Journal Articles
2020
The use of innominate artery cannulation for antegrade cerebral The use of innominate artery cannulation for antegrade cerebral
perfusion in aortic dissection perfusion in aortic dissection
E. C. Payabyab
J. M. Hemli
Zucker School of Medicine at Hofstra/Northwell
A. Mattia
A. Kremers
S. K. Vatsia
Zucker School of Medicine at Hofstra/Northwell
See next page for additional authors
Follow this and additional works at: https://academicworks.medicine.hofstra.edu/publications
Part of the Cardiology Commons
Recommended Citation Recommended Citation
Payabyab EC, Hemli JM, Mattia A, Kremers A, Vatsia SK, Scheinerman SJ, Mihelis EA, Hartman AR,
Brinster DR. The use of innominate artery cannulation for antegrade cerebral perfusion in aortic
dissection. . 2020 Jan 01; 15(1):Article 7017 [ p.]. Available from:
https://academicworks.medicine.hofstra.edu/publications/7017. Free full text article.
This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic
Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara
Zucker School of Medicine Academic Works. For more information, please contact [email protected].

Authors Authors
E. C. Payabyab, J. M. Hemli, A. Mattia, A. Kremers, S. K. Vatsia, S. J. Scheinerman, E. A. Mihelis, A. R.
Hartman, and D. R. Brinster
This article is available at Donald and Barbara Zucker School of Medicine Academic Works:
https://academicworks.medicine.hofstra.edu/publications/7017
RES E A R C H A R T I C L E Open Access
The use of innominate artery cannulation
for antegrade cerebral perfusion in aortic
dissection
Eden C. Payabyab
1,2*
, Jonathan M. Hemli
3
, Allan Mattia
4
, Alex Kremers
1,5
, Sohrab K. Vatsia
3
,
S. Jacob Scheinerman
3
, Efstathia A. Mihelis
3
, Alan R. Hartman
5
and Derek R. Brinster
3
Abstract
Background: Direct cannulation of the innominate artery for selective antegrade cerebral perfusion has been
shown to be safe in elective proximal aortic reconstructions. We sought to evaluate the safety of this technique in
acute aortic dissection.
Methods: A multi-institutional retrospective review was undertaken of patients who underwent proximal aortic
reconstruction for Stanford type A dissection between 2006 and 2016. Those patients who had direct innominate
artery cannulation for selective antegrade cerebral perfusion were selected for analysis.
Results: Seventy-five patients underwent innominate artery cannulation for ACP for Stanford Type A Dissections.
Isolated replacement of the ascending aorta was performed in 36 patients (48.0%), concomitant aortic root
replacement was required in 35 patients (46.7%), of whom 7 had a valve-sparing aortic root replacement, ascending
aorta and arch replacement was required in 4 patients (5%). Other procedures included frozen elephant trunk (n =
11 (14.7%)), coronary artery bypass grafting (n = 20 (26.7%)), and peripheral arterial bypass (n = 4 (5.3%)). Mean
hypothermic circulatory arrest time was 19 ± 13 min. Thirty-day mortality was 14.7% (n = 11). Perioperative stroke
occurred in 7 patients (9.3%).
Conclusions: This study is the first comprehensive review of direct innominate artery cannulation through median
sternotomy for selective antegrade cerebral perfusion in aortic dissection. Our experience suggests that this strategy
is a safe and effective technique compared to other reported methods of cannulation and cerebral protection for
delivering selective antegrade cerebral perfusion in these cases.
Keywords: Aortic dissection, Aortic arch, Direct innominate cannulation, Cerebral perfusion, Outcomes
© The Author(s). 2020 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if
changes were made. The images or other third party material in this article are included in the article's Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the
data made available in this article, unless otherwise stated in a credit line to the data.
* Correspondence: ecp9004@nyp .org
Meeting Presentation: Presented at the American Association of Thoracic
Surgery Aortic Symposium. New York, New York, April 2018
1
Division of Cardiac Surgery, Virginia Commonwealth University Health
Systems, Richmond, VA, UK
2
Department of Cardiothoracic Surgery, New York Presbyterian-Weill Cornell
Medicine, New York, NY, USA
Full list of author information is available at the end of the article
Payabyab et al. Journal of Cardiothoracic Surgery (2020) 15:205
https://doi.org/10.1186/s13019-020-01249-1
Background
Stanford type A dissec tions carry a high mortality with
reports ranging from 17 to 26% [1–4]. Timely operative
intervention improves outcomes with delays increasing
mortality 1–2% every hour in the first 48 h. Repair of the
aortic dissection requires complex circulatory manage-
ment and cerebral protection during circulatory arrest.
Strategies to improve outcomes include hypothermia
alone or in conjunction with antegrade cerebral perfu-
sion (ACP) or retrograde cerebral perfusion (RCP).
Moderate hypothermia with ACP has been shown to be
a safe an d effective strategy from neuroprotection in aor-
tic arch reconstruction including operative interventions
for aortic dissections [5–8].
Several techniques for administering selective ACP
(SACP) have been described including right axillary
artery cannulation with concomitant occlusion of the
base of the innominate artery [9], direct placement of
balloon-tipped catheters into the ostia of the arch vessels
[10], and cannulation of the innominate artery via a
side-graft [11, 12]. Neurologic events with these tech-
niques range from 3.4% in elective aortic arch operations
to 12% in acute Stanford type A dissections. An alterna-
tive technique for SACP, utilizing direct innominate
artery cannulation, has been shown to be safe in elective
arch reconstruction with reported stroke and mortality
rates of 1% [13, 14].
The outcomes of direct innominate artery cannulation
for SACP in acute aortic dissection have yet to be
reported. We sought to evaluate the safety and efficacy
of this technique in acute Stanford type A dissections.
Methods
Patients
We performed a multi-institutional comprehensive
review of all patients who underwent repair of Stanford
type A dissection between 2006 and 2016. Seventy-five
patients had direct cannulation of the innominate artery
for SACP during their dissection repair. The study
protocol was approved by the institutional board reviews
of the Northwell Health System and Virginia Common-
wealth University Health System.
Surgical technique
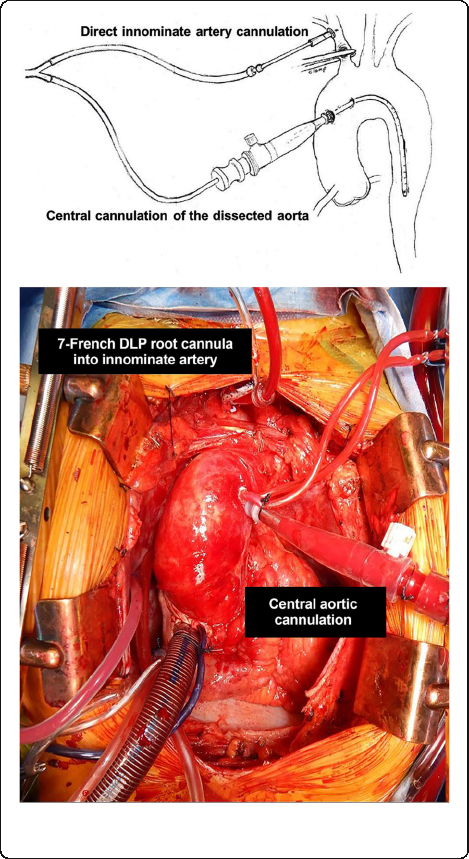
The dissected ascending aorta is cannulated directly to
initiate cardiopulmonary bypass utilizing transesophageal
echo guidance to place a long percutaneous arterial
cannula placed with Seldinger technique as previously
described [15]. The innominate artery is then cannulated
directly with a 7-French standard-tip DLP aortic root
cannula and connected to the arterial limb of the cardio-
pulmonary bypass circuit utilizing standard 3/8″ tubing,
with a customized 1/4″ branched limb that has a perfu-
sion adapter to attach to the innominate artery cannula.
Figure 1. Once the patient’s core temperature reaches
the desired target (typically moderate hypothermia at
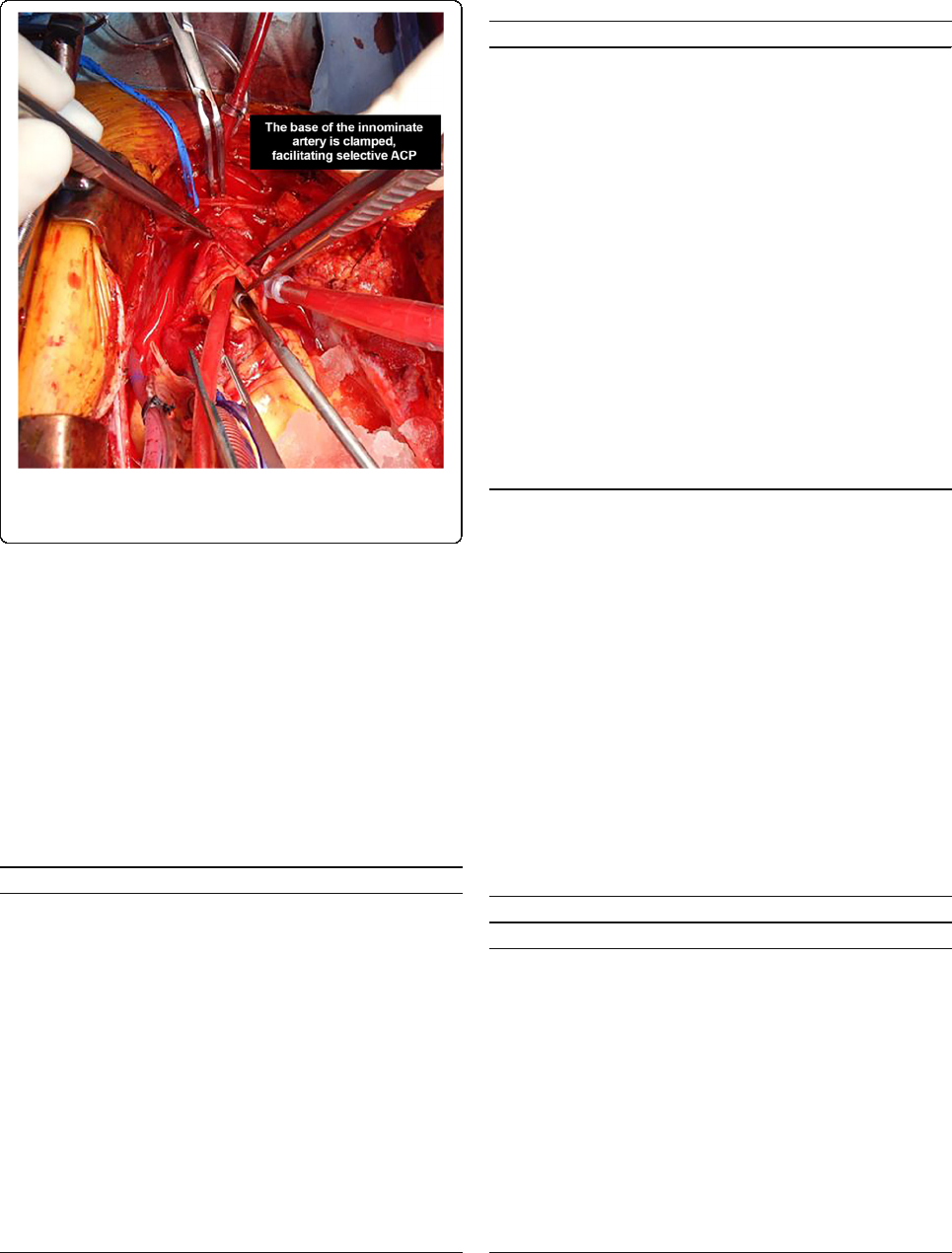
28 °C), the base of the innominate artery is clamped
proximal to the innominate artery cannulation site. The
origin of the left common carotid artery is also isolated
and clamped, keeping the Circle of Willis pressurized
and thereby preventing a ‘steal’ phenomenon. Figure 2.
Cerebral perfusion is measured by an invasive arterial
line situated in the right radial artery, and adjusted
according to arterial pressure and continuous non-invasive
monitoring of cerebral oxygen saturations using near-
infrared spectroscopy.
Results
Preoperative patient demographics are presented in Table 1.
Operative data including details of proximal aortic recon-
struction, concomitant procedures and intraoperative times
Fig. 1 Direct cannulation of innominate artery with 7-French
standard-tip DLP aortic root cannula
Payabyab et al. Journal of Cardiothoracic Surgery (2020) 15:205 Page 2 of 6
are described in Table 2. Perioperative outcomes are listed
in Table 3.
Perioperative stroke occurred in 7 patients (9.3%).
Four patients experienced neurological deficits including
dysphagia and motor dysfunction with complete reso-
lution of symptoms within 30 days. The remaining three
patients (4%), who presented in extremis, unable to asses
neurologic exam and found to have hemopericardium,
had no improvement in neurologic status resulting in
poor prognosis and family withdrawal of care. In the
patients presenting to the operating room with no
neurologic deficits and clinically intact there was 5.3%
postoperative neurological complication rate.
Perioperative mortality was 14.7% (11 patients) which
included 3 patients who experienced a neurological
complication. Of our perioperative mortalitie s, 5 patients
presented in extremis. Amongst the 6 patients present-
ing hemodynamically stable the postoperative mortality
was 8%. (Intraoperative death occurred in three patients,
with one unable to wean from bypass and two experien-
cing uncontrollable hemorrhage. These patients were
hypotensive on presentation, with two requiring CPR
Fig. 2 The origin of the left common carotid artery is also isolated
and clamped, keeping the Circle of Willis pressurized and thereby
preventing a ‘steal’ phenomenon
Table 1 Patient Demographics
Patient Demographics
Variable n (%)
Female gender 28 (37.3)
Age, years (mean ± SD) 58.9 ± 15.8
BMI, kg/m
2
(mean ± SD) 29.9 ± 9.6
Hypertension 37 (49.3)
History of smoking 35 (46.7)
Dyslipidemia 25 (33.3)
Heart failure 10 (13.3)
Cerebrovascular disease 10 (13.3)
Peripheral vascular disease 8 (10.7)
History of prior MI 8 (10.7)
Chronic lung disease 8 (10.7)
Marfan syndrome 1 (1.3)
SD Standard deviation, MI Myocardial infarction
Table 2 Operative Data
Operative Data
Variable n (%)
Proximal aortic reconstruction
Isolated replacement of ascending aorta 36 (48.0)
Ascending Aorta and arch replacement 4 (5)
Aortic root replacement 35 (46.7)
Composite valve-graft conduit 32 (42.7)
Valve-sparing 3 (4.0)
Total arch replacement 7 (9.3)
Concomitant procedures
Frozen elephant trunk 11 (14.7)
CABG 20 (26.7)
Peripheral arterial bypass 4 (5.3)
Cardiopulmonary bypass time, minutes (mean ± SD) 166.1 ± 71.9
Aortic cross-clamp time, minutes (mean ± SD) 108.1 ± 47.7
Circulatory arrest time, minutes (mean ± SD)* 19.2 ± 13.0
Lowest intraoperative temperature, °C (mean ± SD) 24.8 ± 11.1
CABG Coronary artery bypass grafting, SD Standard deviation
Table 3 Perioperative Outcomes
Perioperative Outcomes
Variable n (%)
Stroke 7 (9.3)
Re-operation for bleeding 6 (8.0)
Perioperative MI 1 (1.3)
Deep sternal wound infection 2 (2.7)
New renal failure requiring dialysis 11 (14.7)
Prolonged intubation 37 (49.3)
Tracheostomy 5 (6.7)
Multi-system organ failure 8 (10.7)
Limb ischemia 4 (5.3)
Postop length of stay, days (median ± SD) 10.0 ± 9.1
30-day mortality 11 (14.7)
MI Myocardial infarction, SD Standard deviation.
Payabyab et al. Journal of Cardiothoracic Surgery (2020) 15:205 Page 3 of 6
and all found to have hemopericardium. A 63-year-old
male who underwent an ascending arch, aortic root
replacement, hemiarch and CABG required ECMO for
ventricular assistance secondary to ventricular fibrilla-
tion. He required a re-exploration on postoperative day
one for bleeding, required a left ventricular repair, and
interventions for ventricular fibrillation. Given the likely
poor outcome the patient’s family withdrew care. A 51-
year-old male that presented with malperfusion and
Type I dissection underwent replacement of ascending
aorta with resuspension of the aortic valve, hemi-arch
replacement, placement of descending thoracic aortic
stent graft and ascending aorta to left femoral artery
bypass. Postoperatively the patient required continued
administration of blood products and vasopressors due
to coagulopathic bleeding and hypotension. The patient
experienced multisystem organ failure leading to death. A
79-year-old female underwent a complex aortic root
replacement, ascending aorta and proximal arch replace-
ment and a 2 vessel CABG who experienced disseminated
intravascular coagulopathy and postoperative liver failure
leading to multiorgan system failure and eventual death.
Discussion
Early mortality in patients undergoing surgical repair of
type A aortic dissection is reported as high as 31%. (3)
Developing an efficient and safe surgical technique to
cannulate and provide cerebral perfusion is essential to
successfully perform a repair of type A aortic dissection
pathology. Several cannulation techniques have been
described for the use of arterial inflow in the surgical
repair of type A aortic dissections, all with potential ben-
efits and drawbacks. Femoral artery cannulation, carries
the potential complication of cerebral embolization and
organ malperfusion. The use of the axillary artery for
arterial inflow via a side-graft or direct cannulation has
the disadvantage of needing a second incision and the
additional time to cannulate the axillary artery [9, 16].
Direct cannulation of the innominate artery for full
cardiopulmonary bypass is an altern ative cannulation
site described [11 , 13, 17]. Preventza et al describe in-
nominate artery cannulation with the use of a side graft
as an alternate technique to peripheral cannulation for
surgical repair, having a low stroke and mortality rate
[12]. An advantage of these techniques in the use of these
sites for SACP during circulatory arrest. The use of central
cannulation has also been shown to be safe in the surgical
repair of type A aortic dissections [15, 18, 19].
Evaluation of the different cannulation strategies by
various groups show similar outcomes. Kamiya et al [20]
reviewed 235 patients who underwent operative inter-
vention for type A aortic dissection. They compared the
patients who underwent cannulation of the ascending
aorta and femoral artery and found no difference in
long-term outcomes between the two groups. Stamou
et al [21 ] compared early postoperative outcomes in 305
patients at multiple institutions who underwent axillary
versus femoral cannulation over ten years. They found
no difference in operative mortality.
The use of antegrade cerebral perfusion (ACP) has
been shown to reduce neurologic morbidity after
hypothermic circulatory arrest in proximal aortic recon-
struction [22, 23]. Several techniques for administering
selective ACP have been described including right axil-
lary cannulation with concomitant occlusion of the base
of the innominate artery, direct placement of balloon tip
catheters into the ostia of the arch vessels under direct
vision after circulatory arrest and cannulation of the in-
nominate artery after circulatory arrest via a side-graft.
Neurologic events with these techniques are reported to
be up 12% in acute type A dissections [9, 10, 24]. Our
use of direct innominate artery cannulation for SACP
(9.3%) are similar. Of the 7 patients, 3 presented in
extremis unable to be evaluated neurologically. In evalu-
ating patients who presented neurologically intact, our
neurologic events (4%) are decreased compared to other
techniques.
An alternate technique for SACP, utilizing direct innom-
inate artery cannulation has been described. Garg et al [25]
describe a technique in which central aortic cannulation for
elective aortic surgery. is performed followed by direct
innominate artery cannulation with a 14F pediatric venous
cannula for SACP after hypothermia. They reported
outcomes of 50 patients who underwent replacement of the
ascending aorta using an open distal anastomosis or hemi-
arch replacement. The operative mortality was 2% with a
stroke rage of 2%. A similar technique for elective aortic
surgery described by Jassar et al [13] utilizes direct cannula-
tion of the innominate artery for SACP. Their technique
includes arterial cannulation of the ascending aorta and use
of a short tipped 9-Fr cardioplegia catheter for direct
innominate cannulation following hypothermia and circula-
tory arrest. Our method is similar apart from their use of a
larger cannula to directly cannulate the innominate artery.
They evaluated 100 elective hemiarch reconstructions with
results that showed a 30-day in-hospital mortality and
stroke rate of 1%.
Conclusions
The use of direct innominate artery cannulation with an
accessory cannula for SACP in elective ascending aortic
repairs is comparable to alternative methods. To our
knowledge the use of this method in acute type A aortic
dissection have yet to be reported. We performed a
multi-institutional review of 75 patients over ten years.
All these patients underwent direct cannulation of the
innominate artery with a 7-French standard-tip DLP
aortic root cannula. Our patient cohort included seven
Payabyab et al. Journal of Cardiothoracic Surgery (2020) 15:205 Page 4 of 6
patients who presented with extension of the dissection
into the innominate artery, which did not preclude the
use of the technique. Our 30-day mortality was 14.7%
and a perioperative stroke rate of 9.3%. These outcomes
compare to those reported in other contemporary series
of acute dissection repair, including IRAD data.
Our study has limitations of being a retrospective and
non-comparative review. The experience is multi-
intuitional but is limited to a single surgeon experience.
Within these limitations, our experience suggests that
direct innominate artery cannulation is a simple, fast,
safe, and effective method of administrating SACP
during hypothermic circulatory arrest for patients with
acute type A dissection.
Abbreviations
SACP: Selective antegrade cerebral perfusion; ACP: Antegrade cerebral
perfusion; RCP: Retrograde cerebral perfusion
Acknowledgements
Not applicable.
Authors’ contributions
ECP: design of the work, acquisition, analysis, interpretation of data and
drafted the work; JMH: contribution to the conception, acquisition, analysis,
interpretation of data; revised work; AM: acquisition, analysis, interpretation
of data; AK: acquisition, analysis, interpretation of data; SKV: analysis,
interpretation of data; JS: contribution to the conception, interpretation of
data; EAM: design of the work, acquisition, analysis, interpretation of data;
ARH: contribution to the conception, interpretation of data; DRB: conception
and design of work, acquisition, analysis, interpretation of data and revision
of wok. The author(s) read and approved the final manuscript.
Funding
No sources of funding to declare.
Availability of data and materials
The datasets used or analyzed during the current study are available from
the corresponding author on reasonable request.
Ethics approval and consent to participate
Institutional board reviews of the Northwell Health System and Virginia
Commonwealth University Health System.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Author details
1
Division of Cardiac Surgery, Virginia Commonwealth University Health
Systems, Richmond, VA, UK.
2
Department of Cardiothoracic Surgery, New
York Presbyterian-Weill Cornell Medicine, New York, NY, USA.
3
Department of
Cardiovascular and Thoracic Surgery, Lenox Hill Hospital / Northwell Health,
New York, NY, USA.
4
Department of Cardiovascular and Thoracic Surgery,
North Shore University Hospital / Northwell Health, Manhasset, NY, USA.
5
Rush University, Chicago, IL, USA.
Received: 24 March 2020 Accepted: 21 July 2020
References
1. Pape, LA, Awais M, Woznicki, EM, Suzuki T, Trimarchi S, Evangelista A, et al.
Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17- Year
Trends From the International Registry of Acute Aortic Dissection. J Am Coll
Cardiol. 2015;66:4.
2. Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al.
Contemporary results of surgery in acute type A aortic dissection: The
International Registry of Acute Aortic Dissection experience. The Journal of
thoracic and cardiovascular surgery. 2005; 129:1.
3. Berretta P, Patel HJ, Gleason TG, Sundt TM, Myrmel T, Desai N, et al. IRAD
experience on surgical type a acute dissection pateints: results and
predictors of mortality. Annals of cardiothoracic surgery. 2016;5:4.
4. Conzelmann LO, Weigang E, Mehlhor U, Abugameh A, Hoffamnn I, Blettner
M, et al. Mortality in patients with acute aortic dissection type a: analysis of
pre- and intraoperative risk factors from the German registry for acute aortic
dissection type a (GERAADA). European journal of cardio-thoracic surgery :
official journal of the European Association for Cardio-thoracic Surgery.
2016;49:2.
5. Leshnower BG, Myung RJ, Kilgo PD, Vassiliades TA, Vega JD, Thourani VH,
et al. Moderate hypothermia and unilateral selective antegrade cerebral
perfustion: a contemporary cerebral protection strategy for aortic arch
surgery. Ann Thorac Surg. 2010;90:2.
6. Leshnower BG, Myung RJ, Chen EP. Aortic arch surgery using moderate
hypothermia and unilateral selective antegrade cerebral perfusion. Annals of
cardiothoracic surgery. 2013;2:3.
7. Leshnower BG, Kilgo PD, Chen EP. Total arch replacement using moderate
hypothermic circulatory arrest and unilateral selective antegrade cerebral
perfusion. J Thorac Cardiovasc Surg. 2014;147:5.
8. Keeping WB, Leshnower BG, Hunting JC, Binongo J, Chen EP. Hypothermia
and selective Antegrade cerebral perfusion is safe for arch repair in type a
dissection. Ann Thorac Surg. 2017;104:3.
9. Wong Dr. Coselli JS, Palmero L, Bozinovski J, Carter SA, Murariu D, et al.
Axillary artery cannulation in surgery for acute or subacute ascending aortic
dissections. The Annals of thoracic surgery. 2010;90:3.
10. Okita Y, Okada K, Omura A, Kano H, Minami H, Inoue T, et al. Surgical
techniques of total arch replacement using selective antergrade cerebral
perfusion. Annals of cardiothoracic surgery. 2013;2:2.
11. Huang FJ, Wu Q, Ren CW, Lai YQ, Yang S, Rui QJ, et al. Cannulation of
the innominate a rtery with a side graft in a rch surgery. Ann Thorac
Surg. 2010;89:3.
12. Preventza O, Bakaeen FG, Stephens EH, Trocciola SM, de la Cruz KI, Coselli
JS. Innomiate artery cannulation: an alternative to femoral or axillary
cannulation for arterial inflow in proximal aortic surgery. The Journal of
thoracic and cardiovascular surgery. 2013;145:3 Suppl.
13. Jassar AS, Vallabhajosyula P, Bavaria JE, Gutsche J, Desai ND, Williams ML,
et al. Direct innominate artery cannulation: an alternate technique for
antegrade cerebral perfusion during aortic hemiarch reconstruction. J
Thorac Cardiovasc Surg. 2016;151:4.
14. Payabyab EC, Mattia A, Kremers A, Vatsia SK, Hemli JM, Scheinerman SJ,
Mihelis EA, Hartman AR, Brinster DR. Direct innominate artery cannulation: a
safe alternative for antegrade cerebral perfusion in aortic dissection. New
York, New York, April: American Association of Thoracic Surgery Aortic
Symposium; 2018.
15. Brinster DR, Parrish DW, Meyers KS, Reddy P, Kasirajan V. Central aortic
cannulation for Stanford type a aortic dissection with the use of three-
dimensional and two-dimensional transesophageal echocardiography. J
Card Surg. 2014;29:5.
16. Schachner T, Nagiller J, Zimmer A, Laufer G, Bonatti J. Technical problems
and complications of axillary artery cannulation. European journal of cardio-
thoracic surgery : official journal of the European Association for Cardio-
thoracic Surgery. 2005;27:4.
17. Garg V, Peterson MD, Chu MW, Ouzounian M, MacArthur RG, Bozinovski J,
et al. Axillary versus innominate artery cannulation for antegrade cerebral
perfustion in aortic surgery: design of the aortic surgery cerebral protection
evaluation (ACE) CardioLink-3 randomised trial. BMJ Open. 2019;7:6.
18. Reece TB, Tribbel CG, Smith RL, Singh RR, Stiles BM, Peeler BB, et al. Central
cannulation is safe in acute aortic dissection repair. J Thorac Cardiovasc
Surg. 2007;133:2.
19. Osumi M, Wada H, Morita Y, Shimizu M, Sukehiro Y, Amako M, et al. Safety
and efficacy of ascending aorta cannulation during repair of acute type A
aortic dissection (PA29–04): “ Presented at the 65
th
Annual Scientific Meeting
of the Japanese Association for the Thoracic Surgery”. General thoracic and
cardiovascular surgery. 2014;62:5.
20. Kamiya H, Kallenbach K, Halmer D, Ozsoz M, Ilg K, Lichtenberg A, et al.
Comparison of ascending aorta verus femoral artery cannulation for acute
aortic dissection type A. Circulation. 2009;120:11Suppl.
Payabyab et al. Journal of Cardiothoracic Surgery (2020) 15:205 Page 5 of 6

21. Stamou SC, Gartner D, Kouchoukos NT, Lobdell KW, Khabbaz K, Murphy E,
et al. Axillary Versus Femoral Arterial Cannulation During Repair of Type A
Aortic Dissection?: An Old Problem Seeking New Solutions. Aorta (Stamford,
Conn). 2016;4:4.
22. Angeloni E, Melina G, Refice SK, Roscitano A, Capuao F, Comito C, et al.
Unilateral versus bilateral Antegrade cerebral protection during aortic
surgery: an updated meta-analysis. Ann Thorac Surg. 2015;99:6.
23. Preventza O, Simpson KH, Cooley DA, Cornwell L. Bakaeen FG, Omer S, et al.
Unilateral versus bilateral cerebral perfusion for acute type A aortic
dissection The Annals of thoracic surgery. 2015;99:1.
24. Preventza O, Garcia A, Tuluca A, Henry M, Cooley DA, Simpson K, et al.
Innominate artery cannulation for proximal aortic surgery: outcomes and
neurological events in 263 patients. European journal of cardio-thoracic
surgery: official journal of the European Association for the Cardio-thoracic
Surgery. 2015;48:6.
25. Garg V, Tsirigotis DN, Dickson J, Dalamagas C, Latter DA, Verma S, et al.
Direct innominate artery cannulation for selective antegrade cerebral
perfusion during deep hypothermic circulatory arrest in aortic surgery. J
Thorac Cardiovasc Surg. 2014;148:6.
Publisher’sNote
Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations.
Payabyab et al. Journal of Cardiothoracic Surgery (2020) 15:205 Page 6 of 6
