than one rate or base is involved for a given type of indirect
cost, then list them as separate entries. If you do not have a current indirect (F&A) rate(s) approved
by a federal agency, indicate “None—will negotiate” and include information for a proposed rate.
Use the Budget Justification if additional space is needed.
Indirect (F&A) Rate (%):
Indicate the most recent Indirect (F&A) cost rate(s) established with the cognizant federal office, or
in the case of for-profit organizations, the rate(s) established with the appropriate agency. If you
have a cognizant/oversight agency and are selected for an award, you must submit your indirect
rate proposal to the NIH awarding IC or to the PHS awarding office for approval. If you do not
have a cognizant/oversight agency, contact the awarding agency. This field should be entered
using a rate such as “55.5.”
Indirect (F&A) Base ($):
Enter the amount of the base for each indirect cost type.
Funds Requested ($):
Enter the funds requested for each indirect cost type.
Cognizant Agency (Agency Name, POC Name and Phone Number):
Enter the name of the cognizant Federal Agency and the name and phone number of the
individual responsible for negotiating your rate (your point of contact). If no cognizant agency is
known, enter “None.”
Indirect (F&A) Rate Agreement Date:
If you have a negotiated rate agreement, enter the agreement date.
R - 78

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.320 - PHS 398 Modular Budget Form
Total Indirect (F&A) Costs:
This field will be automatically calculated based on the sum of the "Funds Requested" fields from
all of the Indirect (F&A) Costs.
C. Total Direct and Indirect (F&A) Costs (A+B)
Funds Requested ($):
This field will be automatically calculated based on the sum of the “Total Direct Costs” and “Total
Indirect (F&A) Costs” fields.
Cumulative Budget Information
1. Total Costs,Entire Project Period
All values for the “Total Costs, Entire Project Period” section are automatically calculated and the
fields are pre-populated. They present the summations of the amounts you entered for each of the
individual budget periods. Therefore, no data entry is allowed or required in the “Total Costs,
Entire Project Period” section.
If any of the amounts displayed in this “Total Costs, Entire Project Period” section appear to be
incorrect, you may correct it by adjusting one or more of the values that contribute to that total. To
make any such corrections, you will need to revisit the appropriate budget period form(s).
2. Budget Justifications
Personnel Justification:
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page. Hyperlinks and URLs are
not allowed in this section unless specified in the funding opportunity announcement.
Content:
List all personnel, including names, percent effort (use the Person Months metric), and roles on the
project.
Do not provide individual salary information. You must use the current legislatively imposed salary
limitation when estimating the number of modules. For guidance on current salary limitations,
contact your office of sponsored programs.
Administrative, Secretarial, and Clerical Support Salaries: In most circumstances, the salaries
of administrative, secretarial, or clerical staff at educational institutions and nonprofit
organizations are included as part of indirect costs. However, examples of situations where direct
charging of these salaries may be appropriate may be found at 45 CFR 75.403.
Inclusion of such costs may be appropriate only if all of the following conditions are met:
R - 79
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.320 - PHS 398 Modular Budget Form
1. Administrative or clerical services are integral to a project or activity;
2. Individuals involved can be specifically identified with the project or activity;
3. Such costs are explicitly included in the budget or have prior written approval of the
federal awarding agency; and
4. The costs are not also recovered as indirect costs.
Requests for direct charging for administrative, secretarial, or clerical personnel must be
appropriately justified here in the “Personnel Justification.” For each individual classified as
administrative/secretarial/clerical, provide the name; percent effort; role; and a justification
documenting how they meet all four conditions. NIH ICs may request additional information for
these positions in order to assess allowability.
Graduate student compensation: NIH grants also limit compensation for graduate students.
Compensation includes salary or wages, fringe benefits, and tuition remission. While actual
institutional-based compensation should be requested and justified, this may be adjusted at the
time of award. This limit should also be used when estimating the number of modules. For more
guidance on this policy, see the NIH Grants Policy Statement, Section 2.3.7.9: Graduate Student
Compensation.
Consortium Justification:
Format:
Attach this information as a PDF file. See the NIH's Format Attachment page.
Content:
Provide an estimate of total consortium/subaward costs (direct costs plus indirect [F&A] costs) for
each budget period, rounded to the nearest $1,000.
List the individuals/organizations with whom consortium or contractual arrangements have been
made and indicate whether the collaborating institution is foreign or domestic.
List all personnel, including names, percent effort (use the Person Months metric), and roles on the
project.
Do not provide individual salary information.
Additional Narrative Justification:
Note: Additional explanation within the Additional Narrative Justification is not needed in
applications to FOAs with direct cost limits that do not spread evenly across budget periods (e.g.,
R21 NOFOs that allow $275,000 in direct costs over two years).
Special Instructions for Applications Submitted with a Data Management and Sharing
(DMS) Plan: If a Data Management and Sharing (DMS) Plan is required in the proposed
application, (see instructions for the “Other Plan(s)” attachment on the PHS 398 Research Plan
Form and the PHS 398 Career Development Award Supplemental Form, as applicable), the
Additional Narrative Justification is required.
Format:
Attach this information as a PDF file. See the NIH's Format Attachment page. Hyperlinks and URLs
are not allowed in this section unless specified in the funding opportunity announcement.
R - 80
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.320 - PHS 398 Modular Budget Form
Content:
If the requested budget requires any additional justification (e.g., variations in the number of
modules requested, applications submitting a DMSplan), include that information in the
Additional Narrative Justification attachment. If you have a quote(s), you may include it here
(information in the quote may be not used to supplement information provided in page-limited
sections of the application, such as the Research Strategy).
Additional justification should include explanations for any variations in the number of modules
requested annually. Also, this section should describe any direct costs that were excluded from the
total direct costs (such as equipment, tuition remission) and any work being conducted off-site,
especially if it involves a foreign study site or an off-site F&A rate.
Note: Additional explanation for variations in the number of modules requested annually is not
needed in applications to NOFOs with direct cost limits that do not spread evenly across budget
periods (e.g., R21 NOFOs that allow $275,000 in direct costs over two years).
Special Instructions for Applications Submitted with a Data Management and Sharing
(DMS) Plan:
NIH recognizes that making data accessible and reusable for other researchers may incur costs. If a
Data Management and Sharing Plan is required in the proposed application (see instructions for
the “Other Plan(s)” attachment on the PHS 398 Research Plan Form and the PHS 398 Career
Development Award Supplemental Form, as applicable), the Data Management and Sharing
justification must be clearly labeled as “Data Management and Sharing Justification” followed by
the estimated dollar amount (total direct costs). If no cost will be incurred, enter "0" for the
estimated dollar amount. Also include a brief justification of the proposed activities that will incur
costs. Provide a brief summary of type and amount of scientific data to be preserved and shared
and the name of the established repository(ies) where they will be preserved and shared. Indicate
general cost categories such as curating data and developing supporting documentation, local
data management considerations, preserving and sharing data through established repositories,
etc., including an amount for each category and a brief explanation. The recommended length of
the justification should be no more than half a page.
Allowable and Unallowable Costs: Allowable costs submitted in budget requests must be
incurred during the performance period, even for scientific data and metadata preserved and
shared beyond the award period. Budget requests must NOT include: Infrastructure costs that are
included in institutional overhead (for instance, NIH Grants Policy Statement Section 7.3 Facilities
and Administrative costs); costs associated with the routine conduct of research, including costs
associated with collecting or gaining access to research data; or costs that are double charged or
inconsistently charged as both direct and indirect costs. For more information, see Budgeting for
Data Management & Sharing on the NIH Scientific Data Sharing website and additional details to
help Develop Your Budget.
R - 81

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
R.400 - PHS 398 Research Plan Form
The PHS 398 Research Plan form is used only for
research, multi-project, and SBIR/STTR applications.
This form includes fields to upload several attachments,
including the Specific Aims and Research Strategy.
The Research Plan, together with the rest of your
application, should include sufficient information needed
for evaluation of the project, independent of any other
documents (e.g., previous application). Be specific and
informative, and avoid redundancies.
View larger image
Quick Links
Introduction
1. Introduction to Application (for Resubmission and
Revision applications)
Research Plan Section
2. Specific Aims
3. Research Strategy
4. Progress Report Publication List
Other Research Plan Section
5. Vertebrate Animals
6. Select Agent Research
7. Multiple PD/PI Leadership Plan
8. Consortium/Contractual Arrangements
9. Letters of Support
10. Resource Sharing Plan(s)
11. Other Plan(s)
12. Authentication of Key Biological and/or Chemical Resources
Appendix
13. Appendix
Your application should represent a sound approach to the investigation of an important biomedical
research, behavioral research, technological, engineering, or scientific question, and be worthy of
support under the stated criteria of the FOA. It should be self-contained and written with the care and
thoroughness accorded to papers for publication.
R - 82

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Review the application carefully to ensure you have included information essential for evaluation. The
scientific and technical merit of the proposed research is the primary concern for all research supported
by the National Institutes of Health (NIH) and other PHS agencies.
Read all the instructions in the FOA before completing this form to ensure that your application meets
all IC-specific criteria.
Who should use the PHS398 Research Plan Form:
Use the PHS 398 Research Plan Form only if you are submitting a research, multi-project, or SBIR/STTR
application.
Applicants must follow all policies and requirements related to formatting, page limits, and
proprietary information. See the following pages for more information:
l
Format Attachments
l
Page Limits
l
NIH Grants Policy Statement, Section 2.3.11.2: Confidentiality of Information
l
NIH Grants Policy Statement, Section 2.3.11.2.2: The Freedom of Information Act
Introduction
1. Introduction to Application (for Resubmission and Revision applications)
Who must complete the “Introduction to Application” attachment:
An "Introduction to Application" attachment is required only if the type of application is
resubmission or revision or if the FOA specifies that one is needed. An introduction is not allowed
for new or renewal applications.
Descriptions of different types of applications are listed here: NIH Types of Applications.
Format:
Follow the page limits for the introduction in the NIH Table of Page Limits unless otherwise
specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page. Hyperlinks and URLs
may not be used in this section unless specified as allowed in the funding opportunity
announcement.
Content:
Resubmission applications: See specific instructions on the content of the introduction on the
NIH's Resubmission Applications page.
Note: For resubmission applications changing from a single PD/PI to multiple PD/PIs,
changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change in the introduction and include the required Multiple PD/PI
Leadership Plan. A rationale for a change from a multiple PD/PI to a single PD/PI application
must also be provided in the introduction.
R - 83
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Competing Revisions: See specific instructions on the content of the introduction on the NIH's
Competing Revisions page.
Research Plan Section
2. Specific Aims
Who must complete the "Specific Aims" attachment:
The “Specific Aims” attachment is required unless otherwise specified in the FOA.
Format:
Follow the page limits for the Specific Aims in the NIH Table of Page Limits unless otherwise
specified in the FOA. A “Specific Aims” attachment that exceeds the page limit will be flagged as an
error by the Agency upon submission.
Attach this information as a PDF file. See NIH's Format Attachments page. Hyperlinks and URLs
may not be used in this section unless specified as allowed in the funding opportunity
announcement.
Content:
State concisely the goals of the proposed research and summarize the expected outcome(s),
including the impact that the results of the proposed research will have on the research field(s)
involved.
List succinctly the specific objectives of the research proposed (e.g., to test a stated hypothesis,
create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice,
address a critical barrier to progress in the field, or develop new technology).
3. Research Strategy
Who must complete the "Research Strategy" attachment:
The “Research Strategy” attachment is required.
Format:
Follow the page limits for the Research Strategy in the NIH Table of Page Limits, unless otherwise
specified in the FOA. Although multiple sections of information are required in the Research
Strategy as detailed below, the page limit applies to the entirety of the single "Research Strategy"
attachment.
Attach this information as a PDF file. See NIH's Format Attachments page. Hyperlinks and URLs
may not be used in this section unless specified as allowed in the funding opportunity
announcement.
Content:
Organize the Research Strategy in the specified order and use the instructions provided below
unless otherwise specified in the FOA. Start each section with the appropriate heading –
Significance, Innovation, Approach.
R - 84
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Cite published experimental details in the Research Strategy attachment and provide the full
reference in R.220 - R&R Other Project Information Form, Bibliography and Reference Cited.
Note for Applications Proposing the Use of Human Fetal Tissue: If the use of human fetal
tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy Statement) is
included in the proposed application you must include specific information in the Approach
section of the Research Strategy attachment. See specific instructions below in Section 3.
Approach. This information must be provided regardless of whether Human Subjects research is
proposed or not.
Applications proposing HFTthat do not address these requirements will be administratively
withdrawn. For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section
2.3.7.11 Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research
and Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
Note for Applications Proposing the Involvement of Human Subjects and/or Clinical Trials:
l
Do not duplicate information in the Research Strategy and the PHS Human Subjects and
Clinical Trials Information form. Use the Research Strategy attachment to discuss the overall
strategy, methodology, and analyses of your proposed research. Use the PHS Human Subjects
and Clinical Trials Information form to provide detailed information for human subjects studies
and clinical trials.
l
The PHS Human Subjects and Clinical Trials Information form will capture detailed study
information, including eligibility criteria; inclusion of women, minorities, and individuals across
the lifespan; protection and monitoring plans; and statistical design and power.
l
You are encouraged to refer to information in the PHS Human Subjects and Clinical Trials
Information form as appropriate in your discussion of the Research Strategy (e.g., see Question
2.4 Inclusion of Women and Minorities).
Note for Applicants with Multiple Specific Aims: You may address the Significance, Innovation,
and Approach either for each Specific Aim individually or for all of the Specific Aims collectively.
1. Significance
l
Explain the importance of the problem or critical barrier to progress that the proposed
project addresses.
l
Describe the strengths and weaknesses in the rigor of the prior research (both published
and unpublished) that serves as the key support for the proposed project.
l
Explain how the proposed project will improve scientific knowledge, technical capability,
and/or clinical practice in one or more broad fields.
Additional Instructions for Research:
Describe how the concepts, methods, technologies, treatments, services, or
preventative interventions that drive this field will be changed if the proposed aims
are achieved.
2. Innovation
l
Explain how the application challenges and seeks to shift current research or clinical
practice paradigms.
R - 85
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
l
Describe any novel theoretical concepts, approaches or methodologies, instrumentation
or interventions to be developed or used, and any advantage over existing
methodologies, instrumentation, or interventions.
l
Explain any refinements, improvements, or new applications of theoretical concepts,
approaches or methodologies, instrumentation, or interventions.
3. Approach
l
Describe the overall strategy, methodology, and analyses to be used to accomplish the
specific aims of the project. Describe plans to address weaknesses in the rigor of the prior
research that serves as the key support for the proposed project. Describe the
experimental design and methods proposed and how they will achieve robust and
unbiased results. Include how the data will be collected, analyzed, and interpreted, and
reference any Resource Sharing Plans and the Data Management and Sharing (DMS) Plan,
as appropriate. Resources and tools for rigorous experimental design can be found at the
Enhancing Reproducibility through Rigor and Transparency website.
l
For trials that randomize groups or deliver interventions to groups, describe how your
methods for analysis and sample size are appropriate for your plans for participant
assignment and intervention delivery. These methods can include a group- or cluster-
randomized trial or an individually randomized group-treatment trial. Additional
information is available at the Research Methods Resources webpage.
l
Discuss potential problems, alternative strategies, and benchmarks for success anticipated
to achieve the aims.
l
If the project is in the early stages of development, describe any strategy to establish
feasibility, and address the management of any high risk aspects of the proposed work.
l
Explain how relevant biological variables, such as sex, are factored into research designs
and analyses for studies in vertebrate animals and humans. For example, strong
justification from the scientific literature, preliminary data, or other relevant
considerations, must be provided for applications proposing to study only one sex. Refer
to the NIH Guide Notice on Sex as a Biological Variable in NIH-funded Research for
additional information.
l
Point out any procedures, situations, or materials that may be hazardous to personnel and
the precautions to be exercised. A full discussion on the use of select agents should
appear in the Select Agent Research attachment below.
l
If research on Human Embryonic Stem Cells (hESCs) is proposed but an approved cell line
from the NIH hESC Registry cannot be chosen, provide a strong justification for why an
appropriate cell line cannot be chosen from the registry at this time.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application
l
Use the specific heading: “Human Fetal Tissue Research Approach”.
l
Describe the proposed characteristics, procurement, and procedures for the research use of
HFT. The description should be sufficiently detailed to permit meaningful evaluation by NIH.
R - 86

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
l
Justify the use of HFT in the proposed research by indicating the following:
l
Why the research goals cannot be accomplished by using an alternative to HFT.
l
What methods were used (e.g. literature review, preliminary data) to determine that
alternatives could not be used.
l
Results from a literature review used to provide justifications.
l
Plans for the treatment of HFT and the disposal of HFT when research is complete.
l
Description of planned written, voluntary, informed consent process for cell/tissue
donation, or description and documentation of process if cells/tissue were already
obtained.
Applications proposing HFT that do not address these requirements will be administratively
withdrawn. For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section
2.3.7.11 Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research
and Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
As applicable, also include the following information as part of the Research Strategy,
keeping within the three sections (Significance, Innovation, and Approach) listed above.
Preliminary Studies for New Applications:
For new applications, include information on preliminary studies. Discuss the PD/PI’s preliminary
studies, data, and or experience pertinent to this application. Except for
Exploratory/Developmental Grants (R21/R33), Small Research Grants (R03), and Academic
Research Enhancement Award (AREA) Grants (R15), preliminary data can be an essential part of a
research grant application and can help to establish the likelihood of success of the proposed
project. Early stage investigators should include preliminary data.
Progress Report for Renewal and Revision Applications:
Note that the Progress Report falls within the Research Strategy and is therefore included in the
page limits for the Research Strategy.
For renewal/revision applications, provide a Progress Report. Provide the beginning and ending
dates for the period covered since the last competitive review. In the Progress Report, you should:
l
Summarize the specific aims of the previous project period and the importance of the
findings, and emphasize the progress made toward their achievement.
l
Explain any significant changes to the specific aims and any new directions, including
changes resulting from significant budget reductions.
l
Discuss previous participant enrollment (e.g., recruitment, retention, inclusion of women,
minorities, children, etc.) for any studies meeting the NIH definition for clinical research.
Use the Progress Report section to discuss, but not duplicate information collected
elsewhere in the application.
Do not include a list of publications, patents, or other printed materials in the Progress Report.
That information will be included in the "Progress Report Publication List" attachment.
Renewal Applications: For renewal applications changing from a single PD/PI to multiple
PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change and include the required Multiple PD/PI Leadership Plan. A rationale for a
change from a multiple PD/PI to a single PD/PI application must also be provided.
R - 87
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
4. Progress Report Publication List
Who must complete the “Progress Report Publication List” attachment:
A “Progress Report Publication List” attachment is required only if the type of application is
renewal.
Descriptions of different types of applications are listed here: NIH's Types of Applications.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page. Use of hyperlinks and
URLs in this section is not allowed unless specified in these instructions or in the funding
opportunity announcement.
Content:
List the titles and complete references to all appropriate publications, manuscripts accepted for
publication, patents, and other printed materials that have resulted from the project since it was
last reviewed competitively.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions on citing interim research products
and claiming them as products of your NIH award.
Provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed
Central (PMC) reference number (e.g., PMCID234567) for each of the following:
l
Articles that fall under the Public Access Policy,
l
Articles that were authored or co-authored by the applicant and arose from NIH support,
l
Articles that were authored or co-authored by the applicant and arose from AHRQ
funding provided after February 19, 2016 (see the Guide Notice on Policy for Public
Access to AHRQ-Funded Scientific Publications).
If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of
their authors, indicate “PMC Journal – In Process.” NIHmaintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free,
online format may include URLs or PubMed ID (PMID) numbers along with the full reference.
Active hyperlinks are not allowed.
Other Research Plan Section
5. Vertebrate Animals
Who must complete the “Vertebrate Animals” attachment:
Include a “Vertebrate Animals” attachment if you answered “Yes” to the question “Are Vertebrate
Animals Used?” on the R.220 - R&R Other Project Information Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Do not use this attachment to circumvent the page limits of the Research Strategy.
R - 88
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Content:
If live vertebrate animals are involved in the project, address each of the following criteria:
1. Description of Procedures: Provide a concise description of the proposed procedures to
be used that involve live vertebrate animals in the work outlined in the “Research
Strategy” attachment. The description must include sufficient detail to allow evaluation of
the procedures. Identify the species, strains, ages, sex, and total numbers of animals by
species, to be used in the proposed work. If dogs or cats are proposed, provide the source
of the animals.
2. Justifications: Provide justification that the species are appropriate for the proposed
research. Explain why the research goals cannot be accomplished using an alternative
model (e.g. computational, human, invertebrate, in vitro).
3. Minimization of Pain and Distress: Describe the interventions including analgesia,
anesthesia, sedation, palliative care and humane endpoints that will be used to minimize
discomfort, distress, pain, and injury.
Each of the criteria must be addressed. Failure to adequately address the criteria may negatively
affect the application’s impact score. In addition to the 3 criteria above, you should also:
l
Identify all project performance (or collaborating) sites and describe the proposed
research activities with vertebrate animals that will be conducted at those sites.
l
Explain when and how animals are expected to be used if plans for the use of animals
have not been finalized.
See the following pages for more information:
l
NIH’s Office of Laboratory Animal Welfare website
l
NIH's Vertebrate Animals Section Worksheet
l
See the NIH Grants Policy Statement, Section 4.1.1: Animal Welfare Requirements (an
applicable Animal Welfare Assurance will be required if the grantee institution does not have
one)
6. Select Agent Research
Who must complete the “Select Agent Research” attachment:
Include a “Select Agent Research” attachment if your proposed activities involve the use of select
agents at any time during the proposed project period, either at the applicant organization or at
any performance site.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
For more information:
Select agents are hazardous biological agents and toxins that have been identified by HHS or
theU.S. Department of Agriculture (USDA) as having the potential to pose a severe threat to public
health and safety, to animal and plant health, or to animal and plant products. The Centers for
Disease Control and Prevention (CDC) and the Animal and Plant Health Inspection Service (APHIS)
R - 89

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Select Agent Programs jointly maintain a list of these agents. See the Federal Select Agent
Program website.
See also the NIH Grants Policy Statement, Section 4.1.24.1.1: Select Agents.
Content:
Excluded select agents: If the activities proposed in the application involve only the use of a
strain(s) of select agents which has been excluded from the list of select agents and toxins as per
42 CFR 73.3, the select agent requirements do not apply. Use this “Select Agent Research”
attachment to identify the strain(s) of the select agent that will be used and note that it has been
excluded from this list. The CDC maintains a list of exclusions, which is available on the Select
Agents and Toxins Exclusions website.
Applying for a select agent to be excluded: If the strain(s) is not currently excluded from the list
of select agents and toxins but you have applied or intend to apply to HHS for an exclusion from
the list, use this section to indicate the status of your request or your intent to apply for an
exclusion and provide a brief justification for the exclusion.
All applicants proposing to use select agents: Address the following three points for each site
at which select agent research will take place. Although no specific page limitation applies to this
section, be succinct.
1. Identify the select agent(s) to be used in the proposed research.
2. Provide the registration status of all entities* where select agent(s) will be used.
l
If the performance site(s) is a foreign institution, provide the name(s) of the country
or countries where select agent research will be performed.
l
*An “entity” is defined in 42 CFR 73.1 as “any government agency (Federal, State, or
local), academic institution, corporation, company, partnership, society, association,
firm, sole proprietorship, or other legal entity.”
3. Provide a description of all facilities where the select agent(s) will be used.
l
Describe the procedures that will be used to monitor possession, use, and transfer of
select agent(s).
l
Describe plans for appropriate biosafety, biocontainment, and security of the select
agent(s).
l
Describe the biocontainment resources available at all performance sites.
7. Multiple PD/PI Leadership Plan
Who must complete the “Multiple PD/PI Leadership Plan” attachment:
Any applicant who designates multiple PD/PIs (on the R.240 - R&R Senior/Key Person Profile
(Expanded) Form) must include a Multiple PD/PI Leadership Plan. For applications designating
multiple PD/PIs, all such individuals must be assigned the PD/PI role on the R.240 - R&R
Senior/Key Profile (Expanded) Form, even those at organizations other than the applicant
organization.
Do not submit a Multiple PD/PI Leadership Plan if you are not submitting a multiple PD/PI
application.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
R - 90
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Content:
A rationale for choosing a multiple PD/PI approach should be described. The governance and
organizational structure of the leadership team and the research project should be described,
including communication plans, processes for making decisions on scientific direction, and
procedures for resolving conflicts. The roles and administrative, technical, and scientific
responsibilities for the project or program should be delineated for the PD/PIs and other
collaborators.
If budget allocation is planned, the distribution of resources to specific components of the project
or the individual PD/PIs should be delineated in the Multiple PD/PI Leadership Plan. In the event of
an award, the requested allocations may be reflected in a footnote on the Notice of Grant Award.
Resubmission Applications: For resubmission applications changing from a single PD/PI to
multiple PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must
provide a rationale for the change in the introduction and include the required Multiple PD/PI
Leadership Plan.
Renewal Applications: For renewal applications changing from a single PD/PI to multiple
PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change in the progress report within the research strategy and include the
required Multiple PD/PI Leadership Plan.
For more information:
For background information on the multiple PD/PI initiative, see NIH's Multiple Principal
Investigators page.
8. Consortium/Contractual Arrangements
Who must complete the “Consortium/Contractual Arrangements” attachment:
Include a “Consortium/Contractual Arrangements” attachment if you have consortiums/contracts
in your budget.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Explain the programmatic, fiscal, and administrative arrangements to be made between the
applicant organization and the consortium organization(s). If consortium/contractual activities
represent a significant portion of the overall project, explain why the applicant organization, rather
than the ultimate performer of the activities, should be the grantee.
Note: The signature of the authorized organization representative in R.200 - SF 424 (R&R),
Authorized Representative signifies that the applicant and all proposed consortium participants
understand and agree to the following statement:
The appropriate programmatic and administrative personnel of each organization involved in
this grant application are aware of the agency’s consortium agreement policy and are prepared
to establish the necessary inter-organizational agreement(s) consistent with that policy.
For more information:
Refer to the NIH Grants Policy Statement, Section 15: Consortium Agreements for more
information.
R - 91
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
9. Letters of Support
Format:
Combine all letters of support into a single PDF file and attach this information here. Do not place
these letters in the Appendix.
Follow the attachment guidelines on NIH's Format Attachments page. Use of hyperlinks and URLs
in Letters of Support is not allowed unless specified in the funding opportunity announcement.
Content:
Attach a file with all letters of support, including any letters necessary to demonstrate the support
of consortium participants and collaborators such as Senior/Key Personnel and Other Significant
Contributors included in the grant application.
Letters should stipulate expectations for co-authorship, and whether cell lines, samples, or other
resources promised in the letter are freely available to other investigators in the scientific
community or will be provided to the particular investigators only.
For consultants, letters should include rate/charge for consulting services and level of effort /
number of hours per budget period anticipated. In addition, letters ensuring access to core
facilities and resources should stipulate whether access will be provided as a fee-for-service.
Material Transfer Agreements may be included in this section.
Letters must focus on the topics listed above and not contain data / figures / tables / graphs,
preliminary data, methods, background and significance details that are expected to be found in
Research Strategy section of the application. Letters of Support serve to describe terms of a
collaboration or consultation and also are not de facto letters of reference from persons not
actively participating in the project. Applications with letters containing such excess information
may be withdrawn from the review process.
Letters are not required for personnel (such as research assistants) not contributing in a
substantive, measurable way to the scientific development or execution of the project.
Do not include consultant biographical sketches in the “Letters of Support” attachment, as
consultant biosketches should be in the “Biographical Sketch” section.
10. Resource Sharing Plan(s)
Note: Effective for due dates on or after January 25, 2023, Data Management and
Sharing (DMS) Plans are now included in Section 11. Other Plan(s). Plans for Genomic Data
Sharing should be provided as part of the Data Management and Sharing Plan.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Sharing Model Organisms: Regardless of the amount requested, all applications where the
development of model organisms is anticipated are expected to include a description of a specific
plan for sharing and distributing unique model organisms or state why such sharing is restricted or
not possible. For more information, see the NIH Grants Policy Statement, Section 8.2.3.2: Sharing
Model Organisms.
R - 92

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Research Tools:
NIH considers the sharing of unique research resources developed through NIH-sponsored
research an important means to enhance the value and further the advancement of the research.
When resources have been developed with NIH funds, and the associated research findings
published or provided to NIH, it is important that they be made readily available for research
purposes to qualified individuals within the scientific community. For more information, see the
Research Tools Policy on the NIH Scientific Data Sharing Website and the NIH Grants Policy
Statement, Section 8.2.3: Sharing Research Resources.
11. Other Plan(s)
Who Must Complete This Section: Refer to the list of NIH activity codes subject to the DMS
Policy and your Funding Opportunity Announcement to determine if your application is required
to provide an attachment and address a Data Management and Sharing (DMS) Plan. Applicants
proposing to conduct research that will generate scientific data are subject to the NIH Data
Management and Sharing Policy and must attach a Data Management and Sharing (DMS) Plan.
Scientific data is defined as the recorded factual material commonly accepted in the scientific
community as of sufficient quality to validate and replicate research findings, regardless of
whether the data are used to support scholarly publications. Scientific data includes any data
needed to validate and replicate research findings. Scientific data does not include laboratory
notebooks, preliminary analyses, completed case report forms, drafts of scientific papers, plans for
future research, peer reviews, communications with colleagues, or physical objects such as
laboratory specimens.
The NIH Genomic Data Sharing Policy expects applicants seeking funding for research that
generates large-scale human or non-human genomic data to provide a plan for sharing of these
data as part of their DMS Plan.
Applicants subject to both the NIH Data Management and Sharing Policy and the NIH Genomic
Data Sharing Policy must attach a single Plan including elements for both policies. For more on
applicability of each policy, see research subject to the NIH Data Management and Sharing Policy
and the research subject to the NIH Genomic Data Sharing Policy.
Format: Attach this information as a PDF file. See NIH's Format Attachments page.
A sample format is provided on the Data Management and Sharing Plan Format Page to assist
applicants with preparation of this attachment. Do not include hyperlinks in this attachment.
Recommended not to exceed two pages.
Content: Follow the expectations of the NIH Policy for Data Management and Sharing and
address the Elements of an NIH Data Management and Sharing Plan described below.
Additional expectations: A Data Management and Sharing Plan should reflect the proposed
approach at the time the application is prepared. For some programs and data types, NIH and/or
NIH Institutes, Centers, Offices, or programs have developed additional data sharing requirements
(e.g., specifying which scientific data to share, relevant standards, repository selection, timelines)
that apply and should be reflected in a Plan. These additional requirements may be listed on NIH
Institute and Center Data Sharing Policies or in specific funding opportunity announcements. Note
that some NIH Institutes, Centers, Officers, or programs have developed additional expectations
for sharing genomic data that may be listed on NIH Institute and Center Genomic Data Sharing
Expectations or in specific funding opportunity announcements.
R - 93
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Elements of a Data Management and Sharing Plan:
Data Type: Briefly describe the scientific data to be managed, preserved, and shared,
including a general summary of the types and estimated amount of scientific data to be
generated and a description of which scientific data from the project will be preserved and
shared as well as the rationale for doing so. Briefly list the metadata, other relevant data, and
any associated documentation (e.g., study protocols and data collection instruments) that will
be made accessible to facilitate interpretation of the scientific data.
Related Tools, Software and/or Code: State whether specialized tools are needed to access or
manipulate shared scientific data to support replication or reuse, and name(s) of the needed
tool(s) and software. If specialized tools or software are needed, provide the name(s) of the
needed tool(s) and software and specify how they can be accessed.
Standards: State what common data standards will be applied to the scientific data and
associated metadata to enable interoperability of datasets and resources (e.g., data formats,
data dictionaries, data identifiers, definitions, unique identifiers, and other data
documentation), and provide the name(s) of the data standards that will be applied and
describe how these data standards will be applied to the scientific data generated by the
research proposed in this project. If applicable, indicate that no consensus standards exist.
Data Preservation, Access, and Associated Timelines: Provide plans and timelines for data
preservation and access, including the name of the repository(ies) where scientific data and
metadata arising from the project will be archived (do not include hyperlinks); how the
scientific data will be findable and identifiable, i.e., via a persistent unique identifier or other
standard indexing tools; and when (i.e., no later than time of an associated publication or end
of the performance period, whichever comes first) the scientific data will be made available to
other users (e.g., the larger research community, institutions, and/or the broader public) and
for how long. See Selecting a Data Repository on the NIH Scientific Data Sharing website.
Access, Distribution, or Reuse Considerations: NIH expects that in drafting Plans, researchers
maximize the appropriate sharing of scientific data generated from NIH-funded or conducted
research, consistent with privacy, security, informed consent, and proprietary issues. Describe
and justify any applicable factors affecting subsequent access, distribution, or reuse of
scientific data related to informed consent, privacy and confidentiality protections, any
restrictions imposed by federal, Tribal, or state laws, regulations, or policies, or existing or
anticipated agreements, or any other considerations that may limit the extent of data sharing.
See Frequently Asked Questions for examples of justifiable reasons for limiting sharing of
data. State whether access to the scientific data will be controlled (i.e., made available by a
data repository only after approval).
Genomic Data Sharing Policy: For proposed research subject to the GDS Policy, state
whether data, including genomic summary results, will be made available through controlled
or unrestricted access; see instructions for describing Genomic Summary Results in Data
Management and Sharing Plans.
If generating scientific data derived from humans, describe how the privacy, rights, and
confidentiality of human research participants will be protected (e.g., through de-
identification, Certificates of Confidentiality, and other protective measures). See NIH’s
Scientific Data Sharing page for additional information on protecting human research
participant privacy when sharing data.
Genomic Data Sharing Policy: For proposed research generating human genomic data
within the scope of the GDS Policy, applicants should complete the Data Management and
R - 94

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Sharing Plan anticipating sharing according to the assurances of the Institutional
Certification.
If there is any element of the Institutional Certification that the institution (in consultation
with the IRB) has determined cannot be met, please state which element and provide a
detailed explanation for why the element cannot be met. In such cases, the data management
and sharing plan should describe how genomic data will be shared to the maximal extent
possible (for example, sharing data in a summary format).
Oversight of Data Management and Sharing: Describe how compliance with the Plan will be
monitored and managed, frequency of oversight, and by whom at the applicant institution
(e.g., titles, roles).
For more information on developing a Data Management and Sharing Plan, see Writing a
Data Management and Sharing Plan on the NIH Scientific Data Sharing website.
For more information on the DMS Policy, including expectations for data management and
sharing, protecting research participant privacy, and identifying data repositories, see the NIH Data
Management and Sharing Policy on the NIH Scientific Data Sharing website and the NIH Grants
Policy Statement, Section 8.2.3.1: Data Sharing Policy. See also Frequently Asked Questions for
additional information on the DMS Policy on these and other topics.
For more information on the GDS Policy see the NIH Genomic Data Sharing Policy on the NIH
Scientific Data Sharing website and the NIH Grants Policy Statement, Section 8.2.3.3: Genomic
Data Sharing (GDS) Policy/ Policy for Genome-Wide Association Studies (GWAS).
12. Authentication of Key Biological and/or Chemical Resources
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
If applicable to the proposed science, briefly describe methods to ensure the identity and validity
of key biological and/or chemical resources used in the proposed studies. A maximum of one page
is suggested.
For more Information:
Key biological and/or chemical resources are characterized as follows.
l
Key biological and/or chemical resources may or may not have been generated with NIH
funds and: 1) may differ from laboratory to laboratory or over time; 2) may have qualities
and/or qualifications that could influence the research data; and 3) are integral to the
proposed research. These include, but are not limited to, cell lines, specialty chemicals,
antibodies, and other biologics.
l
Standard laboratory reagents that are not expected to vary do not need to be included in
the plan. Examples are buffers and other common biologicals or chemicals.
l
See NIH's page on Rigor and Reproducibility for more information.
R - 95

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.400 - PHS 398 Research Plan Form
Appendix
13. Appendix
Refer to the FOA to determine whether there are any special appendix instructions for your
application. See the updated NIH Guide Notice on the Appendix Policy.
Format:
A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 allowable appendix
attachments are needed, combine the remaining information into attachment #10.
Use filenames for attachments that are descriptive of the content.
A summary sheet listing all of the items included in the Appendix is encouraged but not required.
When including a summary sheet, it should be included in the first appendix attachment.
Content:
The only allowable appendix materials are:
l
Blank data collection forms, blank survey forms, and blank questionnaire forms - or
screenshots thereof
l
Simple lists of interview questions
Note: In your blank forms and lists, do not include items such as: data, data
compilations, lists of variables or acronyms, data analyses, publications, manuals,
instructions, descriptions or drawings/figures/diagrams of data collection methods or
machines/devices.
l
Blank informed consent/assent forms
l
Other items only if they are specified in the FOA as allowable appendix materials
No other items are allowed in the Appendix. Simply relocating disallowed materials to other parts
of the application will result in a noncompliant application.
Some FOAs may have different instructions for the Appendix. Always follow the instructions in
your FOA if they conflict with these instructions.
Note: Applications will be withdrawn and not reviewed if they do not follow the appendix
requirements in these instructions or in your FOA.
Information that expands upon or complements information provided in any section of the
application – even if it is not required for the review – is not allowed in the Appendix unless it is
listed in the allowed appendix materials above or in your FOA. For example, do not include
material transfer agreements (MTA) in the appendix unless otherwise specified in the FOA.
For more information:
l
The NIH Guide Notice on Reminder: NIH Applications Must Be Complete and Compliant
With NIH Policy and Application Instructions At Time of Submission.
l
Failure of reviewers to address non-required appendix materials in their reviews is not an
acceptable basis for an appeal of initial peer review. For more information, see the NIH
R - 96

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
R.500 - PHS Human Subjects and Clinical
Trials Information
The PHS Human Subjects and Clinical Trials Information
form is used to collect information on human subjects
research, clinical research, and/or clinical trials, including
study population characteristics, protection and
monitoring plans, and a protocol synopsis.
This form accommodates the full spectrum of all types of
clinical trials, including, but not limited to, behavioral,
exploratory/development, mechanistic, pilot/feasibility,
early phase, efficacy, effectiveness, group-randomized,
and others.
Read all the instructions in the Funding Opportunity
Announcement (FOA) before completing this form to
ensure your application meets all IC-specific criteria.
"Section II. Award Information" of the FOA will indicate
whether clinical trials are or are not allowed and whether
clinical trial research experience is or is not allowed. The
designation of your FOA will determine how to use these instructions, and subsequently, how
to fill out this form.
The PHS Human Subjects and Clinical Trials Information form, together with the rest of your
application, should include sufficient information for the evaluation of the project, independent
of any other documents (e.g., previous application). Be specific, describe each study clearly, and
avoid redundancies. Be especially careful to avoid redundancies with your research strategy.
View larger image
Quick Links
PHS Human Subjects and Clinical Trials Information
Use of Human Specimens and/or Data
If No to Human Subjects
If Yes to Human Subjects
Other Requested Information
Study Record(s)
Delayed Onset Study(ies)
Study Record: PHS Human Subjects and Clinical Trials Information
Section 1 - Basic Information
R - 98

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
1.1 Study Title (each study title must be unique)
1.2 Is this Study Exempt from Federal Regulations?
1.3 Exemption Number
1.4 Clinical Trial Questionnaire
1.5 Provide the ClinicalTrials.gov Identifier (e.g. NCT87654321) for this trial, if applicable.
Section 2 - Study Population Characteristics
2.1 Conditions or Focus of Study
2.2 Eligibility Criteria
2.3 Age Limits
2.3.a Inclusion of Individuals Across the Lifespan
2.4 Inclusion of Women and Minorities
2.5 Recruitment and Retention Plan
2.6 Recruitment Status
2.7 Study Timeline
2.8 Enrollment of First Participant
2.9 Inclusion Enrollment Report(s)
Section 3 - Protection and Monitoring Plans
3.1 Protection of Human Subjects
3.2 Is this a multi-site study that will use the same protocol to conduct non-exempt human
subjects research at more than one domestic site?
3.3 Data and Safety Monitoring Plan
3.4 Will a Data and Safety Monitoring Board be appointed for this study?
3.5 Overall Structure of the Study Team
Section 4 - Protocol Synopsis
4.1 Study Design
4.2 Outcome Measures
4.3 Statistical Design and Power
4.4 Subject Participation Duration
4.5 Will the study use an FDA-regulated intervention?
4.6 Is this an applicable clinical trial under FDAAA?
4.7 Dissemination Plan
Section 5 - Other Clinical Trial-related Attachments
5.1 Other Clinical Trial-related Attachments
R - 99

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Complete the PHS Human Subjects and Clinical Trials Information form after you have completed the
R.220 - R&R Other Project Information Form.
This form accommodates the full spectrum of all types of clinical trials, including, but not limited to,
exploratory/development, mechanistic, pilot/feasibility, early phase, efficacy, effectiveness, group-
randomized, and others.
Who should use the PHS Human Subjects and Clinical Trials Information form:
The designation of your FOA will determine how to use these instructions, and subsequently, how to fill
out this form.
All applicants must use the PHS Human Subjects and Clinical Trials Information form regardless of your
answer to the question “Are human subjects involved?” on the R.220 - R&R Other Project Information
Form.
Note for studies involving only the secondary use of identifiable biospecimens or data: For
studies where the only involvement of human subjects is the use of identifiable biospecimens or data
originally collected for another purpose, complete the PHS Human Subjects and Clinical Trials
Information form with information specific to the current study and not the original collection unless
the information associated with the original collection is pertinent to the proposed study. If information
about the original collection is necessary, provide context and clearly distinguish between the current
study and historical information.
Using the PHS Human Subjects and Clinical Trials Information form:
Everyone must complete the "Use of Human Specimens and/or Data" section of the PHS Human
Subjects and Clinical Trials Information form. However, your answer to the “Are human subjects
involved?” question will determine which other sections of the PHS Human Subjects and Clinical Trials
Information form you must complete. Once you have completed the "Use of Human Specimens and/or
Data" section, follow instructions on the form that are specific to your answer to the "Are human
subjects involved?" question on the R.220 - R&R Other Project Information Form:
l
if you answered "Yes" to the question "Are human subjects involved?" on the R.220 - R&R
Other Project Information Form, see the "If Yes to Human Subjects" section for instructions.
l
if you answered "No" to the question "Are human subjects involved?" on the R.220 - R&R
Other Project Information Form, see the "If No to Human Subjects" section for instructions.
The PHS Human Subjects and Clinical Trials Information form allows you to add Study Record(s) and/or
Delayed Onset Study(ies), as applicable.
Within each Study Record, you will add detailed information at the study level. Do not duplicate studies
within your application. Each study within the application should be unique and should have a unique
study title. Each Study Record is divided into numbered sections:
l
Section 1 - Basic Information
l
Section 2 – Study Population Characteristics (includes Inclusion Enrollment Report)
l
Section 3 – Protection and Monitoring Plans
l
Section 4 – Protocol Synopsis
l
Section 5 – Other Clinical Trial-related Attachments
R - 100
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Note: The PHS Human Subjects and Clinical Trials Information form will capture detailed information at
the study level. Although you are encouraged to refer to information in the PHS Human Subjects and
Clinical Trials Information form in your discussion of the Research Strategy, do not duplicate
information between the Research Strategy attachment and the PHS Human Subjects and Clinical Trials
Information form.
For more information on what a “study” is for the purposes of the PHS Human Subjects and Clinical
Trials Information form, see the relevant FAQ on the Applying Electronically FAQ page.
The PHS Human Subjects and Clinical Trials Information form is dynamic and may eliminate sections
that are not relevant to your application. The dynamic form behavior may not be enabled on all
submission methods.
Note: Some fields in this form match fields within ClinicalTrials.gov and are identified as such within
these instructions. Additional information about the fields can be found on the ClinicalTrials.gov
Protocol Registration Data Element Definitions website.
Additional Instructions for Research:
R25 applicants who are proposing to provide clinical trial research experience
for their participants (i.e., participants will not be leading an independent
clinical trial): You will generally follow the standard instructions to complete the
PHS Human Subjects and Clinical Trials Information form, but follow relevant
Research instructions where they are given. Make sure you are applying to a FOA
that allows Clinical Trial Research Experience (this is noted in “Section II. Award
Information” of the FOA). Additionally, your mentor or co-mentor is required to
include a statement to document leadership of the clinical trial. The statement must
include the following:
l
Source of funding;
l
ClinicalTrials.gov identifier (e.g., NCT87654321), if applicable;
l
A description of how the mentor's expertise is appropriate to guide participants in
any proposed clinical trials research experience; and
l
A statement/attestation that the mentor will be responsible for the clinical trial.
o
The mentor must have primary responsibility for leading and overseeing the
trial and must describe how she/he will provide this oversight.
o
Include details on the specific roles / responsibilities of the mentor and
participants.
This statement must be included in the “Other Attachment” attachment in the R.220
– R&R Other Project Information Form.
R36 applicants who are proposing to gain clinical trial research experience
under a mentor’s supervision (i.e., you will not be leading an independent
clinical trial): You will generally follow the standard instructions to complete the
PHS Human Subjects and Clinical Trials Information form, but follow relevant
Research instructions where they are given. Make sure you are applying to a FOA
that allows Clinical Trial Research Experience (this is noted in “Section II. Award
Information” of the FOA). Additionally, your mentor or co-mentor is required to
include a statement to document leadership of the clinical trial. The statement must
include the following:
R - 101
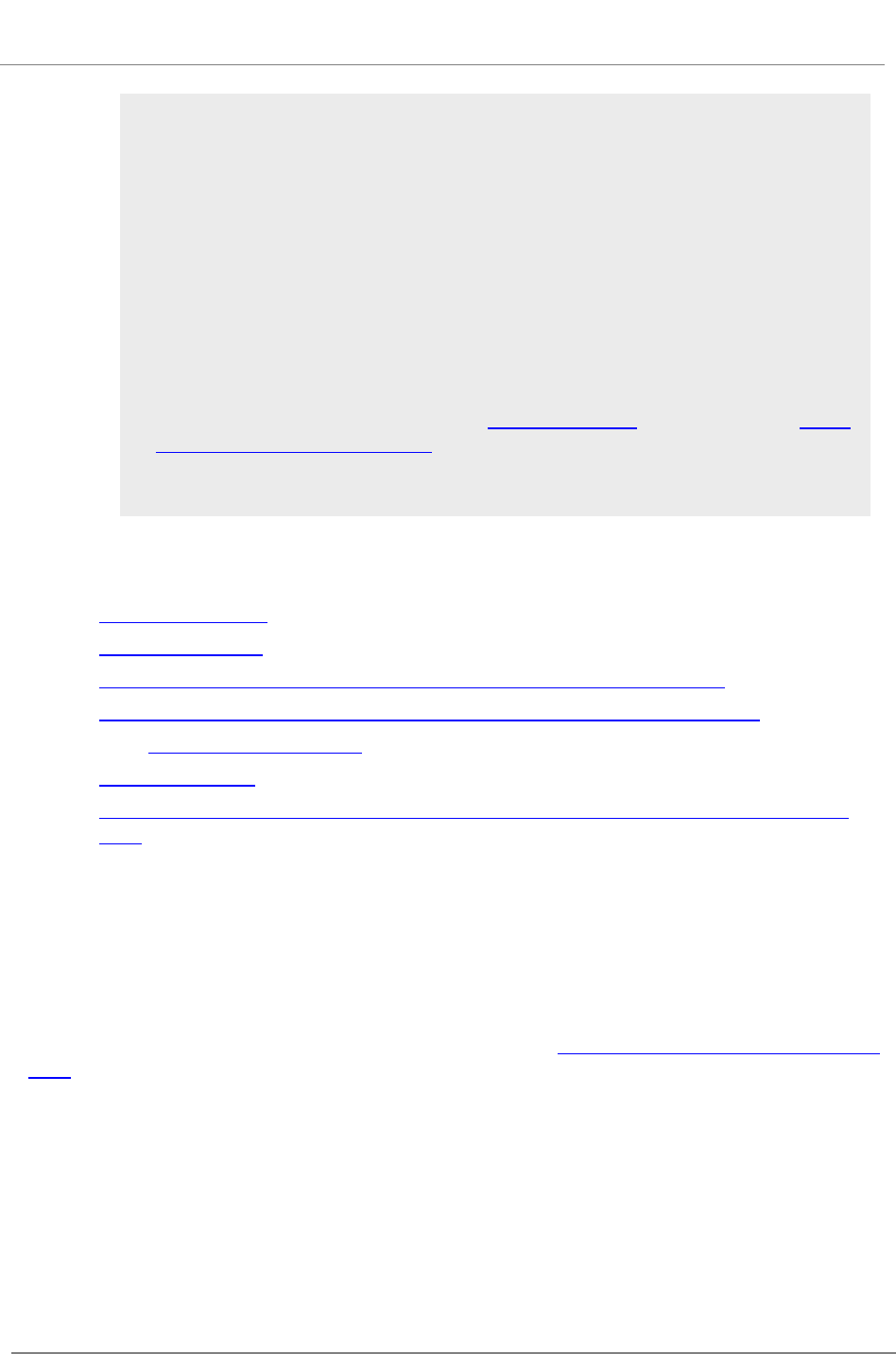
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
l
Source of funding;
l
ClinicalTrials.gov identifier (e.g., NCT87654321), if applicable;
l
A description of how your expertise is appropriate to guide the applicant in any
proposed clinical trials research experience; and
l
A statement/attestation that the mentor will be responsible for the clinical trial.
o
The mentor must have primary responsibility for leading and overseeing the
trial and must describe how she/he will provide this oversight (be careful not
to overstate the candidate’s responsibilities).
o
Include details on the specific roles/responsibilities of the applicant and
mentor.
This statement must be included in the “Other Attachment” attachment in the R.220
- Other Project Information Form..
All other Research applicants: Follow the standard instructions to complete the
PHS Human Subjects and Clinical Trials Information form.
Applicants must follow all policies and requirements related to formatting, proprietary
information, human subjects, and clinical trials. See the following pages for more information:
l
Format Attachments
l
Rules for Text Fields
l
NIH Grants Policy Statement, Section 2.3.11.2: Confidentiality of Information
l
NIH Grants Policy Statement, Section 2.3.11.2.2: The Freedom of Information Act
l
NIH's Human Subjects Research website
l
NIH's Clinical Trials website
l
Policy on Good Clinical Practice Training for NIHAwardees Involved in NIH-funded Clinical
Trials
Note: There are no page limits for any attachments in the PHS Human Subjects and Clinical Trials
Information form.
PHS Human Subjects and Clinical Trials
Information
Applicants must complete the human subjects questions on the R.220 - R&R Other Project Information
Form prior to completing this form.
R - 102
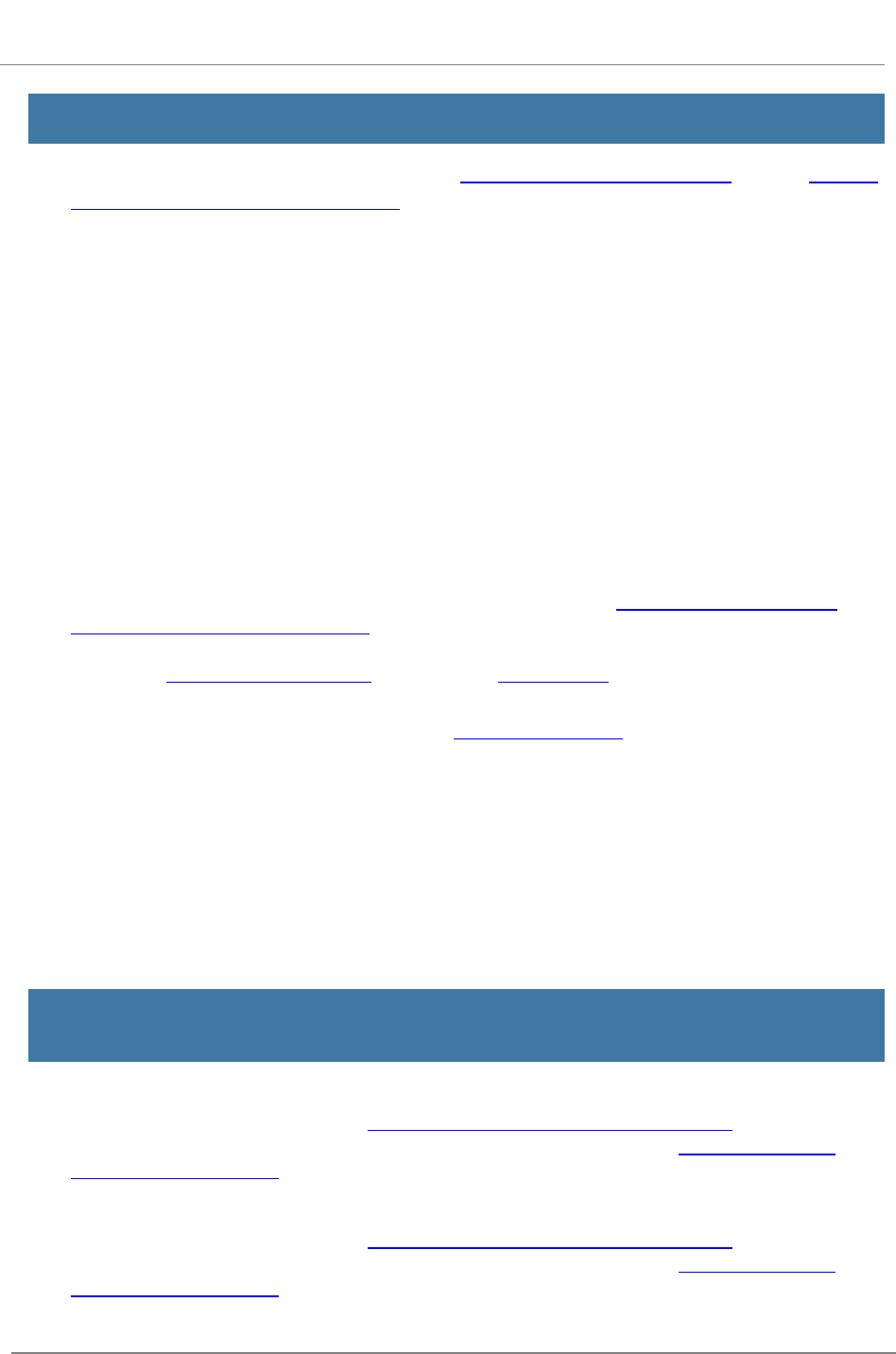
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Use of Human Specimens and/or Data
Regardless of your answer to the question “Are Human Subjects Involved?” on the R.220 -
R&R Other Project Information Form, answer the following question(s) about the use of
human specimens and/or human data.
Does any of the proposed research in the application involve human specimens and/or data?
Select “Yes” or “No” to indicate whether the proposed research involves human specimens and/or
data.
Note: Applications involving the use of human specimens or data may not be considered to be
research involving human subjects, depending on the details of the materials to be used.
Provide an explanation for any use of human specimens and / or data not considered to be
human subjects research.
If you answered “No” to the “Does any of the proposed research in the application involve human
specimens and/or data?” question, you do not need to attach an explanation here.
If you answered “Yes” to the “Does any of the proposed research in the application involve human
specimens and/or data?” question, you must provide an explanation for any use of human
specimens and/or data not considered to be human subjects research. To help determine whether
your research is classified as human subjects research, refer to the Research Involving Private
Information or Biological Specimens flowchart. Do not describe use of human specimens and / or
data considered to be human subjects research here. For any human specimens and/or data that is
considered human subjects research, you will add a Study Record. Do not duplicate the
information in your explanation in any of your Study Records.
Attach the explanation as a PDF file. See NIH’s Format Attachments page.
This explanation should include:
l
information on who is providing the data/biological specimens and their role in the
proposed research;
l
a description of the identifiers that will be associated with the human specimens and data;
l
a list of who has access to subjects’ identities; and
l
information about the manner in which the privacy of research participants and
confidentiality of data will be protected.
Please complete the human subjects section of the Research & Related Other
Project Information form prior to completing this form.
Are Human Subjects Involved? Yes/No
This field is pre-populated from the R.220 - R&R Other Project Information Form. If the value in
this field appears to be incorrect, you may correct it by adjusting it on the R.220 - R&R Other
Project Information Form.
Is the Project Exempt from Federal regulations? Yes/No
This field is pre-populated from the R.220 - R&R Other Project Information Form. If the value in
this field appears to be incorrect, you may correct it by adjusting it on the R.220 - R&R Other
Project Information Form.
R - 103
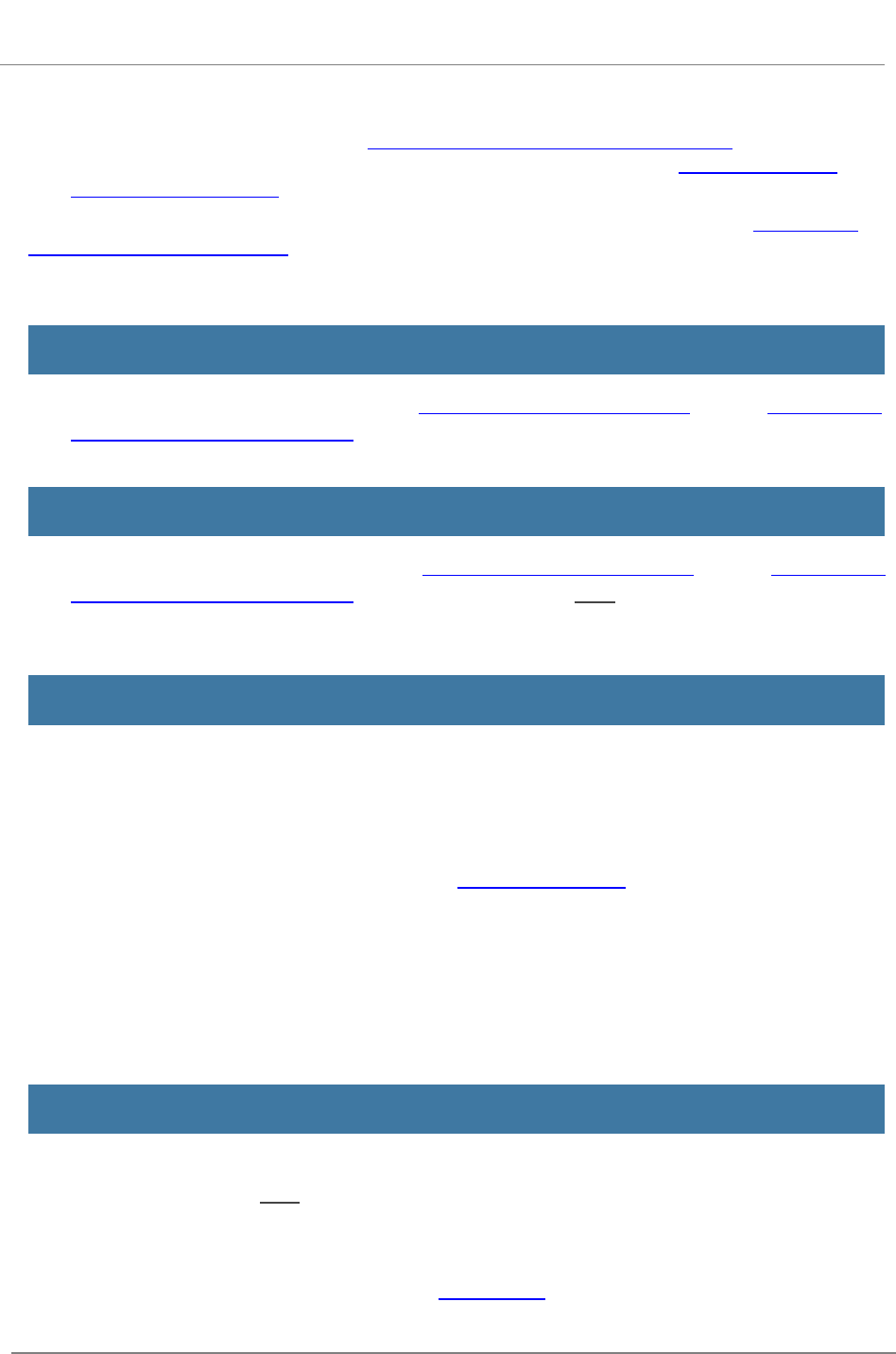
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Exemption number: 1, 2, 3, 4, 5, 6, 7, 8
This field is pre-populated from the R.220 - R&R Other Project Information Form. If the value in
this field appears to be incorrect, you may correct it by adjusting it on the R.220 – R&R Other
Project Information Form.
Note: If you change your answer to the “Are Human Subjects Involved” question on the R.220 - R&R
Other Project Information Form after you have started entering information into the PHS Human
Subjects and Clinical Trials Information form, your data in the PHS Human Subjects and Clinical Trials
Information form may be lost.
If No to Human Subjects
If you answered "No" to the question “Are Human Subjects Involved?” on the R.220 - R&R
Other Project Information Form, skip the rest of the PHS Human Subjects Clinical Trials
Information form unless otherwise directed by your FOA.
If Yes to Human Subjects
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R.220 - R&R
Other Project Information Form, add a Study Record for each proposed study involving
human subjects by selecting “Add New Study” or “Add New Delayed Onset Study,” as
appropriate.
Other Requested Information
Who may provide Other Requested Information:
Follow the instructions below and any instructions in your FOA to determine whether you are
permitted to include the “Other Requested Information” attachment.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page. Hyperlinks and URLs are
not allowed unless specified in the funding opportunity announcement.
Content:
Content is limited to what is described in your FOA or in these instructions. Do not use the “Other
Requested Information” attachment to include any other information.
Renewal applications: When preparing a renewal (or resubmission of a renewal), you can provide
a list of ongoing studies or ClinicalTrials.gov identifiers (e.g., NCT87654321).
Study Record(s)
Adding Study Record Attachment(s):
Add a study record for each proposed study involving human subjects. Projects involving public
health surveillance activities described in 45 CFR 46.102(l)(2) must complete one or more Study
Records describing those public health surveillance activities as if the exclusion does not apply. If
specific plans for your study involving human subjects can be described in the application but will
not begin immediately (i.e., your study has a delayed start), you must add a Study Record for that
R - 104

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
study. If your study anticipates involving human subjects within the period of award but specific
plans cannot be described in the application (i.e., delayed onset), see the instructions for Delayed
Onset Study(ies).
For all submission methods, the Study Record is used to collect human subjects study data. Note:
The steps to add a Study Record attachment(s) may vary with the submission method. For
example, from the ASSIST Human Subjects and Clinical Trials tab, use the ‘Add New Study’ button
to access the data entry screens to enter Study Record information directly into ASSIST. With other
submission methods, you may have to extract a blank copy of the Study Record, complete it
offline, and then attach it to your application.
Note on Grouping Studies into Study Records: While there may be more than one way to split
or group studies into Study Records, you are encouraged to group studies that use the same
human subjects population and same research protocols into a single Study Record, to the extent
that the information you provide is accurate and understandable to NIH staff and reviewers.
If information in any attachment is identical across studies, include the complete information only
in the first Study Record for which the information is relevant. In the subsequent Study Records for
which the identical information is needed, upload an attachment that says, “See information for
attachment X in Study Record entitled [include study title]." No other information is needed in the
attachment. Do not submit attachments that are duplicated from one Study Record to another.
Note that you should not name Study Records by number. Examples of attachments that may be
identical across studies include, but are not limited to, the 3.1 Protection of Human Subjects and
3.5 Overall Structure of the Study Team attachments.
See the NIH Glossary definitions of Study and Study Record.
The PHS Human Subjects and Clinical Trials Information form accommodates up to 150 separate
Study Records.
Format:
All attachments must be PDF files. If you extract a Study Record, it will already be in a fillable PDF
format. Please use this PDF file and do not alter the format of the Study Record file. Use unique
filenames for each human subject study record. The filename for each attachment within a study
must be unique within the application (i.e., do not use the same filename in multiple Study
Records). Use of hyperlinks and URLs is not allowed unless specified in the funding opportunity
announcement.
Content:
Follow the instructions in the “Study Record: PHS Human Subjects and Clinical Trials Information”
section below.
Delayed Onset Study(ies)
If you anticipate conducting research involving human subjects but cannot describe the study at
the time of application (i.e., your study is a delayed onset human subject study), enter a Delayed
Onset Study Record as instructed below.
Generally, for any study that you include as a delayed onset study in this section, you will provide a
study title, indicate whether the study is anticipated to include a clinical trial, and include a
justification attachment. Since by definition, information for a delayed onset study is not available
at the time of application, you will not be given the option to complete a full Study Record for a
delayed onset study. For delayed onset studies, the Delayed Onset Study Record is sufficient.
R - 105
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Notes on delayed onset studies:
l
Delayed onset does NOT apply to a study that can be described but will not start
immediately (i.e., delayed start). Refer to the NIH Glossary definition of Delayed Onset
Study and Delayed Start.
l
If you anticipate multiple delayed onset studies, you can include them together in a single
Delayed Onset Study Record.
Study Title
This field is required.
The Study Title can have a maximum of 600 characters.
Enter a brief, unique title that describes the study the participants will be involved in. Each study
within your application must have a unique Study Title. The first 150 characters will display in the
application image bookmarks.
Note on multiple delayed onset studies: If you are including multiple delayed onset studies in
one delayed onset study entry, you may enter “Multiple Delayed Onset Studies” as the title of this
record.
Anticipated Clinical Trial?
This field is required.
Check this box if you anticipate that this study will be a clinical trial. For help determining whether
your study meets the definition of clinical trial, see the Clinical Trial Questionnaire below.
Read your FOA carefully to determine whether clinical trials are allowed in your application.
Note on multiple delayed onset studies: If you are including multiple delayed onset studies in
one delayed onset study entry, and you anticipate that any of these studies will be a clinical trial,
check the “Anticipated Clinical Trial?” checkbox.
Justification Attachment
This attachment is required.
Attach the justification as a PDF file. See NIH’s Format Attachments page. Use of hyperlinks and
URLs is not allowed unless specified in the funding opportunity announcement.
l
All delayed onset studies must provide a justification explaining why human subjects
study information is not available at the time of application.
l
If NIH’s Policy on the Dissemination of NIH-Funded Clinical Trial Information will apply to
your study, this justification must also include the dissemination plan.
Note on multiple delayed onset studies: If you are including more than one delayed onset
study in any given delayed onset study entry, address all the included studies in a single
justification attachment.
R - 106
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Study Record: PHS Human Subjects and Clinical
Trials Information
Section 1 - Basic Information
Who must complete “Section 1 – Basic Information:”
“Section 1 – Basic Information” is required for all studies involving human subjects.
1.1 Study Title (each study title must be unique)
The “Study Title” field is required.
The Study Title can have a maximum of 600 characters.
Enter a brief title that describes the study the participants will be involved in. If there is more than
one study (i.e., you are including more than one Study Record and/or delayed onset study in your
application), each one must have a unique study title. The first 150 characters will display in the
bookmarks of the application image.
Note: When registering a clinical trial in ClinicalTrials.gov, all study titles across your organization
must be unique.
Note: This field matches a ClinicalTrials.gov field (Official Title).
1.2 Is this Study Exempt from Federal Regulations?
An answer to the “Is this Study Exempt from Federal Regulations?” question is required.
Indicate whether the study is exempt from Federal regulations for the Protection of Human
Subjects.
For more information, see the NIH's Definition of Human Subjects Research website.
1.3 Exemption Number
The “Exemption Number” field is required if you selected “Yes” to the “Is this Study Exempt from
Federal Regulations?” question.
Select the appropriate exemption number(s) for this particular study. Multiple selections are
permitted. Regardless of whether these exemptions may apply to you in the future, you must fill
out your application following the instructions below.
For more information:
The categories of research that qualify for exemption are defined in the Common Rule for the
Protection of Human Subjects. These regulations can be found at 45 CFR 46.
Need help determining the appropriate exemption number?
l
Refer to NIH's Human Subjects FAQs.
l
See the NIH's Human Subjects Frequently Asked Questions section on Exemptions.
R - 107
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
The Office for Human Research Protections (OHRP) guidance states that appropriate use of
exemptions described in 45 CFR 46 should be determined by an authority independent from the
investigators (for more information, see OHRP's Frequently Asked Questions). Institutions often
designate their Institutional Review Board (IRB) to make this determination. Because NIH does not
require IRB approval at the time of application, the exemptions designated often represent the
opinion of the PD/PI, and the justification provided for the exemption by the PD/PI is evaluated
during peer review. See NIH Grants Policy Statement Section 4.1.15 for more information.
1.4 Clinical Trial Questionnaire
The Clinical Trial Questionnaire is required.
Note for basic and mechanistic studies involving human participants: The NIH definition of a
clinical trial encompasses a broad range of studies, including studies using human participants
that aim to understand fundamental aspects of phenomena, the pathophysiology of a disease, or
the mechanism of action of an intervention. This includes many mechanistic studies and studies
submitted to Basic Experimental Studies with Humans FOAs.
Answer “Yes” or “No” to the following questions to determine whether this study involves a clinical
trial. Answer the following questions based only on the study you are describing in this Study
Record.
Note: The answer to question “1.4.a Does the study involve human participants?” will be pre-
populated with “Yes” for all study records. You will not be able to change this answer.
1.4.a. Does the study involve human participants? Yes/No
1.4.b. Are the participants prospectively assigned to an intervention? Yes/No
1.4.c. Is the study designed to evaluate the effect of the intervention on the participants?
Yes/No
1.4.d. Is the effect that will be evaluated a health-related biomedical or behavioral out-
come? Yes/No
If you answered “Yes” to all the questions in the Clinical Trial Questionnaire, this study meets the
definition of a clinical trial.
Refer to the table below for information about what sections of this form are required, based on
your answers to Question 1.4 "Clinical Trial Questionnaire."
Form Section
If you answered "yes"
to all the questions in
the Clinical Trial
Questionnaire
If you answered "no"
to any of the questions
in the Clinical Trial
Questionnaire
Section 2 - Study Population
Characteristics
Required
Required
Section 3 - Protection and
Monitoring Plans
Required
Required
R - 108
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Form Section
If you answered "yes"
to all the questions in
the Clinical Trial
Questionnaire
If you answered "no"
to any of the questions
in the Clinical Trial
Questionnaire
Section 4 - Protocol Synopsis
Required
Do not complete
Section 5 - Other Clinical Trial-
related Attachments
Required if specified in the
FOA
Do not complete
Additional Instructions for Research:
R25 applicants who are proposing to provide clinical trial research experience
for their participants (i.e., participants will not be leading an independent
clinical trial): Even if you answered “Yes” to all the questions in the Clinical Trial
Questionnaire, only certain fields of the PHS Human Subjects and Clinical Trials
Information form are required (and other fields are not allowed) because the study is
not an independent clinical trial. Do not provide information in “Section 4 – Protocol
Synopsis” or in “Section 5 – Other Clinical Trial-related Attachments” of the Study
Record. Inputting information into these sections will result in errors and will prevent
your application from being accepted.
R36 applicants who are proposing to gain clinical trial research experience
under a mentor’s supervision (i.e., you will not be leading an independent
clinical trial): Even if you answered “Yes” to all the questions in the Clinical Trial
Questionnaire, only certain fields of the PHS Human Subjects and Clinical Trials
Information form are required (and other fields are not allowed) because the study is
not an independent clinical trial. Do not provide information in “Section 4 – Protocol
Synopsis” or in “Section 5 – Other Clinical Trial-related Attachments” of the Study
Record. Inputting information into these sections will result in errors and will prevent
your application from being accepted.
For more information:
l
NIH Glossary’s definition of an NIH-defined clinical trial
l
NIH's Definition of a Clinical Trial page
l
NIH Definition of Clinical Trials Case Studies page
l
FAQs on the NIH Clinical Trial Definition
l
NIH’s decision tool will help determine whether your human subjects research study is an
NIH-defined clinical trial
l
Your study may also be subject to additional regulations. Read NIH’s Requirements for
Registering & Reporting NIH-funded Clinical Trials in ClinicalTrials.gov.
R - 109
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
1.5. Provide the ClinicalTrials.gov Identifier (e.g., NCT87654321) for this trial, if
applicable
If a clinical trial has already been entered into ClinicalTrials.gov, enter the ClinicalTrials.gov
identifier (e.g., NCT87654321) for this trial. Enter the identifier only if you are proposing to work on
that specific clinical trial. If you are only getting samples and/or data from a clinical trial that has
already been entered into ClinicalTrials.gov, do NOT enter the identifier.
If you are building on an existing study (e.g., ancillary study), enter the ClinicalTrials.gov identifier
only for the ancillary study (if registered separately), not the parent study.
Note: The number you enter in this field should match the ClinicalTrials.gov identifier assigned by
ClinicalTrials.gov.
Section 2 - Study Population Characteristics
Who must complete “Section 2 - Study Population Characteristics:”
All of “Section 2 – Study Population Characteristics” is required (see exceptions for Question 2.7 Study
Timeline and for Question 2.8 Enrollment of First Subject) for all human subjects studies unless the
following applies to you:
l
If you selected only Exemption 4 and no other exemptions on the "1.3 Exemption Number"
question, then “Section 2 – Study Population Characteristics” is not required.
2.1 Conditions or Focus of Study
At least 1 entry is required, and up to 20 entries are allowed (enter each entry on its own line). Each
entry is limited to 255 characters.
Identify the name(s) of the disease(s) or condition(s) you are studying, or the focus of the study. If
available, use appropriate descriptors from NLM's Medical Subject Headings (MeSH) so the
application can be categorized. Include an entry for each condition.
Note: This field matches a ClinicalTrials.gov field (Primary Disease or Condition Being Studied in
the Trial, or the Focus of the Study).
2.2 Eligibility Criteria
List the study’s inclusion and exclusion criteria. To provide a bulleted list, use a dash (or other
character) followed by a space (“- “) at the start of each bullet. Be sure to check the formatting in
the assembled application image. Further explanation or justification should be included in the
Recruitment and Retention plan.
Your text entry is limited to 15,000 characters (but typically needs only 500 characters).
Note: This field matches a ClinicalTrials.gov field (Eligibility Criteria).
For more information about formatting text entry fields, see NIH's Rules for Text Fields page and
the ClinicalTrials.gov's Protocol Registration and Results System User's Guide.
R - 110
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
2.3 Age Limits
Minimum Age
Enter the numerical value for the minimum age a potential participant can be to be eligible for the
study. Provide the relevant units of time (i.e., years, months, weeks, days, hours, or minutes). If
there is no lower limit or no lower limit is known, enter “N/A (No Limit)” and do not enter a unit of
time.
Maximum Age
Enter the numerical value for the maximum age a potential participant can be to be eligible for the
study. Provide the relevant units of time (i.e., years, months, weeks, days, hours, or minutes). If
there is no upper limit or no upper limit is known, enter “N/A (No Limit)” and do not enter a unit of
time.
Note: This field matches a ClinicalTrials.gov field (Age Limits).
2.3.a Inclusion of Individuals Across the Lifespan
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Discuss each of the points listed below. Also include any additional information requested in the
FOA.
You will also have to complete an Inclusion Enrollment Report (IER). Note that you may need to
include multiple IERs for each study. Refer to the instructions for the IER below for more
information.
Inclusion of Individuals Across the Lifespan
For the purposes of the Inclusion of Individuals Across the Lifespan, exclusion of any specific age
or age range group (e.g., children or older adults) should be justified in this section. In addition,
address the following points:
l
Individuals of all ages are expected to be included in all NIH-defined clinical research
unless there are scientific or ethical reasons not to include them. Discuss whether
individuals will be excluded based on age and provide a rationale for the minimum and
maximum age of study participants, if applicable. Additionally, if individuals will be
excluded based on age, provide a scientific or ethical rationale for their exclusion. See the
NIH Policy and Guidelines on the Inclusion of Individuals Across the Lifespan as
Participants in Research Involving Human Subjects for additional information about
circumstances that may justify the exclusion of individuals based on age.
l
Include a description of the expertise of the investigative team for working with
individuals of the ages included, the appropriateness of the available facilities to
accommodate individuals in the included age range, and how the age distribution of
participants will contribute to a meaningful analysis relative to the purpose of the study.
When children are involved in research, the policies under HHS’ 45 CFR 46, Subpart D - Additional
Protections for Children Involved as Subjects in Research apply and must be addressed in the
Protection of Human Subjects attachment.
R - 111

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Existing Datasets or Resources. If you will use an existing dataset, resource, or samples that may
have been collected as part of a different study, you must address inclusion, following the
instructions above. Generally, you must provide details about the sex/gender, race, and ethnicity
of the existing dataset/resource and justify the details as appropriate to the scientific goals of the
proposed study.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Monitoring Inclusion When Working with Existing Datasets and/or
Resources.
For more information, see:
l
NIH Policy Implementation Page on Inclusion Across the Lifespan
l
Inclusion Across the Lifespan: Guidance for Applying the Policy infographic
l
NIH FAQs on Inclusion Across the Lifespan
l
HHS’ 45 CFR 46 Subpart D – Additional Protections for Children
l
NIH Grants Policy Statement, Section 4.1.15.7: Inclusion of Individuals Across the Lifespan
as Participants in Research Involving Human Subjects
2.4 Inclusion of Women and Minorities
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Discuss each of the points listed below and include any additional information requested in the
FOA.
You will also have to complete an Inclusion Enrollment Report (IER). Note that you may need to
include multiple IERs for each study. Refer to the instructions for the IER below for more
information.
Inclusion of Women and Minorities
Address the following points:
l
Describe the planned distribution of subjects by sex/gender, race, and ethnicity.
l
Describe the rationale for selection of sex/gender, racial, and ethnic group members in
terms of the scientific objectives and proposed study design. The description may include,
but is not limited to, information on the population characteristics of the disease or
condition under study.
l
Describe proposed outreach programs for recruiting sex/gender, racial, and ethnic group
members.
l
Inclusion and Excluded Groups: Provide a reason for limiting inclusion of any group by
sex/gender, race, and/or ethnicity. In general, the cost of recruiting certain groups and/or
geographic location alone are not acceptable reasons for exclusion of particular groups.
See the Inclusion of Women and Minorities as Participants in Research Involving Human
Subjects - Policy Implementation Page for more information.
R - 112
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Existing Datasets or Resources. If you will use an existing dataset, resource, or samples that may
have been collected as part of a different study, you must address inclusion, following the
instructions above. Generally, you must provide details about the sex/gender, race, and ethnicity
of the existing dataset/resource and justify the details as appropriate to the scientific goals of the
proposed study.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Monitoring Inclusion When Working with Existing Datasets and/or
Resources.
NIH-Defined Phase III Clinical Trials. If the proposed research includes an NIH-Defined Phase III
Clinical Trial, the “Inclusion of Women and Minorities” attachment MUST address plans for how
sex/gender, race, and ethnicity will be taken into consideration in the design and valid analysis of
the trial. See the instructions for “Valid Analysis” and “Plans to test for Differences in Effect among
Sex/gender, Racial, and/or Ethnic Groups” below.
Additional information about valid analysis is available on the NIH Policy and Guidelines on The
Inclusion of Women and Minorities as Subjects in Clinical Research page.
Valid Analysis (for NIH-Defined Phase III Clinical Trials only):
Address the following issues for ensuring valid analyses:
l
Inclusive eligibility criteria – in general, the cost of recruiting certain groups and/or
geographic location alone are not acceptable reasons for exclusion of particular
groups;
l
Allocation of study participants of both sexes/genders and from different racial and/or
ethnic groups to the intervention and control groups by an unbiased process such as
randomization;
l
Unbiased evaluation of the outcome(s) of study participants; and
l
Use of unbiased statistical analyses and proper methods of inference to estimate and
compare the intervention effects by sex/gender, race, and/or ethnicity, particularly if
prior evidence strongly suggests that such differences exist.
Plan to Test for Differences in Effect among Sex/gender, Racial, and/or Ethnic Groups (for NIH-
Defined Phase III Clinical Trials only):
Applicants also should address whether they plan to test for differences in effect among
sex/gender, racial, and/or ethnic groups and why such testing is or is not appropriate.
This plan must include selection and discussion of one of the following analysis plans:
l
Plans to conduct analyses to detect significant differences in intervention effect
among sex/gender, racial, and/or ethnic subgroups when prior studies strongly
support these significant differences among one or more subgroups, or
l
Plans to include and analyze sex/gender, racial, and/or ethnic subgroups when prior
studies strongly support no significant differences in intervention effect between
subgroups. (Representation of sex/gender, racial, and ethnic groups is not required as
subject selection criteria, but inclusion is encouraged.), or
l
Plans to conduct valid analyses of the intervention effect in sex/gender, racial, and/or
ethnic subgroups (without requiring high statistical power for each subgroup) when
the prior studies neither support nor negate significant differences in intervention
effect among subgroups.
R - 113

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
For more information, see:
l
NIH's Policy Implementation Page on the Inclusion of Women and Minorities
l
HHS’ 45 CFR 46 Subpart B – Additional Protections for Pregnant Women, Fetuses, and
Neonates
l
NIH Grants Policy Statement, Section 4.1.15.8: Inclusion of Women and Minorities as
Subjects in Clinical Research and Reporting Sex/Gender, Racial, and Ethnic Participation
2.5 Recruitment and Retention Plan
Who must complete the "Recruitment and Retention Plan" attachment:
The “Recruitment and Retention Plan” attachment is required unless the following applies to you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Describe how you will recruit and retain participants in your study. You should address both
planned recruitment activities as well as proposed engagement strategies for retention.
2.6. Recruitment Status
Who must complete the "Recruitment Status" question:
The “Recruitment Status” question is required unless the following applies to you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
Content:
From the dropdown menu, select the "Recruitment Status" that best describes the proposed study,
based upon the status of the individual sites. If any facility in a multi-site study has an individual
site status of “recruiting,” then choose “recruiting” for this question. Only one selection is allowed.
Choose from the following options:
l
Not yet recruiting
l
Recruiting
l
Enrolling by invitation
l
Active, not recruiting
l
Completed
l
Suspended
l
Terminated (Halted Prematurely)
l
Withdrawn (No Participants Enrolled)
R - 114

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Note: This field matches a ClinicalTrials.gov field (Overall Recruitment Status).
2.7. Study Timeline
Who must complete the "Study Timeline" attachment:
The “Study Timeline” attachment is required if you answered "Yes" to all the questions in the
"Clinical Trial Questionnaire" (i.e., your study is a clinical trial).
The "Study Timeline" attachment is optional if either of the following apply to you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
l
You answered "No" to any of the questions in the "Clinical Trial Questionnaire" (i.e., your
study is not a clinical trial).
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Provide a description or diagram describing the study timeline. The timeline should be general
(e.g., "one year after notice of award"), and should not include specific dates.
Note: Additional milestones or timelines may be requested as just-in-time information or post-
award.
2.8. Enrollment of First Participant
Who must complete the "Enrollment of First Participant" question:
Do not complete this field if you will answer "Yes" to the question "Using an Existing Dataset or
Resource" in the Inclusion Enrollment Report.
The “Enrollment of First Participant” question is otherwise required unless the following applies to
you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
Content:
Enter the date (MM/DD/YYYY) of the enrollment of the first participant into the study. From the
dropdown menu, select whether this date is anticipated or actual.
2.9. Inclusion Enrollment Report(s)
Who must complete the Inclusion Enrollment Report(s):
An Inclusion Enrollment Report is required for all human subjects studies unless, on Question 1.3
“Exemption Number,” you selected only Exemption 4 and no other exemptions.
R - 115
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Using the Inclusion Enrollment Report:
Each proposed study, unless it falls under Exemption 4, must contain at least one Inclusion Enrollment
Report (IER). However, more than one IER per study is allowed.
Once you have added an IER for a given study, you may edit, remove, or view it.
Note: You can add a maximum of 20 IERs per Study Record. These can be a combination of planned
and cumulative reports.
Multi-site studies: Generally, if the application includes a study recruiting subjects at more than one
site/location, investigators may create one IER or separate, multiple IERs to enable reporting by study or
by site, depending on the scientific goals of the study and whether monitoring of inclusion enrollment
would benefit from being combined or separated. At a minimum, participants enrolled at non-U.S. sites
must be reported separately from participants enrolled at U.S. sites, even if they are part of the same
study. Please review the FOA to determine whether there are any other specific requirements about
how to complete the IER.
Duplicative Inclusion Reports: It is important that the IER for a given study be associated with only
one application and be provided only once in a given application (e.g., do not submit the same IER on
both the data coordinating center and the research site). If submitting individual application(s) as part
of a network or set of linked applications, please provide the IER with the individual site applications
unless otherwise directed by the FOA.
Renewal applications: When preparing a renewal (or resubmission of a renewal), investigators should
provide a narrative description regarding the cumulative enrollment from the previous funding period
(s) as part of the progress report section of the research strategy attachment in the application. The IER
should NOT be used for this purpose. If a given study will continue with the same enrollment or
additional enrollment, or if new studies are proposed, provide a new IER for each as described in the
instructions below.
Resubmission applications: If IERs were provided in the initial submission application, and if those
studies will be part of the resubmission application, complete the IER and submit again with the
resubmission application, regardless of whether the enrollment has changed or not. Also, provide any
new (additional) IERs.
Revision applications: Provide an IER if new studies are planned as part of the Revision and they meet
the NIH definition for clinical research.
For more information:
Refer to the Inclusion of Women and Minorities as Participants in Research Involving Human Subjects -
Policy Implementation Page.
1. Inclusion Enrollment Report Title
The “Inclusion Enrollment Report Title” field is required.
The “Inclusion Enrollment Report title can have a maximum of 600 characters.
Enter a unique title for each IER. The title should indicate specific criteria that uniquely identify
each report. If the Project Title is pre-populated, you may edit it so that each IER title is unique.
2. Using an Existing Dataset or Resource?
The “Using an Existing Dataset or Resource” question is required.
R - 116
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
If the study involves analysis of an existing dataset or resource (e.g., biospecimens) only, answer
“Yes” to this question. If the study involves prospective recruitment or new contact with
participants answer “No” to this question. Use separate IERs for studies involving use of existing
datasets or resources only and for studies that involve prospective recruitment or new contact with
study participants.
For additional guidance on what is considered an existing dataset, refer to the NIH FAQs on
Monitoring Inclusion When Working with Existing Datasets and/or Resources.
3. Enrollment Location Type (Domestic/Foreign)
The “Enrollment Location Type” field is required.
Select whether the participants described in the IER are based at a U.S. (Domestic) or at a non-U.S.
(Foreign) site. Participants at U.S. and non-U.S. sites must be reported separately (i.e., on separate
IERs), even if it is for the same study.
For additional guidance on how to complete the IER if you will be working with non-U.S.
populations, refer to these FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
4. Enrollment Country(ies)
The “Enrollment Country(ies)” field is optional.
Indicate the country or countries in which participants will be enrolled. Multiple U.S. sites can be
reported together in one IER. Foreign countries can be reported together in one IER. However, you
must use separate IERs for U.S. and non-U.S. sites. You can add up to 200 countries per IER.
5. Enrollment Location(s)
The “Enrollment Location(s)” field is optional.
Indicate the type of enrollment location (e.g., hospital, university, or research center), not the name
of the enrollment location.
Enrollment locations are typically where the research is conducted, and can be different from the
recruitment site.
6. Comments
Your comments are limited to 500 characters.
Enter information you wish to provide about this IER. This includes, but is not limited to,
addressing information about distinctive subpopulations if relevant to the scientific hypotheses
being studied. If inclusion monitoring is conducted on another study or NIH grant (e.g., data
coordinating center or research site), please indicate here.
Revision applications: If there are no updates to the IER(s) in your original grant application, do
not include an IER in your Revision application. Instead, provide a comment in this field to the
effect that previous IER(s) are still applicable. If you are revising the IER(s) in your original grant
application, provide a comment here to that effect.
Planned
Who must complete planned enrollment tables:
All studies must enter planned enrollment counts unless your proposed study will use only an
existing dataset or resource. Planned enrollment generally means that individuals will be recruited
R - 117
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
into the study and/or that individuals have already been recruited and continue to be part of the
study.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
For more information on racial categories, see the NIH Glossary definition of Racial Categories.
For more information on ethnic categories, see the NIH Glossary definition of Ethnic Categories.
Racial Categories
American Indian/Alaska Native:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both American
Indian/Alaska Native and Not Hispanic or Latino. Enter the expected number of females and males
(in the respective fields) who are both American Indian/Alaska Native and Hispanic or Latino.
Asian:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Asian and
Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields)
who are both Asian and Hispanic or Latino.
Native Hawaiian or Other Pacific Islander:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Native
Hawaiian or Other Pacific Islander and Not Hispanic or Latino. Enter the expected number of
females and males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander
and Hispanic or Latino.
Black or African American:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Black or
African American and Not Hispanic or Latino. Enter the expected number of females and males (in
the respective fields) who are both Black or African American and Hispanic or Latino.
White:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both White and
Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields)
who are both White and Hispanic or Latino.
More than One Race:
These fields are required.
Enter the expected number of females and males (in the respective fields) who both identify with
more than one racial category and are Not Hispanic or Latino. Enter the expected number of
females and males (in the respective fields) who both identify with more than one racial category
and are Hispanic or Latino.
R - 118

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Total:
The total fields at the bottom will be automatically calculated and reflect the totals of all racial
categories for females, males, and individuals of unknown/not reported sex/gender who are Not
Hispanic or Latino and of all racial categories for females, males, and individuals of unknown/not
reported sex/gender who are Hispanic or Latino. The “Total” fields in the right column will be
automatically calculated to total all individuals.
Cumulative (Actual)
Who must complete cumulative (actual) enrollment tables:
You must enter cumulative enrollment counts if your proposed study will use an existing dataset
or resource.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
For more information on racial categories, see the NIH Glossary definition of Racial Categories.
For more information on ethnic categories, see the NIH Glossary definition of Ethnic Categories.
Racial Categories
American Indian/Alaska Native:
These fields are required.
Enter the number of females and males (in the respective fields) who are both American
Indian/Alaska Native and Not Hispanic or Latino. Enter the number of females and males (in the
respective fields) who are both American Indian/Alaska Native and Hispanic or Latino. Use the
“Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Asian:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Asian and Not
Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who
are both Asian and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e.,
race and/or ethnicity is unknown).
Native Hawaiian or Other Pacific Islander:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Native Hawaiian or
Other Pacific Islander and Not Hispanic or Latino. Enter the expected number of females and
males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander and
Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is
unknown).
Black or African American:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Black or African
American and Not Hispanic or Latino. Enter the expected number of females and males (in the
respective fields) who are both Black or African American and Hispanic or Latino. Use the
“Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
R - 119

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
White:
These fields are required.
Enter the number of females and males (in the respective fields) who are both White and Not
Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who
are both White and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e.,
race and/or ethnicity is unknown).
More than One Race:
These fields are required.
Enter the number of females and males (in the respective fields) who both identify with more than
one racial category and are Not Hispanic or Latino. Enter the expected number of females and
males (in the respective fields) who both identify with more than one racial category and are
Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is
unknown).
Unknown or Not Reported:
These fields are required.
Enter the number of females, males, and individuals of unknown/not reported sex/gender (in the
respective fields) whose race is unknown/not reported and who are Not Hispanic or Latino. Enter
the number of females, males, and individuals of unknown/not reported sex/gender (in the
respective fields) whose race is unknown/not reported and who are Hispanic or Latino. Enter the
number of females, males, and individuals of unknown/not reported sex/gender (in the respective
fields) who are both of unknown/not reported race and of unknown/not reported ethnicity. Use
the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Total:
The total fields at the bottom will be automatically calculated and reflect the totals of all racial
categories for females, males, and individuals of unknown/not reported sex/gender who are Not
Hispanic or Latino and of all racial categories for females, males, and individuals of unknown/not
reported sex/gender who are Hispanic or Latino. Use the “Unknown/Not Reported” fields as
needed (i.e., race and/or ethnicity is unknown). The “Total” fields in the right column will be
automatically calculated to total all individuals.
Section 3 – Protection And Monitoring Plans
Who must complete “Section 3 – Protection and Monitoring Plans:”
All of “Section 3 – Protection and Monitoring Plans” is required for all studies involving human subjects,
unless otherwise noted.
3.1 Protection of Human Subjects
The “Protection of Human Subjects” attachment is required.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
R - 120

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Do not use the “Protection of Human Subjects” attachment to circumvent the page limits of the
Research Strategy.
For Human Subjects Research Claiming Exemptions: If you are claiming that your human
subjects research falls under any exemptions, justify why the research meets the criteria for the
exemption(s) that you have claimed. This justification should explain how the proposed research
meets the criteria for the exemption claimed. Do not merely repeat the criteria or definitions
themselves.
For Studies that involve Non-Exempt Human Subjects Research: For any proposed non-
exempt study involving human subjects, NIH requires a Protection of Human Subjects attachment
that is commensurate with the risks of the study, its size, and its complexity. Organize your
attachment into four sections, following the headings and specified order below, and discuss each
of the points listed below. Start each section with the appropriate section heading – Risks to
Human Subjects, Adequacy of Protection Against Risks, Potential Benefits of the Proposed
Research to Research Participants and Others, and Importance of the Knowledge to be Gained.
Also include any additional information requested in the FOA.
1. Risks to Human Subjects
a. Human Subjects Involvement, Characteristics, and Design
l
Briefly describe the overall study design.
l
Describe the subject population(s) to be included in the study; the procedures for assignment
to a study group, if relevant; and the anticipated numbers of subjects for each study group.
l
List any collaborating sites where human subjects research will be performed, and describe the
role of those sites and collaborating investigators in performing the proposed research.
b. Study Procedures, Materials, and Potential Risks
l
Describe all planned research procedures (interventions and interactions) involving study
subjects; how research material, including biospecimens, data, and/or records, will be obtained;
and whether any private identifiable information will be collected in the proposed research
project.
l
For studies that will include the use of previously collected biospecimens, data or records,
describe the source of these materials, whether these can be linked with living individuals, and
who will be able to link the materials.
l
Describe all the potential risks to subjects associated with each study intervention, procedure
or interaction, including physical, psychological, social, cultural, financial, and legal risks; risks
to privacy and/or confidentiality; or other risks. Discuss the risk level and the likely impact to
subjects.
l
Where appropriate, describe alternative treatments and procedures, including their risks and
potential benefits. When alternative treatments or procedures are possible, make the rationale
for the proposed approach clear.
2. Adequacy of Protection Against Risks
a. Informed Consent and Assent
l
Describe the process for obtaining informed consent. Include a description of the
circumstances under which consent will be sought and obtained, who will seek it, the nature of
R - 121
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
the information to be provided to prospective subjects, and the method of documenting
consent. When appropriate, describe how potential adult subjects’ capacity to consent will be
determined and the plans for obtaining consent from a legally authorized representative for
adult subjects not able to consent.
o
For research involving children: If the proposed studies will include children, describe
the process for meeting HHS regulatory requirements for parental permission and child
assent (45 CFR 46.408). See the HHS page on Research with Children FAQs and the NIH
page on Requirements for Child Assent and Parent/Guardian Permission.
l
If a waiver of some or all of the elements of informed consent will be sought, provide
justification for the waiver. Do not submit informed consent document(s) with your application
unless you are requested to do so.
b. Protections Against Risk
l
Describe planned strategies for protecting against or minimizing all potential risks identified,
including strategies to manage and protect the privacy of participants and confidentiality of
research data.
l
Where appropriate, discuss plans for ensuring necessary medical or professional intervention in
the event of adverse effects on participants.
l
Describe plans for handling incidental findings, such as those from research imaging, screening
tests, or paternity tests.
c. Populations that are vulnerable to coercion or undue influence and pregnant women, fetuses
and neonates, if relevant to your study
Explain the rationale for the involvement of populations that are vulnerable to coercion or undue
influence, such as children, prisoners, individuals with impaired decision-making capacity, or
economically or educationally disadvantaged persons or others who may be considered vulnerable
populations. 'Prisoners' includes all subjects involuntarily incarcerated (for example, in detention
centers). Additionally, explain the rationale for the involvement of pregnant women, human fetuses and
neonates.
Pregnant Women, Fetuses, and Neonates or Children
If the study involves subjects afforded additional protections under Subparts B and D (pregnant women,
fetuses, and neonates or children), provide a clear description of the risk level and additional
protections necessary to meet the HHS regulatory requirements.
l
HHS’ Subpart B - Additional Protections for Pregnant Women, Fetuses, and Neonates
l
HHS’ Subpart D - Additional Protections for Children
l
OHRP Guidance on Subpart D Special Protections for Children as Research Subjects and the
HHS 407 Review Process
Prisoners
If the study involves vulnerable subjects afforded additional protections under Subpart C (prisoners),
describe how proposed research meets the additional regulatory requirements, protections, and plans
to obtain OHRP certification for the involvement of prisoners in research.
Refer to HHS regulations, and OHRP guidance:
R - 122

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
l
HHS’ Subpart C - Additional Protections Pertaining to Prisoners as Subjects
l
OHRP Subpart C Guidance on Involvement of Prisoners in Research
3. Potential Benefits of the Proposed Research to Research Participants and Others
l
Discuss the potential benefits of the research to research participants and others.
l
Discuss why the risks to subjects are reasonable in relation to the anticipated benefits to
research participants and others.
l
Note: Financial compensation of subjects should not be presented as a benefit of participation
in research.
4. Importance of the Knowledge to be Gained
l
Discuss the importance of the knowledge to be gained as a result of the proposed research.
l
Discuss why the risks to subjects are reasonable in relation to the importance of the knowledge
that reasonably may be expected to result.
For more information:
l
Refer to the NIH’s Human Subjects Research website.
3.2 Is this a multi-site study that will use the same protocol to conduct non-
exempt human subjects research at more than one domestic site?
Select "Yes" or "No" to indicate whether this is a multi-site study that will use the same protocol to
conduct non-exempt human subjects research at more than one domestic site.
Select “N/A” only if any of the following apply (do not select “N/A” if none of the following apply):
l
You answered “Yes” to “Question 1.2 Is this Study Exempt from Federal Regulations?
(Yes/No)”
l
You are a training grant applicant.
Applicants who check “Yes” and are subject to the revised Common Rule are expected to use a
single Institutional Review Board (sIRB) to conduct the ethical review required by HHS regulations
for the Protections of Human Subjects Research unless review by a sIRB would be prohibited by
law (including tribal law passed by the official governing body of an American Indian or Alaska
Native tribe).
Applicants who check “Yes” and are subject only to the NIH sIRB policy are expected to use a
single Institutional Review Board (sIRB) to conduct the ethical review required by HHS regulations
for the Protections of Human Subjects Research unless review by a sIRB would be prohibited by a
federal, tribal, or state law, regulation, or policy.
Note: The NIH sIRB policy applies to participating domestic sites. Foreign sites
participating in NIH-funded, multi-site studies are not expected to follow this policy.
For more information:
l
HHS regulations and requirements for the Protections of Human Subjects can be found at
45 CFR 46.
l
See NIH’s Single IRB Policy for Multi-site Research for more information.
R - 123

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
l
See the FAQ about answering “No” for this question on the Applying Electronically FAQ
page.
Single IRB Plan Attachment
For NIHApplicants, the single IRBplan is no longer required. See additional information in
the content section below.
For AHRQ applicants, if this is a research project that involves more than one institution and that
will be conducted in the United States, Applicants are expected to use a single Institutional Review
Board (sIRB) to conduct the ethical review required by HHS regulations for the Protections of
Human Subjects Research, and include a single IRB plan as instructed below, unless review by a
sIRB would be prohibited by a federal, tribal, or state law, regulation, or policy.
Note: The sIRB requirement applies to participating sites in the United States. Foreign sites
participating in AHRQ-funded, cooperative research studies are not expected to follow this
requirement.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Although one sIRB plan attachment per application is sufficient, you must include a file for each
study within your application. All filenames within your application must be unique. You may either
attach the same sIRB plan (with different filenames) to different studies or attach a file that refers
to the sIRB plan in another study within your application. For example, you may attach a file that
says “See sIRB plan in the 'My Unique Study Name' study.”
Content:
For NIHapplicants, the single IRB plan is no longer required. Do not provide an attachment.
The applicant must provide a statement naming the sIRB of record in the Just-in-Time submission
prior to award.
For more information:
l
NIH's Single IRB Policy for Multi-site Research page
l
NIH's FAQs on Single IRB Policy for Multi-site Research
l
NIH's Office of Science Policy's FAQs on NIH Policy on the Use of a Single IRB for Multi-
Site Research Costs
l
NIH's Office of Science Policy's FAQs on Implementation of the sIRB policy
For AHRQ applicants, the single IRB plan should include the following elements:
l
Describe how you will comply with the single IRB review requirement under the Revised
Common Rule at 45 CFR 46.114 (b) (cooperative research). If available, provide the name of the
IRB that you anticipate will serve as the sIRB of record.
l
Indicate that all identified participating sites will agree to rely on the proposed sIRB and that
any sites added after award will rely on the sIRB.
l
Briefly describe how communication between sites and the sIRB will be handled.
R - 124
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
l
Indicate that all participating sites will, prior to initiating the study, sign an
authorization/reliance agreement that will clarify the roles and responsibilities of the sIRB and
participating sites.
l
Indicate which institution or entity will maintain records of the authorization/reliance
agreements and of the communication plan.
l
Note: Do not include the authorization/reliance agreement(s) or the communication plan(s)
documents in your application.
l
Note: If you anticipate research involving human subjects but cannot describe the study at the
time of application, include information regarding how the study will comply with the single
Institutional Review Board (sIRB) requirement prior to initiating any multi-site study in the
delayed onset study justification.
For Studies with Legal-, Regulatory-, or Policy-based Claims for Exception as described by
the sIRB Policy: Indicate that review by a sIRB will not be possible for all or some sites (specify
which sites) because local IRB review is required by an existing federal/state/tribal law or policy.
Include a specific citation to the relevant law, policy, or regulation.
For more information:
• AHRQ Guide Notice on Single IRB
• AHRQ Protection of Human Subjects page
3.3 Data and Safety Monitoring Plan
A “Data and Safety Monitoring Plan” attachment is required if you answered “Yes” to all the
questions in the “Clinical Trial Questionnaire.” The “Data and Safety Monitoring Plan” attachment
is optional for all other human subjects research.
For human subjects research that does not involve a clinical trial: Your study, although it is
not a clinical trial, may have significant risks to participants, and it may be appropriate to include a
data and safety monitoring plan. If you choose to include a data and safety monitoring plan, you
may follow the content criteria listed below, as appropriate.
For AHRQ Applicants, Data and Safety Monitoring (DSM) plans are required in all non-exempt
research applications when support is sought to study the effect of a health-related intervention
on outcomes in human subjects where there is greater than minimal risk.
If you seek AHRQ support to conduct non-exempt research to study the effect of a health-related
intervention on outcomes in human subjects where there is greater than minimal risk, a “Data and
Safety Monitoring Plan” attachment is required.
Refer to AHRQ Data and Safety Monitoring Policy
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
For any proposed clinical trial, NIH requires a data and safety monitoring plan (DSMP) that is
commensurate with the risks of the trial, its size, and its complexity. Provide a description of the
R - 125
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
DSMP, including:
l
Indicate how many people and what type of entity will provide the monitoring. Include
such details as whether a single person, multiple people, or a data safety monitoring
board will provide monitoring. Also indicate what type of entity will provide the
monitoring (e.g., PD/PI, Independent Safety Monitor/Designated Medical Monitor,
Independent Monitoring Committee, Safety Monitoring Committee, Data and Safety
Monitoring Board, etc.).
l
The overall framework for safety monitoring and what information will be monitored.
l
The frequency of monitoring, including any plans for interim analysis and stopping rules
(if applicable).
l
The process by which Adverse Events (AEs), including Serious Adverse Events (SAEs) such
as deaths, hospitalizations, and life threatening events and Unanticipated Problems (UPs),
will be managed and reported, as required, to the IRB, the person or group responsible for
monitoring, the awarding IC and the Food and Drug Administration.
l
The individual(s) or group that will be responsible for trial monitoring and advising the
appointing entity. Because the DSMP will depend on potential risks, complexity, and the
nature of the trial, a number of options for monitoring are possible. These include, but are
not limited to, monitoring by a:
o
PD/PI: While the PD/PI must ensure that the trial is conducted according to the
approved protocol, in some cases (e.g., low risk trials, not blinded), it may be
acceptable for the PD/PI to also be responsible for carrying out the DSMP.
o
Independent safety monitor/designated medical monitor: a physician or other expert
who is independent of the study.
o
Independent Monitoring Committee or Safety Monitoring Committee: a small group
of independent experts.
o
Data and Safety Monitoring Board (DSMB): a formal independent board of experts
including investigators and biostatisticians. NIH requires the establishment of DSMBs
for multi-site clinical trials involving interventions that entail potential risk to the
participants, and generally, for all Phase III clinical trials, although Phase I and Phase II
clinical trials may also need DSMBs. If a DSMB is used, please describe the general
composition of the Board without naming specific individuals.
For more information:
l
NIH Grants Policy Statement, Section 4.1.15.6: Data and Safety Monitoring
l
NIHData and Safety Monitoring Policies
l
NIH Policies and IC Guidance for Data and Safety Monitoring of Clinical Trials
3.4 Will a Data and Safety Monitoring Board be appointed for this study?
The “Data Safety and Monitoring Board” question is required if you answered “Yes” to all the
questions in the “Clinical Trial Questionnaire.” This question is optional for all other human
subjects research.
R - 126
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Check the appropriate box to indicate whether a Data Safety and Monitoring Board (DSMB) will be
appointed for this study.
3.5 Overall Structure of the Study Team
The “Overall Structure of the Study Team” attachment is optional. Refer to your specific FOA for
specific instructions on the "Overall Structure of the Study Team" attachment.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Provide a brief overview of the organizational/administrative structure and function of the study
team, particularly the administrative sites, data coordinating sites, enrollment/participating sites,
and any separate laboratory or testing centers. The attachment may include information on study
team composition and key roles (e.g., medical monitor, data coordinating center), the governance
of the study, and a description of how study decisions and progress are communicated and
reported.
Note: Do not include study team members’ individual professional experiences (i.e., biosketch
information).
Section 4 – Protocol Synopsis
Who must complete “Section 4 – Protocol Synopsis:"
If you answered "Yes" to all the questions in the "Clinical Trial Questionnaire:" All the questions
in the “Protocol Synopsis” section are required.
If you answered “No” to any question in the “Clinical Trial Questionnaire:” Do not provide
information in this section. Inputting information in this section will result in errors and will prevent your
application from being accepted.
Additional Instructions for Research:
R25 applicants who are proposing to provide clinical trial research experience
for their participants (i.e., participants will not be leading an independent
clinical trial): Do not provide information in “Section 4 - Protocol Synopsis.”
Inputting information in this section will result in errors and will prevent your
application from being accepted.
R36 applicants who are proposing to gain clinical trial research experience
under a mentor’s supervision (i.e., you will not be leading an independent
clinical trial): Do not provide information in “Section 4 - Protocol Synopsis.”
Inputting information in this section will result in errors and will prevent your
application from being accepted.
R - 127
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
4.1. Study Design
4.1.a. Detailed Description
Enter a narrative description of the protocol. Studies differ considerably in the methods used to
assign participants and deliver interventions. Describe your plans for assignment of participants
and delivery of interventions. You will also need to show that your methods for sample size and
data analysis are appropriate given those plans. For trials that randomize groups or deliver
interventions to groups, special methods are required; additional information is available at the
Research Methods Resources webpage. The Narrative Study Description is not meant to be a
repeat of the Research Strategy.
The narrative description is limited to 32,000 characters (but typically needs only 5,000 characters),
should be written in layperson’s terms, and may repeat some of the information in the Research
Strategy.
Note: This field matches a ClinicalTrials.gov field (Detailed Description).
For more information about formatting text entry fields, see NIH's Rules for Text Fields page.
4.1.b. Primary Purpose
Enter or select from the dropdown menu a single "Primary Purpose" that best describes the clinical
trial. Choose from the following options:
l
Treatment
l
Prevention
l
Diagnostics
l
Supportive Care
l
Screening
l
Health Services Research
l
Basic Science
l
Device Feasibility
l
Other (If you select “Other,” provide a description in the space provided. Your response is
limited to 255 characters.)
Note: This field matches a ClinicalTrials.gov field (Primary Purpose).
4.1.c. Interventions
Complete the “Interventions” fields for each intervention to be used in your proposed protocol. If
an arm of the study to which subjects will be assigned (as discussed in 4.1.a. Detailed Description)
includes more than one intervention (e.g., drug plus educational intervention), complete this
section for each intervention. You can add up to 20 interventions.
Intervention Type: Enter or select from the dropdown menu the intervention type the clinical trial
will administer during the proposed award. Choose from the following options:
l
Drug (including placebo)
l
Device (including sham)
l
Biological/Vaccine
l
Procedure/Surgery
R - 128
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
l
Radiation
l
Behavioral (e.g., Psychotherapy, Lifestyle Counseling)
l
Genetic (including gene transfer, stem cell, and recombinant DNA)
l
Dietary Supplement (e.g., vitamins, minerals)
l
Combination Product
l
Diagnostic Test
l
Other
Name: Enter the name of the intervention. The name is limited to 200 characters.
Description: Enter a description of the intervention. The description is limited to 1,000 characters.
Note: This field matches a ClinicalTrials.gov field. (Interventions, including Intervention Type and
Intervention Name(s)).
For more information on how to answer this question for behavioral research trials, refer to the
relevant FAQ on the Applying Electronically FAQ page.
4.1.d. Study Phase
Enter or select from the dropdown menu a "Study Phase" that best describes the clinical trial. If
your study involves a device or behavioral intervention, choose “N/A”.
Choose from the following options:
l
Early Phase 1 (or Phase 0)
l
Phase 1
l
Phase 1/2
l
Phase 2
l
Phase 2/3
l
Phase 3
l
Phase 4
l
N/A
Is this an NIH-defined Phase III clinical trial? Yes/No
Select "Yes" or "No" to indicate whether the study includes an NIH-defined Phase III clinical trial.
Device and behavioral intervention studies may select “Yes” here even if the answer above is
“Other”.
For more information on how to answer this question for devices or behavioral interventions,
refer to the relevant FAQ on the Applying Electronically FAQ page.
4.1.e. Intervention Model
Enter or select from the dropdown menu a single "Intervention Model" that best describes the
clinical trial. If you select “Other,” provide a description in the space provided. Choose from the
following options:
l
Single Group
l
Parallel
l
Cross-Over
R - 129
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
l
Factorial
l
Sequential
l
Other (If you select “Other,” provide a description in the space provided. Your response is
limited to 255 characters.)
Note: This field matches a ClinicalTrials.gov field (Interventional Study Model).
For more information: Definitions of intervention models may be found in ClinicalTrials.gov’s
Glossary of Common Site Terms or in the ClinicalTrials.gov’s description of Study Design.
4.1.f. Masking
Select "Yes" or "No" to indicate whether the protocol uses masking. Note that masking is also
referred to as “blinding.”
If you answered “Yes” to the “Masking” question, select one or more types of masking that best
describes the protocol. Choose from the following options:
l
Participant
l
Care Provider
l
Investigator
l
Outcomes Assessor
Note: This field matches a ClinicalTrials.gov field (Masking).
4.1.g. Allocation
Enter or select from the dropdown menu a single "Allocation" that best describes how subjects will
be assigned in your protocol. If allocation is not applicable to your clinical trial, select “N/A” (e.g.,
for a single-arm trial). Choose from the following options:
l
N/A
l
Randomized
l
Non-randomized
Note: This field matches a ClinicalTrials.gov field (Allocation).
4.2. Outcome Measures
Complete the “Outcome Measures” fields for each primary, secondary, and other important
measures to be collected during your proposed clinical trial. You may have more than one primary
outcome measure, and you can add up to 50 outcome measures.
Name: Enter the name of the individual outcome measure. The outcome measure must be unique
within each Study Record.
Type: Enter or select from the dropdown menu the type of the outcome measure. Choose from
the following options:
l
Primary – select this option for the outcome measures specified in your protocol that are
of greatest importance to your study
l
Secondary – select this option for outcome measures specified in your protocol that are of
lesser importance to your study than your primary outcomes
R - 130

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
l
Other – select this option for additional key outcome measures used to evaluate the
intervention.
Time Frame: Indicate when a measure will be collected for analysis (e.g., baseline, post-
treatment).
Brief Description: Describe the metric used to characterize the outcome measure if the metric is
not already included in the outcome measure name. Your description is limited to 999 characters.
NIH-Defined Phase III Clinical Trials: If the proposed research includes an NIH-Defined Phase III
Clinical Trial, then outcomes for required analyses by sex/gender, race, and ethnicity should be
entered.
Additional information about valid analysis is available on the NIH Policy and Guidelines on The
Inclusion of Women and Minorities as Subjects in Clinical Research page.
Note: This field matches a ClinicalTrials.gov field (e.g., Primary Outcome Measure Information,
which includes Title, Description, and Time Frame).
For more information:
l
Refer to the relevant FAQ for question 4.2 Outcome Measures on the Applying
Electronically FAQ page.
4.3. Statistical Design and Power
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Specify the number of subjects you expect to enroll, the expected effect size, the power, and the
statistical methods you will use with respect to each outcome measure you listed in 4.2 Outcome
Measures.
You will need to show that your methods for sample size and data analysis are appropriate given
your plans for assignment of participants and delivery of interventions. For trials that randomize
groups or deliver interventions to groups, special methods are required; additional information is
available at the Research Methods Resources webpage.
4.4 Subject Participation Duration
Enter the time (e.g., in months) it will take for each individual participant to complete all study
visits. If the participation duration is unknown or not applicable, write “unknown” or “not
applicable.” The subject participation duration is limited to 255 characters.
4.5 Will the study use an FDA-regulated intervention?
Select "Yes" or "No" to indicate whether the study will use an FDA-regulated intervention (see the
definition of “FDA Regulated Intervention” under the Oversight section of the ClinicalTrials.gov
Protocol Registration Data Element Definitions for Interventional and Observational Studies page).
R - 131

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
4.5.a. If yes, describe the availability of Investigational Product (IP) and Investigational New
Drug (IND)/Investigational Device Exemption (IDE) status:
This attachment is required if you answered “Yes” to the “Will the study use an FDA-regulated
intervention?” question.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
This attachment’s typical length is approximately 3,000 characters.
Content:
Provide a summary describing the availability of study agents and support for the acquisition and
administration of the study agent(s).
Please indicate, if applicable, the IND/IDE status of the study agent, including whether a clinical
investigation is exempt from the IND/IDE requirement. Also indicate whether the investigators
have had any interactions with the FDA (e.g., indicate if the FDA has stated that research may
proceed). If the study agent currently has an IND/IDE number, provide that information.
Do not include the IND/IDE application, manufacturer’s product specifications, study protocol, or
protocol amendments in this attachment.
Additional information such as FDA letters or correspondence with the FDA may be requested in
the FOA.
Note: The awarding component may request consultation with the FDA and the IND/IDE sponsor
about the proposed clinical trial after peer review and prior to award.
4.6 Is this an applicable clinical trial under FDAAA?
Select "Yes" or "No" to indicate whether the study is an applicable clinical trial (ACT) under the
Food and Drug Administration Amendments Act (FDAAA).
For more information:
l
NIH Glossary’s definition of an applicable clinical trial
l
FAQs on the ClinicalTrials.gov & FDAAA
l
ClinicalTrials.gov FAQs
4.7 Dissemination Plan
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Although one Dissemination Plan per application is sufficient, you must include a file for each
study within your application. All filenames within your application must be unique. You may either
attach the same Dissemination Plan to different studies or attach a file that refers to the
Dissemination Plan in another study within your application. For example, you may attach a file
that says “See Dissemination Plan in the 'My Unique Study Name' study.”
R - 132
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
Content:
Explain briefly your plan for the dissemination of NIH-funded clinical trial information and address
how the expectations of the policy will be met. The plan must contain sufficient information to
assure the following:
l
the applicant will ensure that clinical trial(s) under the award are registered and results
information is submitted to ClinicalTrials.gov as outlined in the policy and according to
the specific timelines stated in the policy;
l
informed consent documents for the clinical trial(s) will include a specific statement
relating to posting of clinical trial information at ClinicalTrials.gov; and
l
the recipient institution has an internal policy in place to ensure that clinical trials
registration and results reporting occur in compliance with policy requirements.
Note: Do not include informed consent documents in the Dissemination Plan attachment.
Note: If your human subjects study meets the definition of “Delayed Onset,” include the
Dissemination Plan attachment in the delayed onset study justification.
For more information:
l
See the NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information
l
See the NIH Guide Notice on the Delayed Enforcement and Short-Term Flexibilities for
Some Requirements Affecting Prospective Basic Science Studies Involving Human
Participants
l
See the NIH Grants Policy Statement, Section 4.1.3.1 NIH Policy on Dissemination of NIH-
Funded Clinical Trial Information.
Section 5 – Other Clinical Trial-related Attachments
Who must complete “Section 5 – Other Clinical Trial-related Attachments:"
If you answered “Yes” to all the questions in the “Clinical Trial Questionnaire:” Include an
attachment only if your FOA specifies that an attachment(s) is required or permitted; otherwise, do not
include any Other Clinical Trial-related attachments.
If you answered “No” to any question in the “Clinical Trial Questionnaire:” Do not provide
information in this section. Inputting information in this section will result in errors and will prevent your
application from being accepted.
Additional Instructions for Research:
R25 applicants who are proposing to provide clinical trial research experience
for their participants (i.e., participants will not be leading an independent
clinical trial): Do not provide information in “Section 5 – Other Clinical Trial-related
Attachments.” Inputting information in this section will result in errors and will
prevent your application from being accepted.
R36 applicants who are proposing to gain clinical trial research experience
under a mentor’s supervision (i.e., you will not be leading an independent
R - 133

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.500 - PHS Human Subjects and Clinical Trials Information
clinical trial): Do not provide information in “Section 5 – Other Clinical Trial-related
Attachments.” Inputting information in this section will result in errors and will
prevent your application from being accepted.
5.1 Other Clinical Trial-related Attachments
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
A maximum of 10 PDF attachments is allowed in the “Other Clinical Trial-related Attachments”
section.
Content:
Provide additional trial-related information only if your FOA specifically requests it. Include only
attachments requested in the FOA, and use requested filenames. If a specific filename is not given
in the FOA, use a meaningful filename since it will become a bookmark in the assembled
application image. Each attachment included in the application must have a unique filename. Do
not use the same file name in multiple study records. If the FOA requires a specific filename, add
unique numbers at the end of the filenames for each study record (e.g. study_filename1, study_
filename2). File name sizes are limited to 50 characters.
R - 134
Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.600 - PHS Assignment Request Form
R.600 - PHS Assignment Request Form
The PHS Assignment Request Form may be used to
communicate specific application assignment and review
preferences to the Division of Receipt and Referral (DRR)
and to Scientific Review Officers (SROs).
This information will not be part of your assembled
application, and it will neither be made available to
program staff nor provided to reviewers. It is used
specifically to convey additional, optional information
about your preference(s) for assignment and review of
your application to DRR and SROs.
View larger image
Completing the PHS Assignment Request Form:
This form is optional. Use it only if you wish to communicate
specific awarding component assignments or review preferences. There is no requirement that all fields
or all sections be completed. You have the flexibility to make a single entry or to provide extensive
information using this form.
Note on Application Assignments: The Division of Receipt and Referral (DRR), Center for Scientific
Review (CSR) is responsible for assigning applications to awarding components such as NIH
Institutes/Centers (ICs) and other PHS agencies for funding consideration. DRR also assigns applications
to NIH Scientific Review Groups (SRGs) and Special Emphasis Panels (SEPs).
Awarding Component Assignment Suggestions (optional)
To facilitate accurate communication of any assignment preferences to NIH referral and review
staff, use the short abbreviation (e.g., NCI for the National Cancer Institute).
All assignment suggestions will be considered; however, not all assignment suggestions can be
honored. Applications are assigned based on relevance of your application to an individual
awarding component mission and scientific interests in addition to administrative requirements
such as IC participation in the funding opportunity announcement used to submit your
application.
Descriptions of the scientific areas covered by all NIH ICs and links to other PHS agency
information can be found on the PHS Assignment Information website.
You do not need to make entries in all three boxes of the “Awarding Component Assignment
Suggestions” section.
Suggested Awarding Component(s):
You may enter up to three preferences for primary assignment in the boxes in the “Suggested
Awarding Component(s)” row. Note: Your application will be assigned based on the most
R - 135

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.600 - PHS Assignment Request Form
appropriate match between it, the terms of the FOA, and the mission of each possible awarding
component, with your preference(s) taken into consideration when possible.
Study Section Assignment Suggestions (optional)
To facilitate accurate communication of any review assignment preferences to NIH referral and
review staff, use the short abbreviation of the SRG/SEP you would prefer. For example, enter
“CAMP” for the NIH Cancer Molecular Pathobiology study section or enter “ZRG1HDMR” for the
NIH Healthcare Delivery and Methodologies SBIR/STTR panel for informatics. Be careful to remove
all hyphens, parentheses, and spaces when you type in the suggestion. Freeform text (such as
"special emphasis panel" or "member conflict SEP") should not be entered.
All suggestions will be considered; however, not all assignment suggestions can be honored.
More information about how to identify CSR and NIH SRGs and SEPs, including their short
abbreviations, can be found on CSR Study Sections and Special Emphasis Panel. A list of all NIH
SRGs and SEPs is also available.
While the majority of NIH research grant and fellowship applications are reviewed by CSR, some
are assigned to individual IC review groups and some are clustered for review in SRGs/SEPs,
depending on existing locus of review agreements within NIH and other PHS agencies. This limits
flexibility for honoring assignment preferences.
You do not need to make an entry in all three boxes of the "Study Section Assignment
Suggestions" section.
Suggested Study Sections:
You may enter up to three preferences for SRGs/SEPs in the boxes in the “Suggested Study
Sections” row. Use one box per individual SRG/SEP preference suggestion. All review preferences
will be considered. Note: Your application will be assigned based on the most appropriate match
between it, the terms of the FOA, and the guidelines for each SRG/SEP, with your preference(s)
taken into consideration when possible.
Note: This information is not applicable if you are submitting an application to an RFA.
Rationale for assignment suggestions (optional)
Enter the rationale (i.e., why you think the assignment is appropriate) for your Awarding
Component and Study Section suggestions.
Your answer can have a maximum of 1000 characters.
List individuals who should not review your application and why (optional)
You may list specific individuals, if any, who should not review your application and why they
should not review your application. Provide sufficient information (e.g., name, organizational
affiliation) so that the SRO can correctly identify the individual. Be prepared to provide additional
information to the SRO if needed. Simply stating “Dr. John Smith is in conflict with my application”
is not helpful.
Your answer can have a maximum of 1000 characters.
R - 136

Research Instructions for NIH and Other PHSAgencies - Forms Version H Series
R.600 - PHS Assignment Request Form
Identify scientific areas of expertise needed to review your application (optional)
You may list up to five general or specific types of expertise needed for the review of your
application. Limit your answers to areas of expertise – do not enter names of individuals you would
like to review your application.
Each field can have a maximum of 40 characters.
R - 137
Form Screenshots
R.- i
Form Screenshots
Quick Links
SF 424 (R&R) Form
PHS 398 Cover Page Supplement Form
R&R Other Project Information Form
Project/Performance Site Location(s) Form
R&R Senior/Key Person Profile (Expanded) Form
R&R Budget Form
R&R Subaward Budget Attachment(s) Form
PHS 398 Modular Budget Form
PHS 398 Research Plan Form
PHS Human Subjects and Clinical Trials Information
PHS Assignment Request Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
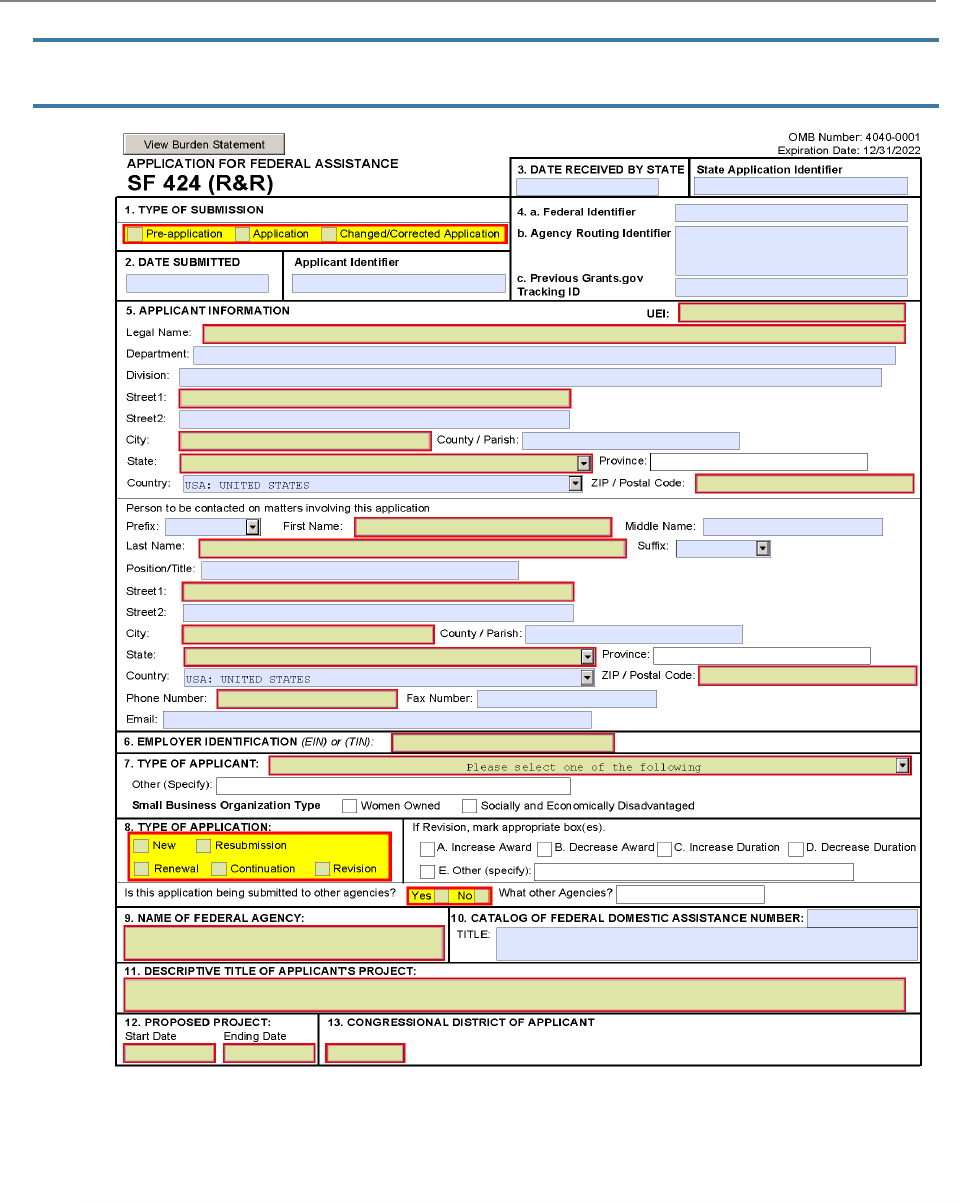
Form Screenshots
R.- ii
SF 424 (R&R)Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
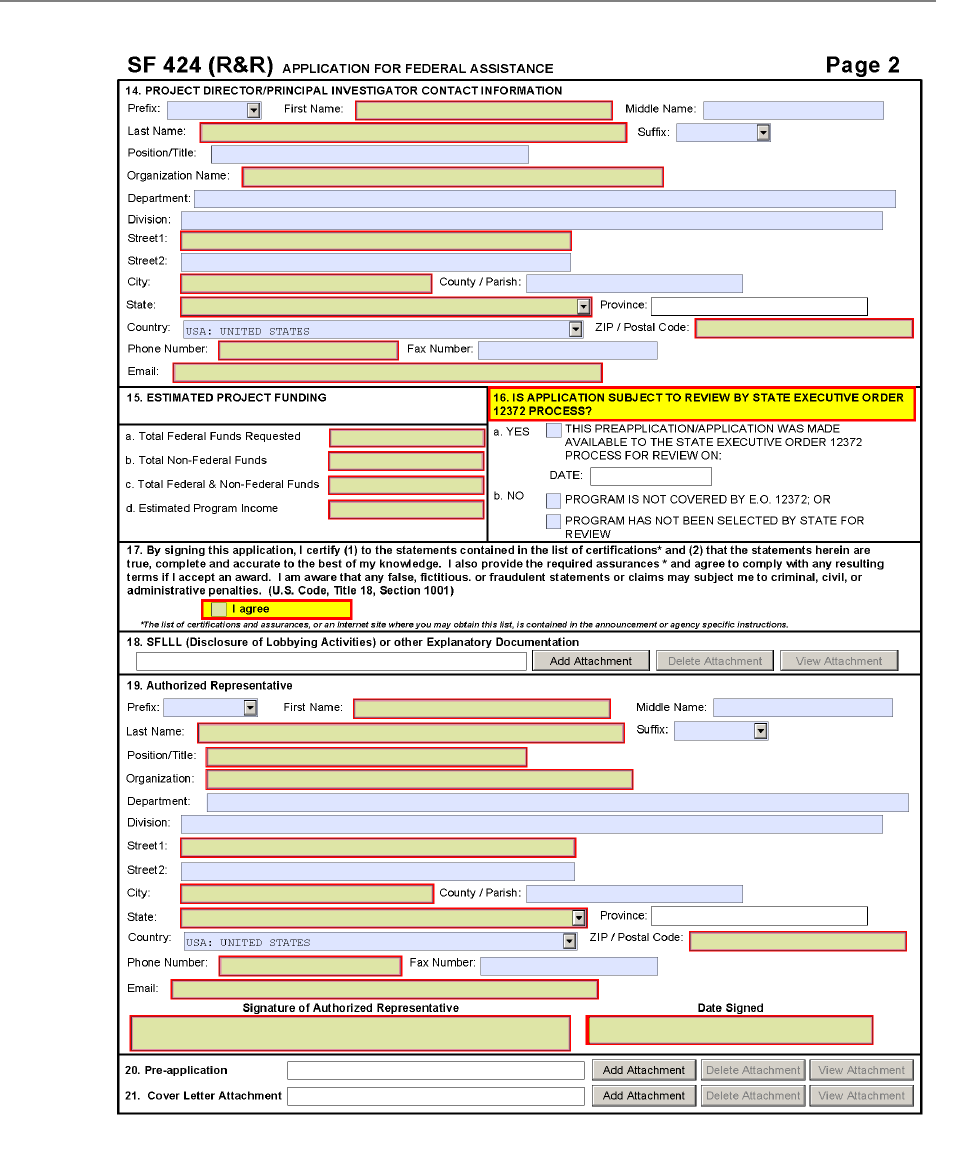
Form Screenshots
R.- iii
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
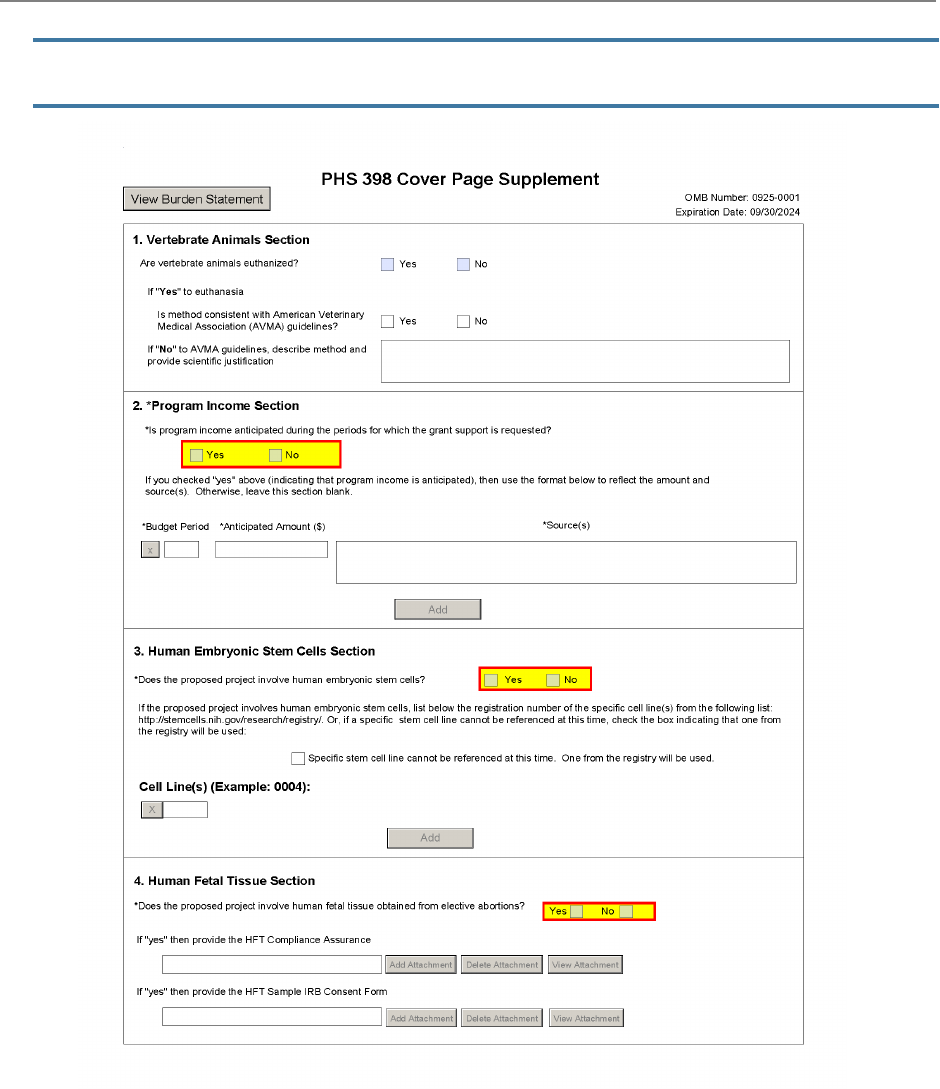
Form Screenshots
R.- iv
PHS 398 Cover Page Supplement Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- v
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
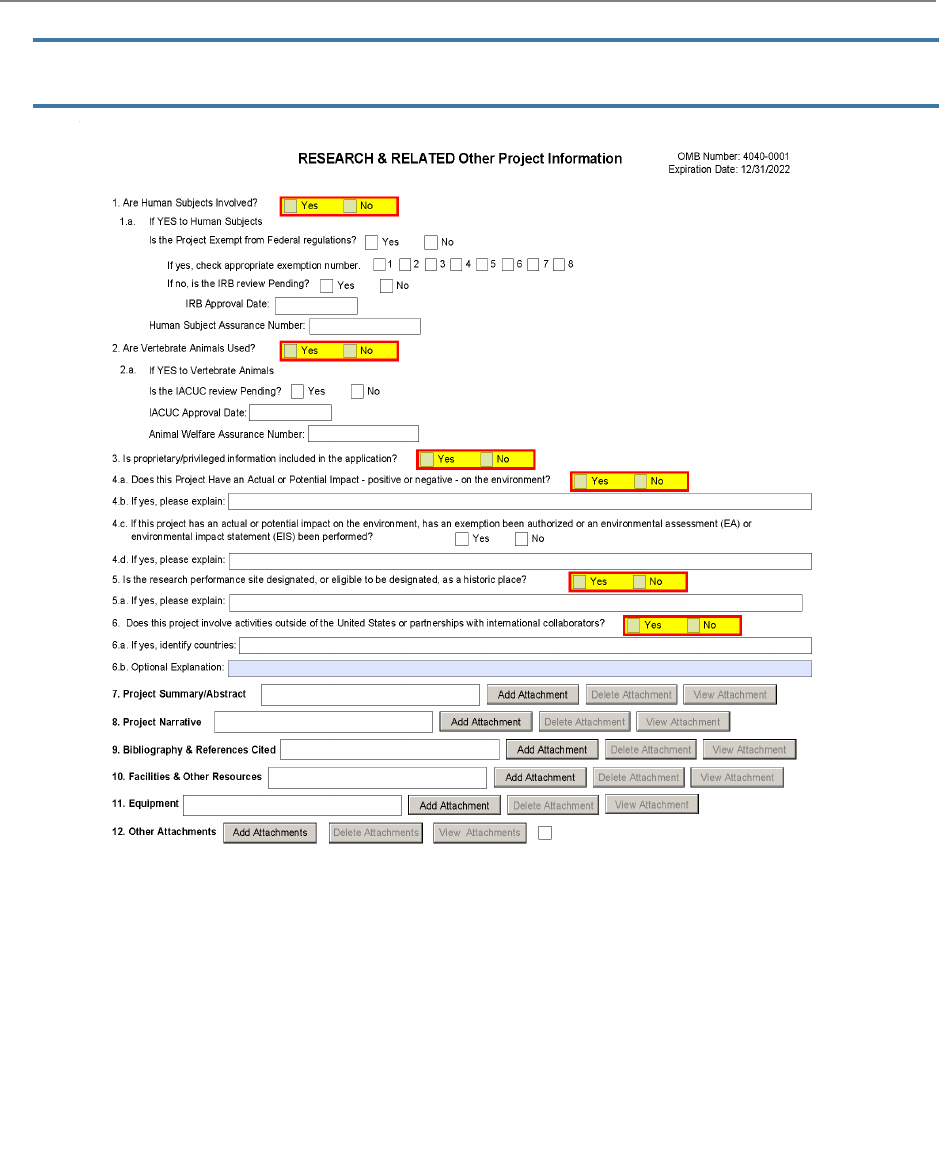
Form Screenshots
R.- vi
R&R Other Project Information Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
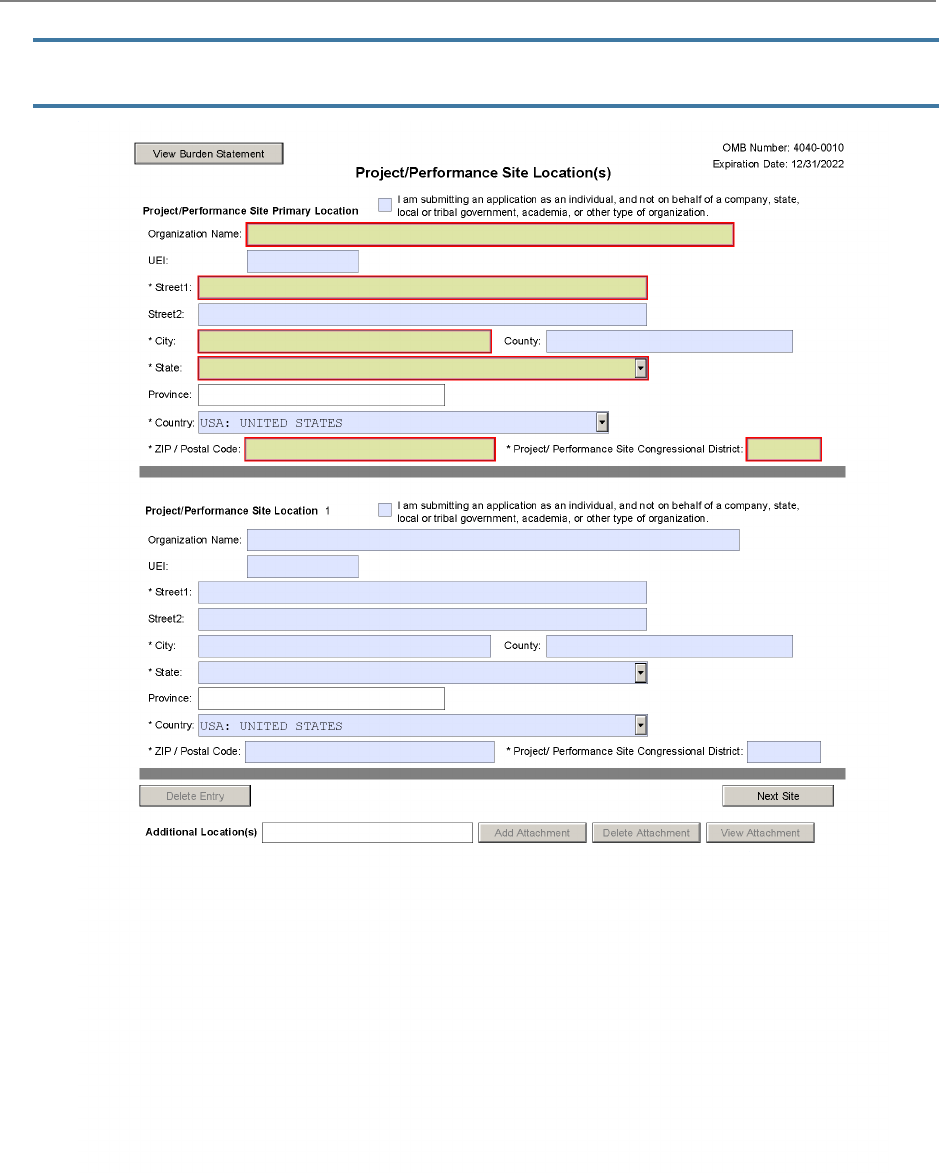
Form Screenshots
R.- vii
Project/Performance Site Location(s) Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
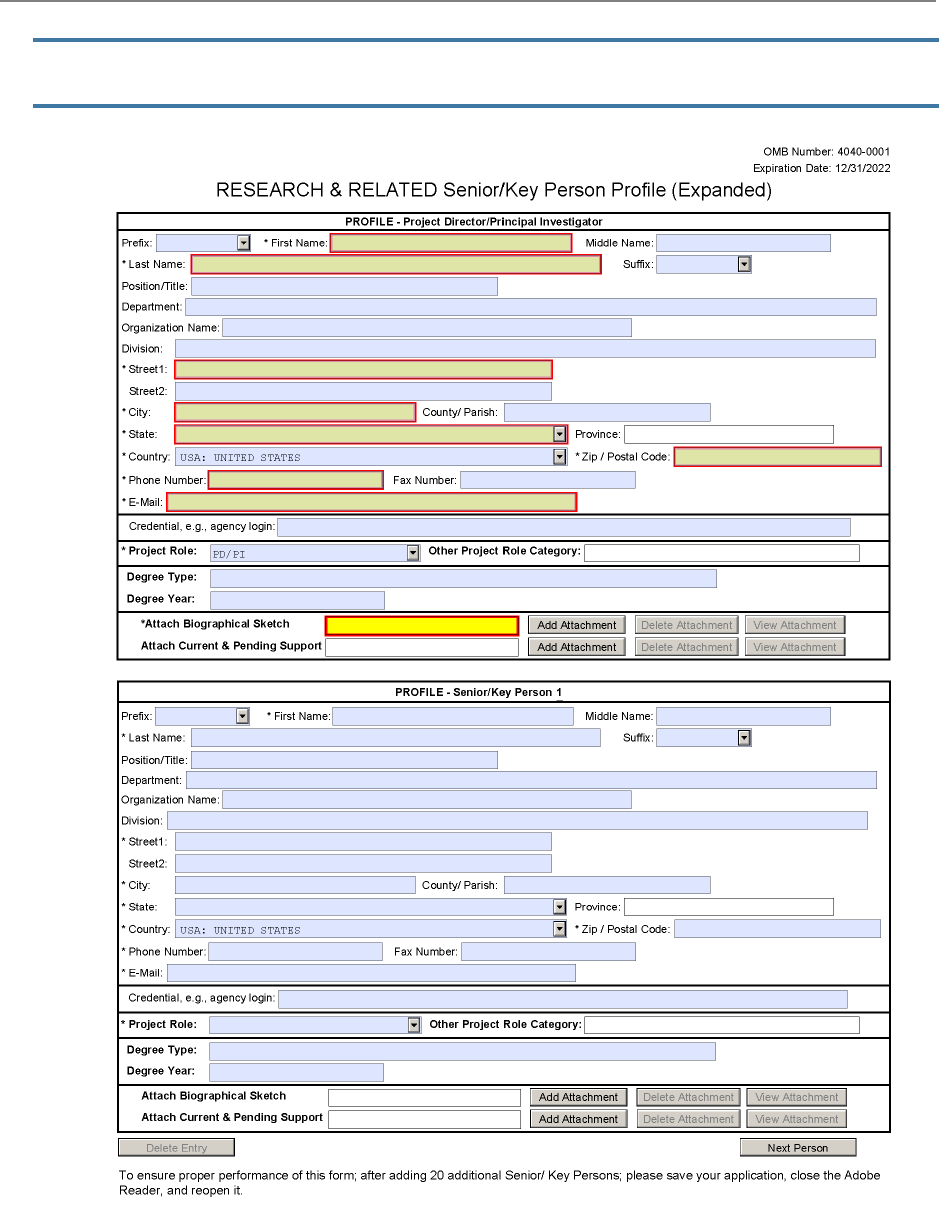
Form Screenshots
R.- viii
R&R Senior/Key Person Profile (Expanded) Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- ix
R&R Budget Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
Form Screenshots
R.- x
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- xi
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- xii
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- xiii
R&R Subaward Budget Attachment(s) Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
Form Screenshots
R.- xiv
PHS 398 Modular Budget Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- xv
PHS 398 Research Plan Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- xvi
PHS Human Subjects and Clinical Trials Information
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- xvii
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
Form Screenshots
R.- xviii
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
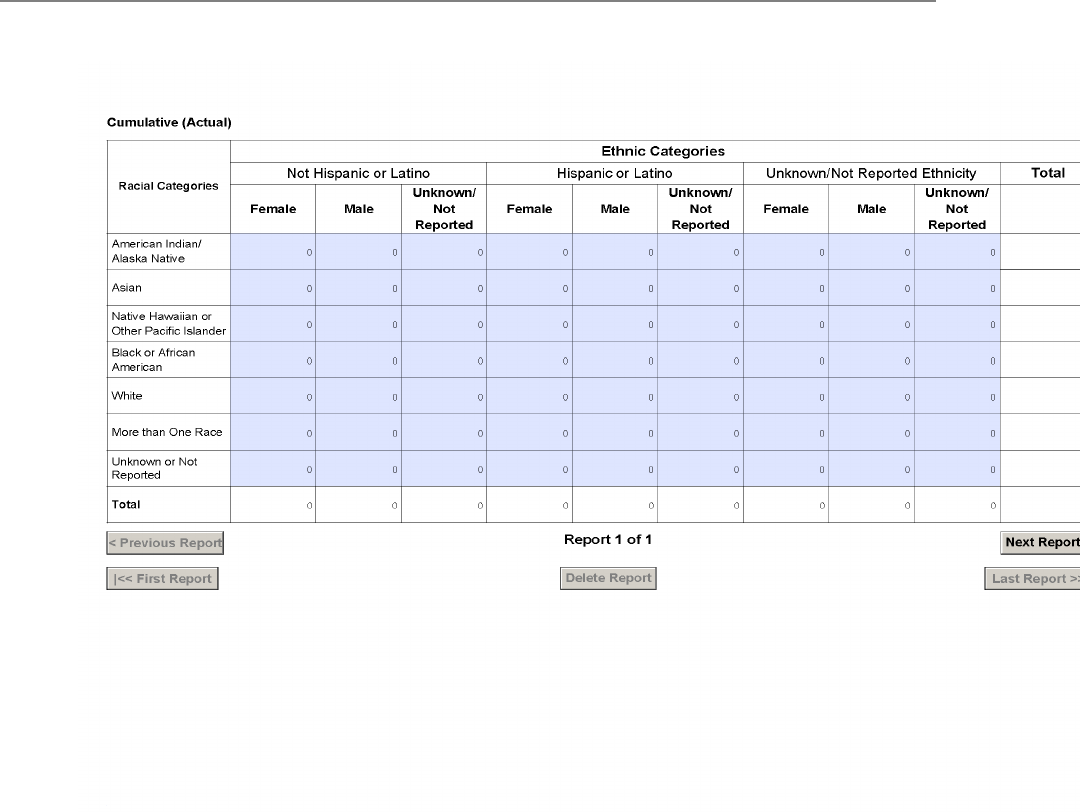
Form Screenshots
R.- xix
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series
Form Screenshots
R.- xx
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
R.- xxi
PHS Assignment Request Form
Research Instructions for NIH and Other PHS Agencies - Forms Version H Series

