
Recommendations from the 2023
International Evidence-based
Guideline for the Assessment and
Management of Polycystic
Ovary Syndromey
Helena J. Teede,
a,b
Chau Thien Tay,
a,b
Joop Laven,
b,c
Anuja Dokras,
d
Lisa J. Moran,
a,b
Terhi T. Piltonen,
e
Michael F. Costello,
b,f
Jacky Boivin,
g
Leanne M. Redman,
h
Jacqueline A. Boyle,
b,i
Robert. J. Norman,
b,j
Aya Mousa,
a
and Anju E. Joham
a,b
on behalf of the International PCOS Network
#
a
Monash Centre for Health Research and Implementation, Monash University and Monash Health, Melbourne, Victoria,
Australia;
b
National Health and Medical Research Council Centre for Research Excellence in Women’s Health in
Reproductive Life, Australia;
c
Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and
Gynaecology, Erasmus Medical Centre, Rotterdam, The Netherlands;
d
Obstetrics and Gynecology, University of
Pennsylvania, Philadelphia, Pennsylvania, U.S.A.;
e
Department of Obstetrics and Gynaecology, Medical Research Center
Oulu, Research Unit of Clinical Medicine, University of Oulu and Oulu University Hospital, Oulu, Finland;
f
University of
New South Wales, New South Wales, Australia;
g
Cymru Fertility and Reproductive Research, School of Psychology,
Cardiff University, Cardiff, Wales, United Kingdom;
h
Pennington Biomedical Research Center, Louisiana State
University, Baton Rouge, Louisiana, U.S.A.;
i
Eastern Health Clinical School, Monash University, Melbourne, Victoria,
Australia;
j
Robinson Research Institute, University of Adelaide, Adelaide, South Australia, Australia
Abstract
STUDY QUESTION: What is the recommended assessment and management of those with polycystic ovary syndrome (PCOS), based
on the best available evidence, clinical expertise, and consumer preference?
SUMMARY ANSWER: International evidence-based guidelines address prioritized questions and outcomes and include 254
recommendations and practice points, to promote consistent, evidence-based care and improve the experience and health outcomes
in PCOS.
WHAT IS KNOWN ALREADY: The 2018 International PCOS Guideline was independently evaluated as high quality and integrated
multidisciplinary and consumer perspectives from six continents; it is now used in 196 countries and is widely cited. It was based
on best available, but generally very low to low quality, evidence. It applied robust methodological processes and addressed shared pri-
orities. The guideline transitioned from consensus based to evidence-based diagnostic criteria and enhanced accuracy of diagnosis,
whilst promoting consistency of care. However, diagnosis is still delayed, the needs of those with PCOS are not being adequately
met, evidence quality was low and evidence-practice gaps persist.
STUDY DESIGN, SIZE, DURATION: The 2023 International Evidence-based Guideline update reengaged the 2018 network across
professional societies and consumer organizations with multidisciplinary experts and women with PCOS directly involved at all
stages. Extensive evidence synthesis was completed. Appraisal of Guidelines for Research and Evaluation-II (AGREEII)-compliant
processes were followed. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was
applied across evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength and
diversity and inclusion were considered throughout.
PARTICIPANTS/ MATERIALS, SETTING, METHODS: This summary should be read in conjunction with the full Guideline for
detailed participants and methods. Governance included a six-continent international advisory and management committee, five
guideline development groups, and paediatric, consumer, and translation committees. Extensive consumer engagement and
guideline experts informed the update scope and priorities. Engaged international society-nominated panels included paediatrics,
endocrinology, gynaecology, primary care, reproductive endocrinology, obstetrics, psychiatry, psychology, dietetics, exercise
y
This article is simultaneously published in Fertility and Sterility, Journal of Clinical Endocrinology and Metabolism, European Journal of Endocrinology and
Human Reproduction.
#
Participants of the International PCOS Network are listed in the Appendix.
Correspondence: Helena J. Teede, Locked Bag 29, Clayton, Australia, 3168, VIC, Australia (E-mail: helena.teede@ monash.edu).
Fertility and Sterility® Vol. -, No. -, - 2023 0015-0282/$36.00
This article has been co-published with permission in Fertility and Sterility, Human Reproduction, European Journal of Endocrinology, and The Journal of
Clinical Endocrinology and Metabolism. © The Author(s) 2023. Published by Elsevier Inc. on behalf of the American Society of Reproductive Medicine.
This is an open access article under the CC-BY-NC license (http://creativecommons.org/licenses/by-nc/4.0/). The articles are identical except for minor
stylistic and spelling differences in keeping with each journal's style.
https://doi.org/10.1016/j.fertnstert.2023.07.025
VOL. - NO. - / - 2023 1

physiology, obesity care, public health and other experts, alongside consumers, project management, evidence synthesis, statisticians
and translation experts. Thirty-nine professional and consumer organizations covering 71 countries engaged in the process. Twenty
meetings and five face-to-face forums over 12 months addressed 58 prioritized clinical questions involving 52 systematic and 3
narrative reviews. Evidence-based recommendations were developed and approved via consensus across five guideline panels,
modified based on international feedback and peer review, independently reviewed for methodological rigour, and approved by the
Australian Government National Health and Medical Research Council (NHMRC).
MAIN RESULTS AND THE ROLE OF CHANCE: The evidence in the assessment and management of PCOS has generally improved in
the past five years, but remains of low to moderate quality. The technical evidence report and analyses (6000 pages) underpins 77
evidence-based and 54 consensus recommendations, with 123 practice points. Key updates include: i) further refinement of
individual diagnostic criteria, a simplified diagnostic algorithm and inclusion of anti-M
€
ullerian hormone (AMH) levels as an
alternative to ultrasound in adults only; ii) strengthening recognition of broader features of PCOS including metabolic risk factors,
cardiovascular disease, sleep apnea, very high prevalence of psychological features, and high risk status for adverse outcomes
during pregnancy; iii) emphasizing the poorly recognized, diverse burden of disease and the need for greater healthcare professional
education, evidence-based patient information, improved models of care and shared decision making to improve patient experience,
alongside greater research; iv) maintained emphasis on healthy lifestyle, emotional wellbeing and quality of life, with awareness
and consideration of weight stigma; and v) emphasizing evidence-based medical therapy and cheaper and safer fertility management.
LIMITATIONS, REASONS FOR CAUTION: Overall, recommendations are strengthened and evidence is improved, but remain gener-
ally low to moderate quality. Significantly greater research is now needed in this neglected, yet common condition. Regional health
system variation was considered and acknowledged, with a further process for guideline and translation resource adaptation provided.
WIDER IMPLICATIONS OF THE FINDINGS: The 2023 International Guideline for the Assessment and Management of PCOS provides
clinicians and patients with clear advice on best practice, based on the best available evidence, expert multidisciplinary input and con-
sumer preferences. Research recommendations have been generated and a comprehensive multifaceted dissemination and translation
programme supports the Guideline with an integrated evaluation program.
STUDY FUNDING/COMPETING INTEREST(S): This effort was primarily funded by the Australian Government via the National
Health Medical Research Council (NHMRC) (APP1171592), supported by a partnership with American Society for Reproductive Med-
icine, Endocrine Society, European Society for Human Reproduction and Embryology, and the Society for Endocrinology. The
Commonwealth Government of Australia also supported Guideline translation through the Medical Research Future Fund
(MRFCRI000266). HJT and AM are funded by NHMRC fellowships. JT is funded by a Royal Australasian College of Physicians
(RACP) fellowship. Guideline development group members were volunteers. Travel expenses were covered by the sponsoring organi-
zations. Disclosures of interest were strictly managed according to NHMRC policy and are available with the full guideline, technical
evidence report, peer review and responses (www.monash.edu/medicine/mchri/pcos). Of named authors HJT, CTT, AD, LM, LR, JBoyle,
AM have no conflicts of interest to declare. JL declares grant from Ferring and Merck; consulting fees from Ferring and Titus Health
Care; speaker’s fees from Ferring; unpaid consultancy for Ferring, Roche Diagnostics and Ansh Labs; and sits on advisory boards
for Ferring, Roche Diagnostics, Ansh Labs, and Gedeon Richter. TP declares a grant from Roche; consulting fees from Gedeon Richter
and Organon; speaker’s fees from Gedeon Richter and Exeltis; travel support from Gedeon Richter and Exeltis; unpaid consultancy for
Roche Diagnostics; and sits on advisory boards for Roche Diagnostics. MC declares travels support from Merck; and sits on an advisory
board for Merck. JBoivin declares grants from Merck Serono Ltd.; consulting fees from Ferring B.V; speaker’s fees from Ferring Arz-
neimittell GmbH; travel support from Organon; and sits on an advisory board for the Office of Health Economics. RJN has received
speaker’s fees from Merck and sits on an advisory board for Ferring. AJoham has received speaker’s fees from Novo Nordisk and Boeh-
ringer Ingelheim. The guideline was peer reviewed by special interest groups across our 39 partner and collaborating organizations, was
independently methodologically assessed against AGREEII criteria and was approved by all members of the guideline development
groups and by the NHMRC. (Fertil Steril
2023;-:-–-. This article has been co-published with permission in Fertility and Sterility,
Human Reproduction, European Journal of Endocrinology, and The Journal of Clinical Endocrinology and Metabolism. The Author(s)
2023. Published by Elsevier Inc. on behalf of the American Society of Reproductive Medicine. This is an open access article under the
CC-BY-NC license (http://creativecommons.org/licenses/by-nc/4.0/). The articles are identical except for minor stylistic and spelling
differences in keeping with each journal's style.
Key Words: Polycystic ovary syndrome, guideline, evidence-based, assessment, management, GRADE
WHAT DOES THIS MEAN FOR THOSE WITH
PCOS?
Building on the 2018 International Evidence-based Guideline
for the Assessment and Management of Polycystic Ovary
Syndrome (PCOS), this Guideline updates and expands clin-
ical questions, aiming to ensure that women with PCOS
receive optimal, evidence-based care that meets their needs
and improves health outcomes. The guideline and translation
program were developed with full consumer participation at
all stages including priority topics and outcomes for those
with PCOS. The aim is to support women and their healthcare
providers to optimize diagnosis, assessment and management
of PCOS. There is an emphasis on improved education and
awareness of healthcare professionals, partnership in care,
and empowerment of women with PCOS. Personal character-
istics, preferences, culture and values are considered, in addi-
tion to resource availability across different settings. With
effective translation, the Guideline will address priorities
identified by women with PCOS, upskill healthcare
professionals, empower consumers, improve care and out-
comes, identify key research gaps, and promote vital future
research.
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common endo-
crinopathy affecting reproductive-aged women, with impacts
across the lifespan from adolescence to post menopause.
PCOS prevalence is between 10 to 13% as demonstrated in
2 VOL. - NO. - / - 2023

the guideline process.
1,2
PCOS aetiology is complex; clinical
presentation is heterogeneous with reproductive, metabolic,
and psychological features.
1,2
Women internationally experi-
ence delayed diagnosis and dissatisfaction with care.
3-5
Clinical practice in the assessment and management of
PCOS remains inconsistent, with ongoing key practice
evidence gaps. Following on from the 2018 International
Evidence-based Guideline for the Assessment and Manage-
ment of Polycystic Ovary Syndrome,
6,7
independently evalu-
ated as high quality, this extensive update integrates current
literature with previous systematic reviews and extends to
new clinical questions prioritized by consumers. Ultimately,
we aim to update, extend and translate rigorous, comprehen-
sive evidence-based guidelines for diagnosis, assessment and
treatment, to improve the lives of those with PCOS worldwide.
To do so, the Guideline leverages substantive government
and society investment and brings together extensive con-
sumer engagement and international collaboration with lead-
ing societies and organizations, multidisciplinary experts, and
primary care representatives. This comprehensive evidence-
based Guideline is constructed from a rigorous, Appraisal of
Guidelines for Research and Evaluation-II (AGREEII)-
compliant, evidence-based guideline development process. It
provides a single source of international evidence-based rec-
ommendations to guide clinical practice with the opportunity
for adaptation in relevant health systems. Together with an
extensive translation program, the aim is to reduce worldwide
variation in care and promote high quality clinical service pro-
vision to improve health outcomes and quality of life in women
with PCOS. The Guideline is supported by a multifaceted inter-
national translation programme with co-designed resources to
enhance the skills of healthcare professionals and to empower
women with PCOS, with an integrated comprehensive evalua-
tion program. Here, we summarize recommendations from the
2023 International Evidence-based Guideline for the Assess-
ment and Management of PCOS.
MATERIALS AND METHODS
Best practice evidence-based guideline development methods
were applied and are detailed in the full Guideline and the tech-
nical reports, which are available online (www.monash.edu/
medicine/mchri/pcos).
8
In brief, extensive healthcare profes-
sional and consumer or patient engagement informed the
Guideline priority areas. International society-nominated
panels from across three leading entities, four partner organiza-
tions and thirty-two collaborating entities included consumers
and experts in paediatrics, endocrinology, gynaecology, pri-
mary care, reproductive endocrinology, psychology, dietetics,
exercise physiology, sleep, bariatric/ metabolic surgery, public
health, other co-opted experts, project management, evidence
synthesis and translation. Governance included an interna-
tional advisory and a management committee, five guideline
development groups (GDGs) with 56 members, and paediatric,
consumer, and translation committees. The five GDGs covered
i) Screening, diagnostic and risk assessment and life stage; ii)
Psychological features and models of care; iii) Lifestyle man-
agement; iv) Management of nonfertility features; and v)
Assessment and management of infertility. The leading en-
tities; the Australian National Health and Medical Research
Council (NHMRC) Centres for Research Excellence in Women’s
Health in Reproductive Life and in Polycystic Ovary Syndrome,
led by Monash University, partnered with the American Society
for Reproductive Medicine, the Endocrine Society, the Euro-
pean Society of Endocrinology and the European Society of
Human Reproduction and Embryology and collaborated with
32 other entities. With international meetings over 12 months
fifty-five prioritized clinical questions involved 52 systematic
and three narrative reviews, generating evidence-based and
consensus recommendations with accompanying practice
points. Committee members nominated by partner and collab-
orator organizations provided international peer review, and
independent experts reviewed methods which were then sub-
mitted to NHMRC for independent review. The target audience
includes multidisciplinary healthcare professionals, consumers
or patients, policy makers, and educators. The Guideline in-
cludes a focus on equity, cultural and ethnic diversity, avoid-
ance of stigma and inclusivity (see full guideline for details).
Processes aligned with all elements of the AGREE-II tool
for quality guideline assessment,
9
with extensive evidence syn-
thesis and meta-analysis. Integrity assessment was integrated
into guideline evidence synthesis processes and followed the
Research Integrity in Guideline Development (RIGID) frame-
work, with studies assessed against criteria from the Research
Integrity Assessment (RIA) tool and the Trustworthiness in
RAndomised Controlled Trials (TRACT) checklist.
10-12
Evidence synthesis methods are outlined in the full guideline
and followed best practice
9,13,14
Guideline recommendations
are presented by category, terms used, evidence quality and
Grading of Recommendations, Assessment, Development and
Evaluation (GRADE) framework considerations. Category
includes evidence-based (sufficient evidence in PCOS) or
consensus (insufficient evidence in PCOS, hence evidence in
general or relevant populations was considered) recommenda-
tions and accompanying practice points (implementation con-
siderations) (Table 1).
TABLE 1
Categories of PCOS guideline recommendations
EBR Evidence Based Recommendations:
Evidence sufficient to inform a
recommendation made by the
guideline development group.
CR Consensus Recommendations: In
the absence of adequate
evidence, a consensus
recommendation has been
made by the guideline
development group, also
informed by evidence from the
general population.
PP Practice Points: Evidence not
sought. A practice point has
been made by the guideline
development group where
important issues arose from
discussion of evidence-based or
consensus recommendations.
PCOS, polycystic ovary syndrome.
Teede. International PCOS Guideline 2023. Fertil Steril 2023.
VOL. - NO. - / - 2023 3
Fertility and Sterility®

The terms include ‘‘ should’’, ‘‘could’’ and ‘‘ should not’’ ,
which are informed by the nature of the recommendation (ev-
idence or consensus), the GRADE framework and the evidence
quality and are independent descriptors reflecting GDG
judgement. They refer to overall interpretation and practical
application of the recommendation, balancing benefits and
harms. ‘‘Should’’ is used where benefits of the recommenda-
tion exceed harms and where the recommendation can be
trusted to guide practice. Conditional recommendations are
reflected using the terms ‘‘ could’’ or ‘‘ should/could consider’’
which are used where evidence quality was limited or avail-
able studies demonstrate little clear advantage of one
approach over another, or the balance of benefits to harms
was unclear. ‘‘ Should not’’ applies when there is a lack of
appropriate evidence, or harms may outweigh benefits.
Evidence quality was categorized according to the GRADE
framework, with judgments about the quality of the included
studies and/or synthesized evidence incorporating risk of
bias, inconsistency, indirectness, imprecision and any other
considerations (e.g., publication bias) that may influence evi-
dence quality. These judgments considered study number and
design, statistical data and importance of outcomes (Table 2).
The quality of evidence reflects the confidence that the estimate
of the effect is adequate to support each recommendation,
13
largely determined by the expert evidence synthesis team.
GRADE acknowledges that evidence quality is a continuum;
any discrete categorization involves some arbitrary decisions;
nevertheless, the advantages of simplicity, transparency, and
clarity outweigh these limitations.
13
The GRADE framework enabled structured and trans-
parent consideration across evidence quality, feasibility,
acceptability, cost, implementation, and ultimately recom-
mendation strength
13
and was completed at face to face
guideline group meetings for all clinical questions (Table 3).
15
Notably, certainty of evidence varied across outcomes
within each question. Here evidence certainty reflects the
lowest certainty for the critical outcomes. Evidence was often
stronger for the top ranked outcome, and high quality
randomized controlled trials (RCTs) were often present,
despite overall low quality of evidence. These nuances were
considered by the GDG for all question as per the technical
report, with any apparent discrepancy between recommenda-
tion strength and evidence certainty justified in the full
Guideline. Finally, we note that this is a living Guideline
with annual evidence review in rapidly evolving areas.
The recommendations (Table 4) apply the category, descrip-
tive terms, GRADE of the recommendations and the quality of
the evidence. The full Guideline, technical evidence and admin-
istrativereportsareavailableonline(www.monash.edu/
medicine/mchri/pcos). The Guideline outlines the clinical need
for the question, the clinical question, the evidence summary,
the recommendations and practice points, and a summary of
the justification developed by the GDGs using the GRADE
framework. Extensive international peer review from across
the39organizationswasthenconsideredbyeachGDGandrec-
ommendations were reconsidered applying the GRADE frame-
work if justified. The comprehensive evidence reviews,
profiles, and GRADE frameworks supporting each recommenda-
tion can be found in the Technical Report. The administrative
report on guideline development, disclosure of interest process
and declarations, peer review feedback and responses can also
be found online. Here, we present the evidence-based and
consensus recommendations and practice points (Table 4). This
summary, the full Guideline and technical reports are supported
by a comprehensiv e co-des igned trans lation program to
optimize dissemination and impact with resources freely avai l-
able online (www.monash.edu/med icine/ mchri/p cos).
Two algorithms are provided to support recommenda-
tions on diagnosis (Figure 1) and infertility management
(Figure 2).
DISCUSSION
The International Evidence-based Guideline for the Assessment
and Management of PCOS and the related translation program
aims to provide a high quality, reliable source of international
evidence-based recommendations to guide consistent clinical
practice and to empower women with evidence-based informa-
tion. All recommendationswere formulated after an assessment
of the best available evidence, multidisciplinary clinical exper-
tise, consumer preferences and structured review by five GDGs.
The guideline provides 77 evidence-based and 54 consensus
recommendations, with 123 practice points underpinned by a
technical report on evidence synthesis and GRADE detailed
TABLE 2
Quality (certainty) of evidence categories (adapted from GRADE)
High 4444 Very confident that the true
effect lies close to that of the
estimate of the effect.
Moderate 444 Moderate confidence in the
effect estimate. The true
effect is likely to be close to
the estimate of the effect,
but there is a possibility that
it is different.
Low 44 Limited confidence in the effect
estimate. The true effect may
be substantially different
from the estimate of the
effect.
Very Low 4 Very little confidence in the
effect estimate. The true
effect is likely to be
substantially different from
the estimate of the effect.
GRADE, Grading of Recommendations, Assessment, Development, and Evaluation.
Teede. International PCOS Guideline 2023. Fertil Steril 2023.
TABLE 3
The Grading of Recommendations, Assessment, Development, and
Evaluation (GRADE) framework recommendation strength
❖ Conditional recommendation against the
option.
❖❖ Conditional recommendation for either the
option or the comparison.
❖❖❖ Conditional recommendation for the
option.
❖❖❖❖ Strong recommendation for the option.
Teede. International PCOS Guideline 2023. Fertil Steril 2023.
4 VOL. - NO. - / - 2023

TABLE 4
Recommendations for the assessment and management of polycystic ovary syndrome (PCOS). Monash University on behalf of the NHMRC
Centre for Research Excellence in Women's Health in Reproductive Life, 2023.
NO. TYPE RECOMMENDATION GRADE/QUAITY
1 Screening, diagnostic and risk assessment and life-stages
General principles
PP All diagnostic assessments are recommended for use in accordance with the
diagnostic algorithm (Algorithm 1).
1.1 Irregular cycles and ovulatory dysfunction
1.1.1 CR Irregular menstrual cycles are defined as:
Normal in the first year post menarche as part of the pubertal transition.
1to< 3 years post menarche: < 21 or > 45 days.
3 years post menarche to perimenopause: < 21 or > 35 days or < 8 cycles
per year.
1 year post menarche > 90 days for any one cycle.
Primary amenorrhea by age 15 or > 3 years post thelarche (breast devel-
opment).
When irregular menstrual cycles are present a diagnosis of PCOS should be
considered and assessed according to these PCOS Guidelines.
❖❖❖❖
1.1.2 PP The mean age of menarche may differ across populations.
1.1.3 PP In adolescents with irregular menstrual cycles, the value and optimal timing of
assessment and diagnosis of PCOS should be discussed with the patient
and their parent/s or guardian/s, considering diagnostic challenges at this
life stage and psychosocial and cultural factors.
1.1.4 PP For adolescents who have features of PCOS, but do not meet diagnostic
criteria, an ‘‘ increased risk’’ could be considered and reassessment advised
at or before full reproductive maturity, 8 years post menarche. This
includes those with PCOS features before combined oral contraceptive pill
(COCP) commencement, those with persisting features and those with
significant weight gain in adolescence.
1.1.5 PP Ovulatory dysfunction can still occur with regular cycles and if anovulation
needs to be confirmed serum progesterone levels can be measured.
1.2 Biochemical hyperandrogenism
1.2.1 EBR Healthcare professionals should use total and free testosterone to assess
biochemical hyperandrogenism in the diagnosis of PCOS; free
testosterone can be estimated by the calculated free androgen index.
❖❖❖❖
4
1.2.2 EBR If testosterone or free testosterone is not elevated, healthcare professionals
could consider measuring androstenedione and dehydroepiandrosterone
sulfate (DHEAS), noting their poorer specificity and greater age associated
decrease in DHEAS.
❖❖❖
4
1.2.3 EBR Laboratories should use validated, highly accurate tandem mass spectrometry
(LC-MS/MS) assays for measuring total testosterone and if needed, for
androstenedione and DHEAS. Free testosterone should be assessed by
calculation, equilibrium dialysis or ammonium sulfate precipitation.
❖❖❖❖
44
1.2.4 EBR Laboratories should use LC-MS/MS assays over direct immunoassays (e.g.,
radiometric, enzyme-linked, etc.) for assessing total or free testosterone,
which have limited accuracy and demonstrate poor sensitivity and
precision for diagnosing hyperandrogenism in PCOS.
❖❖❖❖
44
1.2.5 PP For the detection of hyperandrogenism in PCOS, the assessment of
biochemical hyperandrogenism is of greatest value in patients with
minimal or no clinical signs of hyperandrogenism (i.e., hirsutism).
1.2.6 PP It is very difficult to reliably assess for biochemical hyperandrogenism in
women on the combined oral contraceptive pill (COCP) as the pill
increases sex hormone-binding globulin and reduces gonadotrophin-
dependent androgen production. If already on the COCP, and assessment
of biochemical androgens is imperative, the pill should be withdrawn for a
minimum of three months and contraception should be managed
otherwise during this time.
1.2.7 PP Repeated androgen measures for the ongoing assessment of PCOS in adults
have a limited role.
1.2.8 PP In most adolescents, androgen levels reach adult ranges at 12-15 years of age
1.2.9 PP If androgen levels are markedly above laboratory reference ranges, causes of
hyperandrogenaemia other than PCOS, including ovarian and adrenal
neoplastic growths, congenital adrenal hyperplasia, Cushing’s syndrome,
ovarian hyperthecosis (after menopause), iatrogenic causes, and
syndromes of severe insulin resistance, should be considered. However,
some androgen-secreting neoplasms are associated with only mild to
moderate increases in androgen levels. The clinical history of time of onset
and/or rapid progression of symptoms is critical in assessing for an
androgen-secreting tumour.
VOL. - NO. - / - 2023 5
Fertility and Sterility®

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
1.2.10 PP Reference ranges for different methods and laboratories vary widely, and are
often based on an arbitrary percentile or variances of the mean from a
population that has not been fully characterized and is highly likely to
include women with PCOS. Normal values should be determined either by
the range of values in a well characterized healthy control population or by
cluster analysis of general population values.
1.2.11 PP Laboratories involved in androgen measurements in females should consider:
Determining laboratory normal values by either the range of values in a well
characterized healthy control population or by cluster analysis of the values
of a large general population.
Applying the most accurate methods where available.
Using extraction/chromatography immunoassays as an alternative to mass
spectrometry only where adequate expertise is available.
Future improvements may arise from measurement of 11-oxygenated
androgens, and from establishing cut-off levels or thresholds based on
large-scale validation in populations of different ages and ethnicities.
1.3 Clinical hyperandrogenism
1.3.1 EBR The presence of hirsutism alone should be considered predictive of
biochemical hyperandrogenism and PCOS in adults.
❖❖❖
4
1.3.2 EBR Healthcare professionals could recognize that female pattern hair loss and
acne in isolation (without hirsutism) are relatively weak predictors of
biochemical hyperandrogenism.
❖❖❖
4
1.3.3 CR A comprehensive history and physical examination should be completed for
symptoms and signs of clinical hyperandrogenism, including acne, female
pattern hair loss and hirsutism in adults, and severe acne and hirsutism in
adolescents.
❖❖❖❖
1.3.4 CR Healthcare professionals should be aware of the potential negative
psychosocial impact of clinical hyperandrogenism and should consider the
reporting of unwanted excess hair growth and/or female pattern hair loss
as being important, regardless of apparent clinical severity.
❖❖❖
1.3.5 CR A modified Ferriman Gallwey score (mFG) of 4 – 6 should be used to detect
hirsutism, depending on ethnicity, acknowledging that self-treatment is
common and can limit clinical assessment.
❖❖❖❖
1.3.6 CR Healthcare professionals should consider that the severity of hirsutism may
vary by ethnicity but the prevalence of hirsutism appears similar across
ethnicities.
❖❖❖
1.3.7 PP Healthcare professionals should:
Be aware that standardized visual scales are preferred when assessing hir-
sutism, such as the mFG scale in combination with a photographic atlas.
Consider the Ludwig or Olsen visual scales for assessing female pattern hair
loss.
Note that there are no universally accepted visual instruments for assessing
the presence of acne.
Recognize that women commonly treat clinical hyperandrogenism
cosmetically, diminishing their apparent clinical severity.
Appreciate that self-assessment of unwanted excess hair growth, and
possibly acne and female pattern hair loss, has a high degree of validity and
merits close evaluation, even if overt clinical signs of hyperandrogenism are
not readily evident on examination.
Note that only terminal hairs need to be considered in defining hirsutism,
and these can reach >5 mm if untreated, vary in shape and texture, and are
generally pigmented.
Note that new-onset severe or worsening hyperandrogenism, including
hirsutism, requires further investigation to rule out androgen-secreting
tumours and ovarian hyperthecosis.
Monitor clinical signs of hyperandrogenism, including hirsutism, acne and
female pattern hair loss, for improvement or treatment adjustment during
therapy.
1.4 Ultrasound and polycystic ovarian morphology
1.4.1 EBR Follicle number per ovary (FNPO) should be considered the most effective
ultrasound marker to detect polycystic ovarian morphology (PCOM) in
adults.
❖❖❖❖
44
1.4.2 EBR Follicle number per ovary (FNPO), follicle number per cross-section (FNPS) and
ovarian volume (OV) should be considered accurate ultrasound markers
for PCOM in adults.
❖❖❖❖
44
1.4.3 CR PCOM criteria should be based on follicle excess (FNPO, FNPS) and/or ovarian
enlargement.
❖❖❖❖
6 VOL. - NO. - / - 2023

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
1.4.4 CR Follicle number per ovary (FNPO) R 20 in at least one ovary should be
considered the threshold for PCOM in adults.
❖❖❖❖
1.4.5 CR Ovarian volume (OV) R 10ml or follicle number per section (FNPS) R 10 in at
least one ovary in adults should be considered the threshold for PCOM if
using older technology or image quality is insufficient to allow for an
accurate assessment of follicle counts throughout the entire ovary.
❖❖❖❖
1.4.6 PP There are no definitive criteria to define polycystic ovary morphology (PCOM)
on ultrasound in adolescents, hence it is not recommended in adolescents.
1.4.7 PP When an ultrasound is indicated, if acceptable to the individual, the
transvaginal approach is the most accurate for the diagnosis of PCOM.
1.4.8 PP Transabdominal ultrasound should primarily report ovarian volume (OV) with
a threshold of R 10 ml or follicle number per section (FNPS) R 10 in either
ovary in adults given the difficulty of assessing follicle counts throughout
the entire ovary with this approach.
1.4.9 PP In patients with irregular menstrual cycles and hyperandrogenism, an ovarian
ultrasound is not necessary for PCOS diagnosis.
1.4.10 PP Thresholds for PCOM should be revised regularly with advancing ultrasound
technology, and age-specific cut-off values for PCOM should be defined.
1.4.11 PP There is a need for training in careful and meticulous follicle counting per
ovary and clear standardized protocols are recommended for PCOM
reporting on ultrasound including at a minimum:
Last menstrual period (or stage of cycle).
Transducer bandwidth frequency.
Approach/route assessed.
Total number of 2 – 9 mm follicles per ovary.
Measurements in three dimensions (in cm) or volume of each ovary.
Other ovarian features and/or pathology including ovarian cysts, corpus
lutea, dominant follicles (R10 mm) (which should not be included in
ovarian volume calculations).
Reliance on the contralateral ovary FNPO for diagnosis of PCOM, where a
dominant follicle is noted.
Uterine features and/or pathology including endometrial thickness and
pattern.
1.5 Anti-M
€
ullerian Hormone in the diagnosis of PCOS
1.5.1 EBR Serum anti-M
€
ullerian hormone (AMH) could be used for defining PCOM in
adults.
❖❖❖
444
1.5.2 EBR Serum AMH should only be used in accordance with the diagnostic algorithm,
noting that in patients with irregular menstrual cycles and
hyperandrogenism, an AMH level is not necessary for PCOS diagnosis.
❖❖❖❖
444
1.5.3 EBR We recommend that serum AMH should not be used as a single test for the
diagnosis of PCOS.
❖❖❖❖
444
1.5.4 EBR Serum AMH should not yet be used in adolescents. ❖❖❖❖
444
1.5.5 PP Either serum AMH or ultrasound may be used to define PCOM; however, both
tests should not be performed to limit overdiagnosis.
1.5.6 PP Laboratories and healthcare professionals need to be aware of factors that
influence AMH in the general population including:
Age: Serum AMH generally peaks between the ages of 20-25 years in the
general population.
Body mass index (BMI): Serum AMH is lower in those with higher BMI in the
general population.
Hormonal contraception and ovarian surgery: Serum AMH may be sup-
pressed by current or recent COCP use.
Menstrual cycle day: Serum AMH may vary across the menstrual cycle.
1.5.7 PP Laboratories involved in AMH measurements in females should use
population and assay specific cut-offs.
1.6 Ethnic variation
1.6.1 EBR Healthcare professionals should be aware of the high prevalence of PCOS in
all ethnicities and across world regions, ranging from 10-13% globally
using the Rotterdam criteria.
❖❖❖❖
44
1.6.2 EBR Healthcare professionals should be aware that PCOS prevalence is broadly
similar across world regions, but may be higher in South East Asian and
Eastern Mediterranean regions.
❖❖❖❖
44
1.6.3 PP Healthcare professionals should be aware that the presentation of PCOS may
vary across ethnic groups.
1.7 Menopause life stage
VOL. - NO. - / - 2023 7
Fertility and Sterility®

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
1.7.1 CR A diagnosis of PCOS could be considered as enduring / lifelong. ❖❖❖
1.7.2 CR Healthcare professionals could consider that both clinical and biochemical
hyperandrogenism persist in the postmenopause for women with PCOS.
❖❖❖
1.7.3 CR PCOS diagnosis could be considered postmenopause if there is a past
diagnosis, or a long-term history of oligo-amenorrhoea with
hyperandrogenism and/or PCOM, during the earlier reproductive years
(age 20-40).
❖❖❖
1.7.4 CR Further investigations should be considered to rule out androgen-secreting
tumours and ovarian hyperthecosis in postmenopausal women presenting
with new-onset, severe or worsening hyperandrogenism including
hirsutism.
❖❖❖
1.8 Cardiovascular disease risk
1.8.1 EBR Women with PCOS should be considered at increased risk of cardiovascular
disease and potentially of cardiovascular mortality, acknowledging that
the overall risk of cardiovascular disease in pre-menopausal women is low.
❖❖❖
4
1.8.2 EBR All women with PCOS should be assessed for cardiovascular disease risk
factors.
❖❖❖❖
4
1.8.3 CR All women with PCOS, regardless of age and BMI, should have a lipid profile
(cholesterol, low density lipoprotein cholesterol, high density lipoprotein
cholesterol and triglyceride level) at diagnosis. Thereafter, frequency of
measurement should be based on the presence of hyperlipidaemia and
additional risk factors or global cardiovascular risk.
❖❖❖❖
1.8.4 CR All women with PCOS should have blood pressure measured annually and
when planning pregnancy or seeking fertility treatment, given the high
risk of hypertensive disorders in pregnancy and the associated
comorbidities.
❖❖❖❖
1.8.5 CR Funding bodies should recognize that PCOS is highly prevalent with
multisystem effects including cardiometabolic disease and should diversify
and increase research support accordingly.
❖❖❖❖
1.8.6 CR Cardiovascular general population guidelines could consider the inclusion of
PCOS as a cardiovascular risk factor.
❖❖❖
1.8.7 CR Healthcare professionals, women with PCOS and other stakeholders should
all prioritize preventative strategies to reduce cardiovascular risk.
❖❖❖❖
1.8.8 PP Consideration should be given to the differences in cardiovascular risk factors,
and cardiovascular disease, across ethnicities (see 1.6.1) and age, when
determining frequency of risk assessment.
1.9 Impaired glucose tolerance and type 2 diabetes risk
1.9.1 EBR Healthcare professionals and women with PCOS should be aware that,
regardless of age and BMI, women with PCOS have an increased risk of
impaired fasting glucose, impaired glucose tolerance and type 2 diabetes.
❖❖❖❖
44
1.9.2 EBR Glycaemic status should be assessed at diagnosis in all adults and adolescents
with PCOS.
❖❖❖❖
44
1.9.3 CR Glycaemic status should be reassessed every one to three years, based on
additional individual risk factors for diabetes.
❖❖❖❖
1.9.4 CR Healthcare professionals, women with PCOS and other stakeholders should
prioritize preventative strategies to reduce type 2 diabetes risk.
❖❖❖❖
1.9.5 CR Funding bodies should recognize that PCOS is highly prevalent, has
significantly higher risk for diabetes, and should be funded accordingly.
❖❖❖❖
1.9.6 CR Diabetes general population guidelines should consider the inclusion of PCOS
as an independent risk factor for diabetes.
❖❖❖❖
1.9.7 PP Healthcare professionals, adults and adolescents with PCOS and their first-
degree relatives, should be aware of the increased risk of diabetes and the
need for regular glycaemic assessment.
1.9.8 PP Women with type 1 and type 2 diabetes have an increased risk of PCOS and
screening should be considered in individuals with diabetes.
Glycaemic testing
1.9.9 EBR Healthcare professionals and women with PCOS should recommend the 75-g
oral glucose tolerance test (OGTT) as the most accurate test to assess
glycaemic status in PCOS, regardless of BMI.
❖❖❖❖
4
1.9.10 EBR If an OGTT cannot be performed, fasting plasma glucose and/or glycated
haemoglobin (HbA1c) could be considered, noting significantly reduced
accuracy.
❖❖❖
4
8 VOL. - NO. - / - 2023

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
1.9.11 EBR An OGTT should be considered in all women with PCOS and without pre-
existing diabetes, when planning pregnancy or seeking fertility treatment,
given the high risk of hyperglycaemia and the associated comorbidities in
pregnancy. If not performed preconception, an OGTT could be offered at
the first prenatal visit and all women with PCOS should be offered the test
at 24-28 weeks gestation.
❖❖❖
4
1.9.12 PP Insulin resistance is a pathophysiological factor in PCOS, however, clinically
available insulin assays are of limited clinical relevance and are not
recommended in routine care (refer to 3.1.10).
1.10 Obstructive Sleep Apnea
1.10.1 EBR Healthcare professionals should be aware that women with PCOS have
significantly higher prevalence of obstructive sleep apnea compared to
women without PCOS, independent of BMI.
❖❖❖❖
444
1.10.2 EBR Women with PCOS should be assessed for symptoms of obstructive sleep
apnea (i.e., snoring in combination with waking unrefreshed from sleep,
daytime sleepiness or fatigue) and if present, screen with validated tools or
refer for assessment.
❖❖❖❖
444
1.10.3 PP Simple obstructive sleep apnea screening questionnaires (such as the Berlin
questionnaire, validated in the general population) can assist in identifying
obstructive sleep apnea in women with PCOS, noting that diagnosis
requires a formal sleep study.
1.10.4 PP Goals of treatment should target obstructive sleep apnea related symptom
burden.
1.11 Endometrial hyperplasia and cancer
1.11.1 EBR Healthcare professionals should be aware that premenopausal women with
PCOS have markedly higher risk of developing endometrial hyperplasia
and endometrial cancer.
❖❖❖❖
4
1.11.2 PP Women with PCOS should be informed about the increased risk of
endometrial hyperplasia and endometrial cancer, acknowledging that the
overall chance of developing endometrial cancer is low, therefore routine
screening is not recommended.
1.11.3 PP Long-standing untreated amenorrhea, higher weight, type 2 diabetes and
persistent thickened endometrium are additional to PCOS as risk factors
for endometrial hyperplasia and endometrial cancer.
1.11.4 PP Women with PCOS should be informed of preventative strategies including
weight management, cycle regulation and regular progestogen therapy.
1.11.5 PP When excessive endometrial thickness is detected, consideration of a biopsy
with histological analysis and withdrawal bleed is indicated.
1.12 Risks in first degree relatives
1.12.1 EBR Healthcare professionals could consider that fathers and brothers of women
with PCOS may have an increased prevalence of metabolic syndrome, type
2 diabetes, and hypertension.
❖❖❖
4
1.12.2 PP The cardiometabolic risk in female first degree relatives of women with PCOS
remains inconclusive.
2 Prevalence, screening and management of psychological features and
models of care
General principles
PP Psychological features are common and important component of PCOS that
all healthcare professionals should be aware of.
PP Funding bodies should recognize that PCOS is highly prevalent, has
significantly higher psychological disorders which should be prioritized
and funded accordingly.
2.1 Quality of Life
2.1.1 EBR Healthcare professionals and women should recognize the adverse impact of
PCOS and/or PCOS features on quality of life in adults.
❖❖❖❖
44
2.1.2 PP Women with PCOS should be asked about their perception of PCOS related-
symptoms, impact on quality of life, key concerns, and priorities for
management.
2.2 Depression and Anxiety
2.2.1 EBR Healthcare professionals should be aware of the high prevalence of moderate
to severe depressive symptoms and depression in adults and adolescents
with PCOS and should screen for depression in all adults and adolescents
with PCOS, using regionally validated screening tools.
❖❖❖❖
4444
VOL. - NO. - / - 2023 9
Fertility and Sterility®

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
2.2.2 EBR Healthcare professionals should be aware of the high prevalence of moderate
to severe anxiety symptoms and anxiety disorders in adults and should
screen for anxiety in all adults with PCOS, using regionally validated
screening tools.
❖❖❖❖
4444
2.2.3 CR If moderate or severe depressive or anxiety symptoms are detected,
practitioners should further assess, refer appropriately, or offer treatment.
❖❖❖❖
2.2.4 PP Severity of symptoms and clinical diagnosis of depression or anxiety should
guide management.
The optimal interval for anxiety and depression screening is not known. A
pragmatic approach could include screening at diagnosis with repeat
screening based on clinical judgement, risk factors, comorbidities, and life
events, including the perinatal period.
Screening for mental health disorders comprises assessment of risk factors,
symptoms, and risk of self-harm and suicidal intent.
2.3 Psychosexual function
2.3.1 CR Healthcare professionals could consider the multiple factors that can influence
psychosexual function in PCOS including higher weight, hirsutism, mood
disorders, infertility and PCOS medications.
❖❖❖
2.3.2 CR Permission to discuss psychosexual function should be sought noting that the
diagnosis of psychosexual dysfunction requires both low psychosexual
function combined with related distress.
❖❖❖❖
2.4 Body Image
2.4.1 EBR Healthcare professionals should be aware that features of PCOS can have a
negative impact on body image.
❖❖❖❖
44
2.5 Eating disorders
2.5.1 EBR Eating disorders and disordered eating should be considered in PCOS,
regardless of weight, especially in the context of weight management and
lifestyle interventions (see sections 2.4 and 3.6).
❖❖❖
44
2.5.2 PP If disordered eating or eating disorders are suspected, appropriately qualified
practitioners should further assess via a full diagnostic interview.
If an eating disorder or disordered eating is detected, appropriate
management and support should be offered.
2.6 Information resources, models of care, cultural and linguistic
considerations
2.6.1 Information needs
2.6.1.1 EBR Tailored information, education and resources that are high-quality, culturally
appropriate and inclusive should be provided to all with PCOS.
❖❖❖❖
444
2.6.1.2 EBR Information, education and resources are a high priority for patients with
PCOS and should be provided in a respectful and empathic manner.
❖❖❖❖
444
2.6.1.3 CR Entities responsible for healthcare professional education should ensure that
information and education on PCOS is systemically embedded at all levels
of healthcare professional training to address knowledge gaps.
❖❖❖❖
2.6.1.4 PP The diversity of the population should be considered when adapting practice
paradigms.
Healthcare professional education opportunities should be optimised at all
stages of graduate and postgraduate training and continuing professional
development and in practice support resources.
2.6.1.5 PP Women should be counselled on the risk of misinformation and guided to
evidence-based resources.
2.6.2 Models of care
2.6.2.1 CR Models of care should prioritize equitable access to evidence-based primary
care with pathways for escalation to integrated specialist and
multidisciplinary services as required.
❖❖❖❖
2.6.2.2 PP Strategies to deliver optimal models of care could include healthcare
professional education, care pathways, virtual care, broader health
professional engagement (e.g., nurse practitioners) and coordination
tools.
2.6.3 Support to manage PCOS
2.6.3.1 CR Public health actors should consider increasing societal awareness and
education on PCOS to reduce stigma and marginalization.
❖❖❖
2.6.3.2 PP Culturally appropriate resources and education on PCOS across the life span
for families of those with the condition should be considered.
10 VOL. - NO. - / - 2023

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
2.6.4 Patient care
2.6.4.1 EBR Healthcare professionals should employ shared decision-making and support
patient agency or ability to take independent actions to manage their
health and care.
❖❖❖❖
444
2.6.4.2 EBR The importance of being knowledgeable about PCOS, of applying evidence-
based practices when sharing news on diagnosis, treatment, and health
implications, and of ascertaining and focusing on patient priorities, should
be recognized.
❖❖❖❖
444
2.6.4.3 CR Healthcare system leaders should enable system wide changes to support
healthcare professional training, knowledge and practice in sharing news
optimally, shared decision making and patient agency, including ensuring
adequate consultation time and accessible resources.
❖❖❖❖
2.6.4.4 PP Evidence-based strategies for shared decision making and for sharing news
(such as the SPIKES framework) are readily available and should be used to
inform PCOS care.
All healthcare professionals partnering with women with PCOS should be
knowledgeable in sharing news, in shared decision-making, and in
supporting patient self-management.
Evidence-based strategies and resources can be used to support patient
activation, which refers to modifiable knowledge, skills, ability,
confidence, and willingness to self-manage one’s own health and care.
2.7 Psychological therapy
2.7.1 CR Women with PCOS diagnosed with depression, anxiety, and/or eating
disorders should be offered psychological therapy guided by regional
general population guidelines and the preference of the woman with
PCOS.
❖❖❖❖
2.7.2 CR Women with PCOS with disordered eating, body image distress, low self-
esteem, problems with feminine identity, or psychosexual dysfunction
should be offered evidence-based treatments (e.g., cognitive behaviour
therapy) where appropriate.
❖❖❖❖
2.8 Antidepressant and anxiolytic treatment
2.8.1 CR Psychological therapy could be considered first-line management, and
antidepressant medications considered in adults where mental health
disorders are clearly documented and persistent, or if suicidal symptoms
are present, based on general population guidelines.
❖❖❖
2.8.2 PP Lifestyle intervention and other therapies (e.g., COCP, metformin, laser hair
removal) that target PCOS features should be considered, given their
potential to improve psychological symptoms.
Where pharmacological treatment for anxiety and depression is offered in
PCOS, healthcare professionals should apply caution:
to avoid inappropriate treatment with antidepressants or anxiolytics.
to limit use of agents that exacerbate PCOS symptoms, including weight
gain.
Healthcare professionals should be aware that not managing anxiety and
depression may impact adherence to PCOS treatment / management.
3 Lifestyle management
3.1 Effectiveness of lifestyle interventions
3.1.1 EBR Lifestyle intervention (exercise alone or multicomponent diet combined with
exercise and behavioural strategies) should be recommended for all
women with PCOS, for improving metabolic health including central
adiposity and lipid profile.
❖❖❖❖
4
3.1.2 CR Healthy lifestyle behaviours encompassing healthy eating and/or physical
activity should be recommended in all women with PCOS to optimize
general health, quality of life, body composition and weight management
(maintaining weight, preventing weight gain and/or modest weight loss).
❖❖❖❖
3.1.3 PP Healthcare professionals should be aware that lifestyle management is a core
focus in PCOS management.
3.1.4 PP Lifestyle management goals and priorities should be co-developed in
partnership with women with PCOS, and value women’s individualized
preferences.
3.1.5 PP There are benefits to a healthy lifestyle even in the absence of weight loss.
VOL. - NO. - / - 2023 11
Fertility and Sterility®

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
3.1.6 PP In those with higher weight, weight management can be associated with
significant clinical improvements and the following key points need to be
considered including:
A lifelong focus on prevention of further weight gain.
If the goal is to achieve weight loss, a tailored energy deficit could be pre-
scribed for women, considering individual energy requirements, body
weight and physical activity levels.
The value of improvement in central adiposity (e.g., waist circumference,
waist-hip ratio) or metabolic health.
The need for ongoing assessment and support.
3.1.7 PP Healthcare professionals should be aware of weight stigma when discussing
lifestyle management with women with PCOS (see 3.6).
3.1.8 PP Healthy lifestyle and optimal weight management, in the context of
structured, intensive, and ongoing clinical support, appears equally
effective in PCOS as in the general population.
3.1.9 PP In those who are not overweight, in the adolescent and at key life points, the
focus should be on healthy lifestyle and the prevention of excess weight
gain.
3.1.10 PP Insulin resistance is a pathophysiological factor in PCOS, however, clinically
available insulin assays are of limited clinical relevance and should not be
used in routine care (refer to 1.9.12).
3.2 Behavioural Strategies
3.2.1 CR Lifestyle interventions could include behavioural strategies such as goal-
setting, self-monitoring, problem solving, assertiveness training,
reinforcing changes, and relapse prevention, to optimize weight
management, healthy lifestyle and emotional wellbeing in women with
PCOS.
❖❖❖
3.2.2 PP Behavioural support could include: goal-setting, problem solving, self-
monitoring and reviewing, or SMART goals (Specific, Measurable,
Achievable, Realistic and Timely).
3.2.3 PP Comprehensive healthy behavioural or cognitive behavioural interventions
could be considered to increase support, engagement, retention,
adherence, and maintenance of healthy lifestyle and improve health
outcomes in women with PCOS.
3.3 Dietary Intervention
3.3.1 EBR Healthcare professionals and women should consider that there is no
evidence to support any one type of diet composition over another for
anthropometric, metabolic, hormonal, reproductive or psychological
outcomes.
❖❖❖
4
3.3.2 CR Any diet composition consistent with population guidelines for healthy eating
will have health benefits and, within this, healthcare professionals should
advise sustainable healthy eating tailored to individual preferences and
goals.
❖❖❖❖
3.3.3 PP Tailoring of dietary changes to food preferences, allowing for a flexible,
individual and co-developed approach to achieving nutritional goals, and
avoiding unduly restrictive and nutritionally unbalanced diets, are
important, as per general population guidelines.
3.3.4 PP Barriers and facilitators to optimize engagement and adherence to dietary
change should be discussed, including psychological factors, physical
limitations, socioeconomic and sociocultural factors, as well as personal
motivators for change. The value of broader family engagement should be
considered. Referral to suitably trained allied healthcare professionals
needs to be considered when women with PCOS need support with
optimizing their diet.
3.4 Exercise Intervention
3.4.1 EBR Healthcare professionals and women could consider that there is a lack of
evidence supporting any one type and intensity of exercise being better
than another for anthropometric, metabolic, hormonal, reproductive or
psychological outcomes.
❖❖❖
4
3.4.2 CR Any physical activity consistent with population guidelines will have health
benefits and, within this, healthcare professionals should advise
sustainable physical activity based on individual preferences and goals.
❖❖❖❖
12 VOL. - NO. - / - 2023

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
3.4.3 CR Healthcare professionals should encourage and advise the following in
concordance with general population physical activity guidelines:
All adults should undertake physical activity as doing some physical activity
is better than none.
Adults should limit the amount of time spent being sedentary (e.g., sitting,
screen time) as replacing sedentary time with physical activity of any in-
tensity (including light intensity) provides health benefits.
For the prevention of weight gain and maintenance of health, adults (18-64
years) should aim for a minimum of 150 to 300 minutes of moderate in-
tensity activities or 75 to 150 minutes of vigorous intensity aerobic activity
per week or an equivalent combination of both spread throughout the
week, plus muscle strengthening activities (e.g., resistance/flexibility) on
two non-consecutive days per week.
For promotion of greater health benefits including modest weight-loss and
prevention of weight-regain, adults (18-64 years) should aim for a
minimum of 250 min/week of moderate intensity activities or 150 min/
week of vigorous intensities or an equivalent combination of both, plus
muscle strengthening activities (e.g., resistance/flexibility) ideally on two
non-consecutive days per week.
Adolescents should aim for at least 60 minutes of moderate- to vigorous-
intensity physical activity per day, including activities that strengthen muscle
and bone at least three times per week.
❖❖❖❖
3.4.4 PP Physical activity is any bodily movement produced by skeletal muscles that
requires energy expenditure. It includes leisure time physical activity,
transportation (e.g., walking or cycling), occupational (i.e., work),
household chores, playing games, sports or planned exercise, or activities
in the context of daily, family and community activities.
3.4.5 PP Aerobic activity is best performed in bouts of at least 10 minutes duration,
aiming to achieve at least 30 minutes daily on most days.
3.4.6 PP Barriers and facilitators to optimize engagement and adherence to physical
activity should be discussed, including psychological factors (e.g., body
image concerns, fear of injury, fear of failure, mental health), personal
safety concerns, environmental factors, physical limitations,
socioeconomic factors, sociocultural factors, and personal motivators for
change. The value of broader family engagement should be considered.
Referral to suitably trained allied healthcare professionals needs to be
considered for optimizing physical activity in women with PCOS.
3.4.7 PP Self-monitoring, including with fitness tracking devices and technologies for
step count and exercise intensity, could be considered as an adjunct to
support and promote active lifestyles and minimize sedentary behaviours.
3.5 Factors affecting weight gain in PCOS
3.5.1 EBR Healthcare professionals and women with PCOS could consider that there is a
lack of consistent evidence of physiological or behavioural lifestyle
differences, related to weight, in women with PCOS compared to women
without PCOS.
❖❖❖
4
3.5.2 PP Whilst the specific mechanisms are unclear, it is recognized that many women
with PCOS will have underlying mechanisms that drive greater
longitudinal weight gain and higher BMI which may:
Underpin greater challenges with weight management.
Highlight the importance of lifelong healthy lifestyle strategies and pre-
vention of excess weight gain.
Assist women with PCOS and healthcare professionals in forming realistic,
tailored lifestyle goals.
3.6 Weight Stigma
3.6.1 EBR Many women with PCOS experience weight stigma in healthcare and other
settings and the negative biopsychosocial impacts of this should be
recognized.
❖❖❖❖
44
3.6.2 CR Healthcare professionals should be aware of their weight biases and the
impact this has on their professional practice and on women with PCOS.
❖❖❖❖
3.6.3 CR Health policy makers, managers and educators should promote awareness of
weight stigma and invest in weight stigma education and minimization
strategies.
❖❖❖❖
VOL. - NO. - / - 2023 13
Fertility and Sterility®

TABLE 4
Continued.
NO. TYPE RECOMMENDATION GRADE/QUAITY
3.6.4 PP Healthcare professionals should be aware of weight-inclusive practices which
promote acceptance of and respect for body size diversity and focus on
improvement of health behaviours and health outcomes for people of all
sizes. In PCOS this includes:
Acknowledging that whilst higher weight is a risk factor for PCOS and its
complications, it is only one indicator of health and broader factors should
be assessed.
Asking permission to discuss and measure weight and using strategies to
minimize discomfort (e.g., blind weighing).
Recognizing that the terms ‘‘overweight’’ and ‘‘obese/obesity’’ can be
stigmatizing with suggested alternatives including ‘‘ higher weight’’ .
If weighing, explaining how weight information will be used to inform risks,
prevention and treatment and how not knowing may impact on
recommendations.
Ensuring appropriate equipment is available for women of all sizes.
Offering options of weight-centric care (promoting intentional weight loss)
or weight-inclusive care (promoting healthy lifestyle change without
focusing on intentional weight loss) tailored to individual goals and
preferences.
Offering all women best practice assessment, treatment and support
regardless of weight, acknowledging that weight may be a non-modifiable
risk factor when using lifestyle modification alone.
3.6.5 PP Increasing awareness of weight stigma among family members of women
and adolescents with PCOS should be considered.
4 Management of non-fertility features
4.1 Pharmacology treatment principles in PCOS
PP Shared decision making between the patient (and parent/s or guardian/s, if
the patient is a child) and the healthcare professional is required.
PP An individual’s characteristics, preferences and values must be elicited and
considered when recommending any intervention alone or in
combination.
PP Understanding how individual adults and adolescents value treatment
outcomes is essential when prescribing medications.
PP Medical therapy is generally not approved for use specifically in PCOS and
recommended use is therefore evidence-based, but off-label. Healthcare
professionals need to inform adults, adolescents and their parents/s or
guardian/s and discuss the evidence, possible concerns and side effects.
Regulatory agencies should consider approval of evidence-based
medications for use in PCOS.
4.2 Combined Oral Contraceptive Pills
4.2.1 EBR Combined oral contraceptive pills (COCP) could be recommended in
reproductive age adults with PCOS for management of hirsutism and/or
irregular menstrual cycles.
❖❖❖
4
4.2.2 EBR The COCP could be considered in adolescents at risk or with a clear diagnosis
of PCOS for management of hirsutism and/or irregular menstrual cycles.
❖❖❖
4
4.2.3 EBR Healthcare professionals could consider that there is no clinical advantage of
using high dose ethinylestradiol (R 30 mg) versus low dose
ethinylestradiol (< 30mg) when treating hirsutism in adults with PCOS.
❖❖❖
4
4.2.4 EBR General population guidelines should be considered when prescribing COCP
in adults and adolescents with PCOS as specific types or doses of
progestins, estrogens or combinations of COCP cannot currently be
recommended.
❖❖❖
4
4.2.5 EBR The 35mg ethinyl estradiol plus cyproterone acetate preparations should be
considered as second-line therapy over other COCPs, balancing benefits
and adverse effects, including venous thromboembolic risks.
❖❖❖
4
4.2.6 EBR Progestin only oral contraceptives may be considered for endometrial
protection, based on general population guidelines, acknowledging that
evidence in women with PCOS is limited.
❖❖❖
4
14 VOL. - NO. - / - 2023

TABLE 4
Continued.
4.2.7 PP When prescribing COCPs in adults and adolescents with PCOS, and
adolescents at risk of PCOS
It is important to address main presenting symptoms and consider other
treatments such as cosmetic therapies.
Shared decision-making (including accurate information and reassurance
on the efficacy and safety of COCP) is recommended and likely to improve
adherence.
Natural estrogen preparations and the lowest effective estrogen doses
(such as 20-30mg of ethinyl estradiol or equivalent), need consideration,
balancing efficacy, metabolic risk profile, side effects, cost, and availability.
The relatively limited evidence on COCPs specifically in PCOS needs to be
appreciated with practice informed by general population guidelines.
The relative and absolute contraindications and side effects of COCPs
need to be considered and be the subject of individualized discussion.
PCOS specific features, such as higher weight and cardiovascular risk
factors, need to be considered.
4.3 Metformin
4.3.1 EBR Metformin alone should be considered in adults with PCOS and a BMI R 25
kg/m2 for anthropometric, and metabolic outcomes including insulin
resistance, glucose, and lipid profiles.
❖❖❖
4
4.3.2 EBR Metformin alone could be considered in adolescents at risk of or with PCOS
for cycle regulation, acknowledging limited evidence.
❖❖❖
4
4.3.3 CR Metformin alone may be considered in adults with PCOS and BMI < 25 kg/
m2, acknowledging limited evidence.
❖❖❖
4.3.4 PP Where metformin is prescribed the following need to be considered:
Shared decision making needs to consider feasibility and effectiveness of
active lifestyle intervention. Women should be informed that metformin
and active lifestyle intervention have similar efficacy.
Mild adverse effects, including gastrointestinal side-effects are generally
dose dependent and self-limiting.
Starting at a low dose, with 500mg increments 1-2 weekly and extended-
release preparations may minimize side effects and improve adherence.
Suggested maximum daily dose is 2.5g in adults and 2g in adolescents.
Use appears safe long-term, based on use in other populations, however
indications for ongoing requirement needs to be considered.
Use may be associated with low vitamin B12 levels, especially in those with
risk factors for low vitamin B12 (e.g., diabetes, post bariatric / metabolic
surgery, pernicious anaemia, vegan diet etc.), where monitoring should be
considered.
4.4 Metformin and combined oral contraceptive pills
4.4.1 EBR COCP could be used over metformin for management of hirsutism in
irregular menstrual cycles in PCOS.
❖❖❖
4
4.4.2 EBR Metformin could be used over COCP for metabolic indications in PCOS. ❖❖❖
4
4.4.3 EBR The combination of COCP and metformin could be considered to offer
little additional clinical benefit over COCP or metformin alone, in
adults with PCOS with a BMI % 30 kg/m
2
.
❖❖❖
4
4.4.4. PP In combination with the COCP, metformin may be most beneficial in high
metabolic risk groups including those with a BMI >30 kg/m
2
, diabetes
risk factors, impaired glucose tolerance or high-risk ethnic groups.
4.4.5 PP Where COCP is contraindicated, not accepted or not tolerated, metformin
may be considered for irregular menstrual cycles. For hirsutism, other
interventions may be needed.
4.5 Anti-obesity pharmacological agents
4.5.1 CR Anti-obesity medications including liraglutide, semaglutide, both glucagon-
like peptide-1 (GLP-1) receptor agonists and orlistat, could be considered,
in addition to active lifestyle intervention, for the management of higher
weight in adults with PCOS as per general population guidelines.
❖❖❖
4.5.2 PP Healthcare professionals should ensure concurrent effective contraception
when pregnancy is possible for women who take GLP-1 receptor
agonists, as pregnancy safety data are lacking.
4.5.3 PP Gradual dose escalation for GLP-1 receptor agonists is recommended to
reduce gastrointestinal adverse effects.
4.5.4 PP Shared decision making, when discussing GLP-1 receptor agonist use with
women with PCOS, needs to consider side effects, and the potential need
for long-term use in weight management, given the high risk for weight
regain after discontinuation, and the lack of long-term safety data.
4.6 Anti-androgen pharmacological agents
VOL. - NO. - / - 2023 15
Fertility and Sterility®

TABLE 4
Continued.
4.6.1 EBR In combination with effective contraception, anti-androgens could be
considered to treat hirsutism in women with PCOS, if there is a
suboptimal response after a minimum of six months of COCP and/or
cosmetic therapy.
❖❖❖
4
4.6.2 CR Given the negative psychological impact of female pattern hair loss, anti-
androgens in combination with COCP could be trialed, acknowledging
the lack of evidence in the PCOS population.
❖❖❖
4.6.3 PP Whenever pregnancy is possible, healthcare professionals must educate and
counsel women and adolescents, parents/s or guardian/s, regarding the
risks of incomplete development of external genital structures of male
fetuses (undervirilization) when anti-androgens are used. To prevent this,
women who can get pregnant should be strongly counselled to use
effective contraception (e.g., intrauterine device or COCPs).
4.6.4 PP Anti-androgens could be considered to treat hirsutism, in the presence of
another effective form of contraception, for women with
contraindications for COCP therapy or when COCPs are poorly tolerated.
4.6.5 PP
When prescribing anti-androgens, based on general population
recommendations, healthcare professionals should consider that:
Spironolactone at 25-100mg / day appears to have lower risks of adverse
effects.
Cyproterone acetate at doses R 10mg is not advised due to an increased
risk including for meningioma.
Finasteride has an increased risk of liver toxicity.
Flutamide and bicalutamide have an increased risk of severe liver toxicity.
The relatively limited evidence on anti-androgens in PCOS needs to be
appreciated with small numbers of studies and limited numbers of
participants.
4.7 Inositol
4.7.1 EBR Inositol (in any form) could be considered in women with PCOS based on
individual preferences and values, noting limited harm, potential for
improvement in metabolic measures, yet with limited clinical benefits
including in ovulation, hirsutism or weight.
❖❖❖
4
4.7.2 EBR Metformin should be considered over inositol for hirsutism and central
adiposity, noting that metformin has more gastrointestinal side effects
than inositol.
❖❖❖
4
4.7.3 PP Women taking inositol and other complementary therapies are encouraged
to advise their healthcare professional.
4.7.4 PP Specific types, doses or combinations of inositol cannot currently be
recommended in adults and adolescents with PCOS, due to a lack of
quality evidence.
4.7.5 PP Shared decision making should include discussion that regulatory status and
quality control of inositol in any form (like other nutrient supplements) can
differ from those for pharmacological products and doses and qualities
may vary.
4.7.6 PP Policy makers and healthcare professionals have a responsibility to ensure
women have access to unconflicted, evidence-based information to
inform shared-decision making, whilst also acknowledging and
respecting individual values and preferences, including for
complementary therapies.
4.8 Mechanical laser and light therapies for hair reduction
4.8.1 EBR Mechanical laser and light therapies should be considered for reducing facial
hirsutism and for related depression, anxiety, and quality of life in women
with PCOS.
❖❖❖
4
4.8.2 EBR A greater number of laser treatment sessions may be required in women with
PCOS, compared to women with idiopathic hirsutism, to achieve hair
reduction.
❖❖❖
4
4.8.3 CR Adverse effects appear limited in the hands of experienced and suitably
qualified providers, and women should be encouraged to seek hair
reduction therapies from such providers.
❖❖❖❖
4.8.4 PP Where laser hair removal is prescribed, the following need to be considered:
Wavelength and delivery of laser treatment varies by skin and hair colour.
Laser is relatively ineffective in women with blond, grey or white hair.
The addition of combined oral contraceptive pills (COCP), with or without
anti-androgens, to laser treatment may provide greater hair reduction and
maintenance compared to laser alone.
Low and high fluence laser appear to have similar efficacy in reducing facial
hair, while low fluence laser has reduced associated pain.
16 VOL. - NO. - / - 2023

TABLE 4
Continued.
4.8.5 PP Mechanical hair removal with Intense Pulse Light (IPL) could be considered,
albeit benefits may be less pronounced compared to laser treatment.
There is no evidence to support the efficacy of home-based IPL kits.
4.8.6 PP Policy makers should consider funding this evidence-based effective therapy
for women with PCOS to alleviate distressing symptoms of hirsutism, and
related negative impact on quality of life, body image, and psychological
health.
4.9 Bariatric/ metabolic surgery
4.9.1 CR Bariatric/ metabolic surgery could be considered to improve weight loss,
hypertension, diabetes (prevention and treatment), hirsutism, irregular
menstrual cycles, ovulation, and pregnancy rates in women with PCOS.
❖❖❖
4.9.2 CR Bariatric/ metabolic surgery in women with PCOS should be informed by
general population guidelines.
❖❖❖❖
4.9.3 CR PCOS is a metabolic condition and could be considered an indication at a
lower BMI threshold for bariatric/ metabolic surgery similarly to other
metabolic conditions including diabetes.
❖❖❖
4.9.4 CR Women should be strongly counselled on the likelihood of rapid return of
fertility and the need to commit to effective contraception, ideally prior to
surgery. Even when pregnancy is desired, contraception should be
continued until a stable weight is achieved, usually after one year, to avoid
significantly increased risk of growth restriction, prematurity, small for
gestational age, pregnancy complications, and prolonged hospitalization
of the infant.
❖❖❖❖
4.10 Pregnancy outcomes
4.10.1 EBR Women with PCOS have higher risk pregnancies, and healthcare
professionals should ensure that PCOS status is identified during
antenatal care, and appropriate monitoring and support is provided.
❖❖❖❖
4
4.10.2 EBR
Healthcare professionals should recognize that pregnant women with PCOS
have an increased risk of:
Higher gestational weight gain.
Miscarriage.
Gestational diabetes.
Hypertension in pregnancy and preeclampsia.
Intrauterine growth restriction, small for gestational age babies and low
birth weight.
Preterm delivery.
Caesarean section.
❖❖❖❖
4
4.10.3 EBR Assisted reproductive technology in women with PCOS should be considered
as not conferring additional risk of miscarriage, preterm birth, impaired
fetal growth, and caesarean section, over that observed in women
without PCOS.
❖❖❖
4
4.10.4 EBR Women with PCOS should be considered as not having an increased risk of
large for gestational age babies, macrosomia and instrumental delivery.
❖❖❖
4
4.10.5 PP Early lifestyle intervention should be offered to pregnant women with PCOS,
given the risk of higher baseline weight, excess gestational weight gain
and pregnancy complications.
4.10.6 PP Blood pressure measurement should be performed when planning
pregnancy or seeking fertility treatment, given the high risk of
hypertensive disorders in pregnancy and the associated comorbidities in
women with PCOS.
4.10.7 PP An OGTT should be offered to all women with PCOS when planning
pregnancy or seeking fertility treatment, given the high risk of
hyperglycaemia and the associated comorbidities in pregnancy. If not
performed in the preconception phase, an OGTT should be offered at the
first antenatal visit and repeated at 24-28 weeks gestation.
4.11 Metformin in pregnancy
4.11.1 EBR
Healthcare professionals should be aware that metformin in preg-
nant women with PCOS has not been shown to prevent:
Gestational diabetes.
Late miscarriage (12 weeksþ1 day to 21 weeks þ6 days gesta-
tional age).
Hypertension in pregnancy.
Pre-eclampsia.
Macrosomia or birthweight R 4000 g.
❖❖❖❖
44
VOL. - NO. - / - 2023 17
Fertility and Sterility®

TABLE 4
Continued.
4.11.2 EBR Metformin could be considered in some circumstances (e.g., risk for
preterm birth) to reduce preterm delivery and limit excess
gestational weight gain, in pregnant women with PCOS.
❖❖❖
444
4.11.3 PP Women should be counselled that the consequences of metformin
exposure on long-term offspring health remain unclear and there
is a suggestion of increased childhood weight, although causality
is not certain.
4.11.4 PP Side effects of metformin are mostly mild, transient gastrointestinal
symptoms and are not worse in pregnancy.
5 Assessment and treatment of infertility
General principles
PP All fertility treatment in PCOS should be guided by the fertility treatment
algorithm (Algorithm 2).
PP Those with PCOS should be reassured that pregnancy can often be
successfully achieved either naturally or with assistance.
PP Prenatal vitamins supplementation should be commenced with ovulation
induction therapy aligned to routine preconception care.
PP Pregnancy should be excluded prior to ovulation induction therapy.
PP The use of ovulation induction agents, including letrozole, metformin and
clomiphene citrate, is off-label in many countries. Where off-label use of
ovulation induction agents is allowed, healthcare professionals need to
inform women and discuss the evidence, possible concerns, and side
effects.
PP There should be ongoing monitoring of patients for adverse effects, and
infants for congenital anomalies, in all studies conducted with ovulation
induction agents and these should be reported in any published papers.
5.1 Preconception risk factors
5.1.1 EBR Women with PCOS should be counselled on the adverse impact of excess
weight on clinical pregnancy, miscarriage, and live birth rates, following
infertility treatment.
❖❖❖❖
4
5.1.2 CR Consistent with routine preconception care, in women with PCOS planning
pregnancy, weight, blood pressure, smoking, alcohol, diet and nutritional
status, folate supplementation (higher dose in those with BMI >30 kg/
m2), exercise, sleep and mental, emotional and sexual health should be
considered and optimized to improve reproductive and pregnancy
outcomes and overall health.
❖❖❖❖
5.1.3 PP A reproductive life plan and age-appropriate education on optimizing
reproductive health is recommended in adolescents and women with
PCOS, including healthy lifestyle, prevention of excess weight gain, and
optimizing preconception risk factors.
5.1.4 PP Healthcare professionals are encouraged to seek permission and, if given, to
assess weight and BMI and initiate a dialogue on the importance of
weight and lifestyle on women's health before pregnancy. This requires
caution to avoid weight stigma and needs to consider the cultural, social,
and environmental determinants of health (see 3.6).
5.1.5 PP Chronic conditions such as diabetes, high blood pressure, anxiety, depression
and other mental health conditions, should be optimally managed and
women should be counselled regarding the risk of adverse pregnancy
outcomes.
5.2 Tubal patency testing
5.2.1 CR In women with PCOS and infertility due to anovulation alone with normal
semen analysis, the risks, benefits, costs and timing and techniques of
tubal patency testing in relation to the cost and complexity of the
treatment, should be considered on an individual basis, depending on
personal history and population prevalence, prior to starting ovulation
induction with timed intercourse or intrauterine insemination.
❖❖❖
5.3 Letrozole
5.3.1 EBR Letrozole should be the first-line pharmacological treatment for ovulation
induction in infertile anovulatory women with PCOS, with no other
infertility factors.
❖❖❖❖
4444
5.3.2 PP The use of letrozole is still off- label in many countries.
Where it is not allowed, clinicians should use other ovulation induction
agents.
5.3.3 PP Letrozole should not be given where there is any possibility of a pre-existing
pregnancy, though there is no evidence for increased teratogenicity
compared to other ovulation induction agents.
18 VOL. - NO. - / - 2023

TABLE 4
Continued.
5.4 Clomiphene citrate and metformin
5.4.1 Metformin versus placebo
5.4.1.1 EBR Metformin could be used alone, in women with PCOS with anovulatory
infertility and no other infertility factors, to improve clinical pregnancy and
live birth rates, whilst informing women that there are more effective
ovulation agents.
❖❖❖
44
5.4.1.2 PP Women should be counselled as to potential mild gastrointestinal side-effects
with metformin.
5.4.1.3 PP Healthcare and resource burden including monitoring, travel and costs are
lower with metformin.
5.4.1.4 PP Consideration of age and screening for other fertility factors needs to be
discussed before prescribing metformin.
5.4.2 Clomiphene citrate verses metformin
5.4.2.1 EBR Clomiphene citrate could be used in preference to metformin in women with
PCOS with anovulatory infertility and no other infertility factors, to
improve ovulation, clinical pregnancy and live birth rates.
❖❖❖
44
5.4.2.2 PP The risk of multiple pregnancy is increased with clomiphene citrate use (alone
or in combination with metformin) and therefore clomiphene cycles may
require ultrasound monitoring.
5.4.3 Clomiphene citrate and metformin verses clomiphene citrate alone
5.4.3.1 EBR Clomiphene citrate combined with metformin could be used rather than
clomiphene citrate alone in women with PCOS with anovulatory infertility
and no other infertility factors to improve ovulation and clinical pregnancy
rates.
❖❖❖
44
5.4.4 Clomiphene citrate and metformin versus metformin alone
5.4.4.1 EBR Clomiphene citrate combined with metformin could be used rather than
metformin alone in women with PCOS with anovulatory infertility and no
other infertility factors to improve live birth rates.
❖❖❖
44
5.4.4.2 PP Monitoring of combined cycles will need to be equivalent to clomiphene
citrate alone.
5.4.5 Clomiphene citrate versus Letrozole
5.4.5.1 EBR Letrozole should be used rather than clomiphene citrate in women with PCOS
with anovulatory infertility and no other infertility factors to improve
ovulation, clinical pregnancy and live birth rates.
❖❖❖❖
4
5.4.5.2 PP Current evidence demonstrates no difference in fetal abnormality rates
between letrozole or clomiphene citrate ovulation induction or natural
conception.
5.5 Gonadotrophins
5.5.1 EBR Gonadotrophins alone could be considered rather than clomiphene citrate in
therapy naïve women with PCOS with anovulatory infertility and no other
infertility factors to improve ovulation, clinical pregnancy and live birth
rates (refer to PP 5.5.6).
❖❖❖
44
5.5.2 EBR Gonadotrophins alone could be used over gonadotrophins combined with
clomiphene citrate in women with PCOS who are anovulatory and infertile
with clomiphene citrate resistance or failure, and no other infertility
factors.
❖❖❖
44
5.5.3 EBR Gonadotrophins could be considered rather than the combination of
clomiphene citrate and metformin in women with PCOS who are
anovulatory and infertile, with clomiphene citrate-resistance and no other
infertility factors.
❖❖❖
4
5.5.4 EBR Either gonadotrophins or laparoscopic ovarian surgery could be used in
women with PCOS who are anovulatory and infertile, with clomiphene
citrate-resistance and no other infertility factors, following counselling on
higher live birth rate and higher multiple pregnancy rates with
gonadotrophins.
❖❖
44
5.5.5 EBR Gonadotrophins could be second-line pharmacological therapy for women
with PCOS who are anovulatory and infertile, with no other infertility
factors and who have failed first line oral ovulation induction.
❖❖❖
44
5.5.6 PP Where gonadotrophins are to be prescribed, the following should be
considered:
Cost of the intervention for ovulation induction.
Expertise required for the use of the intervention for ovulation induction.
The degree of intensive ultrasound monitoring that is required.
A low dose step-up gonadotrophin protocol should be used to optimize the
chance of monofollicular development.
Implications of potential multiple pregnancy.
5.5.7 PP There appears to be no difference in the clinical efficacy of the available
gonadotrophin preparations.
VOL. - NO. - / - 2023 19
Fertility and Sterility®

TABLE 4
Continued.
5.5.8 PP When using gonadotrophins, best clinical practice is to avoid multiple
pregnancy. Considerations here include cancelling cycles when there is
more than a total of two follicles greater than 14mm in diameter and
advising avoidance of unprotected intercourse.
5.5.9 PP Live birth rate, clinical pregnancy rate per patient and ovulation rate per cycle
are higher with gonadotrophins than with clomiphene citrate.
5.5.10 PP A low dose gonadotrophin protocol should be used to optimize the chance of
monofollicular growth and minimize multiple pregnancy.
5.5.11 PP Cycle monitoring and drug costs coupled with multiple injection will influence
choice in gonadotrophin use.
5.6 Laparoscopic ovarian surgery
5.6.1 EBR Laparoscopic ovarian surgery could be second-line therapy for women with
PCOS who are anovulatory and infertile, with clomiphene citrate
resistance and no other infertility factors.
❖❖❖
44
5.6.2 PP When using laparoscopic ovarian surgery, the following should be
considered:
Comparative cost of the intervention for ovulation induction.
Expertise required for the safe use of the intervention for ovulation
induction.
Both intraoperative and postoperative risks, which are higher in women
who are above healthy weight.
5.7 In vitro fertilization and in vitro maturation
5.7.0.1 CR In the absence of an absolute indication for in vitro fertilization (IVF)/
intracytoplasmic sperm injection (ICSI), IVF could be offered in women
with PCOS and anovulatory infertility, if first- or second-line ovulation
induction therapies have failed.
❖❖❖
5.7.0.2 PP In women with anovulatory PCOS, the use of IVF is effective and when elective
single embryo transfer is used, multiple pregnancies can be minimized.
5.7.0.3 PP Women with PCOS undergoing IVF/ICSI treatment should be counselled prior
to starting treatment about the increased risk of ovarian hyperstimulation
syndrome and options to reduce the risk should be offered.
5.7.1 Gonadotrophin releasing hormone protocol
5.7.1.1 PP Gonadotrophin releasing hormone (GnRH) antagonist protocol cannot be
recommended over GnRH agonist long protocol for women with PCOS
undergoing IVF/ICSI to improve clinical pregnancy or live birth rate.
5.7.1.2 PP The use of a GnRH antagonist protocol for women with PCOS undergoing
IVF/ICSI is recommended as it enables the use of an agonist trigger, with
the freezing of all embryos generated if required, without compromising
the cumulative live birth rate, to reduce the risk of significant ovarian
hyperstimulation syndrome.
5.7.2 Trigger type
5.7.2.1 CR Triggering final oocyte maturation with a GnRH agonist and freezing all
suitable embryos is recommended, in an IVF/ICSI cycle with a GnRH
antagonist protocol, where a fresh embryo transfer is not intended or
where there is an increased risk of ovarian hyperstimulation syndrome.
❖❖❖❖
5.7.3 Choice of follicle stimulating hormone
5.7.3.1 CR Either urinary or recombinant follicle stimulating hormone (FSH) could be
used in women with PCOS undergoing (controlled) ovarian (hyper)
stimulation for IVF/ICSI, with insufficient evidence to recommend a
particular type of FSH preparation.
❖❖❖
5.7.4 Exogenous luteinising hormone
5.7.4.1 CR Exogenous recombinant luteinising hormone (LH) treatment should not be
routinely used in combination with FSH therapy in women with PCOS
undergoing controlled ovarian hyperstimulation for IVF/ ICSI.
❖
5.7.5 Adjunct metformin
5.7.5.1 EBR Adjunct metformin therapy could be used before and/or during FSH ovarian
stimulation in women with PCOS undergoing IVF/ICSI treatment with
GnRH agonist long protocol, to reduce the risk of developing ovarian
hyperstimulation syndrome and miscarriage.
❖❖❖
⨁⨁
5.7.5.2 PP Good practice in PCOS and IVF is the use of a GnRH antagonist protocol as it
gives the flexibility of using a GnRH agonist trigger, freeze all strategy to
reduce the risk of ovarian hyperstimulation syndrome. However, if using a
GnRH agonist long protocol then metformin could be considered.
If using metformin, the following could be considered:
Commence metformin at the start of GnRH agonist treatment.
Gradually titrate metformin up to a dose of between 1000mg to 2500mg
daily in order to minimize side effects.
Stopping metformin therapy at the time of the pregnancy test or period,
unless the metformin therapy is otherwise indicated.
5.7.6 In vitro maturation
20 VOL. - NO. - / - 2023

FIGURE 1
Step 1: Irregular cycles + clinical hyperandrogenism
(exclude other causes)* = diagnosis
Step 2: If no clinical hyperandrogenism
Test for biochemical hyperandrogenism (exclude other causes)* = diagnosis
Step 3: If ONLY irregular cycles OR hyperandrogenism
Adolescents ultrasound is not indicated = consider at risk of PCOS and reassess later
Adults - request ultrasound for PCOM*, if posive (exclude other causes)* = diagnosis
* Exclusion of other causes = TSH, prolacn, 17-OH progesterone, FSH or if clinically indicated
exclude other causes (e.g. Cushing's Syndrome, adrenal tumours). For hypogonadotrophic
hypogonadism, usually due to low body fat or intensive exercise, exclude clinically and with LH/ FSH.
PCOM = polycysc ovarian morphology on ultrasound
Algorithm 1 - Diagnostic algorithm for polycystic ovary syndrome (PCOS). ª Monash University on behalf of the NHMRC Centre for Research
Excellence in Women's Health in Reproductive Life, 2023.
ª International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023, Helena Teede et al. Monash
University (monash.edu/medicine/mchri/pcos), 2023, by permission of Monash University, on behalf of the NHMRC Centre for Research
Excellence in Women’s Health in Reproductive Life. This image/content is not covered by the terms of the Creative Commons licence of this
publication. For permission re reuse, please contact the rights holder.
*Exclusion of other causes ¼ TSH, prolactin, 17-OH progesterone, FSH or if clinically indicated exclude other causes (e.g. Cushing’s syndrome,
adrenal tumours). For hypogonadotrophic hypogonadism, usually due to low body fat or intensive exercise, exclude clinically and with LH and
FSH levels. TSH, thyroid stimulating hormone.
Teede. International PCOS Guideline 2023. Fertil Steril 2023.
TABLE 4
Continued.
5.7.6.1 EBR The use of in vitro maturation (IVM) and ICSI) could be considered in women
with PCOS as an alternative to a stimulated IVF / ICSI cycle, where an
embryo is frozen and replaced in a subsequent embryo transfer cycle,
acknowledging there is no risk of ovarian hyperstimulation syndrome, but
a lower cumulative live birth rate.
❖❖
⨁⨁⨁
5.7.6.2 CR The use of IVM and ICSI could be considered prior to stimulated IVF/ ICSI
cycles acknowledging both benefits and limitations.
❖❖
5.7.6.3 PP IVM should only be considered in services with sufficient expertise, and
advocacy is needed for regional or national centres of expertise.
5.7.6.4 PP IVM could be offered as an option in women with prior severe ovarian
hyperstimulation syndrome and where the risk of severe ovarian
hyperstimulation syndrome is deemed unacceptably high, provided that
expertise in IVM techniques exists.
5.7.6.5 PP Evidence suggests that IVM/ ICSI is less effective than standard IVF/ICSI in
terms of clinical pregnancy per patient and live birth rate per patient.
5.8 Inositol
5.8.1 EBR Inositol in any form alone, or in combination with other therapies, should be
considered experimental therapy in women with PCOS with infertility,
with benefits and risks currently too uncertain to recommend the use of
these agents as fertility therapies.
❖❖❖
⨁
5.8.2 PP There is limited evidence with uncertain results, on the effect of inositol on
ovulation, clinical pregnancy and live birth rates.
5.8.3 PP Side effects and safety are not known for inositol.
5.8.4 PP Women need to be aware that these agents can have limited regulation with
variable dose, quality, consistency, and combination with other agents.
5.8.5 PP Women’s personal goals and preferences should be considered when
discussing complimentary therapies.
5.9 Anti-obesity pharmacological agents
5.9.1 CR We recommend using anti-obesity agents in PCOS for reproductive outcomes
only in research settings to establish the efficacy and safety.
See Table 1 for definition of CR, EBR and PP.
ª International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023, Helena Teede et al. Monash University (monash.edu/medicine/mchri/pcos), 2023,
by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in Women’s Health in Reproductive Life. This image/content is not covered by the terms of the Creative
Commons licence of this publication. For permission re reuse, please contact the rights holder.
Teede. International PCOS Guideline 2023. Fertil Steril 2023.
VOL. - NO. - / - 2023 21
Fertility and Sterility®
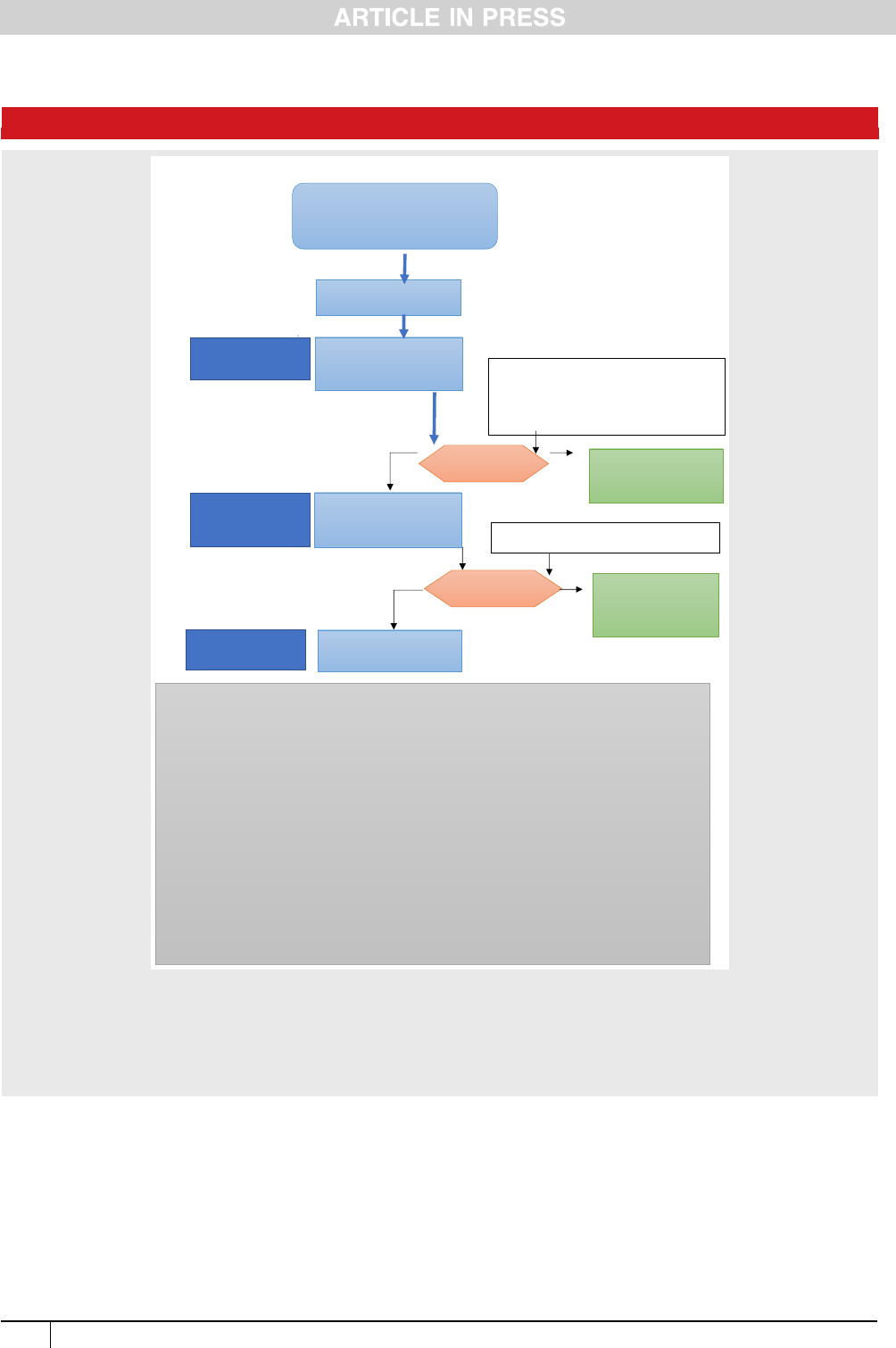
considerations (6000 pages). The evidence has generally
improved over the past five years but remains of low to moder-
ate quality, requiring significant research investment into this
neglected, yet common condition.
Key recommendations and updates include that PCOS
should be diagnosed using the 2018 International Evidence-
based Guideline criteria, which built on the consensus based
2003 Rotterdam criteria. This requires the presence of two
of the following: i) clinical / biochemical hyperandrogenism;
ii) ovulatory dysfunction; and iii) polycystic ovaries on ultra-
sound; and here in 2023, alternatively anti-M
€
ullerian hor-
mone (AMH) can now be used instead of ultrasound, with
the exclusion of other aetiologies. Importantly, where irreg-
ular menstrual cycles and hyperandrogenism are present,
diagnosis is simplified and ultrasound or AMH are not
required for diagnosis. In adolescents, both hyperandrogen-
ism and ovulatory dysfunction are required, with ultrasound
and AMH not recommended due to poor specificity. AMH was
FIGURE 2
\
Letrozole
c
, increasing
dose as required
Central Blue Pathway follows best practice evidence and is preferred
Polycystic Ovary Syndrome
Diagnosis: International Guideline
criteria, building on Rotterdam criteria
Full baseline investigations
a
Ovulation
detected?
a. Baseline investigations (see narrative):
i. Diagnosis of PCOS - Endocrine profile and pelvic ultrasound scan
ii. Assessment of BMI, BP & glycemic status (OGTT / HbA1c)
iii. Routine preconception assessments (Rubella immunity, infection screen etc..), advice
and supplementation.
iv. Additional investigations: semen analysis and consider tubal patency assessment
b. Healthy lifestyle encompassing healthy eating and regular physical activity should be
recommended in all those with PCOS to limit adverse impacts on fertility and fertility
treatment outcomes and to optimize health during pregnancy
c. Off label prescribing: Letrozole, metformin and other pharmacological treatments are
generally off label in PCOS, as pharmaceutical companies have not applied for approval in
this condition. However, recommended off label use is evidence-based and allowed in
many countries. Where it is allowed, health professionals should inform women and discuss
the evidence, possible concerns and side effects of treatment.
d. Compared to letrozole, metformin has lower efficacy, cost and multiple pregnancy rate and
gonadotrophins have higher efficacy, cost and multiple pregnancy rate. Both may be an
alternative first line choice for informed women.
e. In vitro fertilization (IVF) - Third line unless other infertility factors (e.g. male, tubal). PCOS
specific protocols to minimise risk of ovarian hyperstimulation syndrome, consider invitro
maturation if available.
Yes
Repeated cycles– shared
decision-making
considering age and
resources then to IVF
No
No
Gonadotrophins
c
with
US monitoring. Adjusting
dose as required.
In vitro fertilization
e
Can consider Laparoscopic ovarian surgery,
noting need for facilities and experience
Can consider with full explanation of risks, benefits,
efficacy and costs: Clomiphene citrate + metformin
(preferred to clomiphene alone), clomiphene,
metformin (low cost/low efficacy/no monitoring) or
gonadotrophins (high cost/ multiple pregnancy/
high efficacy/ monitoring)
c,d
Second line
Medical
Treatment
Ovulation
detected?
Repeated cycles –
shared decision-making
considering age and
resources then to IVF
Yes
First line Medical
Treatment
Third line Medical
Treatment
Optimise preconception
health and lifestyle
b
OR
OR
Algorithm 2 - Infertility algorithm for polycystic ovary syndrome (PCOS). ª Monash University on behalf of the NHMRC Centre for Research
Excellence in Women's Health in Reproductive Life, 2023.
ª International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023, Helena Teede et al. Monash
University (monash.edu/medicine/mchri/pcos), 2023, by permission of Monash University, on behalf of the NHMRC Centre for Research
Excellence in Women’s Health in Reproductive Life. This image/content is not covered by the terms of the Creative Commons licence of this
publication. For permission re reuse, please contact the rights holder.
Teede. International PCOS Guideline 2023. Fertil Steril 2023.
22 VOL. - NO. - / - 2023

highlighted as a rapidly evolving area in 2018 and evidence is
now strong enough to make this new recommendation. This
will significantly change practice and offers women a low
cost, convenient option, without evidence of overdiagnosis.
Insulin re sistance is recognized as a key feature of
PCOS, yet routinely available measures of insulin resi stance
are inaccurate and clini cal measurement is not currently
recommended. Once diagnosed, assessment and manage-
ment should address reproductive, m etabolic, cardiova scu-
lar, dermatologic, sleep, and psychologic al features. A
lifelong health pla n i s re commended including a f ocus o n
healthy lifestyle, prevention of excess wei ght gain, optim i-
zation of fertility and p reconception risk factors, and pre-
vention and treatment of diverse clini cal features. These
include metabolic risk factors, diabetes, c ardiovascula r di s-
ease, and sleep disorders, which are all increased in PCOS.
PCOS should be c onsidered a high-risk condition i n p reg-
nancy with women identifie d and monitored. A n increased
premenopausal r isk of endo metrial cancer should also be
recognized, whilst absolute risks remain low .
Symptoms of depression and anxiety are significantly
increased and should be screened for in all women with PCOS,
with psychological assessment and therapy as indicated. Greater
awareness of psychological features including eating disorde rs
and impacts on body image and quality of life is needed.
Dissatisfaction with PCOS diagnosis and care is high and
significant improvement in education and awareness is
strongly recommended for women and healthcare profes-
sionals including high quality, evidence-based resources.
Shared decision making and self-empowerment are funda-
mental and integrated models of care should be codesigned,
funded and evaluated.
Supported healthy lifestyle remains vital throughout the
lifespan in PCOS, with a strong focus on overall health, preven-
tion of weight gain and, if required, on weight management.
Recognizing the benefits of many diet and physical activity
regimens, there is no one specific regimen that has benefits
over others in PCOS. Weight bias and stigma should be mini-
mized and healthcare professionals should seek permission to
weigh women, with explanation of weight-related risks.
Combined oral contraceptive pills are the first line phar-
macological treatment for menstrual irregularity and hyper-
androgenism, with no specific recommended preparation
and a preference for lower ethinyl estradiol dose preparations
and those with less side-effects. Metformin is recommended
primarily for metabolic features and has greater efficacy
than inositol, which offers limited clinical benefits in PCOS.
Metformin is not routinely recommended for use in pregnant
women with PCOS. Mechanical laser therapy is effective for
hair reduction in some subgroups, whilst anti-androgens
have a limited role where other therapies are ineffective or
contraindicated. Anti-obesity agents and bariatric/ metabolic
surgery may be considered based on general population
guidelines, balancing potential for benefits and side effects.
Letrozole is the preferred first line pharmacological
infertility therapy, with clomiphene in combination
with metformin; gonadotrophins or ovarian surgery pri-
marily having a role as second line therapy. In vitro
fertilization (IVF) could be offered, potentially with
in vitro maturation, as third line therapy, where other
ovulation induction therapies have failed and in the
absence of an absolute indication for IVF in women
with PCOS and anovulatory infertility. Given the under-
lying risk for pregnancy complications in PCOS, single
embryo transfer should be preferred.
Overall, evidence in PCOS is low to moderate quality.
Based on high prevalence and significant health impact,
greater priority, education, models of care, funding, and
research are recommended. Guideline translation will be
extensive including multilingual education outputs and
evidence-based resources for consumers (the ASKPCOS
app), healthcare professionals and policy makers.
The guideline recommendations are protected under copy-
right, however a clear process for adaption of guideline recom-
mendations to regional context is available by contacting the
author for correspondence online (www.monash.edu/
medicine/mchri/pcos). The translation program will be free
and internationally accessible, building on the existing range
of codesigned resources including the patient focused,
evidence-bas ed PCOS APP (AskPCOS), used in 186 countries
and based on a rigorously developed question prompt list.
Multi-faceted patient codesigned resources will aim to enhance
health literacy with comprehensive PCOS-related health infor-
mation available in multiple formats and in 15-20 languages.
Internationally accessible resources include education modules
for healthcare professionals at different career stages and disci-
plines, healthcare professional accredit ed courses, practice re-
sources and tools, webinars with international expert panels,
and e-health information resources that will be available online
(www.monash.edu/medicine/mchri/pcos). Importantly, the
Guideline and translation of the Guideline is expected to
improve patient experiences through the provision of timely
and accurate diagnosis, of accessible evidence-based informa-
tion and of improved multi-discipl inar y support. Ultimately,
this international initiative may serve as an exemplar for large
scale collaborative engagement, pooling of resources, avoidance
of duplication and inconsistency with consensus-based state-
ments, and codesign of best quality consistent guidelines with
processes for local adaption and healthcare impact. Key elements
include extensive collaboration, broad stakeholder representa-
tion, consumer partn ership, distributive leadership, adequate
funding, robust project management and governance, adher-
ence to best practice and integrated comprehensive translation,
and evaluation. We sincerely thank the partner and collabo-
rating organizations, consumer groups and members of the
GDGs for their substantive commitment to the international
partnership to optimize health outcomes for women with this
common, heterogeneous, and much neglected condition.
DATA AVAILABILITY
All data extracted and analyzed in the guideline is available in
a repository and can be accessed via https://doi.org/10.
26180/23625288.v1
Conflict of Interest: Disclosures of interest were declared at
the outset and updated throughout the guideline process,
aligned with National Health Medical Research Council
(NHMRC) guideline processes. These are available online
VOL. - NO. - / - 2023 23
Fertility and Sterility®

(www.monash.edu/medicine/mchri/pcos). Of named authors
HJT, CTT, AD, LM, LR, JBoyle, AM have no conflicts of inter-
est to declare. JL declares grant from Ferring and Merck;
consulting fees from Ferring and Titus Health Care; speaker’s
fees from Ferring; unpaid consultancy for Ferring, Roche Di-
agnostics and Ansh Labs; and sits on advisory boards for Fer-
ring, Roche Diagnostics, Ansh Labs, and Gedeon Richter. TP
declares a grant from Roche; consulting fees from Gedeon
Richter and Organon; speaker’s fees from Gedeon Richter
and Exeltis; travel support from Gedeon Richter and Exeltis;
unpaid consultancy for Roche Diagnostics; and sits on advi-
sory boards for Roche Diagnostics. MC declares travels sup-
port from Merck; and sits on an advisory board for Merck.
JBoivin declares grants from Merck Serono Ltd.; consulting
fees from Ferring B.V; speaker’s fees from Ferring Arzneimit-
tell GmbH; travel support from Organon; and sits on an advi-
sory board for the Office of Health Economics. RJN has
received speaker’s fees from Merck and sits on an advisory
board for Ferring. AJoham has received speaker’s fees from
Novo Nordisk and Boehringer Ingelheim.
Acknowledgements: We gratefully acknowledge contribu-
tion of our partners and collaborating organizations:
The Australian National Health and Medical Research
Council (NHMRC) Centre for Research Excellence in Women’s
Health in Reproductive Life (CRE WHiRL) (APP1171592),
Centre for Research Excellence in Polycystic Ovary Syndrome
(CRE PCOS) (APP1078444) and the members of these Centres
who coordinated this international guideline effort.
Our partner and co-funding organizations are:
American Society for Reproductive Medicine (ASRM)
Endocrine Society (ENDO)
European Society for Endocrinology (ESE)
European Society of Human Reproduction and Embry-
ology (ESHRE)
Collaborating and engaged societies and consumer
providing in-kind support include:
Androgen Excess and Polycystic Ovary Syndrome Soci-
ety (AEPCOS)
Asia Pacific Paediatric Endocrine Society (APPES)
Asia Pacific Initiative on Reproduction (ASPIRE)
Australia and New Zealand Society for Paediatric Endo-
crinology and Diabetes (ANZSPED)
Australian Diabetes Society (ADS)
Brazilian Society of Endocrinology and Metabolism (SBEM)
British Fertility Society (BFS)
Canadian Society of Endocrinology and Metabolism (CSEM)
Dietitians Association Australia (DA)
Endocrine Society Australia (ESA)
European Society for Paediatric Endocrinology (ESPE)
Exercise and Sports Science Aust ralia (ESSA)
Fertility Society Australia and New Zealand (FSA)
International Federation of Fertility Societies (IFFS)
International Federation of Gynecology and Obstetrics
(FIGO)
International Society of Endocrinology (ISE) - 40 partner so-
cieties
Italian Society of Gynaecology and Obstetrics
Japanese Society for Paediatric Endocrinology (JSPE)
Latin American Society for Paediatric Endocrinology (SLEP)
Nordic Federation of Societies of Obstetrics and Gynaecol-
ogy (NFOG)
PCOS Challenge Inc: The National Polycystic Ovary Syn-
drome Association
PCOS Society of India
PCOS Vitality
Paediatric Endocrine Society (PES)
Royal Australasian College of Physicians (RACP)
Royal Australian New Zealand College of Obstetricians and
Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Radiologists
(RANZCR)
Royal College of Obstetricians and Gynaecologists (RCOG)
South African Society of Gynaecology and Obstetrics (SA-
SOG)
Society for Endocrinology
Verity UK
Victorian Assisted Reproductive Technology Association
(VARTA)
Other relevant organizations are welcome to apply to
partner in guideline translation.
Authors’ Roles: HJT led the guidelines from funding,
engaging partners, coordinating processes, prioritizing clin-
ical questions, co-chairing guideline meetings, coordinating
peer review responses and leading writing, approval and pub-
lication processes. Listed authors held senior leadership roles
as chair or deputy chair of the five GDGs or leadership of the
evidence team with roles from the management committee,
chair/ co-chair of GDG or the early career evidence network,
involvement at all stages, responding to feedback, providing
input into and endorsing the guideline. All other included au-
thors were actively engaged as partner nominees and multi-
disciplinary GDG or consumer experts. The evidence
synthesis network was led by CTT AM, across search strate-
gies, training, Covidence processes, quality appraisal and
GRADE, meta-analysis, evidence integrity processes (with
BM) and preparing the technical report. The listed members
of this network led evidence synthesis across the clinical
questions and had input into the technical report.
Funding: The Australian National Health Medical Research
Council (NHMRC) (APP1171592) primarily funded this work.
The American Society for Reproductive Medicine, Endocrine So-
ciety, the European Society of Human Reproduction and Embry-
ology and the European Society for Endocrinology provided
partnership funding. Collaborating organizations provided in-
kind support. The Co mmonwealth Government of Australia
also supported Guideline Translation through the Medical
Research Future Fund (MRFCRI000266). HJT and AM are funded
by NHMRC fellowships and JT by an RACP fellowship.
REFERENCES
1. Teede H, Deeks A, M oran L. Polycystic ovary syndrome: a complex condition
with psychological, reproductive and metabolic manifestations that impacts
on health across the lifespan. BMC Med 2010;8:41.
24 VOL. - NO. - / - 2023

2. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nature reviews
Disease primers 2016;2:16057.
3. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack
of information associated with dissatisfaction in women with polycystic
ovary syndrome. J Clin Endo & Metab 2017;102:604-12.
4. Dokras A, Saini S, Gibson-Helm M, Schulkin J, Cooney L, Teede H. Gaps
in knowledge among physicians regarding diagnostic criteria and man-
agement of polycystic ovary syndrome. Fertil Steril 2017;107(6),
1380_6.e1.
5. Teede H, Gibson-Helm M, Norman RJ, Boyle J. Polycystic ovary syndrome:
perceptions and attitudes of women and primary health care physicians
on features of PCOS and renaming the syndrome. J Clin Endocrinol Metab
2014;99(1), E107_11 .
6. Teede J Helena, Marie Misso, Michael Costello, et al. International evidence-
based guideline for the assessment and management of polycystic ovary
syndrome; 2018, Available at www.monash.edu/medicine/mchri/pcos.
7. Teede HJ, Misso ML, C ostello MF, et a l. R ecommendations from the
international evidence-based guideline for the assessment and man-
agement of p olycystic ovary syndrome. Hum Reprod 2 018;33(9),
1602_18.
8. Misso ML, Teede HJ. Evidence based guideline (EBG) development: A prac-
tical guide in Knowledge Transfer: Practices, Types and Challenges. In: D I,
editor. Knowledge Transfer: Practices, Types and Challenges. New York:
Nova Science Publishers, Inc.; 2012:141–74 .
9. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline
development, reporting and evaluation in health care. CMAJ 2010;
182(18):E839–42.
10. Mousa A, Tay CT, Teede H. Technical Report for the International
Evidence-based Guideline f or the Assessm ent and Management of
Polycystic Ovary Syndrome. Monash University. 2023. Available at
https://doi.org/10.26180/23625288.v1
11. Weibel S, Popp M, Reis S, Skoetz N, Garner P, Sydenham E. Identifying and
managing problematic trials: A research integrity assessment tool for ran-
domized controlled trials in evidence synthesis. Research Synthesis Methods
2023;14(3):357–69.
12. Mol BW, Lai S, Rahim A, et al. Checklist to assess Trustworthiness in RAnd-
omised Controlled Trials (TRACT checklist): concept proposal and pilot.
Research Integrity and Peer Review 2023;8(1):6.
13. National Health and Medical Research Council. NHMRC levels of evidence
and grades for recommendations for developers of guidelines. Australia;
2009.
14. National Health and Medical Research Council. NHMRC standards
and procedures for externally developed guidelines. Australia;
2007.
15. GRADE working group. Grading of Recommendations Assessment, Devel-
opment and Evaluation (GRADE) guidelines.
APPENDIX
Members of the PCOS Network:
The inter national advisory panel, guideline technical
team, paediatric, c onsumer an d transl ation commit tees,
the Indigenous cultural advisor and the extended early
career support network who assisted with evi dence synthe-
sis, c an be foun d online (www.monash.edu/medicine/
mchri/pcos).
Guideline development members (in addition to listed
authors)
Wiebke Arlt, University of Birmingham, UK
Ricardo Azziz, University of Alabama at Birmingham,
USA
Adam Balen, Leeds Teaching Hospital; British Fertility
Society, UK
Lisa Bedson, Repromed, Australia
Lorna Berry, Polycystic Ovary Syndrome Association of
Australia, Australia
Jacky Boivin, Cardiff University, UK
Jacqueline Boyle, Monash University, Australia
Leah Brennan, Latrobe University, Australia
Wendy Brown, Monash University, Australia
Tania Burgert, University Missouri – Kansas School of
Medicine, USA
Maureen Busby, PCOS Vitality, Ireland
Carolyn Ee, Western Sydney University, Australia
Rhonda M. Garad, Monash University, Australia
Melanie Gibson-Helm, Te T
atai Hauora o Hine, Victoria
University of Wellington; NZ
Cheryce Harrison, Monash University, Australia
Roger Hart, The University of Western Australia; City
Fertility, Australia
Kim Hopkins, PCOS Challenge: National Polycystic Ovary
Syndrome Association, USA
Angelica Lind
en Hirschberg, Karolinska Institutet, Karo-
linska University Hospital, Sweden
Tuong Ho, HOPE Research Centre, My Duc Hospital, Viet-
nam
Kathleen Hoeger, University of Rochester, USA
Cailin Jordan, Genea Hollywood Fertility, Australia
Richard S. Legro, Penn State Clinical and Translational
Institute, USA
Rong Li, Peking University Third Hospital, China
Marla Lujan, Cornell University, USA
Ronald Ma, Chinese University of Hong Kong, Hong Kong
/ China
Darren Mansfield,1 Monash and Epworth Health, Monash
University, Australia
Kate Marsh, Northside Nutrition & Dietetics, Australia
Edgar Mocanu, Rotunda Hospital, Ireland
Ben Mol, Monash University, Australia
Rachel Mormon, Verity – PCOS Charity, UK
Robert Norman, University of Adelaide, Australia
Sharon Oberfield, Columbia University Medical Center, USA
Malika Patel, University of Cape Town; Groote Schuur Hos-
pital, South Africa
Loyal Pattuwage, Cochrane Australia, Monash University,
Australia
Alexia Pe
~
na, The Robinson Research Institute at the Univer-
sity of Adelaide, Australia
Leanne Redman, Pennington Biomedical Research Center,
USA
Luk Rombauts, Monash University, Australia
Daniela Romualdi, Fondazione Policlinico Universitario
Agostino Gemelli, Italy
Duru Shah, PCOS Society of India; Cen tre for Women’s
Health and Fertility, India
Poli Mara Spritzer, Federal University of Rio Grande Do Sul,
Brazil
Elisabet Stener-Victorin, Karolinska Institutet, Sweden
Fahimeh Ramezani Tehrani, Shahid Beheshti University of
Medical Sciences, Iran
Shakila Thangaratinam, University of Birmingham, UK
VOL. - NO. - / - 2023 25
Fertility and Sterility®

Mala Thondan, Harp Family Medical, Australia
Eszter Vanky, Norwegian University of Science and Tech-
nology; Norway
Chandrika Wijeyaratne, University of Colombo, Sri Lanka
Selma Witchel, Children's Hospital of Pittsburgh of UPMC,
University of Pittsburgh, USA
Dongzi Yang, Reproductive Medical Centre, Sun Yat-Sen
Memorial Hospital, China
Bulent Yildiz, Hacettepe University, Turkey
International early career evidence synthesis network
leads
Simon Alesi, Monash University, Australia
Snigdha Alur-Gupta, University of Rochester, USA
Jodie Avery, University of Adelaide, Australia
Mahnaz Bahri Khomami, Monash University, Australia
Jamie Benham, University of Calgary, Canada
Hugh Bidstrup, Australian Catholic University, Australia
Su Jen Chua, Monash University, Australia
Laura Cooney, University of Wisconsin, USA
Thisara Coster, Monash University, Australia
Carolyn Ee, Western Sydney University, Australia
Victoria Fitz, Harvard University, USA
Madeline Flanagan, Monash University, Australia
Maria Forslund, University of Gothenburg, Sweden
Geranne Jiskoot, Erasmus MC, Netherlands
Maryam Kazemi, Icahn School of Medicine at Mount
Sinai, USA
Punith Kempegowda, University of Birmingham, UK
Yvonne Louwers, Erasmus MC, Netherlands
Marla Lujan, Cornell University, USA
Johanna Melin, University of Helsinki, Finland
Eka Melson, University of Leicester, UK
Yitayeh Belsti Mengistu, Monash University, Australia
Negar Naderpoor, Monash University, Australia
Adriana Neven, Monash University, Australia
Hester Pastoor, Erasmus MC, Netherlands
Thais Rocha, University of Birmingham, UK
Angelo Sabag, Western Sydney University, Australia
Anuradhaa Subramanian, University of Birmingham,
UK
Katrina Tan, Monash Health, Australia
26 VOL. - NO. - / - 2023
