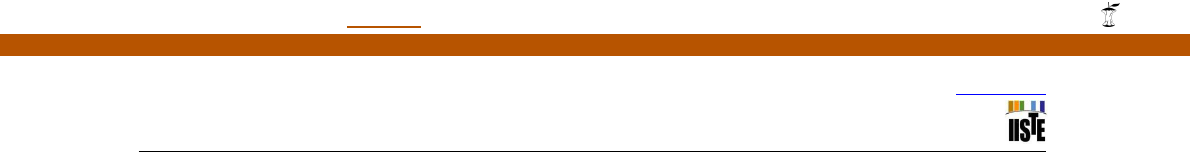
Developing Country Studies www.iiste.org
ISSN 2224-607X (Paper) ISSN 2225-0565 (Online)
Vol.6, No.1, 2016
87
Isolation, Identification and Authentication of Root Nodule
Bacteria (Rhizobia) in Promoting Sustainable Agricultural
Productivity: A Review
Solomon Legesse
School of Agriculture, Animal and Range Science Course Team, Madawalabu University, Bale-Robe, Ethiopia
Abstract
The ability of indigenous rhizobia to nodulate a legume crop effectively, or to act as barrier to the successful
establishment of inoculant strains, is critical to successful establishment and growth of legumes. Effective groups
serve both as a guide to inoculant preparation and as a basis for predicting the need for inoculation. This review
included all aspects of rhizobial work, starting from isolation and characterization of root nodule bacteria from
the field to authentication of the symbiotic effectiveness test under sterile sand condition. This review shows the
process on how to evaluate the symbiotic effectiveness along with growth responses to varied conditions of pH,
temperature, antibiotics as well as carbon and nitrogen sources. Goal of Rhizobium scientists is to discover new
and better strains for use in legume inoculants. This pursuit entails the collection of isolates, strain
characterization, assessment of symbiotic capacity and comparison to strains currently included within
inoculants. During the process of collection, care should be taken not to simply collect strains that were obtained
through past inoculation, and the elite strains identified through exploration and characterization should be
distinguished as original through strain identification procedures. This was a very valuable review for
researchers dealing with improving soil fertility and supporting sustainable crop production with the minimum
use of costly and environmentally unfavorable chemical fertilizers.
Keywords: Agriculture, Authentication, Identification, Isolation, Rhizobium
Introduction
In all regions of the world where food consumption exceeds production or where nitrogenous fertilizer has to be
imported, leguminous crops have a special relevance because of their unique ability to fix atmospheric nitrogen
and being self-sufficient they are capable of supplying nitrogen to corresponding crops and the soil. This
property of legumes makes them increasingly attractive. Therefore, greater use of legumes can have a significant
beneficial impact in tropical countries where population increase, food production are considerably not
proportional and the cost of artificial fertilizer is prohibitively expensive resulting in least adequate importation
of nitrogen fertilizers (Somasegaran and Hoben, 1985).
Root infection by rhizobia is a multistep process that is initiated by pre-infection events in the
rhizosphere. Rhizobia respond by positive chemotaxis to plant root exudates and move toward localized sites on
the legume roots (Dowling and Broughton, 1986). Goal of Rhizobium scientists is to discover new and better
strains for use in legume inoculants. This pursuit entails the collection of isolates, strain characterization,
assessment of symbiotic capacity and comparison to strains currently included within inoculants. During the
process of collection, care should be taken not to simply collect strains that were obtained through past
inoculation, and the elite strains identified through exploration and characterization should be distinguished as
original through strain identification procedures. Symbiotic performance is a key but the ability of rhizobia to
survive stress conditions or to utilize less expensive growth media are also important considerations. The process
of Rhizobium exploration and characterization is somewhat arduous, and efforts must remain focused upon
relatively few legumes of interest in an unbiased manner so that elite strains emerging from a work must be
recognizably superior (Bala et al., 2010).
Having engaged in high input agriculture - adopting the “green revolution” of the 1960s - most
developing countries today find themselves in a quagmire in continuing to support and subsidize their farming
communities to sustain this model of crop production. The continuous, indiscriminate application of chemical
fertilizer and other agro-chemicals has largely contributed to the elimination of beneficial soil microorganisms
and the farmers are struggling to sustain high productivity on virtually ‘dead soils’. This is evident in most
tropical countries, where crop productivity has either stagnated or even declined despite the addition of chemical
fertilizer. Furthermore, addition of chemical fertilizer to such degraded soils, depleted of microorganisms and
associated organic matter and therefore unable to retain the added nutrients, results in large quantities of
chemicals being washed into waterways, leading to environmental pollution. Nutrient loading by such leachates
results in eutrophication of large water bodies (tanks, reservoirs, ponds and lakes), often producing algal blooms
that are harmful to animals and humans.
Therefore, both on economic and ecological grounds, the time has come to review the continuation of
the “green revolution” model and to seek alternatives of environmentally benign, economically sustainable
brought to you by COREView metadata, citation and similar papers at core.ac.uk
provided by International Institute for Science, Technology and Education (IISTE): E-Journals

Developing Country Studies www.iiste.org
ISSN 2224-607X (Paper) ISSN 2225-0565 (Online)
Vol.6, No.1, 2016
88
systems of agriculture. Foremost among such alternatives is Biological Nitrogen Fixation (BNF) which has the
potential to reduce and/or replace the continuous use of nitrogen fertilizer, which is very often the nutrient
limiting the productivity of several annual crops. As there are many leguminous crops that in symbiosis with root
nodule bacteria are capable of BNF, this area of study has become critical for their utilization in agriculture. The
ability of indigenous rhizobia to nodulate a legume crop effectively, or to act as barrier to the successful
establishment of inoculant strains, is critical to successful establishment and growth of legumes. Effective groups
serve both as a guide to inoculant preparation and as a basis for predicting the need for inoculation. Hence, this
review on the isolation, identification and authentication of root nodule bacteria (rhizobia) in promoting
sustainable agricultural productivity was most timely and appropriate for researchers in developing countries,
where large populations of rural, resource-poor farmers are engaged in crop production under trying
circumstances.
Soil and seed sample
Collection of soil and seed samples are the first step in trapping and identification of rhizobia isolates. Soil
samples should be collected from a soil where leguminous plants were grown with 10-15 cm depth. Then the
sample were pooled and collected in alcohol sterilized polyethylene plastic bags for further process in green
house condition. For both trapping method and authentication test selected seed varites of legume crops
preparation is also mandatory in this stage (Solomon and Fassil, 2014).
Trapping of Nodules
Nodulation were induced by ‘plant trap’ method in green house condition as described by Vincent (1970).The
soil samples were filled in 3 kg capacity plastic pots that had been surface sterilized (using 70% alcohol for
5sec.). Similar sized seeds of the selected seed verity were also surface sterilized with 70% ethanol for 5 seconds
and with 3 % (v/v) solution of sodium hypo-chlorate for 3-minutes, and washed thoroughly with five changes of
sterile distilled water. Then the seeds were sown in each pot under greenhouse conditions. After germination the
seedlings were thinned down to appropriate number per pot. There by plants were watered every two days for 45
days. After 45 days the pink and undamaged nodules were collected at flowering stage of the plants. Then, the
nodules were transferred in to sterile empty petri-dishes using forceps sterilized by dipping in alcohol and
flaming.
Isolation of rhizobia from Nodules
Collected nodules were surface sterilized with 95% ethanol for 10 seconds, and transferred to 3% (v/v) solution
of sodium hypo-chlorate for 3-4 minutes. The surface sterilized nodules were then rinsed in five changes of
sterile distilled water to completely rinse the sterilizing chemicals (Lupwayi and Haque, 1994). Then nodules
were transferred into sterile Petri-dishes and crushed with alcohol flamed sterile glass rod in a drop of normal
saline solution (0.85% NaCl) inside a laminar air flow hood (Somasegaran and Hoben, 1994). Then after 0.1ml
(loopful) of the suspensions were streaked on plate containing Yeast Extract Mannitol Agar (YEMA) and
incubated at 28 ± 2
0
C from 3-5 days. Yeast Extract Mannitol Agar (YEMA) (Vincent, 1970) composition
contains:-
Mannitol ----------------------------------------------10 g/l
K
2
HP0
4
-----------------------------------------------0.5 g/l
MgS0
4
.7H
2
0 -----------------------------------------0.2 g/l
NaCl ---------------------------------------------------0.1 g/l
Yeast Extract------------------------------------------0.5 g/l
Agar----------------------------------------------------15 g/l
Distilled Water ---------------------------------------1000 ml
PH ------------------------------------------------------7±0.1
They were Autoclaved at 121
0
C for 15 minutes.
Purification and preservation of isolates
After 3-5 days of growth, single dome-shaped colonies were picked with sterile inoculating loop and streaked on
sterile YEMA plates and incubated at 28±2
0
C. The purity and uniformity of colony types were carefully
examined through repeated re-streaking and a single well isolated colony was picked and transferred to YEMA
slant containing 0.3% (W/V) CaCO
3
in a culture tube and incubated at 28±2
0
C. When sufficient growth was
observed, the culture was transferred to be preserved at 4
0
C for future use (Vincent, 1970). The isolated native
strains were then characterized on the basis of morphological and physiological characters according to Jordan,
(1984).

Developing Country Studies www.iiste.org
ISSN 2224-607X (Paper) ISSN 2225-0565 (Online)
Vol.6, No.1, 2016
89
Presumptive screening of pure cultures
According to Somasegaren and Hoben, (1994), each isolate should examine for presumptive purity using
Peptone-Glucose Test (PGT), gram staining and growth response to YEMA-CR medium.
Congo red absorption test
Stock solution of Congo red was prepared by dissolving 0.25g of Congo red in 100ml of sterile distilled water.
From stock solution, 10ml was added to a liter of YEMA and autoclaved. Loop full of test isolates were streaked
on the medium and covered with aluminum foil to a dark condition and incubated at 28±2
0
C for 3 to 7 days to
detect Congo red absorption by the colonies (Vicent, 1970).
Peptone-glucose test
According to the procedure of Lupwayi and Haque (1994), Peptone Glucose Test was prepared by dissolving 5g
of glucose, 10g of peptone, 15g of agar and 10ml of bromocresol purple (BCP) in a liter of distilled water and
the pH was adjusted to 6.8 with 1N NaoH and HCl. Stock solution of BCP was prepared by dissolving 1g of
BCP in 100ml of ethanol. Three days old yeast extract manitol broth culture containing approximate number of
cells (10
4
cells ml
-1
) was streaked on to the Peptone Glucose Medium to observe the growth after having
incubated at 28±2
0
C for 3 to 7 days.
Gram reaction test
As indicated in Lupwayi and Haque (1994), all isolates were tested in gram reaction (gram negative or not) for
rapid means of contaminants identification.
Designation of the isolates
All the isolates requires designation of their identification with abbreviation and/or numbers at this stage
(Solomon and Fassil, 2014).
Cultural characteristics
Colony morphology
The morphological characteristics of the isolates were determined according to Lupwayi and Haque (1994). A
loopful of 48 hrs old grown broth culture from each isolate was inoculated onto YEMA and incubated at 28±2
0
C
for 3-7days. After 7days, colony diameter, morphology and colony texture were recorded as indicated in
Martinez-Romero et al., (1991).
Acid-Base production Test
To determine the ability of the Rhizobial isolates to produce acid or alkaline in the medium, YEMA containing
bromothymol blue (BTB) (0.025 w/v) was used. A loopfull of the isolates from a 48 hrs old culture broth was
streaked on to the YEMA-BTB medium and incubated for 3-7 days so as to record the color changes of the
medium (Jordan, 1984).
Growth and Eco physiological tests
For each biochemical and physiological test, inoculation of a loopfull of 48hrs old broth culture was streaked on
to the YEMA medium. The inoculated YEMA plates were incubated at 28 ± 2
0
C for 3-5 days (Somasegaren and
Hoben, 1994). Ultimately, the growth of each rhizobial isolate was determined as (+) for positive growth and (-)
for no growth (Solomon and Fassil, 2014).
Temperature tolerance
The growth of each isolate at different incubation temperatures was evaluated by inoculating each isolate on
YEMA plates. As indicated in Lupwayi and Haque, (1994), the inoculated plates were incubated at a temperature
of 4
0
C, 10
0
C, 15
0
C, 35
0
C, 40
0
C and above or below 4
0
C.
Salt tolerance
The ability of the isolates to grow at different level of salt concentrations was determined by inoculating each
isolate on the YEMA media containing 0.1%, 0.3%, 0.5%, 0.8%, 1%, 2%, 3%, 4%, 5% and above or below
0.1% of NaCl as indicated in Lupwayi and Haque (1994).
pH tolerance
The capacity of each rhizobial isolate to grow on acidic and alkaline media was determined by inoculating each
isolate on YEMA adjusted at a pH of 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and above or below 4.0, using NaOH
and HCl adjustments as described by Bernal and Graham (2001).

Developing Country Studies www.iiste.org
ISSN 2224-607X (Paper) ISSN 2225-0565 (Online)
Vol.6, No.1, 2016
90
Phosphorous solubilization
All isolates were tested for their ability to solubilize tri-calcium phosphate according to Lupway and Haque
(1994). The Basal Sperber Agar (BSA) medium that is used to test the ability of isolates to solubilize tri-calcium
phosphate was prepared using the following ingredients.
Yeast extract----------------------------10g/l
CaCl2 ------------------------------------0.1g/l
MgSo4.7H2O---------------------------0.25g/l
Ca3 (Po4)2 -------------------------------2.5g/l
Glucose----------------------------------10g/l
Agar--------------------------------------10g/l
After mixing these components, the Basal Sperber agar medium was autoclaved at 121
0
C for 15 minutes and
48hr old culture broth was streaked on to the Basal Sperber agar medium and incubated at 28±2
0
C to measure
the diameter of halo zone on each culture medium for about seven days. Colony diameter and solubilization
(halo) zone were measured; there by Solubilization Index (SI) was calculated as stated in Edi-premono et al.
(1996).
Amino acid utilization
Different types of amino acids including methionine, L-glutamate, L-tryptophane, L-tyrosine, lysine, aspargine,
alanine, L-phenylalanine, L-leucine, Proline, Urea, Argenine, L-arginine and other types of aminoacides were
used in this experiment in order to determine the ability of the isolates to utilize the amino acids as a nitrogen
source. These amino acids were added at a final concentration of 0.5g/l to a basal media source that lack
ammonium sulfate (1g of KH
2
PO
4
; 1g K
2
HPO
4
; 0.01g FeCl
3
.6H
2
O; 0.2g MgSO
4
.7H
2
O; 0.1g CaCl
2
; 1g manitol
and 15g agar) and supplemented with 1g/l of mannitol. The membrane filter sterilized amino acids were added to
the autoclaved and cooled (approximately 55
0
C) basal media as indicated in Amargar et al. (1997). Finally 48hr
old rhizobial suspensions were inoculated in to these basal media and incubated at 28±2 C for 3-5 days.
Carbohydrate utilization
Isolates were checked for their ability to utilize different carbohydrate sources at this stage. For this matter,
different sources of carbohydrate can be checked up. This may include monosaccharides (D-glucose, D-fructose,
D- galactose, D-arabinose, D-mannose and xylose), disaccharides (maltose, lactose and sucrose) and sugar
alcohols (sorbitol, inositol and glycerol). The test were carried out according to Somasegaran and Hoben (1994).
Ten percent distilled water solution of each carbohydrate (w/v but v/v for glycerol) was prepared and heat stable
carbohydrates (D-glucose, D-fructose, lactose and sucrose) were autoclaved together with the medium, but heat
labile carbohydrates (D-arabinose, D- mannose, sorbitol, D-galactose, maltose, xylose, dextrose, inositol and
glycerol) were filter sterilized using disposable membrane filter of 0.22μm sizes and added to the basal medium
(essentially similar to YEMA except the reduction of yeast extract from 0.5g/l to 0.05g/l) after sterilization when
the medium temperature was reduced to 50
O
C. 90ml of the carbohydrate-free basal medium together with each
test carbohydrate was dispensed in to several 250ml Erlenmeyer flasks. The media were then poured in to
sterilized plates and kept inverted for 24 hours in a laminar flow hood to check for contamination. Finally, a loop
full of 72 hours old YEM broth culture of each rhizobial isolate was streaked on the plates of incorporated
carbohydrates under test and incubated at 28
O
C for 10 days and growth was recorded as (+) for positive growth
and (-) for no growth in relation to the positive control YEMA plates.
Intrinsic Antibiotic Resistance (IAR)
The intrinsic antibiotic resistance of isolates to different antibiotics at different concentration was evaluated by
streaking each isolate on YEMA containing freshly prepared filter sterilized antibiotics using 0.22 µm sized
membrane filters. As described in Lupwayi and Haque (1994), the stock solution of each antibiotic was prepared
by dissolving 2g of each antibiotic in 100ml of water. The required concentration was aseptically added to the
media using a single pipette for each antibiotic. Stock solution of each antibiotic was filter sterilized using a
milipore filter (0.22 µm) and aseptically added to autoclaved YEMA (kept at 50
O
C in water bath) at the final
concentrations of 2.5µg/ml, 5µg/ml and 10 µg/ml, which is 12.5, 25 and 50 µl of antibiotic solution per 100 ml
medium, respectively, and finally poured separately in to plates. Erythromycin was dissolved in ethanol, whereas
the other four were dissolved in sterilized water. The antibiotics with their final concentration were listed as
follows:-
Developing Country Studies www.iiste.org
ISSN 2224-607X (Paper) ISSN 2225-0565 (Online)
Vol.6, No.1, 2016
91
Table IV: List of antibiotics with their final concentration used for the test
Group
Antibiotic
Final conc. (μg/ml)
I (
inhibitors of Protein Synthesis)
Erythromycin (Eryth)
2.5, 5, 10,20
Streptomycin (Strp)
II (Inhibitors of DNA and RNA synthesis)
Nalidixic acid (Nalid)
2.5, 5, 10,20
III (Inhibitors of protein synthesis and cell
wall membrane permeability)
Chloramphinicol (Chlo)
2.5, 5, 10,20
Ampicilin (Amp)
Generation time
Each isolate was inoculated in to a 10ml YEM broth (YEMB), vortex-dispersed and shaken on orbital shaker at
125 rev. min-1 for 3 days. Then, 1ml of each broth culture (cell suspensions) was inoculated into 100ml
sterilized YEM broth in 250ml Erlenmeyer flask and kept on orbital shaker at 125 rev. min
-1
. Turbidity was
measured by taking optical density (OD540nm) reading of the YEM broth cultures just at the time of inoculation
(0hr) and every 6hrs interval by using spectrophotometer (UV-7804C, Ultraviolet Visible spectrophotometer)
after calibrating it to zero with sterile un inoculated YEM broth as a blank. Isolates were immediately taken,
serially diluted (10
-1
-10
-10
) with sterile distilled water). 0.1 ml sample from each solution was dispersed on to the
sterilized YEMA plates and spreaded by using alcohol flamed spreader made out a glass rod to determine the
colony forming units (CFU) (Somasegaren and Hoben, 1994). Mean generation (doubling) time was calculated
from the logarithmic phase of either the optical density (OD) reading of spectrophotometer or viable count of
colony forming units (c.f.u) (White, 1995). The formula below was used to calculate mean generation time:
, Where is generation time, t is time elapsed,
X
o
is first OD reading in logarithmic phase,
X is second OD reading in logarithmic phase
Characterization of symbiotic effectiveness in sand culture
In order to test the definitive purity of all rhizobial isolates and there by their symbiotic effectiveness, nodulation
test was carried out for each of the purified isolates.
Experimental design
Three replicates were used for each treatment and also three replicates of positive control (Nitrogen added) and
negative control (nitrogen free) treatments were used as controls for each treatment. The treatments were
arranged in a randomized complete block design (RCBD) according to Somasegaran and Hoben (1994).
Preparation of sand and pots
Collected sand were socked with sulfuric acid and thoroughly washed with tap water until its PH becomes
neutral. Then the sand was drained and autoclaved at 121
O
C for 1hour. About 1.5kg of the autoclaved sand was
added to surface sterilized normal plastic pots and saturated with sterile distilled water prior to sowing seeds.
Seeds: preparation and sowing
Similar sized seeds of faba bean (vicia faba) were surface sterilized with 70% ethanol for 5 seconds and with 3%
(v/v) solution of sodium hypo-chlorate for 3-minutes, and washed thoroughly with five changes of sterile
distilled water. Then, the seeds of faba bean were plated into sterilized 0.75% (w/v) water agar and incubated at
28
O
C. The germinated seeds were selected and transferred by using sterile forceps into sterile potted sand at a
depth of 1cm after two days. Five seeds were planted into each pot and thinned down into three after a week.
Inoculant: Preparation and inoculation
Starter cultures of the selected test strains were grown in test tubes containing 10ml YEM broth on orbital shaker
at 150 rev/min at room temperature for 3 days. One ml YEM broth culture of each test strain (10
9
cells) was
transferred in to 100ml sterilized YEM broth in 250ml Erlenmeyer flask and placed on orbital shaker at 150
rev/min at room temperature for 4 days. One ml of each 4 days old YEM broth culture (about10
9
cells,
0.93OD540) was inoculated by pipetting on to the base of the seedlings after the emerging of the sown seedlings
according to Vincent (1970).
Green house conditions, nutrient supply and watering
The green house is located on Science Faculty Campus (Addis Ababa University) and its average temperature
during the photoperiod of 12hrs day/night is 28
O
C and 10
O
C respectively. KNO
3
at the final concentration of
0.05% (w/v) and 100ml per pot was added to positive control every week. The nitrogen-free nutrient solution to
supply for all treatments including positive and negative control was prepared according to Broughton and
Developing Country Studies www.iiste.org
ISSN 2224-607X (Paper) ISSN 2225-0565 (Online)
Vol.6, No.1, 2016
92
Dilworth (1970) as indicated below.
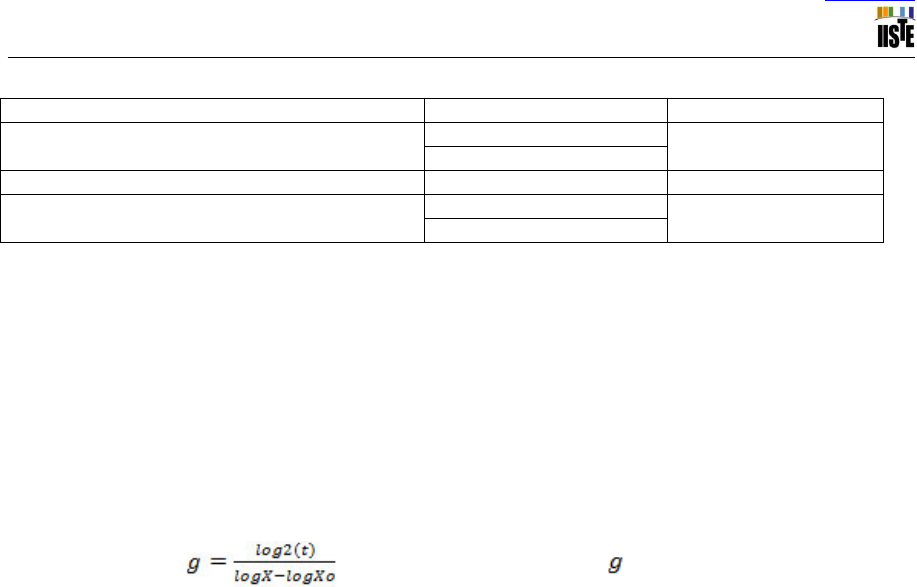
Table V. N-Free nutrient solution
Stock solution
Chemical
g/liter
1
CaCl
2
.2H
2
O
294.0
2
KH
2
PO
4
136.1
3
Fe C
6
H
5
O
7
. 3H
2
O
6.700
MgSO
4
. 7H
2
O
123.3
K
2
SO
4
. H
2
O
87.00
MnSO
4
. H
2
O
0.338
4
H
3
BO
3
0.247
ZnSO
4
. 7H
2
O
0.228
CuSO
4
. 5H
2
O
0.100
CoSO
4
. 7H
2
O
0.056
Na
2
MoO
2
. 2H
2
O
0.048
Source: Adapted from Somasegaren and Hoben (1994)
After preparing the stock solutions, 10 liters of full strength nitrogen-free plant nutrient solution was
prepared by mixing 5 ml of each stock solution with 5 liters of distilled water in a container and further diluting
it to 10 liters by adding another 5 liters of distilled water. The pH of the solution was adjusted to 6.8 with NaOH
and HCl. Hundred ml of quarter-strength N-free nutrient solution was supplied to each pot once every week. All
the pots were watered every two days.
Harvesting the plants and assessing symbiotic effectiveness
The plants were harvested 7 weeks after inoculation, within the time range (6-8 weeks) as indicated by Lupwayi
and Haque (1994).The shoots were collected by cutting the plants at the level of the sand. Then shoots from each
growth unit were placed in paper bags and dried at 70
O
C for 48 hours as described by Somasegaran and Hoben
(1994) and their dry weight was determined. The roots and adhering sand were dislodged in to a coarse sieve
(0.76mm) and were washed with a gentle tap water and observed for nodules. The nodules were collected,
counted and their dry weight was determined in the same way as the shoots. The relative effectiveness of isolates
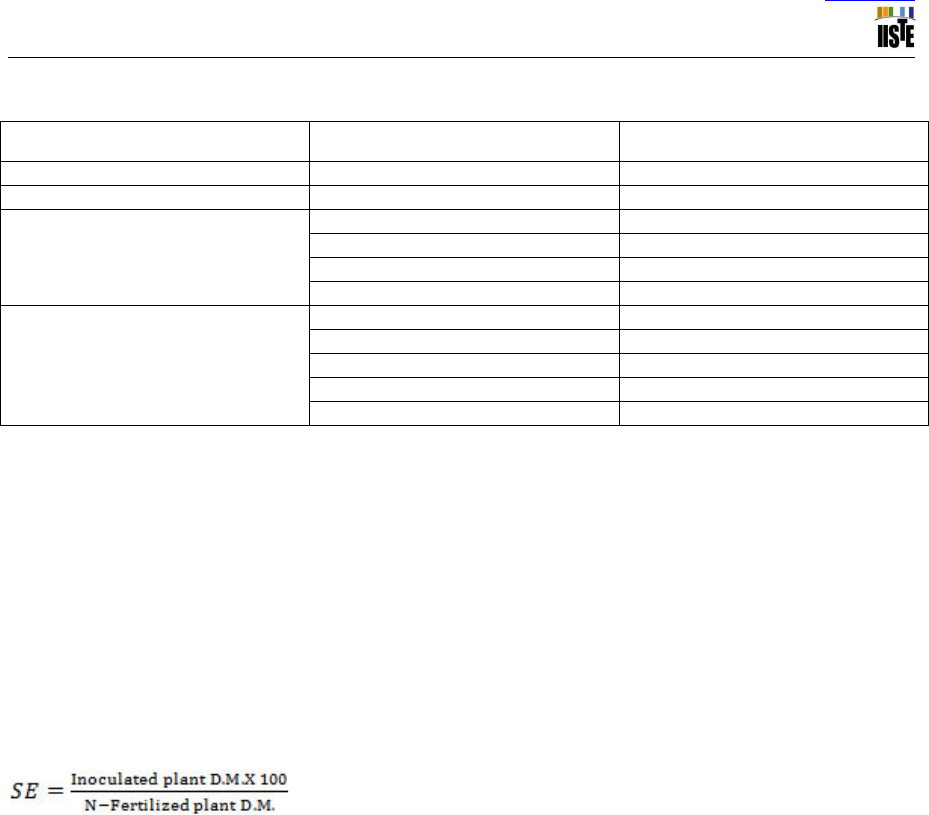
in accumulating plant shoot dry matter was calculated as described in Somasegaren and Hoben (1984) as follows:
, Where, D.M. = dry matter, S.E. = symbiotic effectiveness
The rate of nitrogen fixing effectiveness is evaluated as: Highly effective > 85%, Effective 55-85%, Lowly
effective 35-54% and Ineffective <35%.
Conclusion
Goal of Rhizobium scientists is to discover new and better strains for use in legume inoculants. This pursuit
entails the collection of isolates, strain characterization, assessment of symbiotic capacity and comparison to
strains currently included within inoculants. From this review, it can be concluded that the selection of highly
performed isolates using different consecutive steps of identification and authentication are worthy of further
investigation of field try for rhizobilogical science. Symbiotic performance is a key but the ability of rhizobia to
survive stress conditions or to utilize less expensive growth media are also important considerations. The process
of Rhizobium exploration and characterization is somewhat arduous, and efforts must remain focused upon
relatively few legumes of interest in an unbiased manner so that elite strains emerging from a work must be
recognizably superior.
Reference
Amarger, N., Macheret, V. and Aguerre, G. (1997). Rhizobium gallicum sp. nov. and Rhizobium giardinii sp.
nov., from Phaseolus vulgaris Nodules Int. J.
Syst. Bacteriol. 47:996–1006.Bala, A., Abaidoo, R. and Woomer, P. (2010). Rhizobia Strain Isolation and
Characterisation Protocol, www.N2Africa.org, p.16.
Bernal, G. and Graham, P.H. (2001). Diversity in the rhizobia associated with Phaseolus vulgaris L. in Ecuadore,
and comparisons with Mexican bean rhizobia. Can. J. Microbiol. 47: 526-534.
Broughton, W.J. and Dilworth, M.J. (1970). Control of leghaemoglobin synthesis in Snake beans. Biochemistry
Journal 125: 1075-1080.
Jordan, D. (1984). Rhizobaceae. In: Bergey's Manual of Systematic Bacteriology, pp. 234-256 (Hendricks, D.P.,
Sneath, H.A. and Halt, J.H., eds). Orient Longman, New York.
Lupwayi, N. and Haque, I. (1994). Legume-Rhizobium Technology Manual: Environmental sciences division,
international livestock center for Africa, Addis Ababa, Ethiopia. pp. 1-93.

Developing Country Studies www.iiste.org
ISSN 2224-607X (Paper) ISSN 2225-0565 (Online)
Vol.6, No.1, 2016
93
Martinez-Romero, E., Segovia, L., Mercante, F. M., Franco, A. A., Graham, P. and Pardo, M. A. (1991).
Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L beans and Leucaena sp trees. Int. J.
Syst. Bacteriol. 41:417-426.
Solomon Legesse and Fassil Assefa (2014). Symbiotic and phenotypic characteristics of rhizobia nodulating faba
bean (vicia faba) from tahtay koraro, northwestern zone of Tigray Regional State, Ethiopia. IJTEEE:
2(11), 15-23.
Somasegaran, P. and Hoben, H. J. (1985). Hand Book for Rhizobia – Methods in Legume Rhizobium Technology.
Springer-Verlag, Heidelberg, Germany.Somasegaran, P. and Hoben, H.J. (1994). Handbook for
Rhizobia. Springer-Verlag, p.380.
Vincent, J.M. (1970). A Manual for the Practical Study of Root Nodule Bacteria. Blackwell, Oxford and
Edinburgh, pp.164.
White, D. (1995). The physiology and biochemistry of prokaryotes. Oxford University Press, Oxford, pp.378.
