
Western Carolina University
Controlled Substances
Program
This document provides guidance for WCU personnel who utilize controlled substances in
teaching, research, and pharmacy. This procedure is in place to ensure compliance with
requirements from the U.S. Drug Enforcement Administration (DEA) and the North Carolina
Department of Health and Human Services, Drug Control Unit (NC-DCU).
Office of Safety and Risk Management June 2017

1 | WCU Controlled Substances Program
EMERGENCY TELEPHONE NUMBERS
Phone
Hours
Safety and Risk Management
Office
• Work Related Injuries
(Normal Business Hours)
• Gas Leaks or Odors
• Chemical Spills
• General Inquiries
828-227-7443
8:00am - 5:00pm
Monday-Friday
University Police Department
• Work Related Injuries
(After Normal Business
Hours)
Police Services – 828-227-7301
Emergency Line – 828-227-8911
24 hours
Fire or Smoke
911 or
University Police 828-227-8911
24 hours
Medical Emergencies
911 or
University Police 828-227-8911
24 hours
NC Poison Control Center
1-800-84 TOXIN
(1-800-848-6946)
24 hours

2 | WCU Controlled Substances Program
Table of Contents
Section 1: Definitions .........................................................................................................................3
Section 2: Overview ...........................................................................................................................3
2.1 Schedules ............................................................................................................................................ 3
Section 3: Controlled Substances Program Requirements ....................................................................4
3.1 Registration ......................................................................................................................................... 4
3.2 Ordering .............................................................................................................................................. 5
3.3 Storage and Security ........................................................................................................................... 5
3.4 Use ...................................................................................................................................................... 6
3.5 Disposal ............................................................................................................................................... 6
3.6 Recordkeeping .................................................................................................................................... 6
Section 4: Chemical Control Program ..................................................................................................7
3 | WCU Controlled Substances Program
Section 1: Definitions
Definitions for selected terms used in this policy are included below. For a complete list of
definitions please refer to Title 21 United States Code (USC) Controlled Substances Act, Section
802.
Administer: Direct application of a controlled substance to the body of a patient or research
subject by (a) a practitioner (or, in his presence, by his authorized agent), or (b) the patient or
research subject at the direction and in the presence of the practitioner, whether such
application be by injection, inhalation, ingestion, or any other means.
Dispense: Deliver a controlled substance to an ultimate user or research subject by, or pursuant
to the lawful order of, a practitioner, including the prescribing and administering of a controlled
substance and the packaging, labeling or compounding necessary to prepare the substance for
such delivery. The term “dispenser” means a practitioner who so delivers a controlled
substance to an ultimate user or research subject.
Distribute: Deliver (other than by administering or dispensing) a controlled substance or a
listed chemical. The term “distributor” means a person who so delivers a controlled substance
or listed chemical.
Practitioner: A physician, dentist, veterinarian, scientific investigator, pharmacy, hospital, or
other person licensed, registered, or otherwise permitted, by the United States or the
jurisdiction in which he practices or does research, to distribute, dispense, conduct research
with respect to, administer, or use in teaching or chemical analysis, a controlled substance in
the course of professional practice or research.
Section 2: Overview
Controlled substances are any drugs or chemical substances whose possession and use are
regulated under the United States Controlled Substances Act (U.S. CSA) and the North Carolina
Controlled Substances Act (NC CSA). These chemicals have stimulant, depressant, or
hallucinogenic effects on the higher functions of the central nervous system, and tend to
promote abuse or physiological/psychological dependence.
Because of their potential for abuse, controlled substances have specific regulatory
requirements for the acquisition, storage, security, inventory/recordkeeping, disposal, and
importing or exporting.
2.1 Schedules
Substances regulated under the U.S. CSA are listed in one of five schedules. Schedule I have
the most restrictions and include substances with a high potential for abuse, no currently
accepted medical use in treatment in the U.S., and a lack of accepted safety protocols for use
4 | WCU Controlled Substances Program
under medical supervision. Schedules II through V include substances with decreasing
potential for abuse; currently accepted use in treatment or acceptable medical use in the U.S.;
and decreasing risks for physical or psychological dependence accordingly. Refer to Part 1308 –
Schedules of Controlled Substances on the DEA website for more information and the current
list of controlled substances for each schedule.
North Carolina CSA has added a Schedule VI which includes substances with no currently
accepted medical use in the United States, or a relatively low potential for abuse in terms of
risk to public health and potential to produce psychic or physiological dependence liability
based upon present medical knowledge, or a need for further and continuing study to develop
scientific evidence of its pharmacological effects. A current list of substances is available under
NC Controlled Substances Act G.S. 90-94.
Section 3: Controlled Substances Program Requirements
3.1 Registration
Registration for all controlled substances is required at the State level (NC-DCU) and at the
Federal level (US-DEA). The registration process and compliance requirements are detailed in
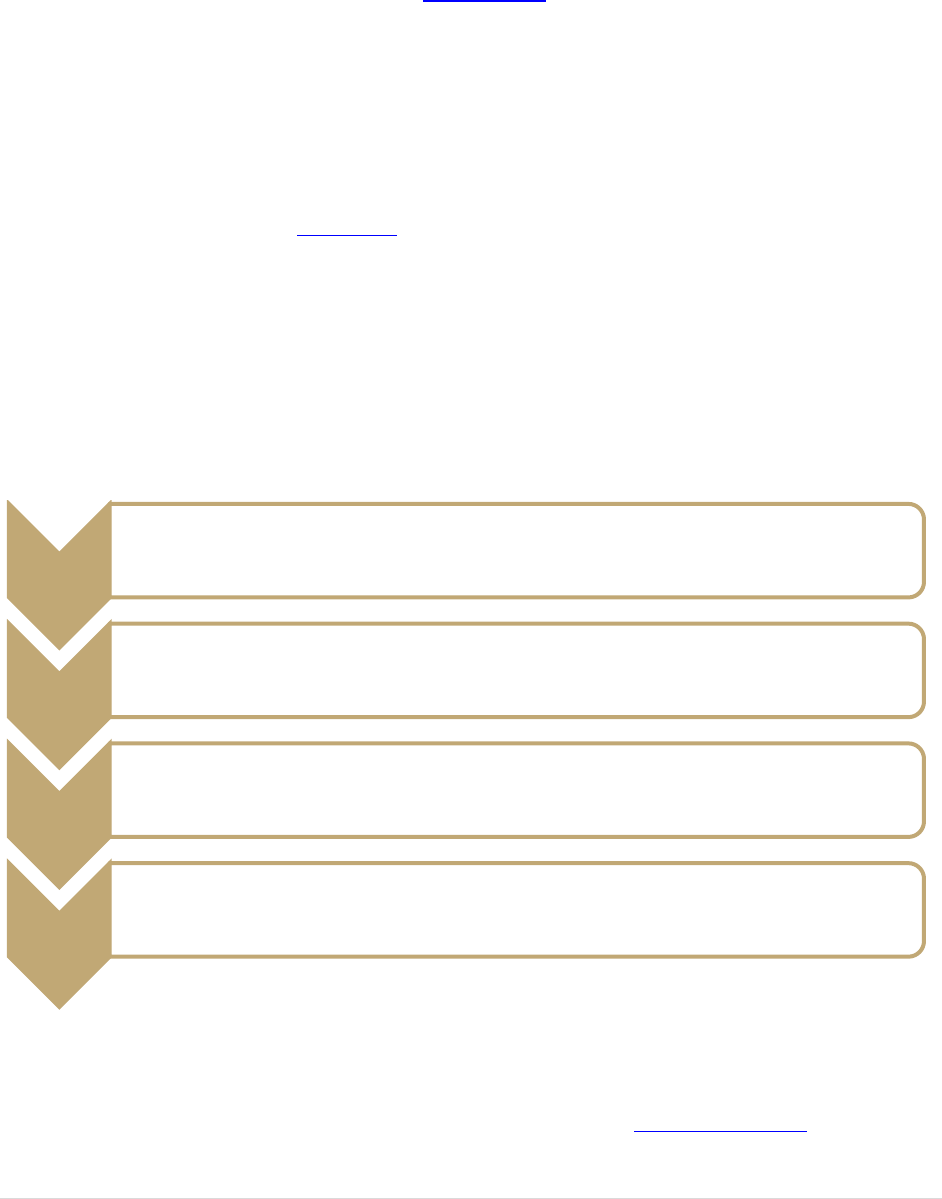
the following flow chart:
Step 1: New and/or existing users of controlled substances at WCU must notify the Office of
Safety and Risk Management. Please submit the Notification of Controlled Substance
Registration Form (available on the Safety Office website) to the Lab Safety Officer. For
questions call 828-227-7443.
Registration
Notification
Form
•Notify the Office of Safety and Risk Management for new or existing applications for a controlled substance
•Submit the WCU Notification of Controlled Substance Registration Form to the Office of Safety and Risk
Management
Register
with
NC-DCU
•Complete the applicable NC DHHS registration form and submit the payment
•Wait for NC-DCU Registation Approval
Register
with
US-DEA
•Complete the applicable DEA registration form and submit the payment
•Wait for US-DEA Registration Approval
Order
•DEA Registration # is required. Additional form (DEA Form 222) required for Schedule I and II substances
•Follow storage, use, recordkeeping, and disposal requirements
•Registration renewal as required by NC-DCU and US-DEA

5 | WCU Controlled Substances Program
Step 2: Register with the North Carolina Department of Health and Human Services, NC-Drug
Control Unit. Compete the applicable registration form and submit the payment by following
the instructions provided on the NC Controlled Substances Regulatory website.
Step 3: Once the initial registration with NC-DCU is approved and certification of registration is
granted, you will need to register with the US-Drug Enforcement Administration (DEA).
Registration is submitted online at the US-DEA website.
Registration Renewal: NC-DCU requires annual renewal for all types of registration. However,
the renewal for Federal DEA is based on the type of application. Refer to the DEA website for
forms and renewal periods for the different types of application.
Departmental Registrants: Departments may register with NC-DCU as a research facility, which
will allow them to share controlled substances within the department and for research
purposes only. Departmental registration would still require a specific person as
applicant/registrant who would be responsible for the proper use, storage, disposal, and
recordkeeping for federal and state requirements. If a department performs research in more
than one building, NC-DCU may require separate registrations for each building. Note:
Schedule I and VI substances require individual registration and cannot be shared with other
individuals. Registration cannot be transferred to a new person once a registrant leaves
campus.
3.2 Ordering
Once registration at the State and Federal level is complete, and you have received your
registration number for controlled substances, you may proceed with ordering. Note that NC-
DCU and the US-DEA can send out inspectors to verify that adequate security is in place before
registration is approved. For any controlled substance ordering, the DEA registration number
will be required. For ordering Schedule I and II substances, you must use the official Order
Form – DEA 222. Forms and information are available from the US-DEA Registration website.
Only registered individuals can order controlled substances.
3.3 Storage and Security
The registrant is responsible for managing the controlled substances in accordance with all
regulatory requirements including security, inventory, and recordkeeping.
Security: All controlled substances must be kept under lock and key and be accessible only to
authorized personnel. Storage cabinets must be built into the structure of the building or
essentially immovable with doors that cannot be forced open or easily removed at the hinges.
Schedule I and VI substances should be stored separately from other schedule substances and
regular laboratory chemicals should not be stored with any controlled substance. Schedule I

6 | WCU Controlled Substances Program
and II substances have the highest security requirements and must be stored in an approved
safe, steel cabinet, or vault.
Schedule I substances may not be issued to or used by anyone other than the registrant. If
additional personnel need to use the schedule I substance, they must register individually with
NC-DCU and DEA.
In the event that controlled substances are lost, stolen, or used in an unauthorized manner, the
registrant must immediately contact campus police (x8911) and the Office of Safety and Risk
Management (x7443). You may be required to submit DEA Form 106: Report of Theft or Loss of
Controlled Substance.
3.4 Use
The individual registered person retains all liabilities for the substance. Transferring controlled
substances (Schedule II – V only) from a registered to a non-registered user is only allowed if
authorized previously in the registration terms. Transferring these substances to other DEA-
registered individuals requires extra filing with the State DEA office, and is discouraged. Note:
Schedule I and VI substances require individual use only and cannot be shared within a
department.
3.5 Disposal
Controlled substances that are expired, unused, or contaminated must be stored securely until
ready for disposal. For disposal, the registrant must contact an NC-DCU agent at 919-733-1765
Only the registrant individual can perform disposal. If the registrant is not available to perform
the disposal (for example the registrant has left the institution), contact the NC-DCU for
guidance.
Breakage and Spillage of Controlled Substances: When there is breakage, damage, spillage, or
some other form of destruction, and the substance is recoverable, disposal must be followed as
stated above. If the substance is not recoverable, the registrant must document the
circumstances in their inventory records. Two individuals who witnessed the event must also
sign the inventory records.
3.6 Recordkeeping
Registrants must maintain complete and accurate inventory records for all controlled
substances. These records must be in or near the primary work area, separate from all other
records and documents, and available for inspection during regular work hours. Maintain the
records for a period of at least 3 years from the date of last entry. In the event of an audit by

7 | WCU Controlled Substances Program
DEA or NC-DCU, you will need to produce these records. Records must include the following
information:
• Receipt of controlled substance indicating the date received, name and address of supplier,
type, strength, and concentration of substance, and amount received. The receiving person
must sign each record.
• Current record for the storage and use of each substance indicating the starting quantity,
use date, building and room, specific research experiment or analysis, type and strength
used, and the quantity used. This record will provide a substance balance log sheet.
• Initial and biennial inventory record to include the name of each substance, form of
substance (solid, inhalant, etc.), number of units or volume, and number of containers.
Substances awaiting disposal must also be included on the inventory until they are
disposed.
Section 4: Chemical Control Program
The DEA’s Chemical Control Program is intended to disrupt the illicit production of controlled
substances by preventing diversion of chemicals used to make drugs. The production of illegal
drugs such as methamphetamine, cocaine, heroin, and MDMA (ecstasy) requires enormous
quantities of precursor chemicals. The Chemical Control Program aims to deny precursor
chemicals to drug trafficking organization while at the same time ensuring an adequate supply
for commercial licit markets. DEA registration, record keeping, and suspicious order reporting
requirements apply to importers, exporters, manufacturers, distributors and certain retailers of
these listed chemicals.
