
INVESTIGATOR INFORMATION HANDBOOK
Prepared by The Division of Laboratory Animal Resources
July 2024
2 (TOC)
Table of Contents
DIVISION OF LABORATORY ANIMAL RESOURCES INVESTIGATOR
ORIENTATION PROGRAM
Page
Division of Laboratory Animal Resources (DLAR) Mission Statement 4
Division of Laboratory Animal Resources Staff 5
DLAR Veterinarians 5
DLAR Health Care Technicians 5
DLAR Animal Care Supervisors 6
DLAR Veterinary Research Support
Training Team, Research Analyst, 7
Surgery Technician, DLAR Administrative Support 8
Facility Access and Security Protocols 9
Visitor Badges and Procedures 9
Training 10
DIVISION OF LABORATORY ANIMAL RESOURCES SUPPORT UNITS
Animal Husbandry and Support
Housing and Animal Facilities 11
Caging for Rodents 11
Working In Rodent Rooms 11
Cage Docking Procedures 11
Breeding Colony Management and Weaning Pups 12
Yellow Transparency Card 12
Micro Isolator Technique 13
Procedures in Rodent Housing Rooms 14
Examples of Rodent Caging 15
Returning Dirty Caging to Cage Wash 16
Inquiries and Problems 16
Unresolved Problems 16
Transportation of Animals 16
Procedure Rooms 18
Service Requests 18
Storage of Drugs or Biologics 18
Special Diets 18
Animal Health Division
Veterinary and Veterinary Health Technician Services 19
How to contact Veterinarian Health Care Technicians 19
Experimental Surgery
Scheduling the Surgery Area 20
IACUC Guidelines for Rodent Surgery 20
Post-Operative Recovery and Care for Mice and Rats 21
Anesthesia 22
Other Services 22
Cryopreservation/Rederivation 22
3 (TOC)
Technical Services
Breeding Colony Management 23
Sample Collection 23
Animal Pathology Division 23
Other Services 23
Animal Procurement
Ordering animals 24
Non-commercial Vendors 24
Quarantine and Acclimation 24
Cage Cards, Census, and Per Diems 25
Training
Requirements for Personnel Who Use Animals in Research at UKY 26
Occupational Health and Safety 27
REGULATORY AGENCIES AND OTHER OVERSIGHT
USDA/Animal Welfare Regulations (Title 9 CFR parts 1 and 2) 29
Public Health Service (PHS) 29
Office of Laboratory Animal Welfare (OLAW) 29
The Guide for Humane Care and Use of Laboratory Animals 30
The Center for Disease Control (CDC) 30
Association for Assessment and Accreditation of Animal Care, Internation
(AAALAC) 30
AVMA Guidelines for Euthanasia 30
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE
Contact Information 31
Animal Protocols 31
Necessary Elements of a Literature Search 31
Alternatives to the Use of Animals 32
USDA Pain Categories 33
Reporting Concerns 35
Veterinary Consultation 35
Resource Material and Forms
Resources and Links
Cage Densities for Mice being housed in DLAR Facilities 36
Mouse Caging 36
Rat Caging 37
Other Species 38
Additional DLAR Resources 38
DLAR Forms
DLAR Access Form 38
Cayuse AO Request Forms 38
DLAR Service Forms 39
DLAR Visitor Form 39
Animal Import/Export Forms 39

4 (TOC)
Division of Laboratory Animal Resources Investigator
Orientation Program
MISSION STATEMENT:
We support research at the University of Kentucky by providing the highest
quality veterinary services and humane care and treatment to all research animals. We
strive to assist in the continued advancement of scientific knowledge for the benefit of
humans and animals. We abide by the ethical principles of humane animal care and
good science in accordance with all regulatory agencies. We serve as a resource for
knowledge and technical expertise and provide an atmosphere of mutual respect and
cooperation with our researchers.
VISION:
The Division of Laboratory Animal Resources will be viewed as an essential
and valued component of the biomedical research enterprise by the faculty
researchers at the University of Kentucky and the unit will be considered among the
top 25% of programs regarding laboratory animal medicine, facility management, and
laboratory animal research.
VALUES:
• The use of vertebrate laboratory animals is essential and appropriate for the
conduct of scientifically valuable research relevant to human or animal health,
the advancement of knowledge, or the good of society.
• Optimal animal care, health, and well-being are essential for valid leading
edge biomedical research when laboratory animals are used.
• Personnel involved in the care and maintenance of animals are essential
partners in, and valuable contributors to, the biomedical research enterprise.
• Comparative medical scientists are valuable contributors to the overall
biomedical research effort at the University of Kentucky.
• Training and instruction in laboratory animal and comparative medicine is
valuable and necessary for the continued rapid advancement of biomedical
research using vertebrate animals.

5 (TOC)
Division of Laboratory Animal Resources Staff
DLAR Veterinarians
DLAR Veterinary Health Care Technicians
Amelia Hall
Research Facility
Manager Clinical
*859-330-0388
amelia.hall@uky.edu
Jeanie Kincer
DVM, DACLAM
Acting Director
859-323-5469
jeanie.kincer@uky.edu
Jessica Perpich
DVM,
Clinical
Veterinarian
jessica.perpich@uky.edu
Nikki Caudill
Veterinary Technician
859-323-6010
nikki.herb@uky.edu

6 (TOC)
DLAR Animal Care Supervisors
Josh Murphy
ACSI
BBSRB/HKRB
859-218-3672
Joshuah.Murphy@uky.edu
Erin Jones
LAT
ACSII
MedCen/Dental-CAF-
Combs/Spindletop
859-257-3562
erin.dehnart@uky.edu
Ariel Masingo
Veterinary Technician
859-323-3093
ariel.masingo@uky.edu
Juan Slaughter
RLAT
Research Facilities
Manager
859-562-3380
jslaughter@uky.edu

8 (TOC)
DLAR Surgery Technicians
DLAR Administrative Support
Melissa (Missy)
Hammons
HR/Payroll/Procurement
859-323-2934
missy.hammons@uky.edu
Cheryl Carmichael
RLATg
Cayuse AO Database
Coordinator
859-323-6006
cheryl.carmichael@uky.edu
Lori Lunsford
Supply Procurement &
Facility Access
859-323-7132
lori.lunsford@uky.edu
Jacob Baker
Animal Procurement &
Facility Access
859-323-5885
Jacob.Baker@uky.edu
9 (TOC)
FACILITY ACCESS AND SECURITY PROTOCOLS
Access to the DLAR animal facilities is limited to authorized personnel only.
Authorized users must not “loan” their badges to others or allow others to use their
credentials at the risk of having their access revoked. “Tailgating” or following others
into the facilities without swiping your badge is not permitted. The DLAR facilities have
a variety of security measures to enter a facility.
• Med Center, Combs and HSRB require badge and Iris Scan for facility entry
and badge out of facility (some rooms in Combs have badge access)
• Lee T. Todd (Bio Pharm) requires a badge and 5-digit PIN followed by the #
sign for facility entry, badge out of facility and badge to enter animal rooms
• Sanders Brown requires badge and 5-digit PIN for facility entry and badge out
of facility
• BBSRB requires badge and Iris Scan for facility entry and 4-digit PIN to enter
animal housing rooms
• MDSB requires a key for facility entry and 4-digit PIN to enter animal housing
rooms
• Spindletop requires a key for facility entry
• HKRB requires badge and Iris Scan for facility entry in and out of facility and
badge to enter animal and procedure rooms
Access to DLAR facilities can be obtained once someone is added to your protocol.
All access requests go through the DLAR main office in HSRB 204. See instructions
for facility access on the DLAR web page under forms:
https://www.research.uky.edu/division-laboratory-animal-resources/dlar-forms
• The EyeLock enrollment for the iris scan is located in HSRB 204. Enrollment
only takes a few minutes. Please schedule a time for your enrollment by
contacting the main DLAR office (HSRB room 204) 323-7132, 323-2934, or
323-5885
• PIN numbers for facility access and Lee T. Todd animal room access are
assigned by the DLAR Main Office. The same 5-digit PIN will work for any of
our facilities requiring a 5-digit PIN.
• BBSRB and MDSB room entry 4-digit PINs are assigned by the area
supervisor. The forms may be accessed on the DLAR Forms page under
DLAR Access.
Visitor Badges and Procedures
Visitor badges are issued to visitors and vendors who will be here for a short while
(from a few hours to a day or two for equipment service). To access any DLAR facility,
there needs to be a Visitor/Vendor Occupational Health form on file in DLAR. This
information page is part of our overall occupational health and safety program to
inform visitors and vendors of any potential exposure to allergen or other hazards that
may or may not be encountered in an animal facility.
10 (TOC)
Visitor Badges have NO entry or room door access capabilities and are only valid for
the day they are issued. If you require a temporary badge for more than one day, it
must be renewed each day.
Visitor Badges are available in the following DLAR locations:
• The Main Office in the Health Sciences Research Building (HSRB) Room 204
between 7:30 AM and 4:30 PM Monday through Friday.
• BBSRB DLAR office (B036) Between 7:00 AM thru 3:15 PM Monday thru Friday
• Lee T. Todd Building DLAR office (030H) between the hours of 7:00 AM and
3:15 PM Monday through Friday.
• H41A training room 7:30 AM – 4:00 PM
The DLAR Visitor/Vendor Occupational Health and Safety Form is available by on The
DLAR Forms page under DLAR Visitors. For more information in regards to
accessing DLAR facilities please review the Guidelines and Policies for Accessing
DLAR Facilities.
Visitors/Vendors to our facilities must be escorted by their hosts or a member of the
hosts’ staff or appropriate personnel.
All photography must be with approval of the Director of the Division of Laboratory
Animal Resources and the Principal Investigator.
Note: Media representatives must also be cleared, and approval obtained through the
Office of the Vice President for Research and UK Public Relations. Media
representatives MUST be accompanied by a member of the DLAR staff or the
Investigator staff. Should you need to make arrangements for your visitors ahead of
time, please contact our Facility Manager, one of our DLAR veterinarians, our office
staff, or our Training Team.
Training
Training is available on a wide range of subjects by contacting the DLAR Training
Group, any of the DLAR Supervisors, or our DLAR Veterinarians. Basic micro-isolator
techniques, working under the laminar airflow workstations and Biosafety cabinets, as
well as animal handling, routine procedures such as oral gavage, injection and blood
collection, are just a few of the techniques available. These can be scheduled as an
individual or group session and can be arranged with our training team. Additional
techniques may be available on request. It is recommended that all new staff
members attend a basic procedures training session. All personnel must participate in
the Occupational Health and Safety program if they will be working with or around
laboratory animals or their tissues. Please contact the DLAR Training Group for more
information regarding training opportunities for your staff.

11 (TOC)
DIVISION OF LABORATORY ANIMAL RESOURCES
SUPPORT UNITS
Animal Husbandry and Support
Housing and Animal Facilities
The location of animal housing will be determined by DLAR with the consideration for
the investigator and his/her needs. Space within the animal facilities may be limited at
times, but every attempt will be made to work with the investigators to ensure
adequate space. All animals must be housed in DLAR facilities unless special
permission has been obtained from the Institutional Animal Care and Use Committee
and the requirements for their care have been addressed. If you will be working
with non-human primates, you will have to consult with the DLAR veterinary
staff for the requirements for being granted access to this area. Additional
training and current negative tuberculin testing and measles, mumps, and
rubella (MMR) titer are required for all personnel.
Caging for Rodents
The majority of rodents housed in the DLAR facilities are in ventilated caging to
maximize biosecurity at the cage level. These cages are changed under animal
transfer systems and Class II Biosafety cabinets facility wide. All manipulations of
caging and animals will be accomplished using the hoods. This will include changing
cages, weaning, pairing for breeding, sexing of weanlings, medical examination and
treatment, and all procedures done within the animal housing areas. Prepared clean
caging is available in the hallway for weaning. Should you need more than just a few
cages for weaning or separating animals, please submit a request to the area
supervisor using the Special Request form. Cages manipulated outside the hoods
will be marked as CONTAMINATED and should be handled last after all other
animals have been manipulated.
Working in Rodent Rooms
All work with mice and rats must be done in the hoods. The micro-isolator lid
should not be opened unless the cage is in the hood and the hood/change station
motor is running. Disinfect all work areas before and after use.
Cage Docking Procedures for IVC Racks
Ensure that a water valve is present and activate it to ensure that water is
flowing from the water valve
When replacing a cage back into the ventilated cage rack, be sure that it is properly
“docked” so that the animals can obtain water from the automated water lines and the
water valve is not compromised.
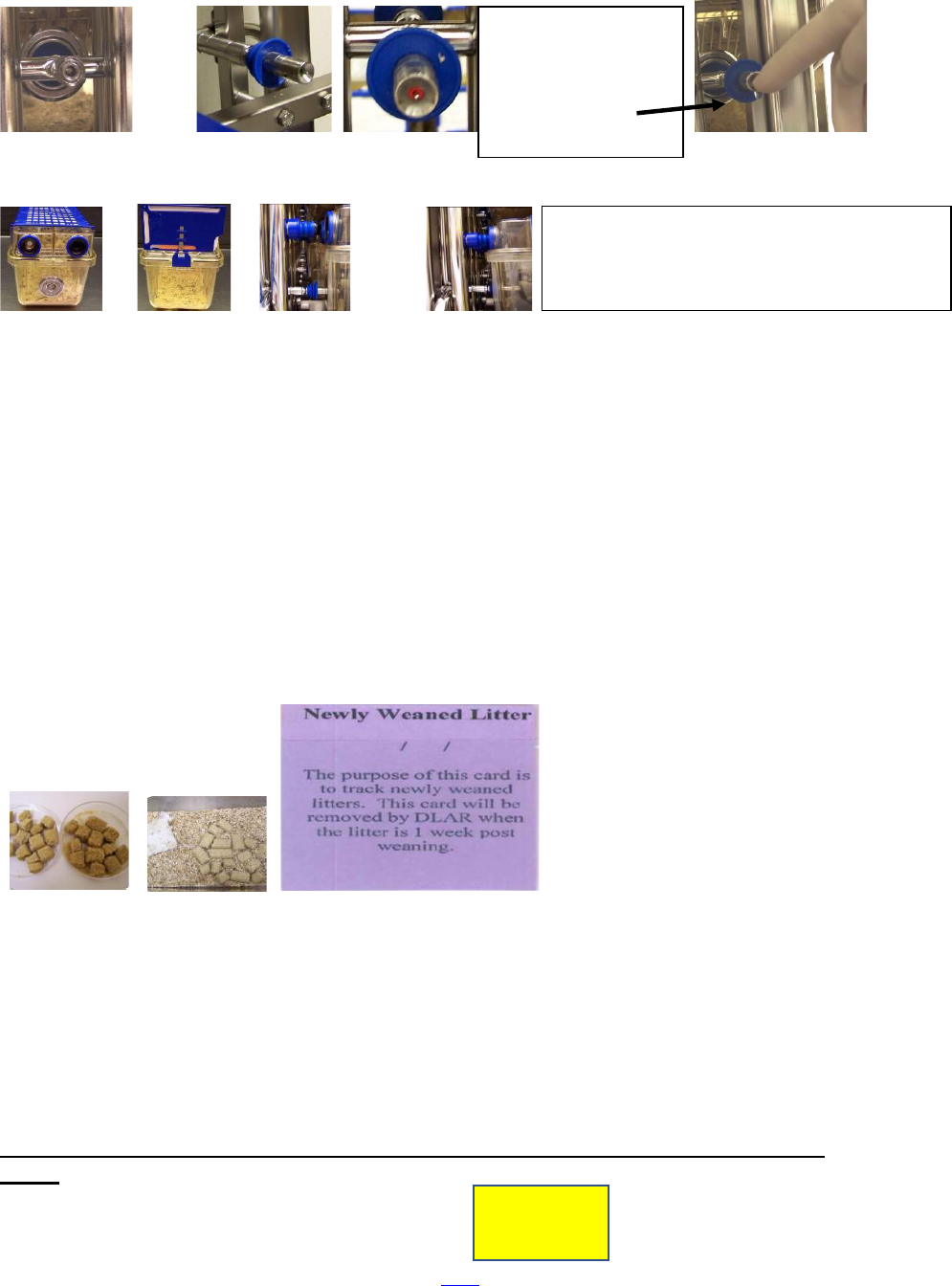
12 (TOC)
No water valve Water valve is in place and ready to use
in place
Back Front NOT properly Properly docked
Docked
Breeding Colony Management and Weaning Pups
Food should be placed on the bottom of the weanling cage to ensure that they have
food available. Young mice may sometimes not be able to bite off pieces of food from
the food hopper since many of them feed from the bottom on pieces their parents
break off. It may be necessary to place moistened food in the bottom of weanling
mouse cages particularly if the weanlings are small. It is important to remember that
weanling mice do not do well alone. They should always have a cage mate until they
have acclimated to being on their own. Place a water bottle on the cage until they
have learned to use the automated water valve. This could take two weeks. Be sure
to mark the cage with a newly weaned card so they can be monitored by the
technicians. Make sure to enter the date of birth and cage card number. This way if
the card falls off the cage, we know which cage to put back on.
Yellow Transparency Cards
If you, as an investigator, will be caring for your animals, you will want to use a special
transparency as an indicator in addition to the DLAR Service Request form that you
will have filled out. These yellow transparencies are used to indicate that there is a
special situation where an investigator has a specific study or sensitive project going
on.
Whole Yellow Transparency
DLAR will not pull out the cage during room checks and will not change the
cage. The animals will be observed without disturbing the cage. Research staff is
responsible for the husbandry of the cage.
Activate the water
valve with gloved
hand to ensure water
is flowing. You should
see water dripping
almost immediately
by
lightly touching the
stem. You should see
water dripping.
Be sure that the cage is pushed all the way back
in order to provide air changes and water. If the
cage is “sticking out” from the line of cages on
the rack you should push it all the way back

13 (TOC)
If you use transparency WITHOUT the corner removed, you, as the investigator,
are responsible for the husbandry of the cage.
Yellow Transparency with Right Corner Cut Off
DLAR is responsible for the husbandry of the cage, however, the PI does not want the
cage pulled for observation.
Micro Isolator Technique
Micro-isolator technique is easily accomplished. There is a presentation on the DLAR
webpage outlining the basics of good Micro-Isolator Technique. This proven method of
working with rodents is highly effective when used properly in the prevention or
containment of murine pathogens. When used improperly or not at all, it jeopardizes
not only the results or health of your colonies, but the health and study results of
potentially the entire facility. Here are a few simple rules to follow when using the
micro-isolator technique.
• Always turn the animal transfer system or Biosafety cabinet on and allow it to
run for a few minutes. This enables the air flow to begin to circulate properly.
• Be sure the shield is at the proper working height. An alarm will sound if not.
• Always wear a dedicated lab coat and sleeves and gloves, be sure that the
cuffs of your sleeves are covered by the cuff of your gloves.
• Always wipe down the work surface with appropriate disinfectant.
• When using a “dip tray” be sure that you have fresh disinfectant
in the tray so you can dip your fingers/hands when working
between different cages of animals.

14 (TOC)
• Do not over fill the work surface or block the vents in the front of the cabinet.
• Place cage inside of hood and spray the outside with disinfectant
(Avoid spraying Cage Card)
• Remove filter top by tilting left or right (a) and place on floor of the hood right side up
(b). An acceptable alternative is to rest the lid against the back or side of the cage (c).
(a) (b) (c)
• Wipe the work surface when you have finished your work with appropriate
disinfectant followed by clean water. while sinks are not
available in all animal rooms, the use of water from a
clean water bottle is acceptable.
Procedures in Rodent Housing Rooms
This section is to provide guidance to investigators regarding performance of
procedures in animal holding rooms versus designated vivarium procedure rooms.
DLAR animal rooms are primarily intended for animal housing. Husbandry and
research procedures, as well as in-house transport of animals, can cause changes in
physiological parameters such as heart rate, blood pressure, plasma corticosterone
levels, and blood glucose levels. Providing stable, consistent environmental
conditions in these rooms minimizes variability in research results and disruption of the
animals’ normal functions such as breeding and sleep patterns.
However, it is understood that both research procedures and husbandry tasks are
necessary parts of the research enterprise. It is also difficult to determine if these
physiologic changes are indicative of a negative impact on the animal since
presumably “positive” stimuli can elicit some of the same responses as “negative”
stimuli. To minimize these variables, the following recommendations are intended to
provide guidance to researchers on performing these tasks in the vivarium.
Certain minor procedures may be performed in the animal housing rooms, such as
injections, blood collection, tissue sampling for genotyping (e.g., tail biopsy, ear
punching), and weighing.

15 (TOC)
However, procedures that may be harmful to other animals in the room such as the
use of volatile chemicals, surgery, euthanasia or generation of excess noise, etc. may
not be performed in animal housing rooms. Such activities should be done in
procedure rooms which are available in DLAR for more complex, noxious, or time-
consuming procedures.
When working with or around more sensitive species, such as rodents or rabbits, work
quietly to avoid disturbing or distressing the animals. Any excessively distressing
procedures, such as invasive or anesthetized blood collection or surgery, are to be
done outside of the animal housing rooms, to avoid unnecessary stimulation of the
other animals in the housing room.
Many DLAR facilities have associated procedure rooms. These rooms must be
scheduled by investigators, through the University of Kentucky Event Management
System (EMS).
Additional considerations:
When you are planning procedures, please also consider the length and timing of your
tasks with consideration for animal husbandry activities as well as those of other
researchers sharing the housing room. The DLAR technicians working in the rooms
and the DLAR Supervisor of the area can provide information on room sanitization and cage
change schedules as well as the location of procedure rooms.
Many species can hear frequencies of sound outside the range of humans. Research
equipment can generate this type of noise, especially those with video display
terminals or processors. To the greatest extent possible, activities that might be noisy
should be conducted in areas separate from those used for animal housing.
If you have unique procedural needs that cannot be performed by the guidelines
above, please also feel free to contact any of the DLAR Veterinarians for further
assistance.
Examples of Rodent Caging
This is a standard static micro-isolator cage. These cages may be used
for regular long-term housing on shelf racks.
The Top Flow IVC cage can convert to a standard micro-isolator cage if
necessary and is able to be placed on a shelf rack. The large filter
enables the cage to allow sufficient air flow to prevent excessive CO2
buildup. These cages can be used in this manner for a number of days if
required.
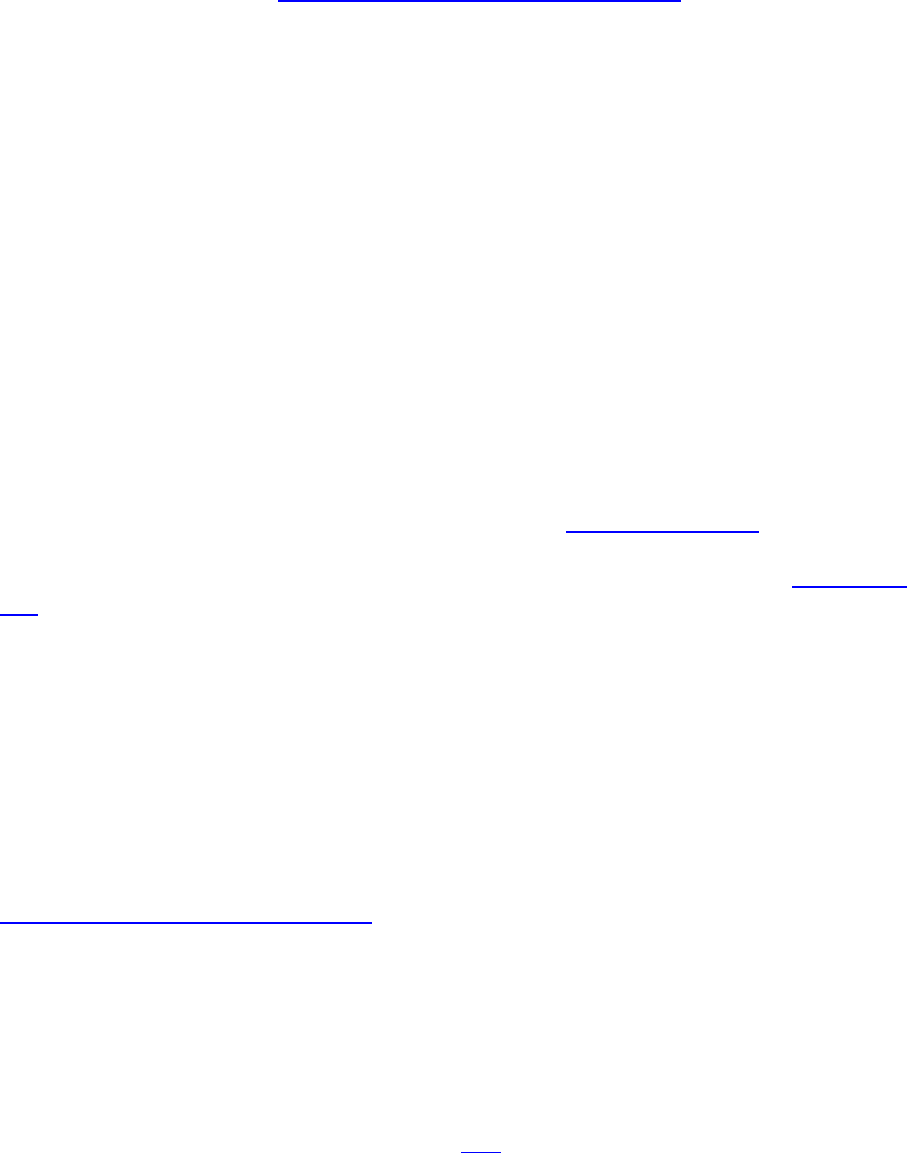
16 (TOC)
Additional types of caging for both rats and mice are used in the DLAR facilities.
Please contact the animal care supervisor in the facility where your animals are
housed for additional information on the housing used with the animals on your study.
Rack configuration, cage types, water valves, Housing Density Policy, and other useful
information, including the use of color-coded Special Alert Cards and Service Request
forms can be found in the Resource Material and Forms Section of this Handbook.
Housing needs for species other than rodents should be discussed with our facility
manager, animal care supervisors, or our DLAR veterinarians.
Returning Dirty Caging to Cage Wash
When returning dirty caging from your lab or animal housing room to the dirty side of
cagewash, cages must be covered to prevent the spread of allergens. Please
remember before you leave the lab or housing area to ALWAYS check INSIDE of the
cage you are returning to be sure there are no animals present BEFORE you place
the cage in the designated area for the facility you are working with. Animals that have
been euthanized must go into the designated cooler or freezer in the facility you are
working in. DO NOT leave them inside the dirty cage when it is returned to cage
wash.
Inquiries and Problems
“We can't fix it if we don’t know it’s broken!” If you have a problem, please let us know.
If you have a concern or a question about husbandry care or animal caging or other
equipment, or you see a potential problem, please do not correct it yourself unless the
animal's life is in danger. Instead, contact one of the DLAR supervisors so that they
can evaluate the problem firsthand. A DLAR supervisor is always on duty.
Instructions for contacting them are located in the DLAR entryway and the DLAR web
site.
Our ability to directly observe the problem greatly aids us in trouble-shooting the
situation and making sure that the problem doesn't happen again.
Unresolved problems
If you have repeated or unresolved problems with the daily care of your animals,
please bring your problem to the attention of our Animal Care Supervisors. They can
often resolve issues in short order, but they must be made aware that a problem
exists. Please allow them to assist you by informing them of the nature of your
difficulties. If the situation is still not addressed to your satisfaction, please contact our
DLAR Facility Operations Manager, their contact information is listed on page 5.
Transportation of Animals
Live animals may not be removed from the animal facility without prior approval of the
Institutional Animal Care and Use Committee. Under no circumstances can
rodents or other laboratory animals be transported in personal vehicles. If
animals must be moved to other facilities for housing or transfer to another

17 (TOC)
investigator, follow the information in your approved animal use protocol or contact a
DLAR Animal Care Supervisor for assistance. Transfers from one location to another
or to another investigator will be completed when a DLAR Services Request Form has
been submitted to the local Area Supervisor.
Animals that are being transported to investigator laboratories MUST be
COMPLETELY covered or in a closed transport cart and can ONLY be transported in
the service/freight elevators. They must not be transported into or through any patient
areas like waiting rooms or clinics under any circumstances. When transporting
animals through patient areas or to another building, animals must be transported in
an enclosed cart. When transporting animals to lab areas within the same building the
animals are housed in; you may use an open cart with cages completely covered by a
drape making certain NO PART of the CAGE is visible. Do not carry a single cage
by hand, it must be placed on or in a cart. There is the possibility that you may
drop the cage and injure the animals, or they may escape. When returning dirty
caging to the animal facilities, be certain the cage is empty and does not contain
animals alive or deceased. Dirty cages must be covered as well to protect personnel
from potential exposure to animal allergens.
Examples of what you CAN use to completely cover cages
Examples of what NOT to use to cover cages while transporting
DLAR has steel enclosed transport carts if investigators must travel between buildings
or in high traffic areas. These carts may be reserved by contacting the Animal Care
Supervisors. Transportation of laboratory animals housed in the DLAR facility by
private vehicle is strictly prohibited. Please refer to the IACUC Policies,
Procedures and Guidelines DOC 133 for more information on transporting animals.
Drapes
Towels
Pillowcase
Closed Cart
Cabinet Type
Cart
DO NOT USE
“YELLOW GOWNS”
DO NOT USE
LAB COAT
DO NOT USE WATER BOTTLE
BASKET COVERS

18 (TOC)
Procedure Rooms
DLAR has a limited number of procedures rooms that can be reserved for use by our
researchers. Please go to https://meetatbigblue.uky.edu and log in with your linkblue
ID and password to access the reservation page. All researchers on approved animal
use protocols can access this system. If you need assistance, please contact your
area supervisor.
Service Requests
The Division of Laboratory Animal Resources is able to meet a number of investigators
needs with regard to the animals housed in our facilities. The requests routinely
carried out by DLAR are transferring ownership, transportation of research animals,
special observations, the administration of medicated water, and a wide range of other
simple procedures associated with routine animal care. DLAR Service Request Form
can be found on the DLAR Website Forms Page under DLAR Service.
DLAR Service Request must have a Start and End Date to be Accepted
Should be submitted AT LEAST 48 hours prior to the effective start date and
must be delivered to the supervisor of the area in which your animals are
housed.
If the Special Request is to start over the weekend, it should be delivered to the
area supervisor no later than the Wednesday prior to the start of the weekend
requested
Service Requests submitted on a Friday afternoon may not appear on the
weekend duty schedule.
Storage of Drugs or Biologics
Due to space considerations, the storage of drugs and biologics in the DLAR facilities
is very limited. Investigators are encouraged to bring these items with them when they
are needed. There is limited space available in some of the Procedure Room on an
assigned cabinet space basis as these become available. DLAR must have a key to
any locked cabinets that you have been assigned. This would include the combination
to any combination lock placed on the cabinets. Please note that ALL drugs and
biologics should be securely stored and ALL packages, bottles, vials and other
containers MUST BE CLEARLY LABELED with substance and expiration date
and contact information to include PI name and Protocol number. NO EXPIRED
MATERIAL is allowed to be stored in DLAR and may not be used in live animals.
Absolutely NO ETHER may be stored or used in DLAR.
Special Diets
All Special diets must be listed in your approved animal use protocol. If there is a
need for Special Diets, it may be ordered and delivered with our regular feed delivery if
ordered from the same vendor. DLAR will notify you when it arrives so that you may
take it to your lab or arrange for it to be stored in the DLAR food storage area. You will
need to coordinate this with our Animal Care Supervisors and Facility Operations
Manager. Their contact information appears on page 5 of this Handbook or on the
DLAR website About Us page DLAR has limited storage for small amounts of food that
needs refrigeration in specially marked refrigerators. All food must be clearly marked

19 (TOC)
with the investigator’s name, manufacture date, storage conditions, expiration date for
the food and the type of food contained in the package. The container must be
secured with a lid or if in a plastic bag, it must be sturdy and closable (“Ziplock®”
freezer bag type). Should the bag tear, it must be replaced. Please try to avoid using
glass whenever possible. Improperly labeled feed will be discarded if no contact
information is available.
Animal Health Division
The health of all the animals in our care is very important to us. We do extensive
health monitoring using sentinel animals or Sentinel EAD (Exhaust Air Dust) media in
each rodent room to ensure the health of our rodent colonies. In addition to our in-
house testing, we submit samples for independent surveillance of the health of the
colonies at UKY.
Veterinary and Veterinary Health Technician Services
Our DLAR Veterinary Staff is ready to assist you in the design of protocols for animal
research, surgical consultations, treatment alternatives, locating animal-related
services not available at UKY, and participation in collaborative research. Please
contact them to arrange for a consultation
We have a staff of full-time registered veterinary technicians whose primary focus is
the well-being of our animals. Under the guidance of our DLAR veterinarians, our
veterinary technicians routinely respond to all reports of animals in distress or reported
to be ill or “not quite right”. The reports generated by our animal care technicians are
attended to as soon as they are reported. Our veterinarians are available for
consultations, to prescribe treatments, and to respond to emergencies.
How to Contact our Veterinary Health Care Technicians:
See Contact information on page 5 of this manual or visit the DLAR About Us page on the
DLAR Website.
Experimental Surgery
Our Experimental Surgery Unit offers 3 fully equipped surgery suites maintained at the
highest standards for sterile surgeries, and a diagnostic/surgical area. One suite is
dedicated for multi-species sterile surgery, one suite may be used for sterile or non-
sterile surgical procedures, and the third suite is a dedicated species sterile surgery.
We have a wide variety of instruments available for use, as well as providing steam,
gas and chemical sterilizations for a fee. The diagnostic area/surgery suite is
dedicated as an imaging room and contains Cardiac Fluoroscopy, digital imaging and
dental x-ray equipment. Should you wish to visit the Experimental Surgery area,
please contact either of our Surgical Technicians. Access to the surgical facility is
restricted. You must have an IACUC approved animal protocol with a surgical
component. Additional approval forms are available from the DLAR office which must
be approved by our veterinarians prior to access being granted.
20 (TOC)
Of special note:
If you will be doing survival surgery on any species of animal(s) (rodents, rabbits, etc.)
please remember that all procedures MUST be on your IACUC approved protocol,
Appropriate analgesics are listed and provided to the animal pre and/or post-
operatively and that all animals are closely monitored during and after surgery until
they are totally ambulatory. No animal is to be left unattended while still in an
anesthetized state. A chart of recommended anesthesia and analgesics is available
on the DLAR webpage address https://www.research.uky.edu/division-laboratory-
animal-resources/animal-health-veterinary-information If you require further
information or guidance, please contact one of our DLAR veterinarians.
Scheduling the Surgical Area
Surgical suites may book as much as 3 months in advance so the earlier
you reserve your dates, the better. We prefer to do survival surgery
toward the beginning of the week if possible. Please contact: our
Experimental Surgery unit. If there are specific questions that you need
an answer to immediately you may contact DLAR Experimental Surgery Team or
phone: 859-323-5829, 859-323-6027, 859-323-0289. The Lab Animal Surgery and
Research Support Rates can be accessed on the DLAR Website. Technical
assistance is available if needed Technician time will be charged as per the Current
Rates for FY 2022-2023.
IACUC Guidelines for Rodent Surgery
Excerpt from IACUC Guidelines for Meeting Policy 102 Surgery Requirements
pertaining to:
Post-surgical anesthetic recovery period
Frequent and documented observation of animals during the post-surgical
anesthetic recovery period is important. The animal, in or out of its cage, must be kept
warm. Warm water pads, blankets, or the blue "diaper" pads work well. The use of
electric heat pads or heat lamps may overheat the animal and their use is
discouraged. If electric heat pads or heat lamps must be used, provision must be
made to make frequent observations and turning of a somnolent animal so that the
animal will not be overheated. Provision must also be made so that an awake animal
can escape the heat source when it becomes too warm. Warmed fluids can be
administered subcutaneously, intravenously, or intra-peritoneally if there is any
suspicion the animal may be dehydrated. A recovering animal should be watched very
closely until securely in sterna recumbency, and able to move around without plugging
its nostrils with bedding. Some rodents left overnight on pads or paper bedding will
eat that bedding.
Post-surgical period
Daily postsurgical observations should, at a minimum, include observations of the
condition of the animal and the surgical site. Sutures and/or staples need to be
21 (TOC)
removed by two weeks following surgery, if the rodent has not already done so. Any
foreign substance, including sutures, catheters, implants, etc., left in the incision for
long period of time can serve as a nidus of irritation and infection. A veterinarian
should examine incisions that do not appear to be healing. Animals found dead during
the post-surgical period should be submitted for diagnostic necropsy. Rapid
identification of infectious diseases, post-surgical infections, surgical problems, etc.,
permits responses by the veterinary or research staff to improve the surgical
outcomes, minimize variability, and enhance the research results.
Post-Operative Recovery and Care of Mice and Rats
Following all procedures, animals will need to be directly and continuously observed
and monitored in a warm environment until they are fully recovered from anesthesia.
Unless prior arrangements for continuous monitoring have been made with DLAR
Veterinary Services for monitoring, research staff are expected to monitor their
animals. Animals are considered recovered from anesthesia when they can ambulate
fully, maintain themselves upright in sternal recumbency and are attempting to move
purposefully ( see https://www.research.uky.edu/office-attending-veterinarian/iacuc-
policies-procedures-and-guidelines. At a minimum, rodents should be observed
frequently postoperatively for recovery, once recovered at about 6 hours and again at
18-24 hours post-operatively for signs of pain, infection or dehiscence (opening up of
the incision). Animals should be observed daily for the first 7 days after surgery. The
surgery and the date should be noted on the cage card. There are special Surgery
cards to be placed by the investigator behind the normal cage card. These special
cards include the contact information, analgesic/treatment record, and observation
information as to Surgical site and Alert response. Dates, and times, and findings of
each post-operative observation and the dates, dosages, and times of each analgesic
administration should be maintained on the cage card in a form clearly legible for the
Facility Veterinarian. Wound clips and non-absorbable skin sutures are to be removed
at 7-14 days post-surgically.
Frequent and documented observation of animals during the post-surgical anesthetic
recovery period is important. The animal, in or out of its cage, must be kept warm.
Warm water pads, blankets, or the blue "diaper" pads work well. There are special
rodent surgery tables also. The use of electric heat pads or heat lamps may overheat
the animal; their use is discouraged. If electric heat pads or heat lamps must be used,
provision must be made to make frequent observations and turning of anesthetized
animal so that the animal will not be overheated. Provision must also be made so that
an awake animal can escape the heat source when it becomes too warm. Warmed
fluids can be administered if there is any suspicion the animal may be dehydrated.
This may be done by giving 1 to 2 ml of warm sterile fluids (0.95% NaCl or equivalent)
per 100 gm of body weight by subcutaneous injection. If blood loss occurred during the
surgical procedure, or if the animal is slow to recover from anesthesia, provide
additional fluids.
It is essential for a recovering animal to be watched very closely until walking or
securely in sternal recumbency, and able to move around without plugging its
22 (TOC)
nostrils with bedding if present. Therefore, it is best to perform surgery as early in
the day as possible. Animal recovery can vary greatly, so you should always plan on a
minimum of two hours post-surgery to allow enough time to monitor your animals.
A postoperative record on DLAR surgery cards must be started and affixed to the
animal's cage. Having the record in the room accomplishes several functions. 1) It
explains the condition of the animals to animal care staff (a sedated animal may
otherwise be thought to be ill), 2) It assures animal care staff and USDA Animal
Welfare inspectors that the animal is being cared for, and 3) It informs animal care
staff how recently the investigator has seen the animal; this knowledge helps them
decide whether or not there is a need to contact the investigator to inform him or her of
the present condition of the animal. Although individual records are desirable, USDA
allows a composite post-operative record to be used for a group of rodents.
Rodent Anesthesia Machines
DLAR has several anesthesia machines available to investigators on a fee
for services basis. The rodent anesthesia machines may be rented by
contacting our Research Analysts. The rental fee includes setup and
training if needed. Please contact the DLAR Research Analysts for further
information regarding the training and to schedule a reservation for the use of these
machines. All users of the DLAR rodent anesthesia machines are encouraged to take
advantage of the training, so you will be familiar with our equipment, to operate it
safely for yourself and your animals during anesthesia.
Other Services
Technical/surgical/monitoring/post-surgical monitoring assistance is available on a fee
for services basis if needed.
Cryopreservation/Rederivation
For these services, please contact Kristin Fox or 859-323-5469
Technical Services
The Division of Laboratory Animal Resources has a team of Research Analysts
available on a fee for services basis. A wide variety of services are available to you
from individual animal micro chipping to surgical assistance, post operative monitoring,
and administration of post-operative medications. Should you require extra assistance
with your studies, or additional expertise, please contact one of our Research Analyst
Glenn Florence or Kristin Fox or one of our DLAR Veterinarians to discuss your needs.
We will make every effort to help, but please note, in some cases due to
scheduling conflicts, it may be difficult to assist on short notice.
Examples of Animal procedures include:
Injections
Blood or urine collection
Tail biopsies
Specialized techniques (implantation, surgical procedures)

23 (TOC)
Tissue collection from euthanized animals
Breeding colony management and maintenance
Special Observations and monitoring
Note: Requests for services must be submitted at least 48 hrs. in advance. Requests
for euthanasia require a signature. DLAR cannot guarantee that the same person will
perform the service every time.
Anesthesia always involves a risk of animal death.
Breeding Colony Management
One of the most labor-intensive tasks for any lab is the management of their breeding
colonies. We offer this service to our investigators on a fee for services basis. Our
research analyst in charge of breeding colony management has a wealth of
experience and has attended the Jackson Laboratories Breeding Colony Management
course in Bar Harbor, Maine. He is also an AALAS Certified Laboratory Animal
Technologist. He is available for consultation and assistance along with our DLAR
veterinarians, to address your breeding colony needs. Please contact Glenn Florence
at 257-1026.
Sample Collection
Our team of research analysts can provide a variety of sample collections such as
blood collection, tissue collection for genotyping, data collection and other services.
Animal Pathology Division
Diagnostic veterinary services are available including tissue or organ collection and full
pathology and histopathologic services by the Laboratory Pathology Unit at the
University of Kentucky Veterinary Diagnostic Laboratory (UKVDL), and Dr. Carney
Jackson, Pathology Consultant to DLAR.
Research pathology services are available through this fee for services unit. Please
coordinate these services through our DLAR Veterinarians and for additional
information on available services. Forms for submission can be found on the DLAR
website at the following address:
https://www.research.uky.edu/division-laboratory-animal-resources/pathology
Other Services
Other services performed by our research analysts include routine weighing,
observation of animals, post-surgical monitoring, assistance with surgical procedures,
dosing, medications, and other data collection your lab may require. These services
must be requested in advance to avoid scheduling conflicts. They are also available
for training in the use of anesthesia machines and basic procedures.

24 (TOC)
Animal Procurement
All animal procurement must be coordinated through the Division of Laboratory Animal
Resources (DLAR).
Animals cannot be procured until an approved protocol is available online in Cayuse
Animal Oversight (Cayuse AO).
All animal procurement, regardless of source or cost, must be processed through
DLAR.
All animal orders must be submitted electronically through Cayuse AO.
All animals being received into or shipped from the facility will be scheduled by DLAR
personnel.
All animals being received into the facility will be required to have a certificate of health
status.
Under no circumstances may animals of any kind, other than those ordered from
approved vendors or animals that are currently housed in DLAR facilities, be brought
into DLAR by research or non-DLAR personnel. This applies to research, pet, stray or
injured animals.
Ordering Animals
All animal orders must be submitted electronically through Cayuse AO.
Mice and Rats: Orders must be placed by Wednesday, 12:00 p.m. for the
following Monday delivery from Harlan and Jackson Laboratory and Tuesday for
delivery from Charles River and Taconic. If animals are ordered from NIA (National
Institute on Aging) they must be in the DLAR office before 10:00 AM on Tuesday for
placement.
Other species: Contact the DLAR office for information
Please contact Cheryl Carmichael in our main office if you have questions.
Non-commercial Vendor Import or Export of Rodents
Please refer to the Import and Export of Animals page on our website. Contact
DLAR’s Import and Export Coordinator Ronda Combs or 859-323-6018.
Quarantine and Acclimation
All animals received from other institutions or vendors other than Charles River,
Jackson Laboratory, Taconic Farms or Envigo are held in quarantine after receipt.
During quarantine, animals are screened for selected pathogens.
The length of quarantine depends on the species origin and/or the intended use of the
animals. Quarantine is a minimum of 4-6 weeks for rodents. Rodent breeding stock
and animals from non-standard vendors, and animals from outside the U.S. may
require an extended quarantine.

25 (TOC)
Quarantine for non-human primates will be the length of time it takes for successful
completion of the required TB testing, approximately 60 days. This process could
require some length of time. Please consult with DLAR veterinarians on this matter.
USDA Animals cannot be used experimentally nor used as breeding stock while
in quarantine. Please plan accordingly.
If you wish to obtain rodents from another institution or you will be sending animals to
another institution, please contact our Animal Import Coordinator, Ronda Combs or
859-323-6018. The Request to Import Animals Form can be found on the Forms page
of the DLAR website
Cage Cards, Census, and Per Diems
All cages must have a proper cage card issued by DLAR. The information on the
cards indicates the following:
• Principle Investigator
• Protocol Number
• Cage Description
• Requisition Number
• Who to Notify
• Date Received
• Strain/ Species
• Account Number
• Age and Date of Birth
• Sex and Weight (if appropriate)
• Identification Number
• Vendor
• And a Miscellaneous category
• Bar Code Number for activation and deactivation of per diems
Of special note, cage cards MUST NOT be reused. Each card is unique. If you need
extra cards for splitting cages or weaning animals, please contact Cheryl Carmichael.
There is no charge for this service. The cage cards contain pertinent information as
well as a bar code. This bar code is critical to accurate animal census and should be
dated and returned to the main office when the cage card is removed, and the animals
are used. If the card is damaged, please request a new card from Cheryl Carmichael
to ensure accurate accounting of the animals. Please do not place tape on the cage
cards or cover the bar codes.
Cage Cards are scanned twice a month. Missing or damaged cards that are not
scanned are still charged per diem unless the bar codes are deactivated. Cage cards
will be removed by reconciliation after the third time it is missed on a scan. The DLAR
staff checks the bar code drop envelopes twice daily during the week and bar code
tops are returned to the DLAR main office for deactivation. The bar code slips are
collected on the weekends and turned into the DLAR main office on Monday.

26 (TOC)
When turning in the portion of the cage card containing the barcode either activation or
deactivation, the date of activation of a new or deactivation of a previous cage card
must be annotated. When activating a new cage card make sure to document the
number of animals in the cage. Following this simple practice will alleviate per diem
charges being unnecessarily charged for cages not in use or bar codes that are
deactivated when they appear in the envelopes during regular collection.
Our current Per Diem chart is located on the DLAR webpage under the Tech Services
Tab Rates.
Training
Requirements for personnel who use animals in research at
University of Kentucky
The University of Kentucky Institutional Animal Care and Use Committee has outlined
minimum requirements for training for investigators. Those requirements in the IACUC
Policies, and Procedures, and Guidelines Doc 106.
• Must complete required online training modules through the AALAS Learning
Library
• Must participate in the Occupational Health and Safety Program
• Must complete requirements to be listed on an approved animal use protocol
• Must be listed on an IACUC approved animal care and use protocol
• Must obtain access into the facilities through the DLAR office
• Must participate in Continuing Education requirements
In addition to the IACUC required training, all individuals accessing the DLAR
facilities must attend the DLAR Orientation given twice monthly.
All personnel working with animals must receive appropriate training in the use of
animals. Assurance of training for research personnel is primarily the responsibility of
the Principal Investigator or protocol director. Training is available through the Division
of Laboratory Animal Resources Veterinary staff and the Training Team on a wide
variety of common procedures and species-specific information.
Individual training sessions can be scheduled on a variety of basic and species-
specific procedures. We have several PowerPoint presentations available for your lab
use on the Training Page of the DLAR Website. DLAR also conducts workshops from
time to time on basic procedures and anatomy of rats and mice. Contact the DLAR
Training Group for more information and other available resources.
DLAR has a variety of books, CD’s, DVD’s and publications in our library located in
H41A of the Chandler Medical Center.

27 (TOC)
Occupational Health and Safety
Do not attempt to go over, under, or through hallways that have
been blocked by yellow chains. These areas are blocked off
when dirty/contaminated nonhuman primate caging is being
moved through the hallways.
Do Not Wear Open Toed Shoes, Sandals or “Flip Flops” or any other shoe that
does not provide adequate foot protection
in The Animal Facility as This Constitutes
a Personal Safety Hazard.
Do not wear shorts, skirts, tight-fitting leggings or nylons, pants or slacks that do not
cover your ankles into the animal rooms as per University of Kentucky
Environmental/Biological Health and Safety and DLAR policy. Follow the EHS
guideline on laboratory safety as DLAR animal rooms fall within those guidelines.
Wear dedicated lab coats, disposable gowns, or dedicated scrubs when entering
animal areas. Remove and discard disposable lab coats and gowns in DLAR when
leaving the facility. Do not wear your dedicated lab coat in public areas of the
hospital as this may put some patients at risk. Clean or launder lab coats
whenever they become soiled or at least weekly.
Do not wear or carry umbrellas, outer coats or jackets, backpacks or purses into the
animal rooms. Please leave personal items in your lab
No consumption of food or beverages, chewing gum or mints, is allowed in the animal
facilities or laboratories, except in designated areas. Opened/uncovered containers of
food or beverages cannot be transported through the hallways or into the animal
housing rooms, experimental surgery or laboratories.
ALWAYS wear gloves and other personal protective clothing as indicated while
handling animals.
Wash your hands before leaving the area or use the hand sanitizers located
throughout the facility
Allergies
Allergies are the most common human health problem associated with laboratory
rodents. People that already have allergies may be more likely to develop rodent

28 (TOC)
allergies. Allergens are proteins in the urine and dander. Prudent practices involve
wearing protective clothing (gown or lab coat, gloves, and mask) and working with
animals in hoods whenever possible. Remember to always wear appropriate personal
protective equipment when working with laboratory animals.
Bites and Scratches
A common human health problem associated with laboratory animals Avoid injury by
learning how to properly handle and restrain animals; if you are unsure about correct
procedures or need assistance, ask at the DLAR office, or contact the DLAR Training
Team to arrange for training in the procedures. If you are bitten or scratched,
regardless of the perceived severity of the injury:
Clean the injured area appropriately, remembering that any bite or scratch
wound can easily become infected
Report the accident to an Animal Care Supervisor as well as your own
Supervisor, lab manager or Principal Investigator
Information to Report:
➢ Cage Card information as EH&S will need this to know if the
animal(s) have been treated with something that could be harmful
to the person.
➢ For NHP Bite Scratches follow the “Nonhuman Primate
Instructions” inside the NHP bite kits.
Contact UK Workers Care for further instructions 1-800-440-6285
Remember that ALL accidents regardless of the severity should be reported to
UK Occupational Health and Safety IMMEDIATELY. If the injury involves an
animal bite or other injury, you must fill out the University’s Accident-
Occupational Injury/illness Report (Form 6). Log in with LinkBlue ID and Follow
the instruction on webpage.
Sharps Containers
Always dispose of all sharps such as needles, empty syringes, scalpel blades, etc. in
sharps containers and remember DO NOT RECAP NEEDLES, they must be placed in
the appropriate sharps container located in the procedure rooms. Do NOT throw trash
such as paper towels, gauze pads, alcohol preps, etc. into the sharps containers.
Remember to keep sharps containers as close to your work area as possible to avoid
having to walk across the room to dispose of sharps. If you cannot locate a sharps
container, please let one of our technicians or supervisors know. Boxes for broken
glass are available in each facility, contact the area supervisor.

29 (TOC)
REGULATORY AGENCIES AND OTHER OVERSIGHT
The United States Department of Agriculture (USDA)
Conducts visits to every institution that uses a USDA covered species in any way in
any IACUC approved protocol. USDA regulations refer to the Animal Welfare Act
governing the transportation, handling, housing and sale of certain species of animals.
https://www.research.uky.edu/division-laboratory-animal-resources/regulatory-
agencies
USDA/Animal Welfare Regulations (Title 9 CFR parts 1 and
2)
The Animal Welfare Regulations cover the use of animals in teaching, testing and research. It
is administered by the United States Department of Agriculture (USDA). The provisions of the
law set standards for the purchase, housing, and use of laboratory animals and requires prior
approval of all animal use by the IACUC. It also requires training of all personnel. The
University of Kentucky must submit an annual report to the USDA, listing numbers of animals
used, species, and how many received pain-relieving drugs. The USDA conducts
unannounced inspections annually to ensure compliance. During these inspections, records
of covered species are checked, selected protocols are reviewed, and The Institutional Animal
Care and Use reports as well as other pertinent data are reviewed by the inspectors. Species
covered under the Animal Welfare Act include:
• Rabbits
• Cats
• Dogs
• Ferrets
• Hamsters
• Gerbils
• Guinea Pigs
• Wild-caught rodents
• Non-human primates
• Laboratory sheep and goats (farm animals not used for food or fiber)
• Laboratory Swine (farm animals not used for food or fiber)
The Public Health Services Policy
Covers all animals used in any way on all IACUC approved protocols regardless of species
View the Public Health Services Policy. Institutions must meet federal guidelines before
applying for federal funds to conduct animal research. These guidelines apply to all vertebrate
animals, and to all animal research, regardless of the source of funding. The University of
Kentucky must submit an annual assurance to the PHS stating that it adheres to these
guidelines.
OLAW - Office of Laboratory Animal Welfare
An office of the National Institutes of Health responsible for implementation of the
Public Health Service Policy. You may view the OLAW website at the following web
address: http://grants.nih.gov/grants/olaw/olaw.htm. This site contains multiple links to
30 (TOC)
various resources available to the investigator outlining many of the policies and
procedures which must be followed to research with vertebrate animals.
National Academy of Science (NAS)/Institute for Laboratory Animal Research (ILAR)
Veterans Administration (VA)/ Department of Defense (DOD)
National Institutes of Health (NIH)
The Guide for the Humane Care and Use of Laboratory
Animals
Outlines requirements for many aspects of care, housing and environmental requirements and
training of personnel who use them. To view The Guide.
The Centers for Disease Control (CDC)
A government organization that is dedicated to protecting health and promoting quality
of life through the prevention and control of disease, injury, and disability. It provides
information on many aspects that can pertain to laboratory animal research. One of its
publications in collaboration with the National Institutes of Health and others is The
Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition. This
publication contains guidelines for the proper containment of biologic agents used in
research and can be viewed at the following web address:
http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf.
The Association for Assessment and Accreditation of
Laboratory Animal Care, International
A voluntary peer review organization that conducts in depth reviews of all aspects of
an institutions' animal care and use program including veterinary care, husbandry
practices, animal housing facilities, training, and institutional policies. The University of
Kentucky Division of Laboratory Animal Resources has had continuous accreditation
since 1966. You can view the AAALAC site at the following web address:
http://www.aaalac.org/.
The AVMA Guidelines on Euthanasia 2020
While not strictly a regulatory agency, it is important to know that humane euthanasia
of all animal species fall under the guidelines and regulations of all the regulatory
agencies governing the humane care and use of laboratory animals in all protocols
approved by the Institutional Animal Care and Use Committee. The AVMA Guidelines
on Euthanasia can be viewed on the IACUC webpage on the UKY OAVs website.

31 (TOC)
INSTITUTIONAL ANIMAL CARE AND USE
COMMITTEE
Mandated by both the Animal Welfare Act and NIH policy. Must review and approve
all proposals for animal use before animals are purchased. Must re-review protocols
annually. Must conduct facility inspections and review the animal use program twice
yearly.
Contact Information for the IACUC
https://www.research.uky.edu/office-attending-veterinarian/about-oav
Animal Protocols
All use of animals requires prior approval by the Institutional Animal Care and
Use Committee.
• Research must not be unnecessarily duplicative.
• Alternatives must be considered before animals are used.
• All procedures that cause more than momentary pain require the use of
anesthetics or analgesics, or scientific justification for withholding of analgesics
or anesthetics.
Necessary Elements of a Literature Search
The literature search is one of the most important parts of any Institutional Animal
Care and Use Protocol and is a requirement of the Institutional Animal Care and Use
Committee. It is important to note that a current literature search is more useful than a
“from the beginning of recorded history” search. The essential features of a good
literature search are:
• Date of search
• Databases searched, or other sources consulted
• Years covered in the search
• Key words or search strategy used
• For compliance with USDA and OLAW requirements, the search must clearly
document good-faith effort to find alternatives to painful or distressful
procedures.
Personal experience can be used, but it cannot be your only source.
References spanning the past 10 years are generally sufficient for most research
projects, a comprehensive literature search needs to be done at the beginning of the
protocol. Subsequent renewals of the same protocol, in most cases, need only an
updated search to include results of studies that have been done from the time of the
previous full search. Included here are several sites to enable you to perform a search
of available of pertinent information related to the area of your study.

32 (TOC)
National Institutes of Health Links:
• MedLine
• PubMed
USDA National Agricultural Library Links:
• Agricola database
• Animal Welfare Information Center (AWIC)
University of Kentucky Medical Library Site Links:
• Animal Welfare Libraries
• ERIC (Educational Resources Information Center)
• PsycINFO
• Ask a Medical Librarian
• Medical Center Library Classes
Web of Science Links:
• CAB Abstracts
• Biosis
• Web of Science
Other Helpful Sites:
• CAAT Center for Alternatives to Animal Testing (Johns Hopkins Bloomberg
School of Public Health)
For assistance contact the following individuals in the University of Kentucky Libraries:
• Jason Keinsley 218-1523 at Medical Center Library (College of Agricultural)
• Mark Ingram 323-6568 at Medical Center Library
• Rick Brewer 323-5296 at Medical Center Library
Additional assistance can be obtained by contacting the Medical Center Library.
In addition, you may contact any of the DLAR Veterinarians or the Office of the
Attending Veterinarian (OAV) to assist with protocol design.
Alternatives to the Use of Animals
Replacement - use non-animal models whenever possible
33 (TOC)
Reduction - use the fewest number of animals consistent with good science
Refinement - use the most humane methods available
For assistance in this subject, please visit the following website for information on
alternatives to the use of animals:
http://awic.nal.usda.gov/nal_display/index.php?tax_level=1&info_center=3&tax_subjec
t=183
You will find resources and information on refinement, alternatives as well as other
useful information.
USDA Pain Categories
According to regulatory agencies, all vertebrate animals used in research, teaching or
testing must be assigned to an appropriate pain category. The chart below outlines
these categories and a few of the examples for each. If you are in doubt as to which
category your animals may fall under, please contact the DLAR veterinarians.
USDA Category B
USDA Category C
USDA Category D
USDA Category E
Breeding or Holding
Colony Protocols
No more than
momentary or slight
pain or distress and
no use of pain-
relieving drugs, or no
pain or distress. For
example:
euthanatized for
tissues; just
observed under
normal conditions;
positive reward
projects; routine
procedures;
injections; and blood
sampling.
Pain or distress
appropriately
relieved with
anesthetics,
analgesics and/or
tranquilizer drugs or
other methods for
relieving pain or
distress.
Pain or distress or
potential pain or
distress that is not
relieved with
anesthetics,
analgesics and/or
tranquilizer drugs or
other methods for
relieving pain or
distress.
(Note: there is no USDA Pain Category A.)
A simple definition of a painful or distressful procedure on an animal in this:
“A procedure that would cause pain or distress in a human.”
It is important to remember and understand that if an animal needs to undergo multiple
procedures, it must be placed in the category indicated for the most painful/distressful
procedure it will be experiencing. Also note that a single animal cannot be placed in
multiple categories.
Pain categories are described as follows:
Category B animals are those that are being “bred”, conditioned, or held for use in
teaching, testing, experiments, research, or surgery but not yet used for such
purposes.” These animals have not been used for any research procedure, however

34 (TOC)
minor. Category B is the place to put breeders and other animals that are not
undergoing any experimental procedures.
Category C animals are not subjected to procedures that involve pain or distress or
would require the use of pain-relieving drugs. Routine procedures such as injections
and blood sampling from veins that produce only mild, transient pain or discomfort are
reported in this category. Another example of Category C procedures is an
observational study of animal behavior. Animals that are euthanized before tissue
collection or other manipulations are also commonly placed in this category, if no other
procedures performed that put them in a higher pain/distress category.
Category D animals are those subjected to potentially painful procedures for which
anesthetics, analgesics, or tranquilizers will be used. The important concept is that
animals are given appropriate anesthesia and/or pain relief to limit their pain and
distress as much as possible.
Examples of category D procedures are
▪ Surgery conducted with appropriate anesthesia and postoperative analgesia.
▪ Rodent retro-orbital eye bleeding performed under anesthesia.
▪ Non-human primate tattooing performed for identification under anesthesia.
▪ Removal of small tumors under local or general anesthesia, and use of
analgesia after an animal’s skin is exposed to ultraviolet light to cause a
“sunburn”; and
▪ Terminal exsanguinations (euthanasia by removal of blood) under anesthesia
Category E animals are those that are subjected to painful or stressful procedures
without the use of anesthetics, analgesics, or tranquilizers. Withholding of anesthetics,
analgesics, or tranquilizers can only be allowed if it is scientifically justified in writing
and approved by the IACUC. Examples of category E procedures are lethal dose
studies (e.g. LD50 studies) that allow animals to die without intervention, pain studies
that would not be possible if pain-relieving agents were administered, and
psychological conditioning experiments that involve painful stimuli such as noxious
electrical shock that cannot immediately be avoided by an animal.
By law, the institution must annually report all category E procedures to the
USDA and include a scientific justification supporting the IACUC’s decision to approve
them. It is important for the information on category E procedures to be complete and
accurate
1.
1. Descriptive text from AALAS Learning Library training module
Reporting Concerns:
All animals owned or used by the University of Kentucky for research or training will
receive proper care and will be used humanely in accordance with approved protocols,
federal laws, and University of Kentucky regulations and guidelines. Any person who
witnesses or suspects abuse of said animals is encouraged to report their concern to
any IACUC member or to:

35 (TOC)
Dr. M. Paul Murphy, Chairperson
UK Institutional Animal Care and Use Committee
(859) 218-3811
Dr. Mark Suckow, University of Kentucky Attending Veterinarian
(859) 257-1117
Dr. Lisa Cassis, University of Kentucky Vice President of Research
(859) 257-5294
Or contact the Office of Research Integrity anonymously at
1-(866)-400-9428 (toll free call)
Written concerns may be sent to the
UK Institutional Animal Care and Use Committee
435 Bowman Hall, 151 Washington Avenue
Lexington, Kentucky 40506-0059
No adverse action will be taken against anyone making a report.
You are NOT required to give your name.
Veterinary Consultation
Veterinary consultations are available from University of Kentucky Veterinarians
to assist you. Additionally, the veterinarians may also serve as collaborators or co-
investigators on your protocols when requested. Please refer to the Cayuse AO
Veterinarian Consultation page for a complete list of University of Kentucky
Veterinarians and their contact information.
Cayuse AO
Cayuse AO is UK's on-line IACUC protocol management and animal ordering
system. Principle Investigators can request a Cayuse AO user account by submitting
a PI Cayuse AO Account Request and hitting the submit button on the form.
Questions regarding user accounts may be directed to Rob Williams at 859-257-
3694. The Office of the Attending Veterinarian (OAV) staff is responsible for
administrative protocol development assistance, processing and routing for review of
new protocols, amendments, 3rd year re-submissions and annual reviews. Please
contact the OAV-IACUC Office with any questions regarding protocols in Cayuse AO.
Contact Cheryl Carmichael at 323-6006, with any questions concerning animal orders,
cage care request, or animal transfers. The Cayuse AO landing page contains a
plethora of information regarding Getting Started, Training, Cayuse FAQs, and Contact
Information.
36 (TOC)
Resource Material and Forms
RESOURCES
The weight and food and water consumption is dictated by age and sex. Further
information can be obtained by contacting the DLAR veterinary staff or the Training
Coordinator. Strain specific information is also available on various vendor websites.
Taconic Farms www.taconic.com
Harlan/Envigo www.envigo.com
Charles River www.criver.com
Information on mice can be found at the Jackson Laboratory website: www.jax.org
AALAS Learning Library On-Line Learning http://www.aalaslearninglibrary.org/ User
names and passwords can be obtained by contacting Angie Croucher in the Office of
Research Integrity.
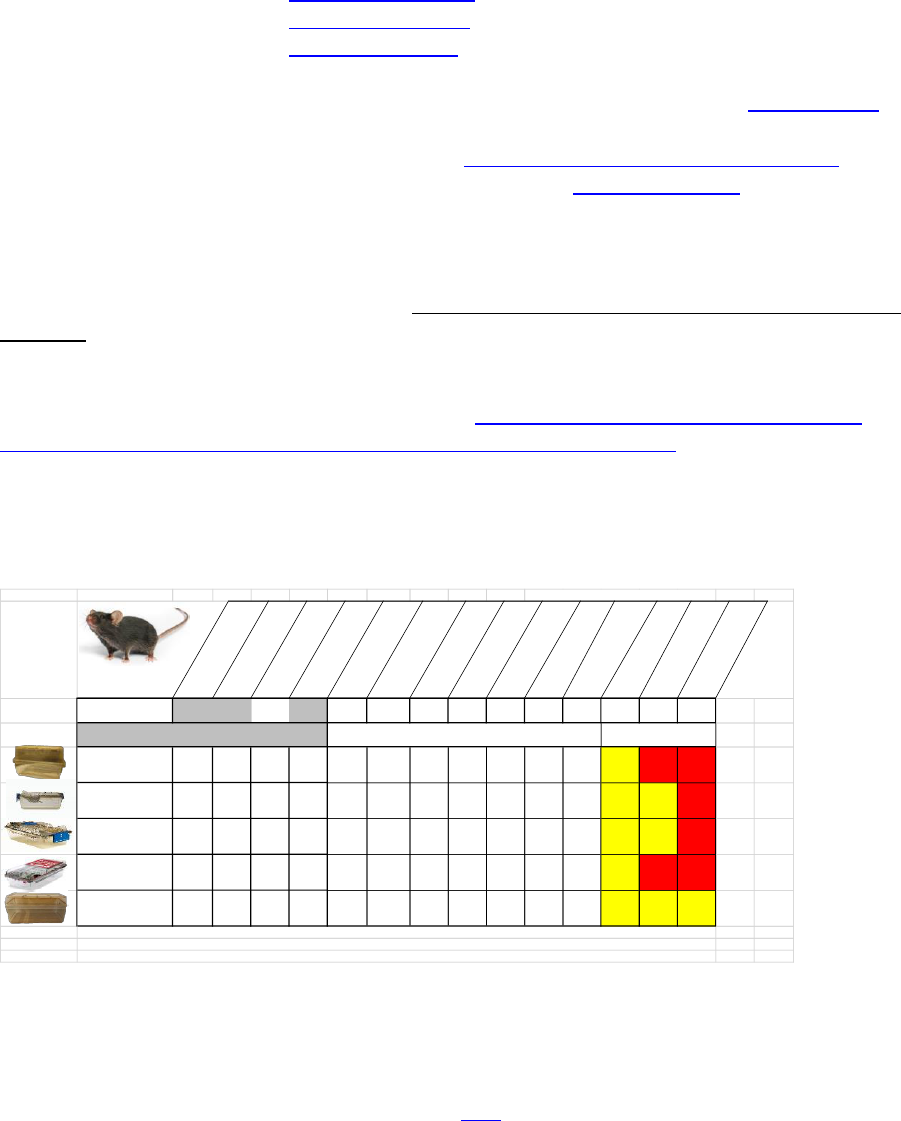
Cage Densities for Mice being housed in DLAR Facilities
To comply with space requirements of The Guide for the Care and Use of Laboratory
Animals and other regulatory agencies, we have outlined the number of mice that can
be housed in our various caging systems. Please refer to IACUC Guidelines and
Standard Operating Procedures 110 Mouse Housing Density. This document can be
found by going directly to the IACUC page https://www.research.uky.edu/office-
attending-veterinarian/iacuc-policies-procedures-and-guidelines and clicking the
appropriate link.
Below are examples of the different types of mouse housing caging used in the DLAR
facilities, including the number of adult animals that can be housed in each type of
caging
Mouse Caging
Maximum Mouse Density
Cage Type
Width In (cm)
Length In (cm)
Height In (cm)
Floor Area in
2
(cm
2
)
Body Weight
<10g [<24 days of age*]
Body Weight
10-15g [24-31 days of age*]
Body Weight
15-25G [32-70 days of age*]
Body Weight
25-35g [>70 days of age*]
Body Weight
35-45g (Obese Strains)
Body Weight>45g
(Obese Strains)
Female + Litter
Female with Male + Litter
Male with 2 Female with
1 Litter (Tri Breeding)
Male with 2 Female with
2 Litter (Tri Breeding)
Required Floor Area
[sq. in (sq. cm0]
5.0
12.7
6.0
(38.7)
8.0
(51.6)
12.0
(77.4)
15.0
(96.7)
19.0
(122.6)
23.0
(148.4)
51.0
(330)
66.0
(426.7)
81.0
(523.4)
107.0
(756.7)
Standard Mouse Cage &
Static Microisolator
7.5
(18.9)
11.75
(29.37)
5.0
(12.7)
67
(451)
11 8 5 4 3 2 1 Yes No No
Techniplast 1284/85L
Top Flow-Flow Blue Line
8.46
(21.5)
15.67
(39.8)
7.36
(18.7)
82.15
(530)
13 10 6 5 4 3 1 Yes Yes No
**Techniplast 1284/85L
Seal-Safe Blue Line
8.46
(21.5)
15.67
(39.8)
7.36
(18.7)
82.15
(530)
13 10 6 5 4 3 1 Yes Yes No
Allentown XJ IVC Cage
(Angled Bottom)
7.64
(19.4)
15
(38.1)
5.16
(13)
77.5
(500)
12 9 6 5 4 3 1 Yes No No
Large (King Box) Static
Microisolator
10.5
(26.67)
19
(48.26)
6
(15.24)
142
(916)
23 17 11 9 7 6 2 Yes Yes Yes
Maximum Number Allowed in Cage
Allowed Yes/No
*Age is based on the C57BL/6NHsd inbreb mouse and are only approximate and varies greatly between species
The body weight is the limiting factor and the approximate age information is provided only for guidance
** Not in regular use-In storage in case ABLS3 suite reopens
37 (TOC)
Chart below is from the new 8
th
edition of the ILAR publication The Guide for the Care
and Use of Laboratory Animals (2010)
Animals
Weight
in
grams
Floor
Area/Animal,
a
in.
2
(cm
2
)
Height,
b
in. (cm)
Comments
Mice in Groups
c
<10
6 in
2
(38.7)
5 (12.7)
Larger animals may require more
space to meet performance
standards
Up to
15
8 in
2
(51.6)
5 (12.7)
Up to
25
12 in
2
(77.4)
5 (12.7)
>25
> (>96.7)
5 (12.7)
Female + litter
51in
2
(33)
(Recommended
space for the
housing group)
5 (12.7)
Rat Caging
The housing density for rats is outlined in The Guide for the Care of Laboratory
Animals and states that housing density is determined by several factors, the size of
the cage and the weight of the animal. Each IVC Rat cage is different in floor space
and the typical rat static micro-isolator can hold two adult rats weighing up to 500
grams each. Breeding animals or breeding pairs may have different space
requirements to allow for optimum growth of young. If you will be working with rats,
please consult the area supervisors as to the number of rats that can be housed in
each cage type to remain in compliance with the IACUC and other regulatory
agencies.
Rat Caging
Maximum Rat Density
Cage Type
Width In (cm)
Length In (cm)
Height In (cm)
Floor Area in
2
(cm
2
)
Body Weight
<100g [<4 Weeks of age*]
Body Weight 100-200g
[7-10 (M-F) Weeks of age*]
Body Weight 200-300
[10-12 (M-F) Weeks of age*]
Body Weight 300-400g
[>12 (M-F) Weeks of age*]
Body Weight
400-500G
Body Weight 500-650g
Body Weight 650-800g
Body Weight >800g
Female + Litter
Female with Male + Litter
Required Floor Area
[sq. in (sq. cm]
7.0
(17.8)
17
(109.6)
23
(148.35)
29
(187.05)
40
(258.0)
60
(387.0)
70
(451.5)
80
(516.1)
90
(580.1)
124.0
(800)
214.0
(1380)
Allowed
Yes/No
Standard Static Cage/
Microisolator
10.5
(25.9)
19
(47.6)
8.0
(20.9)
143
(922.0)
8 6 4 3 2 2 1 1 1 No
Techniplast 1291H
10.47
(26.6)
16.73
(42.5)
7.28
(18.5)
124
(800)
7 5 4 3 2 1 1 1 1 No
Allentown Next Gen 900
11.8
(29.9)
16.2
(41.2)
9.2
(23.4)
142
(916)
8 6 4 3 2 2 1 1 1 No
Techniplast Double
Decker
18.2
(46.2)
15.87
(40.3)
15.89
(40.4)
288.6
(1849)
17 12 10 7 4 4 3 3 2 Yes
*Age is based on the HsdSD Sprague-Dawley ratand are only approximate and varies greatly between species
The body weight is the limiting factor and the approximate age information is provided only for guidance
Maximum Number Allowed in Cage
38 (TOC)
Other Species
DLAR has the ability to house a variety of common laboratory animals including
rabbits, dogs, hamsters, guinea pigs, as well as mice and rats, birds and, on a limited
basis non-human primates, sheep and pigs. When planning your studies, please
consult with our veterinarians for further information.
Additional Resources Available from DLAR:
• Website https://www.research.uky.edu/division-laboratory-animal-resources
• Micro Isolator presentation
• Recommendations for analgesia and anesthesia
• Breeding Colony Management presentation and Workshop, Pup aging chart,
Weaning calendar
• Outline for managing breeding colonies
• List of Special cards and their usage
• links to the various forms and examples
• CD’s on Biomethodology in Mice and Rats, Rodent Survival Surgery
• Aseptic Surgery presentation and Workshop
• Handling and Injection Techniques
• Post-Surgical Monitoring and Basic Suturing workshops
DLAR Forms
DLAR Access Forms
• DLAR Access Instructions
• Research Personnel Request for Access to DLAR Facilities
• DLAR Staff-PPD-Security-Vendor Request for Access to DLAR
Cayuse AO Request Forms
• Principal Investigator Cayuse AO Account Request
• Non-PI Cayuse AO Account Request
Weight
Floor Area/Animal
in2 cm2
Height
in cm
Comments
Rats in
<100
17 109.6
7 17.8
Larger animals may require
Groups
Up to 200
23 148.35
7 17.8
more space to meet performance
Up to 300
29 187.05
7 17.8
standards. This is particularly
Up to 400
40 258.0
7 17.8
true with breeding pairs, trios, or
Up to 500
60 387.0
7 17.8
females with litters
>500
>70 >451.5
7 17.8

39 (TOC)
DLAR Service Forms
• DLAR Service request Form
• DLAR Do Not Feed Form
• DLAR Treatment Request Form
• DLAR Hazardous Agent Use Form
• DLAR Research Supply Acquisition Form
• Pathology/Necropsy request Form
• DLAR Cryopreservation Service Request Form
DLAR Visitor Form
• Occupational Health and Safety Form for Visitors
Please download and print the Occupational Health and Safety form, sign it, and
then either fax it to the DLAR main office at 859-323-6002, or bring it with you at
the time of your visit.
• Guidelines and Policies for Accessing DLAR Facilities
Animal Import/Export Forms
• Instructions for Importing Rodent Colonies to DLAR
• Request to Import Animals from Other Institutions (pdf)
• Request to Export Animals to Other Institutions (pdf)

