
May 2016 | Volume 7 | Article 491
ORIGINAL RESEARCH
published: 27 May 2016
doi: 10.3389/fendo.2016.00049
Frontiers in Endocrinology
| www.frontiersin.org
Edited by:
Nicholas Michael Morton,
University of Edinburgh, Scotland
Reviewed by:
Maximilian Zeyda,
Medical University of Vienna, Austria
Matthew Brook,
University of Edinburgh, UK
*Correspondence:
Antonio Brunetti
Diego Russo
Specialty section:
This article was submitted
to Genomic Endocrinology,
a section of the journal
Frontiers in Endocrinology
Received: 24March2016
Accepted: 09May2016
Published: 27May2016
Citation:
LombardoGE, ArcidiaconoB,
DeRoseRF, LeporeSM, CostaN,
MontalciniT, BrunettiA, RussoD,
DeSarroG and CelanoM (2016)
Normocaloric Diet Restores Weight
Gain and Insulin Sensitivity
in Obese Mice.
Front. Endocrinol. 7:49.
doi: 10.3389/fendo.2016.00049
Normocaloric Diet Restores Weight
Gain and Insulin Sensitivity
in Obese Mice
Giovanni Enrico Lombardo
1
, Biagio Arcidiacono
1
, Roberta Francesca De Rose
1
,
Saverio Massimo Lepore
1
, Nicola Costa
1
, Tiziana Montalcini
2
, Antonio Brunetti
1
*,
Diego Russo
1
*, Giovambattista De Sarro
1
and Marilena Celano
1
1
Department of Health Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy,
2
Department of Medical and
Surgical Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy
An increased incidence of obesity is registered worldwide, and its association with insulin
resistance and type 2 diabetes is closely related with increased morbidity and mortality
for cardiovascular diseases. A major clinical problem in the management of obesity is the
non-adherence or low adherence of patients to a hypocaloric dietetic restriction. In this
study, we evaluated in obese mice the effects of shifting from high-calorie foods to nor-
mal diet on insulin sensitivity. Male C57BL/6JOlaHsd mice (n=20) were fed with high fat
diet (HFD) for a 24-week period. Afterward, body weight, energy, and food intake were
measured in all animals, together with parameters of insulin sensitivity by homeostatic
model assessment of insulin resistance and plasma glucose levels in response to insulin
administration. Moreover, in half of these mice, Glut4 mRNA levels were measured in
muscle at the end of the high fat treatment, whereas the rest of the animals (n=10) were
shifted to normocaloric diet (NCD) for 10weeks, after which the same analyses were
carried out. A signicant reduction of body weight was found after the transition from
high to normal fat diet, and this decrease correlated well with an improvement in insulin
sensitivity. In fact, we found a reduction in serum insulin levels and the recovery of insulin
responsiveness in terms of glucose disposal measured by insulin tolerance test and
Glut4 mRNA and protein expression. These results indicate that obesity-related insulin
resistance may be rescued by shifting from HFD to NCD.
Keywords: insulin resistance, obesity, Glut4, diet, glucose
INTRODUCTION
Modern lifestyle is oen characterized by sedentary activities and overeating. As a consequence,
in the last decades, this has been responsible for the increased incidence and prevalence of obesity
and obesity-induced comorbidities, such as insulin resistance and metabolic syndrome (1, 2) that
may contribute to type 2 diabetes mellitus (T2DM) and cardiovascular disease (3). Several studies
have demonstrated that a healthy lifestyle can lead to weight loss and improve insulin sensitivity
(4–7). In this regard, a crucial role is played by the nutrient composition of the diet, both in terms
of total caloric intake and the variety of its components, with particular attention to the dierent
types of fatty acids (8, 9). Unfortunately, most of anti-obesity interventions are oen limited by
the diculty to maintain a low-calorie dietary regimen, especially when long-term treatments are
required (10,11). us, few anti-obesity programs have been found to be helpful.
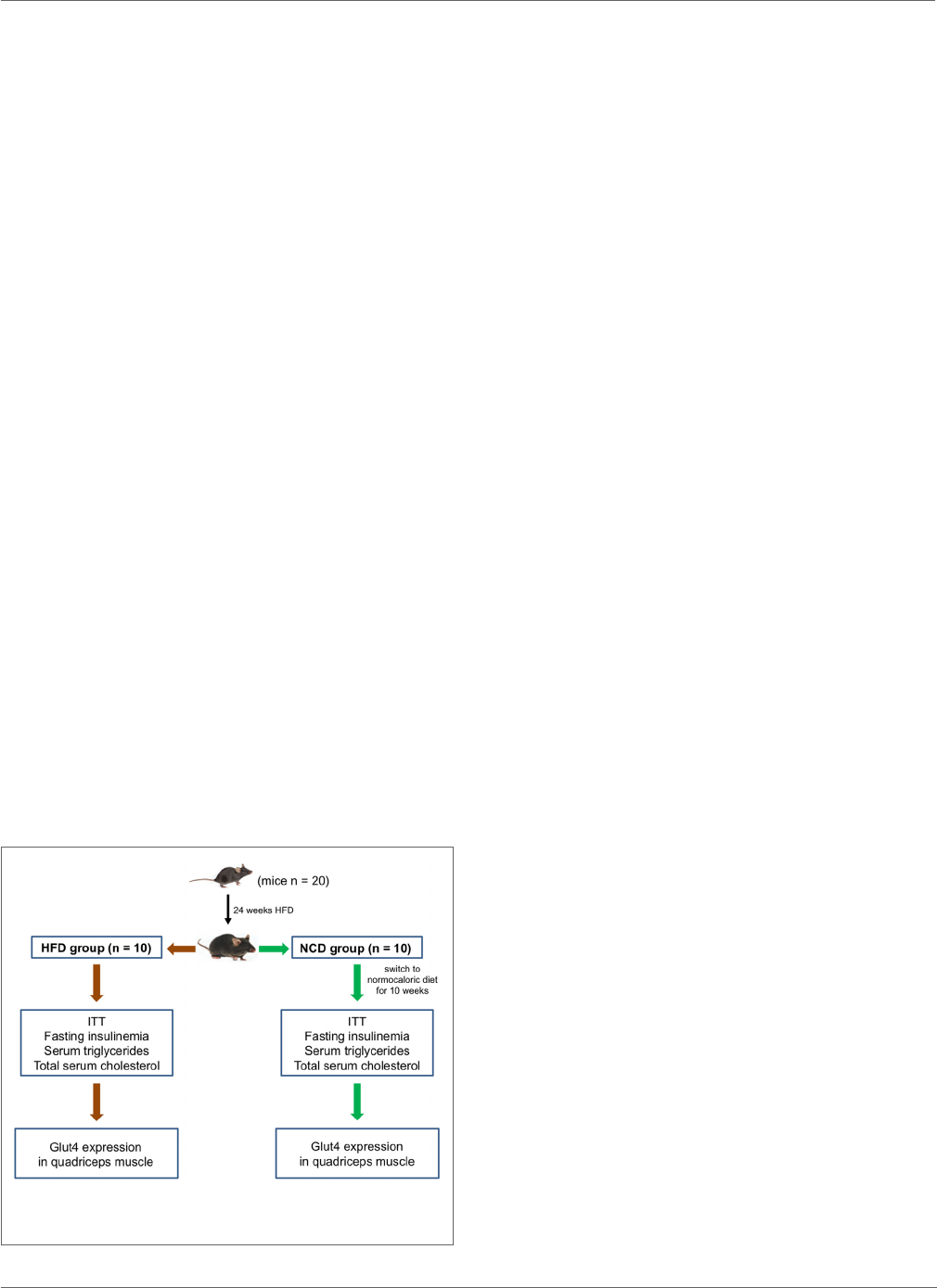
FIGURE 1 | Study design. A schematic representation of the study protocol
and experimental plan is shown.
2
Lombardo et al.
Normocaloric Diet in Obese Mice
Frontiers in Endocrinology | www.frontiersin.org May 2016 | Volume 7 | Article 49
To date, several animal models have been used to evaluate the
eects of various dietetic regimens on body weight and meta-
bolic parameters. A validated experimental model is represented
by mice fed with a high fat diet (HFD), which develop obesity,
insulin resistance, and dyslipidemia (8–13).
In the present study, we evaluated the eects of the transition
from HFD to normocaloric diet (NCD) (regular food with no
additive agents or nutraceutical compounds) on body weight and
insulin responsiveness in C57BL/6JOlaHsd mice, a strain of mice
genetically prone to develop obesity and insulin resistance (14).
MATERIALS AND METHODS
Animals and Study Design
Five-week-old male C57BL/6JOlaHsd mice (n=20), NCD and
HFD, were purchased from Harlan Laboratories S.r.l (Udine,
Italy). Mice were housed in individual cages and maintained on
12-h light/dark cycle at 21±1°C and 50±5% humidity with
free access to water and food adlibitum. Animals were fed with
HFD containing 60.3% kcal fat, 21.3% kcal carbohydrate, and
18.4% kcal protein (HFD group) for 24weeks. Aer this period,
10 mice were euthanized by cervical dislocation and the other
10 were fed with NCD only (Teklad Global 18% kcal fat, 58%
kcal carbohydrate, and 24% kcal protein) (NCD group) for the
subsequent 10weeks. A schematic representation of the study
design is shown in Figure1. Body weight, girth waist, and food
intake were recorded at weekly interval for all animals (15). Liver,
skeletal muscle, and abdominal fat were excised, weighted, and
stored in liquid nitrogen. is study was performed following the
Italian (D.M. 116/92) and ECC regulations (O.J. of E.C.L 358/1
12/18/1986), in accordance with the guide for the care and use of
laboratory animals and approved by the local ethical committee.
Biochemical Analysis
Blood samples were collected aer 12h of fasting. Serum was sep-
arated by centrifugation at 1700g for 10min at room temperature
and stored at −20°C, until use. Total cholesterol and triglycerides
were measured using commercial reagents (Siemens Healthcare
Diagnostics, Milano, Italy) and an automated biochemistry ana-
lyzer (Dimension EXL, Siemens Healthcare Diagnostics). Insulin
levels were measured using ELISA kit (Rat/Mouse Insulin ELISA
Kit, EMD Millipore Corporation, Darmstadt, Germany), accord-
ing to the manufacturers’ instructions.
Insulin Tolerance Test
Insulin tolerance test (ITT) was performed in both HFD and
NCD groups, as previously described (16). Animals were fasted
for 12 h, weighed, and injected intraperitoneally with insulin
(1U/kg body weight Regular
®
, Novorapid, Novonordisk, Roma,
Italy). Blood glucose levels were measured aer 0, 15, 30, 60, and
90 min using an automatic glucometer (Glucocard, Menarini
Diagnostics, Firenze, Italy).
Expression of Glucose Transporter Type 4
Total RNA was isolated from quadriceps skeletal muscle using
TRIzol reagent (Life Technologies, Monza, Italy), following the
manufacturer’s recommended protocol and quantied with a
NanoDrop Spectrophotometer (ermo Fisher Scientic, Inc.,
Waltham, MA, USA). RNA levels were normalized against 18S
ribosomal RNA in each sample, and cDNAs were synthesized
from 1μg of total RNA using the High Capacity cDNA Reverse
Transcription Kit (Life Technologies). Primers for mouse Glut4
and ribosomal protein S9 (RPS9) were designed according to
sequences from the GenBank database. Relative quantication was
made using a real-time thermocycler (Eppendorf Mastercycler ep
realplex, Milano, Italy). In a 20-μl nal volume, 1μl of cDNA solu-
tion was mixed with SYBR Green RealMasterMix (Eppendorf)
and 0.2μM of each sense and antisense primers. SYBR Green
uorescence was measured, and relative quantication was made
against either RPS9 or Gapdh cDNAs, used as internal standards.
All PCR reactions were carried out in triplicates. Glut4 protein
expression was measured in quadriceps muscle from six to eight
mice of each group, using a rabbit anti-Glut4 polyclonal antibody
as previously described (17).
Statistical Analysis
Results are expressed as mean±SD. e independent t-test was
used to evaluate intergroup dierences. All statistical analyses
were performed using GraphPad Prism version 5.0 statistical
soware (GraphPad Soware Inc., San Diego, CA, USA). p values
lower than 0.05 were considered statistically signicant.
RESULTS
Effects of Normocaloric Diet on Body
Weight and Biochemical Parameters
Twenty mice were fed with HFD (HFD group) for 24weeks, reach-
ing a weight of approximately 43g, with fasting plasma glucose
levels between 90.5 and 117.7mg/dL, which were consistent with
a condition of impaired fasting glucose. Aer the 24-week period,
half of the mice were fed with NCD for the following 10weeks
(NCD group). A signicant decrease of body weight was observed
in the NCD group compared to the HFD group (27%, p<0.001),
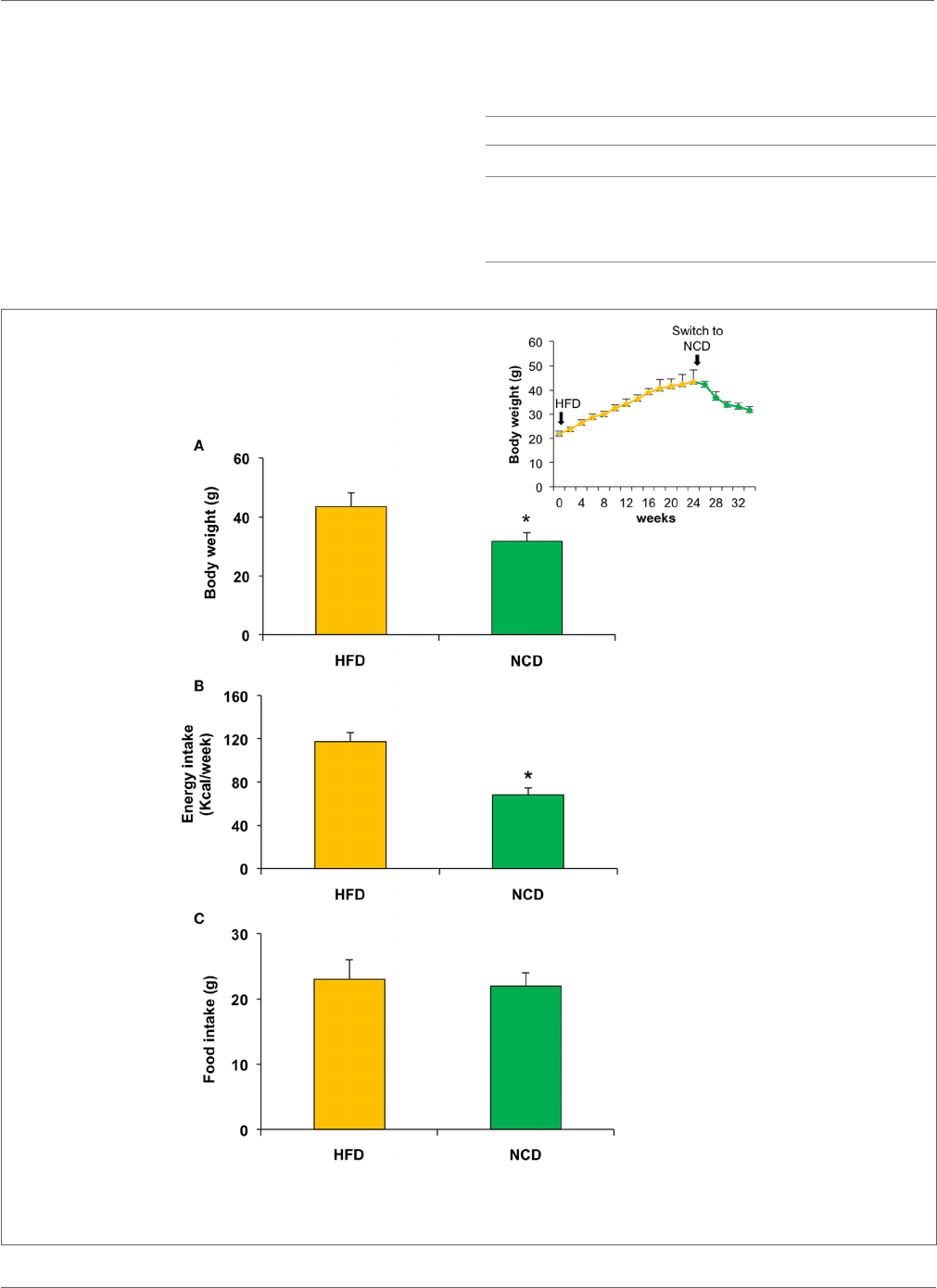
TABLE 1 | Weight and waist in HFD and NCD mice.
HFD NCD p value
Liver (g)
1.24±1.09 1.09±0.09 <0.05
White adipose (g) 1.54±0.31 1.29±0.08 <0.05
Epididymis (g) 0.60±0.16 0.33±0.08 <0.01
Girth waist (cm) 10.55±0.42 9.54±0.17 <0.01
Values are expressed as mean±SD.
FIGURE 2 | Effects on body weight, food, and energy intake in mice fed with high fat diet (HFD) for 24weeks and with normocaloric diet (NCD) for
other 10weeks. A signicant reduction of body weight and energy intake was observed in NCD mice (
A,B), whereas no signicant difference was detected in food
intake (
C). Body weight over the time is shown in the inset. Values are expressed as mean±SD. *p<0.001.
3
Lombardo et al.
Normocaloric Diet in Obese Mice
Frontiers in Endocrinology | www.frontiersin.org May 2016 | Volume 7 | Article 49
as a result of the decrease in energy intake due to the less caloric
supply derived from the NCD rather than the dierent food
intake (Figure2). In the NCD group, we also observed a decrease
in liver size, fat depots, and girth waist (Table1). Moreover, shi-
ing to NCD resulted in a signicant decrease in plasma glucose
levels (p<0.05) and serum insulin levels (p<0.01), as well as
triglycerides (p<0.05) and total cholesterol (p<0.05) (Figure3).
Effects on Insulin Sensitivity
Next, we evaluated the eects of NCD on insulin sensitiv-
ity. ITT performed in mice before and aer NCD showed a
better response to insulin in terms of changes in blood glucose
concentrations in the NCD group than in the HFD group.
In fact, the glucose-lowering eect of exogenous insulin was
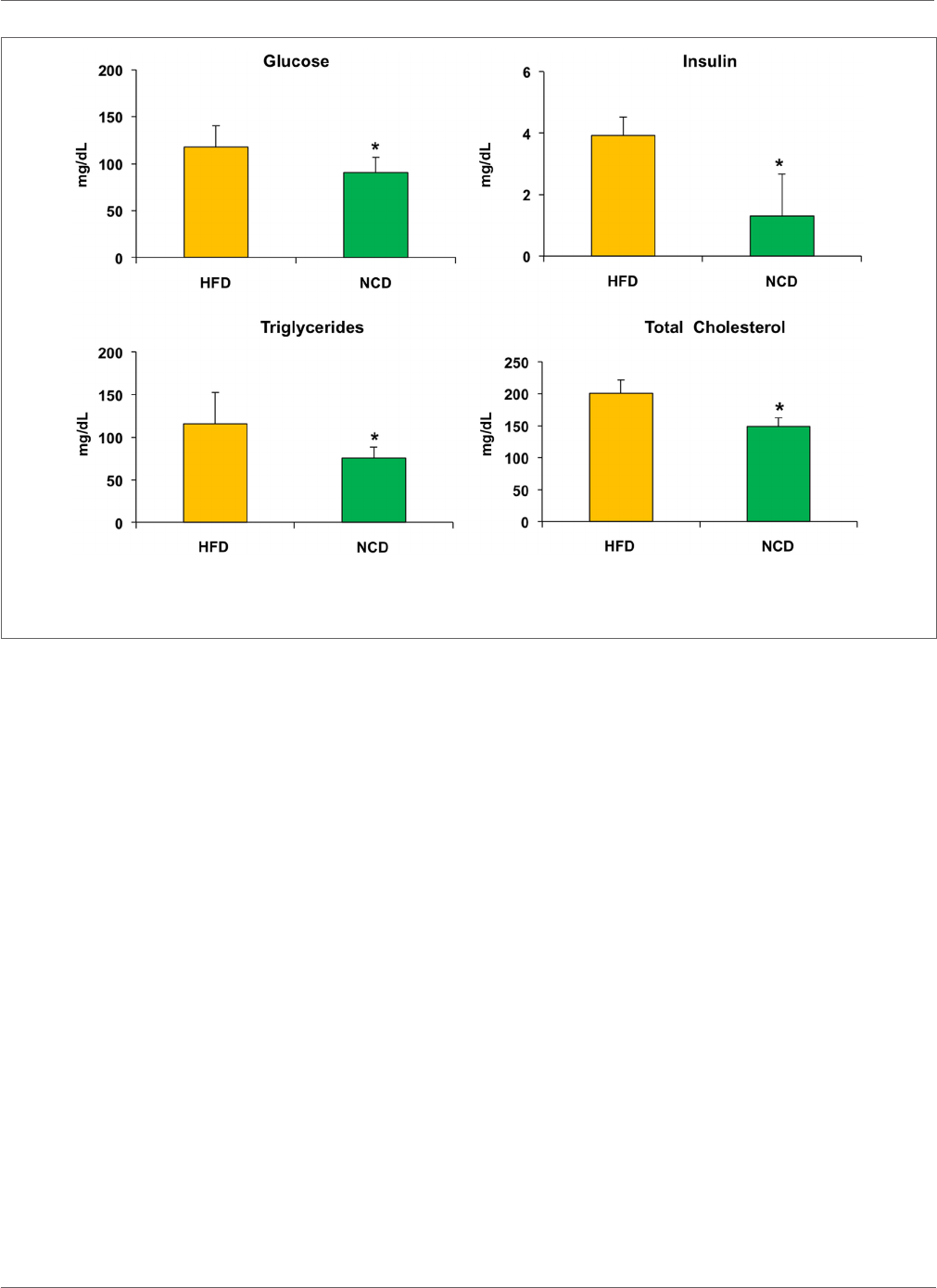
FIGURE 3 | Biochemical parameters. Blood samples were collected as indicated in Section “Materials and Methods.” After 10weeks of feeding with a
normocaloric diet (NCD), mice showed a signicant reduction of plasma glucose levels and serum insulin levels, as well as a reduction in both triglycerides and total
cholesterol when compared to the HFD. Values are expressed as mean±SD. *p<0.05.
4
Lombardo et al.
Normocaloric Diet in Obese Mice
Frontiers in Endocrinology | www.frontiersin.org May 2016 | Volume 7 | Article 49
enhanced in NCD mice during ITT and was reduced in HFD
mice (Figure4). From a mechanistic point of view, the improve-
ment in insulin sensitivity in mice in response to NCD was
dependent, at least in part, on an increase in Glut4 expression
induced in skeletal muscle following the transition from HFD
to NCD. To show such a molecular link between restoration
of insulin sensitivity and NCD, total RNA was extracted from
skeletal muscle of animals before and aer shiing to NCD, aer
insulin stimulation, and Glut4 mRNA and protein levels were
measured. As shown in Figure5, both insulin-stimulated Glut4
mRNA and protein expression were signicantly increased in
skeletal muscle of NCD mice as compared with that of HFD
mice (p<0.05).
DISCUSSION
Obesity is a chronic disorder that can cause other health prob-
lems, such as diabetes, hypertension, hepatic steatosis, obstruc-
tive sleep apnea, and atherosclerosis (18). e association of
obesity with T2DM is well established, due to the negative
inuence of excessive body fat on peripheral insulin action and
hepatic function, leading to insulin resistance (19). Treatment
of obesity includes hypocaloric diet, exercise, and lifestyle
modications, with dietary manipulation still representing the
rst-line therapeutic approach for this common disorder (20,
21). However, it is still debated which is the more appropriated
dietetic regimen to obtain a weight loss, which may be at the
same time rapid, well tolerated, and sustainable for a long period
of time. Although the importance of calorie restriction in this
condition is well recognized, also for the positive psychological
benet for the patient and the family, there is no doubt that
a major problem in treating obesity is still represented by the
relatively low level of adherence of aected subjects to low/very
low-calorie diets (22–24). us, many dietary strategies have
been proposed to overcome such obstacles, but the results are
not satisfactory enough in most of obese patients (25, 26). In
these individuals, we hypothesized that shiing to normoca-
loric balanced diet, formulated to avoid excess fat, rather than
hypocaloric diet – which would obtain a better compliance
especially in view of long-term treatment–might be sucient,
in addition to physical exercise and lifestyle change, to get
more satisfactory results in terms of weight loss and conse-
quent improvement in obesity-related insulin resistance. is
hypothesis is well supported by the present nding in our mouse
model of obesity and obesity-induced insulin resistance. In fact,
shiing from HFD to NCD for 10weeks, caused a signicant
reduction of body weight mainly due to the reduction of vis-
ceral fat, together with the overall reduction of triglycerides,
total cholesterol, and, most importantly, restoration of insulin
sensitivity, as reected by the decline in fasting insulin levels. A
similar approach treating obese mice with NCD has also been
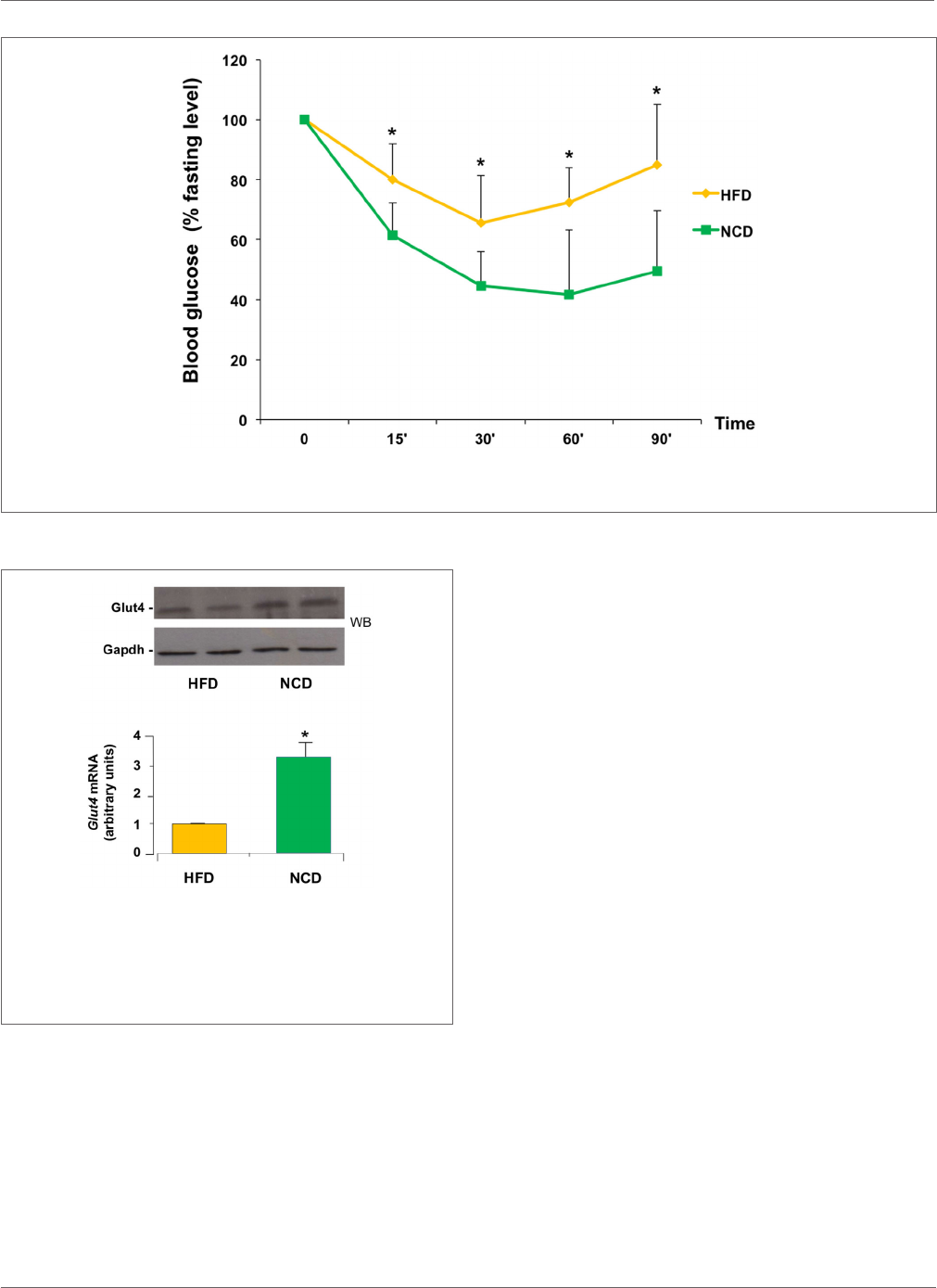
FIGURE 5 | Expression of Glut4. Glut4 mRNA levels were measured by
qRT-PCR in skeletal muscle from HFD and NCD mice, after insulin
stimulation. Results are the mean±SD for six animals per group. *p<0.05
versus HFD mice. A representative Western blot (WB) of Glut4 in quadriceps
muscle from six to eight mice of each group is shown in duplicate in the
autoradiogram. Gapdh, control of protein loading.
FIGURE 4 | Insulin sensitivity. HFD and NCD mice fasted for 12h were injected intraperitoneally with insulin (1U/kg). Blood glucose levels were measured with a
glucometer, as reported in Section “Materials and Methods.” Values are expressed as mean±SD. *p<0.05.
5
Lombardo et al.
Normocaloric Diet in Obese Mice
Frontiers in Endocrinology | www.frontiersin.org May 2016 | Volume 7 | Article 49
used in a few other studies where, however, some nutraceutical
compounds or other ingredients were added to regular food
(27–30). is is slightly dierent than what we did in our study,
in which NCD itself, without any additive agent, was able to
improve insulin sensitivity and Glut4 expression.
Glut4 is the major insulin-dependent glucose transporter in
muscle. Abnormalities at this level are a hallmark of peripheral
insulin resistance (31). In the present study, the improvement
in insulin sensitivity associated with increased Glut4 mRNA
expression in NCD mice provides a possible mechanistic expla-
nation as to how the normal calorie diet can improve insulin
responsiveness and supports the hypothesis that rescue from
insulin resistance and diabetes can be reached without the adop-
tion of a low-calorie diet. If conrmed in obese humans, such
an approach, in association with adequate and individualized
physical exercise programs, might be able to contribute to coun-
teract the long-term failure of the current therapeutic approaches
adopted in these individuals, and this would conrm further the
appropriateness of mouse models for studying human obesity.
However, on the other hand, it is also known that marked inter-
species dierences exist between human and mouse with respect
to behavioral control of food uptake, tissue energy disposal and
storage, weight, and weight loss, which emphasize the inuence
of non-genetic environmental factors and genetic modiers in
determining the phenotypic variations observed in humans and
animal models of obesity. us, caution is required in general-
izing these ndings. As a limitation of the present work, the fact
is that mice of dierent ages were compared in our study.
In conclusion, numerous anti-obesity initiatives have been
adopted up to now, which include lifestyle changes, drug treat-
ments, and surgery. However, because of the limited ecacy
and the occurrence of adverse events in aected treated patients,
alternative and complementary therapies for weight loss have
been investigated, including acupuncture, dietary supplements,
etc. Our ndings in the current work provide valuable informa-
tion about the ecacy of shiing to NCD in restoring weight and
insulin sensitivity in HFD-induced obese mice. Similar studies in
obese humans would reveal whether this strategy, probably better
accepted by patients, may be successful in correcting weight gain
and obesity-related insulin resistance.

6
Lombardo et al.
Normocaloric Diet in Obese Mice
Frontiers in Endocrinology | www.frontiersin.org May 2016 | Volume 7 | Article 49
AUTHOR CONTRIBUTIONS
GEL contributed to animal testing and draing of the manu-
script; RFDR elaborated gures and tables and contributed to
the analysis of the results; SML contributed to animal testing
and draing of the manuscript; BA performed the molecular
analysis; NC performed the operation on the animals and
supervised the animals’ maintenance during the treatment
period; TM and GDS reviewed the nal version of the manu-
script; AB contributed to the conception of the idea and critically
reviewed the manuscript; DR contributed to the conception of
the idea, draed the manuscript, and critically reviewed the nal
manuscript; and MC contributed to animal testing, analysis of
the results, and editing of the manuscript. All authors read and
approved the submitted version.
ACKNOWLEDGMENTS
We thank Professor D. Britti and Dr. F. Trimboli for assistance in
the management of animals.
REFERENCES
1. Lieberman LS. Dietary, evolutionary, and modernizing inuences on the
prevalence of type 2 diabetes. Annu Rev Nutr (2003) 23:345–77. doi:10.1146/
annurev.nutr.23.011702.073212
2.
Forbes JM, Cowan SP, Andrikopoulos S, Morley AL, Ward LC, WalkerKZ,
et al. Glucose homeostasis can be dierentially modulated by varying
individual components of a western diet. J
Nutr Biochem (2013) 24:1251–7.
doi:10.1016/j.jnutbio.2012.09.009
3.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin
resistance and type 2 diabetes. Nature (2006) 444:840–6. doi:10.1038/
nature05482
4.
Paolisso G, Gambardella A, Amato L, Tortoriello R, D’Amore A, Varricchio M,
etal. Opposite eects of short- and long-term fatty acid infusion on insulin
secretion in healthy subjects. Diabetologia (1995) 38:1295–9. doi:10.1007/
BF00401761
5.
Hotamisligil GS. Molecular mechanisms of insulin resistance and the role of
the adipocyte. Int J
Obes Relat Metab Disord (2000) 24:S23–7. doi:10.1038/
sj.ijo.0801497
6.
Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest (2000)
106:171–6. doi:10.1172/JCI10583
7.
Zraika S, Dunlop M, Proietto J, Andrikopoulos S. Eects of free
fatty acids on insulin secretion in obesity. Obes Rev (2002) 3:103–12.
doi:10.1046/j.1467-789X.2002.00062.x
8.
Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the met-
abolic syndrome. Clin Nutr (2004) 23:447–56. doi:10.1016/j.clnu.2004.02.006
9.
Andrikopoulos S. Obesity and type 2 diabetes: slow down! – can meta-
bolic deceleration protect the islet beta cell from excess nutrient-induced
damage? Mol Cell Endocrinol (2010) 316:140–6. doi:10.1016/j.mce.
2009.09.031
10.
Moreno B, Bellido D, Sajoux I, Goday A, Saavedra D, Crujeiras AB.
Comparison of very low-calorie ketogenic diet with a standard low-calorie
diet in the treatment of obesity. Endocrine (2014) 47:793–805. doi:10.1007/
s12020-014-0192-3
11.
Basciani S, Costantini D, Contini S, Persichetti A, Watanabe M, Mariani S,
etal. Safety and ecacy of a multiphase dietetic protocol with meal replace-
ments including a step with very low calorie diet. Endocrine (2015) 48:863–70.
doi:10.1007/s12020-014-0355-2
12.
Andrikopoulos S, Massa CM, Aston-Mourney K, Funkat A, Fam BC, Hull RL,
etal. Dierential eect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on
insulin secretory function in response to a high fat diet. J
Endocrinol (2005)
187:45–53. doi:10.1677/joe.1.06333
13.
Zaman MQ, Leray V, Le Bloc’h J, orin C, Ouguerram K, Nguyen P. Lipid
prole and insulin sensitivity in rats fed with high-fat or high-fructose diets.
Br J
Nutr (2011) 106:S206–10. doi:10.1017/S0007114511004454
14.
Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement
of sex, strain and age factors in high fat diet-induced obesity in
C57BL/6J and BALB/cA mice. Exp Anim (2007) 56:263–72. doi:10.1538/
expanim.56.263
15.
Lepore SM, Morittu VM, Celano M, Trimboli F, Oliverio M, Procopio A,
etal. Oral administration of oleuropein and its semisynthetic peracetylated
derivative prevents hepatic steatosis, hyperinsulinemia, and weight gain in
mice fed with high fat cafeteria diet. Int J
Endocrinol (2015) 2015:431–53.
doi:10.1155/2015/431453
16.
Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, etal. Lack of the
architectural factor HMGA1 causes insulin resistance and diabetes in humans
and mice. Nat Med (2005) 11:765–73. doi:10.1038/nm1254
17.
Chiefari E, Paonessa F, Iiritano S, Le Pera I, Palmieri D, Brunetti G, etal. e
cAMP-HMGA1-RBP4 system: a novel biochemical pathway for modulating
glucose homeostasis. BMC Biol (2009) 7:24. doi:10.1186/1741-7007-7-24
18.
Baeza-Raja B, Sachs BD, Li P, Christian F, Vagena E, Davalos D, et al. p75
neurotrophin receptor regulates energy balance in obesity. Cell Rep (2016)
14:255–68. doi:10.1016/j.celrep.2015.12.028
19.
Chang JW, Chen HL, Su HJ, Lee CC. Abdominal obesity and insulin resistance
in people exposed to moderate-to-high levels of dioxin. PLoS One (2016)
11:e0145818. doi:10.1371/journal.pone
20.
Haslam DW, James WP. Obesity. Lancet (2005) 366:1197–209. doi:10.1016/
S0140-6736(05)67483-1
21.
Donini LM, Cuzzolaro M, Gnessi L, Lubrano C, Migliaccio S, Aversa A, etal.
Obesity treatment: results aer 4 years of a nutritional and psycho-physical
rehabilitation program in an outpatient setting. Eat Weight Disord (2014)
19:249–60. doi:10.1007/s40519-014-0107-6
22.
Barte JC, ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA,
et al. Maintenance of weight loss aer lifestyle interventions for over-
weight and obesity, a systematic review. Obes Rev (2010) 11:899–906.
doi:10.1111/j.1467-789X.2010.00740.x
23.
Wadden TA, Fujioka K, Toubro S, Gantz I, Erondu NE, Chen M, etal. A ran-
domized trial of lifestyle modication and taranabant for maintaining weight
loss achieved with a low-calorie diet. Obesity (2010) 18:2301–10. doi:10.1038/
oby.2010.67
24.
Grams J, Garvey WT. Weight loss and the prevention and treatment of type
2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery:
mechanisms of action. Curr Obes Rep (2015) 4:287–302. doi:10.1007/
s13679-015-0155-x
25.
Melotto S. Clinical pharmacology of eating and not eating. Curr Opin
Pharmacol (2014) 14:1–5. doi:10.1016/j.coph.2013.09.015
26.
Munsters MJ, Saris WH. Body weight regulation and obesity: dietary strategies
to improve the metabolic prole. Annu Rev Food Sci Technol (2014) 5:39–51.
doi:10.1146/annurev-food-030212-182557
27.
Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, etal. Functional het-
erogeneity of CD11c-positive adipose tissue macrophages in
diet-induced
obese mice. J
Biol Chem (2010) 285(20):15333–45. doi:10.1074/jbc.
M110.100263
28.
Guo J, Jou W, Gavrilova O, Hall KD. Persistent diet-induced obesity in male
C57BL/6 mice resulting from temporary obesigenic diets. PLoS One (2009)
4(4):e5370. doi:10.1371/journal.pone.0005370
29.
Dossi CG, Tapia GS, Espinosa A, Videla LA, D’Espessailles A. Reversal
of high-fat diet-induced hepatic steatosis by n-3 LCPUFA: role of PPAR-α
and SREBP-1c. J
Nutr Biochem (2014) 25(9):977–84. doi:10.1016/j.
jnutbio.2014.04.011
30.
Panchenko PE, Voisin S, Jouin M, Jouneau L, Prézelin A, Lecoutre S, etal.
Expression of epigenetic machinery genes is sensitive to maternal obesity and
weight loss in relation to fetal growth in mice. Clin Epigenetics (2016) 8:22.
doi:10.1186/s13148-016-0188

7
Lombardo et al.
Normocaloric Diet in Obese Mice
Frontiers in Endocrinology | www.frontiersin.org May 2016 | Volume 7 | Article 49
31. Al-Shaqha WM, Khan M, Salam N, Azzi A, Chaudhary AA. Anti-diabetic
potential of Catharanthus roseus Linn. and its eect on the glucose transport
gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats.
BMC Complement Altern Med (2015) 15:379. doi:10.1186/s12906-015-0899-6
Conict of Interest Statement: e authors declare that the research was con-
ducted in the absence of any commercial or nancial relationships that could be
construed as a potential conict of interest.
Copyright © 2016 Lombardo, Arcidiacono, De Rose, Lepore, Costa, Montalcini,
Brunetti, Russo, De Sarro and Celano. This is an open-access article distributed
under the terms of the Creative Commons Attribution License (CC BY). The
use, distribution or reproduction in other forums is permitted, provided the
original author(s) or licensor are credited and that the original publication in
this journal is cited, in accordance with accepted academic practice. No use,
distribution or reproduction is permitted which does not comply with these
terms.
