1
General Chemistry II Jasperse
Intermolecular Forces, Ionic bond strength, Phase Diagrams, Heating Curves. Extra Practice Problems
1. Rank the ionic bond strength for the following ionic formulas, 1 being strongest:
Al
2
S
3
MgO MgCl
2
NaCl
Strategy: Identify ion charges.
2. Rank the lattice energy (ionic bond strength) for the following formulas, 1 being strongest:
LiF NaF NaCl NaI KI
Strategy: When Charges are Equal, Use Ion Size to Break Ties.
3. Rank the ionic bond strength for the following ionic formulas, 1 being strongest:
Na
2
O NaBr LiCl Fe
3
N
2
CaO
Strategy: Charge is more important than Ion Size. Use Ion size only to Break Ties.
4. Arrange the following compounds in order of increasing attraction between their ions:
MgO CaO BaO.
5. Which of the following will require the greatest energy input to separate the ions?
a.
MgI
2
d.
MgBr
2
b.
MgF
2
e.
NaCl
c.
MgCl
2
6. Which of the following will have the highest melting point?
a.
NaF
d.
NaI
b.
NaCl
e.
CsCl
c.
NaBr
7. Which of the following requires the lowest melting point?
a.
CaF
2
d.
MgF
2
b.
KCl
e.
LiCl
c.
NaCl
2
8. Arrange the three compounds sodium chloride, magnesium chloride, and aluminum chloride in
order of increasing melting point.
a. NaCl < MgCl2 < AlCl3
b. MgCl2 < NaCl < AlCl3
c. AlCl3 < NaCl < MgCl2
d. AlCl3 < MgCl2 < NaCl
e. NaCl < AlCl3 < MgCl2
9. Rank the attractive power for water to the following, 1 being strongest:
Mg
2+
Na
+
H-Br N
2
10. Ion–dipole forces always require
a.
an ion and a water molecule.
d.
an ion and a polar molecule.
b.
a cation and a water molecule.
e.
a polar and a nonpolar molecule.
c.
an anion and a polar molecule.
11. Classify each of the following as polar (molecular), completely nonpolar (molecular), weakly polar
(molecular), ionic, or metallic.
a. CO
2
b. CH
3
OH
c. O
2
d. NH
3
e. CH
2
Cl
2
f. PCl
3
g. CO
h.
i. SiCl
4
j. Fe
k. NaCl
12. Which of the following compounds is capable of dipole–dipole interactions?
a. CH
4
d. SF
6
b. CO
2
e. NH
4
+
c. H
2
CO
13. Which of the following compounds is capable of hydrogen bonding?
a.
CH
3
OCH
3
d.
H
2
CO
b.
CH
3
COCH
3
e.
CH
3
F
c.
CH
3
CH
2
OH
H
C
H
O
3
14. Based on their boiling points, which of the following compounds has the largest dipole–dipole
interaction? (They are all molecular, variably polar, but without hydrogen-bonding.)
a.
propane (231 K)
d.
methyl chloride (249 K)
b.
dimethyl ether (248 K)
e.
butane (135 K)
c.
acetonitrile (355 K)
15. Classify as having network versus molecular bonding:
a. CH
3
CH
2
CH
2
CH
2
CH
2
SH
b. P(CH
3
)
3
c. K
3
PO
4
d. C
3
H
7
OH
e. Diamond
f. CH
3
CH
2
CH
2
CH
2
Cl
g. CH
3
CH
2
NHCH
2
CH
3
h. H-N=O
i. Fe
2
O
3
j. CO
k. Zn
l. NH
3
16. Which of the following polar compounds is likely to have the highest boiling point?
a.
CH
3
OCH
3
d.
H
2
CO
b.
CH
3
CH
2
OH
e.
CO
c.
(CH
3
)
2
CO
17. Which of the following shows a “hydrogen bond”?
18. Which of the following will have hydrogen bonding?
CH
3
CH
2
CH
2
OH CH
3
CH
2
OCH
3
CH
3
CH
2
NH
2
CH
3
CH
2
SH
C
H O
O
H N
N
H C
H C
N
H N
O
H O
O
H C
C
H O
N
H C
O
H S
S
H O
O
H N

4
19. Rank the following in terms of increasing boiling point: LiCl C
3
H
7
OH C
4
H
8
N
2
a. N
2
< LiCl < C
3
H
7
OH < C
4
H
8
b. LiCl < C
4
H
8
< C
3
H
7
OH < N
2
c. N
2
< C
4
H
8
< C
3
H
7
OH < LiCl
d. LiCl < C
4
H
8
< N
2
< C
3
H
7
OH
e. C
3
H
7
OH < C
4
H
8
< N
2
< LiCl
20. Which is higher boiling, and why? (Both have the same formula, C3H8O)
CH
3
CH
2
OCH
3
CH
3
CH
2
CH
2
OH
21. Rank the boiling points for the following, 1 being highest:
Cl
2
Br
2
I
2
22. Rank the melting points for the following, 1 being highest:
Ca(OH)
2
CH
3
CH
2
CH
2
OH CH
3
CH
2
CH
2
CH
2
OH CH
3
CH
2
OCH
3
23. Rank the evaporation rate (1 being highest)
CH
3
CH
2
CH
2
CH
2
CH
2
OH CH
3
CH
2
CH
2
OH CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
OCH
3
24. Rank the melting points for the following, 1 being highest:
CH
3
CH
2
CH
2
NH
2
LiCl N(CH
3
)
3
CH
3
CH
2
CH
2
CH
2
CH
2
NH
2
25. Rank the following in terms of increasing boiling point:
C
4
H
9
OH C
2
H
5
OH C
4
H
10
CaBr
2
a. C
4
H
10
< CaBr
2
< C
2
H
5
OH < C
4
H
9
OH
b. CaBr
2
< C
4
H
10
< C
2
H
5
OH < C
4
H
9
OH
c. C
4
H
10
< C
4
H
9
OH < C
2
H
5
OH < CaBr
2
d. C
2
H
5
OH < C
4
H
10
< C
4
H
9
OH < CaBr
2
e. C
4
H
10
< C
2
H
5
OH < C
4
H
9
OH < CaBr
2

5
26. Rank the following in terms of increasing boiling point:
CH
3
CH
2
OCH
2
CH
3
CH
3
CH
2
OCH
3
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
CH
2
NH
2
a. CH
3
CH
2
OCH
3
< CH
3
CH
2
CH
2
CH
3
< CH
3
CH
2
OCH
2
CH
3
< CH
3
CH
2
CH
2
CH
2
NH
2
b. CH
3
CH
2
CH
2
CH
3
< CH
3
CH
2
OCH
3
< CH
3
CH
2
OCH
2
CH
3
< CH
3
CH
2
CH
2
CH
2
NH
2
c. CH
3
CH
2
CH
2
CH
2
NH
2
< CH
3
CH
2
OCH
2
CH
3
< CH
3
CH
2
OCH
3
< CH
3
CH
2
CH
2
CH
3
d. CH
3
CH
2
CH
2
CH
2
NH
2
< CH
3
CH
2
CH
2
CH
3
< CH
3
CH
2
OCH
3
< CH
3
CH
2
OCH
2
CH
3
27. For F
2
, C
3
H
7
OH, and Na
2
O, which of the following is true at room temperature?
a. F
2
is a gas, C
3
H
7
OH is a liquid, and Na
2
O is a solid
b. Na
2
O is a gas, F
2
is a liquid, and C
3
H
7
OH is a solid
c. F
2
is a gas, Na
2
O is a liquid, and C
3
H
7
OH is a solid
d. C
3
H
7
OH is a gas, F
2
is a liquid, and Na
2
O is a solid
28. For CO
2
, Zn(NO
3
)
2
, and C
5
H
11
NH
2
, which of the following is true at room temperature?
a. Zn(NO
3
)
2
is a gas, CO
2
is a liquid, and C
5
H
11
NH
2
is a solid
b. CO
2
is a gas, C
5
H
11
NH
2
is a liquid, and Zn(NO
3
)
2
is a solid
c. CO
2
is a gas, Zn(NO
3
)
2
is a liquid, and C
5
H
11
NH
2
is a solid
d. C
5
H
11
NH
2
is a gas, CO
2
is a liquid, and Zn(NO
3
)
2
is a solid
29. The highest vapor pressure is observed for which of the following liquid/temperature
combinations?
a. C
6
H
14
at 275 K
b. C
6
H
14
at 299 K
c. C
5
H
12
at 299 K
d. HOC
4
H
8
OH at 299 K
e. HOC
4
H
8
OH at 275 K
30. Which of the following liquids would have the lowest vapor pressure, factoring in both the impact
of the substance and the temperature?
a. CH
3
NH
2
at 25˚C
b. SiH
4
at 75˚C
c. SiH
4
at 25˚C
d. C
3
H
7
NH
2
at 25˚C
e. C
3
H
7
NH
2
at 75˚C

6
31. Which of the following liquids would have the lowest viscosity, factoring in both the impact of the
substance and the temperature?
a. C
3
H
7
OH at 25˚C
b. C
3
H
7
OH at 75˚C
c. MgBr
2
at 25˚C
d. C
5
H
11
OH at 25˚C
e. C
5
H
11
OH at 75˚C
32. CH
2
F
2
has a dipole moment of 1.93 D and a boiling point of –52°C. CH
2
Cl
2
has a dipole moment of 1.60 D
and a boiling point of 40°C. Why is the boiling point of dichloromethane 92º higher than that of
difluoromethane? Which of the following explains why dichloromethane has the higher boiling point?
a. CH
2
F
2
is more polar and thus must have stronger binding forces. With stronger intermolecular
attraction, of course CH
2
F
2
will have a lower boiling point.
b. CH
2
Cl
2
is ionic while CH
2
F
2
is molecular.
c. CH
2
Cl
2
has hydrogen-bonding while CH
2
F
2
does not.
d. That CH
2
Cl
2
has a higher boiling point proves that is has stronger intermolecular attractions, even
though CH
2
F
2
has a larger dipole moment. Evidently CH
2
Cl
2
has larger London dispersion attraction,
which is more than making up for it’s smaller permanent dipole.
33. HCl (mw=36.5) has a dipole moment of 1.03 D and a boiling point of 190K. HBr (mw=80.9) has a dipole
of 0.79 D and a boiling point of 206K. Which of the following statements is true?
a. HBr is more polar.
b. HCl has stronger intermolecular forces.
c. HCl has stronger London dispersion forces
d. Both molecules have hydrogen bonding.
e. That HBr
has a higher boiling point proves that it is has stronger intermolecular attractions, despite it’s
lesser dipole moment. Evidently with its extra mass it has much stronger
London dispersion attraction,
enough so to overcome the dipole advantage of HCl.
34. Hexane, C
6
H
14
(mw=86) has a boiling point of 68º. Ethanol, CH
3
CH
2
OH (mw=46) has a boiling point of
78º. Mark each of the following statements as TRUE or FALSE.
a. Ethanol must have stronger intermolecular attraction, based on its higher boiling point.
b. Ethanol has a higher boiling point because of greater London dispersion force
c. Both hexane and ethanol have hydrogen bonding.
d. Ethanol has a higher boiling point due to hydrogen bonding.
e. Hydrogen bonding and London dispersion forces are at cross purposes here. (One favors ethanol, the
other favors hexane.) In this case, the hydrogen bonding evidently “wins”.
35. Viscosity is a measure of a substance’s __________
a.
ability to resist changes in its surface area.
d.
compressibility.
b.
surface tension.
e.
color.
c.
resistance to flow.
7
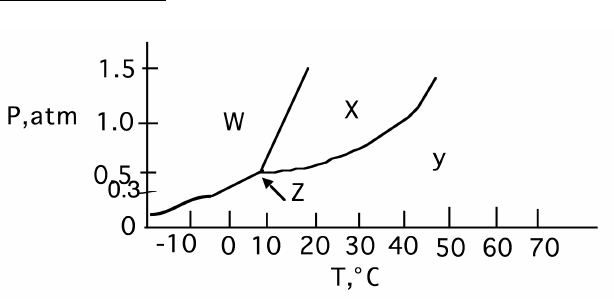
Phase Diagrams
36. Which letter represents:
a. Gas Phase
b. Liquid
c. Solid
d. Triple Point
37. What is the normal bp?
a) 20°
b) 40°
c) 65°
d) 80°
38. What is the normal mp?
a) 12°
b) 40°
c) 80°
39. When a liquid originally at 20° and 1 atm has pressure reduced, at what pressure will it vaporize?
40. When a liquid originally at 20° and 1 atm has pressure increased, at what pressure will it solidify?
41. When solid at 1.0 atm is warmed, does it: a) melt or b) sublime
42. When solid at 0.3 atm is warmed, does it: a) melt or b) sublime
43. Suppose a solid is originally at 0.3 atm and 0ºC. If it is first pressurized to 1.0 atm, and then
subsequently heated to 60ºC, what will happen to it?
a. It will sublime directly to gas
b. It will melt and end up as a liquid
c. It will first melt, and then boil, ending up as a gas
d. It will sublime to gas, then compress to a liquid and end up in the liquid phase
e. No phase change will happen. It will just stay solid.
44. Suppose a solid is originally at 0.3 atm and 0ºC. If it is first heated to 30ºC, then pressurized to 1.0
atm, what will happen to it?
a. It will sublime directly to gas and stay a gas.
b. It will melt and end up as a liquid
c. It will first melt, and then boil, ending up as a gas
d. It will sublime to gas first, then compress to a liquid and end up in the liquid phase
e. No phase change will happen. It will just stay solid.
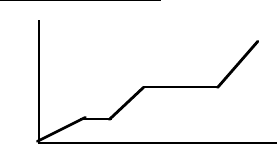
8
Heating Curves
45. Which regions on the on the heating curve shown (Temperature versus heat, “q”) corresponds to:
a. a pure gas increasing in temperature
b. a liquid increasing in temperature
c. a solid increasing in temperature
d. a solid melting
e. a liquid boiling
46. On the above heating curve, which phases are present:
a. in region “a”
b. in region “b”
c. in region “c”
d. in region “d”
e. in region “e”
a
b
c
d
e
q
T
9
General Chemistry II Jasperse
Intermolecular Forces, Ionic bond strength, Phase Diagrams, Heating Curves. Extra Practice Problems
Answers
1. 1-2-3-4 (Al
2
S
3
> MgO > MgCl
2
>NaCl) Ion charge
2. 1-2-3-4-5 (LiF > NaF > NaCl > NaI > KI (Ion size)
3. 3-5-4-1-2 (Fe
3
N
2
> CaO > Na
2
O > LiCl > NaBr) (Ion size first, then size as tiebreaker)
4. 1-2-3 (MgO > CaO > BaO)
5. b
6. a
7. b (this is for corrected version of question. Original version had a confusion factor included)
8. a
9. 1-2-3-4 (Mg
2+
> Na
+
> H-Br > N
2
)
10. d
11. Classify
a. Nonpolar
b. Polar
c. Nonpolar
d. Polar
e. Weakly polar
f. Polar
g. Polar
h. Polar
i. Nonpolar
j. Metal
k. Ionic
12. C
13. C
14. C
15. Classify
a. Molecular
b. Molecular
c. Network
d. Molecular
e. Network
f. Molecular
g. Molecular
h. Molecular
i. Network
j. Molecular
k. Network
l. Molecular
16. B
17. Which show a “hydrogen bond”
–N
…..
H-N-
–O
…..
H-N- –O
…..
H-O-
–O
…..
H-N-
18. CH
3
CH
2
CH
2
OH, CH
3
CH
2
NH
2
19. C
20. CH
3
CH
2
CH
2
OH
21. 3-2-1
10
22. 1-3-2-4 (Ca(OH)
2
> CH
3
CH
2
CH
2
CH
2
OH > CH
3
CH
2
CH
2
OH > CH
3
CH
2
OCH
3
)
23. 4-3-1-2 (CH
3
CH
2
CH
2
CH
3
> CH
3
CH
2
OCH
3
> CH
3
CH
2
CH
2
OH > CH
3
CH
2
CH
2
CH
2
CH
2
OH)
24. 3-1-4-2 (LiCl > CH
3
CH
2
CH
2
CH
2
CH
2
NH
2
> CH
3
CH
2
CH
2
NH
2
> N(CH
3
)
3
)
25. e
26. b
27. a
28. b
29. c
30. d
31. b
32. d
33. e
34. a, d, and e are all true.
35. c
36. Y-X-W-Z
37. B
38. A
39. About 0.5 atm
40. About 1.5 atm
41. Melt
42. Sublime
43. C
44. d
45. e-c-a-b-d
46.
a. solid
b. solid + liquid
c. liquid
d. liquid + gas
e. gas
