
Use of dual nucleic acid
amplification tests for chlamydia
and gonorrhoea on samples
collected for the National
Chlamydia Screening Programme:
Results from a national survey of local
authority commissioners

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
2
About Public Health England
PHE exists to protect and improve the nation's health and wellbeing, and reduce health
inequalities. It does this through advocacy, partnerships, world-class science,
knowledge and intelligence, and the delivery of specialist public health services. PHE is
an operationally autonomous executive agency of the Department of Health.
Public Health England
133-155 Waterloo Road
Wellington House
London SE1 8UG
Tel: 020 7654 8000
www.gov.uk/phe
Twitter: @PHE_uk
Facebook: www.facebook.com/PublicHealthEngland
Authors: Nigel Field, Iain Kennedy, Kate Folkard, Catherine Ison, Stephen Duffell and
Gwenda Hughes
Thank you to Katy Town, Martina Furegato, Nöel Gill and André Charlett for their
review and assistance in preparing the document. We are grateful to the PHE sexual
health facilitators for their support with the local delivery of the survey which enabled
good coverage.
For queries relating to this document, please contact: Nigel Field at
© Crown copyright 2014
You may re-use this information (excluding logos) free of charge in any format or
medium, under the terms of the Open Government Licence v2.0. To view this licence,
visit OGL or email [email protected]. Where we have identified any third
party copyright information you will need to obtain permission from the copyright
holders concerned. Any enquiries regarding this publication should be sent to [insert
email address].
Published August 2014
PHE publications gateway number: 2014 259
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
4
Executive summary
this report summarises findings from a national survey of Local Authorities
(LAs) in England undertaken in 2013 to understand the use of nucleic acid
amplification tests (NAATs) which simultaneously detect both chlamydia and
gonorrhoea (hereafter referred to as ‘dual NAATs’) on samples collected for
chlamydia screening by the National Chlamydia Screening Programme (NCSP)
an online questionnaire was delivered to commissioners of sexual health
services, who were responsible for commissioning chlamydia screening in
young people aged 15-24 years in the 152 local authoritis (LA) in England;
the aim of the survey was to:
o understand the proportion of LAs currently commissioning the use of dual
NAATs on samples collected for chlamydia screening by the NCSP
o map the microbiological and clinical pathways used when gonorrhoea is
identified through dual screening, including confirmation of diagnoses
o provide an evidence base for clear and workable guidance to LA
commissioners, clinicians and other decision-makers to ensure that the
use of dual NAATs is clinically and ethically appropriate, and cost efficient
64% (98/152) of LAs responded to the survey, with no significant differences
found between those responding and not responding
53% (52/98) of LAs reported currently using dual NAATs; gonorrhoea diagnosis
rates based on genitourinary medicine (GUM) clinic data were significantly
higher among LAs reporting use of dual NAATs
where gonorrhoea screening was occurring alongside chlamydia screening, it
was found:
o the consent process for gonorrhoea was generally less clear than for
chlamydia, with patients not always being informed that their sample
would be tested for both infections
o considerable variation in clinical pathways used to manage gonorrhoea
o supplementary testing (using a second assay with a different nucleic acid
target) is recommended, but was not universally delivered
o patients were sometimes notified of the initial screening results, either
prior to supplementary tests being performed or where no such tests were
carried out, which increases the risk that false positive results are
returned to patients, who may be given antibiotics and whose partners
may be notified unnecessarily
the British Society for Sexual Health and HIV (BASHH) treatment guidelines for
gonorrhoea were followed in just over half of LAs where the NCSP-affiliated
service provided treatment
antimicrobials not recommended by BASHH are less likely to be effective and
might result in selection pressures leading to antimicrobial resistance (AMR)
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
5
most LA commissioners received data about gonorrhoea diagnoses made through
their local NCSP
NCSP providers submitted gonorrhoea diagnoses to the national surveillance
database for only half of LAs
RecommendationsThis survey found that use of dual NAATs on samples collected for
chlamydia screening by the NCSP is already widespread, with the result that
gonorrhoea screening is occurring alongside chlamydia screening in many areas in
England. This has been introduced without any change in NCSP policy, nor any
evidence that gonorrhoea screening is necessary or cost effective. There are no
structured, systematic clinical and procedural arrangements in place to manage
gonorrhoea screening of samples collected by the NCSP.
While gonorrhoea testing guidance maintains that there is no evidence to support
widespread unselected screening for gonorrhoea in the UK, in practice it may be
difficult to reverse the trend for using dual NAATs. To mitigate the potential harms
associated with use of dual NAATs in low prevalence settings, the following public
health messages should be emphasised to any LAs using or considering use of dual
NAATs for asymptomatic, community-based screening:
wherever dual NAATs are used for community-based screening, patients should be
provided with appropriate information about gonorrhoea screening (in addition to
chlamydia screening) to help ensure that testing is only performed with informed
consent
where the initial screening test is positive for gonorrhoea in low prevalence settings
(as is likely to be the case for most community-based screening), a supplementary
test, using a second assay with a different nucleic acid target, should be used on
the same sample to prevent false positive results
return of results, treatment and partner notification should only be undertaken
following confirmation of gonorrhoea
patients with confirmed gonorrhoea should be referred to level two or three sexual
health services for further management
clearly defined clinical pathways are required and should be followed to ensure the
best care for patients and their partners
data on gonorrhoea diagnoses should be reported through national surveillance
systems (GUMCADv2) wherever possible so that national trends can be monitored
national gonorrhoea testing guidance has been revised to inform and support
commissioners of sexual health services in making decisions about use of dual
NAATs
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
6
Introduction
The NCSP is a large national public health intervention designed to improve sexual
health in young people in England. The programme offers sexually active,
asymptomatic women and men, aged 15-24 yearsopportunistic screening to diagnose
and control Chlamydia trachomatis (chlamydia) infection.
1
Testing for chlamydia,
including through the NCSP, is undertaken using highly sensitive and specific NAATs.
Technological advancements in NAATs make it possible and inexpensive to
simultaneously test for Neisseria gonorrhoeae (gonorrhoea) alongside chlamydia in a
single assay – called combined or ‘dual NAATs’.
Like chlamydia, gonorrhoea is a sexually transmitted infection (STI) primarily causing
uncomplicated lower genital tract infection, which may lead to complicated or systemic
infection in some cases. Unlike chlamydia, the prevalence of gonorrhoea is very low in
the general population, instead being concentrated in specific groups: GUM attendees,
men who have sex with men, black Caribbeans, and in some regions where outbreaks
have occurred among young heterosexuals with high rates of partner change.
2
Screening of asymptomatic individuals in low risk populations with low gonorrhoea
prevalence, and the potential for cross-reaction with non-gonococcal neisseria species,
3
can result in high rates of false positive results, even when using highly sensitive and
specific NAATs. False positive results may lead to incorrect and stigmatising diagnoses,
partner notification, unnecessary use of antibiotics, and avoidable expense. Revised
guidance from PHE, the British Society for Sexual Health and HIV (BASHH) and the
Royal College of Pathologists (RCPath), accepted by the NCSP, states that while
testing for gonorrhoea is strongly recommended within specialist sexual health clinics
targeting higher risk populations there is no evidence to support widespread unselected
screening for gonorrhoea, and only sparse evidence for selective community screening
in the UK.
4
If screening is undertaken, the guidance recommends the positive predictive
value (PPV) of the testing algorithm should be at least 90%, usually requiring
supplementary testing using a second NAAT with a different nucleic acid target on the
same sample.
4
A survey of English laboratories in 2007 found that 29% of laboratories responding to
the survey were already routinely using dual NAATs for chlamydia and gonorrhoea.
5
A
recent update of this survey suggests this proportion has increased (Ison, pers. comm.).
However, the extent to which dual NAATs are used on samples collected by the NCSP
for chlamydia screening, where gonorrhoea prevalence is likely to be low, is not known.

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
7
Aim
A survey was undertaken of LA sexual health commissioners to:
understand the proportion of LAs currently commissioning the use of dual tests for
chlamydia screening on samples collected by the NCSP
map the microbiological and clinical pathways used to screen, confirm and manage
patients diagnosed with gonorrhoea identified through the use of dual NAATs
provide an evidence base for clear and workable guidance to LA commissioners,
clinicians, and other decision-makers about the use of dual NAATs
Methods
During May to July 2013, an online questionnaire was delivered to commissioners of
sexual health services in the 152 LA areas in England who were responsible for
commissioning chlamydia screening in young people aged 15-24 years.
Questionnaire development
The questionnaire was deployed through the PHE web-based survey tool, ‘Select
Survey’. Such web-based surveys are easy to use and tend to maximise the number of
respondents.
6
The survey used adaptive questioning so that all participants completed
basic information, with subsequent questions determined by their initial answers.
Respondents who reported never commissioning dual NAATs went straight to a
conclusion page, which provided an opportunity to give feedback as free-text
comments. Respondents who currently or had previously commissioned dual NAATs
were asked further questions.
The questionnaire was developed through an iterative process. Key areas were
developed by consensus within the project team. These were: (1) service setting and
sample types, (2) use of dual NAATs, (3) confirmation using supplementary NAAT tests
*
and use of gonorrhoea culture, (4) patient information and consent processes, (5)
clinical pathways for screening and management of gonorrhoea, (6) data management
and surveillance, and (7) contractual arrangements and costs associated with use of
dual NAATs. Specific questions were developed from these themes. Closed questions
were used, with dropdown menus for local authority and laboratory names, to increase
data quality. The questionnaire was initially screened by PHE staff members to test
usability, understanding, clarity, and question flow. It was then tested by the eight NCSP
sexual health facilitators (SHFs), who were asked to comment on clarity, content, and
*
Although true confirmation requires culture to identify Neisseria gonorrhoea, the survey questionnaire
gave the following pragmatic definition for a ‘confirmation test’: ‘a second test used to confirm the
diagnosis of gonorrhoea where the initial screening test is positive for gonorrhoea.’
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
8
wording. The questionnaire was put into an online webpage format and piloted with
commissioners in one area. The final questionnaire consisted of 29 questions and took
approximately 20 minutes to complete (Appendix 1).
Sampling and recruitment
The sampling frame for this survey was the 152 upper tier LAs in England. Each of the
NCSP SHFs wrote by email to their contact list of LA sexual health commissioners,
which covered the whole of England. This email introduced and briefly explained the
project and provided a web link to the survey. The survey was advertised in the
quarterly NCSP newsletter (including the weblink). SHFs also wrote individually to
commissioners not responding within three weeks to remind them of the survey, and
contacted commissioners who they believed not to be using dual NAATs to ensure that
this group was not under represented.
Data handling and statistical analysis
The survey data were extracted to Microsoft Excel and a descriptive analysis was
undertaken to understand the proportion of respondents reporting each outcome. Using
Stata (version 12.1), independent samples t-tests compared area-level characteristics
between LA responders and non-responders and between LAs using dual NAATs and
those not using dual NAATs. In some instances, more than one person responded for
the same LA; where there were inconsistencies between answers or for other reasons,
individual respondents were contacted to check the correct response. For descriptive
analyses of the survey data, the denominator throughout the analysis is the number of
LAs. Where an answer was missing, the LA was generally excluded from the
denominator and the denominator therefore varies according to item non-response.
This work was undertaken as a service evaluation project, with data collected and held
within the requirements of the data protection act and in accordance with PHE data
sharing best practice.

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
9
Results
Survey response
Survey response was good; 64% (98/152) of LAs responded to the survey and stated
whether or not they used dual NAATs (Figure 1). Although response varied by PHE
centre area, the proportion of LAs responding was at least 50% in all but one area,
suggesting good geographical coverage (Figure 1). The area-level characteristics of
responding and non-responding LAs were compared (Table 1) and no significant
differences were found in gonorrhoea diagnosis rates (estimated from diagnoses made
in GUM clinics), area-level deprivation (index of multiple deprivation (IMD)),
7
NCSP
chlamydia positivity, chlamydia diagnosis rates, coverage, or service type (Table 1).
From the data available, there was no evidence of participation bias.
Figure 1. Survey response showing the proportion of LAs responding in each PHE
centre area (n=152)
64
100
57
67
75
50
75
100
33
64
58
67
50
57
64
53
0
25
50
75
100
%

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
10
Table 1. Comparison of area-level characteristics between LAs responding and not
responding to the web survey
1
Number of
LAs
Mean
chlamydia
diagnosis rate
/ 100,000
2
Mean NCSP
coverage
3
Mean
gonorrhoea
rate (GUM)
/ 100,000
4
Responders
98
2151.9
27.3%
42.9
Non-responders
54
1869.8
24.2%
38.9
p-value difference
-
0.06
0.06
0.68
1. In addition, no significant difference was found by NCSP chlamydia positivity rate (p=0.63), service type (the
proportion of NCSP services provided by GUM, primary care, or Sexual and Reproductive Health (SRH) in
2012) (p=0.54), or LA IMD (p=0.89)
2. Chlamydia diagnosis rates (per 100,000 population) include diagnoses made in community-based and GUM
settings collected through the Chlamydia Test Activity Dataset (CTAD) and the GUM Clinic Activity Dataset
(GUMCAD)
3. Chlamydia testing coverage includes tests done in community-based and GUM settings collected through
CTAD and the GUMCAD
4. Gonorrhoea diagnoses (per 100,000 population) include diagnoses made in GUM clinics collected through
GUMCAD.
Use of dual NAATs on NCSP samples
Over half (53% (52/98)) of LAs reported currently using dual NAATs on NCSP samples,
45% (44/98) reported never using dual NAATs, and 2% (2/98) reported previously using
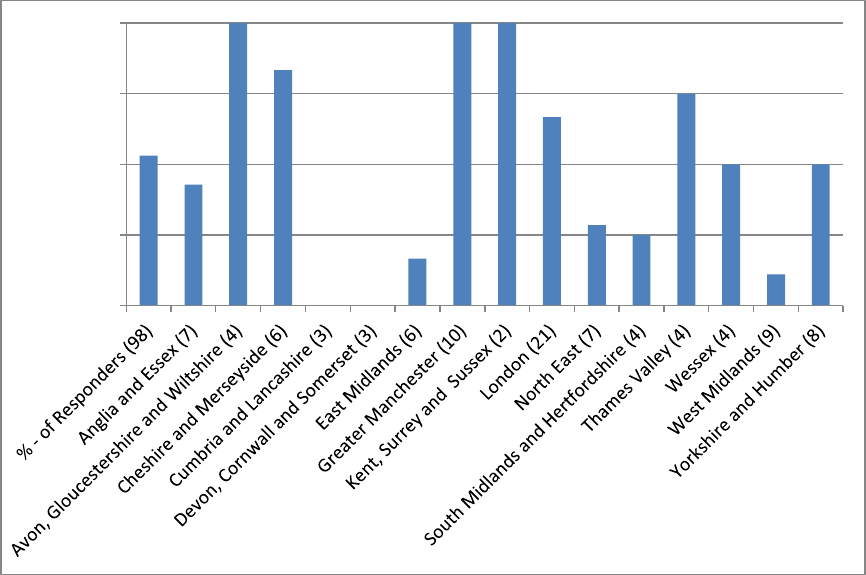
dual NAATs or did not know. Figure 1 shows that the proportion of LAs in each PHE
centre area reporting current use of dual NAATs showed substantial geographical
variation, with 100% of LAs using dual NAATs in some areas (Avon, Gloucestershire,
and Wiltshire, Greater Manchester, and Kent, Surrey and Sussex) and no LAs using
dual NAATs in two areas (Cumbria and Lancashire, and Devon, Cornwall and
Somerset).
Comparing LAs by whether or not they reported current use of dual NAATs, there was
no significant difference in IMD, NCSP chlamydia positivity, chlamydia diagnosis rate or
coverage (Table 1). However, mean gonorrhoea diagnosis rates (estimated from
diagnoses made in GUM clinics) were higher (p=0.03) and a higher proportion of
services were provided by community sexual health services (CSHS) in LAs using dual
NAATs on NCSP samples (p<0.1) (Table 1).
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
11
Figure 2. Proportion of LAs using dual NAATs on NCSP samples, by PHE centre area
(n=98)
For 14% (7/52) of the LAs who reported using dual NAATs, no further answers were
given to the survey and these LAs were excluded from further analyses.
LAs reporting use of dual NAATs on NCSP samples were asked in which settings this
occurred. Dual NAATs were used in a wide range of venues across the country,
including settings such as Contraception and Sexual Health and Sexual and
Reproductive Health (CaSH/SRH) services (98% (44/45)) and primary care (91%
(41/45)), as well as through remote testing by post or Internet (80% (36/45)) and
termination of pregnancy (ToP) services (87% (39/45)) (Appendix 2). Most LAs reported
using dual NAATs for samples collected in at least five different settings (82% (37/45)).
Respondents were asked to select their largest venue using dual NAATs as the setting
to consider when answering the remaining questions in the survey. In 51% (23/45), this
was CaSH/SRH, in 20% (9/45) this was primary care, in 13% (6/45) it was another
setting and 16% (7/45) did not know the answer to this question.
Dual NAATs were used with all sample types, although less frequently with pharyngeal
and rectal samples (24% (11/45)) and urethral samples (18% (8/45)) than with
endocervical (56% (25/45)), urine (91% (41/45)) and vaginal samples (82% (37/45))
(Appendix 2).
53.1
42.9
100.0
83.3
0.0 0.0
16.7
100.0 100.0
66.7
28.6
25.0
75.0
50.0
11.1
50.0
0.0
25.0
50.0
75.0
100.0

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
12
Table 2. Comparison of area-level characteristics between LAs reporting current
commissioning of dual NAATs and those not
1
Number of
LAs
Mean
chlamydia
diagnosis rate
/ 100,000
2
Mean chlamydia
testing
coverage
3
Mean
gonorrhoea
diagnosis rate
/ 100,000
4
Dual NAATs
52
2254.8
28.6%
52.7
No dual NAATs
46
2063.2
26.2%
32.4
p-value difference
-
0.31
0.24
0.03
1. No significant difference was found by NCSP chlamydia positivity rate (p=0.93), LA IMD (p=0.88), or the
proportion of NCSP services provided by GUM or GP, but the proportion of services provided by CSHS was
higher in those LAs using dual NAATs (19.4% vs 8.6%; (p<0.01))
2. Chlamydia diagnosis rates (per 100,000 population) include diagnoses made in community-based and GUM
settings collected through the Chlamydia Test Activity Dataset (CTAD) and the GUM Clinic Activity Dataset
(GUMCAD)
3. Chlamydia testing coverage includes tests done in community-based and GUM settings collected through
CTAD and the GUM Clinic Activity Dataset (GUMCAD)
4. Gonorrhoea diagnoses (per 100,000 population) include diagnoses made in GUM clinics collected through
GUMCAD
Patient information and consent
Informed consent is essential when undertaking clinical diagnostic tests and returning
results to patients. The survey sought to understand whether and how patients were
informed about, and consented to, gonorrhoea screening when undertaken alongside
chlamydia screening. Overall, 36% (15/42) of LAs using dual NAATs provided
gonorrhoea-specific patient information materials to patients, 45% (19/42) provided no
gonorrhoea-specific information materials and 19% (8/42) did not know (Appendix 2). Of
those LAs reporting no gonorrhoea-specific patient information materials, 84% (16/19)
reported that gonorrhoea was discussed within their NCSP patient information leaflet,
while only 5% (1/19) of these LAs provided no gonorrhoea information and 11% (2/19)
did not know (Appendix 2). Informed consent for gonorrhoea testing was assumed on
the basis that information was provided and the testing kit was returned in 71% (25/35)
of LAs, and consent was taken in writing in 14% (5/35). Only 2% (1/52) of LAs reported
that no informed consent was obtained for gonorrhoea testing, although this may
underestimate the true proportion not obtaining consent, because 32% (17/52) of all
LAs using dual NAATs did not answer this question and 4% (2/52) did not know whether
consent was obtained.

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
13
Four LAs that included information on gonorrhoea in their NCSP leaflets uploaded
copies of the documents to the survey website. Two leaflets were produced by NHS
organisations and two by a third sector organisation (Terrence Higgins Trust). Three of
the leaflets discussed chlamydia and gonorrhoea, and one leaflet discussed solely
gonorrhoea. The leaflets mentioning both infections explained appropriately that testing
with dual NAATs was performed on a single sample. Only one leaflet stated that
gonorrhoea testing was optional and explained how to opt out.
Clinical care pathways
Of 52 LAs performing testing using dual NAATs, 41 provided sufficient information to
understand their clinical care pathways in detail. Although confirmation testing
†
involving
a supplementary test was used in 93% (38/41) of LAs, 63% (26/41) of LAs referred
patients to higher level sexual health services on the basis of any initial reactive
screening test results, 17% (7/41) referred only after supplementary testing, 15% (6/41)
of LAs did not refer patients to another service (possibly because the initial service was
level 2), and 10% (4/41) did not know when patients were referred in relation to the
timing of supplementary testing. Where patients were referred, 8% (3/36) of LAs
referred to level 2 sexual health services and the remaining LAs referred to Level 3
(GUM). Patients were informed of the initial reactive test result by the initial testing
service prior to supplementary testing in 73% (29/40) of areas where confirmation was
undertaken. Where patients were referred, the supplementary test results were provided
to patients by the referral service for 75% (27/36) of LAs.
Partner notification was usually initiated by the referral service, with 60% (15/26)
undertaking partner notification only after gonorrhoea was confirmed. This contrasts
with LAs where the initial service provider undertook partner notification: 79% (11/14)
initiated partner notificationimmediately following the initial reactive screening result.
Referral services performed treatment in 71% (29/41) of LAs. Treatment was delayed
until the supplementary test result was known by 33% (4/12) of initial service providers
and 52% (15/29) of referral services, but the difference was not significant. Seventy five
per cent (9/12) of LAs where the initial service provided treatment reported on the
treatment regimen used. All nine of these LAs prescribed combination treatment with
azithromycin 1g; this was with intramuscular ceftriaxone 500mg in four cases, oral
cefixime 400mg in another four cases, and with both ceftriaxone 500mg and
doxycycline 100mg in the final LA.
†
The survey provided the following definition for ‘confirmation test’: ‘a second test used to confirm the
diagnosis of gonorrhoea where the initial screening test is positive for gonorrhoea.’ More complex
definitions involving second DNA targets were thought not likely to be understood well.
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
14
LAs were assessed on whether they met three core criteria for using dual NAATs:
1. was a supplementary test performed?
2. were patients with gonorrhoea referred to level 2 or 3 sexual health services?
3. were the return of gonorrhoea results, treatment, and partner notification undertaken
only after gonorrhoea was confirmed?
Overall, 12% (5/41) of LAs met all three criteria, 71% (29/41) met two criteria, 8% (3/41)
met one criterion and 3% met none of the criteria. Eight per cent (3/41) provided
insufficient data to fully assess the clinical pathways in this way.
Gonorrhoea data collection and reporting
For most LAs using dual NAATs their NCSP commissioners reported receiving data
about gonorrhoea diagnoses (86% (36/42)), although 12% (5/42) received no data
(Appendix 2). The survey asked whether gonorrhoea diagnoses made through NCSP
services are submitted through the national STI reporting systems, called the
genitourinary medicine clinic activity dataset (GUMCADv2). About half of LAs
undertaking testing using dual NAATs reported that their NCSP gonorrhoea data are
submitted to GUMCADv2 (52% (22/43)), 9% (4/43) did not submit data and 16% (7/43)
did not know whether or not data were submitted (Appendix 2).
Costs of using dual NAATs
Commissioners were asked: ‘Has the cost for your NCSP service changed due to the
introduction of dual testing?’ Seventeen per cent (7/42) reported that costs for their
NCSP service had increased as a result of introducing dual NAATs, although most LAs
(69% (29/42) reported that costs had stayed the same and the remainder did not know
(14% (6/42) (Appendix 2). The survey also asked about the tariff for dual NAATs (if a
fee per service or item was in place). However, item non-response was high for this
question and the data are therefore difficult to interpret: 15% (8/52) reported paying less
than £20 per dual test (the lowest price quoted was £7) and 4% (2/52) reported paying
£20-59 per test, while the remaining LAs did not provide an answer.
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
15
Discussion
This is the first study to estimate the extent of testing using dual NAATs on samples
collected for chlamydia screening by the NCSP. We found that just over half of LAs
were already commissioning testing using dual NAATs, although it is likely that in many
cases these arrangements will have been inherited by LAs from their predecessor
Primary Care Trusts.
LAs which were using dual NAATs on NCSP samples were more likely to be areas with
higher rates of gonorrhoea diagnosis made in GUM clinics. Whether this is an indication
of evidence-based policy making, or whether the finding is associated with the
introduction of dual NAATs in these areas, is not known. However, gonorrhoea rates
have historically been high in these LAs, suggesting that some dual testing of NCSP
samples may have been introduced to further improve case detection in areas with
higher gonorrhoea prevalence.
Patient information material about gonorrhoea screening was provided (either
specifically or as part of dual testing information) and consent was taken (implied,
written or verbal) by at least 60% of LAs using dual NAATs. Although very few LAs
reported not providing information or not taking informed consent, many of the
remaining LAs did not answer or did not know the answer to these questions,
suggesting that commissioners may not be including this level of detail in service
specifications.
We found significant variation in care pathways between LAs, with 13 different
pathways described by 41 LAs where data were available. In many LAs (73%),
unconfirmed results were given to patients, and at least 4 LAs did not adhere to national
guidance on first-line antimicrobial therapy for gonorrhoea. Three suggested standards
for use of dual NAATs were adhered to by only 12% of LAs reporting clinical pathway
data. These findings support the need for a standardised clinical pathway for use of dual
NAATs.
Although data about gonorrhoea diagnoses made through testing of NCSP samples
were received by commissioners in most LAs, providers submitted these data to
national surveillance systems in less than half of LAs. There are currently limited data
routinely available to monitor and evaluate the use of gonorrhoea screening in
community-based settings, but this may improve as GUMCADv2 is rolled out across
level 2 sexual health services.
Limitations
The survey response was high and similar across the geographical areas in England,
and there was no evidence of participation bias associated with IMD or NCSP area-level

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
16
characteristics. It therefore seems reasonable to assume that the responding LAs are
representative for England in their use of dual NAATs. However, the responses might
be subject to reporting bias and were dependent on the respondents’ full understanding
of what was being asked, particularly where this required technical knowledge about
molecular tests or clinical pathways. Most questions had item non-response rates of
around 14%, which may reflect respondents’ lack of understanding or knowledge about
service specifications or a reluctance to answer questions that might reveal sub-optimal
practice.
Use of dual NAATs in settings where gonorrhoea prevalence is low
Importance of supplementary testing
Overall, these data suggest that use of dual NAATs on samples collected by the NCSP
is already widespread so that, in many areas, gonorrhoea screening is occurring
through a programme that was only designed to diagnose and control chlamydia.
Gonorrhoea diagnoses were nearly ten fold lower than chlamydia diagnoses in England
and Wales in 2012. Recent data from the third National Survey for Sexual Attitudes and
Lifestyles (Natsal-3) shows that the weighted population prevalence of gonorrhoea was
less than 0.1% among those aged 16-44 years, although the prevalence in community-
based services, such as NCSP settings, is likely to be somewhat higher.
8,9
Table 3
shows worked hypothetical examples of gonorrhoea screening in populations where the
gonorrhoea prevalence is 0.1% and 1.0% using an assay with a sensitivity and
specificity of 99%. If prevalence is 0.1%, this would result in the PPV being 9% for an
unconfirmed reactive screening test for gonorrhoea, equivalent to 91 unconfirmed
gonorrhoea diagnoses for every 100 positive tests. A supplementary test would result in
the PPV rising to 91%. In fact, gonorrhoea prevalence needs to be greater than 8%
before the PPV reaches 90% when using a single assay with 99% sensitivity and
specificity. These data highlight the importance of adhering to testing guidance and
using supplementary tests as part of the clinical diagnostic algorithm.
3,10
Table 3. Modelling the effect of prevalence on PPV and the number of false positives
using a gonorrhoea test with 99% sensitivity and specificity
Screening test
PPV
No. patients
with false +ve /
100 +ve tests
Supplementary
test PPV
No. patients
with false +ve /
+ve 100 tests
Prevalence 0.1%
9%
91
91%
9
Prevalence 1.0%
50%
50
99%
1
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
17
Implications for patients
Where dual NAATs are used, it is important to mitigate the potential risks of screening for
gonorrhoea. For example, there was considerable variation in the clinical pathways used to test
patients and manage results, including that patients were sometimes notified of the initial
screening results, either prior to supplementary tests being performed or where no
supplementary tests were used. There were also several LAs where partner notification and
treatment for gonorrhoea were based solely on the initial test results. Not undertaking
supplementary testing and/or initiating treatment or partner notification prior to supplementary
testing increases the risk of treating and managing patients on the basis of a false positive
result, which may lead to harm and increase unnecessary antibiotic treatment, increasing risk
of developing AMR.
A diagnosis of gonorrhoea may be highly stigmatising and might have considerable negative
social and health consequences for patients and their partners. It is therefore important that
patients are made aware that they are being tested for both infections. It is also important that
the risk of gonorrhoea misdiagnosis is minimised, usually requiring supplementary testing. This
survey found that patients were not always informed that their sample would be tested for both
infections. Consent for both chlamydia and gonorrhoea screening should be obtained where
dual testing is performed; assumed or opt-out consent may not be appropriate.
Gonorrhoea resistance
Over the last 50 years, N. gonorrhoeae has developed resistance to a wide range of
antibiotic treatments in England and Wales. Treatment guidelines are based on known
patterns of susceptibility and aim to reduce practices that increase the likelihood of
resistance developing. In this survey, the treatment guideline (intramuscular ceftriaxone
and oral azithromycin)
11
was followed in just over half of LAs where the initial service
provided treatment. In the other LAs, cefixime/azithromycin combination treatment was
used, which may reflect that cefixime can be administered orally and is therefore easier
for patients to receive and practitioners to provide. However, cefixime is less likely to be
effective and might result in selection pressures promoting AMR.
12
Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
18
References
1. National chlamydia screening programme - NCSP home. at
<http://www.chlamydiascreening.nhs.uk/>
2. Public Health England. Health Protection Report. (2013). at
<http://www.hpa.org.uk/hpr/archives/2013/hpr2313.pdf>
3. Ison, C. GC NAATs: is the time right? Sex. Transm. Infect. 82, 515 (2006).
4. Guidance for the detection of gonorrhoea in England; including guidance on the use
of dual testing for chlamydia and gonorrhoea. Public Health England © Crown
copyright (2014). https://www.gov.uk/government/publications/guidance-for-the-
detection-of-gonorrhoea-in-england
5. Benzie, A. et al. Gonococcal NAATs: what is the current state of play in England and
Wales? Int. J. STD AIDS 21, 246–248 (2010).
6. Dayan, Y., Paine, C. S. & Johnson, A. J. Responding to sensitive questions in
surveys: A comparison of results from Online panels, face to face and self-
completion interviews. (2009). at <http://www.ipsos-
mori.com/Assets/Docs/Publications/Ops_RMC_Responding_Sensitive_Questions_0
8_03_10.pdf>
7. Payne, R. A. & Abel, G. A. UK indices of multiple deprivation - a way to make
comparisons across constituent countries easier. Health Stat. Q. Off. Natl. Stat. 53,
22–37 (2012).
8. Sonnenberg, P. et al. Prevalence, risk factors, and uptake of interventions for
sexually transmitted infections in Britain: findings from the National Surveys of
Sexual Attitudes and Lifestyles (Natsal). The Lancet 382, 1795–1806 (2013).
9. Fifer, H. & Ison, C. A. Nucleic acid amplification tests for the diagnosis of Neisseria
gonorrhoeae in low-prevalence settings: a review of the evidence. Sex. Transm.
Infect. (2014). (In Press)
10. Standards Unit, Microbiology Services Division, HPA. UK Standards for
Microbiology Investigations: detection of Neisseria gonorrhoeae using Molecular
Methods. (2012).
11. Bignell, C., Fitzgerald, M., Guideline Development Group & British Association for
Sexual Health and HIV UK. UK national guideline for the management of
gonorrhoea in adults, 2011. Int. J. STD AIDS 22, 541–547 (2011).
12. Ison, C. A. et al. Decreased susceptibility to cephalosporins among gonococci: data
from the Gonococcal Resistance to Antimicrobials Surveillance Programme
(GRASP) in England and Wales, 2007–2011. Lancet Infect. Dis. 13, 762–768
(2013).

19
Appendix 1: dual testing survey
National Chlamydia Screening Programme –
Chlamydia and Gonorrhoea Dual Testing survey
Introduction
National Chlamydia Screening Programme – Chlamydia and Gonorrhoea Dual
Testing survey
Thank you for agreeing to participate in this survey, which we are asking all
commissioners of sexual health services in England, including chlamydia testing
programmes, to complete. It will take approximately 10-15 minutes. The survey will
automatically save your responses, allowing you to complete the survey at a later
time if necessary.
What is ‘dual testing’?
The term ‘dual testing’, refers to the testing of two infections, chlamydia and
gonorrhoea, at the same time using a single laboratory reaction. The survey is about
‘dual testing’ and gonorrhoea diagnosis in the NCSP (including chlamydia screening
of 15-24 year olds which has been integrated into local services). We are not
seeking information about GUM services.
What is the aim of the survey?
The data collected will be used to update current NCSP and other national
guidelines on the use of 'dual testing':
Link to BASHH guidance for gonorrhoea testing in England and Wales
Please click next to begin the survey

20
Section A
This section asks some basic information about you and services in your area.
1.
What is your job title/role?*
2.
Which organisation do you work for?*
3.
Which local authority areas do you cover?*
Please select each unitary/upper tier local authority you cover from the drop down menu. If you cover more than four local authorities, please enter
the names of the other authorities in the comments box at the end of the survey.
Local Authority 1
Local authority 2
Local authority 3
Local authority 4
Local
authority
-- Please Select --
-- Please Select --
-- Please Select --
-- Please Select --
4
.
Please provide the names and locations of all the laboratories for your area that test chlamydia or 'dual testing' samples.*
If a lab is not listed, please enter it in question 5.
Lab 1
Lab 2
Lab 3
Laboratory
-- Please Select --
-- Please Select --
-- Please Select --
5.
Please provide the names and locations of any laboratories for your area that were not included in question 4. Please include private laboratories,
if applicable*
Please enter each lab name and location on a separate line
Lab 1 name
Lab 1 location
Lab 2 name

21
Lab 2 location
Lab 3 name
Lab 3 location
Lab 4 name
Lab 4 location
6.
Has any ‘dual testing’, where samples are tested for chlamydia and gonorrhoea in the same reaction, been commissioned in your area outside of
GUM clinic settings?*
We currently commission ‘dual testing’ services
We have previously commissioned ‘dual testing’ services but these have stopped
We have never commissioned any ‘dual testing’ services
Please tell us the dates 'dual testing' ran between and briefly state why it was stopped. If you require more space please use the comments box at
the end of the survey.

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
22
Section B
This section asks questions about some of the basic aspects of the service
specification for ‘dual testing’ for chlamydia and gonorrhoea in settings outside of
GUM clinics. If 'dual testing' is no longer commissioned in your area, please answer
about the services that used to be commissioned.
7.
In which settings does ‘dual testing’ for chlamydia and gonorrhoea currently occur
(please select all that apply)?
Select at least 1.
General Practice
Pharmacy
Termination of pregnancy services
Contraception and Sexual Health /Sexual and Reproductive Health
Remote (eg mailout, web based)
Outreach/educational settings
Other, please specify
8.
When responding to the remaining questions in this survey, we would like you
to answer for the setting where the largest proportion of your 'dual testing'
occurs.
Please tell us the setting which undertakes the largest volume of 'dual testing' in your
area (and therefore which the remaining questions relate to).
9.
Please use the slider to indicate what percentage of your 'dual testing' occurs in the
setting with the highest volume of testing?
This should be the same setting as for question 8.
0
10.
What biological specimens are used for ‘dual testing’ (please select all that apply)?
Select at least 1.
Urine
Endocervical swab
Urethral swab
Vaginal swab
Rectal swab
Pharyngeal swab
Don't know
Other, please specify

23
11.
How is consent taken for ‘dual testing’ ?
A written consent form is required, which specifically mentions gonorrhoea
testing
People are informed that gonorrhoea testing will occur, and by completing and
returning the test kit, it is assumed the patient has consented to gonorrhoea
testing
No specific consent for gonorrhoea testing is taken
Other, please specify
12.
Does the patient information leaflet that is provided to people undergoing 'dual
testing' discuss gonorrhoea?
Yes
No
Don't know
13.
Select file to upload:
(click "Browse" button below to locate file)
File size restricted to: 4194304 KB
File type restricted to: no file type restrictions
File name: (limit 255 characters)
File description: (limit 255 characters)
Upload
Files uploaded:
14.
Are any additional gonorrhoea specific patient information materials provided
to people offered 'dual testing'?
Yes
No
Don't know
15.
Select file to upload:
24
(click "Browse" button below to locate file)
File size restricted to: 4194304 KB
File type restricted to: no file type restrictions
File name: (limit 255 characters)
File description: (limit 255 characters)
Upload
Files uploaded:

25
Section C
The next questions ask about the clinical care pathway for clients with a positive test
result for gonorrhoea.
In this survey we are not collecting information about positive chlamydia test results.
A 'confirmation test' is a second test used to confirm the diagnosis of gonorrhoea
where the initial screening test is positive for gonorrhoea.
The next questions ask about the clinical care pathway for clients with a positive
test result for gonorrhoea.
In this survey we are not collecting information about positive chlamydia test
results.
A 'confirmation test' is a second test used to confirm the diagnosis of gonorrhoea
where the initial screening test is positive for gonorrhoea.
16.
If a ‘dual test’ is positive for gonorrhoea, do local service specifications require
further tests to be carried out to confirm the diagnosis of gonorrhoea?
Further tests to confirm the diagnosis of gonorrhoea are performed on the
original sample
Further tests to confirm the diagnosis of gonorrhoea are performed on a
new sample
Further tests to confirm the diagnosis are performed, but it is not specified
if this is on the same sample or a new sample
Further tests to confirm the diagnosis are not included in the service
specification
Don't know
Other, please specify
If no conformation is carried out, what action occors on the basis of the initial
screening result? (for example: notification, treatment, referral?)
17.
If confirmation tests are performed, do these occur
at the same lab or a different lab to where the
original screening test was carried out?
Same commissioned lab
Different commissioned lab
National reference lab
Don't know
26
18.
Is culture carried out following a positive 'dual test'?
Yes
No
Don't know
19.
Who is responsible for the following care steps?
Please select the most appropriate box in each row.
Initial
'dual
testing'
service
provider
A
different
level 2/3
sexual
health
provider
Process
does not
happen
Don't
know
Informing person
of initial screening
result
Informing person
of diagnosis
(confirmation test
result)
Treatment
Partner notification
20.
When in the pathway do the following care steps occur?
Please select the most appropriate box in each row.
When
initial
screening
result is
known
When
confirmation
test result is
known
Process
does not
happen
Don't
know
Person first
notified of result
Treatment
Partner
notification
Referral to other
services

27
21.
Where are people referred if they are diagnosed with gonorrhoea through the
'dual test'?
The initial service provider refers the person directly to Level 2 sexual
health services
The initial service provider refers the person directly to Level 3 sexual
health services
The initial service provider refers the person directly to primary care
services
The person is encouraged to self-refer to appropriate services
Not referred
Don't know
Other, please specify
22.
If someone is referred to other services, are any actions taken to confirm they
attended the other service?
The NCSP service contacts the person to confirm they have attended
The NCSP service contacts the receiving service to confirm that the
person has attended
The NCSP service is contacted by the receiving service, who confirm that
the person has attended
The NCSP service does not confirm that the person has attended
N/A - clients are not referred
23.
If treatment is initiated as part of the ‘dual testing’ service, what treatment is
given?
28
Section D
This section asks about the information on gonorrhoea cases that is collected in
your area as part of NCSP services
24.
What data on gonorrhoea diagnoses made through NCSP services is received by
commissioners of NCSP services in your area?
Please tick all that apply
Individual identifiable line listing
Anonymised/pseudonymised line listing
Aggregated summary usable data by patient demography (ie split by age group,
gender or geography)
Aggregate summary by testing venue
Count of tests/postivity rates only
None
Other, please specify
25.
How are data on gonorrhoea diagnoses through NCSP services held by providers
in your area?
Individual paper patient records
Individual electronic patient records
Combined electronic record (for example line listing)
Don't know
Other, please specify
26.
Are data on gonorrhoea diagnoses made through ‘dual testing’ reported through
GUMCAD/GUMCAD 2 (the national electronic STI reporting systems)?
Yes
No
Don't know

29
Section E
This section asks about the contractual arrangements for any chlamydia and
gonorrhoea ‘dual testing’ service commissioned in your area for the NCSP
27.
Has the cost for your NCSP service changed due to the introduction of 'dual testing'?
Costs have increased
Costs have decreased
Costs have stayed the same
Don't know
28.
Please inform us of the tariff if a fee per item/service is in place. These data will be
used to generate aggregated national averages, and will not be reported
individually.
There is no need to enter a "£" symbol. Please enter data for any option that
applies
Cost per
patient
Cost per
'dual test'
Cost per
standard
chlamydia
only test
Cost per
confirmation
Cost per
treatment
Cost per
referral to
other
services
29.
Do you have any other comments on the use of ‘dual testing’ for chlamydia and
gonorrhoea as part of the NCSP?

30
Finally
Thank you for taking the time to complete this survey. These data will be used to
inform national guidelines about the future use of ‘dual testing’, and we hope the
results of this project will help to support you in making future commissioning
decisions.

Use of dual NAATs on samples collected for the National Chlamydia Screening Programme
31
Appendix 2: additional data table
Use of dual testing assays on samples collected for the National Chlamydia Screening Programme (NCSP):
Results from a national survey of local authority commissioners
Appendix 2 - additional data tables
Q7 In which settings does ‘dual testing’ for chlamydia and gonorrhoea currently occur?
Type of setting CaSH/SRH GP Outreach/Educational Pharmacy Remote (mail/web) ToP
No. LAs (%) 44 (98%) 41 (91%) 31 (69%) 32 (71%) 36 (80%) 39 (87%)
7 LAs did not answer; denominator is 45 LAs
Q8 Where does the largest proportion of your 'dual testing' occur? In which settings does ‘dual testing’ for chlamydia and gonorrhoea currently occur?
Type of setting CaSH/SRH GP Outreach/Educational Other - not specified Not known
No. LAs (%) 23 (51%) 9 (20%) 3 (7%) 3 (7%) 7 (16%)
7 LAs did not answer; denominator is 45 LAs
Q10 What biological specimens are used for ‘dual testing’?
Type of specimen Endocervical Pharyngeal Rectal Urethral Urine Vaginal Other Not known
No. LAs (%) 25 (56%) 11 (25%) 11 (25%) 8 (18%) 41 (91%) 37 (82%) 1 (2%) 3 (6%)
7 LAs did not answer; denominator is 45 LAs
Q11 How is consent taken for ‘dual testing’?
Method of consent
Information provided, consent for
gonorrhoea testing is assumed by
return of kit
Written consent is obtained, which
specifically mentions gonorrhoea testing
Consent for gonorrhoea testing is opt-
out
Verbal consent is obtained for
gonorrhoea testing
No consent is obtained for
gonorrhoea testing
Not known
No. LAs (%) 25 (71%) 5 (14%) 1 (3%) 1 (3%) 1 (3%) 2 (6%)
17 LAs did not answer; denominator is 35 LAs
Q12 Does the patient information leaflet that is provided to people undergoing 'dual testing' discuss gonorrhoea?
LA answer Yes No Not known
No. LAs (%) 15 (36%) 19 (45%) 8 (15%)
10 LAs did not answer; denominator is 42 LAs
Q14 Are any additional gonorrhoea specific patient information materials provided to people offered 'dual testing'?
LA answer Yes No Not known
No. LAs (%) 16 (84%) 1 (5%) 2 (11%)
Denominator is 19 LAs without gonorrhoea-specific patient information materials
Q24 What data on gonorrhoea diagnoses made through NCSP services is received by commissioners of NCSP services?
Method of data receipt
Summary data obtained by patient
demography (gender, age-group etc)
Summary data obtained by testing venue
Anonymised /pseudonymised data
obtained listing individual patient
outcomes
Summary count of tests/postivity rates
without further breakdown
Identifiable data obtained
listing individual patient
outcomes
Bespoke analyses are
obtained
No data are
obtained
Not known
No. LAs (%) 9 (17%) 5 (21%) 2 (5%) 8 (19%) 1 (2%) 11 (26%) 5 (12%) 1 (2%)
10 LAs did not answer; denominator is 42 LAs
Q26 Are data on gonorrhoea diagnoses made through ‘dual testing’ reported through GUMCAD/GUMCAD 2 (the national electronic STI reporting systems)?
LA answer Yes No Not known
No. LAs (%) 22 (51%) 4 (9%) 7 (16%)
9 LAs did not answer; denominator is 43 LAs
Q27 Has the cost for your NCSP service changed due to the introduction of 'dual testing'?
LA answer Increased Stayed the same Not known
No. LAs (%) 7 (16%) 29 (69%) 6 (14%)
10 LAs did not answer; denominator is 42 LAs
