
SAHPGL-MD-07_v3
Page 1 of 28
17 November 2023
GUIDELINE ON QUESTIONS AND ANSWERS: LICENSING OF
MEDICAL DEVICE ESTABLISHMENTS
This document is intended to provide clarity on guidelines and applications for the licensing of medical device establishments.
It reflects the current situation and will be regularly updated with changes in legislation and experience gained. It is important that
applicants adhere to the administrative requirements to avoid delays in the processing and evaluation of applications.
Guidelines and application forms are available from the office of the CEO and the website.
Document History
Final
Version
Reason for Amendment
Effective Date
1
First issue, industrial comments incorporated and published for
implementation
December 2017
2 Administrative changes November 2019
3
- Content structured on the new SAHPRA Guideline Template
- Old Guideline no. 8.10 changed to a new document number SAHPGL-
MD-07
February 2023
DR BOITUMELO SEMETE-MAKOKOTLELA
CHIEF EXECUTIVE OFFICER

SAHPGL-MD-07_v3
Page 2 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Contents
Document History .............................................................................................................................................. 1
Glossary ............................................................................................................................................................. 3
1. INTRODUCTION ......................................................................................................................................... 5
1.1 Purpose ....................................................................................................................................................... 5
1.2 Scope ........................................................................................................................................................... 5
2. LEGAL PROVISION ...................................................................................................................................... 5
3. QUESTIONS ABOUT SUBMISSION REQUIREMENTS ................................................................................... 6
3.1 How to submit a medical device establishment license application? ........................................................ 6
4. QUESTIONS ABOUT THE COST OF A MEDICAL DEVICE ESTABLISHMENT LICENSE .................................. 16
4.1 How much it costs to apply for a medical device establishment license and how to make the .............. 16
5. QUESTIONS ABOUT SUPPORTING DOCUMENTS ..................................................................................... 18
5.1 Documents to be submitted with the license application ........................................................................ 18
6. QUESTIONS ABOUT THE RELATIONSHIP BETWEEN SAHPRA AND OTHER REGULATORY BODIES ........... 18
6.1 The relationship between SAHPRA and other regulatory bodies and partners. ...................................... 19
7. GENERAL QUESTIONS .............................................................................................................................. 20
8. REFERENCES ............................................................................................................................................. 21
9. VALIDITY .................................................................................................................................................. 22
10. ANNEXURES ............................................................................................................................................. 23
10.1 Annexure A: Step by step guideline (Step 1 – 6) ..................................................................................... 23
10.2 Annexure B: Step-by- step process on how to download guidelines required for a Medical Device ..... 26
SAHPGL-MD-07_v3
Page 3 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Glossary
Abbreviation/ Term Meaning
Authorized
Representative
natural person, resident in the Republic of South Africa, who a) has the
written mandate to represent a manufacturer, importer, distributor,
wholesaler, retailer or service provider in the Republic; b) acts on behalf of a
manufacturer, importer, distributor, wholesaler, retailer or service provider
for specified tasks with regard to the latter’s obligations and in whose name
the manufacturer License, distributor License, wholesaler License and or
certificate of registration is issued; and c) is responsible for all aspects of the
medical device or IVD, including performance, quality, safety and compliance
with conditions of registration, clinical trials or clinical investigations;
Classification
The medical devices regulatory framework has a classification system for
medical devices and IVDs, as per the regulations of Act 101 of 1965South
African risk classification as per classification guideline 8.05.
Distributor
Natural or legal person who a) imports or exports a medical device or IVD
which is on the register for medical devices or on the register for IVDs in its
final form, wrapping and packaging, with a view to the medical device or IVD
being placed on the market under the natural or legal person’s own name;
and b) sells the medical device or IVD.
IN VITRO Diagnostic
Medical Devices (IVDs)
Means a medical device, whether used alone or in combination, intended by
the manufacturer for the in vitro examination of specimens derived from the
body solely or principally to provide information for diagnostic, monitoring or
compatibility purposes.
Manufacturer
All operations that include the design, purchasing of material, specification
development, production, fabrication, assembly, processing, reprocessing,
releasing, packaging, repackaging, labelling and refurbishing of a medical
device or IVD, as the case may be, and includes putting a collection of medical
devices or IVDs, and possibly other products, together for a medical purpose
in accordance with quality assurance and related controls
Medical device
any instrument, appliance, material, machine, apparatus, implant or
diagnostic reagent- (a) used or purporting to be suitable for use or
manufactured or sold for use in- (i) the diagnosis, treatment, mitigation,
modification, monitoring or prevention of disease, abnormal physical or
mental states or the symptoms thereof; or (ii) restoring, correcting or
modifying any somatic or psychic or organic function; or (iii) the diagnosis or
prevention of pregnancy, and which does not achieve its purpose through
chemical, pharmacological, immunological or metabolic means in or on the
human body but which may be assisted in its function by such means; or (b)
declared by the Minister by notice in the Gazette to be a medical device, and
includes any part or an accessory of a medical device
Quality Manual
A Quality Manual provides an overview of the documented quality
management system which is in operation and must include information
about the organization, the facilities, the key personnel, the quality assurance
policies, procedures, work instructions, controls and activities which are
undertaken by the organization to demonstrate its ability to provide medical
devices and related services that consistently meet the South African

SAHPGL-MD-07_v3
Page 4 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
regulatory requirements.
Wholesaler
A dealer who purchases medical devices or IVDs from a manufacturer or
distributor and sells them to a retailer.

SAHPGL-MD-07_v3
Page 5 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
1. INTRODUCTION
The Medicines and Related Substances Act, 1965 (Act 101 of 1965) Amendment, No.14 of 2015, makes
provision for the implementation of the regulatory oversight of medical devices in South Africa.
This document is a summary of questions that relate to the South African Health Products Regulatory Authority
(SAHPRA) guidelines for licence of medical device establishments:
a) SAHPGL-MD-06 Guideline for a licence to manufacture, import, export or distribute medical devices
and IVDs
b) 16.04 Licence to act as a wholesaler of medical devices and IVDs
c) Position paper for Amendments
d) SAHPGL-MD-04: Classification guideline
e) SAHPGL-MD-05: Medical device Quality Manual
Application forms:
a) 6.21 Licence Application to manufacture, import, distribute or export medical devices
b) 6.22 Licence Application to import, distribute or export medical devices
c) 6.26 Licence Application to Wholesale Medical Device
And represents the South African Health Products Regulatory Authority current view.
1.1 Purpose
The purpose of the document is to provide questions and answers that relate to medical device
establishment licences
1.2 Scope
The intention is to be a dynamic document that supplements and the above-mentioned documents.
Refer to the SAHPRA website (www.sahpra.org.za) for further information regarding the licensing
procedure.
2. LEGAL PROVISION

SAHPGL-MD-07_v3
Page 6 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
2.1 Section 22C of the medicines and related substance Act 101 0f 1965
3. QUESTIONS ABOUT SUBMISSION REQUIREMENTS
3.1 How to submit a medical device establishment license application?
3.1.1 What are the types of applications for Medical Device Establishment License?
There are three different types of medical device establishment licences that applicants may apply for:
a) Manufacturer license
b) Distributor license
c) Wholesaler license
3.1.2 What type of license can I apply for?
The type of licence to be applied for depends on the activities that you are performing.
If you are doing any packaging, labelling, servicing or refurbishment of medical devices, you will be required
to apply for a Manufacturer’s licence as these activities are considered as manufacturing activities. The
Manufacturing application includes manufacturing activities, as well as import, distribution, export activities
for medical devices/IVD's.
Manufacturers who use a third-party storage must ensure that the third-party company has an approved
license.
If you are importing, exporting or distributing medical devices then you will be required to apply for a
Distributor’s license as these activities are considered as distributor’s activities.
Distributors who use a third-party storage must ensure that the third-party company has an approved
license
If you are procuring the medical devices from a local manufacturer or and you sell them to a retailer or
distributor then you will be required to apply for a wholesaler’s license.
A wholesaler’s license does not permit you to import or export medical devices.
A wholesaler may store the products for an approved distributor or manufacturer.
3.1.3 What is the difference between COVID-19 applications and Business-as-usual applications?
COVID-19 applications are applications which have COVID-19 related medical devices (IVD’s and Non-IVD’s),
this includes rapidly developed COVID-19 test kits, rapidly developed ventilators, PPEs, face masks (surgical
and respirator masks), thermometers, oximeters for example.
SAHPGL-MD-07_v3
Page 7 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Business as usual applications are applications which have medical devices (IVD’s and Non-IVD’s), and
excludes the products mentioned above.
3.1.4 Which documents must be submitted when applying for a Business-as-usual medical device
establishment licence?
All applications should be submitted with a Cover letter that has been prepared on a company letterhead,
signed by the authorised representative and it should be dated.
The Cover letter must be addressed to the CEO and marked for the attention of the Medical Device Unit.
The cover letter must indicate the purpose of the submission and Signed by the authorised representative,
for example: Application for a medical device establishment licence to manufacture, distribute or wholesale
medical devices
The Cover letter must include a list of annexures that are submitted with the application, for example:
• Annex 1: Licence Application
• Annex 2: Proof of Payment
• Annex 3: Curriculum Vitae of the Authorised Representative
• Annex 4: Quality Manual (Manufacturers/Distributors) or Site Master File (Wholesalers)
An electronic version of the completed licence application in MS Excel format (manufacturer or distributor
application) or MS Word format (wholesaler application).
The curriculum vitae of the Authorised Representative and the Quality Manual (for Manufacturers and
Distributors) or the Site Master File (for Wholesalers) must also be submitted.
You are required to submit a PDF version of the licence application, initialled by the Authorised
Representative on each page, along with the proof of payment of the licence application fee.
3.1.5 How do I apply for a Medical Device Establishment license?
Visit the South African Health Products Regulatory Authority (SAHPRA) website at www.SAHPRA.org.za.

SAHPGL-MD-07_v3
Page 8 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Click on the tab “Health products” at the top of the page.
Click on the tab “Medical devices”, which is the fourth tab under the health products tab on the SAHPRA’s
home page.
Select “Application Forms”, which is the first link on the medical devices tab..
Select and download either licence application form:
a) 6.21 – Manufacturer’s Licence;
b) 6.22 – Distributor’s Licence;
c) 6.23 – Wholesaler’s Licence
Complete the relevant application form (in MS Excel format / MS Word) and submit with the relevant
supporting documents (refer to subsection 5 for the supporting documents) and send to the following email
address for business-as-usual applications:
address for COVID-19 related applications: mdcovid@sahpra.org.za.
Also refer to Annexure A below, for a step-by-step guideline.
3.1.6 How do I apply for an amendment to an approved license?
Visit South African Health Products Regulatory Authority (SAHPRA) website at www.SAHPRA.org.za.
Click on the “health products tab” on the top of the page.
Click on the “medical devices tab”, which is the fourth tab under the health products tab on SAHPRA’s home
page.
Select “Application forms”, which is the first link within the medical devices tab
Select and download either license application form:
a) 6.21 - Manufacturer’s license
b) 6.22 - Distributor’s license
c) 6.23 - Wholesaler’s license
Complete the relevant application form ( in MS Excel format / Ms Word) and submit the application form
and supporting documents (refer to subsection 3.1.4 for the supporting documents) and send to the

SAHPGL-MD-07_v3
Page 9 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
following email address for COVID-19 related applications: mdcovid@sahpra.org.za.
Also refer to Annexure A below, for a step-by-step guideline
For all amendment applications, the licensee is required to submit the updated product listing which
includes the already approved products and indicate the changes made on the cover letter.
In the case that the licensee would like to remove a product from the product list, the licensee must omit
this device from the application form.
Once the amendment is approved, the license will reflect the updated product list as submitted by the
licensee.
3.1.7 What is a notification?
Any change that a license holder makes that does not affect the details or listed on the license and does not
change the class of medical device / IVD of which the applicant has been approved and licensed for.
The license holder is required to notify the authority (SAHPRA) of these changes by submitting a notification,
which details these changes.
3.1.8 How do I submit a notification?
The notification cover letter must be prepared on the Company letterhead of the license holder.
The subject of the notification must state: Notification of amendment to section(s) [x] of the
manufacturer/distributor/wholesaler Medical Device Establishment License [ License no] of [Company
name] [MDF Number].
For a notification, the license holder is required to submit an updated product list in the application form
(this updated product list includes the products that have been approved).
In the case that the license holder wants to remove certain medical devices from their product list then the
updated product list should omit these items.
The notification will be approved according to the latest updated product list submitted by the license
holder.
A written declaration by the authorised representative indicating the changes made to the product list (s)
that does not affect the class of medical device(s) which the license holder has been licensed for.
The notification must be dated and signed by the authorised representative.
SAHPGL-MD-07_v3
Page 10 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
The notification must be accompanied by a fully completed medical device establishment license
application form, which will include the updated product list as indicated in the written notification.
An electronic version (PDF version) of the completed licence application that is initialled on each page by the
authorised representative as well as an MS Excel format/ MS Word format of the application form.
All documents are to be submitted to the following email address: mdnotifications@sahra.org.za
3.1.9 What is the difference between an amendment application and a notification?
• A notification is when an applicant wants to make changes to their company previous application
that does not affect their previous license issued.
• A notification must still be reported and approved, there are currently no applicable fees in Medical
Devices.
• A notification may be updating the product listing with medical devices/ IVD’s that are the same
class in which they already have approval for. However, there are exceptions- all updates that
include Covid-19 products will be regarded as an amendment.
An amendment is where any changes that an applicant makes will affect the details or listed on the license
they were issued.
• E.g. Change of address, change in authorized representative, change of contact details or the
authorized representative or license holder, change of company name, change of license holder,
change in the activities listed on the current license.
• There are applicable fees for an amendment, which currently costs R5 300, this is subject to change
as per publication in the government gazette.
• Refer to position statement communication to industry license amendment, For Notifications and
amendments:
https://www.sahpra.org.za/wp-content/uploads/2020/01/Communication-to-
industry_licence_amendment_nov2019.pdf
3.1.10 If the Authorized Representative/ License Contact Holder resign from the company what will be
the process to make changes?
The company must apply for an amendment to their license. This means the company must follow the
amendment application process.

SAHPGL-MD-07_v3
Page 11 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Refer to section 3.1.4 for the amendment process and requirements.
Refer to position statement communication to industry license amendment,
For Notifications and amendments:
https://www.sahpra.org.za/wp-content/uploads/2020/01/Communication-to-
industry_licence_amendment_nov2019.pdf
3.1.11 Can applicant submit new amendment while other is in process?
No, the previous application must be concluded prior to submission of an amendment.
3.1.12 What if my Medical Device Establishment License is incomplete?
There are 2 different screening processes that take place for a medical device establishment license
application- that is the administrative and technical screening.
Applicants may be asked to address deficiencies after administrative screening and or technical screening.
During process of admin screening the medical device license application, the screener will contact the
applicant by email if it is determined that any information necessary for the license application is missing.
The applicant will be provided with observation letter stating deficiencies.
Failure to respond to a deficiency notice will result in the rejection of the application.
Where the expedited regulatory pathway is applied, applicants are given 2 working days to respond to
technical observation letters, however for business as usual applications, applicants are given 10 working
days to respond to the observation letter issued.
During the process of technical screening, the technical evaluator will contact the applicant by email if it is
determined that any information is missing. The applicant will be provided with the observation letter
stating the deficiencies. Failure to respond to a deficiency notice will result in the rejection of the
application. Where the expedited regulatory pathway is applied, applicants are given 2 working days to
respond to technical observation letters, however for business as usual applications, applicants are given 10
working days to respond to the observation letter issued.
For expedited COVID applications the review period is 10-15 working days, depending on the completeness
of the application and the timeous response by the applicants to the observation letters issued to the
applicant.
For business-as-usual applications the review period is 6-8 weeks, depending on the completeness of the
application and the timeous response by the applicants to the observation letters issued to the applicant.
SAHPGL-MD-07_v3
Page 12 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Refer to communication to industry:
Processing of Medical Device Establishment License Applications made to SAHPRA
MD019-Processing-of-licence-applications-v1-22072020.pdf (sahpra.org.za)
3.1.13 How long does it take to get a license for a Medical Device Establishment?
Each license application is evaluated and pronounced upon by the Medical Device Peer Review Meeting. The
timelines for licensing are thus dependent on the date of the submission of the application by the applicant
and the meeting dates of the Medical Device Expert Committee (for Covid-19 test kit applications) and the
Medical Device Peer Review Meetings. Timelines for applications also depends on the timeous response by
applicants and the submission of complete applications.
The average time for an applicant to be issued with a license is 10-15 working days for complete COVID
applications and 6-8 weeks for complete business as usual applications.
Refer to communication to industry:
Processing of Medical Device Establishment License Applications made to SAHPRA
MD019-Processing-of-
licence-applications-v1-22072020.pdf (sahpra.org.za)
3.1.14 Can I amend from Distributor to Manufacturer/ Wholesaler to Distributor?
If a company is already licensed as distributor and intends to change to Manufacturer/ Wholesaler and verse
versa. They will have to submit a new application and pay the full fee for the relevant application.
3.1.15 Why should I withdraw my distributor’s license if I am now applying for a manufacturer’s license?
• The Manufacturing application includes manufacturing activities, as well as import, distribution,
export activities for medical devices/IVD's.
• The Distribution application is for import, export and distribution activities for Medical Devices/IVD's
only.
• The manufacturer’s license encompasses the distributor’s activities therefore if an applicant is
applying for a manufacturer’s license for the same site as the distributor’s license site, it is advisable
to withdraw the distributor’s license.
• Should the applicant retain the distributor’s license then the applicant will then have to pay
retention fees for both licenses, when the retention fees are due.
3.1.16 How should I confirm the classification of a device before applying for a Medical Device
Establishment License?

SAHPGL-MD-07_v3
Page 13 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Visit the South African Health Products Regulatory Authority (SAHPRA) website at www.SAHPRA.org.za
.
Click on the tab “Health products” at the top of the page.
Click on the tab “Medical devices”, which is the fourth tab under the health products tab on the SAHPRA’s
home page.
Select “Guidelines”, which is the third link on the medical devices tab.
Download the guideline titled: Classification of medical devices and IVDs (refer to Annexure B below for a
step-by-step guideline on how to download guidelines).
3.1.17 When do applications get rejected?
Applications may get rejected for the following reasons:
a) the period of review has lapsed, such as when there is no response to a query/ observation within
the stipulated timeframe.
b) The applicant does not comply with the stipulated specifications, requirements and test reports.
c) The applicant submits insufficient information.
d) The applicant submits incorrect or falsified information.
e) As per recommendation from the NHLS, Medical Device Committee, COVID Expert Committee as
well as the Ventilator Expert Committee.
3.1.18 Which documents must be submitted when applying for a medical device establishment licence
with COVID-19 products (this includes mask, respirator’s, thermometers and oximeters)?
All applications should be submitted with a Cover letter that has been prepared on a company letterhead,
signed by the authorised representative and it should be dated.
The Cover letter must be addressed to the CEO and marked for the attention of the Medical Device Unit.
The cover letter must indicate the purpose of the submission and Signed by the authorised representative,
for example: Application for a medical device establishment licence to manufacture, distribute or wholesale
medical devices.
The Cover letter must include a list of the attachments that are submitted with the application, for example:
• Annex 1: Licence Application
• Annex 2: Proof of Payment
• Annex 3: Curriculum Vitae of the Authorised Representative

SAHPGL-MD-07_v3
Page 14 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
• Annex 4: Quality Manual (Manufacturers/Distributors) or Site Master File (Wholesalers)
• Annex 5: ISO 13485 certification from the original manufacturer
• Annex 6: Evidence of premarket registration from one of the 6 jurisdictions recognised by SAHPRA.
• Annex 7: SANS certificate or NRCS certificate.
• Annex 8: Packaging and labelling information.
• Annex 9: Instructions for use of the medical device
Refer to the following communication issued by SAHPRA:
Licensing and Regulatory requirements for the manufacture and distribution of medical and respirator masks
during COVID-19.
MD025_Alternative-licensing-and-regulatory-pathway-for-masks_September2020_vF.docx.pdf
(sahpra.org.za)
An electronic version of the completed licence application in MS Excel format (manufacturer or distributor
application) or MS Word format (wholesaler application).
The curriculum vitae of the Authorised Representative and the Quality Manual (for Manufacturers and
Distributors) or the Site Master File (for Wholesalers) must also be submitted.
You are required to submit a PDF version of the licence application, initialled by the Authorised
Representative on each page, along with the proof of payment of the licence application fee.
3.1.19 Which documents must be submitted with the application when applying for a medical device
establishment license with COVID-19 products (this includes Covid-19 test kits and ventilators)?
All applications should be submitted with a Cover letter that has been prepared on a company letterhead,
signed by the authorized representative and it should be dated.
The Cover letter must be addressed to the CEO and marked for the attention of the Medical Device Unit.
The cover letter must indicate the purpose of the submission and Signed by the authorized representative,
for example: Application for a medical device establishment license to manufacture, distribute or wholesale
medical devices.
The Cover letter must include a list of the attachments that are submitted with the application, for example:
• Annex 1: License Application
• Annex 2: Proof of Payment
• Annex 3: Curriculum Vitae of the Authorized Representative
• Annex 4: Quality Manual (Manufacturers/Distributors) or Site Master File (Wholesalers)
• Annex 5: ISO 13485 certification from the original manufacturer

SAHPGL-MD-07_v3
Page 15 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
• Annex 6: Evidence of premarket registration from one of the 6 jurisdictions recognized by SAHPRA.
• Annex 7: SANS certificate or NRCS certificate.
• Annex 8: Packaging and labelling information.
• Annex 9: Instructions for use of the medical device.
• Annex 10: Technical dossier.
Refer to the following documents for the communication issued by SAHPRA to industry:
Regulatory requirements for the supply of medical devices considering the COVID-19 pandemic
MD001-Regulatory-Requirements-for-Medical-Devices-COVID-19-v2-22072020.pdf (sahpra.org.za)
Regulatory Requirements for the manufacture, distribution or wholesale of Covid-19 serological test kits
MD002-Regulatory-Requirements-for-Serological-Test-Kits-v2-22072020.pdf (sahpra.org.za)
Regulatory Requirements for the manufacture, distribution or wholesale of Covid-19 molecular test kits
MD014-Regulatory-Requirements-for-Molecular-Test-Kits-v1-22072020.pdf (sahpra.org.za)
Regulatory requirements, Technical Specifications, License Conditions and Authorisations for use of
Unregistered Rapidly Developed Invasive and Non-Invasive Ventilators for Covid-19
MD010_Guidance_Rapidly-developed-ventilators_v1-26052020.pdf (sahpra.org.za)
3.1.20 What is a section 36 application?
Refers to section 36 of Medicines and Relates Substances Act 101 of 1965, that allows companies to apply
for exemptions to the operation of any or all provisions of this Act, under specific conditions or
circumstances.
Refer to the publication of this Act on the SAHPRA website:
Medicines and Related Substances Act no 101 of 1965,
Government_Gazette_Medicines_and_Devices_Act_Jun_2017-1.pdf (sahpra.org.za)
SAHPGL-MD-07_v3
Page 16 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
3.1.21 Who should I contact if I have more questions or require more information?
4. QUESTIONS ABOUT THE COST OF A MEDICAL DEVICE ESTABLISHMENT LICENSE
4.1 How much it costs to apply for a medical device establishment license and how to make
the payment?
4.1.1 How much does it cost to apply for a Medical Device Establishment Licence?
The fees payable are determined in consultation with National Treasury and are published in the
Government Gazette.
The following fees, as published in Government Gazette No. 1379 on 22 December 2020 are currently
applicable:
a) Manufacturer’s Licence Fee – R25 200
b) Distributor’s Licence Fee – R15 000
c) Wholesaler’s Licence Fee – R15 000
• The fee for a medical device establishment licence application is payable upon application and proof
of payment should be submitted together with the completed licence application.
• Retention fees are due every year in June from the first anniversary of the date on which the license
was issued.
• These fees are applicable to existing license holders who are required to pay these fees in order to
retain their license, should the license holder wish to cancel their license then a formal letter has to
be submitted by the license holder, to the authority (SAHPRA) indicating that they wish to withdraw
their license.
NOTE: Fees may be updated from time to time. The onus is on the applicant to ensure that payment is made
in line with the current fees’ structures, as published in the Government Gazette.
4.1.2 How do I make a payment to SAHPRA?
Payments to the SAHPRA must be made through electronic funds transfer (EFT).
The SAHPRA banking details are:
Account name: SOUTH AFRICAN HEALTH PRODUCTS REGULATORY AUTHORITY
Account type: CHEQUE ACCOUNT

SAHPGL-MD-07_v3
Page 17 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Account number: 40-5939-2080
Bank: ABSA
Bank branch code: 632005
Swift Code for application fee for a new license application: MD NLIC *company name*
Swift Code for application fee for an amendment application: MD AMD * company name*
Swift code for license collection fee: MD *company name*
Swift code for retention fee: MD RET *company name*
Refer to the latest fees published in the government gazette on: Published-SAHPRA-Fees.pdf
Refer to SAHPRA payment guideline for payment references on: https://www.sahpra.org.za/wp-
content/uploads/2021/06/SAHPRA_Payment_Guideline.pdf
4.1.3 What is the process if applicant has paid surplus?
1. The applicant must apply for a refund with the finance department.
2. The process for refund application follows below.
3. Please provide the following information or documents to SAHPRA:
a) Letter from the company explaining the reason for the refund request
b) Proof of payment - (A letter from the bank is not acceptable)
c) Valid Tax Clearance Certificate
d) Banking details
e) Cipro Certificates and ID copies of directors [CK]
Or
If the refund is requested by an individual:
a) An affidavit or letter stating the reason for refund request
b) Certified copy of ID
c) Proof of payment
d) Banking details

SAHPGL-MD-07_v3
Page 18 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
.
Subject line: Marked “application for refund”
5. QUESTIONS ABOUT SUPPORTING DOCUMENTS
5.1 Documents to be submitted with the license application
5.1.1 Who needs to submit a Quality Manual?
a) Manufacturers
b) Distributors
A guideline on how to prepare a quality manual is available on the SAHPRA website. To download the
guideline, please follow the steps below (refer to Annexure B below for step-by-step guideline on how to
download guidelines).
Visit South African Health Products Regulatory Authority (SAHPRA) website at www.SAHPRA.org.za.
Click on the “health products tab” on the top of the page.
Click on the “medical devices tab”, which is the fourth tab under the health products tab on SAHPRA’s home
page.
Select “Guidelines”, which is the third link in the medical devices tab
Select and download “Medical Device Quality manual”
5.1.2 What is an ISO 13485?
A certificate issued by the International Organisation for Standardization to a company that complies with the
requirements for a quality management system in the medical device industry.
5.1.3 Who needs to submit ISO 13485 and when will it be compulsory?
The ISO 13485 needs to be submitted by manufactures and distributors and it will be compulsory upon
communication from SAHPRA.
6. QUESTIONS ABOUT THE RELATIONSHIP BETWEEN SAHPRA AND OTHER
REGULATORY BODIES AND PARTNERS

SAHPGL-MD-07_v3
Page 19 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
6.1 The relationship between SAHPRA and other regulatory bodies and partners.
6.1.1 What is the relationship between SAHPRA & NHLS HTA Unit in the regulation of diagnostic
devices?
The NHLS conducts performance validation of COVID-19 test kits on behalf of SAHPRA.
Refer to the communication issued by SAHPRA:
Frequently asked questions: performance evaluation of point-care COVID-19 serology antibody test kits.
MD024-FAQs_POC_COVID-19_Antibody_Serology_Test_Kit_10092020_vF.pdf (sahpra.org.za)
6.1.2 Will this be limited to Covid-19 diagnostic devices or there will role of the NHLS will be extended
further beyond the pandemic?
The role of the NHLS might be extended once registration of medical devices starts, communication will be
shared accordingly.
6.1.3 What happens during the validation processes: SAHPRA and NHLS
Validation of COVID-19 test kits on behalf of SAHPRA looking at the test performance of the test kit.
NHLS will submit a report to SAHPRA, indicating that it recommends or not recommend the test and section
21 authorisation is issued to the applicant.
Refer to the communication issued by SAHPRA:
Frequently asked questions: performance evaluation of point-care COVID-19 serology antibody test kits.
MD024-FAQs_POC_COVID-19_Antibody_Serology_Test_Kit_10092020_vF.pdf (sahpra.org.za)
6.1.4 What is the role of Port Health?
The role of port health is to serve as a first line of defence to protect the citizens of South Africa and visitors
against the entry of communicable diseases associated with cross border movement of people, conveyances,
baggage, cargo and imported consignments.
6.1.5 Where must I go if my products are being held at Port Health?
For any queries related to port health shipment, refer to regulatory compliance unit.
Please contact Mokgadi Daphney Fafudi
Email: [email protected]
Tel: 012 015 5434
6.1.6 What is the role of the medical device unit in relation to Port Health?
SAHPGL-MD-07_v3
Page 20 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
The role of the medical device unit is to ensure that all companies who are importing or exporting medical
devices are licensed and are listed on the licenses issued register.
6.1.7 What is the relationship between the six jurisdictions recognised by SAHPRA / What role do these
authorities play in South African Medical Devices regulatory affairs?
SAHPRA recognises and follows these six jurisdictions as a reliance model which ensures that Class C and D
medical devices / IVDs have at least one approval from the six jurisdictions.
SAHPRA still makes the final decision to approve or reject an application based on the South African medical
device regulations as well as the Medicines and Related Substances Act 101 of 1965, therefore approval
from one of the six jurisdictions does not guarantee approval from SAHPRA as SAHPRA has its own
requirements based on the South African market,.
These six jurisdictions recognised by SAHPRA are IMDRF countries, namely:
• Australia
• Canada
• Japan
• United States of America
• Brazil
7. GENERAL QUESTIONS
7.1 Can someone be Authorized Representative to more than one company?
Yes, It is only acceptable in two scenarios, one for an authorized representative that acts on behalf of the
companies within the same address/province and also for companies where the Regulatory oversight is at
the head office.
Refer to document:
Guideline for a License to Manufacture, Import, Export or Distribute Medical Devices and IVDs
GUIDANCE DOCUMENT: (sahpra.org.za)
7.2 Can the company apply for different type of licenses on the same premises?
Yes, such as in the case where the company has sub-unit companies or departments within the company.

SAHPGL-MD-07_v3
Page 21 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
7.3 Can applicant make any changes of application that was already screened/ evaluated?
No. any changes made after the application is screened will be considered amendments and the applicant
will have to follow the amendment application procedure once the application in process has been
concluded.
7.4 What happens if one of the shareholders leaves the company?
Not relevant if the shareholder is neither the authorised representative nor the license holder.
7.5 Can establishments (hospitals, pharmacies etc.) use Covid-19 antibody tests to diagnose active Covid-
19 cases?
No. These tests determine if the body has started to fight a SARS-CoV2 infection rather that detecting the
actual SARS-CoV2 virus.
COVID-19 antibody tests are used to guide decision making regarding patient management, decision making
regarding the need for quarantine, isolation and contact tracing.
Refer to communication issued by SAHPRA:
Testing for COVID-19
MD003-Testing-for-COVID-19-v1-22072020.pdf (sahpra.org.za)
8. REFERENCES
The following related documents are referenced:
8.1 SAHPGL-MD-06 Guideline for a licence to manufacture, import, export or distribute medical devices
and IVDs
8.2 16.04 Licence to act as a wholesaler of medical devices and IVDs
8.3 Position paper for Amendments
8.4 SAHPGL-MD-04: Classification guideline
8.5 SAHPGL-MD-05: Medical device Quality Manual
8.6 6.21 Licence Application to manufacture, import, distribute or export medical devices
8.7 6.22 Licence Application to import, distribute or export medical devices
8.8 6.26 Licence Application to Wholesale Medical Device

SAHPGL-MD-07_v3
Page 22 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
9. VALIDITY
This guideline is valid for a period of 5 years from the effective date of revision and replaces the old guideline
on Questions & Answers: Licensing of Medical Devices/ IVDs, old document number 8.10. It will be reviewed
on this timeframe or as and when required.
SAHPGL-MD-07_v3
Page 23 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
10. ANNEXURES
10.1 Annexure A: Step by step guideline (Step 1 – 6)
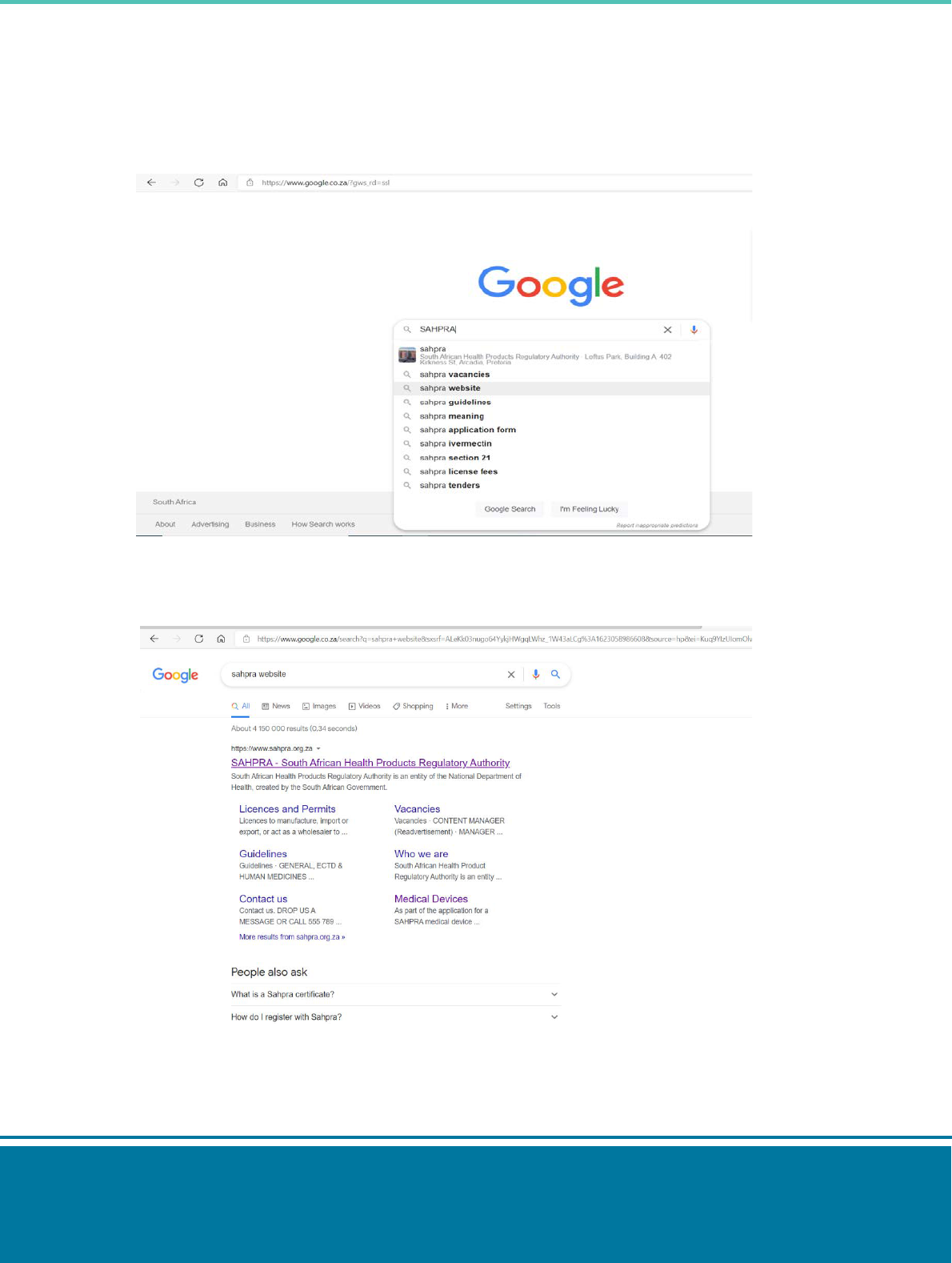
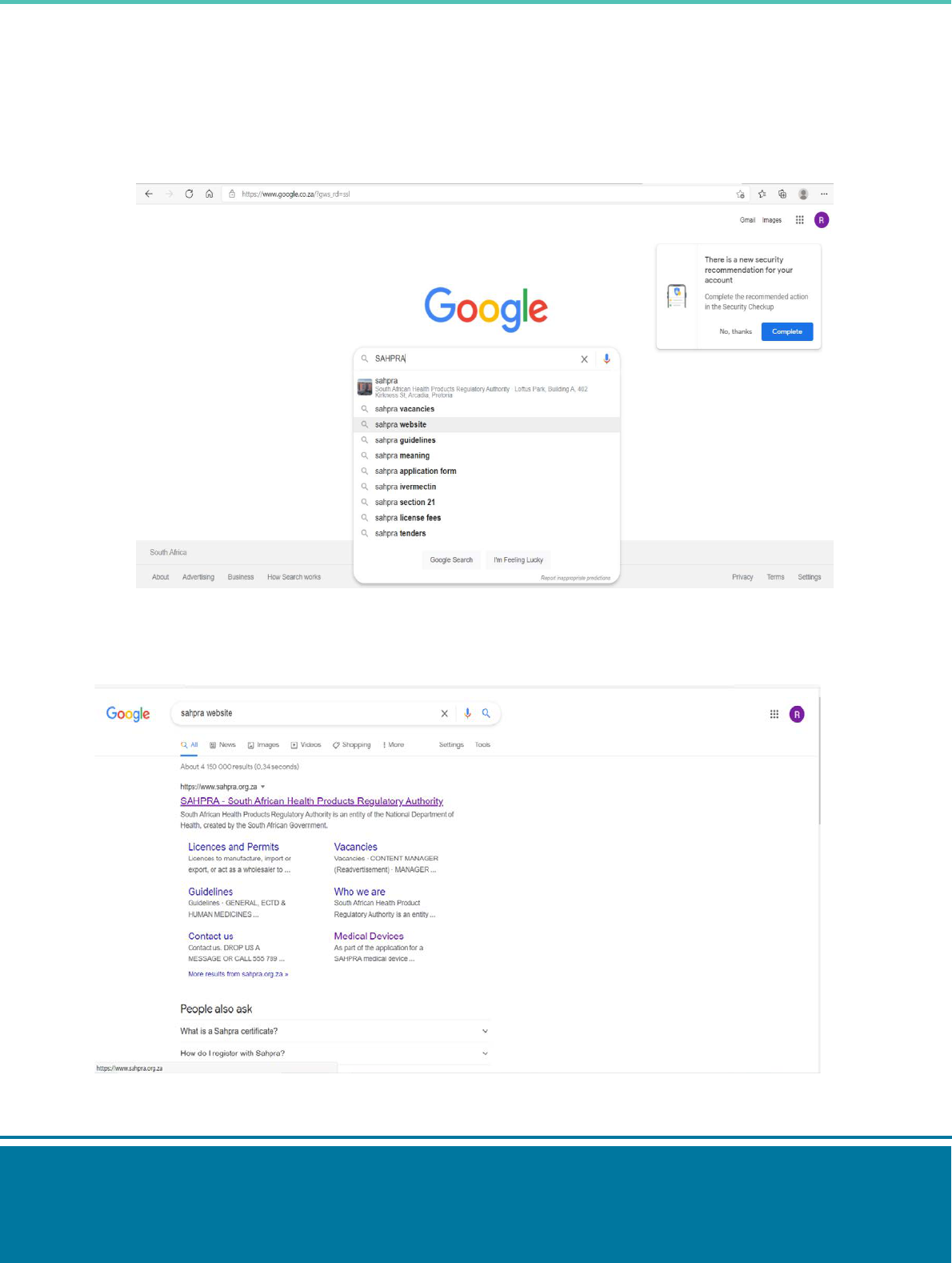
Step 1: type ‘SAHPRA Website’ on your search engine.
Step 2: Select the SAHPRA link
SAHPGL-MD-07_v3
Page 24 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
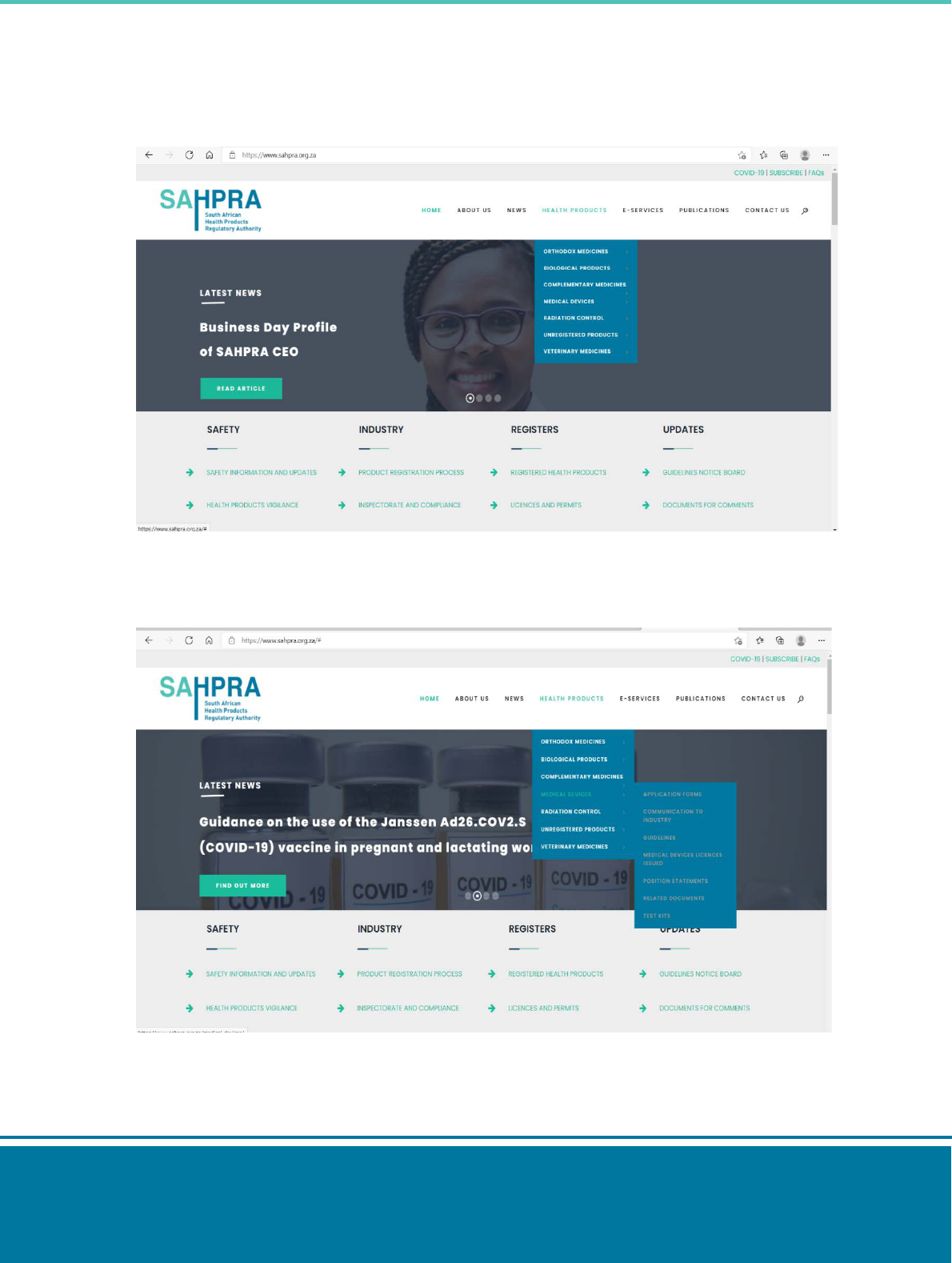
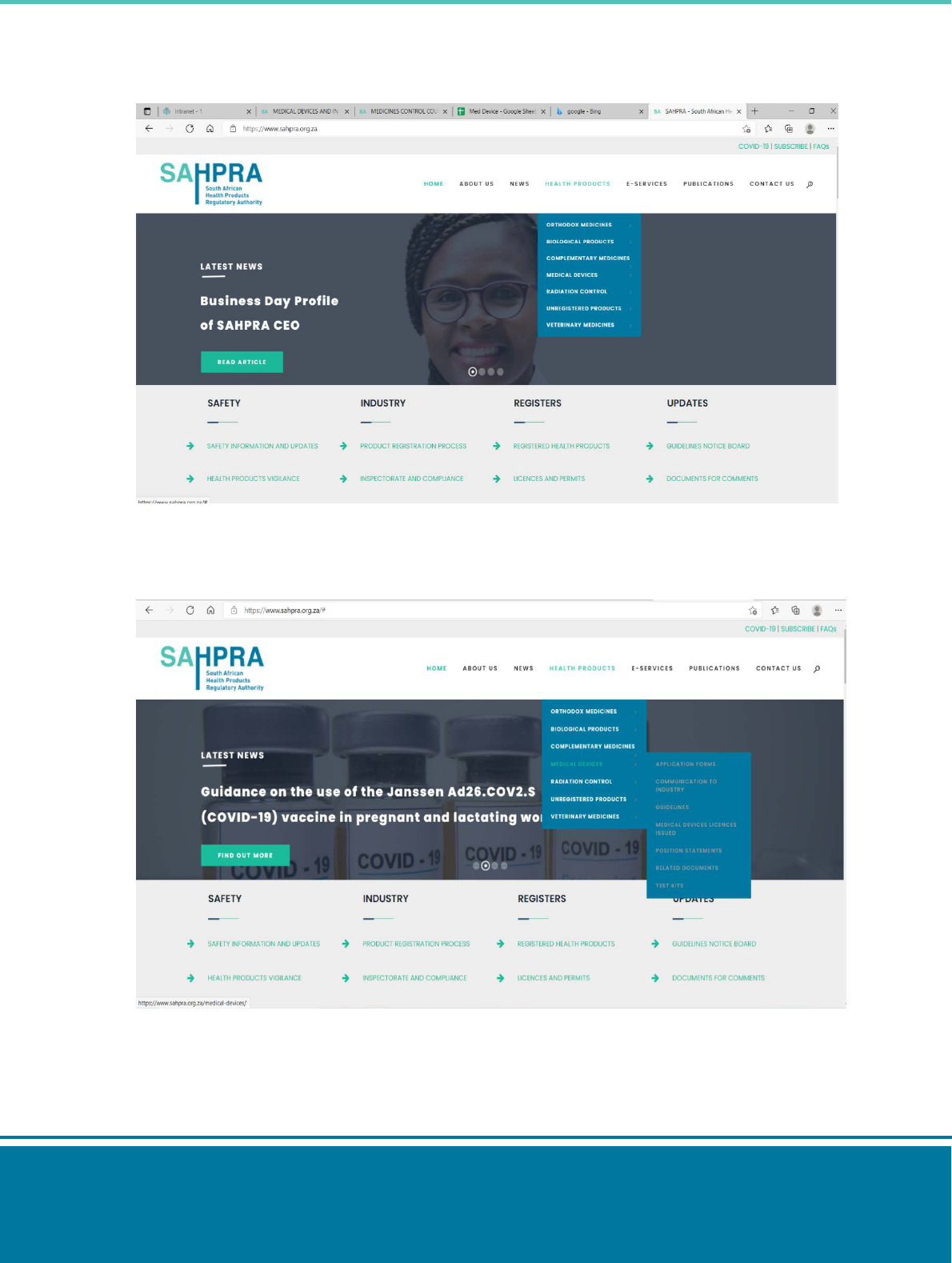
Step 3: On the SAHPRA website, select the health products tab.
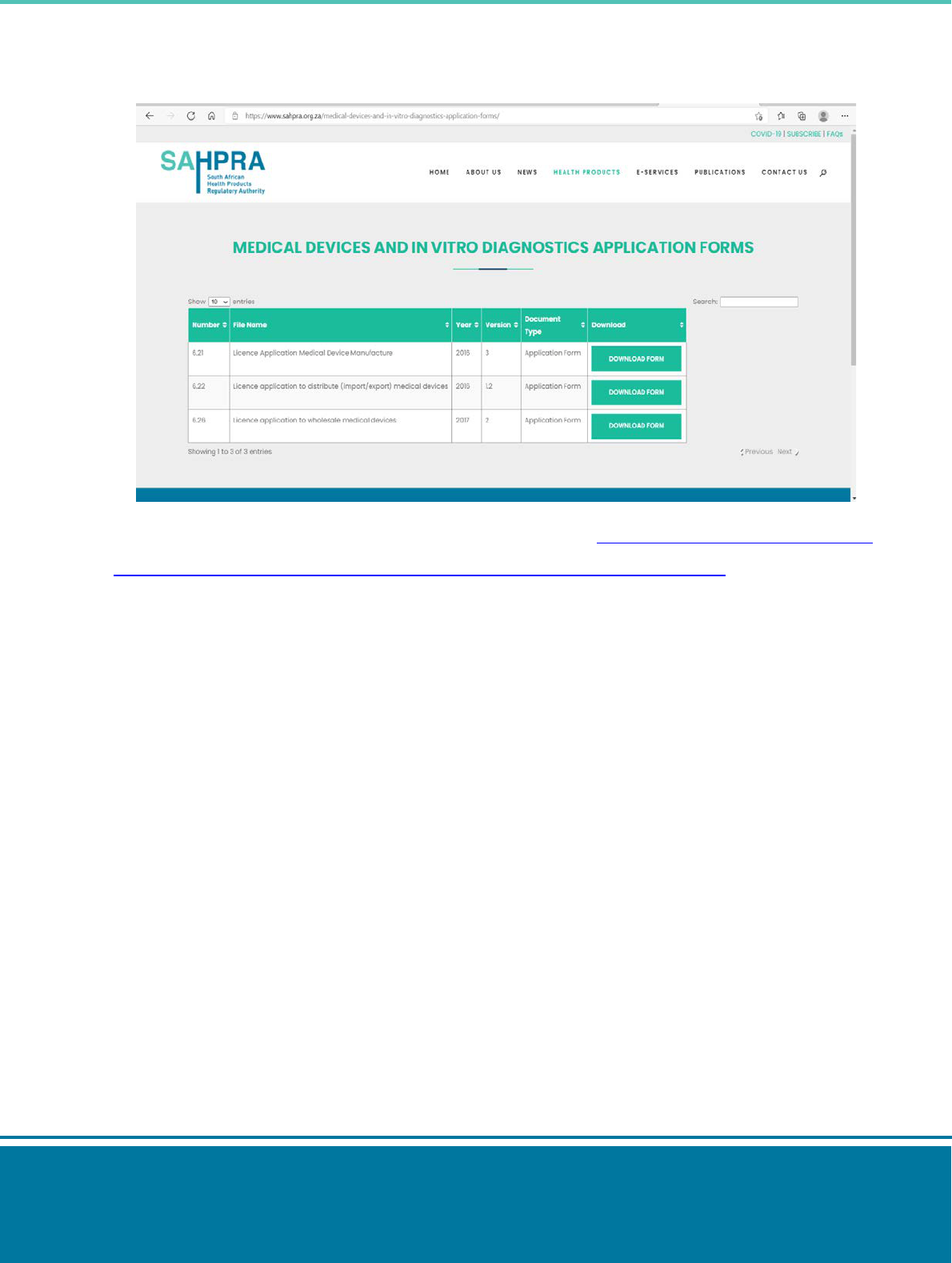
Step 4: Select Medical devices tab > application forms
SAHPGL-MD-07_v3
Page 25 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Step 5: Select the appropriate application form. Download and complete the application form.
Step 6: Submit the application form and supporting documents to: mdc[email protected]g.za
for COVID-19
related applications and mdadmin@sahpra.org.za for business as usual applications
NB: Supporting documents required:
1. Cover letter
2. Proof of payment (application fee)
3. Site master file for wholesale application
4. Quality manual for distributor or manufacturer application
5. CV of the authorised representative
6. PDF application form that is initialled on each page by the authorised representative
7. Declaration on the application form must be signed by the authorised representative
SAHPGL-MD-07_v3
Page 26 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
10.2 Annexure B: Step-by- step process on how to download guidelines required for a Medical
Device Establishment License application (Step 1 – 6)
Step 1: type ‘SAHPRA Website’ on your search engine.
Step 2: Select the SAHPRA website:
SAHPGL-MD-07_v3
Page 27 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
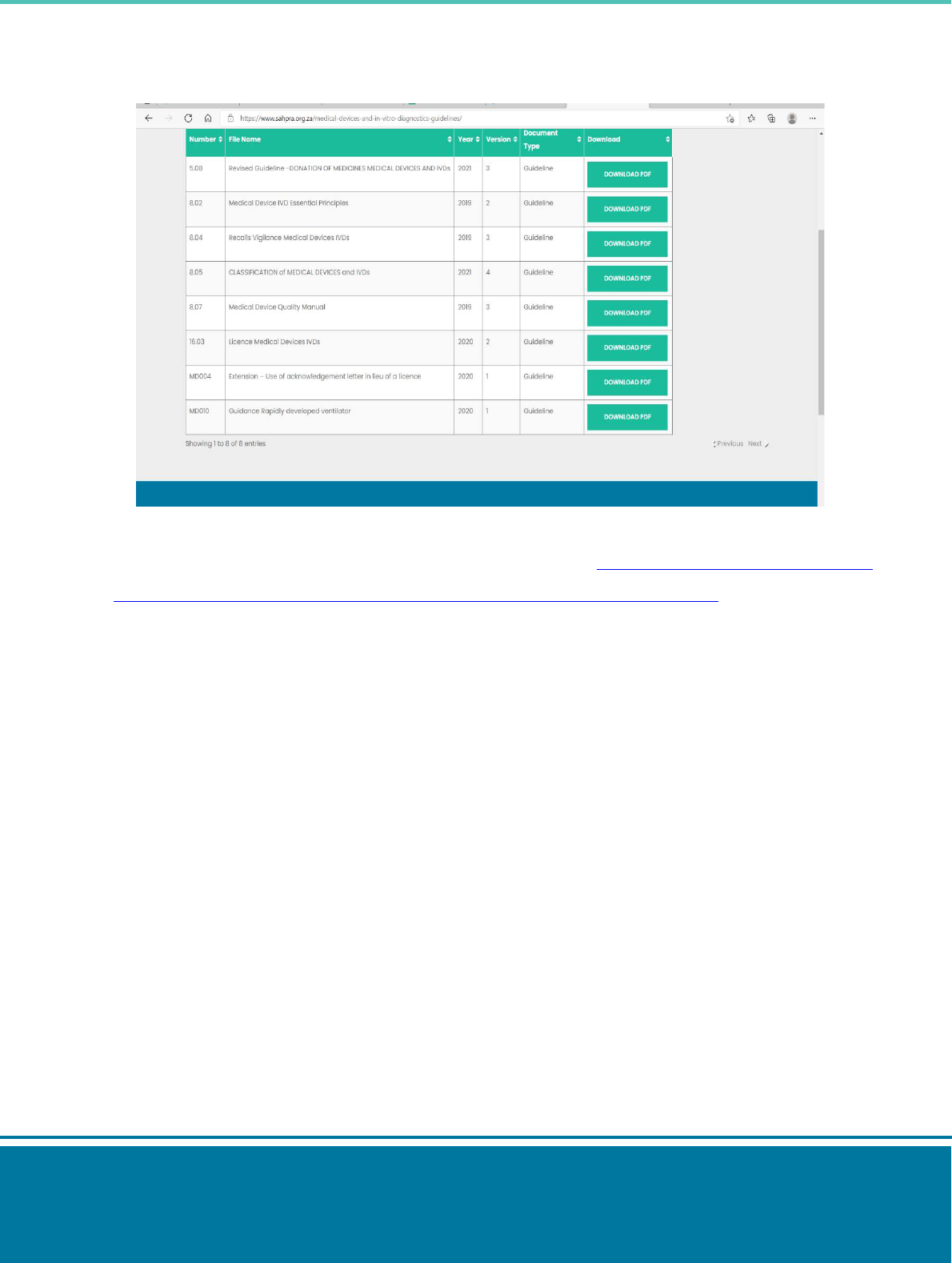
Step 3: On the SAHPRA website, select the health products tab.
Step 4: Select medical devices tab > guidelines
SAHPGL-MD-07_v3
Page 28 of 28
Guideline on Questions and Answers: Licensing of Medical Device Establishments
17 November 2023
Step 5: download the applicable guideline to use when compiling the supporting documents.
Step 6: Submit the application form and supporting documents to: mdc[email protected]g.za
for COVID-19
related applications or mdadmin@sahpra.org.za for business as usual applications.
NB: Supporting documents required:
1. Cover letter
2. Proof of payment (application fee)
3. Site master file for wholesale application
4. Quality manual for distributor or manufacturer application
5. CV of the authorised representative
6. PDF application form that is initialled on each page by the authorised representative
7. Declaration on the application form must be signed by the authorised representative
8. Copy of existing license and cover letter
