
CS310021-D
6/27/2024
Report
y Suspected cases of reportable vaccine-preventable diseases or outbreaks to
the local or state health department
y Clinically signicant adverse events to the Vaccine Adverse Event Reporting System at
www.vaers.hhs.gov or 800-822-7967
Questions or comments
Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Spanish,
8 a.m.–8 p.m. ET, Monday through Friday, excluding holidays.
Helpful information
y Complete Advisory Committee on Immunization Practices (ACIP) recommendations:
www.cdc.gov/vaccines/hcp/acip-recs/index.html
y ACIP Shared Clinical Decision-Making Recommendations:
www.cdc.gov/vaccines/acip/acip-scdm-faqs.html
y General Best Practice Guidelines for Immunization
www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
y Vaccine information statements: www.cdc.gov/vaccines/hcp/vis/index.html
y Manual for the Surveillance of Vaccine-Preventable Diseases
(including case identication and outbreak response):
www.cdc.gov/vaccines/pubs/surv-manual
How to use the adult immunization schedule
1
Determine
recommended
vaccinations
by age
(Table1)
2
Assess need
for additional
recommended
vaccinations by
medical
condition or
other indication
(Table2)
3
Review vaccine
types, dosing
frequencies and
intervals, and
considerations for
special situations
(Notes)
4
Review
contraindications
and precautions
for vaccine types
(Appendix)
5
Review new
or updated
ACIP guidance
(Addendum)
Recommended by the Advisory Committee on Immunization Practices (www.cdc.gov/vaccines/
acip) and approved by the Centers for Disease Control and Prevention (www.cdc.gov), American
College of Physicians (www.acponline.org), American Academy of Family Physicians (www.aafp.
org), American College of Obstetricians and Gynecologists (www.acog.org), American College
of Nurse-Midwives (www.midwife.org), American Academy of Physician Associates (www.aapa.
org), American Pharmacists Association (www.pharmacist.com), and Society for Healthcare
Epidemiology of America (www.shea-online.org).
Vaccines in the Adult Immunization Schedule*
Vaccine Abbreviation(s) Trade name(s)
COVID-19 vaccine
1vCOV-mRNA
Comirnaty®/Pzer-BioNTech COVID-19 Vaccine
Spikevax®/Moderna COVID-19 Vaccine
1vCOV-aPS Novavax COVID-19 Vaccine
Haemophilus inuenzae type b vaccine Hib
ActHIB®
Hiberix®
PedvaxHIB®
Hepatitis A vaccine HepA
Havrix®
Vaqta®
Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix®
Hepatitis B vaccine HepB
Engerix-B®
Heplisav-B®
PreHevbrio®
Recombivax HB®
Human papillomavirus vaccine HPV Gardasil 9®
Inuenza vaccine (inactivated) IIV4 Many brands
Inuenza vaccine (live, attenuated) LAIV4 FluMist® Quadrivalent
Inuenza vaccine (recombinant) RIV4 Flublok® Quadrivalent
Measles, mumps, and rubella vaccine MMR
M-M-R II®
Priorix®
Meningococcal serogroups A, C, W, Y vaccine
MenACWY-CRM
MenACWY-TT
Menveo®
MenQuad®
Meningococcal serogroup B vaccine
MenB-4C
MenB-FHbp
Bexsero®
Trumenba®
Meningococcal serogroup A, B, C, W, Y vaccine
MenACWY-TT/
MenB-FHbp
Penbraya™
Mpox vaccine Mpox Jynneos®
Pneumococcal conjugate vaccine
PCV15
PCV20
Vaxneuvance™
Prevnar 20™
Pneumococcal polysaccharide vaccine PPSV23 Pneumovax 23®
Poliovirus vaccine IPV Ipol®
Respiratory syncytial virus vaccine RSV
Arexvy®
Abrysvo™
Tetanus and diphtheria toxoids Td
Tenivac®
Tdvax™
Tetanus and diphtheria toxoids and acellular
pertussis vaccine
Tdap
Adacel®
Boostrix®
Varicella vaccine VAR Varivax®
Zoster vaccine, recombinant RZV Shingrix
* Administer recommended vaccines if vaccination history is incomplete or unknown. Do not restart or add doses to vaccine
series if there are extended intervals between doses. The use of trade names is for identication purposes only and does not
imply endorsement by the ACIP or CDC.
Download the CDC Vaccine Schedules app for providers at
www.cdc.gov/vaccines/schedules/hcp/schedule-app.html.
Scan QR code
for access to
online schedule
Recommended Adult Immunization Schedule
for ages 19years or older
UNITED STATES
2024

Vaccine 19–26 years 27–49 years 50–64 years ≥65 years
COVID-19 1 or more doses of updated (2023–2024 Formula) vaccine (See Notes)
Inuenza inactivated (IIV4) or
Inuenza recombinant (RIV4)
1 dose annually
Inuenza live, attenuated
(LAIV4)
1 dose annually
Respiratory Syncytial Virus
(RSV)
Seasonal administration during pregnancy. See Notes. >60 years
Tetanus, diphtheria, pertussis
(Tdap or Td)
1 dose Tdap each pregnancy; 1 dose Td/Tdap for wound management (see notes)
1 dose Tdap, then Td or Tdap booster every 10 years
Measles, mumps, rubella
(MMR)
1 or 2 doses depending on indication
(if born in 1957 or later)
For healthcare personnel,
see notes
Varicella
(VAR)
2 doses
(if born in 1980 or later)
2 doses
Zoster recombinant
(RZV)
2 doses for immunocompromising conditions (see notes) 2 doses
Human papillomavirus
(HPV)
2 or 3 doses depending on age at
initial vaccination or condition
27 through 45 years
Pneumococcal
(PCV15, PCV20, PPSV23)
See Notes
See Notes
Hepatitis A
(HepA)
2, 3, or 4 doses depending on vaccine
Hepatitis B
(HepB)
2, 3, or 4 doses depending on vaccine or condition
Meningococcal A, C, W, Y
(MenACWY)
1 or 2 doses depending on indication, see notes for booster recommendations
Meningococcal B
(MenB)
2 or 3 doses depending on vaccine and indication, see notes for booster recommendations
Haemophilus inuenzae type b
(Hib)
1 or 3 doses depending on indication
Mpox
oror
Recommended vaccination for adults who meet age requirement,
lack documentation of vaccination, or lack evidence of immunity
Recommended vaccination for adults with an
additional risk factor or another indication
Recommended vaccination based on shared
clinical decision-making
No recommendation/
Not applicable
19 through 23 years
2, 3, or 4 doses depending on vaccine or condition
Table 1
Recommended Adult Immunization Schedule by Age Group, United States, 2024

Table 2
Recommended Adult Immunization Schedule by Medical Condition or Other Indication, United States, 2024
Always use this table in conjunction with Table 1 and the Notes that follow. Medical conditions or indications are often not mutually exclusive. If multiple medical conditions or indications are present, refer to
guidance in all relevant columns. See Notes for medical conditions or indications not listed.
VACCINE Pregnancy
Immunocompromised
(excluding HIV
infection)
HIV infection CD4
percentage and count
Men who have sex
with men
Asplenia,
complement
deciency
Heart or lung
disease
Kidney failure,
End-stage
renal disease
or on dialysis
Chronic liver
disease;
alcoholism
a
Diabetes
Healthcare
Personnel
b
<15% or
<200mm
3
≥15% and
≥200mm
3
COVID-19 See Notes
IIV4 or RIV4 1 dose annually
LAIV4
1 dose annually
if age 19–49 years
RSV
Seasonal
administration.
See Notes
See Notes See Notes
Tdap or Td
Tdap: 1 dose
each pregnancy
1 dose Tdap, then Td or Tdap booster every 10 years
MMR *
VAR * See Notes
RZV See Notes
HPV * 3 dose series if indicated
Pneumococcal
HepA
Hep B See Notes
Age ≥ 60 years
MenACWY
MenB
Hib HSCT: 3 doses
c
Asplenia:
1 dose
Mpox See Notes See Notes See Notes
Recommended for all adults
who lack documentation of
vaccination, OR lack evidence
of immunity
Not recommended for all
adults, but recommended
for some adults based on
either age OR increased
risk for or severe outcomes
from disease
Recommended based
on shared clinical
decision-making
Recommended for all adults,
and additional doses may be
necessary based on medical
condition or other indications.
See Notes.
Precaution: Might be
indicated if benet of
protection outweighs
risk of adverse reaction
Contraindicated or not
recommended
*Vaccinate after pregnancy,
if indicated
No Guidance/
Not Applicable
1 dose annually if age 19–49 years
a. Precaution for LAIV4 does not apply to alcoholism. b. See notes for inuenza; hepatitis B; measles, mumps, and rubella; and varicella vaccinations. c. Hematopoietic stem cell transplant.
For vaccination recommendations for persons ages
18 years or younger, see the Recommended Child and
Adolescent Immunization Schedule, 2024: www.cdc.gov/
vaccines/schedules/hcp/child-adolescent.html
Additional Information
y For calculating intervals between doses, 4 weeks =
28 days. Intervals of ≥4 months are determined by
calendar months.
y Within a number range (e.g., 12–18), a dash (–) should
be read as “through.”
y Vaccine doses administered ≤4 days before the
minimum age or interval are considered valid. Doses
of any vaccine administered ≥5 days earlier than the
minimum age or minimum interval should not be
counted as valid and should be repeated. The repeat
dose should be spaced after the invalid dose by the
recommended minimum interval. For further details,
see Table 3-2, Recommended and minimum ages
and intervals between vaccine doses, in General Best
Practice Guidelines for Immunization at www.cdc.gov/
vaccines/hcp/acip-recs/general-recs/timing.html.
y Information on travel vaccination requirements and
recommendations is available at www.cdc.gov/travel/.
y For vaccination of persons with immunodeciencies,
see Table 8-1, Vaccination of persons with primary and
secondary immunodeciencies, in General Best Practice
Guidelines for Immunization at www.cdc.gov/vaccines/
hcp/acip-recs/general-recs/immunocompetence.html
y For information about vaccination in the setting of a
vaccine-preventable disease outbreak, contact your
state or local health department.
y The National Vaccine Injury Compensation Program
(VICP) is a no-fault alternative to the traditional legal
system for resolving vaccine injury claims. All vaccines
included in the adult immunization schedule except
PPSV23, RSV, RZV, Mpox, and COVID-19 vaccines are
covered by the National Vaccine Injury Compensation
Program (VICP). Mpox and COVID-19 vaccines are
covered by the Countermeasures Injury Compensation
Program (CICP). For more information, see www.hrsa.
gov/vaccinecompensation or www.hrsa.gov/cicp.
COVID-19 vaccination
Routine vaccination
Age 19 years or older
y Unvaccinated:
- 1 dose of updated (2023–2024 Formula) Moderna or
Pzer-BioNTech vaccine
- 2-dose series of updated (2023–2024 Formula)
Novavax at 0, 3–8 weeks
y Previously vaccinated* with 1 or more doses of any
COVID-19 vaccine: 1 dose of any updated (2023–2024
Formula) COVID-19 vaccine administered at least 8
weeks after the most recent COVID-19 vaccine dose.
Special situations
Persons who are moderately or severely
immunocompromised**
y Unvaccinated:
- 3-dose series of updated (2023–2024 Formula)
Moderna at 0, 4, 8 weeks
- 3-dose series of updated (2023–2024 Formula)
Pzer-BioNTech at 0, 3, 7 weeks
- 2-dose series of updated (2023–2024 Formula)
Novavax at 0, 3 weeks
y Previously vaccinated* with 1 dose of any
Moderna: 2-dose series of updated (2023–2024
Formula) Moderna at 0, 4 weeks (minimum interval
between previous Moderna dose and dose 1: 4 weeks)
y Previously vaccinated* with 2 doses of any
Moderna: 1 dose of updated (2023–2024 Formula)
Moderna at least 4 weeks after most recent dose.
y Previously vaccinated* with 1 dose of any Pzer-
BioNTech: 2-dose series of updated (2023–2024
Formula) Pzer-BioNTech at 0, 4 weeks (minimum
interval between previous Pzer-BioNTech dose and
dose 1: 3 weeks).
y Previously vaccinated* with 2 doses of any Pzer-
BioNTech: 1 dose of updated (2023–2024 Formula)
Pzer-BioNTech at least 4 weeks after most recent
dose.
y Previously vaccinated* with 3 or more doses of any
Moderna or Pzer-BioNTech: 1 dose of any updated
(2023–2024 Formula) COVID-19 vaccine at least 8
weeks after the most recent dose.
y Previously vaccinated* with 1 or more doses of
Janssen or Novavax with or without dose(s) of any
Original monovalent or bivalent COVID-19 vaccine:
1 dose of any updated (2023–2024 Formula) of
COVID-19 vaccine at least 8 weeks after the most
recent dose.
There is no preferential recommendation for the use
of one COVID-19 vaccine over another when more
than one recommended age-appropriate vaccine is
available.
Current COVID-19 vaccine information available at
www.cdc.gov/covidschedule. For information on
Emergency Use Authorization (EUA) indications for
COVID-19 vaccines, see www.fda.gov/emergency-
preparedness-and-response/coronavirus-disease-2019-
covid-19/covid-19-vaccines.
*Note: Previously vaccinated is dened as having
received any Original monovalent or bivalent COVID-19
vaccine (Janssen, Moderna, Novavax, Pzer-BioNTech)
prior to the updated 2023 –2024 formulation.
**Note: Persons who are moderately or severely
immunocompromised have the option to receive
one additional dose of updated (2023 –2024 Formula)
COVID-19 vaccine at least 2 months following the
last recommended updated (2023– 2024 Formula)
COVID-19 vaccine dose. Further additional updated
(2023–2024 Formula) COVID-19 vaccine dose(s) may
be administered, informed by the clinical judgement
of a healthcare provider and personal preference and
circumstances. Any further additional doses should be
administered at least 2 months after the last updated
(2023–2024 Formula) COVID-19 vaccine dose.
Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Notes

Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Notes
Haemophilus inuenzae type b vaccination
Special situations
y Anatomical or functional asplenia (including sickle
cell disease): 1 dose if previously did not receive
Hib vaccine; if elective splenectomy, 1 dose preferably
at least 14 days before splenectomy.
y Hematopoietic stem cell transplant (HSCT):
3-dose series 4 weeks apart starting 6–12 months
after successful transplant, regardless of
Hib vaccination history.
Hepatitis A vaccination
Routine vaccination
y Any person who is not fully vaccinated and requests
vaccination (identication of risk factor not required):
2-dose series HepA (Havrix 6–12 months apart or
Vaqta 6–18 months apart [minimum interval:
6 months]) or 3-dose series HepA-HepB (Twinrix at 0,
1, 6 months [minimum intervals: dose 1 to
dose 2: 4 weeks / dose 2 to dose 3: 5 months])
Special situations
y Any person who is not fully vaccinated and who is at
risk for hepatitis A virus infection: 2-dose series HepA
or 3-dose series HepA-HepB as above. Risk factors for
hepatitis A virus infection include:
- Chronic liver disease (e.g., persons with
hepatitis B, hepatitis C, cirrhosis, fatty liver disease,
alcoholic liver disease, autoimmune hepatitis,
alanine aminotransferase [ALT] or aspartate
aminotransferase [AST] level greater than
twice the upper limit of normal)
- HIV infection
- Men who have sex with men
- Injection or noninjection drug use
- Persons experiencing homelessness
- Work with hepatitis A virus in research
laboratory or with nonhuman primates
with hepatitis A virus infection
- Travel in countries with high or intermediate
endemic hepatitis A (HepA-HepB [Twinrix] may
be administered on an accelerated schedule of
3 doses at 0, 7, and 21–30 days, followed by a
booster dose at 12 months)
- Close, personal contact with international adoptee
(e.g., household or regular babysitting) in rst 60 days
after arrival from country with high or intermediate
endemic hepatitis A (administer dose 1 as soon
as adoption is planned, at least 2 weeks before
adoptee’s arrival)
- Pregnancy if at risk for infection or severe outcome
from infection during pregnancy
- Settings for exposure, including health care settings
targeting services to injection or noninjection drug
users or group homes and nonresidential day care
facilities for developmentally disabled persons
(individual risk factor screening not required)
Hepatitis B vaccination
Routine vaccination
y Age 19 through 59 years: complete a 2- or 3- or
4-dose series
- 2-dose series only applies when 2 doses of
Heplisav-B* are used at least 4 weeks apart
- 3-dose series Engerix-B, PreHevbrio*, or Recombivax
HB at 0, 1, 6 months [minimum intervals: dose 1 to
dose 2: 4 weeks / dose 2 to dose 3: 8 weeks / dose 1
to dose 3: 16 weeks])
- 3-dose series HepA-HepB (Twinrix at 0, 1, 6 months
[minimum intervals: dose 1 to dose 2:
4 weeks / dose 2 to dose 3: 5 months])
- 4-dose series HepA-HepB (Twinrix) accelerated
schedule of 3 doses at 0, 7, and 21–30 days, followed
by a booster dose at 12 months
*Note: Heplisav-B and PreHevbrio are not
recommended in pregnancy due to lack of safety data
in pregnant persons.
y Age 60 years or older without known risk factors
for hepatitis B virus infection may receive a
HepB vaccine series.
y Age 60 years or older with known risk factors for
hepatitis B virus infection should receive a
HepB vaccine series.
y Any adult age 60 years of age or older who requests
HepB vaccination should receive a HepB vaccine
series.
- Risk factors for hepatitis B virus infection include:
Chronic liver disease e.g., persons with hepatitis C,
cirrhosis, fatty liver disease, alcoholic liver disease,
autoimmune hepatitis, alanine aminotransferase
(ALT) or aspartate aminotransferase (AST) level
greater than twice the upper limit of normal
HIV infection
Sexual exposure risk e.g., sex partners of hepatitis
B surface antigen (HBsAg)-positive persons, sexually
active persons not in mutually monogamous
relationships, persons seeking evaluation or
treatment for a sexually transmitted infection,
men who have sex with men
Current or recent injection drug use
Percutaneous or mucosal risk for exposure
to blood e.g., household contacts of HBsAg-
positive persons, residents and sta of facilities for
developmentally disabled persons, health care and
public safety personnel with reasonably anticipated
risk for exposure to blood or blood-contaminated
body uids; persons on maintenance dialysis
(including in-center or home hemodialysis and
peritoneal dialysis), persons who are predialysis, and
patients with diabetes*
Incarceration
Travel in countries with high or intermediate
endemic hepatitis B
*Age 60 years or older with diabetes: Based on
shared clinical decision making, 2-, 3-, or 4-dose series
as above.

Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Notes
Special situations
y Patients on dialysis: complete a 3- or 4-dose series
- 3-dose series Recombivax HB at 0, 1, 6 months
(Note: Use Dialysis Formulation 1 mL = 40 mcg)
- 4-dose series Engerix-B at 0, 1, 2, and 6 months
(Note: Use 2 mL dose instead of the normal adult
dose of 1 mL)
Human papillomavirus vaccination
Routine vaccination
y All persons up through age 26 years: 2- or 3-dose
series depending on age at initial vaccination or
condition
- Age 9–14 years at initial vaccination and received 1
dose or 2 doses less than 5 months apart:
1 additional dose
- Age 9–14 years at initial vaccination and received
2 doses at least 5 months apart: HPV vaccination
series complete, no additional dose needed
- Age 15 years or older at initial vaccination: 3-dose
series at 0, 1–2 months, 6 months (minimum
intervals: dose 1 to dose 2: 4 weeks / dose 2 to dose 3:
12 weeks / dose 1 to dose 3: 5 months; repeat dose if
administered too soon)
y No additional dose recommended when any HPV
vaccine series of any valency has been completed
using the recommended dosing intervals.
Shared clinical decision-making
y Adults age 27–45 years: Based on shared clinical
decision-making, complete a 2-dose series (if initiated
age 9-14 years) or 3-dose series (if initiated ≥15 years)
For additional information on shared clinical decision-
making for HPV; see www.cdc.gov/vaccines/hcp/admin/
downloads/isd-job-aid-scdm-hpv-shared-clinical-
decision-making-hpv.pdf
Special situations
y Age ranges recommended above for routine and
catch-up vaccination or shared clinical decision-
making also apply in special situations
- Immunocompromising conditions, including HIV
infection: 3-dose series, even for those who initiate
vaccination at age 9 through 14 years.
- Pregnancy: Pregnancy testing is not needed before
vaccination. HPV vaccination is not recommended
until after pregnancy. No intervention needed if
inadvertently vaccinated while pregnant.
Inuenza vaccination
Routine vaccination
y Age 19 years or older: 1 dose any inuenza vaccine
appropriate for age and health status annually.
- Age 65 years or older: Any one of quadrivalent
high-dose inactivated inuenza vaccine (HD-
IIV4), quadrivalent recombinant inuenza vaccine
(RIV4), or quadrivalent adjuvanted inactivated
inuenza vaccine (aIIV4) is preferred. If none of these
three vaccines are available, then any other age-
appropriate inuenza vaccine should be used.
y For the 2023–2024 season, see www.cdc.gov/mmwr/
volumes/72/rr/rr7202a1.htm
y For the 2024–2025 season, see the 2024–2025 ACIP
inuenza vaccine recommendations.
Special situations
y Close contacts (e.g., caregivers, healthcare
workers) of severely immunosuppressed persons
who require a protected environment: should not
receive LAIV4. If LAIV4 is given, they should avoid
contact with/caring for such immunosuppressed
persons for 7 days after vaccination.
Note: Persons with an egg allergy can receive any
inuenza vaccine (egg-based and non-egg based)
appropriate for age and health status.
Measles, mumps, and rubella vaccination
Routine vaccination
y No evidence of immunity to measles, mumps, or
rubella: 1 dose
- Evidence of immunity: Born before 1957 (except for
health care personnel, see below), documentation
of receipt of MMR vaccine, laboratory evidence of
immunity or disease (diagnosis of disease without
laboratory conrmation is not evidence of immunity)
Special situations
y Pregnancy with no evidence of immunity to rubella:
MMR contraindicated during pregnancy;
after pregnancy (before discharge from
health care facility), 1 dose
y Nonpregnant persons of childbearing age with no
evidence of immunity to rubella: 1 dose
y HIV infection with CD4 percentages ≥15% and CD4
count ≥200 cells/mm
3
for at least 6 months and
no evidence of immunity to measles, mumps, or
rubella: 2-dose series at least 4 weeks apart; MMR
contraindicated for HIV infection with CD4 percentage
<15% or CD4 count <200 cells/mm
3
y Severe immunocompromising conditions:
MMR contraindicated
y Students in postsecondary educational institutions,
international travelers, and household or close,
personal contacts of immunocompromised persons
with no evidence of immunity to measles, mumps,
or rubella: 2-dose series at least 4 weeks apart if
previously did not receive any doses of MMR or 1 dose
if previously received 1 dose MMR
y In mumps outbreak settings, for information about
additional doses of MMR (including 3rd dose of MMR), see
www.cdc.gov/mmwr/volumes/67/wr/mm6701a7.htm

Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Notes
y Health care personnel:
- Born before 1957 with no evidence of immunity
to measles, mumps, or rubella: Consider 2-dose
series at least 4 weeks apart for protection against
measles or mumps or 1 dose for protection against
rubella
- Born in 1957 or later with no evidence of immunity
to measles, mumps, or rubella: 2-dose series at
least 4 weeks apart for protection against measles
or mumps or at least 1 dose for protection against
rubella
Meningococcal vaccination
Special situations for MenACWY
y Anatomical or functional asplenia (including sickle
cell disease), HIV infection, persistent complement
component deciency, complement inhibitor
(e.g., eculizumab, ravulizumab) use: 2-dose series
MenACWY (Menveo or MenQuad) at least 8 weeks
apart and revaccinate every 5 years if risk remains
y Travel in countries with hyperendemic or epidemic
meningococcal disease, or microbiologists routinely
exposed to Neisseria meningitidis: 1 dose MenACWY
(Menveo or MenQuad) and revaccinate every 5 years
if risk remains
y First-year college students who live in residential
housing (if not previously vaccinated at age
16 years or older) or military recruits: 1 dose
MenACWY (Menveo or MenQuad)
y For MenACWY booster dose recommendations for
groups listed under “Special situations” and in an
outbreak setting (e.g., in community or organizational
settings, or among men who have sex with men) and
additional meningococcal vaccination information,
see www.cdc.gov/mmwr/volumes/69/rr/rr6909a1.htm
Shared clinical decision-making for MenB
y Adolescents and young adults age 16–23 years
(age 16–18 years preferred) not at increased risk
for meningococcal disease: Based on shared clinical
decision-making, 2-dose series MenB-4C (Bexsero)
at least 1 month apart or 2-dose series MenB-FHbp
(Trumenba) at 0, 6 months (if dose 2 was administered
less than 6 months after dose 1, administer dose 3
at least 4 months after dose 2); MenB-4C and
MenB-FHbp are not interchangeable (use same
product for all doses in series).
For additional information on shared clinical decision-
making for MenB, see www.cdc.gov/vaccines/hcp/
admin/downloads/isd-job-aid-scdm-mening-b-shared-
clinical-decision-making.pdf
Special situations for MenB
y Anatomical or functional asplenia (including sickle
cell disease), persistent complement component
deciency, complement inhibitor (e.g., eculizumab,
ravulizumab) use, or microbiologists routinely
exposed to Neisseria meningitidis:
2-dose primary series MenB-4C (Bexsero) at least
1 month apart or 3-dose primary series
MenB-FHbp (Trumenba) at 0, 1–2, 6 months
(if dose 2 was administered at least 6 months after
dose 1, dose 3 not needed; if dose 3 is administered
earlier than 4 months after dose 2, a fourth dose
should be administered at least 4 months after dose
3); MenB-4C and MenB-FHbp are not interchangeable
(use same product for all doses in series); 1 dose MenB
booster 1 year after primary series and revaccinate
every 2–3 years if risk remains.
y Pregnancy: Delay MenB until after pregnancy unless
at increased risk and vaccination benets outweigh
potential risks.
y For MenB booster dose recommendations for groups
listed under “Special situations” and in an outbreak
setting (e.g., in community or organizational settings
and among men who have sex with men) and
additional meningococcal vaccination information,
see www.cdc.gov/mmwr/volumes/69/rr/rr6909a1.htm
Note: MenB vaccines may be administered
simultaneously with MenACWY vaccines if indicated,
but at a dierent anatomic site, if feasible.
Adults may receive a single dose of Penbraya as an
alternative to separate administration of MenACWY and
MenB when both vaccines would be given on the same
clinic day. For adults not at increased risk, if Penbraya
is used for dose 1 MenB, MenB-FHbp (Trumenba)
should be administered for dose 2 MenB. For adults
at increased risk of meningococcal disease, Penbraya
may be used for additional MenACWY and MenB doses
(including booster doses) if both would be given on
the same clinic day and at least 6 months have elapsed
since most recent Penbraya dose.
Mpox vaccination
Special situations
y Any person at risk for Mpox infection: 2-dose series,
28 days apart.
Risk factors for Mpox infection include:
- Persons who are gay, bisexual, and other MSM,
transgender or nonbinary people who in the past 6
months have had:
A new diagnosis of at least 1 sexually transmitted
disease
More than 1 sex partner
Sex at a commercial sex venue
Sex in association with a large public event in
a geographic area where Mpox transmission is
occurring
- Persons who are sexual partners of the persons
described above
- Persons who anticipate experiencing any of the
situations described above
Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Notes
y Pregnancy: There is currently no ACIP recommendation
for Jynneos use in pregnancy due to lack of safety data
in pregnant persons. Pregnant persons with any risk
factor described above may receive Jynneos.
y Healthcare personnel: Except in rare circumstances
(e.g. no available personal protective equipment),
healthcare personnel who do not have any of the
sexual risk factors described above should not receive
Jynneos.
For detailed information, see: www.cdc.gov/vaccines/
acip/meetings/downloads/slides-2023-10-25-26/04-
MPOX-Rao-508.pdf
Pneumococcal vaccination
Routine vaccination
y Age 65 years or older who have:
- Not previously received a dose of PCV13, PCV15,
or PCV20 or whose previous vaccination history is
unknown: 1 dose PCV15 OR 1 dose PCV20.
If PCV15 is used, administer 1 dose PPSV23 at least
1 year after the PCV15 dose (may use minimum
interval of 8 weeks for adults with an
immunocompromising condition,* cochlear
implant, or cerebrospinal uid leak).
- Previously received only PCV7: follow the
recommendation above.
- Previously received only PCV13: 1 dose PCV20 OR
1 dose PPSV23.
If PCV20 is selected, administer at least 1 year after
the last PCV13 dose.
If PPSV23 is selected, administer at least 1 year after
the last PCV13 dose (may use minimum interval of
8 weeks for adults with an immunocompromising
condition,* cochlear implant, or cerebrospinal
uid leak).
- Previously received only PPSV23: 1 dose PCV15 OR
1 dose PCV20. Administer either PCV15 or PCV20 at
least 1 year after the last PPSV23 dose.
If PCV15 is used, no additional PPSV23 doses are
recommended.
- Previously received both PCV13 and PPSV23 but
NO PPSV23 was received at age 65 years or older:
1 dose PCV20 OR 1 dose PPSV23.
If PCV20 is selected, administer at least 5 years after
the last pneumococcal vaccine dose.
If PPSV23 is selected, see dosing schedule at
www.cdc.gov/vaccines/vpd/pneumo/downloads/
pneumo-vaccine-timing.pdf.
- Previously received both PCV13 and PPSV23, AND
PPSV23 was received at age 65 years or older: Based
on shared clinical decision-making, 1 dose of PCV20
at least 5 years after the last pneumococcal vaccine
dose.
y For guidance on determining which pneumococcal
vaccines a patient needs and when, please refer to the
mobile app, which can be downloaded here: www.cdc.
gov/vaccines/vpd/pneumo/hcp/pneumoapp.html.
Special situations
y Age 19–64 years with certain underlying medical
conditions or other risk factors** who have:
- Not previously received a PCV13, PCV15, or PCV20
or whose previous vaccination history is unknown:
1 dose PCV15 OR 1 dose PCV20.
If PCV15 is used, administer 1 dose PPSV23
at least 1 year after the PCV15 dose (may use
minimum interval of 8 weeks for adults with
an immunocompromising condition,* cochlear
implant, or cerebrospinal uid leak).
- Previously received only PCV7: follow the
recommendation above.
- Previously received only PCV13: 1 dose PCV20 OR
1 dose PPSV23.
If PCV20 is selected, administer at least 1 year after
the PCV13 dose.
If PPSV23 is selected, see dosing schedule at
www.cdc.gov/vaccines/vpd/pneumo/downloads/
pneumo-vaccine-timing.pdf
- Previously received only PPSV23: 1 dose PCV15 OR
1 dose PCV20. Administer either PCV15 or PCV20 at
least 1 year after the last PPSV23 dose.
If PCV15 is used, no additional PPSV23 doses are
recommended.
- Previously received PCV13 and 1 dose of PPSV23:
1 dose PCV20 OR 1 dose PPSV23.
- If PCV20 is selected, administer at least 5 years after
the last pneumococcal vaccine dose.
- If PPSV23 is selected, see dosing schedule at
www.cdc.gov/vaccines/vpd/pneumo/downloads/
pneumo-vaccine-timing.pdf
y For guidance on determining which pneumococcal
vaccines a patient needs and when, please refer to the
mobile app which can be downloaded here: www.cdc.
gov/vaccines/vpd/pneumo/hcp/pneumoapp.html
*Note: Immunocompromising conditions
include chronic renal failure, nephrotic syndrome,
immunodeciencies, iatrogenic immunosuppression,
generalized malignancy, HIV infection, Hodgkin disease,
leukemia, lymphoma, multiple myeloma, solid organ
transplant, congenital or acquired asplenia, or sickle cell
disease or other hemoglobinopathies.
**Note: Underlying medical conditions or other
risk factors include alcoholism, chronic heart/liver/
lung disease, chronic renal failure, cigarette smoking,
cochlear implant, congenital or acquired asplenia,
CSF leak, diabetes mellitus, generalized malignancy,
HIV infection, Hodgkin disease, immunodeciencies,
iatrogenic immunosuppression, leukemia, lymphoma,
multiple myeloma, nephrotic syndrome, solid
organ transplant, or sickle cell disease or other
hemoglobinopathies.
Poliovirus vaccination
Routine vaccination
y Adults known or suspected to be unvaccinated
or incompletely vaccinated: administer remaining
doses (1, 2, or 3 IPV doses) to complete a 3-dose
primary series.* Unless there are specic reasons to
believe they were not vaccinated, most adults who
were born and raised in the United States can assume
they were vaccinated against polio as children.

Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Notes
Special situations
y Adults at increased risk of exposure to poliovirus
who completed primary series*: may administer one
lifetime IPV booster
*Note: Complete primary series consists of at least 3
doses of IPV or trivalent oral poliovirus vaccine (tOPV) in
any combination.
For detailed information, see: www.cdc.gov/vaccines/
vpd/polio/hcp/recommendations.html
Respiratory syncytial virus vaccination
Routine vaccination
y Pregnant at 32 weeks 0 days through 36 weeks and
6 days gestation from September through January
in most of the continental United States*: 1 dose
RSV vaccine (Abrysvo™). Administer RSV vaccine
regardless of previous RSV infection.
- Either maternal RSV vaccination or infant
immunization with nirsevimab (RSV monoclonal
antibody) is recommended to prevent respiratory
syncytial virus lower respiratory tract infection in
infants.
y All other pregnant persons: RSV vaccine not
recommended
There is currently no ACIP recommendation for RSV
vaccination in subsequent pregnancies. No data are
available to inform whether additional doses are
needed in later pregnancies.
Special situations
y Age 60 years or older: Based on shared clinical
decision-making, 1 dose RSV vaccine (Arexvy® or
Abrysvo™). Persons most likely to benet from
vaccination are those considered to be at increased
risk for severe RSV disease.** For additional
information on shared clinical decision-making for RSV
in older adults, see www.cdc.gov/vaccines/vpd/rsv/
downloads/provider-job-aid-for-older-adults-508.pdf
For further guidance, see www.cdc.gov/mmwr/
volumes/72/wr/mm7229a4.htm
*Note: Providers in jurisdictions with RSV seasonality
that diers from most of the continental United
States (e.g., Alaska, jurisdiction with tropical climate)
should follow guidance from public health authorities
(e.g., CDC, health departments) or regional medical
centers on timing of administration based on local RSV
seasonality. Refer to the 2024 Child and Adolescent
Immunization Schedule for considerations regarding
nirsevimab administration to infants.
**Note: Adults age 60 years or older who are at
increased risk for severe RSV disease include those
with chronic medical conditions such as lung diseases
(e.g., chronic obstructive pulmonary disease, asthma),
cardiovascular diseases (e.g., congestive heart failure,
coronary artery disease), neurologic or neuromuscular
conditions, kidney disorders, liver disorders,
hematologic disorders, diabetes mellitus, and moderate
or severe immune compromise (either attributable to
a medical condition or receipt of immunosuppressive
medications or treatment); those who are considered
to be frail; those of advanced age; those who reside in
nursing homes or other long-term care facilities; and
those with other underlying medical conditions or
factors that a health care provider determines might
increase the risk of severe respiratory disease.
Tetanus, diphtheria, and pertussis vaccination
Routine vaccination
y Previously did not receive Tdap at or after age
11 years*: 1 dose Tdap, then Td or Tdap every 10 years
Special situations
y Previously did not receive primary vaccination series
for tetanus, diphtheria, or pertussis: 1 dose Tdap
followed by 1 dose Td or Tdap at least 4 weeks later,
and a third dose of Td or Tdap 6–12 months later (Tdap
is preferred as rst dose and can be substituted for any
Td dose), Td or Tdap every 10 years thereafter.
y Pregnancy: 1 dose Tdap during each pregnancy,
preferably in early part of gestational weeks 27–36.
y Wound management: Persons with 3 or more doses
of tetanus-toxoid-containing vaccine: For clean and
minor wounds, administer Tdap or Td if more than
10 years since last dose of tetanus-toxoid-containing
vaccine; for all other wounds, administer Tdap or Td if
more than 5 years since last dose of tetanus-toxoid-
containing vaccine. Tdap is preferred for persons who
have not previously received Tdap or whose Tdap
history is unknown. If a tetanus-toxoid-containing
vaccine is indicated for a pregnant woman, use Tdap.
For detailed information, see www.cdc.gov/mmwr/
volumes/69/wr/mm6903a5.htm
*Note: Tdap administered at age 10 years may be
counted as the adolescent dose recommended at age
11–12 years
Varicella vaccination
Routine vaccination
y No evidence of immunity to varicella: 2-dose series
4–8 weeks apart if previously did not receive varicella-
containing vaccine (VAR or MMRV [measles-mumps-
rubella-varicella vaccine] for children); if previously
received 1 dose varicella-containing vaccine, 1 dose at
least 4 weeks after rst dose.
- Evidence of immunity: U.S.-born before 1980
(except for pregnant persons and health care
personnel [see below]), documentation of 2 doses
varicella-containing vaccine at least 4 weeks apart,
diagnosis or verication of history of varicella or
herpes zoster by a health care provider, laboratory
evidence of immunity or disease.
Special situations
y Pregnancy with no evidence of immunity to
varicella: VAR contraindicated during pregnancy;
after pregnancy (before discharge from health care
facility), 1 dose if previously received 1 dose varicella-
containing vaccine or dose 1 of 2-dose series
(dose 2: 4–8 weeks later) if previously did not receive
any varicella-containing vaccine, regardless of
whether U.S.-born before 1980.

2/29/2024 Centers for Disease Control and Prevention
|
Recommended Adult Immunization Schedule, United States, 2024
Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Notes
y Health care personnel with no evidence of immunity
to varicella: 1 dose if previously received 1 dose
varicella-containing vaccine; 2-dose series 4–8 weeks
apart if previously did not receive any varicella-
containing vaccine, regardless of whether U.S.-born
before 1980.
y HIV infection with CD4 percentages ≥15% and CD4
count ≥200 cells/mm
3
with no evidence of immunity:
Vaccination may be considered (2 doses 3 months
apart); VAR contraindicated for HIV infection with CD4
percentage <15% or CD4 count <200 cells/mm
3
y Severe immunocompromising conditions:
VAR contraindicated.
Zoster vaccination
Routine vaccination
y Age 50 years or older*: 2-dose series recombinant
zoster vaccine (RZV, Shingrix) 2–6 months apart
(minimum interval: 4 weeks; repeat dose if
administered too soon), regardless of previous
herpes zoster or history of zoster vaccine live
(ZVL, Zostavax) vaccination.
*Note: Serologic evidence of prior varicella is not
necessary for zoster vaccination. However, if serologic
evidence of varicella susceptibility becomes available,
providers should follow ACIP guidelines for varicella
vaccination rst. RZV is not indicated for the prevention
of varicella, and there are limited data on the use of
RZV in persons without a history of varicella or varicella
vaccination.
Special situations
y Pregnancy: There is currently no ACIP
recommendation for RZV use in pregnancy.
Consider delaying RZV until after pregnancy.
y Immunocompromising conditions (including
persons with HIV regardless of CD4 count)**: 2-dose
series recombinant zoster vaccine (RZV, Shingrix)
2–6 months apart (minimum interval: 4 weeks;
repeat dose if administered too soon). For detailed
information, see www.cdc.gov/shingles/vaccination/
immunocompromised-adults.html
**Note: If there is no documented history of varicella,
varicella vaccination, or herpes zoster, providers should
refer to the clinical considerations for use of RZV in
immunocompromised adults aged ≥19 years and the
ACIP varicella vaccine recommendations for further
guidance: www.cdc.gov/mmwr/volumes/71/wr/
mm7103a2.htm

Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
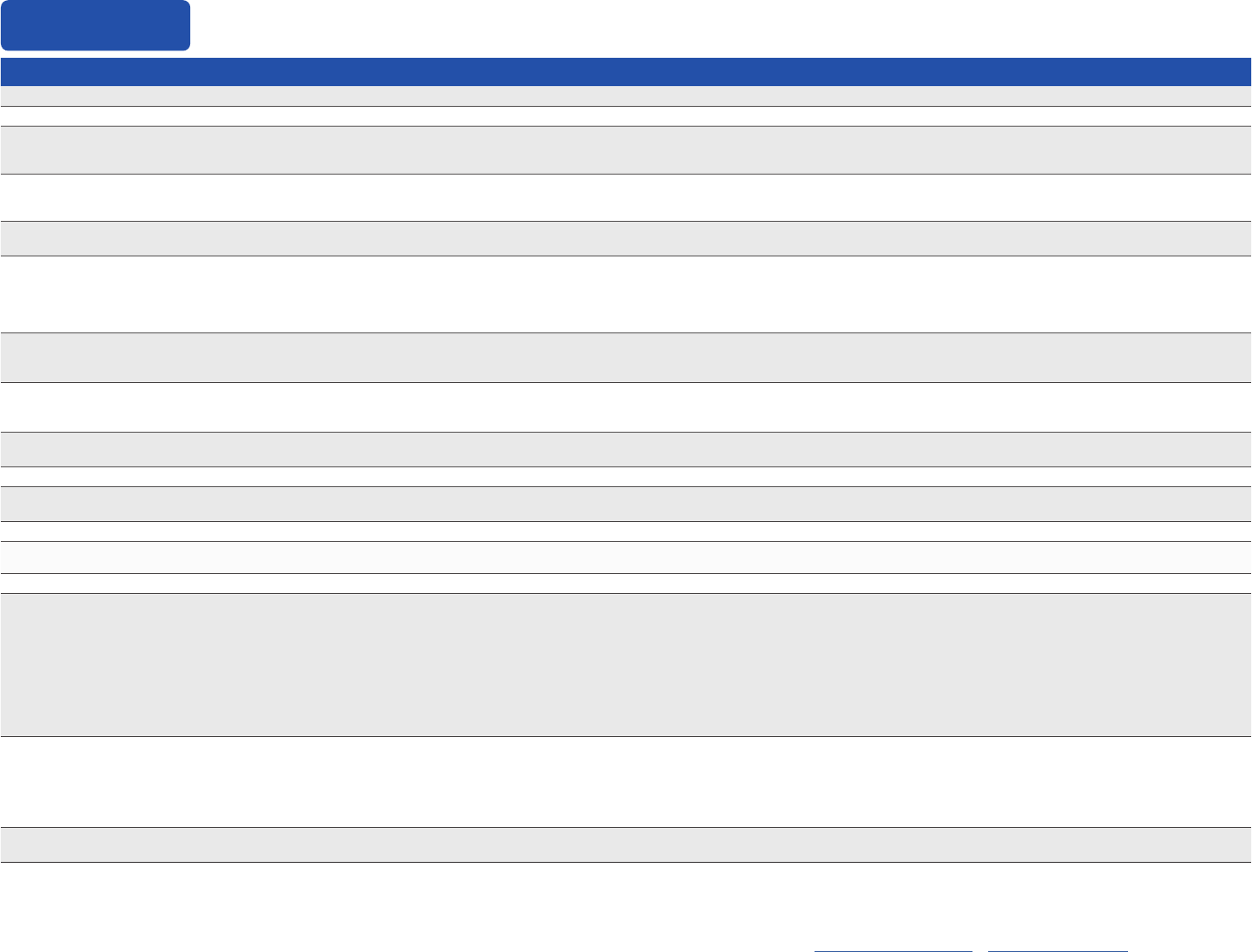
Appendix
Vaccines and Other
Immunizing Agents
Contraindicated or Not Recommended
1
Precautions
2
COVID-19 mRNA vaccines
[Pzer-BioNTech, Moderna]
• Severeallergic reaction (e.g., anaphylaxis) after a previous dose or to a component of
an mRNA COVID-19 vaccine
4
• Diagnosed non-severe allergy (e.g., urticaria beyond the injection site) to a component of
an mRNA COVID-19 vaccine
4
; or non-severe, immediate (onset less than 4 hours) allergic
reaction after administration of a previous dose of an mRNA COVID-19 vaccine
• Myocarditis or pericarditis within 3 weeks after a dose ofany COVID-19 vaccine
• Multisystem inammatory syndrome in children (MIS-C) or multisystem inammatory
syndrome in adults (MIS-A)
• Moderate or severe acute illness, with or without fever
COVID-19 protein subunit
vaccine
[Novavax]
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a component of a
Novavax COVID-19 vaccine
4
• Diagnosed non-severe allergy (e.g., urticaria beyond the injection site) to a component of
Novavax COVID-19 vaccine
4
; or non-severe, immediate (onset less than 4 hours) allergic
reaction after administration of a previous dose of a Novavax COVID-19 vaccine
• Myocarditis or pericarditis within 3 weeks after a dose ofany COVID-19 vaccine
• Multisystem inammatory syndrome in children (MIS-C) or multisystem inammatory
syndrome in adults (MIS-A)
• Moderate or severe acute illness, with or without fever
Inuenza, egg-based,
inactivated injectable (IIV4)
• Severe allergic reaction (e.g., anaphylaxis) after previous dose of any inuenza vaccine
(i.e., any egg-based IIV, ccIIV, RIV, or LAIV of any valency)
• Severe allergic reaction (e.g., anaphylaxis) to any vaccine component
3
(excluding egg)
• Guillain-Barré syndrome (GBS) within 6 weeks after a previous dose of any
type of inuenza vaccine
• Moderate or severe acute illness with or without fever
Inuenza, cell culture-based
inactivated injectable (ccIIV4)
[Flucelvax Quadrivalent]
• Severe allergic reaction (e.g., anaphylaxis) to any ccIIV of any valency, or to any
component
3
of ccIIV4
• Guillain-Barré syndrome (GBS) within 6 weeks after a previous dose of any
type of inuenza vaccine
• Persons with a history of severe allergic reaction (e.g., anaphylaxis) after a previous dose of
any egg-based IIV, RIV, or LAIV of any valency. If using ccIV4, administer in medical setting
under supervision of health care provider who can recognize and manage severe allergic
reactions. May consult an allergist.
• Moderate or severe acute illness with or without fever
Inuenza, recombinant
injectable (RIV4)
[Flublok Quadrivalent]
• Severe allergic reaction (e.g., anaphylaxis) to any RIV of any valency, or to any component
3
of RIV4 • Guillain-Barré syndrome (GBS) within 6 weeks after a previous dose of any
type of inuenza vaccine
• Persons with a history of severe allergic reaction (e.g., anaphylaxis) after a previous dose of
any egg-based IIV, ccIIV, or LAIV of any valency. If using RIV4, administer in medical setting
under supervision of health care provider who can recognize and manage severe allergic
reactions. May consult an allergist.
• Moderate or severe acute illness with or without fever
Inuenza, live attenuated
(LAIV4)
[Flumist Quadrivalent]
• Severe allergic reaction (e.g., anaphylaxis) after previous dose of any inuenza vaccine
(i.e., any egg-based IIV, ccIIV, RIV, or LAIV of any valency)
• Severe allergic reaction (e.g., anaphylaxis) to any vaccine component
3
(excluding egg)
• Anatomic or functional asplenia
• Immunocompromised due to any cause including, but not limited to, medications and
HIV infection
• Close contacts or caregivers of severely immunosuppressed persons who require a
protected environment
• Pregnancy
• Cochlear implant
• Active communication between the cerebrospinal uid (CSF) and the oropharynx,
nasopharynx, nose, ear, or any other cranial CSF leak
• Received inuenza antiviral medications oseltamivir or zanamivir within the previous
48 hours, peramivir within the previous 5 days, or baloxavir within the previous 17 days.
• Guillain-Barré syndrome (GBS) within 6 weeks after a previous dose of any
type of inuenza vaccine
• Asthma in persons aged 5 years or older
• Persons with underlying medical conditions (other than those listed under
contraindications) that might predispose to complications after wild-type inuenza virus
infection [e.g., chronic pulmonary, cardiovascular (except isolated hypertension), renal,
hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus)]
• Moderate or severe acute illness with or without fever
1. When a contraindication is present, a vaccine shouldNOTbe administered. Kroger A, Bahta L, Hunter P.ACIP General Best Practice Guidelines for Immunization.
2. When a precaution is present, vaccination should generally be deferred but might be indicated if the benet of protection from the vaccine outweighs the risk for an adverse reaction. Kroger A, Bahta L, Hunter P.ACIP General
Best Practice Guidelines for Immunization.
3. Vaccination providers should check FDA-approved prescribing information for the most complete and updated information, including contraindications, warnings, and precautions. SeePackage inserts for U.S.-licensed vaccines.
4. Seepackage insertsandFDA EUA fact sheetsfor a full list of vaccine ingredients. mRNA COVID-19 vaccines contain polyethylene glycol (PEG).
Contraindications and Precautions to Commonly Used Vaccines
Adapted from Table 4-1 inAdvisory Committee on Immunization Practices (ACIP) General Best Practice Guidelines for Immunization:Contraindication and Precautions, Prevention and Control of Seasonal Inuenza with
Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023–24 Inuenza Season | MMWR (cdc.gov), Contraindications and Precautions for COVID-19 Vaccination, and
Contraindications and Precautions for Jynneos Vaccination
Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Appendix
Vaccine Contraindicated or Not Recommended
1
Precautions
2
Haemophilus inuenzae type b (Hib) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Moderate or severe acute illness with or without fever
Hepatitis A (HepA) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
including neomycin • Moderate or severe acute illness with or without fever
Hepatitis B (HepB) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
including yeast
• Pregnancy: Heplisav-B and PreHevbrio are not recommended due to lack of safety data in pregnant persons.
Use other hepatitis B vaccines if HepB is indicated
4
• Moderate or severe acute illness with or without fever
Hepatitis A-Hepatitis B vaccine
(HepA-HepB)
[Twinrix]
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
including neomycin and yeast • Moderate or severe acute illness with or without fever
Human papillomavirus (HPV) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Pregnancy: HPV vaccination not recommended
• Moderate or severe acute illness with or without fever
Measles, mumps, rubella (MMR) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Severe immunodeciency (e.g., hematologic and solid tumors, receipt of chemotherapy, congenital immunodeciency,
long-term immunosuppressive therapy or patients with HIV infection who are severely immunocompromised)
• Pregnancy
• Family history of altered immunocompetence, unless veried clinically or by laboratory testing as immunocompetent
• Recent (≤11 months) receipt of antibody-containing blood product (specic
interval depends on product)
• History of thrombocytopenia or thrombocytopenic purpura
• Need for tuberculin skin testing or interferon-gamma release assay (IGRA) testing
• Moderate or severe acute illness with or without fever
Meningococcal ACWY (MenACWY)
(MenACWY-CRM) [Menveo]
(MenACWY-TT) [MenQuad]
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• For MenACWY-CRM only: severe allergic reaction to any diphtheria toxoid–or CRM197–containing vaccine
• For MenACWY-TT only: severe allergic reaction to a tetanus toxoid-containing vaccine
• Moderate or severe acute illness with or without fever
Meningococcal B (MenB)
MenB-4C [Bexsero]
MenB-FHbp [Trumenba]
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Pregnancy
• For MenB-4C only: Latex sensitivity
• Moderate or severe acute illness with or without fever
Meningococcal ABCWY
(MenACWY-TT/MenB-FHbp) [Penbraya]
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Severe allergic reaction to a tetanus toxoid-containing vaccine
• Moderate or severe acute illness, with or without fever
Mpox [Jynneos] • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Moderate or severe acute illness, with or without fever
Pneumococcal conjugate
(PCV15, PCV20)
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Severe allergic reaction (e.g., anaphylaxis) to any diphtheria-toxoid–containing vaccine or to its vaccine component
3
• Moderate or severe acute illness with or without fever
Pneumococcal polysaccharide (PPSV23) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Moderate or severe acute illness with or without fever
Poliovirus vaccine, inactivated (IPV) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Pregnancy
• Moderate or severe acute illness with or without fever
Respiratory syncytial virus vaccine (RSV) • Severe allergic reaction (e.g., anaphylaxis) to a vaccine component • Moderate or severe acute illness with or without fever
Tetanus, diphtheria, and acellular
pertussis (Tdap)
Tetanus, diphtheria (Td)
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• For Tdap only: Encephalopathy (e.g., coma, decreased level of consciousness, prolonged seizures), not attributable to
another identiable cause, within 7 days of administration of previous dose of DTP, DTaP, or Tdap
• Guillain-Barré syndrome (GBS) within 6 weeks after a previous dose of tetanus-
toxoid–containing vaccine
• History of Arthus-type hypersensitivity reactions after a previous dose of
diphtheria-toxoid– containing or tetanus-toxoid–containing vaccine; defer
vaccination until at least 10 years have elapsed since the last tetanus-toxoid–
containing vaccine
• Moderate or severe acute illness with or without fever
• For Tdap only: Progressive or unstable neurological disorder, uncontrolled
seizures, or progressive encephalopathy until a treatment regimen has been
established and the condition has stabilized
Varicella (VAR) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Severe immunodeciency (e.g., hematologic and solid tumors, receipt of chemotherapy, congenital immunodeciency,
long-term immunosuppressive therapy or patients with HIV infection who are severely immunocompromised)
• Pregnancy
• Family history of altered immunocompetence, unless veried clinically or by laboratory testing as immunocompetent
• Recent (≤11 months) receipt of antibody-containing blood product (specic
interval depends on product)
• Receipt of specic antiviral drugs (acyclovir, famciclovir, or valacyclovir) 24 hours
before vaccination (avoid use of these antiviral drugs for 14 days after vaccination)
• Use of aspirin or aspirin-containing products
• Moderate or severe acute illness with or without fever
Zoster recombinant vaccine (RZV) • Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
3
• Moderate or severe acute illness with or without fever
• Current herpes zoster infection
1. When a contraindication is present, a vaccine should NOT be administered. Kroger A, Bahta L, Hunter P. ACIP General Best Practice Guidelines for Immunization. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.html
2. When a precaution is present, vaccination should generally be deferred but might be indicated if the benet of protection from the vaccine outweighs the risk for an adverse reaction. Kroger A, Bahta L, Hunter P. ACIP General Best Practice Guidelines
for Immunization. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.html
3. Vaccination providers should check FDA-approved prescribing information for the most complete and updated information, including contraindications, warnings, and precautions. Package inserts for U.S.-licensed vaccines are available at www.fda.
gov/vaccines-blood-biologics/approved-products/vaccines-licensed-use-united-states.
4. For information on the pregnancy exposure registries for persons who were inadvertently vaccinated with Heplisav-B or PreHevbrio while pregnant, please visit heplisavbpregnancyregistry.com/ or www.prehevbrio.com/#safety.

Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2024
Addendum
Vaccine Recommendations Eective Date of Recommendation*
COVID-19 • ACIP recommends persons ≥65 years of age should receive an additional dose of 2023–2024 Formula COVID-19 vaccine.
• For detailed information, see: www.cdc.gov/covidschedule
February 28, 2024
COVID-19 (Moderna,
Pzer-BioNTech, Novavax)
• ACIP recommends 2024-2025 COVID-19 vaccines as authorized or approved by FDA in persons ≥6 months of age. June 27, 2024
Inuenza • ACIP rearms the recommendation for routine annual inuenza vaccination of all persons aged ≥6 months who do not have
contraindications.
• ACIP recommends high-dose inactivated (HD-IIV3) and adjuvanted inactivated (aIIV3) inuenza vaccines as acceptable options for
inuenza vaccination of solid organ transplant recipients aged 18 through 64 years who are on immunosuppressive medication regimens,
without a preference over other age-appropriate IIV3s or RIV3.
June 27, 2024
Pneumococcal conjugate
vaccine
• ACIP recommends PCV21 as an option for adults aged ≥19 years who currently have a recommendation to receive a dose of PCV. June 27, 2024
Respiratory syncytial
virus vaccine (RSV)
• ACIP recommends adults 75 years of age and older receive a single dose of RSV vaccine.
a,b
• ACIP recommends adults 60–74 years of age and older who are at increased risk of severe RSV disease receive a single dose of RSV
vaccine.
a,b
June 26, 2024
a
RSV vaccination is recommended as a single lifetime dose only. Persons who have already received RSV vaccination are NOT recommended to receive another dose.
b
These recommendations supplant the current recommendation that adults 60 years of age and older may receive RSV vaccination, using shared clinical decision-making. Adults 60–74 years of age who are not at
increased risk of severe RSV disease are NOT recommended to receive RSV vaccination.
C
CDC will publish Clinical Considerations that describe chronic medical conditions and other risk factors for severe RSV disease for use in this risk-based recommendation.
In addition to the recommendations presented in the previous sections of this immunization schedule, ACIP has approved the following recommendations by majority vote since October 26, 2023. The
following recommendations have been adopted by the CDC Director and are now ocial. Links are provided if these recommendations have been published in Morbidity and Mortality Weekly Report (MMWR).
*The eective date is the date when the CDC director adopted the recommendation and when the ACIP recommendation became ocial.
