
December 2019
United States Government Accountability Office
Science, Technology Assessment, and Analytics
Report to Congressional Requesters
TECHNOLOGY ASSESSMENT
Artificial Intelligence
in Health Care
Benefits and Challenges of Machine Learning in
Drug Development
With field background content from the National Academy
of Medicine
GAO-20-215SP
Jointly published with
The cover image displays a stylized representation of data inputs from a variety of sources—including patient data from
health records or clinical trials, lab research data, textual data from scientific and medical literature, and data on
compounds and their properties—to a computer representing machine learning algorithms. Those algorithms then
assist with various aspects of the drug development process, eventually resulting in a marketed drug.
This report is being jointly published by the Government Accountability Office (GAO) and the National Academy of
Medicine (NAM). Part One of this joint publication presents material excerpted and adapted by NAM from its 2020
NAM Special Publication Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril. Part Two is the
full presentation of GAO’s Technology Assessment Artificial Intelligence in Health Care: Benefits and Challenges of
Machine Learning in Drug Development. Although GAO and NAM staff consulted with and assisted each other
throughout this work, reviews were conducted by NAM and GAO separately and independently, and authorship of the
text of Part One and Part Two of the report lies solely with NAM and GAO, respectively.
With the exception of Part One of this joint publication, this is a work of the U.S. government and is not subject to
copyright protection in the United States. All but Part One may be reproduced and distributed in its entirety without
further permission from GAO. However, because the joint publication may contain copyrighted images or other
material, permission from the copyright holder may be necessary if you wish to reproduce this material separately.
The National Academy of Medicine is the author of Part One and waives its copyright rights for that material.

GAO-20-215SP i
Foreword
The U.S. health care system is at an important crossroads as it faces major demographic shifts,
burgeoning costs, and transformative technologies. Although the growth in health care costs has
moderated recently, total annual health care spending in the United States is projected to reach nearly
$6 trillion by 2027. Federal spending for health care programs—which accounts for more than a quarter
of all health care spending—has grown faster than the overall economy in recent years, a trend
projected to continue. Every day more than 10,000 Americans turn age 65, becoming eligible for
Medicare. These demographic realities help illustrate the critical need to better address the
effectiveness and efficiency of our nation’s health care delivery systems.
Artificial intelligence and machine learning (AI/ML) is a set of technologies that includes automated
systems able to perform tasks that normally require human intelligence, such as visual perception,
speech recognition, and decision-making. AI/ML has promising applications in health care, including
drug development. For example, it may have the potential to help identify new treatments, reduce
failure rates in clinical trials, and generally result in a more efficient and effective drug development
process. However, applying AI/ML technologies within the health care system also raises ethical, legal,
economic, and social questions.
The Government Accountability Office (GAO) and the National Academy of Medicine (NAM), individually
and in collaboration, have taken up the charge to explore AI/ML in health care, assess its implications,
and identify key options available for optimizing its use. In recognition of mutual interests and
obligations, and to reinforce and complement each other’s work, NAM and GAO have cooperated on the
development of two publications. The first is NAM’s Special Publication: Artificial Intelligence in Health
Care: The Hope, the Hype, the Promise, the Peril, adapted excerpts of which are presented as Part One of
this joint publication. Any recommendations in Part One are those of NAM alone. The second is GAO’s
Technology Assessment: Artificial Intelligence in Health Care: Benefits and Challenges of Machine
Learning in Drug Development, presented as Part Two.
This cooperative effort included two expert meetings, bringing diverse, interdisciplinary, and cross-
sectoral perspectives to the discussions. We are grateful to the exceptionally talented staff of NAM and
GAO as well as the experts, all of whom worked hard with enthusiasm, great skill, flexibility, clarity, and
drive to make this joint publication possible.
Sincerely,
Timothy M. Persons, PhD
Chief Scientist and Managing Director,
Science, Technology Assessment, and Analytics
U.S. Government Accountability Office
J. Michael McGinnis, MD, MA, MPP
Leonard D. Schaeffer Executive Officer, and
Executive Director, NAM Leadership Consortium

GAO-20-215SP ii
Executive Summary
This report is being jointly published by the Government Accountability Office (GAO) and the National
Academy of Medicine (NAM). Part One of this joint publication presents material excerpted and adapted
by NAM from its 2020 Special Publication Artificial Intelligence in Health Care: The Hope, the Hype, the
Promise, the Peril. Part Two is the full presentation of GAO’s Technology Assessment Artificial
Intelligence in Health Care: Benefits and Challenges of Machine Learning in Drug Development. Although
GAO and NAM staff consulted with and assisted each other throughout this work, reviews were
conducted by NAM and GAO separately and independently, and authorship of the text of Part One and
Part Two of this Executive Summary and the following report lies solely with NAM and GAO,
respectively.
OVERVIEW OF PART ONE – NAM Special Publication: Artificial Intelligence in Health
Care: The Hope, the Hype, the Promise, the Peril
The National Academy of Medicine’s Special Publication: Artificial Intelligence in Health Care: The Hope,
the Hype, the Promise, the Peril surveys current knowledge to present an accessible guide for relevant
health care stakeholders such as artificial intelligence, machine learning (AI/ML) model developers,
clinical implementers, clinicians, patients, and regulation and policy makers.
1
In this publication, an NAM
expert working group comprised of leaders from various disciplines—public health, informatics,
biomedical ethics, and implementation science—provides a sampling of present-day AI applications with
a look to near-term possibilities, highlights the associated challenges and limitations, and outlines
fundamental ethical, legal, regulatory, and societal considerations for the successful development and
implementation of health care AI.
A key component shaping the publication was a January 2019 NAM convening of more than 60 experts
from a range of stakeholder communities to consider how the draft could best ensure coverage of the
most significant issues facing the development, deployment, or use of AI/ML in health care; that the
1
Matheny, M., S. Thadaney, M. Ahmed, and D. Whicher, editors. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril.
Washington, DC: National Academy of Medicine. Part One of this Joint Publication presents material excerpted and adapted by NAM from its
2020 Special Publication Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril. Although GAO staff and leadership
were consulted throughout the development process, authorship for the text lies solely with the National Academy of Medicine, the editors,
and the authors (identified in the relevant sections and at the end of Part One).

GAO-20-215SP iii
solutions and approaches described and reviewed provided fair and balanced guidance for those
interested in developing and deploying AI/ML models in health care settings; and the ways the content
of the publication could most facilitate progress in the field. As an active participant, the GAO provided
critical feedback on the content of the publication focusing on these dimensions.
Drawing on those discussions, and supplemented with written comments from external experts, the
NAM publication identified several cross-cutting themes.
Potential Importance of AI/ML to Progress in Health and Health Care
With much of health and health care moving onto digital platforms, there has been a stunning growth in
the volume of information generated through routine health-related processes and from products of
health, health care, and biomedical science research. Especially as insights continue to emerge from
exploration of underlying genetic predispositions to health and disease, the ability to use of AI and ML
tools will soon be essential to assist with the growing field of precision medicine.
Furthermore, the ability to glean insights from the enormous body of data points generated daily from
mobile apps (m-Health) and sensors will require the capacity for simultaneous data processing from
multiple sources. The increasing availability of environmental and geospatial sensors developed on
digital platforms contribute yet additional data universes requiring AI/ML before incorporation into
predictive modeling tools.
AI and Transparency
As AI applications grow in their ability to lend perspective to health and health care decision-making,
there is a compelling need for transparency in algorithms and data sources with the recognition that the
need for algorithmic transparency is context-dependent, based on risk and intended use. For example, a
high impact AI tool with immediate clinical implications warrants more stringent explanation
requirements than a tool with a proven record of accuracy that is low risk and clearly conveys its
recommendations to the end user. “Therefore, AI developers, implementers, users, and regulators
should collaboratively define guidelines for clarifying the level of transparency needed across a
spectrum.”
2
2
Matheny, Thadaney, Ahmed, and Whicher. Artificial Intelligence in Health Care.

GAO-20-215SP iv
As the field advances rapidly, regulators and legislators are required to remain nimble as they balance
the complex interplay among AI innovation, safety, and trust. To avoid stymying AI development while
ensuring proper oversight, regulators must engage myriad stakeholders and experts in the evaluation of
clinical AI based on real-world data. As a harbinger of things to come, U.S. Food and Drug Administration
(FDA) recently issued a framework for evaluating health care AI based on the level of patient risk, AI
autonomy, and the dynamism of the tool.
3
Yet, to the extent that machine-learning models evolve with
new data, issues of liability will continue to unfold with increasing involvement by the courts, regulators,
and insurers.
Mitigating the hype
As the communication on the potential wonders of AI pervades social consciousness, it is easy for
misguided fears and optimism to obscure its legitimate near-term possibilities. Although AI is certainly
limited in its capacity to match the problem solving capacity of humans, AI-enabled automation is poised
for disruptive workplace innovations. Given the necessary reliance on information technology (IT) and
ML to help health professionals keep pace with the rapidly growing knowledge base, medical education
will need a substantial overhaul. This needs to happen with an added focus on the use of AI as a routine
decision-assistance tool. Training programs across multiple professions will require a focus on data
science and the appropriate use of AI products and services. The bridging function to patient and
consumer comfort levels with these emerging technologies will also need to be established to secure
the bond of confidence between clinicians and their patients. Ultimately, the goal is to build
competency in AI and data science to the point that health care AI provides an assistive benefit to
humans rather than replacing them. For this reason, the near-term focus might be better termed
“augmented intelligence.”
Prioritizing Equity and Inclusivity
Among the many considerations in the NAM publication, especially strong emphasis was placed on the
“the appropriate and equitable development and implementation of health care AI.”
1
Prioritizing equity
and inclusion begins with algorithms that have been developed from rich, population-representative
datasets. Despite an abundance of health data, the lack of system interoperability and suboptimal data
3
Food and Drug Administration. 2019. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML) –
Based Software as a Medical Device (SaMD). Available from: https://www.fda.gov/media/122535/download.

GAO-20-215SP v
standardization techniques prevent the effective integration of health data from disparate systems.
Without a robust base of data, AI algorithms fail to achieve successful levels of generalizability and
utility.
Ensuring that equity, and inclusivity remain at the forefront in the deployment of AI requires the active
engagement of system leaders, AI implementers, and regulators as they work to determine whether an
AI tool is suitable for a particular environment and question whether its introduction could exacerbate
existing biases and inequities. To address patient and community needs, health delivery organizations
are in the process of developing (IT) governance strategies that expand linkages to social determinants
and psychosocial data. National-scale efforts are needed to lower the barrier for adoption of these
technologies and minimize the possible creation of a digital divide in underserved communities where IT
capacities are less developed.
OVERVIEW OF PART TWO – GAO Technology Assessment: Artificial Intelligence in Health Care:
Benefits and Challenges of Machine Learning in Drug Development
The GAO report Artificial Intelligence in Health Care: Benefits and Challenges of Machine Learning in
Drug Development is the first in a planned series of technology assessments on the use of AI
technologies in health care that GAO is conducting at the request of Congress.
4
This report discusses
three topics: (1) current and emerging AI technologies available for drug development and their
potential benefits, (2) challenges to the development and adoption of these technologies, and (3) policy
options to address challenges to the use of machine learning in drug development. As one component of
this review, NAM facilitated consultation with colleagues from the National Academies, to work closely
with GAO in organizing a July 2019 meeting of 19 experts to explore these topics. NAM staff provided
expertise, based on their work on the NAM Special Publication Artificial Intelligence in Health Care: The
Hope, the Hype, the Promise, the Peril, to GAO in the identification of experts from federal agencies,
academia, biopharmaceutical companies, machine learning-focused companies, and legal scholars. The
meeting was intended to enhance GAO’s understanding of ML in health care and drug development.
4
Part Two of this Joint Publication presents the GAO Technology Assessment: Artificial Intelligence in Health Care: Benefits and Challenges of
Machine Learning in Drug Development. Although NAM staff and leadership provided assistance and advice in the identification of issues and
experts consulted during the development process (identified in app. II), responsibility for the text, findings, and options lies solely with GAO.
GAO-20-215SP vi
One of the report’s high-level findings is that machine learning holds tremendous potential in drug
development, according to stakeholders from government, industry, and academia. The current drug
development process is lengthy and expensive, and can take 10 to 15 years to develop a new drug and
bring it to market. ML techniques are already used throughout the drug development process and have
the potential to expedite the discovery, design, and testing of drug candidates, decreasing the time and
cost required. These improvements could save lives and reduce suffering by getting drugs to patients in
need more quickly.
The technology assessment demonstrates the breadth of machine learning research and applications
with examples from the first three steps of the drug development process—drug discovery, preclinical
research, and clinical trials. In drug discovery, researchers are using ML to identify new drug targets,
screen known compounds for new therapeutic applications, and design new drug candidates, among
other applications. In preclinical research, ML can augment preclinical testing of drug candidates and
predict toxicity before human testing. Researchers are also beginning to use ML to improve clinical trial
design, a point where many drug candidates fail. These efforts include applying ML to patient selection
and recruitment, and to identify patient populations who may react better to certain drugs, thus
advancing towards the promise of precision medicine.
The technology assessment also identifies challenges that hinder the adoption and impact of machine
learning in drug development, according to stakeholders, experts, and the literature. Gaps in research in
biology, chemistry, and ML limit the understanding of and impact in this area. A shortage of high-quality
data, which are required for ML to be effective, is another challenge. It is also difficult to access and
share these data because of costs, legal issues, and a lack of incentives for sharing. Furthermore, a low
supply of skilled and interdisciplinary workers creates hiring and retention challenges for drug
companies. Lastly, uncertainty about regulation of machine learning used in drug development may limit
investment in this field. Some of these challenges are similar to those identified in the NAM special
publication, such as the lack of high-quality, structured data, and others are unique to drug
development.
GAO describes options for policymakers—which GAO defines broadly to include federal agencies, state
and local governments, academic and research institutions, and industry, among others—to use in
addressing these challenges. In addition to the status quo, GAO identifies five policy options centered
around research, data access, standardization, human capital, and regulatory certainty.
GAO-20-215SP vii
Table of Contents
Part One - Artificial Intelligence in Health Care: Field Background (National Academy of
Medicine) ............................................................................................................................. 1
Introduction ...................................................................................................................................... 2
1 Definitions of Key AI Terms ........................................................................................................... 2
2 A Historical Perspective and Overview of Current AI .................................................................... 4
3 How Artificial Intelligence Is Changing Health and Health Care .................................................... 6
4 Potential Tradeoffs and Unintended Consequences of AI .......................................................... 11
5 Best Practices for Machine-Learning Model Development and Validation ................................ 15
6 Deploying AI in Clinical Settings .................................................................................................. 18
7 Conclusion ................................................................................................................................... 22
Bibliography .................................................................................................................................... 24
Authors of NAM Special Publication .............................................................................................. 29
Part Two - Artificial Intelligence in Health Care: Benefits and Challenges of Machine Learning
in Drug Development (U.S. Government Accountability Office) ............................................ 30
Introduction .................................................................................................................................... 34
1 Background .................................................................................................................................. 37
1.1 The drug discovery, development, and approval process ............................................... 37
1.2 Machine learning in AI innovation ................................................................................... 39
1.3 Data generated and used in health care ......................................................................... 41
1.4 Economic considerations of drug development .............................................................. 42
2 Status and Potential Benefits of Machine Learning in Drug Development ................................. 44
2.1 Drug discovery ................................................................................................................. 45
2.2 Preclinical research .......................................................................................................... 48
2.3 Clinical trials ..................................................................................................................... 49
3 Challenges Hindering the Use of Machine Learning in Drug Development ................................ 52
3.1 Gaps in research .............................................................................................................. 53
3.2 Data quality ...................................................................................................................... 55
3.3 Data access and sharing................................................................................................... 56
3.4 Low supply of skilled and interdisciplinary workers ........................................................ 57
3.5 Regulatory challenges and federal commitment ............................................................ 57
GAO-20-215SP viii
4 Policy Options to Address Challenges to the Use of Machine Learning in Drug Development .. 59
5 Agency and expert comments ..................................................................................................... 66
Appendix I: Objectives, scope, and methodology .......................................................................... 68
Appendix II: Expert participation. ................................................................................................... 72
Appendix III: GAO contact and staff acknowledgments ................................................................. 74
National Academy of Medicine GAO-20-215SP 1
PART ONE
Artificial Intelligence in
Health Care:
Field Background
National Academy of Medicine (NAM)
Part One of this Joint Publication presents material excerpted and adapted by NAM from
its 2020 Special Publication: Artificial Intelligence in Health Care: The Hope, the Hype, the
Promise, the Peril. Although GAO staff and leadership were consulted throughout the
development process, authorship of the text lies solely with the National Academy of
Medicine, the editors, and the authors (identified in the relevant sections and at the end
of Part One).
National Academy of Medicine GAO-20-215SP 2
PART I: NAM FIELD OVERVIEW
Introduction: The emergence of artificial intelligence (AI) as a tool for better health care offers
unprecedented opportunities to improve patient and clinical team outcomes, reduce costs, and
impact population health. Many are already in use in health care. Nonetheless, the authors of
the National Academy of Medicine’s Special Publication titled Artificial Intelligence in Health
Care: The Hope, the Hype, the Promise, the Peril not only underscore the promise, but also call
out the issues for care and caution.
The material presented here has been adapted from the NAM’s Special Publication and serves to
provide a broad overview of current and near-term AI solutions; the challenges, limitations, and
best practices for AI model development, adoption, and maintenance; the current legal and
regulatory landscape for AI tools in health care; and prioritizes the need for equity, inclusion,
and a human rights lens as we proceed together into a more technological future.
1. Definitions of Key AI Terms: The term artificial intelligence (AI), colloquially and in the
scientific literature, takes on a range of meanings, from specific forms of AI, such as machine
learning, to a hypothetical AI could be considered conscious or sentient. A formal definition of
AI starts with the Oxford English Dictionary: “The capacity of computers or other machines to
exhibit or simulate intelligent behavior; the field of study concerned with this.” More nuanced
definitions of AI might also consider what goal the AI is attempting to achieve and how it is
pursuing that goal. In general, AI systems range from those that attempt to accurately model
human reasoning to solve a problem, to those that ignore human reasoning and exclusively use
large volumes of data to generate a framework to answer the question(s) of interest, to those
that attempt to incorporate elements of human reasoning but do not require accurate modeling
of human processes. The graphic below summarizes the domains of artificial intelligence
(Figure 1).
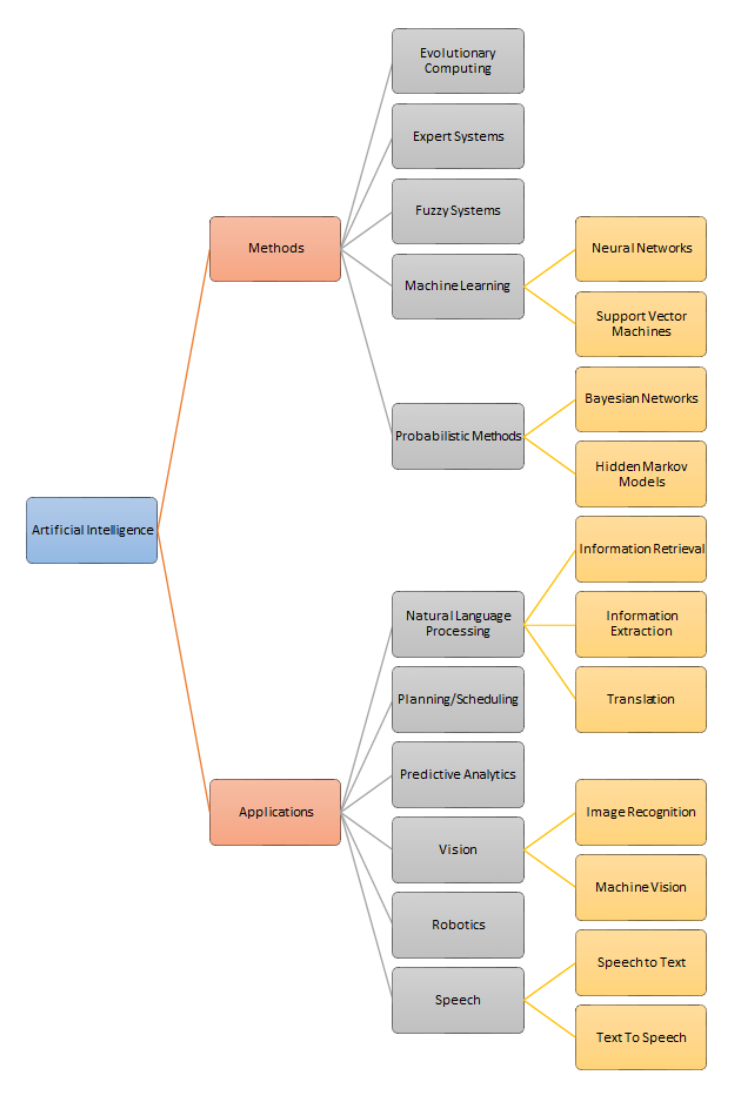
National Academy of Medicine GAO-20-215SP 3
Figure 1 | A summary of the domains of artificial intelligence
SOURCE: Adapted with permission from a figure in Mills, M. 2015. Artificial Intelligence in Law—
The State of Play in 2015? Legal IT Insider. https://www.legaltechnology.com/latest-
news/artificial-intelligence-in-law-the-state-of-play-in-2015.

National Academy of Medicine GAO-20-215SP 4
Machine learning is a family of statistical and mathematical modeling techniques that uses a
variety of approaches to automatically learn and improve the prediction of a target state,
without explicit programming (Witten et al., 2016). Different methods, such as Bayesian
networks, random forests, deep learning, and artificial neural networks, each use different
assumptions and mathematical frameworks for how data is ingested, and learning occurs
within the algorithm. Regression analyses, such as linear and logistic regression, are also
considered machine learning methods, although many users of these algorithms distinguish
them from commonly defined machine learning methods (e.g., random forests, Bayesian
Networks [BNs], etc.).
Natural language processing (NLP) enables computers to understand and organize human
languages (Manning and Schütze, 1999). NLP needs to model human reasoning because it
considers the meaning behind written and spoken language in a computable, interpretable, and
accurate way. NLP incorporates rule-based and data-based learning systems, and many of the
internal components of NLP systems are themselves machine learning algorithms with pre-
defined inputs and outputs, sometimes operating under additional constraints. Examples of
NLP applications include assessment of cancer disease progression and response to therapy
among radiology reports (Kehl et al., 2019), and identification of post-operative complication
from routine EHR documentation (Murff et al., 2011).
Expert systems are a set of computer algorithms that seek to emulate the decision-making
capacity of human experts (Feigenbaum, 1992; Jackson, 1998; Leondes, 2002; Shortliffe and
Buchanan, 1975). These systems rely largely on a complex set of Boolean and deterministic
rules. An expert system is divided into a knowledge base, which encodes the domain logic, and
an inference engine, which applies the knowledge base to data presented to the system to
provide recommendations or deduce new facts.
Authors: Michael Matheny, MD, MS, MPH, Sonoo Thadaney Israni, MBA, Mahnoor Ahmed,
MEng, and Danielle Whicher, PhD, MHS
2. A Historical Perspective and Overview of Current AI: If the term “artificial intelligence”
might be given a birth date, it could be August 31, 1955, when John McCarthy, Marvin L. Minsky,
Nathaniel Rochester, and Claude E. Shannon submitted “A Proposal for the Dartmouth Summer
Research Project on Artificial Intelligence”. The proposal and the resulting conference—the
National Academy of Medicine GAO-20-215SP 5
1956 Dartmouth Summer Research Project on Artificial Intelligence—were the culmination of
decades of thought by many others (Buchanan, 2005; Kline, 2011; Turing, 1950; Weiner, 1948).
Although the conference produced neither formal collaborations nor tangible outputs, it helped
galvanize the field (Moor, 2006).
Thought leaders in this era saw the future clearly, although optimism was substantially
premature. In 1960, J. C. R. Licklider wrote, “The hope is that, in not too many years, human
brains and computing machines will be coupled together very tightly, and that the resulting
partnership will think as no human brain has ever thought and process data in a way not
approached by the information-handling machines we know today” (Licklider, 1960).
By the 1970s, excitement gave way to disappointment because early successes that worked in
well-structured, narrow problems failed to both generalize to broader problem solving and
deliver operationally useful systems. The disillusionment, summarized in the ALPAC (Automatic
Language Processing Advisory Committee)
and Lighthill reports, resulted in an “AI Winter” with
shuttered projects, evaporation of research funding, and general skepticism on the potential for
AI systems (McCarthy, 1974; National Research Council, 1996).
Yet in health care, work continued. Iconic expert systems such as MYCIN (Shortliff, 1974) and
others including Iliad, Quick Medical Reference, and Internist-1, were developed to assist with
clinical diagnosis. AI flowered commercially in the 1980s, becoming a multibillion-dollar
industry advising military and commercial interests (Miller, 1982; Sumner, 1993). However, all
of these prospects ultimately failed to fulfill the hype and lofty promises, resulting in a second
AI Winter from the late 1980s until the late 2000s
During this second AI Winter, the schools of computer science, probability, mathematics, and AI
collaborated to overcome the initial failures of AI. In particular, techniques from probability and
signal processing, such as Hidden Markov Models, Bayesian networks, and Stochastic search
and optimization were incorporated into AI thinking, resulting in the field known as machine
learning.
Around 2010, AI again regained prominence due to the success of machine learning and data
science techniques, as well as significant increases in computational storage and power. These
advances fueled the growth of technology titans such as Google and Amazon. Various ideas have
laid the groundwork for artificial neural networks which have come to dominate the field of

National Academy of Medicine GAO-20-215SP 6
machine learning. (Halevy et al., 2009; Krizhevsky, 2012). The resulting systems are called
“deep learning systems” and show significant performance improvements over prior
generations of algorithms for some use cases.
Modern AI has evolved from an interest in machines that think to ones that sense, think, and act.
It is important to distinguish narrow from general AI. The popular conception of AI is of a
computer, hypercapable in all domains, such as was seen even decades ago in science fiction
with HAL 9000 in 2001: A Space Odyssey (Stanley Kubrick, 1968) or aboard the USS Enterprise
in the Star Trek franchise (Gene Roddenberry, 1966). These are examples of general AIs and, for
now, are fictional. There is an active but niche general AI research community represented by
Deepmind, Cyc, and OpenAI, among others. Narrow AI, in contrast, is an AI specialized at a
single task, such as playing chess, driving a car, or operating a surgical robot.
Still, history has shown that AI has gone through multiple cycles of emphasis and
disillusionment in use. It is critical that all stakeholders are aware and actively seek to educate
and address public expectations and understanding of AI (and associated technologies) in order
to manage hype and establish reasonable expectations, which will enable AI to be applied in
effective ways that have reasonable opportunities for sustained success.
Authors: Jim Fackler, MD and Edmund Jackson, PhD
3. How Artificial Intelligence Is Changing Health and Health Care: The health care industry
has been investing for years in technology solutions with the potential to transform health and
health care There are promising examples, but there are gaps in the evaluation of these tools
including AI, so it can be difficult to assess their impact. The NAM Special Publication reviews
the potential of AI solutions for patients and families; the clinical care team; public health and
population health program managers; business administrators; and researchers (Figure 2).
Here we provide a sample of the potential solutions for patients and families, the clinical care
team, and public health and population health program managers are detailed below.
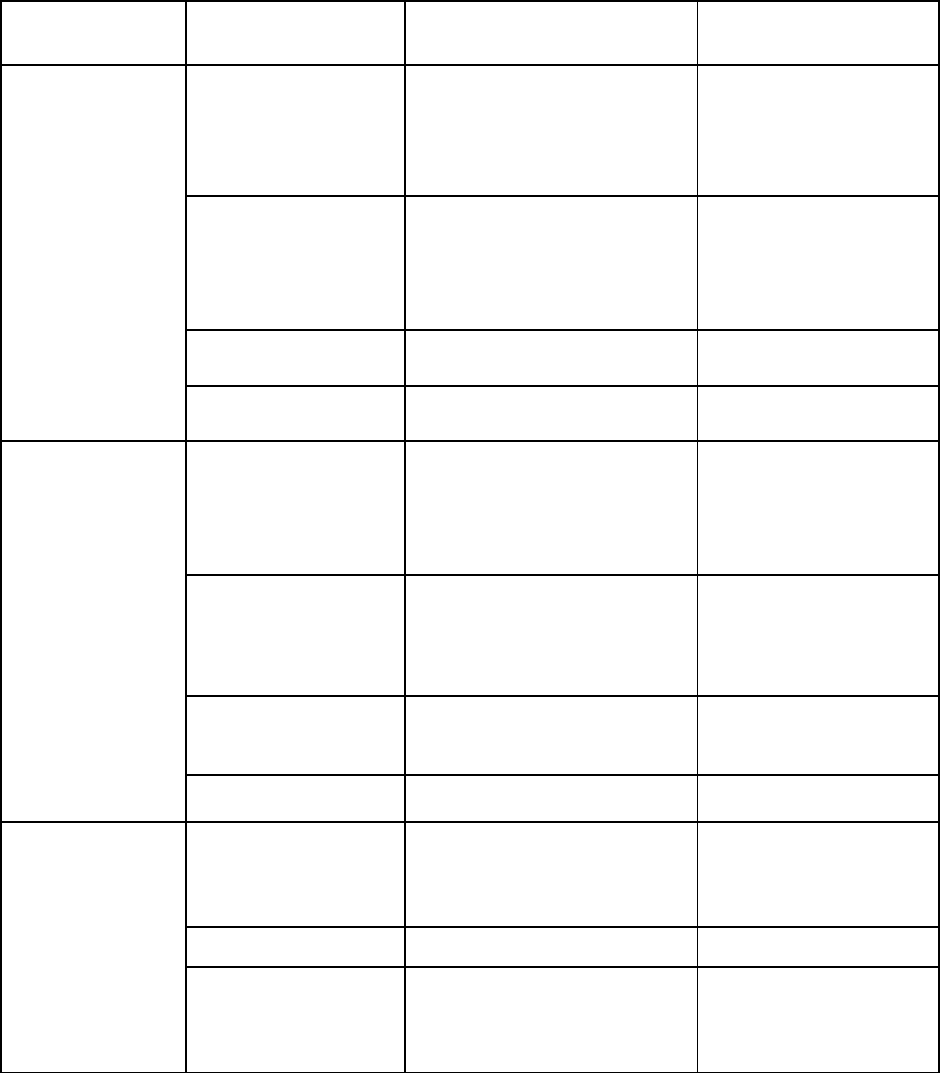
National Academy of Medicine GAO-20-215SP 7
Figure 2 | Examples of AI applications for stakeholder groups
Use Case/User
Group
Category
Illustrative Examples of
Applications
Technology
Patients and
Families
• Health
monitoring
• Benefit/risk
assessment
• Devices and wearables
• Smartphone and tablet
apps, websites
Machine learning,
natural language
processing (NLP),
speech recognition,
chatbots
• Disease
prevention and
management
• Obesity reduction
• Diabetes prevention and
management
• Emotional and mental
health support
Conversational AI, NLP,
speech recognition,
chatbots
• Medication
management
• Medication adherence
Robotic home telehealth
• Rehabilitation
• Stroke rehabilitation
using apps and robots
Robotics
Clinical Care
Teams
• Early detection,
prediction, and
diagnostics tools
• Imaging for cardiac
arrhythmia detection,
retinopathy
• Early cancer detection
(e.g., melanoma)
Machine Learning
• Surgical
Procedures
• Remote-controlled
robotic surgery
• AI-supported surgical
roadmaps
Robotics, machine
learning
• Precision
Medicine
• Personalized
chemotherapy treatment
Supervised machine
learning, reinforcement
learning
• Patient Safety
• Early detection of sepsis
Machine learning
Public Health
Program
Managers
• Identification
of individuals at
risk
• Suicide risk identification
using social media
Deep learning
(convolutional and
recurrent neural
networks)
• Population health
• Eldercare monitoring
Ambient AI sensors
• Population health
• Air pollution
epidemiology
• Water microbe detection
Deep learning,
geospatial pattern
mining, machine
learning
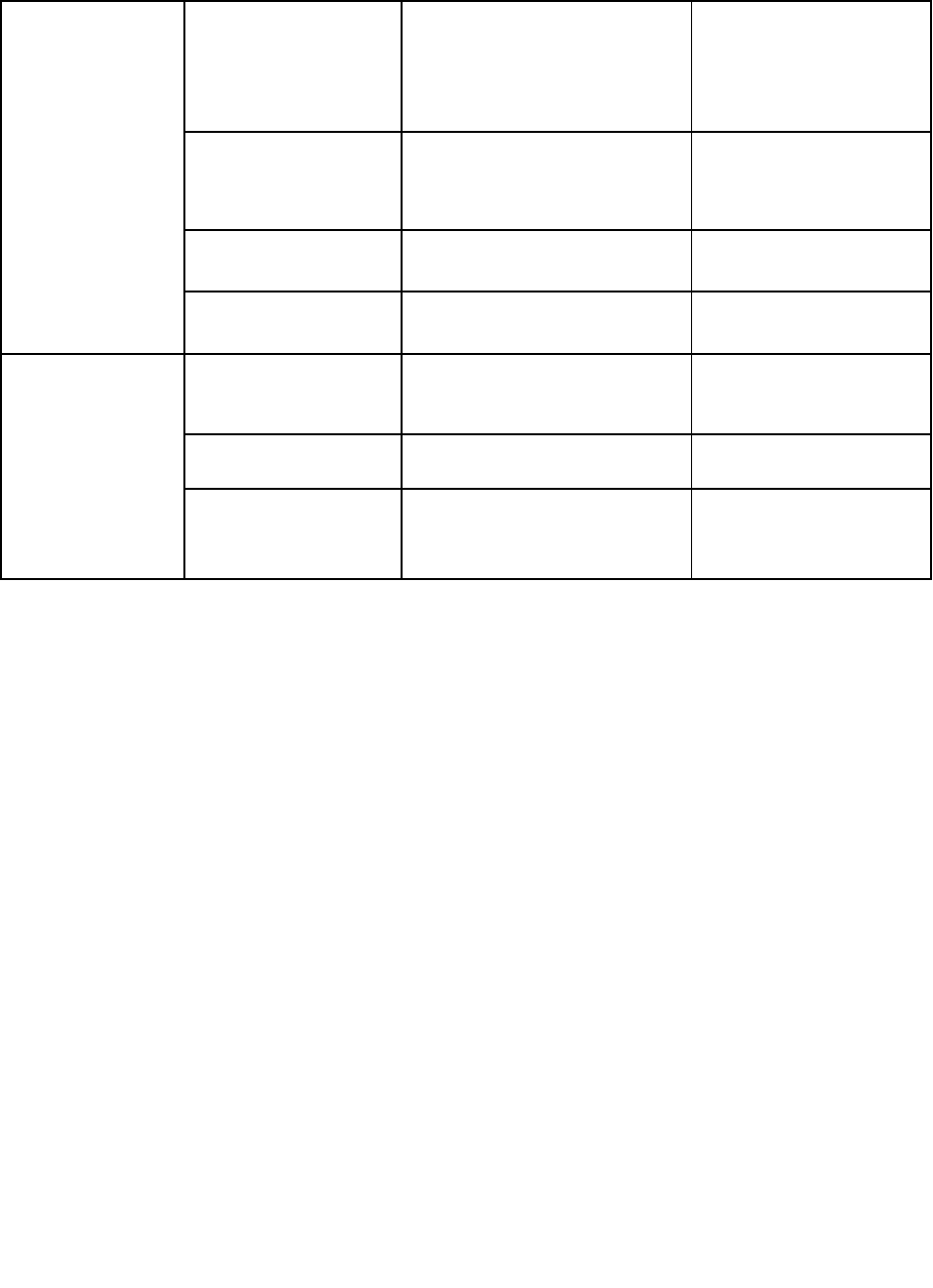
National Academy of Medicine GAO-20-215SP 8
Business
Administrators
• International
Classification
of Diseases, 10th
Rev. (ICD-10)
coding
• Automatic coding of
medical records for
reimbursement
Machine learning, NLP
• Fraud detection
• Health care billing fraud
• Detection of unlicensed
providers
Supervised,
unsupervised, and
hybrid machine learning
• Cybersecurity
• Protection of personal
health information
Machine learning, NLP
• Physician
management
• Assessment of physician
competence
Machine learning, NLP
Researchers
• Genomics
• Analysis of tumor
genomics
Integrated cognitive
computing
• Disease
prediction
• Prediction of ovarian
cancer
Neural networks
• Discovery
• Drug discovery and
design
Machine learning,
computer-assisted
synthesis
AI for Patients and Family: AI could soon play an important role in assisting patients and their
families in the self-management of chronic diseases such as cardiovascular diseases, diabetes,
and depression by assisting patients with taking medications, modifying diet, getting more
physically active, assisting with care management, wound care, device management, and the
delivery of injectables. Conversational agents, which can engage in two-way dialogue with the
user via speech recognition, offer one example of how self-management of these diseases could
be supplemented by AI solutions. Well known examples include Apple’s Siri, Amazon’s Alexa, or
Microsoft’s Cortana. Powered by NLP and natural language understanding, these interfaces may
include text-based dialogue or present a human image (e.g., the image of nurse or coach) or a
non-human image (e.g., a robot or animal) to provide a richer interactive experience.
Conversational agents actually already exist to address depression, smoking cessation, asthma,
and diabetes, although formal evaluation of these agents has been limited (Fitzpatrick et al.,
2017).
In a more passive application for patients and families, AI can use raw data from
accelerometers, gyroscopes, microphones, cameras, and smartphones for health monitoring
and risk prediction. By using machine-learning algorithms to recognize patterns from the raw

National Academy of Medicine GAO-20-215SP 9
data inputs and then categorize these patterns as indicators of an individual’s behavior and
health status, these systems can allow patients to understand and manage their own health and
symptoms, as well as share data with medical providers. Consumer interest is high (~50%) in
using data generated by apps, wearables, and Internet-of-Things devices to predict health risks
(Accenture, 2018). Since 2013, AI start-up companies with a focus on health care and wearables
have raised $4.3 billion to develop smart clothing, for example, bras designed for breast cancer
risk prediction and other clothes for cardiac, lung, and movement sensing (Wiggers, 2018).
AI Solutions for the Clinical Care Team: There are two main areas of opportunity for AI in clinical
care: (1) enhancing and optimizing care delivery and (2) improving information management,
user experience, and cognitive support in EHRs. Prediction, early detection, and risk assessment
for individuals is one of the most fruitful areas of AI applications (Sennaar, 2018). For example,
diagnostic image recognition, which can be supported by AI applications, can differentiate
between benign and malignant melanomas, diagnose retinopathy, identify cartilage lesions
within the knee joint (Liu et al., 2018), detect lesion-specific ischemia, and predict node status
after positive biopsy for breast cancer. Image recognition techniques can differentiate among
competing diagnoses, assist in screening patients, and guide clinicians in radiotherapy and
surgery planning (Matheson, 2018). AI platforms can, relatedly, provide roadmaps to assist
surgical teams in the operating room, reducing risk and making surgery safer (Newmarker,
2018).
Clinicians are testing whether AI will permit them to personalize chemotherapy dosing and
map patient response to a treatment to plan future dosing (Poon et al., 2018), a variation of
precision medicine enabled by AI. AI-driven NLP has been used to identify polyp descriptions in
pathology reports that trigger guideline-based clinical decision support to help clinicians
determine the best surveillance intervals for colonoscopy exams (Imler et al., 2014). Other AI
tools have helped clinicians select the best treatment options for complex diseases like cancer
(Zauderer et al., 2014). Using retrospective data from other patients, AI techniques can predict
treatment responses to different therapy combinations for an individual patient (Brown, 2018).
These types of tools may serve to help select a treatment immediately, and may also provide
new knowledge for future practice guidelines.
As genome-phenome integration is realized, the use of genetic data in AI systems for diagnosis,
clinical care, and treatment planning will probably increase. To truly impact routine care,
National Academy of Medicine GAO-20-215SP 10
though, genetic datasets will need to better represent the diversity of patient populations
(Hindorff et al., 2018).
AI also has the potential to improve the way in which clinicians store and retrieve clinical
documentation in EHRs. AI also has the potential to not only improve existing clinical decision
support modalities, but to support improved cognitive support functions like smarter CDS
alerts and reminders, as well as better access to peer-reviewed literature.
Population and Public Health Management: A spectrum of market-ready AI approaches to
support population health programs already exists. They are used in areas of automated retinal
screening, clinical decision support, predictive population risk stratification, and patient self-
management tools (Contreras and Vehi, 2018; Dankwa-Mullan et al., 2018). Several solutions
have received regulatory approval; for example, the U.S. Food and Drug Administration
approved Medtronic’s Guardian Connect, the first AI-powered continuous glucose monitoring
system. Crowd-sourced, real-world data on inhaler use, combined with environmental data, led
to a policy recommendation model that can be replicated to address many public health
challenges by simultaneously guiding individual, clinical, and policy decisions. (Barrett et al.,
2018) Other areas of potential overlap are standard risk prediction models that apply AI tools
to facilitate recognition of clinically important but unanticipated predictor variables; and how
AI can be used to not only predict risk, but also the presence or absence of a disease in an
individual.
For public health professionals, the focus is on solutions for more efficient and effective
administration of programs, policies, and services; disease outbreak detection and surveillance;
as well as research. The range of AI solutions that can improve disease surveillance is
considerable. For a number of years, researchers have tracked and refined the options for
tracking disease outbreaks using search engine query data. Some of these approaches rely on
the search terms that users type into internet search engines (e.g., Google Flu Trends, etc.).
At the same time, caution is warranted with these approaches. Relying on data not collected for
scientific purposes (e.g., Internet search terms) to predict flu outbreaks has been fraught with
error (Lazer et al., 2014). Non-transparent search algorithms that change constantly cannot be
easily replicated and studied. These changes may occur due to business needs (rather than the
needs of a flu outbreak detection application) or due to changes in the search behavior of

National Academy of Medicine GAO-20-215SP 11
consumers. Finally, relying on such methods exclusively misses the opportunity to combine
them and co-develop them in conjunction with more traditional methods. As Lazer et al. details,
combining traditional and innovative methods (e.g., Google Flu Trends) performs better than
either method alone.
AI and machine learning have also been used to develop a dashboard to provide live insight into
opioid usage trends in Indiana (Bostic, 2018). This tool enabled prediction of drug positivity for
small geographic areas (i.e., hot spots), allowing for interventions by public health officials, law
enforcement, and program managers in targeted ways. A similar dashboarding approach
supported by AI solutions has been used in Colorado to monitor HIV surveillance and outreach
interventions and their impact after implementation (Snyder et al., 2016). This tool integrated
data on regional resources with near real-time visualization of complex information to support
program planning, patient management, and resource allocation.
Authors: Joachim Roski, PhD, MPH, Wendy Chapman, PhD, Jaimee Heffner, PhD, Ranak Trivedi,
PhD, Guilherme Del Fiol, MD, PhD, Rita Kukafka, PhD, Paul Bleicher, MD, PhD, Hossein Estiri,
PhD, Jeffrey Klann, PhD, and Joni Pierce, MBA, MS
4. Potential Tradeoffs and Unintended Consequences of AI: While we optimistically look to a
future where AI-driven solutions can systematically improve health and medicine, AI systems
could also have far-reaching unintended consequences and implications for patient populations,
health systems, and the workforce. To mitigate the effect of these potential consequences, care
must be given to the consideration of how tradeoffs between efficiency and equity impact
populations in delivering against the unmet and unlimited demands of health care.
The Future of Employment and Displacement: While anxiety over job losses due to AI and
automation are likely exaggerated, advancing technology will almost certainly change roles as
certain tasks are automated as seen in other industries. A conceivable future could see AI
eliminating a clinician’s need to perform manual tasks like checking patient vital signs
(especially with self-monitoring devices), collecting laboratory specimens, preparing
medications for pickup, transcribing clinical documentation, completing prior authorization
forms, scheduling appointments, collecting standard history elements, and making routine
diagnoses. However, most clinical jobs and patient needs require much more cognitive

National Academy of Medicine GAO-20-215SP 12
adaptability, problem solving, and communication skills than a computer can muster. Despite
the fear of AI eliminating jobs, industrialization and technology typically yield net productivity
gains to society. For example, many assumed that automated teller machines (ATMs) would
eliminate the need for bank tellers. Instead, the efficiencies gained through the use of ATMs
enabled the expansion of bank branches and resulted in an even greater demand for tellers that
could focus on higher cognitive tasks, such as interacting with customers, rather than simply
counting money (Pethokoukis, 2016).
Need for Education and Workforce Development: A graceful transition into the AI era of health
care that minimizes the unintended consequences of displacement will require deliberate
redesigning of training programs. This ranges from support for a core basis of primary
education in science, technology, engineering, and math literacy in the broader population to
continuing professional education in the face of a changing environment. Health care workers in
the AI future will need to learn how to use and interact with information systems, with
foundational education in information retrieval and synthesis, statistics and evidence-based
medicine appraisal, and interpretation of predictive models in terms of diagnostic performance
measures. Institutional organizations (e.g., National Institutes of Health, health care systems,
professional organizations, universities, and medical schools) should shift focus from skills that
are easily replaced by AI automation to specific education and workforce development
programs for work in the AI future, with emphasis in STEM, data science skills, and human skills
that are hard to replace with technology.
AI System Augmentation of Human Tasks: While much of the popular discussion of AI focuses on
how AI tools will replace human workers, realistically, in the foreseeable future, AI will function
in an augmenting role, adding to the capabilities of the technology’s human partners. As the
volume of data and information available to inform patient care grows exponentially, AI tools
will naturally become part of the clinical care team in much the same way a doctor is supported
by a team of intelligent agents including specialists, nurses, physician assistants, pharmacists,
social workers, and other health professionals (Meskó, Hetényi, and Győrffy, 2018). The
technologies will be able to provide task-specific expertise in the data and information space,
augmenting the capabilities of the physician and the entire team, making their jobs easier and
more effective, and ultimately improving patient care (Herasevich, Pickering, and Gajic, 2018;
Wu, 2019).
National Academy of Medicine GAO-20-215SP 13
Hype versus Hope: One of the greatest near-term risks in the current development of AI tools in
health care is not that it will cause serious unintended harm, but that it simply cannot meet the
expectations stoked by excessive hype. Over the last decade, several factors have led to
increasing interest and escalating hype of AI. Explicit advertising hyperbole may be one of the
most direct triggers for unintended consequences of hype. While such promotion is important
to drive interest and motivate progress, it can become counterproductive in excess. A
combination of technical and subject domain expertise is needed to recognize the credible
potential of AI systems and avoid the backlash that will come from overselling them.
Risks associated with model development and implementation: Since AI systems that will be
deployed in the health care setting are constrained to learn from available observational health
data, high fidelity and reliably measured outcomes are not always achievable. Although data
from EHRs and other health information systems provide a rich longitudinal, multi-dimensional
set of details about an individual’s health, these data are often both noisy and biased as they are
produced for different purposes in the process of documenting care. Poorly constructed or
interpreted models from observational data can harm patients. Health care data scientists must
be careful to apply the right types of modeling approaches based on the characteristics and
limitations of the underlying data.
Although correlation can be sufficient for diagnosing problems and predicting outcomes in
certain cases, methods that primarily learn associations between inputs and outputs can be
unreliable, if not overtly dangerous when used to drive medical decisions. (Schulam and Saria,
2017) There are three common reasons why this is the case. First, performance of association-
based models tend to be susceptible to even minor deviations between the development and
implementation datasets. The learned associations may memorize dataset-specific patterns that
do not generalize as the tool is moved to new environments where these patterns no longer
hold. (Subbaswamy, Schulam, and Saria, 2019) A common example of this phenomenon is shifts
in provider practice with the introduction of new medical evidence, technology, and
epidemiology. If a tool heavily relies on a practice pattern to be predictive, as practice changes,
the tool is no longer valid. (Schulam and Saria, 2017) Second, such algorithms cannot correct for
biases due to feedback loops that are introduced when learning continuously over time.
(Schulam and Saria, 2017) In particular, if the implementation of an AI system changes patient
exposures, interventions, and outcomes (often as intended), it can cause data shifts that
National Academy of Medicine GAO-20-215SP 14
degrade performance. Finally, the proposed predictors may be tempting to treat as factors one
can manipulate to change outcomes but these are often misleading.
One approach is to update models over time so that they continuously adapt to local and recent
data. Such adaptive algorithms offer constant vigilance and monitoring for changing behavior.
However, this may exacerbate disparities when only well-resourced institutions can deploy the
expertise to do so in an environment.
Training reliable models depends on training datasets being representative of the population
where the model will be applied. Learning from real world data---where insights can be drawn
from patients similar to a given index patient---has the benefit of leading to inferences that are
more relevant, but it is important to characterize populations where there is inadequate data to
support robust conclusions. For example, a tool may show acceptable performance on average
across individuals captured within a data set, but may perform poorly for specific
subpopulations because the algorithm has not had enough data to learn from. In genetic testing,
minority groups can be disproportionately adversely affected when recommendations are
made based on data that does not adequately represent them. (Manrai et al., 2016) Test-time
auditing tools that can identify individuals for whom the model predictions are likely to be
unreliable can reduce the likelihood of incorrect decision-making due to model bias. (Schulam
and Saria, 2017)
Machine learning that relies on observational data could also generally have an amplifying
effect on existing behavior, regardless of whether that behavior is beneficial or exacerbates
existing societal biases. For instance, a study found that machine translation systems were
biased against women due to the way in which women were described in the data used to train
the system. (Prates, Avelar, and Lamb, 2018) While some of these algorithms were revised or
discontinued, the underlying issues will continue to be significant problems, requiring constant
vigilance, as well as algorithm surveillance and maintenance to detect and address.
AI Systems Transparency: Transparency is a key theme that underlies deeper issues related to
privacy and consent or notification for patient data use, and to potential concerns on the part of
patients and clinicians around being subject to algorithmically-driven decisions. Consistent
progress in the development and adoption of AI in health care will only be feasible if health care
consumers and health care systems are mutually recognized as trusted data partners.

National Academy of Medicine GAO-20-215SP 15
Tensions exist among the desire for robust data aggregation to facilitate the development and
validation of novel AI models, the need to protect consumer privacy, and the need to
demonstrate respect for consumer preferences through informed consent or notification
procedures. However, lack of transparency about data use and privacy practices could create a
situation in which patients do not clearly consent to their data being used in ways they do not
understand, realize, or accept. Current consent practices for the use of EHR and claims data are
generally based on models focused on HIPAA privacy rules, and some argue that HIPAA needs
updating (Mello and Cohen, 2018). The progressive integration of other sources of patient-
related data (e.g., genetic information, social determinants of health), and the facilitated access
to highly granular and multi-dimensional data are changing the protections provided by
traditional mechanisms, such as HIPAA. For instance, with more data available, re-identification
becomes easier to perform (Cohen and Mello, 2019). Regulations need to be updated and
consent processes will need to be more informative of those added risks.
Authors: Jonathan Chen, MD, PhD, Andrew Beam, PhD, Suchi Saria, PhD, and Eneida Mendonca,
MD, PhD
5. Best Practices for Machine-Learning Model Development and Validation: Machine learning
models should be thoughtfully developed and validated. First, all stakeholders must understand
the needs of clinical practice, so that proposed AI systems address the practicalities of health
care delivery. Second, it is necessary that such models be developed and validated through a
team effort, involving AI experts and health care providers. Throughout the design and
validation process, it is important to be mindful of the fact that the datasets used to train AI are
heterogeneous, complex, and nuanced in ways that are often subtle and institution-specific.
This impacts how AI tools are monitored for safety and reliability, and how they are adapted for
different locations and over time. Third, before deployment at the point of care, AI systems
should be rigorously evaluated to ensure their competency and safety, in a similar process to
that done for drugs, medical devices, and other medical interventions.
Establishing Utility: When considering the use of AI in health care, it is necessary to know how a
member of the care team would act, given a model’s output. While model evaluation typically
focuses on metrics, such as positive predictive value, sensitivity (or recall), specificity, and
National Academy of Medicine GAO-20-215SP 16
calibration, constraints on the action triggered by the model’s output (e.g. continuous rhythm
monitoring might be constrained by availability of Holter monitors) often can have a much
larger influence in determining model utility (Moons et al., 2012). Completing model selection,
then doing a net-benefit analysis, and later factoring work constraints is suboptimal (Shah et al.,
2019). Realizing the benefit of implementation of AI into the work flow requires defining
potential utility upfront. Only by including the characteristics of actions taken on the basis of
the model’s predictions, and factoring in their implications, can a model’s potential usefulness
in improving care be properly assessed.
Learning a Model: After the potential utility of the model has been established, model
developers and model users need to interact closely when learning a model because many
modeling choices are dependent on the model’s context of use (Wiens et al., 2019). For example,
the need for external validity depends on what one wishes to do with the model, the degree of
agency ascribed to the model, and the nature of the action triggered by the model.
It is well known that biased data will result in biased models. Thus, the data that is selected to
learn from matters far more than the choice of the specific mathematical formulation of the
model. Model builders need to pay close attention to the data they train on and to think beyond
the technical evaluation of models. Even in technical evaluation, it is necessary to look beyond
the ROC curves, and examine multiple dimensions of performance. For decision making in the
clinic, additional metrics such as calibration, net reclassification, and a utility assessment are
necessary. Given the nonobvious relationship between a model’s positive predictive value,
recall, and specificity to its utility, it is important to examine simple and obvious parallel
baselines, such as a penalized regression model applied on the same data that are supplied to
more sophisticated models such as deep learning.
The topic of interpretability deserves special discussion because of ongoing debates around
interpretability, or the lack of it (Licitra, Trama, and Hosni, 2017; Lipton, 2016; Voosen, 2017).
To the model builder, interpretability often means the ability to explain which variables and
their combinations, in what manner, led to the output produced by the model (Friedler et al.,
2019). To the clinical user, interpretability could mean one of two things: a sufficient enough
understanding of what is going on, so that they can trust the output and/or be able to get
liability insurance for its recommendations; or enough causality in the model structure to
provide hints as to what mitigating action to take. To avoid wasted effort, it is important to
National Academy of Medicine GAO-20-215SP 17
understand what kind of interpretability is needed in a particular application. A black box
model may suffice if the output is trusted, and trust can be obtained by prospective assessment
of how often the model’s predictions are correct and calibrated.
Data Quality: Bad data quality adversely impacts patient care and outcomes (Jamal, McKenzie,
and Clark, 2009). A recent systematic review shows that the AI models could dramatically
improve if four particular adjustments were made: the use of multicenter datasets,
incorporation of time varying data, assessment of missing data as well as informative censoring,
and development of metrics of clinical utility (Goldstein et al., 2017). As a reasonable starting
point for minimizing data quality issues, the authors of the NAM Special Publication recommend
that data should adhere to the FAIR (findability, accessibility, interoperability, and reusability)
principles in order to maximize the value of data (Wilkinson et al., 2016). An often-overlooked
detail is when and where certain data become available and whether the mechanics of data
availability and access are compatible with the model being constructed.
Stakeholder education and managing expectations: The use of AI solutions presents a wide range
of challenges to law and ethics, most of which are still being worked out. For example, when a
physician makes decisions assisted by AI, it is not always clear where to place blame in the case
of failure. This subtlety is not new to recent technological advancements, and in fact was
brought up decades ago (American Journal of Bioethics, 2010). However, most of the legal and
ethical issues were never fully addressed in the history of computer-assisted decision support,
and a new wave of more powerful AI-driven methods only adds to the complexity of ethical
questions (e.g., the frequently condemned black box model) (Char et al., 2018).
Model builders need to better understand the datasets they choose to learn from. Decision
makers need to look beyond technical evaluations and ask for utility assessments. Media needs
to do a better job in articulating both immense potential and the risks of adopting the use of AI
in health care. Therefore, it is important to promote a measured approach to adopting AI
technology, which would further AI’s role as augmenting rather than replacing human actors.
This framework could allow the AI community to make progress while managing evaluation
challenges (e.g., when and how to employ interpretable models versus black-box models) as
well as ethical challenges that are bound to arise as the technology gets widely adopted.
National Academy of Medicine GAO-20-215SP 18
Authors: Hongfang Liu, PhD, Hossein Estiri, PhD, Jenna Wiens, PhD, Anna Goldenberg, PhD,
Suchi Saria, PhD, and Nigam Shah, MBBS, PhD
6. Deploying AI in Clinical Settings: For AI deployment in health care practice to be successful, it
is critical that the lifecycle of AI use be overseen through effective governance. IT governance
is the set of processes that ensure the effective and efficient use of IT in enabling an
organization to achieve its goals by overseeing the evaluation, selection, prioritization, and
funding, implementation, and tracking of IT projects. Another facet of IT governance that is
relevant to AI is data governance, which institutes methodical process that an organization
adopts to manage its data and ensure the data meet specific standards and business rules
before entering them into a data management system. A health care enterprise that seeks to
leverage AI should consider, characterize, and adequately resolve a number of key
considerations prior to moving forward with the decision to develop and implement an AI
solution (see Figure 3).
Figure 3 | Key Considerations for Instructional Infrastructure and Governance
Consideration
Relevant Governance Questions
Organizational Capabilities
Does the organization possess the necessary technologic (e.g., IT
infrastructure, IT personnel) and organizational (knowledgeable
and engaged workforce, educational and training capabilities) to
adopt, assess and maintain AI driven tools?
Data Environment
What data are available for AI development? Do current systems
possess the adequate capacity for storage, retrieval, and
transmission to support AI tools?
Interoperability
Does the organization support and maintain data at rest and in
motion per national and local standards for interoperability (e.g.,
SMART on FHIR)?
Personnel Capacity
What expertise exists in the health care system to develop and
maintain the AI algorithms?
Cost, Revenue, and Value
What will be the initial and ongoing costs to purchase, install, and
train users, to maintain underlying data models, and to monitor for
variance in model performance?
Is there an anticipated return on investment from the AI
deployment?
What is the perceived value for the institution related to AI
deployment?
National Academy of Medicine GAO-20-215SP 19
Safety and Efficacy
Surveillance
Are there governance and processes in place to provide regular
assessments of the safety and efficacy of AI tools?
Patient/Family/Consumer
Engagement
Does the institution have in place formal mechanisms for
patient/family/consumer such a council or advisory board that can
engage and voice concerns on relevant issues related to
implementation, evaluation etc.?
Cybersecurity and Privacy
Does the digital infrastructure for health care data in the
enterprise have sufficient protections in place to minimize the risk
of breaches of privacy if AI is deployed?
Ethics and Fairness
Is there an infrastructure in place at the institution to provide
oversight and review of AI tools to ensure that the known issues
related to ethics and fairness are addressed and that vigilance for
unknown issues is in place?
Regulatory Issues
Are there specific regulatory issues that must be addressed and if
so, what type of monitoring and compliance programs will be
necessary?
Organizational Approach to Implementation: AI development and implementation should follow
established best practice frameworks in implementation science and software development.
Frameworks for conceptualizing, designing and evaluating this process are discussed in more
detail in the NAM Special Publication, but all implicitly incorporate the most fundamental basic
health care improvement model, often referred to as a plan-do-study-act (PDSA) cycle first
introduced by W.E. Deming more than two decades ago (Deming, 2000). The PDSA cycle relies
on the intimate participation of employees involved in the work, detailed understanding of
workflows, and careful ongoing assessment of implementation that informs iterative
adjustments. Newer methods of quality improvement introduced since Deming represent
variations or elaborations of this approach. All too often, however, quality improvement efforts
frequently fail because they are focused narrowly on a given task or set of tasks using
inadequate metrics without due consideration of the larger environment in which change is
expected to occur (Muller, 2018).
Such concerns are certainly relevant to AI implementation. New technology promises to
substantially alter how medical professionals currently deliver health care at a time when
morale in the workforce is generally poor (Shanafelt et al., 2012). One of the challenges of the
use of AI in health care is that integrating it within the EHR and improving existing decision and
workflow support tools may be viewed as an extension of an already unpopular technology
National Academy of Medicine GAO-20-215SP 20
(Sinsky et al., 2016). Moreover, there are a host of concerns that are unique to AI, some well and
others poorly founded, which might add to the difficulty of implementing AI applications.
In recognition that basic quality improvement approaches are generally inadequate to produce
large-scale change, the field of implementation science has arisen to characterize how
organizations can undertake change in a systematic fashion that acknowledges their
complexity. Some frameworks are specifically designed for evaluating the effectiveness of
implementation, such as the Consolidated Framework for Implementation Research (CFIR) or
the Promoting Action on Research Implementation in Health Services (PARiHS). In general,
these governance and implementation frameworks emphasize sound change management and
methods derived from implementation science that undoubtedly apply to implementation of AI
tools (Damschroder et al., 2009; Rycroft-Malone, 2004).
Clinical Outcome Monitoring: The complexity and extent of local evaluation and monitoring may
necessarily vary depending on the way AI tools are deployed into the clinical workflow, the
clinical situation, and the type of CDS being delivered, as these will in turn define the clinical
risk attributable to the AI tool.
For higher risk AI tools, a focus on clinical safety and effectiveness—from either a non-
inferiority or superiority perspective—is of paramount importance even as other metrics (e.g.,
API data calls, user experience information) are considered. High-risk tools will likely require
evidence from rigorous studies for regulatory purposes and will certainly require substantial
monitoring at the time of and following implementation. For low-risk clinical AI tools used at
point of care, or those that focus on administrative tasks, evaluation may rightly focus on
process of care measures and metrics related to the AI’s usage in practice to define its positive
and negative effects. The authors of the NAM Special Publication strongly endorse
implementing all AI tools using experimental methods (e.g., randomized controlled trials or A/B
testing) where possible. Large-scale pragmatic trials at multiple sites will be critical for the field
to grow but may be less necessary for local monitoring and for management of an AI formulary.
In some instances, due to feasibility, costs, time constraints or other limitations, a randomized
trial may not be practical or feasible. In these circumstances quasi-experimental approaches
such as stepped-wedge designs or even carefully adjusted retrospective cohort studies, may
provide valuable insights.
National Academy of Medicine GAO-20-215SP 21
Monitoring outcomes after implementation will permit careful assessment, in the same manner
that systems regularly examine drug usage or order sets and may be able to utilize data that are
innately collected by the AI tool itself to provide a monitoring platform. Recent work has
revealed that naive evaluation of AI system performance may be overly optimistic, providing a
need for more thorough evaluation and validation.
Clinical AI performance can also deteriorate within a site when practices, patterns, or
demographics change over time. As an example, consider the policy by which physicians order
blood lactate measurements. Historically, it may have been the case that, at a particular
hospital, lactate measurements were only ordered to confirm suspicion of sepsis. A clinical AI
tool for predicting sepsis that was trained using historical data from this hospital would be
vulnerable to learning that the act of ordering a lactate measurement is associated with sepsis
rather than the elevated value of the lactate. However, if hospital policies change and lactate
measurements are more commonly ordered, then the association that had been learned by the
clinical AI would no longer be accurate. Alternatively, if the patient population shifts, for
example to include more drug users, then elevated lactate might become more common and the
value of lactate being measured would again be diminished. In both the case of changing policy
or patient population, performance of the clinical AI application is likely to deteriorate,
resulting in an increase of false positive sepsis alerts.
More broadly, such examples illustrate the importance of careful validation in evaluating the
reliability of clinical AI. A key means for measuring reliability is through validation on multiple
datasets. Classical algorithms that are applied natively or used for training AI are prone to
learning artifacts specific to the site that produced the training data or specific to the training
dataset itself. There are many subtle ways that site-specific or dataset-specific bias can occur in
real world datasets. Validation using external datasets will show reduced performance for
models that have learned patterns that do not generalize across sites (Schulam and Saria,
2017).
In addition to monitoring overall measures of performance, evaluating performance on key
patient subgroups can further expose areas of model vulnerability: High average performance
overall is not indicative of high performance across every relevant subpopulation. Careful
examination of stratified performance can help expose subpopulations where the clinical AI
model performs poorly and therefore poses higher risk. Further, tools that detect individual

National Academy of Medicine GAO-20-215SP 22
points where the clinical AI is likely to be uncertain or unreliable can flag anomalous cases. By
introducing a manual audit for these individual points, one can improve reliability during use
(e.g., Soleimani, Hensman, and Saria, 2018 and Schulam and Saria, 2019). Traditionally,
uncertainty assessment was limited to the use of specific classes of algorithms for model
development. However, recent approaches have led to wrapper tools that can audit some black
box models (Schulam and Saria, 2019). Logging cases flagged as anomalous or unreliable and
performing a review of such cases from time to time may be another way to bolster post
marketing surveillance, and FDA requirements for such surveillance could require such
techniques.
Authors: Stephan D. Fihn, MD, MPH, Suchi Saria, PhD, Eneida Mendonca, MD, PhD, Seth Hain,
MS, Michael Matheny, MD, MS, MPH, Nigam Shah, MBBS, PhD, Hongfang Liu, PhD, and Andrew
Auerbach, MD
7. Conclusion: AI in health care is poised to make transformative and disruptive advances in
health care. It is prudent to balance the need for thoughtful, inclusive health care AI that plans
for and actively manages and reduces potential unintended consequences, while not yielding to
marketing hype and profit motives. The straightforward path for AI is to start with real
problems in health care, explore the best solutions by engaging relevant stakeholders, frontline
users, patients and their families—including AI and non-AI options—and implement and scale
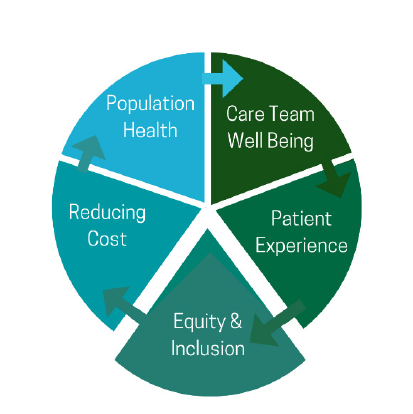
the ones that meet a new Quintuple Aim of equity and inclusion (See Figure 4).
National Academy of Medicine GAO-20-215SP 23
Figure 4 | Advancing the Quintuple Aim
SOURCE: Matheny, M., S. Thadaney, M. Ahmed, and D. Whicher, editors. Artificial Intelligence
and Health Care: The Hope, the Hype, the Promise, and the Perils. Washington, DC: National
Academy of Medicine.
In 21 Lessons for the 21st Century, Yuval Noah Harari writes, “Humans were always far better at
inventing tools than using them wisely” (Harari, 2018, p. 7). It is up to us, the stakeholders,
experts, and users of these technologies, to ensure that they are used in an equitable and
appropriate fashion to uphold the human values that inspired their creation—that is, better
health and wellness for all.
National Academy of Medicine GAO-20-215SP 24
BIBLIOGRAPHY
Accenture. 2018. Consumer Survey on Digital Health: US Results.
https://www.accenture.com/t20180306T103559Z__w__/us-en/_acnmedia/PDF-71/accenture-
health-2018-consumer-survey-digital-health.pdf (accessed November 12, 2019).
Barrett, M., V. Combs, J. G. Su, K. Henderson, M. Tuffli, and AIR Louisville Collaborative. 2018. AIR
Louisville: Addressing asthma with technology, crowdsourcing, cross-sector collaboration, and
policy. Health Affairs (Millwood) 37(4):525–534.
Berg, J. 2010. Review of “The Ethics of Consent, eds. Franklin G. Miller and Alan Wertheimer.”
American Journal of Bioethics 10(7):71–72.
Bostic, B. 2018. Using artificial intelligence to solve public health problems. Beckers Hospital Review.
https://www.beckershospitalreview.com/healthcare-information- technology/using-artificial-
intelligence-to-solve-public-health-problems.html (accessed November 12, 2019).
Brown, N., and T. Sandholm. 2018. Superhuman AI for heads-up no-limit poker: Libratus beats top
professionals. Science 359:418–424.
Buchanan, B. G. 2005. A (very) brief history of artificial intelligence. AI Magazine 26(4):53–60.
Char, D.S., N. H. Shah, and D. Magnus, D., 2018. Implementing machine learning in health care—
addressing ethical challenges. New England Journal of Medicine, 378(11), 981.
Cohen, G., and M. Mello. 2019. Big data, big tech, and protecting patient privacy. JAMA
322(12):1141–1142.
Contreras, I. and J. Vehi. 2018. Artificial intelligence for diabetes management and decision support:
literature review. Journal of medical Internet research, 20(5), .e10775.
Damschroder, L. J., D. C. Aron, R. E. Keith, S. R. Kirsh, J. A. Alexander, and J. C. Lowery. 2009.
Fostering implementation of health services research findings into practice: A consolidated
framework for advancing implementation science. Implementation Science 4(1):50.
Dankwa-Mullan, I., M. Rivo, M. Sepulveda, Y. Park, J. Snowdon, and K. Rhee. 2019. Transforming
diabetes care through artificial intelligence: the future is here. Population health
management, 22(3), 229-242.
Deming, W. E. 2000. The New Economics for Industry, Government, and Education. Cambridge, MA:
MIT Press.
http://www.ihi.org/resources/Pages/Publications/NewEconomicsforIndustryGovernmentEducati
on.aspx (accessed November 13, 2019).
Feigenbaum, E. 1992. Expert Systems: Principles and Practice. The Encyclopedia of Computer Science
and Engineering.
Fitzpatrick, K. K., A. Darcy, and M. Vierhile. 2017. Delivering cognitive behavior therapy to young
adults with symptoms of depression and anxiety using a fully automated conversational agenda
(Woebot): A randomized controlled trial. JMIR Mental Health 4(2):e19.
National Academy of Medicine GAO-20-215SP 25
Friedler, S. A., C. D. Roy, C. Scheidegger, and D. Slack. 2019. Assessing the local interpretability of
machine learning models. arXiv.org.
https://ui.adsabs.harvard.edu/abs/2019arXiv190203501S/abstract (accessed November 13,
2019).
Goldstein, B. A., A. M. Navar, M. J. Pencina, and J. Ioannidis. 2017. Opportunities and challenges in
developing risk prediction models with electronic health records data: A systematic review. Journal
of the American Medical Informatics Association 24(1):198–208.
Halevy, A., P. Norvig, and F. Pereira. 2009. The unreasonable effectiveness of data. IEEE Intelligent
Systems 24:8–12.
https://static.googleusercontent.com/media/research.google.com/en//pubs/archive/35179.pdf
(accessed November 8, 2019).
Harari, Y. N. 2018. 21 Lessons for the 21st Century. Random House: New York, NY, USA.
Herasevich, V., B. Pickering, and O. Gajic. 2018. How Mayo Clinic is combating information overload
in critical care units. Harvard Business Review.
https://www.bizjournals.com/albany/news/2018/04/23/hbr-how-mayo-clinic-is-combating-
information.html (accessed November 13, 2019).
Hindorff, L.A., Bonham, V.L., Brody, L.C., Ginoza, M.E., Hutter, C.M., Manolio, T.A. and Green, E.D.,
2018. Prioritizing diversity in human genomics research. Nature Reviews Genetics, 19(3), 175.
Imler, T. D., J. Morea and T. F. Imperiale. 2014. Clinical decision support with natural language
processing facilitates determination of colonoscopy surveillance intervals. Clinical Gastroenterology
and Hepatology, 12(7), 1130-1136.
Jackson, P. 1998. Introduction to Expert Systems. Boston: Addison-Wesley Longman Publishing Co.,
Inc.
Jamal, A., K. McKenzie, and M. Clark. 2009. The impact of health information technology on the
quality of medical and health care: A systematic review. Health Information Management Journal
38(3):26–37.
Kehl, K.L., H. Elmarakeby, M. Nishino, E. M. Van Allen, E.M. Lepisto, M. J. Hassett, B. E. Johnson, and
D. Schrag. 2019. Assessment of deep natural language processing in ascertaining oncologic
outcomes from radiology reports. JAMA Oncology, 5(10), 1421-1429.
Kline, R. R. 2011. Cybernetics, automata studies, and the Dartmouth Conference on Artificial
Intelligence. IEEE Annals of the History of Computing 33(4):5–16.
Krizhevsky, A., I. Sutskever, and G. E. Hinton. 2012. ImageNet classification with deep convolutional
neural networks. Presented at 25th International Conference on Neural Information Processing
Systems. https://papers.nips.cc/paper/4824-imagenet-classification-with-deep-convolutional-
neural-networks.pdf (accessed November 8, 2019).
Lazer, D., R. Kennedy, G. King, G. and A. Vespignani. 2014. The parable of Google Flu: traps in big
data analysis. Science, 343(6176), 1203-1205.
National Academy of Medicine GAO-20-215SP 26
Lee, K. J. 2018. AI device for detecting diabetic retinopathy earns swift FDA approval. American
Academy of Ophthalmology ONE Network. https://www.aao.org/headline/first-ai-screen-diabetic-
retinopathy-approved-by-f (accessed November 13, 2019).
Leondes, C. T. 2002. Expert Systems: The Technology of Knowledge Management and Decision Making
for the 21st century. San Diego: Academic Press.
Licitra, L., A. Trama, and H. Hosni. 2017. Benefits and risks of machine learning decision support
systems. JAMA 318(23):2354–2354.
Licklider, J. C. R. 1960. Man-computer symbiosis. IRE Transactions on Human Factors in Electronics.
HFE-1:4–11.
Lipton, Z. C. 2016. The mythos of model interpretability. arXiv.org.
https://arxiv.org/abs/1606.03490 (accessed November 13, 2019).
Liu, F., Z. Zhou, A. Samsonov, D. Blankenbaker, W. Larison, A. Kanarek, K. Lian, S. Kambhampati, and
R. Kijowski. 2018. Deep learning approach for evaluating knee MR images: achieving high
diagnostic performance for cartilage lesion detection. Radiology, 289(1), 160-169.
Manning, C. D., and H. Schütze. 1999. Foundations of statistical natural language processing. MIT
press.
Manrai, A. K., B. H. Funke, H. L. Rehm, M. S. Olesen, B. A. Maron, P. Szolovits, D. M. Margulies, J.
Loscalzo, and I. S. Kohane. 2016. genetic misdiagnoses and the potential for health disparities. New
England Journal of Medicine 375:655–665.
Matheny, M., S. Thadaney, M. Ahmed, and D. Whicher, editors. Artificial Intelligence and Health Care:
The Hope, the Hype, the Promise, and the Perils. Washington, DC: National Academy of Medicine.
Matheson, R. 2018a. Machine-learning system determines the fewest, smallest doses that could still
shrink brain tumors. MIT News. http://news.mit.edu/2018/artificial-intelligence-model-learns-
patient-data-cancer-treatment-less-toxic-0810.
McCarthy, J. 1974. Review of “Artificial Intelligence: A General Survey,” by Professor Sir James
Lighthill. Artificial Intelligence 5:317–322.
Mello, M., and I. Cohen. 2018. HIPAA and protecting health information in the 21st century. JAMA
320:231–232.
Meskó, B., G. Hetényi, and Z. Győrffy. 2018. Will artificial intelligence solve the human resource
crisis in healthcare? BMC Health Services Research 18(1): art. 545.
Miller, R. A., H. E. Pople, and J. D. Myers. 1982. Internist-1, an experimental computer-based
diagnostic consultant for general internal medicine. New England Journal of Medicine 307:468–476.
Moons, K. G., A. P. Kengne, D. E. Grobbee, P. Royston, Y. Vergouwe, D. G. Altman, and M. Woodward.
2012. Risk prediction models: II. External validation, model updating, and impact assessment. Heart
98(9):691–698.
National Academy of Medicine GAO-20-215SP 27
Moor, J. 2006. The Dartmouth College Artificial Intelligence Conference: The next fifty years. AI
Magazine 27(4):87–91.
Muller, J. Z. 2018. The Tyranny of Metrics. Princeton NJ: Princeton University Press.
Murff, H. J., F. FitzHenry, M. E. Matheny, N. Gentry, K. L. Kotter, K. Crimin, R. S. Dittus, A. K. Rosen, P.
L. Elkin, S. H. Brown, and T. Speroff. 2011. Automated identification of postoperative complications
within an electronic medical record using natural language processing. JAMA, 306(8), 848-855.
Newmarker, C. 2018. Digital surgery touts artificial intelligence for the operating room. Medical
Design & Outsourcing. https://www.medicaldesignandoutsourcing.com/digital-surgery-touts-
artificial-intelligence- for-the-operating-room/ (accessed November 12, 2019).
NRC (National Research Council). 1996. Language and Machines: Computers in Translation and
Linguistics: A Report. Washington, DC: National Academy Press.
Pethokoukis, J. 2016. What the story of ATMs and bank tellers reveals about the “rise of the robots”
and jobs. AEIdeas Blog. https://www.aei.org/publication/what-atms-bank-tellers-rise-robots-and-
jobs/ (accessed November 12, 2019).
Poon, H., C. Quirk, K. Toutanova, and S. Wen-tau Yih. 2018. AI for precision medicine. Project
Hanover. https://hanover.azurewebsites.net/#machineReading (accessed November 12, 2019).
Prates, M. O. R., P. H. C. Avelar, and L. Lamb. 2018. Assessing gender bias in machine translation—a
case study with Google Translate. arXiv.org. https://arxiv.org/abs/1809.02208 (accessed
November 12, 2019).
Rycroft-Malone, J. 2004. The PARIHS framework—a framework for guiding the implementation of
evidence-based practice. Journal of Nursing Care Quality 19(4):297–304.
Schulam, P., and S. Saria. 2017. Reliable decision support using counterfactual models. Advances in
Neural Information Processing Systems 30 (NIPS 2017), pp. 1697–1708.
https://papers.nips.cc/paper/6767-reliable-decision-support-using-counterfactual-models.pdf
(accessed November 13, 2019).
Schulam, P., and S. Saria. 2019. Can you trust this prediction? Auditing pointwise reliability after
learning. Proceedings of the 22nd International Conference on Artificial Intelligence and Statistics, pp.
1022–1031. http://proceedings.mlr.press/v89/schulam19a/schulam19a.pdf (accessed November
13, 2019).
Sennaar, K. 2018. Machine learning medical diagnostics—4 current applications. Emerj Artificial
Intelligence Research. https://emerj.com/ai-sector-overviews/machine-learning-medical-
diagnostics-4-current- applications/ (accessed November 12, 2019).
Shah, N. H., A. Milstein, and S. C. Bagley. 2019. Making machine learning models clinically useful.
JAMA 322(14):1351–1352. https://doi.org/10.1001/jama.2019.10306.
Shanafelt, T. D., S. Boon, L. Tan, L. N. Dyrbye, W. Sotile, D. Satele, C. P. West, J. Sloan, and M. R.
Oreskovich. 2012. Burnout and satisfaction with work-life balance among US physicians relative to
the general US population. Archives of Internal Medicine 172(18):1377–1385.
National Academy of Medicine GAO-20-215SP 28
Shortliffe, E. H. 1974. MYCIN: A Rule-Based Computer Program for Advising Physicians Regarding
Antimicrobial Therapy Selection. Palo Alto, CA: Stanford Artificial Intelligence Laboratory, Stanford
University.
Shortliffe, E. H., and B. G. Buchanan. 1975. A model of inexact reasoning in medicine. Mathematical
Biosciences 23 (3–4):351–379. doi: 10.1016/0025-5564(75)90047-4.
Sinsky, C., L. Colligan, L. Li, M. Prgomet, S. Reynolds, L. Goeders, J. Westbrook, M. Tutty, and G. Blike.
2016. Allocation of physician time in ambulatory practice: A time and motion study in 4 specialties.
Annals of Internal Medicine 165:753–760.
Snyder, L., D. McEwen, M. Thrun, and A. Davidson. 2016. Visualizing the local experience: HIV Data
to Care Tool. Online Journal of Public Health Informatics 8(1):e39.
Soleimani, H., J. Hensman, and S. Saria. 2018. Scalable joint models for reliable uncertainty-aware
event prediction. IEEE Transactions on Pattern Analysis and Machine Intelligence 40(8):1948–1963.
Subbaswamy, A., P. Schulam, and S. Saria. 2019. Preventing failures due to dataset shift: Learning
predictive models that transport. arXiv.org. https://arxiv.org/abs/1812.04597 (accessed
November 14, 2019).
Sumner, W. I. 1993. Review of Iliad and Quick Medical Reference for primary care providers.
Archives of Family Medicine 2:87–95.
Turing, A. M. 1950. Computing machinery and intelligence. Mind, 59:236, 99.49:433–460.
Voosen, P. 2017. The AI detectives. Science 357(6346):22–27.
https://science.sciencemag.org/content/357/6346/22.summary (accessed November 13, 2019).
Weiner, N. 1948. Cybernetics: Or Control and Communication in the Animal and the Machine.
Cambridge, MA: MIT Press.
Wiens, J., S. Saria, M. Sendak, M. Ghassemi, V. X. Liu, F. Doshi-Velez, K. Jung, K. Heller, D. Kale, M.
Saeed, P. N. Ossorio, S. Thadaney-Israni, and A. Goldenberg. 2019. Do no harm: A roadmap for
responsible machine learning for health care. Nature Medicine. 25(9):1137-1340.
Wiggers, K. 2018. CB Insights: AI health care startups have raised $4.3 billion since 2013.
VentureBeat. https://venturebeat.com/2018/09/13/cb-insights-ai-health-care-startups-have-
raised-4-3-billion-since-2013/ (accessed November 12, 2019).
Wilkinson, M. D., M. Dumontier, I. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J. W.
Boiten, L. B. da Silva Santos, P. E. Bourne, and J. Bouwman. 2016. The FAIR Guiding Principles for
scientific data management and stewardship. Scientific Data 3:art 160018.
Witten, I. H., E. Frank, M. A. Hall, and C. J. Pal. 2016. Data Mining: Practical Machine Learning Tools
and Techniques. Burlington, MA, USA: Morgan Kaufmann.
Zauderer, M. G., A. Gucalp, A. S. Epstein, A. D. Seidman, A. Caroline, S. Granovsky, J. Fu, J. Keesing, S.
Lewis, H. Co, J. Petri, M. Megerian, T. Eggebraaten, P. Bach, and M. G. Kris. 2014. Piloting IBM Watson
Oncology within Memorial Sloan Kettering’s regional network. Journal of Clinical Oncology 32(15
Suppl):e17653-e17653.
National Academy of Medicine GAO-20-215SP 29
AUTHORS of NAM SPECIAL PUBLICATION
MICHAEL MATHENY, Vanderbilt University Medical Center and the Department of Veterans Affairs (Co-
Chair)
SONOO THADANEY-ISRANI, Stanford University (Co-Chair)
ANDREW AUERBACH, University of California San Francisco
ANDY BEAM, Harvard University
PAUL BLEICHER, OptumLabs
WENDY CHAPMAN, University of Melbourne
JONATHAN CHEN, Stanford University
GUILHERME DEL FIOL, University of Utah
HOSSEIN ESTIRI, Harvard Medical School
JAMES FACKLER, Johns Hopkins School of Medicine
STEVE FIHN, University of Washington
ANNA GOLDENBERG, University of Toronto
SETH HAIN, Epic
JAIMEE HEFFNER, Fred Hutchinson Cancer Research Center
EDMUND JACKSON, Hospital Corporation of America
JEFFREY KLANN, Harvard Medical School
RITA KUKAFKA, Columbia University
HONGFANG LIU, Mayo Clinic
DOUG MCNAIR, Cerner
ENEIDA MENDONCA, University of Wisconsin Madison
JONI PIERCE, University of Utah
NICHOLSON PRICE, University of Michigan
JOACHIM ROSKI, Booz Allen Hamilton
SUCHI SARIA, Johns Hopkins University
NIGAM SHAH, Stanford University
RANAK TRIVEDI, Stanford University
JENNA WIENS, University of Michigan
NAM Staff
Development of this publication was facilitated by contributions of the following
NAM staff, under the guidance of J. Michael McGinnis, Leonard D. Schaeffer Executive Officer and
Executive Director of the Leadership Consortium for a Value & Science-Driven Health System:
DANIELLE WHICHER, Senior Program Officer (until September 2019)
MAHNOOR AHMED, Associate Program Officer
JESSICA BROWN, Executive Assistant to the Executive Officer (until September 2019)
FASIKA GEBRU, Senior Program Assistant
JENNA OGILVIE, Deputy Director of Communications

GAO Technology Assessment GAO-20-215SP 30
PART TWO
Artificial Intelligence in
Health Care:
Benefits and Challenges of
Machine Learning in Drug
Development
U. S. Government Accountability Office (GAO)
Part Two of this Joint Publication presents the GAO Technology Assessment: Artificial
Intelligence in Health Care: Benefits and Challenges of Machine Learning in Drug
Development. While GAO worked closely with the National Academy of Medicine giving
feedback on the January 2019 workshop outputs and in preparing the July 2019 meeting
on AI in drug discovery and development, the contents and resulting policy options of
this technology assessment are solely those of GAO and are the responsibility of GAO.
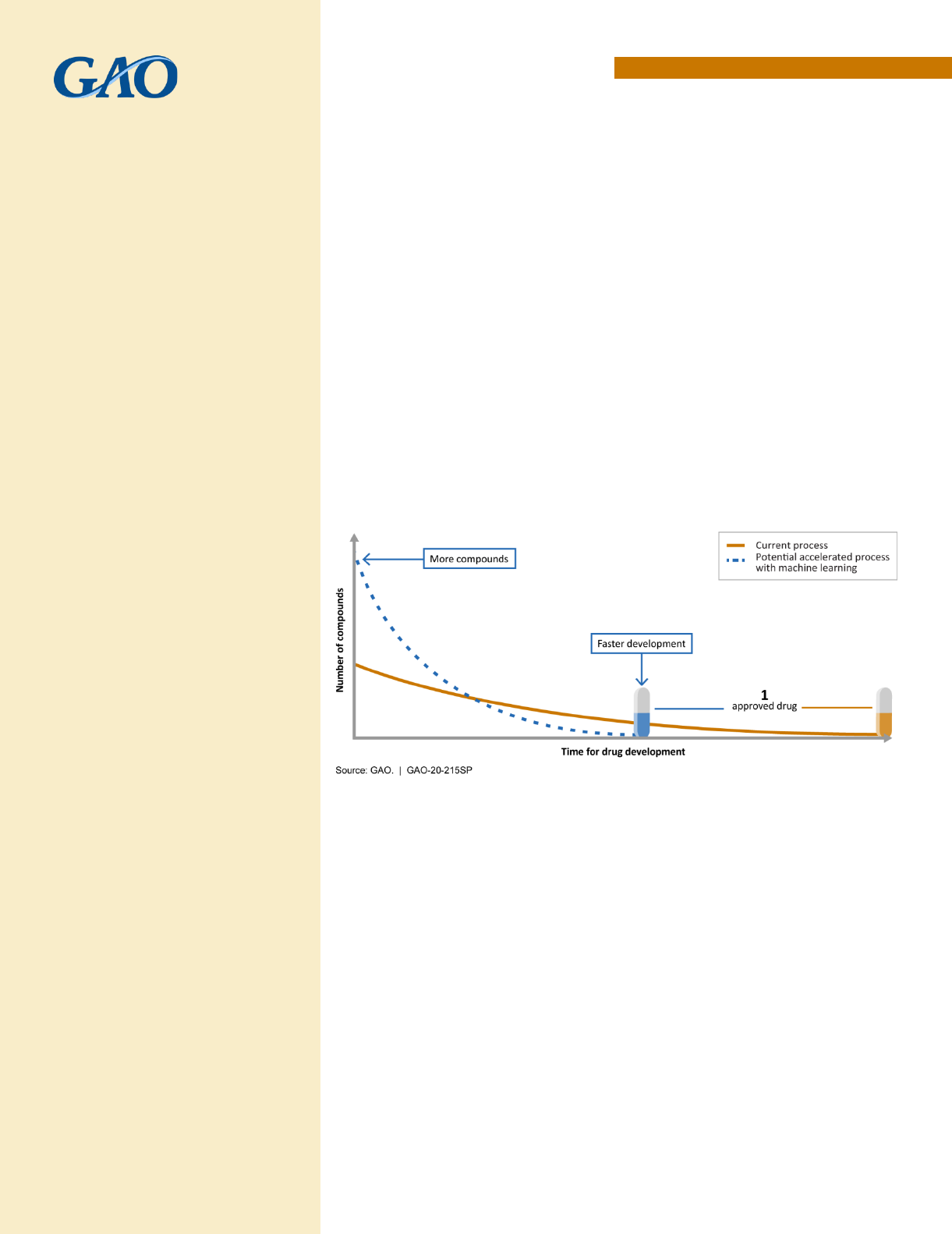
United States Government Accountability Office
Highlights of GAO-20-215SP, a report to
congressional
requesters
December 2019
TECHNOLOGY ASSESSMENT
ARTIFICIAL INTELLIGENCE IN HEALTH CARE
Benefits and Challenges of Machine Learning in
Drug Development
What GAO Found
Machine learning—a field of artificial intelligence (AI) in which software learns from
data to perform a task—is already used in drug development and holds the
potential to transform the field, according to stakeholders such as agency officials,
industry representatives, and academic researchers. Machine learning is used
throughout the drug development process and could increase its efficiency and
effectiveness, decreasing the time and cost required to bring new drugs to market.
These improvements could save lives and reduce suffering by getting drugs to
patients in need more quickly, and could allow researchers to invest more
resources in areas such as rare or orphan diseases.
Machine learning could accelerate drug development
This set of technologies could screen more chemical compounds and zero in on promising drug
candidates in less time than the current process.
Examples of machine learning in the early steps of drug development include:
• Drug Discovery: Researchers are identifying new drug targets, screening
known compounds for new therapeutic applications, and designing new
drug candidates, among other applications.
• Preclinical Research: Researchers are augmenting preclinical testing and
predicting toxicity before testing potential drugs in humans.
• Clinical Trials: Researchers are beginning to improve clinical trial design, a
point where many drug candidates fail. Their efforts include applying
machine learning to patient selection, recruitment, and stratification.
GAO identified several challenges that hinder the adoption and impact of machine
learning in drug development. Gaps in research in biology, chemistry, and machine
learning limit the understanding of and impact in this area. A shortage of high-
quality data, which are required for machine learning to be effective, is another
challenge. It is also difficult to access and share these data because of costs, legal
issues, and a lack of incentives for sharing. Furthermore, a low supply of skilled and
interdisciplinary workers creates hiring and retention challenges for drug
companies. Lastly, uncertainty about regulation of machine learning used in drug
development may limit investment in this field.
View GAO-20-215SP. For more information,
contact
Timothy M. Persons, PhD, at 202-
512
-6888 or personst@gao.gov.
Why GAO Did This Study
Developing and bringing a new drug to
market is lengthy and expensive
. Drug
developers study the benefits and risks
of new compounds before seeking Food
and Drug Administration (FDA) approval.
Only about one out of 10,000 chemical
compounds initially tested for drug
potential makes it through the research
and development pipeline, and is then
determined by FDA to be safe and
effective and approved for marketing in
the United States. Machine learning is
enabling new insights in the field.
GAO was asked to conduct a technology
assessment on the use of AI
technologies in drug development with
an emphasis on foresight and policy
implications. This report discusses (1)
current and emerging AI technologies
available for drug development and
their potential benefits; (2) challenges
to the development and adoption of
these technologies; and (3) policy
options to address challenges to the use
of machine learning in drug
development.
GAO assessed AI technologies used in
the first three steps of the drug
development process—drug discovery,
preclinical research, and clinical trials;
interviewed a range of stakeholder
groups including, government, industry,
academia, and nongovernmental
organizations; convened a meeting of
experts in conjunction with the National
Academies; and reviewed key reports
and scientific literature. GAO is
identifying policy options in this report.
United States Government Accountability Office
GAO developed six policy options in response to these challenges. Five policy options are centered around research, data
access, standardization, human capital, and regulatory certainty. The last is the status quo, whereby policymakers—federal
agencies, state and local governments, academic and research institutions, and industry, among others—would not
intervene with current efforts. See below for details of the policy options and relevant opportunities and considerations.
Policy Options to Address Challenges to the Use of Machine Learning in Drug Development
Opportunities
Considerations
Research (report page 60)
Policymakers could promote
basic research to generate
more and better data and
improve understanding of
machine learning in drug
development.
• Could result in increased scientific and technological
output by solving previously challenging problems.
• Could result in the generation of additional high-
quality, machine readable data.
• Basic research is generally considered a
long term investment and its potential
benefits are uncertain.
• Would likely require assessment of
available resources and may require
reallocation of resources from other
priorities.
Data Access (report page 61)
Policymakers could create
mechanisms or incentives for
increased sharing of high-
quality data held by public or
private actors, while also
ensuring protection of patient
data.
• Could shorten the length of the drug development
process and reduce costs.
• Could help companies identify unsuccessful drug
candidates sooner, conserving resources.
• Would likely require coordination between
various stakeholders and incur setup and
maintenance costs.
• Improper data sharing or use could have
legal consequences.
• Cybersecurity risks could increase, and
those threats would likely take additional
time and resources to mitigate.
• Organizations with proprietary data could
be reluctant to participate.
Standardization (report page
62)
Policymakers could
collaborate with relevant
stakeholders to establish
uniform standards for data
and algorithms.
•
Could improve interoperability by more easily
allowing researchers to combine different data sets.
• Could help efforts to ensure algorithms remain
explainable and transparent, as well as aid data
scientists with benchmarking.
•
Could be time- and labor-intensive because
standards development typically requires
consensus from a multitude of public and
private-sector stakeholders. This process
can result in standards development taking
anywhere from 18 months to a decade to
complete and require multiple iterations.
Human Capital (report page
63)
Policymakers could create
opportunities for more public
and private sector workers to
develop appropriate skills.
•
Could provide a larger pool of skilled workers for
agencies, companies, and other research
organizations, allowing them to better leverage
advances in the use of machine learning in drug
development.
• Interdisciplinary teamwork could improve as workers
with different backgrounds learn to better
communicate with one another.
•
Data science-trained workers could exit the
drug development field in search of higher-
paying opportunities.
• Would likely require an investment of time
and resources. Companies and agencies will
need to decide if the opportunities and
challenges justify the investment or shifting
of existing resources and how best to
provide such training.
Regulatory Certainty (report
page 64)
Policymakers could
collaborate with relevant
stakeholders to develop a
clear and consistent message
regarding regulation of
machine learning in drug
development.
• Could help increase the level of public discourse
surrounding the technology and allow regulators and
the public to better understand its use.
• Drug companies could better leverage the
technology if they have increased certainty
surrounding how, if at all, regulators will review or
approve the machine learning algorithms used in
drug development.
• Would likely require coordination within
and among agencies and other
stakeholders, which can be challenging and
require additional time and costs.
• If new regulations are promulgated,
compliance costs and review times could be
increased.
Status Quo (report page 65)
Policymakers could maintain
the status quo (i.e., allow
current efforts to proceed
without intervention).
• Challenges may be resolved through current efforts.
• Companies are already using machine learning and
may not need action from policymakers to continue
expanding its use.
• The challenges described in this report may
remain unresolved or be exacerbated.
Source: GAO.
GAO Technology Assessment GAO-20-215SP 33
Abbreviations
AI
artificial intelligence
FDA
Food and Drug Administration
HIPAA
Health Insurance Portability and Accountability Act of 1996
IND
investigational new drug application
MELLODDY
Machine Learning Ledger Orchestration for Drug Discovery
MLPDS
Machine Learning for Pharmaceutical Discovery and Synthesis Consortium
NDA
new drug application
NCATS
National Center for Advancing Translational Sciences
NIH
National Institutes of Health
R&D
research and development

GAO Technology Assessment GAO-20-215SP 34
441 G St. N.W.
Washington, DC 20548
Introduction
December 20, 2019
Congressional Requesters
It can take 10 to 15 years and high costs to develop a new drug and bring it to market.
5
During
this time, drug developers conduct tests to study the benefits and risks of new compounds
before seeking Food and Drug Administration (FDA) approval. About one out of every 10,000
chemical compounds initially tested for their drug potential makes it all the way through the
research and development (R&D) pipeline, and is then determined by FDA to be safe and
effective and approved for marketing in the United States. Although high costs and failure rates
make drug development risky, creating a safe and effective new drug can be extremely
rewarding for both the developer and the public. A highly successful new drug can cure or
alleviate diseases affecting millions of people, as well as generate significant revenue—some of
which could support R&D on new treatments for other diseases.
We reported in 2006 the view of some stakeholders that drug industry innovation had
stagnated.
6
Ten to twenty years ago it was widely recognized that the number of new drugs
being produced was generally declining, while R&D expenses were steadily increasing. For
example, we found that the drug industry had reported substantial increases in annual R&D
costs, and that the number of new drug applications (NDA) approved by FDA had not been
commensurate with those investments. At that time, a variety of factors were contributing to
the declining productivity of pharmaceutical R&D, according to experts, including limitations on
the scientific understanding needed to translate chemical and biological discoveries into safe
and effective drugs, business decisions, regulatory uncertainty, and intellectual property issues.
Recent technological developments are bringing new hope to drug development. Advances such
as the sequencing of the human genome and the increasing adoption of electronic health
records have generated vast amounts of data that could assist in the search for drugs to
prevent, treat, or cure serious illnesses. Advanced analytical capabilities, such as machine
learning and related artificial intelligence (AI) technologies, are enabling the industry to convert
these large volumes of complex data into new insights.
5
For example, one study estimated average out-of-pocket cost per new compound that received FDA approval between 2005 and
2013 to be $1.4 billion. See J. A. DiMasi, H. G. Grabowski, and R. W. Hansen, “Innovation in the Pharmaceutical Industry: New
Estimates of R&D Costs,” Journal of Health Economics, vol. 47 (2016). Other studies suggest lower development costs. For example,
another study estimated a median cost to develop cancer drugs of $600 million. See V. Prasad and S. Mailankody, “Research and
Development Spending to Bring Single Cancer Drug to Market and Revenues After Approval,” JAMA Internal Medicine, published
online September 11, 2017, https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/2653012.
6
GAO, New Drug Development: Science, Business, Regulatory, and Intellectual Property Issues Cited as Hampering Drug Development
Efforts, GAO-07-49 (Washington, D.C.: Nov. 17, 2006).
GAO Technology Assessment GAO-20-215SP 35
In view of the potential of AI to help address challenges in drug development, you asked us to
conduct a technology assessment in this area with an emphasis on foresight and policy
implications. This report discusses (1) current and emerging AI technologies available for drug
development, including discovery through clinical trials, and their potential benefits; (2)
challenges to the development and adoption of these technologies; and (3) policy options to
address challenges to the use of machine learning in drug development.
To address these objectives, we assessed available and developing AI technologies that
companies could use during the drug development process as well as the benefits and
challenges associated with their use. To do so, we reviewed key reports and scientific literature
describing current and developing technologies; attended relevant technical conferences and
workshops; and interviewed a variety of stakeholders, including agency officials, drug
companies—both biopharmaceutical and machine learning-focused, academic researchers, and
nongovernmental organizations.
In addition, we collaborated with the National Academies to convene a 2-day meeting of 19
experts on current and emerging machine learning technologies for use in drug development.
We worked with National Academies staff to identify experts from a range of stakeholder
groups including federal agencies, academia, industry, and legal scholars, with expertise
covering all significant areas of our review. During this meeting, we moderated discussion
sessions on several topics related to machine learning in drug development, including research
and example technologies; economic, legal, social, and health factors; and policy and regulatory
implications. Following the meeting, we continued to seek the experts’ advice to clarify and
expand on what we heard. Consistent with our quality assurance framework, we provided the
experts with a draft of our report and solicited their feedback, which we incorporated as
appropriate.
We limited the policy options included in this report to those that met the policy objective and
were within the report scope. We present six policy options in response to the challenges
identified during our work and discuss potential opportunities and considerations of each. The
options are not intended to be inclusive of all potential policy options. To develop the policy
options, we prepared a list of potential policy ideas based on a literature search, stakeholder
interviews, and the expert meeting. We removed ideas that were not likely to achieve the policy
objective or did not fit into the overall scope of our work. We grouped the remaining ideas
based on themes (e.g., human capital, data access). We combined ideas that (1) were
duplicative, (2) could be subsumed into a higher-level policy option, or (3) were examples of
how to implement a policy option rather than the option itself.
We focused our review on selected technologies in the first three steps of the typical drug
development process: drug discovery, preclinical research, and clinical trials. We did not assess
all available or developing technologies; instead, we selected examples to demonstrate the
breadth of machine learning technologies in drug development.
GAO Technology Assessment GAO-20-215SP 36
We conducted our work from February 2019 through December 2019 in accordance with all
sections of GAO’s Quality Assurance Framework that are relevant to technology assessments.
The framework requires that we plan and perform the engagement to obtain sufficient and
appropriate evidence to meet our stated objectives and to discuss any limitations to our work.
We believe that the information and data obtained, and the analysis conducted, provide a
reasonable basis for any findings and conclusions in this product.
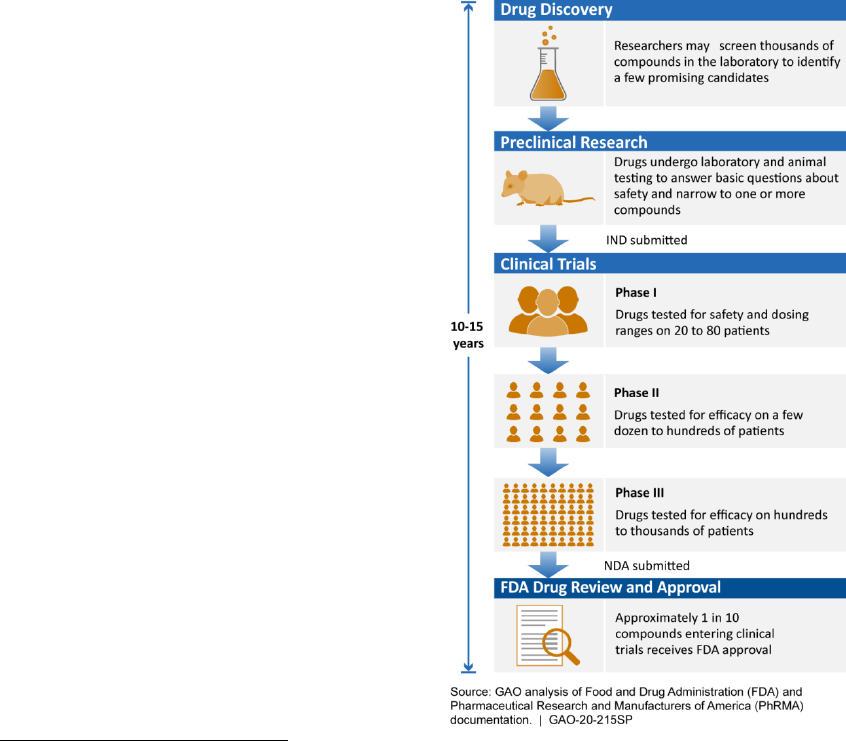
GAO Technology Assessment GAO-20-215SP 37
1 Background
1.1 The drug discovery, development,
and approval process
FDA is responsible for ensuring the safety and
efficacy of drugs marketed in the United
States.
7
According to FDA officials, if AI
technology is submitted to the agency to
support a drug development program or
marketing application, FDA will consider that
technology in its review process. Additionally,
FDA is closely tracking the use of AI in drug
development and is considering its policy
approach to this area, according to officials.
The process of bringing a new drug to market
is long and costly, with only a small fraction of
compounds identified early in the process
eventually receiving FDA approval (see fig. 1).
7
21 U.S.C. § 393(b)(2)(B). Drugs are defined to include, among
other things, articles intended for use in the diagnosis, cure,
mitigation, treatment, or prevention of disease in man or other
animals, and include components of those articles. See 21
U.S.C. §§ 321(g)(1)(B), (D). FDA is also responsible for ensuring
the safety, purity, and potency of biological products. 42 U.S.C.
§ 262(a). Biological products (referred to as biologics in this
report) are materials, such as viruses, therapeutic sera, toxins,
antitoxins, vaccines, or analogous products to prevent, treat, or
cure human diseases or injuries. See 42 U.S.C. § 262(i); 21
C.F.R. § 600.3(h). Most biologics are complex mixtures, and are
derived from living sources (such as humans, animals, and
microorganisms), unlike most drugs, which are chemically
synthesized. Though the FDA approval process is different for
drugs and biologics, for the purposes of this report, we refer to
drugs and biologics collectively as “drugs.”
Figure 1: The typical drug development and
approval process
Note: IND=investigational new drug application, NDA=new
drug application. According to FDA officials, there can be wide
variation in the number of patients involved in the different
clinical trial phases. When a new drug is being tested for a life-
threatening ailment, they said, the drug development process
may be expedited by going through only one or two phases of
clinical trials before an application is submitted to FDA for
marketing approval.
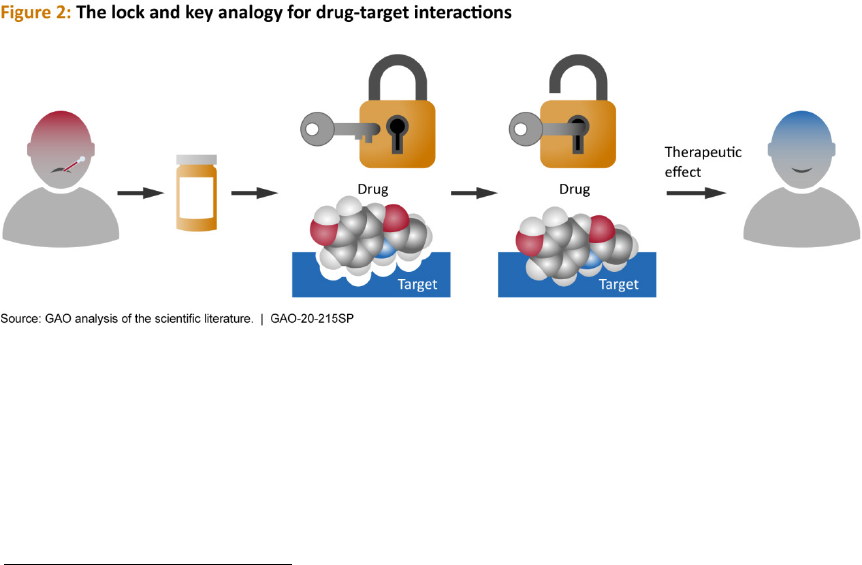
GAO Technology Assessment GAO-20-215SP 38
The drug development process typically
consists of the following five steps:
8
Drug discovery: The drug development
process begins with basic research aimed at
acquiring new knowledge without immediate
commercial application or use. Researchers
conduct basic research to better understand
the underlying mechanisms of disease, thus
increasing the potential for discovering and
developing drugs. Using this knowledge,
researchers seek to identify and validate a
biological target associated with the disease
of interest. A target may be a protein, gene,
or other biological entity that can be acted on
by a drug to achieve a desired therapeutic
outcome. The relationship between target
and small molecule drug is often described
using a lock and key analogy—the drug must
fit appropriately into the binding pocket of
the target to have an effect (fig. 2).
8
This report focuses on the first three of these steps.
Good target identification and validation
enables increased confidence in the
relationship between target and disease.
Once a target is identified and validated,
researchers screen thousands of compounds
from known chemical libraries, or design new
compounds, for the desired biological
response to the target during testing.
Researchers only focus on a small number of
these compounds, which have shown the
most effective response against the target, to
further develop as a potential drug. They
conduct experiments to gather information
on mechanisms of action, side effects,
dosage, delivery, and differential effects
across populations, among others, before
advancing promising compounds to
preclinical research.

GAO Technology Assessment GAO-20-215SP 39
Preclinical research: Before testing a drug
candidate in humans, drug companies test for
toxicity—whether the drug candidate is likely
to be safe in humans—using in vitro (i.e., in
cells or tissues in test tubes or other
chambers) and in vivo (i.e., in animals)
methods. These tests are also used to gather
basic information on the safety and efficacy of
the drug. If the results are promising, the
company may decide to move the drug
candidate forward to the next step—clinical
trials in humans. Generally, before doing so,
the company must submit an investigational
new drug application (IND) to FDA; an IND
must include, among other things,
information from preclinical research and the
clinical trial protocols.
9
Clinical trials: Clinical trials test drug
candidates in human volunteers to gather
data on safety and efficacy in humans.
Typically, clinical trials proceed through
phases I, II, and III, generally beginning with
testing in a small group of healthy volunteers
and then moving on to testing in larger
groups of patients the drug candidate is
intended to treat. Each clinical trial phase is
designed to accomplish something different.
10
FDA drug review and approval: In most cases,
to market a new drug in the United States,
drug companies submit an NDA to FDA, which
includes safety and efficacy data collected
during clinical trials. FDA then reviews and
approves the drug for marketing if the data
show it to be safe and effective for its
intended use.
9
FDA reviews the IND to, among other things, assure the safety
and rights of volunteers who participate in clinical studies. In
general, clinical studies may begin 30 days after the FDA
receives the IND, unless FDA objects. 21 U.S.C. §§ 355(i)(2),
(i)(3); 21 C.F.R. § 312.40.
10
See 21 C.F.R. § 312.21.
Post-approval: After FDA has approved a drug
and the company has begun marketing, FDA
continuously monitors the safety of the drug.
FDA can require companies to conduct post-
approval studies or clinical trials (known as
phase IV clinical trials) to assess a known
serious risk, signals of a serious risk, or to
identify an unexpected serious risk when data
indicate a risk potential.
11
Drug companies
may also undertake these studies
independently to identify modifications to the
drug, such as new delivery mechanisms or
additional indications for use.
1.2 Machine learning in AI innovation
Machine learning systems are a central focus
of the current AI innovation in drug
development.
12
As explained in our previous
work, AI has been conceptualized as having
three waves of development (see fig. 3):
13
• Wave 1 – expert or rules-based systems;
• Wave 2 – statistical learning and
perceiving and prediction systems; and
• Wave 3 – abstracting and reasoning
capability, including explainability.
11
21 U.S.C. § 355(o)(3).
12
AI, which was founded on the idea that machines could be
used to simulate human intelligence, has been defined in a
variety of ways. Researchers have also distinguished between
narrow AI—applications that provide domain-specific expertise
or task completion, and general AI—systems that exhibit
intelligence comparable to that of a human, or beyond.
13
GAO, Artificial Intelligence: Emerging Opportunities,
Challenges, and Implications, GAO-18-142SP (Washington, D.C.:
March 28, 2018).
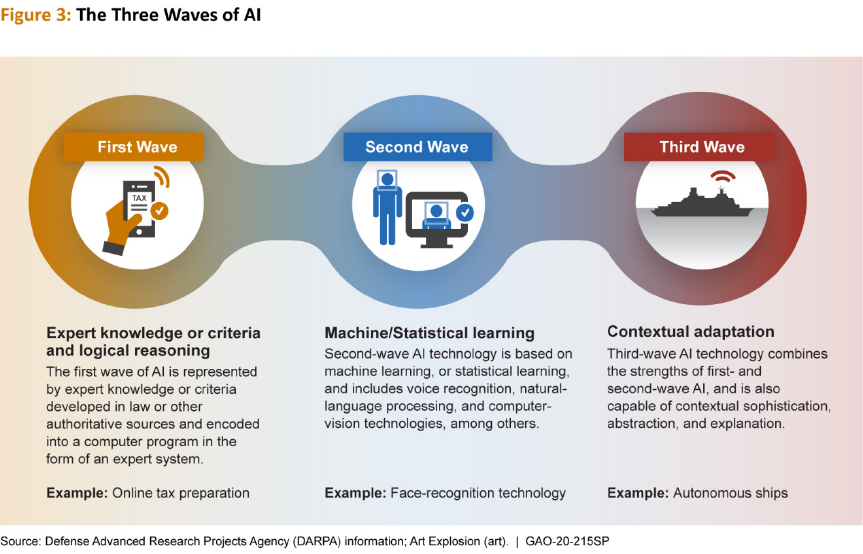
GAO Technology Assessment GAO-20-215SP 40
Machine learning, the basis for second-wave
AI technology, begins with data—generally in
vast amounts—and infers rules or decision
procedures that accurately predict specified
outcomes on the basis of the data provided.
In other words, machine learning systems can
learn from data, known as the training set, in
order to perform a task. Increased availability
of large data sets and computing power has
enabled recent machine learning advances
such as voice recognition by personal
assistants on smart phones, an example of
natural language processing, and image
recognition, an example of computer vision.
Researchers use several methods to train
machine learning algorithms, including:
• Supervised machine learning—the data
scientist presents an algorithm with
labeled data or input; the algorithm
identifies logical patterns in the data and
uses those patterns to predict a specified
answer to a problem. For example, an
algorithm trained on many labeled images
of cats and dogs could then classify new,
unlabeled images as containing either a
cat or a dog.
• Unsupervised machine learning—the data
scientist presents an algorithm with
unlabeled data and allows the algorithm
to identify structure in the inputs, for
example by clustering similar data,
without a preconceived idea of what to
expect. In this technique, for example, an
algorithm could cluster images into
groups based on similar features, such as
a group of cat images and a group of dog
images, without being told that the
images in the training set are those of
cats or dogs.
• Semisupervised learning—the data
scientist provides an algorithm with a

GAO Technology Assessment GAO-20-215SP 41
training set that is partially labeled. The
algorithm uses the labeled data to
determine the pattern and apply labels to
the remaining data.
• Reinforcement learning—an algorithm
performs actions and receives rewards or
penalties in return. The algorithm learns
by developing a strategy to maximize
rewards.
While classical machine learning algorithms
have been used in drug development for
years, recent interest in this area stems from
advances in deep learning.
14
An artificial
neural network is a machine learning
algorithm which, inspired by the brain,
contains an input layer that receives data,
hidden layers that process data, and an
output layer. Deep learning uses deep neural
networks, which contain a large number of
hidden layers. By contrast, classical artificial
neural networks were technologically limited
to one or two hidden layers. The types of
deep neural networks that are seeing success
in other applications are also finding uses in
drug development. For example:
• Techniques that are widely used in
computer vision can also be used to
process biological images such as images
of cells from microscopes.
• Techniques often used with sequential
data—for example, natural language
processing of a text document—can be
used to mine scientific literature or
process molecular data such as the
chemical code in a molecule of DNA.
14
In this report, we use the term “classical machine learning” to
refer to methods not based on deep learning. Examples of
classical machine learning include support vector machines and
random forest.
• Unsupervised learning techniques can be
used to generate new chemical structures
with desirable therapeutic properties.
Deep learning algorithms, as well as many
classical machine learning algorithms, are
considered black-box systems, meaning users
are unable to understand why the system
makes a specific decision or recommendation,
why a decision may be in error, or how an
error can be corrected. Researchers are
actively investigating ways to increase the
interpretability or explainability of these
algorithms.
1.3 Data generated and used in health
care
The generation, collection, access to, and use
of data are important aspects of both health
care and machine learning research and
applications.
15
There are multiple types of
data relevant to drug development, including
data generated through biomedical research
to better understand the biology of diseases
and pharmacology of potential drugs, and the
various forms of patient data generated in the
health care field. Biomedical research data,
such as data on the toxicity of known
compounds or structures of proteins, may be
owned by the organization that generated it
or may be publicly available. Recent patient
data can be found, for example, in electronic
health records, which are digital versions of
medical records that can include a person’s
medical and treatment history, such as
diagnoses, medications, and treatment
15
This report discusses, generally, the many types of data that
could potentially be used for machine learning in drug
development. Specific identification of the legal framework
that governs each type of data is outside the scope of this
report.
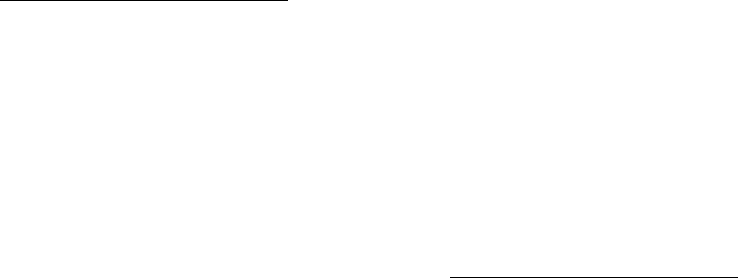
GAO Technology Assessment GAO-20-215SP 42
plans.
16
Both biomedical research and patient
data can be useful for machine learning
training, algorithm design, and drug
development. However, the factors affecting
use of these data differ.
Privacy protections concerning use of
health data
Health data, such as the types of patient data
described above, may include individually
identifiable health information
17
that may be
protected by the Health Insurance Portability
and Accountability Act of 1996 (HIPAA) and its
implementing regulations, known as the
Privacy Rule, as well as by other federal and
state laws.
18
The HIPAA Privacy Rule governs
the use and disclosure of individuals’ health
information and also provides individuals with
privacy rights with regard to their health
information. The Privacy Rule generally
prohibits regulated entities,
19
which may
include health care data warehouses, from
using or disclosing protected health
16
Office of the National Coordinator for Health Information
Technology (ONC) https://www.healthit.gov/faq/what-
electronic-health-record-ehr (accessed 10/17/2019).
17
“Individually identifiable health information” is health
information, including genetic and demographic information
collected from an individual, that (1) is created or received by a
health care provider, health plan, employer, or health care
clearinghouse; (2) relates to the past, present, or future
physical or mental health condition of the individual or the
provision or payment for health care to the individual, and (3)
can be used to identify the individual or with respect to which
there is a reasonable basis to believe the information can be
used to identify the individual. 45 C.F.R. § 160.103.
18
The Privacy Rule preempts any contrary state law unless the
provision of the state law relates to the privacy of individually
identifiable health information and is more stringent than a
standard, requirement, or implementation of the Privacy Rule.
Accordingly, state laws may also play a role in the area of
health data privacy.
19
One expert noted that health data relevant to drug
development may be held in non-HIPAA-covered environments
(for example, non-HIPAA research organizations or technology
companies that are not acting on behalf of HIPAA-covered
entities).
information except as specifically permitted,
such as for research purposes, under the
following conditions:
• with individual authorization,
20
• without individual authorization if the
covered entity obtains documentation
that an institutional review or a privacy
board has granted waiver of the
authorization requirement,
21
• for review preparatory to research,
22
• for a limited data set with a data use
agreement,
23
and
• if the protected health information has
been de-identified.
24
1.4 Economic considerations of drug
development
1.4.1 Grants and tax breaks
The federal government supports new drug
R&D both directly—through grants from
agencies such as the National Institutes of
Health (NIH) and National Science
Foundation—and indirectly through tax
incentives. Specifically, the Internal Revenue
Code includes incentives for research-related
spending, for example: through two income
tax credits—the credit for clinical testing
expenses for certain drugs for rare diseases or
conditions,
25
and the credit for increasing
20
45 C.F.R. § 164.508(a)(1).
21
45 C.F.R. § 164.512(i)(l)(i).
22
45 C.F.R. § 164.512(i)(l)(ii).
23
45 C.F.R. § 164.514(e).
24
45 C.F.R. § 164.502(d)(2). One expert noted that information
that has been de-identified for purposes of HIPAA may
nevertheless be re-identifiable, and this is a source of privacy
concerns for the large data sets used for machine learning.
25
See 26 U.S.C. § 45C.

GAO Technology Assessment GAO-20-215SP 43
research activities
26
—and through deductions
of research and experimental expenditures.
27
1.4.2 Economic incentives for innovation
We previously found that, revenues, costs,
and policy incentives influence drug industry
R&D investment decisions, according to
studies and industry experts.
28
For example,
drug companies may invest more in R&D of
drugs with therapeutic effects for a large
number of patients rather than those
targeting smaller groups because they expect
those investments to generate higher future
streams of revenue.
29
Higher costs of
research and innovation lead companies to
seek to reduce costs by, for example, focusing
on cheaper clinical trials, modifying existing
drugs, and acquiring existing research
projects at lower costs.
Policy incentives, such as patent protection
and market exclusivities, can also influence
investment decisions. Patents and market
26
See 26 U.S.C. § 41.
27
See 26 U.S.C. § 174.
28
GAO, Drug Industry: Profits, Research and Development
Spending, and Merger and Acquisition Deals, GAO-18-40
(Washington, D.C.: Nov. 17, 2017).
29
However, there are also incentives to develop drugs for small
populations, such as the tax credit for clinical testing expenses
for certain drugs for rare diseases or conditions mentioned
above.
exclusivity periods are two ways drug
companies may recoup their R&D
investments by limiting competition for
specified periods of time. Typically, early in
the R&D process, companies developing a
new brand-name drug apply to the U.S.
Patent and Trademark Office for a patent on
the active ingredient in the drug, among other
things.
30
Once the patent is granted, other
drug companies are excluded from making,
using, or selling the patented aspect of the
drug during the patent term.
31
Additionally,
market exclusivity is a specified period of time
during which FDA generally cannot approve a
similar competing version of the drug for
marketing. In general, the availability and
length of an exclusivity period depends on the
type of drug and its approved indication. For
example, new chemical entities are eligible
for five years of market exclusivity upon FDA
approval. However, there is also some
evidence in the economics literature to
suggest that greater competition may be
associated with higher incentives to innovate
in certain circumstances.
30
See 35 U.S.C. §§ 111, 154.
31
Typically, a patent term is 20 years from the date on which
the patent application was filed.
GAO Technology Assessment GAO-20-215SP 44
2 Status and Potential Benefits of Machine Learning in Drug
Development
Machine learning holds the potential to
transform drug development, according to
agency officials, industry representatives, and
academic researchers. Stakeholders stated
that machine learning can make drug
development more efficient and effective,
decreasing the time and cost required to
bring potentially more effective drugs to
market (see fig. 4). Both of these
improvements could save lives and reduce
suffering by getting drugs to patients in need
more quickly. Lower R&D costs could also
allow researchers to invest more resources in
disease areas that are currently not
considered profitable to pursue, such as rare
or orphan diseases.
32
32
Rare diseases became known as orphan diseases because
drug companies were not interested in adopting them to
develop treatments.
Drug companies—including both
biopharmaceutical and machine learning-
focused companies—are already using
machine learning throughout the drug
development process. The five
biopharmaceutical companies we spoke to
said they are incorporating machine learning
techniques at every step of the process, while
the five machine learning-focused companies
we interviewed tend to focus on particular
steps or aspects.
33
The best opportunities for
machine learning are in particularly data-rich
aspects of the process, according to an
industry group. For example, natural language
processing can help researchers mine the vast
and growing scientific literature to prioritize
33
One biopharmaceutical company did not directly answer the
question but described several examples across the process.

GAO Technology Assessment GAO-20-215SP 45
the most relevant publications and identify
patterns in published research. According to
one biopharmaceutical company
representative, these techniques augment
the work of their researchers through more
focused literature research and by connecting
disparate concepts in unanticipated ways.
The specific machine learning techniques that
researchers use generally depends on the
application. Stakeholders emphasized the
importance of using algorithms that are “fit-
for-purpose”—that is, using the best
algorithm for the specific application. As in
other domains, recent advances in deep
learning have generated excitement in the
field of drug development. These methods
offer advantages over classical machine
learning, such as the ability to process larger
data sets, and can demonstrate improved
predictive performance. However, deep
learning comes with a high computational
cost, and does not always outperform
classical machine learning techniques.
Optimizing machine learning algorithms,
especially those for deep learning, for specific
applications is not trivial, and researchers
tend to individualize models for each
application or data set. Deep learning also
requires large quantities of data, which,
according to stakeholders, may not be
available depending on the application. For
example, according to a company working in
clinical trial optimization, they do not use
deep learning because the data they use are
not available in large enough quantities.
According to a scientific review from 2018, it
is too early to determine whether deep
learning is superior to other machine learning
techniques, although deep learning is
superior for certain tasks such as image
analysis and has shown promise in several
applications relevant to drug development.
34
2.1 Drug discovery
Machine learning in drug discovery, the
earliest step of the drug development
process, is an active area of research. One
company we interviewed said that machine
learning during this step will allow them to
find better drugs earlier in the process and
help speed the drugs through the rest of the
process. They estimated that this early-stage
acceleration will allow for R&D cost savings of
between $300 million and $400 million per
successful drug.
Researchers are using machine learning for
several purposes in the drug discovery step,
including identifying new targets, screening
known compounds for new therapeutic
applications, and designing new drug
candidates.
35
Machine learning techniques
are enabling better understanding of disease
biology by allowing researchers to mine and
analyze large biological data sets. Researchers
are, for example, mining biological data to
identify new drug targets. Traditionally,
researchers often discovered targets through
basic research, sometimes relying on a certain
level of serendipity. Poor efficacy is a
common cause of failure when drug
candidates reach clinical trials. One possible
reason is a weak biological link between the
34
H. Chen, O. Engkvist, Y. Wang, M. Olivecrona, and T. Blaschke,
“The rise of deep learning in drug discovery,” Drug Discovery
Today, vol. 23, no. 6 (2018).
35
The examples in this section are not an exhaustive list of
machine learning applications in drug discovery. For example,
researchers are also employing machine learning for predictive
modeling of structure-activity relationships; absorption,
distribution, metabolism, and excretion properties; and
synthesis planning.

GAO Technology Assessment GAO-20-215SP 46
target and the disease the drug candidate is
intended to treat.
Machine learning has the potential to find
targets with stronger links. For example,
researchers recently used a semisupervised
learning approach to predict potential drug
targets using data on genes that have been
found to be associated with various
diseases.
36
The data came from Open Targets,
a public-private partnership that compiles and
openly shares a variety of data that could be
used to link genes to diseases for drug target
identification and prioritization.
37
The
researchers compared four algorithms and
found that an artificial neural network
performed the best, predicting more than
1,000 genes as potential new targets.
Predictive tools such as these could help
companies invest resources in targets with a
strong link to the disease of interest and
therefore increase the likelihood of
developing an effective drug. However, such
predicted targets still need to be validated in
the laboratory, according to the scientific
literature. Additionally, while resources such
as Open Targets provide open access to
biological data, scientific literature indicates
that disease-relevant data are generally
insufficiently available and not evenly
distributed across diseases areas.
One of the most common uses of machine
learning in drug development is virtual
screening of compounds, according to the
scientific literature. There are two principal
strategies used in the laboratory for screening
compounds: target-based screening, which
36
E. Ferrero, I. Dunham, and P. Sanseau, “In silico prediction of
novel therapeutic targets using gene-disease association data,”
Journal of Translational Medicine, vol. 15, no. 182 (2017).
37
Data compiled by Open Targets include genetics, gene
expression, literature, disease pathway, and drug data.
measures the effect compounds have on a
selected target such as a protein, and
phenotypic screening, which measures the
effect compounds have on a whole system
such as a cell or organism. In high throughput
screening, automated systems are used to
simultaneously run assays, or biological tests,
on large numbers of compounds.
Pharmaceutical companies often have large,
proprietary collections of compounds—
known as chemical libraries—for screening.
Virtual screening is complementary to high
throughput screening and can reduce costs
and labor of the screening process.
Researchers can use machine learning or
other computational techniques to prioritize
compounds for further testing, rather than
conducting an expensive screen of the full
library in the laboratory. Additionally, some of
the biological assays discovered through
academic research are very good
representations of disease phenotypes but
are not amenable to high throughput
screening, according to an expert. Machine
learning can help researchers take advantage
of new developments in biology. For example,
one expert described how they used three
separate machine learning models—trained
using data on around 200 compounds
found to be active against the target in
biological assays—to screen 12 million
commercially available compounds for
activity on a protein whose dysfunction is
associated with heart failure. They purchased
200 compounds based on the results of that
screen and found that one-third of those
were active in laboratory tests. In contrast, a
laboratory screen of a massive chemical
library can have a success rate as low as
0.01%. According to the company
representative, after further optimization,
three compounds advanced to preclinical
animal studies. The company completed

GAO Technology Assessment GAO-20-215SP 47
three design cycles in one year, whereas it
could take six years to reach that point in a
traditional drug discovery program.
Advances in computer vision due to deep
learning have translated into applications in
biological imaging, which is widely used
throughout the drug development process.
According to one expert, it is especially useful
in phenotypic drug discovery. Researchers use
techniques such as microscopy, among
others, to understand the effects of a drug
candidate on hosts (humans or animals),
organs, tissues, or cells and their organelles.
High-throughput microscopy imaging, for
example, is often used to screen compounds
for biological activity based on the structural
changes they induce on a cell or its
organelles. According to an expert, the field of
biological imaging is rapidly becoming
quantitative, presenting an opportunity for
data science. Similarly, a company
representative told us that microscopy images
generate rich information but are hard to
interpret, and the use of machine learning
could lead to new insights. For example, an
expert described how, in a screen for
compounds that cause cancer cells to
differentiate into a less harmful form,
computer vision enabled researchers to
precisely measure cell changes in a way that is
not possible by the human eye alone. Many
studies have demonstrated the ability of deep
learning to outperform classical techniques
for image analysis in this field, according to a
scientific review.
38
Deep learning can process
the large amounts of data generated by these
and similar techniques and could potentially
automate laborious tasks. However, the
training of deep neural networks for imaging
38
Chen, Engkvist, Wang, Olivecrona, and Blaschke, “The rise of
deep learning in drug discovery,” 1248.
is time-consuming and computationally
expensive, and large, high-quality data sets
are relatively rare in biological imaging.
In addition to screening known compounds,
researchers may also try to generate new,
previously unknown compounds with the
desired biological properties, a process
known as de novo drug design. These efforts
have benefitted from advances in deep
learning techniques, such as generative
adversarial networks and reinforcement
learning. These networks contain two models:
a generator that creates new molecules and a
discriminator that estimates how likely it is
that a molecule came from the training set or
the generator (see text box). Using
reinforcement learning, the generator is
rewarded when it fools the discriminator (i.e.,
the discriminator labels new molecules as
coming from the training set), and the
discriminator is rewarded when it correctly
labels molecules.
Machine learning models can be generative or discriminative
Generator: A generative model learns the distribution of the
training set in order to create new, or synthetic, data points.
For example, given a training set of molecules with a variety of
toxicities, the model generates new molecules with a desired
toxicity profile.
Discriminator: A discriminative model learns a direct map from
inputs to labels so that it can classify new inputs based on
those labels. For example, given the same training set as above,
the model will aim to predict the toxicity of a given molecule.
Source: GAO analysis of the scientific literature. | GAO-20-
215SP
Models such as these can be used to produce
new molecules with desired physical or
biological properties. For example, a
representative from one machine learning-
focused company told us that using
generative adversarial networks, they were
able to generate very potent inhibitors for a

GAO Technology Assessment GAO-20-215SP 48
particular disease target in less than two
months—a process that would normally take
two to three years. A potential drawback to
this type of technique is that generative
adversarial networks and reinforcement
learning are prone to the problem of mode
collapse, wherein the model only generates a
small number of similar solutions.
Additionally, researchers must ensure that
machine learning-generated molecules are
realistic (i.e., chemically stable and able to be
synthesized) and should also validate
predicted biological or physical properties in
the laboratory.
2.2 Preclinical research
Machine learning can be used to augment
preclinical testing and predict clinical trial
outcomes.
39
While the preclinical step is
intended to test for toxicity, according to FDA,
researchers also use this step to gather basic
information on the safety and efficacy of the
drug. Despite these efforts, failure rates in
clinical trials remain high. Success during
preclinical testing is highly dependent on the
selected animal models, which are meant to
represent specific aspects of a human disease
but cannot reproduce all potential
complexities.
40
One expert stated that it may
eventually be possible to build machine
learning models that are comparable to or
better than animal models. According to
stakeholders, many animal models are poor
predictors of human response to drugs.
Computational methods as an alternative or
39
The examples in this section are not an exhaustive list of
machine learning applications in preclinical research. For
example, researchers are using machine learning to predict
clinical efficacy.
40
T. Denayer, T. Stöhr, M. Van Roy, “Animal models in
translational medicine: Validation and prediction,” New
Horizons in Translational Medicine, vol. 2 (2014).
complement to animal models could reduce
the time and cost of bringing a new drug to
patients by helping better predict clinical trial
outcomes and could address concerns about
the use of animals in research. However,
another expert did not expect the industry to
move away from animal studies because of
the desire to know whether the drug has any
pharmacologically-relevant effects in an
actual, complex in vivo system before moving
to clinical trials.
We recently reported on FDA’s efforts to
foster the development and evaluation of
emerging tools and methods for assessing the
safety of FDA-regulated products.
41
FDA
issued a roadmap on this topic in December
2017 that does not have an explicit goal to
replace, reduce, or refine animal testing but
states that new methods may have the
potential to do so.
42
In that regard, the
roadmap states that FDA will encourage
medical product sponsors to submit a
scientifically valid approach for using a new
method early in the regulatory process and to
engage in frequent communication with the
agency about the suitability of that method.
Previous FDA efforts to promote the use of
alternative methods included, for example,
2012 guidance to industry stating that
companies may use non-animal alternative
methods to test the toxicological safety of
41
GAO, Animal Use in Research: Federal Agencies Should Assess
and Report on Their Efforts to Develop and Promote
Alternatives, GAO-19-629 (Washington, D.C.: Sept. 24, 2019).
FDA-regulated products include human and animal drugs,
medical devices, food and food ingredients, and biological and
tobacco products.
42
Food and Drug Administration, FDA’s Predictive Toxicology
Roadmap (Washington, D.C.: December 2017).

GAO Technology Assessment GAO-20-215SP 49
pharmaceutical drugs if the methods are
appropriate or scientifically justified.
43
Researchers are also exploring the use of
machine learning to predict clinical trial
outcomes related to safety. For example,
researchers developed a model to predict
toxicity before testing drugs in humans.
44
They trained a random forest—a decision-
tree-based machine learning model—to
distinguish between a list of FDA-approved
drugs and a list of drugs that failed for toxicity
in clinical trials.
45
The model considers a
variety of features, including the drug’s
molecular properties and drug-likeness, as
well as properties of the target, to predict the
likelihood of toxicity in clinical trials.
46
According to the researchers, the model is
more predictive than some of the other
methods currently used to assess toxicity. For
example, the model was able to flag several
compounds that had been pulled from the
market as toxic. However, the majority of
clinical trials fail for reasons other than
toxicity, such as efficacy or financial reasons.
Therefore other factors must be considered
to fully predict clinical trial outcomes.
43
Food and Drug Administration, Guidance for Industry: S6
Addendum to Preclinical Safety Evaluation of Biotechnology-
Derived Pharmaceuticals (Washington, D.C.: May 2012).
44
K. Gayvert, N. Madhukar, and O. Elemento, “A data-driven
approach to predicting success and failures of clinical trials,”
Cell Chemical Biology, vol. 23, no. 10 (2016).
45
Random forest is an example of a classical machine learning
algorithm.
46
Drug-likeness is a qualitative property of compounds that is a
measure of similarity to known drugs.
2.3 Clinical trials
Researchers are beginning to use machine
learning to improve clinical trial design, a
point in the process where many potential
drug candidates fail.
47
According to a study by
an industry group, on average 9.6 percent of
drugs that enter phase I clinical trials
ultimately receive FDA approval.
48
For
example, if 100 drug candidates entered
phase I clinical trials, approximately 63
(63.2%) would advance to phase II clinical
trials, with 19 of those (30.7%) advancing to
phase III clinical trials. Of those 19 drugs, 11
(58.1%) would advance to the NDA process,
and, ultimately, 10 of those (85.3%) would be
approved by the FDA (see text box). For
clinical trials, companies are still piloting
machine learning and are not yet publishing
the results, according to an academic
research center. The use of AI in clinical trials
tends to be less mature than earlier steps in
the process because privacy regulations limit
the access to and use of patient data,
according to an industry group. Clinical trials
can be complex and therefore are associated
with a significant portion of overall R&D costs,
according to a recent review.
49
Several
factors in clinical trial design can influence the
likelihood of success, including patient
selection and recruitment.
47
The examples in this section are not an exhaustive list of
machine learning applications in clinical trials. For example,
researchers are also employing machine learning for patient
adherence and monitoring, and to analyze real world evidence
for example data collected via wearable technology.
48
David W. Thomas et al., Clinical Development Success Rates
2006-2015 (Washington, D.C.: BIO, 2016).
49
S. Harrer, P. Shah, B. Antony, and J. Hu “Artificial Intelligence
for Clinical Trial Design,” Trends in Pharmacological Sciences,
vol. 40, no. 8 (2019).

GAO Technology Assessment GAO-20-215SP 50
There are typically three phases of clinical trials before
FDA review
Phase I: This clinical trial phase generally tests the safety of the
drug on about 20 to 80 healthy volunteers. The goal of this
phase is to determine the drug’s most frequent side effects and
how it is metabolized and excreted. If the drug does not show
unacceptable toxicity in the phase I clinical trials, it may move
on to phase II.
Phase II: This clinical trial phase assesses the drug’s safety and
effectiveness on people who have a certain disease or
condition, and typically the assessment is conducted on a few
dozen to hundreds of volunteers. Generally, during this phase
some volunteers receive the drug and others receive a control,
such as a placebo. If there is evidence that the drug is effective
and safety data are acceptable in the phase II clinical trials, it
may move on to phase III.
Phase III: This clinical trial phase generally involves several
hundreds to thousands of volunteers who have a certain
disease or condition and gathers more information about the
drug’s safety and effectiveness, again while being compared to
a control.
Source: GAO, Investigational Drugs: FDA and Drug
Manufacturers Have Ongoing Efforts to Facilitate Access for
Some Patients, GAO-19-630 (Washington, D.C.: Sept. 9, 2019)
and Food and Drug Administration documentation. | GAO-20-
215SP
Selecting and recruiting patients for clinical
trials is a complex process, and enrollment
challenges are the principal cause for clinical
trial delays, according to the scientific
literature. One machine learning-focused
company described how they use classical
machine learning to maximize the probability
of a successful clinical trial—meaning FDA
approval of the drug—by optimizing a
number of design variables, including the
number of patients. Usually, clinical trial
cohorts are not representative of the general
population but rather come from a
subpopulation of suitable patients in whom
researchers believe they will be able to
readily measure drug response. Researchers
may consider patients suitable for a number
of reasons; for example whether the patient
is at the correct stage of disease or has a
specific phenotype.
50
Machine learning tools
can take advantage of the many kinds of data
used to assess suitability, such as genomic
data and electronic health record data, which
are currently fragmented in different
locations and formats. Natural language
processing and computer vision are both
techniques that could harmonize and analyze
these data. However, overfitting of machine
learning models is a potential risk if there is
an imbalance between different training sets,
according to the scientific literature.
51
Machine learning tools are also helping move
clinical research towards precision medicine.
Precision medicine, sometimes referred to as
stratified medicine, is an emerging approach
for disease treatment and prevention that
takes into account individual variability in
genes, environment, and lifestyle for each
person. According to experts, the industry is
moving away from blockbuster drugs and
instead investigating diseases that are more
complex or have smaller patient
populations.
52
Patient stratification—a
process by which patients are grouped by
phenotype or prognosis—is useful in areas
that could be considered as clusters of
smaller diseases rather than one broad
disease, such as oncology and neurology.
53
50
A phenotype, in this case, is a set of observable
characteristics of a patient produced by his or her genetics
interacting with the environment.
51
Overfitting means that the model performs well on the
training set but does not work well on other data sets. It is
similar in concept to how humans may overgeneralize about a
population based on limited information.
52
Blockbuster drugs are those that are intended for large
patient populations and have the potential to reach $1 billion
in annual sales.
53
A prognosis is the prospect of recovery for the patient from
the disease.
GAO Technology Assessment GAO-20-215SP 51
Researchers are using machine learning, both
supervised and unsupervised, for patient
stratification in clinical trials. Neurology, for
example, has one of the highest failure rates
among disease areas in clinical trials,
according to a study by an industry group. An
expert described investigations of the use of
machine learning and genetic information to
predict whether the rate of cognitive decline
in Alzheimer’s disease patient subgroups
correlates with drug response. In the long
term, precision medicine could potentially
improve health outcomes through more
effective targeted therapies for patient
subgroups or individuals. However, certain
subpopulations could be excluded from this
approach if existing biases in health care data
are not overcome. (For more on biases in
health care data, see chapter 3.)

GAO Technology Assessment GAO-20-215SP 52
3 Challenges Hindering the Use of Machine Learning in Drug
Development
Stakeholders, experts, and the literature in
this field identified several major challenges
hindering the use of machine learning in drug
development (see fig. 5). Technological
challenges include gaps in the underlying
scientific data on mechanisms of disease,
structure and behavior of complex molecules,
and how to represent these data to
algorithms. Stakeholders also point to a
shortage of high-quality, unbiased data as
well as difficulty accessing and sharing data
due to high costs and legal issues. It is also
difficult for drug companies to hire and retain
skilled, interdisciplinary workers. Finally,
regulatory uncertainty and a perceived lack of
commitment by the United States compared
to other countries can hinder advancement of
this field.
Figure 5: Challenges hindering the use of machine learning in drug development

GAO Technology Assessment GAO-20-215SP 53
3.1 Gaps in research
Research gaps present a significant challenge
to advancing the use of machine learning in
drug development. These gaps fall into two
broad categories: gaps in understanding of
fundamental biology and chemistry, and gaps
in domain-specific machine learning research.
Experts in the field have noted that
addressing these issues may be
transformational for future applications of
machine learning in drug development.
The federal government has also initiated
research into improving predictive screening
techniques and other machine learning
technologies (see text box).
Existing government research initiatives
Conversations with experts and agency officials
uncovered some existing research programs that seek
to promote increased development and application of
machine learning techniques in biomedical research.
According to an official from NIH, broadly, NIH supports
research on machine learning, including deep learning,
across four primary categories: image analysis, systems
pharmacology, predictive screening, and advanced
methods development. For example, the NIH National
Center for Advancing Translational Sciences (NCATS)
developed a funding opportunity that supports the uses
of computational algorithms to identify new therapeutic
uses of existing drugs and biologics.
However, some
experts expressed concerns that the current research
and training funding model, such as existing NIH study
sections and training grants, were not directed
appropriately to incentivize the incorporation of
machine learning into biomedical research. For
example, an expert expressed concern that a lack of
machine learning expertise within funding agencies
could hinder appropriate review of machine learning-
focused research proposals.
Source: GAO. | GAO-20-215SP
3.1.1 Gaps in fundamental biology and
chemistry research
• Understanding mechanisms of disease:
Experts noted that increased research
into the mechanisms of disease could
help researchers develop better models
which reflect scientific and clinical
realities, thus increasing the accuracy of
machine learning outputs and target
identification in drug development. One
research effort that researchers pointed
to as a potential model is Genomics
England. This project is sequencing the
genomes of 100,000 people to identify
genetic links to disease. Such research
could provide a deeper understanding of
disease mechanisms by revealing, for
example, what genes and proteins are
involved in a disease. This understanding,
in turn, could lead to insights into what
targets might be suitable for drug
development.
• Modeling drug-protein interactions:
Understanding interactions between
proteins and drugs is essential to drug
development; however these interactions
are complex and not always well
understood. For example, drugs interact
with multiple systems in the body—a
concept called polypharmacology.
According to the scientific literature,
animal testing and human clinical trials
are the current gold standard for testing
polypharmacological effects. This may
change as computational methods in this
field advance. Additionally, target
proteins are in rapid, dynamic movement
in the body. Computational methods that
aim to model static drug-protein
interactions therefore have limited
accuracy. Experts agreed that there can
be instances where diseases occur on the

GAO Technology Assessment GAO-20-215SP 54
systems level, and therefore it may not be
a single protein that is being regulated
but rather multiple proteins or even an
entire system or subsystem in the body.
More and better data related to these
interactions could lead to more accurate
deep learning models.
• Understanding the vast universe of
potential compounds: Experts stated that
current chemical libraries contain about
11 billion synthesizable compounds.
While this seems like a large number, it is
only a tiny fraction of the vast universe of
compounds that is theoretically possible.
Therefore, any sample of this set of
known compounds could be outdated
tomorrow and may not provide accurate
models of the range of possible
compound properties. Furthermore,
researchers in the field told us that most
data are on small molecules, with very
little data on the more complex, large
molecules such as biologics. More data on
new compounds, both small and large,
can reduce the unknown and improve the
ability of machine learning algorithms to
identify compounds with the best
biological response to the target of
interest.
• Understanding why drugs fail: As noted
above, very few drug candidates actually
make it to market. More information
about why some drug candidates fail to
make it to market and others succeed, or
whether they have unintended effects,
would help researchers develop machine
learning algorithms that better predict
success of compounds to achieve the
desired effect and reduce the time spent
on inadequate drug candidates.
3.1.2 Gaps in domain-specific machine
learning research
• The representation of molecules in
machine learning: Researchers can
represent molecules to machine learning
algorithms in multiple ways, including
molecular fingerprints and molecular
graphs.
54
Each of these may have
advantages and disadvantages, and it is
not always clear which representation is
the best choice for a given structure.
When the researcher selects the type of
representation, they are making a
subjective choice about the information
supplied to the machine learning model,
which experts stated will have a
significant impact on the resulting output.
More research into how the types of
molecular representations affect the
results of algorithmic selection of
compounds could help inform the
selection of representations and improve
results.
• Generating new chemical structures:
Additional basic research is needed
before machine learning techniques
designed to generate new compounds
will be fully functional in drug
development. For example, as previously
discussed, generative models can be used
to identify new compounds with the
desired biological properties, and
predictive models could then be used to
evaluate those new compounds as drug
candidates. However, those predictive
models may not produce reliable results
when extrapolated to new compounds
54
Molecular fingerprints encode structural or functional
features of molecules in a binary format and a molecular graph
has vertices that represent the atoms and edges that represent
the bonds of a particular molecule.

GAO Technology Assessment GAO-20-215SP 55
that are significantly different from those
on which the models were trained.
• Unsupervised learning: Unsupervised
learning techniques can yield compelling
insights from unlabeled data, such as
electronic health records that do not
contain patient outcomes. For example, a
group of researchers applied an
unsupervised deep learning algorithm to
700,000 such electronic health records,
and reported that the algorithm was able
to consistently and significantly
outperform other predictive methods in
assessing a patient’s future disease profile
for the 78 diseases in the study.
55
However, these techniques have not seen
widespread use in the biomedical
sciences because they can be challenging
to use, with a wide range of hit-or-miss
results and a need to pre-process the data
to remove irrelevant factors. Researchers
stated that these obstacles might be
overcome with additional research into
the predictive elements of certain
diseases and better raw representations
of the data, such as improved
understanding of laboratory results, to
improve the algorithm’s ability to
correlate information and predict future
outcomes.
3.2 Data quality
A shortage of high-quality data is a major
challenge for machine learning in drug
development, according to agency officials
and industry representatives. Machine
learning requires a large amount of accurate
55
R. Miotto, L. Li, B.A. Kidd, and J.T. Dudley, “Deep Patient: An
Unsupervised Representation to Predict the Future of Patients
from Electronic Health Records,” Scientific Reports, vol. 6, no.
26094 (2016).
and representative data. This poses a unique
challenge in drug development, as much of
the data were not originally collected with
machine learning in mind and may not be
machine-readable or model-ready.
Furthermore, according to an industry
representative, data collected across different
organizations and environments come in
different formats, and this lack of
standardization in data quality is a barrier.
Curating these data is a resource-intensive
process, according to stakeholders and the
literature
.
56
One representative from a drug
company told us that 80 percent of their
effort goes into accessing and curating data to
make it usable for their machine learning
applications. The ability to trace data back to
the original experiment is also essential for
machine learning in drug development, and
according to one expert, meaningful machine
learning results require an understanding of
how the data were collected, used, and
analyzed in the research.
Another factor that can reduce data quality is
bias, which can skew and limit machine
learning outputs. For example, publication
bias may result in data skewed toward
positive results, as negative results can be less
valued and remain unpublished. This may
lead to issues when published data are used
for machine learning. Similarly, according to
one drug company, data from failed clinical
trials are often not publicly available and
opening this data up could unleash a wave of
innovation. In addition, patient data may also
be biased as such data are collected mostly
from individuals receiving treatment, causing
an underrepresentation of data from healthy
individuals and an overrepresentation of data
56
Curating refers to the process of collecting, organizing, and
repurposing data.

GAO Technology Assessment GAO-20-215SP 56
from individuals with access to medical care,
according to experts. Lastly, data may
underrepresent individuals or groups based
on race, class, or gender, potentially skewing
machine learning models and causing
differences in the effectiveness of drugs
across subpopulations. Some stakeholders
stated that biases may be exacerbated by
machine learning, though others said that
machine learning can be used to help identify
and alleviate these biases.
3.3 Data access and sharing
As mentioned above, drug companies and
researchers use data from diverse sources in
order to obtain the quantity needed for
machine learning to be effective, but
accessing and sharing these data can be both
costly and challenging. According to one
industry representative, collecting data from
the early drug discovery phase can be cost
prohibitive. This representative said that
certain health-related data may cost tens of
thousands of dollars, as compared to just
cents for other consumer related data that
many technology companies use.
Data sharing also presents unique legal issues.
According to stakeholders, privacy laws such
as HIPAA can make it difficult for drug
companies, especially those that are not
regulated by HIPAA, to share or access data.
One expert, however, said that the privacy
laws and regulations may not be the issue but
rather their interpretation by organizations
that may be hesitant to share data. Two
experts also noted that the public is wary of
the use of their data for commercial profit.
These experts stressed the importance of
being transparent with the public about how
data will be used and how their data may be
used for the greater good. Similarly, experts
told us that rules and processes for patient
consent to data sharing are complicated and
may make it difficult for individuals to give
such consent. In addition, one expert
cautioned that consent can cause selection
biases and could also limit the amount of
usable data, but also noted that there are
legal pathways for accessing and sharing data
for public health purposes, research, and
certain other uses.
Lastly, data sharing may be limited by a lack
of economic incentives for certain
organizations to share. According to a drug
company representative and an academic
researcher, drug companies consider their
data to be valuable, proprietary, and a
competitive edge. According to two legal
researchers, some drug companies are using
mergers and acquisitions to access data
because there is no protection or legal
framework for the data that are transferred
through those transactions.
How partnerships such as the MELLODDY consortium
address data sharing challenges
The Machine Learning Ledger Orchestration for Drug
Discovery (MELLODDY) project is a consortium and
public-private partnership representing 17 partners
from pharmaceutical, technology, and academic fields.
MELLODDY uses a method called federated learning to
train machine learning models across the chemical
libraries of 10 drug companies. In federated learning,
training data are decentralized. The machine learning
model learns from data stored at different geographic
locations, ensuring that each drug company’s private
data set stays within its own secure infrastructure. The
consortium uses block chain architecture technology to
protect proprietary information while at the same time
boosting the predictive performance and applicability of
the drug discovery models by leveraging all available
data.
Source: GAO analysis of Machine Learning Ledger
Orchestration for Drug Discovery documentation. | GAO-20-
215SP

GAO Technology Assessment GAO-20-215SP 57
3.4 Low supply of skilled and
interdisciplinary workers
Experts we spoke with told us that there are
not enough highly skilled workers with
interdisciplinary expertise across data science
and biomedical science. According to one
economist, there is a finite supply of workers
available to do innovative work in this field.
Experts reported challenges with hiring and
retention due to competition from larger
technology companies that can afford to pay
higher salaries. In addition, an expert said
that government agencies, including
regulators, may have trouble competing for
the high-level talent they need to properly
understand and regulate drugs developed
using these technologies. Experts mentioned
the need for alternative and continuous
learning education models to keep up with
the growing demand for such skills, including
reforms to PhD programs, interdisciplinary
programs in high school and college,
vocational schools, online training, and data
science boot camps.
A secondary workforce challenge is the
cultural divide between biomedical scientists
and data scientists. According to
representatives from a drug company, getting
groups of different people to engage together
and speak in a similar language is challenging.
One expert from our meeting said that people
from different disciplines often work in siloed
teams and stressed the importance of
composing interdisciplinary teams with
workers from both of these areas.
How one drug company approaches the cultural
divide between biomedical and data scientists
A representative from one drug company presented
at a conference how they solve the issue of cultural
divides between biomedical and data scientists.
Incoming data scientists are put through a 3-year job
rotation program, where they work in each of the six
drug development departments within the company.
After the 3-year rotation, the data scientists are then
permanently stationed within a department. The
representative acknowledged that this approach
takes time and means that new data experts in the
company are not able to fully immerse themselves in
the job for which they were hired until years later.
However, they noted that the benefits of increased
understanding between the biomedical and data
scientists, improved company culture, and increased
speed of projects are well worth the lead time.
Source: GAO analysis of conference proceedings. | GAO-20-
215SP
3.5 Regulatory challenges and federal
commitment
According to several stakeholders, the
regulatory process for drugs developed using
machine learning is unclear. For example, an
industry group and a legal scholar told us that
there is not enough guidance about what
information or data FDA will require for
approval of machine learning uses in the drug
development process and that regulatory
uncertainty could dissuade drug companies
from investing more resources into machine
learning in drug development. Another legal
scholar we spoke with said that machine
learning offers the potential for revolutionary
improvements in this field but may require
regulatory changes to realize these benefits.
For example, if advancements in
computational methods prove to be more
effective and reliable than animal
experimentation, replacing animal testing
with machine learning applications may
require regulatory changes, according to an

GAO Technology Assessment GAO-20-215SP 58
industry representative and two academic
researchers. As described earlier in this
report, FDA is closely tracking the use of AI in
drug development and is considering its
policy approach to this area, according to
officials.
One representative from a drug company told
us that, in their opinion, the United States is
not as committed to addressing some of
these challenges as other countries, such as
South Korea, and China. For example, this
representative said China is trying to attract
human capital from other countries and is
also the owner of the most data for machine
learning purposes, which provides a large
competitive advantage for Chinese
companies. Another example is South Korea’s
National Cancer Center, which developed the
Korea Cancer Big Data Platform, a multi-
database framework that collects clinical,
genomic, imaging, and biobank data using
secure de-identification technology that
allows for clinical research and practice in
future research.
57
57
Hyo Soung Cha, Jip Min Jung, Seob Yoon Shin, Young Mi Jang,
Phillip Park, Jae Wook Lee, Seung Hyun Chung, and Kui Son
Choi, “The Korea Cancer Big Data Platform (K-CBP) for Cancer
Research,” International Journal of Environmental Research
and Public Health, vol. 16, no. 2290 (2019).

GAO Technology Assessment GAO-20-215SP 59
4 Policy Options to Address Challenges to the Use of Machine
Learning in Drug Development
We identified six policy options in response to
the challenges discussed in the previous
chapter. Those challenges included gaps in
research, data quality concerns, a lack of data
access and sharing, a low supply of skilled and
interdisciplinary workers, and regulatory
challenges. First, we present options that
address research, data access,
standardization, human capital, and
regulatory certainty. Then, we describe how
policymakers—Congress, federal agencies,
state and local governments, academic and
research institutions, and industry, among
others—could choose to maintain the status
quo. In addition, we discuss potential
opportunities and considerations of each
option. We focused on policy options that
were within the report scope.
58
Policymakers
could implement the options in a variety of
ways, including by launching a pilot program.
58
For further information on our scope and methodology,
please see app. I.
While we present options to address the
major challenges we identified, the options
are not intended to be inclusive of all
potential policy options. We intend policy
options to provide policymakers with a
broader base of information for decision-
making. The options are neither
recommendations to federal agencies nor
matters for Congressional consideration. They
are also not listed in any specific rank or
order. We are not suggesting that they be
done individually or combined in any
particular fashion. Additionally, depending on
the options selected, additional work might
need to be done on potential design and legal
issues. We did not conduct work to assess
how effective the options may be, and
express no view regarding the extent to which
legal changes would be needed to implement
them.
GAO Technology Assessment GAO-20-215SP 60
Policy Option: Research
Policymakers could promote basic research to generate more and
better data and improve understanding of machine learning in drug
development.
Potential Opportunities Potential Considerations
• Could result in increased scientific and technological output by solving
previously challenging problems. As we describe above, one such problem is
how best to represent molecular structures to machine learning algorithms.
Policymakers could promote the field in multiple ways, including approaches
such as support for intramural research, grants, or other subsidies.
Policymakers could choose to use one of these approaches or combine
them.
In addition, according to one expert, another approach that could promote
basic scientific research is a “grand challenge”. For example, in 2012, the
biopharmaceutical company Merck hosted a challenge for the best
predictive model for absorption, metabolism, distribution, excretion, and
toxicity modeling of drug candidates.
59
Policymakers could also support collaboration across sectors. The Machine
Learning for Pharmaceutical Discovery and Synthesis Consortium (MLPDS) is
a collaboration between large drug companies such as Pfizer, Merck, and
Novartis with the Chemical Engineering, Chemistry, and Computer Science
departments at the Massachusetts Institute of Technology, and has
published a variety of papers at the intersection of machine learning and
drug development.
• Could result in the generation of additional high-quality, machine readable
data. For example, as described previously, increased research into the
mechanisms of disease could help develop more realistic models.
• Basic research is generally considered a long-term investment and its
potential benefits are uncertain. For example, the new data created by
increased research may not necessarily be high-quality or machine-readable
unless data standards are in place.
60
• Would likely require assessment of available resources and may require
reallocation of resources from other priorities.
61
The potential costs borne
by any one actor could be mitigated if multiple entities combined their
resources.
Source: GAO. | GAO
-20-215SP
59
Merck, Merck Molecular Activity Challenge, accessed November 5, 2019, https://www.kaggle.com/c/MerckActivity.
60
We discuss standardization later in this chapter as its own policy option.
61
We did not perform an economic analysis to attempt to quantify costs that could be incurred.
GAO Technology Assessment GAO-20-215SP 61
Policy Option: Data Access
Policymakers could create mechanisms or incentives for increased
sharing of high-quality secured data held by public or private actors,
while also ensuring protection of patient data.
Potential Opportunities Potential Considerations
• Could shorten the length of the drug development process and reduce costs.
To promote greater availability of data, policymakers could consider forming
or facilitating research consortia that allow for secure data sharing. As
discussed in chapter 3, the MELLODDY consortium is one example of how
different entities are using partnerships to share data. One expert suggested
that rewarding researchers based on how often others used their data
could incentivize the generation of more open and accessible data. For
example, when reviewing grant applications, grant-making organizations
could consider how often others have used the applicant researcher’s
data.
Policymakers could also consider creating a data repository through
encouraging an industry-driven solution, establishing a public-private
partnership, or creating a repository of all data under their control. For
example, the NIH runs the website ClinicalTrials.gov, which hosts data on
over 300,000 research studies. As described earlier, more data could
decrease the time to complete clinical trials. However, one of our experts
said the website contained data not readily usable for machine learning.
• Could help companies identify unsuccessful drug candidates earlier in the
development process, conserving resources. For example, cost reductions
could occur within each step of drug development or as a new compound
moves from one step to another.
62
As described earlier, studies have
estimated the average cost per new FDA-approved drug between $0.6 and
$1.4 billion.
63
• Would likely require coordination between various stakeholders and incur
setup and maintenance costs.
64
Stakeholders, including federal agencies,
would likely need to carefully coordinate across each other’s respective
domains to minimize duplication and overlap.
65
Previous GAO reports
describe how interagency coordination and collaboration can be
challenging.
66
For example, a lack of information on roles and responsibilities
and lack of coordination mechanisms can hinder effective interagency
collaboration. Costs could include computing software and hardware,
energy, and staffing needs. Consortia of academic and public entities could
combine their efforts to create a repository, spreading the time and cost
required across the organizations.
• Improper data sharing or use could have legal consequences. Increased data
sharing could therefore require a careful review of the legal ramifications,
because data are often gathered through a wide variety of mechanisms and
governed by multiple legal frameworks.
• Cybersecurity risks could increase and those threats would likely take
additional time and resources to mitigate. For example, if data were stored
in a central repository and that system was breached, it could cause a large
amount of sensitive data to be exposed at once. In a prior report, we found
that cybersecurity breaches in 2015 caused over 113 million individual health
care records to be compromised.
67
In May 2015, the University of California,
Los Angeles Health network discovered a cyberattack in which individuals
had personally identifiable information compromised, including medical
record numbers, Medicare or health plan ID numbers, and some medical
information.
• Organizations with proprietary data could be reluctant to participate. As we
discussed earlier, drug companies consider their data valuable, proprietary,
and a competitive edge. Data sharing could raise issues related to ownership
or protection of intellectual property. Therefore, incentives may be
necessary to encourage the sharing of data. For example, one expert
suggested extending patent protection for drugs already developed if drug
companies that developed those drugs agreed to release new data.
Source: GAO. | GAO-20-215SP
62
We did not perform an economic analysis to attempt to quantify cost savings.
63
DiMasi, Grabowski, and Hansen, “Innovation in the Pharmaceutical Industry,” and Prasad and Mailankody, “Spending to Bring Single Cancer Drug to Market and
Revenues After Approval.”
64
We did not perform an economic analysis to attempt to quantify costs that could be incurred.
65
We did not estimate the quantity and quality of coordination needed to implement these policy options.
66
GAO, Interagency Collaboration: Key Issues for Congressional Oversight of National Security Strategies, Organizations, Workforce, and Information Sharing, GAO-09-
904SP (Washington, D.C.: Sept. 25, 2009). GAO, Results-Oriented Government: Practices That Can Help Enhance and Sustain Collaboration among Federal Agencies,
GAO-06-15 (Washington, D.C.: Oct. 21, 2005).
67
This number is based on reported breaches of 500 or more individuals. GAO, Electronic Health Information: HHS Needs to Strengthen Security and Privacy Guidance
and Oversight, GAO-16-771 (Washington, D.C.: Aug. 26, 2016).
GAO Technology Assessment GAO-20-215SP 62
Policy Option:
Standardization
Policymakers could collaborate with relevant stakeholders to
establish uniform standards for data and algorithms.
Potential Opportunities Potential Considerations
• Could improve interoperability by more easily allowing researchers to
combine different data sets. Such combinations can serve the needs of
machine learning techniques and reduce bias in the data. Experts we spoke
with described the importance of data standardization for their work. For
example, a standard that defines synthetic data and how they can be used
can help reduce bias by allowing researchers to generate data that could be
used to better represent currently underrepresented communities. Similarly,
a standard data format for uploading and sharing data across platforms
could reduce the need for data scientists to spend time converting data sets
to machine-readable formats.
• Could help efforts to ensure algorithms remain explainable and transparent
to end users, as well as aid data scientists with benchmarking. Officials from
the National Institute of Standards and Technology told us that algorithmic
explainability and transparency could encourage adoption of machine
learning tools for drug development. The creation of documentary standards
could solve such issues by clarifying types of algorithms that can be used for
machine learning in drug development.
68
Further, well-established
performance standards could help data scientists benchmark their
algorithms and make better decisions about when to use certain algorithms
over others.
• Could be time- and labor-intensive because of the need to reach consensus
across a range of public and private-sector stakeholders. Standards
development can take anywhere from 18 months to a decade to complete
and require multiple iterations.
69
For example, one draft set of private-
sector health care AI standards we reviewed began development in March
2019 and is currently on its 14th iteration.
70
Standards development organizations follow similar processes and generally
adhere to principles such as openness, balance of interests, and consensus.
71
Specifically, once an organization agrees to develop a new or revised
standard, it forms a committee of experts from companies, nonprofit
organizations, and government agencies. The representatives serve on a
voluntary basis, and the committee drafts the standard. Generally, a
committee will use a consensus-based process to vote on whether to
approve the draft standard. Each step of the process requires careful
coordination and collaboration across a myriad of stakeholders.
Source: GAO. | GAO
-20-215SP
68
Standards development organizations can specify how a product is designed or made, or establish performance standards that define the product by function rather
than material.
69
GAO, National Institute of Standards and Technology: Additional Review and Coordination Could Help Meet Measurement Service Needs and Strengthen Standards
Activities, GAO-18-445 (Washington, D.C.: July 26, 2018).
70
Consumer Technology Association, Definitions and Characteristics of Artificial Intelligence in Health Care (forthcoming).
71
GAO-18-445.
GAO Technology Assessment GAO-20-215SP 63
Policy Option: Human
Capital
Policymakers could create opportunities for more public and private
sector workers to develop appropriate skills.
Potential Opportunities Potential Considerations
• Could provide a larger pool of skilled workers for agencies and companies,
allowing them to better leverage advances in the use of machine learning in
drug development. For example, expanding data science training
opportunities for regulators could better equip the agencies to understand
machine learning-generated data. Further, if policymakers create
opportunities for training workers with relevant knowledge who are
motivated to go into the drug development field, companies might be more
willing to proceed with efforts to develop machine learning tools for drug
development.
• Interdisciplinary teamwork could improve as workers with different
backgrounds learn to better communicate with one another. One expert
described how interdisciplinary collaboration represented the future of
science and noted that discoveries will be made in the space where
distinctions between disciplines are blurred. As data scientists learn aspects
of biomedical sciences and biomedical scientists learn aspects of data
science, the two groups will better navigate different vocabularies and
problem-solving approaches. For example, one biopharmaceutical company
works to build this collaborative capacity by regularly rotating its workers
across multiple divisions of the company to promote interdisciplinary
understanding; other organizations could use this type of rotation.
Additionally, educational institutions could create interdisciplinary degrees
that meld advanced machine learning techniques with biology, chemistry,
and medical curricula.
• Data science-trained workers could exit the drug development field in search
of higher-paying opportunities. We heard from multiple experts and
companies that it is extremely difficult to recruit highly qualified
interdisciplinary workers because technology companies outside the health
care field can offer significantly higher compensation. Companies and
agencies might mitigate this concern by offering retention incentives or
asking workers to remain with the company for a certain number of years in
exchange for the additional training.
• Would likely require an investment of time and resources.
72
Companies and
agencies will need to decide if the opportunities and challenges described
above justify the investment or shifting of existing resources and how best to
provide such training. For example, a company or agency could invest in a
partnership with an online teaching platform to create a customized solution
rather than developing a new curriculum themselves.
Source: GAO. | GAO
-20-215SP
72
We did not perform an economic analysis to attempt to quantify costs that could be incurred.
GAO Technology Assessment GAO-20-215SP 64
Policy Option: Regulatory
Certainty
Policymakers could collaborate with relevant stakeholders to develop
a clear and consistent message regarding regulation of machine
learning in drug development.
Potential Opportunities Potential Considerations
• Could help increase the level of public discourse surrounding the technology
and allow regulators and the public to better understand its use.
Policymakers can encourage discussion through such mechanisms as holding
meetings, issuing discussion papers, engaging with the public, and scientific
conferences. For example, in April 2019 FDA released a proposed regulatory
framework for modifications to machine learning-based software used as
medical devices.
73
The proposal included examples and questions. FDA then
gave the public until June 2019 to provide feedback on its proposal. It
received over 130 comments from individual citizens, industry groups, health
care companies, and one standards association.
Alternatively, industry could come together to create a self-regulatory
mechanism by which it would monitor the use of machine learning in drug
development.
• Drug companies could better leverage the technology if they have increased
certainty surrounding how, if at all, regulators will review or approve the
machine learning algorithms used in drug development. For example,
regulators could increase certainty in a variety of ways, including issuance of
clarifying documents such as agency guidance or regulations.
• Would likely require coordination within and among agencies and other
stakeholders, which can be challenging and require additional time and
costs.
74
If the process of developing a message is slow, uneven, or
inconsistent, industry might lose confidence in the approval process,
lessening its interest in pursuing machine learning in drug development. For
example, we previously found that inconsistencies among FDA reviewers can
influence approval of generic drugs in the first review cycle.
75
• Compliance costs and review times could increase if new regulations were
promulgated, depending on what those regulations require.
76
For example,
as new regulations are promulgated, companies might be required to
provide additional paperwork, install new or modified capital equipment, or
follow new, more rigorous testing procedures. These costs could be
absorbed by the companies or passed along to consumers in the form of
higher prices. There may also be indirect costs to new regulations, such as a
reduction and redirection in industrial R&D efforts.
Source: GAO. |
GAO-20-215SP
73
FDA, “Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD),”
Washington, D.C.: Apr. 2, 2019. Medical devices include instruments, apparatuses, machines, and implants that are intended for use to diagnose, cure, treat, or
prevent disease, or to affect the structure or any function of the body. See 21 U.S.C. § 321(h).
74
We did not estimate the quantity and quality of coordination needed to implement these policy options.
75
GAO, Generic Drug Applications: FDA Should Take Additional Steps to Address Factors That May Affect Approval Rates in the First Review Cycle, GAO-19-565
(Washington, D.C.: Aug. 7 2019).
76
We did not perform an economic analysis to attempt to quantify costs that could be incurred.
GAO Technology Assessment GAO-20-215SP 65
Policy Option: Status Quo
Policymakers could maintain the status quo (i.e., allow current efforts
to proceed without intervention).
Potential Opportunities Potential Considerations
• Challenges may be resolved through current efforts. For example, with its
2018 Strategic Plan for Data Science, NIH committed to making data
findable, accessible, interoperable, and reusable for all data science activities
and products supported by the agency. If policymakers allow current efforts
time to solve the problems they are targeting, they could avoid potentially
spending time and money switching to suboptimal solutions.
• Companies are already using machine learning in drug development and may
not need action from policymakers to continue expanding the use of such
technologies. As described previously, five biopharmaceutical companies
told us they use machine learning throughout the drug development
process. Further, five additional companies we spoke with focused on using
machine learning in various steps of the drug development process.
• The challenges described earlier in the report may remain unresolved or be
exacerbated. For instance, the status quo includes the challenge of bias and
underrepresentation of certain groups within existing data. Under the status
quo, such groups could be further marginalized by the use of such data to
develop new drugs that may not be as safe or effective for underrepresented
groups.
Source: GAO. | GAO
-20-215SP

GAO Technology Assessment GAO-20-215SP 66
5 Agency and Expert Comments
We provided a draft of this report to the Department of Commerce (National Institute of
Standards and Technology) and Department of Health and Human Services (Food and Drug
Administration, National Institutes of Health) with a request for comments. We incorporated
agency comments into this report as appropriate.
We also provided a draft of this report to 11 participants from our expert meeting and 2
additional experts for review, and incorporated comments received as appropriate.
______________________________________________________________________
As agreed with your offices, unless you publicly announce the contents of this report earlier, we
plan no further distribution until 30 days from the report date. At that time, we will send copies
of the report to the appropriate congressional committees, relevant federal agencies, and other
interested parties. In addition, the report is available at no charge on the GAO website at
http://www.gao.gov.
If you or your staff members have any questions about this report, please contact Timothy M.
Persons at (202) 512-6888 or pe[email protected]. Contact points for our Offices of Congressional
Relations and Public Affairs may be found on the last page of this report. GAO staff who made
key contributions to this report are listed in appendix III.
Timothy M. Persons, PhD
Chief Scientist and Managing Director
Science, Technology Assessment, and Analytics
GAO Technology Assessment GAO-20-215SP 67
List of Requesters
The Honorable Lamar Alexander
Chairman
Committee on Health, Education, Labor, and Pensions
United States Senate
The Honorable Greg Walden
Ranking Member
Committee on Energy and Commerce
House of Representatives
The Honorable Michael C. Burgess, MD
Ranking Member
Subcommittee on Health
Committee on Energy and Commerce
House of Representatives
The Honorable Brett Guthrie
Ranking Member
Subcommittee on Oversight and Investigations
Committee on Energy and Commerce
House of Representatives
GAO Technology Assessment GAO-20-215SP 68
Appendix I: Objectives, Scope, and Methodology
We describe our scope and methodology for
addressing the three objectives outlined
below:
1. What current and emerging artificial
intelligence (AI) technologies are available
for drug development, including discovery
through clinical trials, and what are the
potential benefits of those technologies?
2. What challenges, if any, hinder the
development and adoption of these
technologies?
3. What policy options could address
challenges to the use of machine learning
in drug development?
To address all three research objectives, we
assessed available and developing AI
technologies that companies could use during
the drug development process as well as the
benefits and challenges associated with their
use. To do so, we reviewed key reports and
scientific literature describing current and
developing technologies; attended relevant
technical conferences and workshops;
interviewed a variety of stakeholders,
including agency officials, drug companies—
both biopharmaceutical and machine
learning-focused, academic researchers, and
nongovernmental organizations; and
conducted an expert meeting in conjunction
with the National Academies.
Limitations to scope
We focused our review on selected
technologies in the first three steps of the
typical drug development process: drug
discovery, preclinical research, and clinical
trials. Technologies discussed are examples
and not an exhaustive list of all AI
technologies used in drug development. We
did not assess all available or developing
technologies. We selected narrative examples
to demonstrate the breadth of machine
learning technologies in drug development.
We also did not include AI technologies for
large-scale drug manufacturing.
Literature search
In the course of our work we conducted two
literature searches. To establish background
and identify appropriate technologies and
their benefits and challenges, we reviewed
key articles from the scientific literature. To
support objective 3, we conducted a policy
options literature search using a variety of
databases. For this search, results could
originate from scholarly or peer reviewed
material, government reports, conference
papers, dissertations, working papers, books,
legislative materials, trade or industry articles,
and white papers, but not from general news.
We identified a total of 1,109 results using
search terms such as “machine learning”,
“policy”,“policymaking” and “artificial
intelligence”. We selected 116 of the most
relevant articles for further review based on
our objective, and reviewed the abstracts for
additional search terms to refine the results.
Interviews
We interviewed key stakeholders in the field
of machine learning in drug development,
including representatives or officials from:
• relevant federal agencies including the
National Institute of Standards and

GAO Technology Assessment GAO-20-215SP 69
Technology, the National Institutes of
Health (NIH), and the Food and Drug
Administration (FDA);
• five biopharmaceutical companies;
• five companies focused on using machine
learning in the drug development
process;
• six academic researchers; and
• six nongovernmental organizations
including two industry associations, two
consumer groups, and two think tanks.
77
We selected companies to interview by first
requesting input from relevant stakeholders.
From that initial list, we selected 10
companies that were clearly within the scope
of our review and have a U.S. presence. We
also balanced our selections between
biopharmaceutical and machine-learning
focused companies and across the steps of
the drug development process. Because this is
a small and non-generalizable sample of the
universe of companies using machine learning
for drug development, the results of our
interviews are illustrative and represent
important perspectives, but are not
generalizable.
77
The six academic researchers focus on the following areas: (1)
natural language processing and applications of deep learning
to chemistry and oncology, (2) research and development and
clinical trial management practices and trends, (3) intellectual
property, health law, and regulation, (4) FDA regulation of
machine-learning clinical and patient decision support software
and gene sequencing and editing technologies; health data
privacy and access; genomic civil rights; and citizen science and
citizen-led bioethics standard-setting, (5) the intersection of
trade secrecy incentives and explainability in AI-enabled health
care delivery, and (6) the economics of innovation, intellectual
property, productivity measurement, industrial organization,
and applied econometrics.
Expert meeting
We collaborated with the National Academies
to convene a 2-day meeting of 19 experts on
current and emerging machine learning
technologies for use in drug development. We
worked with National Academies staff to
identify experts from a range of stakeholder
groups including federal agencies, academia,
biopharmaceutical companies, machine
learning-focused companies, and legal
scholars, with expertise covering all significant
areas of our review, including individuals with
research or operational expertise in using
machine learning technology in the drug
development process.
78
We evaluated the
experts for any conflicts of interest. A conflict
of interest was considered to be any current
financial or other interest (such as an
organizational position) that might conflict
with the service of an individual because it
could (1) impair objectivity or (2) create an
unfair competitive advantage for any person
or organization. The 19 experts were
determined to be free of reported conflicts of
interest, except those that were easily
addressed, and the group as a whole was
determined to not have any inappropriate
biases.
79
(See app. II for a list of these experts
and their affiliations.) The comments of these
78
This meeting of experts was planned and convened with the
assistance of the National Academy of Science to better ensure
that a breadth of expertise was brought to bear in its
preparation, however all final decisions regarding meeting
substance and expert participation are the responsibility of
GAO. Any conclusions and recommendations in GAO reports
are solely those of the GAO.
79
For example, one expert had options in a pharmaceutical
company using AI in drug development and sometimes
received honoraria for giving talks as part of an advisory board.
We determined the expert’s relationship did not prevent the
expert from serving on the panel as the discussion was not
planned to revolve around any specific technology,
pharmaceutical company, or vested interest. We did not
interview or otherwise mention the company the expert had
options in within the report or suggest policy options that will
intentionally promote or adversely affect any company.

GAO Technology Assessment GAO-20-215SP 70
experts generally represented the views of
the experts themselves and not the agency,
university, or company with which they were
affiliated, and are not generalizable to the
views of others in the field.
We divided the 2-day meeting into 7
moderated discussion sessions: (1)
technologies to assist with early-stage drug
discovery; (2) technologies to assist with drug
development; (3) technologies to assist with
preclinical research; (4) technologies to assist
with clinical trial research; (5) the economic,
legal, social, and health factors of AI in drug
development; (6) policy and regulatory
implications of AI in drug development; and
(7) policy options that could facilitate drug
development in the United States through the
use of AI technologies. Each session featured
opening presentations by two to three
experts followed by open discussion among
all meeting participants based on key
questions we provided. The meeting was
recorded and transcribed to ensure that we
accurately captured the experts’ statements.
After the meeting, we reviewed the
transcripts to characterize their responses
and to inform our understanding of all three
researchable questions. Following the
meeting, we continued to seek the experts’
advice to clarify and expand on what we had
heard. Consistent with our quality assurance
framework, we provided the experts with a
draft of our report and solicited their
feedback, which we incorporated as
appropriate.
Policy Options
We intend policy options to provide
policymakers with a broader base of
information for decision-making.
80
The
options are neither recommendations to
federal agencies nor matters for
Congressional consideration. They are also
not listed in any specific rank or order. We are
not suggesting that they be done individually
or combined in any particular fashion.
Additionally, we did not conduct work to
assess how effective the options may be, and
express no view regarding the extent to which
legal changes would be needed to implement
them.
Based on our requesters’ interest in U.S.
competitiveness and the use of AI
technologies in drug development, among
other issues, we began our work with an
initial policy objective of facilitating drug
development in the United States through the
use of AI technologies. As our work
progressed, we refined this objective to
identifying options that could help address
challenges to the use of machine learning in
drug development. We limited the policy
options included in this report to those that
met the policy objective and fell within the
report scope. We present six policy options in
response to the challenges identified during
our work and discuss potential opportunities
and considerations of each. While we present
options to address the major challenges we
identified, the options are not intended to be
inclusive of all potential policy options.
To develop the policy options, we prepared a
list of potential policy ideas (97 in total, as
well as the status quo) based on our literature
search, stakeholder interviews, and expert
meeting. We removed ideas that were not
likely to achieve the policy objective or did
80
Policymakers is a broad term including, for example,
Congress, federal agencies, state and local governments,
academic and research institutions, and industry.
GAO Technology Assessment GAO-20-215SP 71
not fit into the overall scope of our work. For
example, we removed policy ideas related to
drug pricing that were not relevant to the use
of machine learning in the drug development
process. We grouped the remaining ideas
based on themes (e.g., human capital, data
access). We combined those that (1) were
duplicative, (2) could be subsumed into a
higher-level policy option, or (3) were
examples of how to implement a policy
option rather than the option itself.
We conducted our work from February 2019
through December 2019 in accordance with
all sections of GAO’s Quality Assurance
Framework that are relevant to technology
assessments. The framework requires that we
plan and perform the engagement to obtain
sufficient and appropriate evidence to meet
our stated objectives and to discuss any
limitations to our work. We believe that the
information and data obtained, and the
analysis conducted, provide a reasonable
basis for any findings and conclusions in this
product.
GAO Technology Assessment GAO-20-215SP 72
Appendix II: Expert Participation
We collaborated with the National Academies to convene a two-day meeting of experts to
inform our work on artificial intelligence in drug development; the meeting was held on July 18-
19, 2019, in Boston, Massachusetts. The experts who participated in this meeting are listed
below. Many of these experts gave us additional assistance throughout our work, including
seven experts who provided additional assistance during our study by sending material for our
review or participating in interviews; and eight experts who reviewed our draft report for
accuracy and provided technical comments.
Brandon Allgood
Chief Technology Officer and Cofounder
Numerate, Inc.
Mohammed AlQuraishi
Departmental Fellow in Systems
Pharmacology
Harvard Medical School
Regina Barzilay
Professor, Department of Electrical
Engineering and Computer Science
Massachusetts Institute of Technology
Anne Carpenter
Institute Scientist and Merkin Institute Fellow
Broad Institute of Harvard and MIT
Will Chen
Head of Computation and Systems Biology
Biogen
Ethan Dmitrovsky
President
Leidos Biomedical Research
Laboratory Director
Frederick National Laboratory for Cancer
Research
Shahram Ebadollahi
Global Head of Data Science and AI
Novartis
Olivier Elemento
Director, Englander Institute for Precision
Medicine
Weill Cornell Medical School
M. Khair ElZarrad
Deputy Director, Office of Medical Policy at
the Center for Drug Evaluation and
Research
Food and Drug Administration
GAO Technology Assessment GAO-20-215SP 73
Barbara Evans
Director, Center for Biotechnology & Law
University of Houston
Sandy Farmer
Principal
NextGenTech Pharma Consultants
Susan Gregurick
Director, Division of Biomedical Technology,
Bioinformatics, and Computational
Biology
National Institute of General Medical
Sciences, National Institutes of Health
Abraham Heifets
Chief Executive Officer
Atomwise, Inc.
Kenneth I. Kaitin
Professor and Director, Tufts Center for the
Study of Drug Development
Tufts University School of Medicine
Michael Keiser
Assistant Professor, Department of
Pharmaceutical Chemistry and the
Institute for Neurodegenerative Diseases
University of California, San Francisco
Patricia McGovern
Vice President and Global Head of Innovation
(Digital) for Regulatory Affairs
Novartis
Arti Rai
Professor and Faculty Director, Center for
Innovation Policy
Duke Law
Bobbie-Jo Webb-Robertson
Chief Scientist Computational Biology,
Biological Sciences Division
Pacific Northwest National Laboratory
Chris Whelan
Senior Scientist
Biogen
GAO Technology Assessment
GAO-17-75 74
Appendix III: GAO Contact and Staff Acknowledgments
GAO contact
Staff acknowledgments
In addition to the contact named above, Karen Howard (Assistant Director), Rebecca Parkhurst
(Analyst-in-Charge), Virginia Chanley, Lacey Coppage, Caitlin Dardenne, Leia Dickerson, Bryce
Fauble, Matt Hunter, Timothy Kinoshita, Anika McMillon, Jon Menaster, Silda Nikaj, Amanda
Postiglione, and Ben Shouse made key contributions to this report. Frederick K. Childers and
Katrina Pekar-Carpenter also contributed to this report.
(103352)

GAO’s Mission
The Government Accountability Office, the audit, evaluation, and investigative arm of Congress,
exists to support Congress in meeting its constitutional responsibilities and to help improve the
performance and accountability of the federal government for the American people. GAO
examines the use of public funds; evaluates federal programs and policies; and provides analyses,
recommendations, and other assistance to help Congress make informed oversight, policy, and
funding decisions. GAO’s commitment to good government is reflected in its core values of
accountability, integrity, and reliability.
Obtaining Copies of GAO Reports and Testimony
The fastest and easiest way to obtain copies of GAO documents at no cost is through GAO’s
website (https://www.gao.gov). Each weekday afternoon, GAO posts on its website newly
released reports, testimony, and correspondence. To have GAO e-mail you a list of newly posted
products, go to https://www.gao.gov and select “E-mail Updates.”
Order by Phone
The price of each GAO publication reflects GAO’s actual cost of production and distribution and
depends on the number of pages in the publication and whether the publication is printed in color
or black and white. Pricing and ordering information is posted on GAO’s website,
https://www.gao.gov/ordering.htm.
Place orders by calling (202) 512-6000, toll free (866) 801-7077, or
TDD (202) 512-2537.
Orders may be paid for using American Express, Discover Card, MasterCard, Visa, check, or money
order. Call for additional information.
Connect with GAO
Connect with GAO on Facebook, Flickr, Twitter, and YouTube.
Subscribe to our RSS Feeds or E-mail Updates.
Listen to our Podcasts and read The Watchblog.
Visit GAO on the web at https://www.gao.gov/podcast/watchdog.html.
To Report Fraud, Waste, and Abuse in Federal Programs
Contact: Website: https://www.gao.gov/fraudnet/fraudnet.htm
Automated answering system: (800) 424-5454 or (202) 512-7470
Congressional Relations
Orice Williams Brown, Managing Director, [email protected], (202) 512-4400, U.S. Government
Accountability Office, 441 G Street NW, Room 7125, Washington, DC 20548
Public Affairs
Chuck Young, Managing Director, youngc1@gao.gov, (202) 512-4800
U.S. Government Accountability Office, 441 G Street NW, Room 7149
Washington, DC 20548
Strategic Planning and External Liaison
James-Christian Blockwood, Managing Director, [email protected], (202) 512-48707
U.S. Government Accountability Office, 441 G Street NW, Room 7814, Washington, DC 20548
