APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
APHA Briefing Note 09/21
SARS-CoV-2 in Animals – Case Definition, Testing
and International Reporting Obligations
Initially issued: 24 March 2020. v3 updated 24 August 2021 and
31 August 2021.
Text highlighted in yellow has changed from version 3.
Purpose
To provide advice to veterinarians and veterinary diagnostic laboratories on testing for
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in animals, including
the case definition for non-domestic species kept in captivity.
The new regulatory framework
1
makes SARS-CoV-2 in mammals reportable in UK since
February 2021. The purpose of this note is to advise on the regulatory and professional
obligations for testing and reporting of positive test results to the Animal and Plant Health
Agency (APHA) in GB, and the Department of Agriculture, Environment and Rural Affairs
(DAERA) in Northern Ireland, as the relevant competent authorities. It also outlines the
UK’s international reporting obligations to the World Animal Health Organisation (OIE).
N.B. This note is applicable to ALL veterinary practitioners including Official Veterinarians
(OVs) and diagnostic laboratories considering SARS-CoV-2 testing.
To be read in conjunction with:
APHA Briefing Note 10/20 Advice for Veterinarians and their Clients on Pets and
COVID-19.
Published guidance on Working safely during coronavirus (COVID-19): guidance
from Step 4 - Guidance - GOV.UK (www.gov.uk)Background
1
The Zoonosis (Amendment) (England) Order 2021
The Zoonosis Amendment (Coronavirus) (Scotland) Order 2021
The Zoonosis (Amendment)(Wales) Order 2021
The Zoonosis (Amendment) Order (Northern Ireland) 2021

APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
1. There is emerging evidence that some animals can become infected with Severe Acute
Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (the causative agent of COVID-
19) following close contact with infected humans (a reverse zoonosis).
2. Globally, given the huge number of people that have been infected, only a small
number of cases of naturally acquired SARS-CoV-2 infections in animals have been
reported, the majority of which had had contact with infected households or people.
The majority of animal species where SARS-CoV-2 has been isolated are
Mustelidae
2
(particularly farmed mink); pet cats and dogs; large cats and; non-human
primates kept in captivity.
3
Given the recent isolation of SARS-CoV-2 in Asian small
clawed otters in captivity, and the theoretical susceptibility of wild Mustelinae to
infection by SARS-CoV-2, they have been included in the case definition below
3. There have been confirmed historical infections in one cat in the UK and one dog in the
British Isles, both from households with COVID-19 infected humans. The cat had been
co-infected with another respiratory virus and made a full recovery; the dog was
euthanised on welfare grounds due to a pre-existing illness. We still consider human-
to-human transmission is responsible for the burden of disease in the UK.
4. Government is aware that there is increasing interest amongst owners, veterinary
practitioners, universities, and veterinary diagnostic laboratories in testing for
SARS-CoV-2 in animals.
5. Testing for SARS-CoV-2 should only be undertaken where it is in the interest of the
health and welfare of the animal.
6. The animals which have tested positive for SARS-CoV-2 to date have generally shown
only mild respiratory signs and gastrointestinal distress. In the absence of a specific
treatment for the virus, testing for SARS-CoV-2 has not and should not result in altering
case management.
7. Collecting samples from animals must only be undertaken with due consideration to
the published guidance on Working safely during coronavirus (COVID-19): guidance
from Step 4 - Guidance - GOV.UK (www.gov.uk) and any additional guidance on this
matter issued by the Royal College of Veterinary Surgeons.
8. The detection of infection with SARS-CoV-2 in animals meets the criteria for reporting
to the World Animal Health Organisation (OIE) (of which the UK is a member country)
as an emerging infection in accordance with the OIE Terrestrial Animal Health Code.
9. SARS-CoV-2 is a reportable disease in animals in the UK. Veterinarians have now a
regulatory obligation to report positive test results to the competent authority (in GB, to
the Animal and Plant Health Agency, APHA. In Northern Ireland, to the Department for
Agriculture, Environment and Rural Affairs, DAERA). This obligation includes the
reporting of any positive results from UK pets, received from a foreign private
laboratory, by the UK PVS submitting the sample to these laboratories located abroad.
If you wish to consider testing an animal for SARS-CoV-2
10. Government does not offer a diagnostic service for SARS-CoV-2 infection in animals.
2
The Mustelinae family belongs to the Mustelidae order and includes animals such as ferrets, polecats, mink,
weasels, stoats, ermine, martens, and wolverines, but this is not an exhaustive list.
3
For an exhaustive list of animal species please visit Coronavirus (COVID-19): advice for people in England with
animals
APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
11. If you wish to consider private testing this should only be undertaken where it is in the
interest of the health and welfare of the animal.
12. It should be noted that under the Veterinary Surgeons Act 1966 sampling and testing
should generally provide a benefit to the animal i.e. be of diagnostic and treatment
value. Sampling to answer research investigations is regulated by The Home Office
under The Animal (Scientific Procedures) Act 1986, though clinical research may also
be conducted, under appropriate ethical review.
13. Government advises that testing should only be considered in animals which meet all
four of the criteria set out in the following case definitions. These have been assessed
based on the current scientific knowledge of SARS-CoV-2 infection in animals:
APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
14. For animals within the remit of Case Definition 1, private testing should take place as
per guidance in paragraphs 20-26. For animals within the Case Definition 2, testing
should take place as per guidance in paragraphs 27-33.
2
The Mustelinae family includes animals such as ferrets, polecats, mink, weasels, stoats, ermine, martens, and
wolverines, but this is not an exhaustive list.
15. In instances where the animal being considered for SARS-CoV-2 testing is from other
mammalian species not included in the case definitions in paragraph 14, the attending
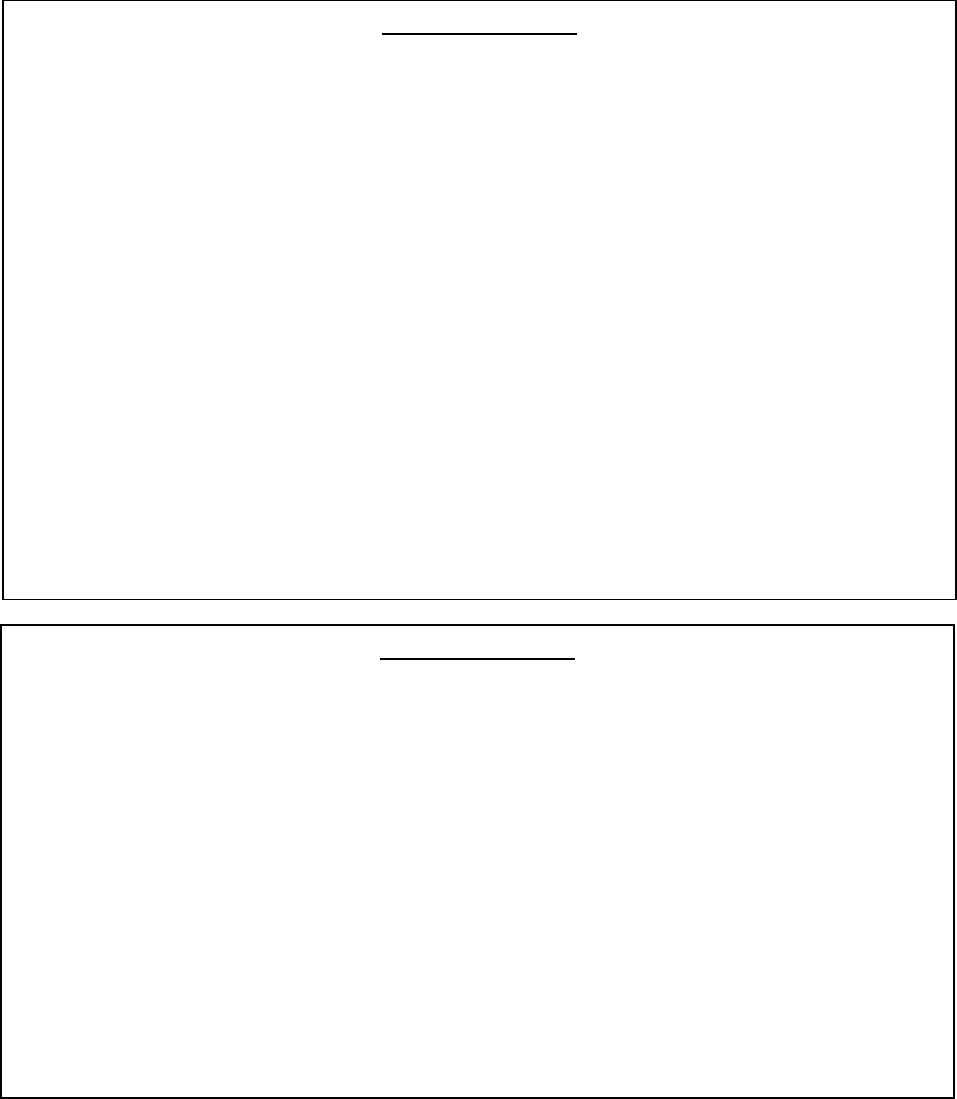
Case Definition 1
The animal is a domestic Felid, Canid or Mustelinae
2
;
AND
It is exhibiting a combination of the following clinical signs as determined by a
veterinary professional:
respiratory infection
gastrointestinal infection
fever
AND
Other common diagnoses have been considered and discounted as determined by a
veterinary professional.
AND
The animal has had confirmed contact with a suspect or known human case of
COVID-19 within three weeks of developing its clinical signs.
Case Definition 2
The animal is a non-domestic species of large felid, non-human primate and any
Mustelinae kept in captivity (including those Mustelinae
2
kept in research facilities)
AND
Has died from unexplained death OR it is exhibiting/has exhibited before death a
combination of the following clinical signs as determined by a veterinary professional:
respiratory infection
gastrointestinal infection
fever
AND
The animal has had contact with a confirmed case of SARS-CoV-2 (human or animal)
within three weeks of its death or developing its clinical signs
APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
veterinarians should contact Officials by telephone (see section 23 for contact
telephone numbers) to discuss the application of the above case definitions.
16. Testing undertaken at private laboratories should be based on the detection of
SARS-CoV-2 viral RNA in animals via polymerase chain reaction (PCR) (typically a
reverse transcriptase quantitative PCR assay (RT-qPCR)) or an equivalent assay.
17. Based on current scientific knowledge oropharyngeal and rectal swabs are the only
suitable specimen types for the detection of SARS-CoV-2 infection in animals. Faecal
and vomitus samples or swabs of the animal’s coat/fur or other environmental swabs
are NOT suitable due to the potential for environmental contamination.
18. Serological assays are coming onto the commercial market for SARS-CoV-2; however,
these assays currently have poor validation, low specificity and sensitivity, and their
use to test animals is not recommended. In addition, serological assays used to detect
antibodies would demonstrate past infection only and therefore would not be of
diagnostic or treatment value to the animal (see note above regarding the Veterinary
Surgeons Act).
19. The receiving laboratory should be capable of retaining the sample or nucleic acid
extraction from the specimen pending the test result. Laboratories should store the
sample in a manner appropriate to maintaining its integrity and traceability. In the event
of a positive result, the sample/nucleic acid extraction may be required to be sent to the
Animal and Plant Health Agency (APHA) Weybridge Laboratory where
secondary/confirmatory testing (in accordance with international standards) will be
undertaken.
What you will need to do if you undertake SARS-CoV-2 testing
of species within Case 1 definition
20. Veterinarians should ensure clients and diagnostic laboratories used are aware of the
following actions when test results are known .
21. If the results are negative: you have no further obligation to report these results to the
competent authority. Veterinarians should continue case management as appropriate
to the animal’s condition.
22. If the results are positive,
a. Reports of a positive result, including those obtained from UK samples sent to
laboratories located abroad, should be communicated to the competent authority.
This should be made immediately by telephone using the number for the
administration in which the tested animal resides (see below). You should be
prepared to provide information on the animal and its testing as outlined in Annex A.
All information provided will be handled with appropriate confidentiality.
i. England : Defra Rural Services Helpline on 03000 200 301
ii. Wales: Animal and Plant Health Agency Regional Office Wales on 0300
3038268
iii. Scotland: your local Field Services Office
Ayr on 03000 600703
Galashiels 03000 600711
Inverness 03000 600709
Inverurie 03000 600708
Perth 03000 600704
APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
iv. Northern Ireland: DAERA on 0300 200 7840 / 0300 2007852 or contact your
local Divisional Veterinary Office.
b. Where relevant, the Official coordinating your report will discuss with you the need
to contact public health officials in your area.
c. You (the private veterinary surgeon (PVS)) should be prepared to take additional
samples from the animal and submit these to the APHA Weybridge laboratory for
secondary testing. Details of how to submit the sample will be provided by the
Official coordinating your report. Samples requested will typically be:
I. Oropharyngeal and rectal swabs
AND
II. 2ml of clotted blood
d. In addition, where possible, you (the PVS) should arrange for the original sample to
be sent from the laboratory at which the positive sample was achieved to the APHA
Weybridge laboratory for confirmatory testing. Details of how to submit the sample
will be provided by the Official coordinating your report.
23. Sampling and postage costs of submitting additional samples will be at your (the
PVS/clients) cost. The laboratory costs of conducting any secondary/confirmatory
testing will be paid for by Government.
24. Results of the secondary/confirmatory testing conducted by APHA Weybridge, and any
other relevant information, will be made available to you (the PVS). It is your (the PVS)
responsibility to share these results with your client. Where relevant, the Official
coordinating your report will discuss with you the need to contact public health officials
in your area.
25. Secondary/confirmatory testing may not be required if the report does not meet the
case definition outlined above. Additional samples from the animal for confirmatory
testing may also not be required if the initial samples were collected at post-mortem, or
the animal has since died or otherwise is not available for retesting. Submission of the
original sample from which the positive test result was obtained may, however, still be
required in these instances. The Official who answers your report call will make this
assessment and inform you of the result (either during the initial call or during a
subsequent call-back) and inform you of any subsequent requirements to submit
samples for secondary/confirmatory testing (as outlined above).
26. Samples from other animals in the household in direct contact with the reported case
may also be considered as suitable for secondary testing. The Official coordinating
your report will inform you of any requests to also submit samples from these animals
for testing at APHA Weybridge.
What you will need to do if you undertake SARS-CoV-2 testing
of species within Case 2 definition
27. If you suspect SARS-CoV-2 in animals within Case definition 2, you (the PVS) should
communicate this to the competent authority. This should be made as soon as possible
by telephone using the number for the administration in which the animal resides (see
below). You should be prepared to provide information on the animal
as outlined in Annex B. All information provided will be handled with appropriate
confidentiality.
APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
i. England : Defra Rural Services Helpline on 03000 200 301
ii. Wales: Animal and Plant Health Agency Regional Office Wales on 0300
3038268
iii. Scotland: your local Field Services Office
Ayr on 03000 600703
Galashiels 03000 600711
Inverness 03000 600709
Inverurie 03000 600708
Perth 03000 600704
iv. Northern Ireland: DAERA on 0300 200 7840 / 0300 2007852 or contact your
local Divisional Veterinary Office.
28. Where relevant, the Official coordinating your report will discuss with you the need to
contact public health officials in your area.
29. You (the private veterinary surgeon (PVS)) should be prepared to take samples from
the animal and submit these to the APHA Weybridge laboratory for testing. Details of
how to submit the sample will be provided by the Official coordinating your report.
Samples requested will typically be:
III. Oropharyngeal and rectal swabs
AND
IV. 2ml of clotted blood
30. Sampling and postage costs of submitting additional samples will be at your (the
PVS/clients) cost. The laboratory costs of conducting any testing will be paid for by
Government.
31. Results of the testing conducted by APHA Weybridge, and any other relevant
information, will be made available to you (the PVS). It is your (the PVS) responsibility
to share these results with your client. Where relevant, the Official coordinating your
report will discuss with you the need to contact public health officials in your area.
32. Secondary/confirmatory testing may not be required if the report does not meet the
case definition outlined above. Additional samples from the animal for testing may also
not be required if the animal has since died or otherwise is not available for testing. The
Official who answers your report call will make this assessment and inform you of the
result (either during the initial call or during a subsequent call-back) and inform you of
any subsequent requirements to submit samples for testing (as outlined above).
33. Samples from other animals in the premises in direct contact with the reported case
(i.e. in the same cage or enclosure) may also be considered as suitable for secondary
testing. The Official coordinating your report will inform you of any requests to also
submit samples from these animals for testing at APHA Weybridge.
APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
Annex A – Epidemiological and Test Information Required at
the Time of the Telephone Report
This information should be collected in advance of making the initial telephone report call
on receipt of a positive private SARS-CoV-2 test result, to avoid unnecessary delays.
Ideally this information should be recorded when considering testing. All information
provided will be handled with appropriate confidentiality
1. The species (and if appropriate, type) of animal from which the sample was taken.
2. The age and sex of the animal from which the sample was taken.
3. History of clinical signs of the animal(s) concerned.
4. The specimen type(s) from which the positive test result(s) were obtained (in addition
to details of any samples where negative results were obtained from the same animal).
5. Any other animals in contact/displaying clinical signs.
6. Were any other animals in the household in which the animal resides previously tested
and if so, with what result? (Including details of any samples where negative results
were obtained from these animals).
7. The address where the specimen(s) were taken, and the name, address, and phone
number/email address of the owner/person in charge of the animal (or property if the
animal is part of a commercial/charitable organisation).
8. The date of sampling and the date the specimens(s) were analysed by the laboratory
(if available), and the name and address of the laboratory.
9. The organism considered to be detected.
10. Details of any other differential diagnostic testing of the animal(s) which has been
undertaken.
11. The name, address and phone number/email address of the veterinarian making the
report.
12. Confirmation that there was no reason to suspect that the specimens(s) were cross-
contaminated (by an infected person or environment) while being taken.
13. The COVID-19 status of the people in the animals’ household (e.g. confirmed human
case(s), suspected human case(s)), including how the onset and duration of
symptoms in people relates to the onset of signs in the animal. We do not need to
know which household member was ill – just that a household member or members
were ill. Where relevant, we will discuss with you the need to contact public health
officials in your area.
14. If known – the assay platform used by the laboratory, and the number of cycles
(commonly expressed as a C
t
or C
q
value) needed or viral copy number used to
generate the positive result (these figures indicates the amount of viral RNA present in
the sample).

APHA is an Executive Agency of the Department for Environment, Food and Rural Affairs and also works on behalf of the Scottish
Government, Welsh Government and Food Standards Agency to safeguard animal and plant health for the benefit of people, the
environment and the economy.
Annex B – Epidemiological Information Required at the Time of
the Telephone Report
This information should be collected in advance of making the initial telephone report call
regarding SARS-CoV-2 in species within Case Definition 2, to avoid unnecessary delays.
Ideally this information should be recorded when considering testing. All information
provided will be handled with appropriate confidentiality
1. The species (and if appropriate, type) of animal
2. The age and sex of the animal
3. History of clinical signs of the animal(s) concerned.
4. Details, if available, of any samples taken from the same animal.
5. Any other animals in contact/displaying clinical signs.
6. Were any other animals in the same premises in which the animal resides previously
tested and if so, with what result? (Including details of any samples where negative
results were obtained from these animals; the date any previous animal confirmed
cases specimens(s) were analysed by the laboratory (if available), and the name and
address of the laboratory)
7. The address where the specimen(s) were taken, and the name, address, and phone
number/email address of the owner/person in charge of the animal (or property if the
animal is part of a commercial/charitable organisation).
8. The date of sampling
9. The organism considered to be detected.
10. If known for animals– the assay platform used by the laboratory, and the number of
cycles (commonly expressed as a C
t
or C
q
value) needed or viral copy number used to
generate the positive result (these figures indicates the amount of viral RNA present in
the sample).
11. Details of any other differential diagnostic testing of the animal(s) which has been
undertaken.
12. The name, address and phone number/email address of the veterinarian making the
report.
13. Confirmation that there was no reason to suspect that the specimens(s) were cross-
contaminated (by an infected person or environment) while being taken.
14. The COVID-19 status of the people in the animals’ premises (e.g. confirmed human
case(s), suspected human case(s)), including how the onset and duration of
symptoms in people relates to the onset of signs in the animal. We do not need to
know who was ill – just that person(s) in close contact with the animals was either ill or
that person(s) was suspected to be in contact with a human COVID-19 case. Where
relevant, we will discuss with you the need to contact public health officials in your
area.
15. If known for people – the assay platform used by the laboratory, and the number of
cycles (commonly expressed as a C
t
or C
q
value) needed or viral copy number used to
generate the positive result (these figures indicates the amount of viral RNA present in
the sample).
