
This Issue
Volume 5, Number 5
May 2014
Creating an Accurate Cause
of Death Statement on a
Death Certificate
1
Figure 1. Death
Registration Process
2
Figure 2. Sample Form of
California Certificate of Death
3
Electronic Cigarettes:
What Health Care
Providers Should Know
6
Figure 1. Diagram of
an Electronic Cigarette
6
Upcoming Training
8
Index of Disease
Reporting Forms
8
Death certificates are important
legal documents. Physicians play
a vital role in ensuring that the
medical information is accurate,
timely, and complete.
Natalia E. Sejbuk, MPH
Elizabeth Friedman, MPH
Loren Lieb, MPH
A
death certificate is a vital legal
document that contains the date,
location, and cause of a person’s
death. The information provided on
the death certificate is as important to
families and the health of the population
as the information entered in medical
records is to individuals. Even so,
physicians, who have primary
responsibility for determining the cause
and manner of death, often receive
little formal training in completing the
death certificate.
A death certificate is vital not only for
settling estates, closing bank accounts,
determining insurance and pension
benefits, and providing evidence
in court, it is also important for
monitoring mortality trends, providing
outcome data for research studies, and
for setting priorities for health-related
funding, research, and interventions. In
some circumstances, death certificates
may also be used for surveillance
of unusual health conditions and
conditions of public health significance.
In most cases, the attending physician
is responsible for determining and
completing the cause-of-death section
on the death certificate. Since
statistical data are derived from this,
it is important to complete the death
registration process as accurately and
promptly as possible.
The Process
On October 1, 2007, Los Angeles County
implemented the web-based California
Electronic Death Registration System
(CA-EDRS) to expedite the death
registration process. This paperless
Creating an Accurate Cause of Death
Statement on a Death Certificate
system enables funeral directors,
physicians, coroners, and hospitals to
submit electronic death certificates
for registration 24 hours per day. This
around-the-clock access assures that
death registration can occur within the
8 days required by California law and
that the cause of death can be reported
by the physician within the required
15 hours (California Health and
Safety Code, Chapter 6, Article 1,
§102775-102805).
The funeral director initiates the
death registration process by gathering
personal and demographic information
about the deceased—this responsibility
makes the funeral director the anchor
of the death registration system. Next,
the attending physician or coroner
completes the medical portion of the
death certificate to determine the
manner in which the individual died.
Medical examiners or coroners are re-
sponsible for investigating any cause of
death that is unexpected, unexplained,
or resulting from injury, poisoning,
or a public health threat. If a case is
referred to the coroner, the coroner
enters cause-of-death information
directly into CA-EDRS under the
“Coroner’s use only” section.
In most cases, however, the attending
physician is responsible for determin-
ing the cause of death. The physician
continued on page 2 >
2
Rx for Prevention LA County Department of Public Health May 2014
CREATING AN ACCURATE CAUSE OF DEATH STATEMENT from page 1
receives a cause-of-death worksheet provided by the funeral
director; once completed, he or she returns it to the funeral
director. The death certificate is then forwarded to the local
Department of Public Health; in LA County, it is the Registrar
for the LA County Department of Public Health. The death
certificate is reviewed and the cause of death is checked for
compliance with the International Classification of Diseases
10th revision (ICD-10) rules and for acceptance of the death
certificate in accordance with state guidelines. Once the death
certificate is accepted by the local Registrar, the physician
must attest to the accuracy of the cause-of-death information
on the certificate. This is done remotely using either a fax
machine to send the physician’s signed attestation to the
funeral home or by telephone, which requires the physician
to dial a toll-free number to provide a voice attestation. The
death certificate can then be submitted to the Public Health
Registrar by the funeral director for legal registration.
Throughout the death registration process, information is
entered directly into CA-EDRS. Los Angeles County data are
incorporated into both state and national databases, which are
used to describe the characteristics of those who died, to
determine life expectancy, and to compare mortality trends
and patterns with other jurisdictions. Mortality data for
Los Angeles County residents are summarized annually in
a report that describes the leading causes of death and pre-
mature death and that examines 10-year mortality trends.
1
Nationally, the Centers for Disease Control and Prevention’s
National Center for Health Statistics (NCHS) compiles data
for the United States that are also published annually.
2
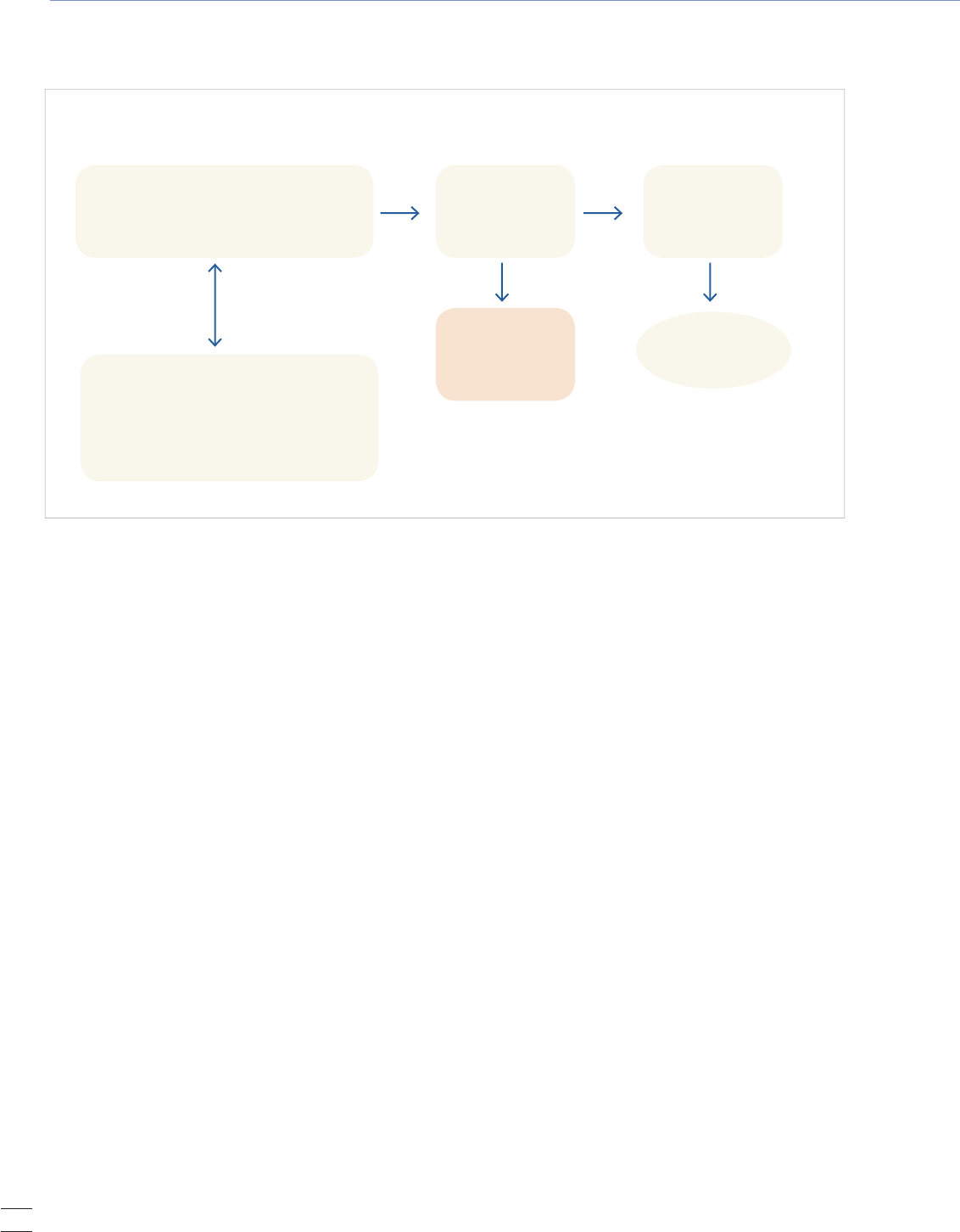
Figure 1 depicts the sequence of events that occur throughout
the course of the death registration process.
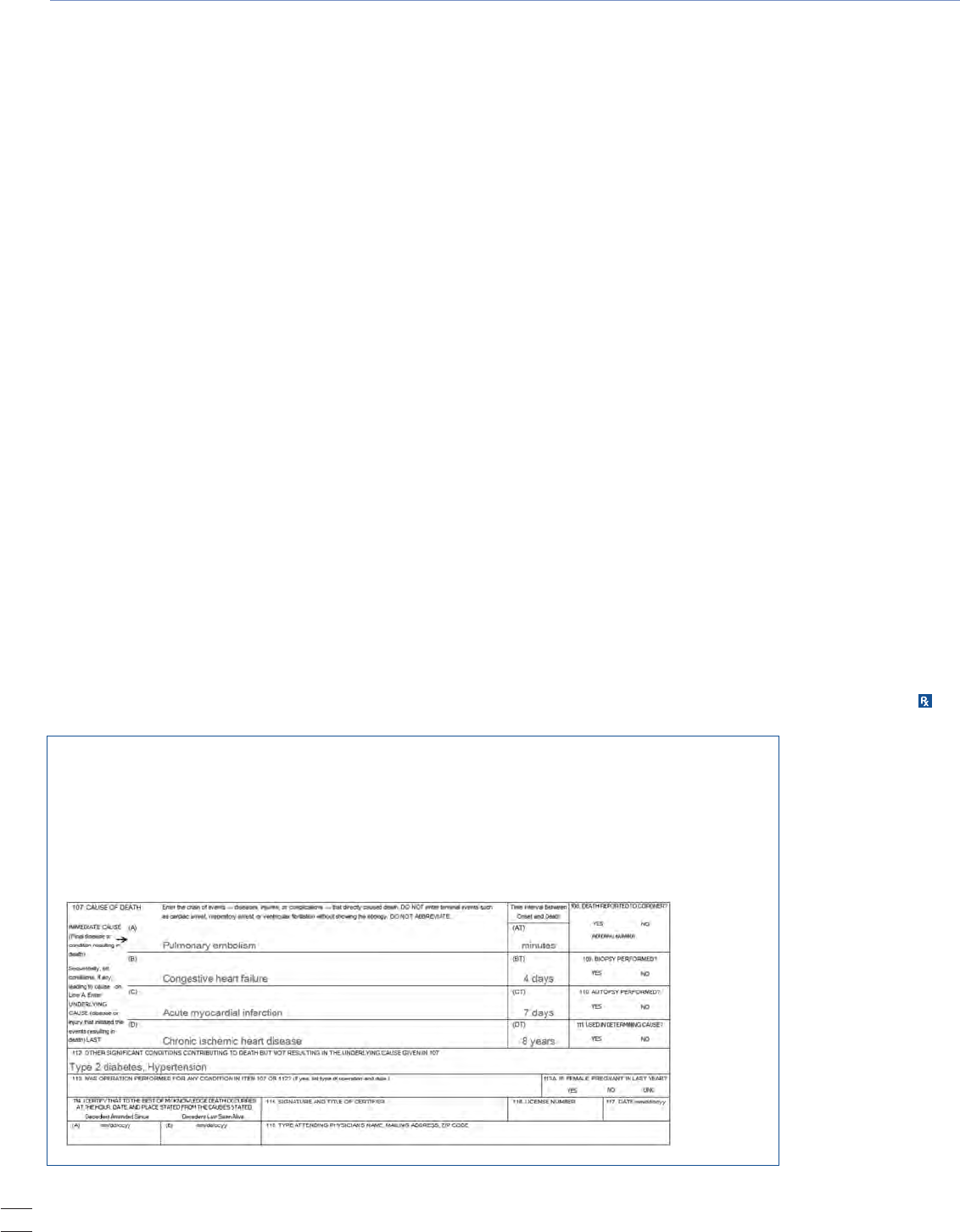
The Cause-of-Death Section: Instructions for Physicians
Section 107 of the California Certificate of Death (Figure 2)
is the most difficult section to complete. It is the physician’s
responsibility to report the cause of death as correctly as pos-
sible based on his or her best medical opinion. The section
consists of two parts. Part I is a sequential list of conditions
leading to the immediate cause of death and the time intervals
between their onset and the death. Part II is a list of other
conditions contributing to, but not directly causing, death.
PART I
Immediate cause of death: Item 107(A) is for the immediate
cause of death. This should be a disease, condition, or injury
that directly resulted in death. A common error is to list a
mechanism of death (for example, cardiac arrest) rather than
a disease (myocardial infarction). Vague terms such as “brain
dead” or “pulmonary arrest” cannot be used on the death
certificate. If cancer is the immediate cause of death, the pri-
mary site, cell type, and specific organ or lobe affected must
be listed. Examples are “adenocarcinoma of sigmoid colon” or
“squamous cell cancer of the breast.” Terms such as “old age”
or “senescence” are not acceptable since they do not actually
cause death. Autopsy cases must always be referred to the
coroner (with the exception of a few teaching medical facili-
ties) and, in some cases, it is appropriate for the coroner to list
the cause of death as “deferred” while waiting for the cause of
death results. If a death certificate is registered as “deferred,”
an amendment needs to be filed by the coroner in CA-EDRS
as soon as the results are available. In rare circumstances,
after investigation, the coroner may list the cause of death as
Figure 1. Death Registration Process
FUNERAL DIRECTOR
• Coordinates the death certicate processing
• Collects personal and demographic data
about decedent
CA State Office of
Vital Records
National Vital
Statistics System
(NVSS)
LOCAL REGISTRAR
Legal registration
MEDICAL CERTIFIER
• Licensed Physician
• Coroner
• Determines and certies the "Cause of
death" as being correct
Certied copy of
death certificate
available to family

SAMPLE
3
May 2014 LA County Department of Public Health Rx for Prevention
Figure 2. Sample Form of California Certificate of Death
4
Rx for Prevention LA County Department of Public Health May 2014
Case Study 1
A 68-year-old woman is admitted to the ICU because of acute chest pain. She has Type 2 diabetes,
hypertension, obesity, and angina. Over the next 24 hours, an acute myocardial infarction is
confirmed. Heart failure develops but improves with management. The woman then experiences a
pulmonary embolus, confirmed by ventilation-perfusion lung scan and blood gases; over the next
2 hours she becomes unresponsive and dies. What should be written in the cause of death section?
“Could not be determined” in Section 119. Abbreviations
must never be used in section 107, and line 107(A) should
never be blank.
Underlying cause of death: Items 107(B-D) are for the
intermediate and underlying causes of death. This is the most
significant piece of information on the certificate since most
mortality analyses are based on the underlying cause of death.
Every condition listed should cause the one above it. Thus,
entering conditions in an illogical order will prompt the
Public Health Registrar to question the cause of death and
the certificate will be returned to the funeral director for
revision. A useful way to make sure the order of the causes
makes sense is to say the phrase “due to” or “as a consequence
of,” moving from line A down to the last filled-in line. For
instance, a death may be due to a pulmonary embolus, as a
consequence of hip surgery, resulting from an injury from a
fall, resulting from a cerebral infarction. Cerebral infarction
is the underlying cause of death. Multiple conditions cannot
be listed on 1 line in this section.
Time intervals: To the right of lines 107(A-D) are items
107(AT-DT) where the time intervals between the conditions
listed and the time of death are to be listed. The more precise
the time the better, but it is acceptable to estimate and
use terms such as “approximately.” If the time interval is
unknown and cannot be estimated, “unknown duration”
can be listed. Something must always be entered on these
lines next to the corresponding conditions; they cannot
be left blank.
PART II
Other significant conditions: Item 112 is where other ill-
nesses or conditions that may have contributed to the death,
but were not the direct cause of it, can be listed. Multiple
conditions may be listed here. There may be uncertainty as
to the direct or contributing causes of death, so it is up to the
physician to use his or her best medical judgment as to the
most likely causes and sequences contributing to death.
The Big Picture
Most physicians at some point in their careers will complete
a death certificate. The cause of death information from each
death becomes a permanent legal record and part of our state
and national mortality databases; therefore, it is important
that physicians, together with all those involved in the death
registration process, make every effort to complete each death
certificate as accurately and completely as possible. Mortality
data are important to physicians since they influence fund-
ing for medical and health research and can influence clinical
practice. They are also critical for establishing public health
priorities. The county’s annual mortality summary provides
information about the leading causes of death and premature
death. For example, in 2010, an average of 155 people died
each day in Los Angeles County, including 35 from coronary
heart disease, 9 from injuries, and 9 from stroke.
1
The remain-
ing deaths resulted from such causes as emphysema, diabe-
tes, pneumonia, liver disease, and cancer. Without properly
completed death certificates, we would not be able to analyze
mortality patterns and make them widely available. Addition-
al resources and contact information are listed on page 5 for
any questions regarding the death registration process.

5
May 2014 LA County Department of Public Health Rx for Prevention
Additional Resources for Physicians
CDC Physician Handbook on Death Certication
www.cdc.gov/nchs/data/misc/hb_cod.pdf
Improving Cause of Death Reporting (online tutorial)
http://www.cdc.gov/primarycare/materials/online-trainings/icdr/player.html
CDC NCHS Mortality Data from the National Vital Statistics System
http://www.cdc.gov/nchs/deaths.htm
California Electronic Death Registration System Website
http://www.edrs.us/edrs/index.jsp
Case Study 2
A 75-year-old female has a 15-year history of Type 2 diabetes, history of hypertension, and an
uncomplicated myocardial infarction 6 years prior. Her daughter found her disoriented in her home
and brought her to the hospital. On admission, she was unresponsive. Laboratory tests disclosed
severe hyperglycemia, hyperosmolarity, azotemia, and mild ketosis without acidosis. A diagnosis
of hyperosmolar nonketotic coma was made. She was vigorously treated, and within 72 hours her
hyperosmolar and hyperglycemic state was resolved. However, she remained anuric with progressive
azotemia. Attempts at renal dialysis were unsuccessful. The patient died 8 days later in severe renal
failure. What should be written in the cause of death section?
Contacts
Los Angeles County Department of Public Health,
Public Health Registrar
Gustavo Feregrino (213) 240-8029
Roland Carrillo (323) 869-8510
Alma Ortega (323) 869-8512
Gregory Mercado (213) 989-7073
LA County Department of Coroner
Dr. Mark A. Fajardo, Chief Medical Examiner Coroner,
(323) 343-0512; After hours, (323) 343-0714
CA-EDRS Help Desk
(916) 552-8123
Natalia E. Sejbuk, MPH, is an Epidemiology Analyst, Office of Health
Assessment and Epidemiology; Elizabeth Friedman, MPH, is an
Epidemiology Analyst, Community Health Services; and Loren Lieb,
MPH, is a Supervising Epidemiologist, Ofce of Health Assessment and
Epidemiology, Los Angeles County Department of Public Health.
REFERENCES
1. Los Angeles County Department of Public Health, Ofce of Health
Assessment and Epidemiology. Mortality in Los Angeles County
2010: Leading causes of death and premature death with trends for
2001-2010. October 2013. Available online at http://publichealth.
lacounty.gov/dca/data/documents/2010MortalityReport.pdf. Print
copies are available from the Ofce of Health Assessment and Epide-
miology at (213) 240-7785.
2. Centers for Disease Control and Prevention. National Vital Statis-
tics Reports, Deaths: Final Data for 2010. May 2013.
6
Rx for Prevention LA County Department of Public Health May 2014
Susan Bradshaw, MD, MPH, ABIHM
Tonya Gorham Gallow, MSW
E
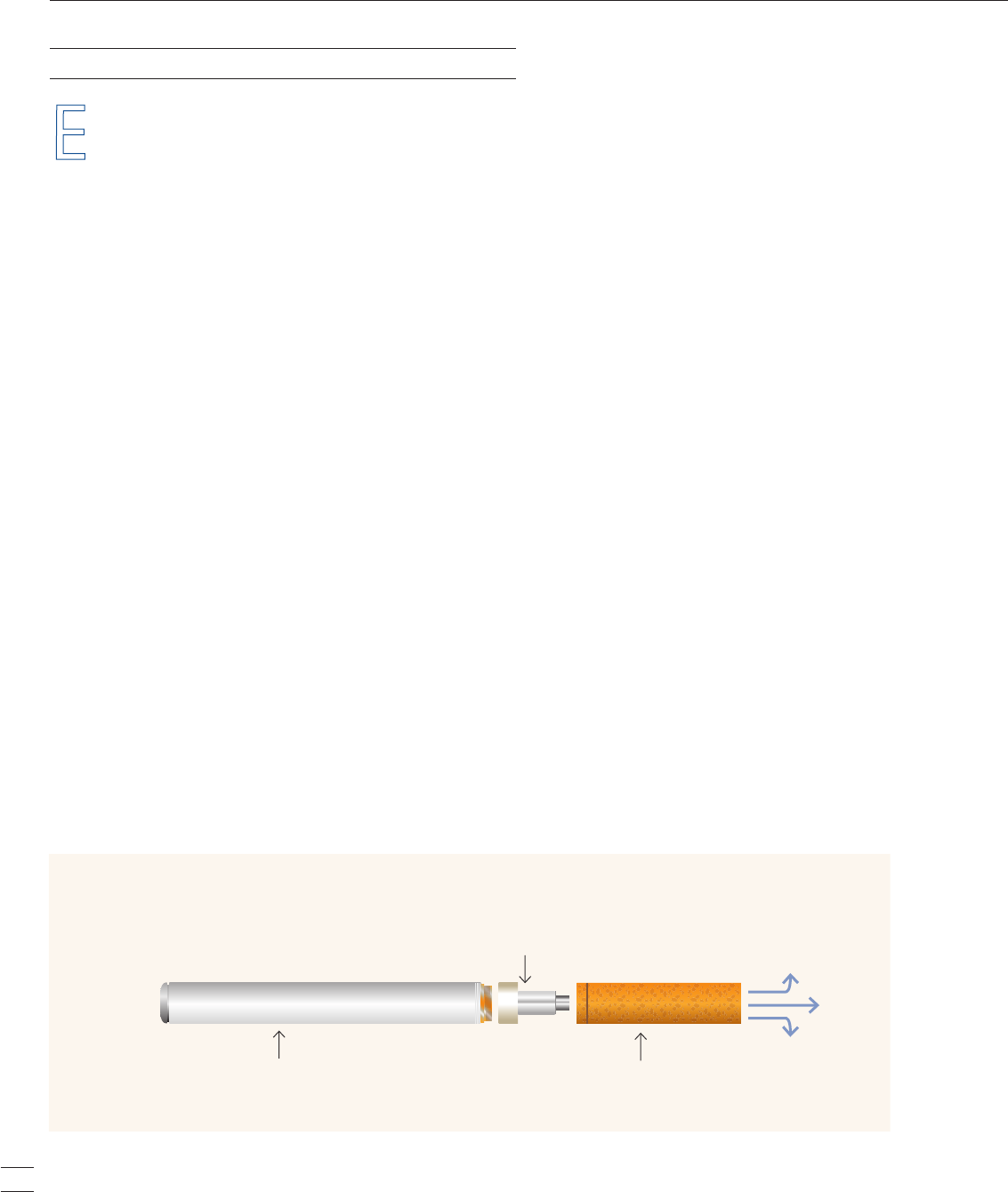
lectronic cigarettes, or e-cigarettes, are battery-operated
devices designed to create a vapor that is inhaled by
the user (“vaping”). The vapor is produced by heating
an internal cartridge that is typically filled with a solution
of nicotine, flavors, and other chemicals. The inhaled
vapor produces a sensation similar to that of inhaled tobacco
smoke.
1-3
E-cigarettes are being widely marketed as a healthier
alternative to conventional cigarettes and as a “safe” smoking-
cessation aid. Although there are anecdotal reports of smokers
who have found e-cigarettes to be helpful in their efforts to
quit smoking, the efficacy of e-cigarettes as an aid in smoking
cessation has not been demonstrated. These products are cur-
rently unregulated and their benefits as well as risks among
youth and adults have not been well-studied.
4
In recent years, there has been an explosion in the popular-
ity of e-cigarettes. Created in China, e-cigarettes became read-
ily available internationally in 2006. Since then, the industry
has grown from a few thousand users to several million
worldwide. In the United States, retail sales of e-cigarettes
doubled from $250 million to $500 million between 2011 and
2012, and sales are expected to quadruple by 2014.
5-6
Of particular concern is the rapid rise in use among youth.
According to the Centers for Disease Control and Prevention
(CDC), the percentage of high-school students in the U.S. who
had ever used e-cigarettes doubled from 4.7% to 10% between
2011 and 2012. During the same 2-year period, the percent-
age of middle-school students who had ever used e-cigarettes
doubled from 1.4% to 2.7%. In 2011, about 21% of adults who
smoked traditional cigarettes had used electronic cigarettes,
up from about 10% in 2010.
7-8
This is unfortunate because
some tobacco control researchers believe e-cigarettes may be
a socially acceptable gateway to nicotine addiction and the
renormalization of tobacco use.
Electronic Cigarettes
What Health Care Providers Should Know
E-cigarette Products
E-cigarettes come in many varieties, including e-pens,
e-cigars, and e-hookah products. They contain e-cigarette
liquid, also known as e-liquid, generally a solution of pro-
pylene glycol, vegetable glycerin, and/or polyethylene glycol
mixed with concentrated flavors; and a variable concentration
of nicotine, including nicotine-free versions. E-liquid is
available in a variety of flavors.
Recent studies have identified serious problems associated
with the lack of product standards and regulation. Manufac-
turers do not always accurately label the amount of nicotine in
their products. The U.S. Food and Drug Administration (FDA)
found that certain cartridges labeled as “No nicotine” actu-
ally contained nicotine, and that other cartridges labeled as
containing identical amounts of nicotine contained markedly
different amounts of nicotine. One study examined 6
brands of products for design, content, quality, and product
information, including warnings. Most of the products
leaked when handled, creating the potential for dermal
nicotine exposure and potential nicotine poisoning.
Health and Safety Risks
The rise in consumption of e-cigarettes is very worrisome
because early studies indicate these products may not be safe.
At least 10 chemicals identified in e-cigarette aerosol (or the
vapor) are on California’s Proposition 65 list of carcinogens
and reproductive toxins.
The compounds that have been identified in e-cigarette
aerosol include acetaldehyde, benzene, cadmium, formalde-
hyde, isoprene, lead, nickel, nicotine, N-nitrosonornicotine,
and toluene. Chemicals found in e-cigarette aerosol include
metals, such as chromium and tin nanoparticles; tobacco-
specific nitrosamines, chemicals known to cause cancer; and
diethylene glycol, a substance commonly found in antifreeze.
The concentrations for most of the above elements in
e-cigarette aerosol were higher or equal to the corresponding
concentrations in conventional cigarette smoke.
Battery
Vapor
Atomization Chamber
Heats the solution, vaporizing it
Nicotine Cartridge
Holds a solution, which may
or may not contain nicotine
Diagram of an Electronic Cigarette
7
May 2014 LA County Department of Public Health Rx for Prevention
Another health concern is the chronic inhalation of pro-
pylene glycol, the main ingredient in e-liquid. Even though
propylene glycol is FDA-approved for oral consumption, the
inhalation of vaporized nicotine in propylene glycol is not.
Short-term exposure causes eye, throat, and airway irrita-
tion, and long-term exposure can result in children develop-
ing asthma. Some studies show that heating propylene glycol
changes its chemical composition, producing small amounts
of propylene oxide, a known carcinogen.
Nicotine toxicity is a significant health concern, given
reports of accidental poisonings from e-cigarette products on
the rise, particularly among children. E-cigarette-related calls
to poison control centers tripled between 2012 and 2013, and
the number of poisonings jumped to 1,351 in 2013, a 300%
increase from 2012. The CDC reported a dramatic rise in the
number of e-cigarette-related phone calls to poison control
centers, from just 1 call per month on average in 2010 to
nearly 200 calls per month in early 2014. More than 50% of
the calls involved children aged 5 and under.
Signs of Nicotine Toxicity
Liquid nicotine is far more dangerous than that found in other
tobacco products because it is absorbed more quickly. Toxi-
cologists identify potential dangers of e-liquids because of
their neurotoxicity and ability to be lethally absorbed quickly
through the skin.
Health care providers should be familiar with signs and
symptoms related to nicotine toxicity. Mild symptoms include
nausea, vomiting, dizziness, drowsiness, increased heart rate,
and increased blood pressure. More severe symptoms include
seizures, decreased heart rate, and decreased blood pressure.
Symptoms from skin or eye exposure include irritation,
redness, severe pain, and inflammation, and may result in
whole-body toxicity.
Recommendations
Given the unknown public health impact and the current
lack of regulation, the Los Angeles County Department of
Public Health recommends a precautionary approach regard-
ing the use of e-cigarettes until further research is available.
The CDC, along with other health agencies, recommend that
health care providers consider the following actions:
• Be well-informed and vigilant that e-cigarettes have the
potential to cause acute adverse health effects and represent
an emerging public health concern.
• Inform patients of potential dangers of e-cigarettes and
encourage parents to talk to their children and to discour-
age use. Advise patients to keep e-cigarettes out of reach of
children, preferably locked in a secure place.
• Inform patients that e-cigarettes have not been approved by
the FDA as a quit-smoking aid. Encourage the use of FDA-
approved smoking-cessation medication among patients
who want to quit. Additional information on strategies and
support for quitting smoking can be found online at
www.LAQuits.com or by calling 1-800-NO-BUTTS.
Update
At press time, the FDA proposed rules to strictly regulate
electronic cigarettes, cigars, pipe tobacco, nicotine gels, water
pipe tobacco, and hookahs. After a 75-day public comment
period (starting April 25, 2014), the proposed rules include
the following:
• Setting the age limit to buy the products to be at least
18 years (states can set it higher)
• Health warnings required on all products
• Sale of the products in vending machines
would be prohibited
• Manufacturers would be required to register all of their
products and ingredients with the FDA
• Manufacturers would only be able to market new products
after an FDA review
• Manufacturers would need to provide scientic evidence
before making any claims of risk reduction tied to use of
their product.
Susan Bradshaw, MD, MPH, ABIHM, is a Physician Specialist, and
Tonya Gorham Gallow, MSW, is Director, Tobacco Control and Preven-
tion Program, Los Angeles County Department of Public Health.
REFERENCES
1. Tobacco Fact Sheet–Electronic Cigarettes (E-Cigarettes). (June
2013). American Legacy Foundation. Retrieved from: http://www.
legacyforhealth.org/content/download/ 582/6926/le/LEG-Fact-
Sheet-eCigarettes-JUNE2013.pdf.
2. Questions and answers on electronic cigarettes or electronic
nicotine delivery systems (ENDS). (July 2013). World Health Organi-
zation, Tobacco Free Initiative. Retrieved from: http://www.who.int/
tobacco/communications/statements/eletronic_cigarettes/en/.
3. E-Cigarettes [fact sheet]. (Oct 2013). American Academy of Pediat-
rics–Julius B. Richmond Center of Excellence. Retrieved from: http://
www2.aap.org/richmondcenter/ pdfs/ECigarette_handout.pdf.
4. Food and Drug Administration. News and events—electronic
cigarettes (e-cigarettes). Silver Spring, Maryland: US Department of
Health and Human Services, Food and Drug Administration; 2014.
Available at http://www.fda.gov/newsevents/publichealthfocus/
ucm172906.htm.
5. Herzog B.E- Cigs Revolutionizing The Tobacco Industry; Wells Far-
go Security Equity Research, June 12, 2013. (Email Correspondence).
6. Mangan D. “E-cigarette sales are smoking hot, set to hit
$1.7 billion.” CNBC. 28 August 2013. Available at: http://
www.cnbc.com/id/100991511.
7. Centers for Disease Control and Prevention. Morbidity and Mortal-
ity Weekly Report: Notes from the Field: Electronic Cigarette Use
Among Middle and High School Students – United States, 2011-
2012. MMWR 2013;62:p729-830.
8. Centers for Disease Control and Prevention. Press Release: About
one in ve U.S. adult cigarette smokers have tried an electronic
cigarette. CDC. 28 February 2013. Available at: http://www.cdc.gov/
media/releases/2013/ p0228_electronic_cigarettes.html.
Index of Disease Reporting Forms
All case reporting forms from the LA County Department of Public Health are
available by telephone or Internet.
Rx for Prevention is published 10 times a year
by the Los Angeles County Department of
Public Health. If you would like to receive this
newsletter by e-mail, go to www.publichealth.
lacounty.gov and subscribe to the ListServ
for
Rx for Prevention.
Office of the Medical Director
241 N. Figueroa St., Suite 275
Los Angeles, CA 90012
Use of trade names and commercial sources in Rx for Prevention is for identification only and does not imply endorsement by the Los Angeles
County Department of Public Health (LACDPH).References to non-LACDPH sites on the Internet are provided as a service to Rx for Prevention
readers and do not constitute or imply endorsement of these organizations or their programs by LACDPH. The Los Angeles County Department of
Public Health is not responsible for the content of these sites. URL addresses listed in Rx for Prevention were current as of the date of publication.
Reportable Diseases & Conditions
Confidential Morbidity Report
Morbidity Unit (888) 397-3993
Acute Communicable Disease Control
(213) 240-7941
www.publichealth.lacounty.gov/acd/
reports/CMR-H-794.pdf
Sexually Transmitted Disease
Confidential Morbidity Report
(213) 744-3070
www.publichealth.lacounty.gov/dhsp/
ReportCase.htm (web page)
www.publichealth.lacounty.gov/dhsp/
ReportCase/STD_CMR.pdf (form)
Adult HIV/AIDS Case Report Form
For patients over 13 years of age
at time of diagnosis
Division of HIV and STD Programs
(213) 351-8196
www.publichealth.lacounty.gov/dhsp/
ReportCase.htm
Pediatric HIV/AIDS Case Report Form
For patients less than 13 years of age
at time of diagnosis
Pediatric AIDS Surveillance Program
(213) 351-8153
Must first call program before reporting
www.publichealth.lacounty.gov/dhsp/
ReportCase.htm
Tuberculosis Suspects & Cases
Confidential Morbidity Report
Tuberculosis Control (213) 745-0800
www.publichealth.lacounty.gov/tb/forms/
cmr.pdf
Lead Reporting
No reporting form. Reports are
taken over the phone.
Lead Program (323) 869-7195
Animal Bite Report Form
Veterinary Public Health (877) 747-2243
www.publichealth.lacounty.gov/vet/
biteintro.htm
Animal Diseases and Syndrome
Report Form
Veterinary Public Health (877) 747-2243
www.publichealth.lacounty.gov/vet/
disintro.htm
LOS ANGELES COUNTY
BOARD OF SUPERVISORS
Gloria Molina, First District
Mark Ridley-Thomas, Second District
Zev Yaroslavsky, Third District
Don Knabe, Fourth District
Michael D. Antonovich, Fifth District
DEPARTMENT OF PUBLIC HEALTH
Jonathan E. Fielding, MD, MPH
Director and Health Officer
Cynthia A. Harding, MPH
Chief Deputy Director
Jeffrey D. Gunzenhauser, MD, MPH
Medical Director
Steven Teutsch, MD, MPH
Chief Science Ofcer
EDITORS IN CHIEF
Jeffrey D. Gunzenhauser, MD, MPH
jgunzenhauser@ph.lacounty.gov
Steven Teutsch, MD, MPH
steutsch@ph.lacounty.gov
MEDICAL COMMUNITY ADVISER
Thomas Horowitz, DO
EDITORIAL BOARD
Melanie Barr, RN, MSN
Stephanie Caldwell, MPH
Kevin Donovan, MPH
Julia Heinzerling, MPH
Christina Jackson, MPH
Anna Long, PhD, MPH
Paula Miller, MPH, CHES
Sadina Reynaldo, PhD
Carrie Tayour, MPH
Rachel A. Tyree, MPH
Summer Nagano,
Managing Editor
Alan Albert & Kathleen Pittman,
Graphic Designers
Maria Ojeda, Administration
Comments or Suggestions? If so, or if you
would like to suggest a topic for a future issue,
e-mail Dr. Jeffrey Gunzenhauser, co-editor, at
jgunzenhauser@ph.lacounty.gov.
Upcoming Training
Immunization Training:
2014 Adult Immunization Schedule
The Los Angeles County Department of
Public Health Immunization Program is
offering a 2-hour CEU training titled
"2014 Adult Immunization Schedule" at
no charge to providers. Topics include adult
immunization schedule updates and recommen-
dations for vaccinating medically high-risk adults
and health care personnel.
To register or learn more about other trainings
sponsored by the Immunization Program,
visit www.publichealth.lacounty.gov/ip/
trainconf.htm or call (213) 351-7800.
