
HIV IN PREGNANCY
Prof. Urmila Singh
Dept. of Obstetrics & Gynaecology
King George Medical University, Lucknow
Presentation is for educational purposes only not for commercial activity

Human Immunodeficiency Virus
• RNA Retrovirus
• Type : HIV 1-India ,
HIV 2-more in Africa
slow & mild in initial stages.
• Very fragile Virus
• Susceptible to heat,
boiling for few seconds kills virus.
1% Na Hypochlorite inactivate virus.
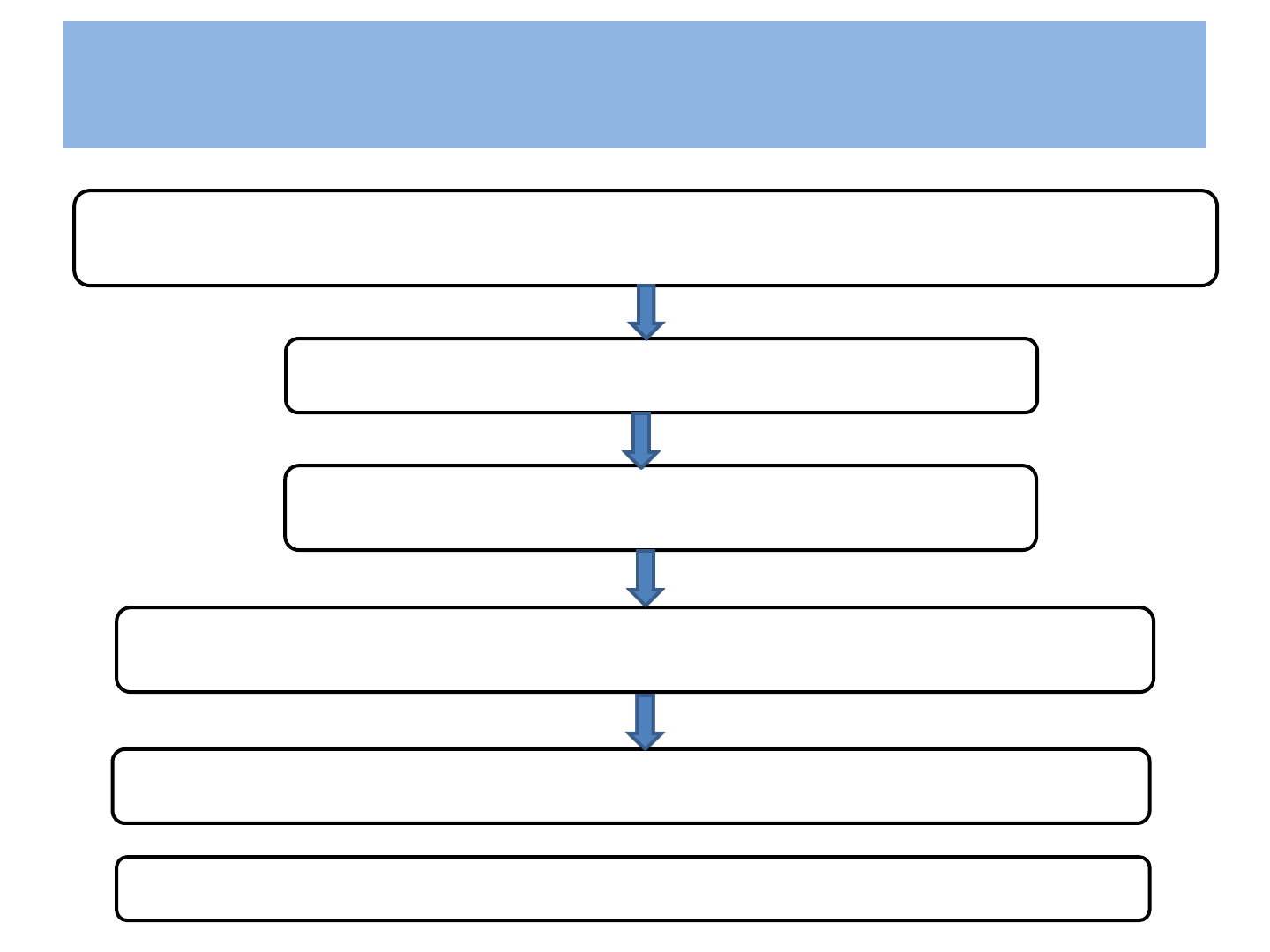
Pathogenesis
Main targets of the Virus are Human CD4` helper’ Lymphocytes
Start Producing new virus particle
Significant fall in CD4 cells
Inability of ß- Lymphocytes to produce antibody to HIV
Causes Progressive Immunosuppression
Ultimately development of AIDS—takes 8 to 10 yrs
HIV Infection
• Incubation period is 1-3 weeks
• After exposure to HIV infection there is a
window period of 12 weeks
in which antibody titre is not detectable .

Routes of Transmission of HIV
• Sexual Route
– Male-to-Female; Female-to-Male
– Male-to-Male
• Parenteral Route
– Transmission of infected blood
– Sharing of infected needles
• Parental Route
– Mother-to-Child
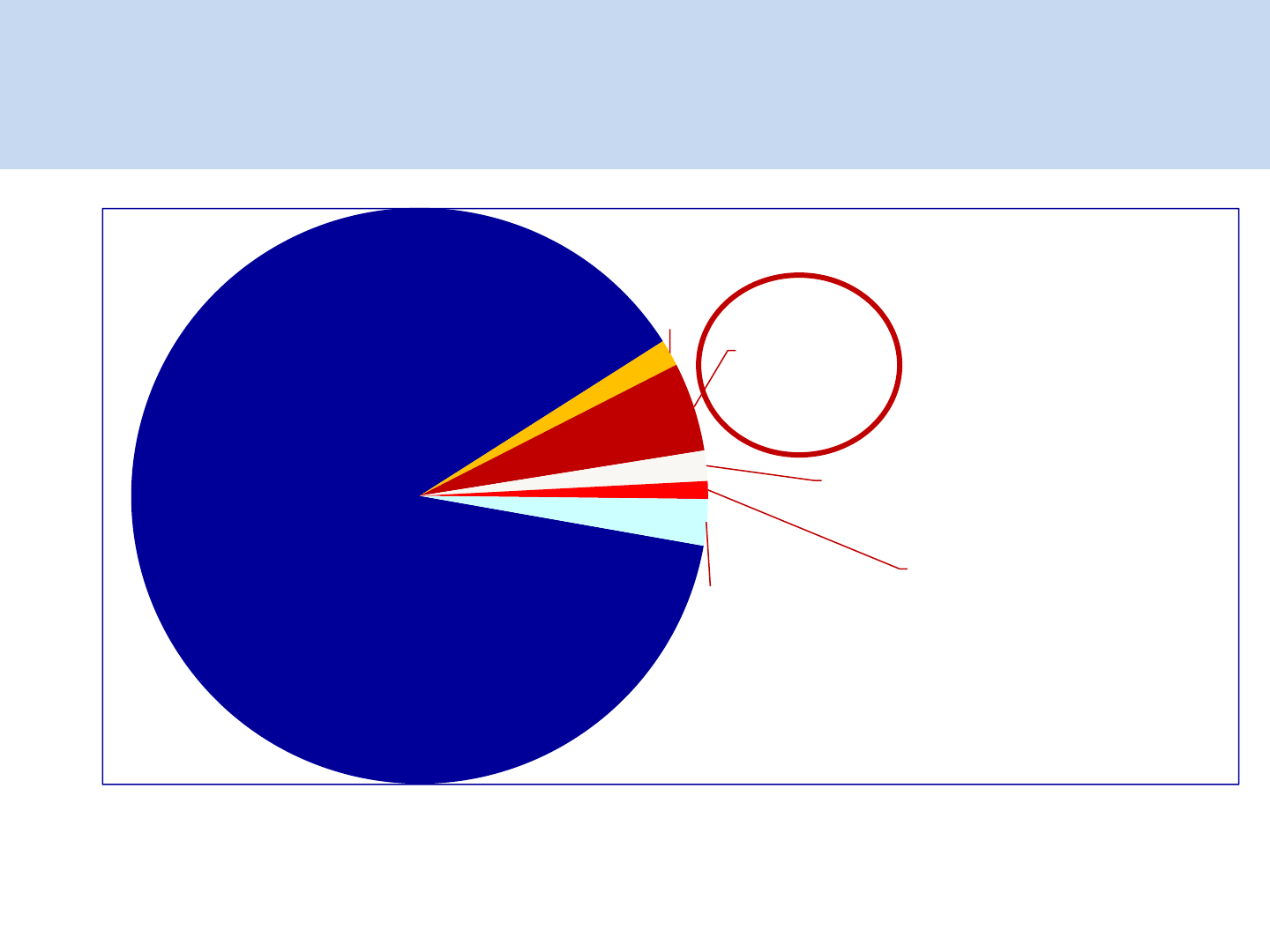
Heterosexual
88.2%
MSM
1.5%
Parent to
Child
5.0%
IDU
1.7%
Blood
Products
1%
Unknown
3.3%
What is the magnitude of problem?
NACO Annual Report 2011-12

Prevention Methods
Sexual Route
– Sexual abstinence
– Being Faithful to sexual partner
– Reducing number of sexual partners
– Correct and consistent use of Condoms
– Less risky sexual practices
Parenteral Route
– Safe blood
– Safe injecting
Parental Route - Mother to Child transmission
- PPTCT

Parent to Child Transmission-PTCT
• Known as vertical or Perinatal Transmission
• In most other countries known as Mother to Child
transmission (MTCT)
• Prevalence of HIV in pregnant women 0.3%
• Vertical transmission rate to fetus is about 30%
• There are proven interventions to reduce likelihood
of PTCT.

PTCT of HIV
Is the main cause of HIV infection in
children
Can occur during
• Pregnancy
• Labour and delivery
• Breast feeding
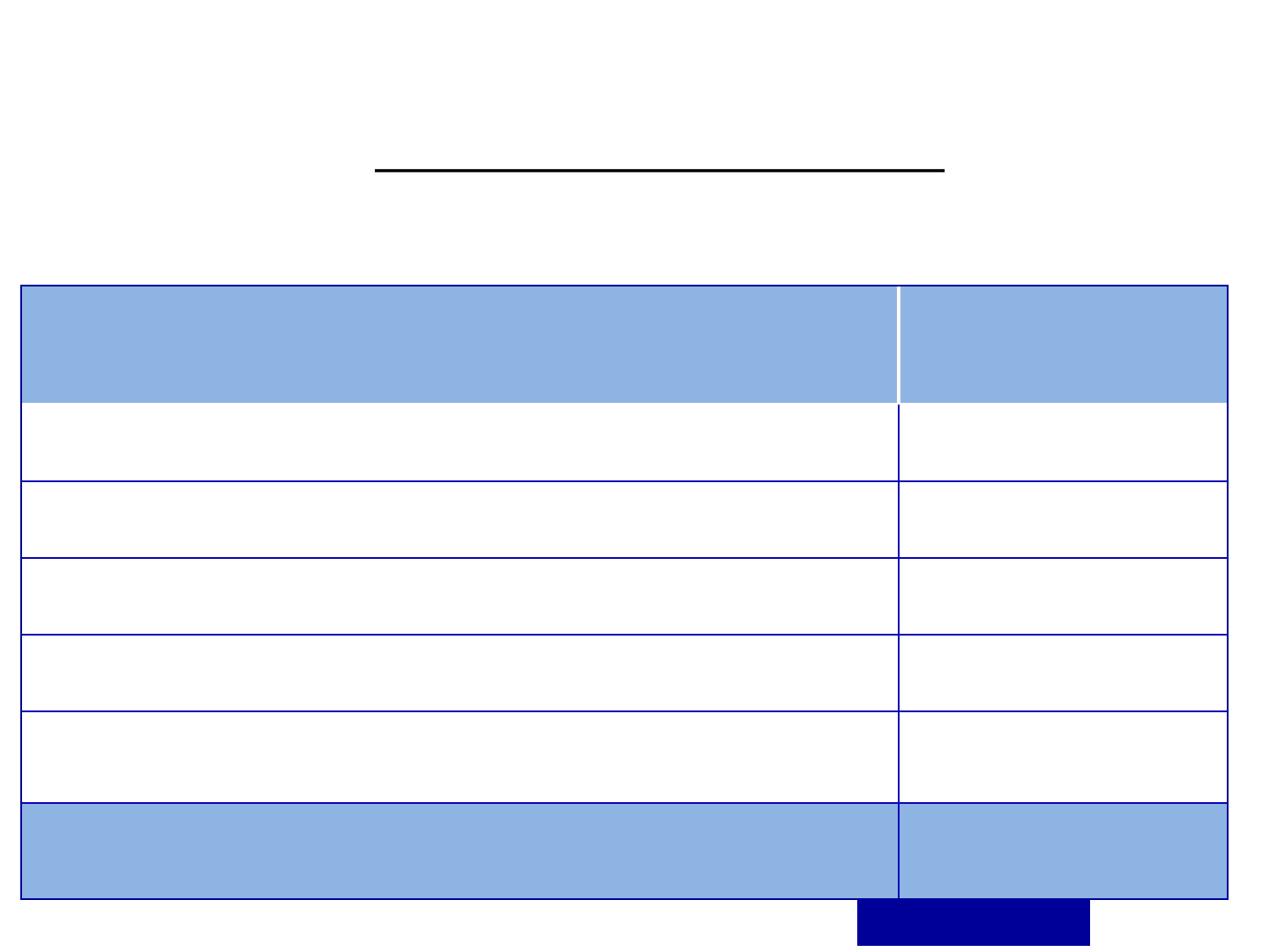
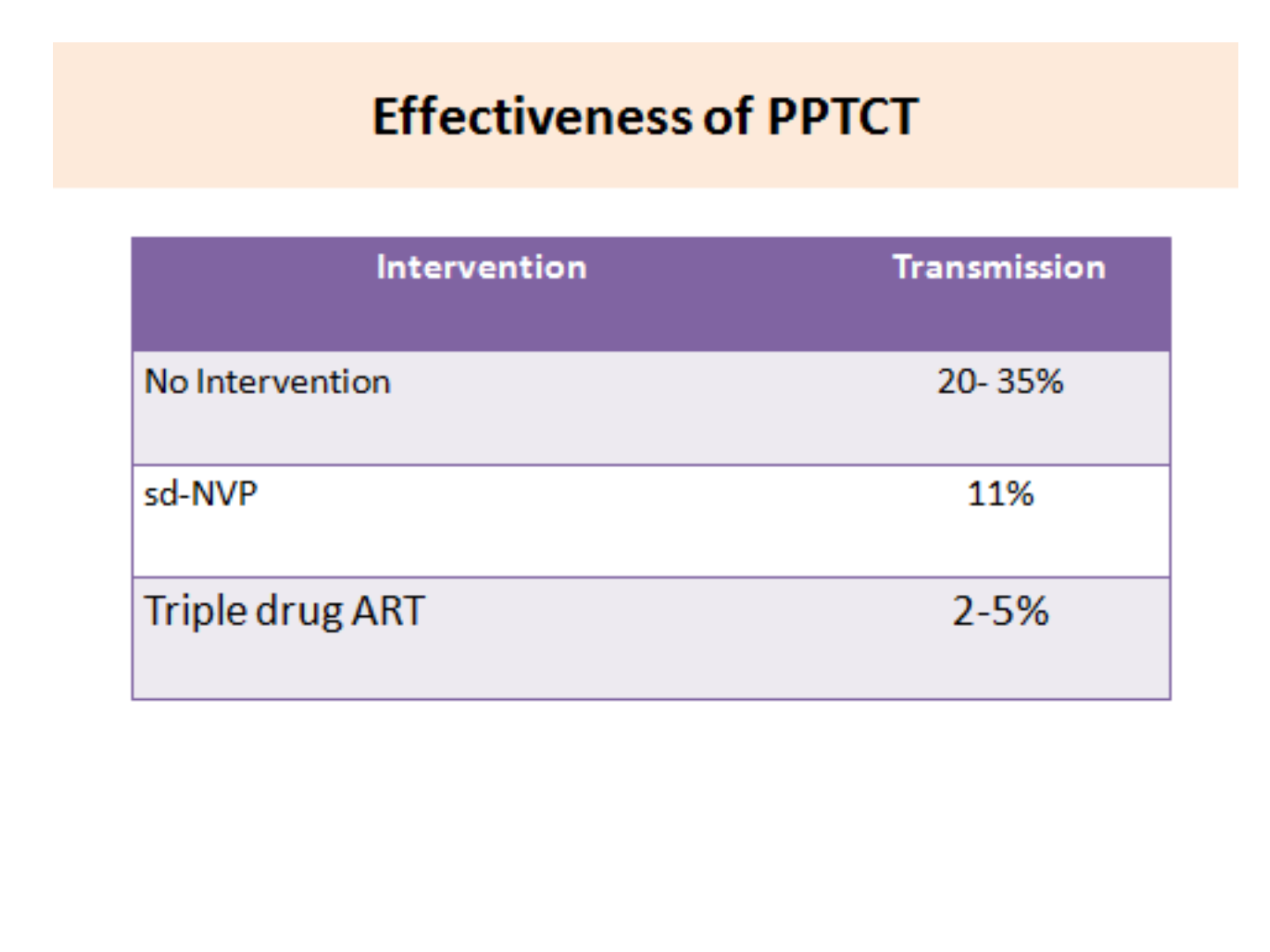
Estimated Risk of Mother to Child transmission
in absence of any intervention
PPTCT Overview
Risk of HIV Transmission
Transmission
Rate
During pregnancy 5-10%
During labour and delivery 10-15%
During breastfeeding 5-20%
Overall without breastfeeding 15-25%
Overall with breastfeeding up-to six months 20-35%
Source: WHO

Maternal risk factors influencing- PTCT
• High viral load (lower the CD4 count , greater the risk
of transmission)
• HIV subtype (HIV – 2 is less pathogenic)
• Advanced clinical stage of HIV disease
• Concurrent STI
• Recent infection
• Viral, bacterial & parasitic (esp malaria) placental
infection
• Malnourishment

Obstetric risk factors influencing- PTCT
• Uterine manipulations (ECV)
• Prolonged rupture of membrane >4 hrs
• Placental disruption (Abruptio, Chorioamnionitis)
• Intrapartum Haemorrhage
• Invasive fetal monitoring, scalp blood sampling
• Invasive delivery techniques (episiotomy,forceps,
metal cup in Vacuum delivery)

Why PPTCT Programme
Parent to Child –Major route for transmission.
If diagnosed / Untreated
• 35% mortality by 1year
• 50% mortality by 2year
• 60% mortality by age 3 year.
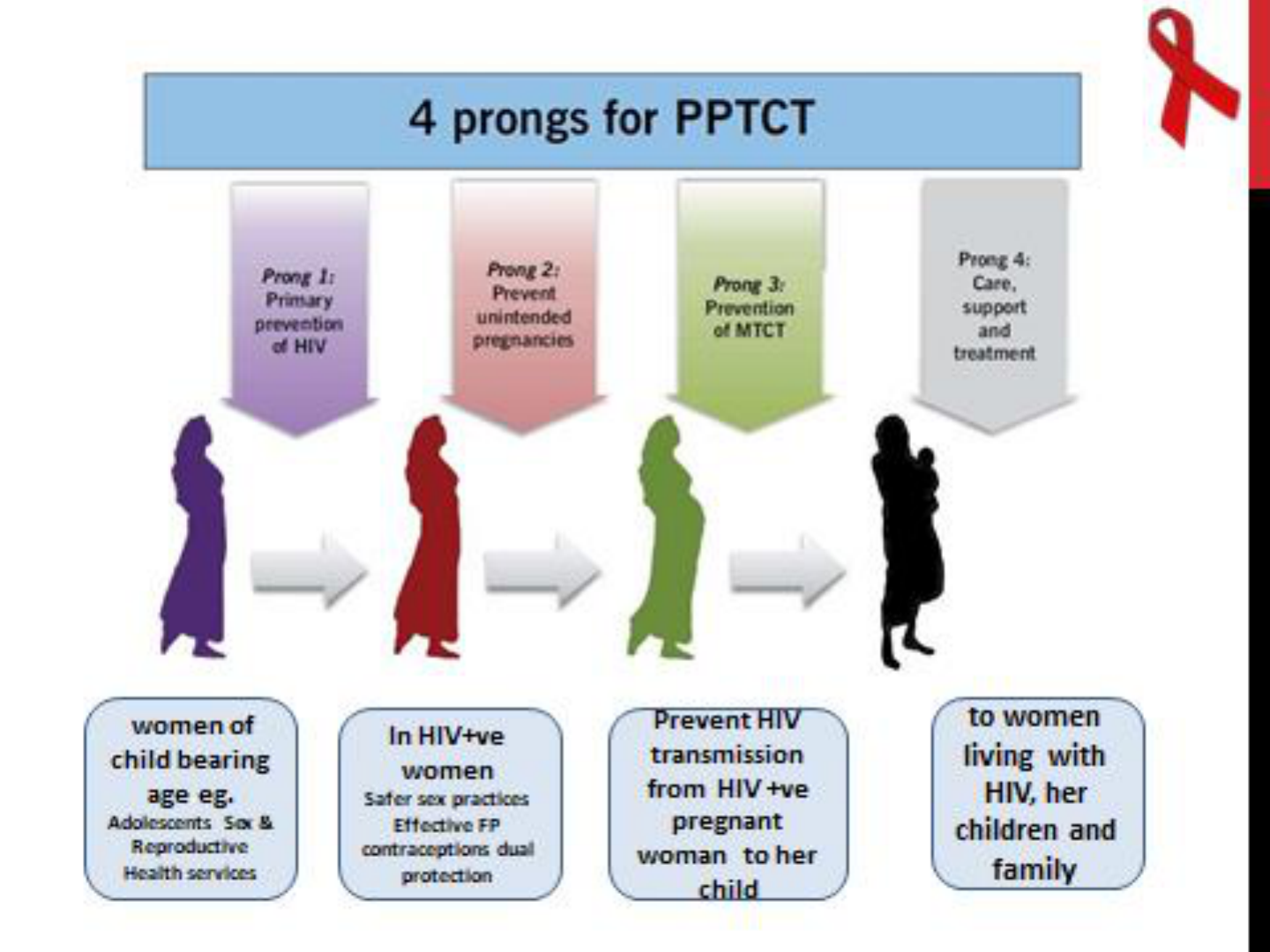

PPTCT Service
Intervention during pregnancy
• Provide HIV information to all pregnant women
attending ANC OPD (Pretest Counseling)
• Voluntary confidential counseling & testing (VCT) to
be given to all pregnant women
• Post test counseling
• Counseling and enable to her to make decision either
to continue or termination of pregnancy
• Availability of safe abortion services & sterilization

HIV positive who wants to continue the
pregnancy
• Ensure Hospital delivery
• Provide similar antenatal care to HIV positive
women as for HIV negative women
• Counseling about infant feeding
• They should have the same number of antenatal
visits and investigations
• Refer to ART center & CD4 count
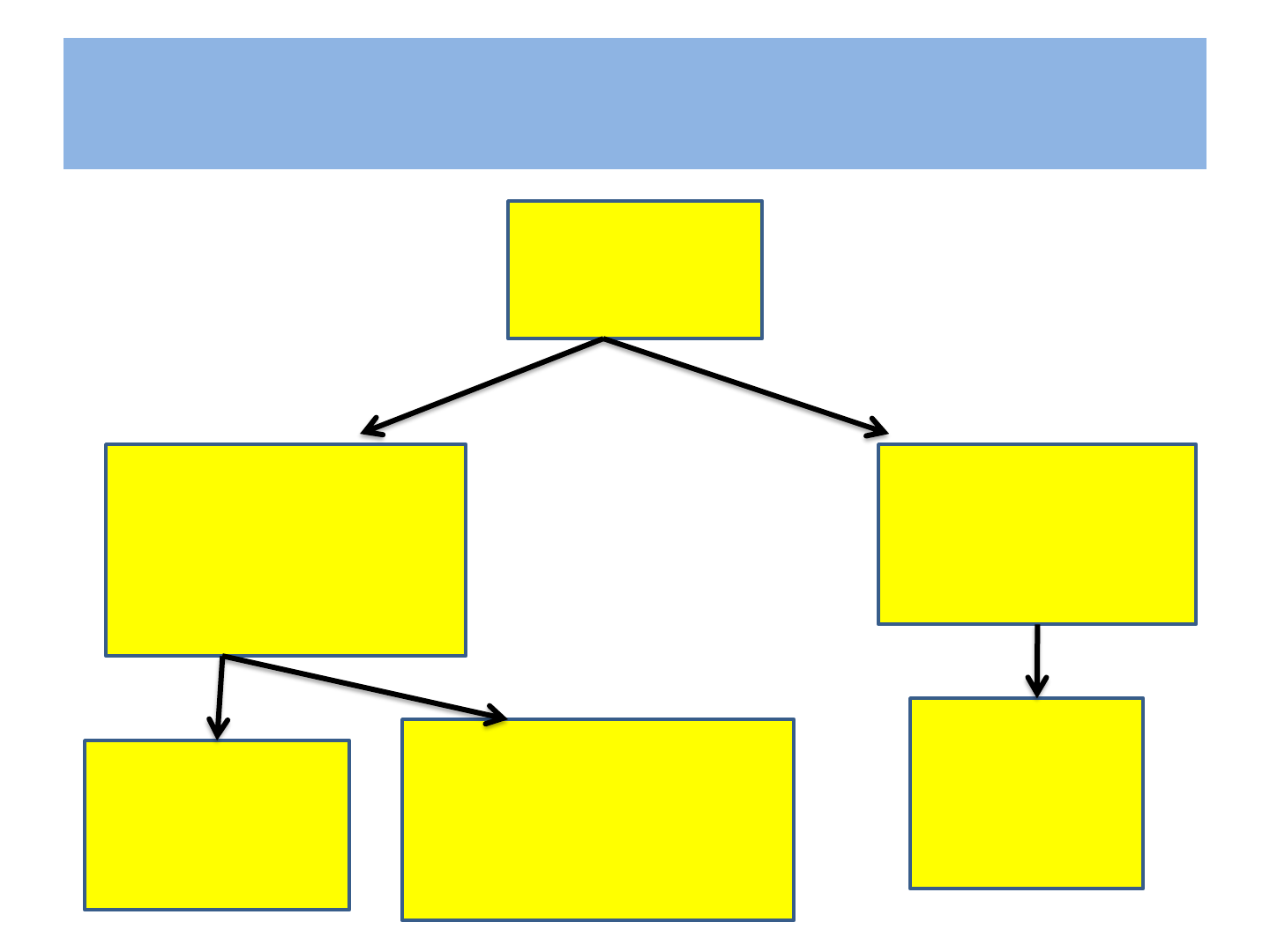
National Guidelines 2010
HIV Infected
pregnant
women
Clinical stage I,II
&CD4 count ≤ 350
cells/mm2 or
stage III &IV
Yes
Initiate ART
No :ARV prophylaxis
Single dose 200mg
of NVP at onset of
labor
Already on Life
long ART
Continue
ART

There have been major changes in
recommendation for ART during pregnancy
by
WHO (2013)
Which has been accepted by NACO (Jan.2014)

National PPTCT Algorithm 2013
HIV infected
pregnant
women
Initiate ART
Already on
Life long ART
Continue ART
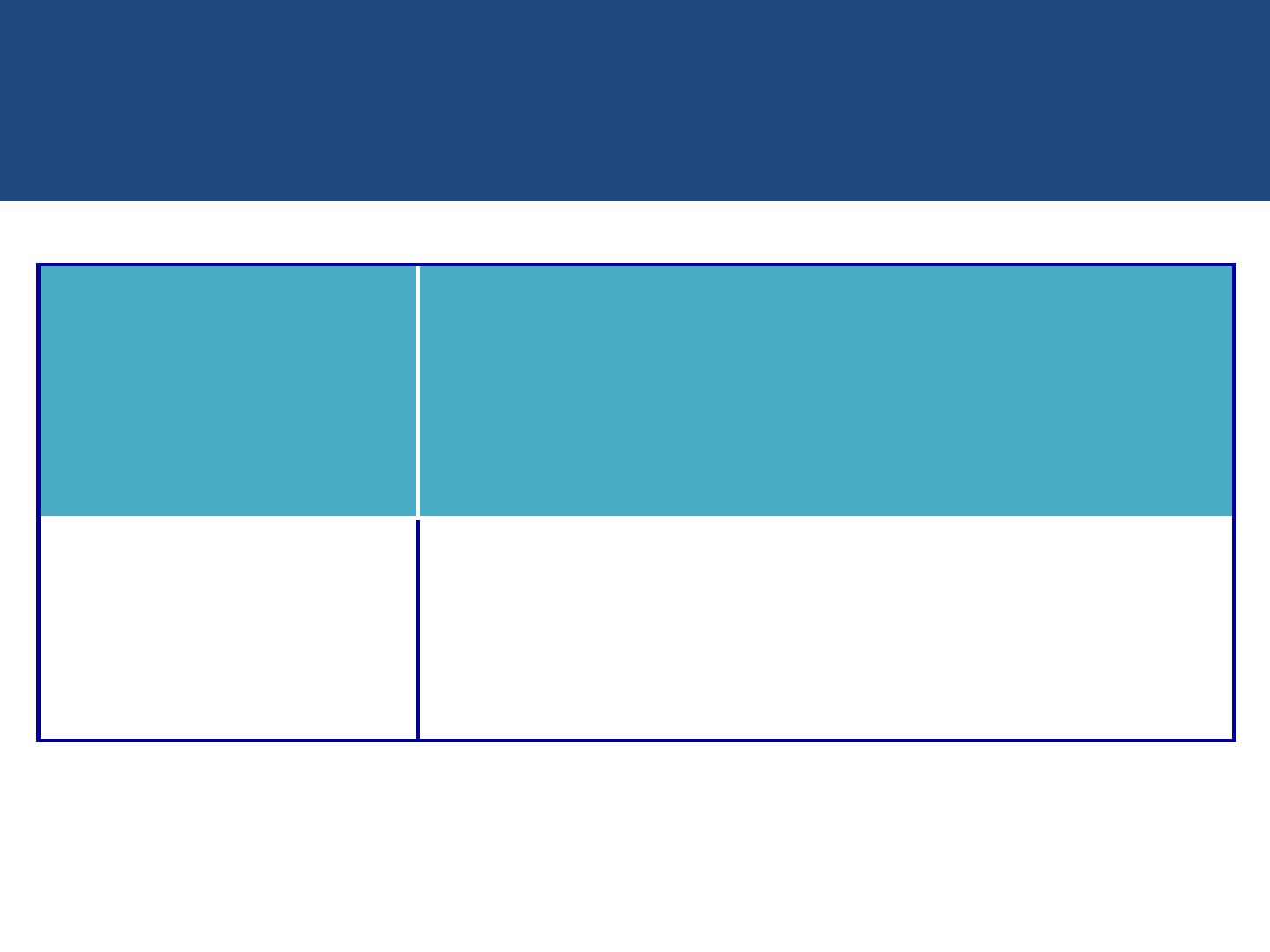
Initiation of ART
in PLHIV with Pregnancy
(India-New PPTCT National guidelines 2013)
WHO
Clinical Staging
CD4 (cells/cu.mm)
I , II, III & IV
(regardless
of clinical
stage)
Start ART irrespective of CD4 Count -
ART lifelong

What is ART?
• Anti-retroviral therapy (ART) is a combination of certain medicines
given to people living with HIV
• This is a combination of at least three drugs from different groups
• It works to control HIV replication in the body and prevent the
destruction of CD
4
Cells
• However, it cannot cure HIV/AIDS
• It is a life long therapy, similar to treatment taken for high BP and
diabetes
• There are certain side-effects, but these are mostly manageable
• ART is given after proper evaluation of patients for eligibility and
counseling for adherence
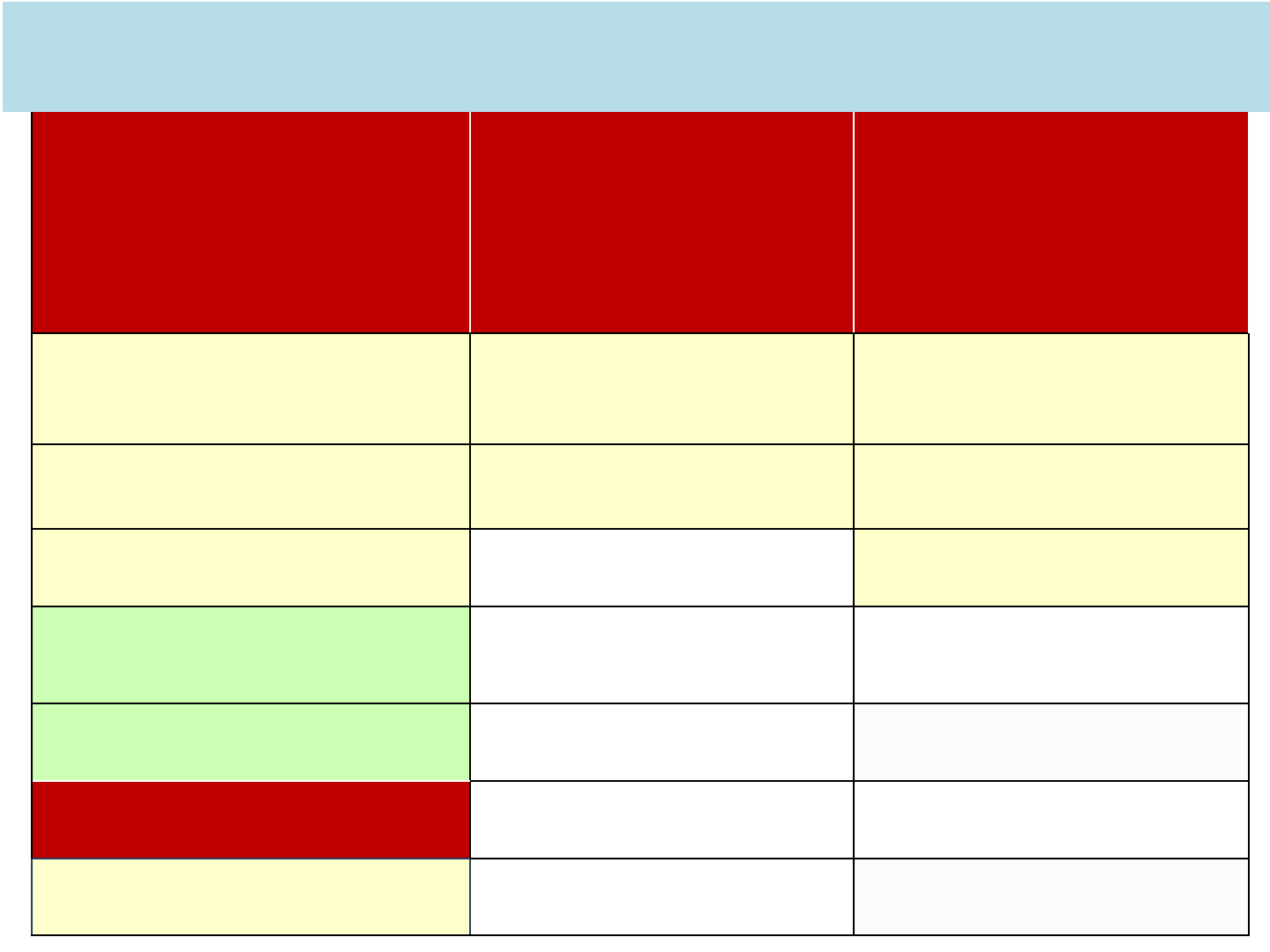
22
Nucleoside Reverse
Transcriptase
Inhibitors
(NsRTI)
NNRTI
Protease Inhibitor
(PI)
Azidothymidine (AZT),
Zidovudine
Nevirapine (NVP)* Lopinavir (LPV)
Stavudine (d4t) Efavirenz (EFV) Ritonavir (RTV)
Lamivudine (3TC) Atazanavir (ATZ)
Didanosine (ddI)
Abacavir (ABC)
NtRTI:
Tenofovir (TDF)
ARV Drugs under National programme
Guidelines for Antiretroviral Therapy
HAART: Highly Active Antiretroviral Therapy
Human Immunodeficiency virus (HIV) infection is currently treated with
combination therapy using at least three drugs from NRTI & NNRTI/PIs
over an indefinite period.
Possible combinations
1. 2 NRTI's + 1 NNRTI
2. 2 NRTI's + 1 PI
3. 2 NRTI's + 1 More NRTI
No MONOTHERAPY or DUAL THERAPY

PPTCT Scenarios
• Pregnant women newly - initiating ART
• Pregnant women already receiving ART
• ART regimen for pregnant women having prior
exposure to NNRTI for PPTCT
• Women presenting Directly-in-labour

Pregnant women newly initiating ART
• Start ART as soon as possible after proper preparedness
counseling and continue ART throughout pregnancy, delivery,
and thereafter life long
• Even if the pregnant women present very late in pregnancy
(including those who present after 36 weeks of gestation) the
ART should be initiated promptly
• This ART shall be initiated at ART centers only, hence all efforts
need to be made to ensure that pregnant women reach ART
centers
All pregnant women at ART centre shall be seen on priority

Pregnant women already receiving ART
• Pregnant women who are already receiving a NVP-based
ART regimen should continue receiving the ART regimen
• Pregnant women who are already receiving EFV-based ART
regimens:
– Should be continued. DO NOT STOP
– There is no indication for abortion/termination of pregnancy in
women exposed to EFV in the first trimester of pregnancy.
ART regimen for pregnant women having prior
exposure to NNRTI for PPTCT
• A small number of HIV-positive pregnant women have had
previous exposure to Single Dose NVP for PPTCT prophylaxis in
prior pregnancies
• Because of the risk of resistance to NNRTI drugs in this
population, an NNRTI-based ART regimen such as TDF/3TC/EFV
may not be effective
• Thus, these women will require a protease inhibitor-based ART
regimen.
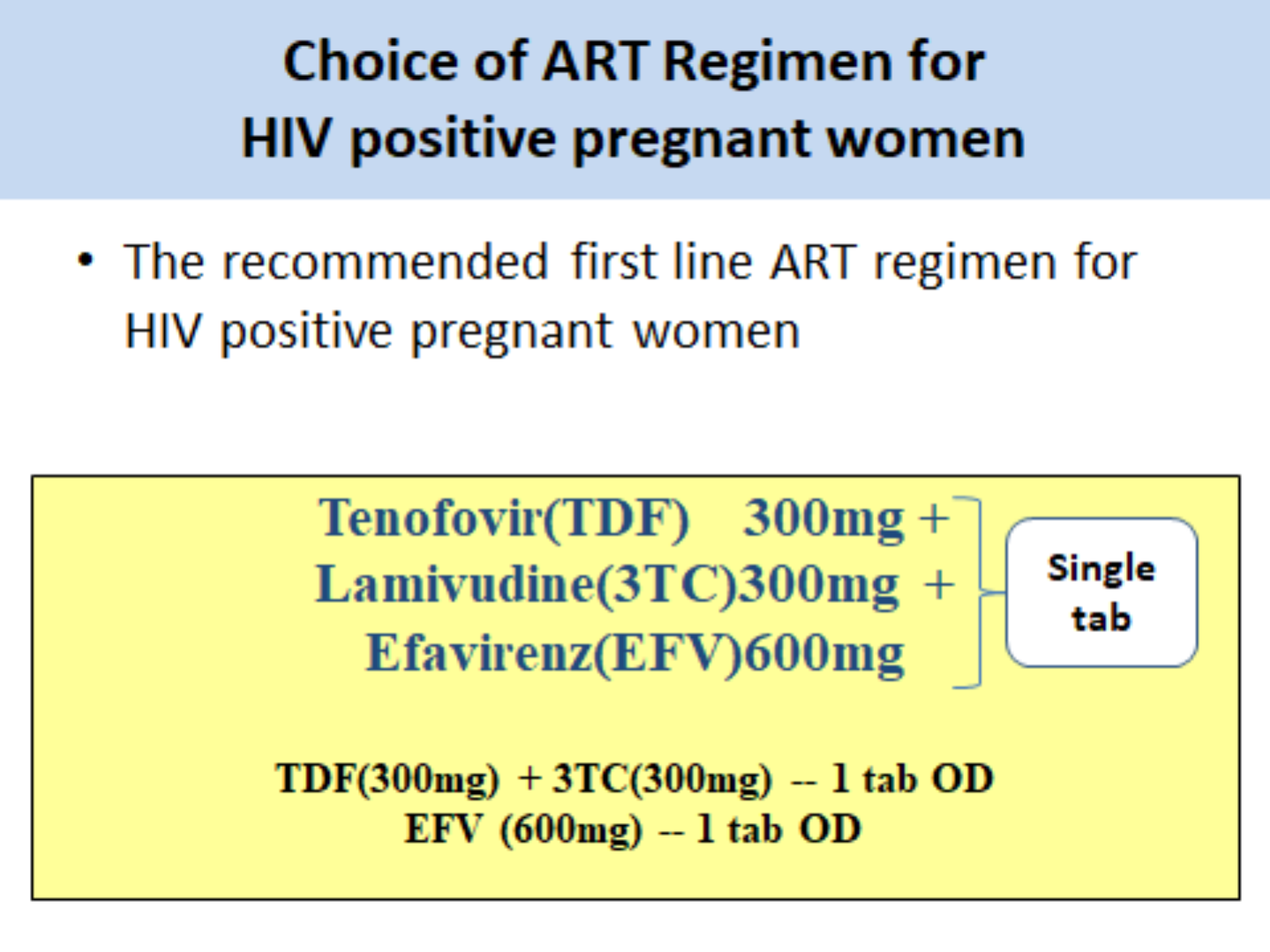
Tenofovir(TDF) 300mg +Lamivudine (3TC) 300mg -- 1 tab OD
LPV(Lopinavir) (400mg)/r(Ritonavir) (100mg)----------1 tab BD

Women presenting Directly-in-labour
NEW PPTCT Prophylactic Regimen
• Women on life long ART should continue to receive
ART as per the usual schedule including during labour
and delivery. Women do not require any other
additional ARV dosing
• HIV status – not known-Whole blood finger prick test-
if negative no further test. If positive give 3drugs ART &
ref. to PPTCT Centre for rapid test
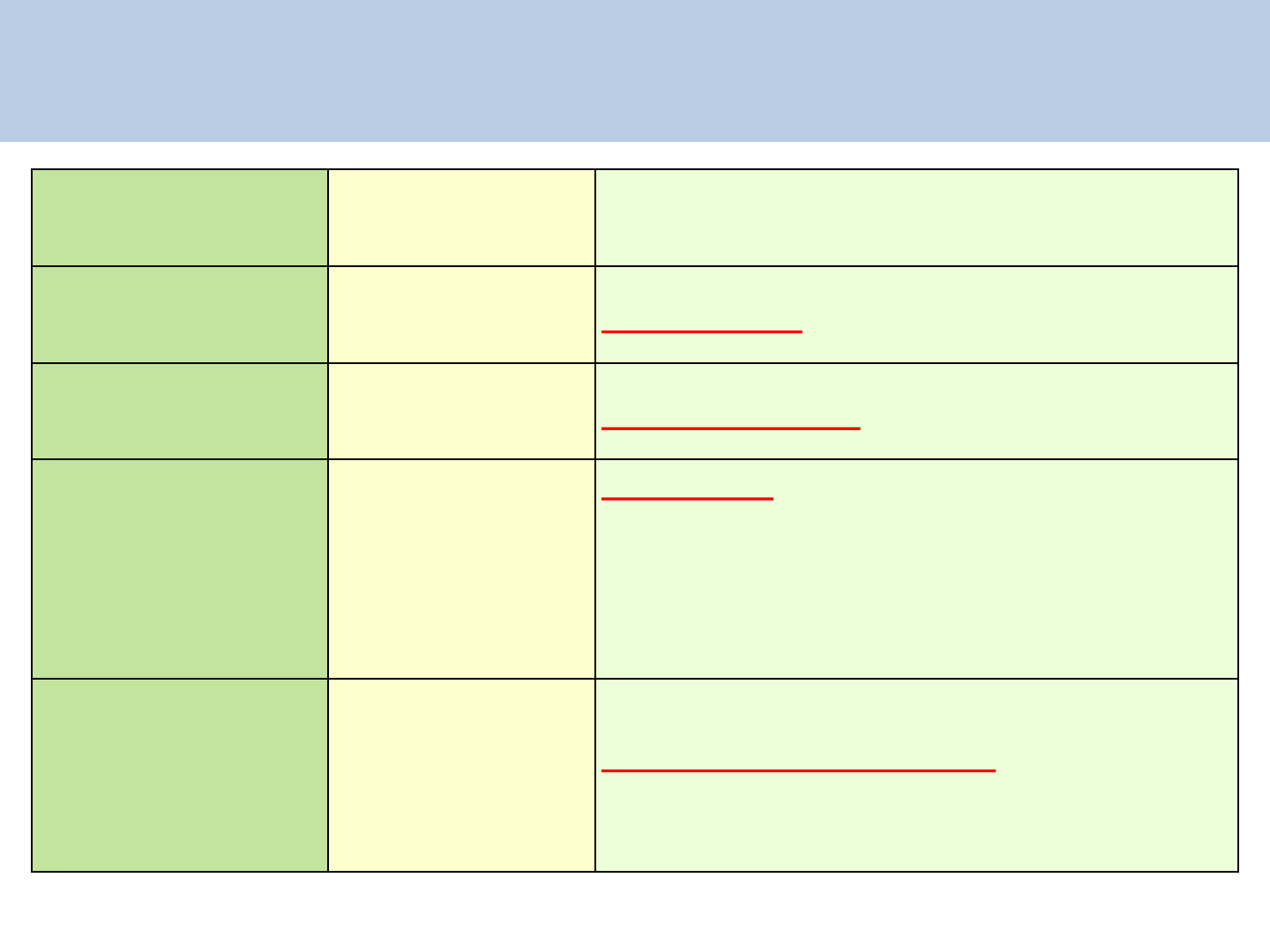
Dosage schedule and side effects with ARV drugs
Name of ARV Dose Schedule Side-effects
Tenofovir (TDF)
NRTI
300mg OD Nephrotoxicity, hypophosphetemia
Lamivudine (3TC)
NRTI
300mg OD Rarely pancreatitis
Efavirenz (EFV)
NNRTI
600 mg HS
CNS toxicity: Vivid dreams, nightmare,
insomnia, dizziness, headache, impaired
concentration, depression, hallucination,
exacerbation of psychiatric disorders (usually
subsides by 2-6 weeks)
Lopinavir/Ritonavir
(LPV/r) PI
400/100 mg BD
Gastro intestinal disturbance, glucose
intolerance, Lipo dystrophy, dyslipdemia

Cotrimaxazole prophylactic therapy (CPT)
for Pregnant Women
• Cotrimoxazole should be started if CD4 count is < 250 cells/mm3
and continued through pregnancy, delivery and breast-feeding as
per national guidelines
• Ensure that pregnant women take their folate supplements
regularly
• Cotrimoxazole prophylaxis therapy (CPT) prevents Opportunistic
Infections (OIs) such as
Pneumocystis pneumonia ,
toxoplasmosis, diarrhea
bacterial infections

Considerations
Regarding Mode of Delivery
• Caesarean section can decrease the risk of vertical
transmission when performed before the onset of labour
and rupture of the membrane
• In India Recommendation by WHO & NACO (2013),
cesarean delivery is to be done only for obstetric
indications, otherwise vaginal delivery can be allowed
• Use of ART can reduce risk of PTCT better and with less
risk than a C-section

Interventions during Labor & Delivery for
reducing MTCT
• Safe delivery practices / Universal Precaution
• Vaginal cleaning with 0.25% chlorhexidine
• Minimize cervical examination
• Always use clean technique
• Avoid
– Routine rupture of membrane
– Prolonged labour
– Instrumental deliveries if instrumental delivery is necessary then
forceps are better than vacuum
– Episiotomy, unnecessary trauma during child birth
• Active management of third stage.

Interventions for Newborn
• Don’t milk the cord
• Cut cord under cover of light gauge
• Do not use suction unless absolutely necessary
• Handle infant with gloves
• Clean inj site with spirit before any inj
• Determine mother’s feeding choice before attaching
the baby to breast

Intervention for safe feeding practices
• Infant feeding as per mother’s choice
• Replacement feeding only when it is acceptable,
affordable, feasible sustainable & safe
• According to 2013 National Guidelines
exclusive breast feeding for 6 months, continue feeding
till 12 months if possible.
• Infectious diseases and malnutrition are the primary
causes of infant deaths in developing countries.
• Avoiding addition of supplements or mixed feeding which
enhances HIV transmission

ARV Prophylaxis for infants born to
HIV +VE Women
• All infants born to HIV +VE pregnant women will receive
Nevirapine syrup once daily for 6 weeks- irrespective of
infant feeding option,
• if ART to mother was started in late pregnancy, during or after
delivery and has not been on adequate period of ART as to be
effective to achieve optimal viral suppression (which is at least
24 weeks), and opt for breast feeding then the infant NVP
should be increased to 12 weeks. ( Extended NVP Regimen )
• This recommendation not applies to those on exclusive
replacement feeding
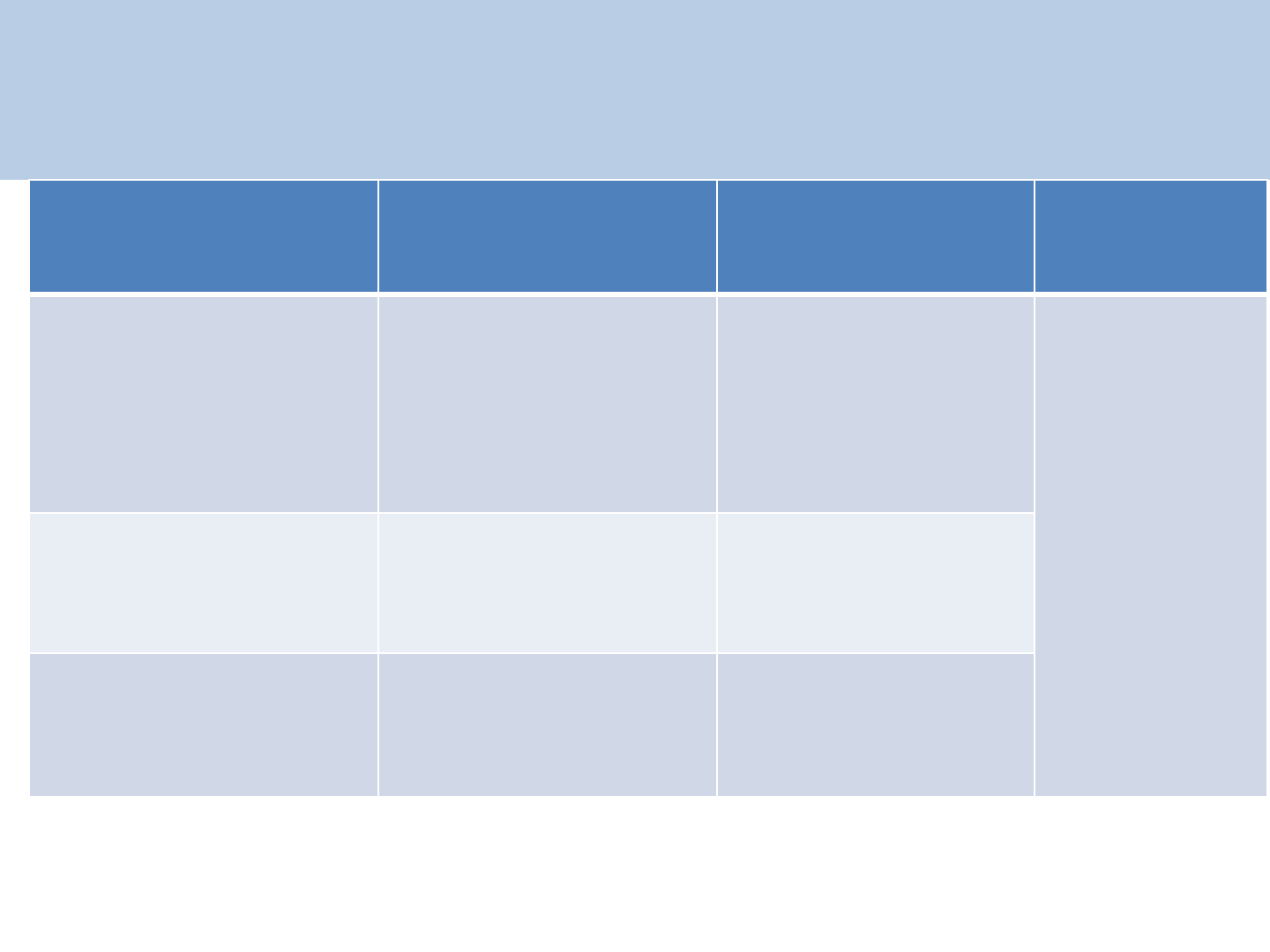
Dose & duration of NVP prophylaxis for HIV
Exposed Infant
(1ml of NVP suspension =10mg NVP)
37
Infants Birth Weight
(gm)
NVP daily dose (mg) NVP daily dose (ml) Duration
Birth weight less than
2000 gm 2 mg /kg. once daily
0.2 ml /kg. once
daily
Up to 6
weeks*
irrespective
of exclusive
breast
feeding or
exclusive
replacement
feeding
Birth weight between
2000 – 2500 gm
10 mg. once daily 1 ml once a day
Birth weight more
than 2500 gm
15 mg. once daily 1.5 ml once a day
*Extended NVP duration up to 12weeks to infant of exclusive breast feeding , mother
has been < 24 weeks duration of ART
ARV prophylaxis for Infant of HIV +VE pregnant
women -Received Single Dose NVP for PPTCT
prophylaxis in prev. pregnancies
Zidovudine (2.5ml) 25mg/day
• Up to 6 weeks irrespective of exclusive breast
feeding or exclusive replacement feeding
• Up to 12 weeks if mother has received ART for <24
weeks duration and infant on breast feeding .

Mother and Infant follow up schedule
• Routine follow up for mother and infant
• Follow up at 6 wks, assess and record status of mother &
infant.
• All HIV exposed infants should receive cotrimaxazole
prophylactic therapy (CPT)
• Follow standard immunization schedule
• DBS- Direct Blood Spot for HIV-1 DNA PCR of child
between 6weeks & < 6 month of age
• Follow up at 6 months HIV test & CPT
• Follow up at 12months HIV test & CPT
• Follow up at 18 months HIV test & CPT
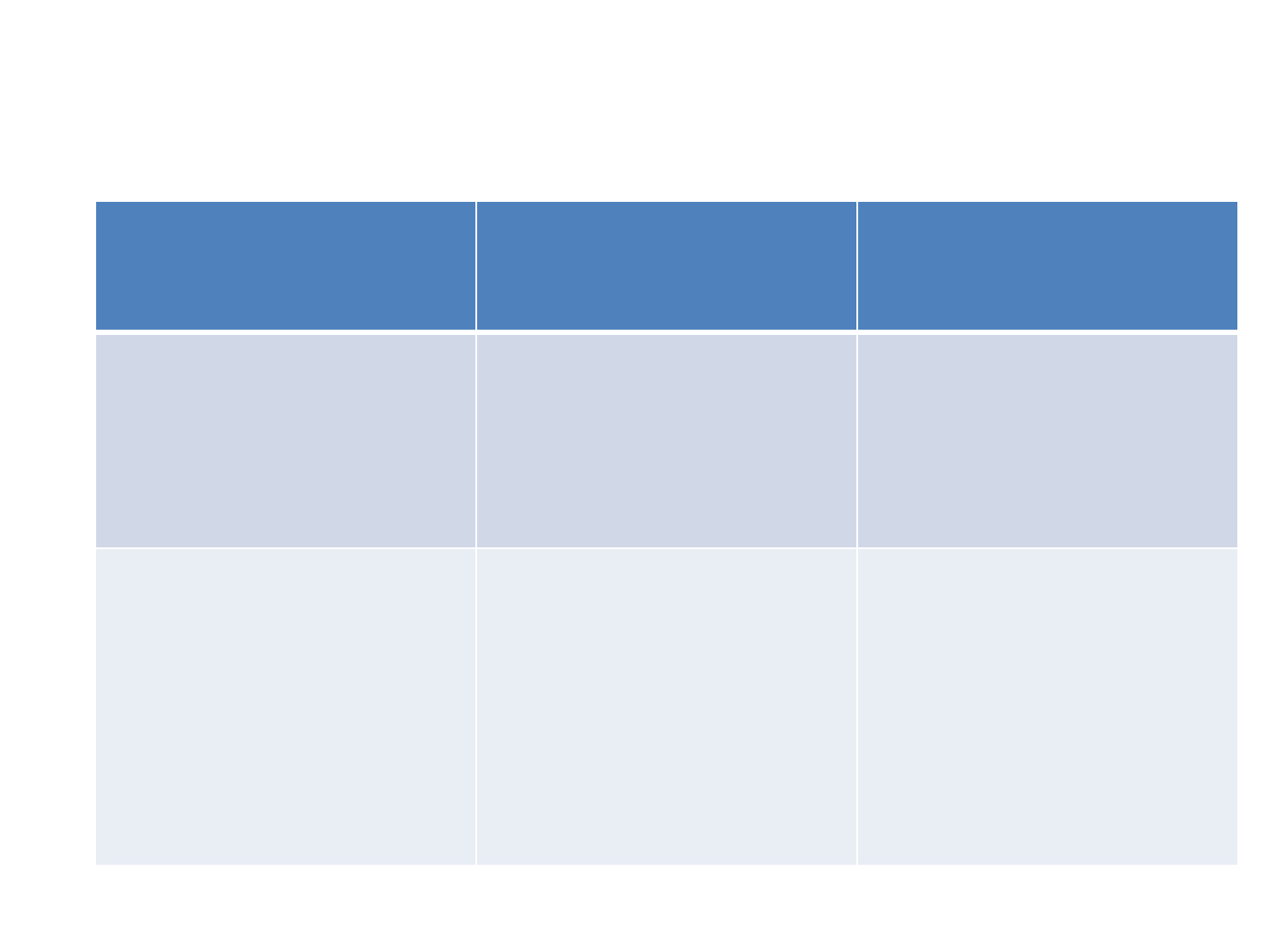
CPT Starting & Stopping criteria for HIV
exposed infants and children
Group Start
Cotrimoxazole
Stop
Cotrimoxazole
All HIV exposed
infants &
children
<18 months
Any time from 6
weeks
of age
at 18 months of age
When HIV infection
has been excluded
Infants &
children
<18 months
diagnosed
HIV+ve
by
HIV DNA PCR / HIV
rapid test at 18
months of age
As above, and
any time afterwards
if not already on
Cotrimoxazole
Continue CPT
till 5
yrs of age
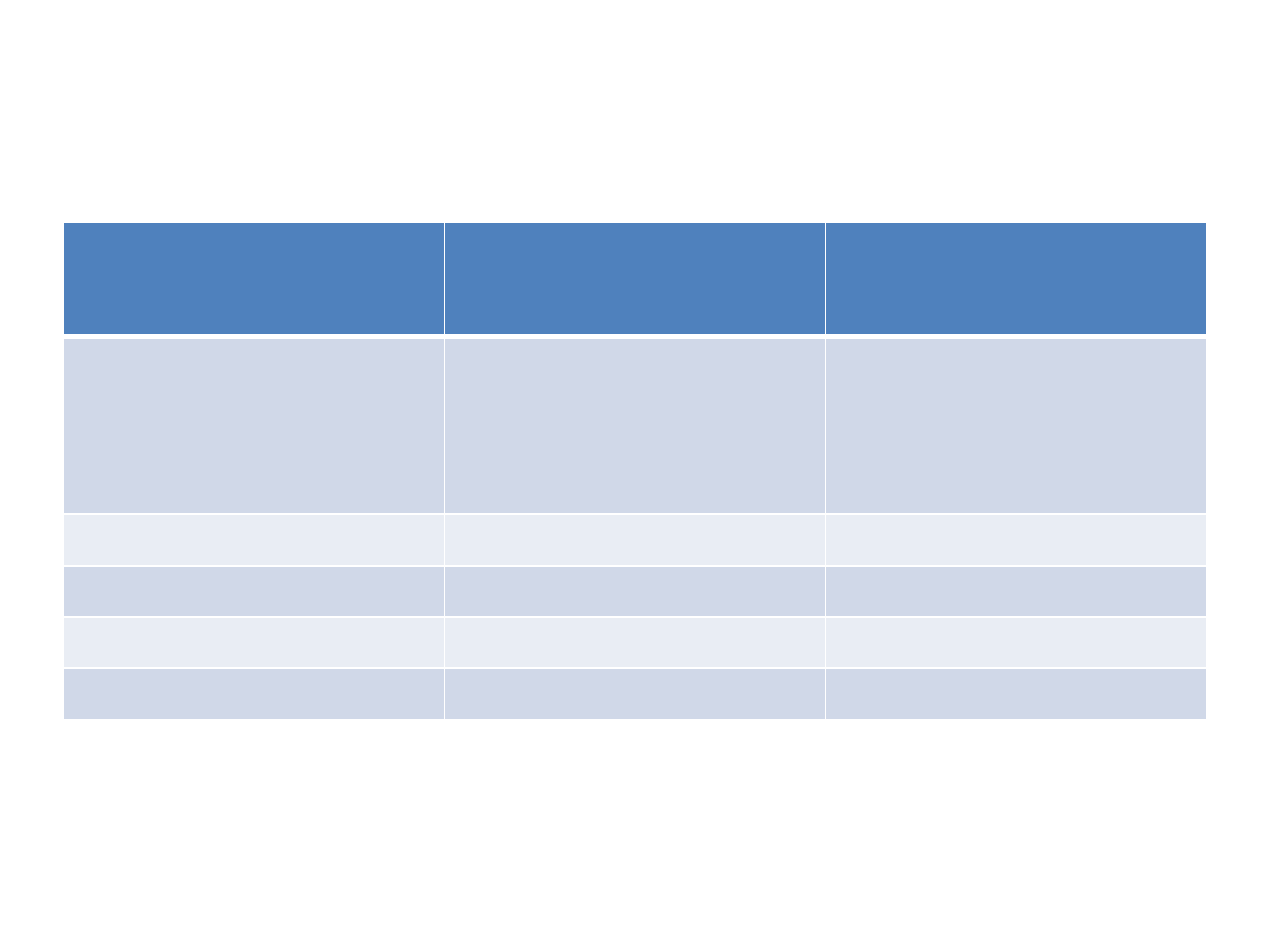
CPT Dosage
Cotrimoxazole
once a day
Weight ( kg) Syrup 5ml
(40mg
trimethoprim &
200mg
sulphamethoxazole
)
1 tablet
(20mg of trimethoprim &
100mg
sulphamethoxazole
)
<5 2.5ml 1 tablet
5-10 5ml 2 tablets
10-15 7.5ml 3 tablets
15-22 10ml 4 tablets

Evidence based newer initiatives
• Initiation of ART
• Exclusive Breast feeding
• Whole blood finger prick test- if negative no further test. If
positive give ART & ref. to PPTCT centre for rapid test
• CPT-Cotrimoxazole prophylactic therapy
• Extended PPTCT regimen
• DBS- Direct Blood Spot for HIV-1 DNA PCR of child between
6weeks & < 6 month of age confirmation by whole blood DNA
PCR
• HIV testing of HIV exposed baby at 6 , 12, and 18 months
Contraception for HIV +ve women
• HIV +ve women are strongly advised to use
dual contrception
• Barrier method to reduce the risk of sexual
transmission of the virus to the partner
In combination with
• Combined oral pills, progestogen only pill and DMPA
can be given in HIV and AIDS (category 1 WHO)
• IUCD is category 2 for HIV infected and
category 3 for AIDS

An HIV Free Generation
THANK YOU
