
1
Cornel Stanciu, MD, CPE, MRO, FASAM, FAPA
Director of Addiction Services at New Hampshire Hospital
Assistant Professor of Psychiatry at Dartmouth’s Geisel School of Medicine
May 16, 2024
Understanding Kratom: Consumption
Patterns and Treatment Strategies for
Kratom Addiction

2
Housekeeping
• This event is brought to you by the Providers Clinical Support System
– Medications for Opioid Use Disorders (PCSS-MOUD). Content and
discussions during this event are prohibited from promoting or selling
products or services that serve professional or financial interests of
any kind.
• The overarching goal of PCSS-MOUD is to increase healthcare
professionals' knowledge, skills, and confidence in providing evidence-
based practices in the prevention, treatment, recovery, and harm
reduction of OUD.

3
AAAP is committed to presenting learners with unbiased, independent,
objective, and evidence-based education in accordance with accreditation
requirements and AAAP policies.
Presenter(s), planner(s), reviewer(s), and all others involved in the planning
or content development of this activity were required to disclose all
financial relationships within the past 24 months
All disclosures have been reviewed, and there are no relevant financial
relationships with ineligible companies to disclose.
All speakers have been advised that any recommendations involving clinical medicine must be based on evidence that is
accepted within the profession of medicine as adequate justification for their indications and contraindications in patient
care. All scientific research referred to, reported, or used in the presentation must conform to the generally accepted
standards of experimental design, data collection, and analysis.
Disclosure to Learners

4
Educational Objectives
• At the conclusion of this activity participants should be able to:
Understand Kratom’s literature, covering its uses, risks (overdose,
addiction), and product toxicities.
Evaluate Kratom’s role in harm reduction, distinguishing between
evidence-based practices and misconceptions.
Implement evidence-based treatments for Kratom addiction,
facilitating informed discussions and effective interventions with
patients.

5
INTRODUCTION

6
• Tropical evergreen
tree/shrub related to the
coffee plant.
• Native to Southeast Asia
• Indigenous to Thailand,
Indonesia, Malaysia and
Papua New Guinea.
• First formally described in
1839 by Dutch colonial
botanist Pieter Korthals.
The Plant - Mytragyna Speciosa Korth

7
• Used for centuries by the indigenous
population to enhance stamina and
combat physical ailments from hard
labor.
• Not regarded as “drug use”, but rather
as a way of life, embedded in
traditions and customs.
• Therapeutically also used for self-
managing pain, cough and diarrhea.
• Leaves are chewed, brewed as tea, or
to a lesser degree smoked, producing
a complex stimulant and opioid-like
effect.
Traditional Use

8
• In time it has also gained popularity as an
opioid substitute.
• Its potential for tolerance and
dependence has long been apparent.
• Reports of significant adverse effects or
mortality in Asia are not extensively
documented.
• A recent trend in the region is its use in
urban settings by young individuals as
part of polydrug concoctions for euphoric
effects.
Use in Southeast Asia

9
• Dry or crushed leaves;
concentrated extracts, powders,
capsules; tablets, liquids, and
gum/resin.
• Readily available at smoke shops
or through online vendors with no
quality control.
• Dramatic increase in importation
since 2016.
Use in the West

10
• Kratom is increasingly used by people who advocate for it as a plant-based
remedy to self-manage pain, mental health symptoms, and opioid withdrawal.
• Growth of Kratom use in the Western world also parallels increasing concerns
over adverse effects, abuse, and addictive potential.
• Adverse effects associated with Kratom products span multiple organ systems
including hepatic, renal, cardiac, endocrine, and neurological.
• Fatalities involving kratom are increasingly documented, however involve co-
ingestions, or polydrug combinations.
Use in the West

11
• The prevalence of Kratom
use in the United States is
not well defined, with
lifetime estimates ranging
from 0.9 to 6.1%
versus 2.9 to 12% in
Thailand.
Epidemiology

12
Epidemiology
• Association data supports a greater burden of mental health and
substance use disorders, especially opioids, among users.
• Lifetime nonmedical opioid use is also associated with a greater
likelihood of Kratom use.
• In SE Asia:
Tolerance occurs within 3 months, and some escalate use 4-10x
within the first few weeks.
55% of regular users become dependent, with some reports
suggesting a relapse rate of 78-83% at 3 months.

13
• Demographics:
Middle-aged (31-50 years of
age), white male.
Married, employed, and insured.
Some college education with an
income of over $35,000 / year.
Duration of use >1 year but < 5
years.
• Reasons for use
Self-manage chronic pain (68%),
anxiety, or depression (65%) and
as an opioid replacement.
40% of users endorse the use of
Kratom to reduce/stop opioid
use, with 74% claiming >6mo
success in abstinence.
41% disclosed use to healthcare
providers.
• Ratings of improvements show a
majority of consumers rate overall
health as “good”.
Survey of consumers

14
Distribution of Consumers
• Heavily concentrated in the South, followed by West and Midwest, and few in
the Northeast
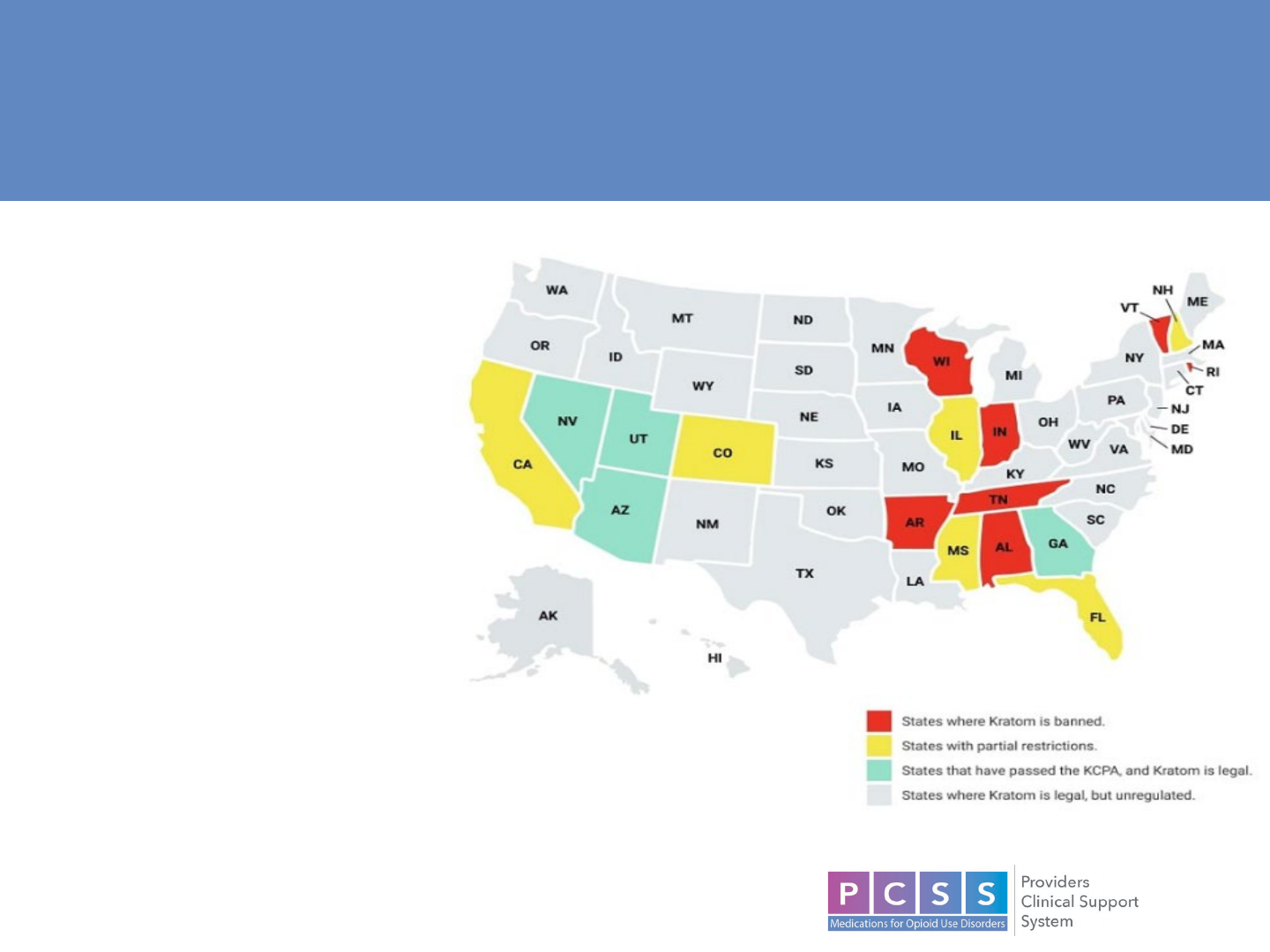
15
• Malaysia, Vietnam – illegal.
• Thailand – illegal until 2021.
• In the US it was legal to grow and
purchase in all states until 2015.
• Uncontrolled under federal
regulation.
• In 2016 the DEA submitted an
intent to schedule under the CSA,
but later that year withdrew.
• The Kratom Consumer Protection
Act exists in some states as
legislation to ensure consumer
access to Kratom with a framework
for regulatory oversight.
Legal Status and Regulation on Sales

16
PHARMACOLOGY

17
• The effects in humans have historically been described as dose-dependent:
Small doses (1-5g)
Stimulatory, caffeine-like, effects.
Larger dosages (>5g)
Sedative, analgesic effects.
Behavioral Pharmacology

18
• Leaf analysis
40 structurally related alkaloids,
flavonoids, terpenoid saponins,
polyphenols, and various glycosides.
• >25 indole alkaloids
− 1-2% by weight
Mitragynine (MG)
− ~60%
7-hydroxymitragynine (7OHMG)
− Extremely small representation of
plants
− Metabolic product of MG
(CYP3A4)
Complex Composition

19
• White vein – stimulating, to help with concentration.
• Green vein – improves mood, and depression.
• Red vein – calming effects, to help relax/sleep.
• Commercial products differ in composition.
“Potency”

20
Phase Model

21
Opioid Receptors
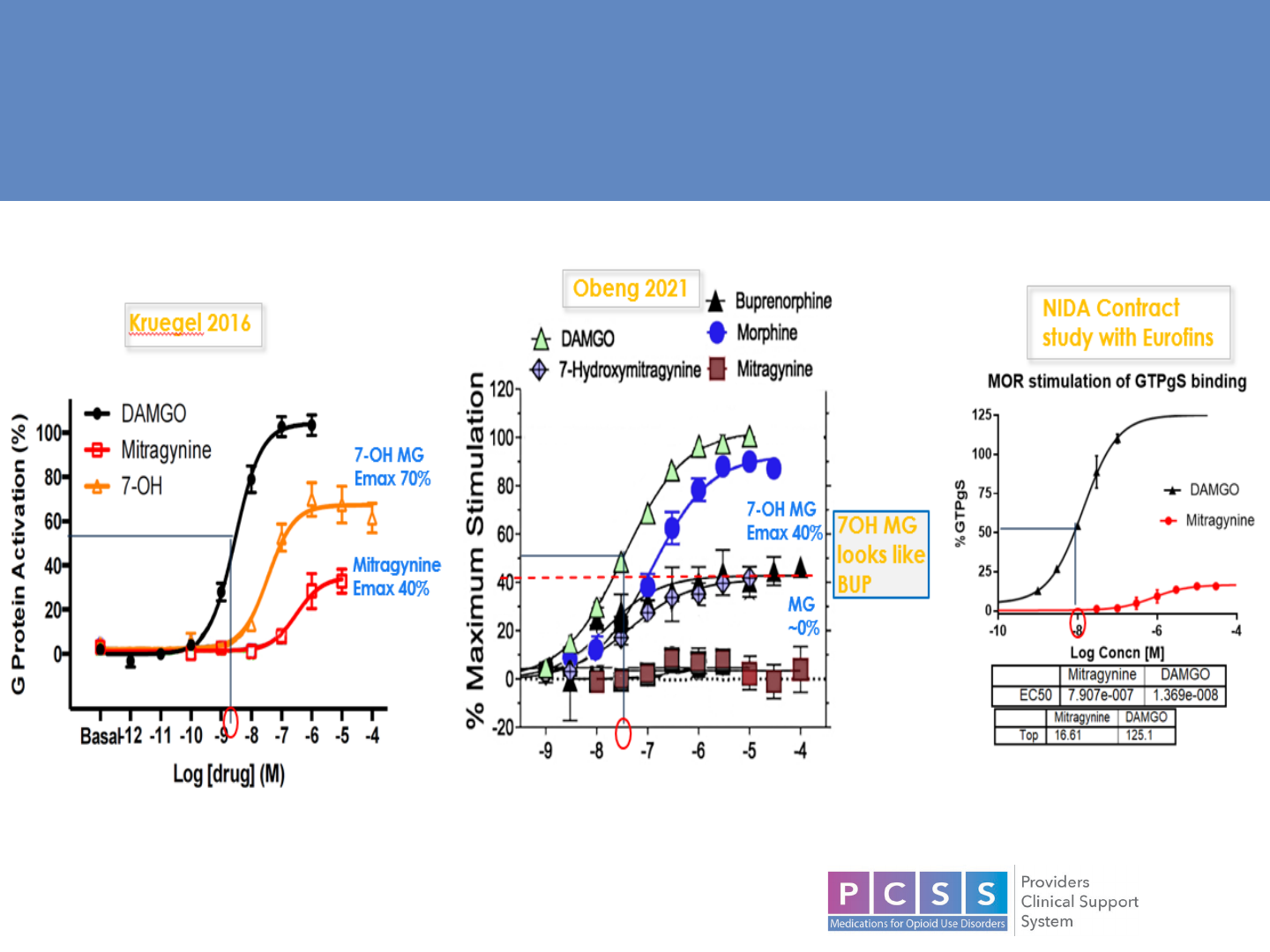
22
Opioid Receptor Efficacy

23
• Similarities to opioids:
Partial agonism at mu
receptors
Binding to opioid Rs initiates G-
protein-coupled receptor
(GPCR) signaling.
• Differences from opioids:
GPCR activation by indole
alkaloids does not initiate the β
-
arrestin pathway
− “biased agonism”
• MG also exerts non-opioid
receptor pain-relieving effects by
stimulating alpha-2 and inhibiting
cyclooxygenase-2 mRNA and
protein expression.
Atypical Interactions with Opioid Receptors

24
Other Receptor Systems

25
• MG relieves neuropathic
pain.
Relief blocked by both
opioid and adrenergic
blockers.
MG
YOH = Yohimbine

26
ADVERSE EFFECTS

27
• Ascending doses of Kratom alkaloids result in an increase in:
− Blood pressure
− Liver function tests
− Creatinine
− Death preceded by convulsions, not respiratory depression
• Herb: drug interactions:
MG inhibits CYP 2C9, 2D6, 3A4 as well as glucuronidation by UDP-
glucuronosyltransferases.
• Labilities:
MG less reinforcing than morphine and may be tolerance-sparing when
co-administered.
7OHMG substitutes for morphine, re-institutes conditioned place
preference (CPP, measure of rewarding effects in animal models).
Animal Studies

28
Human Case Reports
• After > 1 year of regular use:
Weight loss; Insomnia; Constipation; Skin hyperpigmentation; Extreme fatigue.

29
• Kratom overdoses resemble adrenergic toxicity more so than opioids:
Nausea and tachycardia predominate.
Respiratory depression is rare.
Clinical effects
Total
n (%
a
)
Clinical effects
Total
n (%
a
)
Single-substance exposure total 1174
Neurological
Gastrointestinal
Agitated/irritable 269 (22.9) Nausea 171 (14.6)
Drowsiness/lethargy 168 (14.3) Vomiting 155 (13.2)
Confusion 125 (10.6) Abdominal pain 76 (6.5)
Seizures (single/multiple) 113 (9.6)
Respiratory
Tremor 79 (6.7) Respiratory depression 42 (3.6)
Dizziness/vertigo 62 (5.3)
Hematologic/hepatic
Hallucinations/delusions 61 (5.2) AST, ALT > 100 59 (5.0)
Cardiovascular
Tachycardia 251 (21.4)
Hypertension 119 (10.1)
Poison Control Centers

30
Kratom-Related Deaths
• Co-ingestions and other active use disorders predispose patients to death
(found in 87% of cases).
• It is challenging to identify which deaths are attributed to kratom alone.
Stanciu, C.N., et al. (2023). "Kratom Overdose Risk:
A Review." Curr Addict Rep, 10(1), 9–28. DOI:
10.1007/s40429-022-00464-1

31
MANAGEMENT

32
• ~41% of Kratom consumers disclose consumption to healthcare providers.
In assessments, use non-judgmental, non-stigmatizing, questions
− “Do you use any herbal medicines, like valerian root or kratom?”
• Not detected by routine toxicology or conventional confirmatory drug
screening tests.
• HPLC or Mass spectrometry is required for detection & identification.
Detection

33
• Toxicity -- supportive management in most cases.
Acute hepatitis -- N-acetylcysteine (as in any other drug-induced hepatitis).
Seizures or neurological symptoms -- anti-epileptics.
Kidney injury, cardiovascular events, or other emergency presentations
addressed with appropriate measures.
• Overdose
Kratom-only overdoses resemble stimulant overdoses (cardiovascular,
seizure).
− Poison Control Center reports show low levels of meiosis, sedation, and
respiratory depression.
Co-ingestions are common
− Reports describe mixed outcomes with reversal agents (naloxone); no
clinical trials exist.
Toxicity

34
• Mimics opioid withdrawal, but milder:
Withdrawal intensity positivity correlated to:
− Daily amount consumed.
− Duration and frequency of use.
Starts ~12-24 hours from last use, can last
up to 4 days
− Symptomatic management of a
hyperadrenergic state (i.e.. Clonidine).
− Good response to opioid receptor agonists
(Methadone), or partial agonists
(Buprenorphine).
Cravings and relapse risk is high.
Withdrawal

35
“Kratom Use Disorder”
• ~25.5% of consumers, however mild-mod severity (tolerance, withdrawal) and
no functional impact.
• Vulnerability factors: male, young, consuming Kratom frequently, and having
psychiatric and substance use disorders.

36
• Our team’s efforts to establish a “standard of care”.
• Since 2021 several reports emerged.
Mixed evidence of amount used, and Buprenorphine dose needed.
• Those requiring MOUD for maintenance could represent a small
group.
Maintenance Treatment

37
CASES

38
Case 1 - Andrew
• 33yo Caucasian male h/o anxiety, depression (on Escitalopram 20mg and
Propranolol 10mg BID), OUD in sustained remission x 12 y
• Started 2.5y ago, initially 2 caps per dose, progressively increased to 8 caps
within a few weeks to manage various mood states.
• Distinct effects from kratom vs. Percocet: increased energy, uplifted mood, and
motivation.
• Diminishing effects over time, he continued to consume 12-15 caps daily,
transitioning to powdered kratom washed with water 1.5 y ago.
• Significant lifestyle disruptions in the last year, including marital strain, work
interference, and financial burden.
• Struggled with increasing intervals between doses, experiencing withdrawal
symptoms (cold sweats, "brain zaps", and cravings after 6-7 hr. abstinence).
• Detox and eventually transitioning to naltrexone using SOWS alongside
clonidine, loperamide and gabapentin failed - could not abstain for >12 hr.
• Successfully transitioned to buprenorphine - 2mg daily with an additional 2mg
dose as needed in the evening for craving management.

39
Case 2 - Fred
• 37yo Caucasian male h/o OUD in sustained remission admitted to inpatient
psych due to depression, SI.
• General workup was unremarkable (EKG, labs, vs); Urine tox + THC.
• Disclosed a 6-mo h/o Kratom use, escalating to 11 capsules daily (3 in AM, 3
mid-day, 5 in PM), supplemented with an unknown quantity powder in water.
• Introduced to it as an antidepressant and had initially started with 1 cap in the
morning however within days he increased his dosage to 3 caps to combat
decreased effectiveness and maintain energy levels.
• Constipation is the only side effect, recent attempts to quit after learning about
reports on the Internet of various adverse effects were hindered by cravings and
"rebound depression.”
• Last use 24hr prior to admission and he experienced progressively worsening
nausea and flu-like symptoms which were first noticed 6 hours ago.
• COWS = 11, Methadone 5mg x1 administered, COWS = 2.
• Next day COWS remained 2, Clonidine 0.1mg x1 for anxiety, (HR 87 and BP
142/95).
• Aripiprazole 5mg (past response), fifth day he underwent a naltrexone challenge
and received a monthly long-acting injectable.

40
Case 3 - Mark
• 43yo Caucasian male h/o anxiety, chronic pain, ADHD
• Anxiety is generalized with occasional panic attacks, has a therapist
• Past injuries, and back pain necessitating spinal fusion.
• Self-medicating for pain with kratom (~5 g of powder daily) for >5 years.
• Improves pain allowing him to be mobile without the use of any pharmacotherapies –
which his orthopedic surgeon has discussed in the past.
• In addition to the pain benefit he also has noticed that kratom ingestion also tends to
“bring the temperature down” on his anxiety.
• No adverse effects and his dose and frequency have remained the same as when
he initiated.
• Has had stretches of up to 1wk of no kratom and never experienced withdrawal, or
cravings despite pain exacerbation.
• Trintellix 20mg and Vyvanse 30mg
• Organic evaluation unremarkable (CMP, CBC, TSH), MG level 133 ng/mL was
quantified.
*psychoeducation, motivational enhancement ongoing

41
• The exact prevalence of kratom use is unknown, however clinicians
are encountering more and more consumers.
• Despite consumers claiming benefits/harm reduction, it is possible for
some to meet DSM criteria for a “use disorder” diagnosis.
• Kratom is a complex botanical, and its alkaloids interact with many
receptor systems - including opioids.
• There is a spectrum of adrenergic and opiodendric involvement
impacting each user, and withdrawal, and maintenance treatment
requires individualized treatment.
Summary

42
References
• Abuse, N. I. on D. (--). What are the long-term effects of methamphetamine misuse? National Institute on Drug Abuse.
https://www.drugabuse.gov/publications/research-reports/methamphetamine/what-are-long-term-effects-methamphetamine-misuse
• Akindipe, T., Wilson, D., & Stein, D. J. (2014). Psychiatric disorders in individuals with methamphetamine dependence: Prevalence and
risk factors. Metabolic Brain Disease, 29(2), 351–357.
https://doi.org/10.1007/s11011-014-9496-5
• Chan, B., Freeman, M., Kondo, K., Ayers, C., Montgomery, J., Paynter, R., & Kansagara, D. (2019). Pharmacotherapy for
methamphetamine/amphetamine use disorder—A systematic review and meta-analysis. Addiction, 114(12), 2122–2136.
https://doi.org/10.1111/add.14755
• Coffin, P. O., Santos, G.-M., Hern, J., Vittinghoff, E., Santos, D., Matheson, T., Colfax, G., & Batki, S. L. (2018). Extended-release
naltrexone for methamphetamine dependence among men who have sex with men: A randomized placebo-controlled trial. Addiction,
113(2), 268–278. https://doi.org/10.1111/add.13950
• Colfax, G. N., Santos, G.-M., Das, M., Santos, D. M., Matheson, T., Gasper, J., Shoptaw, S., & Vittinghoff, E. (2011). Mirtazapine to
Reduce Methamphetamine Use: A Randomized Controlled Trial. Archives of General Psychiatry, 68(11), 1168–1175.
https://doi.org/10.1001/archgenpsychiatry.2011.124
• Elkashef, A., Kahn, R., Yu, E., Iturriaga, E., Li, S.-H., Anderson, A., Chiang, N., Ait-Daoud, N., Weiss, D., McSherry, F., Serpi, T.,
Rawson, R., Hrymoc, M., Weis, D., McCann, M., Pham, T., Stock, C., Dickinson, R., Campbell, J., … Johnson, B. A. (2012). Topiramate
for the treatment of methamphetamine addiction: A multi-center placebo-controlled trial: Topiramate for methamphetamine addiction.
Addiction, 107(7), 1297–1306.
https://doi.org/10.1111/j.1360-0443.2011.03771.x
• Heinzerling, K. G., Swanson, A.-N., Hall, T. M., Yi, Y., Wu, Y., & Shoptaw, S. J. (2014). Randomized, placebo-controlled trial of
bupropion in methamphetamine-dependent participants with less than daily methamphetamine use: Bupropion for methamphetamine
dependence. Addiction, 109(11), 1878–1886. https://doi.org/10.1111/add.12636
• Jayaram-Lindström, N., Hammarberg, A., Beck, O., & Franck, J. (2008). Naltrexone for the Treatment of Amphetamine Dependence: A
Randomized, Placebo-Controlled Trial. American Journal of Psychiatry, 165(11), 1442–1448.
https://doi.org/10.1176/appi.ajp.2008.08020304
• Kish, S. J. (2008). Pharmacologic mechanisms of crystal meth. CMAJ, 178(13), 1679–1682. https://doi.org/10.1503/cmaj.071675
• Konstenius, M., Jayaram-Lindström, N., Guterstam, J., Beck, O., Philips, B., & Franck, J. (2014). Methylphenidate for attention deficit
hyperactivity disorder and drug relapse in criminal offenders with substance dependence: A 24-week randomized placebo-controlled
trial. Addiction (Abingdon, England), 109(3), 440–449. https://doi.org/10.1111/add.12369

43
References
• Logan, B. K. (2002). Methamphetamine—Effects on Human Performance and Behavior. 19.
• Logan—2002—Methamphetamine—Effects on Human Performance and.pdf. (n.d.). Retrieved November 21, 2021, from
http://www.biblioteca.cij.gob.mx/Archivos/Materiales_de_consulta/Drogas_de_Abuso/Articulos/methamphetamine.pdf
• Longo, M., Wickes, W., Smout, M., Harrison, S., Cahill, S., & White, J. M. (2010). Randomized controlled trial of dexamphetamine maintenance for
the treatment of methamphetamine dependence. Addiction, 105(1), 146–154.
https://doi.org/10.1111/j.1360-0443.2009.02717.x
• Matsumoto, T., Kamijo, A., Miyakawa, T., Endo, K., Yabana, T., Kishimoto, H., Okudaira, K., Iseki, E., Sakai, T., & Kosaka, K. (2002).
Methamphetamine in Japan: The consequences of methamphetamine abuse as a function of route of administration. Addiction, 97(7), 809–817.
https://doi.org/10.1046/j.1360-0443.2002.00143.x
• Methamphetamine Psychosis: Epidemiology and Management—ProQuest. (n.d.-a). Retrieved November 7, 2021, from
https://www.proquest.com/docview/1641841832/fulltext/C1BC91A83BD045E1PQ/1?accountid=10422
• Methamphetamine Psychosis: Epidemiology and Management—ProQuest. (n.d.-b). Retrieved November 7, 2021, from
https://www.proquest.com/docview/1641841832/fulltext/C1BC91A83BD045E1PQ/1?accountid=10422
• Meyers, R. J., Roozen, H. G., & Smith, J. E. (2011). The Community Reinforcement Approach. Alcohol Research & Health, 33(4), 380–388.
• Miller, S. (2019). The ASAM Principles of Addiction Medicine (6th ed.). Wolters Kluwer.
• NIDA. 2021, April 13. What are the long-term effects of methamphetamine misuse?. Retrieved from https://www.drugabuse.go. (n.d.).
• Signs Someone is Using Methamphetamine—How to Tell | The Recovery Village. (n.d.). The Recovery Village Drug and Alcohol Rehab. Retrieved
November 21, 2021, from
https://www.therecoveryvillage.com/meth-addiction/faq/know-someone-crystal-meth/
• Snapshot. (n.d.). Retrieved November 7, 2021, from https://www.drugabuse.gov/publications/research-reports/methamphetamine/what-are-long-
term-effects-methamphetamine-misuse
• The pattern of tissue loss in methamphetamine users whose brains were mapped by MRI scans. (n.d.). UCLA. Retrieved November 21, 2021,
from https://newsroom.ucla.edu/file?fid=52e6ed77f6091d799c00001e
• Thompson, P. M. (2004). Structural Abnormalities in the Brains of Human Subjects Who Use Methamphetamine. Journal of Neuroscience, 24(26),
6028–6036.
https://doi.org/10.1523/JNEUROSCI.0713-04.2004
• Thompson—2004—Structural Abnormalities in the Brains of Human Su.pdf. (n.d.). Retrieved November 21, 2021, from
https://www.jneurosci.org/content/jneuro/24/26/6028.full.pdf
• Trivedi, M. H., Walker, R., Ling, W., dela Cruz, A., Sharma, G., Carmody, T., Ghitza, U. E., Wahle, A., Kim, M., Shores-Wilson, K., Sparenborg, S.,
Coffin, P., Schmitz, J., Wiest, K., Bart, G., Sonne, S. C., Wakhlu, S., Rush, A. J., Nunes, E. V., & Shoptaw, S. (2021). Bupropion and Naltrexone in
Methamphetamine Use Disorder. New England Journal of Medicine, 384(2), 140–153. https://doi.org/10.1056/NEJMoa2020214

44
PCSS-MOUD Mentoring Program
• PCSS-MOUD Mentor Program is designed to offer general information to
clinicians about evidence-based clinical practices in prescribing
medications for opioid use disorder.
• PCSS-MOUD Mentors are a national network of providers with expertise in
addictions, pain, and evidence-based treatment including
medications for opioid use disorder (MOUD).
• 3-tiered approach allows every mentor/mentee relationship to be unique
and catered to the specific needs of the mentee.
• No cost.
For more information visit:
https://pcssNOW.org/mentoring/

46
PCSS-MOUD is a collaborative effort led by the
American Academy of Addiction Psychiatry (AAAP) in partnership with:
_________________________________________
Addiction Policy Forum American College of Medical Toxicology
Addiction Technology Transfer Center* American Dental Association
African American Behavioral Health Center of Excellence American Medical Association*
American Academy of Addiction Psychiatry* American Orthopedic Association
American Academy of Child and Adolescent Psychiatry American Osteopathic Academy of Addiction Medicine*
American Academy of Family Physicians American Pharmacists Association*
American Academy of Neurology American Psychiatric Association*
American Academy of Pain Medicine American Psychiatric Nurses Association*
American Academy of Pediatrics* American Society for Pain Management Nursing
American Association for the Treatment of Opioid
Dependence
American Society of Addiction Medicine*
American Association of Nurse Practitioners Association for Multidisciplinary Education and
Research in Substance Use and Addiction*
American Chronic Pain Association Coalition of Physician Education
American College of Emergency Physicians* College of Psychiatric and Neurologic Pharmacists
Black Faces Black Voices
47
PCSS-MOUD is a collaborative effort led by the
American Academy of Addiction Psychiatry (AAAP) in partnership with:
Columbia University, Department of Psychiatry*
Partnership for Drug-Free Kids
Council on Social Work Education* Physician Assistant Education Association
Faces and Voices of Recovery Project Lazarus
Medscape Public Health Foundation (TRAIN Learning Network)
NAADAC Association for Addiction Professionals* Sickle Cell Adult Provider Network
National Alliance for HIV Education and Workforce
Development
Society for Academic Emergency Medicine*
National Association of Community Health Centers Society of General Internal Medicine
National Association of Drug Court Professionals Society of Teachers of Family Medicine
National Association of Social Workers* The National Judicial College
National Council for Mental Wellbeing* Veterans Health Administration
National Council of State Boards of Nursing Voices Project
National Institute of Drug Abuse Clinical Trials Network World Psychiatric Association
Northwest Portland Area Indian Health Board Young People In Recovery
_________________________________________

48
Educate. Train. Mentor
www.pcssNOW.org
pcss@aaap.org
@PCSSProjects
www.facebook.com/pcssprojects/
Funding for this initiative was made possible by cooperative agreement no. 1H 79TI086770 from SAMHSA. The views expressed in written conference materials or
publications and by speakers and moderators do no t necessarily reflect the o fficial policies of the Department of Health and Human Services; nor does mention of trade
names, co mmercial practices, or organizations imply endorsement by the U.S. Government.

