1
RETAIL FOOD SAMPLE COLLECTION GUIDANCE
Introduction and Purpose
This guidance is intended as general aseptic sample guidance for Retail Food
Safety Inspectors. There are multiple types of samples and sampling
procedures that are not covered in this document, such as environmental,
water, and surveillance sampling. This document focuses on sampling
related to epidemiologic investigations and may require the coordination,
organization, and response of multiple agencies. Once sample is obtained,
the inspector ships it to an approved lab for processing.
Recommended Trainings
• ComplianceWire: Food Microbiological Control 10 - Aseptic Sampling MIC13
• FDA ORA LearnED: CC8035W - Sampling
• AFDO: FD170 – Application of Inspection and Investigation Techniques
Equipment Needed
Keep a stock of the following sampling supplies:
• Security bags with a security strip
• Whirl-pak bags (large and small)
• Plastic bags for blue ice
• Blue ice or ice cubes
• Sterile Gloves
• Clean outer clothing, such as laboratory coat
• Sterile Scoop/Tongue Depressors/Wrapped Spoon
• Sterile plastic cups
• Security tape
• Sharpie pen
• Packaging tape/scissors
• Foam-lined cooler shipping boxes
• Applicable forms (G-22/G-23, Chain of Custody, etc.)
Sampling Process Steps:
1. Determine which product to sample and schedule the sample with the
receiving lab.
2. Notify establishment management.
3. Collect the sample.
4. Document the sample.
5. Pack and ship the sample and form.
6. Respond to the results.
2
Before Leaving for the Investigation:
1. Go over the objectives of the assignment. Determine potential types of
samples to be collected.
2. Plan to collect controls like gloves or bags, in order to ensure
equipment is not contaminated.
3. Arrange for the submission of samples with laboratories. Confirm with
laboratory prior to collection of any sample. If utilizing an approved
Texas DSHS laboratory, then sample must be collected by a Registered
Sanitarian (R.S). See laboratory guidance below for more information.
4. Check expiration dates on all sampling materials to make sure they
are not expired.
5. Print out adequate Chain of Custody, laboratory submission, and other
required forms. TX DSHS lab forms can be found HERE.
6. Schedule sample collection with Person-In-Charge (PIC) of Retail Food
Establishment. (Unless imminent health hazard or unannounced need
exists)
Laboratory Guidance
Food samples shall never be obtained prior to lab approval. Different labs
have different protocols and requirements. It is important to obtain lab
approval and understand the lab’s specific requirements prior to heading into
the field for a sample collection. Texas DSHS labs require the sample
collection to be completed by a Registered Sanitarian (R.S.), while other
accepted labs may not require an R.S. for sample collection. If sample
collection is not possible due to these requirements or other barriers, contact
Texas DSHS for sample collection coordination. Depending on the nature or
state of the outbreak, FDA labs or other approved labs may be utilized. For
more information about Texas DSHS laboratory services, see HERE.
Aseptic Sampling
Aseptic sampling is a technique used to ensure the person collecting the
sample is not increasing the microbial load of a product sample by using
sterile sampling implements/containers and a prescribed sampling method.
Inspectors use proper hygiene and wear protective gear such as hairnet,
beard restraint, clean clothing/lab coat, sterile single-use gloves, and any
required protective gear provided by the firm (e.g. hard hat, sleeve-guards,
face mask), and proper handwashing during the sample collection process.
When obtaining food samples, it is recommended to utilize teams of 2 or
more. See video HERE for aseptic sampling sample collection techniques.

3
Basic Aseptic Sampling Techniques:
• Use only sterile equipment and containers.
• When opening sterile sampling containers, work rapidly so that
contaminants from the environment do not compromise the sample or
equipment. Open sterile sampling containers only to admit the sample
and close the container immediately. Do not touch the inside or
opening of the sterile container.
• If it is necessary to open product containers to collect a sample, open
the container in a way that does not contaminate the product. Be sure
to wash hands and wear sterile gloves to collect the sample
aseptically.
• Take steps to minimize exposure of product and sampling equipment
to the environment. Dust in the air surrounding the container can
carry pathogenic bacteria.
• Use a fresh sterile glove for each sample submitted under a new
number. See video HERE on donning and doffing gloves.
Sample Collection Procedures:
1. Utilize aseptic sampling techniques, when applicable.
2. Wash hands thoroughly to mid forearm for at least 20 seconds and dry
with paper towel.
3. Use properly fitting sterile gloves and be mindful not to cross-
contaminate samples. Remove disposable glove from packaging,
avoiding contact with the outer surface of the glove as much as
possible. Insert hands without puncturing the glove.
4. Open the “whirl-pack” or other sterile container with your gloves on.
5. Using a sterile instrument such as a wrapped spoon or tongue
depressor, fill the container with amount of product requested by lab
during pre-investigation steps. Collect the sample from multiple
locations and depths of the product in order to ensure it is a
representative sample.
6. Close the sample without touching the interior of the container and
seal. Use seal tape to seal food container or Whirl-Pak bag. Be mindful
to not cover any pertinent or important information on containers with
seal tape.
7. Place sealed sample into security bag. Seal security strip.
8. Label each container and security bag with identifying information such
as: type of food, date and time collected, establishment name, sample

4
number, and initials/signature of the person taking the sample. You
can also give it your own unique ID# such as LHD-FiLi-1 (Local Health
Department name - First Initial Last Initial - Sample Number 1).
9. Place samples in insulated carrier with ice packs to transport and
refrigerate as soon as possible. If using wet ice, then double bag to
ensure prevention of melted ice contaminating sample.
10. Provide PIC of Retail Food Establishment with a copy of the
documentation for the receipt of samples (Receipt of Samples Form,
Inspection Form Documentation, etc.).
11. Transport the samples to the pre-approved lab within 24 hours.
Longer time frames may be acceptable if sample integrity is
maintained, and the receiving lab approves the longer duration.
12. Maintain a chain of custody for each food sample taken at all times.
13. Reports and chain of custody forms must be maintained in the
facility’s file when the investigation is completed.
14. Complete any laboratory required documentation, such as G-22/G-23.
Shipping Recommendations
Before sample collection, review the shipping requirements and determine
which shipping location is going to be used. Contact the laboratory manager
if a sample must be shipped in a manner not addressed in the courier’s
shipping requirements. Ensure samples are properly packed to prevent
breakage, spillage, and/or possible contamination of samples. Each food
sample must be put in its own security bag.
Shipping Temperatures
Refrigerated Samples:
• Use a type of refrigerant, or similar product, to maintain refrigerated
temperatures.
• Place refrigerants in sealed plastic bags to protect samples from
possible contamination should the container break, the ice melt, or the
refrigerant penetrate the sample. Use Styrofoam insulated shipping
cartons for shipping samples to the laboratory.
• All micro samples, including environmental swabbing, must be
refrigerated regardless of storage conditions at the firm.
Frozen Samples:
• Pre-chill sterile containers before collecting frozen samples.
• Transfer liquids in glass to expandable containers before freezing. If
the liquid will be frozen in glass, leave sufficient headspace to allow
expansion.

5
• If freezer facilities are not available or if the sample is to be shipped,
pack with ice, or ice substitute, in insulated cartons.
• Do not use dry ice unless advised to do so by lab. Most microbiology
labs will not accept any samples sent with dry ice. All samples must be
packed with ice substitute or wet ice only.
Insect Samples:
• Samples with live insects shall be frozen.
Appendix – Required and Recommended Sample Forms
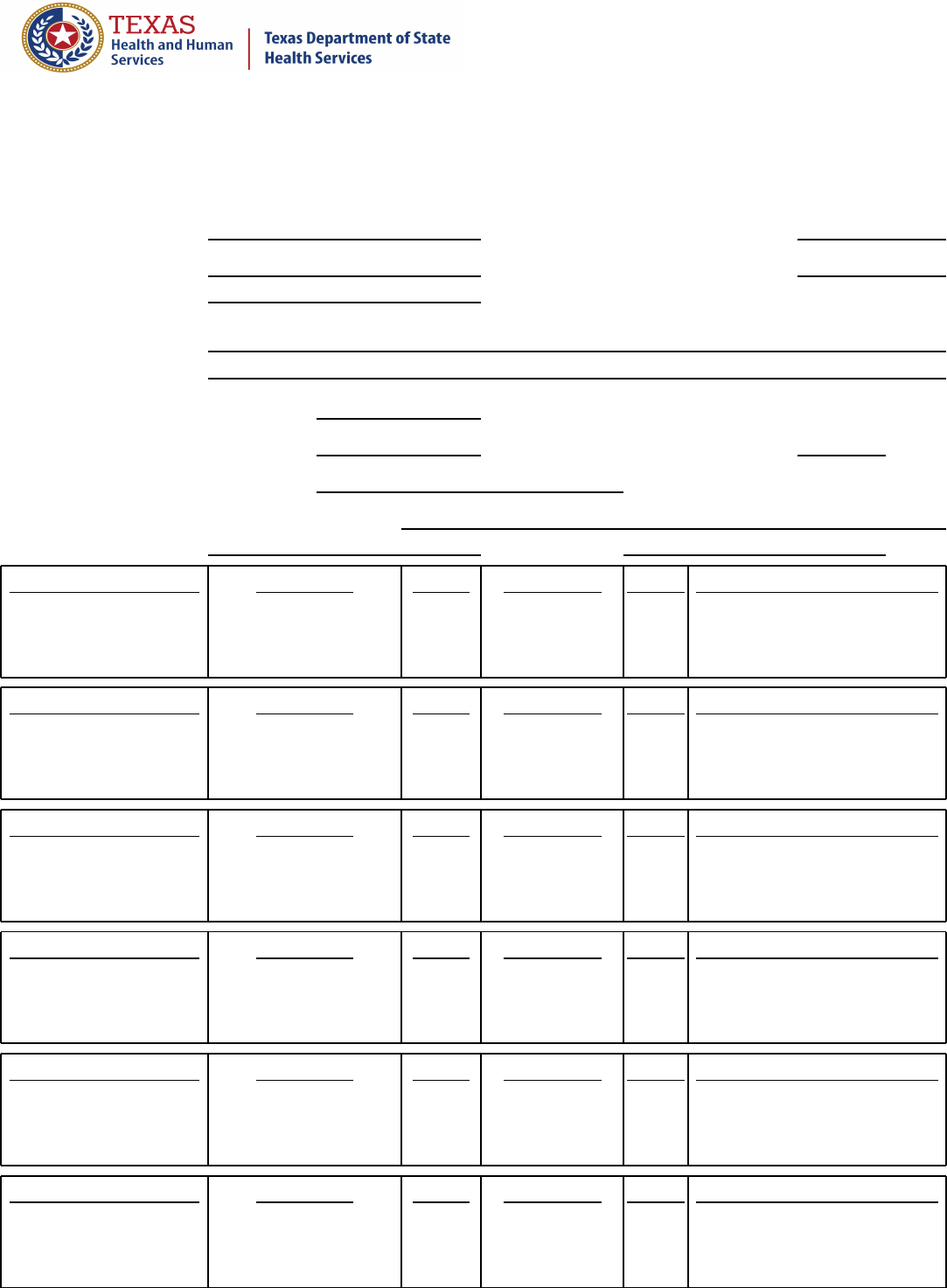
Sample Collection Chain of Custody
DEPARTMENT OF STATE HEALTH SERVICES
P.O. Box 149347
Austin, Texas 78714-9347
Firm Name:
Sample Number:
Firm Address:
Security Number:
Sample Description:
Date Sample Collected:
Sample Collection Start Time:
Sample Collection End Time:
Investigator Name:
Initial Delivery or Storage Location:
Date:
Time:
Transfer Date and Time
Released By
Initials
Received By
Initials
Reason for Change of Custody
Transfer Date and Time
Released By
Initials
Received By
Initials
Reason for Change of Custody
Transfer Date and Time
Released By
Initials
Received By
Initials
Reason for Change of Custody
Transfer Date and Time
Released By
Initials
Received By
Initials
Reason for Change of Custody
Transfer Date and Time
Released By
Initials
Received By
Initials
Reason for Change of Custody
Transfer Date and Time
Released By
Initials
Received By
Initials
Reason for Change of Custody
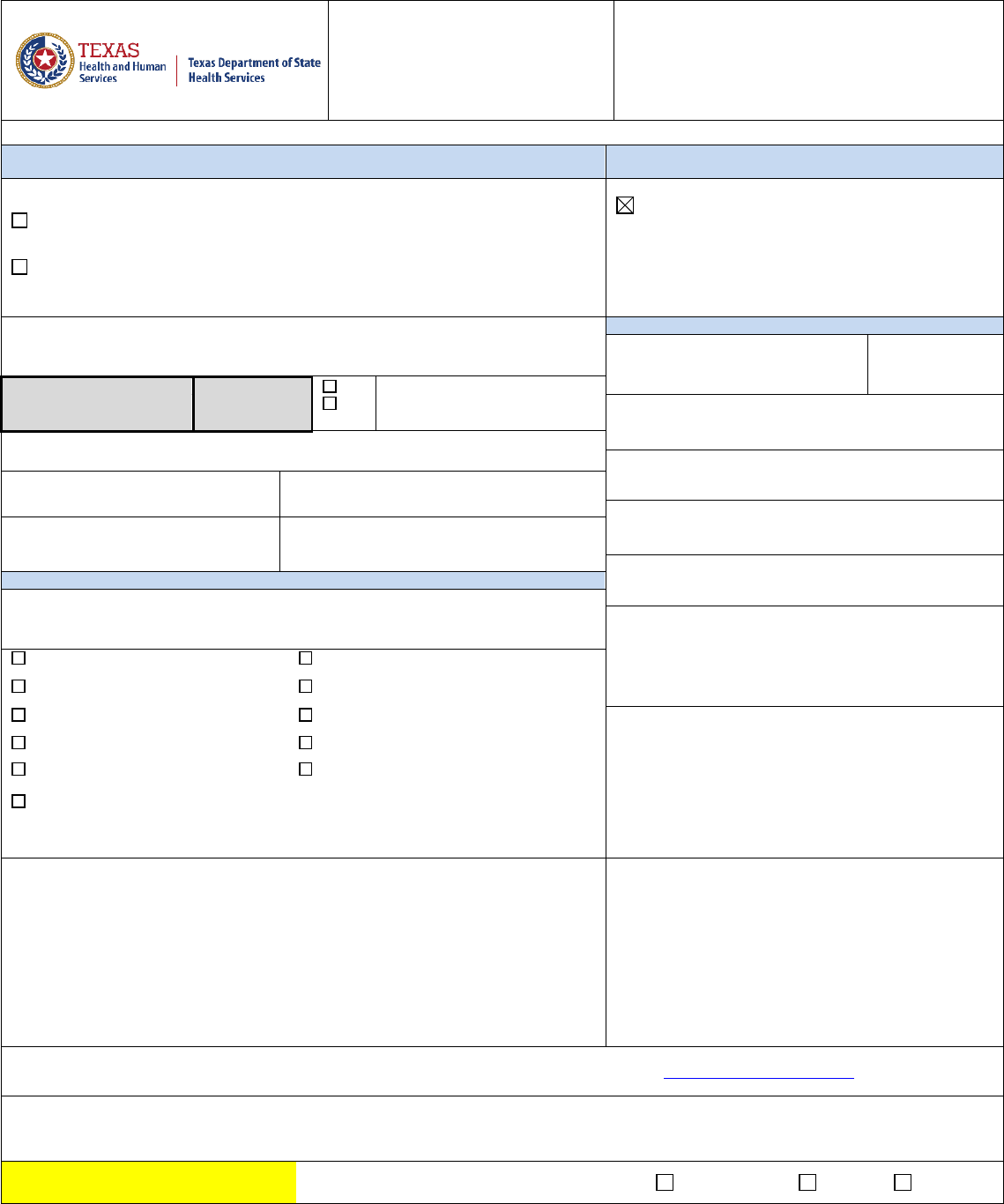
Blank copy 7927921 Approved and current. Effective starting 3/26/2021. 102753.207 (version 1.0) 7b Chain of Custody
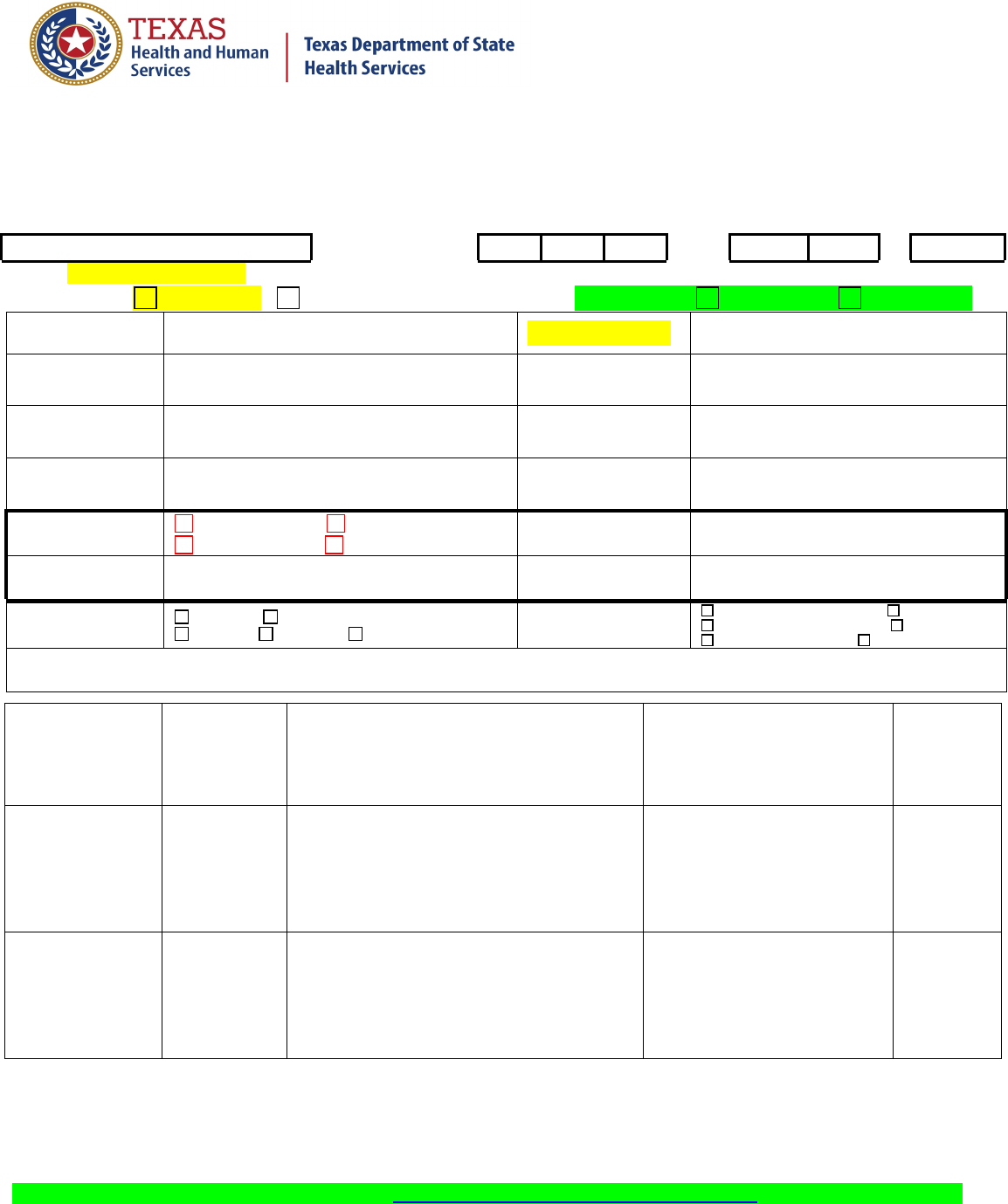
Consumer Protection
Division
Form No. G-22-(Food) Laboratory Services Section Main Lab Number: 512-458-7318 or 888-963-7111
Email results to: DL DSHS Foods Management Group ([email protected]) with “Final or Presumptive”
plus the Foods Group ‘’Sample Number” in the subject line of the e-mail.
Revised 04/26/2018
1
:
Foods Sample Number(s)
Date and Time of
Collection
Month
Day
Year
Hour
Minute
AM/PM
Inspection is: CONTRACT Other: Sample type: Surveillance Compliance
Firm Name:
Inspection #:
Firm
Address:
Reference #:
(PSQA enters)
Firm City,
Zip:
Collected by
Name:
Number of
Samples:
Area:
COMPLETE ONLY
IF NEW FIRM:
Sole Proprietor Partnership
Corporation 501 (c)(3) Tax Exempt
Phone number:
Firm owned by:
Corp officer/ owner’s
name:
Sample category:
Bacterial Chemical
Physical Filth Other:
Check one:
2401 Food Manufacturer 2404 Registrant
2402 Food Warehouse Operator 2405 Salvage
2403 Food Wholesaler 2504 Multiple Products
Brief product description:
Sample
Number
(Sub)
Test
Desired
Complete Description
(Manufacturer, address,
product name, weight,
packaging, physical form, etc.)
Lab Identification
Number
Test
Results
Blank copy 7927922 Approved and current. Effective starting 3/26/2021. 102753.206 (version 1.0) 7c G-22- Lab
Consumer Protection
Division
Form No. G-22-(Food) Laboratory Services Section Main Lab Number: 512-458-7318 or 888-963-7111
Email results to: DL DSHS Foods Management Group ([email protected]) with “Final or Presumptive”
plus the Foods Group ‘’Sample Number” in the subject line of the e-mail.
Revised 04/26/2018
2
Blank copy 7927922 Approved and current. Effective starting 3/26/2021. 102753.206 (version 1.0) 7c G-22- Lab
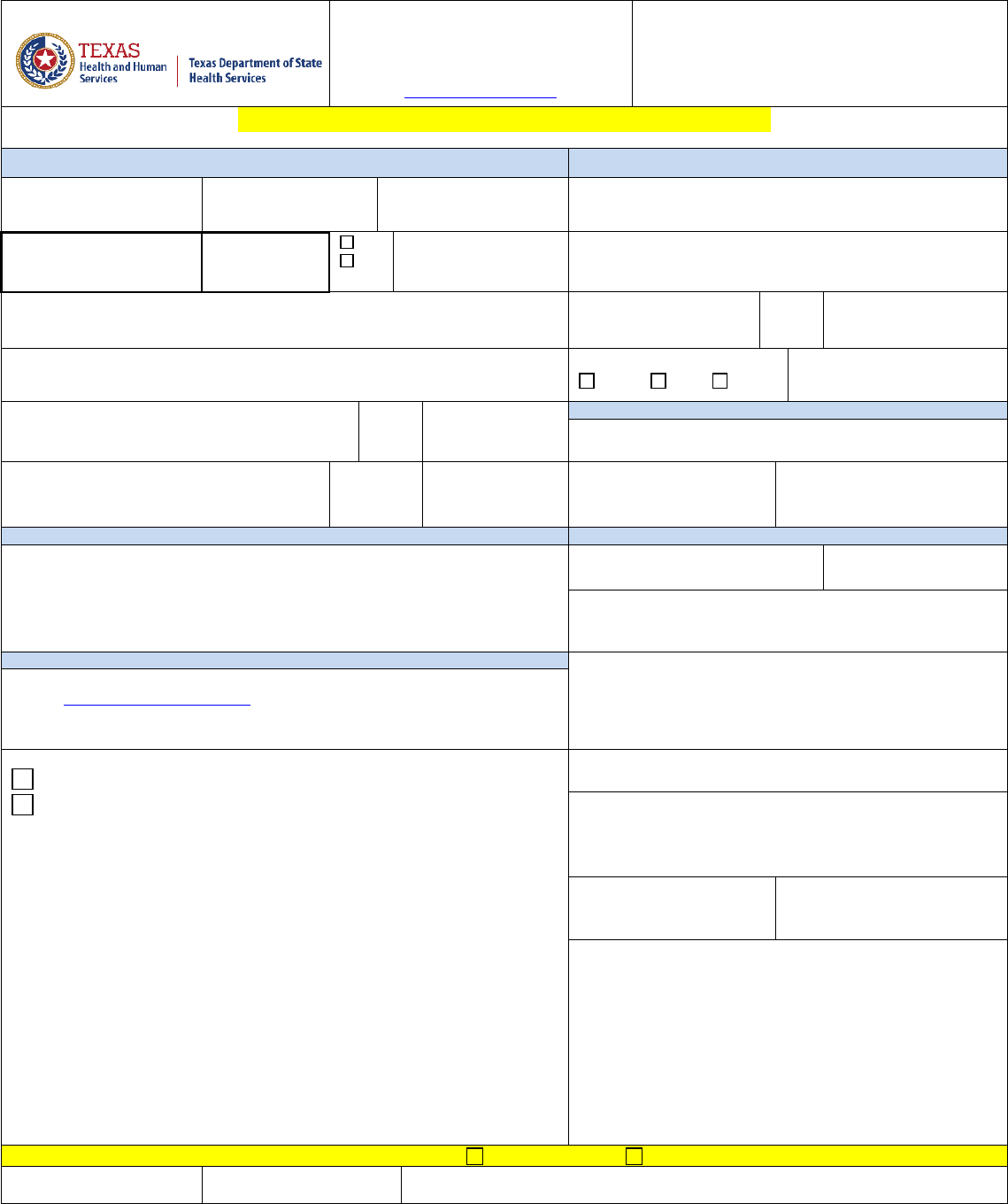
**SAMPLE - DO NOT USE**
Specimen Acquisition: (512) 776-7598
G-23-Food Sample Specimen
Submission Form (JAN 2022)
C
AP# 3024401 CLIA# 45D0660644
www.d
shs.texas.gov/lab
****For DSHS Use Only***
**ONE FORM PER SPECIMEN REQUIRED**
Section 1. SAMPLE INFORMATION –(**REQUIRED)
Section 3. PAYOR SOURCE -- (REQUIRED)
Reason for Testing
Routine
Food Borne Outbreak
(If this box is checked, please complete Section 4 of this form)
IDEAS
Sample Description:
Section 4. OUTBREAK LINKED SAMPLES
Outbreak Location: (City)
PH Region
Date of Collection ** (REQUIRED)
Time of Collection **
AM**
Collected By **
PM**
Brand:
Facility/ Submitter Name
Code:
Sample Number:
Submitter Number:
Product:
Contact Phone #
Contact Fax #
Seal:
Section 2. TESTING INFORMATION
***** EACH TEST REQUIRES ≥ 4 oz SAMPLE-REPEAT, EACH TEST*****
Please Indicate Desired Testing
Size:
Food Analysis: Campylobacter
Food Analysis: Listeria
Food Analysis: Cronobacter
Food Analysis: Salmonella
Food Analysis: Cyclospora, PCR
Food Analysis: Shigella
Condition:
Food Analysis: E. coli O157
Food Analysis: Staphylococcus enterotoxin
Food Analysis: non-O157 STEC
Food Analysis: Yersinia
Food Analysis: Other _________________________________________________
Remarks:
Brief description of patient’s symptoms:
Details of test and specimen requirements can be found in the Laboratory Services Section’s web site at http://www.dshs.texas.gov/lab/.
Date Received
FOR LABORATORY USE ONLY
Specimen Received: Room Temp.
Cold Frozen
Laboratory Services Section: 1100 West 49
th
St Austin, Tx 78756
**SAMPLE - DO NOT USE**
Specimen Acquisition: (512) 776-7598
G-22 Specimen Submission Form
(Jan 2022)
NELAC# T104704297
www.dshs.texas.gov/lab
****For DSHS Use Only***
THE SUBMITTER WILL BE BILLED FOR ALL TESTING
DSHS is not responsible for 3
rd
party payment arrangements
Section 1. SUBMITTER/BILLING INFORMATION – (** REQUIRED)
Section 4. REPORTING INFORMATION
Indicate where & how you would like the results sent
Sample Identifier
Submitter Number
Establishment or Location
Name
Date of Collection ** (REQUIRED)
Time of Collection **
AM**
Collected By/Contact **
Address
PM**
Agency / Submitter Name
City
State
Zip Code
Address
Preferred Reporting Method
Fax Number or email:
Mail Fax Email
City
State
Zip Code
Section 5. PROGRAM INFORMATION when applicable
Program Name
Laboratory Identification # /
TCEQ NELAC Certificate #
Phone #
Fax #
Program Identification Number
Program Sample Identifier
Section 2. SAMPLE INFORMATION -- (** REQUIRED)
Section 6. SPLIT SAMPLE FLUORIDES
Sample Type/Description**:
System ID #:
Date Collected
Name of Water System
Section 3. ENVIRONMENTAL TESTING INFORMATION
Collected By:
***** To Ensure Proper Collection Please Refer to Laboratory Services Section’s web
site at http://www.dshs.texas.gov/lab
for Container, Sample Size, and Requirements
Specific to the Test Requested *****
Phone #
Reagent Water Suitability Test
List Other Test(s) Requested:
Sample Location / Comments:
Water System Test Results
DSHS Lab Test Results(Do Not Write Below)
Fluoride ___________mg/L Fluoride ____________mg/L
Notes / Comments
FOR LABORATORY USE ONLY
Specimen Received:
Room Temp.
Cold _____________ ˚C
Date Received
Date Reported
Laboratory Services Section: 1100 West 49
th
St Austin, Tx 78756
