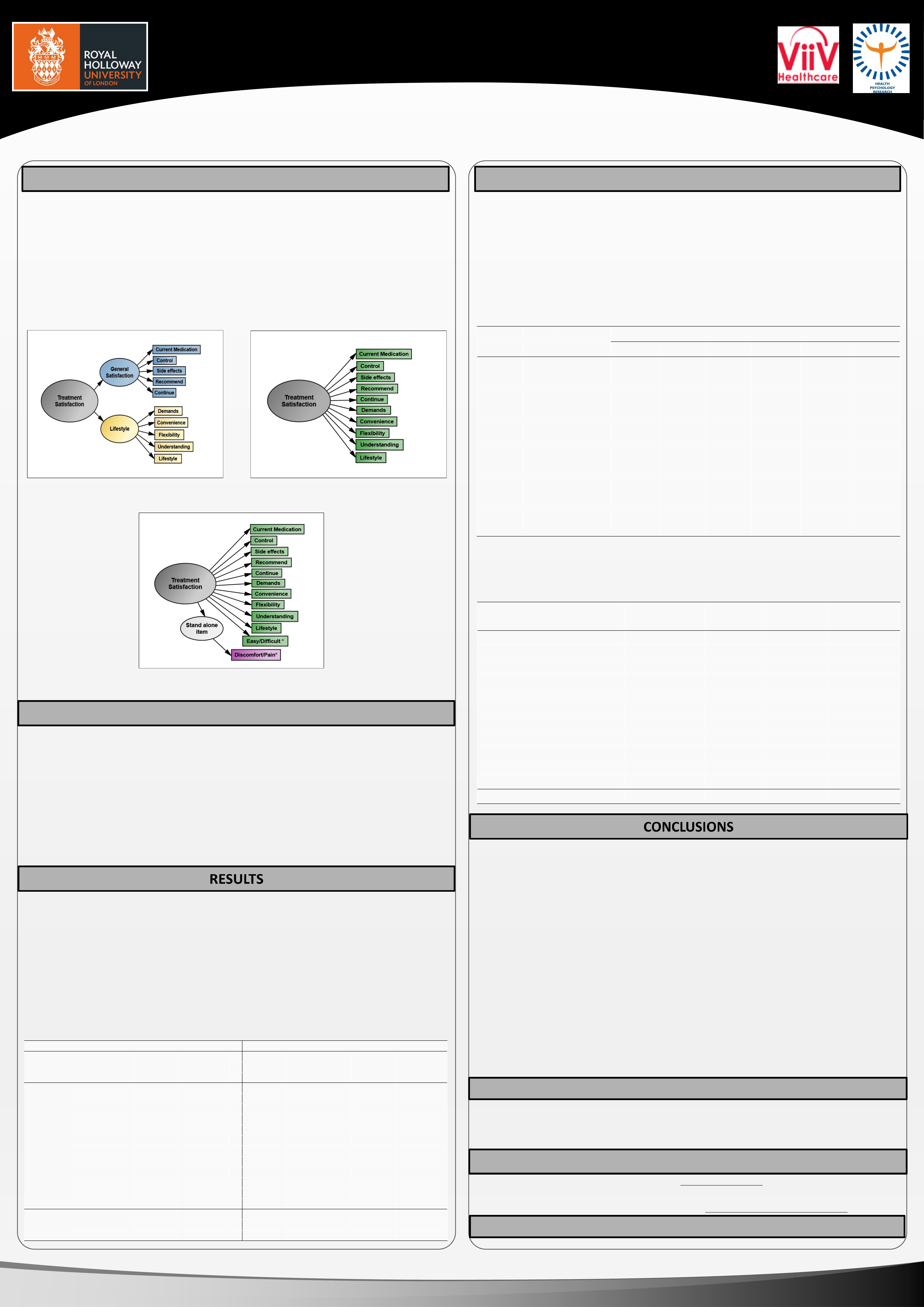
The HIV Treatment Satisfaction Questionnaire (HIVTSQ) is designed to measure treatment
satisfaction in people living with HIV. The original 10-item HIVTSQ (Woodcock & Bradley 2001,
2006) included two five-item subscales (Figure 1a) combinable into a 10-item scale.
Given the dramatic changes in HIV treatment in the last 15 years, including recent
developments of injectable treatment, the HIVTSQ was reviewed in qualitative interviews
which suggested two new items concerning discomfort/pain and ease/difficulty (Figure 1c).
This poster reports on psychometric evaluation of the revised HIVTSQ.
BACKGROUND
ENQUIRIES
Datasets from two studies were available for psychometric evaluation. Study 1 employed a
survey design, with participants from the UK (N=128) and the US (N=127) recruited via the
internet by Opinion Health, who completed mailed questionnaires or telephone interviews.
Study 2 included participants' (N=302) data from two time-points from the LATTE-2 trial
(NCT02120352: evaluating injectable treatment). All participants were HIV positive. Analyses of
the original 10 items and with the additional two items included exploratory factor analysis
(EFA) using SPSS and confirmatory factor analysis (CFA) using Mplus.
a
Health Psychology Research Unit, Royal Holloway, University of London, UK,
b
ViiV Healthcare Ltd, 980 Great West Road, Brentford, London, TW8 9GS UK.
c
Health Psychology Research Ltd, Royal Holloway, University of London, Egham, Surrey, UK.
Jacquelyn Romaine
a
, Miranda Murray
b
& Clare Bradley
a, c
Psychometric evaluation of the revised HIV Treatment Satisfaction
Questionnaire (HIVTSQ)
METHOD
Corresponding author: Professor Clare Bradley, Email: c.br[email protected] Health Psychology Research Unit,
Orchard Building, Royal Holloway, University of London, Egham, Surrey, TW20 0EX, UK.
Information on these and other Questionnaires: Please visit www.healthpsychologyresearch.com
The revised HIVTSQ includes 12 items. Items 1-11 are included in the scale score. Item 12
(discomfort/pain) is a stand-alone item. The high correlation between discomfort/pain and side
effects suggests that discomfort/pain is regarded as a side effect. However inclusion of this item
will ensure that this aspect of treatment is taken into account: this is important now that
intramuscular injectable treatments are available. The subscales seen previously with the 10-
item version were no longer apparent in either study, with lifestyle factors now loading together
with control and side effects when either the original 10 items or the revised 12 items were
analysed. This is perhaps a reflection of the development of new treatments that are better at
controlling the condition and result in lifestyle factors becoming more salient. The original 10
items continue to work well, enabling comparisons with results from previous studies using the
10-item HIVTSQ. The one-factor model of the HIVTSQ is an up-to-date appropriate measure of
treatment satisfaction for individuals living with HIV.
RESULTS
UK Participants US Participants
Item
Number
Aspects of
Treatment
Satisfaction
Factor
Loadings
Cronbach's
Alpha if Item
Deleted
Item
Number
Aspects of
Treatment
Satisfaction
Factor
Loadings
Cronbach's
Alpha if Item
Deleted
10
Continue
0.863 0.905 8
Lifestyle
0.770 0.881
11
Easy/difficult
0.862 0.905 9a
Recommend
0.753 0.883
4
Demands
0.854 0.905 10
Continue
0.732 0.884
8
lifestyle
0.811 0.907 3
Side effects
0.720 0.884
1
Current
0.769 0.910 11
Easy/difficult
0.719 0.885
12
Discomfort/pain
0.751 0.910 1
Current
0.707 0.887
5
Convenient
0.738 0.910 12
Discomfort/pain
0.682 0.887
3
Side effects
0.717 0.911 5
Convenient
0.664 0.886
9a
Recommend
0.637 0.914 4
Demands
0.626 0.888
2
Control
0.600 0.916 6
Flexible
0.571 0.895
6
Flexible
0.485 0.925 7a
Understand HIV
0.460 0.896
7a
Understand HIV
0.336 0.924 2
Control
0.433 0.897
Number of Items in Scale
12
Number of Items in Scale
12
Variance
51.70
Variance
43.80
Alpha
0.919
Alpha
0.896
Table 1: Exploratory Factor Analysis: Factor Loadings and Alpha Coefficients: Dataset 1
Confirmatory Factor Analysis (CFA) of the 12-item model (using Week-16 LATTE-2 data) revealed
a less than optimal model fit, suggesting a problematic association between the side-effect item
and new discomfort/pain item. CFA of an alternative 11-item model (dropping discomfort/pain)
revealed a good fit. Cross-validation using endpoint data from LATTE-2 and UK data from Study
1 also revealed that removal of discomfort/pain while retaining easy/difficult resulted in an
influential improvement in the model.
REFERENCES
Parameters
Standardised
Regression Weights
Standard Error
P value
Cronbach Alpha if
Item Deleted
TSQ 1: Current Treatment
0.784 0.029 < 0.001 0.868
TSQ 2: Control
0.611 0.041 < 0.001 0.877
TSQ 3: Side effects
0.523 0.047 < 0.001 0.881
TSQ 4: Demands
0.831 0.024 < 0.001 0.864
TSQ 5: Convenient
0.880 0.018 < 0.001 0.861
TSQ 6: Flexible
0.710 0.028 < 0.001 0.888
TSQ 7a: Understand HIV
0.607 0.044 < 0.001 0.877
TSQ 8: Fits lifestyle
0.877 0.018 < 0.001 0.860
TSQ
9a: Recommend 0.815 0.029 < 0.001 0.867
TSQ 10: Continue
0.807 0.028 < 0.001 0.868
TSQ 11: Easy or difficult
0.812 0.024 < 0.001 0.866
Overall alpha total
0.881
Table 3: Confirmatory Factor Analysis: Model Factor Loadings & Reliability: One Factor 11-item Model
(LATTE-2 Data Week -16)
Table 2: Confirmatory Factor Analysis: Model Fit Results
Fit Indices
Data
Model Description
c
2
CFI TLI RMSEA CI WRMR
LATTE
-2:
Week
-16
One latent factor
12 observed variables
468.737 0.927 0.911 0.159
0.146
-
0.173
1.564
LATTE
-2:
Week
-16
One latent factor
11 observed variables
116.833 0.986 0.982 0.074
0.058
-
0.090
0.724
LATTE
-2:
Week 32
One latent factor
12 observed variables
318.596 0.953 0.943 0.136
0.122
-
0.151
1.360
LATTE
-2:
Week 32
One latent factor
11 observed variables
130.681 0.984 0.981 0.086
0.069
-
0.103
0.868
UK Data
One latent factor
12 observed variables
157.580 0.967 0.960 0.124
0.102
-
0.147
0.816
UK Data
One latent factor
11 observed variables
92.844 0.982 0.978 0.094
0.067
-
0.121
0.639
Woodcock, A. & Bradley, C. (2001). Validation of the HIV Treatment Satisfaction Questionnaire (HIVTSQ). Quality
of Life Research, 10: 517-531.
Woodcock, A. & Bradley, C. (2006). Validation of the revised 10-item HIV Treatment Satisfaction Questionnaire
status version (HIVTSQs) and new change version (HIVTSQc). Value in Health 9: (5) 320-333.
Figure 1a HIVTSQ Original: Item details and structure of the
original 10-item HIVTSQ including two 5-item subscales
Figure 1c HIVTSQ Revised: Item details and structure of the 12-item HIVTSQ. The two new items are marked with * .
The HIVTSQ-12 has a single-factor structure including the original 10-items plus easy/difficult. Discomfort/pain is a stand alone item.
Figure 1b: Factor structure of the original 10 HIVTSQ items
extracted from the revised HIVTSQ (12 items) used in the
current studies: no evidence of subscales found previously
The underlying factor structure of the 12-item HIVTSQ was examined using EFA and Study 1
(separately in UK and US) data. All analyses revealed one-factor solutions. In order to see
whether the new items alone were affecting the factor structure, analyses were also conducted
excluding the two new items. A one-factor structure (Figure 1b) was again revealed, suggesting
that changes in treatments and patient experience have affected the pattern of responses
rather than the addition of the new items and that the questionnaire is now best scored as a
single scale.
ACKNOWLEDGEMENTS
This research was funded by GSK/ViiV Healthcare.
c
2
: chi-square (perfect fit = 0), CFI: Comparative fit index (0.90 reasonable fit, 0.95 good fit), TLI: Tucker-Lewis Index (0.90
reasonable fit, 0.95 good fit), RMSEA: Root Mean Square Error of Approximation (< 0.05 close fit, > 0.05 but < 0.08 fair fit, > 0.08
but < 0.10 mediocre fit, over 0.10 poor fit), WRMR: Weighted Root Mean Square Residual (< 1 good fit).
