HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Infection Preventionist Training for Skilled Nursing Facilities
Healthcare-Associated Infections Program
Center for Health Care Quality
California Department of Public Health
State and Federal
Regulatory Requirements

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Objectives
• Describe national, state, and local regulatory bodies
that oversee infection prevention and HAI public
reporting
• Describe the purpose of CDPH All Facilities Letters
(AFL)
• Review current infection control-related regulations
2

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
HAI Public Reporting Policies Driven by
Call for Transparency
• Disclosure to the public is intended as a driver for infection
prevention; encourages healthcare providers to take action
• Reporting publicly allows the consumer to assess quality of
healthcare for each facility
• Informing the public can drive demand for higher quality
healthcare
• Reporting HAIs became law for hospitals in 2006 & 2008
• Requirement to report HAIs to CMS became law in 2014 for
SNFs
CMS SNF Quality Reporting Program Public Reporting
(www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-
Instruments/NursingHomeQualityInits/Skilled-Nursing-Facility-Quality-Reporting-
Program/SNF-Quality-Reporting-Program-Public-Reporting
3
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Regulatory Agency Overview
Federal State Local
Centers for Medicare and
Medicaid Services (CMS)
California Department of
Public Health
• Licensing & Certification
• Reportable Diseases and
conditions
• Medical Waste
Local health office and
health department
Occupational Health and
Safety Administration
(OSHA)
Cal-OSHA Environmental Health
Communicable Disease
reporting
4

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
State Oversight:
CDPH Licensing and Certification (L&C)
• Headquartered in Sacramento, CA
• 18 district offices
• >600 health facility evaluator nurses (HFEN)
• Licenses facilities to operate in California, including
• General acute care hospitals (GACH)
• Skilled nursing facilities (SNF)
5

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Federal Oversight: Centers for Medicare and
Medicaid Services (CMS)
• Provision of health insurance through Medicare and Medicaid
• Surveys and certification health care facilities, including nursing
homes, home health agencies, and hospitals
• Requirement by Social Security Administration (SSA) to meet
conditions of participation (CoP) by the facility, in order to
receive Medicare and Medicaid funds (SSA Section 1861)
Electronic Code of Federal Regulations
(www.ecfr.gov/cgi-bin/text-
idx?SID=7db07035274456a040371c5839d77a1d&mc=true&tpl=/ecfrbrowse/Title42/42t
ab_02.tpl)
6
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Accreditation Agencies
• Certifies compliance with CMS conditions of participation
• Private, independent accreditation organizations
• The Joint Commission (TJC)
• National Integrated Accreditation for Healthcare
Organizations (NIAHO; DNV Healthcare)
• Healthcare Facilities Accreditation Program (HFAP)
• No citations come from these directly
• Act as CMS surveyors
• Information from these surveyors is provided to CMS, who
determines penalties
7

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Accreditation Agencies – Ambulatory
Surgery Centers
• American Association of Ambulatory Surgery Centers
(AAASC)
• American Association for Accreditation of Ambulatory
Surgical Facilities (AAAASF)
• Accreditation Association for Ambulatory Health Care
(AAAHC)
8

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Relationships
• The Joint Commission Certifies or “deems” to CMS that
facilities licensed in California they have surveyed met
federal requirements
• Inspections by L&C reported to CMS via a contract with CMS
• Enforcement after L&C surveys of state laws (HSC 1188) and
regulations (CCR Title 22)
• Surveys by Consolidated Accreditation and Licensing (CALS)
conducted jointly with TJC
9

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
General Acute Care Relicensing Survey
• Purpose is to promote quality of care in hospitals, verify
compliance with state regulations and statutes, and ensure
a program wide consistency in the hospital survey
methodology.
• Implemented March 2016
• Survey every 3 years, 3-5 day survey
• Evaluates hospital’s compliance with statutory and
regulatory requirements
• Surveyors will select patients from various service areas
• 6%-10% of the current inpatient census will be
selected for record review (minimum of 30)
CDPH General Acute Care Relicensing Survey
(www.cdph.ca.gov/Programs/CHCQ/LCP/Pages/GeneralAcuteCareRelicensing
Survey.aspx)
10

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
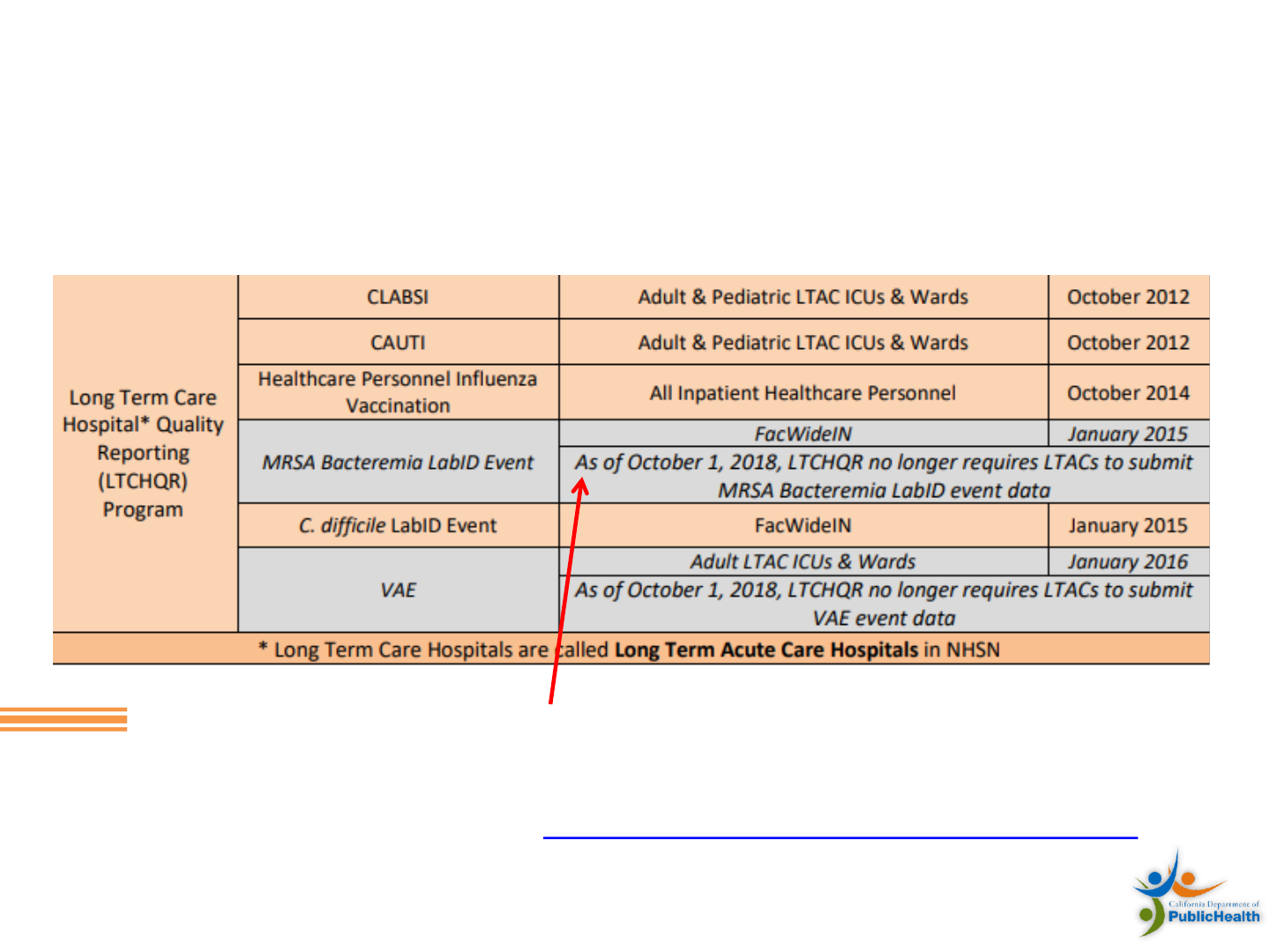
Skilled Nursing Facility Surveys
11
• Surveys of SNFS are conducted by CDPH at least every 6–16
months
• Assess compliance with state and federal standards
• Focus on facilities with outbreaks
• SNF will be surveyed out of schedule, sooner than usual
• Complaints of abuse and infectious disease concerns
• SNF will be visited immediately
• Revisits by other agencies if high risk findings are
reported
CDPH L&C Regulations
(www.cdph.ca.gov/Programs/CHCQ/LCP/Pages/Regulations.aspx)
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
California Law and Regulations Terminology
• Bills are passed by California legislature
• If signed by governor, bill becomes a statute or law
• Laws related to health become part of the California Health
and Safety Code (HSC)
• Regulations are written by the appropriate State Executive
Branch, agency or department such as CDPH to:
• Carry out what a bill authorizes or directly requires a State
department to do
• Clarify requirements of a bill (less common)
12

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
CDPH All Facility Letters (AFL)
• Communicates with healthcare facilities about laws and
regulations
• Sent to inform facilities of
• new requirement or technologies
• change of requirement in healthcare
• clarify an existing law/regulation
• Enforcement actions
• Scope of practice clarifications
• General information that affects health facility
• The absence of an AFL does not absolve a facility from
complying with the law
13

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Antibiotic Stewardship (ABS) for SNF
• AFL 15-30: Requires adoption of ABS policy for each SNF
• SB 361 (oal.ca.gov/): Failure to comply will result in enforcement
actions per HSC section 1423
• Authority by HSC section 1275.4
• ABS to align with the 7 Core Elements of Antibiotic Stewardship for
Nursing Homes:
1. Leadership commitment – Safe antibiotic use support
2. Accountability – phy
sician, nursing, or pharmacist leads
3. Drug expertise – pha
rmacist or consultant pharmacists
4. Action – at least one policy or procedure for antibiotic use
5. Tr
acking – at least one process measure of use or outcome from
an
tibiotic use
6. Reporting – regul
ar feedback on antibiotic use
7. Education – res
ources to clinicians, nursing, residents, families
CDC Nursing Homes and Assisted Living LTCFs
(www.cdc.gov/longtermcare/prevention/antibiotic-stewardship.html)
15

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
AFL 20-84 (SNF)
• Refers to AB 81, AB 2644 - CDPH incorporating Quality and Accountability
Supplemental Payment (QASP) effective January 1, 2021
• Incorporates infection prevention program and COVID-
19 mitigation
requirements into QASP
• QASP assesses SNF quality and bases payment on quality measures
• Requires a full time Infection Preventionist
• Training requirements – mi
nimum of 14 hours initial IP training
• Attend IP education – mi
nimum of 10 hours annually
• Designated IP (or shared by 2 to cover full time) for each facility
• Describes the IP Program Functions
• Management, staff education, regulatory requirements,
p
erformance Improvement and committees, occupational health,
IPC policies and protocols
AFL 20-84
(www.cdph.ca.gov/Programs/CHCQ/LCP/Pages/AFL-20-84.aspx)
16
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
AFL 20-85 (SNF)
Notifies SNF of AB 2644 requiring full time IP
• Requires full time IP
• Must be LVN or RN (may job share)
• Hours specified for IP LVN or RN hours cannot include hours spent
in dir
ect care services
• Provide annual training for HCP in the facility
• Reporting communicable diseases during a declared emergency
• Disease or suspect disease related death reported within 24 hours
• Refers to AFL 20-
43.3 Daily Reporting requires data entry into
NHSN
AFL 20-85
(www.cdph.ca.gov/Programs/CHCQ/LCP/Pages/AFL-20-85.aspx)
17

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
California Health and Safety Code (HSC)
• HAI requirements were passed as Senate Bills 739, 1058,
158 and 1311 in 2006, 2008 and 2014, respectively
• HSC sections that contain HAI requirements:
• 1188.45–1188.95:
reporting and prevention
requirements, including an antimicrobial stewardship
program
• 1255.8: MRSA patient testing
• 1279.7: Hand hygiene program, connector language
California laws and regulations:
Office of Administrative Law
(www.oal.ca.gov)
Official CA Legislative Information
(www.leginfo.ca.gov)
18

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
California Title 22 Regulations
• Division 5 Licensing and Certification of Health Facilities
• Chapter 1 General Acute Care Hospital
• Article 7 Administration
• Chapter 2 Acute Psychiatric Hospital
• Chapter 3 Skilled Nursing Facilities
• Chapter 4 Intermediate Care Facilities
• Chapter 7 Primary Care Clinics
• Chapter 7.1 Specialty Clinics
• Article 6 Hemodialyzer Reuse
• Chapter 12 Correctional Treatment
19

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
California Code of Regulations: Title 22*
• Requires a written infection control program for the
surveillance, prevention, and control of infections
• Covered by policies and procedures:
• Management of transmission risks
• Education
• Surveillance plan, including outbreak management
• Biohazardous equipment and materials identification
• Oversight of the program is part of a multidisciplinary
committee
• Care of residents with infectious diseases
+
*Title 22, Div 5, Chap 1, Article 7, Sec 70739
+
Title 22, Div 5, Chap 3, Article 3, Sec 72321
20
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Reportable Diseases and Conditions -Title 17
• All cases of reportable diseases shall be reported to the local
health officer
• Reportable conditions may vary by local health jurisdiction
• California Confidential Morbidity Report (CMR) form is used
to report all conditions except TB
• Consult with local health for their particular requirements,
forms, method of reporting
Reporting of Communicable Diseases
(govt.westlaw.com/calregs/Document/I805AD6B0FB1711DEACA9F33E9EE53480?viewType=F
ullText&originationContext=documenttoc&transitionType=CategoryPageItem&contextData=(sc.Default)
CDPH Reportable Disease and Conditions Morbidity Report (PDF)
(www.cdph.ca.gov/CDPH%20Document%20Library/ControlledForms/cdph110a.pdf)
21
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
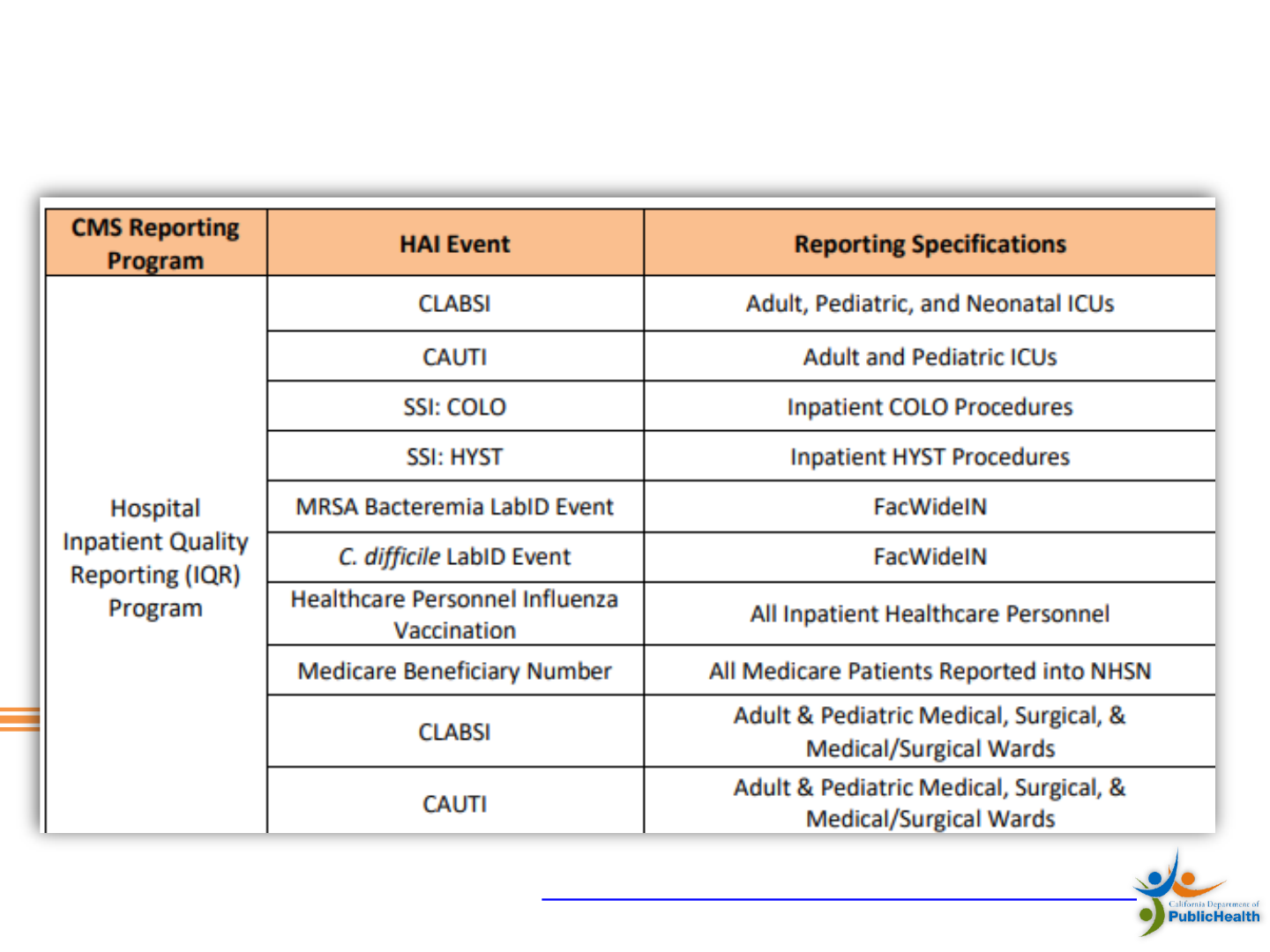
California HAI Reporting Requirements
• Follow California acute care hospital requirements and NHSN
rules for reporting healthcare associated infections (HAI)
• CLABSI – all in-patient hospital locations
• CLIP - for lines inserted in ICUs
• MRSA and VRE – all positive blood stream infections for all
inpatients, ED and 24 hour observation units
• CDI – using LabID, all inpatient, ED and 24 hour
observation units
• SSI - for 28 procedure categories
22
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Cal-OSHA
• Protection of the worker with guidance to keep workers
healthy and safe in the state of California
• Department of Industrial Relations
• Division of Occupational Safety and Health
• Develops regulations for workplace safety and health
• Bloodborne Pathogen Standard
• Aerosol-Transmissible Diseases Standard
• Respiratory Protection Standard
• Regulations must be as stringent (or more) than federal
regulations
Cal/OSHA
(www.dir.ca.gov/dosh/)
23

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
California Medical Waste Management Act*
• Ensures proper handling and disposal of medical waste
throughout California
• Biohazardous waste
a) Laboratory waste, including human or animal specimen
cultures from medical and pathology laboratories
b) Human surgery specimens or tissue
c) Waste containing discarded materials contaminated
with excretion, exudate, or secretions from
humans…required to be isolated by infection control
staff, attending physician and surgeon, …or local health
officer
*Health and Safety Code 117600 and 117635
24

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Federal Regulations -CMS Title 42
• Subchapter G Standards and Certification
• Part 482 Conditions of Participation For Hospitals
• Part 483 Requirements For States and LTC
• Part 484 Home Health Services
• Part 493 Laboratory Requirements
• Part 494 Conditions for Coverage for End-stage Renal
Disease Facilities
25

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
§ 482.21 Quality Assurance
§ 482.22 Medical Staff
§ 482.23 Nursing services
§ 482.24 Medical record
s
er
vices
§ 482.25 Pharmaceutical
ser
vices
§ 482.26 Radiologic
ser
vices
§ 482.27 Laboratory
ser
vices
§ 482.28 Food and Dietetic
ser
vices
§ 482.31 Utilization review
§ 482.41 Physical
en
vi
ronment
§ 482.42 Infection Control
§ 482.43 Discharge
pl
anni
ng
§ 482.45 Organ, tissue, and
ey
e procurement
26
Part 42 Subpart C: Basic Hospital Functions

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Part 42 Subpart B: Requirements for LTCF
§483.5 Definitions
§483.10 Resident Rights
§483.12 Freedom from Abuse,
Neglect, and Exploitation
§483.15 Admission Transfer and
Discharge Rights
§483.20 Resident Assessment
§483.21 Comprehensive Person-
Centered Care Plans
§483.24 Quality of Life
§483.25 Quality of Care
§483.30 Physician Services
§483.35 Nursing Services
§483.40 Behavioral health services
§483.45 Pharmacy Services
§483.50 Laboratory Radiology and
Other Diagnostic Services
§483.55 Dental Services
§483.60 Food and Nutrition Services
§483.65 Specialized Rehabilitative
Services
§483.70 Administration
§483.75 Quality Assurance and
Performance Improvement
§483.80 Infection Control
F Tag 880 lives here
§483.85 Compliance and Ethics
Program
§483.90 Physical Environment
§483.95 Training Requirements
27
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
CMS Conditions of Participation (CoP) -
Interpretive Guidelines for Infection Control
• Develop and maintain Infection control program
• Provide a safe, sanitary environment
• Prevent the development and transmission of communicable diseases
• Surveillance must be systematic (i.e., infections must be logged)
• Support by leadership:
• Ensure problems identified by infection control are addressed
• Take responsibility for corrective action plans when problems are
ide
ntified
CMS Nursing Homes
(www.cms.gov/Medicare/Provider-Enrollment-and-
Certification/GuidanceforLawsAndRegulations/Nursing-Homes)
CMS Appendix A - Survey Protocol, Regulations and Interpretive Guidelines for Hospitals (PDF)
(www.cms.gov/Regulations-and-
Guidance/Guidance/Manuals/downloads/som107ap_a_hospitals.pdf)
28
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Finding Federal Regulations
Centers for Medicare and Medicaid Services (CMS)
(www.cms.gov)
CMS Regulations & Guidance
(www.cms.gov/home/regsguidance.asp)
CMS Hospital Center
(www.cms.gov/Center/Provider-Type/Hospital-Center?redirect=/center/hospital.asp)
CMS 1716-P LTC Prospective Payment – Proposed Rule
(www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2020-
IPPS-Proposed-Rule-Home-Page-Items/FY2020-IPPS-Proposed-Rule-Regulations)
CMS Conditions of Participations (CoPs)
(www.cms.gov/Regulations-and-
Guidance/Legislation/CFCsAndCoPs/index?redirect=/CFCsAndCoPs/06_Hospitals.asp)
CMS Interpretive Guidelines (PDF) revised 2-21-2020
(www.cms.gov/Regulations-and-
Guidance/Guidance/Manuals/downloads/SOM107AP_a_hospitals.pdf)
29
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
CMS Value-Based Purchasing Program
• Participating facilities in CMS quality/incentive reporting
programs are required to track and report HAI to NHSN; NHSN
shares data with CMS
• Penalties from CMS to facilities who do not show improvement
of healthcare acquired conditions
• Conditions include CLABSI, CDI, and CAUTI
• Up to 2% of Medicare claims dollars can be withheld
CMS Value Based Purchasing
(www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-
Based-Programs/HVBP/Hospital-Value-Based-Purchasing)
SNF Value-Based Purchasing Program: FAQs (PDF)
(www.cms.gov/files/document/snf-vbp-faqs-august-2020.pdf)
32

HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Summary
• There are many mandates and influencers that affect
infection prevention practices
• The IP must be familiar and stay informed with mandates
and influencers to facilitate compliance in their facility
33
HEALTHCARE-ASSOCIATED INFECTIONS PROGRAM
Questions?
For more information,
please contact
HAIProgr[email protected]a.gov
Include “SNF IP Training
Class” in the subject line
Post Test
Now that you have
completed this module,
Click on the “Post Test”
link when it pops up
To Return to
Learning Stream
and take the post test
If the Post Test link does not pop up,
you will be sent a link via e-mail
34